Abstract
Background:
The American Joint Commission on Cancer will remove mitotic rate from its staging guidelines in 2018.
Objective:
Using a large nationally-representative cohort, we examined the association between mitotic rate and lymph node positivity among thin melanomas.
Methods:
149,273 thin melanomas in the National Cancer Database were examined for their association between high-risk features of mitotic rate, ulceration, and Breslow depth, with lymph node status.
Results:
Among 17,204 thin melanomas with data on Breslow depth, ulceration, and mitotic rate who underwent a lymph node biopsy, there was a strong linear relationship between odds of having a positive lymph node and mitotic rate (R2 = 0.96, p <0.0001, β = 3.31). The odds of having a positive node increased by 19% with each 1-point increase in mitotic rate (Odds ratio 1.19, 95% confidence interval 1.17 – 1.21). Cases with negative nodes had a mean mitotic rate of 1.54 ± 2.07 mitoses/mm2, compared to 3.30 ± 3.54 mitoses/mm2 for those with positive nodes (p < 0.0001)
Limitations:
The data collected do not allow for survival analyses
Conclusion:
Mitotic rate was strongly associated with the odds of having a positive lymph node and should continue to be reported on pathology.
Capsule Summary
Mitotic rate will be removed from melanoma staging guidelines in 2018
Mitotic rate is strongly associated with lymph node status in patients with thin cutaneous melanoma
Pathology reports should continue to report mitotic rate for cutaneous melanoma
Clinicians should continue to use mitotic rate when referring thin melanomas for lymph node biopsy
INTRODUCTION
There has been a marked global trend of increasing numbers of thin (≤1.00 mm) melanomas1–5. With this shifting epidemiology, there is an increased need for identifying high risk thin melanomas that will have worse outcomes6. Roughly 70% of all new melanomas are ≤1.00 mm thick, however these are still responsible for a quarter of melanoma deaths.7 The American Joint Commission on Cancer (AJCC) has developed staging guidelines since 1972 to help risk -stratify patients with cancer to guide treatments. The updated melanoma staging criteria are planned to go into effect in January 20188,9. Currently, high risk features of thin melanomas include ulceration, and a mitotic rate ≥18.
The addition of mitotic rate to the staging criteria was met with several criticisms, and the 2018 update removes this variable for staging purposes9. Robust data found mitotic rate to be a predictor of overall survival and led to its inclusion in the staging criteria.10–14 Others noted, however, that survival among the thinnest melanomas (0.01 – 0.50 mm) with an elevated mitotic rate was still better than thicker ones (0.51 – 1.00 mm) but no elevated mitotic rate that mitotic rate.8,15 This study sought to evaluate the relationship between mitotic rate, ulceration, and positive sentinel nodes using a large national database.
METHODS
This study was approved by the Institutional Review Board of Vanderbilt University Medical Center and used the National Cancer Database (NCDB) melanoma file. The NCDB is a national registry that collects registry data from all Commission on Cancer accredited facilities. It is reported to register >70% of all cancers occurring in the United States16. Data captured includes clinical and pathologic tumor-node-metastasis (TNM) staging, pathologic features such as Breslow depth, ulceration, and mitotic rate, as well as demographic information. Mitotic rate entered as “ <1” was considered a rate of zero as no numeric value could be obtained from the database, and per NCDB data collection, mitotic rate of 11 or greater was coded as 11. The NCDB begins registering cases among individuals age 18 and older, and those older than age 90 are coded as age 90.
In preliminary analyses of the data, there was considerable discrepancy between the clinical and pathologic staging entered. There were also 105,311 cases listed as “Tx” for both clinical and pathologic staging, while an additional 2,375 cases were missing both of these variables. In discussion with the NCDB, this potentially arose from the data collection rubric that states that the first values for each listed in the chart are to be used. For example, if the first staging entered in the chart came after the initial skin biopsy but prior to excision or sentinel lymph node biopsy, the staging might be listed T1aNxMx. Upon excision, there potentially could be upstaging if the melanoma was found to invade more deeply, have ulceration, or an elevated mitotic rate. The most common discrepancy was for those entered initially as TxNxMx, and then listed as a T1. As the NCDB is not linked to patient records to allow clarification of staging, it was decided that the staging would be derived from Breslow depth, mitotic rate, and presence or absence of ulceration. The rationale for this was that all information should be available from the initial pathology report and would be unlikely to be missing. The clinical decision to undergo a SLNB is made upon receipt of the initial biopsy report as well. Therefore it seemed most rational to use the data that would be available at this branch point for analyses that would guide the decision to undergo SLNB. Because analyses focused on mitotic rate, all 17,000 cases were reclassified based on the 2010 staging guidelines.
Differences between groups were tested using Chi squared analysis, Student’s t-test, or ANOVA for categorical or continuous variables, respectively, or the Mann-Whitney U or Kruskal-Wallis test for non-normally distributed continuous variables. Pearson’s correlation coefficient was used to measure the linear association between mitotic rate and lymph node positivity. Multiple logistic regression was used to calculate odds ratios (OR) and 95% confidence intervals (CI). Because T-stage was derived from variables already in the model, an additional adjustment for this was not included in the models. A two sided p-value < 0.05 was considered statistically significant. All analyses were conducted using SAS v9.1 (SAS Institute, Cary, NC) or R v3.3.1 (R Project for Statistical Computing, Vienna, Austria).
RESULTS
There were 419,787 melanoma cases in the NCDB from 2004-2013. Of these, 171,366 had a recorded Breslow depth of 0.01 - 1.00mm. We excluded 9,497 who were missing ulceration data, and an additional 12,596 who were missing mitotic rate after 2010. Cases before the updated guidelines were not mandated to record these data, and so we did not excluded those before 2010 that were missing data on mitotic rate. The final sample size included 149,273 thin melanomas (Figure 1). Of these, 122,347 were T1a, and 26,926 were T1b based on the 2010 AJCC guidelines (Table 1). The mean age was 58.6, there were more males (54.1%) than females (45.9%), and the cases were predominantly Caucasian (97.4%). A total of 52,865 patients underwent sentinel lymph node biopsy (SLNB), with detection of 5,188 positive nodes (9.8%). There were data on mitotic rate available on 17,204 of these, with 1,746 positive nodes (10.1%).
Figure 1.
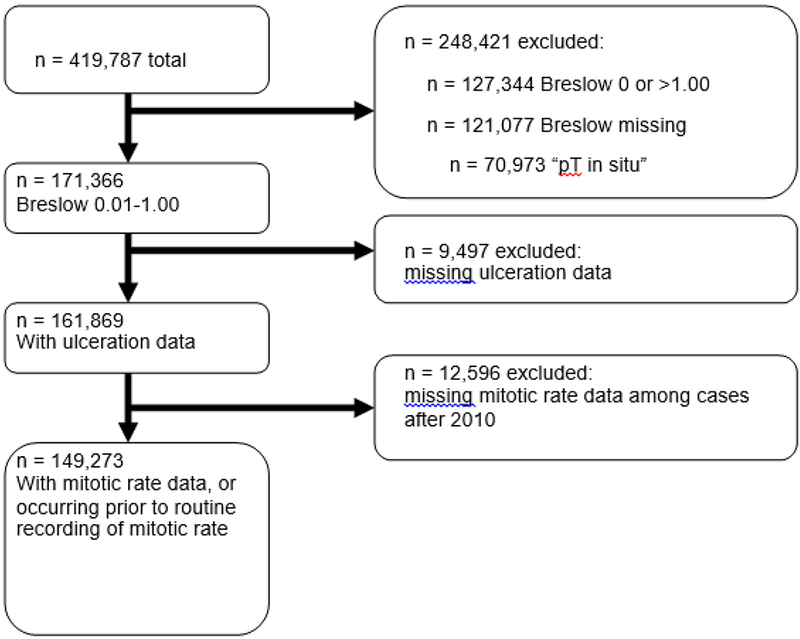
Derivation of study population from the entire melanoma cohort.
Table 1.
Characteristics of the included cohort
| N = 149,273 (%) | |
|---|---|
| Age (Mean ± SD1) | 58. 6 ± 16.3 |
| Gender | |
| Male | 80,730 (54.1%)) |
| Female | 68,543 (45.9%) |
| Race | |
| Caucasian | 145,436 (97.4%) |
| African American | 531 (0.4%) |
| Other | 3,306 (2.2%) |
| T stage | |
| T1a | 122,347 (82.0%) |
| T1b | 26,926 (18.0%) |
| Lymph nodes sampled | |
| 0 | 95,842 (64.2%) |
| 1-3 | 37,497 (25,1%) |
| >3 | 15,368 (10.3%) |
| Unknown | 566 (0.4%) |
| Lymph nodes positive | |
| No | 47,627 (89.1%) |
| Yes | 5188 (9.7%) |
| Unknown | 616 (1.2%) |
Standard deviation
The mean mitotic rate increased with the number of lymph nodes sampled (Table 2). Those who were found to have a positive node had a statistically significantly greater mean mitotic rate of 3.46 +/− 3.55, compared to 1.54 +/− 2.07 for those who were found to have negative SLNB (Mann-Whitney U, p < 0.0001, Table 2).
Table 2.
Mean mitotic rate based on number of lymph nodes sampled, and lymph node positivity.
| Number of lymph nodes sampled | Mean mitotic rate (SD1) | p-value2 |
|---|---|---|
| 0 (n = 31,044) | 0.46 (1.30) | <0.0001 |
| 1-3 (n = 12,380) | 1.62 (2.17) | |
| >3 (n = 4,838) | 2.02 (2.71) | |
| Unknown (n = 114) | 1.05 (2.14) | |
| Lymph node status | ||
| Positive (n = 1,746) | 3.46 (3.54) | <0.0001 |
| Negative (n = 15,458) | 1.54 (2.07) |
Standard deviation
Mann-Whitney U test or Kruskal-Wallis test
There was a highly statistically significant association between rate of lymph node positivity and mitotic rate (R2 = 0.96, p <0.0001, β = 3.31; Figure 2, Table 3). Examining only those with either a clinical or pathologic T1 from the chart as well as Breslow depth ≤1.00, the trend remained highly significant, though not as steep (R2 = 0.87, p <0.0001, β = 2.06). In logistic models adjusting for age, gender, Breslow depth, ulceration, and race, cases with mitotic rate ≥1 were more than twice as likely to have a positive lymph node than those with a mitotic rate <1 (OR 2.13, 95% CI 1.92 – 2.37, Table 4). Treating mitotic rate as a continuous variable, there was a 19% increased odds of a positive node per mitosis (OR 1.19, 95% CI 1.17 - 1.21, Table 4). There were no significant differences in 30- or 90-day survival between those with an elevated mitotic rate who did or did not undergo lymph node sampling (30-day: 99.7% vs. 99.6%, p = 0.51; 90-day: 98.4% vs. 98.9%, p = 0.27).
Figure 2.
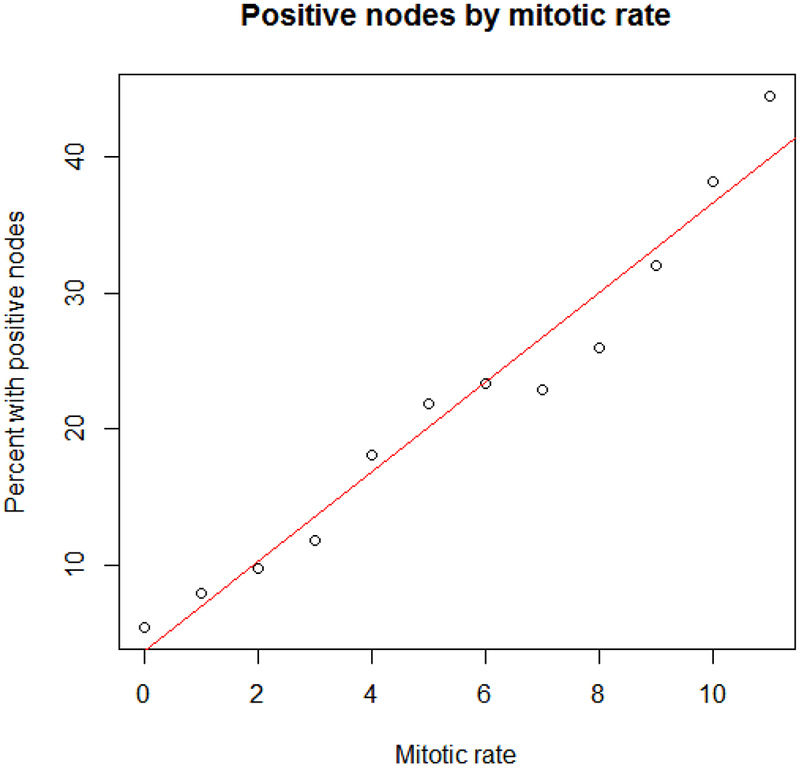
Percent with positive nodes by mitotic rate among thin melanomas. The red line represents a simple linear regression of mitotic rate by percent positive nodes.
Table 3.
Number of total thin melanomas and number and percent with positive lymph nodes based on mitotic rate.
| Mitotic rate | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ≥11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Positive | 5.4 | 7.9 | 9.7 | 11.8 | 18.0 | 21.8 | 23.4 | 22.8 | 26.0 | 32.1 | 38.3 | 44.5 |
| Positive node | 301 | 429 | 262 | 135 | 113 | 102 | 68 | 34 | 41 | 25 | 70 | 166 |
| Total | 5587 | 5444 | 2704 | 1142 | 627 | 468 | 291 | 149 | 158 | 78 | 183 | 373 |
Table 4.
Logistic models examining variables associated with positive lymph nodes.
| OR1 (95% CI2) | OR1 (95% CI2) | |
|---|---|---|
| Mitotic Rate | ≥1: 2.13 (1.92 – 2.37) <1: 1.00 (reference) |
1.19 (1.17 – 1.21) (per mitosis /mm2) |
| Ulceration | 3.53 (3.13 – 3.97) | 2.94 (2.59 – 3.33) |
| Breslow Depth | 0.991 (0.989 – 0.992) | 0.992 (0.990 – 0.993) |
| Age | 0.994 (0.990 – 0.997) | 0.993 (0.989 – 0.996) |
| Gender | Female: 0.79 (0.71 – 0.88) Male: 1.00 (reference) |
Female: 0.82 (0.73 – 0.91) Male: 1.00 (reference) |
| Race | Caucasian 1.00 (reference) AA3: 1.75 (0.92 – 3.31) Other: 0.67 (0.42 – 1.07) |
Caucasian 1.00 (reference) AA3: 1.66 (0.87 – 3.19) Other: 0.63 (0.40 – 1.02) |
Both models adjusted for all variables listed. Mitotic rate treated as a dichotomous outcome in the first column, and continuous outcome in the second column.
Odds ratio
Confidence interval
African American
There were 6,696 cases with a mitotic rate ≥1 who did not undergo a SLNB. Of these, nine were coded as having a non-zero N stage, and 32 had evidence of metastatic disease. The rest were coded as N0M0 (39.9%), or NxMx (60.0%).
Among the 121,077 cases excluded due to missing Breslow depth, there were 2,505 cases listed as being T1, T1a or T1b by either clinical or pathologic staging. Of these, 245 had data on mitotic rate, with 66 cases being ≥1. Data on nodal positivity was available for 52 of these, with five positive nodes, only one of which was in a case with an elevated mitotic rate. Among the 9,497 cases excluded due to missing ulceration data, there were 228 with data for both mitotic rate and lymph node status. There were 25 cases with positive nodes: 2 of 48 cases among those without an elevated rate (4.2%) and 23 of 180 among those with mitotic rate ≥1 (12.8%)(p = 0.09).
DISCUSSION
In a national database containing nearly 150,000 thin melanomas, and 17,000 cases with SLNB and data on mitotic rate, the odds of having a positive sentinel lymph node was strongly correlated with mitotic rate. Of those with an elevated mitotic rate, cases undergoing SLNB with a mitotic rate of 1 had a positive node 7.9% of the time, ranging to 44.5% with positive nodes among those with a mitotic rate of ≥11. Using the 2010 AJCC staging guidelines cutoff of a mitotic rate ≥1, this would capture 82.8% of all of the positive nodes detected in this dataset. Although those with a mitotic rate of 1-3 had a lower odds of positive SLNB, these represent a much more common event, and this group makes up the majority of cases ultimately found to have a positive SLN. Offering a SLNB to those with a mitotic rate ≥ 2, for example, would identify only 58.2% of those with a positive node, while a threshold of ≥3 would identify only 43.2%. These results are powerful in that the initial pathology report of a thin melanoma that has been biopsied to its base contains sufficient information to make a strong estimate regarding the risk of having a positive node.
This is not the first study to observe that mitotic rate was associated with positive node17–20. Most recently, Wat et al. observed a significant association between mitotic rate and lymph node positivity among intermediate thickness (1.01-2.00mm) melanomas, but not among thin melanomas18. The study included 1,072 cases, with only 166 thin melanomas, and 15 with a positive node. Ten percent of those with an elevated mitotic rate had a positive SLNB, compared to 3.6% of those without an elevated rate, however, suggesting the study was underpowered to detect a significant difference in this subgroup. Another group observed a statistically significant association between mitotic rate and both lymph node status and survival among 1,524 intermediate thickness melanomas, but did not examine thin melanomas21. Most notably, one single center study of 1,764 melanomas did observe a significant association between tumor mitotic rate and overall survival in unadjusted analyses22. Other studies have failed to observe statistically significant associations between mitotic rate and lymph node positivity among thin melanomas, however these were likely underpowered, as the largest had only 484 cases23–26. Our study included more than 17,000 thin melanoma cases who underwent a SLNB and had data on mitotic rate, finding a strong correlation between mitotic rate and lymph node positivity.
Several studies have examined the impact of the 2010 AJCC staging guidelines. One single center study found that 29% of cases were changed between T1a and T1b; all of those with positive lymph nodes were listed as T1b in both staging schemes27. Another single-center study observed a non-significant decrease in thin melanomas with an elevated mitotic rate after the adoption of the 2010 guidelines28. Thus a criticism of the addition of mitotic rate to the staging criteria was that it did no better at identifying high-risk thin melanomas. Moreover, it was suggested that an elevated mitotic rate should not warrant a SLNB as it was not significantly associated with survival in a systematic review15. Many of the studies cited in this review did, however, show a trend toward lower survival with an elevated mitotic rate8,29–31. Most of these had a much lower sample size than the current study, and it is likely they were simply under-powered to detect a statistical difference. Balch et. al observed that mitotic rate was the second strongest predictor of survival after Breslow depth among 10,233 melanomas, however8.
An additional criticism of the use of mitotic rate for staging purposes has been that there were no treatments to be offered in this subgroup that can prolong survival15. Since the publication of this criticism, however, the field of melanoma immunotherapy with checkpoint inhibitors has expanded greatly, and have now shown survival benefit among patients with nodal and not just distant metastases32. Moreover, a positive node no longer should mandate an immediate completion, sparing the associated risk of lymphedema, as for most patients no survival benefit has been shown from the dissection33. There are, however, some data showing that patients with >2mm metastases, extracapsular extension, and multiple positive nodes may benefit from completion lymphadenectomy34.
The inverse association with positive nodes and Breslow depth was curious, as the overwhelming bulk of literature shows a worse prognosis with increasing Breslow depth. To determine if this inverse association existed in the thin melanomas only, we reran the analysis using all cases with available data in the NCDB with all Breslow depths included (n = 52,569). In this larger set, an increasing Breslow depth was associated with an increased odds of having a positive node (OR 1.02, 95% CI 1.02 – 1.02, p < 0.0001), which is consistent with the findings of the AJCC. This finding suggests that the inverse association was only among thin melanomas. Looking more closely at the thin melanomas only, there was a marked predominance of T1b melanomas thinner than 0.75mm; this is in agreement with international trends of seeing thinner melanomas.1–5 The inverse association with Breslow depth is thus potentially related to the changing epidemiology of melanoma overall rather than a systemic error in the data. Alternatively, many thin melanomas may have been treated outside of Commission on Cancer-approved facilities, and only those with positive nodes may have been referred to these centers and therefore were registered in the database.
This study had several limitations. Most notably, the NCDB is not linked to individual pathology reports or case records, and so collecting follow-up data for survival analyses was not possible. As such these results can suggest an association, but cannot be used as predictors of prognosis. Next, there was a high degree of missing data. We excluded more than 12,000 cases occurring after 2010 that were missing data on mitotic rate. This group had similar age, gender, race, and Breslow depth distribution compared to those included, and logistic models for positive lymph node status yielded similar values to those from the included cases, making it unlikely these exclusions would have created a spurious association. We also excluded cases missing data on Breslow depth or ulceration. Low numbers with available data precluded conducting analyses on the group missing Breslow depth. Among those missing ulceration only, however, there was a nonsignificant three-fold increase in the proportion with positive nodes among those cases with elevated mitotic rate compared to those mitotic rate <1, suggesting these exclusions did not affect the overall conclusions.
Not all cases with positive nodes had high-risk features. There were 301 cases with a mitotic rate <1 and a positive node. Of these, only 33 had ulceration, and 5 occurred prior to 2010 with no data on mitotic rate collected. Of those with a mitotic rate <1 and a positive node, 97 of the 301 had clinical evidence of nodal or metastatic spread, based on the clinical N or M stage being non-zero, 51 were missing clinical TNM staging, and the remaining 153 had no evidence of high-risk features recorded.
A recent study identified decimal errors in SEER data that falsely suggested a higher mortality among thin melanomas35. Upon linking to the records, these errors were corrected and the spike in mortality disappeared. While it is not possible to link to individual records to search for these sorts of errors, when we excluded cases with a T-stage listed in the chart as anything but T1, we observed a very similar trend. It would be highly unlikely that patients had both a decimal error in their Breslow depth and a downstaging error in their data entry to such a systematic degree as to create the association observed.
The 8th edition of the AJCC melanoma staging guidelines are planned to go into effect January 1, 2018. These guidelines will no longer contain mitotic rate as a criteria based on a number of smaller studies that have failed to identify a significant association between mitotic rate and survival. In this large study of nearly 150,000 thin melanomas with more than 17,000 cases with mitotic rate data, we observed a highly statistically significant association between mitotic rate and SLNB positivity rate. These results suggest that mitotic rate should continue to be reported. Additional studies are needed to confirm the association between mitotic rate and having a positive node.
Acknowledgments
Funding: none
REFERENCES
- 1.Ambrosini-Spaltro A, Dal Cappello T, Deluca J, Carriere C, Mazzoleni G, K E. Melanoma incidence and Breslow tumour thickness development in the central Alpine region of South Tyrol from 1998 to 2012: a population-based study. J Eur Acad Dermatol Venereol 2015;29:243–8. [DOI] [PubMed] [Google Scholar]
- 2.Hardwicke J, Brunt AM, Rylands G, Rayatt S. Ten-year audit of melanoma in a central England population. Acta Derm Venereol 2011;91:440–3. [DOI] [PubMed] [Google Scholar]
- 3.van der Leest R, Zoutendijk J, Nijsten T, Mooi WJ, van der Rhee JI, de Vries E, Hollestein LM. Increasing time trends of thin melanomas in The Netherlands: What are the explanations of recent accelerations? Eur J Cancer 2015;51:2833–41. [DOI] [PubMed] [Google Scholar]
- 4.Baade P, Meng X, Youlden D, Aitken J, Youl P. Time trends and latitudinal differences in melanoma thickness distribution in Australia, 1990-2006. Int J Cancer 2012;130:170–8. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Saraiya M, Patel P, Cherala SS, Barnholtz-Sloan J, Kim J, Wiggins CL, Wingo PA. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol 2011;65:S17–25. [DOI] [PubMed] [Google Scholar]
- 6.Mihic-Probst D, Shea C, Duncan L, de la Fouchardiere A, Landman G, Landsberg J, ven den Oord J, Lowe L, Cook MG, Yun SJ, Clarke L, Messina J, Elder DE, Barnhill RL. Update on Thin Melanoma: Outcome of an International Workshop. Adv Anat Pathol 2016;23:24–9. [DOI] [PubMed] [Google Scholar]
- 7.Karakousis G, Gimotty PA, Bartlett EK, Myung-Shin S, Neuwirth MG, Fraker D, Czerniecki BJ, Faries MB. Thin Melanoma with Nodal Involvement: Analysis of Demographic, Pathologic, and Treatment Factors with Regard to Prognosis. Annals Surg Oncol 2017;24:952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balch C, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009;27:6199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR., Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR (Eds.), ed. AJCC Cancer Staging Manual, 8th Edition: Springer International Publishing; 2017. [Google Scholar]
- 10.Gimotty P, Guerry D. Prognostication in thin cutaneous melanomas. Arch Pathol Lab Med 2010;134:1758–63. [DOI] [PubMed] [Google Scholar]
- 11.Gimotty P, Guerry D, Ming ME, Elenitsas R, Xu X, Czerniecki B, Spitz F,, Schuchter L ED. Thin primary cutaneous malignant melanoma: a prognostic tree for 10-year metastasis is more accurate than American Joint Committee on Cancer staging. . J Clin Oncol 2004;22:3668–76. [DOI] [PubMed] [Google Scholar]
- 12.Gimotty P, Elder DE, Fraker DL, Botbyl J, Sellers K, Elenitsas R, Ming ME,, Schuchter LSF, Czerniecki BJ, Guerry D. . Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol 2007;25:1129–34. [DOI] [PubMed] [Google Scholar]
- 13.Karakousis G, Gimotty PA, Botbyl JD, Kesmodel SB, Elder DE, Elenitsas R, Ming MEGD, Fraker DL, Czerniecki BJ, Spitz FR. Predictors of regional nodal disease in patients with thin melanomas. Ann Surg Oncol 2006;13:533–41. [DOI] [PubMed] [Google Scholar]
- 14.Murali R, Haydu LE, Quinn MJ, Saw RP, Shannon K, Spillane AJ, Stretch JR, Thompson JF, Scolyer RA. Sentinel lymph node biopsy in patients with thin primary cutaneous melanoma. Ann Surg 2012;255:128–33. [DOI] [PubMed] [Google Scholar]
- 15.Kirkland E, Zitelli JA. Mitotic rate for thin melanomas: should a single mitotic figure warrant a sentinel lymph node biopsy? Dermatol Surg 2014;40:937–45. [DOI] [PubMed] [Google Scholar]
- 16.Boffa D, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, Thoburn K, Gress D,, McKellar DPSL, Facktor MA, Winchester DP. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017. [DOI] [PubMed] [Google Scholar]
- 17.Ranieri J, Wagner JD, Wenck S, Johnson CS, Coleman JJ 3rd. The prognostic importance of sentinel lymph node biopsy in thin melanoma. Ann Surg Oncol 2006;13:927–32. [DOI] [PubMed] [Google Scholar]
- 18.Wat H, Senthilselvan A, Salopek TG. A retrospective, multicenter analysis of the predictive value of mitotic rate for sentinel lymph node (SLN) positivity in thin melanomas. J Am Acad Dermatol 2016;74:94–101. [DOI] [PubMed] [Google Scholar]
- 19.Oliveira Filho R, Ferreira LM, Biasi LJ, Enokihara MM, Paiva GR,, J W Vertical growth phase and positive sentinel node in thin melanoma. Braz J Med Biol Res 2003;36:347–50. [DOI] [PubMed] [Google Scholar]
- 20.Kesmodel S, Karakousis GC, Botbyl JD, Canter RJ, Lewis RT, Wahl PM, Terhune KPAA, Elder DE, Ming ME, Guerry D, Gimotty PA, Fraker DL, Czerniecki BJ, FR S. Mitotic rate as a predictor of sentinel lymph node positivity in patients with thin melanomas. Ann Surg Oncol 2005;12:449–58. [DOI] [PubMed] [Google Scholar]
- 21.Mandalà M, Galli F, Cattaneo L, Merelli B, Rulli E, Ribero S, Quaglino P, De Giorgi V, Pigozzo J, Sileni VC, Chirco A, Ferrucci PF, Occelli M, Imberti G, Piazzalunga D, Massi D, Tondini C, Queirolo P; Italian Melanoma Intergroup. Mitotic rate correlates with sentinel lymph node status and outcome in cutaneous melanoma greater than 1 millimeter in thickness: A multi-institutional study of 1524 cases. J Am Acad Dermatol 2017;76:264–73. [DOI] [PubMed] [Google Scholar]
- 22.Rutkowski P, Szydłowski K, Nowecki ZI, Sałamacha M, Goryń T, Mitręga-Korab B, Pieńkowski A, Dziewirski W, Zdzienicki M. The long-term results and prognostic significance of cutaneous melanoma surgery using sentinel node biopsy with triple technique. World J Surg Oncol 2015;13:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong S, Brady MS, Busam KJ, Coit DG. Results of sentinel lymph node biopsy in patients with thin melanoma. Ann Surg Oncol 2006;13:302–9. [DOI] [PubMed] [Google Scholar]
- 24.Cooper C, Wayne JD, Damstetter EM, Martini M, Gordon J, Guitart J, West DP,, Nardone BRA, Gerami P A 10-year, single-institution analysis of clinicopathologic features and sentinel lymph node biopsy in thin melanomas. J Am Acad Dermatol 2013;69:693–9. [DOI] [PubMed] [Google Scholar]
- 25.Murali R, Haydu LE, Quinn MJ, Saw RP, Shannon K, Spillane AJ, Stretch JR, Thompson JFSR. Sentinel lymph node biopsy in patients with thin primary cutaneous melanoma. Ann Surg 2012;255:128–33. [DOI] [PubMed] [Google Scholar]
- 26.Venna S, Thummala S, Nosrati M, Leong SP, Miller JR 3rd, Sagebiel RW, M K-S. Analysis of sentinel lymph node positivity in patients with thin primary melanoma. J Am Acad Dermatol 2013;68:560–7. [DOI] [PubMed] [Google Scholar]
- 27.Chu V, Tetzlaff MT, Torres-Cabala CA, Prieto VG, Bassett R Jr, Gershenwald JE, McLemore MS, Ivan D, Wang WL, Ross MI, Curry JL. Impact of the 2009 (7th edition) AJCC melanoma staging system in the classification of thin cutaneous melanomas. Biomed Res Int 2013;2013:898719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larson A, Rothschild B, Walls AC, Granter SR, Qureshi AA, Murphy GF, Laga AC. Impact of the 2009 AJCC staging guidelines for melanoma on the number of mitotic figures reported by dermatopathologists at one institution. J Cutan Pathol 2015;42:536–41. [DOI] [PubMed] [Google Scholar]
- 29.Thompson J, Soong SJ, Balch CM, Gershenwald JE, Ding S, Coit DG, Flaherty KT,, Gimotty PAJT, Johnson MM, Leong SP, Ross MI, Byrd DR, Cascinelli N,, Cochran AJEA, McMasters KM, Mihm MC Jr, Morton DL, Sondak VK . Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol 2011;29:2199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azzola M, Shaw HM, Thompson JF, Soong SJ, Scolyer RA, Watson GF, Colman MH,, Y Z. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: an analysis of 3661 patients from a single center. Cancer 2003;97:1488–98. [DOI] [PubMed] [Google Scholar]
- 31.Gimotty P, Elder DE, Fraker DL, Botbyl J, Sellers K, Elenitsas R, Ming ME,, Schuchter LSF, Czerniecki BJ, Guerry D. Identification of high-risk patients among those diagnosed with thin cutaneous melanomas. J Clin Oncol 2007;25:1129–34. [DOI] [PubMed] [Google Scholar]
- 32.Eggermont A, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H,, Hamid ORC, Ascierto PA, Richards JM, Lebbé C, Ferraresi V, Smylie M,, Weber JSMM, Bastholt L, Mortier L, Thomas L, Tahir S, Hauschild A, Hassel JCHF, Taitt C, de Pril V, de Schaetzen G, Suciu S, Testori A. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med 2016;375:1845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faries M, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS,, Jahkola TBT, Testori A, Beitsch PD, Hoekstra HJ, Moncrieff M, Ingvar C, , Wouters MWJMSM, Levine EA, Agnese D, Henderson M, Dummer R, Rossi CR, et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N Engl J Med 2017;8:2211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Bilimoria KY. Weighing the value of completion nodal dissection for melanoma. J Surg Oncol 2016;114:281–7. [DOI] [PubMed] [Google Scholar]
- 35.Gimotty P, Shore R, Lozon NL, Whitlock J, He S, Vigneau FD, Dickie L, Elder DE, Xu X, Schwartz AG, Guerry D. Miscoding of Melanoma Thickness in SEER: Research and Clinical Implications. J Invest Dermatol 2016;136:2168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


