ABSTRACT
Proanthocyanidins are phytonutrients formed by oligomerization or polymerization of subunits catechin, epicatechin, and their gallic acid esters. Proanthocyanidins are a component of many plants and thus form an integral part of the human diet. Oligomeric proanthocyanidins are currently marketed as medicinal products that target vascular disorders and chronic pathological conditions, many of which are age-associated. Proanthocyanidins are also characterized by their effects on energy homeostasis. Not dissimilar to their chemically synthesized counterparts, naturally extracted proanthocyanidins act via inhibition of lipases, stimulation of energy expenditure, or suppression of appetite. Here we review the current knowledge-base and highlight challenges and future impacts regarding involvement of proanthocyanidins in global lipid metabolism, with a focus on the molecular mechanisms and pathological conditions that progress with aging.
Keywords: proanthocyanidin, procyanidin, obesity, lipid, polyphenol, flavonoid, grape seeds, aging
Introduction
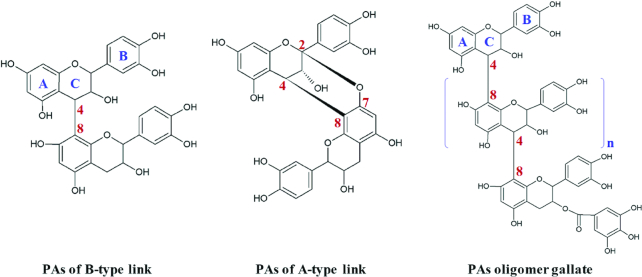
Proanthocyanidins (condensed tannins), when first described in the 1920s, were referred to as “leuco-anthocyanins” because of their ability to hydrolyze, in the presence of acids, into the colored flavylium ion caynidin (1). Belonging to a polyphenol family, proanthocyanidins exist ubiquitously in many edible plant sources including barley, hops, maize, apples, grapes, strawberries, cocoa, almonds, cinnamon, peanuts, and tea. Grape seeds in particular provide a diverse source of proanthocyanidins, owing to the abundance of galloylated oligomers and type-A linked species (2) (Figure 1). Consumption of proanthocyanidins has been linked to a range of health benefits by combating aspects of cardiovascular diseases, certain cancers, diabetes, inflammation, neurodegeneration, allergies, and geriatric disorders (Figure 2) (Supplementary Table 1).
FIGURE 1.
Basic scaffold and numbering system of proanthocyanidins (PAs), and their B-type compared with A-type links.
FIGURE 2.
Potential health benefits of proanthocyanidins (PAs).
Proanthocyanidins are thought to be the most structurally complex flavonoid, comprising the elementary monomeric subunit of falvan-3-ols to form the bioactive oligomers (degree of polymerization, DP = 2–5) and polymers (DP > 5). This subunit is composed of 15 carbons with 2 aromatic rings connected by a heterocyclic ring (Figure 1). Oxidation occurs between C4 of the pyrone ring and C6 or C8 of the attached A and B rings, which facilitates the polymerization, a connection that is referred to as B-type (dimeric) and C-type (trimeric) linkage. The ether bond formed between C2→C7 or C2→C5 is an A-type linkage. Three chiral centers influence the 3-dimensional structure of the molecule and thereby its hydrophilicity, protein binding properties, and bioactivities. Esterification and glycosylation have been identified to occur predominantly at the C3 position. The diverse DP, multivariate stereoisomer formations, and complex hydroxylation patterns of proanthocyanidins lead to the wide range of bioactivities that are specific to the individual moiety and composition. For instance, B-type enriched proanthocyanidins derived from the cinnamon species Cinnamomum cassia regulate lipid accumulation in adipose tissue and liver. In comparison, A-type proanthocyanidins extracted from Cinnamomum tamala increase the concentration of insulin in the blood and pancreas (3). In general, oligomeric proanthocyanidins are thought to be most bioactive in terms of regulating lipid metabolism (4–7), in particular the galloyl ester structure (8–11). The heterogeneous building blocks and complex linkage patterns of proanthocyanidins make their fractionation and purification challenging.
In general, monomeric catechins and their gallates can be absorbed intact in the small intestine, although the less hydrophilic epicatechin gallate can only be detected in trace amounts (12). The plasma half-life of dietary catechin ranges from 2 to 4 h, with epicatechin being slightly longer, and gallated forms and ring-fission products the longest (13). Dimeric and trimeric proanthocyanidins can be absorbed readily by passive transport, crossing the tight junctions via the paracellular route. Higher oligomeric proanthocyanidins decompose in a time-dependent manner, however, leading to rapid accumulation of resultant monomers, dimers, and traces of trimers (12, 14). However, a different conclusion was drawn when proanthocyanidins were administered to rats and monitored over a 24-h period. The majority of proanthocyanidins remained in their original forms along the gastrointestinal tract with trace amounts of metabolites occurring in the duodenum/jejunum and ileum, which contradicts the notion that oligomeric proanthocyanidins are depolymerized into monomeric units (15). Based on the distribution of methylated metabolites, it has been hypothesized that the dose is a determining factor of the primary site of metabolism, with large doses being metabolized in the liver and small doses metabolized by the intestinal mucosa. Nonabsorbable proanthocyanidins higher than octamers were also found to influence absorption of proanthocyanidin oligomers. These proanthocyanidins bind competitively to mucosal proteins of the digestive tract, thereby allowing the proanthocyanidin oligomers to be absorbed (16).
The well-recognized cardioprotective properties of proanthocyanidins have been linked to the “French Paradox,” which refers to epidemiological studies uncovering that French people have, on average, a lower incidence of coronary heart disease despite a higher consumption of saturated fat, but incidentally also a higher intake of proanthocyanidins via wine consumption (17). The vasodilatory bioactivities of proanthocyanidins may be attributed to beneficial regulation of lipid homeostasis which will be reviewed. The decreased intestinal chylomicron secretion (18–22) and lipid absorption (23–29), stimulated fatty acid oxidation (30–34), repressed hepatic lipogenesis (35–45), and low-density lipoprotein secretion (46–50) by proanthocyanidins, and their interactions with epigenetic factors (51–53) and extrahepatic sites (54–60) have been shown to adjust triglyceride, lipoprotein, and cholesterol concentrations in vitro and in vivo. In addition, proanthocyanidins positively regulate multiple signaling pathways that are shared between lipid disorders, for example obesity, and common aging-associated diseases, which will also be discussed (61–107).
Methods
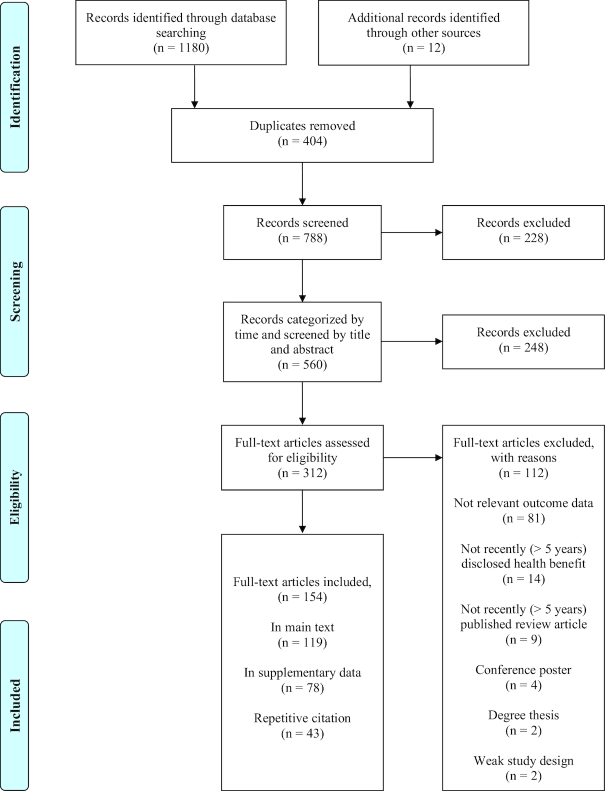
Information for this review was compiled by searching PubMed (https://www.ncbi.nlm.nih.gov/pubmed), Scopus (https://www.scopus.com), Google Scholar (https://scholar.google.com), and the reference lists of articles to identify additional sources. Databases were scanned from all years of study until December 2017 for in vitro, animal, or human studies on lipid metabolism and from 2012 to 2017 for studies on aging-related diseases. The databases were searched systematically using the terms “proanthocyanidin,” “procyanidin,” in combination with “lipid,” “obesity,” “cholesterol,” “hypolipidemia,” “bioavailability,” “epidemiological study,” “aging,” “cancer,” “cardio,” “diabetes,” “oxidation,” “inflammation,” and “degeneration.” This review was reported following the PRISMA (http://prisma-statement.org/) statement (Figure 3).
FIGURE 3.
Screening process for a systematic review of research investigating the metabolic regulation mechanisms of proanthocyanidins and links with other aging-associated diseases.
Proanthocyanidins and the lipid regulation link
Proanthocyanidins restrict absorption of lipids
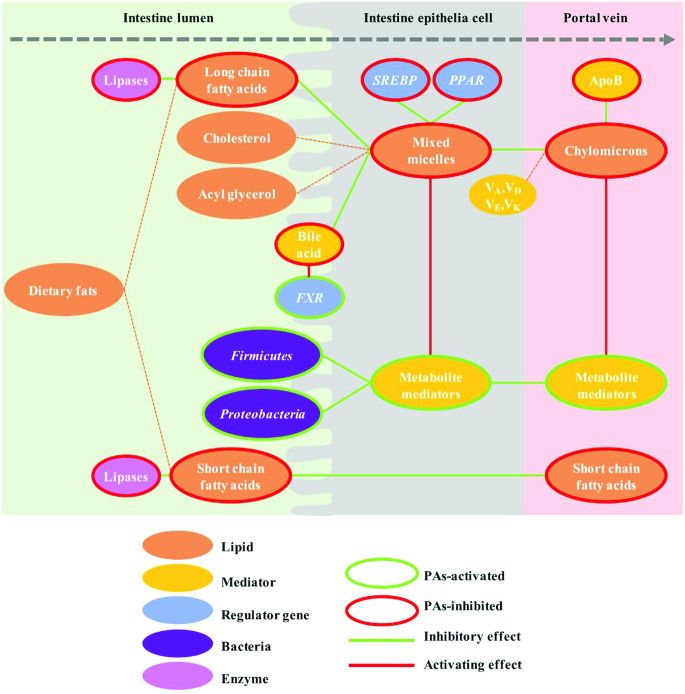
Glycerol, short- and medium-chain fatty acids are directly absorbed from the intestinal lumen into the portal vein for further utilization in the liver. In contrast, long-chain fatty acids, free cholesterol, and β-acyl glycerol, together with bile salts, form mixed micelles. The micellar solubility of cholesterol was shown to be reduced by proanthocyanidin dimers and trimers (procyanidin B2, B5, C1) (18) and the cholesterol micelle can be degraded further by procyanidin A1 and a trimer [epicatechin-(4β→6)-epicatechin-(2β→O→7, 4β→8)-catechin] (19), suggesting that proanthocyanidins may inhibit absorption of cholesterol and bile acids (BAs). Having penetrated the intestinal epithelial cells as mixed micelles, triglycerides are resynthesized and packed, with cholesterol and fat-soluble vitamins, into chylomicrons and exported to the circulatory system, which in turn facilitates transport of lipids through the basolateral intestine epithelial cells. Proanthocyanidins (of grape and apple origin) were also linked to delayed absorption of triglyceride and cholesterol thereby reducing secretion of chylomicrons (20, 21). Likewise, a significant reduction of apoB-48 secretion (the marker of chylomicrons) was linked to consumption of red wine or polyphenols extracted from apples (22) (Figure 4).
FIGURE 4.
Proanthocyanidins downregulate absorption of dietary lipids in gastrointestinal tract. ApoB, Apolipoprotein B; FXR, Farnesoid X receptor; PPAR, Peroxisome proliferator-activated receptor; SREBP, Sterol receptor element binding protein.
Proanthocyanidins coordinate digestion of neutral lipids
When hydrophobic fat globules are broken up into smaller emulsion droplets, they are hydrolyzed by water-soluble lipases including lingual lipase, gastric lipase, and pancreatic lipase (PL), and assisted by phospholipase A2, cholesterol esterase, and isomerase. The resultant FFAs are subsequently digested to acetyl-CoA via β-oxidation. A decrease in fat digestion and absorption, which can be caused by dysfunctional gastric and pancreatic cells or their lipase synthesis, can trigger weight loss. In contrast, the suboptimal secretion of bile salts, as derivatives of cholesterol, can have a negative impact on formation of micelles and thus digestion of dietary fat, thereby inducing hyperlipidemia. The following sections will focus on the involvement of proanthocyanidin in this lipid-digesting cascade (Figure 5).
FIGURE 5.
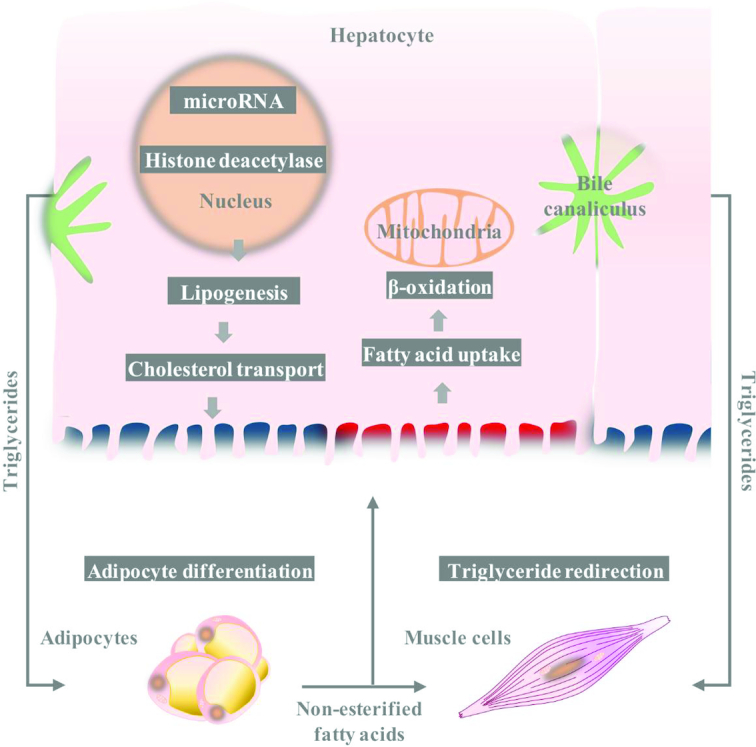
Proanthocyanidins regulate global lipid profiles in hepatocyte and extrahepatic sites, targeting key anabolic and catabolic cascades.
Inhibition of lipases
Several in vitro experiments have suggested that proanthocyanidins inhibit lipases, which in turn reduces fat absorption, and thus proanthocyanidins are potential alternatives to pharmaceutical treatments for obesity (e.g., orlistat). For example, the effects of grape seed procyanidin extract (GSPE) on PL, lipoprotein lipase, and hormone-sensitive lipase were evaluated. The isoproterenol-stimulated lipolytic activity of cultured 3T3-L1 cells was lower when exposed to GSPE, which reduced circulating FFAs. It was suggested that compounds within the extract, including their antioxidative metabolites, may exert synergistic effects (23). Cholesterol esterase was also shown to be significantly inhibited by GSPE in a dose-dependent manner. It is thought that GSPE and BAs fuse into insoluble complexes in the intestine, which inhibits formation of cholesterol micelles in the intestinal lumen, increases their fecal excretion, and thus reduces lipid digestion (24). GSPE also exerts a strong inhibitory effect against pancreatic α-amylase, involving both aggregate formation and enzymatic inhibition (25, 26). In detail, proanthocyanidins (purity 98%) inhibit PL in a concentration-dependent manner by aggregating PL to form PL-proanthocyanidin complexes. The inhibition is likely mediated by the hydrophobic interaction and hydrogen bonding between PL and proanthocyanidins with increasing DP. Thus, formation of the PL-proanthocyanidin complex is thought to be reversible (27). A similar action was also observed in vitro with proanthocyanidin-enriched apple extracts and cocoa extracts. Pancreatic α-amylase, PL, and phospholipase A2 were dose-dependently inhibited by proanthocyanidins (DP = 2–10, B type), characterized by an inverse correlation between log IC50 and DP (28). In contrast, gallic acid, catechins, and epicatechins were not active (29). However, the PL and the substrates applied were not very pure and this brings into question to what extent these findings mimic physiological reality.
Stimulation of β-oxidation and glycerolipid/FFA cycle
Hamsters fed a diet supplemented with GSPE for a period of 15 d were marked by significantly reduced adipose tissue depots, lower FFAs in the plasma, and decreased TG accumulation within the mesenteric white adipose tissue. Experiments at the transcriptional level uncovered that exposure to GSPE regulates β-oxidation relevant genes in retroperitoneal white adipose tissue, including very long-chain acyl-CoA dehydrogenase, carnitine palmitoyltransferase, peroxisomal proliferator-activated receptor α (Ppara), hormone-sensitive lipase, adipose triglyceride lipase, glycerol-3-phosphate acyltransferase, diacylglycerol acyltransferase, and glycerokinase-related genes (30). Offspring of rats fed a standard diet supplemented with GSPE had reduced amounts of circulating C-reactive proteins and lower respiratory quotient values, increased amounts of total and phosphorylated 5’-AMP-activated protein kinase (AMPK), and overexpression of fatty acid uptake genes (fatty acid transport protein 1 and CD36) and β-oxidation genes (Ppara and hydroxyacyl-CoA dehydrogenase) in skeletal muscle. Proanthocyanidins were therefore concluded to shift the rate of β-oxidation via AMPK regulation and to program healthy male offspring towards an improved circulating inflammatory profile and greater lipid utilization in adulthood (31). Flavangenol (pine bark extracts, Toyo Shinyaku Co, Ltd) in the main form of procyanidin B1 was reported to suppress fat accumulation in Western diet-fed Tsumura Suzuki obese diabetes mice and FFA-loaded human liver HepG2 cells (32). It induces mRNA expression of fatty acid oxidative enzymes including PPARA, acyl-CoA oxidase and carnitine palmitoyltransferase. This aligns well with the traditional therapy for hyperlipidemia, which typically applies fibrate drugs that bind to PPARA in the liver and induce expression of fatty acid oxidative genes (33). The liver is also believed to be crucial and probably the main organ in which proanthocyanidins modulate lipid metabolism (34). Moreover, Flavangenol significantly suppressed intracellular fat accumulation in the liver and therefore was deemed to have potentially beneficial effects in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis (32).
Proanthocyanidins decrease hepatic lipogenesis
In the lipogenesis pathway, glycerol 3-phosphate accepts 2 acyl groups to form phosphatidic acid or diacylglycerol 3-phosphate. Phosphatidic acid, incidentally the more common precursor, is then converted to triglyceride via dephosphorylation and acyl transfer reactions. Proanthocyanidins are thought to be actively involved in the regulation of lipogenesis, cholesterol synthesis, and the accompanying production of VLDLs.
The hypolipidemic property of proanthocyanidin-enriched peanut skin extract was investigated in rats fed a Western diet. The presence of proanthocyanidins decreased mRNA expression of fatty acid synthase, sterol receptor element binding protein (SREBP)-1c and acetyl-CoA carboxylase. Expression of genes regulating lipid uptake, such as Pparg, was upregulated, but Ppara was downregulated, which in turn decreased TGs and cholesterol in plasma and liver (35). Analogous effects were observed in vitro, where HepG2 cells were treated with sera collected from rats administered proanthocyanidins, yielding a bioactive form of proanthocyanidins (e.g., conjugated metabolites). GSPE and proanthocyanidins obtained from French maritime pine bark reduced de novo fat synthesis, but cocoa did not (36). Proanthocyanidin-mediated inhibition of TGs and apoB was attributed to induction of the nuclear receptor small heterodimer partner (NR0B2/SHP) via lipogenic genes including SREBP-1c, carnitine palmitoyltransferase-1A, and apoA-5 (37). Through use of mouse mutants for the SHP and liver X receptor α and β, it was confirmed that proanthocyanidins decrease plasma TGs by activation of the farnesoid X receptor (FXR), transient upregulation of SHP, and subsequent repression of SREBP-1c (38).
GSPE is also thought to mediate hypolipidemia through effects on microRNA (miR). Expression of miR-33 and miR-122, miR-33 target ATP-binding cassette gene, and miR-122 target fatty acid synthase gene were repressed by GSPE in vitro in rat hepatocytes (39). Likewise, persimmon originated polymeric proanthocyanidins, as measured by the mean DP of 26, and its characteristic subunits A-type (+)-epicatechin gallate and (–)-epigallocatechin gallate dimers significantly reduced cellular lipid accumulation in the human liver cell line L02 targeting miR-122 and miR-33b (40). miR-122 is a highly specific liver miRNA, which modulates fatty acid synthesis genes (e.g., SREBP-1c, fatty acid synthase) and fatty acid β-oxidation genes (e.g., NADPH-cytochrome P450 reductase-1). Inhibition of miR-122 by antisense oligonucleotides remarkably improved liver steatosis in diet-induced obese mice (41). miR-33a and miR-33b together with their host genes, are involved in modulation of fatty acid and cholesterol homeostasis (42). Their inhibition notably decreased fatty acid synthesis and increased fatty acid oxidation in nonhuman primates. In contrast to other reports (31, 43, 44), the inhibitory effect of persimmon tannin on cellular TG accumulation was shown to be independent of the AMPK pathway. Recently, GSPE in the main form of procyanidin B2 was reported to significantly reduce intercellular lipid accumulation in induced 3T3-L1 cells by targeting the miR-483-5p and PPARG pathway (45), although the exact molecular mechanism regarding regulation of proanthocyanidins over miRs remains unclear. It was noted that polyphenols can bind to components involved in miR biogenesis, as exemplified by the HIV-1 transactivating response RNA-binding protein. Thus, inhibition of transactivating response RNA-binding protein phosphorylation may suppress maturation of miR via the miR-generating complex; alternatively, proanthocyanidins bind directly to miRs to modulate their stability or degradation.
Proanthocyanidins increase cholesterol catabolism
Homeostasis of cholesterol is mainly regulated by the liver. Although biosynthesis of cholesterol is assisted by both cholesterol carrier LDL and VLDL, 1 of the major catabolic pathways involves use of cholesterol as a precursor to synthesize BAs. Increased hepatic LDL receptor mRNA, which is induced by GSPE, aids in removal of LDL or VLDL from circulating blood (46). GSPE also upregulated expression of sterol 12α-hydroxylase, a steroid catabolic gene linked to formation of cholic acid, as well as FXR related to BA biosynthesis (47). In addition, expression of the reverse cholesterol transport-related transporter ABC-A was shown to be induced by GSPE, thereby preventing transformation into foam cells (48). Consumption of red wine elevated apoA-1 as the main regulator of HDL and thus increased cholesterol efflux (49). The observation that apoA-1 binds to proanthocyanidins (derived from a mixture of monomers and oligomers from grape seed) suggests that the reverse cholesterol transport can be modified by proanthocyanidins (50). Taken together these imply that food sources rich in proanthocyanidins may be putative alternatives to standard pharmaceutical treatments (e.g., statins) as a preventive measure against hypercholesterolemia.
Proanthocyanidins are epigenetic regulators
Epigenetics is associated with crucial heritable alterations that play important roles in metabolic syndromes. The epigenetic regulation is vulnerable to environmental and nutritional influences. It was recently noted that natural product materials, such as dietary flavonoids enriched in proanthocyanidins, are potent and beneficial modulators of epigenetic cascades (51).
Histones can be modified post-translationally by acetylation, methylation, or phosphorylation, which can affect chromatin structure and gene expression patterns. For example, the histone deacetylases (HDACs) catalyze removal of acetyl groups from histones, compact the chromatin unit, and downregulate the transcription of certain genes effectively. More specifically, class I HDAC3 inhibits butyrate, and activates PPARA and its downstream genes, which are crucial to hepatic lipid metabolism and fatty acid catabolism. A recent study demonstrated that GSPE, mainly composed of monomeric and dimeric proanthocyanidins, repressed HDAC activity, but also increased PPARA phosphorylation and signaling, and reduced the concentration of serum triglycerides in C57BL/6 mice. Upregulated FXR expression and elevated apoA-5 activity were linked to improved hepatic lipid catabolism (52). A recent study also demonstrated that uptake of GSPE by rats significantly enhanced production of S-adenosylmethionine, a major biological methyl donor that is required by DNA methyltransferase 1 to execute epigenetic modification. However, instead of weight loss, increased adiposity index and white adipose tissue depots of the offspring were reported (53).
Proanthocyanidins target extrahepatic sites
Effects on adipocytes and adipokines
Proanthocyanidins exert local effects on adipocyte tissues in vivo. GSPE was shown to target mesenteric adipose tissue in Wistar lean rats and subcutaneous adipose tissue in Zucker fa/fa obese rats. PPARG, as the master regulator of adipogenesis, together with 11β-hydroxysteroid dehydrogenase 1 were remarkably downregulated in subcutaneous adipose tissue by GSPE. Primarily nonmodified structures of proanthocyanidins were found in adipose depots in the form of catechin, epicatechin, and their dimers (54). A moderate dose of GSPE downregulated expression of inducible NO synthase and IL-6, and upregulated adiponectin in adipocytes of obese female Zucker rats (55). Lyophilized cranberries (LCB) enriched with proanthocyanidins were shown to inhibit preadipocyte differentiation in an in vitro model. LCB significantly downregulated mRNA expression of fatty acid-binding protein (adipocyte protein 2), lipoprotein lipase, fatty acid synthase, hormone-sensitive lipase, and perilipin 1 in 3T3-L1 adipocytes during the differentiation process. Expression of adiponectin gene was also increased by LCB in a dose-dependent manner (56).
Effects on skeletal muscle cells
Skeletal muscle was reported to play an important role in overall utilization and oxidation of fatty acids via activation of β-oxidation, an increased mitochondrial functionality and oxidative capacity. In obese C57BL/6 mice, a high-fat diet induced deposition of fat to white adipose tissue, and weight gain could be reduced by supplementation with proanthocyanidins derived from cacao liquor (CLPr). Upregulation of uncoupling protein 1 in adipose and skeletal muscle increased the corresponding energy expenditure by fat oxidation and heat generation. Plasma cholesterol, high-fat diet induced increase of leptin, and decrease of adiponectin were also prevented by proanthocyanidins, which redirected circulating TGs to skeletal muscles for fat oxidation and ATP synthesis (43).
Effects on gut microbiota
Gut microbiota (with their associated metabolites) have been linked to the onset and progression of environmental stress and aging-linked diseases, and are potential mediators of glucose and fatty acid metabolism (57). In addition to the local effects on intestinal function, the gut microbiota has been associated with inflammation in peripheral tissues thereby affecting host metabolism (58). Natural products, especially polyphenols originating from food sources, have the capacity to influence the profiles of gut microbiota (59). Supplementation of GSPE prevented a systemic inflammation reaction in specific-pathogen-free mice fed a high-fat diet, likely via suppression of the c-Jun N-terminal kinases (JNK) and NF-κB signaling pathways. A reduction of epididymal fat weight to body weight and improved insulin sensitivity was noted in GSPE-fed mice. A comparison of gut microbiota suggested that the positive effects of GSPE might be linked to fat mobilization associated with certain phyla of bacteria, such as Firmicutes and Proteobacteria. The relative stability of proanthocyanidins in the colon adds further support to the general notion that intestinal bacteria play an important role in proanthocyanidin-modulated fat metabolism (60).
Proanthocyanidins regulate common pathways underlying aging-associated diseases
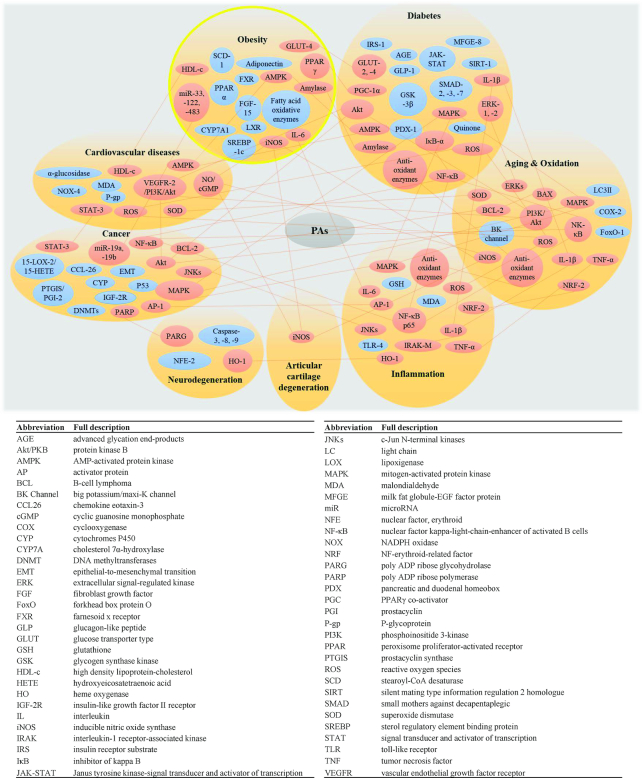
Extension of the human life span is accompanied by increased risk of cell dysfunction and organ degeneration, causing pathologic conditions. Aging-associated diseases have rapidly emerged as the leading cause of death in developed countries (61). Proanthocyanidins have the potential to ameliorate aging-related pathological conditions, such as obesity, diabetes, cancer, neurodegeneration, cardiovascular disease, and arthritis (Figure 6). An advanced knowledge-base regarding molecular processes and shared mechanisms that target the underlying aging-associated disease will help to optimize nutritional application of proanthocyanidins.
FIGURE 6.
Molecular genetic approaches enable study of mechanisms of proanthocyanidin-mediated health benefits, revealing shared regulatory genes among age-associated diseases.
Proanthocyanidins affect AMPK and metabolic pathways
The link between AMPK and the insulin sensitive glucose transporter-4
As mentioned, GSPE was able to reprogram lipid utilization in rats by upregulating both total and phosphorylated 5' AMPK and fatty acid oxidation (62). AMPK is a sensor of cellular energy status. Activated by metabolic stress, AMPK regulates glucose and fatty acid uptake to compensate for cellular energy and is considered a key player in progression of metabolic syndromes such as obesity and diabetes mellitus (63). Downstream of AMPK, insulin sensitive glucose transporters (GLUTs) are membrane proteins that facilitate cellular glucose transport. Of the 14 GLUT members identified so far, GLUT-4 is particularly sensitive to proanthocyanidins. CLPr, for instance, promoted GLUT-4 translocation, enhanced glucose uptake, and improved glucose tolerance in muscle cells (64). In particular, CLPr of low DP (DP ≤ 3) improved hyperglycemia by regulating GLUT-4 translocation through an AMPK-dependent pathway in skeletal muscle (65). In comparison, proanthocyanidins from soybean seed coat activated both insulin and AMPK signaling to induce GLUT-4 translocation in the muscle of mice (66). Procyanidin A2 restored expression of GLUT-2 and GLUT-4 in isolated islets pre-exposed to a harmful chemical bisphenol A, thereby preventing progression of hyperglycemia and type 2 diabetes (67). Proanthocyanidins’ positive regulation of glucose homeostasis also resulted in amelioration of high-fat-related hyperglycemia (43).
The AMPK/silent mating type information regulation 2 homologue-1-PPARG co-activator-1α link
PPAR refers to a family of nuclear receptor proteins. As summarized previously, proanthocyanidins improve global lipid profiles by coordinating PPARA and PPARG signaling-associated anabolic and catabolic metabolism. PPARG co-activator-1α also interacts with multiple transcription factors and influences numerous pathological conditions. Procyanidin B2 was reported to improve diabetic nephropathy by regulating AMPK/silent mating type information regulation 2 homologue-1-PPARG co-activator-1α cascade both in vitro and in vivo (68). Specifically, procyanidin B2 suppressed high glucose-induced podocyte apoptosis, improved apoptosis associated mitochondrial dysfunction and mtDNA copy number decrease, and protected cells from diabetic nephropathy-like phenotypes (69).
Proanthocyanidins are involved in survival and apoptosis pathways
The MAPK/extracellular signal-regulated kinase-NF-κB link
The MAPK/extracellular signal-regulated kinase (ERK) pathway comprises multiple proteins regulating core gene transcription. Proanthocyanidins extracted from Orostachys japonicus suppressed viability of cancer cells through activation of MAPK signaling (70). Proanthocyanidin-regulated MAPK/ERK in combination with NF-κB also mediate Y-box binding protein 1 nuclear translocation associated with a multidrug resistance phenotype, thus enhancing efficiency of cancer chemotherapy (71). MAPK also plays an active role in innate and adaptive immunity. By activating MAPK and NF-κB signaling, trimeric procyanidin C1 elevated activation of macrophage and polarization of T helper type 1 in murine splenocytes (72). Procyanidin B2, when used as an agent against inflammation-related diseases, was shown to markedly reduce lipopolysaccharide-induced proinflammatory cytokines (e.g., TNF-α and ILs) (73, 74). Although NF-κB is often simulated by proanthocyanidins, IκB-α, an inhibitor of NF-κB, is downregulated by proanthocyanidins in endothelial progenitor cells, which in turn drives the proanthocyanidin-enforced anti-inflammatory response (75).
The MAPK/ERK-nuclear factor erythroid 2-related factor link
MAPK/ERK-nuclear factor erythroid 2-related factor (Nrf2) is crucial to proanthocyanidin-mediated antioxidation, anti-inflammation, and attenuation of neurological deficits. Procyanidin B2, for example, activated nuclear translocation of Nrf2 and signaling proteins involved in the process MAPK and ERK, protecting human colonic cells against oxidative stress (76). Proanthocyanidin dimers also reversed pathogenic suppression of the nuclear translocation of Nrf2 and heme oxygenase expression in the ipsilateral ischemic area of brain, preventing blood-brain barrier disruption against ischemic stroke (77). It has been suggested that procyanidin B2 might competitively bind to the Nrf upstream element toll-like receptor-4/myeloid differentiation factor-2 complex, executing biological functions (78). In HepG2 cells, polymeric procyanidin fractions (from defatted grape seeds) also upregulated Nrf2, heme oxygenase, and antioxidant response element-driven phase II detoxification enzymes, and protected the cells from reactive oxygen species (ROS) (79).
The phosphatidylinositol 3’-kinase-serine/threonine-specific protein kinase link
Phosphatidylinositol 3’-kinase (PI3K)-dependent serine/threonine-specific protein kinase (Akt) is a crucial regulator of cellular survival, coordinating glycolysis, protein synthesis, lipogenesis, glycogen synthesis, and glucose transport. Disrupted regulation of the PI3K-Akt pathway leads to pathological conditions including cancer, diabetes, or cardiovascular diseases. An epidemiological study revealed that proanthocyanidins derived from fruits and vegetables exert a positive role in suppression of human colorectal cancer cell growth. Proanthocyanidin hexamers, in particular, decreased PI3K/Akt signaling and cell survival proteins Bad and GSK-3β (80). Grape seed extracted B2–3-3”-di-O-gallate targeted vascular endothelial growth factor receptor-2, associated PI3K/Akt and integrin signaling, inhibited growth, and induced death in both human umbilical vein endothelial cells and human prostate microvascular endothelial cells (81). In diabetic patients, administration of proanthocyanidins reduced apoptosis and oxidative stress (induced by high glucose in endothelial progenitor cells), likely via a comparable mechanism, namely, upregulation of vascular endothelial growth factor receptor-2-Akt and NF-κB and downregulation of an inhibitor of NF-κB (75). Diabetic nephropathy is 1 of the major complications seen in diabetes. A quantitative proteomic analysis in diabetic db/db mice revealed that procyanidin B2 reduced Akt, ERK, and GSK-3β, and ameliorated diabetic nephropathy in vivo (82). Trimeric procyanidin C1, which is thought to improve endothelial functions and cardiovascular diseases, stimulated PI3K/Akt signaling-mediated NO production in rat aortic endothelial cells (83).
Advanced glycation end-products
Advanced glycation end-products (AGEs) are a hallmark of the aging process. They are highly heterogeneous in composition and are formed through complex nonenzymatic glycation reactions, including multistep reactions between reactive sugars and free amino acids residues of peptides or proteins. Many factors seem to influence the accumulation and removal of AGE formation, including genetics, environment, and lifestyle, but notably also pathological conditions such as diabetes. Binding of AGEs to specific receptors for AGEs stimulates molecular signaling pathways including MAPK-ERK, NF-κB, and subsequent transcription of many proinflammatory genes and oxidative stress responses (84). Procyanidins from the litchi pericarp inhibited formation of AGEs in a simulated model system containing lactose as a reducing disaccharide and lysine as a reactive amino acid (85). Thus, the inhibitory effect of proanthocyanidins on AGEs may be valuable in therapy for diabetic complications (86, 87)
Apoptosis regulator BCL-2 mediated mitochondrial pathway
Apoptotic proteins (e.g., BCL-2 associated X protein, BAX; BCL-2 antagonist/killer 1) induce mitochondrial membrane permeabilization, and mitochondrial release of cytochrome C and ROS, which trigger a mitochondrial-dependent apoptosis cascade. Located on the outer membrane of mitochondria, BCL-2 plays an antiapoptotic role by inhibiting this process. In addition, BCL-2 regulates mitochondrial dynamics controlling metabolic activities. The antioxidant potential of GSPE, for example, was related to its effect on BCL-2 and BAX. Exposure to cadmium induced lipid peroxidation and antagonized renal apoptosis in vivo, which was attenuated after administration of GSPE (88). Procyanidin B2 also markedly inhibited CCl4-induced hepatocyte apoptosis, suppressed upregulation of BAX, and restored levels of BCL-2 like 1 gene (89).
The activator protein 1 and signal transducer and activator of transcription 3 link
Activator protein 1 (AP-1) is a transcriptional factor that works with apoptotic and survival pathway elements to control a range of cellular processes including differentiation, proliferation, and apoptosis. Both synthesized and extracted B2–3-3”-di-O-gallate inhibited transcription of AP-1, NF-κB, and the nuclear translocation of signal transducer and activator of transcription 3 (STAT-3) in prostate cancer cell lines (90). Procyanidin B2 inhibited activation of pyrin domain containing 3 protein inflammasome by suppressing the AP-1 pathway, and also downregulated lipopolysaccharide-induced caspase-1 activation and IL-1β secretion in human vascular endothelial cells (91). Proanthocyanidins of grape origin suppressed chronic hypoxia-induced expression of phosphor-STAT-3, attenuated hypoxia-induced increase in right ventricular systolic pressures, and reduced secretion of inflammatory factors including malondialdehyde and NADPH oxidase in Sprague-Dawley rats, thus potentially preventing hypoxic pulmonary hypertension (92). In addition, proanthocyanidins were shown to relieve neurological impairment in diabetic Sprague-Dawley rats with focal cerebral ischemia. The altered expression of STAT-1 modulated Janus kinase–signal transducers and activators of transcription signaling, which in turn may contribute to neuron protective activity (93).
Proanthocyanidins are involved in other aging-associated pathways
Proinflammatory cytokines and inflammation cascades
As revealed by many studies, proanthocyanidins significantly downregulate proinflammatory marker NADPH oxidase, inducible nitric oxide synthase, cyclooxygenase-2, and proinflammatory cytokines IL-6 and TNF-α in vitro and in vivo (55, 89, 94, 95, 96). The anti-inflammatory potential of proanthocyanidins is of particular importance given the shared roles of chronic inflammation in obesity, diabetes, and other metabolic diseases (97), and has been linked recently with a positive impact on maintenance of chondrocytes and osteoarthritis. The risk of osteoarthritis increases when the balance of cartilage degradation and synthesis is disrupted. Procyanidin B3, in addition to being collagen protective, has been shown to inhibit apoptosis in primary chondrocytes, suppress H2O2 or IL-1β induced inducible nitric oxide synthase, and to prevent IL-1β-caused suppression of chondrocyte differentiation marker gene expression (98).
The epigenetic enzyme DNA methyltransferases
Epigenetic enzymes are emerging as pharmacological targets in treatment of aging-associated diseases. DNA methyltransferases (DNMTs), 1 of the most well studied epigenetic enzymes, catalyze DNA methylation and consequentially alter gene expression pattern. An in vitro screen of 32 EGCG analogues demonstrated that procyanidin B2 attenuates activity of DNMTs and subsequently enhanced expression of DNMT targets, namely E-cadherin, Maspin, and BRCA1 (99). This epigenetic regulatory effect by proanthocyanidins is thought to be linked to the epithelial-to-mesenchymal transition (EMT), a biological process in which epithelial cells are reprogrammed into multipotent stromal cells. EMT is closely associated with cancer metastasis. Epigenetic factors, for instance, DNMT targets E-cadherin, inducing the destiny of a cell during EMT. Procyanidin C1 was reported to inhibit TGF-β-induced EMT, thereby suppressing cancer metastasis (100). On the other hand, EMT triggers fibrosis of renal tubular epithelial cells, driving the onset of diabetic nephropathy and ultimately renal failure. In a diabetes model, excessive glucose induced EMT, elevated α-smooth muscle actin, and reduced E-cadherin in HK-2 human renal proximal tubular epithelial cells. Procyanidin B2 was able to reverse this transcriptional change and act as a protective agent (101).
Endothelium factors prostacyclin and NO
Prostacyclin and NO are endothelium-derived molecules functioning as vasodilators or inhibitors of platelet activation. Proanthocyanidins induce secretion of prostacyclin and NO in spontaneously hypertensive rats in an endothelium-dependent manner, thus positively regulating blood pressure. This antihypertensive effect of GSPE was abolished to some extent by indomethacin and NG-Nitro-L-arginine methyl ester (L-NAME), inhibitors of prostacyclin and NO synthesis, respectively (102). The beneficial effect of proanthocyanidins on arterial blood pressure was also revealed in a hypertension disease model associated with metabolic syndrome (103). In another study GSPE was shown to upregulate prostacyclin synthase in human lung premalignant and malignant cells, suggesting its potential as a chemopreventive agent against different cancers (104). The protective action of proanthocyanidin trimer C1 on the cardiovascular system was partially attributed to its effect on the voltage-gated potassium channel (BK channel) and downstream NO production which mediated hyperpolarization in rat aortic endothelial cells. The BK channel allows voltage-gated flux of potassium ions to repolarize the cell membrane potential in response to depolarization or increased calcium. NO production relies partially on a calcium-dependent BK channel. In turn, dysfunctional BK channels can cause systemic disorders, especially prominent neurological and cardiovascular pathological features (83).
Cytochrome P450 and ROS-induced damage
Belonging to the hemoprotein superfamily, cytochrome P450 (CYPs) comprises a family of oxidase enzymes that are actively involved in oxidation and biosynthesis of BAs. GSPEs were noted for their beneficial effect on coronary artery diseases by improving BAs, cholesterol, and triglyceride circulation. Specifically, GSPE coadministered with cholestyramine robustly induced expression of cytochrome P450 7A1 (CYP7A1), a rate-limiting enzyme of the classical biosynthesis of hepatic BAs (46). GSPE administered alone downregulated expression of ileal fibroblast growth factor 15, a crucial regulator of CYP1A1, which in turn suppressed synthesis and recirculation of BAs, contributing to reduced triglyceride deposits (47). Aligned to this, the stimulating effect of procyanidin A2 on the enzymatic activity of 5 CYPs (CYP1A2, CYP3A4, CYP2E1, CYP2C9, and CYP2D6) was confirmed in vitro in a human hepatocyte system (105). In addition, proanthocyanidins protect against inflammation via ROS-mediated mechanisms (79, 89, 106). Cinnamon extracts enriched with trimeric proanthocyanidins were able to protect pancreatic β-cells against diabetes. The trimeric molecule restored H2O2-induced lipotoxicity and oxidative stress in INS-1 pancreatic β-cells, reduced ROS, and improved cell viability and function (107). Likewise, proanthocyanidins extracted from grape seeds reduced the production of ROS, potentially preventing hypoxic pulmonary hypertension via a combined antioxidant and antiproliferative action (92).
Conclusions
Although the knowledge-base surrounding proanthocyanidin involvement in lipid homeostasis has grown over the past decade, a conclusive interpretation of the functional mechanism remains challenging. Numerous studies have proposed positive, neutral, or negative effects (108–110), an inconsistency that, in part, may be attributed to the poorly described composition of raw plant extracts and thus the nonstandardized administration of proanthocyanidins. In the majority of cases only crude proanthocyanidin mixtures were tested but common sense dictates that purified compounds are required to allow definitive conclusions to be drawn about the effectiveness and activity of individual proanthocyanidins. This is complicated by the fact that proanthocyanidins are highly active, but unfortunately also not overly stable. Their polyphenol hydroxyl groups allow them to trigger enzymatic and spontaneous oxidation reactions, and to form reversible complexes with proteins (111). Proanthocyanidin polymers can aggregate with macromolecules and carbohydrates (112). Although the degradation rate of proanthocyanidins increases at low pH, and acetaldehyde condensation of proanthocyanidins (in particular the galloyl dimer, trimer C1 and T2) occurs by means of acid catalysis, the presence of (+)-catechin can increase the stability of proanthocyanidins. It is therefore of paramount importance to explore further how best to store and administer proanthocyanidins. Numerous epidemiological studies have suggested that proanthocyanidins may be able to offer some protection against cardiovascular diseases (113–115) and carcinogenesis (116, 117), as well as reduce free radical and peroxidative loads in humans (118, 119), but only limited data are available concerning involvement of proanthocyanidins in fat metabolism, obesity, and associated conditions/diseases (17). Oligomeric proanthocyanidins, in particular with a DP ranging from 2 to 5, seem to be bioavailable and bioactive. There is, however, a need to unravel the molecular genetic drivers and targets, the structural hallmarks, as well as the pharmacokinetic destiny in vivo before we can understand the efficiency and potential of proanthocyanidins in interventional therapies.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—YN and SRS: jointly wrote, read, and approved the final paper.
Notes
Supported by the King's-China Scholarship Council scheme (YN).
Author disclosures: YN and SRS, no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AGE, advanced glycation end-product; Akt, serine/threonine-specific protein kinase; AMPK, 5’-AMP-activated protein kinase; AP, activator protein; BA, bile acid; BAX, BCL2 associated X protein; CLPr, proanthocyanidins derived from cacao liquor; CYP, cytochrome P450; DNMT, DNA methyltransferases; DP, degree of polymerization; EMT, epithelial-to-mesenchymal transition; ERK, extracellular signal-regulated kinases; FXR, farnesoid X receptor; GLUT, insulin sensitive glucose transporters; GSPE, grape seed procyanidin extract; HDAC, histone deacetylase; LCB, lyophilized cranberries; miR, microRNA; Nrf, nuclear factor erythroid related factor; PI3K, phosphatidylinositol 3’-kinase; PL, pancreatic lipase; ROS, reactive oxygen species; SREBP, sterol receptor element binding protein; STAT, signal transducer and activator of transcription.
References
- 1. Rosenheim O. Observations on anthocyanins. I: the anthocyanins of the young leaves of the grape vine. Biochem J. 1920;14:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Passos CP, Cardoso SM, Domingues MRM, Domingues P, Silva CM, Coimbra MA. Evidence for galloylated type-A procyanidins in grape seeds. Food Chem. 2007;105:1457–67. [Google Scholar]
- 3. Chen L, Sun P, Wang T, Chen K, Jia Q, Wang H, Li Y. Diverse mechanisms of antidiabetic effects of the different procyanidin oligomer types of two different cinnamon species on db/db mice. J Agric Food Chem. 2012;60:9144–50. [DOI] [PubMed] [Google Scholar]
- 4. Dorenkott MR, Griffin LE, Goodrich KM, Thompson-Witrick KA, Fundaro G, Ye L, Stevens JR, Ali M, O'Keefe SF, Hulver MW et al.. Oligomeric cocoa procyanidins possess enhanced bioactivity compared to monomeric and polymeric cocoa procyanidins for preventing the development of obesity, insulin resistance, and impaired glucose tolerance during high-fat feeding. J Agric Food Chem. 2014;62:2216–27. [DOI] [PubMed] [Google Scholar]
- 5. Montagut G, Baiges I, Valls J, Terra X, del Bas JM, Vitrac X, Richard T, Mérillon JM, Arola L, Blay M et al.. A trimer plus a dimer-gallate reproduce the bioactivity described for an extract of grape seed procyanidins. Food Chem. 2009;116:265–70. [Google Scholar]
- 6. Rodriguez K, Ricketts ML. Determination of the bioactive components in a grape seed procyanidin extract responsible for enhanced farnesoid X receptor transactivation. FASEB J. 2017;31(1 Suppl):135–4. [Google Scholar]
- 7. Kimura H, Ogawa S, Niimi A, Jisaka M, Katsube T, Yokota K. Inhibition of fat digestion by highly polymeric proanthocyanidins from seed shells of Japanese horse chestnut (Aesculus turbinata). J Jpn Soc Food Sci. 2009;56:483–9. [Google Scholar]
- 8. Hsu CL, Yen GC. Phenolic compounds: evidence for inhibitory effects against obesity and their underlying molecular signaling mechanisms. Mol Nutr Food Res. 2008;52:53–61. [DOI] [PubMed] [Google Scholar]
- 9. Nie Y, Littleton B, Kavanagh T, Abbate V, Bansal SS, Richards D, Hylands P, Stürzenbaum SR. Proanthocyanidin trimer gallate modulates lipid deposition and fatty acid desaturation in Caenorhabditis elegans. FASEB J. 2017;31:4891–902. [DOI] [PubMed] [Google Scholar]
- 10. Hsu CL, Yen GC. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br J Nutr. 2007;98:727–35. [DOI] [PubMed] [Google Scholar]
- 11. Kusano R, Andou H, Fujieda M, Tanaka T, Matsuo Y, Kouno I. Polymer-like polyphenols of black tea and their lipase and amylase inhibitory activities. Chem Pharm Bull (Tokyo). 2008;56:266–72. [DOI] [PubMed] [Google Scholar]
- 12. Spencer JPE, Chaudry F, Pannala AS, Srai SK, Debnam E, Rice-Evans C. Decomposition of cocoa procyanidins in the gastric milieu. Biochem Biophys Res Commun. 2000;272:236–41. [DOI] [PubMed] [Google Scholar]
- 13. Rice-Evans CA, Packer L. Flavonoids in health and disease. CRC Press: Boca Raton; 2003, 363–90. [Google Scholar]
- 14. Spencer JPE, Schroeter H, Rechner AR, Rice-Evans C. Bioavailability of flavan-3-ols and procyanidins: gastrointestinal tract influences and their relevance to bioactive forms in vivo. Antioxid Redox Signal. 2001;3:1023–39. [DOI] [PubMed] [Google Scholar]
- 15. Donovan JL, Rios L, Morand C, Scalbert A, Remesy C. Procyanidins are not bioavailable in rats fed a single meal containing a grapeseed extract or the procyanidin dimer B3. Br J Nutr. 2002;87:299–306. [DOI] [PubMed] [Google Scholar]
- 16. Shoji T, Masumoto S, Moriichi N, Akiyama H, Kanda T, Ohtake Y, Goda Y. Apple procyanidin oligomers absorption in rats after oral administration: analysis of procyanidins in plasma using the Porter method and high-performance liquid chromatography/tandem mass spectrometry. J Agric Food Chem. 2006;54:884–92. [DOI] [PubMed] [Google Scholar]
- 17. Blade C, Arola L, Salvado MJ. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol Nutr Food Res. 2010;54:37–59. [DOI] [PubMed] [Google Scholar]
- 18. Yasuda A, Natsume M, Sasaki K, Baba S, Nakamura Y, Kanegae M, Nagaoka S. Cacao procyanidins reduce plasma cholesterol and increase fecal steroid excretion in rats fed a high-cholesterol diet. Biofactors. 2008;33:211–23. [DOI] [PubMed] [Google Scholar]
- 19. Tamura T, Inoue N, Ozawa M, Shimizu-Ibuka A, Arai S, Abe N, Koshino H, Mura K. Peanut-skin polyphenols, procyanidin A1 and epicatechin-(4β→6)-epicatechin-(2β→O→7, 4β→8)-catechin, exert cholesterol micelle-degrading activity in vitro. Biosci Biotechnol Biochem. 2013;77:1306–9. [DOI] [PubMed] [Google Scholar]
- 20. Pal S, Naissides M, Mamo J. Polyphenolics and fat absorption. Int J Obes Relat Metab Disord. 2004;28:324–6. [DOI] [PubMed] [Google Scholar]
- 21. Vidal R, Hernandez-Vallejo S, Pauquai T, Texier O, Rousset M, Chambaz J, Demignot S, Lacorte JM. Apple procyanidins decrease cholesterol esterification and lipoprotein secretion in Caco-2/TC7 enterocytes. J Lipid Res. 2005;46:258–68. [DOI] [PubMed] [Google Scholar]
- 22. Pal S, Ho S, Takechi R. Red wine polyphenolics suppress the secretion of ApoB48 from human intestinal Caco-2 cells. J Agric Food Chem. 2005;53:2767–72. [DOI] [PubMed] [Google Scholar]
- 23. Moreno DA, Poulev A, Brasaemle DL, Fried SK, Raskin I. Inhibitory effects of grape seed extract on lipases. Nutrition. 2003;19:876–9. [DOI] [PubMed] [Google Scholar]
- 24. Adisakwattana S, Moonrat J, Srichairat S, Chanasit C, Tirapongporn H, Chanathong B, Ngamukote S, Mäkynen K, Sapwarobol S. Lipid-lowering mechanisms of grape seed extract (Vitis vinifera L.) and its antihyperlidemic activity. J Med Plants Res. 2010;4:2113–20. [Google Scholar]
- 25. Hassan HM. Inhibitory effects of red grape seed extracts on pancreatic-amylase and lipase. Glob J Biotechnol Biochem. 2014;9:130–6. [Google Scholar]
- 26. Gonçalves R, Mateus N, de Freitas V. Inhibition of α-amylase activity by condensed tannins. Food Chem. 2011;125:665–72. [Google Scholar]
- 27. Wang S, Dong S, Zhang R, Shao H, Liu Y. Effects of proanthocyanidins on porcine pancreatic lipase: conformation, activity, kinetics and thermodynamics. Process Biochem. 2014;49:237–43. [Google Scholar]
- 28. Gu Y, Hurst WJ, Stuart DA, Lambert JD. Inhibition of key digestive enzymes by cocoa extracts and procyanidins. J Agric Food Chem. 2011;59:5305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sugiyama H, Akazome Y, Shoji T, Yamaguchi A, Yasue M, Kanda T, Ohtake Y. Oligomeric procyanidins in apple polyphenol are main active components for inhibition of pancreatic lipase and triglyceride absorption. J Agric Food Chem. 2007;55:4604–9. [DOI] [PubMed] [Google Scholar]
- 30. Caimari A, del Bas JM, Crescenti A, Arola L. Low doses of grape seed procyanidins reduce adiposity and improve the plasma lipid profile in hamsters. Int J Obes. 2013;37:576–83. [DOI] [PubMed] [Google Scholar]
- 31. Yamashita Y, Okabe M, Natsume M, Ashida H. Comparison of anti-hyperglycemic activities between low- and high-degree of polymerization procyanidin fractions from cacao liquor extract. J Food Drug Anal. 2012;20:284. [Google Scholar]
- 32. Shimada T, Tokuhara D, Tsubata M, Kamiya T, Kamiya-Sameshima M, Nagamine R, Takagaki K, Sai Y, Miyamoto KI, Aburada M. Flavangenol (pine bark extract) and its major component procyanidin B1 enhance fatty acid oxidation in fat-loaded models. Eur J Pharmacol. 2012;677:147–53. [DOI] [PubMed] [Google Scholar]
- 33. Han L, Shen WJ, Bittner S, Kraemer FB, Azhar S. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part I: PPAR-α. Future Cardiol. 2017;13:259–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aragonès G, Suárez M, Ardid-Ruiz A, Vinaixa M, Rodríguez MA, Correig X, Arola L, Bladé C. Dietary proanthocyanidins boost hepatic NAD+ metabolism and SIRT1 expression and activity in a dose-dependent manner in healthy rats. Sci Rep. 2016;6:24977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bansode RR, Randolph P, Hurley S, Ahmedna M. Evaluation of hypolipidemic effects of peanut skin-derived polyphenols in rats on Western-diet. Food Chem. 2012;135:1659–66. [DOI] [PubMed] [Google Scholar]
- 36. Guerrero L, Margalef M, Pons Z, Quiñones M, Arola L, Arola-Arnal A, Muguerza B. Serum metabolites of proanthocyanidin-administered rats decrease lipid synthesis in HepG2 cells. J Nutr Biochem. 2013;24:2092–9. [DOI] [PubMed] [Google Scholar]
- 37. Del Bas JM, Ricketts ML, Baiges I, Quesada H, Ardevol A, Salvadó MJ, Pujadas G, Blay M, Arola L, Bladé C. Dietary procyanidins lower triglyceride levels signaling through the nuclear receptor small heterodimer partner. Mol Nutr Food Res. 2008;52:1172–81. [DOI] [PubMed] [Google Scholar]
- 38. Del Bas JM, Ricketts ML, Vaqué M, Sala E, Quesada H, Ardevol A, Salvadó MJ, Blay M, Arola L et al.. Dietary procyanidins enhance transcriptional activity of bile acid‐activated FXR in vitro and reduce triglyceridemia in vivo in a FXR‐dependent manner. Mol Nutr Food Res. 2009;53:805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baselga-Escudero L, Bladé C, Ribas-Latre A, Casanova E, Salvadó MJ, Arola L, Arola-Arnal A. Grape seed proanthocyanidins repress the hepatic lipid regulators miR-33 and miR-122 in rats. Mol Nutr Food Res. 2012;56:1636–46. [DOI] [PubMed] [Google Scholar]
- 40. Zou B, Nie R, Zeng J, Ge Z, Xu Z, Li C. Persimmon tannin alleviates hepatic steatosis in L02 cells by targeting miR-122 and miR-33b and its effects closely associated with the A type ECG dimer and EGCG dimer structural units. J Funct Foods. 2014;11:330–41. [Google Scholar]
- 41. Yang ZH, Cappello T, Wang L. Emerging role of microRNAs in lipid metabolism. Acta Pharm Sin B. 2015;5:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rayner KJ, Suárez Y, Dávalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernández-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328:1570–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamashita Y, Okabe M, Natsume M, Ashida H. Prevention mechanisms of glucose intolerance and obesity by cacao liquor procyanidin extract in high-fat diet-fed C57BL/6 mice. Arch Biochem Biophys. 2012;527:95–104. [DOI] [PubMed] [Google Scholar]
- 44. Casanova E, Baselga-Escudero L, Ribas-Latre A, Cedó L, Arola-Arnal A, Pinent M, Bladé C, Arola L, Salvadó MJ. Chronic intake of proanthocyanidins and docosahexaenoic acid improves skeletal muscle oxidative capacity in diet-obese rats. J Nutr Biochem. 2014;25:1003–10. [DOI] [PubMed] [Google Scholar]
- 45. Zhang J, Huang Y, Shao H, Bi Q, Chen J, Ye Z. Grape seed procyanidin B2 inhibits adipogenesis of 3T3-L1 cells by targeting peroxisome proliferator-activated receptor γ with miR-483-5p involved mechanism. Biomed Pharmacother. 2017;86:292–6. [DOI] [PubMed] [Google Scholar]
- 46. Heidker RM, Caiozzi GC, Ricketts ML. Grape seed procyanidins and cholestyramine differentially alter bile acid and cholesterol homeostatic gene expression in mouse intestine and liver. PLoS One. 2016;11:e0154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hintz R, Caiozzi G, Ricketts ML. A grape seed procyanidin extract lowers serum triglyceride levels via selective modulation of intestinal FXR-target gene expression and inhibition of enterohepatic bile acid recirculation (1045.35). FASEB J. 2014;28(1 Suppl):1045–35. [Google Scholar]
- 48. Terra X, Fernández-Larrea J, Pujadas G, Ardèvol A, Bladé C, Salvadó J, Arola L, Blay M. Inhibitory effects of grape seed procyanidins on foam cell formation in vitro. J Agric Food Chem. 2009;57:2588–94. [DOI] [PubMed] [Google Scholar]
- 49. Senault C, Luc G, Hauw P, Rigaud D, Fumeron F. Beneficial effects of a moderate consumption of red wine on cellular cholesterol efflux in young men. Nutr Metab Cardiovasc Dis. 2000;10:63–9. [PubMed] [Google Scholar]
- 50. Brunet MJ, Salvado MJ, Arola L. Human apoA-I and rat transferrin are the principal plasma proteins that bind wine catechins. J Agric Food Chem. 2002;50:2708–12. [DOI] [PubMed] [Google Scholar]
- 51. Cherblanc FL, Robert RW, Di Fruscia P, Srimongkolpithak N, Fuchter MJ. Perspectives on natural product epigenetic modulators in chemical biology and medicine. Nat Prod Rep. 2013;30:605–24. [DOI] [PubMed] [Google Scholar]
- 52. Downing LE, Ferguson BS, Rodriguez K, Ricketts ML. A grape seed procyanidin extract inhibits HDAC activity leading to increased Pparα phosphorylation and target-gene expression. Mol Nutr Food Res. 2017;61:1600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Del Bas JM, Crescenti A, Arola-Arnal A, Oms-Oliu G, Arola L, Caimari A. Grape seed procyanidin supplementation to rats fed a high-fat diet during pregnancy and lactation increases the body fat content and modulates the inflammatory response and the adipose tissue metabolism of the male offspring in youth. Int J Obes. 2015;39:7. [DOI] [PubMed] [Google Scholar]
- 54. Ardévol A, Motilva MJ, Serra A, Blay M, Pinent M. Procyanidins target mesenteric adipose tissue in Wistar lean rats and subcutaneous adipose tissue in Zucker obese rat. Food Chem. 2013;141:160–6. [DOI] [PubMed] [Google Scholar]
- 55. Pallarès V, Cedó L, Castell-Auví A, Pinent M, Ardévol A, Arola L, Blay M. Effects of grape seed procyanidin extract over low-grade chronic inflammation of obese Zucker fa/fa rats. Food Res Int. 2013;53:319–24. [Google Scholar]
- 56. Kowalska K, Olejnik A, Rychlik J, Grajek W. Cranberries (Oxycoccus quadripetalus) inhibit lipid metabolism and modulate leptin and adiponectin secretion in 3T3-L1 adipocytes. Food Chem. 2015;185:383–8. [DOI] [PubMed] [Google Scholar]
- 57. Janssen AWF, Kersten S. Potential mediators linking gut bacteria to metabolic health: a critical view. J Physiol. 2017;595:477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Anhê FF, Varin TV, Le Barz M, Desjardins Y, Levy E, Roy D, Marette A. Gut microbiota dysbiosis in obesity-linked metabolic diseases and prebiotic potential of polyphenol-rich extracts. Curr Obes Rep. 2015;4:389–400. [DOI] [PubMed] [Google Scholar]
- 60. Liu W, Zhao S, Wang J, Shi J, Sun Y, Wang W, Ning G, Hong J, Liu R. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol Nutr Food Res. 2017;61:1601082. [DOI] [PubMed] [Google Scholar]
- 61. Ahima RS. Connecting obesity, aging and diabetes. Nat Med. 2009;15:996. [DOI] [PubMed] [Google Scholar]
- 62. Crescenti A, del Bas JM, Arola-Arnal A, Oms-Oliu G, Arola L, Caimari A. Grape seed procyanidins administered at physiological doses to rats during pregnancy and lactation promote lipid oxidation and up-regulate AMPK in the muscle of male offspring in adulthood. J Nutr Biochem. 2015;26:912–20. [DOI] [PubMed] [Google Scholar]
- 63. Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signalling. Circ Res. 2007;100:328–41. [DOI] [PubMed] [Google Scholar]
- 64. Yamashita Y, Okabe M, Natsume M, Ashida H. Cacao liquor procyanidin extract improves glucose tolerance by enhancing GLUT4 translocation and glucose uptake in skeletal muscle. J Nutr Sci. 2012;1:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamashita Y, Okabe M, Natsume M, Ashida H. Comparison of anti-hyperglycemic activities between low- and high-degree of polymerization procyanidin fractions from cacao liquor extract. J Food Drug Anal. 2012;20:284. [Google Scholar]
- 66. Yamashita Y, Wang L, Nanba F, Ito C, Toda T, Ashida H. Procyanidin promotes translocation of glucose transporter 4 in muscle of mice through activation of insulin and AMPK signaling pathways. PLoS One. 2016;11:e0161704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ahangarpour A, Afshari G, Mard SA, Khodadadi A, Hashemitabar M. Preventive effects of procyanidin A2 on glucose homeostasis, pancreatic and duodenal homebox 1, and glucose transporter 2 gene expression disturbance induced by bisphenol A in male mice. J Physiol Pharmacol. 2016;67:243–52. [PubMed] [Google Scholar]
- 68. Bao L, Cai X, Zhang Z, Li Y. Grape seed procyanidin B2 ameliorates mitochondrial dysfunction and inhibits apoptosis via the AMP-activated protein kinase-silent mating type information regulation 2 homologue 1-PPARγ co-activator-1α axis in rat mesangial cells under high-dose glucosamine. Br J Nutr. 2015;113:35–44. [DOI] [PubMed] [Google Scholar]
- 69. Cai X, Bao L, Ren J, Li Y, Zhang Z. Grape seed procyanidin B2 protects podocytes from high glucose-induced mitochondrial dysfunction and apoptosis via the AMPK-SIRT1-PGC-1α axis in vitro. Food Funct. 2016;7:805–15. [DOI] [PubMed] [Google Scholar]
- 70. Lee WS, Yun JW, Nagappan A, Jung JH, Yi SM, Kim DH, Kim HJ, Kim G, Ryu CH, Shin SC, Hong SC. Flavonoids from Orostachys japonicus A. Berger induces caspase-dependent apoptosis at least partly through activation of p38 MAPK pathway in U937 human leukemic cells. Asian Pac J Cancer Prev. 2015;16:465–9. [DOI] [PubMed] [Google Scholar]
- 71. Zhao BX, Sun YB, Wang SQ, Duan L, Huo QL, Ren F, Li GF. Grape seed procyanidin reversal of p-glycoprotein associated multi-drug resistance via down-regulation of NF-κB and MAPK/ERK mediated YB-1 activity in A2780/T cells. PLoS One. 2013;8:e71071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sung NY, Yang MS, Song DS, Byun EB, Kim JK, Park JH, Song BS, Lee JW, Park SH, Park HJ et al.. The procyanidin trimer C1 induces macrophage activation via NF-κB and MAPK pathways, leading to Th1 polarization in murine splenocytes. Eur J Pharmacol. 2013;714:218–28. [DOI] [PubMed] [Google Scholar]
- 73. Sung NY, Yang MS, Song DS, Kim JK, Park JH, Song BS, Park SH, Lee JW, Park HJ, Kim JH et al.. Procyanidin dimer B2-mediated IRAK-M induction negatively regulates TLR4 signaling in macrophages. Biochem Biophys Res Commun. 2013;438:122–8. [DOI] [PubMed] [Google Scholar]
- 74. Connor CA, Adriaens M, Pierini R, Johnson IT, Belshaw NJ. Procyanidin induces apoptosis of esophageal adenocarcinoma cells via JNK activation of c-Jun. Nutr Cancer. 2014;66:335–41. [DOI] [PubMed] [Google Scholar]
- 75. Liu Y, Liao WJ, Zhu Z, Zeng H, He HQ, Sun XL, Xu XF, Huang L, Wang WM, Zhou XY et al.. Effect of procyanidine on VEGFR-2 expression and transduction pathway in rat endothelial progenitor cells under high glucose conditions. Genet Mol Res. 2016;15:GMR.15016925. [DOI] [PubMed] [Google Scholar]
- 76. Rodríguez-Ramiro I, Ramos S, Bravo L, Goya L, Martín MÁ. Procyanidin B2 induces Nrf2 translocation and glutathione S-transferase P1 expression via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress. Eur J Nutr. 2012;51:881–92. [DOI] [PubMed] [Google Scholar]
- 77. Wu S, Yue Y, Li J, Li Z, Li X, Niu Y, Xiang J, Ding H. Procyanidin B2 attenuates neurological deficits and blood-brain barrier disruption in a rat model of cerebral ischemia. Mol Nutr Food Res. 2015;59:1930–41. [DOI] [PubMed] [Google Scholar]
- 78. Xing J, Li R, Li N, Zhang J, Li Y, Gong P, Gao D, Liu H, Zhang Y. Anti-inflammatory effect of procyanidin B1 on LPS-treated THP1 cells via interaction with the TLR4-MD-2 heterodimer and p38 MAPK and NF-κB signaling. Mol Cell Biochem. 2015;407:89–95. [DOI] [PubMed] [Google Scholar]
- 79. Kim Y, Choi Y, Ham H, Jeong HS, Lee J. Polymeric procyanidin fraction from defatted grape seeds protects HepG2 cells against oxidative stress by inducing phase II enzymes via Nrf2 activation. Food Sci Biotechnol. 2013;22:485–91. [Google Scholar]
- 80. Choy YY, Fraga M, Mackenzie GG, Waterhouse AL, Cremonini E, Oteiza PI. The PI3K/Akt pathway is involved in procyanidin‐mediated suppression of human colorectal cancer cell growth. Mol Carcinog. 2016;55:2196–209. [DOI] [PubMed] [Google Scholar]
- 81. Kumar R, Deep G, Wempe MF, Agarwal R, Agarwal C. Procyanidin B2 3, 3″-di-O-gallate inhibits endothelial cells growth and motility by targeting VEGFR2 and integrin signaling pathways. Curr Cancer Drug Targets. 2015;15(1):14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang Z, Li BY, Li XL, Cheng M, Yu F, Cai Q, Wang JF, Zhou RH, Gao HQ, Shen L. Proteomic analysis of kidney and protective effects of grape seed procyanidin B2 in db/db mice indicate MFG-E8 as a key molecule in the development of diabetic nephropathy. Biochim Biophys Acta. 2013;1832:805–16. [DOI] [PubMed] [Google Scholar]
- 83. Byun EB, Ishikawa T, Suyama A, Kono M, Nakashima S, Kanda T, Miyamoto T, Matsui T. A procyanidin trimer, C1, promotes NO production in rat aortic endothelial cells via both hyperpolarization and PI3K/Akt pathways. Eur J Pharmacol. 2012;692:52–60. [DOI] [PubMed] [Google Scholar]
- 84. Thomas MC. Advanced glycation end products. In Diabetes and the kidney. 170, Karger Publishers: Basel; 2011, 66–74. [DOI] [PubMed] [Google Scholar]
- 85. Li S, Qin X, Cheng J, Zhu Z, He J, Liu G. Inhibitory effect of litchi pericarp procyanidins on advanced glycation end products formation in model system of α-Lactose/L Lysine. TCSAE. 2016;32:299–305. [Google Scholar]
- 86. Muthenna P, Raghu G, Akileshwari C, Sinha SN, Suryanarayana P, Reddy GB. Inhibition of protein glycation by procyanidin‐B2 enriched fraction of cinnamon: delay of diabetic cataract in rats. IUBMB Life. 2013;65:941–50. [DOI] [PubMed] [Google Scholar]
- 87. Muthenna P, Raghu G, Kumar PA, Surekha MV, Reddy GB. Effect of cinnamon and its procyanidin-B2 enriched fraction on diabetic nephropathy in rats. Chem Biol Interact. 2014;222:68–76. [DOI] [PubMed] [Google Scholar]
- 88. Chen Q, Zhang R, Li WM, Niu YJ, Guo HC, Liu XH, Hou YC, Zhao LJ. The protective effect of grape seed procyanidin extract against cadmium-induced renal oxidative damage in mice. Environ Toxicol Pharmacol. 2013;36:759–68. [DOI] [PubMed] [Google Scholar]
- 89. Yang BY, Zhang XY, Guan SW, Hua ZC. Protective effect of procyanidin B2 against CCl4-induced acute liver injury in mice. Molecules. 2015;20:12250–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tyagi A, Raina K, Shrestha SP, Miller B, Thompson JA, Wempe MF, Agarwal R, Agarwal C. Procyanidin B2 3,3″-di-O-gallate, a biologically active constituent of grape seed extract, induces apoptosis in human prostate cancer cells via targeting NF-κB, Stat3, and AP1 transcription factors. Nutr Cancer. 2015;66:736–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yang H, Xiao L, Yuan Y, Luo X, Jiang M, Ni J, Wang N. Procyanidin B2 inhibits NLRP3 inflammasome activation in human vascular endothelial cells. Biochem Pharmacol. 2014;92:599–606. [DOI] [PubMed] [Google Scholar]
- 92. Jin H, Liu M, Zhang X, Pan J, Han J, Wang Y, Lei H, Ding Y, Yuan Y. Grape seed procyanidin extract attenuates hypoxic pulmonary hypertension by inhibiting oxidative stress and pulmonary arterial smooth muscle cells proliferation. J Nutr Biochem. 2016;36:81–8. [DOI] [PubMed] [Google Scholar]
- 93. Song CG, Yang X, Min LQ, Liu CX, Zhao CS. The effect of procyanidin on expression of STAT1 in type 2 diabetes mellitus SD rats with focal cerebral ischemia. Neuro Endocrinol Lett. 2014;35:68–72. [PubMed] [Google Scholar]
- 94. Pallarès V, Fernández-Iglesias A, Cedó L, Castell-Auví A, Pinent M, Ardévol A, Salvadó MJ, Garcia-Vallvé S, Blay M. Grape seed procyanidin extract reduces the endotoxic effects induced by lipopolysaccharide in rats. Free Radic Biol Med. 2013;60:107–14. [DOI] [PubMed] [Google Scholar]
- 95. Park JC, Lee SH, Hong JK, Cho JH, Kim IH, Park SK. Effect of dietary supplementation of procyanidin on growth performance and immune response in pigs. Asian-Australas J Anim Sci. 2014;27:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Martinez‐Micaelo N, González‐Abuín N, Pinent M, Ardévol A, Blay M. Procyanidin B2 inhibits inflammasome‐mediated IL-1β production in lipopolysaccharide‐stimulated macrophages. Mol Nutr Food Res. 2015;59:262–9. [DOI] [PubMed] [Google Scholar]
- 97. Yin W, Li B, Li X, Yu F, Cai Q, Zhang Z, Cheng M, Gao H. Anti-inflammatory effects of grape seed procyanidin B2 on a diabetic pancreas. Food Funct. 2015;6:3065–71. [DOI] [PubMed] [Google Scholar]
- 98. Aini H, Ochi H, Iwata M, Okawa A, Koga D, Okazaki M, Sano A, Asou Y. Procyanidin B3 prevents articular cartilage degeneration and heterotopic cartilage formation in a mouse surgical osteoarthritis model. PLoS One. 2012;7:e37728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shilpi A, Parbin S, Sengupta D, Kar S, Deb M, Rath SK, Pradhan N, Rakshit M, Patra SK. Mechanisms of DNA methyltransferase-inhibitor interactions: procyanidin B2 shows new promise for therapeutic intervention of cancer. Chem Biol Interact. 2015;233:122–38. [DOI] [PubMed] [Google Scholar]
- 100. Kin R, Kato S, Kaneto N, Sakurai H, Hayakawa Y, Li F, Tanaka K, Saiki I, Yokoyama S. Procyanidin C1 from cinnamomi cortex inhibits TGF-β-induced epithelial-to-mesenchymal transition in the A549 lung cancer cell line. Int J Oncol. 2013;43:1901–6. [DOI] [PubMed] [Google Scholar]
- 101. Li D, Zhao T, Meng J, Jing Y, Jia F, He P. Procyanidin B2 inhibits high glucose-induced epithelial-mesenchymal transition in HK-2 human renal proximal tubular epithelial cells. Mol Med Rep. 2015;12:8148–54. [DOI] [PubMed] [Google Scholar]
- 102. Quiñones M, Guerrero L, Fernández-Vallinas S, Pons Z, Arola L, Aleixandre A, Muguerza B. Involvement of nitric oxide and prostacyclin in the antihypertensive effect of low-molecular-weight procyanidin rich grape seed extract in male spontaneously hypertensive rats. J Funct Foods. 2014;6:419–27. [Google Scholar]
- 103. Pons Z, Margalef M, Bravo FI, Arola-Arnal A, Muguerza B. Acute administration of single oral dose of grape seed polyphenols restores blood pressure in a rat model of metabolic syndrome: role of nitric oxide and prostacyclin. Eur J Nutr. 2016;55:749–58. [DOI] [PubMed] [Google Scholar]
- 104. Mao JT, Smoake J, Park HK, Lu QY, Xue B. Grape seed procyanidin extract mediates antineoplastic effects against lung cancer via modulations of prostacyclin and 15-HETE eicosanoid pathways. Cancer Prev Res (Phila). 2016;9:925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ulrichova J, Jourova L, Tomankova V, Kosina P, Kopecna-Zapletalova M, Anzenbacherova E. Effect of the procyanidin A2 on activity of cytochromes P450 in human hepatocytes. Planta Med. 2013;79:PJ2. [Google Scholar]
- 106. Kim Y, Choi Y, Ham H, Jeong HS, Lee J. Protective effects of oligomeric and polymeric procyanidin fractions from defatted grape seeds on tert-butyl hydroperoxide-induced oxidative damage in HepG2 cells. Food Chem. 2013;137:136–41. [DOI] [PubMed] [Google Scholar]
- 107. Sun P, Wang T, Chen L, Yu BW, Jia Q, Chen KX, Fan HM, Li YM, Wang HY. Trimer procyanidin oligomers contribute to the protective effects of cinnamon extracts on pancreatic β-cells in vitro. Acta Pharmacol Sin. 2016;37:1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Vigna G, Costantini F, Aldini G, Carini M, Catapano A, Schena F, Tangerini A, Zanca R, Bombardelli E, Morazzoni Pet al. Effect of a standardized grape seed extract on low-density lipoprotein susceptibility to oxidation in heavy smokers. Metabolism. 2003;52:1250–7. [DOI] [PubMed] [Google Scholar]
- 109. Tomaru M, Takano H, Osakabe N, Yasuda A, Inoue KI, Yanagisawa R, Ohwatari T, Uematsu H. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice. Nutrition. 2007;23:351–5. [DOI] [PubMed] [Google Scholar]
- 110. Hogan C, Canning C, Sun S, Sun X, Kadouh H, Zhou K. Dietary supplementation of grape skin extract improves glycemia and inflammation in diet-induced obese mice fed a Western high fat diet. J Agric Food Chem. 2011;59:3035–41. [DOI] [PubMed] [Google Scholar]
- 111. Soares S, Mateus N, Freitas DF. Interaction of different polyphenols with bovine serum albumin (BSA) and human salivary alpha-amylase (HSA) by fluorescence quenching. J Agric Food Chem. 2007;55:6726–35. [DOI] [PubMed] [Google Scholar]
- 112. Khanal RC, Howard LR, Prior RL. Effect of heating on the stability of grape and blueberry pomace procyanidins and total anthocyanins. Food Res Int. 2010;43:1464–9. [Google Scholar]
- 113. Kruger MJ, Davies N, Myburgh KH, Lecour S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res Int. 2014;59:41–52. [Google Scholar]
- 114. Tresserra-Rimbau A, Rimm EB, Medina-Remón A, Martínez-González MA, De la Torre R, Corella D, Salas-Salvadó J, Gómez-Gracia E, Lapetra J, Arós F et al.. Inverse association between habitual polyphenol intake and incidence of cardiovascular events in the PREDIMED study. Nutr Metab Cardiovasc Dis. 2014;24:639–47. [DOI] [PubMed] [Google Scholar]
- 115. Nunes MA, Pimentel F, Costa AS, Alves RC, Oliveira MBP. Cardioprotective properties of grape seed proanthocyanidins: an update. Trends Food Sci Technol. 2016;57:31–9. [Google Scholar]
- 116. Lei L, Yang Y, He H, Chen E, Du L, Dong J, Yang J. Flavan-3-ols consumption and cancer risk: a meta-analysis of epidemiologic studies. Oncotarget. 2016;7:73573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sak K. Intake of individual flavonoids and risk of carcinogenesis: overview of epidemiological evidence. Nutr Cancer. 2017;69:1119–50. [DOI] [PubMed] [Google Scholar]
- 118. Bagchi D, Swaroop A, Preuss HG, Bagchi M. Free radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: an overview. Mutat Res. 2014;768:69–73. [DOI] [PubMed] [Google Scholar]
- 119. Montlahuc C, Julia C, Touvier M, Fezeu L, Hercberg S, Galan P, Kesse-Guyot E, Chevret S. Association between dietary polyphenols intake and an oxidative stress biomarker: interest of multiple imputation for handling missing covariates and outcomes. BMC Nutr. 2016;2:71. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.