ABSTRACT
Dietary patterns with substantial proportions of energy from plant sources have been associated with favorable biomarkers of low-grade inflammation. Less is known of the relation between vegetarian-based dietary patterns and markers of inflammation and immune status. This systematic review and meta-analysis aimed to determine the relation between vegetarian-based dietary patterns and inflammatory and immune markers (C-reactive protein, tumour necrosis factor α, fibrinogen, natural killer cells, leukocytes, lymphocytes, thrombocytes, interleukins, and immunoglobulins). PubMed, Medline, and Cochrane scientific databases were searched to identify relevant studies. Random effects meta-analyses were conducted to assess the weighted mean differences (WMDs) for each outcome variable between vegetarian and non-vegetarian groups. Thirty observational and 10 intervention studies were included in the review. Pooled effects of vegetarian-based dietary patterns were associated with significantly lower concentrations of CRP (WMD: −0.61 mg/L; 95% CI: −0.91, −0.32 mg/L; P = 0.0001), fibrinogen (WMD: −0.22 g/L; 95% CI: −0.41, −0.04 mg/L; P = 0.02), and total leukocyte (WMD: −0.62 × 103/μL; 95% CI −1.13 × 103, −0.10 × 103/μL; P = 0.02) compared with those following non-vegetarian dietary patterns in observational studies. Insufficient data were identified for a meta-analysis of intervention studies. This study provides evidence that vegetarian-based dietary patterns are associated with lowered serum C-reactive protein, fibrinogen, and total leukocyte concentrations. Future research should focus on large-scale intervention trials, contrasting differences in inflammation and immune status and function between vegetarian and non-vegetarian-based populations.
Keywords: inflammation, immune function, vegetarian, vegan, diet, dietary patterns, CRP, IL-6, meta-analysis, systematic review
Introduction
Nutritional epidemiology has seen a shift away from single nutrient analyses to a complementary approach in the form of dietary pattern analysis (1). Evaluating dietary patterns may provide a more holistic and clinically relevant approach to assessing diet-disease relations as nutrients are not eaten in isolation and synergistic effects of multiple components can have a concerted effect (2). Vegetarian-based dietary patterns are typically higher in fruits, vegetables, whole grains, nuts, seeds, and legumes, all of which are naturally higher in phytochemicals and some vitamins compared to non-vegetarian dietary patterns (3, 4). A variety of vegetarian-based eating patterns exist based on the inclusion or exclusion of animal products. For example, individuals who omit all animal products are classically described as vegan, whilst those who include eggs and dairy products are referred to as lacto-ovo-vegetarian (LOV) (5). Consumption of these dietary patterns are protective against many chronic diseases, including coronary heart disease, type 2 diabetes mellitus (T2DM), some cancers, and are associated with lower all-cause mortality (6–9).
An array of mechanisms are likely responsible for the protective effects observed in vegetarian-based dietary patterns, including improved inflammatory and immune responses. These systems can be modulated by various dietary patterns and food components, demonstrating that plant-based foods can provide favorable outcomes (10–13). When considering inflammation, and immune status, it is important to recognize that these systems are inherently linked and work synergistically. For instance, C-reactive protein (CRP), a nonspecific systemic marker of inflammation, may be elevated in response to cytokines released by phagocytes during an infection or when tissue is damaged (14).
Without a sufficient exogenous supply of nutrients the immune system will be jeopardized (15). In addition, the impact of “non-nutritive” components of food on immune function has been acknowledged (16–18). For example, polyphenolic compounds are shown to improve lymphocyte responsiveness and natural killer cell function (19), while carotenoids can have an immune-modulating effect (20). When considering the implications of these findings, it should be noted that we do not consume these components in isolation (2). As such, exploration of the impact of consuming a whole dietary pattern that is likely to be high in these components seems indicated.
The influence of diet on inflammation has also been examined and clear associations found (12, 21). The inflammatory response is a complex biological response used for protection against mechanical, environmental, and pathological challenges, and is associated with intracellular signaling molecules which can influence both immune and inflammation responses (22, 23). Research has demonstrated links between chronic low-grade inflammation and increased risk of various diseases, with inflammation hypothesized as an underlying pathophysiologic mechanism. For instance, chronic elevation of the inflammatory markers CRP, IL-6, and fibrinogen is shown to predict the risk of cardiovascular disease (CVD) (24), all-cause mortality (25), T2DM (26), and some cancers (27).
There is evidence to suggest that plant-based diets may have favorable effects on inflammation. Consumption of dietary patterns with substantive nutrients obtained from plant rather than animal sources has been shown to attenuate markers of chronic inflammation such as CRP, IL-6, and fibrinogen (12, 13, 21, 28). Similarly, a meta-analysis recently suggested that vegetarianism was associated with lowered serum CRP concentrations and may be a useful dietary approach to manage “inflammaging,” or the increased levels of chronic inflammation associated with aging (29). However, the review may be affected by their inclusion of participants who use statins [which can affect inflammatory markers such as CRP (30, 31)] and inclusion of intervention groups which may have incorporated consumption of some meat. In addition, consideration of the evidence base from randomized controlled trials (RCTs) is required to explore the effect of consuming a plant-based diet, hereinafter referred to as a vegetarian-based diet, on specific inflammatory and immune markers.
This systematic literature review aims to determine if vegetarian-based eating patterns in humans are associated with, or able to modulate, inflammation or immune biomarkers compared with those following non-vegetarian dietary patterns. A meta-analysis will further explore the effect of vegetarian-based eating patterns on common inflammation and immune biomarkers compared with non-vegetarian dietary patterns.
Methods
Study protocol
The systematic review followed the requirements of the Preferred Reporting of Systematic Reviews and Meta-Analyses (PRISMA) statement (32) and was registered with the International Prospective Register of Systematic Reviews (PROSPERO, CRD42016039043; 12 May 2016). A systematic search of the PubMed, MEDLINE, and Cochrane Central Register of Controlled Trials scientific databases (all years to December 2017) was conducted to answer the research question. Scientific database searches were conducted by 1 reviewer (JC). The search strategy used the following key words, with Medical Subject Heading terms used where available: (“Immunoglobulin*” OR “IgE” OR “IgD” OR “IgM” OR “IgA” OR “IgG” OR “Platelet*” OR “Basophil*” OR “Eosinophil*” OR “t lymphocyte subsets” OR “t cell*” OR “b lymphocyte subsets” OR “B cell*” OR “Monocyte*” OR “Neutrophil*” OR “Lymphocyte*” OR “Leukocyte*” OR “white blood cell*” OR “NK” OR “natural killer t cell*” OR “natural killer cell*” OR “immunity” OR “immune” OR “tumor necrosis factor” OR “tumour necrosis factor” OR “TNF” OR “interleukin” OR “IL-6” OR “fibrinogen” OR “CRP” OR “c reactive protein” OR “C-Reactive Protein” OR “inflammat*”) AND (“plant based” OR “plant-based” OR “vegan*” OR “*vegetarian” OR “vegetarian*”). An example of the search strategy in its entirety is shown in Supplemental Table 1. This review considered any dietary pattern including animal meats (including fish) to be non-vegetarian based, and dietary patterns excluding all animal meats to be vegetarian based.
Inclusion criteria
Studies were included if they examined the relationship (observational studies) or effect (intervention studies) of vegetarian-based dietary patterns compared with a non-vegetarian-based control dietary pattern on an outcome of interest [CRP, ILs (all), TNF (all), fibrinogen, natural killer cells, white blood cell counts (leukocytes, lymphocytes, neutrophils, monocytes, eosinophils, basophils, thrombocytes), immunoglobulins (IgG, IgA, IgE, IgD, and IgM)], and were conducted in human populations of all ages.
Observational studies were defined a priori to include any studies in which there was no direct intervention, and could include cross-sectional, case-control, prospective cohort, and retrospective cohort studies. They had to additionally involve participants who had adhered to a vegetarian-based diet (vegetarian group only) for ≥1 y. This timeframe was chosen to represent a habitual dietary pattern.
Intervention studies were also defined a priori to include any studies where a vegetarian-based diet was used as an intervention with a control group and could include RCT, non-RCTs, and pre-post studies. Intervention studies had to additionally study the vegetarian-based diet for a period >4 wk. This timeframe was selected as changes in some serum inflammatory markers such as IL-6 and CRP can take several weeks to become physiologically apparent (33–35).
Exclusion criteria
Observational and intervention studies were excluded for the following reasons: 1) they were not published in the English language; 2) they were conference abstracts, editorials, book series, errata, or conference proceedings; 3) they did not complete between-group analyses or provide raw data to allow these to be calculated; 4) they were animal or cellular models; 5) they were analyzing consumption of single foods or food groups rather than dietary patterns (e.g., exploring legume intake rather than vegetarian diets); 6) they used drugs that could alter biomarker outcomes, i.e. metformin (CRP) (30, 31); 7) they were assessing antibodies to food antigens rather than disease or general blood immunoglobulins; 8) they included any type of animal meat (including fish) in the vegetarian-based groups; or 9) they examined a single diet component/supplement only (e.g., cheese compared with vegan cheese alternative).
Intervention studies were additionally omitted if: 1) they used lifestyle interventions in conjunction with diet intervention, i.e. exercise or stress management; or 2) they used intervention diets containing any type of meat or did not report to controlling/discouraging meat intake.
Duplicate articles were initially removed with the use of EndNote referencing software (version X7, 2013; Thomson Reuters) with any remaining duplicates removed manually. Articles were firstly screened based on title and abstract. Full-text articles were obtained if the abstract was unavailable, or if it was unclear if the article met the inclusion criteria. Screening was performed by reviewer JCC with articles of concern discussed amongst the research team (YCP, EPN, GEP) until consensus was reached. Where results from the same study were reported in multiple articles, the most recent article was included to avoid duplication of results. Reference lists of included articles were hand searched to identify additional relevant articles.
Data extraction
Data extraction was performed by reviewer JCC in consultation with the research team, and included information related to author, date, study design, level of evidence, study population (including age, gender, country, and comorbidities), sample size, length of vegetarianism (observational studies), type of vegetarianism, details of intervention and control groups (intervention studies), outcomes investigated, and significant differences in biomarkers. Study authors were contacted for additional details if the required data were not available in the published article.
Statistical analysis
Meta-analyses were performed when >3 studies reported on a biomarker, median/mean with SD could be obtained or calculated from raw data, and the units of measurement could be made uniform. Meta-analyses were conducted separately for observational and intervention study results. Review Manager software (Review Manager version 5.3; The Nordic Cochrane Centre, Cochrane Collaboration, 2014) was used to estimate the pooled effect of inflammation and immune markers between vegetarian and non-vegetarian diets. Random effect meta-analyses were conducted to determine weighted mean differences (WMDs) by assigning a weight to each study on the basis of an individual study's inverse variance (36), and 95% CIs were used for each outcome. If a study involved >1 intervention group meeting the inclusion criteria, data for all intervention groups were combined as recommended by the Cochrane Handbook (37). For the intervention analysis, crossover studies were initially analyzed as parallel studies through the use of a paired analysis, the most conservative approach to managing crossover studies (37). Paired analyses of crossover studies with correlation coefficients of 0.25, 0.5, and 0.75 were then conducted as sensitivity analyses to determine if this influenced the results (37). The I2 statistic was used to evaluate heterogeneity, with a score 50–90% likely indicating substantial heterogeneity, and a score of 75–100% considerable heterogeneity (37). Where ≥10 studies reported on a biomarker outcome, funnel plots were generated and Egger's test was applied to assess studies for small study effects (38) with the use of StatsDirect statistical software (version 3.1, 2013; StatsDirect Ltd) (39).
Where median and ranges were reported, the Hozo et al. (40) formula was used to calculate SD and mean (when the population was <25 persons). When IQR was given, IQR/1.35 was used to calculate the SD (37). Where insufficient information was described in the published article and raw data were provided by authors, statistical analyses were performed with SPSS software (version 21, 2012; SPSS Inc.). Shapiro-Wilk tests on raw data determined if biomarker outcomes were normally distributed. One-way ANOVA (parametric) or Kruskal-Wallis (nonparametric) tests determined if differences existed between dietary patterns for inclusion in the summary table. P values <0.05 were considered to be statistically significant.
Sensitivity analyses were performed by excluding each study individually to investigate the influence on overall estimates (37). Additionally, sensitivity analyses were conducted by excluding studies where participants suffered from a chronic condition. When sufficient data were available on the type of vegetarianism (LOV or vegan dietary patterns) (≥3 studies), subgroup analyses were performed.
Risk of bias
Study quality for the nonrandomized studies was assessed independently with the use of a modified version of the Newcastle-Ottawa Scale by 2 reviewers (JCC, EPN). Where discrepancies occurred, a third reviewer (YCP) was consulted until a consensus was reached. The Newcastle-Ottawa Scale score for each study was based on the primary outcome of the present study (CRP) if available. For intervention studies, risk of bias was assessed with the use of the Cochrane Collaboration's tool (37). To determine the quality of the body of evidence, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method was applied to both observational and intervention studies (41).
Results
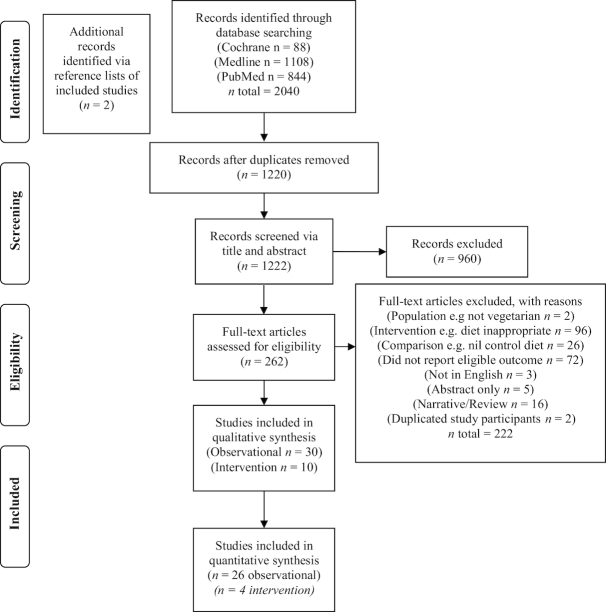
The literature search identified 2040 articles. After the exclusion criteria were applied, 39 studies [30 observational articles (42–71) describing 29 studies (2 separate articles were identified reporting on same study participants, with different outcome markers) and 8 intervention studies] were included in the review. A further 2 studies were identified via hand searching of reference lists, resulting in a total of 10 intervention studies (72–81). Figure 1 displays the complete process of study selection, including identification, screening, eligibility, and inclusion.
FIGURE 1.
Flowchart of study selection.
Observational studies
Description of the included studies
The included studies were cross-sectional or matched-cohort studies (Table 1). Types of vegetarianism included LOV (8), lacto-vegetarian (2), vegan (5), and combinations of these with comparison groups typically consuming mixed omnivorous non-vegetarian diets. Participants in 2 of the included studies had chronic conditions, with 1 receiving dialysis therapy (59, 70), and participants in the other study having CVD, diabetes mellitus, or a combination of both (63). One study (63) reported on participants whose ages ranged between 2 and 18 y old, whereas the remainder reported on adults aged ≥18 y (Table 1). Studies were conducted in a range of continents, including Asia (44–46, 59, 62, 63, 66–71), Africa (49), North America (47, 50, 51, 54, 60), South America (42, 48, 52, 57), and Europe (43, 53, 55, 56, 58, 61, 64, 65). Study quality ranged from 2–6 out of a possible 7 with the use of the modified Newcastle-Ottawa Score tool (Supplemental Table 2).
TABLE 1.
Characteristics of observational studies examining the association of participants following vegetarian-based or non-vegetarian dietary patterns and common biomarkers of inflammation and immune function1
| Study (ref.) | Design/level of evidence | Population/gender | Comorbidities | Country | Age, y (mean or range) | Years on vegetarian diet | Biomarker investigated | Study quality/7 | Matched (NS different at baseline) | Difference in biomarker (significance P < 0.05) |
|---|---|---|---|---|---|---|---|---|---|---|
| Acosta-Navarro et al. (42) | Cross-sectional | V = 44 | Nil | Brazil | V3 = 45.5 | >4 | hs-CRP | 5 | Age | ● hs-CRP (NS)4 |
| Navarro et al.2 (48) | NV = 44 | NV = 46.8 | Leukocytes | Gender | ● Leukocytes significantly ↓ in V group | |||||
| M | Neutrophils | Smoking status | ● Neutrophils significantly ↓ in V group | |||||||
| hx of disease | ||||||||||
| Ambroszkiewicz et al. (43) | Cross-sectional | LOV = 43 | Nil | Poland | 4.5–9.0 | >4.5 | CRP | 4 | Age | ● CRP (NS) |
| NV = 46 | BMI | |||||||||
| M and F | ||||||||||
| Chen et al. (45) | Cross-sectional | V = 99 | Nil | Taiwan | V = 51.24 | >1 | Leukocytes | 4 | Age | ● CRP significantly ↑ in NV group |
| NV = 99 | NV = 49.38 | hs-CRP | BMI | |||||||
| M and F | ||||||||||
| Chen et al. (44) | Cross-sectional | LOV = 173 | Nil | Taiwan | LOV = 54.00 | >1 | hs-CRP | 5 | BMI | ● hs-CRP (NS) |
| NV = 190 | NV = 49.94 | Leukocytes | ● Leukocytes (NS) | |||||||
| F | Thrombocytes | ● Thrombocytes (NS) | ||||||||
| Chuang et al. (46) | Matched cohort/cross-sectional | V = 686 | Nil | Taipei and Taiwan | V = 45.2 | Long-term | CRP | 5 | Age | ● CRP significantly ↑ in NV group |
| NV = 3423 | O = 45.1 | Location | ||||||||
| M and F | Sex | |||||||||
| Dong and Scott (47) | Cross-sectional | Ve = 13 | Nil | USA | Ve: M = 57; F = 40 | >1 | Leukocytes | 1 | Nil | ● Significance not reported |
| LV = 28 | LV: M = 45; F = 42 | |||||||||
| LOV = 15 | LOV: M = 43; F = 35 | |||||||||
| NV = 4 | NV: M = 31; F = 55 | |||||||||
| M and F | ||||||||||
| Famodu et al. (49) | Cross-sectional | Ve = 8 | Nil | Nigeria | Ve = 47.1 | Long-term | Fibrinogen | 3 | Age BMI | ● Fibrinogen significantly ↑ in NV group compared with LOV and Ve group |
| LOV = 28 | LOV = 49.0 | ● Fibrinogen significantly ↑ in LOV group compared with Ve group | ||||||||
| NV = 40 | NV = 48.7 | |||||||||
| M and F | ||||||||||
| Fontana et al. (50) | Matched cohort/cross-sectional | Ve = 21 | Nil | USA | Ve = 53.1 | >2 | hs-CRP | 2 | Age | ● CRP significantly ↑ in NV group |
| NV = 21 | NV = 53.1 | |||||||||
| M and F | ||||||||||
| Fontana et al. (51) | Matched cohort/cross-sectional | Ve = 18 | Nil | USA | 54.2 | 1.5–10 (range) | hs-CRP | 2 | Age | ● hs-CRP significantly ↑ in NV group |
| NV = 18 | Sex | |||||||||
| M and F | SES | |||||||||
| Franco-de-Moraes (52) | Cross-sectional | Ve = 66 | Nil | Brazil | Ve = 49.6 | >1 | TNF-α | 3 | Age | ● TNF-α (NS) |
| LOV = 102 | LOV = 49.6 | CRP | Sex | ● hs-CRP significantly ↑ in NV group | ||||||
| NV = 100 | NV = 49.1 | IL-10 | ● IL-10 (NS) | |||||||
| Gorczyca et al. (53) | Cross-sectional | V = 22 | Nil | Poland | V = 4 | >1 | IgA | 3 | Age | ● IgA (NS) |
| NV = 18 | NV = 9 (range 2–18) | IgM | Body weight | ● IgM (NS) | ||||||
| M and F | IgG | Height | ● IgG (NS) | |||||||
| Haddad et al. (54) | Cross-sectional | Ve = 25 | Nil | USA | Ve = 36.0 | >1 | Leukocytes | 3 | AgePhysical activity level | ● Leukocytes significantly ↓ in Ve group |
| NV = 20 | O = 33.5 | Lymphocytes | Blood lipid concentrations | ● Lymphocytes significantly ↓ in Ve group | ||||||
| M and F | Neutrophils | ● Neutrophils (NS) | ||||||||
| Monocytes | ● Monocytes (NS) | |||||||||
| Eosinophils | ● Eosinophils (NS) | |||||||||
| Basophils | ● Basophils (NS) | |||||||||
| Thrombocytes | ● Thrombocytes significantly ↓ in Ve group | |||||||||
| IgA | ● IgA (NS) | |||||||||
| IgG | ● IgG (NS) | |||||||||
| IgM | ● IgM (NS) | |||||||||
| CRP | ● CRP (NS) | |||||||||
| NK cell cytotoxic activity | ● NK cell cytotoxic activity (NS) | |||||||||
| Krajcovicova-Kudlackova and Blazicek (55) | Cross-sectional | LOV = 133 | Nil | Slovakia | LOV = 46.2 | >1 | hs-CRP | 2 | Age | ● hs-CRP significantly ↑ in NV group. |
| NV = 137 | NV = 47.2 | |||||||||
| M and F | ||||||||||
| Malter et al. (56) | Cross-sectional | V = 22 | Nil | Germany | V = 28–50 | >1 | Thrombocytes | 2 | Age | ● Thrombocytes (NS) |
| O = 22 | Leucocytes | Gender | ● Leucocytes (NS) | |||||||
| M | Lymphocytes | ● Lymphocytes (NS) | ||||||||
| Monocytes | ● Monocytes (NS) | |||||||||
| Basophilic granulocytes | ● Basophilic granulocytes (NS) | |||||||||
| Eosinophilic granulocytes | ● Eosinophilic granulocytes (NS) | |||||||||
| NK cell cytotoxic activity | ● NK cell activity of peripheral blood lymphocytes significantly ↑ in V group. | |||||||||
| Mezzano et al. (57) | Cross-sectional | V = 26 | Nil | Chile | V = 39 | >1 | Platelet count | 3 | Age | ● Thrombocytes significantly ↓ in NV group |
| NV = 26 | Fibrinogen | Sex | ● Fibrinogen significantly ↑ in NV group | |||||||
| M and F | CRP | SES | ● CRP (NS) | |||||||
| Montalcini et al. (58) | Cross-sectional | LOV = 26 | Nil | Italy | LOV = 32.6 | ≥3 | IL-2 | 5 | Age | ● IL-2 (NS) |
| NV = 26 | NV = 30.5 | IL-4 | BMI | ● IL-4 (NS) | ||||||
| M and F | IL-6 | Gender | ● IL-6 (NS) | |||||||
| IL-8 | ● IL-8 (NS) | |||||||||
| IL-10 | ● IL-10 (NS) | |||||||||
| TNF-α | ● TNF-α (NS) | |||||||||
| IL-1α | ● IL-1α (NS) | |||||||||
| IL-1β | ● Interleukin-β significantly ↑ in LOV group | |||||||||
| Ou et al. (59) | Case control/cross-sectional | V = 21 | Patients on dialysis therapy for >6 mo | Taiwan | V = 56.27 | ≥1.5 | hs-CRP | 2 | Age | ● hs-CRP (NS) |
| NV = 42 | O = 56.29 | Sex | ||||||||
| M and F | ||||||||||
| Paalani et al. (60) | Cross-sectional | V = 216 | Nil | USA | 68.8 | >1 | CRP | 4 | Not reported | ● CRP significantly ↑ in NV group |
| NV = 289 | IL-6 | |||||||||
| M and F | IL-10 | |||||||||
| TNF-α | ||||||||||
| Pinto et al. (61) | Matched cohort/cross-sectional | Ve = 23 | Nil | UK | Ve = 49 | >2 | IL-6 | 6 | Age | ● IL-6 (NS) |
| NV = 24 | NV = 54 | Sex | ||||||||
| F | BMI | |||||||||
| Pongstaporn et al. (62) | Cross-sectional | V = 179 | Nil | Thailand | V = 18+ | >1 | Leukocytes | 2 | Nil | ● Leukocytes significantly ↓ in Ve group |
| NV = 58 | Thrombocytes | ● Thrombocytes (NS) | ||||||||
| M and F | Neutrophils | ● Neutrophils (NS) | ||||||||
| Lymphocytes | ● Lymphocytes (NS) | |||||||||
| Refsum et al. (63) | Cross-sectional | V = 78 | 100 CVD (42 of which DM) | India | V = 27–55 | Long-term | Thrombocytes | 3 | Nil | ● Thrombocytes (NS) |
| NV = 126 | 104 without CVD (41 DM) | |||||||||
| M and F | ||||||||||
| Sebekova et al. (64) | Cross-sectional | LOV = 90 | Nil | Slovakia | LOV = 37.7 | >2 | Hs-CRP | 2 | Age | ● Hs-CRP (NS) |
| NV = 46 | O = 37.1 | Leukocytes | Gender | ● Leukocytes (NS | ||||||
| M and F | BMI | |||||||||
| Sebekova et al. (65) | Cross-sectional | Ve = 19 | Nil | Slovakia | Ve = 39.6 | Ve = 7.2 | CRP | 2 | Age | ● CRP (NS) |
| LOV = 19 | LOV = 36.1 | LOV = 8.2 | ||||||||
| NV = 9 | NV = 30.5 | |||||||||
| M and F | ||||||||||
| Su et al. (66) | Cross-sectional | LOV = 49 | Nil | Taiwan | LOV = 58.6 ± 6.0 | 10.8 | hs-CRP | 3 | Age | ● hs-CRP (NS) |
| NV = 41 | O = 57.2 ± 5.4 | Gender | ||||||||
| F | ||||||||||
| Suwannuruks et al. (67) | Cross-sectional | LOV = 50 | Nil | Thailand | LOV 18–50 | >1 | Fibrinogen | 1 | Nil | ● Fibrinogen (NS) |
| NV = 30 | Leukocytes | ● Leukocytes (NS) | ||||||||
| M and F | Thrombocytes | ● Thrombocytes (NS) | ||||||||
| Szeto et al. (68) | Cross-sectional | LOV = 30 | Nil | Hong Kong | LOV = 44.2 | 5–55 (range) | hs-CRP | 2 | Age | ● CRP significantly ↑ in NV group |
| NV = 30 | NV = 44.0 | Sex | ||||||||
| M and F | ||||||||||
| Tungtrongchitr et al. (69) | Cross-sectional | LV = 132NV = 47M and F | Nil | Thailand | LV: M = 35.5; F = 33NV: M = 32.5; F = 32Median | >1 | Leukocytes | 2 | Age | ● Leukocytes (NS) |
| Neutrophils | Sex | ● Neutrophils (NS) | ||||||||
| Lymphocytes | SES | ● Lymphocytes significantly ↓ in LV group | ||||||||
| Monocytes | Ethnic origin | ● Monocytes (NS) | ||||||||
| Eosinophil | ● Eosinophils significantly ↓ in female LV group compared with male LV and NV group | |||||||||
| Basophil | ● Eosinophils significantly ↑ in male LV group compared with female LV group and NV group | |||||||||
| Thrombocytes | ● Basophils (NS) | |||||||||
| ● Thrombocytes (NS) | ||||||||||
| Wu et al. (70) | Cross-sectional | V = 19 | Patients receiving dialysis therapy >6 mo | Taiwan | V = 63.3 | Long before HD—note mean length of HD = 5.9 | hs-CRP | 4 | Age | ● hs-CRP significantly ↑ in NV group |
| NV = 299 | NV = 57.5 | Leukocytes | Sex | ● Leukocytes significantly ↓ in V group | ||||||
| M and F | Mean HD length | |||||||||
| Yang et al. (71) | Matched cohort/cross-sectional | V = 171 | Nil | China | V = 32.6 | > 1 | CRP | 4 | age | ● CRP (NS) |
| NV = 12 | NV = 34.2 | |||||||||
| M |
(hs-)CRP, (high-sensitivity) C-reactive protein; CVD, cardiovascular disease; DM, diabetes mellitus; F, female, HD, hemodialysis; Hx, History; LOV, lacto-ovo-vegetarian; LV, lacto-vegetarian; M, male, NR, not reported; NS, not significant; NV, non-vegetarian; SES, socioeconomic status; Ve, vegan.
Two separate papers identified reporting on same study participants, with different outcome markers; slight difference in Navaro et al.’s population: V = 43, NV = 41; age, V = 45.0 y; NV = 46.5 y; and study quality = 4 between studies.
V, participants followed combinations of Ve, LV, or LOV diets.
NS, P > 0.05.
CRP concentrations were significantly lower in 9 out of 19 studies in the vegetarian-based groups, with no difference in 10 out of 19 studies (42–46, 50–52, 54, 55, 57, 59, 60, 64–66, 68, 70, 71). Leukocyte counts were significantly lower in 6 out of 11 studies in the vegetarian-based groups with no difference in 5 out of 11 studies (44, 45, 47, 48, 54, 56, 62, 64, 67, 69, 70) (Table 2). Four studies reported on lymphocyte counts with vegetarian groups displaying significantly lower counts in 2 of the studies (54, 56, 62, 69). Only 2 studies reported on NK cell cytotoxic activity as a function of applied immune-competence and found improved function in the vegetarian-based group (56) or no difference between groups (54). One study reported lower neutrophil counts in vegetarian-based groups (48), whereas the other 3 studies found no difference between groups (54, 62, 69). Fibrinogen was observed to be lower in vegetarian-based groups in 2 out of 3 studies (49, 57, 67). Table 2 shows the number of included studies identified for each biomarker and summarizes the number of studies reporting significant and nonsignificant differences in outcomes between vegetarian and non-vegetarian groups.
TABLE 2.
Overview of included studies reporting on biomarkers and significant differences between participants following vegetarian-based or non-vegetarian dietary patterns in observational studies1
| Biomarker | Studies included | Differences between groups (significance, P < 0.05) |
|---|---|---|
| Lymphocytes (54, 56, 62, 69) | 4 | ↓ in V group in 2/4 studies; NS 2/4 studies |
| Neutrophils (48, 54, 62, 69) | 4 | ↓ in NV group in 1/4 studies; NS 3/4 studies |
| Basophils (54, 56, 69) | 3 | NS 3/3 studies |
| Monocytes (54, 56, 69) | 3 | NS 3/3 studies |
| Eosinophils3 (54, 56, 69) | 3 | NS 3/3 studies |
| NK cell cytotoxic activity (54, 56) | 2 | ↑ in V group in 1/2 studies; NS 1/2 studies |
| Leukocytes (44, 45, 47, 48, 54, 56, 62, 64, 67, 69, 70) | 11 | ↓ in V group in 6/11 studies; NS 5/11 studies |
| Thrombocytes (44, 54, 56, 62, 63, 67, 69) | 7 | ↓ in V group in 1/7 studies; ↑ in V group in 1/7 studies; NS 5/7 studies |
| CRP (42–46, 50–52, 54, 55, 57, 59, 60, 64–66, 68, 70, 71) | 19 | CRP ↓ in veg group in 9/19; NS 10/19 studies |
| TNF-α4 (52, 58, 60) | 3 | NS2 |
| Fibrinogen (49, 57, 67) | 3 | ↑ in NV group in 2/3 studies; NS 1/3 studies |
| Interleukins | ||
| IL-10 (52, 58, 60) | 3 | NS |
| IL-6 (58, 60, 61) | 3 | NS |
| IL-2, IL-4, IL-8, IL-1α,IL-1β (58) | 1 | IL-1β ↑ in V group in 1/1 study |
| Immunoglobulins | ||
| IgA, IgM, IgG (53, 54) | 2 | NS |
NV, non-vegetarian; V, vegetarian-based.
NS, not significant between groups (P > 0.05).
Tungtrongchitr et al. (69) compared medians between groups and genders. Eosinophils were significantly ↓ in the NV group compared with the male LV group but significantly ↑ compared with the female LV group.
Significance not reported in 1 study.
Relation between vegetarian-based diets on inflammatory and immune biomarkers
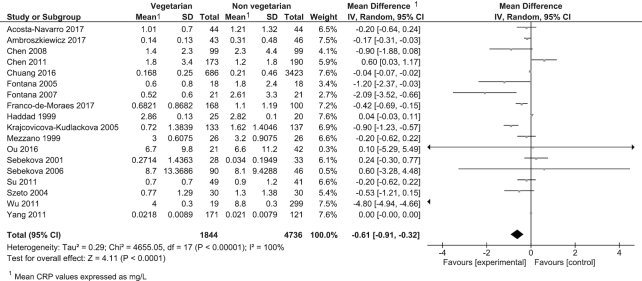
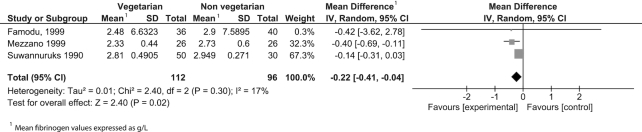
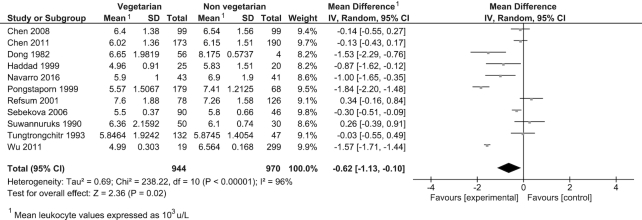
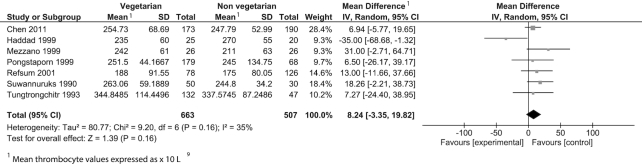
Twenty-six observational studies were included in the meta-analysis reporting on 4 outcomes: CRP, thrombocytes, leukocytes, and fibrinogen (Table 3). Consumption of a vegetarian-based dietary pattern was associated with significantly lower CRP (P = 0.001; Figure 2), fibrinogen (P = 0.02; Figure 3), and leukocyte (P = 0.02; Figure 4) levels compared with those following a mixed omnivorous non-vegetarian comparison diet. No significant difference was observed for thrombocytes between groups (P = 0.16; Figure 5). The quality of body of evidence for the observational studies was deemed to be “very low” after a 1-level downgrade was applied for each outcome as per the GRADE guidelines (41) (Supplemental Table 3). Funnel plots were generated for CRP and leukocyte concentrations. Egger's test indicated no significant asymmetry (Supplemental Figure 1).
TABLE 3.
Meta-analysis summary of observational studies comparing CRP, thrombocytes, fibrinogen, and leukocytes between vegetarian-based and non-vegetarian-based dietary patterns1
| Outcome | Number of analyses | Number of vegetarian participants | Number of control participants | Effect Estimate (95% CI) | P value | Inconsistency (I2) | GRADE quality |
|---|---|---|---|---|---|---|---|
| CRP, mg/L | 18 | 1844 | 4736 | −0.61 (−0.91, −0.32) | 0.001 | 100% | Very low |
| Thrombocytes, × 109/L | 7 | 663 | 507 | 8.24 (−3.35, 19.82) | 0.16 | 35% | Very low |
| Fibrinogen, g/L | 3 | 112 | 96 | −0.22 (−0.41, −0.04) | 0.02 | 17% | Very low |
| Leukocytes, 103/μL | 11 | 944 | 970 | −0.62 (−1.13, −0.10) | 0.02 | 96% | Very low |
1CRP, C-reactive protein.
FIGURE 2.
Difference in CRP values between participants following vegetarian-based dietary patterns and non-vegetarian dietary patterns (cross-sectional studies). Diamond indicates WMD with 95% CI. CRP, C-reactive protein; WMD, weighted mean difference.
FIGURE 3.
Difference in fibrinogen values between participants following vegetarian-based dietary patterns and non-vegetarian dietary patterns (cross-sectional studies). Diamond indicates WMD with 95% CI. WMD, weighted mean difference.
FIGURE 4.
Difference in leukocyte values between participants following vegetarian-based dietary patterns and non-vegetarian dietary patterns (cross-sectional studies). Diamond indicates WMD with 95% CI. WMD, weighted mean difference.
FIGURE 5.
Difference in thrombocyte values between participants following vegetarian-based dietary patterns and non-vegetarian dietary patterns (cross-sectional studies). Diamond indicates WMD with 95% CI. WMD, weighted mean difference.
Sensitivity analysis and subgroup analysis
When sensitivity analyses were applied, the pooled effect on CRP remained significant. The pooled effect on leukocytes became nonsignificant when Pongstaporn et al. (62) was omitted (P = 0.08). Conversely, thrombocytes were significantly higher in the vegetarian group with the omission of Haddad et al. (54) (P = 0.01) (Supplemental Figure 2). Lower leukocyte and CRP levels in vegetarian-based populations continued to be found when sensitivity analyses were applied excluding studies with participants receiving hemodialysis treatment, or suffering from cardiovascular disease (CVD) or T2DM (P = 0.01) (Supplemental Figure 3).
Because of the considerable heterogeneity observed (I2 = 100%) for CRP concentrations between dietary groups, meta-analyses were performed on specific dietary groups in an attempt to identify the source of heterogeneity. No significant subgroup differences were observed between vegan, LOV groups, and non-vegetarian groups for CRP. Neither subgroup analysis accounted for the high heterogeneity (I2 for both vegan and LOV groups 87%; Supplemental Figure 4).
Intervention studies
Ten intervention studies were identified exploring the effect of vegetarian-based eating patterns on common markers of inflammation or immune function (72–81). They included 7 parallel and 3 crossover intervention study designs. Of the included studies, 7 were randomized (72–77, 81), and the remaining 3 were unable to be confirmed as being randomized or nonrandomized (78–80) as authors could not be contacted. Vegetarian-based intervention diets included LOV (n = 3), LV (n = 1), and vegan (n = 6) with varying macronutrient percentages (Table 4). Control diets varied, and included a well-balanced mixed diet from the 5 food groups (72), a conventional T2DM diet recommended by the European Association for the Study of Diabetes (73), habitual mixed diets (74, 75, 77, 79, 80), and an American Heart Association diet (fat total 30%, 7% saturated fat, <300 mg of cholesterol, <1500 mg of sodium daily) (76). Intervention diet duration ranged from 4 to 56 wk. Studies were from North America (76, 81) and Europe (72–75, 77–80). The populations examined in the included studies were mixed. For instance, in 4 studies the participants had rheumatoid arthritis, 1 study population exhibited T2DM, in 1 study the participants were overweight or obese (class 1; as measured by BMI), and in 1 study the participants were children >95% of BMI for age. The biomarkers investigated varied between studies (Table 4).
TABLE 4.
Characteristics of interventional studies examining the effect of vegetarian dietary patterns and non-vegetarian dietary patterns on common biomarkers of inflammation and immune function1
| Study/year | Study design/level of evidence | Population/gender | Comorbidities | Country | Age, y (mean or range) | Duration of vegetarian diet, wk | Intervention vegetarian dietary pattern | Control non-vegetarian | Biomarker investigated | Matched (baseline participant characteristics matched) | Difference in biomarker (significance, P < 0.05) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Elkan et al. (72) | RCT (level II)2 | Ve = 30 | Patients with RA (2 and 10 y duration) | Sweden | Ve = 49.9 | 52 | Ve | Well-balanced mixed diet from 5 food groups | CRP | Age | hs-CRP significantly ↓ within LV group |
| NV = 28 | NV = 50.8 | Gluten free | (CHO 55–60%, pro 10–15%, fat <30% with <10% saturated) | Weight | |||||||
| M and F | (CHO 60%, pro 10%, fat 30%) | BMI | |||||||||
| Disease duration | |||||||||||
| Concomitant treatment | |||||||||||
| Hunt and Roughead (81) | RCT (level II; crossover) | n = 21 | Nil | USA | 33.2 | 8 (nil washout) | LOV | LOV with ∼184 g meat (3 parts beef and 1 part chicken)/d | CRP | NA (crossover) | CRP (NS) |
| F | ↑ amounts of legumes, whole grains, breads/cereals fruits and vegetables | ||||||||||
| Cessation of supplements | |||||||||||
| Kahleova et al. (73) | RCT (level II) | LV = 37 | Patients with T2DM | Czech Republic | LV = 54.6 | 12 | LV | Conventional T2DM diet as per DNSG of the EASD | hs-CRP | Significant differences between groups not reported at baseline | hs-CRP significantly ↓ in LV group |
| NV = 37 | NV = 57.7 | Animal products were limited to 1 low-fat yogurt a day | (CHO 50%, pro 20%,fat <30% with ≤7% saturated, <200 mg cholesterol ⁄ d) | Fibrinogen | Fibrinogen (NS) | ||||||
| M and F | (CHO 60%, pro 15%, fat 25%) | ||||||||||
| Kjeldsen-Kragh et al.3 (74, 75) | RCT (Level II) | Ve = 27 | Classic or definite RA | Norway | Ve = 53 | 56 | Ve | Habitual mixed diet | CRPThrombocytesLeukocytesTNF-αIgMIgAIgG | Significant differences between groups not reported at baseline | hs-CRP significantly ↓ in Ve groupThrombocytes significantly ↓ in Ve groupLeukocytes significantly ↓ in Ve groupTNF-α (NS) IgM significantly ↓ in Ve groupIgA (NS) IgG significantly ↓ Ve group (after 1 month |
| NV = 26 | NV = 56 | Gluten-free Ve for 3.5 mo | Thrombocytes | ||||||||
| M and F | Followed by LOV for 9.5 m | Leukocytes | |||||||||
| TNF-α | |||||||||||
| igM | |||||||||||
| igA | |||||||||||
| igG | |||||||||||
| Macknin et al. (76) | RCT (level II) | Ve = 14 | ChildrenBMI >95th percentile for age/sex, cholesterol >169 mg/dL | USA | Children Ve = 15.0O = 15.0 | 4 | Ve | American Heart Association diet | hs-CRP | No significant difference in biomarkers at baseline | hs-CRP significantly ↓ in children on Ve |
| NV = 14 | AdultsVe = 46.5 O = 46.0 | Avoidance of added fat and limited intake of nuts and avocado | (Fat total 30%, 7% saturated fat, <300 mg cholesterol, <1500 mg of sodium daily) | IL-6 | IL-6 (NS) | ||||||
| Overweight children with 1 × accompanying parent | |||||||||||
| M and F | |||||||||||
| Nenonen et al. (77) | RCT (level II) | Ve = 22 | Chronic and active RA | Finland | Ve = 49.1 | 12 | Ve | Habitual mixed diet | CRP | Height | CRP (NS) |
| NV = 21 | CRP >10 mg/L | NV = 55.6 | Rich in lactobacilli | Weight | |||||||
| M and F | BMI | ||||||||||
| Duration of RA | |||||||||||
| Seropositivity | |||||||||||
| Medication | |||||||||||
| Richter et al. (78) | Nonrandomized crossover design (level III-2)4 | n = 8 | Nil | Denmark | 21–28 | 12 (2 × 6 (crosover; 4 wk washout) | LOV | High amounts of animal protein (CHO 57%, pro 14%, lipids 29%) | Monocyte concentrations | NA (crossover) | Monocytes |
| Well-trained athletes | High in vegetable protein sources | Monocytes (CD14+) | Monocytes (CD14+) (NS) | ||||||||
| M | (CHO 57%, pro 14%, lipids 29%) | NK cells (CD16+) | NK cells (CD16+) (NS) | ||||||||
| Pan T cells (CD3+) | Pan T cells (CD3+) (NS) | ||||||||||
| T suppressor cells (CD8+) | T suppressor cells (CD8+) (NS) | ||||||||||
| T helper cells (CD4+) | T helper cells (CD4+) (NS) | ||||||||||
| Sköldstam et al. (80) | RCT (level II) | LOV = 15 | Classical RA | Sweden | LOV = 35–56 | 12 | LOV | Habitual mixed diet | LeukocytesT lymphocytesB lymphocytesIgGIgAIgM | Not reported | Leukocytes (NS)T lymphocytes (NS)B lymphocytes (NS)IgG (NS)IgA (NS)IgM significantly ↑ within LOV group |
| NV = 10 | NV = 43–66 | Nil alcohol, tobacco, coffee/teaLimited salt, sugar, white flour and grain products | T lymphocytes | T lymphocytes (NS) | |||||||
| M and F | B lymphocytes | B lymphocytes (NS) | |||||||||
| IgG | IgG (NS) | ||||||||||
| IgA | IgA (NS) | ||||||||||
| IgM | IgM significantly ↑ within LOV group | ||||||||||
| T lymphocytes (NS) | |||||||||||
| Sköldstam (79) | Nonrandomized crossover design (Level III-2) | n = 20 | Classical or definite RA | Sweden | 35–68 | 16 | Ve | Habitual mixed diet | CRP | NA (crossover) | CRP (NS) |
| NR |
1(hs-)CRP, high-sensitivity C-reactive protein; CHO, carbohydrates; F, female; LOV, lacto-ovo-vegetarian; LV, lacto-vegetarian; M, male; NR, not reported; NS, not significant (P > 0.05); NV, non-vegetarian; pro, Protein; RA, rheumatoid arthritis; T2DM, type 2 diabetes mellitus; Ve, vegan.
RCT (level II), randomized controlled trial.
Same study/participants—different outcomes investigated.
Level III-2, a comparative study with concurrent controls: nonrandomized, experimental trial.
CRP levels were found to be significantly lower in vegetarian-based groups than in non-vegetarian groups in 4 out of 7 studies, with no significant difference in 3 out of 7 intervention studies (Table 5). Lymphocytes, monocytes, pan T cells (CD3+), T suppressor cells (CD8+), T helper cells (CD4+), NK cells, TNF-α, fibrinogen, IL-6, and IgA were reported by only 1 intervention study, with no significant difference between vegetarian and non-vegetarian groups found. Table 5 shows a summary of the included intervention studies and corresponding biomarker outcomes with significant and nonsignificant differences between study groups. The quality of body of evidence for the intervention studies was rated as “very low” according to GRADE (Supplemental Figure 5) (41).
TABLE 5.
Overview of included studies reporting on biomarkers and significant differences between vegetarian- and non-vegetarian-based dietary patterns in intervention studies1
| Biomarker | Studies included | Differences between groups (significance, P < 0.05) |
|---|---|---|
| Lymphocytes (80) | 1 | NS |
| Monocytes (78) | 1 | NS |
| Monocytes (CD14+) (78) | 1 | NS |
| Pan T cells (CD3+) (78) | 1 | NS |
| T suppressor cells (CD8+) (78) | 1 | NS |
| T helper cells (CD4+) (78) | 1 | NS |
| NK cells (78) | 1 | NS |
| Leukocytes (74, 80) | 2 | ↓ in V group in 1/2 studies; NS 1/2 studies |
| Thrombocytes (74) | 1 | ↓ in V group in 1/1 studies |
| CRP (72–74, 76, 77, 79, 81) | 7 | ↓ in V group in 4/7; NS 3/7 studies |
| TNF-α (75) | 1 | NS |
| Fibrinogen (73) | 1 | NS |
| Interleukins | ||
| IL-6 (76) | 1 | NS |
| Immunoglobulins | ||
| IgM (75, 80) IgA (75, 80) IgG (75, 80) | 2 | ↓ in V group in 1/2 studies; ↑ within V group in 1/2 studies; NS ↓ in V group in 1/2 studies; NS 1/2 studies |
NS, not significant (P > 0.05); V, vegetarian-based.
Pooled effects and subgroup analysis of vegetarian-based diets on inflammatory and immune biomarkers
Of the 10 studies identified, only 4 were eligible for a meta-analysis examining vegetarian-based dietary patterns and their effect on CRP (vegetarian, n = 116; non-vegetarian, n = 114). Due to the small population pool, varied population demographics (patients with rheumatoid arthritis, women, children with a BMI >95th percentile for age/sex with cholesterol >169 mg/dL and patients with T2DM), and varying intervention diets, the meta-analysis has been included as supplementary data to avoid potentially misleading conclusions common in nutritional meta-analyses (82) (Supplemental Figure 6). The Cochrane risk of bias assessment (Supplemental Table 4) and risk of bias graph (Supplemental Figure 7) are available as supplementary data. As a result of insufficient data, studies, or both, it was not possible to perform meta-analyses for the other outcomes.
Discussion
To the authors’ knowledge, this review and meta-analysis is the first to explore both the association and effect of consuming a vegetarian-based dietary pattern on biomarkers of inflammation and immune status. The results of the analysis of observational studies suggest that individuals following vegetarian-based diets may have lower levels of CRP and fibrinogen, 2 prominent markers of inflammation, than their non-vegetarian-based counterparts. Given that CRP is implicated in the development of atherosclerosis (83) and is an independent risk predictor of cardiovascular events (84, 85), the results of this review may partly explain the lowered incidence of cardiovascular events observed in vegetarian populations (86, 87). The lowered leukocyte and fibrinogen concentrations observed in vegetarian-based eating patterns appears to be favorable as elevated leukocyte and fibrinogen biomarkers have been associated with increased risk of all-cause mortality (88), T2DM (89), metabolic syndrome (90), and coronary heart disease (91).
Our results are in contrast to those of a recent meta-analysis, which found nonsignificant differences in CRP concentrations between vegetarian- and non-vegetarian-based dietary patterns (Hedges’ g = −0·15; 95% CI: 0.35, 0.05) (29). There are several explanations for the inconsistency. Firstly, the present review excluded studies where statins were used by participants as these are known to reduce inflammation (30, 31), whereas the previous analysis included 1 study where statin use was significantly different between groups (92). Secondly, the previous review (29) included studies that included small amounts of animal flesh in the vegetarian group (93) or where the vegetarian dietary pattern was not adequately described (94), whereas these studies were excluded from our review. We also only included studies with a duration of vegetarianism of ≥1 y, aligning with the suggestion that there may be a time interval between starting a vegetarian diet and a reduction in CRP (29). Finally, this review has included recently published studies not available at the time of the previous review (42, 43, 52).
Despite 10 intervention studies being identified for inclusion in this review, many biomarkers of interest were not reported upon, or were only explored in a single study, thereby limiting conclusions regarding the effect of vegetarian-based dietary patterns on these outcomes. CRP was explored in 7 studies, however, with significantly lowered concentrations following consumption of a vegetarian-based diet observed in 4/7 studies, which aligns with the results of the observational meta-analysis presented here. The limited body of evidence identified in the intervention studies highlights the need for further RCTs to confirm the results of the observational meta-analysis.
An array of nutrients and “nonnutritive” components of the vegetarian diet may be responsible for the trend for lowered inflammation biomarkers following consumption of a vegetarian-based dietary pattern (95). Consumption of flavonoids such as quercetin, kaempferol, malvidin, peonidin, daidzein, and genistein have been inversely associated with serum CRP even after adjustment for covariates including vitamin C, vitamin E, carotenes, and fruit and vegetable consumption (96). The antioxidant properties of flavonoids have been hypothesized to prevent LDL oxidation—an early inflammatory event in the development of atherosclerosis (97). Similarly, carotenoids are potent antioxidants embedded within the lipid bilayer that scavenge free radicals, and have been inversely associated with markers of inflammation (98, 99). Both flavonoids and carotenoids are typically found in higher concentrations in those following vegetarian-based dietary patterns (100), and may contribute to the observed attenuation of inflammation in vegetarian-based groups. Phytochemicals, which tend to be more plentiful in vegetarian-based eating patterns (100), may act as antioxidant, antibacterial, antifungal, anti-inflammatory, antiallergic, hypotensive, chemopreventive agents (11, 101), and may modulate inflammatory and immune function (11, 17, 18). Quantifying phytochemical intakes between vegetarian and non-vegetarian groups may be a target for future research.
Type and quantity of dietary fat intake may also influence low-grade inflammation concentrations. Several studies have linked dietary saturated fatty acids with increased serum high-sensitivity CRP and fibrinogen levels (102, 103). Saturated fatty acid intake is typically higher in non-vegetarian-based dietary patterns due to the increased consumption of animal-based products (100) and may contribute to the increased concentration of serum CRP and fibrinogen observed in non-vegetarian-based populations. Vegetarian-based populations typically consume a greater proportion of their dietary fat in the form of unsaturated fatty acids than non-vegetarians (104), a trend that is inversely associated with inflammation (105).
It is important to note that overweight and obesity are associated with elevated inflammation markers including TNF-α and IL-6 (106). Vegetarian-based populations typically exhibit lower BMIs than non-vegetarian populations (107), which may in part account for the lower CRP, fibrinogen, and total leukocyte concentrations in the vegetarian-based than in the non-vegetarian-based populations observed in this review.
Due to the limited number of studies, quantitative analysis was not possible for many biomarkers in both observational and intervention studies including interleukins (all), TNF-α, NK cell activity, lymphocytes, neutrophils, monocytes, eosinophils, basophils, IgG, IgA, IgD, IgE, and IgM. Future research should concentrate on investigating potential differences in these biomarkers with a particular focus on immune biomarkers and function between dietary groups given the encouraging, but limited, findings of this review, which included lowered total leukocyte and lymphocyte (in 2 out of 4 studies) concentrations in addition to improved NK cell activity in 1 out of 2 studies in vegetarian-based groups. Interestingly, of the 2 out of 4 studies which reported lowered total lymphocyte concentrations in vegetarian-based groups, both lymphocyte counts were within normal reference ranges [Haddad et al. (54): 3.04 ± 0.83 × 109/L, normal reference range 1.170–4.698 × 109/L (108); and Tungtrongchitr (69) et al.: 30% and 33% white blood cells (median), normal reference range 18–54% (108)]. If lymphocyte counts are reduced in vegetarian-based populations, yet NK cell cytotoxic activity is improved, the overall effect on immune function may be favorable. Further exploration of lymphocyte concentrations and NK cell activity in vegetarian-based populations is required.
Although our review was comprehensive and systematic in nature, some limitations must be noted. Our analysis was limited by the small number of studies assessing the effect of vegetarian-based dietary patterns on fibrinogen (n = 3) and thrombocytes (n = 7) in observational studies, and on CRP (n = 5) in intervention studies. Furthermore, cross-sectional studies provide a high risk of bias and lower levels of study quality (compared to RCTs) (37). However, inclusion of cross-sectional studies was warranted in this review to provide an estimate of vegetarian-based eating patterns and their relation with a wide range of outcomes across a large population sample. In the case of this review, many studies used unit reporting methods which could not be converted to a common unit, preventing their use in the meta-analysis, had limited sample sizes, and often failed to control for risk factors that may have influenced inflammatory markers (e.g. BMI, physical activity, and smoking status), which may have increased the risk of bias in these studies. Moreover, many of the observational studies lacked detail on the types and quality of diet in both vegetarian and non-vegetarian groups, which presents challenges when interpreting the results of these studies. As mentioned, there was substantial variation between population groups and a small population sample pool in the intervention study quantitative analysis, limiting the generalizability of the results. Furthermore, it was unclear if 3 of the intervention studies were randomized or not.
There are also several strengths of this review. This meta-analysis is the first, to the authors’ knowledge, to systematically and quantitatively assess the relation between vegetarian-based dietary patterns and biomarkers of inflammation and immune status in both observational and intervention studies. Previous studies have investigated the effects of specific nutrients and foods on markers of low-grade inflammation; however, nutrients and foods are seldom eaten in isolation (13, 95). A further strength of this review is that dietary patterns were considered as a whole, thereby taking into account the complex synergistic and antagonistic biochemical interactions, and enhancing the applicability of the findings to real-life eating patterns (1).
Conclusion
This study systematically assessed the evidence from observational and intervention studies in order to compare common biomarkers of inflammation and immune status in vegetarian-based and mixed non-vegetarian dietary patterns. Vegetarian-based dietary patterns appeared to be favorable in all quantitative syntheses; however, results should be interpreted with caution due to the limited number of studies and substantial variation between studies. Future research should focus on large-scale intervention studies, exploring differences in immune function between vegetarian-based and non-vegetarian-based groups. This is justified given the increased consumption of “nonnutritive” immune-modulating phytochemicals typically consumed in vegetarian-based dietary patterns. Furthermore, because it appears there are favorable inflammatory profiles in vegetarian-based populations, it is plausible that immune function may also be improved given the inherent link between the two physiologic systems.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ contributions were as follows—all authors: contributed to the conceptual idea of the review; JCC, EPN, and YCP: collected and analyzed the data; JCC, EPN, and YCP: interpreted the data; and all authors: contributed to the manuscript preparation, and have read and approved the final manuscript.
Notes
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Author disclosures: JCC is the proprietor of Purely Plants (www.purelyplantsdietetics.com) and has an interest in advocating plant-based diets.
Supplemental Tables 1–4 and Supplemental Figures 1–7 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CRP, C-reactive protein; CVD, cardiovascular disease; LOV, lacto-ovo-vegetarian; LV, lacto-vegetarian; PRISMA, Preferred Reporting of Systematic Reviews and Meta-analyses; PROSPERO, Prospective Register of Systematic Reviews; RCT randomized controlled trial; T2DM, type 2 diabetes mellitus; WMD, weighted mean difference.
References
- 1. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 2. Jacobs DR Jr, Gross MD, Tapsell LC. Food synergy: an operational concept for understanding nutrition. Am J Clin Nutr. 2009;89(5):1543S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rajaram S. The effect of vegetarian diet, plant foods, and phytochemicals on hemostasis and thrombosis. Am J Clin Nutr. 2003;78(3):552S–8S. [DOI] [PubMed] [Google Scholar]
- 4. Melina V, Craig W, Levin S. Position of the Academy of Nutrition and Dietetics: vegetarian diets. J Acad Nutr Diet. 2016;116(12):1970–80. [DOI] [PubMed] [Google Scholar]
- 5. Tuso PJ, Ismail MH, Ha BP, Bartolotto C. Nutritional update for physicians: plant-based diets. Perm J. 2013;17(2):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ornish D, Scherwitz LW, Billings JH, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA, Kirkeeide RL, Hogeboom C. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280(23):2001–7. [DOI] [PubMed] [Google Scholar]
- 7. Orlich MJ, Fraser GE. Vegetarian diets in the Adventist Health Study 2: a review of initial published findings. Am J Clin Nutr. 2014;100(Suppl 1):353S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tantamango-Bartley Y, Jaceldo-Siegl K, Jing F, Fraser G. Vegetarian diets and the incidence of cancer in a low-risk population. Cancer Epidemiol Biomarkers Prev. 2012;22(2):286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barnard ND, Cohen J, Jenkins DJ, Turner-McGrievy G, Gloede L, Jaster B, Seidl K, Green AA, Talpers S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006;29(8):1777–83. [DOI] [PubMed] [Google Scholar]
- 10. Devereux G. The immune system: an overview. In: Calder PC, Field CJ, Gill HS, editors. Nutrition and Immune Function. Wallingford: CABI Publishing; 2002. pp. 1–20. [Google Scholar]
- 11. Gupta C, Prakash D. Phytonutrients as therapeutic agents. J Complement Integr Med. 2014;11(3):151–69. [DOI] [PubMed] [Google Scholar]
- 12. Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–27. [DOI] [PubMed] [Google Scholar]
- 13. Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, Esposito K, Jönsson LS, Kolb H, Lansink M. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(S3):S1–S78. [DOI] [PubMed] [Google Scholar]
- 14. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–54. [DOI] [PubMed] [Google Scholar]
- 15. Calder PC, Jackson AA. Undernutrition, infection and immune function. Nutr Res Rev. 2000;13(1):3–29. [DOI] [PubMed] [Google Scholar]
- 16. Bermon S, Castell LM, Calder PC, Bishop N, Blomstrand E, Mooren FC, Kruger K, Kavazis AN, Quindry JC, Senchina DS. Consensus statement immunonutrition and exercise. Exerc Immunol Rev. 2017;23:8–50. [PubMed] [Google Scholar]
- 17. Murakami A, Ohigashi H. Targeting NOX, INOS and COX‐2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 2007;121(11):2357–63. [DOI] [PubMed] [Google Scholar]
- 18. Mainardi T, Kapoor S, Bielory L. Complementary and alternative medicine: herbs, phytochemicals and vitamins and their immunologic effects. J Allergy Clin Immunol. 2009;123(2):283–94. [DOI] [PubMed] [Google Scholar]
- 19. Bub A, Watzl B, Blockhaus M, Briviba K, Liegibel U, Müller H, Pool-Zobel BL, Rechkemmer G. Fruit juice consumption modulates antioxidative status, immune status and DNA damage. J Nutr Biochem. 2003;14(2):90–8. [DOI] [PubMed] [Google Scholar]
- 20. Watzl B, Bub A, Brandstetter BR, Rechkemmer G. Modulation of human T-lymphocyte functions by the consumption of carotenoid-rich vegetables. Br J Nutr. 1999;82(5):383–9. [DOI] [PubMed] [Google Scholar]
- 21. Neale E, Batterham M, Tapsell LC. Consumption of a healthy dietary pattern results in significant reductions in C-reactive protein levels in adults: a meta-analysis. Nutr Res. 2016;36(5):391–401. [DOI] [PubMed] [Google Scholar]
- 22. Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012;34(1):43–62. [DOI] [PubMed] [Google Scholar]
- 23. Suzuki K, Nakaji S, Yamada M, Totsuka M, Sato K, Sugawara K. Systemic inflammatory response to exhaustive exercise. Cytokine kinetics. Exerc Immunol Rev. 2002;8:6–48. [PubMed] [Google Scholar]
- 24. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL. Markers of inflammation and cardiovascular disease. Circulation. 2003;107(3):499–511. [DOI] [PubMed] [Google Scholar]
- 25. Marsik C, Kazemi-Shirazi L, Schickbauer T, Winkler S, Joukhadar C, Wagner OF, Endler G. C-reactive protein and all-cause mortality in a large hospital-based cohort. Clin Chem. 2008;54(2):343–9. [DOI] [PubMed] [Google Scholar]
- 26. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–34. [DOI] [PubMed] [Google Scholar]
- 27. Il'yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2413–8. [DOI] [PubMed] [Google Scholar]
- 28. Eichelmann F, Schwingshackl L, Fedirko V, Aleksandrova K. Effect of plant‐based diets on obesity‐related inflammatory profiles: a systematic review and meta‐analysis of intervention trials. Obes Rev. 2016;17(11):1067–79. [DOI] [PubMed] [Google Scholar]
- 29. Haghighatdoost F, Bellissimo N, de Zepetnek JOT, Rouhani MH. Association of vegetarian diet with inflammatory biomarkers: a systematic review and meta-analysis of observational studies. Public Health Nutr. 2017;20(15):2713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carter A, Bennett C, Bostock J, Grant P. Metformin reduces C-reactive protein but not complement factor C3 in overweight patients with type 2 diabetes mellitus. Diabet Med. 2005;22(9):1282–4. [DOI] [PubMed] [Google Scholar]
- 31. Morin-Papunen L, Rautio K, Ruokonen A, Hedberg P, Puukka M, Tapanainen JS. Metformin reduces serum C-reactive protein levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(10):4649–54. [DOI] [PubMed] [Google Scholar]
- 32. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am J Clin Nutr. 2009;90(1):23–32. [DOI] [PubMed] [Google Scholar]
- 34. McAuley KA, Hopkins CM, Smith KJ, McLay RT, Williams SM, Taylor RW, Mann JI. Comparison of high-fat and high-protein diets with a high-carbohydrate diet in insulin-resistant obese women. Diabetologia. 2005;48(1):8–16. [DOI] [PubMed] [Google Scholar]
- 35. Browning L, Krebs J, Moore C, Mishra G, O'Connell M, Jebb S. The impact of long chain n‐3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab. 2007;9(1):70–80. [DOI] [PubMed] [Google Scholar]
- 36. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 37. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0[updated March 2011]. The Cochrane Collaboration. 2011.
- 38. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. StatsDirect. StatsDirect Statistical Software. StatsDirect Ltd; 2013. [Google Scholar]
- 40. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407–15. [DOI] [PubMed] [Google Scholar]
- 42. Acosta-Navarro J, Antoniazzi L, Oki AM, Bonfim MC, Hong V, Acosta-Cardenas P, Strunz C, Brunoro E, Miname MH, Filho WS et al.. Reduced subclinical carotid vascular disease and arterial stiffness in vegetarian men: the CARVOS Study. Int J Cardiol. 2017;230:562–6. [DOI] [PubMed] [Google Scholar]
- 43. Ambroszkiewicz J, Klemarczyk W, Mazur J, Gajewska J, Rowicka G, Strucinska M, Chelchowska M. Serum hepcidin and soluble transferrin receptor in the assessment of iron metabolism in children on a vegetarian diet. Biol Trace Elem Res. 2017;180(2):182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen CW, Lin CT, Lin YL, Lin TK, Lin CL. Taiwanese female vegetarians have lower lipoprotein-associated phospholipase A2 compared with omnivores. Yonsei Med J. 2011;52(1):13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen CW, Lin YL, Lin TK, Lin CT, Chen BC, Lin CL. Total cardiovascular risk profile of Taiwanese vegetarians. Eur J Clin Nutr. 2008;62(1):138–44. [DOI] [PubMed] [Google Scholar]
- 46. Chuang SY, Chiu TH, Lee CY, Liu TT, Tsao CK, Hsiung CA, Chiu YF. Vegetarian diet reduces the risk of hypertension independent of abdominal obesity and inflammation: a prospective study. J Hypertens. 2016;34(11):2164–71. [DOI] [PubMed] [Google Scholar]
- 47. Dong A, Scott SC. Serum vitamin B12 and blood cell values in vegetarians. Ann Nutr Metab. 1982;26(4):209–16. [DOI] [PubMed] [Google Scholar]
- 48. Navarro JA, de Gouveia LA, Rocha-Penha L, Cinegaglia N, Belo V, Castro MM, Sandrim VC. Reduced levels of potential circulating biomarkers of cardiovascular diseases in apparently healthy vegetarian men. Clin Chim Acta. 2016;461:110–3. [DOI] [PubMed] [Google Scholar]
- 49. Famodu AA, Osilesi O, Makinde YO, Osonuga OA, Fakoya TA, Ogunyemi EO, Egbenehkhuere IE. The influence of a vegetarian diet on haemostatic risk factors for cardiovascular disease in Africans. Thromb Res. 1999;95(1):31–6. [DOI] [PubMed] [Google Scholar]
- 50. Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term low-calorie low-protein vegan diet and endurance exercise are associated with low cardiometabolic risk. Rejuvenation Res. 2007;10(2):225–34. [DOI] [PubMed] [Google Scholar]
- 51. Fontana L, Shew JL, Holloszy JO, Villareal DT. Low bone mass in subjects on a long-term raw vegetarian diet. Arch Intern Med. 2005;165(6):684–9. [DOI] [PubMed] [Google Scholar]
- 52. Franco-de-Moraes AC, de Almeida-Pititto B, da Rocha Fernandes G, Gomes EP, da Costa Pereira A, Ferreira SRG. Worse inflammatory profile in omnivores than in vegetarians associates with the gut microbiota composition. Diabetol Metab Syndr. 2017;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gorczyca D, Prescha A, Szeremeta K. Impact of vegetarian diet on serum immunoglobulin levels in children. Clin Pediatr (Phila). 2013;52(3):241–6. [DOI] [PubMed] [Google Scholar]
- 54. Haddad EH, Berk LS, Kettering JD, Hubbard RW, Peters WR. Dietary intake and biochemical, hematologic, and immune status of vegans compared with non-vegetarians. Am J Clin Nutr. 1999;70(3 Suppl):S586–93. [DOI] [PubMed] [Google Scholar]
- 55. Krajcovicova-Kudlackova M, Blazicek P. C-reactive protein and nutrition. Bratisl Lek Listy. 2005;106(11):345–7. [PubMed] [Google Scholar]
- 56. Malter M, Schriever G, Eilber U. Natural killer cells, vitamins, and other blood components of vegetarian and omnivorous men. Nutr Cancer. 1989;12(3):271–8. [DOI] [PubMed] [Google Scholar]
- 57. Mezzano D, Munoz X, Martinez C, Cuevas A, Panes O, Aranda E, Guasch V, Strobel P, Munoz B, Rodriguez S et al.. Vegetarians and cardiovascular risk factors: hemostasis, inflammatory markers and plasma homocysteine. Thromb Haemost. 1999;81(6):913–7. [PubMed] [Google Scholar]
- 58. Montalcini T, De Bonis D, Ferro Y, Care I, Mazza E, Accattato F, Greco M, Foti D, Romeo S, Gulletta E et al.. High vegetable fats intake is associated with high resting energy expenditure in vegetarians. Nutrients. 2015;7(7):5933–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ou SH, Chen MY, Huang CW, Chen NC, Wu CH, Hsu CY, Chou KJ, Lee PT, Fang HC, Chen CL. Potential role of vegetarianism on nutritional and cardiovascular status in Taiwanese dialysis patients: a case-control study. PLoS One. 2016;11(6):e0156297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Paalani M, Lee JW, Haddad E, Tonstad S. Determinants of inflammatory markers in a bi-ethnic population. Ethn Dis. 2011;21(2):142–9. [PMC free article] [PubMed] [Google Scholar]
- 61. Pinto AM, Sanders TA, Kendall AC, Nicolaou A, Gray R, Al-Khatib H, Hall WL. A comparison of heart rate variability, n-3 PUFA status and lipid mediator profile in age- and BMI-matched middle-aged vegans and omnivores. Br J Nutr. 2017;117(5):669–85. [DOI] [PubMed] [Google Scholar]
- 62. Pongstaporn W, Bunyaratavej A. Hematological parameters, ferritin and vitamin B12 in vegetarians. J Med Assoc Thai. 1999;82(3):304–11. [PubMed] [Google Scholar]
- 63. Refsum H, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, Guttormsen AB, Joglekar A, Sayyad MG, Ulvik A et al.. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr. 2001;74(2):233–41. [DOI] [PubMed] [Google Scholar]
- 64. Sebekova K, Boor P, Valachovicova M, Blazicek P, Parrak V, Babinska K, Heidland A, Krajcovicova-Kudlackova M. Association of metabolic syndrome risk factors with selected markers of oxidative status and microinflammation in healthy omnivores and vegetarians. Mol Nutr Food Res. 2006;50(9):858–68. [DOI] [PubMed] [Google Scholar]
- 65. Sebekova K, Krajcoviova-Kudlackova M, Schinzel R, Faist V, Klvanova J, Heidland A. Plasma levels of advanced glycation end products in healthy, long-term vegetarians and subjects on a western mixed diet. Eur J Nutr. 2001;40(6):275–81. [DOI] [PubMed] [Google Scholar]
- 66. Su TC, Torng PL, Jeng JS, Chen MF, Liau CS. Arterial function of carotid and brachial arteries in postmenopausal vegetarians. Vasc Health Risk Manag. 2011;7:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Suwannuruks R, Apibal S, Srisulapanon S, Archararit N. Blood cell production and hemostatic mechanism in Thai vegetarians. J Med Assoc Thai. 1990;73(12):670–3. [PubMed] [Google Scholar]
- 68. Szeto YT, Kwok TC, Benzie IF. Effects of a long-term vegetarian diet on biomarkers of antioxidant status and cardiovascular disease risk. Nutrition. 2004;20(10):863–6. [DOI] [PubMed] [Google Scholar]
- 69. Tungtrongchitr R, Pongpaew P, Prayurahong B, Changbumrung S, Vudhivai N, Migasena P, Schelp FP. Vitamin B12, folic acid and haematological status of 132 Thai vegetarians. Int J Vitam Nutr Res. 1993;63(3):201–7. [PubMed] [Google Scholar]
- 70. Wu TT, Chang CY, Hsu WM, Wang IK, Hsu CH, Cheng SH, Liang CC, Chang CT, Huang CC. Nutritional status of vegetarians on maintenance haemodialysis. Nephrology (Carlton). 2011;16(6):582–7. [DOI] [PubMed] [Google Scholar]
- 71. Yang SY, Zhang HJ, Sun SY, Wang LY, Yan B, Liu CQ, Zhang W, Li XJ. Relationship of carotid intima-media thickness and duration of vegetarian diet in Chinese male vegetarians. Nutr Metab (Lond). 2011;8(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Elkan AC, Sjöberg B, Kolsrud B, Ringertz B, Hafström I, Frostegård J. Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: a randomized study. Arthritis Res Ther. 2008;10(2):R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kahleova H, Matoulek M, Malinska H, Oliyarnik O, Kazdova L, Neskudla T, Skoch A, Hajek M, Hill M, Kahle M et al.. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with type 2 diabetes. Diabet Med. 2011;28(5):549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kjeldsen-Kragh J, Haugen M, Borchgrevink CF, Laerum E, Eek M, Mowinkel P, Hovi K, Førre O. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet. 1991;338(8772):899–902. [DOI] [PubMed] [Google Scholar]
- 75. Kjeldsen-Kragh J, Mellbye OJ, Haugen M, Mollnes TE, Hammer HB, Sioud M, Førre O. Changes in laboratory variables in rheumatoid arthritis patients during a trial of fasting and one-year vegetarian diet. Scand J Rheumatol. 1995;24(2):85–93. [DOI] [PubMed] [Google Scholar]
- 76. Macknin M, Kong T, Weier A, Worley S, Tang AS, Alkhouri N, Golubic M. Plant-based, no-added-fat or American Heart Association diets: impact on cardiovascular risk in obese children with hypercholesterolemia and their parents. J Pediatr. 2015;166(4):953–9.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nenonen MT, Helve TA, Rauma AL, Hanninen OO. Uncooked, lactobacilli-rich, vegan food and rheumatoid arthritis. Br J Rheumatol. 1998;37(3):274–81. [DOI] [PubMed] [Google Scholar]
- 78. Richter EA, Kiens B, Raben A, Tvede N, Pedersen BK. Immune parameters in male atheletes after a lacto-ovo vegetarian diet and a mixed Western diet. Med Sci Sports Exerc. 1991;23(5):517–21. [PubMed] [Google Scholar]
- 79. Sköldstam L. Fasting and vegan diet in rheumatoid arthritis. Scand J Rheumatology. 1986;15:219–23. [DOI] [PubMed] [Google Scholar]
- 80. Sköldstam L, Larsson L, Lindström FD. Effects of fasting and lactovegetarian diet on rheumatoid arthritis. Scand J Rheumatol. 1979;8:249–55. [DOI] [PubMed] [Google Scholar]
- 81. Hunt JR, Roughead ZK. Nonheme-iron absorption, fecal ferritin excretion, and blood indexes of iron status in women consuming controlled lactoovovegetarian diets for 8 wk. Am J Clin Nutr. 1999;69(5):944–52. [DOI] [PubMed] [Google Scholar]
- 82. Barnard ND, Willett WC, Ding EL. The misuse of meta-analysis in nutrition research. JAMA. 2017;318(15):1435–6. [DOI] [PubMed] [Google Scholar]
- 83. Silva D, de Lacerda AP. High-sensitivity C-reactive protein as a biomarker of risk in coronary artery disease. Rev Port Cardiol (English Ed). 2012;31(11):733–45. [DOI] [PubMed] [Google Scholar]
- 84. Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97(20):2007–11. [DOI] [PubMed] [Google Scholar]
- 85. Boekholdt SM, Hack CE, Sandhu MS, Luben R, Bingham SA, Wareham NJ, Peters RJ, Jukema JW, Day NE, Kastelein JJ. C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: the EPIC-Norfolk prospective population study 1993–2003. Atherosclerosis. 2006;187(2):415–22. [DOI] [PubMed] [Google Scholar]
- 86. Huang T, Yang B, Zheng J, Li G, Wahlqvist ML, Li D. Cardiovascular disease mortality and cancer incidence in vegetarians: a meta-analysis and systematic review. Ann Nutr Metab. 2012;60(4):233–40. [DOI] [PubMed] [Google Scholar]
- 87. Le LT, Sabaté J. Beyond meatless, the health effects of vegan diets: findings from the Adventist cohorts. Nutrients. 2014;6(6):2131–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tamakoshi K, Toyoshima H, Yatsuya H, Matsushita K, Okamura T, Hayakawa T, Okayama A, Ueshima H; NIPPON DATA90 Research Group. White blood cell count and risk of all-cause and cardiovascular mortality in nationwide sample of Japanese. Circ J. 2007;71(4):479–85. [DOI] [PubMed] [Google Scholar]
- 89. Gkrania-Klotsas E, Ye Z, Cooper AJ, Sharp SJ, Luben R, Biggs ML, Chen L-K, Gokulakrishnan K, Hanefeld M, Ingelsson E. Differential white blood cell count and type 2 diabetes: systematic review and meta-analysis of cross-sectional and prospective studies. PLoS One. 2010;5(10):e13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jung C-H, Lee W-Y, Kim B-Y, Park SE, Rhee E-J, Park C-Y, Oh K-W, Mok J-O, Kim C-H, Park S-W. The risk of metabolic syndrome according to the white blood cell count in apparently healthy Korean adults. Yonsei Med J. 2013;54(3):615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279(18):1477–82. [DOI] [PubMed] [Google Scholar]
- 92. Lee YJ, Wang MY, Lin MC, Lin PT. Associations between vitamin B-12 status and oxidative stress and inflammation in diabetic vegetarians and omnivores. Nutrients. 2016;8(3):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Krajcovicova-Kudlackova M, Babinska K, Blazicek P, Valachovicova M, Spustova V, Mislanova C, Paukova V. Selected biomarkers of age-related diseases in older subjects with different nutrition. Bratisl Lek Listy. 2011;112(11):610–3. [PubMed] [Google Scholar]
- 94. Tiahou G, Dupuy A-M, Jaussent I, Sees D, Cristol J-P, Badiou S. Determinants of homocysteine levels in Ivorian rural population. Int J Vitam Nutr Res. 2009;79(56):319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Galland L. Diet and inflammation. Nutr Clin Pract. 2010;25(6):634–40. [DOI] [PubMed] [Google Scholar]
- 96. Chun OK, Chung S-J, Claycombe KJ, Song WO. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in US adults. J Nutr. 2008;138(4):753–60. [DOI] [PubMed] [Google Scholar]
- 97. Lind L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis. 2003;169(2):203–14. [DOI] [PubMed] [Google Scholar]
- 98. Walston J, Xue Q, Semba R, Ferrucci L, Cappola A, Ricks M, Guralnik J, Fried L. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2005;163(1):18–26. [DOI] [PubMed] [Google Scholar]
- 99. Pattison DJ, Symmons DP, Lunt M, Welch A, Bingham SA, Day NE, Silman AJ. Dietary β-cryptoxanthin and inflammatory polyarthritis: results from a population-based prospective study. Am J Clin Nutr. 2005;82(2):451–5. [DOI] [PubMed] [Google Scholar]
- 100. Craig WJ, Mangels AR. Position of the American Dietetic Association: vegetarian diets. J Am Diet Assoc. 2009;109(7):1266–82. [DOI] [PubMed] [Google Scholar]
- 101. Dillard CJ, German JB. Phytochemicals: nutraceuticals and human health. J Sci Food Agric. 2000;80(12):1744–56. [Google Scholar]
- 102. Clarke R, Shipley M, Armitage J, Collins R, Harris W. Plasma phospholipid fatty acids and CHD in older men: Whitehall study of London civil servants. Br J Nutr. 2008;102(2):279–84. [DOI] [PubMed] [Google Scholar]
- 103. Arya S, Isharwal S, Misra A, Pandey RM, Rastogi K, Vikram NK, Dhingra V, Chatterjee A, Sharma R, Luthra K. C-reactive protein and dietary nutrients in urban Asian Indian adolescents and young adults. Nutrition. 2006;22(9):865–71. [DOI] [PubMed] [Google Scholar]
- 104. Davis BC, Kris-Etherton PM. Achieving optimal essential fatty acid status in vegetarians: current knowledge and practical implications. Am J Clin Nutr. 2003;78(3):640S–6S. [DOI] [PubMed] [Google Scholar]
- 105. Kalogeropoulos N, Panagiotakos DB, Pitsavos C, Chrysohoou C, Rousinou G, Toutouza M, Stefanadis C. Unsaturated fatty acids are inversely associated and n-6/n-3 ratios are positively related to inflammation and coagulation markers in plasma of apparently healthy adults. Clin Chim Acta. 2010;411(7-8):584–91. [DOI] [PubMed] [Google Scholar]
- 106. Bastard J-P, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17(1):4–12. [PubMed] [Google Scholar]
- 107. Spencer E, Appleby P, Davey G, Key T. Diet and body mass index in 38 000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int J Obes. 2003;27(6):728. [DOI] [PubMed] [Google Scholar]
- 108. Valiathan R, Deeb K, Diamante M, Ashman M, Sachdeva N, Asthana D. Reference ranges of lymphocyte subsets in healthy adults and adolescents with special mention of T cell maturation subsets in adults of South Florida. Immunobiology. 2014;219(7):487–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.