Abstract
Bacterial CRISPR-Cas systems protect their host from bacteriophages and other mobile genetic elements. Mobile elements, in turn, encode various anti-CRISPR (Acr) proteins to inhibit the immune function of CRISPR-Cas. To date, Acr proteins have been discovered for type I (subtypes I-D, I-E, and I-F) and type II (II-A and II-C) but not other CRISPR systems. Here we report the discovery of 12 acr genes, including inhibitors of type V-A and I-C CRISPR systems. Notably, AcrVA1 inhibits a broad spectrum of Cas12a (Cpf1) orthologues including MbCas12a, Mb3Cas12a, AsCas12a, and LbCas12a when assayed in human cells. The acr genes reported here provide useful biotechnological tools and mark the discovery of acr loci in many bacteria and phages.
One Sentence Summary:
A widespread anti-CRISPR gene enables the discovery of novel type V-A CRISPR-Cas12a inhibitors that block gene editing in human cells.
The discovery of bacterial CRISPR-Cas systems that prevent infection by bacterial viruses (phages) has opened a paradigm for bacterial immunity while yielding exciting tools for targeted genome editing. CRISPR systems destroy phage genomes, and in turn, phages express anti-CRISPR (Acr) proteins that directly inhibit Cas effectors (1, 2). Six distinct types (I-VI) of CRISPR systems are spread widely across the bacterial world (3), but Acr proteins have only been discovered for type I and II CRISPR systems (1, 3–6). Given the prevalence and diversity of CRISPR systems, we predict that Acr proteins against other types await discovery.
Anti-CRISPR proteins do not have conserved sequences or structures and only share their relatively small size, making de novo prediction of acr function challenging (6). However, acr genes often cluster together with other acr genes or are adjacent to highly conserved anti-CRISPR associated genes (aca genes) in “acr loci” (7, 8). In this work, we sought to identify acr genes in bacteria and phages that are not homologous to previously identified acr or aca genes.
Acr proteins were first discovered in Pseudomonas aeruginosa, inhibiting type I-F and I-E CRISPR systems (1, 9). P. aeruginosa strains also encode a third CRISPR subtype (type I-C), which lacks known inhibitors (10). We engineered P. aeruginosa to target phage JBD30 with type I-C CRISPR-Cas (fig. S1A) and used it in parallel with existing type I-E (strain SMC4386) and I-F (strain PA14) CRISPR strains to screen for additional acr candidates.
Homologs of aca1 were searched for in Pseudomonas genomes, and 7 gene families not previously tested for anti-CRISPR function were identified upstream of aca1 (Fig. 1A). Three genes inhibited the type I-E CRISPR-Cas system (acrIE5–7), one inhibited type I-F (acrIF11), restoring the plaquing of a targeted phage, and two genes had no inhibitory activity (orf1, orf2) (Fig. 1B, fig. S1B, table S1, S2). Another gene exhibited dual I-E and I-F inhibition, and domain analysis revealed a chimera of previously identified acrIE4 and acrIF7 (acrIE4-F7). No type I-C inhibitors were identified. The type I-F inhibitor acrIF11 was commonly represented in both the P. aeruginosa mobilome and in over 50 species of diverse Proteobacteria (fig. S2, Table S2). acrIF11 is often associated with genes encoding DNA-binding motifs, which we have designated aca4–7 (fig. S2, table S1, S3, S4). To confirm that these aca genes can be used to facilitate acr discovery, we used aca4 to discover an additional Pseudomonas anti-CRISPR, acrIF12 (Fig. 1A, 1B).
Figure 1: The discovery of a widespread type I inhibitor.

(A) Schematic of type I-E and type I-F anti-CRISPRs with anti-CRISPR associated (aca1, aca4) genes in Pseudomonas sp. mobile genetic elements, with dotted lines indicating the “guilt-by-association” relationships used to discover new acr genes in Pseudomonas sp. and Moraxella sp. from known acr genes (top two rows). (B) Phage plaque assays to assess CRISPR-Cas inhibition. Ten-fold serial dilutions of a type I-E or type I-F CRISPR-targeted phage (JBD8 or DMS3m, respectively) titered on lawns of Pseudomonas aeruginosa with naturally active type I-E or type I-F CRISPR-Cas systems expressing candidate inhibitors. ΔCRISPR strains measure phage replication in the absence of CRISPR immunity (top row).
Given the widespread nature of acrIF11, we next used it to discover Acr proteins against CRISPR systems where they have not yet been found: type I-C, a minimal Class 1 system and type V-A CRISPR-Cas12a (Cpf1), a Class 2 single effector system that has high efficiency in genome editing (11–13). To find AcrIC and AcrVA proteins, we first searched for genomes encoding CRISPR spacers that match a target protospacer elsewhere in the same genome (Fig. 2A). The tolerance of this “self-targeting” in viable bacteria indicates potential inhibition of the CRISPR system (4), since genome cleavage would result in bacterial death.
Figure 2: Type I-C and type V-A anti-CRISPR proteins identified in Moraxella.
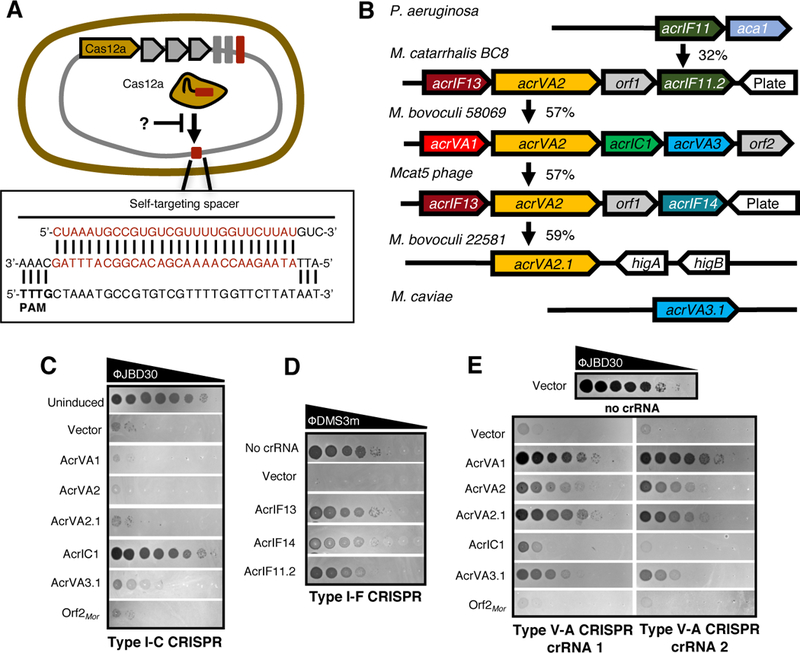
(A) Schematic of Moraxella bovoculi intragenomic self-targeting, wherein spacers encoded by CRISPR-Cas12a system and their target protospacers exist within the same genome.
(B) Schematic showing type V-A (acrVA1-VA3), type I-C (acrIC1), and type I-F (acrIF11-IF14) inhibitors in Moraxella. orf1 and orf2 are genes of unknown function. Vertical arrows indicate the % protein sequence identity. Phage plaque assays with ten-fold serial dilutions of the indicated phage to assess inhibition of CRISPR-Cas type I-C (C), type I-F (D), and type V-A (E). Bacterial clearance (black) indicates phage replication. “Uninduced” panel (C) and “no crRNA” (D, E) indicate full phage titer.
The Gram negative bovine pathogen Moraxella bovoculi (14, 15) is a Cas12a–containing organism (11) where four of the seven genomes feature Type V-A self-targeting (table S5), and one strain (58069) also features self-targeting by type I-C (table S6). Although no previously described acr or aca genes were present in this strain, an acrIF11 homolog was found in phages infecting the human pathogen M. catarrhalis (16), a close relative of M. bovoculi. Genes adjacent to acrIF11 in M. catarrhalis had homologs in the self-targeting M. bovoculi strains (Fig. 2B), and together these genes were selected as candidate acr genes. Each gene was first tested against the type I-C and I-F systems introduced above, as both subtypes are found in Moraxella. Gene AAX09_07415 (now acrIC1) inhibited the type I-C system, explaining the tolerance of self-targeting in strain 58069 (Fig. 2C). Additionally, gene E9U_08473 (acrIF13) from the Moraxella catarrhalis BC8 prophage completely inhibited I-F function, as did AKI27193.1 (acrIF14), found in phage Mcat5 at the same genomic position as acrIF11 in BC8 (Fig. 2B, 2D). Notably, these Acr proteins possess broad spectrum activity as the type I-C and I-F systems in Moraxella and Pseudomonas only share an average pairwise identity of 30% and 36%, respectively (fig. S3)
Due to the limited tools available for the genetic manipulation of Moraxella sp., the remaining genes were tested for type V-A anti-CRISPR function in P. aeruginosa PAO1 engineered to express MbCas12a and a crRNA targeting P. aeruginosa phage JBD30. Two distinct crRNAs were used, showing strong reduction of titer by >4 orders of magnitude (Fig. 2E). The first gene in the M. bovoculi 58069 acr locus, AAX09_07405 (acrVA1), restored phage titers nearly to levels seen with the crRNA-minus control, indicating it robustly inhibits Cas12a. This is in good agreement with the independent discovery of AcrVA1 reported in a companion paper (Watters et al.). The adjacent gene, acrVA2, also inhibited targeting, as did its orthologue (acrVA2.1) (Fig. 2E). An additional gene from this locus, acrVA3, possessed subtle anti-Cas12a activity but was toxic to cells and adversely affected JBD30 phage growth independently of Cas12a (fig. S5). We therefore tested an orthologue with 43% sequence identity, B0181_04965 (acrVA3.1), which showed stronger Cas12a inhibition with no toxicity or adverse effects on phage growth (Fig. 2E, fig. S5). Surprisingly, acrVA3.1 also showed partial restoration of phage titer during type I-C targeting, suggesting that it may inhibit the type I-C as well as type V-A system (Fig. 2C, 2E). Although these two CRISPR subtypes do not share any protein components, a dual-specificity inhibitor may use distinct protein interaction interfaces or modulate an undiscovered host process required for CRISPR immunity. In sum, we used the anti-CRISPR “key” acrIF11 to unlock acr loci encoding seven distinct acr genes inhibiting type I-C, I-F, and V-A CRISPR. Below, we focus on the evolutionary analysis of type V-A inhibitors, and their function in mammalian cells.
acrVA1 encodes a 170-amino acid protein found only in Moraxella sp. and Eubacterium eligens (fig. S6), both type V-A CRISPR-containing organisms. By contrast, acrVA2 (322 aa) and acrVA3 (168 aa) orthologues are found broadly distributed throughout multiple classes of bacteria. For example, acrVA2 orthologues are present in Lachnospiraceae and Leptospira (fig. S7), which contain type V-A CRISPR, as well as in Moraxella, Leptospira, and Lactobacillus phages. Distant orthologues of acrVA2 were also identified on plasmids and conjugative elements in E. coli (fig. S7), although the significance of a bacterium lacking type V-A CRISPR encoding a putative acrVA gene is unknown. Orthologues of acrVA3 were identified in many Proteobacteria, and Eubacterium and Clostridium species, which encode type V-A CRISPR (fig. S8).
Given the inhibitory effect of acrVA1–3.1 on MbCas12a in bacteria, we sought to determine whether AcrVA proteins could block MbCas12a activity in human cells. Human U2-OS-EGFP cells (17) transiently expressing MbCas12a, EGFP-targeting crRNA, and human codon-optimized acrVA1–3.1 were assessed for EGFP disruption using flow cytometry. Co-expression of MbCas12a and crRNA resulted in ~60–70% disruption of EGFP expression relative to background (Fig. 3A). AcrVA1 expression reduced EGFP disruption to background levels, indicating inhibition of MbCas12a, while the other acrVA genes showed little evidence of activity here (Fig. 3A). We additionally found that acrVA1 inhibited another Cas12a orthologue, (Mb3Cas12a), while having no impact on SpyCas9 editing in the same assay (Fig. 3B). Titration of the Acr plasmid relative to the Cas12a expression plasmid revealed comparable dose-dependent responses to inhibition between MbCas12a or Mb3Cas12a with AcrVA1 and SpyCas9 with AcrIIA4 (fig. S9). Furthermore, AcrVA1 was found to be a broad-spectrum inhibitor of other commonly used Cas12a orthologues (11), providing strong inhibition of AsCas12a and LbCas12a, and modest inhibition of FnCas12a (Fig. 3C).
Figure 3: AcrVA1 blocks Cas12a-mediated gene editing in human cells.
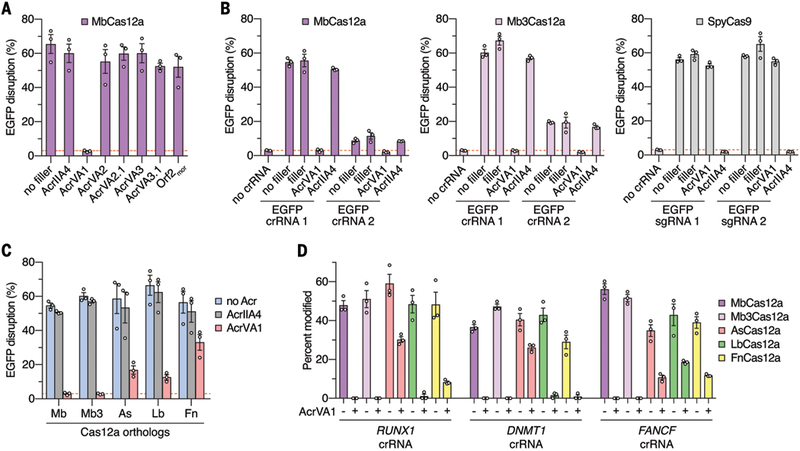
(A-C) Human cell U2-OS-EGFP disruption experiments to assess AcrVA-mediated inhibition of Cas12a activities. (A) Inhibition of MbCas12a activity with various AcrVA constructs; the “no filler” condition contained only plasmids for Cas12a and crRNA expression. (B) Comparisons between the inhibitory activities of AcrVA1 and AcrIIA4 against MbCas12a, Mb3Cas12a, and SpyCas9. Controls using “filler” plasmid in lieu of Acr plasmids were included to equalize amounts of DNA. (C) Assessment of AcrVA1 activity against Cas12a orthologues, with AcrIIA4 used as control. Background EGFP disruption is indicated by the red dashed line; error bars indicate s.e.m. for n = 3. (D) Inhibition of activity of Cas12a orthologues against endogenous sites in human cells (RUNX1, DNMT1, or FANCF genes). Gene modification assessed by T7E1 assay 72 hours post-transfection; error bars indicate s.e.m. for n = 3.
Finally, to determine whether AcrVA1 could inhibit Cas12a-mediated modification of endogenous loci in human cells, U2-OS cells were co-transfected with plasmids expressing acrVA1, Cas12a and crRNAs targeting endogenous genes (RUNX1, DNMT1, or FANCF) and assessed for gene disruption by T7 endonuclease I (T7E1) assay. We found that AcrVA1 completely inhibited gene disruption by MbCas12a and Mb3Cas12a, with modest to strong inhibition of As-, Lb-, and FnCas12a orthologues (Fig. 3D, fig. S10).
Here we report the discovery of a broadly distributed type I-F Acr protein (AcrIF11) that served as a marker for acr loci and led to the identification of type I-C and V-A CRISPR inhibitors. One of these acrVA genes (acrVA1) potently inhibits Cas12a in bacteria and in human cells, providing a new tool for Cas12a regulation. Our findings show that mobile genetic elements can tolerate bacteria with more than one CRISPR-Cas type by possessing multiple Acr proteins in the same locus. The strategy described herein enabled the identification of many widespread anti-CRISPR proteins, which may prove useful in future anti-CRISPR discovery.
Supplementary Material
Acknowledgments:
We thank Jason M. Peters and Carol A. Gross (UCSF) for providing the pUC18T-Lac entry vector for cloning MbCas12a. We thank Dustin Loy (University of Nebraska) and Mike Clawson (USDA) for providing Moraxella bovoculi strain 58069 and 22581 for gDNA extraction.
Funding: Work in the Bondy-Denomy lab was supported by the University of California, San Francisco Program for Breakthrough in Biomedical Research, funded in part by the Sandler Foundation, and an NIH Office of the Director Early Independence Award (DP5-OD021344). Anti-CRISPR discovery efforts were specifically supported by DARPA Safe Genes grant HR0011–17-2–0043. Work in the Joung lab was supported by the Desmond and Ann Heathwood MGH Research Scholar Award (to J.K.J.) and the National Institutes of Health (NIH) Awards K99 CA218870 (B.P.K.) and R35 GM118158 (J.K.J.).
Footnotes
Competing interests: A patent has been filed pertaining to AcrVA genes and their applications. J.K.J. has financial interests in Beam Therapeutics, Blink Therapeutics, Editas Medicine, Endcadia, Inc., Monitor Biotechnologies (formerly known as Beacon Genomics), Pairwise Plants, Poseida Therapeutics, and Transposagen Biopharmaceuticals. J.K.J.’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Data and materials availability: All data are available in the main text or the supplementary materials. Plasmids described in this work are available through Addgene. Phages and bacterial strains will be made available upon request to Joseph.Bondy-Denomy@ucsf.edu.
References and Notes
- 1.Bondy-Denomy J, Pawluk A, Maxwell KL, Davidson AR, Bacteriophage genes that inactivate the CRISPR/Cas bacterial immune system. Nature. 493, 429–432 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bondy-Denomy J et al. , Multiple mechanisms for CRISPR-Cas inhibition by anti-CRISPR proteins. Nature. 526, 136–139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koonin EV, Makarova KS, Zhang F, Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 37, 67–78 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauch BJ et al. , Inhibition of CRISPR-Cas9 with Bacteriophage Proteins. Cell. 168, 150–158.e10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pawluk A et al. , Naturally Occurring Off-Switches for CRISPR-Cas9. Cell. 167, 1829–1838.e9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borges AL, Davidson AR, Bondy-Denomy J, The Discovery, Mechanisms, and Evolutionary Impact of Anti-CRISPRs. Annu Rev Virol. 4, 37–59 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pawluk A, Davidson AR, Maxwell KL, Anti-CRISPR: discovery, mechanism and function. Nat. Rev. Microbiol. 16, 12–17 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Pawluk A et al. , Inactivation of CRISPR-Cas systems by anti-CRISPR proteins in diverse bacterial species. Nat Microbiol. 1, 16085 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Pawluk A, Bondy-Denomy J, Cheung VHW, Maxwell KL, Davidson AR, A new group of phage anti-CRISPR genes inhibits the type I-E CRISPR-Cas system of Pseudomonas aeruginosa. MBio. 5, e00896–e00896–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Belkum A et al. , Phylogenetic Distribution of CRISPR-Cas Systems in Antibiotic-Resistant Pseudomonas aeruginosa. MBio 6, e01796–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zetsche B et al. , Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 163, 759–771 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonfara I, Richter H, Bratovič M, Le Rhun A, Charpentier E, The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature. 532, 517–521 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Kleinstiver BP et al. , Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. Nat. Biotechnol. 34, 869–874 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angelos JA, Spinks PQ, Ball LM, George LW, Moraxella bovoculi sp. nov., isolated from calves with infectious bovine keratoconjunctivitis. Int. J. Syst. Evol. Microbiol. 57, 789–795 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Dickey AM et al. , Large genomic differences between Moraxella bovoculi isolates acquired from the eyes of cattle with infectious bovine keratoconjunctivitis versus the deep nasopharynx of asymptomatic cattle. Vet. Res. 47, 31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ariff A et al. , Novel Moraxella catarrhalis prophages display hyperconserved non-structural genes despite their genomic diversity. BMC Genomics. 16, 860 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyon D et al. , FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 30, 460–465 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Söding J, Biegert A, Lupas AN, The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33, W244–8 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cady KC, Bondy-Denomy J, Heussler GE, Davidson AR, O’Toole GA, The CRISPR/Cas adaptive immune system of Pseudomonas aeruginosa mediates resistance to naturally occurring and engineered phages. J. Bacteriol. 194, 5728–5738 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadopoulos JS, Agarwala R, COBALT: constraint-based alignment tool for multiple protein sequences. Bioinformatics. 23, 1073–1079 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Desper R, Gascuel O, Theoretical foundation of the balanced minimum evolution method of phylogenetic inference and its relationship to weighted least-squares tree fitting. Mol. Biol. Evol. 21, 587–598 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Letunic I, Bork P, 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 46, D493–D496 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleinstiver BP et al. , Engineered CRISPR-Cas9 nucleases with altered PAM specificities. Nature. 523, 481–485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai SQ et al. , Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat. Biotechnol. 32, 569–576 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohland N, Reich D, Cost-effective, high-throughput DNA sequencing libraries for multiplexed target capture. Genome Res. 22, 939–946 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


