ABSTRACT
The importance of an adequate periconceptional maternal folate status to prevent fetal neural tube defects has been well demonstrated and resulted in the recommendation for women to use folic acid supplements during the periconception period. The importance of maternal folate status for offspring neurodevelopment and brain health is less well described. We reviewed the current evidence linking maternal folate status before conception and during pregnancy with neurodevelopment and cognition of the offspring. We discuss both animal and human studies. Preclinical research revealed the importance of maternal folate status for several key processes required for normal neurodevelopment and brain functioning in the offspring, including DNA synthesis, regulation of gene expression, synthesis of phospholipids and neurotransmitters, and maintenance of healthy plasma homocysteine concentrations. Human observational studies are inconclusive; about half have shown a positive association between maternal folate status and cognitive performance of offspring. Whereas some studies suggest a positive association between maternal folate intake and cognition of offspring during childhood, data from interventional studies are too limited to conclude that there is a direct effect. Future preclinical studies are needed to help us characterize the behavioral effects, understand the underlying mechanisms, and to establish an optimal dosage and time window of folate supplementation. Moreover, more conclusive data from well-designed human observational studies and randomized controlled trials are needed to determine whether current recommendations for folic acid supplementation during pregnancy cover the needs for normal cognitive development in the offspring.
Keywords: maternal, prenatal, developmental, folate, folic acid, brain, cognition, neuropsychological
Introduction
The essential micronutrient folate, also known as vitamin B-9, is a water-soluble vitamin complex naturally occurring in, for example, green leafy vegetables. Its name is derived from the Latin word for leaf (folium). Folate acts as a cofactor and regulatory molecule in multiple crucial biological processes in our body. As most mammals, including rodents and humans, cannot synthesize folate de novo, adequate dietary intake of folate is important throughout life. Adequate folate intake is particularly important for pregnant women, in whom folate requirements are 5–10-fold higher compared to non-pregnant women. These increased requirements are critical to support optimal growth and development of both maternal and fetal tissues (1, 2). More specifically, as the developing embryo receives folate from the mother through the placenta (3), an adequate maternal folate supply is necessary to maintain active transfer of folate from the maternal circulation to fetal vessels via polarized folate transporters (4).
Early research demonstrated megaloblastic anemia as a common consequence of folate deficiency in pregnant women (5). As supplementation with folic acid, the synthetic form of folate, has been shown to reduce the prevalence of folate deficiency during pregnancy (6, 7), pregnant women were subsequently recommended to use 200–400 μg folic acid/d (8). Later studies showed that periconceptional folic acid supplementation also protects against neural tube defects (NTDs) in the fetus (9, 10). Besides its importance for prevention of NTDs, maternal periconceptional folate status has also been linked to other health outcomes in the offspring. For instance, low maternal folate status has been associated with increased risks on preterm birth, low birth weight, and fetal growth retardation (1, 2, 11), as well as structural malformations, such as congenital heart defects and oral clefts (2, 12). In addition, maternal folate deficiency is suggested to affect offspring neurodevelopment and brain health, possibly resulting in neurodevelopmental impairments, including autism spectrum disorders and schizophrenia (13–17).
Hence, women planning to become pregnant are advised to take daily supplements containing 400–800 μg folic acid until the end of the first trimester of pregnancy (18). In addition, fortification of foods with folic acid, which has been mandated in over 80 countries including the United States, Canada, and South Africa (but not in Europe), has been associated with a decline in the prevalence of NTDs (19). Recent folate intake data, collected in a population of mostly highly educated 18–40-y-old Dutch women (i.e., Europe) with a pregnancy wish, demonstrated a usual median (IQR) total folate intake of 713 (672) folate equivalents (FE) µg/d. Dietary sources covered 262 (102) FE µg of the daily folate intake; 56% of the women took a folate supplement. In this study, total folate intake was moderately correlated with plasma folate (r: 0.55) (20), which is well within the acceptable range for an intake-status correlation of r: 0.4–0.7 (21). Fourteen percent of the Dutch women had plasma folate concentrations <10 nmol/L (20), a commonly used cutoff value for functional folate adequacy (22). Although national surveys also show gestational folate deficiencies among women in Costa Rica, Venezuela, West Africa, and Asia, global data on the prevalence of folate deficiency during pregnancy are currently lacking (23, 24).
In contrast to the Dutch study mentioned above, considerably higher total and dietary folate intakes [median (IQR): 2181 (2114–2299) and 463 (401–530) FE µg/d, respectively] have been observed among pregnant Canadians, which probably relates to the use of folic acid–fortified foods as well as the high proportion of women using prenatal supplements containing high dosages of folic acid (97%) (25). Concerns have been raised that excessive folic acid fortification and supplementation might pose potential health risks (26), such as masking symptoms and aggravating cognitive impairments in vitamin B-12-deficient elderly (27) and insulin resistance and adiposity in children of mothers with high folate concentrations and low concentrations of vitamin B-12 during gestation (28).
Thus, it is evident that an adequate folate intake during pregnancy can impact normal growth and development of the infant (29, 30) as well as later health outcomes. However, the importance of maternal folate status for offspring neurodevelopment (31) and brain functioning is less well described. Below, we address why folate is an essential element in the various physiological processes important for neurodevelopment and brain functioning (see Figure 1).
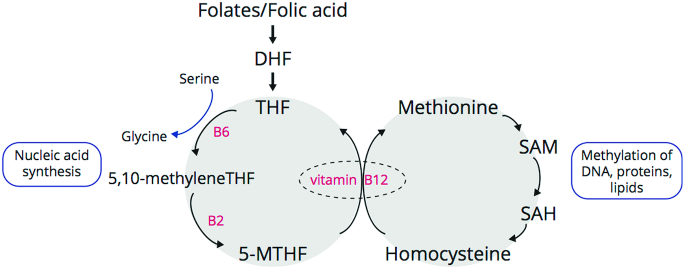
FIGURE 1.
Folate and methionine cycle (simplified). Folates from food and folic acid from fortification/supplementation are converted in the liver by the enzyme dihydrofolate reductase to form dihydrofolate (DHF) and then tetrahydrofolate (THF). THF enters the folate cycle where its vitamin B-6-dependent conversion to 5,10-methylenetetrahydrofolate (5,10-MTHF) is required for nucleic acid synthesis. The vitamin B-2-dependent enzyme methylenetetrahydrofolate reductase catalyzes reduction of 5,10-MTHF to 5-methyltetrahydrofolate (5-MTHF), in an irreversible reaction. As 5-MTHF is converted by methionine synthase into THF in a vitamin B-12-dependent reaction, vitamin B-12 deficiency can cause a functional folate deficiency. The interconnected methionine cycle is depicted on the right: 5-MTHF is required for the vitamin B-12-dependent formation of homocysteine into methionine. Methionine, once converted to S-adenosyl methionine (SAM) acts as a methyl donor in many biological methylation reactions. SAH, S-adenosylhomocysteine.
First, as a coenzyme, folate plays a role in biosynthesis of purine and pyrimidine nucleotides and is therefore crucial for synthesis of DNA and cell division. Second, as a methyl donor in the 1-carbon-metabolism, folate is required for generation of the universal methyl donor S-adenosyl methionine (SAM). SAM is not only critical for all cellular methylation reactions, including epigenetic regulation of gene expression via DNA and histone methylation, but is also implicated in phospholipid synthesis and as such required for the formation of, for example, myelin (32). Third, folate is required for synthesis of various neurotransmitters including serotonin, catecholamines, and melatonin (33). Fourth, as folate is needed as a cofactor for conversion of homocysteine into methionine, it is key for regulation of homocysteine serum concentrations (34), a sulfur-containing amino acid known to be neurotoxic at high concentrations (35). Crucial for this is the action of methylenetetrahydrofolate reductase (MTHFR), the enzyme that catalyzes reduction of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate.
Disturbances in folate metabolism can have a dietary or a genetic cause. The most common genetic disturbance of folate metabolism is a deficiency in MTHFR activity (36). Severe MTHFR deficiency, associated with developmental delay, seizures, and neurological abnormalities, is relatively rare (36). However, mild MTHFR deficiency, caused by a 677C > T (A222V) polymorphism is present in 10–15% of many Caucasian populations, and this mild MTHFR deficiency (±30% residual enzyme activity) is associated with increased risk of neurodevelopmental disorders and psychopathologies including schizophrenia (37).
Considering the importance of folate for all the physiological processes critical for normal neurodevelopment and brain functioning as mentioned above, folate deficiency as well as folate overabundance might have adverse health outcomes. The primary goal of this review is to provide a comprehensive overview of current evidence on the impact of maternal folate status during predominantly (pre-)conception and gestation on offspring neurodevelopment and cognitive function.
Current status of knowledge
Animal studies: maternal folate affects various biological processes in the developing offspring brain
First, preclinical studies on the effects of maternal folate intake during gestation on brain structure and function in rodents are discussed. See Table 1 for the effects of folate deficiency and Table 2 for the effects of folate excess on brain development. These tables also include specifics on the folic acid dose and timing. The daily recommended dose for rodents is 2 mg folic acid/kg diet. In most rodent studies, folic acid intake modification starts several weeks before mating (ranging from 1–8 wk) and continues either until birth, until weaning or until adulthood (age 3–7 mo).
TABLE 1.
Folate deficiency and offspring brain development in rodents1
| Article (year) (ref) | Species | Strain | Period of folate deficiency (animals per group) | Amounts of folic acid | Age of testing | Method | Outcome of deficient vs. normal folate diet in rodents |
|---|---|---|---|---|---|---|---|
| Craciunescu et al. (2004) (38) | Mice | C57Bl/6 J (♂) | From E11 until E17 (n = 3) | 0 vs. 2 mg folic acid/kg diet | E17 | Immunohistochemistry BrdU labeling Hematoxylin/eosin staining | Decreased neural progenitor cells in ventricular zones of septum, caudate putamen, and neocortex Increased apoptotic cells in septum Increased calretinin-positive cells in ventral forebrain |
| Craciunescu et al. (2010) (39) | Mice | C57Bl/6 J (♂) | From E11 until E17 (n = 9–11) | 0 vs. 2 mg folic acid/kg diet | E17 | Histology Immunohistochemistry | Decreased neural progenitor cells in ventricular zones of septum, hippocampus, striatum, anterior neocortex, and midposterior cortex Increased apoptotic cells in septum and hippocampus |
| Jadavji et al. (2015) (42) | Mice | C57BL/6 J (♀/♂) | 6 wk before mating until weaning (n = 6–7) | 0.3 vs. 2 mg folic acid/kg diet | 3 wk | Brain weights Brain morphology Western blots LC-MS | No difference in relative brain weight, hippocampal morphology, and cell proliferation Decreased betaine concentration in hippocampus, but no difference in choline and phosphatidylcholine concentrations Increased ChAT protein levels in hippocampus |
| Langie et al. (2013) (49) | Mice | C57Bl/6 J (♀/♂) | 4 wk before mating until weaning (n = 3–5) | 0.4 vs. 2 mg folic acid/kg diet | P22–25, 6 mo | Comet-based assay Real-time RT-PCRBisulfite sequencing LC-MS/MS | Increased BER-related incision activity at P22–25; decreased at 6 mo Increased methylation of Ogg1 at CpG11 in subcortical regions at 6 mo No effect on expression of other BER-related genes and global DNA methylation level at 6 mo |
| Xiao et al. (2005) (41) | Mice | CD1 (♀/♂) | 8 wk before mating until birth (n = 49–58) | 0.033, 0.066, 0.132, 0.177, 0.265, 0.529 mg folic acid/kg diet | E17 | Western blots Immunohistochemistry Microscopy | Decreased FR expression accompanied with a net loss of cells and architectural abnormalities |
1BER, base excision repair; BrdU, 5-bromo-2’-deoxyuridine; ChAT, choline acetyltransferase; E, embryonic day; FR, folate receptor; LC-MS, liquid chromatography-mass spectrometry; LC-MS/MS, liquid chromatography-tandem mass spectrometry; Ogg1, 8-oxoguanine glycosylase 1; P, postnatal day; ref, reference; ♀, female; ♂, male
TABLE 2.
Folate excess and offspring brain development in rodents1
| Article (year) (ref) | Species | Strain | Period of folate excess (animals per group) | Amounts of folic acid | Age of testing | Method | Outcome of excess vs. normal folate diet in rodents |
|---|---|---|---|---|---|---|---|
| Barua et al. (2014) (50) | Mice | C57BL/6 J (♀/♂) | 1 wk before mating until birth or until age 7 mo (n = 20) | 4 vs. 0.4 mg folic acid/kg diet | P1 | Microarray analysis Real time RT-PCR Western blots | Altered expression of several transcriptional factors, developmental, imprinted, and neural genes and proteins in cerebral hemispheres in a sex-specific manner |
| Barua et al. (2016) (51) | Mice | C57BL/6 J (♀/♂) | 1 wk before mating until birth (n = 12) | 20 vs. 2 mg folic acid/kg diet | P1 | Real-time PCR Western blots ELISA-based assay | Altered expression of several transcriptional factors, developmental, imprinted, and neural genes and proteins in cerebral hemispheres in a sex-specific manner No effect on overall DNA methylation level |
| Craciunescu et al. (2004) (38) | Mice | C57Bl/6 J (♂) | From E11 to E17 (n = 3) | 20 vs. 2 mg folic acid/kg diet | E17 | Immunohistochemistry BrdU labelling Hematoxylin/eosin staining | No difference in number of neural progenitor cells in ventricular zones of septum, caudate putamen, and neocortex, apoptotic cells in septum and calretinin positive cells in ventral forebrain |
| Roy et al. (2012) (52) | Rats | Wistar Albino (♀/♂) | From mating until birth at E20 (n = 8) | 8 vs. 2 mg folic acid/kg diet | E20 | Colorimetric assays Gas chromatography | Decreased DHA concentrations No difference in MDA and AA concentrations |
| Roy et al. (2014) (53) | Rats | Wistar Albino (♀/♂) | From mating until birth at E20 or until weaning (n = 8) | 8 vs. 2 mg folic acid/kg diet | E20, P21 | Colorimetric assays | Decreased GPX levels at E20; no difference at P21 No difference in SOD levels at E20 and P21 |
| Sable et al. (2011) (43) | Rats | Wistar Albino (♀/♂) | From mating until birth at E20 (n = 8) | 8 vs. 2 mg folic acid/kg diet | E20 | Brain weights ELISA Real-time PCR | No difference in relative brain weights No difference in protein and expression levels of BDNF and NGF |
| Sable et al. (2012) (44) | Rats | Wistar Albino (♀/♂) | From mating until birth at E20 or until weaning (n = 8) | 8 vs. 2 mg folic acid/kg diet | P21 | Brain weights Gas chromatography ELISA | No difference in relative brain weight Decreased AA concentrations in postnatal folate-replete pups, but not in postnatal folate-excess pups Decreased BDNF protein levels No difference in DHA concentrations and NGF protein levels |
| Sable et al. (2013) (47) | Rats | Wistar Albino (♀/♂) | From mating until birth at E20 or until age 3 mo (n = 8) | 8 vs. 2 mg folic acid/kg diet | 3 mo | Brain weights Sandwich ELISA Gas chromatography | No difference in absolute and relative weights of cortex and hippocampus Decreased BDNF protein levels in cortex and hippocampus Decreased NGF protein levels in cortex; no difference in hippocampus Decreased AA concentrations in cortex; no difference in hippocampus No difference in DHA concentrations in cortex and hippocampus |
| Sable et al. (2014) (48) | Rats | Wistar Albino (♀/♂) | From mating until birth at E20 or until age 3 mo (n = 8) | 8 vs. 2 mg folic acid/kg diet | 3 mo | Real-time PCR | No effect on mRNA levels of BDNF, NGF, TrkB, and CREB in cortex |
| Sable et al. (2015) (54) | Rats | Wistar Albino (♀/♂) | From mating until birth at E20 or until age 3 mo (n = 8) | 8 vs. 2 mg folic acid/kg diet | E20, P21, 3 mo | ELISA-based assay | No effect on global DNA methylation at E20 and P21 Increased global DNA methylation level in cortex at 3 mo; no difference in hippocampus |
1AA, arachidonic acid; BDNF, brain derived neurotrophic factor; BrdU, 5-bromo-2’-deoxyuridine; CREB, cAMP response element-binding protein; E, embryonic day; GPX, glutathione peroxidase; MDA, malondialdehyde; NGF, nerve growth factor; P, postnatal day; ref, reference; SOD, superoxide dismutase; TrkB, tyrosine receptor kinase B; ♀, female; ♂, male
Neurogenesis and apoptosis
As folate is required for DNA synthesis, all proliferating cells depend on sufficient folate concentrations. Indeed, maternal folate intake during late pregnancy has been shown to affect neurogenesis and apoptosis in fetal mice brains (38, 39). Mouse offspring of dams fed a diet with excessive amounts of folate (20 mg/kg diet) during late pregnancy displayed no differences in cell proliferation, apoptosis, and neuronal differentiation in areas of the fetal forebrain compared with offspring from dams fed a normal diet (38). On the contrary, pups of dams fed a folate-deficient diet (0 mg/kg diet) displayed a reduction in neural progenitor cells in different structures of the fetal forebrain, including the neocortex, an area responsible for complex behaviors such as cognition (40). Furthermore, the number of apoptotic cells was almost doubled in the septum and hippocampus of offspring of folate-depleted mothers, which suggests that folate deficiency affects the development of these 2 brain regions involved in neuronal pathways important for learning and memory. A net reduction in the number of cells in folate-deficient fetal mice brains (41) was accompanied with decreased folate receptor expression and changes in cellular architecture. Conversely, others found no differences in cell proliferation, relative brain weight and hippocampal morphology between mice pups exposed to low (0.3 mg/kg diet) and normal (2 mg/kg diet) amounts of folate during gestation and lactation. However, a trend towards increased apoptosis in the hippocampus was observed (42). Furthermore, folate-deficient (0 mg/kg diet) mice had a higher number of differentiating (calretinin-positive) neurons in the forebrain, suggesting increased neuronal differentiation in response to maternal folate deficiency during late pregnancy (38). In rats, relative brain weights were similar in folate-excess (8 mg/kg diet) and folate-replete (2 mg/kg diet) offspring at birth (43) and at weaning (44).
Neurotrophic factors
A series of studies have specifically looked at the effect of an imbalance in maternal folate status on the protein and mRNA expression levels of neurotrophic factors in the offspring's brain. Neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) are important regulators of neuronal survival, development, and plasticity. BDNF is an important modulator of synaptic plasticity and plays a role in memory and spatial learning (45), whereas NGF is important for cognitive function through its role in cholinergic and glutamatergic transmission (46).
In comparison to offspring of rats fed a normal diet during pregnancy, no differences in protein and expression levels of BDNF and NGF were found in offspring of folate-supplemented (8 mg/kg diet) rats at birth (43). After birth, offspring continued with the same diet or were shifted to a control diet with normal amounts of folate (2 mg/kg diet). Although no differences in NGF levels were found at weaning, BDNF levels were decreased in 3-wk-old rats in both postnatal diet groups. It is suggested that such a decline in BDNF could impair mechanisms important for memory and spatial learning processes (44). Alterations in neurotrophin levels were also observed when pups were followed until age 3 mo (47). In the hippocampus, BDNF levels were decreased in rats, independently of the postnatal diet. In addition, a decline in both BDNF and NGF was observed in the cortex. Reduced NGF levels have been shown to affect pathways crucial in cognitive processes, such as long-term potentiation (46). However, investigation of the gene expression of these molecules in the cortex revealed no differences (48). Furthermore, the mRNA levels of transmembrane receptor tyrosine receptor kinase B (TrkB) and cAMP response element-binding protein (CREB), which are involved in a BDNF-induced signaling cascade critical for learning and memory, were similar in folate-excess and folate-replete rats.
Considering the potential interactions with brain neurotrophins, the effect of maternal folate excess on brain oxidative stress levels have been addressed in this series of studies. In neonatal brains, the concentrations of the oxidative stress biomarker malondialdehyde were comparable between offspring of mothers fed a normal folate diet (2 mg/kg diet) and those of mothers fed a high-folate diet (8 mg/kg diet) (52). No differences were observed in brain concentrations of antioxidant enzyme superoxide dismutase (SOD) (53). In contrast, the concentrations of glutathione peroxidase (GPX), another antioxidant enzyme, were reduced in response to maternal folate supplementation. A decline in antioxidant enzyme concentrations such as GPX may lead to oxidative damage, which is associated with cognitive impairments (55). However, examination of the pups at postnatal day 21 revealed no differences in GPX concentrations.
DNA repair
Maternal folate deficiency has also been shown to affect DNA repair. Offspring of mice fed a folate-deficient diet (0.4 mg/kg diet) throughout pregnancy and lactation showed increased base excision repair (BER) activity in the brain at weaning (49). However, this effect was reversed in adulthood; a decreased BER activity in 6-mo-old adult offspring of folate-deficient dams was observed. This decline in BER activity is suggested to cause neurodegeneration by increasing sensitivity to oxidative damage, as indicated by increased concentrations of the oxidative stress biomarker 8-oxo-7,8-dihydro-2'deoxyguanosine (8-oxodG) and increased methylation of the BER gene 8-oxoguanine glycosylase 1 (Ogg1) in subcortical brain regions in these 6-mo-old mice. No effects were observed on expression of other BER genes and global DNA methylation.
Gene expression and DNA methylation
Prenatal folate also modulates the expression of several genes and proteins involved in neuronal function in offspring. In mice, in utero supplementation with 4 mg/kg diet resulted in altered expression of multiple genes, in a range between 62-fold upregulation to 18-fold downregulation (50). In the cerebral hemispheres of pups, changes in the expression of important developmental and neural genes, including those involved in neurogenesis, neurotransmission, synaptic plasticity, and γ-aminobutyric acid-glutamate and dopamine-serotonin pathways, were induced by a maternal diet high in folate. Some of the dysregulations were common in both males and females, but a large number of genes were differentially expressed, indicating sex-specific alterations in expression pattern. A recent study with even higher amounts of folate (20 mg/kg diet) found similar effects (51). For instance, the expression of nuclear factor 1 X-type, runt-related transcription factor 1, and vestigial like family member 2 were modulated by maternal folate excess. These transcriptional factors important for brain development were downregulated in male pups, whereas female pups showed increased expression of these factors, again suggesting an interaction between maternal folate dose and sex. However, no significant effect was found on overall global methylation level in brains of both sexes. Likewise, no differences in global DNA methylation levels were found in rat brains at birth and at age 3 wk (54). In contrast, a region-specific analysis at 3 mo revealed an increased global methylation level in the cortex of offspring of dams fed a high-folate diet (8 mg/kg diet), even when offspring were fed a diet with normal amounts of folate (2 mg/kg diet) after birth. This effect was not observed in offspring hippocampus, which indicates an effect of folate in a region-specific manner. The authors suggest that the observed hypermethylation in the cortex could affect the expression of genes important for cognitive functioning, more specifically decision making and cognitive behavior in which the cortex is considered to be involved.
Brain FA content and myelin formation
Importantly, 1-carbon metabolism and fatty acid (FA) metabolism interact (56). Methyl groups generated in the 1-carbon cycle are required for synthesis and transport of FAs. Furthermore, when methylation capacity is compromised, for example, during folate imbalance, this impairs generation of phosphatidylcholine, which is crucial for delivery of polyunsaturated fatty acids (PUFAs) from the liver to other peripheral tissues (57). Folate deficiency has been linked to altered FA composition of plasma and platelets (58) and several preclinical studies have investigated the offspring's brain FA content after modification of maternal folate during gestation.
Offspring exposed to prenatal folate excess (8 mg/kg diet) showed lower brain DHA concentrations at birth, whereas no differences were found in brain arachidonic acid (AA) concentrations (52). DHA, an ω-3 PUFA that is highly enriched in the brain, is vital for proper brain development and function. Therefore, low DHA concentrations may contribute to impaired cognitive performance, which has also been suggested by other studies (59). Continuation of a high-folate diet (8 mg/kg diet) during lactation resulted in no significant differences in offspring DHA and AA brain concentrations compared to the control group (2 mg/kg diet) (44). Interestingly, pups that shifted to a control diet after birth showed decreased concentrations of AA. At age 3 mo, both postnatal diets groups showed a reduction in AA concentrations in the hippocampus, whereas no differences were found in AA concentrations in the cortex (47). AA is crucial for normal development of the brain, including the hippocampus, and in this way low AA may affect hippocampal functions, such as learning and memory (60). No effects were seen on the concentrations of DHA in the brains of both rats fed a normal diet (2 mg/kg diet) and rats fed a high-folate (8 mg/kg diet) diet after birth.
In the brain, the biochemical pathways connecting folate and choline have been associated with the maintenance of membrane and myelin integrity (61). Methylated phospholipids, including, for example, phosphatidylcholine and sphingomyelin, are required for membrane formation and form essential components of myelin; the brain's white matter that facilitates signal conduction in axons. Indeed, a brain-specific folate deficiency caused by a heritable folate transport defect, is described to cause a lack of membrane phospholipids and ultimately disrupted myelin formation, associated with progressive movement disturbance, psychomotor decline, and epilepsy (61). Recently, it has been shown that folate supplementation during pregnancy and lactation accelerates the maturation of oligodendrocytes; the cells responsible for myelin production. In addition, pharmacological inhibition of folate metabolism has opposite effects, including oligodendrocyte death and differentiation effects, which could be rescued by folate supplementation (62). Together, these findings indicate that folate is important for myelin development and maintenance.
Interaction with other nutrient concentrations
As described above, folate is metabolically related to other essential micronutrients, including B vitamins and choline. Maternal folate depletion (0.3 mg/kg diet) was shown to contribute to changes in choline metabolism in mice (42). Pups aged 3 wk, from folate-deficient dams showed lower betaine concentrations and higher choline acetyltransferase levels in the hippocampus. This might be of relevance because the choline and folate metabolism are strongly interrelated (63) and both nutrients are known to be important for normal functioning of the hippocampus, a brain area involved in learning and memory. Importantly, within the 1-carbon metabolism, folate is required for remethylation of homocysteine into the essential amino acid methionine, as such a lack of folate can result in increased homocysteine concentrations (34). Homocysteine is known to be neurotoxic in high concentrations (35), and hyperhomocysteinemia has been associated with cognitive decline (64).
Folate and offspring behavior in animals
As evident from the literature reviewed above, maternal folate status is important for various biological processes in the developing (fetal) brain, but what are the functional consequences of this? Below, we will discuss the effects of maternal folate intake and status on cognition in the offspring. Note, we specifically focus on dietary maternal folate, hence studies in which folate status was altered indirectly (e.g., as a result of MTHFR genotype or folate receptor antibodies) are not considered in this section.
Studies on folate deficiency and excess and offspring behavior in rodents are displayed in Table 3. Prenatal folate deficiency that is restored at birth, was shown to have adverse behavioral outcomes in mice offspring at age 9–12 wk (65). Female mice received a folate-deficient (0.177 mg/kg diet or 0.265 mg/kg diet) or folate-replete (0.529 mg/kg diet) diet from 8 wk before mating until birth; at birth they were all switched to a folate-replete diet. Although several developmental tests during the pre-weaning period revealed no differences between the offspring of folate-deficient and folate-replete dams, behavioral testing at age 9–12 wk showed that the mice with the lowest prenatal amount of folate exhibited more anxiety-related behavior in the elevated plus maze. Prenatal folate-deficient mice spent significantly less time in the open arms and more time in the closed arms compared to the group with a normal diet. Moreover, total activity in the elevated plus maze test was higher in the low-folate group, which implicates that maternal folate deficiency during pregnancy may cause alterations in anxiety-related behavior in adult offspring. Furthermore, low maternal folate status has been shown to affect short-term memory in 3-wk-old mice (42). Specifically, pups of dams fed a folate-deficient diet showed impaired visual short-term memory in an object recognition task. In contrast, no effect of folate deficiency during pregnancy and lactation on spatial short-term memory was observed.
TABLE 3.
Folate deficiency, folate excess, and offspring behavior in rodents1
| Article (year) (ref) | Species | Strain | Period of folate manipulation (animals per group) | Amounts of folic acid | Age of testing | Method | Outcome of deficient or excess vs. normal folate diet in rodents |
|---|---|---|---|---|---|---|---|
| Ferguson et al. (2005) (65) | Mice | CD1 (♀/♂) | 8 wk before mating until birth (n = 10) | 0.177, 0.265, 0.529 mg folic acid/kg diet | P4–83 | Righting reflex, negative geotaxis, forelimb hanging, motor coordination, open field, elevated plus maze | Increased anxiety-related behavior No differences found in other outcomes |
| Jadavji et al. (2015) (42) | Mice | C57BL/6 J (♀/♂) | 6 wk before mating until weaning (n = 6–7) | 0.3 vs. 2 mg folic acid/kg diet | 3 wk | Novel object recognition task, Y-maze | Decreased visual short-term memory No differences found in spatial short-term memory |
| Barua et al. (2014) (50) | Mice | C57BL/6 J (♀/♂) | 1 wk before mating until birth or until age 7 mo (n = 20) | 4 vs. 0.4 mg folic acid/kg diet | P2–6, 2–6 mo | USV, social approach, elevated plus maze, marble-burying, self-grooming, buried food, trace fear conditioning, open field | Increased anxiety-related behavior Increased activity in males No difference in social behavior or learning and memory |
| Sable et al. (2013) (47) | Rats | Wistar Albino (♀/♂) | From mating until birth at E20 or until age 3 mo (n = 8) | 8 vs. 2 mg folic acid/kg diet | 3 mo | Morris water maze | No difference in time taken to reach the platform |
| Bahous et al. (2017) (66) | Mice | C57BL/6 J (♀/♂) | 5 wk before mating until E17.5 or age 3 wk | 20 vs. 2 mg folic acid/kg diet | E17.5, 3 wk | Ladder beam test, open field, novel object recognition, Y-maze | Impaired short-term memory in novel object recognition test, no differences found in other behavioral outcomes |
1E, embryonic day; P, postnatal day; ref, reference; USV, ultrasonic vocalization; ♀, female; ♂, male
Not only maternal folate deficiency, but also maternal folate excess has been suggested to alter cognitive function in offspring. In 1 study, dams were fed a normal (0.4 mg/kg diet) or a high-folate (4 mg/kg diet) diet during pregnancy, and pups continued with the same diet after birth (50). In the first week of life, offspring were tested for ultrasonic vocalizations in response to separation from their mother. Pups of dams exposed to the high-folate diet made more and longer calls than pups of dams exposed to a normal folate diet, indicating more anxious behavior because of maternal folate excess. In addition, several other behavioral tests were conducted in offspring between 2 and 6 mo after birth. In the open field test, male mice receiving a pre- and postnatal high-folate diet were more active compared to males receiving a normal diet. There was no difference in activity between the 2 dietary groups in female offspring, suggesting a sex-specific effect. In the other assessments, similar behavior was observed in mice exposed to normal and excessive amounts of folate. Likewise, no difference in cognitive performance as tested by the Morris water maze test was found in rats (47). A recent study indicates that folate supplementation (20 mg/kg diet) throughout pregnancy impaired short-term memory of objects in 3-wk-old male offspring, compared to offspring of dams that received a standard diet (2 mg/kg diet). However, no differences were found in the open field test and Y-maze test, suggesting that there are no effects of maternal folate excess on anxiety-like behavior and spatial memory (66).
Thus, although the importance of maternal folate for several key biological processes required for normal neurodevelopment and brain functioning in the offspring is well described, there is currently only limited preclinical evidence that maternal folate status has a (lasting) effects on cognitive function.
Maternal folate, brain development, and childhood cognitive outcomes in humans
Below, we describe the details of human observational studies on maternal RBC and plasma folate status (Table 4), maternal dietary and supplemental folate intake (Table 5) and childhood cognition—as well as the experimental studies on this matter (Table 6)—at different developmental stages. Two articles described associations of plasma folate (n = 1) or supplemental folate intake (n = 1) with cognition-related outcomes in the embryo or neonate. A total of 5 studies examined the association of maternal plasma folate (n = 1) or folate intake (n = 5) and cognitive performance in infants and/or toddlers. Maternal folate intake (n = 4) and plasma folate (n = 1) in association with cognitive outcomes in preschoolers were examined in 4 studies. In the group of school-age children, associations between RBC and/or plasma folate and cognition were examined in 2 studies; folate intake and the association with cognition was investigated in 2 studies. Moreover, this was the only age group for which we identified a randomized controlled trial (RCT). Two studies were conducted in preadolescent populations, where 1 studied plasma folate concentrations and 1 folic acid intakes. The characteristics of these studies, including, for example, the considered covariates, are depicted in Supplemental Table 1 and Supplemental Table 2.
TABLE 4.
Human observational studies on maternal folate status and offspring cognitive outcomes1
| Article (year) (ref) | Type of study | Location | Participants | Maternal folate determination | Folate status | Method | Main findings |
|---|---|---|---|---|---|---|---|
| Schlotz et al. (2010) (69) | Prospective cohort | United Kingdom | 139 mother-child pairs | 14 weeks of gestation | Median maternal RBC folate in early pregnancy: 10552nmol/L | SDQ (age 8 y) | Lower maternal RBC folate in early pregnancy was associated with an increased risk of childhood hyperactivity (adjusted β: −0.24; CI not provided; P = 0.013) and peer problems (adjusted β: −0.28; CI not provided; P = 0.04) (with suggestive mediation by fetal head growth), but not with emotional and conduct problems |
| Steenweg-de Graaff et al. (2012) (70) | Prospective cohort | Netherlands | 3209 mother-child pairs | <18 weeks of gestation | Maternal plasma folate <7 nmol/L: 4.1% | CBCL 1½-5 (age 3 y) | Maternal folate deficiency (<7 nmol/L) was associated with a higher risk of child emotional problems (adjusted OR: 1.57; 95% CI: 1.03, 2.38; P = 0.03) |
| Tamura et al. (2005) (71) | Prospective; mothers participated in a zinc intervention study | United States | 355 mother-child pairs | 19, 26, and 37 weeks of gestation | Mean maternal plasma folate: 19 wk, 35 nmol/L; 26 wk, 34.7 nmol/L; 37 wk, 32.5 nmol/L Mean maternal RBC folate: 19 wk, 873 nmol/L; 26 wk, 1070 nmol/L; 37 wk, 1096 nmol/L | DAS, Visual Sequential Memory, Auditory Sequential Memory, Knox Cube, Gross Motor Scale, Grooved Pegboard (age 5 y) | No differences in neurodevelopmental outcomes between low- (plasma folate <11.0 nmol/L or RBC folate <440 nmol/L) and normal-folate groups No association of maternal folate concentration quartiles with neurodevelopmental outcomes |
| van Mil et al. (2014) (67) | Prospective cohort | Netherlands | 463 mother-child pairs | <18 weeks of gestation | Mean maternal plasma folate in early pregnancy: 19.1 nmol/L | Bilsulfite sequencing (newborn blood collected at delivery) | No association of maternal plasma folate with newborn overall DNA methylation levels of fetal growth and neuronal developmental genes |
| Veena et al. (2010) (72) | Prospective cohort | India | 536 mother-child pairs | 30 weeks of gestation | Mean maternal plasma folate: 34.7 nmol/L | K-ABC-II, Kohs’ Block Design, WISC-III (age 9.5 y) | Higher maternal folate status was associated with improved learning and long-term memory (adjusted β: 0.10; 95% CI: 0.01, 0.19; P < 0.05), visuo-spatial ability (adjusted β: 0.10; 95% CI: 0.01, 0.19; P < 0.05), and attention and concentration (adjusted β: 0.10; 95% CI: 0.02, 0.18; P < 0.05), but not with short-term memory, reasoning ability, and language production |
| Wu et al. (2012) (73) | Prospective; mothers participated in a DHA intervention study | Canada | 154 mother-child pairs | 16 and 36 weeks of gestation | Mean maternal plasma folate 16 wk: 36.4 nmol/L | BSID-III (age 18 mo) | No association of maternal folate status with cognitive outcomes |
1BSID, Bayley Scales of Infant Development; CBCL, Child Behavior Checklist; DAS, Differential Ability Scale; K-ABC, Kaufman Assessment Battery for Children; ref, reference; SDQ, Strengths and Difficulties Questionnaire; WISC, Wechsler Intelligence Scale for Children
Values are calculated on the basis of reported values. Folate conversion factor: 1 μg/L = 2.265 nmol/L (74).
TABLE 5.
Human observational studies on maternal folate intake and offspring cognitive outcomes1
| Article (year) (ref) | Type of study | Location | Participants | Maternal folate determination | Folate intake | Method | Main findings |
|---|---|---|---|---|---|---|---|
| Boeke et al. (2013) (75) | Prospective cohort | United States | 895 mother-child pairs | First and second trimesters | Mean maternal total folate intake: first trimester, 972 μg/d; second trimester, 1268 μg/d | WRAML-2 Design and Picture Memory subtests, KBIT-2 (age 7 y) | No association of maternal folate intake with cognitive outcomes |
| Chatzi et al. (2012) (76) | Prospective cohort | Greece | 553 mother-child pairs | 14–18 weeks of gestation | Maternal folic acid use: 5000 μg/d, 68%; > 5000 μg/d, 24%; 0 μg/d, 8% | BSID-III (age 18 mo) | Maternal supplemental folic acid intake of 5000 μg/d was associated with improved repetitive (adjusted β: 4.7; 95% CI: 0.5, 8.5; P < 0.05) and expressive communication skills (adjusted β: 3.5; 95% CI: 0.6, 7.9; P < 0.05) (with no additional improvement with intakes >5 mg/d), but not with other cognitive outcomes |
| del Rio Garcia et al. (2009) (77) | Prospective cohort | Mexico | 253 mother-child pairs | First trimester | Maternal folate intake <400 μg/d: 67.2% | BSID-II (age 1, 3, 6, and 12 mo) | Maternal folate intake of <400 μg/d was associated with a reduction in mental development in children of mothers who were carriers of the MTHFR677 TT genotype (adjusted β: −1.8; 95% CI: −3.6, −0.04; P < 0.05) |
| Forns et al. (2012) (78) | Prospective cohort | Spain | 393 mother-child pairs | Not reported | Maternal folic acid use with or without other vitamins: 66.84% | CPT-II (age 11 y) | Maternal folic acid supplementation was associated with a reduced omission error rate (adjusted IRR: 0.80; 95% CI: 0.64, 1.00; P = 0.046), but not with commission error rate and hit reaction time |
| Julvez et al. (2009) (79) | Prospective cohort | Spain | 420 mother-child pairs | End of first trimester | Maternal folic acid use without other vitamins: 34%; with other vitamins: 24% | MCSA, CPSCS, DSM-IV criteria ADHD (age 4 y) | Maternal folic acid supplementation was associated with improved verbal (adjusted β: 3.98; 95% CI: 0.66, 7.31; P < 0.05), motor (adjusted β: 4.55; 95% CI: 1.28, 7.81; P < 0.01), and verbal-executive skills (adjusted β: 3.97; 95% CI: 0.67, 7.24; P < 0.05), improved social competence (adjusted β: 3.97; 95% CI: 0.81, 7.14; P < 0.05), and fewer inattention symptoms (adjusted OR: 0.46; 95% CI: 0.22, 0.95; P < 0.05), but not with perceptive performance and memory |
| Koning et al. (2015) (68) | Prospective cohort | Netherlands | 135 pregnant women | <8 weeks of gestation | Maternal folic acid use: pre-conceptionally, 80%; post-conceptionally, 20% | Transvaginal 3D-ultrasounds (8–12 weeks of gestation) | Pre-conceptional initiation of folic acid supplementation was associated with increased embryonic cerebellar size (TCD, adjusted β: 0.257; SE: 0.117; P = 0.032; LCD, adjusted β: 0.171; SE: 0.067; P = 0.013; RCD, adjusted β: 0.156; SE: 0.062; P = 0.015) and proportional cerebellar growth (TCD/CRL, adjusted β: 0.015; SE: 0.005; P = 0.003; LCD/CRL, adjusted β: 0.012; SE: 0.003; P = 0.001; RCD/CRL, adjusted β: 0.011; SE: 0.003; P = 0.001) in ongoing pregnancies compared to post-conceptional initiation |
| Polańska et al. (2015) (80) | Prospective cohort | Poland | 538 mother-child pairs | 8–12, 20–24, and 30–34 weeks of gestation, and at birth | Maternal folic acid use before and/or within first trimester: 60.9% | BSID-III (age 12 and 24 mo) | No association of maternal folic acid supplementation with child neurodevelopment |
| Roth et al. (2011) (81) | Prospective cohort | Norway | 38954 mother-child pairs | 17 weeks of gestation | Maternal folic acid use within 4 wk before to 8 wk after conception without other supplements: 18.9%; with other supplements: 50.2% | Language Grammar Rating Scale, ASQ on gross motor skills (age 3 y) | Maternal folic acid supplementation was associated with a reduced risk of severe (adjusted OR: 0.55; 95% CI: 0.35, 0.86; P < 0.05) and moderate language delay (adjusted OR: 0.82; 95% CI: 0.69, 0.97; P < 0.05), but not gross motor skills |
| Roza et al. (2010) (82) | Prospective cohort | Netherlands | 4214 mother-child pairs | <18 weeks of gestation | Maternal folic acid use: start pre-conceptionally, 44.8%; start within first 10 weeks of gestation, 28.0% | CBCL 1½-5 (age 18 mo) | Maternal folic acid supplementation during embryogenesis was associated with a lower risk of internalizing (adjusted OR of inadequate folic acid use: 1.65; 95% CI: 1.24, 2.19; P = 0.001) and externalizing problems (adjusted OR of inadequate folic acid use: 1.45; 95% CI: 1.17, 1.80; P = 0.001) |
| Schlotz et al. (2010) (69) | Prospective cohort | United Kingdom | 139 mother-child pairs | 14 and 28 weeks of gestation | Median maternal total folate intake: early pregnancy, 465.5 μg/d; late pregnancy, 323.1 μg/d | SDQ (age 8 y) | Lower maternal folate intake in early pregnancy was associated with an increased risk of childhood hyperactivity (adjusted β: −0.24; CI not provided; P = 0.022) and peer problems (adjusted β: −0.28; CI not provided; P = 0.009) (with suggestive mediation by fetal head growth), but not with emotional and conduct problems |
| Steenweg-de Graaff et al. (2012) (70) | Prospective cohort | Netherlands | 3209 mother-child pairs | <18 weeks of gestation | Maternal folic acid use: start pre-conceptionally, 47.5%; start within first 10 weeks of gestation, 28.7% | CBCL 1½-5 (age 3 y) | Maternal folic acid supplementation during embryogenesis was associated with a lower risk of child emotional problems (adjusted OR of inadequate folic acid use: 1.45; 95% CI: 1.14; P < 0.05) |
| Valera-Gran et al. (2014) (83) | Prospective cohort | Spain | 2213 mother-child pairs | 10–13 and 28–32 weeks of gestation | Maternal folic acid use: <400 μg/d, 57.3%; 400–1000 μg/d, 17.5%; 1000–5000 μg/d, 21.7%; > 5000 μg/d, 3.5% | BSID-I (age 1 y) | Maternal folic acid supplementation with <400 μg/d or 1000–5000 μg/d was associated with improved mental development compared to 400–1000 μg/d (adjusted β: 2.30; 95% CI: 0.38, 4.22; P < 0.05 and adjusted β: 1.53; 95% CI: −0.68, 3.75; P < 0.05, respectively) Maternal folic acid supplementation with >5000 μg/d was associated with reduced psychomotor development compared to 400–1000 μg/d (adjusted β: −4.35; 95% CI: −8.34, 0.36; P < 0.05) |
| van Mil et al. (2014) (67) | Prospective cohort | Netherlands | 463 mother-child pairs | <18 weeks of gestation | Maternal folic acid use: start pre-conceptionally, 55.9%; start within first 10 weeks of gestation, 31.4% | Bilsulfite sequencing (newborn blood collected at delivery) | No association of maternal folic acid supplementation with newborn overall DNA methylation levels of fetal growth and neuronal developmental genes |
| Villamor et al. (2012) (84) | Prospective cohort | United States | 1210 mother-child pairs | First and second trimester | Mean maternal total folate intake: first trimester, 949 μg/d; second trimester, 1272 μg/d | PPVT-III, WRAVMA (age 3 y) | Maternal folate intake during first trimester was associated with improved receptive language (adjusted β: 1.6; 95% CI: 0.1, 3.1; P = 0.04), but not with visual and motor abilities No association of maternal folate intake during second trimester with cognitive outcomes |
| Wehby et al. (2008) (85) | Prospective cohort | United States | 6774 mother-child pairs | Not reported | Maternal folic acid use for ≥3 d/wk during 3 mo before pregnancy and/or during the following 3 mo: 3.2% | DDST items (age 3 y) | Maternal folic acid supplementation was associated with a lower risk of gross-motor delay (adjusted OR: 0.51; 95% CI: 0.28, 0.93; P < 0.05), but a trend toward an increased risk on the personal-social domain (adjusted OR: 1.78; 95% CI: 0.94, 3.38; P < 0.1); no difference in language and fine motor development |
1ADHD, attention-deficit hyperactivity disorder; ASQ, Ages and Stages Questionnaire; BSID, Bayley Scales of Infant Development; CBCL, Child Behavior Checklist; CPSCS, California Preschool Social Competence Scale; CPT, Continuous Performance Test; CRL, crown-to-rump length; DDST, Denver Developmental Screening Test; DSM, Diagnostic and Statistical Manual of Mental Disorders; IRR, incidence rate ratio; KBIT, Kaufman Brief Intelligence Test; LCD, left cerebellar diameter; MCSA, McCarthy Scales of Children's Abilities; MTHFR, methylene tetrahydrofolate reductase; PPVT, Peabody Picture Vocabulary Test; RCD, right cerebellar diameter; ref, reference; SDQ, Strengths and Difficulties Questionnaire; TCD, transcerebellar diameter; WRAML, Wide Range Assessment of Memory and Learning; WRAVMA, Wide Range Assessment of Visual Motor Abilities; 3D, 3-dimensional
TABLE 6.
Human interventional studies on maternal folate status/intake and offspring cognitive outcomes1
| Article (year) (ref) | Type of study | Location | Intervention | Participants | Folate status | Method | Main findings |
|---|---|---|---|---|---|---|---|
| Campoy et al. (2011) (86) | Double-blind RCT | Germany, Hungary, and Spain | Daily intake of 400 μg 5-MTHF or a placebo from 20 weeks of gestation until delivery | 35 mother-child pairs in 5-MTHF group (I); 45 in placebo group (II) | Mean maternal plasma folate (nmol/L) 20 weeks: (I) 26.592, (II) 29.78 30 weeks: (I) 26.482, (II) 17.01 At delivery: (I) 28.952, (II) 15.24 Mean cord plasma folate at delivery (nmol/L): (I) 34.412, (II) 31.852 | K-ABC (age 6.5 y) | No differences in cognitive outcomes between 5-MTHF and placebo group No association of folate status during pregnancy and at delivery with cognitive outcomes |
| Catena et al. (2016) (87) | Double-blind RCT | Germany, Hungary, and Spain | Daily intake of 400 μg 5-MTHF or a placebo from 20 weeks of gestation until delivery | 27 mother-child pairs in 5-MTHF group (I); 32 in placebo group (II) | Mean maternal plasma folate (nmol/L) 20 wk: (I) 11.69, (II) 11.16 30 wk: (I) 10.88, (II) 6.94 At delivery: (I) 12.37, (II) 6.33 Mean maternal whole blood folate (nmol/L) 20 wk: (I) 269.77, (II) 258.86 30 wk: (I) 228.52, (II) 190.92 At delivery: (I) 238.24, (II) 170.01 Mean cord plasma folate at delivery (nmol/L): (I) 14.46, (II) 13.10 | ANT, EEG, sLORETA (age 8.5 y) | 5-MTHF group showed improved ability to solve conflicts (median conflict reaction time: 60 vs. 101 ms; P = 0.01), but a trend toward a reduced readiness to respond compared to placebo group (median global speed: 872 vs. 818 ms; P = 0.07); no difference in orienting scores Maternal whole blood folate at 30 weeks of gestation was negatively associated with overall response speed (adjusted r: −0.18; P < 0.05) Cord plasma folate at delivery was positively associated with ERPs in the conflict condition (adjusted r: 0.21; P < 0.05) No other associations of folate status during pregnancy and at delivery with cognitive outcomes |
1ANT, Attention Network Test; EEG, electroencephalography; K-ABC, Kaufman Assessment Battery for Children; LC-MS/MS, liquid chromatography-tandem mass spectrometry; RCT, randomized controlled trial; sLORETA, standardized low-resolution brain electromagnetic tomography; 5-MTHF, 5-methyltetrahydrofolate.
Values are calculated on the basis of reported values. Folate conversion factor: 1 μg/L = 2.265 nmol/L (74).
Embryo and neonate
No significant associations were observed between maternal plasma folate in early pregnancy and newborn methylation levels of fetal growth and neurodevelopmental genes in the Generation R Study (n = 463) (67). In the Rotterdam Predict Study, analysis of high resolution transvaginal 3D ultrasounds (n = 135) revealed a positive association between initiation of folic acid supplementation within the periconceptional period and embryonic cerebellar size. In embryos at 8–12 weeks of pregnancy, the trans cerebellar (adjusted β: 0.257; SE: 0.117; P = 0.032), left cerebellar (adjusted β: 0.171; SE: 0.067; P = 0.013), and right cerebellar (adjusted β: 0.156; SE: 0.062; P = 0.015) diameters as function of the crown-rump length (CRL) were higher when women started folic acid supplementation pre-conceptionally. In addition, pre-conceptional initiation of folic acid use was associated with increased embryonic proportional cerebellar growth, as calculated by dividing the right cerebellar diameter (RCD) and left cerebellar diameter (LCD) by CRL [trans cerebellar diameter (TCD)/CRL, adjusted β: 0.015; SE: 0.005; P = 0.003; LCD/CRL, adjusted β: 0.012; SE: 0.003; P = 0.001; RCD/CRL, adjusted β: 0.011; SE: 0.003; P = 0.001] (68).
Infants and toddlers (0-2 y)
Maternal plasma folate concentrations at 16 and 36 weeks of gestation were not associated with Bayley Scales of Infant Development (BSID) mental and psychomotor scores among 154 Canadian 18-mo-old toddlers (73). In agreement, maternal folic acid supplement use that started in the periconceptional period was also not associated with BSID scores in 1- and 2-y-old toddlers included in the Polish Mother and Child Cohort study (n = 538) (80). Conversely, inadequate maternal dietary folate intake (i.e., <400 mg/d) during the first trimester was associated with lower BSID mental scores during the first year of life in 253 Mexican infants of mothers carrying the MTHFR-677 TT genotype (adjusted β: −1.8; 95% CI: −3.6, −0.04); no significant associations were observed in children of mothers with the other MTHFR genotypes (77). In the ‘Rhea’ cohort, including 553 18-mo-old Greek children, maternal supplemental folic acid intake of 5000 μg/d in early pregnancy was associated with higher receptive (adjusted β: 4.7; 95% CI: 0.5, 8.5) and expressive (adjusted β: 3.5; 95% CI: 0.6, 7.9) communication subtests scores of the BSID. Folic acid intakes exceeding 5000 μg/d were not associated with any of the BSID domains (76). Interestingly, the multicenter prospective mother-child cohort Infancia y Medio Ambiente showed that 1-y-old Spanish children (n = 2213) of mothers using <400 or 1000–5000 μg folic acid/d, assessed at 10–13 and 28–32 weeks of gestation, had higher BSID mental development scores than children of mothers taking the recommended folic acid dosage of 400 μg/d (adjusted difference: 2.30; 95% CI: 0.38, 4.22; and 1.53; 95% CI: −0.68, 3.75). Children of mothers exceeding supplemental intakes of >5000 μg folic acid/d had lower BSID psychomotor scores (adjusted difference: −4.35; 95% CI: −8.34, 0.36) (83). These “contrasting” findings may suggest an inverted U-shaped link between maternal folate and childhood cognitive outcomes. In the Generation R Study, 18-mo-old toddlers (n = 4214) of mothers who did not use folic acid supplements during the periconceptional period had a higher risk of both internalizing and externalizing problems as assessed with the Child Behavior Checklist, adjusted OR: 1.65; 95% CI: 1.24, 2.19; and 1.45; 95% CI: 1.17, 1.80. Fetal head size did not mediate these associations (82).
Preschoolers (2–4 y)
The Generation R Study also showed inverse associations of inadequate folic acid supplement use and folate deficiency (plasma folate <7 nmol/L) in early pregnancy with higher risk of emotional problems at age 3 y (n = 3209), adjusted ORs: 1.45; 95% CI: 1.14, 1.84 and 1.57; 95% CI: 1.03, 2.38, but not behavioral problems. Stratification and interaction analyses did not point towards a role for maternal MTHFR genotype in the association between plasma folate and offspring emotional problems (70). In the Norwegian Mother and Child Cohort Study (n = 38,954), maternal folic acid supplement use initiated before the 8th week of gestation was associated with a reduced risk of severe (only 1 word or unintelligible utterances) (adjusted OR: 0.55; 95% CI: 0.35, 0.86) language delay in 3-y-old children as reported by the parents using the language grammar scale of the Ages and Stages Questionnaire. This association was not observed in children of mothers who started with folic acid supplements after 8 weeks of gestation (81). In agreement, Project Viva (n = 1210) showed that each 600-μg/d increase in first, but not second, trimester folate intake from food and supplements was associated with a 1.6-point (95% CI: 0.1–3.1) increase in a child's receptive language score in 3-y-old American children; this association was even stronger for 600 μg/d of folic acid from supplements only, that is 1.7 points (95 CI: 0.2, 3.3) (84). In contrast, no associations were observed between periconceptional folic acid supplement use and language development or fine motor development, as assessed with the Denver screening test, in 3-y-old American children included in the National Maternal Infant Health Survey (n = 6774) (85). However, whereas maternal folic acid use in this cohort was associated with a lower risk of gross-motor delay, adjusted OR: 0.51; 95% CI: 0.28, 0.93 (85), no association between early maternal folic acid use and child gross motor development was observed in the Norwegian Mother and Child Cohort Study (81). In addition, no associations were shown between folate intake in the first and/or second trimester and fine motor and visual abilities among 3-y-old American mother-child pairs in the project Viva cohort (n = 1210) (84).
School-age children (4–8 y)
Positive associations between maternal folic acid supplement use, assessed at the end of the first trimester, and social competences (adjusted β: 3.97; 95% CI: 0.81, 7.14), inattention symptoms (adjusted OR: 0.46; 95% CI: 0.22, 0.95), verbal skills (β: 3.98; 95% CI: 0.66, 7.31), motor skills (4.55; 95% CI: 1.28, 7.81), and verbal-executive skills (adjusted β: 3.97; 95% CI: 0.67, 7.24) were observed in 420 4-y-old Spanish children included in the Menorca cohort. No differences in perceptive performance and memory were observed (79). Moreover, an American study (n = 355) among socio-economically disadvantaged mothers did not show any differences in mental and psychomotor development between 5-y-old offspring when comparing different groups of RBC or plasma folate as assessed at 3 different time points during pregnancy (71). Correspondingly, no significant associations were observed between maternal folate intake in the first and second pregnancy trimesters and childhood visual memory and intelligence at age 7 in Project Viva including 895 American mother-child pairs (75). Lower RBC folate concentrations or intakes in early pregnancy were also not associated with emotional problems in 139 8-y-old children from the United Kingdom. Conversely, lower early pregnancy RBC folate concentrations and intakes were associated with an increased probability of childhood hyperactivity (β: −0.24; P = 0.013 for status and −0.24; P = 0.022 for intake) and peer problems (β: −0.28; P = 0.004 for status and −0.28; P = 0.009 for intake), where mediation analyses suggested a link with fetal growth. In contrast, lower maternal folate intake in late pregnancy was not associated with offspring hyperactivity and peer problems (69). For this age-group we also identified a European multicenter RCT with cognitive follow-up data at 6.5 and 8.5 y old, in which women were assigned to 400 μg folic acid/d or a placebo during the second half of pregnancy. At age 6.5 y, Kaufman Assessment Battery for Children scores did not point towards cognitive differences between the folic acid-supplemented group and the placebo group (86). However, 8.5-y-old children of mothers supplemented with folic acid from 20 weeks of gestation until delivery performed better on the Attention Network Test (median conflict reaction time folic acid compared with control group: 60 compared with 101 ms; P = 0.01), suggesting improved executive function. In agreement, cord plasma folate concentrations at delivery were positively correlated with brain activity during the executive function test (adjusted r: 0.21; P < 0.05). Conversely, higher maternal whole blood folate concentrations at 30 weeks of pregnancy were inversely correlated with a lower overall response speed (adjusted r: −0.18; P < 0.05). Other measures of folate status were not associated with offspring executive function and alertness. Moreover, no effect was found on spatial orienting of attention scores (87).
Preadolescents (9–12 y)
Among 9.5-y-old Indian offspring (n = 536) participating in the Mysore Parthenon birth cohort, each unit increase in maternal plasma folate concentration in late pregnancy was associated with better children's learning ability and long-term memory (model 2 β: 0.10; 95% CI: 0.01, 0.18), visuospatial ability (model 2 β: 0.09; 95% CI: 0.005, 0.18), and attention and concentration (model 2 β: 0.12; 95% CI: 0.04, 0.20). Further adjustment for children's current head circumference, BMI, education, and vitamin B-12 and plasma folate concentrations at 9.5 y did not alter the significance of these results (72). Moreover, the Menorca's birth-cohort (n = 393) showed that 11-y-old children of mothers who reported use of folic acid supplements during any time of the pregnancy had a lower omission error rate (adjusted IRR: 0.80; 95% CI: 0.64, 1.00; P = 0.046), suggesting a beneficial link with child's attention. No differences were found in offspring impulsivity and visual processing, as measured with the commission error rate and the hit reaction time (78).
Discussion
In this review of animal and human studies we provided an overview of the current evidence on the association between periconceptional and gestational maternal folate status and offspring brain structure and function. The included animal experimental studies indicate an essential role of maternal folate intake for the offspring's developing brain. Both folate deficiency and folate excess have been linked to morphological and physiological deviations in normal brain development. In addition, offspring's DNA methylation and gene expression levels seem to be influenced by maternal folate status. However, the limited number of studies investigating the cognitive abilities in rodent offspring exposed to a folate imbalanced diet observed only little or no effects. Among the included human observational studies, the findings were mixed. Studies investigating the association between maternal folate status and offspring cognitive function in humans were inconsistent, with about half of the studies showing beneficial associations between adequate maternal folate status and offspring cognitive performance, and half of the studies showing no significant associations. The majority of the observational studies on maternal folate intake suggest a positive association with child cognitive function. Only 1 RCT on maternal folic acid supplementation during late pregnancy and offspring cognitive performance was identified, providing insufficient evidence for a causal relation between maternal folate and childhood cognitive performance. In contrast to the animal studies, no effect of maternal folate status or intake on newborn DNA methylation level of fetal growth and neurodevelopmental genes was observed in the only human study on this subject matter.
When reviewing the current evidence, and comparing the included studies, several considerations must be noted. First, the included animal studies varied largely with respect to their study design. All rodent studies on folate deficiency were conducted in mice, whereas studies on folate excess were conducted in both mice and rats. However, whereas it is challenging to compare data of distinct animal species, for example because of differences in nutrient requirements and metabolism, it enlarges the possibility of extrapolation to other animals and humans. Furthermore, the included animal studies greatly varied with respect to the degree of folate deficiency or excess. For example, in some studies folate excess was defined as twice the recommended dose (4 mg folic acid/kg food), in other studies 10 times the recommended dose (20 mg folic acid/kg food) was given. Other factors that varied between the experimental designs of the studies reviewed include the timing of the folate imbalanced diet as well as the age of examination of the cognitive outcomes. In addition, most animal studies started with the folate intervention before mating and continued until birth or even until weaning. Therefore, it remains difficult to determine the exact effects of folate imbalance during the various periods of brain development. Lastly, it remains uncertain to what extent the results of these animal studies are predictive for humans.
With respect to human studies, it should be noted that almost all the included studies were conducted in developed countries with predominantly Caucasian mother-child pairs, which limits the generalizability to other populations. Second, there is substantial heterogeneity in the assessment of folate status and intake as well as the timing of the assessment (Tables 5 and 6). Moreover, although validated by folate status in a few studies, use of folic acid supplements was often based on self-report, which may have introduced measurement error. Moreover, some studies lacked information about the dosage of the folic acid supplement. In addition, despite several countries distributing folic acid–fortified foods, which substantially contribute to the maternal total folate intake, only a limited number of studies considered dietary folate intakes. Third, discrepancies in study outcomes may also relate to the methodology used for assessment of child cognitive functions. Whereas some studies used extensive and validated cognitive tests, others collected their data with only a limited number of test items or used questionnaires completed by parents instead of trained researchers, reducing the reliability of the reported findings. Fourth, it cannot be completely ruled out that the observed effects attributed to folate are partially caused by intake of other nutrients, in particular, when no distinction was made between maternal use of folic acid with or without other supplements and results were not adjusted for intakes of other nutrients. Fifth, high attrition rates are a point of concern in several included studies, such as a study in which the analysis sample comprised less than half of the study population at baseline (75). Moreover, results might be influenced by selective dropouts. Although the majority of studies adjusted for several confounders, residual confounding factors may still be present. Sixth, differences in findings between studies may also relate to differences in the statistical approach used to analyze the data, for instance through use of different cutoff values for folate deficiency and/or the included covariates (Tables 5 and 6). Lastly, the many intersecting pathways of the folate metabolism with 1-carbon metabolism, are often not taken into account. In future studies, it would be important to assess, next to folate concentrations, also the concentrations of other essential components of this pathway, such as vitamin B-12, vitamin B-6, choline, and homocysteine. In particular, the possible health risks of maternal vitamin B-12 deficiency in combination with folate excess warrant further investigation, as such a disbalance has been shown to increase the risk factors for type 2 diabetes (e.g., insulin resistance and adiposity) in the offspring (28).
To determine whether there is a critical time window of adequate folate intake, future animal studies may further advance this research field by exposing dams to folate deficiency or excess during specific periods of gestation and/or early neonatal life. Although this clearly needs further investigation, it is likely that folate requirements for the brain are also dependent on the neurodevelopmental time-window; the optimal dose of folate at 1 time point, might be suboptimal at another. The brain requirements largely depend on which areas are developing at any given time. As such, the effects of folate deficiency or excess on, for example, proliferation, DNA methylation, and neurotransmitter synthesis are likely to be regionally distributed within the brain. Folate is also important for brain-wide processes such as myelination, which accelerates during late gestation and early postnatal life. For future research it is important to note that sensitive neurodevelopmental time-windows deviate between humans and rodents. As an example, the hippocampal formation, which is critically important for learning and memory and shown to be affected by folate status, starts to develop in humans in the 15th week of gestation and its development continues postnatally, resembling the adult hippocampal structure by age 4 y (88). In rodents, hippocampal development is most sensitive for nutritional insults during neuron formation at embryonic days 11–18 and during rapid synapse formation around 16–30 d postnatally (89). The timing and duration of folate imbalance in combination with the brain's need for folate at that particular time will determine the severity of the effect. Furthermore, investigation of the dose-response relations is required to identify the optimal dosage of folic acid supplementation. Given the limited number of animal studies examining the effects of pre- and/or postnatal folate deficiency or excess on offspring cognitive performance, more studies should focus on/or incorporate behavioral outcomes besides morphological, physiological, and epigenetic outcomes. In addition, more well-designed human studies are required to clarify the associations of maternal folate status and intake with offspring cognitive functioning. Well-designed observational studies and RCTs should be performed to determine whether current recommendations for folic acid supplementation during pregnancy cover the requirements for normal childhood cognitive development. Establishing the optimal folic acid dosage at the different stages of pregnancy is critical because excessive folic acid fortification and/or supplementation strategies might pose adverse health outcomes. Folic acid has a higher bioavailability than natural folates; whereas the bioavailability of folates in food is around 50%, for folic acid taken in combination with food this is around 85% and when taken on an empty stomach this is even close to 100% (74). High intake of folic acid raises blood concentrations of naturally occurring folates and unmetabolized folic acid (26), as it must be reduced to dihydrofolate before it can enter the 1-carbon metabolism. High folic acid intake also leads to high intracellular folate concentrations. The exact consequences of this on the various intracellular folate-dependent processes are not fully understood yet. However, many folate-requiring enzymes are inhibited by excess substrate (90), meaning that the activity of these enzymes follows an inverted U-shape, where both very low and very high concentrations of folates exert a similar effect. In addition, folic acid competes with tetrahydrofolates (THFs, the reduced form of folate used in all folate-dependent biochemical processes in our body) for binding with enzymes and binding proteins. Importantly, as folate receptors (FRα) have a higher affinity for folic acid compared to MTHF, high folic acid concentrations can occupy FRαs in the choroid plexus and thereby prevent transport of MTHF into the brain (91). Folate binding protein in the placenta also has a very high affinity for folic acid, suggesting that exposure to very high concentrations of unmetabolized folic acid, theoretically, may interfere with the transport of reduced folates across the placenta. It should be noted that no such effects have been observed at maternal folic acid supplementation at a dose of 400 μg/d continued throughout 40 weeks of gestation (92). Actually, supplementation with 400 μg/d throughout pregnancy has been shown to increase maternal and neonatal folate status, without causing accumulation of unmetabolized folic acid in the cord, which represents only 10% of that found in the maternal circulation. It is likely this is the result of metabolization of folic acid to reduced folates in maternal tissues before reaching the fetus. As the fetus has only a limited capacity to store folate, it must use what is immediately available via the placenta (2).
To establish the optimal dosage for supplementation, future studies could benefit from more accurate and frequent folate intake measurements, using detailed interviews about folic acid supplement use in combination with habitual dietary folate intakes, particularly in countries with folate-enriched foods. Reliability can be further improved by validation of reported folate intakes with blood folate concentrations, as already done by others (76, 82). In addition, use of extensive and validated cognitive tests performed by trained researchers is recommended to increase reliability and enable comparisons between studies. Furthermore, variation in study setting and population could add to our knowledge of differences in the effects of maternal folate status or intake on offspring cognitive function. In future research, brain imaging techniques, such as MRI and electroencephalography, could be used to investigate brain structure and function in relation to cognitive performance. Finally, more research is required to deepen our understanding of the impact of folate on the expression of genes and proteins involved in cognitive development via epigenetic modifications.
Conclusion
The current evidence suggests that folate may play a role in offspring cognitive development and function. Animal studies indicate morphological, physiological, and genetic alterations in the offspring as a result of prenatal and/or postnatal exposure to folate deficiency or excess. However, the small number of rodent studies on offspring cognitive abilities suggests only subtle behavioral effects. In humans, some data suggest a positive link between sufficient maternal folate status and intake and offspring cognitive function. However, a lack of consistency in assessments of folate exposure and cognitive function, and insufficient support from human interventional studies, make it difficult to draw harsh conclusions. Some important questions remaining to be answered are: i) the effects of folate intakes higher than recommended, ii) the potential differences between synthetic folic acid intake and intake of natural folates, and iii) the folate requirements in later periods of pregnancy. Future well-designed studies are recommended for better understanding of the importance of folate during pregnancy in offspring cognitive function.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows: EFGN and EMBB were responsible for the final content of the manuscript. PCS and EMBB collaborated to develop the concept of the manuscript. All authors contributed to the literature search and writing of the manuscript. All authors read and approved the final paper.
Notes
Financial support: none.
Author disclosures: EFGN, PCS, and EMB-B, no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AA, arachidonic acid; BDNF, brain-derived neurotrophic factor; BER, base excision repair; BSID, Bayley Scales of Infant Development; CREB, cAMP response element-binding protein; CRL, crown-rump length; FE, folate equivalents; FRα, folate receptors; GPX, glutathione peroxidase; LCD, left cerebellar diameter; MTHFR, methylenetetrahydrofolate reductase; NGF, nerve growth factor; NTD, neural tube defect; RCD, right cerebellar diameter; RCT, randomized controlled trial; SAM, S-adenosyl methionine; SOD, superoxide dismutase; TCD, transcerebellar diameter; THF, tetrahydrofolate; TrkB, tyrosine receptor kinase B; 8-oxodG, 8-oxo-7,8-dihydro-2'-deoxyguanosine.
References
- 1. Scholl TO, Johnson WG.. Folic acid: influence on the outcome of pregnancy. Am J Clin Nutr. 2000;71:1295s–303s. [DOI] [PubMed] [Google Scholar]
- 2. Tamura T, Picciano MF. Folate and human reproduction. Am J Clin Nutr. 2006;83:993–1016. [DOI] [PubMed] [Google Scholar]
- 3. Desai A, Sequeira JM, Quadros EV. The metabolic basis for developmental disorders due to defective folate transport. Biochimie. 2016;126:31–42. [DOI] [PubMed] [Google Scholar]
- 4. Solanky N, Requena Jimenez A, D'Souza SW, Sibley CP, Glazier JD. Expression of folate transporters in human placenta and implications for homocysteine metabolism. Placenta. 2010;31:134–43. [DOI] [PubMed] [Google Scholar]
- 5. Chanarin I, Mollin D, Anderson BB. Folic acid deficiency and the megaloblastic anaemias. Proc R Soc Med. 1958;; 51:757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Willoughby MLN, Jewell FG. Folate status throughout pregnancy and in postpartum period. BMJ. 1968;4:356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chanarin I, Rothman D, Ward A, Perry J. Folate status and requirement in pregnancy. BMJ. 1968;2:390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Research Council (U.S.) Committee on Maternal Nutrition. Maternal nutrition and the course of pregnancy: summary report: National Academies; 1970. [Google Scholar]
- 9. Czeizel AE, Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–5. [DOI] [PubMed] [Google Scholar]
- 10. Medical Research Council. Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–7. [PubMed] [Google Scholar]
- 11. Fekete K, Berti C, Trovato M, Lohner S, Dullemeijer C, Souverein OW, Cetin I, Decsi T. Effect of folate intake on health outcomes in pregnancy: a systematic review and meta-analysis on birth weight, placental weight and length of gestation. Nutr J. 2012;11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bailey LB, Berry RJ. Folic acid supplementation and the occurrence of congenital heart defects, orofacial clefts, multiple births, and miscarriage. Am J Clin Nutr. 2005;81:1213S–7S. [DOI] [PubMed] [Google Scholar]
- 13. DeVilbiss EA, Gardner RM, Newschaffer CJ, Lee BK. Maternal folate status as a risk factor for autism spectrum disorders: a review of existing evidence. Br J Nutr. 2015;114:663–72. [DOI] [PubMed] [Google Scholar]
- 14. Picker JD, Coyle JT. Do maternal folate and homocysteine levels play a role in neurodevelopmental processes that increase risk for schizophrenia?. Harv Rev Psychiatry. 2005;13:197–205. [DOI] [PubMed] [Google Scholar]
- 15. Gao Y, Sheng C, R-h Xie, Sun W, Asztalos E, Moddemann D, Zwaigenbaum L, Walker M, Wen SW. New perspective on impact of folic acid supplementation during pregnancy on neurodevelopment/autism in the offspring children – a systematic review. PLoS One. 2016;11:e0165626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McGarel C, Pentieva K, Strain JJ, McNulty H. Emerging roles for folate and related B-vitamins in brain health across the lifecycle. Proc Nutr Soc. 2015;74:46–55. [DOI] [PubMed] [Google Scholar]
- 17. Chmielewska A, Dziechciarz P, Gieruszczak-Białek D, Horvath A, Pieścik-Lech M, Ruszczyński M, Skórka A, Szajewska H. Effects of prenatal and/or postnatal supplementation with iron, PUFA or folic acid on neurodevelopment: update. Br J Nutr. 2016:1–6.. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18. Viswanathan M, Treiman KA, Kish-Doto J, Middleton JC, Coker-Schwimmer EJ, Nicholson WK. Folic acid supplementation for the prevention of neural tube defects: an updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:190–203. [DOI] [PubMed] [Google Scholar]
- 19. Crider KS, Bailey LB, Berry RJ. Folic acid food fortification - its history, effect, concerns, and future directions. Nutrients. 2011;3:370–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Looman M, van den Berg C, Geelen A, Samlal RAK, Heijligenberg R, Klein Gunnewiek JMT, Balvers MGJ, Leendertz-Eggen CL, Wijnberger LDE, Feskens EJM et al.. Supplement use and dietary sources of folate, vitamin D, and n-3 fatty acids during preconception: the GLIMP2 Study. Nutrients. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Willett WC. Nutritional epidemiology. 3rd ed New York: Oxford University Press; 2013. [Google Scholar]
- 22. de Benoist B. Conclusions of a WHO Technical Consultation on folate and vitamin B12 deficiencies. Food Nutr Bull. 2008;29:S238–44. [DOI] [PubMed] [Google Scholar]
- 23. McLean E, de Benoist B, Allen LH. Review of the magnitude of folate and vitamin B12 deficiencies worldwide. Food Nutr Bull. 2008;29:S38–51. [DOI] [PubMed] [Google Scholar]
- 24. Gernand AD, Schulze KJ, Stewart CP, West Jr KP, Christian P. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol. 2016;12:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dubois L, Diasparra M, Bedard B, Colapinto CK, Fontaine-Bisson B, Morisset AS, Tremblay RE, Fraser WD. Adequacy of nutritional intake from food and supplements in a cohort of pregnant women in Quebec, Canada: the 3D Cohort Study (Design, Develop, Discover). Am J Clin Nutr. 2017;106:541–8. [DOI] [PubMed] [Google Scholar]
- 26. Smith AD, Kim YI, Refsum H. Is folic acid good for everyone?. Am J Clin Nutr. 2008;87:517–33. [DOI] [PubMed] [Google Scholar]
- 27. Selhub J, Rosenberg IH. Excessive folic acid intake and relation to adverse health outcome. Biochimie. 2016;126:71–8. [DOI] [PubMed] [Google Scholar]
- 28. Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, Bhat DS, Naik SS, Coyaji KJ, Joglekar CV et al.. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bailey LB, Caudill MA.. Folate. Edition ed Present Knowledge in Nutrition: Wiley-Blackwell, 2012:321–42. [Google Scholar]
- 30. Bailey LB, Gregory JF. Folate metabolism and requirements. J Nutr. 1999;129:779–82. [DOI] [PubMed] [Google Scholar]
- 31. Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. 2014;72:267–84. [DOI] [PubMed] [Google Scholar]
- 32. Djukic A. Folate-responsive neurologic diseases. Pediatr Neurol. 2007;37:387–97. [DOI] [PubMed] [Google Scholar]
- 33. Hutto BR. Folate and cobalamin in psychiatric illness. Compr Psychiatry. 1997;38:305–14. [DOI] [PubMed] [Google Scholar]
- 34. Scott JM, Weir DG.. Folic acid, homocysteine and one-carbon metabolism: a review of the essential biochemistry. J Cardiovasc Risk. 1998;5:223–7. [PubMed] [Google Scholar]
- 35. Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, Mattson MP. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. 2000;20:6920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP et al.. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–3. [DOI] [PubMed] [Google Scholar]
- 37. Mitchell ES, Conus N, Kaput J. B vitamin polymorphisms and behavior: evidence of associations with neurodevelopment, depression, schizophrenia, bipolar disorder and cognitive decline. Neurosci Biobehav Rev. 2014;47:307–20. [DOI] [PubMed] [Google Scholar]
- 38. Craciunescu CN, Brown EC, Mar MH, Albright CD, Nadeau MR, Zeisel SH. Folic acid deficiency during late gestation decreases progenitor cell proliferation and increases apoptosis in fetal mouse brain. J Nutr. 2004;134:162–6. [DOI] [PubMed] [Google Scholar]
- 39. Craciunescu CN, Johnson AR, Zeisel SH. Dietary choline reverses some, but not all, effects of folate deficiency on neurogenesis and apoptosis in fetal mouse brain. J Nutr. 2010;140:1162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Franco SJ, Muller U. Shaping our minds: stem and progenitor cell diversity in the mammalian neocortex. Neuron. 2013;77:19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xiao S, Hansen DK, Horsley ET, Tang YS, Khan RA, Stabler SP, Jayaram HN, Antony AC. Maternal folate deficiency results in selective upregulation of folate receptors and heterogeneous nuclear ribonucleoprotein-E1 associated with multiple subtle aberrations in fetal tissues. Birth Defects Res A Clin Mol Teratol. 2005;73:6–28. [DOI] [PubMed] [Google Scholar]
- 42. Jadavji NM, Deng L, Malysheva O, Caudill MA, Rozen R. MTHFR deficiency or reduced intake of folate or choline in pregnant mice results in impaired short-term memory and increased apoptosis in the hippocampus of wild-type offspring. Neuroscience. 2015;300:1–9. [DOI] [PubMed] [Google Scholar]
- 43. Sable P, Dangat K, Kale A, Joshi S. Altered brain neurotrophins at birth: consequence of imbalance in maternal folic acid and vitamin B12 metabolism. Neuroscience. 2011;190:127–34. [DOI] [PubMed] [Google Scholar]
- 44. Sable PS, Dangat KD, Joshi AA, Joshi SR. Maternal omega 3 fatty acid supplementation during pregnancy to a micronutrient-imbalanced diet protects postnatal reduction of brain neurotrophins in the rat offspring. Neuroscience. 2012;217:46–55. [DOI] [PubMed] [Google Scholar]
- 45. Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory?. Front Mol Neurosci. 2010;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Conner JM, Franks KM, Titterness AK, Russell K, Merrill DA, Christie BR, Sejnowski TJ, Tuszynski MH. NGF is essential for hippocampal plasticity and learning. J Neurosci. 2009;29:10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sable PS, Kale AA, Joshi SR. Prenatal omega 3 fatty acid supplementation to a micronutrient imbalanced diet protects brain neurotrophins in both the cortex and hippocampus in the adult rat offspring. Metabolism. 2013;62:1607–22. [DOI] [PubMed] [Google Scholar]
- 48. Sable P, Kale A, Joshi A, Joshi S. Maternal micronutrient imbalance alters gene expression of BDNF, NGF, TrkB and CREB in the offspring brain at an adult age. Int J Dev Neurosci. 2014;34:24–32. [DOI] [PubMed] [Google Scholar]
- 49. Langie SA, Achterfeldt S, Gorniak JP, Halley-Hogg KJ, Oxley D, van Schooten FJ, Godschalk RW, McKay JA, Mathers JC. Maternal folate depletion and high-fat feeding from weaning affects DNA methylation and DNA repair in brain of adult offspring. FASEB J. 2013;27:3323–34. [DOI] [PubMed] [Google Scholar]
- 50. Barua S, Chadman KK, Kuizon S, Buenaventura D, Stapley NW, Ruocco F, Begum U, Guariglia SR, Brown WT, Junaid MA. Increasing maternal or post-weaning folic acid alters gene expression and moderately changes behavior in the offspring. PLoS ONE. 2014;9:e101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barua S, Kuizon S, Brown WT, Junaid MA. High gestational folic acid supplementation alters expression of imprinted and candidate autism susceptibility genes in a sex-specific manner in mouse offspring. J Mol Neurosci. 2016;58:277–86. [DOI] [PubMed] [Google Scholar]
- 52. Roy S, Kale A, Dangat K, Sable P, Kulkarni A, Joshi S. Maternal micronutrients (folic acid and vitamin B(12)) and omega 3 fatty acids: implications for neurodevelopmental risk in the rat offspring. Brain Dev. 2012;34:64–71. [DOI] [PubMed] [Google Scholar]
- 53. Roy S, Sable P, Khaire A, Randhir K, Kale A, Joshi S. Effect of maternal micronutrients (folic acid and vitamin B12) and omega 3 fatty acids on indices of brain oxidative stress in the offspring. Brain Dev. 2014;36:219–27. [DOI] [PubMed] [Google Scholar]
- 54. Sable P, Randhir K, Kale A, Chavan-Gautam P, Joshi S. Maternal micronutrients and brain global methylation patterns in the offspring. Nutr Neurosci. 2015;18:30–6. [DOI] [PubMed] [Google Scholar]
- 55. Onodera K, Omoi NO, Fukui K, Hayasaka T, Shinkai T, Suzuki S, Abe K, Urano S. Oxidative damage of rat cerebral cortex and hippocampus, and changes in antioxidative defense systems caused by hyperoxia. Free Radic Res. 2003;37:367–72. [DOI] [PubMed] [Google Scholar]
- 56. da Silva RP, Kelly KB, Al Rajabi A, Jacobs RL. Novel insights on interactions between folate and lipid metabolism. Biofactors. 2014;40:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Devlin A, Green T. Mechanisms of altered fatty acid and phospholipid levels in hyperhomocysteinemia. Clin Lipidol. 2009;4:159–66. [Google Scholar]
- 58. Pita ML, Delgado MJ. Folate administration increases n-3 polyunsaturated fatty acids in rat plasma and tissue lipids. Thromb Haemost. 2000;84:420–3. [PubMed] [Google Scholar]
- 59. Weiser MJ, Butt CM, Mohajeri MH. Docosahexaenoic acid and cognition throughout the lifespan. Nutrients. 2016;8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hadley KB, Ryan AS, Forsyth S, Gautier S, Salem N. The essentiality of arachidonic acid in infant development. Nutrients. 2016;8:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Steinfeld R, Grapp M, Kraetzner R, Dreha-Kulaczewski S, Helms G, Dechent P, Wevers R, Grosso S, Gartner J. Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am J Hum Genet. 2009;85:354–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Weng Q, Wang J, Wang J, Tan B, Wang J, Wang H, Zheng T, Lu QR, Yang B, He Q. Folate metabolism regulates oligodendrocyte survival and differentiation by modulating AMPKα activity. Sci Rep. 2017;7:1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zeisel SH. The fetal origins of memory: the role of dietary choline in optimal brain development. J Pediatr. 2006;149:S131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Smith AD, Refsum H.. Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr. 2016;36:211–39. [DOI] [PubMed] [Google Scholar]
- 65. Ferguson SA, Berry KJ, Hansen DK, Wall KS, White G, Antony AC. Behavioral effects of prenatal folate deficiency in mice. Birth Defects Res A Clin Mol Teratol. 2005;73:249–52. [DOI] [PubMed] [Google Scholar]
- 66. Bahous RH, Jadavji NM, Deng L, Cosin-Tomas M, Lu J, Malysheva O, Leung KY, Ho MK, Pallas M, Kaliman P et al.. High dietary folate in pregnant mice leads to pseudo-MTHFR deficiency and altered methyl metabolism, with embryonic growth delay and short-term memory impairment in offspring. Hum Mol Genet. 2017;26:888–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van Mil NH, Bouwland-Both MI, Stolk L, Verbiest MM, Hofman A, Jaddoe VW, Verhulst FC, Eilers PH, Uitterlinden AG, Steegers EA et al.. Determinants of maternal pregnancy one-carbon metabolism and newborn human DNA methylation profiles. Reproduction. 2014;148:581–92. [DOI] [PubMed] [Google Scholar]
- 68. Koning IV, Groenenberg IA, Gotink AW, Willemsen SP, Gijtenbeek M, Dudink J, Go AT, Reiss IK, Steegers EA, Steegers-Theunissen RP. Periconception maternal folate status and human embryonic cerebellum growth trajectories: the Rotterdam Predict Study. PLoS One. 2015;10:e0141089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schlotz W, Jones A, Phillips DI, Gale CR, Robinson SM, Godfrey KM. Lower maternal folate status in early pregnancy is associated with childhood hyperactivity and peer problems in offspring. J Child Psychol Psychiatry. 2010;51:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Steenweg-de Graaff J, Roza SJ, Steegers EA, Hofman A, Verhulst FC, Jaddoe VW, Tiemeier H. Maternal folate status in early pregnancy and child emotional and behavioral problems: the Generation R Study. Am J Clin Nutr. 2012;95:1413–21. [DOI] [PubMed] [Google Scholar]
- 71. Tamura T, Goldenberg RL, Chapman VR, Johnston KE, Ramey SL, Nelson KG. Folate status of mothers during pregnancy and mental and psychomotor development of their children at five years of age. Pediatrics. 2005;116:703–8. [DOI] [PubMed] [Google Scholar]
- 72. Veena SR, Krishnaveni GV, Srinivasan K, Wills AK, Muthayya S, Kurpad AV, Yajnik CS, Fall CH. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10-year-old children in South India. J Nutr. 2010;140:1014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wu BT, Dyer RA, King DJ, Richardson KJ, Innis SM. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS One. 2012;7:e43448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline: National Academies Press (US), 1998. [PubMed] [Google Scholar]
- 75. Boeke CE, Gillman MW, Hughes MD, Rifas-Shiman SL, Villamor E, Oken E. Choline intake during pregnancy and child cognition at age 7 years. Am J Epidemiol. 2013;177:1338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chatzi L, Papadopoulou E, Koutra K, Roumeliotaki T, Georgiou V, Stratakis N, Lebentakou V, Karachaliou M, Vassilaki M, Kogevinas M. Effect of high doses of folic acid supplementation in early pregnancy on child neurodevelopment at 18 months of age: the mother-child cohort ‘Rhea’ study in Crete, Greece. Public Health Nutr. 2012;15:1728–36. [DOI] [PubMed] [Google Scholar]
- 77. del Rio Garcia C, Torres-Sanchez L, Chen J, Schnaas L, Hernandez C, Osorio E, Portillo MG, Lopez-Carrillo L. Maternal MTHFR 677C>T genotype and dietary intake of folate and vitamin B(12): their impact on child neurodevelopment. Nutr Neurosci. 2009;12:13–20. [DOI] [PubMed] [Google Scholar]
- 78. Forns J, Torrent M, Garcia-Esteban R, Caceres A, Pilar Gomila M, Martinez D, Morales E, Julvez J, Grimalt JO, Sunyer J. Longitudinal association between early life socio-environmental factors and attention function at the age 11 years. Environ Res. 2012;117:54–9. [DOI] [PubMed] [Google Scholar]
- 79. Julvez J, Fortuny J, Mendez M, Torrent M, Ribas-Fito N, Sunyer J. Maternal use of folic acid supplements during pregnancy and four-year-old neurodevelopment in a population-based birth cohort. Paediatr Perinat Epidemiol. 2009;23:199–206. [DOI] [PubMed] [Google Scholar]
- 80. Polańska K, Muszyński P, Sobala W, Dziewirska E, Merecz-Kot D, Hanke W. Maternal lifestyle during pregnancy and child psychomotor development - Polish Mother and Child Cohort study. Early Hum Dev. 2015;91:317–25. [DOI] [PubMed] [Google Scholar]
- 81. Roth C, Magnus P, Schjolberg S, Stoltenberg C, Suren P, McKeague IW, Davey Smith G, Reichborn-Kjennerud T, Susser E. Folic acid supplements in pregnancy and severe language delay in children. JAMA. 2011;306:1566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Roza SJ, van Batenburg-Eddes T, Steegers EA, Jaddoe VW, Mackenbach JP, Hofman A, Verhulst FC, Tiemeier H. Maternal folic acid supplement use in early pregnancy and child behavioural problems: the Generation R Study. Br J Nutr. 2010;103:445–52. [DOI] [PubMed] [Google Scholar]
- 83. Valera-Gran D, Garcia de la Hera M, Navarrete-Munoz EM, Fernandez-Somoano A, Tardon A, Julvez J, Forns J, Lertxundi N, Ibarluzea JM, Murcia M et al.. Folic acid supplements during pregnancy and child psychomotor development after the first year of life. JAMA Pediatr. 2014;168:e142611. [DOI] [PubMed] [Google Scholar]
- 84. Villamor E, Rifas-Shiman SL, Gillman MW, Oken E. Maternal intake of methyl-donor nutrients and child cognition at 3 years of age. Paediatr Perinat Epidemiol. 2012;26:328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wehby GL, Murray JC. The effects of prenatal use of folic acid and other dietary supplements on early child development. Matern Child Health J. 2008;12:180–7. [DOI] [PubMed] [Google Scholar]
- 86. Campoy C, Escolano-Margarit MV, Ramos R, Parrilla-Roure M, Csabi G, Beyer J, Ramirez-Tortosa MC, Molloy AM, Decsi T, Koletzko BV. Effects of prenatal fish-oil and 5-methyltetrahydrofolate supplementation on cognitive development of children at 6.5 y of age. Am J Clin Nutr. 2011;94:1880S–8S. [DOI] [PubMed] [Google Scholar]
- 87. Catena A, Munoz-Machicao JA, Torres-Espinola FJ, Martinez-Zaldivar C, Diaz-Piedra C, Gil A, Haile G, Gyorei E, Molloy AM, Decsi T et al.. Folate and long-chain polyunsaturated fatty acid supplementation during pregnancy has long-term effects on the attention system of 8.5-y-old offspring: a randomized controlled trial. Am J Clin Nutr. 2016;103:115–27. [DOI] [PubMed] [Google Scholar]
- 88. Seress L, Abraham H, Tornoczky T, Kosztolanyi G. Cell formation in the human hippocampal formation from mid-gestation to the late postnatal period. Neuroscience. 2001;105:831–43. [DOI] [PubMed] [Google Scholar]
- 89. Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–99. [DOI] [PubMed] [Google Scholar]
- 90. Nijhout HF, Reed MC, Budu P, Ulrich CM. A mathematical model of the folate cycle: new insights into folate homeostasis. J Biol Chem. 2004;279:55008–16. [DOI] [PubMed] [Google Scholar]
- 91. Zhao R, Diop-Bove N, Visentin M, Goldman ID. Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr. 2011;31:177–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Pentieva K, Selhub J, Paul L, Molloy AM, McNulty B, Ward M, Marshall B, Dornan J, Reilly R, Parle-McDermott A et al.. Evidence from a randomized trial that exposure to supplemental folic acid at recommended levels during pregnancy does not lead to increased unmetabolized folic acid concentrations in maternal or cord blood. J Nutr. 2016;146:494–500. [DOI] [PubMed] [Google Scholar]