Abstract
Background.
Cognitive decline after cardiac surgery occurs frequently and persists in a significant proportion of patients. Preclinical studies and human trials suggest that intravenous lidocaine may confer protection in the setting of neurologic injury. We hypothesized that lidocaine administration would reduce cognitive decline after cardiac surgery compared to placebo.
Methods.
Following IRB approval, 478 patients undergoing cardiac surgery were enrolled into this multi-center, prospective, randomized, double-blinded, placebo-controlled, parallel group trial. Subjects were randomized to lidocaine 1 mg/kg bolus after the induction of anesthesia followed by a continuous infusion [48 mcg/kg/min for the first hour, 24 mcg/kg/min for the second hour, and 10 mcg/kg/min for the next 46 hours] or saline with identical volume and rate changes to preserve blinding. Cognitive function was assessed preoperatively and at 6 weeks and 1 year postoperatively using a standard neurocognitive test battery. The primary outcome was change in cognitive function between baseline and 6 weeks postoperatively, adjusting for age, years of education, baseline cognition, race, and procedure type.
Results.
Among the 420 allocated subjects who returned for 6-week follow-up (lidocaine: N=211; placebo: N=209), there was no difference in the continuous cognitive score change (adj. mean difference (95% CI) 0.02 (−0.05, 0.08); p=0.626). Cognitive deficit (>1 standard deviation decline in at least 1 cognitive domain) at 6-weeks occurred in 41% (87/211) in the lidocaine group vs. 40% (83/209) in the placebo group (adj. OR (95% CI) 0.94 (0.63, 1.41); p=0.766). There were no differences in any quality of life outcomes between treatment groups. At the 1-year follow-up there continued to be no difference in cognitive score change, cognitive deficit, or quality of life.
Conclusions.
Intravenous lidocaine administered during and after cardiac surgery did not reduce postoperative cognitive decline at 6 weeks.
9. Summary Statement
In a large cohort of non-diabetic cardiac surgical patients, intravenous lidocaine failed to alter cognitive outcomes at 6 weeks after surgery.
Introduction
Neurocognitive impairment occurs frequently in the increasingly elderly patient population undergoing cardiac surgery. At the time of hospital discharge, up to 50% of cardiac surgical patients may experience postoperative cognitive dysfunction (POCD)1. Despite a general improvement in the initial months following surgery, cognitive dysfunction may persist up to 5 years postoperatively1, resulting significant in loss of functional independence and reduced quality of life.2
The mechanisms underlying POCD after cardiac surgery are not yet fully understood, and, despite the investigation of several possibilities3–9, a potential preventative or therapeutic agent remains elusive. Previous clinical research has reported a beneficial effect of the drug lidocaine on POCD after both cardiac and non-cardiac surgery.10–12 Lidocaine is a class 1B antiarrhythmic and local anesthetic that crosses the blood-brain barrier. Potential mechanisms for a neuroprotective effect of lidocaine have been postulated to include reduced cerebral metabolism13, deceleration of ischemic ion fluxes14,15, preservation of cerebral blood flow16, and modulation of inflammatory mediators17–21. Based on these potential mechanisms, lidocaine would appear to be an ideal drug for preventing the biphasic pattern of neurologic injury by both 1) maintaining cerebral blood flow and preventing adverse ion flux during ischemia and 2) ameliorating the secondary inflammatory changes associated with reperfusion. Data published by our group further suggested that intravenous lidocaine administered intraoperatively and for 48-hours might have a protective effect against postoperative cognitive dysfunction in non-diabetic cardiac surgical patients.22 Notably, in this previous study, diabetic subjects receiving lidocaine were more likely to suffer cognitive decline, possibly because of higher lidocaine doses and altered lidocaine metabolism.22
Based on these preclinical and clinical data, we hypothesized that the administration of intravenous lidocaine intraoperatively and postoperatively for a total of 48 hours would reduce the incidence of postoperative cognitive dysfunction in non-diabetic patients at 6 weeks after cardiac surgery with cardiopulmonary bypass compared to placebo (i.e., superiority). We secondarily assessed neurocognitive outcomes at 1-year postoperatively along with quality of life measures at both postoperative time points.
Materials and Methods
Study Population
Subsequent to approval by respective Institutional Review Boards and informed consent, 478 adults ≥ 50 years of age and scheduled to undergo coronary artery bypass grafting (CABG), valve surgery, or CABG plus valve surgery with cardiopulmonary bypass (CPB) were enrolled into this multi-center, prospective, randomized, double-blinded, placebo controlled, parallel group clinical trial between July 2009 and June 2016. Subjects were enrolled at Duke University Health System (N=448), Cornell Weil Medical Center (N=2), and Sentara Norfolk General Hospital (N=28). Prospective patients were approached by study staff. Patients were excluded if the procedure included planned circulatory arrest or if patients had a history of diabetes mellitus (documented history, elevated fasting blood glucose, or hemoglobin A1c ≥ 6.5%), symptomatic cerebrovascular disease (e.g., prior stroke) with residual deficit, higher alcohol consumption (>2 drinks per day), drug abuse (any illicit drug use in the past 3 months), psychiatric illness (any clinical diagnosis requiring therapy), renal failure (serum creatinine >2 mg/dL), hepatic insufficiency (liver function tests >1.5 times the upper limit of normal), severe pulmonary insufficiency (requiring home oxygen therapy), left ventricular ejection fraction <20%, preoperative intraaortic balloon pump requirement, liver/heart/lung transplant, current pregnancy, and those who were unable to read and thus complete the cognitive testing or who scored <24 on the baseline Mini Mental State examination or ≥27 on the baseline Center for Epidemiologic Studies Depression (CES-D) Scale. Informed consent was obtained by study staff.
Subjects were randomized to two treatment groups: 1) lidocaine group: bolus of 1 mg/kg of lidocaine administered after induction of anesthesia and followed immediately by a continuous infusion at 48 mcg/kg/min for the first hour, 24 mcg/kg/min for the second hour, and 10 mcg/kg/min for the next 46 hours; or 2) placebo group: normal saline administered as a bolus and an infusion for 48 hours with identical volume and rate changes as the treatment group such that blinding of both the patient and study/clinical team was preserved. This weight-based lidocaine infusion regimen is based on previous pharmacokinetics work by our group to determine the optimal infusion strategy to maintain therapeutic lidocaine levels while avoiding potentially toxic levels (>5 mcg/mL) in patients undergoing CPB.23 A group assignment schedule was prepared for each site using Nquery software version 7 (SAS, Cary, NC, USA) by the study statistician and stored in consecutively numbered sealed envelopes until allocation by study staff.
Patient Management
Anesthesia was induced with midazolam, fentanyl, and propofol, and isoflurane was used for maintenance. All patients underwent nonpulsatile, hypothermic (30°C - 32°C) CPB with a membrane oxygenator (Prim-O2X, Sorin Group Inc., USA; Synthesis, Sorin Group Inc., USA; or Terumo FX15 or FX25, Terumo Inc., USA) and arterial line filter by a centrifugal pump (Revolution pump head and S5 machine, Sorin Group Inc., USA) primed with crystalloid and using bio-active tubing (SMART-X, Sorin Group Inc., USA). Serial hematocrit levels were maintained at ≥0.21. Before initiating CPB, heparinization (300 – 400 U/kg) was performed to a target activated coagulation time of >480 s. Perfusion was maintained at flow rates of 2 – 2.4 L/min/m2 throughout CPB to maintain a mean arterial pressure of 50 – 80 mmHg. Arterial blood gases were measured every 15 – 30 min to maintain the PaCO2 at 35 – 40 mmHg unadjusted for temperature and the PaO2 at 150 – 250 mmHg. Cell salvage (BRAT, Cobe CV Inc., USA or Elite, Haemonectics Inc., USA) was utilized for the majority of cases.
Plasma lidocaine levels were sampled at baseline (prior to bolus), at end of CPB, and at 24 and 48 hours after bolus; analysis was performed by the Duke University Clinical Laboratories. Results were made available by fax only to a study team member in order to monitor lidocaine toxicity; plasma levels > 5 mcg/ml required discontinuation of the study drug. Blinding of the patient, medical care teams, and neurocognitive testers was preserved at all times. If the study drug was discontinued because of high lidocaine levels, the patient and the medical care team were no longer blinded (n=1).
Neurocognitive Testing
Neurocognitive testing was performed at baseline (preoperatively), 6 weeks, and 1 year after surgery by experienced psychometricians blinded to treatment group. In accordance with the consensus statement on assessment of neurobehavioral outcomes after cardiac surgery24, the following tests were included in the assessment battery: 1) Hopkins Verbal Learning Test25, 2) Randt Short Story Memory Test26, 3) Modified Visual Reproduction Test from the Wechsler Memory Scale27, 4) Digit Span and Digit Symbol and Vocabulary subtests from the Wechsler Adult Intelligence Scale-Revised27, and 5) Trail Making Test, Parts A and B28.
Quality of Life and Neurologic Outcomes
To assess health-related quality of life outcomes, the following battery was administered at baseline, 6 weeks, and 1 year postoperatively: 1) Duke Activity Status Index (DASI) – a measure of functional status derived for use in cardiovascular populations, 2) Center for Epidemiologic Studies Depression Scale (CES-D), 3) the State Trait Anxiety Inventory (STAI), 4) the Hopkins Symptom Checklist (SCL-90), 5) the Short Form 36 (SF 36), 6) Symptom Limitations Scale, 7) Duke Older American Resources and Services Procedures – Instrumental Activities of Daily Living (OARS-IADL), 8) Cognitive Difficulties Scale, 9) Perceived Social Support Scale, and 10) Social Anxiety Scale.
Change in neurologic outcomes was assessed using the National Institutes of Health Stroke Scale (NIHSS) and the Western Perioperative Neurologic Scale (WPNS) and comparing baseline values to 6-week and 1-year postoperative follow-up values.
Statistical Analyses
To characterize cognitive function over time while minimizing potential redundancy in the cognitive measures, a factor analysis with oblique rotation (a linear transformation of the data, which allows for correlated factors) was performed on the 14 cognitive test scores from baseline. Scoring coefficients (weights) of each test on each factor were determined using the rotated factor solution from the factor analysis conducted on 452 study patients with completely observed cognitive test scores at the baseline time point. Factors of each patient at all time points were computed using the same scoring coefficients, so that the cognitive domain structure remained consistent and comparable over time. The number of factors was determined based on cumulative variance explained of at least 80%, examination of scree plot for elbow location, and previous experience with this cognitive battery. Factor analysis suggested a 5-factor solution, which accounted for 80% of the variability in the original test scores and represents 5 cognitive domains: 1) structured verbal memory (i.e., the ability to recall from a list), 2) unstructured verbal memory (i.e., the ability to remember from a narrative), 3) visual memory, 4) executive function, and 5) attention and concentration. Two outcome measures were calculated to represent postoperative cognitive function: 1) continuous outcome: the change in cognitive score calculated by subtracting the baseline cognitive index (the 5-domain mean) from the follow-up cognitive index (a change score of 0 indicates no change from baseline while a negative score indicates cognitive decline and a positive score indicates cognitive improvement); 2) binary outcome (cognitive deficit): defined as a decline of >1 standard deviation in at least 1 domain from before to after surgery.
Categorical and continuous demographic characteristics were compared between treatment groups with two-sided Pearson Chi-Square, Fisher Exact, independent t-tests and Wilcoxon rank sum tests. Normality was assessed via Shapiro-Wilks tests and if evidence of non-normality was present non-parametric analysis was performed. The effect of lidocaine treatment on the cognitive change score was tested using an a priori defined multivariable linear regression modeling accounting for known risk factors of age, years of education, baseline cognition, and procedure type. The following potentially influential covariates were examined for balance between the treatment groups: sex, race, duration of surgery and bypass time, depression, and anxiety. If any clinically important group differences on covariates were identified, we added those factors to the multivariable model to reduce potential confounding of treatment effect estimates. Interactions between treatment group and each of the covariates were also examined. Similarly, the effect of lidocaine on cognitive deficit was tested using multivariable logistic regression accounting age, years of education, baseline cognition, and procedure type, as well as any factor found to be out of balance despite randomization. All analyses were performed with SAS® version 9.4 (SAS, Cary, NC, USA); p < 0.05 was considered significant.
The primary outcome was change in cognitive function – as both continuous cognitive change score and the binary outcome of cognitive deficit - between baseline and 6 weeks postoperatively as assessed by multivariable linear regression adjusted for a priori defined covariates representing known risk factors for cognitive decline. Secondary outcomes included change in cognitive function at 1 year postoperatively and 6-week and 1-year changes in quality of life and neurologic outcomes compared between treatment groups. We also investigated adverse event rates and length of stay differences between the treatment groups.
For patients returning at follow-up time points but missing test scores required for determination of primary or secondary endpoints, we pursued multiple imputation based on all collected patient and surgical factors as well as observed cognitive, quality of life and neurological test scores (imputation performed in SRCWare V 0.2).29 We created 10 imputation datasets and used standard methods to pool across imputed sets.30 The primary analyses were conducted according to the intention-to-treat principle. We conducted 4 types of sensitivity analyses to assess the impact of analysis strategies on our conclusions as follows: 1) on per-protocol cases to assess impact of protocol deviations (inadvertent bolus lidocaine given on induction of anesthesia [n=8], unblinding and administration of lidocaine for arrhythmia treatment [n=5], termination of study drug for elevated serum lidocaine level [n=1], physician request to terminate study drug [n=2], and error in study drug administration e.g., interruption in infusion during transfer from intensive care unit to step-down unit [n=12]), 2) on complete cases, 3) on the full set of eligible randomized patients to assess impact of imputation strategy and dropout, and 4) by assigning the worst cognitive performance scores to patients who had suffered an early postoperative stroke and thus lacked 6-week neurocognitive testing to assess impact of unobserved outcomes for patients with expected cognitive impairment. For sensitivity analysis #3 we created a new set of multiple imputation data sets including all randomized and treated patients who met inclusion criteria using the same methods and inputs as we did for the primary analysis.
In our previous work, the lidocaine treatment group showed a change in cognitive index of 0.16 compared to a placebo group change of 0.09, with a common standard deviation of 0.25. Based on these data, we estimated that a sample size of 202 patients in each group would have 80% power to detect a difference in means at alpha=0.05, with two-sided significance level. Assuming a loss to follow-up of 15%, we planned to enroll 476 patients to achieve an analysis sample of 404.
This trial was conducted in accordance with the original trial protocol as registered on clincialtrials.gov (NCT00938964). No changes to the outcome definitions were made during or after completion of the trial; however, the registration of the trial outcomes on clincialtrials.gov was revised on several occasions in response to changes in reporting requirements.
Results
From July 6, 2009 to June 2, 2016, a total of 550 patients were consented to participate in the study, and 478 met all inclusion criteria and were randomized (Figure 1), with 237 allocated to placebo and 241 allocated to lidocaine treatment. Fifty-eight patients met exclusion criteria or were lost to follow-up after randomization, leaving 209 placebo patients and 211 lidocaine patients for evaluation at 6 weeks postoperatively. A further 43 patients were lost to follow-up between 6 weeks and 1 year postoperatively, leaving 192 placebo and 185 lidocaine patients for evaluation at 1 year postoperatively.
Figure 1.
CONSORT diagram showing flow of study participants.
Demographic and clinical characteristics of the randomized subjects are listed in Table 1. Despite randomization, the lidocaine group had a statistically higher proportion of Caucasian patients, a lower ejection fraction, and lower years of education compared to placebo patients; all other characteristics were similar between treatment groups. Supplemental Tables 1 and 2 describe the demographic characteristics of patients lost to follow-up at the 6-week and 1- year time points.
Table 1.
Cohort summary and randomization group comparison of patients returning at 6-weeks.
| Lidocaine (N=211) | Placebo (N=209) | |
|---|---|---|
| Age, years (SD) | 67 (9.1) | 67 (9.5) |
| Gender, N (% Female) | 60 (28.4) | 49 (23.4) |
| Race, N (% Caucasian) | 200 (94.8) | 186 (89.0) |
| Weight, kg (SD) | 83.8 (16.5) | 82.8 (17.4) |
| History of hypertension, N (%) | 124 (59.0) | 128 (61.2) |
| Previous myocardial infarction, N (%) | 31 (14.7) | 38 (18.2) |
| History of atrial fibrillation, N (%) | 56 (26.5) | 50 (23.9) |
| History of neurological incident | 23 (10.9) | 19 (9.1) |
| Ejection fraction (Q1, Q3) | 55 (50, 55) | 55 (55, 60) |
| Ejection fraction < 30%, N (%) | 2 (0.9) | 5 (2.4) |
| Years of education (Q1, Q3) | 15 (12, 16) | 16 (13, 17) |
| Preoperative statin use, N (%) | 121 (57.3) | 131 (62.7) |
| Preoperative platelet inhibitor use, N (%) | 149 (70.6) | 150 (71.8) |
| Preoperative cognitive index (SD)* | 0.02 (0.7) | 0.01 (0.7) |
| Lidocaine level at baseline, mcg/mL (SD) + | 0.09 (0.3) | 0.06 (0.2) |
| Baseline CES-D (SD) * | 9.0 (6.7) | 8.4 (6.8) |
| Baseline STAI (SD) * | 36.7 (11.4) | 36.2 (10.7) |
| Surgical Procedure, N (%) | ||
| CABG | 61 (28.9) | 62 (29.7) |
| CABG + Valve | 21 (10.0) | 23 (11.0) |
| Valve | 129 (61.1) | 124 (59.3) |
| Previous CABG/Valve Surgery, N (%) | 24 (11.4) | 24 (11.5) |
| CPB time, min (Q1, Q3) | 157 (127, 198) | 166 (124, 214) |
| Cross-clamp time, min (Q1, Q3) | 100 (74, 120) | 101 (67, 121) |
| Number of bypass vessels (Q1, Q3) ‡ | 3 (2, 4) | 3 (2, 4) |
Missing data imputed using SRCWARE and combined statistics presented here were generated using SAS proc MIANALYZE.
Baseline values are non-zero numbers because the analytical measurement range of the lidocaine assay is 0.6 – 8.0 mcg/mL, thus values below this linear range (including 0 values) may be reported as <0.6 mcg/mL.
Among those that received grafts.
Data are reported as mean (SD) or median (Q1, Q3) as appropriate.
CES-D: Center for Epidemiologic Studies – Depression Scale, STAI: State Trait Anxiety Inventory, CPB: cardiopulmonary bypass.
6-Week Change in Cognition (Primary Outcome)
Among the 420 subjects who returned for follow-up testing, the continuous cognitive change score was not significantly different between the treatment groups [mean (standard deviation, SD) - lidocaine: 0.074 (0.32) vs. placebo: 0.072 (0.37); p=0.957]. Cognitive deficit at 6 weeks after surgery was present in 41% (87/211) of subjects randomized to lidocaine and in 40% (83/209) of subjects randomized to placebo (p=0.700). Multivariable analysis accounting for the covariable effects of age, years of education, baseline cognition, Caucasian race, and procedure type found similar results [adj. mean difference (95% CI) between groups: 0.02 (−0.05, 0.08); p=0.626] (Table 2). Similarly, logistic regression analysis of the binary POCD outcome indicated no treatment difference [adj. odds ratio (95% CI): 0.94 (0.63, 1.41); p=0.766] (Table 2). There was no evidence of any significant interaction effects between treatment and covariates on either cognitive index change or binary POCD outcomes. As expected, lidocaine levels were significantly higher in the lidocaine group and without achieving toxic levels (Figure 2) at all post-bolus measurement points [mean (95% CI) serum lidocaine levels: end-bypass, placebo 0.02 mcg/mL (0 – 0.03) vs. lidocaine 1.82 mcg/mL (1.75 – 1.89); 24 h post-bypass, placebo 0.03 mcg/mL (0 – 0.06) vs. lidocaine 2.18 mcg/mL (2.08 – 2.28); 48 h post-bypass, placebo 0.01 mcg/mL (0 – 0.01) vs. lidocaine 2.86 mcg/mL (2.71 – 3.01)].
Table 2.
Multivariable linear and logistic regression predicting cognitive change and cognitive deficit, respectively, at 6-weeks postoperatively.
| Cognitive Change Index | Cognitive Deficit | |||
|---|---|---|---|---|
| Variable | Parameter Estimate (95% CI) | P-Value | Odds Ratio (95% CI) | P-Value |
| Age (per year) | −0.01 (−0.02, −0.01) | <0.001 | 1.05 (1.02–1.08) | 0.001 |
| Years of Education (per year) | 0.01 (−0.003, 0.02) | 0.127 | 0.88 (0.82–0.96) | 0.002 |
| Preoperative cognitive index | −0.17 (−0.22, −0.11) | <0.001 | 1.45 (0.98–2.13) | 0.063 |
| Caucasian race | −0.05 (−0.17, 0.07) | 0.419 | 1.70 (0.73–3.99) | 0.222 |
| Valve vs. CABG | −0.07 (−0.14, 0.001) | 0.054 | 1.39 (0.87–2.20) | 0.168 |
| CABG+Valve vs. CABG | −0.09 (−0.20, 0.02) | 0.122 | 1.74 (0.84–3.58) | 0.134 |
| Lidocaine treatment | 0.02 (−0.05, 0.08) | 0.626 | 0.94 (0.63, 1.41) | 0.766 |
Figure 2.
Serum concentration of lidocaine (mean, 95% CI) at the four measurement timepoints.
1-Year Change in Cognition
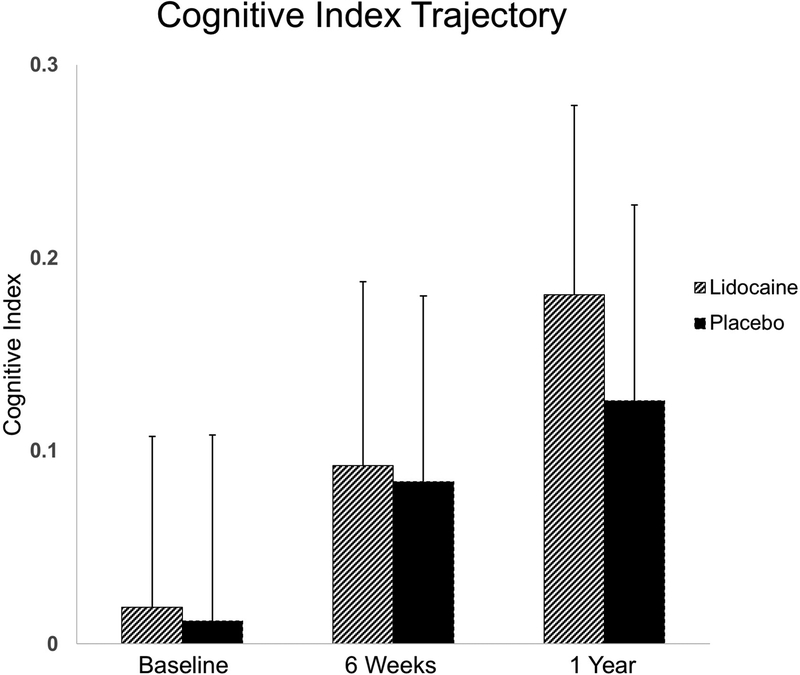
At one year after surgery, 377 subjects underwent cognitive testing. The continuous cognitive change score was not significantly different between the treatment groups [mean (SD) – lidocaine: 0.09 (0.34) vs. placebo: 0.07 (0.34); p=0.471]. While we observed a lower rate of cognitive deficit in lidocaine subjects compared to placebo subjects the difference failed to reach statistical significance (48.1% vs 56.8%, p=0.092). Multivariable linear regression adjusting for age, years of education, baseline cognition, Caucasian race, and procedure type revealed no difference in outcome between treatment groups [adj. mean difference (95% CI) between groups: 0.04 (−0.03, 0.10); p=0.255] (Table 3). Logistic regression analyses of the binary outcome also indicated a nonsignificant reduction in risk [adj. odds ratio (95% CI): 0.69 (0.45, 1.04); p=0.077]. Again, there was no evidence of any significant interaction effects between treatment and covariates on cognitive index change or binary POCD outcomes. The trajectory of postoperative cognition over time did not differ between treatment groups (Figure 3).
Table 3.
Multivariable linear and logistic regression predicting cognitive change and cognitive deficit, respectively, at 1-year postoperatively.
| Cognitive Change Index | Cognitive Deficit | |||
|---|---|---|---|---|
| Variable | Parameter Estimate (95% CI) | P-Value | Odds Ratio (95% CI) | P-Value |
| Age (per year) | −0.01 (−0.02, −0.01) | <0.001 | 1.03 (1.00, 1.06) | 0.023 |
| Years of education (per year) | 0.02 (0.01, 0.03) | <0.001 | 0.95 (0.88, 1.02) | 0.190 |
| Preoperative cognitive index | −0.20 (−0.26, −0.14) | <0.001 | 1.13 (0.76, 1.68) | 0.553 |
| Caucasian race | 0.02 (−0.11, 0.15) | 0.725 | 0.70 (0.30, 1.63) | 0.410 |
| Valve vs. CABG | −0.07 (−0.14, 0.004) | 0.065 | 0.93 (0.58, 1.49) | 0.775 |
| CABG+Valve vs. CABG | −0.04 (−0.16, 0.08) | 0.471 | 0.95 (0.44, 2.05) | 0.893 |
| Lidocaine treatment | 0.04 (−0.03, 0.10) | 0.255 | 0.69 (0.45, 1.04) | 0.077 |
Figure 3.
Cognitive index trajectory (mean, 95% CI) from baseline to 6-weeks and 1-year postoperatively.
Domain-specific cognitive scores for 6 weeks and 1 year are provided in Supplemental Table 3.
Rate of Adverse Events
Adverse events were not significantly different between treatment groups; among the randomized patients serious adverse events were recorded in 43.6% of lidocaine and 43.5% of placebo subjects (p=0.981). Nearly all of the adverse events occurred during the index surgical hospitalization: in-hospital adverse events occurred in 41.9% of lidocaine patients and 41.8% of placebo patients compared to post-hospital adverse events which occurred in 4.6% each of lidocaine and placebo patients. Stroke occurred prior to discharge in 2 patients in the lidocaine group and 6 patients in the placebo group (p=0.173): 6 were embolic/ischemic, 1 was hemorrhagic, and 1 was a transient ischemic attack. The rate of atrial fibrillation was 31.1% in the lidocaine group compared to 31.7% in the placebo group (p=0.902). Among randomized patients, the length of hospital stay was 6 days [IQR: 5, 7] in the lidocaine group and 6 days [IQR: 5, 8] in the placebo group (p=0.737). In-hospital and 1-year mortality rates were not different between lidocaine and placebo groups (1.2% vs. 0.0%, p=0.249; and 2.5% vs. 3.0%, p=0.755, respectively). All serious adverse events are outlined in Supplemental Table 4.
Quality of Life and Neurologic Outcomes
Comparisons of the various quality of life and neurologic outcomes are summarized for the 6-week (Table 4) and 1-year (Table 5) postoperative time points. There were no differences in any of the outcomes between patients who received lidocaine and placebo.
Table 4.
Summary of 6-week change scores in quality of life and neurological outcomes measures.
| Variable | Lidocaine (mean ± SD) | Placebo (mean ± SD) | P-Value* | |
|---|---|---|---|---|
| Quality of Life | CESD (−) | 0.57 ± 7.5 | 0.16 ± 8.3 | >0.999 |
| DASI (+) | −10.98 ± 15.4 | −11.67 ± 16.8 | >0.999 | |
| STAI (−) | −7.12 ± 12.1 | −6.31 ± 11.4 | >0.999 | |
| Symptoms Limitation (−) | −0.67 ± 4.0 | −0.80 ± 3.9 | >0.999 | |
| OARS-IADL (−) | 2.46 ± 4.2 | 2.10 ± 3.9 | >0.999 | |
| Cognitive Difficulties (−) | −3.00 ± 14.6 | −3.21 ± 15.9 | >0.999 | |
| Social Activities (−) | 0.95 ± 3.4 | 1.59 ± 3.2 | 0.084 | |
| Social Support (+) | 1.23 ± 14 | −0.49 ± 13.9 | 0.814 | |
| Work Activities (−) | 2.71 ± 4.9 | 3.00 ± 4.3 | >0.999 | |
| General Health Perception (−) | −0.004 ± 1.3 | −0.03 ± 1.2 | >0.999 | |
| Neurologic Outcomes | NIHSS (−) | 0.05 ± 0.5 | 0.04 ± 0.5 | >0.999 |
| WPNS (+) | 0.04 ± 1.1 | −0.01 ± 1.3 | >0.999 | |
A minus sign (−) indicates that a lower score is better; a plus sign (+) indicates that a higher score is better. CESD: Center for Epidemiologic Studies – Depression Scale, DASI: Duke Activity Status Index, STAI: State Trait Anxiety Inventory, OARS-IADL: Duke Older American Resources Services Procedures – Instrumental Activities of Daily Living, NIHSS: National Institutes of Health Stroke Scale, WPNS: Western Perioperative Neurologic Scale.
Adjusted for multiple comparisons.
Table 5.
Summary of 1-year change scores in quality of life and neurological outcomes measures.
| Variable | Lidocaine (mean ± SD) | Placebo (mean ± SD) | P-Value* | |
|---|---|---|---|---|
| Quality of Life | CESD (−) | −1.27 ± 7.7 | −0.89 ± 7.9 | >0.999 |
| DASI (+) | 6.3 ± 18.3 | 6.96 ± 16.9 | >0.999 | |
| STAI (−) | −6.7 ± 12.7 | −6.39 ± 10.3 | >0.999 | |
| Symptoms Limitation (−) | −1.39 ± 3.8 | −1.48 ± 3.8 | >0.999 | |
| OARS-IADL (−) | −0.15 ± 2.1 | −0.31 ± 2.6 | >0.999 | |
| Cognitive Difficulties (−) | −0.46 ± 16.1 | −1.02 ± 17 | >0.999 | |
| Social Activities (−) | −0.2 ± 3.2 | 0.03 ± 3.4 | >0.999 | |
| Social Support (+) | 0.71 ± 13.4 | −1.16 ± 12.9 | 0.696 | |
| Work Activities (−) | −1.37 ± 4.3 | −1.42 ± 4.1 | >0.999 | |
| General Health Perception (−) | −0.28 ± 1.2 | −0.43 ± 1.1 | >0.999 | |
| Neurologic Outcomes | NIHSS (−) | 0.05 ± 0.7 | 0.07 ± 0.6 | >0.999 |
| WPNS (+) | 0.02 ± 1.1 | −0.02 ± 1.2 | >0.999 | |
A minus sign (−) indicates that a lower score is better; a plus sign (+) indicates that a higher score is better. CESD: Center for Epidemiologic Studies – Depression Scale, DASI: Duke Activity Status Index, STAI: State Trait Anxiety Inventory, OARS-IADL: Duke Older American Resources Services Procedures – Instrumental Activities of Daily Living, NIHSS: National Institutes of Health Stroke Scale, WPNS: Western Perioperative Neurologic Scale.
Adjusted for multiple comparisons.
Sensitivity Results
Sensitivity analyses were performed for the primary outcome of cognitive index score change at 6-weeks postoperatively for the following: 1) per protocol cases, 2) complete cases, 3) all eligible and randomized cases, and 4) assignment of worst cognitive performance scores to patients who had suffered an early postoperative stroke and thus lacked 6-week neurocognitive testing data. None of these sensitivity analyses identified significant differences in cognitive index score change or binary outcome of cognitive deficit between treatment groups (Table 6). Of note, when all eligible and randomized patients were included (n=471) and all missing values are imputed, we found that the 1-year rate of POCD was 49.2% in the lidocaine group compared to 58.4% in the placebo group (p=0.045). However, this difference was no longer significant after multivariable adjustment for age, level of education, procedure type, Caucasian race, and baseline cognition [OR (95% CI) 0.76 (0.50, 1.15); p=0.197].
Table 6.
Sensitivity analyses.
| Cognitive Change Index | Cognitive Deficit | |||
|---|---|---|---|---|
| Sensitivity analysis | Adjusted Mean Difference (95% CI) | P-Value | Adjusted Odds Ratio (95% CI) | P-Value |
| 1) Per-protocol cases (N=406) | 0.02 (−0.05, 0.08) | 0.638 | 0.90 (0.60, 1.37) | 0.632 |
| 2) Complete cases (N=393) | 0.02 (−0.05, 0.08) | 0.621 | 0.88 (0.58, 1.35) | 0.563 |
| 3) All eligible and randomized cases (N=470)* | 0.01 (−0.05, 0.08) | 0.642 | 0.97 (0.66, 1.45) | 0.896 |
| 4) Assignment of worst cognitive performance (N=423) | 0.05 (−0.03, 0.13) | 0.19 | 0.92 (0.61, 1.38) | 0.676 |
1 patient was missing level of education.
Discussion
In this prospective, placebo-controlled, parallel group, randomized study of the administration of intravenous lidocaine for 48 hours during and after cardiac surgery with CPB, we found that lidocaine did not reduce the incidence (binary outcome) or magnitude of cognitive decline (continuous cognitive change score) at 6 weeks postoperatively. Additionally, intravenous lidocaine had no effect on 1-year cognitive outcomes or on additional measures of neurologic outcomes and quality of life.
Since the early 1980s, preclinical evidence has suggested a neuroprotective effect of lidocaine. Evans et al. were the first to demonstrate a protective effect of lidocaine against ischemia from cerebral arterial gas embolism in an animal model.31,32 This was followed by multiple preclinical studies corroborating a protective effect of conventional doses of intravenous lidocaine, given both pre- and post-injury and in multiple models of neurologic insult (reviewed elsewhere33).
Among the potential mechanisms for a neuroprotective effect of lidocaine, the most obvious is its sodium channel blocking effects. Cerebral ischemia produces a depletion of neuronal energy stores with a subsequent loss of ionic hemostasis and consequent depolarization, leading to downstream excitotoxicity and the initiation of apoptosis due to calcium influx. In an animal model of neuronal ischemia, Astrup et al.13 demonstrated that large doses of lidocaine (100–160 mg/kg) reduced the cerebral metabolic rate by 15–20% beyond that achieved by barbiturates, thus preserving cellular energy stores. They attributed their findings to lidocaine’s ability to block anoxic sodium influx/potassium efflux that leads to cellular edema and loss of function. Similarly, in rat hippocampal slices exposed to varying concentrations of lidocaine within the therapeutic range, anoxic depolarization was less frequent and delayed when it did occur.14,34 With conventional dosing of lidocaine (0.2 mg/kg/min) in rabbits undergoing cerebral ischemia, anoxic depolarization was delayed.15 As a consequence of reduced anoxic depolarization in response to lidocaine, secondary neurotoxic events, such as intracellular edema,35 cytosolic calcium accumulation,36 and glutamate37 and aspartate38 release, can be attenuated.
Others have purported a neuroprotective effect from lidocaine due to its anti-inflammatory properties, which ties in with studies that have suggested that the magnitude of the inflammatory response to surgery may be a risk factor for POCD.39,40 In models of tissue injury, lidocaine has been shown to prevent leukocyte accumulation and endothelial adherence along with consequent microvascular thrombus when applied preventatively and to restore microvascular blood flood by disadhering leukocytes from the endothelium when applied post-injury.41,42 As part of this study and reported elsewhere43, paired jugular venous and arterial samples were drawn on the first 202 randomized patients to measure transcerebral gradients of activated platelets and platelet-leukocyte conjugates. In that cohort, we observed a significant reduction in the transcerebral activation of platelet-monocyte conjugates after aortic cross-clamp release in patients who received lidocaine, which we hypothesized might reflect reduced cerebral inflammation during CPB. Despite these results, the data reported here do not show a protective effect of lidocaine on cognition. Analysis of the 202 patients with platelet-leukocyte data showed no difference in cognitive outcomes between lidocaine and placebo groups, suggesting that any drug-related changes in circulating inflammatory cell markers do not impact cognitive outcomes; however, this pre-defined sub-study was not powered to study cognitive outcomes. Cerebrospinal inflammatory markers were also not measured and may better correlate with cognitive outcomes.
Despite the promise demonstrated in preclinical studies, human trials have been conflicting. The first study of intravenous lidocaine for neuroprotection in cardiac surgery was published by Mitchell et al.44 Sixty-five patients undergoing open heart valve surgery were randomized to 48 hours of intravenous lidocaine vs. placebo, and cognition was compared at 3 postoperative follow-up time points with baseline measures. At the 10-day and 10-week follow-up points, the number of patients with a 1-SD decrement in 1 cognitive test was significantly lower in the lidocaine group. Wang et al. published a similar study in patients undergoing CABG, demonstrating that significantly fewer patients who received lidocaine compared to placebo manifested cognitive deficit at 9 days postoperatively.12 A large randomized trial of lidocaine vs. placebo in cardiac surgical patients performed by Mathew et al. was unable to demonstrate a protective effect of lidocaine on cognition in the global study cohort.22 However, diabetes and high lidocaine dose were found to be detrimental to cognition, and secondary analyses pointed to a potentially protective effect of pharmacokinetically-driven dosing of lidocaine in non-diabetic cardiac surgical patients. Finally, a follow-up study by Mitchell et al. contradicted the results of their original trial, showing no benefit of a 12-hour lidocaine infusion on cognition in 158 patients at 10 and 25 weeks after cardiac surgery.10
Some have postulated that the variability seen in human trials may reflect differences in procedure types, with benefit seen primarily in those studies involving open-chamber procedures with higher risk of gas emboli to the brain. This appears plausible given the benefit seen in preclinical models of cerebral injury induced by gas embolism and human data demonstrating enhanced neurologic recovery with intravenous lidocaine in divers suffering decompression sickness.45–47 Our patient population underwent both open and closed-chamber procedures, with a preponderance of Valve and Valve+CABG surgeries in both treatment groups (71% in lidocaine vs. 70% in placebo). Yet multivariable regression failed to demonstrate an interaction between procedure type and treatment group. In the per-protocol analysis, there was a signal for worse cognition with valve surgery compared to CABG [cognitive index change difference Valve vs. CABG (95% CI): −0.07 (−0.15, 0); p=0.041]; however, there was no treatment effect.
The results of this study are in line with the general consensus in the literature that postoperative cerebral injury is a complex phenomenon that is not easily ameliorated. Available evidence suggests a wide range of risk factors and potential mechanisms underlying cognitive decline after general and cardiac surgery, including preoperative cognitive impairment, genetic predisposition, cerebral microembolism or hypoperfusion during CPB, central nervous and systemic inflammatory responses, hemodilution, hyperglycemia, hyperthermia, and the unmasking of Alzheimer’s disease and acceleration of amyloid deposition associated with inhalational anesthetics (reviewed elsewhere)48. Not surprisingly, individual drugs investigated for neuroprotection during cardiac surgery have thus far produced disappointing results, including several drugs targeting similar physiologic pathways as lidocaine.49
POCD studies often suffer from the lack of controls and inconsistent measurement and definitions of cognitive decline. Our large, multicenter study was placebo-controlled and involved rigorous neurocognitive testing. We examined cognitive function as both a dichotomous outcome and as a continuous measure; we failed to find an impact of lidocaine on cognitive change by either measure. Loss to follow-up was a limitation of the current study, although the rate was not different between groups. We attempted to adjust for loss to follow-up due to stroke (i.e., poorest neurologic outcome) by assignment of poor cognitive scores to stroke patients in secondary analyses without a change in the results. There were several other important demographic differences in patients that dropped out before the 6-week or 1-year evaluation time points (described in Supplemental Tables 1 and 2). Globally, patients lost to follow-up were more likely to be female, have a lower preoperative ejection fraction, have a lower preoperative cognitive index score, and have had CABG surgery; however, these characteristics were evenly distributed across patients lost to follow-up in both treatment groups.
Additional limitations include the methodology of defining cognitive dysfunction. As yet, there is no standard definition or criteria for diagnosing POCD, although the International Perioperative Neurotoxicity Group is currently working toward a consensus definition of POCD. We utilized a standard battery of neuropsychological test, as outlined by a consensus statement on the assessment of neurobehavioral outcomes after cardiac surgery24. The binary outcome of POCD was defined as a decline of >1 SD in at least 1 domain; both a > 1 and > 2 SD decline are commonly used in the POCD literature. Furthermore, we reported the continuous cognitive change score from baseline to highlight the magnitude of cognitive change in addition the incidence of the binary cognitive deficit outcome.
Finally, our study was limited by sensitivity for detection of cognitive deficit at the 1-year follow-up point. While there appears to be a potential divergence in the cognitive trajectory between lidocaine-treated and placebo patients at the 1-year time point (Figure 3), we were underpowered for this outcome. To detect a difference between the observed POCD rate between lidocaine and placebo (48.1% and 56.8%, respectively), a future study would require 517 patients per treatment group (2-sided chi-square test at alpha = 0.05).
In summary, intravenous administration of lidocaine intraoperatively and for a total of 48 hours in patients undergoing both open and closed chamber cardiac surgery with CPB did not protect against POCD or improve cognitive scores at 6-weeks or 1-year postoperatively. Lidocaine also failed to improve additional measures of postoperative neurologic outcomes or quality of life indices.
Supplementary Material
6. Acknowledgements:
10. Funding: This study was supported by grants to Dr. Mathew from the National Institutes of Health (HL096978, HL108280, HL109971, HL130443).
Appendix
**Neurologic Outcome Research Group (NORG)
Director: Joseph P. Mathew, M.D., Co-Director: James A. Blumenthal, Ph.D.
Anesthesiology: Miles Berger, M.D., Ph.D., Jorn A. Karhausen, M.D., Miklos D. Kertai, M.D., Rebecca Y. Klinger, M.D., M.S., Vijay Krishnamoorthy, MD, PhD, Yi-Ju Li, Ph.D., Joseph P. Mathew, M.D., Mark F. Newman, M.D, Mihai V. Podgoreanu, M.D., Mark Stafford-Smith, M.D., Madhav Swaminathan, M.D., Niccolo Terrando, Ph.D., David S. Warner, M.D., Bonita L. Funk, R.N., CCRP, Rachele Brassard, BSW, Tiffany Bisanar, R.N., B.S.N., Mary Cooter, MS, Yanne Toulgoat-Dubois, B.A., Peter Waweru, CCRP.
Behavioral Medicine: Michael A. Babyak, Ph.D., James A. Blumenthal, Ph.D., Jeffrey N. Browndyke, Ph.D., Kathleen A. Welsh-Bohmer, Ph.D.
Cardiology: Michael H. Sketch, Jr., M.D.
Neurology: Ellen R. Bennett, Ph.D., Carmelo Graffagnino, M.D., Daniel T. Laskowitz, M.D., Warren J. Strittmatter, M.D.
Perfusion Services: Kevin Collins, B.S., C.C.P., Greg Smigla, B.S., C.C.P., Ian Shearer, B.S., C.C.P.
Surgery: Thomas A. D'Amico, M.D., Mani A. Daneshmand, M.D., R. Jeffrey G. Gaca, M.D., Donald D. Glower, M.D., Jack Haney, M.D., R. David Harpole, M.D., Mathew G. Hartwig, M.D., G. Chad Hughes, M.D., Jacob A. Klapper, M.D., Shu S. Lin, M.D., Andrew J. Lodge, M.D., Carmelo A. Milano, M.D., Ryan P. Plichta, M.D, Jacob N. Schroeder, M.D., Peter K. Smith, M.D., Betty C. Tong, M.D.
Footnotes
11. Conflicts of Interest: None declared.
4. ClinicalTrials.gov Registration: NCT00938964
5. Prior Presentation: Data presented in part at the Society for Cardiovascular Anesthesiologists Annual Meeting, 2015.
References
- 1.Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA, Neurological Outcome Research G, the Cardiothoracic Anesthesiology Research Endeavors I: Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med 2001; 344: 395–402 [DOI] [PubMed] [Google Scholar]
- 2.Phillips-Bute B, Mathew JP, Blumenthal JA, Grocott HP, Laskowitz DT, Jones RH, Mark DB, Newman MF: Association of neurocognitive function and quality of life 1 year after coronary artery bypass graft (CABG) surgery. Psychosom Med 2006; 68: 369–75 [DOI] [PubMed] [Google Scholar]
- 3.Nussmeier NA, Arlund C, Slogoff S: Neuropsychiatric complications after cardiopulmonary bypass: cerebral protection by a barbiturate. Anesthesiology 1986; 64: 165–70 [DOI] [PubMed] [Google Scholar]
- 4.Arrowsmith JE, Harrison MJ, Newman SP, Stygall J, Timberlake N, Pugsley WB: Neuroprotection of the brain during cardiopulmonary bypass: a randomized trial of remacemide during coronary artery bypass in 171 patients. Stroke 1998; 29: 2357–62 [DOI] [PubMed] [Google Scholar]
- 5.Forsman M, Olsnes BT, Semb G, Steen PA: Effects of nimodipine on cerebral blood flow and neuropsychological outcome after cardiac surgery. Br J Anaesth 1990; 65: 514–20 [DOI] [PubMed] [Google Scholar]
- 6.Legault C, Furberg CD, Wagenknecht LE, Rogers AT, Stump DA, Coker L, Troost BT, Hammon JW: Nimodipine neuroprotection in cardiac valve replacement: report of an early terminated trial. Stroke 1996; 27: 593–8 [DOI] [PubMed] [Google Scholar]
- 7.Kong RS, Butterworth J, Aveling W, Stump DA, Harrison MJ, Hammon J, Stygall J, Rorie KD, Newman SP: Clinical trial of the neuroprotectant clomethiazole in coronary artery bypass graft surgery: a randomized controlled trial. Anesthesiology 2002; 97: 585–91 [DOI] [PubMed] [Google Scholar]
- 8.Fish KJ, Helms KN, Sarnquist FH, van Steennis C, Linet OI, Hilberman M, Mitchell RS, Jamieson SW, Miller DC, Tinklenberg JS: A prospective, randomized study of the effects of prostacyclin on neuropsychologic dysfunction after coronary artery operation. J Thorac Cardiovasc Surg 1987; 93: 609–15 [PubMed] [Google Scholar]
- 9.Grieco G, d’Hollosy M, Culliford AT, Jonas S: Evaluating neuroprotective agents for clinical anti-ischemic benefit using neurological and neuropsychological changes after cardiac surgery under cardiopulmonary bypass. Methodological strategies and results of a double-blind, placebo-controlled trial of GM1 ganglioside. Stroke 1996; 27: 858–74 [DOI] [PubMed] [Google Scholar]
- 10.Mitchell SJ, Merry AF, Frampton C, Davies E, Grieve D, Mills BP, Webster CS, Milsom FP, Willcox TW, Gorman DF: Cerebral protection by lidocaine during cardiac operations: a follow-up study. Ann Thorac Surg 2009; 87: 820–5 [DOI] [PubMed] [Google Scholar]
- 11.Chen K, Wei P, Zheng Q, Zhou J, Li J: Neuroprotective effects of intravenous lidocaine on early postoperative cognitive dysfunction in elderly patients following spine surgery. Med Sci Monit 2015; 21: 1402–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Wu X, Li J, Xiao F, Liu X, Meng M: The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg 2002; 95: 1134–41, [DOI] [PubMed] [Google Scholar]
- 13.Astrup J, Sorensen PM, Sorensen HR: Inhibition of cerebral oxygen and glucose consumption in the dog by hypothermia, pentobarbital, and lidocaine. Anesthesiology 1981; 55: 263–8 [DOI] [PubMed] [Google Scholar]
- 14.Weber ML, Taylor CP: Damage from oxygen and glucose deprivation in hippocampal slices is prevented by tetrodotoxin, lidocaine and phenytoin without blockade of action potentials. Brain Res 1994; 664: 167–77 [DOI] [PubMed] [Google Scholar]
- 15.Ayad M, Verity MA, Rubinstein EH: Lidocaine delays cortical ischemic depolarization: relationship to electrophysiologic recovery and neuropathology. J Neurosurg Anesthesiol 1994; 6: 98–110 [DOI] [PubMed] [Google Scholar]
- 16.Dutka AJ, Mink R, McDermott J, Clark JB, Hallenbeck JM: Effect of lidocaine on somatosensory evoked response and cerebral blood flow after canine cerebral air embolism. Stroke 1992; 23: 1515–20; [DOI] [PubMed] [Google Scholar]
- 17.Hollmann MW, Durieux ME: Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology 2000; 93: 858–75 [DOI] [PubMed] [Google Scholar]
- 18.Hollmann MW, Gross A, Jelacin N, Durieux ME: Local anesthetic effects on priming and activation of human neutrophils. Anesthesiology 2001; 95: 113–22 [DOI] [PubMed] [Google Scholar]
- 19.Picardi S, Cartellieri S, Groves D, Hahnenkamp K, Gerner P, Durieux ME, Stevens MF, Lirk P, Hollmann MW: Local anesthetic-induced inhibition of human neutrophil priming: the influence of structure, lipophilicity, and charge. Reg Anesth Pain Med 2013; 38: 9–15 [DOI] [PubMed] [Google Scholar]
- 20.MacGregor RR, Thorner RE, Wright DM: Lidocaine inhibits granulocyte adherence and prevents granulocyte delivery to inflammatory sites. Blood 1980; 56: 203–9 [PubMed] [Google Scholar]
- 21.Lan W, Harmon D, Wang JH, Ghori K, Shorten G, Redmond P: The effect of lidocaine on in vitro neutrophil and endothelial adhesion molecule expression induced by plasma obtained during tourniquet-induced ischaemia and reperfusion. Eur J Anaesthesiol 2004; 21: 892–7 [DOI] [PubMed] [Google Scholar]
- 22.Mathew JP, Mackensen GB, Phillips-Bute B, Grocott HP, Glower DD, Laskowitz DT, Blumenthal JA, Newman MF, Neurologic Outcome Research Group of the Duke Heart C: Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke 2009; 40: 880–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu YW, Somma J, Newman MF, Mathew JP: Population pharmacokinetics of lidocaine administered during and after cardiac surgery. J Cardiothorac Vasc Anesth 2011; 25: 931–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murkin JM, Newman SP, Stump DA, Blumenthal JA: Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg 1995; 59: 1289–95 [DOI] [PubMed] [Google Scholar]
- 25.Rasmusson DX, Bylsma FW, Brandt J: Stability of performance on the Hopkins Verbal Learning Test. Arch Clin Neuropsychol 1995; 10: 21–6 [PubMed] [Google Scholar]
- 26.Randt C, Brown E: Adminstration manual: Randt memory test. New York, Life Sciences Associates, 1983 [Google Scholar]
- 27.Wechsler D: The Wechsler Adult Intelligence Scale-Revised (Manual), Psychological Corporation, 1981 [Google Scholar]
- 28.Reitan R: Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills 1958; 8: 271–6 [Google Scholar]
- 29.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P: A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol 2001; 27: 85–95 [Google Scholar]
- 30.Little RJ, Rubin DB: Statistical analysis with missing data, 2nd Ed edition. New Jersey, J Wiley & Sons, 2002 [Google Scholar]
- 31.Evans DE, Catron PW, McDermott JJ, Thomas LB, Kobrine AI, Flynn ET: Effect of lidocaine after experimental cerebral ischemia induced by air embolism. J Neurosurg 1989; 70: 97–102 [DOI] [PubMed] [Google Scholar]
- 32.Evans DE, Kobrine AI, LeGrys DC, Bradley ME: Protective effect of lidocaine in acute cerebral ischemia induced by air embolism. J Neurosurg 1984; 60: 257–63 [DOI] [PubMed] [Google Scholar]
- 33.Mitchell SJ, Merry AF: Lignocaine: neuro-protective or wishful thinking? J Extra Corpor Technol 2009; 41: P37–42 [PMC free article] [PubMed] [Google Scholar]
- 34.Fried E, Amorim P, Chambers G, Cottrell JE, Kass IS: The importance of sodium for anoxic transmission damage in rat hippocampal slices: mechanisms of protection by lidocaine. J Physiol 1995; 489 ( Pt 2): 557–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopachin RM: Intraneuronal ion distribution during experimental oxygen/glucose deprivation. Routes of ion flux as targets of neuroprotective strategies. Ann N Y Acad Sci 1999; 890: 191–203 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Lipton P: Cytosolic Ca2+ changes during in vitro ischemia in rat hippocampal slices: major roles for glutamate and Na+-dependent Ca2+ release from mitochondria. J Neurosci 1999; 19: 3307–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujitani T, Adachi N, Miyazaki H, Liu K, Nakamura Y, Kataoka K, Arai T: Lidocaine protects hippocampal neurons against ischemic damage by preventing increase of extracellular excitatory amino acids: a microdialysis study in Mongolian gerbils. Neurosci Lett 1994; 179: 91–4 [DOI] [PubMed] [Google Scholar]
- 38.Diaz L, Gomez A, Bustos G: Lidocaine reduces the hypoxia-induced release of an excitatory amino acid analog from rat striatal slices in superfusion. Prog Neuropsychopharmacol Biol Psychiatry 1995; 19: 943–53 [DOI] [PubMed] [Google Scholar]
- 39.Peng L, Xu L, Ouyang W: Role of peripheral inflammatory markers in postoperative cognitive dysfunction (POCD): a meta-analysis. PLoS One 2013; 8: e79624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terrando N, Eriksson LI, Ryu JK, Yang T, Monaco C, Feldmann M, Jonsson Fagerlund M, Charo IF, Akassoglou K, Maze M: Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol 2011; 70: 986–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishina K, Mikawa K, Takao Y, Shiga M, Maekawa N, Obara H: Intravenous lidocaine attenuates acute lung injury induced by hydrochloric acid aspiration in rabbits. Anesthesiology 1998; 88: 1300–9 [DOI] [PubMed] [Google Scholar]
- 42.Luostarinen V, Evers H, Lyytikainen MT, Scheinin Wahlen A: Antithrombotic effects of lidocaine and related compounds on laser-induced microvascular injury. Acta Anaesthesiol Scand 1981; 25: 9–11 [DOI] [PubMed] [Google Scholar]
- 43.Klinger RY, Cooter M, Berger M, Podgoreanu MV, Stafford-Smith M, Ortel TL, Welsby IJ, Levy JH, Rinder HM, Newman MF, Mathew JP, Neurologic Outcomes Research Group of The Duke Heart C: Effect of intravenous lidocaine on the transcerebral inflammatory response during cardiac surgery: a randomized-controlled trial. Can J Anaesth 2016; 63: 1223–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell SJ, Pellett O, Gorman DF: Cerebral protection by lidocaine during cardiac operations. Ann Thorac Surg 1999; 67: 1117–24 [DOI] [PubMed] [Google Scholar]
- 45.Drewry A, Gorman DF: Lidocaine as an adjunct to hyperbaric therapy in decompression illness: a case report. Undersea Biomed Res 1992; 19: 187–90 [PubMed] [Google Scholar]
- 46.Cogar WB: Intravenous lidocaine as adjunctive therapy in the treatment of decompression illness. Ann Emerg Med 1997; 29: 284–6 [DOI] [PubMed] [Google Scholar]
- 47.Mitchell SJ, Benson M, Vadlamudi L, Miller P: Cerebral arterial gas embolism by helium: an unusual case successfully treated with hyperbaric oxygen and lidocaine. Ann Emerg Med 2000; 35: 300–3 [DOI] [PubMed] [Google Scholar]
- 48.Berger M, Burke J, Eckenhoff R, Mathew J: Alzheimer’s disease, anesthesia, and surgery: a clinically focused review. J Cardiothorac Vasc Anesth 2014; 28: 1609–23 [DOI] [PubMed] [Google Scholar]
- 49.Berger M, Nadler JW, Browndyke J, Terrando N, Ponnusamy V, Cohen HJ, Whitson HE, Mathew JP: Postoperative Cognitive Dysfunction: Minding the Gaps in Our Knowledge of a Common Postoperative Complication in the Elderly. Anesthesiol Clin 2015; 33: 517–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.