Abstract
Background:
Despite a rich literature on human laboratory paradigms of subjective response to alcohol (SR), craving for alcohol, and alcohol self-administration, few studies have examined the interplay across these three constructs. The present study addresses this gap in the literature by examining the interplay between SR, craving, and self-administration in the human laboratory.
Methods:
Data were culled from a medication study (NCT02026011) in which heavy drinking participants of East Asian ancestry completed two double-blinded and counterbalanced experimental sessions. In each experimental session, participants received a priming dose of IV alcohol to a target breath alcohol concentration (BrAC) of 0.06 g/dl and measures of SR (stimulation and sedation) and alcohol craving were collected across rising BrACs. The IV alcohol challenge was immediately followed by a 1-hour alcohol self-administration period.
Results:
Mixed model analyses found a positive and significant relationship between the slope of stimulation and the slope of craving during the alcohol challenge. The relationship between sedation and craving, however, was not significant. The slope of craving during the alcohol challenge significantly predicted a higher number of mini-drinks consumed and lower latency to first drink. Further, mediation analyses found that craving was a significant mediator of the relationship between stimulation and total number of mini-drinks consumed, but the same pattern was not found for sedation.
Conclusions:
Insofar as alcohol self-administration represents the endpoint of interest for a host of experimental and clinical research questions, the present study suggests that alcohol craving represents a more proximal predictor of self-administration than measures of alcohol-induced stimulation. It is recommended that human laboratory models interpret measures of SR and craving in light of their relative predictive utility for drinking outcomes.
Keywords: Subjective response, craving, self-administration, human laboratory, NCT02026011
INTRODUCTION
Human laboratory studies in the field of alcohol research have been comprised primarily of three broad categories of experimental manipulations and associated outcomes. The first is the alcohol administration, or alcohol challenge, paradigm in which individuals are systematically administered alcohol and assessed for their subjective responses (SR) to alcohol. The second category consists of studies of alcohol craving, which are commonly assessed through cue-exposure paradigms in which participants are exposed to alcohol cues (or to alcohol itself) and asked to provide subjective ratings of craving for alcohol. The third category consists of alcohol self-administration paradigms in which individuals are given the opportunity to consume alcohol in the laboratory. As discussed in detail elsewhere, each paradigm has its own strengths and weaknesses (Bujarski and Ray, 2016), and can be leveraged to address a multitude of research questions (Ray et al., 2016). While the aforementioned experimental methods are widely used in the field of alcohol research, surprisingly few studies have examined the associations between subjective response to alcohol, craving for alcohol, and alcohol self-administration within individuals. The present study addresses this question in a sample of non-treatment seeking heavy drinkers of East Asian descent. Specifically, data culled for the present study were obtained in a behavioral pharmacology trial with heavy drinkers of East Asian descent as it provides a unique opportunity to combine the three phenotypes of interest at the within-subjects level, namely subjective response and alcohol craving during alcohol administration and alcohol self-administration (Ray et al., 2018b).
Alcohol administration studies have documented substantial variability in individuals’ subjective responses to alcohol and have shown that such differences impact the predisposition to alcohol use and misuse (Schuckit, 1984, Schuckit and Smith, 1996, King et al., 2014). Subjective response to alcohol represents a multifaceted (Ray et al., 2009, Bujarski et al., 2015b) and replicable construct (Roche et al., 2014, King et al., 2015). Moreover, alcohol administration methods include both intravenous and oral alcohol administration, with the first benefiting from tight controls over breath alcohol concentration (BrAC) and the later benefiting from greater ecological validity (Bujarski and Ray, 2016). While there are few direct comparisons of the two methods, an early study found them comparable in eliciting subjective response with the exception of craving which was significantly higher in the oral alcohol administration model (Ray et al., 2007). Regarding the ability of subjective response to alcohol, measured under controlled laboratory conditions, to predict one’s risk to develop alcohol-related problems, elegant longitudinal studies have suggested two distinct and relatively independent, pathways of risk. The first pathway suggests lower levels of response to the sedative and unpleasant effects of alcohol, and the second suggests higher sensitivity to the stimulant and pleasurable effects of alcohol (King et al., 2011, King et al., 2014, Schuckit, 1994, Schuckit and Smith, 1996). A recent study by our laboratory found that craving for alcohol during alcohol administration was a robust predictor of subsequent self-administration, such that greater self-reported craving was associated with higher levels of self-administration (Bujarski et al., 2018). In the same study we found that alcohol-induced sedation was associated with lower levels of self-administration (Bujarski et al., 2018), which is consistent with longitudinal findings indicating that sedation may be protective against the development of an alcohol use disorder (AUD) (King et al., 2014).
In the context of alcohol challenge studies, craving is defined as a subjective state of “wanting” the alcohol that is brought on (or induced) by alcohol administration. Craving for alcohol can also be captured through cue- or stress-induction paradigm (Plebani et al., 2012). Together, these models produce measures of craving for alcohol that are either alcohol-induced, cue-induced, or stress-induced, which in turn parallels nicely with the preclinical literature on alcohol/drug reinstatement (Egli, 2018). Recent studies examining the factor structure of responses to an alcohol challenge suggested that alcohol craving is distinct from other dimensions of SR, namely stimulation, sedation, and negative mood (Bujarski et al., 2015b, Ray et al., 2009). Subjective response in turn is often predictive of craving, although that may vary as a function of sample and alcohol use severity (Bujarski et al., 2015a, Bujarski and Ray, 2014). In the current investigation we examine craving for alcohol elicited by alcohol administration (i.e., alcohol-induced craving), which we recently reported to be associated with tonic measures of alcohol craving (Hartwell and Ray, 2018).
Alcohol self-administration in the laboratory represents an important tool for capturing clinically meaningful outcomes within controlled experimental conditions. A host of models have been developed and validated, starting with basic paradigm of asking individuals to choose between alcoholic drinks or monetary reinforcement which has been well-applied to testing AUD medications (O’Malley et al., 2002, McKee et al., 2009). This paradigm has been modified to capture impaired control (Leeman et al., 2013) and more recently, an intravenous (IV) alcohol-self administration version has been used to test determinants of binge drinking in the laboratory (Gowin et al., 2017) as well as the effects of AUD severity and allostasis on self-administration (Bujarski et al., 2018). Insofar as alcohol self-administration represents the most clinically relevant outcome in human laboratory models for AUD, the present study examines the associations between SR and alcohol-induced craving as within-subject determinants of alcohol self-administration. As argued by Wardell et al. (2005) and elsewhere (Curran and Bauer, 2011), within-person tests can generate stronger inferences in psychological research, including human laboratory models of AUD.
In sum, there is a rich literature on human laboratory paradigms of subjective response to alcohol, craving for alcohol, and alcohol self-administration applied to a range of research questions from AUD etiology to treatment development (Bujarski and Ray, 2016). Few studies have examined the interplay across these three constructs. A recent study by Wardell, Ramchandani, and Hendershot (2015), conducted within-person analysis of subjective responses and craving during a self-administration task as predictors of ongoing self-administration behavior in a sample of heavy drinking young adults. Results were such that stimulation was positively associated with alcohol intake and sedation was negatively associated with intake during the self-administration period. Importantly, the effects of SR (both stimulation and sedation) on self-administration were partially mediated by alcohol craving (Wardell et al., 2015). However, it is important to note that the Wardell et al. study asked participants to self-administer alcohol “to achieve a level of intoxication that was pleasurable, but to avoid experiencing unpleasant effects.” Those instructions are distinct from self-administration models where participants are asked to decide whether to consume alcohol or to receive a monetary compensation (Bujarski and Ray, 2016). Nonetheless, they are consistent with the well-validated Computer Assisted Self-administration of Ethanol (CASE) model, which captures self-regulation of the subjective effects of alcohol (Zimmermann et al., 2013). The present study extends upon these findings by examining a sample of non-treatment seeking heavy drinkers of East Asian descent for the associations between SR (stimulation and sedation) and craving during an IV alcohol administration (target BrAC=0.06 g/dl) and subsequent oral alcohol self-administration. The self-administration task implemented in this study consisted of asking participants to decide whether to have a drink containing alcohol versus receiving a monetary compensation. Based on the work of Wardell, Ramchandani and Hendershot (2015), as well as the broader literature on these phenotypes, we hypothesize that SR will be associated with craving and self-administration (i.e., sedation will be negatively associated while stimulation will be positively associated) and that alcohol craving will be a more proximal determinant of self-administration within a drinking session. To address the study goals analyses examined the direct and indirect (i.e., mediated) effects of subjective response and craving on the outcome of self-administration.
METHODS
Design Overview
Data were culled from a recently completed medication study (NCT02026011) in which participants completed two double-blinded and counterbalanced experimental sessions: one after taking naltrexone (50 mg/day) for five days and one after taking matched placebo for five days. In each experimental session, participants received a priming dose of IV alcohol up to the breath alcohol concentration (BrAC) target of 0.06 g/dl which allowed us to capture measures of SR (stimulation and sedation) and alcohol craving across rising BrACs. The alcohol challenge was immediately followed by a 1-hour oral alcohol self-administration period in which participants could choose to either consume up to four mini-drinks, or receive one dollar for every mini drink remaining. The outcomes during the self-administration period were (a) total number of mini-drinks consumed, (b) latency to first drink, and (c) abstinence. The present analyses used data from the placebo condition only.
Participants
Participants were recruited from the greater Los Angeles community through fliers, online and print advertisements, and social media between December 2013 and September 2016. Inclusion criteria were as follows: (1) a score of 8 or higher on the Alcohol-Use Disorders Identification Test (AUDIT; Allen et al., 1997), indicating a hazardous drinking pattern; (2) East Asian ethnicity (i.e., Chinese, Korean, Japanese, or Taiwanese); and (3) between the ages of 21 and 55. In all, 87 (29 females) non-treatment-seeking heavy drinkers were randomized in this trial. Participants with a history of depression with suicidal ideation, lifetime psychotic disorder, lifetime substance use disorder (except marijuana), or ≥10 on the Clinical Institute Withdrawal Assessment-revised (CIWA-R), indicating clinically significant alcohol withdrawal (Sullivan et al., 1989) were excluded. All female participants tested negative for pregnancy and all subjects had a BrAC of zero before each session. The study was approved by the University of California Los Angeles Institutional Review Board.
Screening Procedures
Initial assessment of the eligibility criteria was conducted through a telephone interview. At the time of the telephone interview, participants were excluded if they reported current involvement in treatment for alcohol use or had received treatment in the last 30 days. Potential participants were also excluded if they reported a current interest in seeking treatment for alcohol use. Eligible participants were invited to the laboratory for additional in-person screening. Upon arrival, participants read and signed an informed consent form upon receiving a full explanation of all study procedures. Participants then completed a series of individual differences measures and interviews, including a demographics questionnaire and the Timeline Follow-back (TLFB; Sobell et al., 1986) to assess for quantity and frequency of drinking over the past 30 days. All participants were required to test negative on a urine drug test (except for marijuana) and to have a BrAC of 0.00g/dl. Eligible participants attended a physical examination to determine medical eligibility. A total of 199 participants (78 women) were screened in the laboratory, 106 of whom were eligible and therefore invited to complete the physical exam. Common reasons for exclusion at the in-person screening session were a positive toxicology test for drugs of abuse and presence of an exclusionary psychiatric diagnosis. Of the 106 individuals invited to the physical exam 5 were ineligible for medical reasons and 14 of whom decided not to participate in the trial, leaving 87 participants who enrolled and were randomized. Of the 87 individuals randomized, 77 completed at least one alcohol administration session, and 72 completed the entire study. The analytic dataset for this study included individuals who completed at least one infusion, and thus included 77 participants.
Alcohol Administration
Testing sessions consisted of two portions, intravenous alcohol administration and oral alcohol self-administration. Participants were asked to fast for two hours before arrival and were given a standardized meal before the alcohol administration began. Smokers were allowed to smoke a cigarette immediately prior to the alcohol infusion procedures to mitigate the effect of nicotine withdrawal. Participants were seated in a recliner chair, and the IV was placed in their non-dominant arm. After completing the baseline assessment, participants received intravenous infusions of alcohol. The intravenous route of administration was chosen in order to reduce and control BAC variability between subjects (Li et al., 2001, O’connor et al., 1998, Ramchandani et al., 1999). The IV alcohol administration used methods previously developed by our laboratory (Ray and Hutchison, 2004, Ray et al., 2017). Infusion rates were 0.166 ml/min × weight (in kg) for males and 0.126 ml/min × weight for females. Target BrACs were as follows: 0.02, 0.04, and 0.06 g/dl. Upon reaching each of the target levels of BrAC, participants’ infusion rates were reduced to half, to maintain stable BrAC during testing. Across medication conditions, the alcohol challenge session lasted an average of 1 hour and 36 minutes (SD=20 minutes). Upon completion of the alcohol infusion, the IV was removed and participants immediately began an oral self-administration session (1-hour long) following standard procedures (O’Malley et al., 2002, Krishnan-Sarin et al., 2007). Across medication conditions, the time that elapsed between the infusion session and the self-administration session was 5 minutes to allow for removal of the IV. Participants were offered four mini-drinks of their preferred beverage and allowed to watch a movie. In total, the mini-drinks allowed participants to consume up to .04 g/dl (i.e., .01 g/dl per mini-drink) of alcohol over the one-hour period. Drink sizes were determined by participant’s gender, weight, height, and alcohol content (Brick, 2006). Participants had one hour to either consume the mini-drinks or receive one dollar for every mini drink remaining. Participants notified the research assistant before consuming a mini-drink and were breathalyzed before drinking as well as every 10 minutes during the self-administration period. As a precaution, if BrAC ≥.100 g/dl, participants had to wait until BrAC dropped before consuming the drink; however, this event was not encountered in the study. Immediately after the self-administration period, participants were given a meal and asked to stay at the laboratory for a 4-hour period allowing their BrAC to drop below 0.020 g/dl (or to 0.000 g/dl if driving). Participants were aware prior to infusion that they would be required to stay in the laboratory for a full 4 hours, regardless of their decision to drink or to abstain.
Measures
The following measures were used in this study: (1) The Biphasic Alcohol Effects Scale (BAES), represents a valid and reliable measure of subjective response to alcohol, capturing two distinct domains of stimulation and sedation (Erblich and Earleywine, 1995, Martin et al., 1993); (2) The Alcohol Urge Questionnaire (AUQ) consists of eight items assessing the urge to drink, each rated on a seven-point Likert scale and demonstrating high internal consistency (Bohn et al., 1995, MacKillop, 2006); and (3) The following measures were obtained from the self-administration session (a) total number of mini-drinks consumed, (b) latency to first drink, and (c) BrAC assessed every 10-minutes. All measures and outcomes used in the present study are consistent with the previous report from this dataset (Ray et al., 2018b).
Data Analysis
All analyses presented herein were conducted using a multilevel mixed modeling framework (Singer, 1998) in SAS version 9.4. For each multilevel model, BrAC (coded 0, 1, 2, 3 for baseline, 0.02, 0.04, and 0.06 g/dl BrAC) and behavioral response (i.e., sedation, stimulation, craving, or self-administration) were within subject measures (nested within subjects). The first set of models tested the association between SR (i.e., stimulation and sedation, each tested separately) and alcohol craving. In these models we first ran a mixed model predicting SR (each separately) with BrAC rates as random slopes and random medication effects to adjust for variation across person effects. The following covariates were controlled for in all models: medication, randomization order for medication, OPRM1 status, gender, ethnicity, drinking days past 30 days, drinks per drinking day past 30 days, and alcohol metabolites (ALDH2 (rs671) and ADH1B (rs1229984). Next, we estimated the deviation of each individual’s slope from the mean slope of BrAC and added deviation plus mean slope value to obtain each person’s slope value. Notably, baseline levels were included in all slope estimates. Thus, each estimation of SR slope represents the individual magnitude of alcohol-induced stimulation and sedation while leveraging the Bayesian estimation benefits of a multilevel modeling framework (Raudenbush and Bryk, 2002). Finally, we used each person’s slope to predict alcohol craving during the alcohol challenge. Since we controlled for various covariates in models creating person-level slopes, we did not re-control for these covariates in models of individual slope predicting alcohol craving. All other models however continued to control for these covariates. Alcohol craving was analyzed in the same manner as SR, such that each estimation of SR represented the individual magnitude alcohol-induced craving. The second set of models tested the associations between stimulation, sedation, and craving (each tested separately) with self-administration outcomes (i.e., total number of mini-drinks consumed and latency to first drink). The aforementioned approach to estimating each individual’s slope values was implemented for stimulation, sedation, and alcohol craving. After that, these individualized slopes were used as predictors of self-administration. Total number of mini-drinks consumed during the self-administration session was modeled as a continuous outcome in our mixed models. Accelerated Failure Time (AFT) models were conducted to examine these effects on latency to first drink as these models have been shown to be advantageous for survival-time outcomes in mediational analyses (Gelfand et al., 2016). In these models, participants who did not drink were censored. Additionally, as an exploratory aim, average BrAC across the self-administration session was analyzed as an outcome. To test for craving as a mediator of our self-administration outcomes, the Sobell test of mediated effect was used (Sobel, 1982). If there was not direct effect of subjective response on self-administration outcomes, craving was still examined as a mediator due to mediational support that it is not necessary to have a direct effect to have an indirect effect through a mediating variable (Hayes, 2009, Mackinnon et al., 2004, Shrout and Bolger, 2002).
Mixed models predicting self-administration outcomes from subjective response included robust estimation for standard errors to account for heteroscedasticity amongst dependent variables (White, 1980). As reported previously, there were no effects of medication or OPRM1 genotype on any of the outcomes (Ray et al., 2018b). However, we controlled for a number of covariates: medication, randomization order for medication, OPRM1 status, gender, ethnicity, drinking days past 30 days, drinks per drinking day past 30 days, and alcohol metabolites (ALDH2 (rs671) and ADH1B (rs1229984).,
RESULTS
Sample Characteristics
Of the 77 participants enrolled in this study, 36.4% (N=28) were female. The average age was 26.8 (SD 6.15; Range: 21–47), and the ethnic background was 32.5% (N=25) Chinese descent, 45.5% (N=35) Korean descent, 10.4% (N=8) Japanese descent, and 11.6% (N=9) Taiwanese descent. Participants reported an average of 13.55 drinking days in the past 30 days (SD=6.80; Range: 4–30) and a mean of 4.91 drinks per drinking episode in the past 30 days (SD=2.57: Range: 1.4–15.1). The average AUDIT score was 14.29 (SD=5.39; Range: 8–32) and 32.5% (N=25) of the sample reported using marijuana at least once in the past 30 days. Regarding DSM-5 AUD criteria, the sample breakdown was as follows: 39% (N=30) did not meet criteria for an alcohol use disorder, 44.2% (N=34) met criteria for AUD mild, 9% (N=7) met criteria for AUD moderate, and 7.8% (N=6) met criteria for AUD severe. Scores on subjective response measures are presented in Table 1 and self-administration outcomes are reported in Table 2.
Table 1.
Observed Means and Standard Deviations During Alcohol Challenge Session
| Alcohol Challenge Session | BrAC Timepoint | Mean (SD) |
|---|---|---|
| Sedation | Baseline | 1.348 (1.353) |
| 0.02 g/dl | 2.324 (1.620) | |
| 0.04 g/dl | 2.769 (1.796) | |
| 0.06 g/dl | 2.873 (2.011) | |
| Stimulation | Baseline | 1.747 (1.617) |
| 0.02 g/dl | 1.997 (1.695) | |
| 0.04 g/dl | 2.491 (1.858) | |
| 0.06 g/dl | 2.826 (2.102) | |
| Craving | Baseline | 1.706 (0.870) |
| 0.02 g/dl | 1.931 (1.050) | |
| 0.04 g/dl | 2.180 (1.161) | |
| 0.06 g/dl | 2.305 (1.291) |
Note: BrAC values averaged across medication conditions
Table 2.
Self-Administration Session Outcomes
| Variable | Medication Condition | Frequencies |
|---|---|---|
| Abstinent | Placebo | 35 |
| Naltrexone | 44 | |
| aTotal # of mini-drinks | Placebo | 1.189 (1.143) |
| Naltrexone | 0.933 (1.350) | |
| a,bLatency to 1st mini-drink | Placebo | 4 min. (4 min.) |
| Naltrexone | 6 min. (8 min.) |
Mean (SD)
Only includes participants who chose to drink during self-administration session
Association between Subjective Response and Craving (a path)
Mixed model analyses using the slope of stimulation to predict the slope of alcohol craving indicated a positive association (b=0.29, SE=0.06, t=5.17, p<.0001) such that a steeper slope of stimulation during the alcohol administration predicted higher slope of craving. Results for the slope of sedation predicting the slope of alcohol craving, however, did not support a significant association between these constructs (b=-0.03, SE=0.06, t=-0.56, p=0.58). In initial models generating individual slopes for sedation, drinking days was the only significant covariate (p = 0.02). There were no significant covariates for stimulation (p > 0.36) and for craving the following covariates were significant: gender (p < 0.01), ethnicity (p < 0.01), drinking days (p = 0.03), and ALDH2 genotype (p = 0.02).
Association between Subjective Response and Self-Administration (c path)
Total Number of Mini-Drinks Consumed:
Mixed model analyses used the slope of stimulation to predict number of mini-drinks consumed during the self-administration period. Results revealed no significant association between the progression of alcohol-induced stimulation and number of mini-drinks consumed (b=0.23, SE=0.35, t=0.65, p=0.52). For the slopes of alcohol-induced sedation, there was a significant negative association between sedation and number of mini-drinks in this sample (b=-0.69, SE=0.27, t=-2.52, p=.01), such that a steeper slope of alcohol-induced sedation was associated with fewer mini-drinks consumed during the self-administration period. There were no significant covariates for alcohol-induced stimulation (p > 0.08) and OPRM1 genotype was the only significant covariate for alcohol-induced sedation (p = 0.03).
Latency to First Drink:
Accelerated failure time models were conducted to examine the effects of SR and on latency to first drink. Participants who were abstinent were censored with Accelerated Failure Time (AFT) models. There was a significant association between alcohol-induced sedation and latency to first drink such that individuals who were more sedated had longer time to first drink (Wald χ2=6.65, p=0.01). However, there was no significant association between stimulation and latency to first drink (Wald χ2=0.05, p=0.83) during the self-administration period. In stimulation models, significant covariates were drinks per drinking day (p = 0.01), ethnicity (p < 0.01), and gender (p = 0.05). Significant covariates in sedation models were OPRM1 genotype (p = 0.02), drinks per drinking day (p < 0.01), ethnicity (p = 0.03), and gender (p = 0.05).
Association between Subjective Response, Craving, and Self-Administration (b and c’ path)
Total Number of Drinks Consumed:
When craving was added to the models, the slope of stimulation remained a non-significant predictor of number of drinks consumed (b=-0.28, SE=0.34, t=-0.82, p=0.42). The relationship between the slope of craving during the alcohol administration and number of drinks consumed was positive and statistically significant (b=1.97, SE=0.54, t=3.67, p<.001) such that greater craving was associated with consuming more mini-drinks. When craving was added to models of sedation predicting total number of mini-drinks, sedation continued to exert a significant negative association (b=-0.66, SE=0.26, t=-2.59, p=.01) such that a steeper slope of alcohol-induced sedation was associated with fewer mini-drinks consumed during the self-administration period. Craving was also a significant predictor of number of mini-drinks consumed (b=1.74, SE=0.44, t=3.96, p<0.001) in the same manner as stimulation models. Across stimulation and sedation models, the only significant covariate was OPRM1 genotype (p < 0.01).
Latency to First Drink:
Accelerated failure time (AFT) models were conducted to examine the effects of SR and craving on latency to first drink. There was no observed significant association between alcohol-induced stimulation (Wald χ2=1.23, p=0.27,). There was a significant positive association between alcohol-induced sedation (Wald χ2=7.93p=0.01) and latency to first drink during the self-administration period. There was also a significant and positive association between alcohol craving during the alcohol administration and latency to first drink in stimulation models (Wald χ2=6.03, p=.01) and sedation models (Wald χ2=6.18, p<.01). Significant covariates across stimulation models were OPRM1 genotype (p = 0.01), drinks per drinking day (p = 0.03), and ethnicity (p = 0.02). In sedation models, significant covariates were OPRM1 genotype (p < 0.01) and drinks per drinking day (p < 0.01).
Test of Mediated Effect
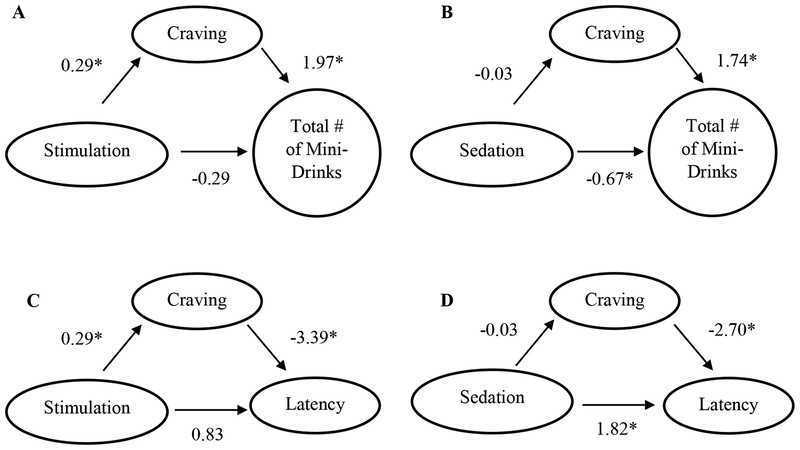
The Sobell test of mediated effects was used to analyze if craving was a mediator of our self-administration outcomes. For total number of mini-drinks, craving was not a statistically significant mediator for sedation models (z-score=-0.55, p=0.58). However, for stimulation models, craving was a significant mediator (z-score=2.94, p<0.01). For latency to 1st drink, craving was not a significant mediator for sedation (z-score=0.55, p=0.59) or stimulation models (z-score=-0.51, p=0.61). Path diagrams for the mediational models tested are presented in Figure 1. Parameter estimates for each mediation model are reported in Table 3.
Figure 1.
Path diagrams depicting results of mediation models. A) stimulation predicting total number of mini-drinks mediated by craving. B) sedation predicting total number of mini-drinks mediated by craving. C) stimulation predicting latency to first mini-drink (seconds) mediated by craving. D) sedation predicting latency to first mini-drink (seconds) mediated by craving. * p<0.05
Table 3.
Parameter Estimates for Mediation Models
| Model | Estimate (Std. Err.) | p-value |
|---|---|---|
| Outcome: Total # of Mini-drinks | ||
| Predictor: Sedation | ||
| ba | −0.034 (0.060) | 0.578 |
| bb | 1.735 (0.439) | <0.001 |
| bc (direct effect) | −0.686 (0.272) | 0.014 |
| bc’ | −0.661 (0.255) | 0.012 |
| Predictor: Stimulation† | ||
| ba | 0.289 (0.056) | <0.001 |
| bb | 1.969 (0.537) | <0.001 |
| bc (direct effect) | 0.228 (0.348) | 0.516 |
| bc’ | −0.280 (0.342) | 0.416 |
| Outcome: Latency to 1st Mini-drink | ||
| Predictor: Sedation | ||
| ba | −0.034 (0.060) | 0.578 |
| bb | −2.700 (1.086) | 0.013 |
| bc (direct effect) | 1.780 (0.691) | 0.010 |
| bc’ | 1.823 (0.648) | 0.005 |
| Predictor: Stimulation | ||
| ba | 0.289 (0.056) | <0.001 |
| bb | −3.385 (1.378) | 0.014 |
| bc (direct effect) | −0.150 (0.677) | 0.825 |
| bc’ | 0.827 (0.747) | 0.268 |
| Outcome: Average BrAC | ||
| Predictor: Sedation | ||
| ba | −0.034 (0.060) | 0.578 |
| bb | 0.018 (0.005) | <0.001 |
| bc (direct effect) | −0.006 (0.002) | 0.020 |
| bc’ | −0.005 (0.002) | 0.011 |
| Predictor: Stimulation† | ||
| ba | 0.289 (0.056) | <0.001 |
| bb | 0.015 (0.005) | 0.002 |
| bc (direct effect) | 0.008 (0.004) | 0.028 |
| bc’ | 0.004 (0.003) | 0.184 |
Note: All estimates presented are unstandardized coefficients. The term bc’ represents the effect of the predictor on the outcome while controlling for craving mediator. The term bb represents the effect of the mediator when the predictor is in the models. For latency (seconds) to 1st drink outcome, estimates derived from Accelerated Failure Time (AFT) models, whereas all other outcomes utilized mixed models. In all analyses, the following variables were controlled for: medication, randomization order for medication, OPRM1 status, gender, ethnicity, drinking days past 30 days, drinks per drinking day past 30 days, and alcohol metabolites (ALDH2 (rs671) and ADH1B (rs1229984). Significance status of these covariates is reported within the manuscript.
denotes significant mediated effect of craving
Exploratory Analyses
Exploratory analyses were conducted to examine average BrAC achieved over the course of the self-administration period. Participants averaged a BrAC of 0.042 (SD = 0.011) across medication conditions. Slope of sedation was a significant predictor of average BrAC (b=-0.005, p=0.02) and remained significant when craving was added to the model (b=-0.005, p=0.01) indicating that a steeper slope of alcohol-induced sedation was associated with lower BrAC across the self-administration session. OPMR1 genotype was a significant covariate across both models (p < 0.05), and drinks per drinking day became a significant covariate when adding craving to the model (p = 0.04). The slope of stimulation was a positive significant predictor of BrAC (b=0.008, p=0.03) suggesting steeper slope of alcohol-induced stimulation was associated with greater BrAC across the self-administration session. Significant covariates were OPRM1 genotype (p = 0.02) and drinks per drinking day (p = 0.02). However, when craving was added to this model, stimulation was no longer significant (b=0.004, p=0.18) and significant covariates in the aforementioned model remained significant (p < 0.02). Sobel test of the mediated effect found that craving did not significantly mediate the relationship between sedation and BrAC (z-score=-0.55, p=0.58), but was a significant mediator in the relationship between stimulation and BrAC (z-score=2.77, p<0.01).
DISCUSSION
Despite a rich literature on human laboratory paradigms of subjective response to alcohol, craving for alcohol, and alcohol self-administration, few studies have examined the interplay across these constructs. The present study addresses this gap in the literature by examining the interplay between SR, craving, and self-administration using a within-subjects approach in a sample of non-treatment seeking heavy drinkers of East Asian descent who completed human laboratory paradigms of alcohol challenge followed by oral alcohol self-administration (Ray et al., 2018b). Specifically, several psychological processes which are inherently within-person processes, such as the relationship between how one feels when s/he drinks and how much s/he wants to drink in the future, are presumed to be explained in between-subjects models, when in fact, within-subject analyses provide a more representative test of the process at hand (Curran and Bauer, 2011). Results from this study suggested a positive and significant relationship between alcohol-induced stimulation and alcohol craving during the alcohol challenge. The relationship between sedation and craving, however, was not significant. Importantly, when testing SR and craving as determinants of self-administration in the laboratory, there was a consistent pattern whereby the slope of craving during the alcohol challenge significantly predicted a higher number of mini-drinks consumed and lower latency to first drink. Regarding SR, only sedation predicted total number of mini-drinks, such that greater alcohol-induced sedation was associated with fewer mini-drinks consumed. Moreover, the mediation analyses undertaken suggested that craving was a significant mediator of the relationship between stimulation and total number of mini-drinks consumed. A similar pattern was found for the exploratory outcome of average BrAC during the self-administration period, whereby craving mediated the effect of alcohol-induced stimulation on average BrAC. An interesting finding was that stimulation had a direct effect on average BrAC but not on total number of mini-drinks consumed. One possible reason for this is measurement variance, such that the number of mini-drinks consumed was limited to 4 with our participants primarily either not drinking or consuming all 4 drinks, whereas with BrAC a normal distribution was observed. Additionally, BrAC has a tendency to be more often used as an outcome measure in self-administration studies than number of mini-drinks consumed (Wardell et al., 2015, Hendershot et al., 2016), so it is possible that BrAC represents a more reliable indicator of self-administration outcomes.
Overall, the present study results are generally consistent with those of Wardell, Ramchandani, and Hendershot (2015), reporting that stimulation was positively associated with alcohol intake and sedation was negatively associated with intake, while the effects of SR (both stimulation and sedation) on self-administration were in turn partially mediated by alcohol craving. Importantly, these findings significantly extend the work of Wardell and colleagues (2015) by using a decision-making self-administration task, as opposed to a task where participants are instructed to self-administer to experience the pleasure effects of alcohol and to avoid the unpleasant effects. This is critical because the instructional set implies that all participants will self-administer to some degree, whereas in our study participants only self-administered alcohol if they chose to do so. In other words, the self-administration model employed herein focuses on motivation and relative reinforcing value of alcohol, whereas the CASE model employed by Wardell and colleagues (2015) captures self-regulation of pleasant and unpleasant responses to alcohol (Zimmermann et al., 2013). However, unlike Wardell and colleagues (2015), we did not find craving to be a significant mediator in the relationship between sedation and self-administration outcomes. We speculate one reason for this difference is that sedation, as assessed by the BAES, has been shown to be highly correlated with negative affect (Ray et al., 2010), and it is possible that our results are population-specific with our sample reporting higher sedation scores that may have been more indicative of negative affect, in which craving may not be a mediator.
These results are also consistent with our recent work demonstrating that craving consistently predicted subsequent self-administration in the laboratory, even in the context of a progressive ratio translational task (Bujarski et al., 2018).
In this study, craving was the only variable to consistently predict drinking outcomes in the laboratory, and in some cases had an indirect effect on the relationship between stimulation and self-administration outcomes. Taken together, we interpret these results as indicating that craving appears to be a more proximal predictor of drinking behavior, specifically this arises in the relationship between stimulation and self-administration outcomes (total number of mini-drinks and average BrAC). Generally speaking, craving and sedation were consistent predictors of the self-administration outcomes assessed in this study. On the basis of these findings, we recommend that human laboratory models that combine SR and craving interpret craving results as more closely associated with putative drinking outcomes than SR. This may be particularly relevant in the context of human laboratory studies applied to medication development for AUD, whereby SR and craving are often examined as early efficacy indicators (Ray et al., 2018a, Litten et al., 2016).
The present study should be interpreted in light of strengths and limitations. Strengths include the within-subjects design and well characterized sample across two established human laboratory models, namely controlled IV alcohol challenge and oral alcohol self-administration. The fact that the alcohol challenge preceded the self-administration period represents a strength of the study as the SR and craving measures were obtained prior to self-administration, thus strengthening causal inferences. Study limitations include the sample comprised solely of individuals of East Asian descent, which may behave uniquely with regard to alcohol phenotypes (Wall et al., 1992), thereby limiting the generalizability of the findings. We did control for alcohol metabolizing genes (ALDH2 and ADH1B) throughout our analyses as these genes have been associated with subjective responses to alcohol (Wall et al., 1992). Additionally, our sample means for each SR suggest that our sample experienced less stimulation and greater sedation during the alcohol infusion which may have also influenced our results. Further, the medication component may have led individuals motivated to change their drinking to self-select into the study with the expectation that the medication may influence their drinking. Upon enrollment, the medication component may have also influenced drinking outcomes during the self-administration portion. The IV alcohol administration methods may have biased the results by altering expectancies, both for medication effects for the effects of IV alcohol. The low overall rates of self-administration in this sample, is also a limitation which warrants examination of these effects in samples displaying higher levels of consumption in the laboratory. The lack of a placebo alcohol condition is limiting factor as SR outcomes may have been influenced by alcohol-expectancy factors and future studies should employ a fully controlled double blind design for the alcohol component. Additionally, our representation of AUD severity leads to a study limitation whereby a majority of our participants either had no AUD or mild AUD, thus limiting generalizability. If our sample was of more severe AUD participants, we may expect stronger associations between our SR outcomes and self-administration outcomes than what we observed. The sample size poses a limitation, particularly to the mediation analyses undertaken. Analyses of the interplay between SR, craving, and self-administration using more naturalistic methods, such as a bar lab and/or ecological momentary assessment (EMA) (Ramirez and Miranda, 2014) are also recommended. Additionally, considering cue- and stress-induced craving as determinants of self-administration would inform the field as the present study focused exclusively on alcohol-induced craving. Lastly, given the ethical issues associated with alcohol administration paradigms to clinical populations (Enoch et al., 2009), it is noteworthy that our group (Bacio et al., 2014) and others (Pratt and Davidson, 2005, Sommer et al., 2015) have demonstrated that alcohol administration in the laboratory does not increase subsequent alcohol intake in the natural environment.
In conclusion, the present study examined the interplay between SR, craving, and self-administration. Insofar as drinking behavior (i.e., alcohol self-administration) represents the endpoint of interest for a host of experimental and clinical research questions, the present study suggests that alcohol craving represents a more proximal determinant than stimulation in predicting self-administration within a drinking session in the laboratory,. It is recommended that human laboratory models interpret measures of SR and craving in light of their relative predictive utility for drinking outcomes. Further, the field must continue to strive towards identifying optimal behavioral and biological endpoints for experimental and clinical research on AUD, including human laboratory models.
Acknowledgements
This research was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) to LAR (R01AA021744). Support for this study was also provided by a grant from the UCLA Clinical and Translational Science Institute (CTSI), grants UL1RR033176 and UL1TR000124. LAR has received study medication from Pfizer Medicinova and consulted for GSK and Mitsubishi Tanabe. None of the authors have conflicts of interest to disclose.
REFERENCES
- Allen JP, Litten RZ, Fertig JB, Babor T (1997) A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcoholism: Clinical and Experimental Research 21:613–619. [PubMed] [Google Scholar]
- Bacio GA, Lunny KF, Webb JN, Ray LA (2014) Alcohol use following an alcohol challenge and a brief intervention among alcohol-dependent individuals. Am J Addict 23:96–101. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA (1995) Development and Initial Validation of a Measure of Drinking Urges in Abstinent Alcoholics. Alcoholism: Clinical and Experimental Research 19:600–606. [DOI] [PubMed] [Google Scholar]
- Brick J (2006) Standardization of alcohol calculations in research. Alcohol Clin Exp Res 30:1276–1287. [DOI] [PubMed] [Google Scholar]
- Bujarski S, Hutchison KE, Prause N, Ray LA (2015a) Functional significance of subjective response to alcohol across levels of alcohol exposure. Addict Biol. [DOI] [PubMed] [Google Scholar]
- Bujarski S, Hutchison KE, Roche DJ, Ray LA (2015b) Factor Structure of Subjective Responses to Alcohol in Light and Heavy Drinkers. Alcohol Clin Exp Res 39:1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Jentsch JD, Roche DJO, Ramchandani VA, Miotto K, Ray LA (2018) Differences in the subjective and motivational properties of alcohol across alcohol use severity: application of a novel translational human laboratory paradigm. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Ray LA (2014) Subjective response to alcohol and associated craving in heavy drinkers vs. alcohol dependents: an examination of Koob’s allostatic model in humans. Drug Alcohol Depend 140:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, Ray LA (2016) Experimental psychopathology paradigms for alcohol use disorders: Applications for translational research. Behav Res Ther 86:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, Bauer DJ (2011) The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol 62:583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M (2018) Advancing Pharmacotherapy Development from Preclinical Animal Studies. Handb Exp Pharmacol. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Johnson K, George DT, Schumann G, Moss HB, Kranzler HR, Goldman D (2009) Ethical considerations for administering alcohol or alcohol cues to treatment-seeking alcoholics in a research setting: can the benefits to society outweigh the risks to the individual? A commentary in the context of the National Advisory Council on Alcohol Abuse and Alcoholism -- Recommended Council Guidelines on Ethyl Alcohol Administration in Human Experimentation (2005). Alcohol Clin Exp Res 33:1508–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich J, Earleywine M (1995) Distraction does not impair memory during intoxication: support for the attention-allocation model. Journal of studies on alcohol 56:444–448. [DOI] [PubMed] [Google Scholar]
- Gelfand LA, MacKinnon DP, DeRubeis RJ, Baraldi AN (2016) Mediation Analysis with Survival Outcomes: Accelerated Failure Time vs. Proportional Hazards Models. Frontiers in psychology 7:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Sloan ME, Stangl BL, Vatsalya V, Ramchandani VA (2017) Vulnerability for Alcohol Use Disorder and Rate of Alcohol Consumption. Am J Psychiatry 174:1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell EE, Ray LA (2018) Relationship between tonic and phasic craving for alcohol. Addictive behaviors reports 7:71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2009) Beyond Baron and Kenny: Statistical Mediation Analysis in the New Millennium. Commun Monogr 76:408–420. [Google Scholar]
- Hendershot CS, Claus ED, Ramchandani VA (2016) Associations of OPRM1 A118G and alcohol sensitivity with intravenous alcohol self-administration in young adults. Addict Biol 21:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Hasin D, O’Connor SJ, McNamara PJ, Cao D (2015) A Prospective 5-Year Re-Examination of Alcohol Response in Heavy Drinkers Progressing in Alcohol Use Disorder (AUD). Biological Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D (2011) Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry 68:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D (2014) Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry 75:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O’Malley SS (2007) Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol Psychiatry 62:694–697. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Corbin WR, Nogueira C, Krishnan-Sarin S, Potenza MN, O’Malley SS (2013) A human alcohol self-administration paradigm to model individual differences in impaired control over alcohol use. Exp Clin Psychopharmacol 21:303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Yin SJ, Crabb DW, O’Connor S, Ramchandani VA (2001) Genetic and environmental influences on alcohol metabolism in humans. Alcoholism: Clinical and Experimental Research 25:136–144. [PubMed] [Google Scholar]
- Litten RZ, Falk DE, Ryan ML, Fertig JB (2016) Discovery, Development, and Adoption of Medications to Treat Alcohol Use Disorder: Goals for the Phases of Medications Development. Alcohol Clin Exp Res 40:1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J (2006) Factor Structure of the Alcohol Urge Questionnaire Under Neutral Conditions and During a Cue-elicited Urge State. Alcoholism: Clinical and Experimental Research 30:1315–1321. [DOI] [PubMed] [Google Scholar]
- Mackinnon DP, Lockwood CM, Williams J (2004) Confidence Limits for the Indirect Effect: Distribution of the Product and Resampling Methods. Multivariate behavioral research 39:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM (1993) Development and validation of the biphasic alcohol effects scale. Alcoholism: Clinical and Experimental Research 17:140–146. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E (2009) Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry 66:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’connor S, Morzorati S, Christian J, Li TK (1998) Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcoholism: Clinical and Experimental Research 22:202–210. [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ (2002) Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 160:19–29. [DOI] [PubMed] [Google Scholar]
- Plebani JG, Ray LA, Morean ME, Corbin WR, MacKillop J, Amlung M, King AC (2012) Human laboratory paradigms in alcohol research. Alcohol Clin Exp Res 36:972–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WM, Davidson D (2005) Does participation in an alcohol administration study increase risk for excessive drinking? Alcohol 37:135–141. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’connor S (1999) A physiologically‐based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcoholism: Clinical and Experimental Research 23:617–623. [PubMed] [Google Scholar]
- Ramirez J, Miranda R Jr. (2014) Alcohol craving in adolescents: bridging the laboratory and natural environment. Psychopharmacology (Berl) 231:1841–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS (2002) Hierarchical linear models: Applications and data analysis methods, Sage. [Google Scholar]
- Ray LA, Bujarski S, Roche DJ (2016) Subjective Response to Alcohol as a Research Domain Criterion. Alcohol Clin Exp Res 40:6–17. [DOI] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Roche DJO, Magill M (2018a) Overcoming the “Valley of Death” in Medications Development for Alcohol Use Disorder. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Bujarski S, Shoptaw S, Roche DJO, Heinzerling K, Miotto K (2017) Development of the neuroimmune modulator ibudilast for the treatment of alcoholism: a randomized, placebo-controlled, human laboratory trial. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Green R, Roche DJO, Bujarski S, Hartwell EE, Lim AC, Rohrbaugh T, Ghahremani D, Hutchison K, Miotto K (2018b) Pharmacogenetic Effects of Naltrexone in Individuals of East Asian Descent: Human Laboratory Findings from a Randomized Trial. Alcohol Clin Exp Res 42:613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE (2004) A Polymorphism of the μ-Opioid Receptor Gene (OPRM1) and Sensitivity to the Effects of Alcohol in Humans. Alcoholism: Clinical and Experimental Research 28:1789–1795. [DOI] [PubMed] [Google Scholar]
- Ray LA, MacKillop J, Leventhal A, Hutchison KE (2009) Catching the alcohol buzz: an examination of the latent factor structure of subjective intoxication. Alcohol Clin Exp Res 33:2154–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Mackillop J, Monti PM (2010) Subjective responses to alcohol consumption as endophenotypes: advancing behavioral genetics in etiological and treatment models of alcoholism. Subst Use Misuse 45:1742–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Meskew-Stacer S, Hutchison KE (2007) The relationship between prospective self-rating of alcohol sensitivity and craving and experimental results from two alcohol challenge studies. J Stud Alcohol Drugs 68:379–384. [DOI] [PubMed] [Google Scholar]
- Roche DJ, Palmeri MD, King AC (2014) Acute alcohol response phenotype in heavy social drinkers is robust and reproducible. Alcohol Clin Exp Res 38:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA (1984) Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry 41:879–884. [DOI] [PubMed] [Google Scholar]
- Schuckit MA (1994) Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry 151:184–189. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL (1996) An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry 53:202–210. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N (2002) Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods 7:422–445. [PubMed] [Google Scholar]
- Singer JD (1998) Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of educational and behavioral statistics 23:323–355. [Google Scholar]
- Sobel ME (1982) Asymptotic Confidence Intervals for Indirect Effects in Structural Equation Models. Sociological Methodology 13:290–312. [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E (1986) The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addictive Behaviors 11:149–161. [DOI] [PubMed] [Google Scholar]
- Sommer C, Seipt C, Spreer M, Blumke T, Markovic A, Junger E, Plawecki MH, Zimmermann US (2015) Laboratory alcohol self-administration experiments do not increase subsequent real-life drinking in young adult social drinkers. Alcohol Clin Exp Res 39:1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM (1989) Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA‐Ar). British Journal of Addiction 84:1353–1357. [DOI] [PubMed] [Google Scholar]
- Wall TL, Thomasson HR, Schuckit MA, Ehlers CL (1992) Subjective feelings of alcohol intoxication in Asians with genetic variations of ALDH2 alleles. Alcohol Clin Exp Res 16:991–995. [DOI] [PubMed] [Google Scholar]
- Wardell JD, Ramchandani VA, Hendershot CS (2015) A multilevel structural equation model of within- and between-person associations among subjective responses to alcohol, craving, and laboratory alcohol self-administration. J Abnorm Psychol 124:1050–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H (1980) A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica: Journal of the Econometric Society:817–838. [Google Scholar]
- Zimmermann US, O’Connor S, Ramchandani VA (2013) Modeling alcohol self-administration in the human laboratory. Curr Top Behav Neurosci 13:315–353. [DOI] [PubMed] [Google Scholar]