Abstract
Glucagon-like-peptide-1 (GLP-1)-receptor agonists have been proposed as putative treatment for substance use disorders (SUD). The objective of this systematic review is to characterize the effects of GLP-1-receptor agonists on SUD-related behavioural effects of drugs, nicotine, and alcohol.
The review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). A search was performed in PubMed and EMBASE on June 16, 2018. The inclusion criteria were primary studies investigating the use of GLP-1-receptor agonists on behavioural endpoints related to SUD.
Seventeen studies were included, investigating the effect of the GLP-1-receptor agonists exendin-4, fluoro-exendin-4, liraglutide, AC3174 and GLP-1 (7–36) on SUD-related behavioural effects of ethanol, cocaine, amphetamine, and/or nicotine. All studies used rodents as subjects. Nine of the studies dealt with ethanol, six with cocaine, two with amphetamine, and two with nicotine. Most studies investigated acute treatment effects, finding a significant effect in all but one experiment. A few studies investigated more chronic effects, but only on ethanol. All the studies reported sustained effects. Eleven studies tested more than one dose, finding a dose-related response in ten out of thirteen experiments. Six studies report a central effect through intra-cerebral administration or by using mice in which the central GLP-1-receptors had been inactivated. In conclusion, a solid body of evidence documents acute effects of GLP-1-receptor agonist treatment on behavioural effects of alcohol, nicotine, amphetamine and cocaine. Documentation of effect of more chronic GLP-1-receptor stimulation on these behaviours is limited.
Keywords: GLP-1, abuse, substance use disorder, smoking, ethanol, stimulants
1. INTRODUCTION
Substance use disorders (SUD) are a worldwide problem. The harmful use of alcohol is estimated to result in 3.3 million deaths every year (“WHO | Global status report on alcohol and health 2014,” 2016). Tobacco causes more than 7 million deaths every year (“WHO | WHO report on the global tobacco epidemic 2017,” 2017). Regarding the use of illicit drugs, about 31 million people suffer from substance use disorders defined as “drug use that is harmful to the point where the user needs treatment” (UNDOC, 2018) and roughly 450,000 people died as a result of drug use in 2015 (“WHO | Information sheet on opioid overdose,” 2018). Although a number of medical interventions against alcohol-, tobacco- and drug use disorders exist (Ali et al., 2017; Soyka & Müller, 2017), we believe that there is still an urgent need for more effective treatments of SUD than those available today.
Glucagon-like-peptide-1 (GLP-1) is a peptide with both hormonal and neurotransmitter functions. It is produced in the intestinal tract as well as in the brain and regulates both blood sugar and feeding behaviour (Holst, 2013). GLP-1 receptor agonists have been approved and used for the treatment of type 2 diabetes mellitus since 2006 (Drucker et al., 2017) and have also been approved for the treatment of obesity (Dar et al., 2015). GLP-1 plays a role in brain areas involved in both alcohol consumption and feeding behaviour (Jerlhag, 2018; Sørensen et al., 2015). GLP-1 receptors have been identified in brain areas associated with reward and addiction, such as the ventral tegmental area (VTA) and the nucleus accumbens (NAc) (Alhadeff et al. 2012; Cork et al., 2015; Göke et al., 1995; Heppner et al., 2015; Merchenthaler et al., 1999. This makes the GLP-1 receptor a possible target for treatment of SUD.
The effects of GLP-1-receptor agonist treatment on SUD-related behavioural effects of alcohol, nicotine, and some drugs have been investigated. The aim of this systematic review was to characterize the effect of GLP-1-receptor agonist treatment on alcohol, nicotine and drug SUD.
2. METHODS
2.1. Protocol and registration
The systematic review was structured according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, Altman, & PRISMA Group, 2010).
2.2. Eligibility criteria
Studies were included if they reported on the use of GLP-1-receptor agonists on SUD-related effects of either alcohol, nicotine, or drugs. Only primary literature (no reviews, protocols, abstracts, or commentaries) in English was included (abstracts were located for articles in Hungarian, Japanese, German, and Spanish, which are not understood by the authors and there was no budget for translators). There were no limitations regarding date of publication, study design, demographics, or subjects (i.e., both animal and human studies were included).
2.3. Information sources
Databases searched included PubMed and Excerpta Medica Database (EMBASE). The search strategy was developed together with a biomedical research librarian and the final search date was 16.07.2018.
2.4. Literature search
The following search terms were used: GLP-1, glucagon-like-peptide, exenatide, victoza, byetta, liraglutide, liraglutid, dependence, dependency, addiction, craving, abuse, substance-related disorders, addictive, amphetamine, tobacco, nicotine, smoking, tobacco use, tobacco use disorder, opioids, opioid, morphine, opium, opiate alkaloids, opiate, narcotics, hypnotics, sedatives, morphine derivatives, crack, coke, cocaine, alcohol, intoxicant, booze.
The same search terms were used in PubMed and EMBASE. The search in EMBASE was limited to only include journal articles.
2.5. Study selection
One reviewer conducted the study identification, exported the citations and removed duplicates. Titles and/or abstracts of studies retrieved were screened by two independent reviewers. Disagreement on the eligibility of the studies was resolved with discussion until consensus was reached. The literature search was supplemented with relevant articles from the reference list of the included articles (snowballing).
2.6. Data collection
Data was extracted from articles by one reviewer and checked twice for accuracy. Data items included authors, year of publication, study type, population, type of animal, abuse substance, type of SUD-related behavioural assays, treatment (acute/repeated), GLP-1-receptor agonist used, dosage of GLP-1-receptor agonist, administration method of GLP-1-receptor agonist, access to food and water, and results.
2.7. Risk of bias in individual studies
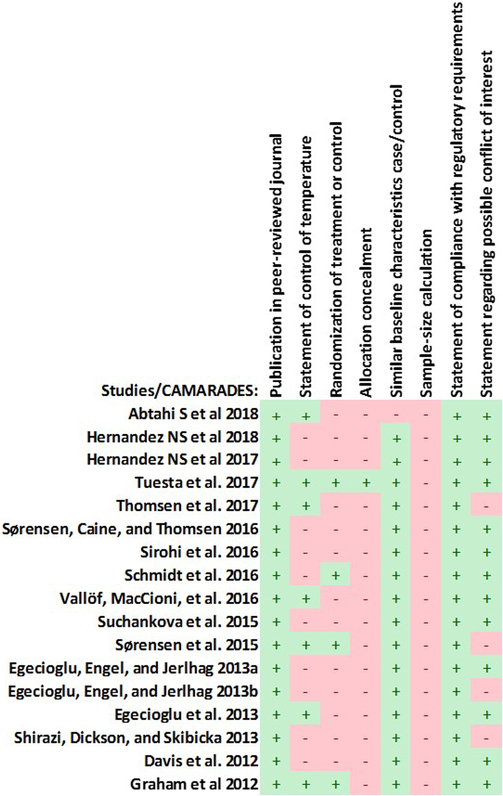
The risk of bias for non-randomised observational studies was assessed with a modified version of the validated CAMARADES’s risk of bias tool for animal studies (Hooijmans et al., 2014).
3. RESULTS
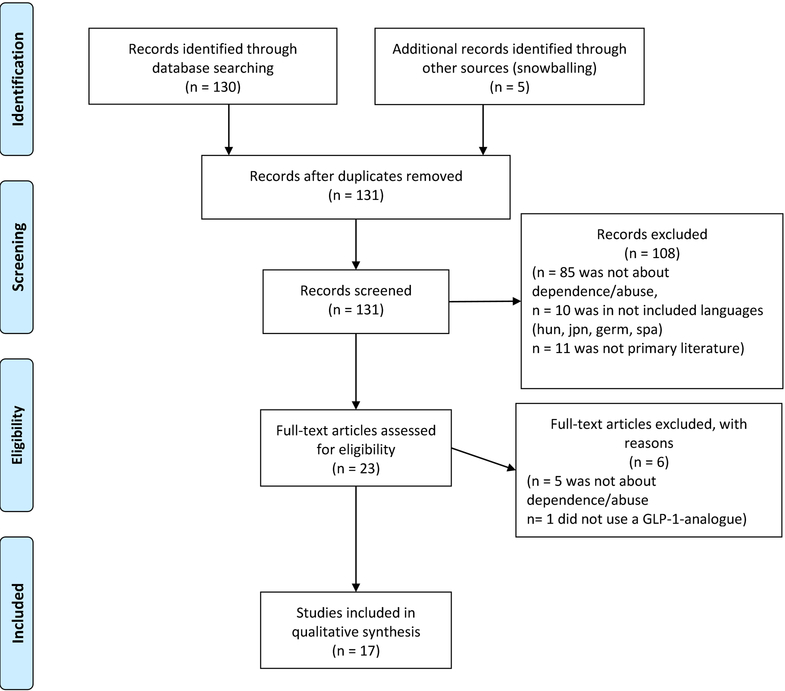
The number of articles screened, assessed for eligibility, reasons for exclusion, and articles found through snowballing is depicted in the flowchart in Figure 1.
Figure 1.
PRISMA flow diagram of articles included in the systematic review as well as the main reasons for rejection. hun: Hungarian, jpn: Japanese, germ: German, span: Spanish.
Seventeen studies were included in this review (Abtahi et al., 2018; Davis et al., 2012; Egecioglu et al., 2013a, 2013b; Egecioglu et al., 2012; Graham et al., 2013; Hernandez et al., 2018, 2019; Schmidt et al., 2016; Shirazi et al., 2013; Sirohi et al., 2016; Sørensen et al., 2016; Sørensen et al., 2015; Suchankova et al., 2015; Thomsen, et al., 2017; Tuesta et al., 2017; Vallöf et al., 2016), fourteen studies found through PubMed and EMBASE and two found through snowballing. The characteristics of the included studies are depicted in Tables 1–4. The bias assessment is presented in Figure 2.
Table 1.
: Characteristics of studies and assays using ethanol as abuse substance. s.c.: subcutaneous, i.p.: intraperitoneal, i.v.: Intravenous, VTA: ventral tegmental area, NAcC: nucleus accumbens core, NAcS: nucleus accumbens shell, Ex-4: exendin-4, lir: liraglutide, f-Ex-4: fluoro-Exendin-4, GLP-1: GLP-1 (7–36), AC3174: AC3174 ([Leu14]exendin-4) SA: self-administration, TBC: two-bottle-choice, CPP: conditioned place preference,
| Author | Study type | Population | Specific type of animal | Abuse substance | Type of assay | Treatment | GLP-1-recetor-agonist used | Dose of GLP-1-receptor-agonist | Administration method GLP-1-receptor-agonist | Access to food | Access to water | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abtahi et al 2018 | Within-subjects | Rats | Sprague-Dawley, female | Ethanol | TBC | Acute | Ex-4 | 0.025and 0,05 μg | Intra-NAcC + Intra-NAcS | No restrictions | No restrictions | Intra-NAcC: no effect compared to vehicle. Intra-NAcS: 0.05 μg Ex-4 decreased intake compared to vehicle. No effect of 0.025 μg Ex-4. |
| Thomsen et al. 2017 | Between-subjects | Mice | C57BL/6J, male | Ethanol | TBC | Repeated | Ex-4 | 1.5 μg/kg/day | s.c. | No restrictions | No restrictions | No deprivation effect in Ex-4 treated mice compared to baseline but deprivation effect in vehicle group. Decreased alcohol drinking in Ex-4 treated mice compared to vehicle. Washout period: both groups drank comparable amounts with comparable preference, compared to baseline and compared to each other. |
| Sørensen et al. 2016a | Between-subjects | Mice | C57BL/6J, male | Ethanol | SA | Acute | Ex-4 | 3.2 and 1.8 μg/kg | i.p. | No restrictions | No restrictions | 3.2 μg/kg Ex-4 reduced intake compared to vehicle. |
| Sirohi et al. 2016 | Between-subjects | Mice | Central GLP_1R ko KD nestin and FLOX, male | Ethanol | TBC | Acute | Ex-4 | 30 μg/kg | i.p. | No restrictions | No restrictions | Ex-4 decreased intake in mice with intact GLP-1 receptors compared to vehicle, no effect in GLP-1R KD mice compared to vehicle. |
| Vallöf et al. 2015 | Between-subjects | Mice | NMRI, male | Ethanol | CPP | Repeated | Lira | 0.1 mg/kg | s.c. | No restrictions | No restrictions | Liraglutide attenuated the acquisition of ethanol CPP compared to vehicle. |
| Between-subjects | Mice | NMRI, male | Ethanol | CPP | Acute | Lira | 0.1 mg/kg | s.c. | No restrictions | No restrictions | No significant difference between Liraglutide group and vehicle group on the expression of CPP. | |
| Between-subjects | Rats | Wistar, male | Ethanol | TBC | Acute | Lira | 0.1 mg/kg | s.c. | No restrictions | No restrictions | Liraglutide decreased intake and preference compared to vehicle. | |
| Between-subjects | Rats | Wistar, male | Ethanol | TBC | Acute | Lira | 0.05 mg/kg | s.c. | No restrictions | No restrictions | Liraglutide decreased intake in high consuming group compared to vehicle, no significant effect on preference or in low consuming group. | |
| Between-subjects | Rats | Wistar, male | Ethanol | TBC | Acute | Lira | 0.1 mg/kg | s.c. | No restrictions | No restrictions | No deprivation effect in Liraglutide-treated rats compared to baseline or vehicle. Liraglutide decreased intake and preference compared to vehicle. | |
| Between-subjects | Rats | Wistar, male | Ethanol | TBC | Repeated | Lira | 0.1 mg/kg | s.c. | No restrictions | No restrictions | Liraglutide reduced intake and preference compared to vehicle in test session 1 and 2. No effect in test session 3. No information about effects during washout period. | |
| Between-subjects | Rats | Alcohol-preferring sP, male | Ethanol | SA | Repeated | Lira | 0.05 and 0.1 mg/kg | s.c. | No restrictions | No restrictions | Reduction in number of lever presses and intake with both doses on day 2. High dose remained relatively stably reduced through day 3–5. Washout (4 days): high dose effect remained 2–3 days. | |
| Suchankova et al. 2015 | Between-subjects | Mice | C57BL/6J, male | Ethanol | TBC | Repeated | AC3174 | 0.03, 0.10 and 0.30 μg/kg | i.p. | No restrictions | No restrictions | AC3174 reduced intake (all doses) compared with control in the last cycle only. Washout: cycle 1: Doses 0.1 and 0.3 μg/kg AC3174 reduced intake. Cycle 2: no effect compared to vehicle. |
| Shirazi et al. 2013 | Between-subjects | Mice | NMRI, male | Ethanol | CPP | Acute | GLP-1 | 0.02 mg/kg | i.p. | * | * | GLP-1 attenuated the expression of CPP compared to vehicle. |
| Between-subjects | Rats | Wistar, male | Ethanol | TBC | Acute | Ex-4 | 0.3 and 1.0 mg/kg | i.p. | No restrictions | No restrictions | Both Ex-4 doses reduced intake. | |
| Within-subjects (Latin Square Design) | Rats | Wistar, male | Ethanol | TBC | Acute | GLP-1 | 0.1 mg/kg | i.p. | No restrictions | No restrictions | GLP-1 decreased intake at 1h compared to vehicle. | |
| Between-subjects | Rats | Wistar, male | Ethanol | TBC | Acute | Ex-4 | 0.1 μg | intra-VTA | No restrictions | No restrictions | GLP-1 decreased intake at 16h (not significantly at 1h) compared to vehicle. | |
| Between-subjects | Rats | Wistar, male | Ethanol | TBC | Acute | GLP-1 | 0.1 μg | intra-VTA | No restrictions | No restrictions | GLP-1 decreased intake at 16h (not at 1h) compared to vehicle. | |
| Egecioglu et al. 2012 | Between-subjects | Mice | NMRI, male | Ethanol | CPP | Acute | Ex-4 | 2.4 μg/kg | i.p. | Restricted during experimental setup | Restricted during experimental setup | Ex-4 attenuated the expression of CPP compared to vehicle. |
| Between-subjects | Mice | NMRI, male | Ethanol | CPP | Repeated | Ex-4 | 2.4 μg/kg | i.p. | Restricted during experimental setup | Restricted during experimental setup | Ex-4 attenuated the acquisition of CPP compared to vehicle. | |
| Within-subjects (Latin Square Design) | Rats | Wistar, male | Ethanol | TBC | Acute | Ex-4 | 0.3and 1.2 μg/kg | i.p. | No restrictions | No restrictions | Higher dose Ex-4 decreased intake compared to vehicle. No effect of lower dose on intake. Both doses reduced preference. | |
| Within-subjects (Latin Square Design) | Rats | Wistar, male | Ethanol | SA | Acute | Ex-4 | 1.2 μg/kg | i.p. | No restrictions | No restrictions | Ex-4 reduced number of active lever presses and breakpoint compared to vehicle. | |
| Davis et al. 2012 | Between-subjects | Rats | RYGB operated/Sham operated P-rats, male | Ethanol | TBC | Acute | Ex-4 | 1.0 and 4.0 μg/kg | * | (After surgical period/recovery) ad libitum high-fat diet | (After surgical period/recovery) No restrictions | Both Ex-4 doses decreased intake in sham-operated rats, no effect in RYGB-treated rats. |
: not disclosed in the study.
Table 4:
Characteristics of studies and assays using nicotine as abuse substance. s.c.: subcutaneous, i.p.: intraperitoneal, i.v.: Intravenous, Ex-4: exendin-4, SA: self-administration, CPP: conditioned place preference, IPN: intra-interpeduncular nucleus, ICSS: intracranial self-stimulation.
| Author | Study type | Population | Specific type of animal | Abuse substance | Type of assay | Treatment | GLP-1-recetor-agonist used | Dose of GLP-1-receptor-agonist | Administration method GLP-1-receptor-agonist | Access to food | Access to water | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tuesta et al. 2017 | Between-subjects | Mice | C57BL6 with null mutation of GLP1r gene/J (wild type littermates), male and female | Nicotine | SA | Acute | Ex-4 | 10 μg/kg | i.p. | Food restricted to 85–90% of free-feeding body weight | No restrict-ions | Ex-4 decreased nicotine intake compared to vehicle. |
| Between-subjects | Rats | Wistar, male | Nicotine | SA | Acute | Ex-4 | 0.1 μg | intra- IPN | Food restricted to 85–90% of free-feeding body weight | No restrict-ions | Ex-4 decreased nicotine intake compared to vehicle. | |
| Between-subjects | Rats | Wistar with intracranial electrode, male | Nicotine | SA | Acute | Ex-4 | 0.1 μg | intra- IPN | Food restricted to 85–90% of free-feeding body weight | No restrict-ions | Ex-4 reduced nicotine intake compared to vehicle. Also abolished the lowering of post-nicotine ICSS thresholds. | |
| Egecioglu et al. 2013b | Between-subjects | Mice | NMRI, male | Nicotine | CPP | Acute | Ex-4 | 2.4 μg/kg | i.p. | Restricted during experimental setup | Restricted during experimental setup | Ex-4 attenuated the expression of CPP compared to vehicle. |
Figure 2.
Modified CAMARADES bias assessment
The Seventeen studies investigated an SUD-related effect of GLP-1-receptor agonist treatment on ethanol, cocaine, amphetamine, and/or nicotine, respectively. The studies used different assays to describe different aspects of the effect of GLP-1-receptor agonists on SUD: Conditioned Place Preference (CPP) measures rewarding (or aversive) effects of drugs and is dependent on memory consolidation. Two-bottle-choice (TBC) paradigms use voluntary drinking and measure reinforcing effects of alcohol or other oral solutions. Self-administration (SA) assays are operant conditioning tasks that investigate reinforcing effects of drugs of abuse via oral or intravenous (i.v.) routes, in which the response contingency (the “price” and availability of the drug) can be manipulated. CPP uses passive drug exposure, TBC and SA measure active drug taking by the subject.
As GLP-1-receptor-agonist, 15 studies used exendin-4 (Ex-4, a.k.a. exenatide) (Abtahi et al., 2018; Davis et al., 2012; Egecioglu et al., 2013a, 2013b, 2012; Graham et al., 2013; Hernandez et al., 2018, 2019; Schmidt et al., 2016; Shirazi et al., 2013; Sirohi et al., 2016; Sørensen et al., 2016, 2015; Thomsen et al., 2017; Tuesta et al., 2017), one study also used GLP-1 (7–36) (Shirazi et al., 2013), two studies used fluoro-exendin-4 (f-Ex-4) (Hernandez et al., 2018, 2019), one study used AC3174 (Suchankova et al., 2015) and one study used liraglutide exclusively (Vallöf et al., 2016).
3.1. Effects of GLP-1-receptor agonist treatment: Ethanol
Ethanol:
Nine of the studies dealt with SUD-related effects of ethanol (see Table 1), with a total of 22 experiments. Nine of the experiments used mice and 13 used rats. Study types consisted of between-subjects (n = 18) and within-subjects (n = 4). The assays consisted of CPP (n = 5; all mice), TBC (n = 14; 3 mice, 11 rats), oral SA (n = 2; rats), and i.v. SA (n = 1; mice). Seven of the studies (11 experiments) used Ex-4, one study used liraglutide (7 experiments), one used AC3174 (1 experiment) and one study used GLP-1 (7–36) (3 experiments) as well as Ex-4 (2 experiments).
In ten of the experiments the GLP-1-receptor agonist was administered intraperitoneally (i.p.), in eight subcutaneously (s.c.), in two directly into the VTA and in one directly into the NAc core (NAcC) and NAc shell (NAcS); in one experiment the authors do not provide information about administration method.
Seven experiments comprised more than one dose and the doses varied considerably across the studies. The i.p.-administered Ex-4 doses varied from 0.03 μg/kg to 1 mg/kg. AC3174 doses were 0.03 μg/kg, 0.10 μg/kg and 0.30 μg/kg (i.p.). Doses of liraglutide were 0.05 mg/kg and 0.1 mg/kg (s.c.). The study using GLP-1 (7–36) tested 0.02 mg/kg (mice) and 0.1 mg/kg (rats) i.p. and 0.1 μg intra-VTA (rats).
Sixteen experiments investigated the acute effect of GLP-1-receptor agonist treatment, in which a GLP-1-receptor agonist or vehicle was administered before access to ethanol (SA and TBC) or before a session in which the animals were placed between chambers at post-conditioning (expression of CPP). Nine experiments used Ex-4 as the GLP-1-receptor agonist (1 CPP, 6 TBC, 2 SA; Abtahi et al., 2018; Davis et al., 2012; Egecioglu et al., 2012; Shirazi et al., 2013; Sirohi et al., 2016; Sørensen et al., 2016). Four experiments used liraglutide (1 CPP, 3 TBC; Vallöf et al., 2016) and three experiments used GLP-1 (7–36) (1 CPP, 2 TBC; Shirazi et al., 2013).
In one SA experiment (mice), nose poking under an FR 1 schedule was reinforced with 75 mg/kg/infusion ethanol i.v. and Ex-4 administered i.p. 30 min before a 2-h ethanol-access session (Sørensen et al., 2016).
In another SA experiment (rats), lever pressing under a progressive ratio (PR) schedule (i.e., testing with an increasing response requirement for each subsequent reward) was reinforced with 0.1 ml 20% ethanol solution. Ten minutes before the test session, Ex-4 was administered i.p. (Egecioglu et al., 2012).
In three TBC experiment (rats), the acute effect of liraglutide on ethanol intake was measured, administered s.c. 60 min before a 24-h ethanol-access session (20% solution) (Vallöf et al., 2016). In one, liraglutide was administered 60 min before the test session. In the second, the rats were divided into high-alcohol consuming and low-alcohol-consuming groups (cut-off 2.5 g/kg) before administration of liraglutide 60 min before test session. In the third experiment, the acute effect of liraglutide on the deprivation effect of ethanol was investigated, administering liraglutide before the re-introduction of ethanol after 10 days of deprivation (Vallöf et al., 2016).
In two TBC experiments (rats) the acute effect of GLP-1 (7–36) administered before a 24-h ethanol-access session (20% solution) was tested (Shirazi et al., 2013). In one GLP-1 (7–36) was administered i.p. 30 min before the test-session. In the second GLP-1 (7–36) intra-VTA was administered before the test session with no information about time from administration to test-session.
Six TBC experiments tested the acute effect of Ex-4.
In one TBC experiment (mice), Ex-4 was administered i.p. after a 24-h baseline ethanol intake measurement (10% solution) and before a 90-minute test-session. No information provided about time from Ex-4 to test-session (Sirohi et al., 2016).
In a second TBC experiment (rats), Ex-4 was administered i.p. 10 minutes before a 24-h ethanol-session (20% solution; (Egecioglu et al., 2012).
In a third TBC experiment (rats), Ex-4 was administered in Roux-en-Y Gastric Bypass (RYGB)-operated/sham-operated rats before a 24-h ethanol-session (10% solution) with intake measurements within the first 120 minutes. No information was provided about route of administration or time from administration to test-session (Davis et al., 2012).
In a fourth TBC experiment (rats), Ex-4 was administered i.p. 30 minutes before a 24-h ethanol-session (20% solution, (Shirazi et al., 2013).
In a fifth TBC experiment (rats), Ex-4 was administered intra-VTA before a 24-h ethanol-session (20% solution). No information provided about time from administration to test session (Shirazi et al., 2013).
In the sixth TBC experiment (rats), Ex-4 was administered intra-NAcC and intra-NAcS at onset of the ethanol-access session (8% solution; (Abtahi et al., 2018).
In a CPP experiment (mice), a single dose of Ex-4 was administered i.p. 10 min before placement between compartments on the post-conditioning test day, i.e., testing for an effect on expression of CPP. Conditioning was four days of 20-min sessions with 1.75 g/kg ethanol i.p., ethanol paired with the non-preferred compartment (biased design; (Egecioglu et al., 2012).
In another CPP experiment (mice) using the same setup, liraglutide was administered s.c. 60 min before the post-conditioning test (Vallöf et al., 2016).
In a third CPP experiment (mice), with the same setup, but with seven conditioning days, GLP-1 (7–36) was administered before the post-conditioning test. No information provided about time from administration to test-session (Shirazi et al., 2013).
In summary, all studies investigating an acute effect of GLP-1-receptor agonist treatment showed a effect in all but one experiment (CPP in (Vallöf et al., 2016)) In three experiments the results varied by agonist dose. In addition, one experiment reported variation by subject subgrouping: in the TBC in (Vallöf et al., 2016) testing liraglutide in high-alcohol consuming and low-alcohol-consuming groups, a significant effect was only found in the high-consuming group.
Five studies investigated the SUD-related effects of repeated treatments with GLP-1-receptor agonists on ethanol, in six experiments. Ex-4 was used as the GLP-1-receptor agonist in two experiments (1 CPP, 1 TBC; Egecioglu et al., 2012; Thomsen et al., 2017); AC3174 was used in one experiment (TBC; Suchankova et al., 2015), and liraglutide in three experiment (1 CPP, 1 TBC, 1 SA; Vallöf et al., 2016).
In the two CPP experiments (Egecioglu et al., 2012; Vallöf et al., 2016), the GLP-1-receptor agonists (liraglutide (Vallöf et al., 2016) and Ex-4 (Egecioglu et al., 2012), respectively) was administered before each of the 20-min conditioning sessions with ethanol i.p. (four days), ethanol paired with the non-preferred compartment (biased design). No treatment was given before the post-conditioning test, i.e., experiments tested for an effect of GLP-1 receptor agonists on acquisition of CPP.
In one TBC experiment liraglutide was administered s.c. 60 min before test sessions for eight consecutive days with intermittent unlimited access to ethanol (20% ethanol solution three 24-h sessions/week). This was followed by a three-day post-treatment period (Vallöf et al., 2016).
The design in the second TBC experiment was 9 repeated cycles of chronic intermittent exposure (16h/day, 4 days) to alcohol/water vapour in inhalation chambers. Each cycle was followed by 72h forced abstinence, then 5 days of 2h/day access to 15% ethanol. Treatment with different doses of AC3174 was administered in both the ethanol- and water-vapour groups in cycle 5–7, 15 min before ethanol access (Suchankova et al., 2015).
In the third TBC experiment, repeated treatments after a deprivation period was tested. A dose of Ex-4 was administered from day 3 of the 10 days of deprivation and continued until 8 days after ethanol was re-introduced (8% ethanol solution). The Ex-4 treatment was followed by a 7-day washout period during which vehicle was administered to both groups (Thomsen et al., 2017).
In an SA experiment (rats), lever pressing under an FR 4 schedule of reinforcement with ethanol (15% solution) was used. Thirty minutes before the 30-min test sessions, pre-treatment with liraglutide was administered s.c. for 5 consecutive days, followed by a 4-day post-treatment period with daily alcohol sessions (Vallöf et al., 2016).
In summary, all the studies investigating repeated treatments all found a significant SUD-related effect of the GLP-1 receptor agonists, but with varied results. The acquisition of CPP was blocked by both Ex-4 and liraglutide (Egecioglu et al., 2012; Vallöf et al., 2016).
(Vallöf et al., 2016) found an effect in their SA experiment on the second day after treatment had begun with both doses, but only the higher dose showed a continued effect after 3–5 days.
The three TBC experiments testing repeated treatments had various results: (Thomsen et al., 2017) found a significant effect of Ex-4 on all 8 treatment days, (Vallöf et al., 2016) found an effect of liraglutide in the two first test sessions but none in the third, and (Suchankova et al., 2015) only found an effect in cycle 7 (the 3rd cycle of AC3174 treatment).
The three studies with a follow-up period described above (Suchankova et al., 2015; Thomsen et al., 2017; Vallöf et al., 2016) tested a long-lasting effect of liraglutide, Ex-4, and AC3174, respectively, in four experiments.
In the TBC experiment in (Suchankova et al., 2015), using repeated cycles of intermittent exposure, vehicle was injected in both groups in the last two of the nine cycles as an evaluation of the drug washout effect. Authors found a dose-dependent lasting effect in cycle 8 with the larger doses having a continued significant effect, which disappeared in cycle 9.
In the TBC experiment in (Vallöf et al., 2016), behaviour was recorded for 3 washout days after the eight days of treatment. Authors do not comment on the effects on ethanol consumption.
The SA experiment in (Vallöf et al., 2016) continued for 4 days after ended liraglutide treatment, finding a dose-related effect with a prolonged effect of only the larger dose of liraglutide on lever pressing for 3 days and on the amount of alcohol consumed for 2 days.
(Thomsen, et al., 2017) had a seven-day washout period in their TBC administering 1.5 μg/kg/day of Ex-4 with effect on alcohol consumption only on the first day of the washout period.
Seven experiments tested more than one dose (4 TBC, 2 SA; 5 Ex-4, 1 liraglutide); four of these showed an effect related to dose.
(Sørensen et al., 2016) found a significant effect of an acute dose of 3.2 μg/kg Ex-4, but no effect with 1.8μg/kg (which was only tested on a small number of animals).
(Egecioglu et al., 2012) found a reduction in ethanol intake after administration of 1.2μg/kg Ex-4, but no reduction in ethanol intake after 0.3 μg/kg compared to vehicle.
(Davis et al., 2012) found that treatment with both 1 and 4 μg/kg of Ex-4 significantly decreased ethanol intake in sham-operated rats but had no effect in RYGB-operated rats.
(Abtahi et al., 2018) found that 0.05 μg of Ex-4 administered intra-NAcS significantly attenuated intake, but no effect of 0.025 μg Ex-4.
(Shirazi et al., 2013) found that 0.3 mg/kg and 1.0 mg/kg Ex-4 i.p. both significantly reduced 1-h alcohol intake.
(Suchankova et al., 2015) found no significant effect of AC3174 (all doses) in cycle 5–6 but a significantly reduced ethanol intake (all doses) in cycle 7, but only mice treated with 0.1 or 0.3 μg/kg AC3174 still consumed significantly less ethanol compared with vehicle in the first washout cycle.
(Vallöf et al., 2016) found in an SA experiment with 5 consecutive days of repeated liraglutide treatments that both 0.05 mg/kg and 0.1 mg/kg significantly decreased ethanol intake on the second day, but only animals treated with 0.1 mg/kg retained a significant reduction through days 3–5.
3.2. Effects of GLP-1-receptor agonist treatment: Cocaine
Cocaine:
Six of the included studies dealt with SUD-related effects of GLP-1-receptor agonist treatment on cocaine (see Table 2), with a total of 14 experiments, five of which used mice and nine used rats. Study types consisted of within-subjects (n = 8) and between-subjects (n = 6). The assays consisted of CPP (n = 2; mice) and i.v. SA (n = 12; 3 mice, 9 rats). All studies used Ex-4 and two also used f-Ex-4. Three experiments administered this intra-VTA (rats), one experiment intra-NAcC and intra-NAcS (rats), and the rest i.p.
Table 2:
Characteristics of studies and assays using cocaine as abuse substance. i.p.: intraperitoneal, VTA: ventral tegmental area, NAcC: nucleus accumbens core, NAcS: nucleus accumbens shell, Ex-4: exendin-4, f-Ex-4: fluoro-Exendin-4, SA: self-administration, CPP: conditioned place preference.
| Author | Study type | Population | Specific type of animal | Abuse substance | Type of assay | Treatment | GLP-1-recetor-agonist used | Dose of GLP-1-receptor-agonist | Administration method GLP-1-receptor-agonist | Access to food | Access to water | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hernandez et al 2018 | Within-subjects | Rats | Sprague-Dawley, male | Cocaine | SA | Acute | f-Ex-4 | 3.0 μg/kg | i.p. | No restrictions | No restrictions | 3.0 μg/kg-Ex-4 reduced cocaine prime-induced reinstatement of responding compared to vehicle. |
| Within-subjects | Rats | Sprague-Dawley, male | Cocaine | SA | Acute | f-Ex-4 | 0.01, 0.1 and 0.2 μg/kg | i.p. | No restrictions | No restrictions | 0.1 μg/kg and 0.2 μg/kg f-Ex-4 reduced prime-induced reinstatement of responding compared to vehicle. No effect of 0.01 μg/kg f-Ex-4. | |
| Within-subjects | Rats | Sprague-Dawley, male | Cocaine | SA | Acute | f-Ex-4 | 0.2 μg/kg | i.p. | No restrictions | No restrictions | 0.2 μg/kg f-Ex-4 reduced cue-induced reinstatement of responding compared to vehicle. | |
| Within-subjects | Rats | Sprague-Dawley, male | Cocaine | SA | Acute | Ex-4 | 0.005 and 0.05 μg/100nL | intra-VTA | No restrictions | No restrictions | 0.05 μg/100nL Ex-4 reduced prime-induced reinstatement of responding compared to vehicle. No effect of 0.005 μg/100nL Ex-4. | |
| Within-subjects | Rats | Sprague-Dawley, male | Cocaine | SA | Acute | f-Ex-4 | 3.0 μg/kg | i.p. | No restrictions | No restrictions | 3.0 μg/kg f-Ex-4 reduced prime-induced reinstatement of responding in intra-VTA veh/f-Ex-4-group compared to vehicle. No effect of peripheral f-Ex-4 when administered with intra-VTA Ex-(9–39). | |
| Hernandez et al. 2019 | Within-subjects | Rats | Sprague-Dawley, male | Cocaine | SA | Acute | f-Ex-4 | 0.1 and 0.2 μg/kg | i.p. | No restrictions | No restrictions | 0.1 μg/kg and 0.2 μg/kg f-Ex-4 reduced prime-induced reinstatement of responding compared to vehicle. |
| Within-subjects | Rats | Sprague-Dawley, male | Cocaine | SA | Acute | Ex-4 | 0.005 and 0.05 μg/500nL | Intra-NAcC + Intra-NAcS | No restrictions | No restrictions | Intra-NAcC: both doses EX-4 reduced prime-induced reinstatement of responding compared to vehicle; intra-NAcS: 0.05 μg/500nL Ex-4 reduced prime-induced reinstatement of responding compared to vehicle. No effect of 0.005 μg/500nL Ex-4. | |
| Schmidt et al. 2016 | Between-subjects | Rats | Sprague-Dawley, male | Cocaine | SA | Acute | Ex-4 | 0.005 and 0.05 μg | intra-VTA | No restrictions | No restrictions | High dose Ex-4 reduced total active lever responses, breakpoints, and total infusions compared to vehicle. |
| Between-subjects | Rats | Sprague-Dawley, male | Cocaine | SA | Acute | Ex-4 | 0.05 μg | intra-VTA | No restrictions | No restrictions | Reduced total active lever presses, breakpoints, and total infusions on both cocaine doses, compared to vehicle. | |
| Sørensen et al. 2015 | Within-subjects (Latin Square Design) | Mice | C57BL/6, male | Cocaine | SA | Acute | Ex-4 | 10 μg/kg | i.p. | No restrictions | No restrictions | Ex-4 decreased cocaine reinforcers earned at all cocaine doses compared to vehicle. |
| Between-subjects | Mice | NMRI, male | Cocaine | SA (single session) | Acute | Ex-4 | 10, 30 and 100 μg/kg | i.p. | No restrictions | No restrictions | Decreased cocaine reinforcers earned with 30 and 100 μg/kg Ex-4compared to vehicle. No effect of 10μg/kg Ex-4. | |
| Between-subjects | Mice | NMRI, male | Cocaine | SA (single session) | Acute | Ex-4 | 30 μg/kg | i.p. | No restrictions | No restrictions | Ex-4 reduced cocaine reinforcers earned compared to vehicle. | |
| Egecioglu et al. 2013a | Between-subjects | Mice | NMRI, male | Cocaine | CPP | Acute | Ex-4 | 2.4 μg/kg | i.p. | Restricted during experiments | Restricted during experiments | Ex-4 attenuated the expression of CPP compared to vehicle. |
| Graham et al. 2012 | Between-subjects | Mice | C57BI/6J, male | Cocaine | CPP | Repeater treatments | Ex-4 | 10, 30 and 100 μg/kg | i.p. | No restrictions | No restrictions | Ex-4 attenuated the acquisition of CPP compared to vehicle. |
The dosage of Ex-4 administered i.p. varied from 2.4 μg/kg to 100 μg/kg, and four studies (7 experiments) included more than one dose. The doses administered intra-VTA were 0.05 μg and 0.005 μg respectively, with one experiment testing both doses.
All experiments but one investigated the acute effects of GLP-1-receptor agonist treatment on cocaine SUD-related behavioural effects. GLP-1-receptor agonist/vehicle was administered before access to cocaine (SA) or before a session in which the animals were placed between chambers on the post-conditioning test day (expression of CPP).
In one SA experiment (mice), nose poking was reinforced under an FR 1 schedule with 0.03 mg/kg/cocaine infusion i.v. in a single session per mouse (i.e., acquisition of cocaine SA), sessions started with one experimenter-administered infusion of cocaine. Different doses of Ex-4 were administered i.p. 90 min. before the session (Sørensen et al., 2015).
In the second SA experiment (mice), the same setup was used with different doses of cocaine (0.01, 0.03 or 0.3 mg/kg/infusion) and an Ex-4 dosage i.p. (Sørensen et al., 2015).
In the third SA experiment (mice), nose poking was reinforced with different doses of cocaine (0.03, 0.1, 0.3, 1.0, 3.2 mg/kg/infusion) under an FR 1 schedule in daily sessions, testing the effect of a dose of Ex-4 administered i.p. 60 minutes before two 3-h/day sessions for each cocaine dose (Sørensen et al., 2015).
In the fourth SA experiment (rats), lever pressing under a PR schedule of 0.75 mg/kg/infusion cocaine reinforcement was used testing Ex-4 administered intra-VTA immediately before the session (Schmidt et al., 2016).
In the fifth SA experiment (rats), the same setup as above was used with different doses of cocaine (0.19 or 1.5 mg/kg/infusion) under the PR schedule and with an Ex-4 infusion intra-VTA (Schmidt et al., 2016).
In the sixth SA experiment (rats), cocaine reinstatement was tested with lever pressing under an FR 5 schedule. After establishing and then extinguishing cocaine-taking behaviour, rats pre-treated with f-Ex-4 i.p. had an acute priming injection of cocaine (10 mg/kg i.p.) and were placed in the operant conditioning chambers for a 2-h reinstatement session in which saline was available as the reinforcer (Hernandez et al., 2018).
In the seventh SA experiment (rats), the same setup as above was used, testing different doses of f-Ex-4 (Hernandez et al., 2018).
In the eighth SA experiment (rats), the same setup as above was used, testing the effects of different doses of intra-VTA infusions of Ex-4 10 minutes before the test session (Hernandez et al., 2018).
In the ninth SA experiment (rats), the same setup as above was used, testing the effects of f-Ex-4 i.p. (1 h before the test session) after intra-VTA administration of a GLP-1-receptor antagonist/vehicle (Hernandez et al., 2018).
In the tenth SA experiment (rats), cue-induced reinstatement of cocaine seeking was tested with lever pressing under an FR 5 schedule. After extinguished cocaine-taking behaviour rats were treated with f-Ex-4 i.p. 60 min before the session, during which lever presses resulted in cue light presentation and a saline infusion (Hernandez et al., 2018).
In the eleventh SA experiment (rats), reinstatement of cocaine seeking was tested with lever pressing under an FR 5 schedule. After extinguished cocaine-taking behaviour, rats were pre-treated with f-Ex-4 i.p. 60 min before an acute priming injection of cocaine (10 mg/kg i.p.) and were placed in the operant conditioning chambers for a 2-h reinstatement session (Hernandez et al., 2019).
In the twelfth SA experiment (rats), the same setup as above was used, testing the effects of different doses of Ex-4 administered intra-NAcC and intra-NAcS 10 minutes before the test session (Hernandez et al., 2019).
In the CPP experiment (mice), a single dose of Ex-4 was administered 10 min before the post-conditioning test, after four days of 20-min conditioning sessions with 10 mg/kg i.p cocaine in the non-preferred compartment (i.e., testing effects on expression of CPP) (Egecioglu et al., 2013a).
In summary, all studies showed a significant effect of Ex-4 and f-Ex-4.
One study investigated the addicion-related effects of repeated treatments with GLP-1 receptor agonists on cocaine in one CPP experiment (Graham et al., 2013). Different doses of Ex-4 were administered 30 min before four of the eight 20-min conditioning sessions and no treatment was given before the post-conditioning test i.e. the experiment tested for an effect of GLP-1 receptor agonists on acquisition of CPP.
All doses of Ex-4 blocked the acquisition of CPP.
3.3. Effects of GLP-1-receptor agonist treatment: Amphetamine
Amphetamine:
Two of the included studies dealt with abuse-related effects of GLP-1-receptor agonist treatment on amphetamine (Table 3), with a total of two experiments, both using mice. Study types were between-subjects and the assays were CPP, both administering Ex-4 i.p. as acute treatment.
Table 3:
Characteristics of studies and assays using amphetamine as abuse substance. i.p.: intraperitoneal, Ex-4: exendin-4, CPP: conditioned place preference.
| Author | Study type | Population | Specific type of animal | Abuse substance | Type of assay | Treatment | GLP-1-recetor-agonist used | Dose of GLP-1-receptor-agonist | Administration method GLP-1-receptor-agonist | Access to food | Access to water | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sirohi et al. 2016 | Between-subjects | Mice | GLP-1R KD nestin (GLP-1R selectively ablated from CNS) and FLOX, male | Amphetamine | CPP | Acute | Ex-4 | 30 μg/kg | i.p. | No restrictions | No restrictions | EX-4 blocked the expression of CPP in mice with intact GLP-1 receptors but had no effect in GLP-1R KD mice. |
| Egecioglu et al. 2013a | Between-subjects | Mice | NMRI, male | Amphetamine | CPP | Acute | Ex-4 | 2.4 μg/kg | i.p. | Restricted during experiments | Restricted during experiments | Ex-4 attenuated the expression of CPP compared to vehicle. |
In the first CPP experiment (mice), a single dose of Ex-4 was administered 10 min before the post-conditioning test, after four days of 20-min conditioning sessions with 2.0 mg/kg amphetamine i.p. paired with the non-preferred compartment (testing effects on expression of CPP; (Egecioglu et al., 2013a).
In the second CPP experiment (mice) a dose of Ex-4 i.p. was administered using the same setup with a dose of 1.0 mg/kg amphetamine i.p. (Sirohi et al., 2016).
The amphetamine-induced CPP was significantly attenuated by a single injection of Ex-4 in both studies.
3.4. Effects of GLP-1-receptor agonist treatment: Nicotine
Nicotine:
Two of the included studies dealt with abuse-related effects of GLP-1-receptor agonist treatment on nicotine (Table 4), with a total of four experiments. All four experiments were between-subjects study type, and the assays consisted of one CPP (mice) and three SA (1 with mice, 2 with rats). In two experiments, Ex-4 was administered i.p., in two directly into the nucleus tractus solitarius (NTS). All the studies used Ex-4. All four experiments investigated the acute effect of GLP-1-receptor agonist treatment. Two of the included studies dealt with abuse-related effects of GLP-1-receptor.
In the first SA experiment (mice), lever pressing under an FR 5 schedule of 0.01 mg/kg/infusion nicotine i.v. reinforcement was used. Before the test session, Ex-4 was administered i.p. (Tuesta et al., 2017).
In the second (rats) and third (rats with intracranial electrode), SA experiments lever pressing under an FR 5 schedule of 0.03 mg/kg/infusion i.v. nicotine reinforcement was used. Before the test session, Ex-4 was administered intra-interpeduncular nucleus (IPN; (Tuesta et al., 2017).
In the CPP experiments, a single dose of Ex-4 was administered 10 min before placement between compartments on the post-conditioning test, after four days of 20-min conditioning sessions with 0.5 mg/kg nicotine i.p. paired with the non-preferred compartment (testing expression of CPP; Egecioglu et al., 2013b).
All the studies showed a significant effect of Ex-4 with a significant decrease in nicotine intake in the SA experiments, and attenuated nicotine CPP.
4. DISCUSSION
4.1. Summary of evidence
All 17 included studies found an effect of GLP-1-receptor agonist treatment on SUD-related behavioural effects of ethanol, cocaine, amphetamine or nicotine, respectively. The effect was found with all tested GLP-1-receptor agonists. Thus, confidence is increased by findings being replicated using several compounds, dosing regimens, and subject species by at least a few independent research groups. It is still a relatively small number of studies, from a limited number of groups, but the findings warrant further replication and investigation of the reported effects.
Twelve studies (Abtahi et al., 2018; Davis et al., 2012; Egecioglu et al., 2012; Graham et al., 2013; Hernandez et al., 2018, 2019; Schmidt et al., 2016; Shirazi et al., 2013; Sørensen et al., 2016, 2015; Suchankova et al., 2015; Vallöf et al., 2016) tested more than one dose of Ex-4, liraglutide, f-Ex-4, or AC3174, but only on ethanol and cocaine. A dose-related response was found in ten out of 14 experiments, suggesting that the effect is dose-related. This was also seen in regard to the washout periods, (Vallöf et al., 2016) and (Suchankova et al., 2015) found that the higher dose tested had a longer-lasting effect. This implies that it will be important to find the correct dosage if translated to human studies, to have a maximum effect of the treatment balanced with minimal adverse effects.
Most of the studies, including all experiments with amphetamine and nicotine, tested only the effects of acute GLP-1-receptor agonist administration, finding an acute abuse-related effect in 34 out of 35 experiments. Thus GLP-1 receptor agonists have acute anti-addiction-like effects in animal models using mice and rats. However, a treatment that is proven to work in an acute setting can prove ineffective or can even increase intake of the abused substance with chronic treatment (Czoty et al., 2016; Haney & Spealman, 2008; Thomsen et al., 2017). To verify a more chronic effect on SUD, the effect of repeated treatments must be tested further. Only few studies tested the sub-chronic or chronic (weeks) effect of GLP-1-receptor agonist treatment, and this was only tested on ethanol (Egecioglu et al., 2013b, 2012; Suchankova et al., 2015; Thomsen et al., 2017; Vallöf et al., 2016) and one experiment with cocaine (Graham et al., 2013). Although a continued effect was seen in all six studies, the results varied, indicating that the continued effect could be dose-related or a consequence of differences between the GLP-1-receptor agonists. Thus, the results indicate a continued effect with repeated treatments although more evidence is needed before this can be concluded more firmly. Those findings on alcohol-related effects also do not necessarily extend to other drugs of abuse, and studies are needed on the chronic effects of GLP-1 receptor agonists on SUD-related behavioural effects of e.g. stimulants or nicotine.
Three studies also tested for a prolonged effect of Ex-4, liraglutide and AC3174, respectively, observing washout periods after ended treatment, all on alcohol. Results varied but generally showed little or no effect (Suchankova et al., 2015; Thomsen et al., 2017; Vallöf et al., 2016). None of the studies reported a rebound effect (i.e., increase in alcohol intake above baseline or vehicle levels), rather, the intake returned to baseline levels after ended treatment. This is an important aspect in terms of potential usefulness of GLP-1-receptor agonists in a clinical setting, although the risk of relapse after ended treatment remains. Future research should include repeated treatments for a longer period including a continued test period after ended treatment to confirm and extend this finding across different abused substances.
Six studies (nine experiments) (Abtahi et al., 2018; Hernandez et al., 2018, 2019; Schmidt et al., 2016; Shirazi et al., 2013; Tuesta et al., 2017) tested a central administration of GLP-1-receptor agonists on the SUD-related behavioural measures (ethanol, cocaine, and nicotine) by administering infusions directly into either IPN, VTA, NAcC, or NAcS. An effect was found at all the administration sites although effects varied regarding NAcC vs. NAcS (Abtahi et al., 2018; Hernandez et al., 2019), and dose-dependent effects were reported for VTA (Hernandez et al., 2018; Schmidt et al., 2016; Shirazi et al., 2013). This suggests a centrally-mediated effect of GLP-1-receptor agonists, which is supported by a study using knock-out mice in two experiments, in which the effect of Ex-4 was eliminated in the group of mice that lacked central GLP-1 receptors but had intact peripheral GLP-1 receptors (Sirohi et al., 2016).
Most of the studies used Ex-4 as the GLP-1-receptor agonist, which makes it difficult to draw conclusions regarding possible differences between the various available GLP-1-receptor agonists. Of the five agonists tested, all showed a positive effect. Two intravenous cocaine self-administration experiments used restrained mice, but the findings were replicated in freely moving mice (Sørensen et al. 2015, therefore potential stress from restraint was not likely a factor necessary for GLP-1 analogs to have an effect. It should be noted that all studies used rodents, therefore research with non-human primates and/or humans is required to translate the findings into clinically applicable treatment strategies. Also, all but one study used exclusively male subjects, and future research should include female subjects to verify if the effect generalizes to both sexes. The best-characterized effects are on alcohol, making alcohol use disorder an attractive choice for initial translation to human research. The scarcer but positive findings on cocaine, amphetamine and nicotine generate an interesting need for further knowledge – on these substances as well as others (e.g. opioids and benzodiazepines).
4.2. Strengths and Limitations
The strengths of this systematic review include thoroughly prepared search, the use of two independent reviewers, and that the data extraction was conducted twice.
The review also has some limitations. As in all reviews, the results are limited by the amount of data possible to extract. Only 131 studies were identified, even though there were no restrictions regarding time span or population. Only animal studies were identified. Languages were restricted to English and the search was only conducted in two databases.
Overall, the studies had a high degree of heterogeneity, which limits the possibilities for comparing the studies. Further, the bias assessment indicated a high or unclear risk in some of the studies, although methodology for bias assessment in laboratory animal studies needs to be further tested for validity and reliability and may need refinement (Krauth et al., 2013). Hence, findings of this review should be considered with some caution. However, the primary outcome, characterization of the GLP-1-receptor agonist effect on SUD-related behaviours, was not prone to bias.
4.3. Conclusions
In conclusion, we found 17 studies investigating the effect of GLP-1-receptor agonists on SUD-related behaviours using ethanol, cocaine, amphetamine, and nicotine. Through different types of assays and with different doses of GLP-1-receptor agonists and abuse substances, all studies found an abuse-related effect. A dose-related response was found in most experiments investigating multiple doses. Effects of GLP-1-receptor agonists were observed both after systemic administration and after direct intracerebral administration of GLP-1-receptor agonists, suggesting that effects on SUD-related behaviours are mediated in the brain.
The few studies testing a repeated treatment all found a persistent effect, although results varied, which could be caused by differences in dosage or GLP-1-receptor agonist. The few studies testing a prolonged effect after ended treatment also had varied results, but none found a rebound effect.
A more detailed knowledge of the chronic and prolonged effects and of the different GLP-1 receptor agonists, as well as the effect on other abused substances, i.e., cocaine, amphetamine, nicotine, opioids, and benzodiazepines, should be investigated. Based on the results presented in this review, it is warranted to test the effects of GLP-1 receptor agonists in patients with SUD. At present, we are performing such a study in patients with alcohol use disorder (Antonsen et al., 2018).
HIGHLIGHTS:
Documented acute effects of GLP-1-receptor agonists on drugs, alcohol, nicotine
All studies found an effect on SUD-related measures
Fewer studies tested chronic dosing but all found sustained effects of treatment
A few studies used intracerebral administration, suggest centrally mediated effect
Data lacking on opioids, benzodiazepines, cannabinoids
Funding:
MT was supported by the Psychiatric Center Copenhagen Research Foundation and by grant R01AA025071 from the National Institutes of Health while working on the manuscript.
Footnotes
Conflicts of interest:
The authors declare no competing financial interests in relation to the work.
REFERENCES
- Abtahi S, Howell E, & Currie PJ (2018). Accumbal ghrelin and glucagon-like peptide 1 signaling in alcohol reward in female rats. NeuroReport, 29(12), 1046–1053. 10.1097/WNR.0000000000001071 [DOI] [PubMed] [Google Scholar]
- Alhadeff AL, Rupprecht LE, & Hayes MR (2012). GLP-1 Neurons in the Nucleus of the Solitary Tract Project Directly to the Ventral Tegmental Area and Nucleus Accumbens to Control for Food Intake. Endocrinology, 153(2), 647–658. 10.1210/en.2011-1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Tahir B, Jabeen S, & Malik M (n.d.). Methadone Treatment of Opiate Addiction: A Systematic Review of Comparative Studies. Innovations in Clinical Neuroscience, 14(7–8), 8–19. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/29616150 [PMC free article] [PubMed] [Google Scholar]
- Antonsen KK, Klausen MK, Brunchmann AS, le Dous N, Jensen ME, Miskowiak KW, … Fink-Jensen A (2018). Does glucagon-like peptide-1 (GLP-1) receptor agonist stimulation reduce alcohol intake in patients with alcohol dependence: study protocol of a randomised, double-blinded, placebo-controlled clinical trial. BMJ Open, 8(7), e019562 10.1136/bmjopen-2017-019562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, & Trapp S (2015). Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Molecular Metabolism, 4(10), 718–731. 10.1016/j.molmet.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, & Rush CR (2016). Evaluation of the "Pipeline" for Development of Medications for Cocaine Use Disorder: A Review of Translational Preclinical, Human Laboratory, and Clinical Trial Research. Pharmacological Reviews, 68(3), 533–562. 10.1124/pr.115.011668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar S, Tahrani AA, & Piya MK (2015). The role of GLP-1 receptor agonists as weight loss agents in patients with and without type 2 diabetes. Practical Diabetes, 32(8), 297–300b. 10.1002/pdi.1978 [DOI] [Google Scholar]
- Davis JF, Schurdak JD, Magrisso IJ, Mul JD, Grayson BE, Pfluger PT, … Benoit SC (2012). Gastric bypass surgery attenuates ethanol consumption in ethanol-preferring rats. Biological Psychiatry, 72(5), 354–360. 10.1016/j.biopsych.2012.01.035 [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Habener JF, & Holst JJ (2017). Discovery, characterization, and clinical development of the glucagon-like peptides. Journal of Clinical Investigation, 127(12), 4217–4227. 10.1172/JCI97233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, & Jerlhag E (2013a). The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PloS One, 8(7), e69010 10.1371/journal.pone.0069010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, & Jerlhag E (2013b). The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PloS One, 8(10), e77284 10.1371/journal.pone.0077284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, & Jerlhag E (2012). The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology, 38(8), 1259–1270. 10.1016/j.psyneuen.2012.11.009 [DOI] [PubMed] [Google Scholar]
- Göke R, Larsen PJ, Mikkelsen JD, & Sheikh SP (1995). Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. The European Journal of Neuroscience, 7(11), 2294–2300. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8563978 [DOI] [PubMed] [Google Scholar]
- Graham DL, Erreger K, Galli A, & Stanwood GD (2013). GLP-1 analog attenuates cocaine reward. Molecular Psychiatry, 18(9), 961–962. 10.1038/mp.2012.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, & Spealman R (2008). Controversies in translational research: drug self-administration. Psychopharmacology, 199(3), 403–419. 10.1007/s00213-008-1079-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner KM, Kirigiti M, Secher A, Paulsen SJ, Buckingham R, Pyke C, … Grove KL (2015). Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology, 156(1), 255–267. 10.1210/en.2014-1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez NS, Ige KY, Mietlicki-Baase EG, Molina-Castro GC, Turner CA, Hayes MR, & Schmidt HD (2018). Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology, 43:2000–2008. 10.1038/s41386-018-0010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez NS, O’Donovan B, Ortinski PI, & Schmidt HD (2019). Activation of glucagon-like peptide-1 receptors in the nucleus accumbens attenuates cocaine seeking in rats. Addiction Biology, 24:170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ (2013). Incretin hormones and the satiation signal. International Journal of Obesity, 37(9), 1161–1168. 10.1038/ijo.2012.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijmans CR, Rovers MM, Bm De Vries R, Leenaars M, Ritskes-Hoitinga M, & Langendam MW (2014). SYRCLE’s risk of bias tool for animal studies. 10.1186/1471-2288-14-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E (2018). GLP-1 signaling and alcohol-mediated behaviors; preclinical and clinical evidence. Neuropharmacology 10.1016/j.neuropharm.2018.01.013 [DOI] [PubMed] [Google Scholar]
- Kalkhoran S, Benowitz NL, & Rigotti NA (2018). Prevention and Treatment of Tobacco Use: JACC Health Promotion Series. Journal of the American College of Cardiology, 72(9), 1030–1045. 10.1016/j.jacc.2018.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauth D, Woodruff TJ, & Bero L (2013). Instruments for assessing risk of bias and other methodological criteria of published animal studies: a systematic review. Environmental Health Perspectives, 121(9), 985–992. 10.1289/ehp.1206389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, & Shughrue P (1999). Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. The Journal of Comparative Neurology, 403(2), 261–280. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9886047 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & PRISMA Group. (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International Journal of Surgery (London, England), 8(5), 336–341. 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, … Hayes MR (2016). Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 41(7), 1917–1928. 10.1038/npp.2015.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi RH, Dickson SL, Skibicka KP, & Caramelli D (2013). Gut Peptide GLP-1 and Its Analogue, Exendin-4, Decrease Alcohol Intake and Reward. PLoS ONE, 8(4). 10.1371/journal.pone.0061965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Schurdak JD, Seeley RJ, Benoit SC, & Davis JF (2016). Central & peripheral glucagon-like peptide-1 receptor signaling differentially regulate addictive behaviors. Physiology & Behavior, 161, 140–144. 10.1016/j.physbeh.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Sørensen G, Caine SB, & Thomsen M (2016). Effects of the GLP-1 Agonist Exendin-4 on Intravenous Ethanol Self-Administration in Mice. Alcoholism, Clinical and Experimental Research, 40(10), 2247–2252. 10.1111/acer.13199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G, … Fink-Jensen A (2015). The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiology & Behavior, 149, 262–268. 10.1016/j.physbeh.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M (2017). Treatment of opioid dependence with buprenorphine: current update. Dialogues in Clinical Neuroscience, 19(3), 299–308. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/29302227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, & Müller CA (2017). Pharmacotherapy of alcoholism - an update on approved and off-label medications. Expert Opinion on Pharmacotherapy, 18(12), 1187–1199. 10.1080/14656566.2017.1349098 [DOI] [PubMed] [Google Scholar]
- Suchankova P, Yan J, Schwandt ML, Stangl BL, Caparelli EC, Momenan R, … Leggio L (2015). The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Translational Psychiatry, 5(6), e583 10.1038/tp.2015.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Butler P, Negus SS, & Caine SB (2017). Effects of Acute and Chronic Treatments with Dopamine D 2 and D 3 Receptor Ligands on Cocaine versus Food Choice in Rats. Journal of Pharmacology and Experimental Therapeutics, 362(1), 161–176. 10.1124/jpet.117.241141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Dencker D, Wörtwein G, Weikop P, Egecioglu E, Jerlhag E, … Molander A (2017). The glucagon-like peptide 1 receptor agonist Exendin-4 decreases relapse-like drinking in socially housed mice. Pharmacology Biochemistry and Behavior, 160, 14–20. 10.1016/j.pbb.2017.07.014 [DOI] [PubMed] [Google Scholar]
- Tuesta LM, Chen Z, Duncan A, Fowler CD, Ishikawa M, Lee BR, … Kenny PJ (2017). GLP-1 acts on habenular avoidance circuits to control nicotine intake. Nature Neuroscience, 20(5), 708–716. 10.1038/nn.4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDOC. (2018). World drug report. Analysis of drug markets. Trends in Organized Crime (Vol. 3). Retrieved from https://www.unodc.org/wdr2018/ [Google Scholar]
- Vallöf D, Maccioni P, Colombo G, Mandrapa M, Jörnulf JW, Egecioglu E, … Jerlhag E (2016). The glucagon-like peptide 1 receptor agonist liraglutide attenuates the reinforcing properties of alcohol in rodents. Addiction Biology, 21(2), 422–437. 10.1111/adb.12295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO | Global status report on alcohol and health 2014. (2016). WHO. Retrieved from http://www.who.int/substance_abuse/publications/global_alcohol_report/en/
- WHO | Information sheet on opioid overdose. (2018). WHO. Retrieved from http://www.who.int/substance_abuse/information-sheet/en/
- WHO | WHO report on the global tobacco epidemic 2017. (2017). WHO. Retrieved from http://www.who.int/tobacco/global_report/2017/en/