Abstract
Depressed individuals exhibit biased attention to negative emotional information. However, much remains unknown about (1) the neurocognitive mechanisms of attention bias (e.g., qualities of negative information that evoke attention bias, or functional brain network dynamics that may reflect a propensity for biased attention) and (2) distinctions in the types of attention bias related to different dimensions of depression (e.g., ruminative depression). Here, in 50 women, clinical depression was associated with facilitated processing of negative information only when such information was self-descriptive and task-relevant. However, among depressed individuals, trait rumination was associated with biases towards negative self-descriptive information regardless of task goals, especially when negative self-descriptive material was paired with self-referential images that should be ignored. Attention biases in ruminative depression were mediated by dynamic variability in frontoinsular resting-state functional connectivity. These findings highlight potential cognitive and functional network mechanisms of attention bias specifically related to the ruminative dimension of depression.
Keywords: cognitive bias, attention bias, rumination, depression, functional connectivity
Cognitive models of depression propose that negative beliefs about the self are central to depressive disorders, driving negative interpretations and automatic thinking that bias goaldirected attention (Beck, 2008). Such models have received robust support, and have informed the development of psychosocial interventions focused on redirecting attention in the presence of negative cognitions (Eisendrath et al., 2016; Hollon & Ponniah, 2010). However, clinical research has also revealed a lack of precision in our understanding of cognitive or neural mechanisms of attention biases. In particular, evidence is mixed regarding the specific domains of attention that are biased in depression, the qualities of negative information that bias attention, and functioning of neural systems that may be associated with a propensity towards biased attention. Furthermore, there is considerable heterogeneity in the cognitive biases exhibited by depressed individuals (Everaert, Koster, & Derakshan, 2012). One source of this heterogeneity in attention biases may be heterogeneity in depression phenotypes. That is, dimensional features such as trait rumination, which vary across individuals with depression, may interact with mood to produce distinct profiles of attention bias. The current study thus seeks to gain a better understanding of the specificity and nature of attention biases related to ruminative depression, a critical step for more precisely characterizing mood disorder at an individual level.
Understanding Component Mechanisms of Attention Bias in Depression
Meta-analytic evidence indicates that depression (current or past diagnosis, or elevated symptoms of depression) is associated with slowed responses when naming the ink color of emotionally negative words, and speeded responses to negative targets or cues that are spatially congruent with negative words or images (Epp, Dobson, Dozois, & Frewen, 2012; Peckham, McHugh, & Otto, 2010; Winer & Salem, 2016). Neuroimaging studies have provided converging evidence for attention biases in depression, e.g., showing that elevated symptoms of depression are related to increased activity and functional connectivity among prefrontal cognitive systems and midline regions involved in self-directed attention in response to negative distractors on an emotion word Stroop (R. H. Kaiser, Andrews-Hanna, Spielberg, et al., 2015). These findings provide evidence for attention biases in depression, and reveal important distinctions in the impact of biases on performance (e.g., enhanced performance when negative information is consistent with task goals but impaired performance when negative information is inconsistent with goals). However, effect sizes have been inconsistent across meta-analyses and individual studies, suggesting that attention biases may be less reliable or more complex than originally suspected.
To address mixed findings for attention bias in depression, more recent theories point to established models in cognitive neuroscience emphasizing that attention is not a unitary construct, but includes subprocesses such as orienting, selecting, engaging, and disengaging from stimuli (Petersen & Posner, 2012; Posner & Boies, 1971), which may be differentially associated with depression. One theory proposes that attention biases in depression are specifically active at later stages of processing, e.g., facilitating elaboration of (and difficulty disengaging from) negative information once it has captured attention (De Raedt & Koster, 2010). This idea is supported by evidence that individuals with depression show attention biases for negative information when such information is either presented at longer (>500ms) but not shorter (<250ms) durations in the dot-probe paradigm, or is followed by longer (≥1300ms) but not shorter (<250ms) delays to a target in the exogenous cueing paradigm (E. H. W. Koster, De Raedt, Goeleven, Franck, & Crombez, 2005; Ernst H. W. Koster, De Raedt, Leyman, & De Lissnyder, 2010; Mogg, Bradley, & Williams, 1995; Sylvester, Hudziak, Gaffrey, Barch, & Luby, 2016). In addition, the idea that attention biases are specific to later stages of processing is supported by evidence that depression is related to increased dwell time looking at negative material but no differences in initial orienting (Caseras, Gamer, Bradley, & Mogg, 2007; Leyman, De Raedt, Vaeyens, & Philippaerts, 2011; Matthews & Antes, 1992). However, metaanalyses have yielded equivocal support for attention biases at later (elaborated) as well as earlier (orienting) stages of processing in depression, or failed to find an association between attention bias and stimulus duration (Armstrong & Olatunji, 2012; Peckham et al., 2010). Thus, while important distinctions may exist in the attention subprocesses that are biased in depression, evidence for those distinctions is not yet conclusive.
In addition to distinguishing which domains of attention are biased in depression, a complementary goal is to distinguish the types of information that evoke such bias, i.e., what is it about negative emotional information that captures or holds attention? At least three potential answers exist for this question. One is that depressed individuals are drawn towards negative emotional content because it matches their current mood state. This mood-congruence hypothesis is supported by evidence that experimentally-induced negative mood in healthy individuals can induce attention biases towards negative material that are similar to those exhibited in depression (Bradley, Mogg, & Lee, 1997; Gilboa-Schechtman, Revelle, & Gotlib, 2000; Gotlib & McCann, 1984; Isaac et al., 2012; Ridout, Noreen, & Johal, 2009). However, these effects have not been consistently replicated (Chepenik, Cornew, & Farah, 2007; McCabe, Gotlib, & Martin, 2000; Newman & Sears, 2015). In addition, attention biases in depression have been observed with other forms of negative information (e.g., anger) that are putatively unrelated to mood state (Lonigan & Vasey, 2009; Mogg et al., 1995; Oehlberg, Revelle, & Mineka, 2012; Platt, Murphy, & Lau, 2015). Together, these findings suggest that depression-related attention biases are not exclusively explained by mood congruency.
A second explanation is that depressed individuals are more sensitive to self-referential information (regardless of emotional content), and biases towards negative information are coincident to the fact that depressed individuals happen to have a self-concept that is more negative than non-depressed individuals. Across clinical and non-clinical samples, self-relatedness of stimuli has been shown to facilitate recall and perceptual integration of information (reviewed in (Sui & Humphreys, 2015)) and boost activity and functional connectivity in brain systems including medial prefrontal cortex (MPFC), insula, hippocampus, and areas of anterior and posterior cingulate cortex (ACC, PCC) (Craik et al., 1999; Fossati et al., 2003; Macrae, Moran, Heatherton, Banfield, & Kelley, 2004; Murray, Debbane, Fox, Bzdok, & Eickhoff, 2015; Murray, Schaer, & Debbane, 2012). These neural systems, many of which are grouped in a functional network known as the default network, show increased functional connectivity during autobiographical thinking (Young, Siegle, Bodurka, & Drevets, 2016) and other forms of self-focused attention (reviewed in (Qin & Northoff, 2011)). Critically, default network and frontoinsular regions also exhibit resting-state hyperconnectivity in major depression (R. H. Kaiser, Andrews-Hanna, Wager, & Pizzagalli, 2015). Although caution to avoid reverse inference is warranted when interpreting these converging patterns, one theory is that amplified activity and coordination among frontoinsular-default networks is a marker of attention biases towards self-focused thinking in depressed individuals.
A third explanation for negative attention biases in depression points to the interaction between self-relatedness and emotional valence of information: e.g., that attention is biased towards positive (but not negative) self-referential information in healthy people, and towards negative (but not positive) self-referential information in depressed people. Consistent with this assumption, research in healthy individuals has demonstrated faster judgements of, and increased medial prefrontal activity in response to, positive as compared with negative self-referential information (Moran, Macrae, Heatherton, Wyland, & Kelley, 2006; Watson, Dritschel, Obonsawin, & Jentzsch, 2007). In contrast, depressed individuals exhibit a reversed pattern of reduced prefrontal and hippocampal response to positive self-referential information (Quevedo et al., 2016), and amplified response to negative self-referential information (Macdonald & Kuiper, 1985; Shestyuk & Deldin, 2010). These findings complement evidence for enhanced self-focused attention and frontoinsular-default network activity in depression, but suggest that the combination of self-relatedness and negative emotionality is responsible for evoking attention bias.
The hypotheses outlined above have been only partially tested, because although many experiments have manipulated the emotional content of stimuli, few have directly manipulated the self-referential quality of stimuli (reviewed in (Wisco, 2009)). Instead, researchers have commonly defined the self-referential nature of stimuli post-hoc on the basis of task performance (e.g., reaction time or neural response to unselected emotional words while words are being judged on their self-referential quality (Alloy, Abramson, Murray, Whitehouse, & Hogan, 1997; Connolly, Abramson, & Alloy, 2016; Gencoz, Voelz, Gencoz, Pettit, & Joiner, 2001)). Although this approach has merit, the same response biases that are of interest in this research can also confound the comparison of depressed and non-depressed participants on specific categories of self-referential information, e.g., healthy individuals may endorse few negative words as self-descriptive, whereas depressed individuals may endorse many negative words as self-descriptive, yielding unbalanced sets of stimuli for further experimentation or statistical analysis (discussion in (Connolly et al., 2016)). In sum, research optimized for testing attention to emotionally negative (or positive) and self-descriptive (or non-self-descriptive) information is needed to understand the qualities of emotional information that evoke attention bias.
Attention Bias Across Clinical Phenotypes: Rumination and Depression
Experimental paradigms designed to unpack the neurocognitive mechanisms of attention bias are necessary to refine our understanding of how and when attention biases occur in depression. However, such research, when conducted exclusively using categorical case-control designs, may be insufficient for understanding individual differences in attention bias. Depression is a complex and heterogeneous family of disorders, with varying symptom presentations, etiologies, and functional impairments exhibited across individuals (R.H. Kaiser, 2017). Thus, attention biases may not characterize different depressed individuals to the same extent. Here, the dimension of trait rumination, defined by the tendency towards negative and repetitive self-focused thinking (Susan Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008), may be particularly relevant. Consistent with this notion, non-depressed individuals prone to rumination exhibit attention biases that overlap with those observed in depression (Beckwe & Deroost, 2016; Hilt & Pollak, 2013), and higher levels of rumination among depressed individuals are associated with more extreme attention biases (Donaldson, Lam, & Mathews, 2007). This convergence suggests the possibility that trait rumination may explain or exacerbate attention biases associated with depression.
However, not all depressed individuals are prone to rumination, and not all individuals prone to rumination are depressed. Accordingly, it is possible is that different symptom dimensions interact to produce distinct phenotypes of depression characterized by unique profiles of attention bias. In particular, whereas both depression and trait rumination have been separately linked to biased elaboration (or difficulty disengaging from) negative information (Joormann, Levens, & Gotlib, 2011; Joormann, Nee, Berman, Jonides, & Gotlib, 2010; R. H. Kaiser, Andrews-Hanna, Metcalf, & Dimidjian, 2015), some evidence suggests that depressed ruminators also exhibit biases orienting to or selecting negative information (De Lissnyder, Derakshan, De Raedt, & Koster, 2011; Joormann, Dkane, & Gotlib, 2006; Whitmer & Banich, 2007). Thus, depressed ruminators may be uniquely characterized by both preferential attention to, and elaboration of, negative self-referential thoughts (related discussion in (Everaert et al., 2012)). Such biases match the clinical profile of ruminative depression (i.e., elevated trait rumination co-occurring with depression), in which rumination is experienced as intrusive and difficult to escape (Papageorgiou & Wells, 2001).
On the level of brain functioning, ruminative depression has been associated with increased resting-state functional connectivity (RSFC) among regions of the default network (Berman et al., 2011) and highly variable RSFC between medial prefrontal cortex (MPFC) regions of default network and anterior insula (R.H. Kaiser et al., 2016). As noted above, the default network comprises midline, inferior temporal, and parietal regions involved in self-generated, self-focused, or autobiographical thinking (Andrews-Hanna, Smallwood, & Spreng, 2014), whereas the anterior insula is considered to be a hub of the “salience network” involved in allocating resources to other networks (including default network) on the basis of salient cues or thoughts (Menon & Uddin, 2010; Sridharan, Levitin, & Menon, 2008). Prior research suggests that variability in cross-network RSFC may be related to regulatory relationships in which key regions of one network are engaged in up- or down-regulating activity in a second network at rest or in response to cognitive demands (Hutchison & Morton, 2015; Hutchison et al., 2013). Thus, increased variability in RSFC between regions of the salience (insula) and default (MPFC) networks may reflect an individual’s heightened tendency to recruit these cross-network regulatory systems, i.e., increased tendency for insula to be engaged to up- or down-regulate activity in default network (Sridharan et al., 2008). On the level of cognitive processing, such increased frontoinsular variability may reflect a tendency for biased allocation of resources towards self-focused thinking, or efforts to regulate self-focused thinking. Together, this suggests a model in which ruminative depression is related to attention biases via altered functioning of frontoinsular and default network regions.
Present Study
The present study aimed to provide insight into the cognitive mechanisms and functional network correlates of depression, and in particular, the ruminative dimension of depression. Toward this goal, we developed a behavioral task designed to separately manipulate the self-referential quality and emotional valence of information, and that varied the depth of elaboration for (self-referential or emotional aspects of) information (see (Elliott, Rubinsztein, Sahakian, & Dolan, 2000; Etkin, Egner, Peraza, Kandel, & Hirsch, 2006) for descriptions of other tasks with partially overlapping procedures). In this task, participants judged either the self-descriptiveness or the emotional valence of words (pre-selected to be self-descriptive or non-self-descriptive, crossed by negative or positive emotion), while ignoring background images that were selfreferential (own face) or non-self-referential (other face). Therefore, the content of the word must always be elaborated upon in order to complete the task, although the level of elaboration of specific features of the word (self-descriptiveness or valence) depends on the relevance of that feature to task goals (e.g., self-descriptiveness should be more deeply processed when the goal is to judge self-descriptiveness than when the goal is to judge emotional valence). Meanwhile, the self-referential content of the images should be ignored entirely in order to complete the task.
Together, performance biases related to task-relevant word content are interpreted as evidence for later-stage attention biases (difficulty disengaging from elaborated information), whereas performance biases related to task-irrelevant word content or images are interpreted as earlier stage attention biases (difficulty ignoring information that should not be deeply elaborated upon [irrelevant word content] or towards which attention should not be oriented at all [images]).
This design allowed us to test several competing hypotheses. First, regarding the type of information that evokes attention biases in depression, or in ruminative depression: if attention biases in this task are evoked by mood congruence, facilitated performance (faster response speed) should be observed when judging negative words, regardless of word self-descriptiveness (hypothesis 1a). However, if attention biases in this task are evoked by self-descriptiveness, facilitated performance should be observed when judging self-descriptive words, regardless of word valence (hypothesis 1b). Alternately, if attention biases in this task are evoked by the conjunction of negative emotion and self-descriptiveness, facilitated performance should specifically be observed when judging negative, self-descriptive words (interaction of word valence-by-self-descriptiveness) (hypothesis 1c). Second, regarding the type of attention processing that is biased in depression, or in ruminative depression: if attention biases are specific to later stages of processing (information that is elaborated), performance facilitation effects on this task will be strongest when task goals (e.g., judge self-descriptiveness) match the type of stimulus content that evokes attention bias (e.g., self-descriptive word) (hypothesis 2a). In contrast, if attention biases are also evident at earlier stages of processing, performance facilitation would also be observed when bias-evoking content was task-irrelevant (e.g., judging emotional valence of self-descriptive word) or should be ignored (e.g., judging words while an image of yourself is presented in the background) (hypothesis 2b).
In addition to behavioral measures, we collected resting-state neuroimaging data to evaluate the associations between depression and the dimension of ruminative depression, attention biases, and intrinsic functional connectivity of a frontoinsular circuit linking medial prefrontal regions of the default network and areas of insula. The goals of this analysis were to replicate prior findings of increased dynamic variability in frontoinsular RSFC in ruminative depression, and test the novel hypothesis that frontoinsular network functioning mediates an association between ruminative depression and attention bias (hypothesis 3).
Method
Participants
Participants included 53 unmedicated adult women recruited from the Boston metropolitan area, who either reported current major depressive disorder (MDD group n=31) or no history of depression or other Axis I psychiatric diagnoses (healthy control (HC) group n=22). Participants were restricted to women in light of evidence that rumination is especially prevalent in women (Susan Nolen-Hoeksema, 1994). All participants completed a Structured Clinical Interview for the DSM-IV-TR to evaluate psychiatric history (First, Spitzer, Gibbon, & Williams, 2002). Inclusion in the MDD group required a primary diagnosis of major depression; participants were excluded from this group for lifetime history of substance dependence, psychosis, mania, anorexia, or recent history of substance abuse (past twelve months) or bulimia (past two years; Table S1). Participants recruited to the HC group were excluded for any lifetime psychiatric illness. For both groups, participants were excluded based on history of neurological impairment, head injury, MRI counter-indications, or cognitive or language impairments that interfered with the ability to complete behavioral testing. Groups did not differ on age, and both groups reflected the geographic region in terms of race and ethnicity, and education (Table S1).
Procedures
The study consisted of experimental procedures spanning two sessions approximately four weeks apart (average=27.47 days). In the first session, participants provided ratings of self-descriptiveness of a list of adjectives, and photographs of the participants were taken, in order to create individualized stimuli for the Self-referential Information Processing (SIP) task. Participants also completed a series of self-report questionnaires at this session. In the second session, participants completed the SIP task (one MDD participant did not complete the emotion judgment condition of this task due to technical error), followed by an MRI scan. Of note, the present sample was drawn from an ongoing study with distinct, non-overlapping experimental objectives and analyses that will be reported elsewhere (see Supplement). All procedures were approved by the Institutional Review Board at Partners Healthcare and McLean Hospital, and were conducted in accordance with the provisions of the World Medical Association Declaration of Helsinki. Participants were reimbursed for their participation and travel, and were fully debriefed and provided (upon request) with referral information for sliding-scale psychological services in the area.
Measures
Self-referential information processing.
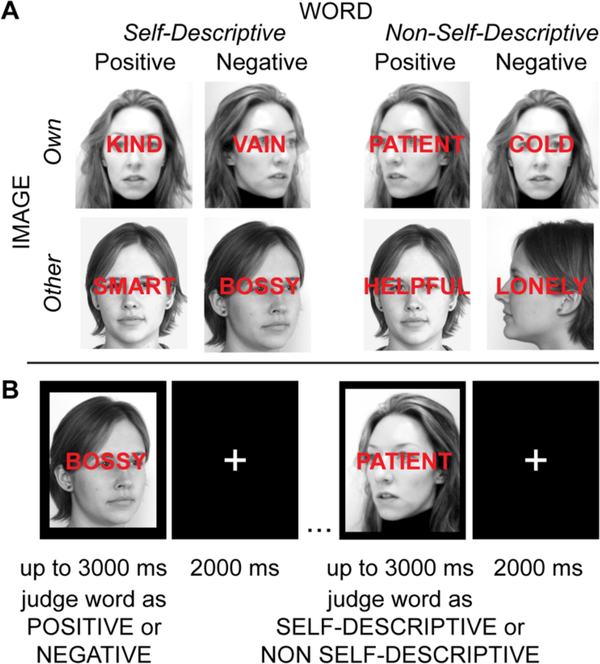
The SIP task includes a set of individualized stimuli consisting of self-descriptive (positive or negative), or non-self-descriptive (positive or negative), adjectives that are superimposed onto images of either the participant’s own face or a gender- and race-matched other person’s face (Fig 1). For the emotion judgment version of the SIP (SIP-EJ), the goal is to respond as quickly as possible to identify the valence of the word, while ignoring the background image. For the self-descriptiveness judgement version of the SIP (SIP-SJ), the goal is to respond as quickly as possible to indicate whether or not the word is self-descriptive, also while ignoring the background image. See Supplement for replication of task effects in an independent sample.
Figure 1. Self-referential Information Processing Task Design.
The Self-referential Information Processing (SIP) task was designed to test the effects of self-referential material on responses when judging either the emotional valence or the self-descriptiveness of emotional words. (A) Stimuli were individualized to the participant, and consisted of emotionally valenced self-descriptive (“Self-Descriptive Positive” or “Self-Descriptive Negative”), or non-self-descriptive (“Non-Self-Descriptive Positive” or “Non-Self-Descriptive Negative”), adjectives superimposed onto images of either the participant’s face (“Own”) or a gender- and race-matched other person’s face (“Other”). Word stimuli were selected from a list of (320) positive and negative adjectives based on the participant’s ratings in a previous session. (B) There were two task goal conditions. In one condition, the participant was instructed to judge the emotional valence of the word as quickly as possible; in the other condition, the participant was asked to judge whether or not the word was self-descriptive, as quickly as possible. Thus, this task aimed to examine direct or moderated effects of self-referential or emotional content on information processing when such content is task-relevant (e.g., self-referential quality of words when judging word self-descriptiveness), task-irrelevant but attended (e.g., valence of words when judging self-descriptiveness), or task-irrelevant and should be ignored (self-referential quality of images across task conditions).
Individualized word stimuli.
To create the individualized word stimulus set, we administered an adjective rating protocol to participants at the first session using E-Prime. Participants were presented serially with 320 positive and negative adjectives drawn from the Dumas word list (Dumas, Johnson, & Lynch, 2002) and rated each word on a 0 (not at all self-descriptive) to 9 (highly self-descriptive) scale. Participants were given these instructions: “Rate each word on how self-descriptive it is of you, in general. Try to be as honest as possible in rating both the positive and negative traits that either do, or do not, describe you. Sometimes it helps to think about how someone who knows you well but who is as unbiased as possible would rate you – like a cousin, classmate, or work colleague.” Participants had up to 10 seconds to rate each word. After the participant completed the adjectives rating protocol, a subset of 96 words was selected from the list based on participant ratings of self-descriptiveness balanced on valence (as determined by published norms, (Dumas et al., 2002)). The 96-word subset was selected to include approximately 24 words in each of four categories: self-descriptive positive words, self-descriptive negative words, non-self-descriptive positive words, and non-self-descriptive negative words (see Supplement, Fig S1). Of note, three (HC) participants were unable to provide words in each category (e.g., either reported all positive words to be self-descriptive or all negative words to be non-self-descriptive) and therefore were removed from task analyses, yielding a final sample of n=50.
Individualized image stimuli.
To obtain individualized images, we took photographs of each participant’s face in five orientations to the camera (right and left 90° view, right and left 45° view, and front facing) with the participant standing three feet from the camera against a white backdrop. Each photograph was cropped to 336x425 pixels, and desaturated to create five black-and-white photographic stimuli for the Own image condition (Own). The matched Other condition (Other) consisted of a standardized set of five black-and-white images of a gender- and race-matched other person in the same resolution, size, face orientation, and brightness (from the MIT CBCL Face Recognition Database, http://cbcl.mit.edu/softwaredatasets/heisele/facerecognition-database.html, or taken in the laboratory by the same specifications).
Task administration.
The SIP task was administered in session two, prior to the MRI scan, using E-Prime and a laptop computer. The SIP-EJ and the SIP-SJ task conditions were counter-balanced in order of presentation across participants. Both task conditions included 16 trial blocks of 12 trials each, each preceded by instructions and 4 practice trials. Trial stimuli consisted of a word (Self-Descriptive Positive, Self-Descriptive Negative, Non-Self-Descriptive Positive, Non-Self-Descriptive Negative) superimposed on an image (Own, Other) for a total of six stimulus conditions (see Supplement for task validation using alternative control conditions). Each word was presented twice for the SIP-EJ and twice for the SIP-SJ; once paired with Own image, once paired with Other image. Trial order was counterbalanced within the SIP-EJ and SIP-SJ, and across conditions. For the SIP-EJ, participants were instructed: “For the words you are about to see, please respond to each word to indicate whether it is POSITIVE or NEGATIVE. Please respond as FAST AS POSSIBLE.” For the SIP-SJ, participants were instructed: “For the words you are about to see, please respond to each word based on whether or not it DESCRIBES YOU. Please respond as FAST AS POSSIBLE.” Participants had up to 3000ms to respond to each stimulus using keys labeled either “positive” or “negative”, or “yes” or “no” (different keys used for response mappings for the SIP-EJ versus the SIP-SJ). In between trials, the participant was presented with a fixation cross for up to 2000ms.
Brooding rumination.
At session one, participants completed the Ruminative Responses Scale (RRS; (Susan Nolen-Hoeksema et al., 2008)). The RRS is a measure of trait rumination that can be decomposed into subscales that provide measures of Brooding (RRS-B), Reflection (RRS-R), and Depression (RRS-D) (Treynor, Gonzalez, & Nolen-Hoeksema, 2003). The RRS-B is believed to represent the maladaptive tendency towards negative, repetitive thinking that is distinct from depressive symptoms (captured in the RRS-D) or a more adaptive form of introspection (captured in the RRS-R) The RRS-B includes five items describing typical responses that an individual makes to stressors or negative emotions, e.g. “go away by yourself and think about why you feel this way”, and each item is rated on a scale of 1 (“almost never”) to 4 (“almost always”). (See Supplement for analyses using other subscales).
Resting-state functional connectivity.
At session two, participants completed an MRI scan that included anatomical scanning and a resting-state functional scan. A Siemens Tim Trio 3T scanner and 32-channel head coil were used to collect a high-resolution T1-weighted anatomical image (TR=2200 ms, TE=4.27 ms, flip angle=7, 144 slices, field of view=230 mm, matrix=192 × 192, voxel size 1.2 × 1.2 × 1.2 mm), and eyes-open resting functional images (TR=3000 ms, TE=30 ms, flip angle=85, 47 slices, field of view=216 mm, matrix=72 × 72, voxel size 3 × 3 × 3 mm, total duration = 6.2 min, total volumes=124). Resting state fMRI data were collected immediately following collection of anatomical data, and prior to other functional scanning.
Analyses
Behavioral analyses.
The outcome variables of interest from the SIP task were reaction time (RT) or proportion of trials within each task condition that were reported to be positive versus negative (for the SIP-EJ) or self-descriptive versus non-self-descriptive (for the SIP-SJ). Behavioral data were processed using R. Reaction time analyses were conducted by calculating an average for each trial type. Incorrect trials, and trials on which RTs were less than 200ms or more than 3 standard deviations above the within-subject mean, were excluded from analyses (consistent with (Henderson, Snyder, Gupta, & Banich, 2012; R. H. Kaiser, Andrews-Hanna, Metcalf, et al., 2015; Snyder et al., 2014). RTs were natural log transformed, and proportion estimates were arcsine transformed, to reduce the skew common to RT or proportion estimate data and which violates the statistical assumption of normal distribution. All RT distributions met normality requirements (Table S2); however, arcsine-transformed proportion data for several conditions were non-normally distributed, suggesting possible ceiling effects and prompting the focus on RT as the outcome variable of interest. (For exploratory analyses using proportion data, see Supplement).
Behavioral analyses were conducted using SPSS to perform mixed design analysis of variance ((M)ANOVA) in which within-subject variables included task condition (Emotion Judgement, Self-descriptiveness Judgement), image type (Own, Other), word self-descriptiveness (Self-Descriptive, Non-Self-Descriptive), and word valence (Positive, Negative); and the between-subject variables were clinical group (MDD = +1, HC = −1), trait brooding (ztransformed RRS-B score), and their interaction. Together, two (M)ANOVAs were performed to test hypotheses 1(a,b,c) and 2(a,b): (1) a (M)ANOVA with task conditions as within-subject variables and clinical group and trait brooding as group-level variables, (2) the same (M)ANOVA, adding the interaction between clinical group and trait brooding. (Of note, a simple (M)ANOVA was performed first to clarify task effects, in the absence of group-level variables).
Analyses were also performed replacing the group variable for depression with symptom severity, as evaluated with the Beck Depression Inventory, 2nd ed. (Beck, Steer, & Garbin, 1988). The analyses yielded results that were nearly identical to those in which depression was included as a categorical variable, likely because clinical status and BDI scores were highly collinear and BDI scores in the present sample had a clear bimodal distribution. Therefore, because BDI scores were clearly bimodal, we elected to operationalize depression as a categorical variable in experimental analyses.
Resting-state functional connectivity analyses.
Functional connectivity analyses were performed with parameters and processing steps identical to those described in (R. H. Kaiser et al., 2016). Static (overall) and dynamic (variability over a sequence of sliding windows) RSFC was calculated between a region of interest (ROI) in medial prefrontal cortex (MPFC, cluster defined by meta-analysis of default network RSFC abnormalities in depression, (R. H. Kaiser, Andrews-Hanna, Wager, et al., 2015)), and ROIs in left and right anterior insular (AI, 4mm radius spherical ROIs at +/− 34, 8, −8, defined by meta-analysis of insular RSFC and implicated in emotion processing and psychopathology (Chang, Yarkoni, Khaw, & Sanfey, 2013)) (Fig S2).
General image preprocessing.
We discarded the first 6 seconds of each subject’s functional data to allow for stabilization of the magnetic field. Preprocessing of functional data was performed in SPM12 using the standard spatial preprocessing steps of slice-time correction, realignment, normalization in Montreal Neurological Institute (MNI) space, and smoothing with a 6-mm kernel.
Head motion and artifact detection.
We used SPM12 to assess head motion by translation and rotation in x, y, z directions. Next, we used Artifact Detection Tools (ART, www.nitrc.org/projects/artifact_detect/) to calculate time points of significant head motion or fluctuations in the magnetic field (>0.5 mm motion from previous frame, global mean intensity >3 standard deviations from mean intensity across functional scans) for each participant. Then, outlier images were modeled in each participant’s first-level general linear model (as a vector the length of the timeseries, with 1 for outlier time points and 0 for non-outlier time points) to censor the influence of outlier timepoints on estimates of functional connectivity while maintaining the temporal structure of the data. Thus, together, motion correction included the regressing out of both residual head motion parameters (three translation and three rotation parameters, plus one composite motion parameter reflecting the maximum scan-to-scan movement), and outlier volumes (as calculated through artifact detection). Analyses were performed to test for associations between motion and experimental variables; framewise motion/outliers were not significantly associated with clinical depression, t(41)=0.14, p=0.89, depressive symptom severity, r(41)=0.03, p=0.83, or brooding, r(41)=−0.09, p=0.59.
Denoising.
Denoising of the timeseries was performed in the CONN toolbox (https://www.nitrc.org/projects/conn/; (Whitfield-Gabrieli & Nieto-Castanon, 2012)) which uses CompCor (Behzadi, Restom, Liau, & Liu, 2007) to estimate physiological noise from white matter and cerebrospinal fluid for each subject using principal component analysis. The first five components were then regressed out of each subject’s functional data on the first level of analysis. In addition, a band-pass filter of 0.0278–0.10 Hz was applied to the time series. This range was selected to remove high-frequency activity related to cardiac and respiratory activity (Cordes et al., 2001), and low-frequency activity with a period that exceeds the duration of sliding windows used in dynamic analyses (Leonardi & Van De Ville, 2015). Accordingly, the corrections performed on the timeseries included: detrending, outlier correction, motion regression, and CompCor correction (which were performed together in a single first-level regression model), followed by band-pass filtering. These corrections produced a residual BOLD time course at each voxel that was used for subsequent analyses.
Dynamic resting-state functional connectivity analysis.
Dynamic RSFC analyses were performed using the CONN toolbox (to estimate RSFC among ROIs for each sliding window), and in-house scripts (to calculate standard deviation in RSFC across windows) written in Matlab (Mathworks, Natick, MA). For first-level dynamic analyses, the time course was segmented into 36-s windows sliding the onset of each window by 18s, for a total of 19 windows (see discussion in (R. H. Kaiser et al., 2016; Leonardi & Van De Ville, 2015). Next, the Fisher’s z-transformed Pearson’s correlation coefficient was computed for each sliding window between the truncated time course of the MPFC and that of all other voxels, yielding a set of sliding-window beta maps for each participant. Dynamic variability in RSFC was estimated by calculating the standard deviation (SD) in beta values at each voxel. For group-level analyses, the SD in beta values was extracted from the left and right AI ROIs using REX (Duff, Cunnington, & Egan, 2007), and a (M)ANOVA was performed in which the between-subject variables were clinical group (MDD = +1, HC = −1), trait brooding (z-transformed RRS-B), and their interaction; hemisphere (left, right) was included as the within-subject variable.
Static resting-state functional connectivity analysis.
Static RSFC analyses were performed using the CONN toolbox (to estimate RSFC among ROIs across the full duration of the scan). For first-level static analyses, the Fisher’s z-transformed Pearson’s correlation coefficient was computed between the full-time course of the MPFC seed and the full-time courses of all other voxels. For group-level analyses, the beta value representing overall correlation in activity was extracted from the left and right AI ROIs using REX, and an (M)ANOVA was performed in which the between-subject variables were clinical group (MDD, = +1, HC = −1), trait brooding (z-transformed RRS-B), and their interaction; hemisphere (left, right) was the within-subject variable.
All group-level fMRI analyses were repeated including age and number of outlier images as covariates; because these variables did not significantly differ between clinical groups or correlate with RRS-B scores, and controlling for these variables did not affect results, simple analyses are reported.
Mediation.
To test the hypothesis that RSFC in a frontoinsular circuit linking insula with MPFC mediates an association between rumination and attention bias among depressed individuals, we used a bootstrapping approach to estimate the indirect effect of brooding rumination (z-transformed RRS-B) on task performance (RT for self-descriptive compared with non-self-descriptive words, as moderated by valence and image type) through dynamic RSFC (SD in correlated activity between MPFC and left AI, and between MPFC and right AI). This analysis was designed to test hypothesis 3. Regression analyses were performed to estimate the regression coefficients.
In sum, a total of three analyses were performed for hypothesis testing (two (M)ANOVAs, and one mediation model). Supporting analyses were performed to demonstrate basic task effects and replication of prior findings, or for post-hoc clarification of significant experimental effects.
Results
General Task Effects
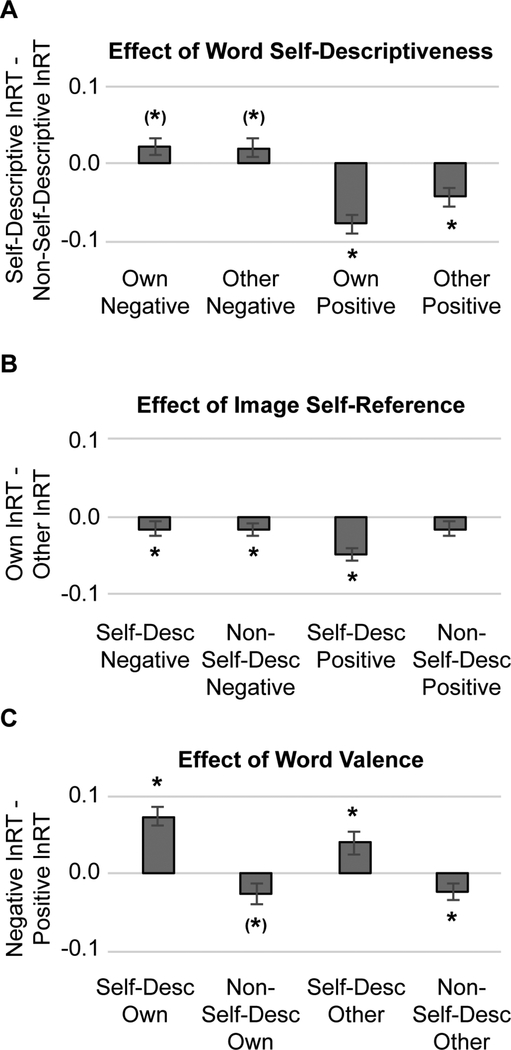
To investigate overall task effects, a 2 (task condition: Emotion Judgement, Self-descriptiveness Judgement) × 2 (image type: Own, Other) × 2 (word self-descriptiveness: Self-Descriptive, Non-Self-Descriptive) × 2 (word valence: Positive, Negative) (M)ANOVA was performed. This analysis revealed significant main effects for all factors. Across the group, participants were faster to judge emotional valence of words than self-descriptiveness of words, F(1,48)=43.94, p<0.01, η2p=0.48, faster to judge self-descriptive than non-self-descriptive words, F(1,48)=12.08, p<0.01, η2p=0.20, faster to judge positive than negative words, F(1,48)=5.57, p=0.02, η2p=0.10, and faster to judge words presented with their own image in the background than another person’s image in the background, F(1,48)=18.19, p<0.01, η2p=0.28. Interactions among stimulus conditions also emerged: the facilitating effects of self-referential content (either word self-descriptiveness, or background image of the participant’s own face) were stronger for positive than for negative words, (F(1,48)=26.12, p<0.01, η2p=0.35 and F(1,48)=4.60, p=0.03, η2p=0.09), and the strongest facilitation effects emerged for self-descriptive positive words paired with the participant’s own image, F(1,48)=5.50, p=0.02, η2p=0.10 (Fig 2, Fig S3-S4).
Figure 2. Main and Moderated Effects of the Self-referential Information Processing Task on Reaction Time (RT).
On average, participants were significantly faster to judge both emotional valence and self-descriptiveness of self-descriptive (compared with non-self-descriptive) positive (compared with negative) words paired with their own (compared with someone else’s) image. (A) Main and moderated effects of word self-descriptiveness: displayed are natural-log transformed RTs for Self-Descriptive – Non-Self-Descriptive word trials. (B) Main and moderated effects of image type: displayed are natural-log transformed RTs for Own – Other image trials. (C) Main and moderated effects of word valence: displayed are natural-log transformed RTs for Negative – Positive word trials. Note: Task effects, *p<0.05, (*)p<0.10.
Moderating Effects of Clinical Depression and Trait Rumination
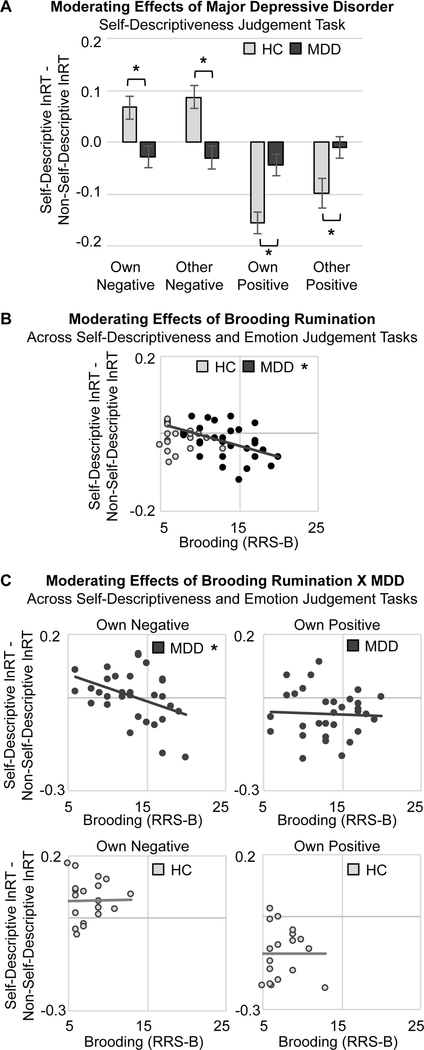
Experimental analyses to test hypotheses were performed by adding the group-level variables of clinical depression (HC = −1, MDD = +1) and brooding rumination (z-transformed RRS-B) to the (M)ANOVA described above. In this (M)ANOVA, first, clinical group moderated the effects of task condition and word type (group X condition X valence X self-descriptiveness interaction), F(1,46)=17.40, p<0.01, η2p=0.27. Follow-up (M)ANOVA revealed that depression effects were specific to the self-descriptiveness judgement task: when participants responded to indicate whether or not a word described them, healthy participants were faster to judge positive self-descriptive words, and slower to judge negative self-descriptive words, but depressed participants showed faster judgement of negative self-descriptive words and also showed blunted facilitation for positive self-descriptive words, F(1,47)=15.23, p<0.01, η2p=0.25 (Fig 3A, Fig S5–S6). These depression effects are consistent with hypotheses 1c (attention biases evoked by the interaction of self-descriptiveness and valence) and 2a (attention biases evoked at later stages of processing with information that is elaborated).
Figure 3. Moderating Effects of Depression, Trait Brooding, and Their Interaction on Reaction Time (RT) for the Self-referential Information Processing Task.
(A) When judging self-descriptiveness of words, individuals with major depressive disorder (MDD) were faster to respond to self-descriptive (relative to non-self-descriptive) negative words than healthy control (HC) individuals, whereas HC individuals were faster to respond to self-descriptive (relative to non-selfdescriptive) positive words. (B) Across the group and controlling for depression status, higher levels of trait brooding rumination (measured with the Ruminative Responses Scale, Brooding subscale, RRS-B) were associated with faster responses to self-descriptive (relative to non-self-descriptive) words, regardless of task goals. (C) Among individuals with depression, those with higher levels of trait brooding exhibited stronger self-referential facilitation effects for negative words, i.e., faster responses to self-descriptive, negative words accompanied by their own image.
Note: Group differences in task effects or significant correlations, *p<0.05, (*)p<0.10.
Second, trait brooding moderated the effects of word self-descriptiveness: regardless of task goals, emotional word content, or accompanying images, participants more prone to brooding were faster to judge self-descriptive (compared with non-self-descriptive) words, F(1,46)=7.94, p<0.01, η2p=0.15 (Fig 3B). Next, adding the interaction of depression and rumination to the model revealed attention bias effects specific to ruminative depression, F(1,45)=3.80, p=0.05, η2p=0.08. Follow-up (M)ANOVAs conducted within the MDD and HC groups showed that there were no significant effects of trait brooding among healthy participants, but among depressed individuals, higher levels of brooding were associated with faster responses to self-descriptive words accompanied by the participant’s own image when words were negative, but not positive, F(1,28)=4.65, p=0.04, η2p=0.14 (Fig 3C, Fig S7). Together, these findings for ruminative depression support hypotheses 1c (attention biases evoked by the interaction of self-descriptiveness and valence); however, they also support hypothesis 2b (attention biases evoked at earlier stages of processing with information that is task-irrelevant and should be ignored).
(Of note, replacing brooding (RRS-B) with depression-specific rumination (RRS-D) in the above analyses failed to yield significant results; replacing brooding with reflective rumination (RRS-R) revealed overlapping attention biases to self-descriptive words, in general, but only brooding showed an interaction with clinical depression in predicting attention bias to negative self-referential material: see Supplement).
Frontoinsular Dynamic and Static Functional Connectivity
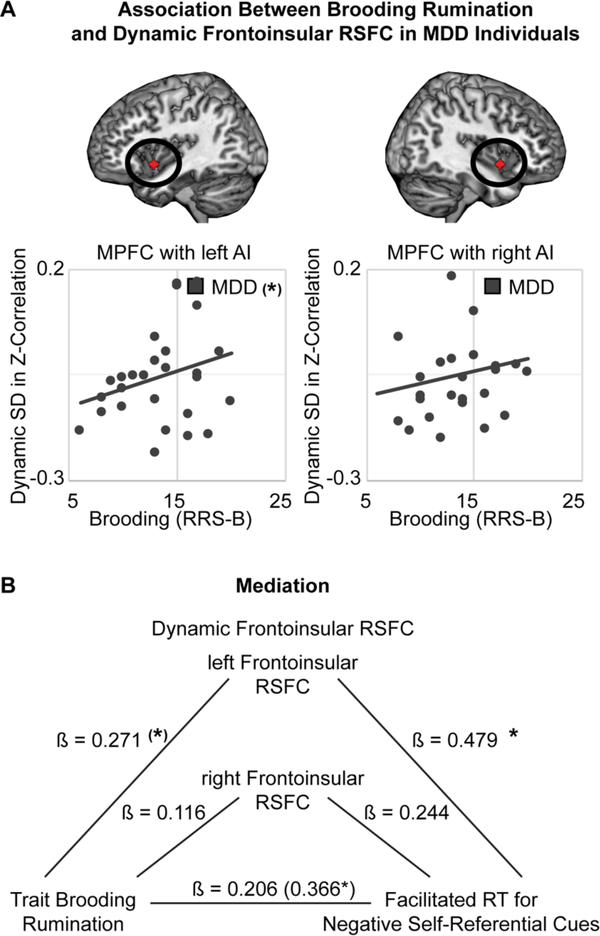
In preparation for mediation analysis, analyses were performed to test for replication of prior findings of altered RSFC between AI and MPFC in depressed ruminators (R. H. Kaiser et al., 2016). Two separate (M)ANOVAs were performed to examine dynamic variability (standard deviation over time) in RSFC between MPFC and AI, or static (overall) RSFC between MPFC and AI. In each (M)ANOVA, the within-subject variable was hemisphere (left, right), and the group-level variables were clinical group (MDD = +1, HC = −1), trait brooding (z-transformed RRS-B), and their interaction. Analyses revealed a significant interaction between depression and brooding in predicting dynamic RSFC, F(1,39)=5.85, p=0.02, η2p=0.13, and a trend for a main effect of brooding, F(1,39)=3.41, p=0.07, η2p=0.08: higher levels of brooding were associated with increased variability in frontoinsular RSFC, and this association was significantly stronger for depressed than for healthy participants (Fig 4A, Fig S8). For analyses examining static frontoinsular RSFC, there were no significant main effects of clinical depression, F(1,39)=2.05, p=0.16, η2p=0.05, or brooding, F(1,39)=2.06, p=0.16, η2p=0.05, and no interaction, F(1,39)=1.53, p=0.22, η2p=0.04 (Fig S8).
Figure 4. Frontoinsular Resting- State Functional Connectivity (RSFC) and Attention Biases in the Ruminative Phenotype of Depression.
(A) Consistent with prior findings in an independent sample (Kaiser et al., 2016), elevated trait brooding rumination (Ruminative Responses Scale, Brooding subscale, RRS-B) was related at a trend level to increased variability in RSFC between regions of interest (ROIs) in medial prefrontal cortex (MPFC) and left anterior insula (AI). (B) Dynamic variability in RSFC between MPFC and left AI significantly mediated the association between trait brooding and attention biases towards negative, self-descriptive and self-referential information (indirect effect across bootstrapping = 0.01, bias-corrected CI: 0.0002 to 0.0441). Note: Dynamic variability in RSFC was operationalized as standard deviation (SD) in Fisher’s Z-transformed correlation coefficients over a series of sliding windows; conservative motion correction procedures were applied to the data, see Methods. Correlations or paths in mediation model, *p<0.05, (*)p<0.10.
Mediation
To test the prediction (hypothesis 3) that RSFC between AI and MPFC mediates an association between ruminative depression and attention biases, a bootstrapping mediation analysis was performed within the MDD group using the INDIRECT SPSS command (Fig 4B). Variables entered in the model included brooding (z-transformed RRS-B: X), attention bias to negative self-referential information (RT contrast corresponding with the task effect of word self-descriptiveness as moderated by valence and image type: Y), and frontoinsular RSFC (dynamic and static RSFC between MPFC and left and right AI: Ms). Consistent with the results of (M)ANOVA analyses above, there was a significant total effect of brooding in predicting facilitated RT to negative self-descriptive words accompanied by the participant’s own image (c path unstandardized b = 0.032, CI: 0.001 to 0.063, standardized ß = 0.366), and a trend-level association between increased brooding and higher dynamic RSFC between MPFC and left anterior insula (left AI: a path unstandardized b = 0.018, CI: −0.006 to 0.042, standardized ß = 0.271; right AI: a path unstandardized b = 0.008, CI: −0.019 to 0.034, standardized ß = 0.116). Higher left frontoinsular dynamic RSFC was significantly associated with more extreme attention bias to negative self-referential information (left AI: b path unstandardized b = 0.671, CI: 0.097 to 1.245, standardized ß = 0.479; right AI: b path unstandardized b = 0.335, CI: −0.207 to 0.876, standardized ß = 0.244). The indirect effect of brooding through left frontoinsular dynamic RSFC on attention bias was significant (average indirect effect across bootstrapping = 0.01, bias-corrected CI: 0.0002 to 0.0441), and the direct effect of brooding on attention bias was no longer significant when including frontoinsular dynamic RSFC as the mediator (c' path unstandardized b = 0.019, CI: −0.016 to 0.054, standardized ß = 0.206).
Discussion
This study aimed to provide insight into the neurocognitive mechanisms of attention bias in depression by asking: what types of information evoke biased attention, what aspects of attention are biased, and how are attention biases reflected in the intrinsic functioning of frontoinsular-default networks? Results showed that first, clinical depression was associated with attention biases specifically towards negative information that was self-referential and elaborated, as evidenced by faster judgements by those with depression when the self-referential quality of the stimulus was relevant to task goals (self-descriptive words judged on self-descriptiveness). Self-related content that should not be elaborated (in the form of background images, or self-descriptiveness of words when judging emotional valence), or non-self-referential content, did not evoke biased performance among depressed as compared with non-depressed individuals. These findings support the hypothesis that it is the interaction between emotional content and self-relatedness that evokes attention bias in depression (Wisco, 2009), and are consistent with prior research suggesting that attention biases in depression are instantiated at later (elaborative) stages of attention processing, leaving intact earlier (selecting, orienting) stages of processing (De Raedt & Koster, 2010; Kircanski & Gotlib, 2015).
Second, results showed that ruminative depression was associated with attention biases that were distinct from those generally associated with depression: controlling for main effects of depression, depressed ruminators were faster to judge negative, self-descriptive information regardless of the task-relevance of such information, and in particular when such information was paired with self-referent images. These patterns are consistent with the idea that ruminative depression is related both to (earlier-stage) biases in orienting to, and (later-stage) biases elaborating upon, negative self-referential information (Joormann, 2010; Whitmer & Banich, 2007).
Third, the present study replicates the association between ruminative depression and dynamic variability in functional connectivity of a frontoinsular circuit linking AI with MPFC (R. H. Kaiser et al., 2016), and further shows that such altered network dynamics mediate the association between ruminative depression and biased attention to self-referential negative information. These patterns support the idea that coordinated functioning of insula and midline cortical systems may underlie the tendency for attention to be captured by negative self-focused thinking. Amplified and variable resting-state functional connectivity between these regions may represent an intrinsic tendency for salient self-referential material (albeit irrelevant to present moment goals) to activate insula to direct resources to midline brain systems and enhance processing of self-referential information (Menon & Uddin, 2010; Sridharan et al., 2008). In the present task we observed enhanced (faster) performance; however, in more complex tasks or daily life, enhanced processing of self-referential material may also come at the cost of goaldirected action and emotional health (E. H. W. Koster, De Lissnyder, & De Raedt, 2013; E. H. W. Koster, De Lissnyder, Derakshan, & De Raedt, 2011).
Of note, there are other interpretations of the present study findings. For example, depression-related differences in task performance were interpreted as evidence for attention biases because depressed women were faster to judge self-descriptive (than non-self-descriptive) negative words, whereas non-depressed women were slowed in judging this material. However, as a group, depressed women were also faster to judge self-descriptive (than non-selfdescriptive) positive words, albeit to a weaker extent than non-depressed women. Therefore, it could be argued that depression-related “biases” are defined by the absence of valence-specific biases that are robustly exhibited by healthy individuals. However, it is noted that among depressed women, brooding rumination was associated with the presence of increased biases towards negative, self-referential material (rather than an absence of performance biases towards positive, self-referential material). Thus, there may be different ways that attention biases manifest in depression and depressive phenotypes, and interpretations for attention biases are performed in consideration of relative patterns of performance between groups or across dimensions.
Several limitations to the present study warrant discussion, as do a number of questions motivated by these findings that should be addressed in future research. First, these results highlight the interactive roles of personal salience and emotional content in driving attention bias. However, stimuli were restricted to particular forms of emotional and self-referent information (adjectives, images); extending experimental stimuli to other forms of self-referent cues (e.g., words evoking autobiographical memories) may provide complementary information about attention bias. Second, attention biases with task-relevant versus task-irrelevant information are here interpreted as evidence for biases evoked at varying stages of processing; however, there may be other explanations. For example, depression versus rumination could differently influence processing of verbal versus image-based self-referential content; task variants that include other types of (image or verbal) stimuli may distinguish these possibilities. In addition, although the present behavioral task was adapted from classic attention interference tasks, other cognitive processes may influence performance. For example, this task (and indeed, many tasks requiring attention to, and judgement of, stimulus content) could involve incidental encoding of information, incidental retrieval of autobiographical memories, or other processing (e.g., schema activation) that could enhance or impede performance. Future research using alternative task designs that yields converging results may provide more precise evidence for biases in attention or specific attention subprocesses.
A third limitation of this study is related to sample characteristics. Here, in light of evidence that ruminative depression is more common in women, we recruited a female sample. It is unknown, from this study, whether effects would generalize to other genders, or if gender-specific attention biases exist. In addition, it is noted that a broader range of trait rumination was reported among depressed women, yielding relatively enhanced statistical power for detecting effects of rumination within this group. Studies specifically designed to assess trait rumination in non-depressed samples may better evaluate attention biases related to rumination in healthy individuals.
Fourth, the focus of the present neurobiological measures was on intrinsic frontoinsular functioning in a circuit linking AI with MPFC, but neural activity in other circuits or in response to explicit task demands may provide complementary information (Ho et al., 2014). For example, future research may compare frontoinsular activity at rest with activity in response to cognitive regulation of self-referential thinking, when attention biases may be most active. It should also be noted that despite the encouraging replication of rumination-related abnormalities in intrinsic network dynamics, much remains unknown about the functional significance of such dynamics. Some recent theoretical and empirical work supports the idea that network dynamics at rest are a reliable property of brain functioning (Hutchison et al., 2013) that may reflect crosstalk among neural circuits (Hutchison & Morton, 2015; R. H. Kaiser et al., 2016). However, other researchers have pointed out that estimates of dynamic variability are vulnerable to contamination by motion or sampling variability (Laumann et al., 2016). Although we took a conservative approach to motion correction in these analyses, and sampling variability is unlikely to explain group-level effects, it is worthwhile to note that the field of network dynamics is new and rapidly evolving. Future research that investigates the convergence of multiple measures of dynamic network functioning (e.g., intrinsic network states, (Calhoun, Miller, Pearlson, & Adali, 2014), co-activation pattern analysis, (Chen, Chang, Greicius, & Glover, 2015)) is needed.
In conclusion, this study provides evidence that attention biases in clinical depression are specific to negative self-referential material, and also reveals distinctions in the nature of attention biases related (generally) to depression or (particularly) to the ruminative dimension of depression. Relative to non-depressed woman, those with depression showed attention biases towards negative information that was self-referential and task-relevant, suggesting biases evoked at later stages of processing with elaborated information. Depressed ruminators were characterized by attention biases to negative self-referential information regardless of task goals and especially when paired with self-referent images, suggesting biases at both later (elaborative) and earlier (orienting) stages of processing. Rumination-related attention biases were mediated by dynamic circuit activity among regions involved in orienting attention towards self-focused thinking. These findings highlight the importance of a research approach that disentangles distinct clinical dimensions in order to understand psychopathology, and suggest future research that asks questions such as how rumination may interact with other symptom presentations (e.g., ruminative phenotype of substance use disorder or anxiety (S. Nolen-Hoeksema & Watkins, 2011)), or how other clinical dimensions (e.g., anhedonia) may interact with depressive symptoms to produce distinct profiles of cognitive bias. In addition, this research supports integration across multiple levels of neurocognitive functioning to understand phenotypes (or “biotypes”) of illness, which may be critical for revising and improving our theories of the active mechanisms of mood pathology. Such an integrative approach may support discovery of treatment targets, e.g., interventions that target frontoinsular-default circuits (with neurofeedback or other techniques) and attention biases (using cognitive therapy techniques or attention bias modification). For example, existing attention bias modification programs teach individuals to direct attention away from negative and towards positive material using standardized computer-based paradigms and stimuli. Although the standardization of such interventions is in many ways a strength, the present results suggest that tailoring bias modification to the attention biases of a particular client – e.g. by incorporating personally salient negative and positive stimuli, or targeting biases at multiple levels of attention processing – may enhance the impact of these treatments. Together, these dimensionally-focused, multi-modal approaches may support a more precise characterization of depression and related mood pathology for individual patients, and enhance clinical outcomes.
Supplementary Material
Acknowledgments
Funding
Supported by the Beverley Sears Award (R.H.K.), Ted Volsky Memorial Award (R.H.K.), and NIMH grant 5R01 MH095809 (D.A.P.). A subset of analyses reported here were presented (by R.H.K.) at the 2014 meeting of the Cognitive Neuroscience Society and the 2015 McLean Hospital Research Day.
Footnotes
Declaration of Conflicting Interests
The authors declared no conflicts of interest with respect to the authorship or the publication of this article. Over the past three years, D.A.P received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Pfizer, and Posit Science for activities unrelated to the current study.
References
- Alloy LB, Abramson LY, Murray LA, Whitehouse WG, & Hogan ME (1997). Selfreferent information-processing in individuals at high and low cognitive risk for depression. Cognition & Emotion, 11(5–6), 539–568. [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, & Buckner RL (2010). Functionalanatomic fractionation of the brain's default network. Neuron, 65(4), 550–562. doi: 10.1016/j.neuron.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J, Smallwood J, & Spreng RN (Eds.). (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance (Vol. 1316). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong T, & Olatunji BO (2012). Eye tracking of attention in the affective disorders: A meta-analytic review and synthesis. Clinical Psychology Review, 32(8), 704–723. doi: 10.1016/j.cpr.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT (2008). The evolution of the cognitive model of depression and its neurobiological correlates. American Journal of Psychiatry, 165(8), 969–977. doi: 10.1176/appi.ajp.2008.08050721 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Garbin MG (1988). Psychometric properties of the Beck Depression Inventory - 25 years of evaluation. Clinical Psychology Review, 8(1), 77–100. doi: 10.1016/0272-7358(88)90050–5 [DOI] [Google Scholar]
- Beckwe M, & Deroost N (2016). Attentional biases in ruminators and worriers. Psychological Research-Psychologische Forschung, 80(6), 952–962. doi: 10.1007/s00426-015-0703-8 [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage, 37(1), 90–101. doi: 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, & Jonides J (2011). Depression, rumination and the default network. Social Cognitive and Affective Neuroscience, 6(5), 548–555. doi: 10.1093/scan/nsq080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, & Lee SC (1997). Attentional biases for negative information in induced and naturally occurring dysphoria. Behaviour Research and Therapy, 35(10), 911–927. doi: 10.1016/s0005-7967(97)00053-3 [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Miller R, Pearlson G, & Adali T (2014). The Chronnectome: Time-Varying Connectivity Networks as the Next Frontier in fMRI Data Discovery. Neuron, 84(2), 262274. doi: 10.1016/j.neuron.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X, Gamer M, Bradley BP, & Mogg K (2007). Biases in visual orienting to negative and positive scenes in dysphoria: An eye movement study. Journal of Abnormal Psychology, 116(3), 491–497. doi: 10.1037/0021-843x.116.3.491 [DOI] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, & Sanfey AG (2013). Decoding the Role of the Insula in Human Cognition: Functional Parcellation and Large-Scale Reverse Inference. Cerebral Cortex, 23(3), 739–749. doi: 10.1093/cercor/bhs065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JE, Chang C, Greicius MD, & Glover GH (2015). Introducing co-activation pattern metrics to quantify spontaneous brain network dynamics. Neuroimage, 111, 476–488. doi: 10.1016/j.neuroimage.2015.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepenik LG, Cornew LA, & Farah MJ (2007). The influence of sad mood on cognition. Emotion, 7(4), 802–811. doi: 10.1037/1528-3542.7.4.802 [DOI] [PubMed] [Google Scholar]
- Connolly SL, Abramson LY, & Alloy LB (2016). Information processing biases concurrently and prospectively predict depressive symptoms in adolescents: Evidence from a selfreferent encoding task. Cognition & Emotion, 30(3), 550–560. doi: 10.1080/02699931.2015.1010488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD Turski PA, Moritz H, . . . Meyerand ME (2001). Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. American Journal of Neuroradiology, 22(7), 13261333. [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, & Kapur S (1999). In search of the self: A positron emission tomography study. Psychological Science, 10(1), 26–34. doi: 10.1111/1467-9280.00102 [DOI] [Google Scholar]
- De Lissnyder E, Derakshan N, De Raedt R, & Koster EHW (2011). Depressive symptoms and cognitive control in a mixed antisaccade task: Specific effects of depressive rumination. Cognition & Emotion, 25(5), 886–897. doi: 10.1080/02699931.2010.514711 [DOI] [PubMed] [Google Scholar]
- De Raedt R, & Koster EHW (2010). Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cognitive Affective & Behavioral Neuroscience, 10(1), 50–70. doi: 10.3758/cabn.10.1.50 [DOI] [PubMed] [Google Scholar]
- Donaldson C, Lam D, & Mathews A (2007). Rumination and attention in major depression. Behaviour Research and Therapy, 45(11), 2664–2678. doi: 10.1016/j.brat.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Duff EP, Cunnington R, & Egan GF (2007). REX: Response exploration for neuroimaging datasets. Neuroinformatics, 5(4), 223–234. doi: 10.1007/s12021-007-9001-y [DOI] [PubMed] [Google Scholar]
- Dumas JE, Johnson M, & Lynch AM (2002). Likableness, familiarity, and frequency of 844 person-descriptive words. Personality and Individual Differences, 32(3), 523–531. doi: 10.1016/s0191-8869(01)00054-x [DOI] [Google Scholar]
- Eisendrath SJ, Gillung E, Delucchi KL, Segal ZV, Nelson JC, McInnes LA, . . . Feldman MD (2016). A Randomized Controlled Trial of Mindfulness-Based Cognitive Therapy for Treatment-Resistant Depression. Psychotherapy and Psychosomatics, 85(2), 99–110. doi: 10.1159/000442260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, & Dolan RJ (2000). Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport, 11(8), 1739–1744. doi: 10.1097/00001756-200006050-00028 [DOI] [PubMed] [Google Scholar]
- Epp AM, Dobson KS, Dozois DJA, & Frewen PA (2012). A systematic meta-analysis of the Stroop task in depression. Clinical Psychology Review, 32(4), 316–328. doi: 10.1016/j.cpr.2012.02.005 [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, & Hirsch J (2006). Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51(6), 871–882. doi: 10.1016/j.neuron.2006.07.029 [DOI] [PubMed] [Google Scholar]
- Everaert J, Koster EHW, & Derakshan N (2012). The combined cognitive bias hypothesis in depression. Clinical Psychology Review, 32(5), 413–424. doi: 10.1016/j.cpr.2012.04.003 [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, & Williams J (2002). Structured Clinical Interview for DSM-IVTR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, & Mayberg H (2003). In search of the emotional self: An fMRI study using positive and negative emotional words. American Journal of Psychiatry, 160(11), 1938–1945. doi: 10.1176/appi.ajp.160.11.1938 [DOI] [PubMed] [Google Scholar]
- Gencoz T, Voelz ZR, Gencoz F, Pettit JW, & Joiner TE (2001). Specificity of information processing styles to depressive symptoms in youth psychiatric inpatients. Journal of Abnormal Child Psychology, 29(3), 255–262. doi: 10.1023/a:1010385832566 [DOI] [PubMed] [Google Scholar]
- Gilboa-Schechtman E, Revelle W, & Gotlib IH (2000). Stroop interference following mood induction: Emotionality, mood congruence, and concern relevance. Cognitive Therapy and Research, 24(5), 491–502. doi: 10.1023/a:1005517326981 [DOI] [Google Scholar]
- Gotlib IH, & McCann CD (1984). CONSTRUCT ACCESSIBILITY AND DEPRESSION - AN EXAMINATION OF COGNITIVE AND AFFECTIVE FACTORS. Journal of Personality and Social Psychology, 47(2), 427–439. doi: 10.1037/0022-3514.47.2.427 [DOI] [PubMed] [Google Scholar]
- Henderson RK, Snyder HR, Gupta T, & Banich MT (2012). When does stress help or harm? The effects of stress controllability and subjective stress response on Stroop performance. Frontiers in Psychology, 3. doi: 10.3389/fpsyg.2012.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilt LM, & Pollak SD (2013). Characterizing the Ruminative Process in Young Adolescents. Journal of Clinical Child and Adolescent Psychology, 42(4), 519–530. doi: 10.1080/15374416.2013.764825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Connolly CG, Henje Blom E, LeWinn KZ, Strigo IA, Paulus MP, . . . Yang TT (2014). Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biological Psychiatry, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon SD, & Ponniah K (2010). A REVIEW OF EMPIRICALLY SUPPORTED PSYCHOLOGICAL THERAPIES FOR MOOD DISORDERS IN ADULTS. Depression and Anxiety, 27(10), 891–932. doi: 10.1002/da.20741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, & Morton JB (2015). Tracking the Brain's Functional Coupling Dynamics over Development. Journal of Neuroscience, 35(17), 6849–6859. doi: 10.1523/jneurosci.4638-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Chang C (2013). Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage, 80, 360–378. doi: 10.1016/j.neuroimage.2013.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac L, Vrijsen JN, Eling P, van Oostrom I, Speckens A, & Becker ES (2012). Verbal and facial-emotional Stroop tasks reveal specific attentional interferences in sad mood. Brain and Behavior, 2(1), 74–83. doi: 10.1002/brb3.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J (2010). Cognitive Inhibition and Emotion Regulation in Depression. Current Directions in Psychological Science, 19(3), 161–166. doi: 10.1177/0963721410370293 [DOI] [Google Scholar]
- Joormann J, Dkane M, & Gotlib IH (2006). Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behavior Therapy, 37(3), 269–280. doi: 10.1016/j.beth.2006.01.002 [DOI] [PubMed] [Google Scholar]
- Joormann J, Levens SM, & Gotlib IH (2011). Sticky Thoughts: Depression and Rumination Are Associated With Difficulties Manipulating Emotional Material in Working Memory. Psychological Science, 22(8), 979–983. doi: 10.1177/0956797611415539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Nee DE, Berman MG, Jonides J, & Gotlib IH (2010). Interference resolution in major depression. Cognitive Affective & Behavioral Neuroscience, 10(1), 2133. doi: 10.3758/cabn.10.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH (2017). Neurocognitive Markers of Depression. Biological psychiatry, 81(4), e29e31. doi: 10.1016/j.biopsych.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Metcalf CA, & Dimidjian S (2015). Dwell or Decenter? Rumination and Decentering Predict Working Memory Updating After Interpersonal Criticism. Cognitive Therapy and Research, 39(6), 744–753. doi: 10.1007/s10608-0159697-1 [DOI] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Spielberg JM, Warren SL, Sutton BP, Miller GA, . . . Banich (2015). Distracted and down: neural mechanisms of affective interference in subclinical depression. Social Cognitive and Affective Neuroscience, 10(5), 654–663. doi: 10.1093/scan/nsu100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, & Pizzagalli DA (2015). Large-Scale Network Dysfunction in Major Depressive Disorder A Meta-analysis of Resting-State Functional Connectivity. Jama Psychiatry, 72(6), 603–611. doi: 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Whitfield-Gabrieli S, Dillon DG, Goer F, Beltzer M, Minkel J, . . . Pizzagalli DA (2016). Dynamic Resting-State Functional Connectivity in Major Depression. Neuropsychopharmacology, 41(7), 1822–1830. doi: 10.1038/npp.2015.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircanski K, & Gotlib IH (2015). Processing of Emotional Information in Major Depressive Disorder: Toward a Dimensional Understanding. Emotion Review, 7(3), 256–264. doi: 10.1177/1754073915575402 [DOI] [Google Scholar]
- Koster EHW, De Lissnyder E, & De Raedt R (2013). Rumination is characterized by valencespecific impairments in switching of attention. Acta Psychologica, 144(3), 563–570. doi: 10.1016/j.actpsy.2013.09.008 [DOI] [PubMed] [Google Scholar]
- Koster EHW, De Lissnyder E, Derakshan N, & De Raedt R (2011). Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical Psychology Review, 31(1), 138–145. doi: 10.1016/j.cpr.2010.08.005 [DOI] [PubMed] [Google Scholar]
- Koster EHW, De Raedt R, Goeleven E, Franck E, & Crombez G (2005). Mood-congruent attentional bias in dysphoria: Maintained attention to and impaired disengagement from negative information. Emotion, 5(4), 446–455. doi: 10.1037/1528-3542.5.4.446 [DOI] [PubMed] [Google Scholar]
- Koster EHW, De Raedt R, Leyman L, & De Lissnyder E (2010). Mood-congruent attention and memory bias in dysphoria: Exploring the coherence among information-processing biases. Behaviour Research and Therapy, 48(3), 219–225. doi: 10.1016/j.brat.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Laumann TO, Snyder AZ, Mitra A. n., Gordon EM,Gratton C, Adeyemo B, . . . Petersen SE (2016). On the stability of BOLD fMRI correlations. Cerebral Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi N, & Van De Ville D (2015). On spurious and real fluctuations of dynamic functional connectivity during rest. Neuroimage, 104, 430–436. doi: 10.1016/j.neuroimage.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Leyman L, De Raedt R, Vaeyens R, & Philippaerts RM (2011). Attention for emotional facial expressions in dysphoria: An eye-movement registration study. Cognition & Emotion, 25(1), 111–120. doi: 10.1080/02699931003593827 [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, & Vasey MW (2009). Negative Affectivity, Effortful Control, and Attention to Threat-Relevant Stimuli. Journal of Abnormal Child Psychology, 37(3), 387–399. doi: 10.1007/s10802-008-9284-y [DOI] [PubMed] [Google Scholar]
- Macdonald MR, & Kuiper NA (1985). EFFICIENCY AND AUTOMATICITY OF SELF-SCHEMA PROCESSING IN CLINICAL DEPRESSIVES. Motivation and Emotion, 9(2), 171–184. doi: 10.1007/bf00991574 [DOI] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, & Kelley WM (2004). Medial prefrontal activity predicts memory for self. Cerebral Cortex, 14(6), 647–654. doi: 10.1093/cercor/bhh025 [DOI] [PubMed] [Google Scholar]
- Matthews GR, & Antes JR (1992). VISUAL-ATTENTION AND DEPRESSION - COGNITIVE BIASES IN THE EYE FIXATIONS OF THE DYSPHORIC AND THE NONDEPRESSED. Cognitive Therapy and Research, 16(3), 359–371. doi: 10.1007/bf01183287 [DOI] [Google Scholar]
- McCabe SB, Gotlib IH, & Martin RA (2000). Cognitive vulnerability for depression: Deployment of attention as a function of history of depression and current mood state. Cognitive Therapy and Research, 24(4), 427–444. doi: 10.1023/a:1005579719849 [DOI] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function, 214(5–6), 655–667. doi: 10.1007/s00429-0100262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, & Williams R (1995). ATTENTIONAL BIAS IN ANXIETY AND DEPRESSION - THE ROLE OF AWARENESS. British Journal of Clinical Psychology, 34, 17–36. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, & Kelley WM (2006). Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience, 18(9), 1586–1594. doi: 10.1162/jocn.2006.18.9.1586 [DOI] [PubMed] [Google Scholar]
- Murray RJ, Debbane M, Fox PT, Bzdok D, & Eickhoff SB (2015). Functional Connectivity Mapping of Regions Associated with Self- and Other-Processing. Human Brain Mapping, 36(4), 1304–1324. doi: 10.1002/hbm.22703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RJ, Schaer M, & Debbane M (2012). Degrees of separation: A quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neuroscience and Biobehavioral Reviews, 36(3), 1043–1059. doi: 10.1016/j.neubiorev.2011.12.013 [DOI] [PubMed] [Google Scholar]
- Newman KR, & Sears CR (2015). Eye Gaze Tracking Reveals Different Effects of a Sad Mood Induction on the Attention of Previously Depressed and Never Depressed Women. Cognitive Therapy and Research, 39(3), 292–306. doi: 10.1007/s10608-014-9669-x [DOI] [Google Scholar]
- Nolen-Hoeksema S (1994). The emergence of gender differences in depression during adolescence. Psychological Bulletin, 115(3), 424–443. doi: 10.1037/0033-2909.115.3.424 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, & Watkins ER (2011). A Heuristic for Developing Transdiagnostic Models of Psychopathology: Explaining Multifinality and Divergent Trajectories. Perspectives on Psychological Science, 6(6), 589–609. doi: 10.1177/1745691611419672 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco B, & Lyubomirsky S (2008). Rethinking rumination. Perspectives on Psychological Science, 3(5), 400–424. doi: 10.1111/j.1745-6924.2008.00088.x [DOI] [PubMed] [Google Scholar]
- Northoff G (2016). Is the self a higher-order or fundamental function of the brain? The "basis model of self-specificity" and its encoding by the brain's spontaneous activity. Cognitive Neuroscience, 7(1–4), 203–222. doi: 10.1080/17588928.2015.1111868 [DOI] [PubMed] [Google Scholar]
- Oehlberg KA, Revelle W, & Mineka S (2012). Time-Course of Attention to Negative Stimuli: Negative Affectivity, Anxiety, or Dysphoria? Emotion, 12(5), 943–959. doi: 10.1037/a0027227 [DOI] [PubMed] [Google Scholar]
- Papageorgiou C, & Wells A (2001). Metacognitive beliefs about rumination in recurrent major depression. Cognitive and Behavioral Practice, 8(2), 160–164. doi: 10.1016/s10777229(01)80021-3 [DOI] [Google Scholar]
- Peckham AD, McHugh RK, & Otto MW (2010). A META-ANALYSIS OF THE MAGNITUDE OF BIASED ATTENTION IN DEPRESSION. Depression and Anxiety, 27(12), 1135–1142. doi: 10.1002/da.20755 [DOI] [PubMed] [Google Scholar]
- Petersen SE, & Posner MI (2012). The Attention System of the Human Brain: 20 Years After In Hyman SE (Ed.), Annual Review of Neuroscience, Vol 35 (Vol. 35, pp. 73–89). Palo Alto: Annual Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt B, Murphy SE, & Lau JYF (2015). The association between negative attention biases and symptoms of depression in a community sample of adolescents. Peerj, 3, 20. doi: 10.7717/peerj.1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, & Boies SJ (1971). COMPONENTS OF ATTENTION. Psychological Review, 78(5), 391-&. doi: 10.1037/h0031333 [DOI] [Google Scholar]
- Qin PM, & Northoff G (2011). How is our self related to midline regions and the defaultmode network? Neuroimage, 57(3), 1221–1233. doi: 10.1016/j.neuroimage.2011.05.028 [DOI] [PubMed] [Google Scholar]
- Quevedo K, Ng R, Scott H, Martin J, Smyda G, Keener M, & Oppenheimer CW (2016). The Neurobiology of Self-Face Recognition in Depressed Adolescents With Low or High Suicidality. Journal of Abnormal Psychology, 125(8), 1185–1200. doi: 10.1037/abn0000200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout N, Noreen A, & Johal J (2009). Memory for emotional faces in naturally occurring dysphoria and induced sadness. Behaviour Research and Therapy, 47(10), 851–860. doi: 10.1016/j.brat.2009.06.013 [DOI] [PubMed] [Google Scholar]
- Shestyuk AY, & Deldin PJ (2010). Automatic and Strategic Representation of the Self in Major Depression: Trait and State Abnormalities. American Journal of Psychiatry, 167(5), 536–544. doi: 10.1176/appi.ajp.2009.06091444 [DOI] [PubMed] [Google Scholar]
- Snyder HR, Kaiser RH, Whisman MA, Turner AEJ, Guild RM, & Munakata Y (2014). Opposite effects of anxiety and depressive symptoms on executive function: The case of selecting among competing options. Cognition & Emotion, 28(5), 893–902. doi: 10.1080/02699931.2013.859568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, & Menon V (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105(34), 12569–12574. doi: 10.1073/pnas.0800005105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, & Humphreys GW (2015). The Integrative Self: How Self-Reference Integrates Perception and Memory. Trends in Cognitive Sciences, 19(12), 719–728. doi: 10.1016/j.tics.2015.08.015 [DOI] [PubMed] [Google Scholar]
- Sylvester CM, Hudziak JJ, Gaffrey MS, Barch DM, & Luby JL (2016). Stimulus-Driven Attention, Threat Bias, and Sad Bias in Youth with a History of an Anxiety Disorder or Depression. Journal of Abnormal Child Psychology, 44(2), 219–231. doi: 10.1007/s10802015-9988-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, & Nolen-Hoeksema S (2003). Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research, 27(3), 247–259. doi: 10.1023/a:1023910315561 [DOI] [Google Scholar]
- Watson LA, Dritschel B, Obonsawin MC, & Jentzsch I (2007). Seeing yourself in a positive light: Brain correlates of the self-positivity bias. Brain Research, 1152, 106–110. doi: 10.1016/j.brainres.2007.03.049 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity. doi: 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Whitmer AJ, & Banich MT (2007). Inhibition versus switching deficits in different forms of rumination. Psychological Science, 18(6), 546–553. doi: 10.1111/j.14679280.2007.01936.x [DOI] [PubMed] [Google Scholar]
- Winer ES, & Salem T (2016). Reward Devaluation: Dot-Probe Meta-Analytic Evidence of Avoidance of Positive Information in Depressed Persons. Psychological Bulletin, 142(1), 18–78. doi: 10.1037/bul0000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisco BE (2009). Depressive cognition: Self-reference and depth of processing. Clinical Psychology Review, 29(4), 382–392. doi: 10.1016/j.cpr.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Young KD, Siegle GJ, Bodurka J, & Drevets WC (2016). Amygdala Activity During Autobiographical Memory Recall in Depressed and Vulnerable Individuals: Association With Symptom Severity and Autobiographical Overgenerality. American Journal of Psychiatry, 173(1), 78–89. doi: 10.1176/appi.ajp.2015.15010119 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.