Abstract
The Hiraoka transmission electron microscopy (TEM) grid staining apparatus, which allowed multiple grids to be batch stained together on a plate, is no longer commercially available. Because the need for such a device still exists, we developed a modified Hiraoka TEM grid staining apparatus by combining a Leica AC20 grid plate holder with a modified 3D printed grid loading holder and trays lined with ParafilmR. This apparatus was then used to test a post-section contrasting method that uses gadolinium triacetate tetrahydrate solution, a non-radioactive uranyl acetate substitute, which produces similar staining results to uranyl acetate when used with lead stain in mouse ocular tissue for TEM.
Keywords: 3D printing, Gadolinium triacetate tetrahydrate, Hiraoka, Mouse ocular ultrastructure, Transmission electron microscopy grid staining, Uranyl acetate replacement
Introduction
Conventional TEM grid batch staining requires methods that both preserve delicate mounted tissue sections in an organized arrangement and allow for the delivery of heavy metal stains for electron contrasting. There are numerous apparatus and techniques for mass staining TEM grids [1–4] that, in comparison to single drop staining, make staining more efficient and preserve thin sections on coated and uncoated grids with less uranyl acetate and lead stain artifact precipitation. For single drop staining, each grid containing ultrathin sections is placed on top of a drop of stain and transferred to other water rinses and solutions with forceps. The Hiraoka batch staining method increases efficiency, reduces stain artifact precipitate and preserves mounted thin sections on either coated grids compared to the single drop staining method [1]. Batch processing TEM grids with the Hiraoka TEM grid staining apparatus (HSA) for staining and autoradiography was published in 1972 [1] and made commercially available until 2014. Here, we have modified and validated a relatively inexpensive substitute for the HSA that we fabricated using 3D software and thermoplastic printing technology [5] to meet our TEM batch staining needs.
Non-radioactive chemical alternatives have been developed for TEM section contrast staining as an alternative to hazardous, low level radioactive uranyl acetate. Japan has restricted the usage of uranium based salts e.g., uranyl acetate even for scientific research [6]. Uranyl acetate registers up to 0.51 mCu (19 kBq) of radioactivity and is highly toxic requiring special handling, solution preparation, and hazardous radioactive waste disposal. A commercial non-radioactive ‘uranyl acetate replacement’ (UAR) containing heavy metal gadolinium triacetate tetrahydrate was tested. This UAR stain solution was applied onto mouse ocular tissues using post-section TEM grid double staining with a lead stain solution in our 3D printed modified HSA.
Materials and Methods
Tissue preparation
Mouse eye samples (set A, n=2) were fixed by intracardiac perfusion with 0.1M phosphate buffered 4% paraformaldehyde then half strength Karnovsky’s fixative (2% formaldehyde + 2.5% glutaraldehyde, in 0.1 M sodium cacodylate buffer, pH 7.4) according to Greenwald and co-worker [7] at 37 oC then eyes were enucleated and immersion fixed at room temperature. Another set of mouse eyes samples (set B, n=3) were directly immersion fixed in half strength Karnovsky’s fixative at room temperature. For subsequent processing on all sample sets (A and B), the anterior was removed from each eye, and resulting posterior eyecups rinsed with 0.1M sodium cacodylate buffer then post-fixed with 2% osmium tetroxide in 0.1M sodium cacodylate buffer for 1.5 hours. Sample B eyecups were en bloc stained with 2% aqueous uranyl acetate solution (Uac, Electron Microscopy Sciences, Cat. #22400, Hatfield, PA, USA) for 30 minutes. All sample sets (A and B) were then dehydrated with graded ethyl alcohol solutions, transitioned with propylene oxide, and infiltrated in tEPON-812 epoxy resin (Tousimis, Rockville, MD, USA) utilizing an automated EMS Lynx 2 EM tissue processor (Electron Microscopy Sciences, Hatfield, PA, USA.) The processed tissues were oriented into tEPON-812 epoxy resin inside flat molds and polymerized in a 60 °C oven. Semi-thin retinal cross-sections were cut at 1 μm and stained with 1% toluidine blue in 1% sodium tetraborate aqueous solution for assessment by light microscopy [8]. Ultrathin sections (80 nm) were cut from each block using a Leica EM UC7 ultramicrotome (Leica Microsystems, Buffalo Grove, IL, USA) and a diamond knife, then collected using a loop tool onto either 2 mm x 1 mm, single slot formvar-carbon coated or 200 mesh uncoated copper grids and air-dried.
3D Printing
The original Hiraoka plate (43 mm x 44 mm) is a flexible transparent plastic material with slits holding up to 40 TEM grids [1]. Over time the original plate wears out, become discolored, and its slits lose their ability to hold grids securely. A suitable substitute is the Leica EM AC20 grid support plate (38×40mm, cat. #16707299) for the EM AC20 automated TEM grid contrasting system (Leica Microsystems, Buffalo Grove, IL, USA) comprised of a flexible silicone material that holds a maximum of 20 TEM grids in a vertical upright position. For most TEM grid staining runs in our laboratory, a grid plate holder facilitating <20 grids for a staining run is sufficient. The design of the 3D printed modified HSA was made to fit on the Leica EM AC20 grid support plate. Online software available free from Tinkercad (http://www.tinkercad.com) was used to generate the 3D file design (.stl format) files based on measurements and scaling from the original HSA and the Leica grid support plate. These 3D files are available for free from the National Institutes of Health (NIH) 3D Print Exchange [9]. Figures 1 and 2 illustrate the 3D printed modified HSA along with the grid loading into slits of the plate and staining technique. The four pieces of the 3D printed modified HSA are:
Figure 1.
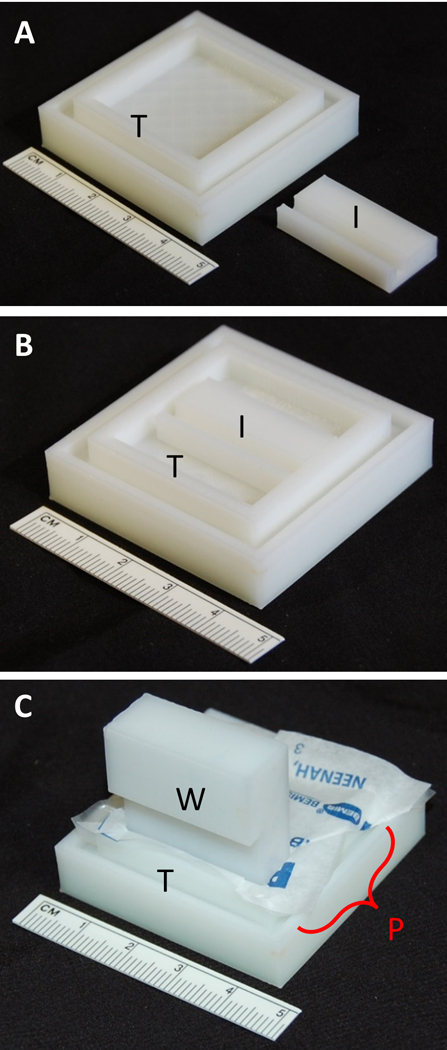
A) 3D-printed Hiraoka staining tray (T) and staining tray space insert (I). B) Space insert positioned in well of tray. C) ParafilmR well mold (W) positioned vertically to line ParafilmR into the 3D printed tray (T) well with the space insert in place for minimizing stain solution volume.
Figure 2.
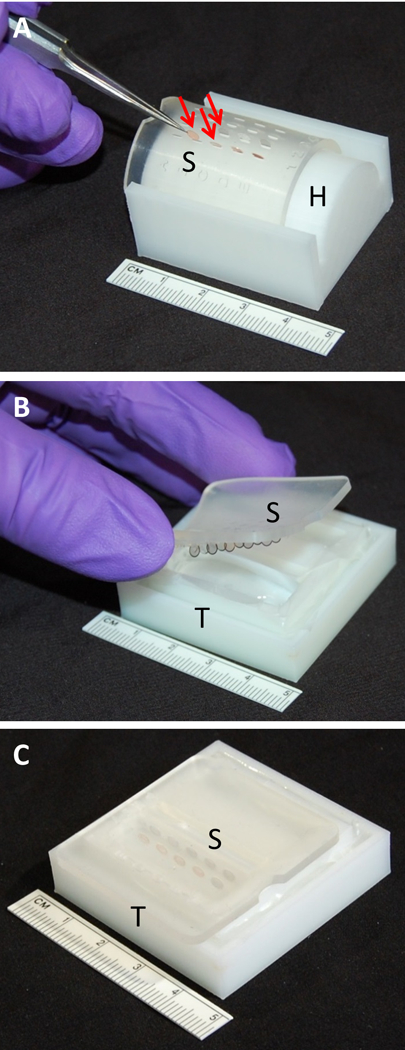
A) Leica EM AC20 grid support plate (S) stretched out to open slits and clipped into modified Hiraoka plate holder (H) with TEM grids (arrows) loaded using fine tipped forceps. B) Grid support plate (S) containing TEM grids placed into staining solution within tray (T) at an angle to avoid bubble formation. C) TEM grids in support plate (S) submerged in stain filled in tray (T.)
Modified Hiraoka staining tray: Design modeled after the tray from the original Hiraoka staining tray that holds a total of 5 ml liquid (Figures 1 & 2). The 3D printed tray fits the original Hiraoka grid plate holder. Modified Hiraoka plate holder: Dimensions of arc and edges were modified to retain the Leica EM AC20 grid support plate that allows loading grids into slits on the plate in a flexed position (Figure 2A.) ParafilmR well mold: Assists in molding ParafilmR sheets into the 3D printed Hiraoka staining tray wells (Figure 1C). This well mold fits in either 3D printed and original Hiraoka trays. Staining tray space insert: Designed to decrease the modified Hiraoka staining tray volume to ~1 to 2 ml by sliding the insert into the tray and then lining the well surface with ParafilmR within the tray. The insert minimizes staining solution volume in the Modified Hiraoka staining tray for a batch run of <10 grids (Figures 1A & 1B.)
A local 3D printing service provider was selected though 3Dhubs (http://www.3Dhubs.com) using a Zortrax M200 3D printer (Zortrax Inc., Olsztyn, Poland) at a 75 μm layer resolution to produce the HSA with acrylonitrile butadiene styrene filament (ABS, Zortrax catalog# z-ABS, 1.75 mm diameter.) All apparatus pieces were 3D printed with polylactic acid filament (PLA, Flashforge catalog#B00W41D9N8, 1.75 mm diameter) using a Flashforge Finder printer (Flashforge, Rowland Heights, CA, USA) at a 100 μm layer resolution. The ABS material was selected for its relative low-cost, durability, water-resistance, and allowance for lining the trays with ParafilmR that can be used to prevent contamination and reduce the need for extensive cleaning after every staining run.
Staining Procedure
UAR working solution containing gadolinium triacetate tetrahydrate, (Electron Microscopy Sciences, Cat. #22405, Hatfield, PA, USA) was diluted 1:4 in reverse osmosis distilled water at pH 6.3 to make a 25% solution, then 0.2 μm filtered (Acrodisc 25mm syringe filter with SuporR membrane, Pall Life Sciences, Ann Arbor, MI, USA) prior to use [10]. A 2% Uac in reverse osmosis distilled water solution at pH 4.2 was freshly prepared and 0.2 μm filtered (Acrodisc 25mm syringe filter with SuporR membrane) prior to use. Modified Sato’s lead stain solution (Sato’s Pb11) at pH 13 was prepared in advance and stored at 4 °C. Sato’s Pb was aliquoted into syringes, sealed with ParafilmR, and stored at 4 °C until the day of use, then warmed to room temperature and 0.2 μm filtered (Acrodisc 25mm syringe filter with SuporR membrane.)
Formvar-carbon coated single slot (2 × 1 mm) and 200 mesh uncoated TEM grids with mounted ultrathin sections were loaded into matrix slits within the Leica EM AC20 grid support plate at defined locations (Figure 2A), then slowly unclipped from the grid plate holder. The grid support plate with the mounted grids was placed onto each filled tray so that the grids would be totally submerged in the staining solutions and subsequent distilled water rinses (Tables 1 and 2) at an angle that prevents air bubbles (Figures 2B & 2C.)
Table 1.
25% UAR + Sato’s Pb Staining Procedure.
| Step | Time | Reagent/Solution |
|---|---|---|
| 1 | 30 seconds | Distilled water |
| 2 | 30 minutes | 25% UAR in distilled water, 0.2 μm filtered |
| 3 | 1 minute | Distilled water |
| 4 | 1 minute | Distilled water |
| 5 | 1 minute | Distilled water |
| 6 | 1 minute | Distilled water |
| 7 | 1 minute | Distilled water |
| 8 | 5 minutes | Sato’s Pb Stain, 0.2 μm filtered |
| 9 | 1 minute | Distilled water |
| 10 | 1 minute | Distilled water |
| 11 | 1 minute | Distilled water |
| 12 | 1 minute | Distilled water |
| 13 | 1 minute | Distilled water |
| 14 | 1 minute | Distilled water |
| 15 | Remove grid plate and wick off excess water drops with lens paper |
Table 2.
2% Uac + Sato’s Pb Staining Procedure.
| Step | Time | Reagent/Solution |
|---|---|---|
| 1 | 30 seconds | Distilled water |
| 2 | 10 minutes | 2% Uac in distilled water, 0.2 μm filtered |
| 3 | 1 minute | Distilled water |
| 4 | 1 minute | Distilled water |
| 5 | 1 minute | Distilled water |
| 6 | 1 minute | Distilled water |
| 7 | 1 minute | Distilled water |
| 8 | 5 minutes | Sato’s Pb Stain, 0.2 μm filtered |
| 9 | 1 minute | Distilled water |
| 10 | 1 minute | Distilled water |
| 11 | 1 minute | Distilled water |
| 12 | 1 minute | Distilled water |
| 13 | 1 minute | Distilled water |
| 14 | 1 minute | Distilled water |
| 15 | Remove grid plate and wick off excess water drops with lens paper |
Transmission Electron Microscopy
Grids were imaged using a FEI Tecnai G2 Spirit transmission electron microscope (FEI, Hillsboro, OR, USA) at 80 kV, interfaced with an AMT XR41 digital CCD camera (Advanced Microscopy Techniques, Woburn, MA, USA) for digital TIFF file image acquisition. Digital 16-bit TEM images were acquired from representative regions using identical microscope spot sizes, intensity settings, and magnifications for 25% UAR-Sato’s Pb stained, 2% Uac-Sato’s Pb stained, and unstained ultrathin sections of retinal and optic nerve tissue.
Results
The 3D printed modified HSA works reliably and proven to be durable in our laboratory after nearly one year of routine use. No structural cracking, warping, or water-based dissolution has been observed in the 3D printed apparatus materials in either ABS or PLA materials. Low stain precipitate background was observed on ultrathin sections mounted with either formvar-carbon coated single slot (2 mm x 1 mm) or uncoated 200 mesh grids. In addition, ultrathin sections remained intact on coated and uncoated grids. The Hiraoka staining tray space insert decreases the staining solution volume requirement to 1 to 2 ml from 5 ml.
The contrast in TEM ultrathin sections of ocular mouse tissues obtained using dual 25% UAR-Sato’s Pb stain (Figure 3A) was similar to dual 2% Uac-Sato’s Pb stain (Figure 3B). Extracellular matrix from the Bruch’s membrane was notably contrasted with dual 25% UAR-Sato’s Pb and 2% Uac-Sato’s Pb stains, compared to unstained ultrathin sections from the same mouse retina samples (Figure 3C.) Assessment of other mouse ocular tissue structures, including the retina and optic nerve, revealed that ultrathin sections stained with either dual 25% UAR- Sato’s Pb or 2% Uac- Sato’s Pb yielded equivalent results (data not presented).
Figure 3.
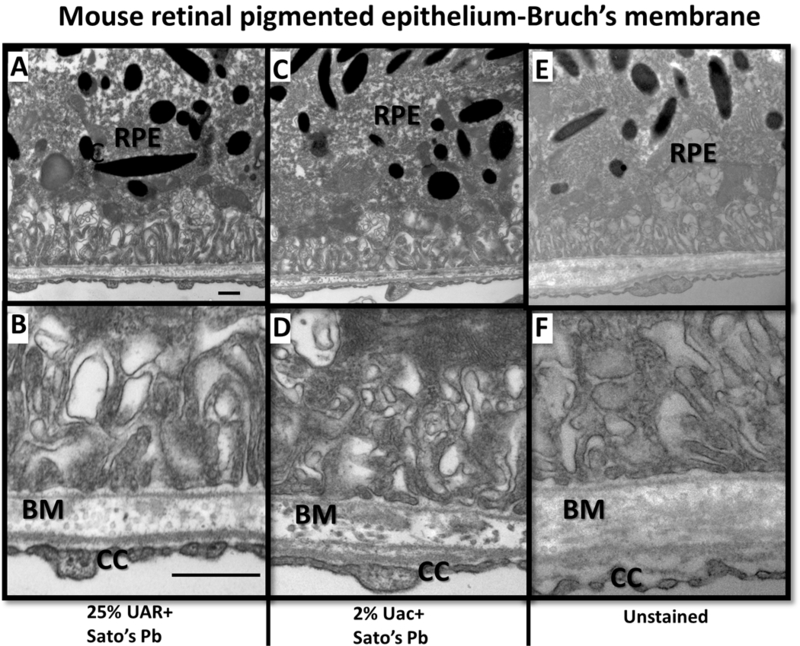
TEM of individual mouse eye sample from set A (without Uac en bloc staining). Retinal pigmented epithelium (RPE) cells and Bruch’s membrane (BM) stained with 25% UAR+Sato’s Pb stain (A & B), 2% Uac+Sato’s Pb stain (C&D) compared to unstained thin section (E&F.) Higher magnification images (B, D, E) of basal RPE and BM interface with surrounding fenestrations of choriocapillary (CC). BM matrix is electron dense in the dual stained 25% UAR+Sato’s Pb stain (A & B), 2%Uac+Sato’s Pb sections. Images A, C, E acquired at 18,500x magnification, scale bar =500 nm. Images B,D,F acquired at 30,000x magnification, scale bar = 500nm.
Discussion
The aim of this technical note is to further the development of custom staining apparatus using 3D printing technology. Also, this note encourages the use of lower toxic, non- radioactive chemicals for contrast staining in biological TEM procedures. The relative ease of use and low cost of 3D design software and thermoplastic printing technology may allow researchers to design and produce commercially unavailable tools and apparatus to suit their unique needs. NIH 3D print exchange offers a sharing platform website for custom labware designs which can facilitate further 3D file improvements to the modified HSA12. There are numerous and more 3D printable materials compared to readily available and economical ABS and PLA. A 3D printing alternative for the modified HSA is to have the apparatus printed in a durable, solvent and corrosion-resistant material and use of laser sculpting sintering technology so one can directly stain within the wells of the trays without a covering.
Commercial TEM grid staining pads are large and have wide spaces between the TEM grid slits (50 mm diameter), requiring large volumes of stain and water rinse solutions [3]. The Chien grid pad may be oriented with the grids facing upwards to apply pooled droplets of stain onto each grid individually [3]. The advantage of using the Leica EM AC20 grid support plate on the trays for lead citrate staining was to prevent carbon dioxide exposure from the air, possibly resulting in lead carbonate precipitate artifact. Existing users of the original HSA may utilize the 3D printed modified HSA with the use of the Leica EM AC20 grid support plate as replacements.
There are distinct advantages of using the 3D printed modified HSA including grid preservation and control of staining process. Batch grid staining is preferable compared to single drop grid staining with disruptive forceps interactions causing risk of precipitate artifact. The 25% UAR-Sato’s Pb and 2% Uac-Sato’s Pb staining procedures (Tables 1 and 2) with the 3D printed modified HSA have been utilized in recently published studies investigating mouse models of vision disease for glaucoma [13] and NMNAT1-Leber Congenital Amaurosis [3]. Further use of this TEM grid staining technique may facilitate the use of UAR in future research studies involving conventional biological TEM. Batch immuno- gold staining methods may be tested using the 3D printed modified HSA, as reported in another custom fabricated grid holder for immuno-gold staining [4]. The Leica EM AC20 automated TEM grid contrasting instrument may not be cost effective for laboratories needing small stain volumes due to the expense of the consumable reagents for <10 grid batches. The Leica EM AC20 instrument is able to use non-radioactive uranyl acetate replacement solutions [14].
The principal compound in UAR is gadolinium triacetate tetrahydrate which contains electron dense gadolinium, a rare earth lanthanide (atomic number 64.) Uac has long been used in electron microscopy for its contrasting and protein fixation characteristics and has low levels of radioactive elemental uranium, an electron-dense actinide (atomic number 92.) A major advantage of using UAR is the lower health and environmental hazards (GHS07 irritation, NFPA health=1, flammability= 0, reactivity= 0) [15] compared to Uac reagent (GHS06 acute toxicity, GHS07 Irritation, GHS08 health hazard, NFPA health=4, flammability=0, reactivity=0 [16].) Another advantage is the cost of using gadolinium triacetate tetrahydrate is less than half the cost of Uac on a per gram basis, let alone the additional cost of uranyl acetate radioactive hazardous waste disposal. Other non-radioactive uranyl acetate substitutes including samarium triacetate[6], oolong tea extract [14,17], platinum blue [14,18], and hafnium chloride [19] have been investigated for post-grid TEM grid staining applications with some substitutes displaying differences in staining characteristics and less intensity [6,14]. Additional studies on non-radioactive replacements for compounds, including lanthanides, should be pursued comparing these to Uac with details of staining characteristics, intensities, and tissue applications. In addition, investigations into using non-radioactive replacements for en bloc staining, performance in different solvents, buffers, and conditions are some future study prospects.
Conclusion
The 3D printed modified HSA and non-radioactive UAR containing gadolinium triacetate tetrahydrate as a Uac substitute may be used for TEM grid staining of conventionally processed biological tissue sections. Options for custom apparatus production and chemical replacements for TEM grid staining may produce efficient tissue processing, cost savings for consumables, safer working conditions for researchers, and decreased levels of hazardous waste.
Acknowledgements
This work was supported by NIH National Eye Institute Core Grant P30EYE003790. The author greatly appreciates the helpful suggestions from Gayle Callis and editorial reviews from Tatjana Jakobs, Scott Greenwald, Donita Garland and Pablo Argueso.
References
- 1.Hiraoka JI. A holder for mass treatment of grids, adapted especially to electron staining and autoradiography. Stain Technol. 1972; 47: 297–301. [DOI] [PubMed] [Google Scholar]
- 2.Wilson SM, Bacic A. Preparation of plant cells for transmission electron microscopy to optimize immunogold labeling of carbohydrate and protein epitopes. Nature Protocols 2012; 7:1716–1727.7 [DOI] [PubMed] [Google Scholar]
- 3.Chien K, van de Velde R, Heusser R. A simple procedure for obtaining clean sections for TEM. Proc 42nd Ann EMSA, ed. Bailey GW, 1984; 42–43. [Google Scholar]
- 4.Lännenpää M, Syväoja JE. Device for handling electron microscopy grids. BioTechniques. 2006; 40:450–452. [DOI] [PubMed] [Google Scholar]
- 5.Pearce JM. Open-Source Lab: How to Build Your Own Hardware and Reduce Research Costs. Amsterdam (The Netherlands): Elsevier; 2014. [Google Scholar]
- 6.Nakakoshi M, Nishioka H, Katayama E. New versatile staining reagents for biological transmission electron microscopy that substitute for uranyl acetate J Electron Microsc (Tokyo) (2011) 60: 401–407. [DOI] [PubMed] [Google Scholar]
- 7.Greenwald SH, Charette JR, Staniszewska M, et al. Mouse models of NMNAT1-Leber Congenital Amaurosis (LCA9) recapitulate key features of the human disease. Am J Pathol. 2016; 186: 1925–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trump GF, Smuckler EA, Benditt EP. A method for staining epoxy sections for light microscopy. 1961. J Ultrastructure Research 5:343–348. [DOI] [PubMed] [Google Scholar]
- 9.Modified Hiraoka TEM Grid Staining Apparatus 3D.stl files, Model ID: 3DPX-007685,3dprint.nih.gov [Internet]. Washington (DC): U.S. Department of Health and Human Services- National Institutes of Health; [cited 2017 July 17]. Available from: https://3dprint.nih.gov/discover/3dpx-007685
- 10.UAR-EMS Technical Data Sheet, Catalog number 22405 [Internet]. Hatfield (Pennsylvania, USA): Electron Microscopy Sciences; [cited 2017 Feb 6]. Available from: https://www.emsdiasum.com/microscopy/technical/datasheet/22405.aspx
- 11.Hanaichi T, Sato T, Hoshin M, et al. A stable lead stain by modification of Sato’s method. Proc. XIth Int. Cong. on Electron Microscopy Kyoto: 1986; 2181–2182. [PubMed] [Google Scholar]
- 12.3dprint.nih.gov [Internet]. Washington (DC): U.S. Department of Health and Human Services- National Institutes of Health; [cited 2016 Nov 11]. Available from: http://3dprint.nih.gov/
- 13.Wang R, Seifert P, Jakobs TC. Astrocytes in the Optic Nerve Head of Glaucomatous Mice Display a Characteristic Reactive Phenotype. IOVS 2017: 58: 924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.http://www.leica-microsystems.com/science-lab/perusing-alternatives-for-automated-staining-of-tem-thin-sections/ [Internet]. Buffalo Grove (IL): Leica-Microsystems Inc.; [cited 2016 Nov 11]. Available from: http://www.leica-microsystems.com/science-lab/
- 15.Uranyl Acetate Replacement Safety Data Sheet, Catalog number 22405 [Internet]. Hatfield (Pennsylvania, USA): Electron Microscopy Sciences; [cited 2017 Feb 1]. Available from: https://www.emsdiasum.com/microscopy/technical/msds/22405.pdf
- 16.Uranyl Acetate Safety Data Sheet, Catalog number 22400 [Internet]. Hatfield (Pennsylvania, USA): Electron Microscopy Sciences; [cited 2017 Feb 1]. Available from: https://www.emsdiasum.com/microscopy/technical/msds/22400.pdf
- 17.Sato S, Adachi A, Sasaki Y, et al. Oolong tea extract as a substitute for uranyl acetate in staining of ultrathin sections. J Microsc. 2007;229:17–20. [DOI] [PubMed] [Google Scholar]
- 18.Inaga S, Katsumoto T, Tanaka K, et al. Platinum blue as an alternative to uranyl acetate for staining in transmission electron microscopy. 7Arch. Histol. Cytol. 2007; 70:43–49. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda K-I, Inoue K, Kanematsu S, et al. Enhanced effects of nonisotopic hafnium chloride in methanol as a substitute for uranyl acetate in TEM contrast of ultrastructure of fungal and plant cells. Microsc. Res. Tech. 2011;74:825–830. [DOI] [PubMed] [Google Scholar]


