Abstract
Anorexia nervosa is a severe psychiatric illness with high mortality. Brain imaging research has indicated altered reward circuits in the disorder. Here we propose a disease model for anorexia nervosa, supported by recent studies, that integrates psychological and biological factors. In that model, we propose that there is a conflict between the conscious motivation to restrict food, and a body-homeostasis driven motivation to approach food in response to weight loss. These opposing motivations trigger anxiety, which maintains the vicious cycle of ongoing energy restriction and weight loss.
Keywords: Anorexia nervosa, Motivation, Conflict, Anxiety, Brain
1. Introduction
At the 2018 annual meeting of the Society for the Study of Ingestive Behaviors in Bonita Springs, we presented recent data from our group on brain reward processing in anorexia nervosa (AN). Here we provide a background to those studies and present a model for the vicious cycle of food restriction and weight loss that occurs in AN despite being underweight.
2. Clinical Presentation of AN
AN is the third most common chronic illness among adolescent females. It has the highest mortality rate among the psychiatric disorders with most deaths occurring between the ages of 16 and 29 years [1-3]. AN is associated with severe emaciation from restriction of food intake, and a perception of being overweight despite severe underweight [4]. The disorder shows a complex interplay between neurobiological, psychological and environmental factors [5], and it is a chronic disorder with frequent relapse, high treatment costs and severe disease burden [6, 7]. Treatment effectiveness is limited [8], and no medication has been approved for AN [9]. Yet, little is known about the pathophysiology or brain biomarkers that characterize AN [10]. Increasing efforts to identify biologic mechanisms that affect behavior in AN is of central importance in gaining a more complete understanding of the disorder, and to develop pharmacological treatments [11, 12].
3. The Core Conflict in AN
The spectrum of individuals who develop AN is broad. This may range from adolescents who simply exercise more to be more competitive in sports or try to eat healthier, to individuals who have been traumatized and have had lifelong body image problems, and yet, they all develop AN with the same core features. While those individuals come from different paths to develop AN, they all have in common a discrepancy between their conscious motivation for how much they want to eat - or rather restrict food intake - versus the body’s need to stay at a healthy and sustainable body weight. Here we will summarize recent research from our group that is investigating motivational processes of food intake in AN together with other human and basic research to develop a psycho-biological model for how core AN behaviors develop and contribute to a continuous cycle of weight loss. Central to this model is the conflict between a conscious motivation to restrict eating and the unconscious messages from the body-homeostasis maintaining mechanisms including reward circuits and neuroendocrine systems to seek out food and to preserve a healthy body weight. This is a data-driven model that integrates empirically studied brain biology with measured behavior and weight gain observed in treatment.
4. AN is Associated with Altered Neuroendocrine and Brain Reward Circuit Function
During the course of AN, a multitude of changes happen in appetite regulating circuits. While the results are not all uniform, leptin as a marker of body fat is low, the appetite stimulating hormone ghrelin is elevated as are the stress markers cortisol and corticotropin releasing factor, and the level of the appetite reducing oxytocin is low in AN [13]. While those hormones and peptides that take part in regulating body homeostasis adapt to the acute nutritional state and typically normalize with weight recovery, they may have important roles in the modulation of the brain reward circuitry [14].
The brain reward system is a well-studied circuitry that has been hypothesized to hold promise as a target for future treatment interventions in eating disorders including AN [14]. Especially important to eating and food reward is the taste pathway that projects inputs from the tongue taste receptors via the thalamus to the insula, which contains the primary taste cortex [15]. The reward system receives further input from frontal cortical regions about desires to consume foods, and it is connected with the hypothalamus to integrate signals from the body periphery such as blood sugar levels to regulate food intake and maintain energy homeostasis of the body [16]. The taste perception leads to learned associations between taste and subjective hedonic experiences to create an internal cognitive and emotional representation of food stimuli that gets activated when we see, smell or taste food [17]. Those associations provide dopamine mediated learning signals to higher-order brain regions to compare the current (food) experience with past experience and store new, or update previously stored, information on how much we value a particular food stimulus. This is to support the decision-making process for what type and amount of food we would like to eat in the future [18, 19]. The above described circuitry to motivate food intake is interrelated with the interoceptive signal of satiety, and a variety of gut hormones and neuropeptides together with specific brain regions contribute to this signal to regulate eating [20, 21].
An increasing number of functional brain imaging studies have indicated altered activation in reward-processing brain regions in AN. It was hypothesized that such abnormalities together with anxious traits could contribute to AN-specific brain pathophysiology and drive extremes of food restriction [22]. Brain reward studies in AN have applied various paradigms using for instance food images to study emotional or hormonal response [23].
Our lab has focused on brain dopamine function to study altered reward circuits in AN. The dopamine pathways are a neuromodulatory system that arises from cells in the midbrain [24]. These midbrain neurons release dopamine, which acts on cortical and subcortical dopamine receptors. Dopamine function contributes to the modulation of motor activity [25], feeding behaviors [26], and reinforcement and reward learning [27]. The dopamine system is particularly interesting to study as we know relatively more about the neuronal dynamics of its activation compared to other neurotransmitter systems and mathematical models have been developed to predict dopaminergic neuron response during presentation of rewarding or salient stimuli [28, 29].
The dopamine system adapts in opposite directions to extremes of food intake [30-33]. Animal models have shown enhanced neuronal dopamine activation following food restriction [34, 35], which led to the hypothesis that brain dopamine circuits sensitize in AN in during food restriction and weight loss and are part of its specific pathophysiology [14, 22]. Food restriction sensitizes both dopamine D1 and D2/D3 receptors in animal studies, which is suggestive of being the underlying mechanism for enhanced dopamine neuron activation in response to weight loss [36, 37].
In the past, low cerebrospinal fluid homovanillic acid, the major dopamine metabolite in ill AN [38], and elevated dopamine D2/3 receptor availability after recovery [39], suggested directly altered dopamine function in AN, although a group currently ill with AN showed normal dopamine D2/3 receptor distribution [40]. Those receptor studies, however, could not inform on brain function in response to or during behavior.
Dopamine related brain function can be studied in humans indirectly using blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) and prediction error model tasks. The prediction error model is a model for how dopamine neurons respond to environmental stimuli and drive motivation to approach rewards and learn from rewarding or salient stimuli [41]. Dopamine neurons exhibit a phasic burst of activation in response to presentation of an unexpected rewarding stimulus, and a decrease in tonic dopamine neuron activity in response to unexpected omission of an expected reward stimulus [42]. This model was first validated in rodents [43] and later adapted for human brain imaging [44, 45]. Those circuits are critically associated with providing signals regarding the presence and amplitude of rewards [16, 28], and code the value of reward stimuli, including the metabolic value of food [46-49]. In earlier studies, adults with AN showed elevated prediction error response (stronger positive response to unexpected receipt and stronger negative response to unexpected omission) to sucrose taste stimuli versus controls [50, 51]. This elevation in both directions was interpreted as a general sensitization of the dopamine neuronal response in AN. This was in contrast to obese individuals who showed lower prediction error response compared to controls, further supporting the adaptation of the dopamine system to the amount of food intake and consistent with basic science [32, 51]. A smaller elevation in prediction error response in long term recovered AN suggested a gradual normalization of brain response with illness recovery [52]. In summary, those studies suggested that dopamine-related reward processing is altered in AN and this led us to expand those studies to integrate behavior and treatment response with brain circuit activation.
5. A Model for Competing Motivations to Eat and Not to Eat in AN
Recently we published a study in a large group of adolescents with AN (n=56), and an age-matched control group (n=52) [53]. In that study we applied a taste prediction error task as above. We also studied dynamic effective brain connectivity, that is what brain region drives another during sugar tasting. The prediction error signal derived from unexpected omission or receipt of sucrose taste reward resulted in heightened response in insula, striatum and orbitofrontal cortex in AN versus controls. This confirmed our previously found elevated prediction error response in AN. Orbitofrontal cortex prediction error signal in the AN group was significantly positively correlated with harm avoidance, a measure for an anxiety trait, which in turn was positively correlated with drive for thinness and body dissatisfaction (Figure 1.). In addition, the prediction error signal correlated negatively with increase in body mass index (BMI, weight in kg/height in m2) during treatment. Salivary cortisol was elevated in AN, consistent with other studies, and showed positive correlation with prediction error response. It was therefore hypothesized that stress and associated cortisol increase may directly contribute to elevation in prediction error brain response in AN. Lastly, prediction error response in AN was positively correlated with dynamic effective connectivity from ventral striatum to hypothalamus, an anxiety driven circuit that quickly inhibits food intake [54]. An additional smaller study in adolescent AN using a monetary reward prediction error task also showed elevated prediction error brain response together with negative correlation with BMI increase during treatment [55]. This suggested that enhanced prediction error response could be an illness state biomarker and independent from the stimulus type.
Figure 1.
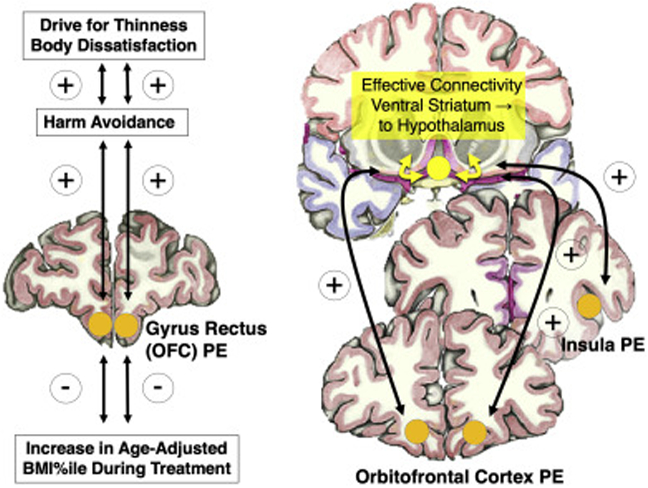
Previous studies have shown that prediction error brain response (PE) was positively correlated with harm avoidance as a measure for anxious temperament in adolescents with anorexia nervosa (AN), which correlated positively with core AN behaviors drive food thinness and body dissatisfaction, but negatively with weight gain in treatment; PE was also positively related to the dynamic effective connectivity from ventral striatum to hypothalamus (adapted from Frank et al., JAMA Psychiatry, 2018); OFC, orbitofrontal cortex.
Based on those empirical data we propose a model for the continuous cycle of energy restriction in AN that integrates cognitive emotional factors such as drive for thinness, body dissatisfaction and fear of weight gain, with the body’s adaptations to weight loss that include neuroendocrine factors as well as the changes in the brain dopamine system that occur with food restriction and weight loss (Figure 2.).
Figure 2.
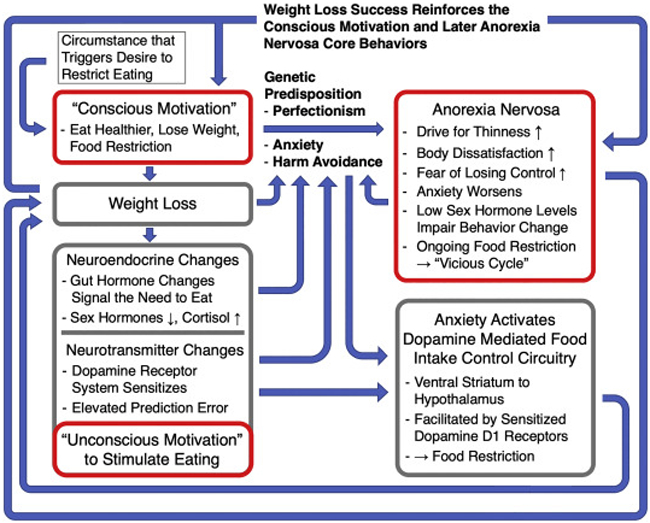
A schematic model that integrates the empiric findings from Figure 1. with previous research on neuroendocrine function in human studies and animal models for the effects of food restriction. After deciding to change eating behavior and lose weight, endocrine changes occur that signal the need to eat; the dopamine system gets activated to support the motivation to seek out food; perfectionism and high anxiety mediate the transition to developing AN core behaviors while the original conscious motivation is sustained. Weight loss briefly alleviates anxiety and reinforces food restriction. However, gut hormones and dopamine that stimulate food seeking, elevate anxiety and subsequently elevate AN core behaviors. Anxiety triggers a food-control circuitry from ventral striatum to hypothalamus that depends on dopamine D1 receptors, which have been sensitized in the context of food restriction. Anxiety gets further elevated in the illness process due to the possibility of loss of control and weight gain, and this becomes a self-reinforcing process. Ongoing food restriction and weight loss perpetuate the cycle.
Initiation of AN behavior:
AN is characterized by weight loss for various reasons. This can range from losing weight in the context of increased exercise to get fitter, to eating “healthier” (leaving out certain high calorie foods from the diet) as for instance often discussed in middle school health class, to overweight individuals who decidedly want to lose weight, to individuals who were maybe abused and alter their eating in that context, and variations in between. This “conscious motivation” to change eating or exercise behavior is then followed by weight loss. Weight loss is subsequently learned to be associated with the original goal of eating healthier, being fitter or thinner, and weight gain then may be perceived as a threat to that goal.
Underlying cognitive-emotional mechanisms:
Not everyone who diets, or exercises more, develops AN. There are reports on specific underlying cognitive-emotional dynamics, for instance feeling in control, that drive food restriction in AN, but to what extent those have developed as part of the illness or have been there premorbidly is not known [56]. What is known is that individuals who develop AN tend to have anxious temperaments, biological traits such as high harm avoidance and perfectionism, which may facilitate development and maintenance of AN core behaviors [57]. Those traits that drive the desire to do things correctly are strong motivators of behavior in general, including eating disorder behaviors. Weight loss as an indicator of success then becomes a conditioned reward and cognitive reinforcer of the behavior. AN runs in families and we postulate a genetic predisposition for this transition from the initial motivation to change eating to core AN behaviors and excessive weight loss. We believe that brain changes occur that drive the extreme preoccupation with food and weight and shape. What the underlying biological mechanisms may be is unclear. Recent genetic research has shown an overlap between AN and obsessive compulsive disorder genes and the two disorders might share biological mechanisms that drive excessive behavior perpetuation [58]. Habit learning has also been hypothesized to be part of AN’s pathophysiology and it is possible that high habit strength (the connection between stimulus and behavior response) together with heightened frontostriatal brain connectivity contribute to ongoing AN behaviors [59-61].
Physiological response to weight loss:
Animal models have shown that the body’s normal response to weight loss is an activation of the feeding system, including many adaptations of gut hormones and neuropeptides that signal to the hypothalamus the body’s nutritional needs, as well as a sensitization of the dopamine system to support food approach [14, 16]. Food restriction is associated with increased brain cortisol, which affects dopamine release and postsynaptic dopamine D1 and D2 receptor function [37, 54, 62-64]. Those bodily feedback mechanisms (biological or “unconscious” motivation to stimulate weight gain) however are in stark contrast to the conscious motivation to restrict food intake and threaten the set goal of healthy eating, fitness, weight loss, etc. This creates an internal conflict and triggers the anxiety about losing control and giving in to the eating drive. Food avoidance and weight loss reduce those fears, but only briefly, until the fear of potential weight gain again dominates.
Chronic AN behavior:
The fear of weight gain supersedes the bodily mechanisms that drive food intake and further food restriction occurs. This positive feedback loop leads to ongoing food restriction, more weight loss, further stimulation of the biological feeding drive, followed by heightened anxiety etc. During the underweight state of AN measures for anxious traits such as harm avoidance are elevated and individuals with AN can often be observed getting more anxious with increasing weight loss, except for brief episodes of anxiety relief when meeting a weight loss goal or exercise [65, 66]. This supports that there may be a reinforcing mechanism between AN core behaviors and anxiety. AN is also associated with low gonadal hormones and basic science has suggested that females at low weight and low sex hormone levels show altered learning including reward learning [67-70]. Low gonadal hormone levels in AN therefore may provide a neurobiological factor to impair behavior change and recovery, but the specific supporting studies in humans are still to be done.
Empirical data that support the model:
Various psychosocial factors have been identified as triggers for the initiation of AN, while family studies support a strong underlying biology that predisposes to developing the illness [71, 72]. Genetic factors that concern brain neurotransmitter function in the context of underweight or comorbid disorders such as obsessive compulsive disorder may play important roles but need further study [58, 73]. The food restriction behaviors become self-reinforcing in the underweight state, facilitated by stress and anxiety, and over time worsening the clinical presentation as suggested in other research [74-77]. Human studies have found that the frontostriatal connectivity, which has been associated with habit learning, was inversely correlated with actual food intake and thus could be a biological correlate for habit learning [78]. Animal models have supported repeatedly that weight loss is associated with physiological changes in the body and the brain reward circuitry, including dopamine release and receptor function, to drive food intake [36, 79, 80]. Our studies using the prediction error model support a similar pattern in humans with elevated dopamine-related reward system sensitivity associated with underweight [51, 53, 55]. The prediction error response was significantly positively correlated with anxiety (harm avoidance) in our AN group, and this anxiety correlated positively with core eating disorder thoughts and behaviors, drive for thinness and body dissatisfaction [53]. We also found evidence that dopamine responsiveness as reflected by the prediction error response may facilitate ongoing food restriction in AN when studying the dynamic connectivity between hypothalamus and ventral striatum. The hypothalamus integrates information about body homeostasis to drive food approach, but a fear mediated dopamine circuit from the ventral striatum to the hypothalamus has also been identified that inhibits food intake via dopamine D1 receptors [54, 81, 82]. We found that in the control group activity was directed from the hypothalamus to the ventral striatum, while in the AN group dynamic connectivity was directed from ventral striatum to the hypothalamus, and prediction error response was positively correlated with this connectivity in AN. We interpret this result that high anxiety triggers a ventral striatal-hypothalamic circuitry that depends on dopamine D1 receptors and inhibits food intake [54, 82]. Those ventral striatal dopamine D1 receptors that take part in the prediction error response, are already sensitized due to weight loss and may be hyper responsive [37, 54, 83]. Clinically, prediction error activation correlated inversely with weight gain in treatment. This is in line with the positive correlation between prediction error response and effective connectivity strength of the ventral striatal-hypothalamic circuitry that controls food intake.
Limitations to the model:
There is a host of endocrine factors that are altered in AN that could also interfere with normal eating and that are largely not integrated in the model due to lack of specific data [14]. In our study, anxiety was correlated with body dissatisfaction and drive for thinness, but we cannot exclude intrinsic abnormalities in body integration or interoception that may also worsen with weight loss and exacerbate the illness [84]. Alterations in brain structure and function may alter satiety processing in AN, which may be part of such a model and will need to be explored in future studies [85, 86]. The well-known cognitive-emotional reinforcers that strengthen the cognitive aspect of desire to lose weight such as feedback from the environment, “you lost weight, you look good” were not assessed in our study. The so-called habit formation that may be part of this model for anorexia nervosa was not measured and including this concept remains conjectural at this point [87]. The model is based on correlations between brain function, clinical outcome and behavior. Whether mediator or moderator analyses can successfully be applied will require a larger data set and further exploration of the various variables. Comorbid depression and anxiety may also have vital parts in the pathophysiology of AN and those effects need further study.
6. Clinical Implications
The here presented model provides several clinical implications. For one, it provides a disease model that is helpful in helping individuals with AN or their caregivers better understand the illness. Second, the integration of brain biology that interferes with recovery increases empathy in both treatment providers and family or caregivers as it reduces blame and the idea that AN is simply a sociocultural disorder [88]. Third, the model supports the important need to promote eating and weight gain to normalize the unconscious motivators for weight gain because normalization of eating will desensitize the feeding system. A study in AN after long term recovery indicated to a high degree normalization of prediction error response, while short term recovered individuals still showed widespread elevations of that signal [52, 55]. Fourth, the model supports an approach to develop alternative cognitive-emotional motivators. Cognitive treatment strategies typically question the drive to lose weight and the motivation for weight loss that results in objectively poor quality of life and treatment admissions. Developing again healthy motivators such as spending time with friends, family, etc. and by learning by experience to enjoy life without the constant pressure of weight loss is probably key that the person with AN is able to start let go. This is done by replacing the AN driven behaviors by the conscious healthy motivators. Cognitive behavior or other psychotherapy forms show only limited evidence in the treatment of AN, but such strategies may be helpful when weight gain has started, and engaging intrinsic conscious motivation is crucial for sustained recovery [89]. Fifth, the model supports a biological treatment direction that targets the dopamine system. The fear of weight gain can be so overwhelming that the use of logic and reasoning may be difficult to implement and altered brain dopamine function has been speculated in anxiety and altered insight in AN [90, 91]. Focus on management of temperamental traits such as high harm avoidance and punishment sensitivity may further aid in this process [92]. Elevated dopamine system sensitivity in AN might suggest applying a dopamine receptor blocking agent. However, many such drugs have been tried with poor success and this may be due to the brain expressing more receptors in response, which interferes with the desired effect of downregulating the system [12]. On the other hand, dopamine agonists could be helpful in supporting learning and behavior change, especially in females who are underweight and in a low estrogen state [93]. The dopamine D2 receptor also has effects on energy homeostasis, leptin signaling and body composition and there could be metabolic mechanisms that promote weight gain as well [94-97]. Thus, we have started to use the dopamine D2 receptor partial agonist aripiprazole with the rationale that it could support psychotherapy and weight gain. Recent data from our group indicate that aripiprazole may in fact be beneficial in weight gain during treatment of AN [98, 99]. However, aripiprazole also acts on serotonin and other neurotransmitter receptors. Whether aripiprazole’s beneficial effects for AN treatment are specifically attributable to the drug’s dopamine D2 receptor agonism is still unknown and requires further study and other neurotransmitters may also have key roles and need to be identified [93].
7. Conclusion
AN is a complex illness that is typically difficult to treat. Our limited understanding of AN’s underlying neurobiology has prevented us from developing pharmacological interventions. Here we proposed a brain-based model of how the specific pathophysiology of AN develops and maintains the desire to lose weight despite being underweight. After deciding to change eating behavior or lose weight, perfectionism and high anxiety may mediate in individuals who are prone to develop AN, the transition to preoccupations with AN core behaviors such as drive for thinness and body dissatisfaction. The body’s adaptations to weight loss that include sensitization of the dopamine system and activation of hypothalamic feeding circuits to stimulate eating, conflicts with the desire to lose weight. The fear of weight gain drives food restriction and leads to the ongoing self-reinforcing or positive feedback loop of emaciation, stimulation of feeding circuits, further fear of losing control over eating followed by starvation. The complexity of the illness and high relapse rate will require a combination of nutritional rehabilitation with more specific biological and psychotherapeutic interventions to improve treatment outcome.
Highlights.
Anorexia nervosa is associated with a drive to restrict food.
Gut hormones and reward circuits stimulate eating.
This creates a discrepancy between conscious and unconscious motivation to eat.
This leads to anxiety that mediates a vicious cycle of weight loss.
Acknowledgments
Funding
The study was supported by NIMH grants MH096777 and MH103436 (principal investigator, Dr. Frank). Ms. DeGuzman was supported by NIH grant T32HD041697 (University of Colorado Neuroscience Program) and NIH/NCATS Colorado CTSA grant TL1 TR001081.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Golden NH Eating disorders in adolescence and their sequelae. Best Pract. Res. Clin. Obstet. Gynaecol 2003,17:57–73. [DOI] [PubMed] [Google Scholar]
- [2].Arcelus J, Mitchell AJ, Wales J, Nielsen S Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch. Gen. Psychiatry 2011,68:724–31. [DOI] [PubMed] [Google Scholar]
- [3].Sullivan PF Mortality in anorexia nervosa. Am. J. Psychiatry 1995,152:1073–4. [DOI] [PubMed] [Google Scholar]
- [4].American Psychiatric Association. Desk reference to the diagnostic criteria from DSM-5. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- [5].Bulik CM Exploring the gene-environment nexus in eating disorders. J. Psychiatry Neurosci 2005,30:335–9. [PMC free article] [PubMed] [Google Scholar]
- [6].Khalsa SS, Portnoff LC, McCurdy-McKinnon D, Feusner JD What happens after treatment? A systematic review of relapse, remission, and recovery in anorexia nervosa. J Eat Disord. 2017,5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hay P, Mitchison D, Collado AEL, Gonzalez-Chica DA, Stocks N, Touyz S Burden and health-related quality of life of eating disorders, including Avoidant/Restrictive Food Intake Disorder (ARFID), in the Australian population. J Eat Disord. 2017,5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Attia E Anorexia nervosa: current status and future directions. Annu. Rev. Med 2010,61:425–35. [DOI] [PubMed] [Google Scholar]
- [9].Powers PS, Bruty H Pharmacotherapy for eating disorders and obesity. Child Adolesc. Psychiatr. Clin. N. Am 2009,18:175–87. [DOI] [PubMed] [Google Scholar]
- [10].Bulik CM, Hebebrand J, Keski-Rahkonen A, Klump KL, Reichborn-Kjennerud T, Mazzeo SE, et al. Genetic epidemiology, endophenotypes, and eating disorder classification. Int. J. Eat. Disord 2007,40 Suppl:S52–60. [DOI] [PubMed] [Google Scholar]
- [11].Crow SJ, Mitchell JE, Roerig JD, Steffen K What potential role is there for medication treatment in anorexia nervosa? Int. J. Eat. Disord 2009,42:1–8. [DOI] [PubMed] [Google Scholar]
- [12].Frank GK, Shott ME The Role of Psychotropic Medications in the Management of Anorexia Nervosa: Rationale, Evidence and Future Prospects. CNS Drugs. 2016,30:419–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Monteleone P, Maj M Dysfunctions of leptin, ghrelin, BDNF and endocannabinoids in eating disorders: beyond the homeostatic control of food intake. Psychoneuroendocrinology. 2013,38:312–30. [DOI] [PubMed] [Google Scholar]
- [14].Monteleone AM, Castellini G, Volpe U, Ricca V, Lelli L, Monteleone P, et al. Neuroendocrinology and brain imaging of reward in eating disorders: A possible key to the treatment of anorexia nervosa and bulimia nervosa. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018,80:132–42. [DOI] [PubMed] [Google Scholar]
- [15].Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010,214:519–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kelley AE, Baldo BA, Pratt WE, Will MJ Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol. Behav 2005,86:773–95. [DOI] [PubMed] [Google Scholar]
- [17].Rolls ET Taste, olfactory and food texture reward processing in the brain and obesity. Int J Obes (Lond). 2010. [DOI] [PubMed] [Google Scholar]
- [18].Kennerley SW, Walton ME, Behrens TE, Buckley MJ, Rushworth MF Optimal decision making and the anterior cingulate cortex. Nat. Neurosci 2006,9:940–7. [DOI] [PubMed] [Google Scholar]
- [19].Kringelbach ML, Rolls ET The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol 2004,72:341–72. [DOI] [PubMed] [Google Scholar]
- [20].Moran TH, Ladenheim EE Physiologic and Neural Controls of Eating. Gastroenterol. Clin. North Am 2016,45:581–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rolls BJ, Rolls ET, Rowe EA, Sweeney K Sensory specific satiety in man. Physiol. Behav 1981,27:137–42. [DOI] [PubMed] [Google Scholar]
- [22].Frank GK The Perfect Storm - A Bio-Psycho-Social Risk Model for Developing and Maintaining Eating Disorders. Front. Behav. Neurosci 2016,10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lloyd EC, Steinglass JE What can food-image tasks teach us about anorexia nervosa? A systematic review. J Eat Disord. 2018,6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kapur S, Remington G Serotonin-dopamine interaction and its relevance to schizophrenia. Am. J. Psychiatry 1996,153:466–76. [DOI] [PubMed] [Google Scholar]
- [25].Alexander G, Crutcher M, DeLong M Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor "prefrontal" and "limbic" functions. Prog. Brain Res 1990,85:119–46. [PubMed] [Google Scholar]
- [26].Halford JC, Cooper GD, Dovey TM The pharmacology of human appetite expression. Curr. Drug Targets 2004,5:221–40. [DOI] [PubMed] [Google Scholar]
- [27].Volkow ND, Fowler JS, Wang GJ Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav. Pharmacol 2002,13:355–66. [DOI] [PubMed] [Google Scholar]
- [28].Schultz W Getting formal with dopamine and reward. Neuron. 2002,36:241–63. [DOI] [PubMed] [Google Scholar]
- [29].Haber SN, Knutson B The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010,35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Avena NM, Rada P, Hoebel BG Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience. 2008,156:865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Carr KD Chronic food restriction: enhancing effects on drug reward and striatal cell signaling. Physiol. Behav 2007,91:459–72. [DOI] [PubMed] [Google Scholar]
- [32].Johnson PM, Kenny PJ Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci 2010,13:635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Volkow ND Addiction Reviews. Introduction. Ann. N. Y. Acad. Sci 2008,1141:xi–xii. [DOI] [PubMed] [Google Scholar]
- [34].Oinio V, Backstrom P, Uhari-Vaananen J, Raasmaja A, Piepponen P, Kiianmaa K Dopaminergic modulation of reward-guided decision making in alcohol-preferring AA rats. Behav. Brain Res 2017,326:87–95. [DOI] [PubMed] [Google Scholar]
- [35].Kelley AE Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci. Biobehav. Rev 2004,27:765–76. [DOI] [PubMed] [Google Scholar]
- [36].Carr KD, Tsimberg Y, Berman Y, Yamamoto N Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience. 2003,119:1157–67. [DOI] [PubMed] [Google Scholar]
- [37].Carr KD, Cabeza de Vaca S, Sun Y, Chau LS Reward-potentiating effects of D-1 dopamine receptor agonist and AMPAR GluR1 antagonist in nucleus accumbens shell and their modulation by food restriction. Psychopharmacology (Berl.) 2009,202:731–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kaye WH, Frank GK, McConaha C Altered dopamine activity after recovery from restricting-type anorexia nervosa. Neuropsychopharmacology. 1999,21:503–6. [DOI] [PubMed] [Google Scholar]
- [39].Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC, et al. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol. Psychiatry 2005,58:908–12. [DOI] [PubMed] [Google Scholar]
- [40].Broft A, Slifstein M, Osborne J, Kothari P, Morim S, Shingleton R, et al. Striatal dopamine type 2 receptor availability in anorexia nervosa. Psychiatry Res. 2015,233:380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].NIMH-RDoC-working-group. Positive Valence Systems: Workshop Proceedings. In: NIMH, editor. Rockville, Maryland: 2011. [Google Scholar]
- [42].Rescorla RA Stimulus generalization: some predictions from a model of Pavlovian conditioning. J. Exp. Psychol. Anim. Behav. Process 1976,2:88–96. [DOI] [PubMed] [Google Scholar]
- [43].Schultz W, Dayan P, Montague PR A neural substrate of prediction and reward. Science. 1997,275:1593–9. [DOI] [PubMed] [Google Scholar]
- [44].D'Ardenne K, McClure SM, Nystrom LE, Cohen JD BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008,319:1264–7. [DOI] [PubMed] [Google Scholar]
- [45].O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ Temporal difference models and reward-related learning in the human brain. Neuron. 2003,38:329–37. [DOI] [PubMed] [Google Scholar]
- [46].Jocham G, Klein TA, Ullsperger M Dopamine-mediated reinforcement learning signals in the striatum and ventromedial prefrontal cortex underlie value-based choices. J. Neurosci 2011,31:1606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ Model-based influences on humans' choices and striatal prediction errors. Neuron. 2011,69:1204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].de Araujo IE, Ren X, Ferreira JG Metabolic sensing in brain dopamine systems. Results Probl. Cell Differ 2010,52:69–86. [DOI] [PubMed] [Google Scholar]
- [49].Olsavsky AK, Shott ME, DeGuzman MC, Frank GKW Neural correlates of taste reward value across eating disorders. Psychiatry Res Neuroimaging. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cowdrey FA, Park RJ, Harmer CJ, McCabe C Increased neural processing of rewarding and aversive food stimuli in recovered anorexia nervosa. Biol. Psychiatry 2011,70:736–43. [DOI] [PubMed] [Google Scholar]
- [51].Frank GK, Reynolds JR, Shott ME, Jappe L, Yang TT, Tregellas JR, et al. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology. 2012,37:2031–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Frank GK, Collier S, Shott ME, O'Reilly RC Prediction error and somatosensory insula activation in women recovered from anorexia nervosa. J. Psychiatry Neurosci 2016,41:304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Frank GKW, DeGuzman MC, Shott ME, Laudenslager ML, Rossi B, Pryor T Association of Brain Reward Learning Response With Harm Avoidance, Weight Gain, and Hypothalamic Effective Connectivity in Adolescent Anorexia Nervosa. JAMA Psychiatry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].O'Connor EC, Kremer Y, Lefort S, Harada M, Pascoli V, Rohner C, et al. Accumbal D1R Neurons Projecting to Lateral Hypothalamus Authorize Feeding. Neuron. 2015,88:553–64. [DOI] [PubMed] [Google Scholar]
- [55].DeGuzman M, Shott ME, Yang TT, Riederer J, Frank GKW Association of Elevated Reward Prediction Error Response With Weight Gain in Adolescent Anorexia Nervosa. Am. J. Psychiatry 2017,174:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dignon A, Beardsmore A, Spain S, Kuan A 'Why I won't eat': patient testimony from 15 anorexics concerning the causes of their disorder. J. Health Psychol 2006,11:942–56. [DOI] [PubMed] [Google Scholar]
- [57].Jacobs MJ, Roesch S, Wonderlich SA, Crosby R, Thornton L, Wilfley DE, et al. Anorexia nervosa trios: behavioral profiles of individuals with anorexia nervosa and their parents. Psychol. Med 2009,39:451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yilmaz Z, Halvorsen M, Bryois J, Yu D, Thornton LM, Zerwas S, et al. Examination of the shared genetic basis of anorexia nervosa and obsessive-compulsive disorder. Mol. Psychiatry 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Coniglio KA, Becker KR, Franko DL, Zayas LV, Plessow F, Eddy KT, et al. Won't stop or can't stop? Food restriction as a habitual behavior among individuals with anorexia nervosa or atypical anorexia nervosa. Eat Behav. 2017,26:144–7. [DOI] [PubMed] [Google Scholar]
- [60].Steinglass JE, Glasofer DR, Walsh E, Guzman G, Peterson CB, Walsh BT, et al. Targeting habits in anorexia nervosa: a proof-of-concept randomized trial. Psychol. Med 2018,48:2584–91. [DOI] [PubMed] [Google Scholar]
- [61].Haynos AF, Hall LMJ, Lavender JM, Peterson CB, Crow SJ, Klimes-Dougan B, et al. Resting state functional connectivity of networks associated with reward and habit in anorexia nervosa. Hum. Brain Mapp 2019,40:652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Beck KD, Luine VN Food deprivation modulates chronic stress effects on object recognition in male rats: role of monoamines and amino acids. Brain Res. 1999,830:56–71. [DOI] [PubMed] [Google Scholar]
- [63].Oswald LM, Wong DF, McCaul M, Zhou Y, Kuwabara H, Choi L, et al. Relationships among ventral striatal dopamine release, cortisol secretion, and subjective responses to amphetamine. Neuropsychopharmacology. 2005,30:821–32. [DOI] [PubMed] [Google Scholar]
- [64].Wise RA, Morales M A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Res. 2010,1314:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bergh C, Sodersten P Anorexia nervosa, self-starvation and the reward of stress. Nat. Med 1996,2:21–2. [DOI] [PubMed] [Google Scholar]
- [66].Klump KL, Strober M, Bulik CM, Thornton L, Johnson C, Devlin B, et al. Personality characteristics of women before and after recovery from an eating disorder. Psychol. Med 2004,34:1407–18. [DOI] [PubMed] [Google Scholar]
- [67].Lipatova O, Wiener N, Andrews K, Kirshenbaum AP, Green JT, Toufexis DJ 17betaestradiol replacement in ovariectomized female rats slows set 1 dorsolateral striatial-dependent learning and enhances learning of set 2 in an extradimensional set-shifting paradigm. Behav. Neurosci 2016,130:44–9. [DOI] [PubMed] [Google Scholar]
- [68].Kunkhyen T, Perez E, Bass M, Coyne A, Baum MJ, Cherry JA Gonadal hormones, but not sex, affect the acquisition and maintenance of a Go/No-Go odor discrimination task in mice. Horm. Behav 2018,100:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Rey CD, Lipps J, Shansky RM Dopamine D1 receptor activation rescues extinction impairments in low-estrogen female rats and induces cortical layer-specific activation changes in prefrontal-amygdala circuits. Neuropsychopharmacology. 2014,39:1282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Richard JE, Lopez-Ferreras L, Anderberg RH, Olandersson K, Skibicka KP Estradiol is a critical regulator of food-reward behavior. Psychoneuroendocrinology. 2017,78:193–202. [DOI] [PubMed] [Google Scholar]
- [71].Munro C, Randell L, Lawrie SM An Integrative Bio-Psycho-Social Theory of Anorexia Nervosa. Clin. Psychol. Psychother 2017,24:1–21. [DOI] [PubMed] [Google Scholar]
- [72].Thornton LM, Mazzeo SE, Bulik CM The heritability of eating disorders: methods and current findings. Curr. Top. Behav. Neurosci 2011,6:141–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Shih PA, Woodside DB Contemporary views on the genetics of anorexia nervosa. Eur. Neuropsychopharmacol 2016,26:663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Tabri N, Murray HB, Thomas JJ, Franko DL, Herzog DB, Eddy KT Overvaluation of body shape/weight and engagement in non-compensatory weight-control behaviors in eating disorders: is there a reciprocal relationship? Psychol. Med 2015,45:2951–8. [DOI] [PubMed] [Google Scholar]
- [75].Gianini LM, Klein DA, Call C, Mayer L, Foltin RW, Walsh BT, et al. The reinforcing effect of exercise in anorexia nervosa: Clinical correlates and relationship to outcome. Eat Disord. 2016,24:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Guarda AS, Schreyer CC, Boersma GJ, Tamashiro KL, Moran TH Anorexia nervosa as a motivated behavior: Relevance of anxiety, stress, fear and learning. Physiol. Behav 2015,152:466–72. [DOI] [PubMed] [Google Scholar]
- [77].Sodersten P, Nergardh R, Bergh C, Zandian M, Scheurink A Behavioral neuroendocrinology and treatment of anorexia nervosa. Front. Neuroendocrinol 2008,29:445–62. [DOI] [PubMed] [Google Scholar]
- [78].Foerde K, Steinglass JE, Shohamy D, Walsh BT Neural mechanisms supporting maladaptive food choices in anorexia nervosa. Nat. Neurosci 2015,18:1571–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Branch SY, Goertz RB, Sharpe AL, Pierce J, Roy S, Ko D, et al. Food restriction increases glutamate receptor-mediated burst firing of dopamine neurons. J. Neurosci 2013,33:13861–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008,62:50–61. [DOI] [PubMed] [Google Scholar]
- [81].Stratford TR, Kelley AE Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J. Neurosci 1999,19:11040–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Castro DC, Cole SL, Berridge KC Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front. Syst. Neurosci 2015,9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Maia TV, Frank MJ From reinforcement learning models to psychiatric and neurological disorders. Nat. Neurosci 2011,14:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gaudio S, Quattrocchi CC Neural basis of a multidimensional model of body image distortion in anorexia nervosa. Neurosci. Biobehav. Rev 2012,36:1839–47. [DOI] [PubMed] [Google Scholar]
- [85].Frank GK, Shott ME, Hagman JO, Mittal VA Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am. J. Psychiatry 2013,170:1152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wierenga CE, Bischoff-Grethe A, Rasmusson G, Bailer UF, Berner LA, Liu TT, et al. Aberrant Cerebral Blood Flow in Response to Hunger and Satiety in Women Remitted from Anorexia Nervosa. Front Nutr. 2017,4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Steinglass JE, Walsh BT Neurobiological model of the persistence of anorexia nervosa. J Eat Disord. 2016,4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Crisafulli MA, Von Holle A, Bulik CM Attitudes towards anorexia nervosa: the impact of framing on blame and stigma. Int. J. Eat. Disord 2008,41:333–9. [DOI] [PubMed] [Google Scholar]
- [89].Yager J, Devlin M, Halmi K, Herzog D, Mitchell J, Powers P, et al. Practice Guideline for the Treatment of Patients With Eating Disorders, 3rd Edition. Guideline Watch: American Psychiatric Association; 2012. [Google Scholar]
- [90].O'Hara CB, Campbell IC, Schmidt U A reward-centred model of anorexia nervosa: a focussed narrative review of the neurological and psychophysiological literature. Neurosci. Biobehav. Rev 2015,52:131–52. [DOI] [PubMed] [Google Scholar]
- [91].Bailer UF, Price JC, Meltzer CC, Wagner A, Mathis CA, Gamst A, et al. Dopaminergic activity and altered reward modulation in anorexia nervosa-insight from multimodal imaging. Int. J. Eat. Disord 2017,50:593–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kaye WH, Wierenga CE, Knatz S, Liang J, Boutelle K, Hill L, et al. Temperament-based treatment for anorexia nervosa. Eur Eat Disord Rev. 2015,23:12–8. [DOI] [PubMed] [Google Scholar]
- [93].Frank GK Could dopamine agonists aid in drug development for anorexia nervosa? Front Nutr. 2014,1:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ramos EJ, Meguid MM, Campos AC, Coelho JC Neuropeptide Y, alpha-melanocyte-stimulating hormone, and monoamines in food intake regulation. Nutrition. 2005,21:269–79. [DOI] [PubMed] [Google Scholar]
- [95].Kim KS, Yoon YR, Lee HJ, Yoon S, Kim SY, Shin SW, et al. Enhanced hypothalamic leptin signaling in mice lacking dopamine D2 receptors. J. Biol. Chem 2010,285:8905–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yoon YR, Baik JH Melanocortin 4 Receptor and Dopamine D2 Receptor Expression in Brain Areas Involved in Food Intake. Endocrinol Metab (Seoul). 2015,30:576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Perez Millan MI, Luque GM, Ramirez MC, Noain D, Ornstein AM, Rubinstein M, et al. Selective disruption of dopamine D2 receptors in pituitary lactotropes increases body weight and adiposity in female mice. Endocrinology. 2014,155:829–39. [DOI] [PubMed] [Google Scholar]
- [98].Frank GK, Shott ME, Hagman JO, Schiel MA, DeGuzman MC, Rossi B The partial dopamine D2 receptor agonist aripiprazole is associated with weight gain in adolescent anorexia nervosa. Int. J. Eat. Disord 2017,50:447–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Trunko ME, Schwartz TA, Duvvuri V, Kaye WH Aripiprazole in anorexia nervosa and low-weight bulimia nervosa: case reports. Int. J. Eat. Disord 2011,44:269–75. [DOI] [PubMed] [Google Scholar]


