Abstract
Osteoporosis, a condition of skeletal decline that undermines quality of life, is treated with pharmacological interventions that are associated with poor adherence and adverse effects. Complicating efforts to improve clinical outcomes, the incidence of obesity is increasing, predisposing the population to a range of musculoskeletal complications and metabolic disorders. Pharmacological management of obesity has yet to deliver notable reductions in weight and debilitating complications are rarely avoided. By contrast, exercise shows promise as a non-invasive and non-pharmacological method of regulating both osteoporosis and obesity. The principal components of exercise — mechanical signals — promote bone and muscle anabolism while limiting formation and expansion of fat mass. Mechanical regulation of bone and marrow fat might be achieved by regulating functions of differentiated cells in the skeletal tissue while biasing lineage selection of their common progenitors — mesenchymal stem cells. An inverse relationship between adipocyte versus osteoblast fate selection from stem cells is implicated in clinical conditions such as childhood obesity and increased marrow adiposity in type 2 diabetes mellitus, as well as contributing to skeletal frailty. Understanding how exercise-induced mechanical signals can be used to improve bone quality while decreasing fat mass and metabolic dysfunction should lead to new strategies to treat chronic diseases such as osteoporosis and obesity.
The evolution of Homo sapiens from hunters to farmers, and then from agrarian cultures to the industrial era1,2, drove profound adaptations to our skeletal phenotype, including a decline in bone quantity and quality3. In parallel, as food has become more accessible and lifestyles less physically demanding, the composition of the human body has shifted towards increased body fat and reduced lean tissue mass, which is partly a consequence of a vestigial survival strategy that stockpiles calories when food is available4. The post-Industrial Revolution era and the sedentary lifestyle that it has promoted has fostered two major diseases — osteoporosis and obesity5–7. Basic, applied and translational sciences emphasize that these diseases can be managed by reintroducing physical activity into our lives8. Exercise, at a minimum, provides additive benefits to pharmacological interventions to improve bone quality and reduce fat mass, and is frequently recommended to those capable of strenuous activity and sustaining high-magnitude loading. However, intense physical activity is often not achievable in ageing populations and for those with underlying musculoskeletal or metabolic conditions for whom exercise is simply not possible or could be dangerous. Alternative approaches that incorporate mechanical stimuli without the need to run a marathon or compete on a football pitch are being studied for clinical application in these less-mobile populations.
Osteoporosis, which is defined as decreased bone quantity and quality, has multiple aetiologies, ranging from age-related conditions (postmenopausal and ageing physiology) to genetic causes (for example, mutations that result in WNT1 deficiency), endocrine or disease-specific treatment modifiers (for example, glucocorti-costeroids and aromatase inhibitors) and unloading (bed rest or paraplegia). Statistically, osteoporosis primarily affects postmenopausal women and elderly men, with 30% of women and 20% of men >50 years old predicted to experience an osteoporosis-related fracture in their lifetime9,10. Age-driven shifts in hormone status11–13, compounded by reduced physical activity, disrupt balanced bone remodelling, leading to elevated bone resorption and suppressed bone formation. The risk of fracture in the ageing population is further compounded by muscle wasting, or sarcopenia, which contributes to muscle weakness and decreased mobility, as well as an increase in instability that precipitates the risk of falls14. Although pharmaceutical approaches for sarcopenia have yet to be approved for use, interventions targeting osteoporosis have been well studied and demonstrate beneficial effects on fracture risk15. However, adverse effects16 can accrue during long-term use of osteoporosis therapeutics17, and a lack of patient adherence to the drug therapy can occur18,19. In addition, poor patient adherence with standard osteoporotic recommendations20 continues to be a barrier to effective treatment in our ageing populations.
Equally disconcerting, almost 40% of adults and nearly 20% of adolescents in the United States have obesity, with a continuing upwards trend: in the past 50 years, the prevalence of obesity has risen by 27% in adults and 47% among children21,22, which has been promoted by sedentary lifestyles and poor nutrition. Obesity markedly increases susceptibility to a range of associated diseases (for example, type 2 diabetes mellitus23 and cardiovascular disease24), physical limitations (such as immobility25 and atypical gait26) and chronic inflammation (for example, osteoarthritis27–29). Obesity not only increases the risk of many solid tumours30–32 but also promotes cancer metastases33. Compounding these problems, accrual of adipose tissue within the bone marrow space can lead to an inflammatory state34, which increases bone resorption, disrupts differentiation of mesenchymal stem cells (MSCs) and haematopoietic stem cells (HSCs)33 and undermines regenerative and immune responses35,36. Decreasing adipose tissue mass or regulating the functions of the adipocytes in the bone marrow might present a target for controlling both bone quality and inflammation, which is of great interest for developing new therapeutic strategies37.
Weight-bearing exercise is a cornerstone in the treatment and prevention of postmenopausal and age-associated osteoporosis. The National Osteoporosis Foundation recommends skeletal loading with both high-impact and low-impact weight-bearing exercises for at least 30 min per day, 5–7 days a week38. Importantly, MSCs, the shared progenitor for bone and adipose cells, seem to be key to the inverse control of cell output, interpreting mechanical signals as stimulatory for bone and inhibitory for adipose39. Furthermore, muscle strengthening exercises are now recommended as complimentary to weight-bearing exercises to improve posture, reduce fall risk and promote musculoskeletal anabolism8. The dynamic ground-reaction forces generated during exercise transduce a range of signals across the skeleton and musculature, subjecting cells, tissues and organs to mechanical strain (deformation) and acceleration. Key beneficial outcomes of exercise include increased lean muscle mass, increased bone mineralization and turnover rate and decreased systemic inflammation.
A central tenet of this Review is that key regulatory signals are generated during exercise and that these factors are first and foremost mechanical in nature. Thus, a goal of this Review is to discuss how these ubiquious signals arising from activity are first perceived by the cell population and then how the cells respond to them, with particular emphasis on the musculoskeletal and adipose systems. In addition, how metabolic and genetic disorders, as well as ageing, can disrupt this process is addressed. Finally, we consider how surrogates for exercise might serve to treat these conditions.
Mechanical influences on bone
Bone adaptation to physiological extremes.
Bone mass and architecture are placed at risk by disuse40 but can respond to exercise with increased mass and strength, leading to the ‘use it or lose it’ tenet of bone adaptation that is often referred to as Wolff ‘s law41. Retrospective studies illustrate the response of bone to physical extremes. Astronauts enduring microgravity lose as much as 2% of their hip BMD each month42, whereas professional tennis players have up to 35% more bone hypertrophy in the serving arm than in the arm that simply tosses the ball into the air43. Furthermore, several site-specific benefits correlate with the specialized tasks of elite sportspeople trained over extended periods44, where enhancement of bone morphology is greater in athletes challenged with intense impact training (for example, football and gymnastics) than in those engaged in ‘smoother’ sports such as cycling or swimming45. What is also clear is that commitment to exercise early in life will maximize potential gains, with strong correlations between physical activity and bone strength being evident from childhood to early adulthood46.
Response to new exercise regimens.
The results of several prospective trials indicate that new loading challenges can induce system-level and site-specific accretions of bone mass. Intense exercise in young army recruits stimulated increases in BMD47, and a 10-month, high-impact strength-building regimen in children significantly (1.9% versus 3.8%, P = 0.002) increased femoral neck BMD48. Despite the apparent anabolic nature of the mechanical signal, moderate exercise regimens generally result in modest (if any) increases in bone mass; for example, a 1-year high-resistance strength-training study in young women (mean age of 23.8 ± 5.0 years) significantly increased muscle strength (14%, P = 0.001) but failed to influence bone mass49. By contrast, high-intensity resistance plus impact training improved both BMD and physical function in postmenopausal women with osteoporosis8. The inherent complexity of exercise-generated mechanical challenges for skeletal tissues50 indicates that some components of the load-bearing regimen might be more influential than others51,52.
Mechanisms.
The ability of mechanical signals to increase musculoskeletal mass and quality is multifactorial, as it involves simultaneously repressing pathways involved in the formation of adipose tissue and the resorption of bone53,54 (FIG. 1). Exercise exerts a range of forces across the appendicular and axial skeleton55; therefore, the musculoskeletal construct acts as a conduit to transduce both peaks of ground-reaction forces and the spectral content of muscle contraction, bombarding the bone tissue with both high-frequency and low-frequency mechanical signals. Transmitted through tissues to the cellular level, mechanical responses are mediated through cytoskeletal proteins and transmembrane-bound integrins that link the extracellular environment with the genetic machinery encased within the nucleus56–61. Propagation of mechanical signals along the WNT–β-catenin pathway enhances osteogenic62,63 (RUNX2, encoding Runt-related transcription factor 2) gene expression and chondrogenic (SOX9) growth while arresting adipogenesis64. By increasing both muscle and bone mass and strength, exercise succeeds in reducing the incidence of bone fracture (reduction in falls and improvement of fracture strength)65, achieved to a degree by upregulating the expression of osteogenic, chondrogenic or myogenic66 growth factors while pathways conducive to PPARγ-driven adipogenesis are downregulated64,67–70.
Fig. 1 |. Exercise and mechanical signals are anabolic to skeletal tissue and muscle and slow excessive bone resorption, counteracting the negative effects of a high-fat diet and sedentary lifestyle on bone and fat.
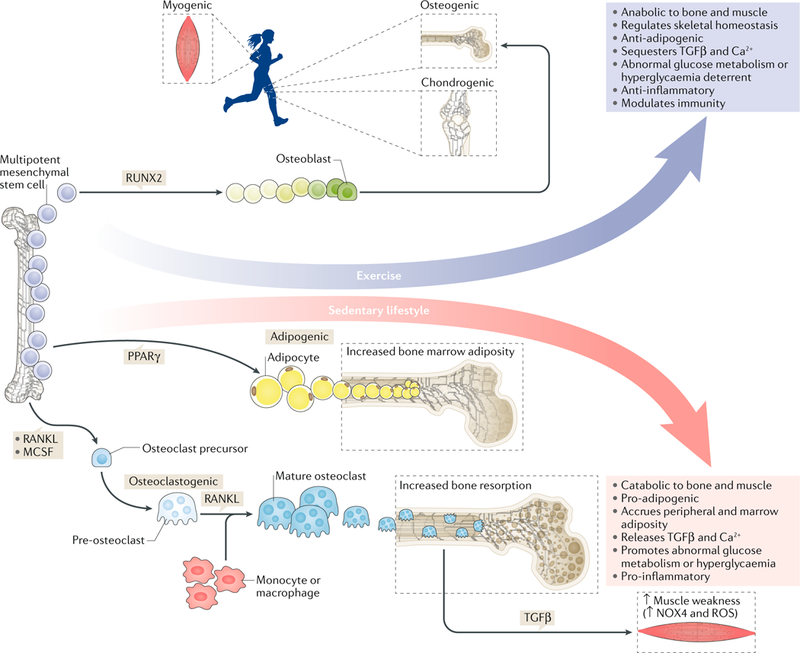
Mesenchymal stem cell lineage selection as a function of mechanical signals drives osteogenic differentiation. Here, exercise is directly responsible for augmenting bone and muscle mass while suppressing the accumulation of fat. By contrast, sedentary lifestyles (the absence of mechanical stimuli, often accompanied by poor diet) increase adipogenic programmes, resulting in increased marrow adiposity that can obstruct the persistence of bone-remodelling cells. In the absence of mechanical load, osteoclast-mediated resorption is accelerated, in part, by the secretion of inflammatory adipokines released into the marrow, which results in the resorption of abnormal levels of bone that are not reciprocated by bone formation, as is the case in normal bone remodelling. Chronic destruction of bone matrix releases transforming growth factor-β (TGFβ), an inflammatory cytokine that leads to an impairment in the calcium gradient across muscle fibres. Therefore, mechanical signals are critical in regulating the dynamic between bone, muscle and fat. Depriving the body of mechanical stimuli in combination with a high-fat diet perpetuates extensive bone loss, muscle weakness and fatty-tissue accumulation around vital organs, which are tissue phenotypes that are conducive to the advancement of osteoporosis, impairments in glucose metabolism and chronic inflammation. NOX4, NADPH oxidase 4; RANKL, receptor activator of nuclear factor-κB ligand; ROS, reactive oxygen species; RUNX2, Runt-related transcription factor 2.
Whereas exercise delivers large quantities of mechanical information to the musculoskeletal system, absence of this regulatory information as a consequence of disuse (such as during chronic bed rest, exposure to microgravity, immobilization due to a cast or reduced physical activity)71,72 results in conditions in which muscles, tendons and ligaments undergo catabolism and bone is rapidly resorbed73–76. Concurrently, studies show that extended bed rest drives increased marrow adipogenesis77, which exacerbates the consequences of inactivity. For example, disuse will increase the expression of PPARγ in MSCs and receptor activator of nuclear factor-κB ligand (RANKL) in bone marrow, which promotes osteoclast-mediated bone resorption, yet both are rapidly suppressed via introduction of mechanical stimuli64,78.
Benefits of exercise in patients.
As outlined, the musculoskeletal system of healthy individuals is highly sensitive to mechanical signals. The mechanosensitivity of bone and muscle also suggests that patients with osteoporosis might benefit from incorporating exercise into a treatment regimen; however, the degree of the regenerative effects of exercise might be limited by cell senescence, radiation damage and the effects of ageing (where stem and progenitor cells that have undergone apoptosis are already removed from the potential pool of replacement cells)79,80. Indeed, activities such as strength training are recognized as critical to achieving and maintaining a robust musculoskeletal system and to reducing the risk of fracture81. These activities have the added benefits of suppressing adiposity, the onset of obesity and the development of obesity-induced diabetes mellitus82.
Postmenopausal women83 and men with low testosterone levels84, who have an increased risk of fracture85 and elevated levels of visceral adiposity, are encouraged to incorporate exercise into their daily regimen to improve bone strength86 and muscle mass87. In children and adolescents with chronic diseases, such as cancer88 or type 1 diabetes mellitus89, concomitant low bone density and suboptimal muscle mass and/or function are prevalent and persist into adulthood90,91. The addition of exercise, when tolerated, is key to conditioning in children with chronic diseases. Exercise is also recommended for individuals with secondary osteoporosis as a result of cancer (such as breast cancer) or as an adverse effect of certain treatments (such as aromatase inhibitors); however, the effects of the disease or the treatment can prevent these individuals from participating in enough exercise to see benefits and might actually cause the fracture it is intended to prevent92. This effect is particularly evident in patients who are too frail to undertake exercise with sufficient impact to improve bone end points; the frail state thus aggravates bone loss. Furthermore, studies have shown that variations in osteocyte sensitivity and their lacunar morphology persist with ageing93,94. However, in vitro studies performed on cells collected from the bones of ageing women (between the ages of 53 and 80 years)95,96 have shown that anabolic responses (for example, production of bone matrix and prostaglandin E2) can be upregulated if the signals are dynamic (that is, a time-varying, as opposed to static, or constant, signal) in nature97.
Age influences the response to exercise.
Indeed, ageing might affect skeletal sensitivity to mechanical information. It is clear that younger people more rapidly accrue bone in response to exercise than do older people81, an observation supported by animal studies98,99. Ageing could also affect the ability of loading to deform bone99 and results in a reduction in the number of available stem cells in older mice100. Other factors that are associated with ageing, such as a change in osteocyte morphology93, might also contribute to reduced load sensation. A great deal of exciting new information regarding the nature of tissue senility suggests fundamentally new ways to think about ageing. It appears that within many tissues, including bone, joint and muscle, some cells become ‘senescent’ and secrete cytokines that lead to disruption of normal physiology101. In the case of bone, senescent osteoblasts secrete a pro-inflammatory panel of factors that lead to resorption and decreased repair102. Indeed, senolytic compounds (such as navitoclax and quercetin) and targeted destruction of senescent cells have demonstrated promise in overcoming apoptotic programmes103 and preventing bone102 and muscle104 loss105. It will be fascinating to determine if exercise can delay the appearance of these ageing-associated cells or modify their secretory profiles.
Ageing, exercise and muscle phenotype
Muscle in ageing.
Fracture risk is coupled to muscle health; thus, in order to address the effect of mechanical input on bone, one should also consider how exercise influences muscle mass and function106. Age107,108, disease, cell senescence80,109, reduced physical activity110 and diminished synthesis of sex steroids (androgens and oestrogens) contribute to reduced muscle cross-sectional area and mass111, alterations in which types of muscle fibre are present, lipid infiltration of muscle, decreased protein synthesis and decreased muscle-specific force, which collectively lead to sarcopenia112,113. The definition of sarcopenia, which might seem obvious to clinicians, is still controversial in clinical studies114 but can be diagnosed using dual-energy X-ray absorptiometry (DXA) to measure skeletal mass and predict disease progression as well as all-cause mortality. Decline in muscle function is also hard to precisely diagnose. Measurable muscle decline begins at approximately 30 years of age, with one report estimating sarcopenia to affect almost 50% of the US population >60 years old115 and other reports suggesting numbers below 20%116. Men and women are equally predisposed to the development of age-related sarcopenia, but muscle decline begins earlier in women than in men117 and might contribute to fall risk, ultimately increasing fracture risk. A consensus definition, as well as more studies, will be necessary to confirm the degree to which sarcopenia affects the health of the ageing population.
Muscle–bone crosstalk.
Crosstalk between bone and skeletal muscle is mediated by mechanical signal transduction and soluble factors118. Skeletal muscle strength is dependent on protein integrity, which is regulated by calcium, specifically ryanodine receptors (RyRs; intracellular calcium channels). These receptors regulate119 potentials maintained across the sarcoplasmic reticulum of muscle fibres, thereby orchestrating contractile forces. Maladaptive post-translational modifications to the RyR1 channel, including oxidation or nitrosylation, lead to the dissociation of its stabilizing subunit calstabin 1. As a result of this lack of stabilization, pathological leaks of Ca2+ occur, which contribute to muscle weakness in ageing, chronic muscle fatigue, heart disease and muscular dystrophy120–122.
Accelerated bone destruction as a consequence of bone metastases123,124 or other high-turnover bone diseases, such as Camurati–Engelmann disease125, releases transforming growth factor-β (TGFβ) stored within the mineralized bone matrix126 into the circulation. In mice and humans, this bone-derived TGFβ upregulates NADPH oxidase 4 (NOX4)-mediated production of reactive oxygen species (ROS), which leads to instability of the RyR1–calstabin 1 complex, leakage of Ca2+ and muscle weakness127. Bisphosphonate inhibitors of osteoclastic bone resorption reduce circulating levels of TGFβ128 and prevent muscle weakness in mice with osteolytic breast cancer bone metastases, which suggests that pathological bone remodelling is involved in muscle weakness in cancer-induced osteolysis as well as other states of bone loss129. As physical activity decreases in those with osteoporosis124, other bone maladies or obesity130,131, MSC fate selection is biased towards adipocytes in lieu of bone68, further degrading musculoskeletal integrity and strength. This process represents a feed-forward cycle in which bone loss promotes muscle weakness, resulting in further reductions in bone and increases in adipose tissue.
Effects of exercise on muscle.
The management of sarcopenia includes improved nutrition, protein and/or pharmacological supplementation and physical training. Use of either synthetic or endogenous growth hormone has had mixed efficacy as unexpected biochemical alterations have led to adverse cardiovascular and endocrine function132–134. These poor results have meant that the pharmaceutical industry has been unwilling to address common age-associated sarcopenia. Incorporating various forms of exercise135,136 and physical activity into our daily lives improves muscle function, offsets age-related changes to muscle morphology136 and improves insulin resistance137,138. Physical activity increases the oxidative capacity of muscle and encourages anabolic growth and function through the mammalian target of rapamycin complex 1 (mTORC1)139,140, PI3K–AKT and NF-κB pathways141. At the molecular level, exercise is a potent inhibitor of the FOXO142 family of muscle-controlled transcription factors that are tightly linked to muscle atrophy143 (such as FOXO3) and attenuation of bone formation through WNT suppression144. Inhibition of FOXO3 is mediated by resistance exercise through activation of the PI3K–AKT–mTOR pathway145,146. Therefore, by default, the introduction of mechanical stimuli through exercise facilitates pathways conducive to the maintenance and growth of bone.
Force production at the level of the skeletal myocyte depends on the proper handling of Ca2+ between the sarcoplasmic reticulum and the cytosol. During excitation contraction coupling, sarcoplasmic-reticulum-sequestered Ca2+ is released through activated RyR1 into the cytoplasm, which permits Ca2+-dependent actin–myosin cross bridging. Disruption of the RyR1 complex caused by oxidative stress has been implicated in muscle weakness122 due to ageing, congestive heart failure, muscular dystrophy and cancer-associated osteolysis127. In the latter setting, the muscle weakness is mediated by release of bone-derived TGFβ and has implications for any state of increased bone resorption, including ageing, sex steroid deprivation, cancer and/or drug-treatment-induced osteoporosis. Thus, the prevention of bone loss through exercise intervention might have positive indirect downstream effects on muscle. Maladaptation of RyR1 has been shown in other disorders of muscle weakness and bone loss. For instance, in humans, muscle atrophy as a consequence of 60 days of bed rest resulted in dysfunctional Ca2+ homeostasis with increased S-nitrosylation of the RyR1 and malfunction of the SERCA1 pump147. This maladaptive nitrosylation of RyR1 was rescued with a combination of resistive exercise and low-intensity vibration (an exercise surrogate) but not with resistive exercise alone147. In addition to reducing bed rest-induced RyR1 S-nitrosylation, low-magnitude mechanical signals increased protein expression of RyR1 (REF.148) and nuclear factor erythroid 2-related factor 2 (NRF2)147, a critical transcriptional regulator of antioxidant protein expression, which protects against oxidative damage. These low-intensity signals also served to protect the actual number of satellite cells (precursors to differentiated skeletal muscle cells) available within the muscle when challenged with endocrinopathy149 or obesity150.
Osteocalcin.
Bone-derived osteocalcin has also been demonstrated to regulate skeletal muscle function, mass and exercise capacity in mice151. At the molecular level, osteocalcin signalling in skeletal muscle promotes the uptake and subsequent catabolism of glucose and free fatty acids152, effects that are similar to those of IL-6 (REF.153). This metabolic response and optimization of energy utilization in myofibrils might contribute to gains in muscle performance following the delivery of mechanical forces to bone. Interestingly, muscle-derived IL-6 increases the production of bioactive osteocalcin154 and, similarly, these same preclinical studies demonstrated that osteocalcin stimulated the expression and secretion of IL-6 from skeletal muscle. These data support a feedforward mechanism of adaptation to exercise mediated by osteoblast expression of osteocalcin and skeletal muscle expression of IL-6 during mechanical loading of the musculoskeletal system.
Types of exercise regimen.
Exercise modalities can halt or reverse muscle loss. For example, resistance and endurance training are both effective countermeasures to slow muscle loss155 and promote gain in mass and neuronal activation155. In addition, resistance training induces muscle hypertrophy156, which increases muscle mass and strength through morphological changes to muscle fibres157. Although resistance training ensures dramatic effects, individuals with sarcopenia and osteoporosis cannot endure (or risk) the higher magnitudes of resistance training158. Alternatively, adherence to exercise regimens might be more achievable through endurance training in ageing adult populations, particularly in those with obesity, which would inhibit weight gain and maintain healthier muscle by stimulating satellite cell proliferation and increasing metabolic muscle output159. Even the low-intensity nature of yoga, which is known to enhance musculature and improve balance160, has been introduced as a means to treat the effects of muscle wasting in patients with cancer-associated cachexia, a strategy that is also encouraged in those with sarcopenia who have restricted mobility161–163.
Muscle and bone outcomes.
The hypertrophy of muscle mass and bone mass are positively correlated to each other in response to mechanical stimuli164 as exemplified by exercise-induced increases in satellite cells, increased fibre size and muscle hypertrophy. The dependency of bone outcomes on muscle is also apparent in embryonic paralysis165 and genetic muscle dysfunction166, as well as in humans with muscular dystrophy who develop skeletal abnormalities. Translating this observation to humans, patients adversely affected by myopathy-inducing pharmacological treatments have benefited from incorporating exercise into the treatment strategy. Exercise surrogates, such as low-intensity vibration, have been hypothesized to play a similar role; low-intensity vibration increases the satellite cell pool149, limits fatty infiltration of skeletal muscle167, downregulates pro-inflammatory gene expression168 and upregulates the expression of anti-inflammatory molecules. Therefore, through mechanical intervention, whether strenuous or as general maintenance of adequate muscle health, bone outcomes are improved.
Factors that promote marrow fat
Marrow adipocytes.
In excess, adipose tissue can contribute to a host of metabolic conditions — the most extreme of these being obesity. However, the degree of adipose accrual does not need to reach a BMI ≥35 kg/m2 to be harmful: fat is found in bone marrow, muscle, joints and liver and has a myriad of functional consequences when present in excess. In the marrow microenvironment, which is a constrained space consisting of MSCs and HSCs, the impact of excess adipose tissue probably depends on age, aetiology and inflammatory status37. Ageing, postmenopausal status, undernutrition, some pharmacological therapies and an absence of physical activity can all drive marrow adiposity169. Marrow adipose tissue (MAT), which first develops in the prenatal skeleton, is estimated to occupy 70% of the marrow space by young adulthood170. Marrow adipocytes secrete a multitude of adipokines (such as adiponectin and IL-6), some of which induce inflammation171 and osteoclastogenesis172, which can disrupt haematopoiesis173 and aggravate bone loss174. For instance, IL-6 induces the expression of RANKL on osteoclasts and their precursors, which increases recruitment of haematopoietic macrophage precursors into the osteoclast lineage and increases bone resorption175. Adiponectin, another adipokine highly expressed by marrow adipocytes176, stimulates RANKL expression on mature osteoclasts and is associated with low BMD in elderly men and women177,178. With these findings in mind, slowing the expansion of adipose tissue throughout the marrow space might protect and preserve the MSC and HSC niche, permitting progenitors to retain their regenerative (MSC) and immune (HSC) functions and counteracting osteoporosis and inflammatory disease.
Exercise suppresses the formation of MAT, even when MAT is stimulated by an anti-diabetic thiazolidinedione drug or a high-fat dietary intervention39. As such, exercise might help preserve the morphology and phenotype of the marrow microniche where osteoprogenitors, as well as HSCs, reside. For example, in contrast to their non-exercised counterparts, 6 weeks of daily running increased bone quantity, improved bone quality and suppressed MAT accumulation in mice fed either regular or high-fat diets (HFDs)39. Furthermore, treatment with a PPARγ agonist (rosiglitazone) increases stem cell adipogenesis in rodents179,180 and humans181. These outcomes are suppressed by dynamic (time-varying) mechanical signals in vitro67. In vivo, treadmill running in rosiglitazone-treated mice suppressed an adipogenic shift in the marrow phenotype180.
Obesity.
Obesity predisposes the body to a wide-range of perturbations and morphological changes, such as adipocyte hypertrophy from excess lipid storage182, including within the marrow space. Multiple studies have demonstrated that MAT increases as total fat mass increases in mouse models of obesity39,183–187. For instance, 6 weeks of a mild HFD (45% kcal from fat) led to a 2.6-fold increase in MAT in young female mice39. Six weeks of higher-fat-supplemented chow (60% kcal from fat) led to a fourfold increase in MAT in young male C57BL/6 mice183. After 12 weeks of a similar diet, which was fed from weaning, MAT increased more than fivefold185. In addition, 3 months of a diet consistng of 45% kcal from fat increased MAT and adipocyte hypertrophy187 (FIG. 2).
Fig. 2 |. Exercise suppresses expansion of marrow adipocytes and strengthens bone in obese mice.
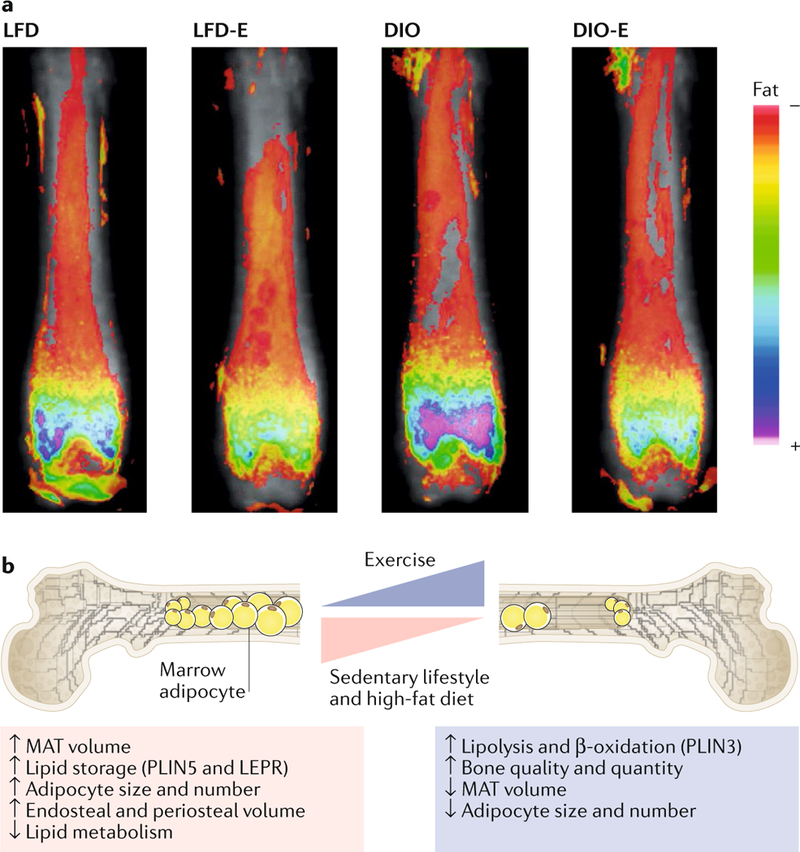
a | Obese (diet-induced obesity (DIO)) and lean (low-fat diet (LFD)) mice were allocated to running exercise (DIO-E and LFD-E, respectively) or sedentary groups for 6 weeks (n = 6 per group). The images are a visualization of femoral marrow adipose tissue (MAT) in mice measured by MRI with advanced image analysis. Each image represents six images superimposed on each other. The heat map demonstrates the relative lipid quantity. b | Schematic representation of marrow adipocytes in the setting of obesity with or without exercise. DIO increases adipocyte size and number and expression of the lipid droplet marker PLIN5, resulting in expansion of cortical endosteal and periosteal bone surfaces. By contrast, exercise increases bone quantity and quality relying on β-oxidation of lipids in the marrow, as supported by a reduced number of adipocytes in the marrow and their cross-sectional area and increased expression of oxidation and lipolysis markers (for example, PLIN3). Part a reproduced with permission from REF.187,Wiley-VCH.
Six months of a diet consisting of 45% kcal from fat in C57BL/6 mice led to a 20–25% increase in total numbers of cells in the bone marrow without affecting the fraction of different cell types; thus, factors secreted by elevated amounts of MAT, such as leptin, increased haematopoietic and lymphopoietic populations, which indicates that an HFD heavily dysregulates immunity188. Similarly, 12 weeks of a diet of 60% kcal from fat led to bone marrow hyperplasia (28% increase in nucleated cells) in Wistar rats189. Conversely, 18 weeks of a diet of 45% kcal from fat led to decreased HSCs and progenitor cells in the marrow, owing partly to both reduced proliferation and increased differentiation of short-term HSCs and progenitor cells into multipotent progenitor cells190. By contrast, 16 weeks of an HFD led to increased long-term HSC populations in the marrow191.
Most studies on obesity suggest that the condition is associated with impaired lymphopoiesis and increased myelopoiesis. In rats, obesity led to an increased number of osteoblasts, segmented neutrophils and eosinophils, whereas no notable difference was observed in the number of marrow-bound lymphocytes189. In mice, the effects of obesity on immunity in the marrow have been associated with reduced numbers of B cells and T cells and an increased number of myeloid cells183,192. By contrast, another mouse study demonstrated a 10–18% increase in lymphocyte progenitors within the marrow during obesity, resulting in an enrichment of total lymphocyte counts in the circulation of 70–125%188. Furthermore, HSCs harvested from a marrow environment that was high in fat have an elevated capacity to produce macrophages191.
Obesity in humans has been linked with elevated systemic inflammation, much of which is directly associated with macrophage infiltration of extra-marrow adipose depots and increased numbers of immune cells in the circulation193–197. Interestingly, a positive correlation between BMI and blood leukocyte count is found in individuals who are insulin resistant195. Increased infiltration of immune cells into the visceral cavity and increased secretion of pro-inflammatory cytokines perpetuate in the obese phenotype34,198–200. In mice, just 2 weeks of an HFD facilitates rapid weight gain and diffuse visceral adiposity68,201. White adipose tissue-mediated secretion of pro-inflammatory cytokines (adipokines)34 and ROS (which drive macrophage and cytotoxic T cell production) further promotes the state of chronic inflammation198 through release of matrix metalloproteinases (MMPs), tumour necrosis factor (TNF) and IL-6, among others. In humans, these factors predispose the individual to developing insulin resistance202 and glucose intolerance, conditions that can lead to the onset of type 2 diabetes mellitus203 and ultimately contribute to deficits in cortical bone density, trabecular microarchitecture and bone size65. Inflammation and increased levels of pro-inflammatory cytokines, such as TNF, IL-1, IL-6 and IL-17, are associated with increased bone loss204–206. Pro-inflammatory cytokines promote bone loss by increasing the expression of macrophage colony-stimulating factor and RANKL by osteoblasts and fibroblasts in the marrow204. Inhibiting the function of IL-1 or TNF, which can be secreted by adipocytes, prevented bone loss in ovariectomized mice207. In addition, multiple animal studies have shown increased adipogenesis resulting from a HFD that led to an obese phenotype exhibited, in parallel, with suppression of osteoblastogenesis68,192,201. Translated to humans, these findings demonstrate that the damage associated with poor diet and obesity to bone and immunity are exacerbated by increased inflammation. Whether the rise in marrow adiposity as a consequence of HFDs contributes to elevated inflammation and dysregulated immunity or outcompetes osteoprogenitors for marrow space, the resulting phenotype is conducive to increased pathological outcomes across a range of systems.
Transducing a mechanical signal
Mechanical forces.
The array of mechanical forces experienced by all cells in bone, including MSCs and HSCs in the marrow, is complex and multifactorial. Spatially, MSCs reside in bone marrow niches near the bone surface and are exposed to matrix deformations55,208–211, accelerations212–217, muscle activity218–220, fluid flow221–225 and changes in intramedullary pressure226–228, each of which cannot be disentangled from the other229. During locomotion, the bone matrix encounters strains in the range of 2,000–3,500 microstrain (µε)208. Owing to bone’s porous structure, local strain concentrations create pressure gradients and induce local fluid flow in and out of the bone matrix, similar to squishing a kitchen sponge. In vivo, even fairly low strains (400 µε) that might correspond to seemingly gentle activities such as walking can produce fluid flow within the lacunar–canalicular network that is as high as 5 Pa (REF.230). In this way, MSCs that reside on or in proximity to bone surfaces are also subjected to exercise-derived fluid flow. Within the marrow, small motions at the interface between marrow and bone, such as those induced by exercise, will generate a fluid shear that is independent of strain-derived fluid flow231. Dynamic shear forces232,233, such as pulsatile fluid flow, can promote osteogenesis in rat calvarial cells and represent a key physical factor in mechanotransduction.
During moderate running, tibial accelerations approach 2.0 g (where 1.0 g (or 9.8 m/s2) is Earth’s gravitational pull), and the ground-reaction force of Olympic sprinters can exceed 3.0 g (that is, three times their body weight)234. In silico studies reveal that when using vibration to introduce subtle mechanical oscillations (with a range of 0.1–0.5g), marrow-filled trabecular compartments235,236 generate fluid shear stresses up to 2.0 Pa (REFS231,237), which is a mechanical signal capable of influencing MSC function237. The viscosity of red marrow was found to be much higher (400 cP) than that of fatty marrow (40 cP)238, which implies that fluid shear at the bone marrow interface, and within the marrow itself, can change dramatically because of marrow’s fluid dynamic properties239. Red and fatty bone marrow can replace one another240, and conditions such as ageing and osteoporosis result in an increase in adipose tissue volume in the marrow while depleting the bone241.
Pathways.
Osteoblasts have more cytoskeletal constructs and interconnections that link to the nucleus than adipocytes; culturing human MSCs and osteoblasts on hard surfaces improves osteogenesis and is associated with the development of a complex cytoskeleton242,243. The application of physical forces in vitro, which dynamically increases cytoskeletal actin structure244, inhibits adipogenesis, which preserves the multipotentiality of MSCs and their ability to enter the osteoblast lineage63,245,246. In vitro application of mechanical strain to MSCs is associated with recruitment of signalling complexes to focal adhesions, where AKT activation inhibits the effect of GSK3β targeting β-catenin for destruction, thereby increasing β-catenin signalling247. AKT activation also leads to increased levels of GTP-bound active RhoA and production of highly connected cytoplasmic actin connections245 that are involved in molecule translocation as well as transmitting forces directly into the nucleus248 (FIG. 3). Thus, the dynamic remodelling of cytoskeletal elements in response to the local bone marrow mechanical environment orchestrates the delivery of this mechanical information from the plasma membrane and/or other sites, such as the nucleus itself, in order to regulate gene expression programmes in cells60.
Fig. 3 |. Mechanotransductive responses of mesenchymal stem cells to dynamic mechanical stimuli are achieved through the internal stiffening of the cell via cytoplasmic-bound actin proteins.
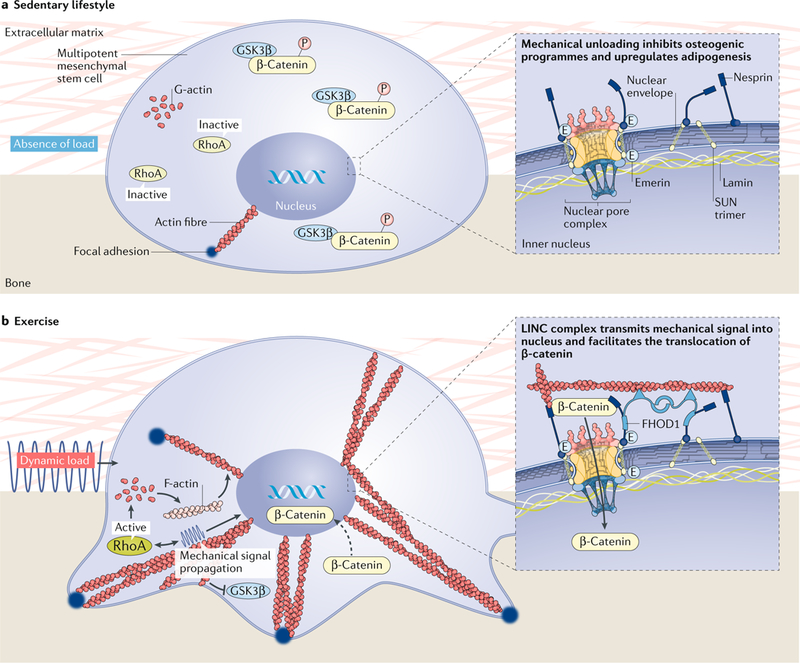
a | The absence of mechanical forces prevents the polymerization of actin fibres, preventing the dephosphorylation of β-catenin, which remains bound to GSK3β. As such, β-catenin does not translocate to the nucleus, resulting in the expression of PPARγ-driven adipogenic pathways. b | By contrast, mechanical stimuli recruit actin fibres to the interface of the cell membrane and the substrate surface. These focal adhesions become stronger and denser in response to dynamic mechanical stimuli, permitting the movement of β-catenin into the nucleus and an ensuing osteogenic response. FHOD1, FH1/FH2 domain-containing protein 1; LINC, linker of nucleoskeleton and cytoskeleton.
Perhaps the most widely recognized mechanoreceptive pathways are those that sense mechanical information at the plasma membrane and transmit it to the nucleus (termed outside-in signalling). Focal adhesions, which are maintained by cytoskeletal tension249 and extracellular force, act as signalling relays for extracellular (and intracellular) cues250. In response to mechanical challenges, structural proteins, such as vinculin, paxillin and talin, are recruited into focal adhesions251–253, whereas others (such as zyxin) leave focal adhesions and localize themselves onto actin fibres to recruit actin nucleation and the branching factor actin-related protein 2 (ARP2)–ARP3 complex254–256. Structural changes in focal adhesions are accompanied by the recruitment of signalling molecules, including focal adhesion kinase (FAK) and SRC kinases as well as AKT, a known activator of Rho GTPases63,245, such as RhoA, RAS and CDC42 (REF.257). RhoA activity increases the cell tension through its effector protein ROCK, which activates myosin light-chain kinases, leading to activation of the dimerized motor protein myosin II258,259. These mechanically driven changes in RhoA–ROCK activity have been implicated in the osteogenic commitment of MSCs as they increase the activity of two early-stage osteogenic markers, osterix and RUNX2 (REF.260). Reinforcing the role of RhoA in MSC osteogenesis, our group recently showed that regulation of RhoA activity through leukaemia-associated Rho guanine nucleotide exchange factor (LARG; also known as ARHGEF12) and Rho GTPase-activating protein 18 (ARHGAP18) regulates osteogenic commitment in MSCs244.
Signalling molecules.
In parallel to cytoskeletal restructuring, mechanical signals also activate a number of signalling molecules, including MAP kinases (such as ERK and JNK) and the WNT effector β-catenin. Perhaps the most studied signalling protein in bone, β-catenin counteracts an adipogenic stimulus when activated, which inhibits adipogenesis of bone-marrow-derived MSCs as demonstrated by reduced levels of lipids and decreased expression of PPARγ and adiponectin60,245,260. Following a mechanical challenge, FAK operates in conjunction with the SRC kinase FYN to activate mTORC2, which then initiates the signal cascade of increased levels of AKT leading to decreased levels of GSK3β, thereby increasing levels of β-catenin247,261. In this way, the increase in the number of focal adhesions after application of an acute mechanical challenge amplifies the downstream response to force, as demonstrated by a greater induction of β-catenin with a subsequent application of force70. Thus, a transient adaptation of the cell increases its sensitivity to follow-on mechanical signals, yet the absence of mechanical signals could systematically dismantle these ‘antennae’ and leave the system unresponsive to input63. Translating this finding to the clinic, strategic delivery of physical interventions during rehabilitation might have the potential to ratchet up the mechanical sensitivity and response of cell populations; however, leaving a system unstimulated for long periods of time (for example, as in long-term bed rest) might undermine the adaptive machinery’s capacity to protect the patient.
The nucleus.
Emerging evidence suggests that the nuclear envelope houses a number of mechanoregulatory proteins and has an active role in both cytoskeletal dynamics and nuclear access to molecular transducers of mechanical information. Mechanically, the cytoskeleton couples to the nucleus through the linker of nucleoskeleton and cytoskeleton (LINC) complex protein262. F-actin binds to a nesprin protein (nesprin 1 or nesprin 2), which are spectrin repeat proteins that pierce the nuclear envelope, connecting via its KASH (Klarsicht, ANC1, SYNE homology) domain to intramembrane leaflet SUN proteins (SUN1 and SUN2)262. As LINC elements, SUN1 and SUN2 partly regulate nuclear mechanical integrity263. Mechanically, the nuclear envelope transmembrane protein emerin is known to accelerate actin polymerization264, shifting between inner and outer nuclear membranes. By contrast, external mechanical challenges to epidermal stem cells cause emerin enrichment at the opposing nuclear envelope, and emerin accumulation is accompanied by the recruitment of non-muscle myosin IIA to promote local actin polymerization that reduces nuclear actin levels and promotes perinuclear actin accumulation265. This finding is consistent with our recent findings that nuclear actin levels and its polymerizing state are powerful determinants of MSC differentiation into osteogenic and adipogenic lineages246,266.
Another protein that is active in the outer nuclear envelope, FH1/FH2 domain-containing protein 1 (FHOD1), binds to the spectrin repeat domain of nesprin 2G to increase the coupling strength between LINC and F-actin267. Supporting the regulatory role of the LINC complex in cytoskeletal dynamics, depletion of nesprin 1 (REF.268) or SUN1 (REF.269) alters focal adhesion kinetics by increasing focal adhesion strength, whereas deletion of SUN2 results in the opposite effect, decreasing focal adhesions270. Co-depletion of LINC elements SUN1 and SUN2, as well as disconnecting LINC through overexpressing the nesprin–SUN binding domain KASH, accelerates MSC osteoblastogenesis and impedes cell mechanosensitivity to subtle mechanical signals, such as low-intensity vibration261. Although this finding suggests that LINC has a role in regulating mechanosensitivity, both LINC-depleted MSCs and cells without nuclei remain responsive to high-magnitude substrate strain and activate FAK at Tyr397 in response to strain. This observation suggests that LINC has a nuanced function in cell mechanosensitivity. Moreover, the LINC complex serves an important role in the nuclear access of important mechanotransducers, such as β-catenin and YAP1 (REFS271,272).
Depleting nesprin 1 inhibits strain-induced nuclear entry of transcriptional co-activator YAP1. Findings published in 2017 indicate that the access of transcriptional co-activator YAP1 to the nucleus is regulated through stretching of nuclear pores during cytoskeletal tension, which facilitates the transfer of transcriptional co-activator YAP1 to the nucleus273. Our group has also demonstrated that LINC has an important role in β-catenin access to the nucleus. β-Catenin does not have a classic nuclear localization signal but, instead, enters through the nuclear leaflets via direct contact with the nuclear pore complex (NPC)274,275. β-Catenin transiently localizes to the LINC element nesprin, which might provide a ‘launching pad’ for subsequent nuclear entry276. Untethering of nesprin 2 from the nuclear envelope via co-depletion of both SUN1 and SUN2 proteins displaces β-catenin and decreases its levels in the nucleus271. Thus, β-catenin generated through exercise appears to be a critical event in transmitting these signals into the nuclear-mediated transcription of osteogenic genes.
Lamin A/C is a well-known intranuclear, mechanoadaptive intermediate filament system housed inside the nucleus. Nuclear levels of lamin A/C positively correlate with resident tissue stiffness in a linear manner242, and lamin A/C plays a major role in regulating nuclear stiffness277,278. Multipotent MSCs, which enter musculoskeletal cell lineages that have mechanically demanding functions, have a more robust lamin A/C network and increased LINC connectivity than multipotent embryonic stem cells242. As embryonic stem cells differentiate into somatic cell lineages, levels of LINC and lamin A/C increase279. In bone, levels of lamin A/C increase when MSCs enter the osteogenic lineage280, a change that contributes to the increased cellular stiffness of osteoblasts242,281. Furthermore, lamin A/C overexpression promotes osteogenic differentiation282. By contrast, levels of lamin A/C decrease when MSCs undergo adipogenesis283, and both partial and complete deletion of lamin A/C promote an adipogenic programme in MSCs284–286. In this way, stiffness of resident tissues, such as the hard tissues of the skeleton, exerts control on the cell itself, both at the material level (higher effective modulus or stiffer bone) and through regulating nuclear stiffness (stiffer cell) to bias MSC differentiation towards osteogenesis and against adipogenesis.
Bone cells.
Resident bone cells exhibit distinct responses to mechanical loading, including increased β-catenin signalling (for example, osteoblasts287 and osteocytes have dendritic processes that are embedded throughout the bone matrix that function as a mechanosensor array)288. Oestrogen has a distinct role in regulating bone homeostasis as osteoblast apoptosis is prevented289 by the phosphorylation of oestrogen receptor-α (ERα) and upregulation of MAPK expression290, the latter perhaps similar in nature to activation of MAPK by mechanical strain78. In states of oestrogen depletion in mice, achieved via ovariectomy, osteoclast activity is heavily upregulated, yet incorporation of mechanical strain via low-intensity vibration still enhances bone formation during fracture callous healing through increased expression of ERα291. In vitro, mechanical strain of pre-osteoblasts increases the matrix mineralization of osteocalcin and osteopontin, which, when extrapolated to exercise challenges at the level of the organism, should improve bone strength292. When stimulated by fluid shear stress, β-catenin signalling increases in osteocytes and osteoblasts293. Finally, bringing us back to how these bone cells might recognize and best respond to mechanical signals through their dynamic cytoskeletal apparatus, we have shown that inclusion of a 3 h refractory period between successive bouts of mechanical challenges, in this case by low-intensity vibration, improves the ability of these mechanical signals to suppress adipogenesis70. To a degree, this effect is achieved, as discussed in a previous section, because the second bout of mechanical force is given after an increase in focal adhesion number and connectivity through a RhoA-based signalling cascade, taking advantage of an adapted cell better suited to perceive the mechanical challenges63. Separating the refractory period even further to 5 h between mechanical bouts, which were delivered in vivo, also increased the MSC population52.
Collectively, these findings indicate that MSCs residing throughout the bone marrow utilize both cytoskeleton remodelling and biochemical transducers to facilitate information flow between two critical mechanosensory centres (focal adhesions and the nuclear envelope) in response to mechanical challenges. In addition, in vivo exposure to multiple mechanical events that are separated by sufficient time for the system to adapt results in the promotion of osteogenic and anti-adipogenic outcomes52,294. In deciphering how exercise regulates MSC fate, consideration should be given as to how the marrow mechanical environment and MSCs residing within bone evolve with age and disease state, as well as treatment of diseases, and adapt to loading demands during exercise. For instance, in the setting of cancer, certain treatments, such as radiation295 and hormone deprivation296,297, significantly increase bone marrow adiposity. The implications of marrow fat for musculoskeletal health are unclear in humans but will be important areas of study. Understanding the role of mechanical signals in improving musculoskeletal function will be important, as elderly individuals and individuals who are infirm, injured or obese are often unlikely to adhere to an exercise prescription no matter how beneficial it might be.
Low-intensity vibration
Mechanical effects of exercise.
Exercise is often presumed to suppress adiposity through metabolic pathways, such as increasing caloric expenditure298, and it is assumed that the longer and more strenuous the activity the more effective it becomes. Indeed, our group showed that exercise (both treadmill and wheel running) in mice (aged 4–8 weeks)39,299, even under conditions conducive to adipogenesis, such as a HFD or treatment with thiazolidinedione, suppressed adiposity. The salutary effect of exercise on the skeleton, however, is generally believed to be energy-independent and is instead regulated by osteoblast or osteocyte signalling, or concerted effort from both cells300, generated by load-induced bone strain55, enhanced fluid flow301, intramedullary pressure302 and/or streaming potentials303. Furthermore, once a threshold of loading is surpassed, no additional influence is realized304. These mechanical parameters correlate more strongly to the dynamics (time-varying) of the load environment (that is, impact305, strain rate209, strain gradients306 and cycle number208) than to load magnitude, a conclusion strengthened when considering that static challenges (that is, upright stance and balance) fail to serve as anabolic stimuli307. Departing from a ‘more is better’ strategy, several groups have reported that extremely small mechanical signals, induced at high frequency using low-intensity vibration, are anabolic to bone212,216,308–315 and suppress the formation of adipose tissue316,317.
Frequency and magnitude of signals.
High-frequency, low-magnitude mechanical signals persist in the functional load regime220; low-magnitude mechanical signals are generated by the dynamics of muscle contraction318. The persistence of such signals is evident when considering that the daily history of bone strain consists of a few large mechanical events (for example, four loading events per day that are >2,000 με)319 as well as hundreds of thousands of daily events that are well below 10 με (REF.320). That low-magnitude mechanical signals, induced using low-intensity vibrations in the absence of weight bearing, can promote bone formation74,321 led to the unexpected finding that low-intensity vibrations also inhibit adipogenesis and systemic adiposity in adult mice68,201,317 while decreasing levels of triglycerides, free fatty acids and liver steatosis322. These findings suggest that adipose development might also have an energyindependent element; the reciprocal relationship of fat to bone323 points to a novel target to control the bone versus fat phenotype — their shared MSC progenitor68,201,324,325. Furthermore, low-intensity vibrations alter the haematopoietic response of obese mice fed an HFD by restoring depleted B cell populations in gonadal fat pads, a mechanism suggested to have arisen through fate selection of HSCs towards B cell lymphopoiesis at the expense of osteoclastogenesis192. Therefore, by targeting both bone marrow MSCs and HSC immune progenitors of the marrow, low-intensity vibrations could mitigate the pernicious consequences of obesity on the immune system while suppressing adiposity. As age increases, however, the challenge of using low-intensity vibrations in adults to treat obesity (or osteoporosis) is that the sensitivity to mechanical stimulation might have already declined326.
Cycles of signals and rest.
Growing evidence suggests that the incorporation of multiple cycles of mechanical signals within a given day, separated by periods of rest, can increase their effects in reducing adipogenesis and increasing osteogenesis. Low-intensity vibrations have the greatest impact in young participants (aged 5–20 years)61,327, an influence that reduces over time in ageing adults (65–85 years). Young mice (aged 5 weeks) receiving HFD chow are receptive to singular bouts of low-intensity vibrations over 12 weeks, which results in increased glucose and insulin metabolism328. However, aged HFD-fed mice (17 weeks old) benefit more in terms of offsetting adiposity and impaired glucose metabolism (hyperinsulinaemia) as a result of two 15 min bouts of low-intensity vibrations than from a singular 30min bout52.
Rest periods, ranging anywhere from 14 s to 8 h, introduced between mechanical inputs increase the anabolic response of bone329. Incorporating refractory periods into the low-intensity vibration loading scheme increases the expression of insulin receptor substrate 1 (IRS1), which is a negative regulator of the PI3K pathway330, in the perigonadal fat pads in HFD-fed mice, eliciting an even stronger effect than that seen in aged mice on control diets exposed to a single bout of low-intensity vibration treatment. In the clinical setting, exercise regimens to treat patients with obesity might elicit the anti-diabetic effects of mechanical loading, but the effects will probably be dependent on the age of the patient — the younger the patient is, the more responsive they will be. Altogether, targeting the immunosuppressive and anti-inflammatory capacity of bone marrow stem cells by inducing proliferation and lineage selection using exercise or exercise surrogates might collectively help address adipose tissue dysfunction.
Effects of low-intensity vibrations.
In humans, low-intensity vibrations promote increased bone mass and quality, both of which contribute to bone strength and resistance to fracture, in children with disabling conditions, including cerebral palsy331,332, Duchenne muscular dystrophy333 and adolescent girls with idiopathic scoliosis332. Low-intensity vibrations are anabolic to bone and muscle in young women (15–20 years old) with osteoporosis327 and augment bone accretion in survivors of childhood cancer334 and patients with Crohn’s disease335. Acute studies (within 5 days) show that normal bone turnover can be restored in young women (aged 16.3 ± 1.9 years) combating anorexia nervosa336 and that markers for bone resorption are suppressed in healthy young women within 3 months313. In each of these studies, however, it is important to note that the salutary influence correlates with adherence; mechanical signals are effective only if you use them.
The design of the low-intensity vibration platform uses closed-loop acceleration feedback to ensure a high-fidelity signal337, a design that can safely229,338 deliver these barely perceptible mechanical signals to participants, including frail elderly individuals339,340 and those with spinal cord injuries341. Low-intensity vibrations are considered a nonsignificant risk by the FDA338, with an intensity considered safe for up to 4 h of exposure per day342. Other instruments providing mechanical stimulation operate in higher-magnitude and lower-frequency domains, making them less practical and even risky for use in patients whose skeletons are frail (such as those with postmenopausal osteoporosis or osteogenesis imperfecta)343. Although low-intensity vibrations cannot be considered a substitute for exercise, these studies indicate that they represent salutary mechanical signals to improve clinical end points in participants with limited exercise capacity344 and might be a means of priming responsiveness to exercise. Increasing sensitivity to, and thus efficacy of, exercise might ultimately make it more accessible to older adults (age ≥60 years) and infirm patients unable to exercise adequately to stimulate these integrated regulatory systems.
Conclusion
Living systems are affected by mechanical signals at the organ and tissue level (bone, muscle and fat) as well as at the level of the cell (MSCs, osteoblasts, myocytes and adipocytes). Gravity has been an inescapable physical signal across all life systems since the beginning of time, whereas other physical factors, such as light, temperature, geography, substrate, external threats or food availability, vary with time, both acutely and over aeons. Biological systems have been challenged to resist gravity, and by evolving to become mechanosensitive, their ability to survive improved345,346. Conversely, sedentary lifestyles, combined with ageing, have led to a degraded musculoskeletal system and increased adiposity. Mounting evidence indicates that these systemic stressors disrupt both MSC and HSC populations, in addition to biasing the fate selection of their progeny35, contributing to a compromised regenerative (MSC) and immune (HSC) system. Empirical evidence suggests that mechanical signals can be used to prevent and/or treat osteoporosis and obesity, guiding mesenchymal and satellite stem cell lineage selection towards an improved musculoskeletal system and suppressed adipose burden, salutary end points that mirror those of exercise and are enabled by an intact cytoskeletal and nuclear connectivity.
Key points.
Ageing and inactivity each contribute towards a local and systemic environment conducive to poor bone quality, increased systemic adiposity, marrow adipogenesis and inflammation.
Mesenchymal stem cells and their lineage-differentiated progeny (for example, osteoblasts) are mechanosensitive, such that increased mechanical signals (such as exercise) stimulate muscle and bone anabolism.
Mechanical signals suppress obesity end points, including fat gain, adipocyte lipid acquisition, chronic inflammation and indices associated with type 2 diabetes mellitus.
Transduction of mechanical signals across the plasma membrane of stem cells into the nucleus activates signalling cascades and cytoskeletal adaptations to initiate osteogenic, chondrogenic and myogenic differentiation and inhibit adipocyte differentiation.
Mechanical signals, such as those induced through low-intensity vibration, need not be large in magnitude, or long in duration, to influence bone or fat phenotypes.
Loading.
In terms of mechanical loading, a singular or compound series of static or dynamic (time-varying) forces applied to a system via gravity or direct application from an external body, causing tension, shear or compression.
Unloading.
A cell or body is considered mechanically unloaded if no static or dynamic strain is present, such as what might occur with bed rest or spaceflight (that is, microgravity).
Ground-reaction forces.
As applicable to biomechanics, ground-reaction forces consist of the normal forces exerted by the ground on the body making contact with it, particularly resulting from a heel strike during walking or running.
Spectral content.
Muscle contractive forces, specifically on bone, resonate within a discrete frequency range.
Load sensation.
Mechanical loads are ‘sensed’ by cells through transduction of external or internal forces across cytoskeletal proteins into the nucleus.
Tissue senility.
The ageing process is associated with the quiescence of regenerative cell populations residing in tissues throughout the body.
Muscle-specific force.
Quantification of the contractile forces generated by muscles can be normalized to muscle size ex vivo.
Fluid shear.
Fluidic forces applied tangentially across cell membranes or tissues.
Dynamic shear forces.
Physiological fluids exert a gradient of pulsatile flow across vessel walls, mineralized bone and cells housed in the bone marrow microenvironment.
Tissue stiffness.
In terms of bone, the stiffness of the tissue is correlated to its ability to resist deformation.
Nuclear stiffness.
Nuclear stiffness refers to its rigidity and is directly related to polymeric structural proteins (that is, microtubules, intermediate filaments and microfilaments) found across the cytoskeleton, of which actin proteins provide substantial reinforcement.
Footnotes
Competing interests
C.T.R. is a founder of Marodyne Medical, Inc. and BTT Health and has several patents issued and pending related to the ability of mechanical signals to control musculoskeletal and metabolic disorders. The other authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Endocrinology thanks G. Duque, and other anonymous reviewers, for their contribution to the peer review of this work.
References
- 1.Ruff CB, Larsen CS & Hayes WC Structural changes in the femur with the transition to agriculture on the Georgia coast. Am. J. Phys. Anthropol 64, 125–136 (1984). [DOI] [PubMed] [Google Scholar]
- 2.Larsen CS Biological changes in human-populations with agriculture. Annu. Rev. Anthropol 24, 185–213 (1995). [Google Scholar]
- 3.Ruff CB Gracilization of the modern human skeleton—the latent strength in our slender bones teaches lessons about human lives, current and past. Am. Sci 94, 508–514 (2006). [Google Scholar]
- 4.Nowlan NC, Jepsen KJ & Morgan EF Smaller, weaker, and less stiff bones evolve from changes in subsistence strategy. Osteoporos. Int 22, 1967–1980 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Bilezikian JP Osteoporosis in men. J. Clin. Endocrinol. Metab 84, 3431–3434 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Hu FB, Li TY, Colditz GA, Willett WC & Manson JE Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA 289, 1785–1791 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Manson JE, Skerrett PJ, Greenland P & VanItallie TB The escalating pandemics of obesity and sedentary lifestyle. A call to action for clinicians. Arch. Intern. Med 164, 249–258 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Watson SL et al. High-intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: the LIFTMOR randomized controlled trial. J. Bone Miner. Res 33, 211–220 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Wright NC et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res 29, 2520–2526 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 285, 785–795 (2001).11176917 [Google Scholar]
- 11.Brown M Skeletal muscle and bone: effect of sex steroids and aging. Adv. Physiol. Educ 32, 120–126 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Compston JE Sex steroids and bone. Physiol. Rev 81, 419–447 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Manolagas SC, O’Brien CA & Almeida M The role of estrogen and androgen receptors in bone health and disease. Nat. Rev. Endocrinol 9, 699–712 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg IH Sarcopenia: origins and clinical relevance. Clin. Geriatr. Med 27, 337–339 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Black DM & Rosen CJ Clinical practice. Postmenopausal osteoporosis. N. Engl. J. Med 374, 254–262 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Rossouw JE et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288, 321–333 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Shane E et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res 29, 1–23 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Siris ES et al. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin. Proc 81, 1013–1022 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Cramer JA, Gold DT, Silverman SL & Lewiecki EM A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos. Int 18, 1023–1031 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Khosla S et al. Addressing the crisis in the treatment of osteoporosis: a path forward. J. Bone Miner. Res 32, 424–430 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Childhood obesity facts CDC http://www.cdc.gov/obesity/data/childhood.html (2018).
- 22.Ogden CL, Carroll MD, Kit BK & Flegal KM Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311, 806–814 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahn SE, Hull RL & Utzschneider KM Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Van Gaal LF, Mertens IL & De Block CE Mechanisms linking obesity with cardiovascular disease. Nature 444, 875–880 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Wearing SC, Hennig EM, Byrne NM, Steele JR & Hills AP The biomechanics of restricted movement in adult obesity. Obes. Rev 7, 13–24 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Ko S, Stenholm S & Ferrucci L Characteristic gait patterns in older adults with obesity—results from the Baltimore Longitudinal Study of Aging. J. Biomech 43, 1104–1110 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messier SP Osteoarthritis of the knee and associated factors of age and obesity: effects on gait. Med. Sci. Sports Exerc 26, 1446–1452 (1994). [PubMed] [Google Scholar]
- 28.Felson DT, Anderson JJ, Naimark A, Walker AM & Meenan RF Obesity and knee osteoarthritis. The Framingham Study. Ann. Intern. Med 109, 18–24 (1988). [DOI] [PubMed] [Google Scholar]
- 29.Hart DJ & Spector TD The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. J. Rheumatol 20, 331–335 (1993). [PubMed] [Google Scholar]
- 30.Calle EE & Kaaks R Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4, 579–591 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Lashinger LM, Ford NA & Hursting SD Interacting inflammatory and growth factor signals underlie the obesity-cancer link. J. Nutr 144, 109–113 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.International Agency for Research on Cancer, Stewart BW. & Wild CP. World cancer report 2014 WHO https://www.who.int/cancer/publications/WRC_2014/en/ (2014).
- 33.Olson OC, Quail DF & Joyce JA Obesity and the tumor microenvironment. Science 358, 1130–1131 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Tilg H & Moschen AR Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol 6, 772–783 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Adler BJ, Kaushansky K & Rubin CT Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat. Rev. Endocrinol 10, 737–748 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Kennedy DE & Knight KL Bone marrow fat induces inflammation that inhibits B lymphopoiesis. J. Immunol 196 (Suppl), 12211 (2016). [Google Scholar]
- 37.Singh L, Tyagi S, Myers D & Duque G Good, bad, or ugly: the biological roles of bone marrow fat. Curr. Osteoporos. Rep 16, 130–137 (2018). [DOI] [PubMed] [Google Scholar]
- 38.National Osteoporosis Foundation. Exercise for your bone health NOF https://cdn.nof.org/wp-content/uploads/2016/02/Exercise-for-Your-Bone-Health.pdf (2013).
- 39.Styner M et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone 64, 39–46 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bortz WM 2nd. Disuse and aging. JAMA 248, 1203–1208 (1982). [PubMed] [Google Scholar]
- 41.Wolff J The Law Of Bone Remodeling (Springer, 1986). [Google Scholar]
- 42.Lang T et al. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J. Bone Miner. Res 19, 1006–1012 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Jones HH, Priest JD, Hayes WC, Tichenor CC & Nagel DA Humeral hypertrophy in response to exercise. J. Bone Joint Surg. Am 59, 204–208 (1977). [PubMed] [Google Scholar]
- 44.Heinonen A et al. Bone mineral density in female athletes representing sports with different loading characteristics of the skeleton. Bone 17, 197–203 (1995). [DOI] [PubMed] [Google Scholar]
- 45.Vlachopoulos D et al. Longitudinal adaptations of bone mass, geometry, and metabolism in adolescent male athletes: the PRO-BONE study. J. Bone Miner. Res 32, 2269–2277 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Gabel L, Macdonald HM, Nettlefold L & McKay HA Physical activity, sedentary time, and bone strength from childhood to early adulthood: a mixed longitudinal HR-pQCT study. J. Bone Miner. Res 32, 1525–1536 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Leichter I et al. Gain in mass density of bone following strenuous physical activity. J. Orthop. Res 7, 86–90 (1989). [DOI] [PubMed] [Google Scholar]
- 48.McKay HA et al. “Bounce at the Bell”: a novel program of short bouts of exercise improves proximal femur bone mass in early pubertal children. Br. J. Sports Med 39, 521–526 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinonen A, Sievanen H, Kannus P, Oja P & Vuori I Effects of unilateral strength training and detraining on bone mineral mass and estimated mechanical characteristics of the upper limb bones in young women. J. Bone Miner. Res 11, 490–501 (1996). [DOI] [PubMed] [Google Scholar]
- 50.Rubin CT, Seeherman H, Qin YX & Gross TS The mechanical consequences of load bearing in the equine third metacarpal across speed and gait: the nonuniform distributions of normal strain, shear strain, and strain energy density. FASEB J 27, 1887–1894 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubin C et al. Differentiation of the bone-tissue remodeling response to axial and torsional loading in the turkey ulna. J. Bone Joint Surg. Am 78, 1523–1533 (1996). [DOI] [PubMed] [Google Scholar]
- 52.Patel VS et al. Incorporating refractory period in mechanical stimulation mitigates obesity-induced adipose tissue dysfunction in adult mice. Obesity 25, 1745–1753 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallace BA & Cumming RG Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif. Tissue Int 67, 10–18 (2000). [DOI] [PubMed] [Google Scholar]
- 54.Warden SJ et al. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J. Bone Miner. Res 20, 809–816 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Rubin CT & Lanyon LE Regulation of bone mass by mechanical strain magnitude. Calcif. Tissue Int 37, 411–417 (1985). [DOI] [PubMed] [Google Scholar]
- 56.Tjandrawinata RR, Vincent VL & Hughes-Fulford M Vibrational force alters mRNA expression in osteoblasts. FASEB J 11, 493–497 (1997). [DOI] [PubMed] [Google Scholar]
- 57.Ko KS & McCulloch CA Intercellular mechanotransduction: cellular circuits that coordinate tissue responses to mechanical loading. Biochem. Biophys. Res. Commun 285, 1077–1083 (2001). [DOI] [PubMed] [Google Scholar]
- 58.Rubin J, Rubin C & Jacobs CR Molecular pathways mediating mechanical signaling in bone. Gene 367, 1–16 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson WR et al. Osteocyte specific responses to soluble and mechanical stimuli in a stem cell derived culture model. Sci. Rep 5, 11049 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uzer G et al. Cell mechanosensitivity to extremely low-magnitude signals is enabled by a LINCed nucleus. Stem Cells 33, 2063–2076 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uzer G, Fuchs RK, Rubin J & Thompson WR Concise review: plasma and nuclear membranes convey mechanical information to regulate mesenchymal stem cell lineage. Stem Cells 34, 1455–1463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Case N & Rubin J Beta-catenin—a supporting role in the skeleton. J. Cell. Biochem 110, 545–553 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sen B et al. Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells. Stem Cells 29, 1829–1836 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sen B et al. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology 149, 6065–6075 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samelson EJ et al. Diabetes and deficits in cortical bone density, microarchitecture, and bone size: Framingham HR-pQCT study. J. Bone Miner. Res 33, 54–62 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murfee WL et al. High-frequency, low-magnitude vibrations suppress the number of blood vessels per muscle fiber in mouse soleus muscle. J. Appl. Physiol 98, 2376–2380 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Case N et al. Mechanical input restrains PPARgamma2 expression and action to preserve mesenchymal stem cell multipotentiality. Bone 52, 454–464 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luu YK et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J. Bone Miner. Res 24, 50–61 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Styner M, Sen B, Xie Z, Case N & Rubin J Indomethacin promotes adipogenesis of mesenchymal stem cells through a cyclooxygenase independent mechanism. J. Cell. Biochem 111, 1042–1050 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sen B et al. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J. Biomech 44, 593–599 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Globus RK, Bikle DD & Morey-Holton E The temporal response of bone to unloading. Endocrinology 118, 733–742 (1986). [DOI] [PubMed] [Google Scholar]
- 72.Bikle DD, Sakata T & Halloran BP The impact of skeletal unloading on bone formation. Gravit. Space Biol. Bull 16, 45–54 (2003). [PubMed] [Google Scholar]
- 73.Rubin C, Xu G & Judex S The anabolic activity of bone tissue, suppressed by disuse, is normalized by brief exposure to extremely low-magnitude mechanical stimuli. FASEB J 15, 2225–2229 (2001). [DOI] [PubMed] [Google Scholar]
- 74.Rubin C et al. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J. Bone Miner. Res 17, 349–357 (2002). [DOI] [PubMed] [Google Scholar]
- 75.Judex S et al. Genetically linked site-specificity of disuse osteoporosis. J. Bone Miner. Res 19, 607–613 (2004). [DOI] [PubMed] [Google Scholar]
- 76.Squire M, Brazin A, Keng Y & Judex S Baseline bone morphometry and cellular activity modulate the degree of bone loss in the appendicular skeleton during disuse. Bone 42, 341–349 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Trudel G et al. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration study. J. Appl. Physiol 107, 540–548 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Rubin J et al. Mechanical strain differentially regulates endothelial nitric-oxide synthase and receptor activator of nuclear kappa B ligand expression via ERK1/2 MAPK. J. Biol. Chem 278, 34018–34025 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Tchkonia T, Zhu Y, van Deursen J, Campisi J & Kirkland JL Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest 123, 966–972 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Childs BG, Durik M, Baker DJ & van Deursen JM Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med 21, 1424–1435 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stattin K, Michaelsson K, Larsson SC, Wolk A & Byberg L Leisure-time physical activity and risk of fracture: a cohort study of 66,940 men and women. J. Bone Miner. Res 32, 1599–1606 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Lindstrom J et al. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 26, 3230–3236 (2003). [DOI] [PubMed] [Google Scholar]
- 83.Hu FB et al. Adiposity as compared with physical activity in predicting mortality among women. N. Engl. J. Med 351, 2694–2703 (2004). [DOI] [PubMed] [Google Scholar]
- 84.Hinton PS, Nigh P & Thyfault J Effectiveness of resistance training or jumping-exercise to increase bone mineral density in men with low bone mass: a 12-month randomized, clinical trial. Bone 79, 203–212 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dalsky GP et al. Weight-bearing exercise training and lumbar bone-mineral content in postmenopausal women. Ann. Intern. Med 108, 824–828 (1988). [DOI] [PubMed] [Google Scholar]
- 86.Nilsson M, Sundh D, Mellstrom D & Lorentzon M Current physical activity is independently associated with cortical bone size and bone strength in elderly Swedish women. J. Bone Miner. Res 32, 473–485 (2017). [DOI] [PubMed] [Google Scholar]
- 87.Ensrud KE & Crandall CJ Osteoporosis. Ann. Intern. Med 167, ITC17–ITC32 (2017). [DOI] [PubMed] [Google Scholar]
- 88.Ness KK et al. Skeletal, neuromuscular and fitness impairments among children with newly diagnosed acute lymphoblastic leukemia. Leuk. Lymphoma 56, 1004–1011 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maratova K et al. Muscle functions and bone strength are impaired in adolescents with type 1 diabetes. Bone 106, 22–27 (2018). [DOI] [PubMed] [Google Scholar]
- 90.Joyce ED et al. Association of muscle strength and bone mineral density in adult survivors of childhood acute lymphoblastic leukemia. Arch. Phys. Med. Rehabil 92, 873–879 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ness KK et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J. Clin. Oncol 31, 4496–4503 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang HP et al. Adherence to prescribed exercise time and intensity declines as the exercise program proceeds: findings from women under treatment for breast cancer. Support. Care Cancer 23, 2061–2071 (2015). [DOI] [PubMed] [Google Scholar]
- 93.Hemmatian H, Bakker AD, Klein-Nulend J & van Lenthe GH Aging, osteocytes, and mechanotransduction. Curr. Osteoporos. Rep 15, 401–411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bonewald LF The amazing osteocyte. J. Bone Miner. Res 26, 229–238 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Joldersma M, Klein-Nulend J, Oleksik AM, Heyligers IC & Burger EH Estrogen enhances mechanical stress-induced prostaglandin production by bone cells from elderly women. Am. J. Physiol. Endocrinol. Metab 280, E436–E442 (2001). [DOI] [PubMed] [Google Scholar]
- 96.Joldersma M, Burger EH, Semeins CM & Klein-Nulend J Mechanical stress induces COX-2 mRNA expression in bone cells from elderly women. J. Biomech 33, 53–61 (2000). [DOI] [PubMed] [Google Scholar]
- 97.Sterck JG, Klein-Nulend J, Lips P & Burger EH Response of normal and osteoporotic human bone cells to mechanical stress in vitro. Am. J. Physiol 274, E1113–E1120 (1998). [DOI] [PubMed] [Google Scholar]
- 98.Rubin CT, Bain SD & McLeod KJ Suppression of the osteogenic response in the aging skeleton. Calcif. Tissue Int 50, 306–313 (1992). [DOI] [PubMed] [Google Scholar]
- 99.Willie BM et al. Diminished response to in vivo mechanical loading in trabecular and not cortical bone in adulthood of female C57Bl/6 mice coincides with a reduction in deformation to load. Bone 55, 335–346 (2013). [DOI] [PubMed] [Google Scholar]
- 100.Strube P et al. Sex-specific compromised bone healing in female rats might be associated with a decrease in mesenchymal stem cell quantity. Bone 45, 1065–1072 (2009). [DOI] [PubMed] [Google Scholar]
- 101.Wiley CD & Campisi J From ancient pathways to aging cells-connecting metabolism and cellular senescence. Cell Metab 23, 1013–1021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farr JN et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat. Med 23, 1072–1079 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu Y et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15, 428–435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chiche A et al. Injury-induced senescence enables in vivo reprogramming in skeletal muscle. Cell Stem Cell 20, 407–414 (2017). [DOI] [PubMed] [Google Scholar]
- 105.Akunuru S & Geiger H Aging, clonality, and rejuvenation of hematopoietic stem cells. Trends Mol. Med 22, 701–712 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin Y et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: a novel mechanism in muscle-bone communication. J. Biol. Chem 292, 11021–11033 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Evans WJ & Campbell WW Sarcopenia and age-related-changes in body-composition and functional-capacity. J. Nutr 123, 465–468 (1993). [DOI] [PubMed] [Google Scholar]
- 108.Lamberts SW, van den Beld AW & van der Lely AJ The endocrinology of aging. Science 278, 419–424 (1997). [DOI] [PubMed] [Google Scholar]
- 109.Hu ZY et al. MicroRNA-29 induces cellular senescence in aging muscle through multiple signaling pathways. Aging 6, 160–175 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Evans WJ Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr 91, 1123S–1127S (2010). [DOI] [PubMed] [Google Scholar]
- 111.Morse CI, Thom JM, Reeves ND, Birch KM & Narici MV In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. J. Appl. Physiol 99, 1050–1055 (2005). [DOI] [PubMed] [Google Scholar]
- 112.[No authors listed.] Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People [Japanese]. Nihon Ronen Igakkai Zasshi 49, 788–805 (2012). [DOI] [PubMed] [Google Scholar]
- 113.Cruz-Jentoft AJ et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han A, Bokshan SL, Marcaccio SE, DePasse JM & Daniels AH Diagnostic criteria and clinical outcomes in sarcopenia research: a literature review. J. Clin. Med 7, 70 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Janssen I, Shepard DS, Katzmarzyk PT & Roubenoff R The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc 52, 80–85 (2004). [DOI] [PubMed] [Google Scholar]
- 116.Cruz-Jentoft AJ et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 43, 748–759 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Phillips SK, Rook KM, Siddle NC, Bruce SA & Woledge RC Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin. Sci 84, 95–98 (1993). [DOI] [PubMed] [Google Scholar]
- 118.Huang J et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/beta-catenin pathway. JBMR Plus 1, 86–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marks AR Intracellular calcium-release channels: regulators of cell life and death. Am. J. Physiol 272, H597–H605 (1997). [DOI] [PubMed] [Google Scholar]
- 120.Wehrens XH et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113, 829–840 (2003). [DOI] [PubMed] [Google Scholar]
- 121.Bellinger AM et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat. Med 15, 325–330 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Andersson DC et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 14, 196–207 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guise TA et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res 12, 6213S–6216S (2006). [DOI] [PubMed] [Google Scholar]
- 124.Mundy GR Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002). [DOI] [PubMed] [Google Scholar]
- 125.Janssens K et al. Mutations in the gene encoding the latency-associated peptide of TGF-beta 1 cause Camurati-Engelmann disease. Nat. Genet 26, 273–275 (2000). [DOI] [PubMed] [Google Scholar]
- 126.Hauschka PV, Mavrakos AE, Iafrati MD, Doleman SE & Klagsbrun M Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J. Biol. Chem 261, 12665–12674 (1986). [PubMed] [Google Scholar]
- 127.Waning DL et al. Excess TGF-beta mediates muscle weakness associated with bone metastases in mice. Nat. Med 21, 1262–1271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mundy GR, Yoneda T & Hiraga T Preclinical studies with zoledronic acid and other bisphosphonates: impact on the bone microenvironment. Semin. Oncol 28, 35–44 (2001). [DOI] [PubMed] [Google Scholar]
- 129.Waning DL & Guise TA Molecular mechanisms of bone metastasis and associated muscle weakness. Clin. Cancer Res 20, 3071–3077 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tuttle LJ, Sinacore DR, Cade WT & Mueller MJ Lower physical activity is associated with higher intermuscular adipose tissue in people with type 2 diabetes and peripheral neuropathy. Phys. Ther 91, 923–930 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bittel DC et al. Adipose tissue content, muscle performance and physical function in obese adults with type 2 diabetes mellitus and peripheral neuropathy. J. Diabetes Compl 29, 250–257 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Papadakis MA et al. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann. Intern. Med 124, 708–716 (1996). [DOI] [PubMed] [Google Scholar]
- 133.Brioche T et al. Growth hormone replacement therapy prevents sarcopenia by a dual mechanism: improvement of protein balance and of antioxidant defenses. J. Gerontol. A Biol. Sci. Med. Sci 69, 1186–1198 (2014). [DOI] [PubMed] [Google Scholar]
- 134.Jorgensen JO et al. Beneficial effects of growth hormone treatment in GH-deficient adults. Lancet 1, 1221–1225 (1989). [DOI] [PubMed] [Google Scholar]
- 135.Mikkelsen UR et al. Skeletal muscle morphology and regulatory signalling in endurance-trained and sedentary individuals: the influence of ageing. Exp. Gerontol 93, 54–67 (2017). [DOI] [PubMed] [Google Scholar]
- 136.Rogers MA & Evans WJ Changes in skeletal muscle with aging: effects of exercise training. Exerc. Sport Sci. Rev 21, 65–102 (1993). [PubMed] [Google Scholar]
- 137.Ryan AS Insulin resistance with aging: effects of diet and exercise. Sports Med 30, 327–346 (2000). [DOI] [PubMed] [Google Scholar]
- 138.Ivy JL Role of exercise training in the prevention and treatment of insulin resistance and non-insulin-dependent diabetes mellitus. Sports Med 24, 321–336 (1997). [DOI] [PubMed] [Google Scholar]
- 139.Baar K & Esser K Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am. J. Physiol 276, C120–C127 (1999). [DOI] [PubMed] [Google Scholar]
- 140.Kubica N, Bolster DR, Farrell PA, Kimball SR & Jefferson LS Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin-dependent manner. J. Biol. Chem 280, 7570–7580 (2005). [DOI] [PubMed] [Google Scholar]
- 141.Ji LL, Gomez-Cabrera MC, Steinhafel N & Vina J Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J 18, 1499–1506 (2004). [DOI] [PubMed] [Google Scholar]
- 142.Senf SM, Dodd SL & Judge AR FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am. J. Physiol. Cell Physiol 298, C38–C45 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kavazis AN, Smuder AJ & Powers SK Effects of short-term endurance exercise training on acute doxorubicin-induced FoxO transcription in cardiac and skeletal muscle. J. Appl. Physiol 117, 223–230 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Iyer S et al. FOXOs attenuate bone formation by suppressing Wnt signaling. J. Clin. Invest 123, 3409–3419 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stitt TN et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 14, 395–403 (2004). [DOI] [PubMed] [Google Scholar]
- 146.Bodine SC et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol 3, 1014–1019 (2001). [DOI] [PubMed] [Google Scholar]
- 147.Salanova M et al. Nitrosative stress in human skeletal muscle attenuated by exercise countermeasure after chronic disuse. Redox Biol 1, 514–526 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salanova M, Schiffl G, Rittweger J, Felsenberg D & Blottner D Ryanodine receptor type-1 (RyR1) expression and protein S-nitrosylation pattern in human soleus myofibres following bed rest and exercise countermeasure. Histochem. Cell Biol 130, 105–118 (2008). [DOI] [PubMed] [Google Scholar]
- 149.Frechette DM, Krishnamoorthy D, Adler BJ, Chan ME & Rubin CT Diminished satellite cells and elevated adipogenic gene expression in muscle as caused by ovariectomy are averted by low-magnitude mechanical signals. J. Appl. Physiol 119, 27–36 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Frechette DM et al. Mechanical signals protect stem cell lineage selection, preserving the bone and muscle phenotypes in obesity. Ann. NY Acad. Sci 1409, 33–50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mera P, Laue K, Wei J, Berger JM & Karsenty G Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol. Metab 5, 1042–1047 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mera P et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab 23, 1078–1092 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Febbraio MA, Hiscock N, Sacchetti M, Fischer CP & Pedersen BK Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 53, 1643–1648 (2004). [DOI] [PubMed] [Google Scholar]
- 154.Karsenty G & Mera P Molecular bases of the crosstalk between bone and muscle. Bone 11, 43–49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Messi ML et al. Resistance training enhances skeletal muscle innervation without modifying the number of satellite cells or their myofiber association in obese older adults. J. Gerontol. A Biol. Sci. Med. Sci 71, 1273–1280 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stewart VH, Saunders DH & Greig CA Responsiveness of muscle size and strength to physical training in very elderly people: a systematic review. Scand. J. Med. Sci. Sports 24, e1–e10 (2014). [DOI] [PubMed] [Google Scholar]
- 157.Thompson LV Effects of age and training on skeletal muscle physiology and performance. Phys. Ther 74, 71–81 (1994). [DOI] [PubMed] [Google Scholar]
- 158.Pedersen BK & Saltin B Evidence for prescribing exercise as therapy in chronic disease. Scand. J. Med. Sci. Sports 16 (Suppl. 1), 3–63 (2006). [DOI] [PubMed] [Google Scholar]
- 159.Hedlund PB, Yanaihara N & Fuxe K Evidence for specific N-terminal galanin fragment binding sites in the rat brain. Eur. J. Pharmacol 224, 203–205 (1992). [DOI] [PubMed] [Google Scholar]
- 160.Kelly OJ & Gilman JC Can unconventional exercise be helpful in the treatment, management and prevention of osteosarcopenic obesity? Curr. Aging Sci 10, 106–121 (2017). [DOI] [PubMed] [Google Scholar]
- 161.Tiedemann A, O’Rourke S, Sesto R & Sherrington CA 12-week Iyengar yoga program improved balance and mobility in older community-dwelling people: a pilot randomized controlled trial. J. Gerontol. A Biol. Sci. Med. Sci 68, 1068–1075 (2013). [DOI] [PubMed] [Google Scholar]
- 162.Courneya KS et al. Predictors of adherence to different types and doses of supervised exercise during breast cancer chemotherapy. Int. J. Behav. Nutr. Phys. Act 11, 85 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cox CL et al. Modifying bone mineral density, physical function, and quality of life in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 65, e26929 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schoenau E, Neu CM, Beck B, Manz F & Rauch F Bone mineral content per muscle cross-sectional area as an index of the functional muscle-bone unit. J. Bone Miner. Res 17, 1095–1101 (2002). [DOI] [PubMed] [Google Scholar]
- 165.Hall BK & Herring SW Paralysis and growth of the musculoskeletal system in the embryonic chick. J. Morphol 206, 45–56 (1990). [DOI] [PubMed] [Google Scholar]
- 166.Sharir A, Stern T, Rot C, Shahar R & Zelzer E Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development 138, 3247–3259 (2011). [DOI] [PubMed] [Google Scholar]
- 167.Hamrick MW, McGee-Lawrence ME & Frechette DM Fatty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Front. Endocrinol 7, 69 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Long P, Liu F, Piesco NP, Kapur R & Agarwal S Signaling by mechanical strain involves transcriptional regulation of proinflammatory genes in human periodontal ligament cells in vitro. Bone 30, 547–552 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pagnotti GM & Styner M Exercise regulation of marrow adipose tissue. Front. Endocrinol 7, 94 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cawthorn WP & Scheller EL Editorial: bone marrow adipose tissue: formation, function, and impact on health and disease. Front. Endocrinol 8, 112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fantuzzi G Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol 115, 911–919; quiz 920 (2005). [DOI] [PubMed] [Google Scholar]
- 172.Xue Y et al. Adipokines in psoriatic arthritis patients: the correlations with osteoclast precursors and bone erosions. PLOS ONE 7, e46740 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Patel VS, Ete Chan M, Rubin J & Rubin CT Marrow adiposity and hematopoiesis in aging and obesity: exercise as an intervention. Curr. Osteoporos. Rep 16, 105–115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Neumann E, Junker S, Schett G, Frommer K & Muller-Ladner U Adipokines in bone disease. Nat. Rev. Rheumatol 12, 296–302 (2016). [DOI] [PubMed] [Google Scholar]
- 175.Kudo O et al. Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone 32, 1–7 (2003). [DOI] [PubMed] [Google Scholar]
- 176.Cawthorn WP et al. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab 20, 368–375 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Basurto L et al. Adiponectin is associated with low bone mineral density in elderly men. Eur. J. Endocrinol 160, 289–293 (2009). [DOI] [PubMed] [Google Scholar]
- 178.Barbour KE et al. The effects of adiponectin and leptin on changes in bone mineral density. Osteoporosis Int 23, 1699–1710 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu L et al. Rosiglitazone inhibits bone regeneration and causes significant accumulation of fat at sites of new bone formation. Calcif. Tissue Int 91, 139–148 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Styner M et al. Exercise regulation of marrow fat in the setting of PPARgamma agonist treatment in female C57BL/6 mice. Endocrinology 156, 2753–2761 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Grey A et al. Pioglitazone increases bone marrow fat in type 2 diabetes: results from a randomized controlled trial. Eur. J. Endocrinol 166, 1087–1091 (2012). [DOI] [PubMed] [Google Scholar]
- 182.Kannel WB, Gordon T & Castelli WP Obesity, lipids, and glucose intolerance. The Framingham Study. Am. J. Clin. Nutr 32, 1238–1245 (1979). [DOI] [PubMed] [Google Scholar]
- 183.Adler BJ, Green DE, Pagnotti GM, Chan ME & Rubin CT High fat diet rapidly suppresses B lymphopoiesis by disrupting the supportive capacity of the bone marrow niche. PLOS ONE 9, e90639 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ambrosi TH et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20, 771–784 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Doucette CR et al. A high fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6J mice. J. Cell. Physiol 230, 2032–2037 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Scheller EL et al. Changes in skeletal integrity and marrow adiposity during high-fat diet and after weight loss. Front. Endocrinol 7, 102 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Styner M et al. Exercise decreases marrow adipose tissue through β-oxidation in obese running mice. J. Bone Miner. Res 32, 1692–1702 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Trottier MD, Naaz A, Li Y & Fraker PJ Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proc. Natl Acad. Sci. USA 109, 7622–7629 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.do Carmo LS et al. A high-fat diet increases interleukin-3 and granulocyte colony-stimulating factor production by bone marrow cells and triggers bone marrow hyperplasia and neutrophilia in Wistar rats. Exp. Biol. Med 238, 375–384 (2013). [DOI] [PubMed] [Google Scholar]
- 190.van den Berg SM et al. Diet-induced obesity in mice diminishes hematopoietic stem and progenitor cells in the bone marrow. FASEB J 30, 1779–1788 (2016). [DOI] [PubMed] [Google Scholar]
- 191.Singer K et al. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol. Metab 3, 664–675 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chan ME, Adler BJ, Green DE & Rubin CT Bone structure and B cell populations, crippled by obesity, are partially rescued by brief daily exposure to low-magnitude mechanical signals. FASEB J 26, 4855–4863 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nanji AA & Freeman JB Relationship between body weight and total leukocyte count in morbid obesity. Am. J. Clin. Pathol 84, 346–347 (1985). [DOI] [PubMed] [Google Scholar]
- 194.Womack J et al. Obesity and immune cell counts in women. Metabolism 56, 998–1004 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ryder E et al. Association of obesity with leukocyte count in obese individuals without metabolic syndrome. Diabetes Metab. Syndr 8, 197–204 (2014). [DOI] [PubMed] [Google Scholar]
- 196.Pecht T, Gutman-Tirosh A, Bashan N & Rudich A Peripheral blood leucocyte subclasses as potential biomarkers of adipose tissue inflammation and obesity subphenotypes in humans. Obes. Rev 15, 322–337 (2014). [DOI] [PubMed] [Google Scholar]
- 197.Xu X et al. Obesity is associated with more activated neutrophils in African American male youth. Int. J. Obes 39, 26–32 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ghigliotti G et al. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation 37, 1337–1353 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nteeba J, Ortinau LC, Perfield JW 2nd & Keating AF Diet-induced obesity alters immune cell infiltration and expression of inflammatory cytokine genes in mouse ovarian and peri-ovarian adipose depot tissues. Mol. Reprod. Dev 80, 948–958 (2013). [DOI] [PubMed] [Google Scholar]
- 200.Caer C et al. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci. Rep 7, 3000 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rubin CT et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc. Natl Acad. Sci. USA 104, 17879–17884 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kahn BB & Flier JS Obesity and insulin resistance. J. Clin. Invest 106, 473–481 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Qatanani M & Lazar MA Mechanisms of obesity-associated insulin resistance: many choices on the menu. Gene Dev 21, 1443–1455 (2007). [DOI] [PubMed] [Google Scholar]
- 204.Souza PP & Lerner UH The role of cytokines in inflammatory bone loss. Immunol. Invest 42, 555–622 (2013). [DOI] [PubMed] [Google Scholar]
- 205.Schett G Effects of inflammatory and anti-inflammatory cytokines on the bone. Eur. J. Clin. Invest 41, 1361–1366 (2011). [DOI] [PubMed] [Google Scholar]
- 206.McLean RR Proinflammatory cytokines and osteoporosis. Curr. Osteoporos. Rep 7, 134–139 (2009). [DOI] [PubMed] [Google Scholar]
- 207.Kimble RB et al. Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology 136, 3054–3061 (1995). [DOI] [PubMed] [Google Scholar]
- 208.Rubin CT & Lanyon LE Regulation of bone formation by applied dynamic loads. J. Bone Joint Surg. Am 66, 397–402 (1984). [PubMed] [Google Scholar]
- 209.O’Connor JA, Lanyon LE & MacFie H The influence of strain rate on adaptive bone remodelling. J. Biomech 15, 767–781 (1982). [DOI] [PubMed] [Google Scholar]
- 210.Rath B, Nam J, Knobloch TJ, Lannutti JJ & Agarwal S Compressive forces induce osteogenic gene expression in calvarial osteoblasts. J. Biomech 41, 1095–1103 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Poliachik SL, Threet D, Srinivasan S & Gross TS 32-wk-old C3H/HeJ mice actively respond to mechanical loading. Bone 42, 653–659 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rubin C, Turner AS, Bain S, Mallinckrodt C & McLeod K Anabolism. Low mechanical signals strengthen long bones. Nature 412, 603–604 (2001). [DOI] [PubMed] [Google Scholar]
- 213.Oxlund BS, Ortoft G, Andreassen TT & Oxlund H Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone 32, 69–77 (2003). [DOI] [PubMed] [Google Scholar]
- 214.Tanaka SM et al. Effects of broad frequency vibration on cultured osteoblasts. J. Biomech 36, 73–80 (2003). [DOI] [PubMed] [Google Scholar]
- 215.Garman R, Gaudette G, Donahue LR, Rubin C & Judex S Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J. Orthop. Res 25, 732–740 (2007). [DOI] [PubMed] [Google Scholar]
- 216.Wren TA et al. Effect of high-frequency, low-magnitude vibration on bone and muscle in children with cerebral palsy. J. Pediatr. Orthop 30, 732–738 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pre D et al. The differentiation of human adipose-derived stem cells (hASCs) into osteoblasts is promoted by low amplitude, high frequency vibration treatment. Bone 49, 295–303 (2011). [DOI] [PubMed] [Google Scholar]
- 218.Robinson TL et al. Gymnasts exhibit higher bone mass than runners despite similar prevalence of amenorrhea and oligomenorrhea. J. Bone Miner. Res 10, 26–35 (1995). [DOI] [PubMed] [Google Scholar]
- 219.Verschueren SM et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J. Bone Miner. Res 19, 352–359 (2004). [DOI] [PubMed] [Google Scholar]
- 220.Judex S & Rubin CT Is bone formation induced by high-frequency mechanical signals modulated by muscle activity? J. Musculoskelet. Neuronal Interact 10, 3–11 (2010). [PMC free article] [PubMed] [Google Scholar]
- 221.McGarry JG, Klein-Nulend J, Mullender MG & Prendergast PJ A comparison of strain and fluid shear stress in stimulating bone cell responses — a computational and experimental study. FASEB J 19, 482–484 (2005). [DOI] [PubMed] [Google Scholar]
- 222.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA & Mikos AG Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc. Natl Acad. Sci. USA 100, 14683–14688 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bancroft GN et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc. Natl Acad. Sci. USA 99, 12600–12605 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Weinbaum S, Cowin SC & Zeng Y A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech 27, 339–360 (1994). [DOI] [PubMed] [Google Scholar]
- 225.Reich KM, Gay CV & Frangos JA Fluid shear stress as a mediator of osteoblast cyclic adenosine monophosphate production. J. Cell. Physiol 143, 100–104 (1990). [DOI] [PubMed] [Google Scholar]
- 226.Qin YX & Hu M Intramedullary pressure induced by dynamic hydraulic pressure stimulation and its potential in treatment of osteopenia. Bone 48, S186 (2011). [Google Scholar]
- 227.Qin YX & Lam HY Intramedullary pressure and matrix strain induced by oscillatory skeletal muscle stimulation and its potential in adaptation. J. Biomech 42, 140–145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhang P, Su M, Liu YL, Hsu A & Yokota H Knee loading dynamically alters intramedullary pressure in mouse femora. Bone 40, 538–543 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chan ME, Uzer G & Rubin CT The potential benefits and inherent risks of vibration as a non-drug therapy for the prevention and treatment of osteoporosis. Curr. Osteoporos. Rep 11, 36–44 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Price C, Zhou X, Li W & Wang L Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: direct evidence for load-induced fluid flow. J. Bone Miner. Res 26, 277–285 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Coughlin TR & Niebur GL Fluid shear stress in trabecular bone marrow due to low-magnitude high-frequency vibration. J. Biomech 45, 2222–2229 (2012). [DOI] [PubMed] [Google Scholar]
- 232.Lim KT et al. Physical stimulation-based osteogenesis: effect of secretion in vitro on fluid dynamic shear stress of human alveolar bone-derived mesenchymal stem cells. IEEE Trans. Nanobioscience 15, 881–890 (2016). [DOI] [PubMed] [Google Scholar]
- 233.Williams JL, Iannotti JP, Ham A, Bleuit J & Chen JH Effects of fluid, shear-stress on bone-cells. Biorheology 31, 163–170 (1994). [DOI] [PubMed] [Google Scholar]
- 234.Vainionpaa A et al. Intensity of exercise is associated with bone density change in premenopausal women. Osteoporosis Int 17, 455–463 (2006). [DOI] [PubMed] [Google Scholar]
- 235.Cabrita GJM et al. Hematopoietic stem cells: from the bone to the bioreactor. Trends Biotechnol 21, 233–240 (2003). [DOI] [PubMed] [Google Scholar]
- 236.Gordon M Stem cells handbook. Bone Marrow Transplant 33, 1165 (2004). [Google Scholar]
- 237.Dickerson DA, Sander EA & Nauman EA Modeling the mechanical consequences of vibratory loading in the vertebral body: microscale effects. Biomech. Model. Mechanobiol 7, 191–202 (2008). [DOI] [PubMed] [Google Scholar]
- 238.Bryant JD, David T, Gaskell PH, King S & Lond G Rheology of bovine bone marrow. Proc. Inst. Mech. Eng. H 203, 71–75 (1989). [DOI] [PubMed] [Google Scholar]
- 239.Blecha LD, Rakotomanana L, Razafimahery F, Terrier A & Pioletti DP Mechanical interaction between cells and fluid for bone tissue engineering scaffold: modulation of the interfacial shear stress. J. Biomech 43, 933–937 (2010). [DOI] [PubMed] [Google Scholar]
- 240.Gimble JM, Robinson CE, Wu X & Kelly KA The function of adipocytes in the bone marrow stroma: an update. Bone 19, 421–428 (1996). [DOI] [PubMed] [Google Scholar]
- 241.Justesen J et al. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2, 165–171 (2001). [DOI] [PubMed] [Google Scholar]
- 242.Swift J et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341, 1240104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Engler AJ, Sen S, Sweeney HL & Discher DE Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). [DOI] [PubMed] [Google Scholar]
- 244.Thompson WR et al. LARG GEF and ARHGAP18 orchestrate RhoA activity to control mesenchymal stem cell lineage. Bone 107, 172–180 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sen B et al. mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells. J. Bone Miner. Res 29, 78–89 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sen B et al. Intranuclear actin regulates osteogenesis. Stem Cells 33, 3065–3076 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Sen B et al. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. J. Biol. Chem 284, 34607–34617 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lombardi ML et al. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J. Biol. Chem 286, 26743–26753 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Parsons JT, Horwitz AR & Schwartz MA Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol 11, 633–643 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Burridge K, Fath K, Kelly T, Nuckolls G & Turner C Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu. Rev. Cell Biol 4, 487–525 (1988). [DOI] [PubMed] [Google Scholar]
- 251.Grashoff C et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Turner CE, Glenney JR Jr & Burridge K. Paxillin: a new vinculin-binding protein present in focal adhesions. J. Cell Biol 111, 1059–1068 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD & Waterman CM Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J. Cell Biol 188, 877–890 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Machesky LM & Insall RH Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol 8, 1347–1356 (1998). [DOI] [PubMed] [Google Scholar]
- 255.Marchand JB, Kaiser DA, Pollard TD & Higgs HN Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nat. Cell Biol 3, 76–82 (2001). [DOI] [PubMed] [Google Scholar]
- 256.Mullins RD, Heuser JA & Pollard TD The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl Acad. Sci. USA 95, 6181–6186 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Jaffe AB & Hall A Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol 21, 247–269 (2005). [DOI] [PubMed] [Google Scholar]
- 258.Riddick N, Ohtani K & Surks HK Targeting by myosin phosphatase-RhoA interacting protein mediates RhoA/ROCK regulation of myosin phosphatase. J. Cell. Biochem 103, 1158–1170 (2008). [DOI] [PubMed] [Google Scholar]
- 259.Bhadriraju K et al. Activation of ROCK by RhoA is regulated by cell adhesion, shape, and cytoskeletal tension. Exp. Cell Res 313, 3616–3623 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Thompson WR et al. Mechanically activated Fyn utilizes mTORC2 to regulate RhoA and adipogenesis in mesenchymal stem cells. Stem Cells 31, 2528–2537 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Case N et al. Mechanical regulation of glycogen synthase kinase 3beta (GSK3beta) in mesenchymal stem cells is dependent on Akt protein serine 473 phosphorylation via mTORC2 protein. J. Biol. Chem 286, 39450–39456 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Stewart-Hutchinson PJ, Hale CM, Wirtz D & Hodzic D Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp. Cell Res 314, 1892–1905 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Neelam S et al. Direct force probe reveals the mechanics of nuclear homeostasis in the mammalian cell. Proc. Natl Acad. Sci. USA 112, 5720–5725 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Holaska JM, Kowalski AK & Wilson KL Emerin caps the pointed end of actin filaments: evidence for an actin cortical network at the nuclear inner membrane. PLOS Biol 2, E231 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Le HQ et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol 18, 864–875 (2016). [DOI] [PubMed] [Google Scholar]
- 266.Sen B et al. Intranuclear actin structure modulates mesenchymal stem cell differentiation. Stem Cells 35, 1624–1635 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kutscheidt S et al. FHOD1 interaction with nesprin-2G mediates TAN line formation and nuclear movement. Nat. Cell Biol 16, 708–715 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chancellor TJ, Lee J, Thodeti CK & Lele T Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys. J 99, 115–123 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Chen CY et al. Accumulation of the inner nuclear envelope protein Sun1 is pathogenic in progeric and dystrophic laminopathies. Cell 149, 565–577 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Thakar K, May CK, Rogers A & Carroll CW Opposing roles for distinct LINC complexes in regulation of the small GTPase RhoA. Mol. Biol. Cell 28, 182–191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Uzer G et al. Sun-mediated mechanical LINC between nucleus and cytoskeleton regulates betacatenin nuclear access. J. Biomech 74, 32–40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Shiu JY, Aires L, Lin Z & Vogel V Nanopillar force measurements reveal actin-cap-mediated YAP mechanotransduction. Nat. Cell Biol 20, 262–271 (2018). [DOI] [PubMed] [Google Scholar]
- 273.Elosegui-Artola A et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410 (2017). [DOI] [PubMed] [Google Scholar]
- 274.Koike M et al. beta-Catenin shows an overlapping sequence requirement but distinct molecular interactions for its bidirectional passage through nuclear pores. J. Biol. Chem 279, 34038–34047 (2004). [DOI] [PubMed] [Google Scholar]
- 275.Tolwinski NS & Wieschaus E A nuclear function for armadillo/beta-catenin. PLOS Biol 2, E95 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Neumann S et al. Nesprin-2 interacts with {alpha}-catenin and regulates Wnt signaling at the nuclear envelope. J. Biol. Chem 285, 34932–34938 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Broers JL et al. Decreased mechanical stiffness in LMNA−/− cells is caused by defective nucleocytoskeletal integrity: implications for the development of laminopathies. Hum. Mol. Genet 13, 2567–2580 (2004). [DOI] [PubMed] [Google Scholar]
- 278.Stephens AD, Banigan EJ, Adam SA, Goldman RD & Marko JF Chromatin and lamin A determine two different mechanical response regimes of the cell nucleus. Mol. Biol. Cell 28, 1984–1996 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Khatau SB et al. The differential formation of the LINC-mediated perinuclear actin cap in pluripotent and somatic cells. PLOS ONE 7, e36689 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Vidal C, Bermeo S, Fatkin D & Duque G Role of the nuclear envelope in the pathogenesis of age-related bone loss and osteoporosis. Bonekey Rep 1, 62 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.McBeath R, Pirone DM, Nelson CM, Bhadriraju K & Chen CS Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 (2004). [DOI] [PubMed] [Google Scholar]
- 282.Bermeo S, Vidal C, Zhou H & Duque G Lamin A/C acts as an essential factor in mesenchymal stem cell differentiation through the regulation of the dynamics of the Wnt/beta-catenin pathway. J. Cell. Biochem 116, 2344–2353 (2015). [DOI] [PubMed] [Google Scholar]
- 283.Constantinescu D, Gray HL, Sammak PJ, Schatten GP & Csoka AB Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells 24, 177–185 (2006). [DOI] [PubMed] [Google Scholar]
- 284.Akter R, Rivas D, Geneau G, Drissi H & Duque G Effect of lamin A/C knockdown on osteoblast differentiation and function. J. Bone Miner. Res 24, 283–293 (2009). [DOI] [PubMed] [Google Scholar]
- 285.Tong J et al. Lamin A/C deficiency is associated with fat infiltration of muscle and bone. Mech. Ageing Dev 132, 552–559 (2011). [DOI] [PubMed] [Google Scholar]
- 286.Li W et al. Decreased bone formation and osteopenia in lamin a/c-deficient mice. PLOS ONE 6, e19313 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Armstrong VJ et al. Estrogen receptor a is required for strain-related beta-catenin signaling in osteoblasts. J. Bone Miner. Res 22, S95 (2007). [Google Scholar]
- 288.Bonewald LF Mechanosensation and transduction in osteocytes. Bonekey Osteovision 3, 7–15 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Bradford PG, Gerace KV, Roland RL & Chrzan BG Estrogen regulation of apoptosis in osteoblasts. Physiol. Behav 99, 181–185 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Jessop HL et al. Mechanical strain and estrogen activate estrogen receptor alpha in bone cells. J. Bone Miner. Res 16, 1045–1055 (2001). [DOI] [PubMed] [Google Scholar]
- 291.Wehrle E et al. The impact of low-magnitude high-frequency vibration on fracture healing is profoundly influenced by the oestrogen status in mice. Dis. Model. Mech 8, 93–104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Guo Y et al. Mechanical strain promotes osteoblast ECM formation and improves its osteoinductive potential. Biomed. Eng. Online 11, 80 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Kamel MA et al. Fluid flow shear stress and prostaglandin E2 activates beta-catenin signaling in MLO-Y4 osteocytic and 2T3 osteoblastic cells. J. Bone Miner. Res 22, S375 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Srinivasan S, Weimer DA, Agans SC, Bain SD & Gross TS Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J. Bone Miner. Res 17, 1613–1620 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wright LE et al. Single-limb irradiation induces local and systemic bone loss in a murine model. J. Bone Miner. Res 30, 1268–1279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Krishnamoorthy D et al. Marrow adipogenesis and bone loss that parallels estrogen deficiency is slowed by low-intensity mechanical signals. Osteoporos. Int 27, 747–756 (2016). [DOI] [PubMed] [Google Scholar]
- 297.Limonard EJ et al. Short-term effect of estrogen on human bone marrow fat. J. Bone Miner. Res 30, 2058–2066 (2015). [DOI] [PubMed] [Google Scholar]
- 298.Tuomilehto J et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med 344, 1343–1350 (2001). [DOI] [PubMed] [Google Scholar]
- 299.Wallace IJ et al. Focal enhancement of the skeleton to exercise correlates with responsivity of bone marrow mesenchymal stem cells rather than peak external forces. J. Exp. Biol 218, 3002–3009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Bonewald LF & Johnson ML Osteocytes, mechanosensing and Wnt signaling. Bone 42, 606–615 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ & Burger EH Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts—correlation with prostaglandin upregulation. Biochem. Biophys. Res. Commun 217, 640–648 (1995). [DOI] [PubMed] [Google Scholar]
- 302.Qin YX, Lin W & Rubin C The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. Ann. Biomed. Eng 30, 693–702 (2002). [DOI] [PubMed] [Google Scholar]
- 303.Qin YX, Kaplan T, Saldanha A & Rubin C Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J. Biomech 36, 1427–1437 (2003). [DOI] [PubMed] [Google Scholar]
- 304.Rubin CT & Lanyon LE Kappa Delta Award paper. Osteoregulatory nature of mechanical stimuli: function as a determinant for adaptive remodeling in bone. J. Orthop. Res 5, 300–310 (1987). [DOI] [PubMed] [Google Scholar]
- 305.Jarvinen TL et al. Randomized controlled study of effects of sudden impact loading on rat femur. J. Bone Miner. Res 13, 1475–1482 (1998). [DOI] [PubMed] [Google Scholar]
- 306.Gross TS, Edwards JL, McLeod KJ & Rubin CT Strain gradients correlate with sites of periosteal bone formation. J. Bone Miner. Res 12, 982–988 (1997). [DOI] [PubMed] [Google Scholar]
- 307.Lanyon LE & Rubin CT Static versus dynamic loads as an influence on bone remodelling. J. Biomech 17, 897–905 (1984). [DOI] [PubMed] [Google Scholar]
- 308.Reyes ML, Hernandez M, Holmgren LJ, Sanhueza E & Escobar RG High-frequency, low-intensity vibrations increase bone mass and muscle strength in upper limbs, improving autonomy in disabled children. J. Bone Miner. Res 26, 1759–1766 (2011). [DOI] [PubMed] [Google Scholar]
- 309.Dumas V et al. Extracellular matrix produced by osteoblasts cultured under low-magnitude, high-frequency stimulation is favourable to osteogenic differentiation of mesenchymal stem cells. Calcif. Tissue Int 87, 351–364 (2010). [DOI] [PubMed] [Google Scholar]
- 310.Raso V, Natale VM, Duarte AJ, Greve JM & Shephard RJ Immunological parameters in elderly women: correlations with aerobic power, muscle strength and mood state. Brain Behav. Immun 26, 597–606 (2012). [DOI] [PubMed] [Google Scholar]
- 311.Wenger KH et al. Effect of whole-body vibration on bone properties in aging mice. Bone 47, 746–755 (2010). [DOI] [PubMed] [Google Scholar]
- 312.Bacabac RG et al. Bone cell responses to high-frequency vibration stress: does the nucleus oscillate within the cytoplasm? FASEB J 20, 858–864 (2006). [DOI] [PubMed] [Google Scholar]
- 313.Sherk VD et al. Acute bone marker responses to whole-body vibration and resistance exercise in young women. J. Clin. Densitom 16, 104–109 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Pagnotti GM et al. Low magnitude mechanical signals mitigate osteopenia without compromising longevity in an aged murine model of spontaneous granulosa cell ovarian cancer. Bone 51, 570–577 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Pagnotti GM et al. Low intensity vibration mitigates tumor progression and protects bone quantity and quality in a murine model of myeloma. Bone 90, 69–79 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.David V et al. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology 148, 2553–2562 (2007). [DOI] [PubMed] [Google Scholar]
- 317.Maddalozzo GF, Iwaniec UT, Turner RT, Rosen CJ & Widrick JJ Whole-body vibration slows the acquisition of fat in mature female rats. Int. J. Obes 32, 1348–1354 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Huang RP, Rubin CT & McLeod KJ Changes in postural muscle dynamics as a function of age. J. Gerontol. A Biol. Sci. Med. Sci 54, B352–B357 (1999). [DOI] [PubMed] [Google Scholar]
- 319.Adams DJ et al. Testing the daily stress stimulus theory of bone adaptation with natural and experimentally controlled strain histories. J. Biomech 30, 671–678 (1997). [DOI] [PubMed] [Google Scholar]
- 320.Fritton SP, McLeod KJ & Rubin CT Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J. Biomech 33, 317–325 (2000). [DOI] [PubMed] [Google Scholar]
- 321.Rubin C et al. Mechanical strain, induced noninvasively in the high-frequency domain, is anabolic to cancellous bone, but not cortical bone. Bone 30, 445–452 (2002). [DOI] [PubMed] [Google Scholar]
- 322.Luu YK et al. Development of diet-induced fatty liver disease in the aging mouse is suppressed by brief daily exposure to low-magnitude mechanical signals. Int. J. Obes 34, 401–405 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Rosen CJ et al. Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone 35, 1046–1058 (2004). [DOI] [PubMed] [Google Scholar]
- 324.Mukherjee S et al. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J. Clin. Invest 118, 491–504 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Luu YK, Capilla E, Pessin JE, Judex S & Rubin CT in 2007 IEEE 33rd Annual Northeast Bioengineering Conference 203–204 (IEEE, Long Island, NY, 2007). [Google Scholar]
- 326.Klein-Nulend J et al. Donor age and mechanosensitivity of human bone cells. Osteoporosis Int 13, 137–146 (2002). [DOI] [PubMed] [Google Scholar]
- 327.Gilsanz V et al. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J. Bone Miner. Res 21, 1464–1474 (2006). [DOI] [PubMed] [Google Scholar]
- 328.McGee-Lawrence ME et al. Whole-body vibration mimics the metabolic effects of exercise in male leptin receptor-deficient mice. Endocrinology 158, 1160–1171 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Robling AG, Burr DB & Turner CH Recovery a resonance-based testing device. Ann. Biomed. Eng. periods restore mechanosensitivity to dynamically loaded bone. J. Exp. Biol 204, 3389–3399 (2001). [DOI] [PubMed] [Google Scholar]
- 330.Guo S Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J. Endocrinol 220, T1–T23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Ward K et al. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J. Bone Miner. Res 19, 360–369 (2004). [DOI] [PubMed] [Google Scholar]
- 332.Lam TP et al. Effect of whole body vibration (WBV) therapy on bone density and bone quality in osteopenic girls with adolescent idiopathic scoliosis: a randomized, controlled trial. Osteoporos. Int 24, 1623–1636 (2013). [DOI] [PubMed] [Google Scholar]
- 333.Bianchi M et al. Effects of low-magnitude high-frequency vibration on bone density, bone resorption and muscular strength in ambulant children affected by Duchenne muscular dystrophy. J. Bone Miner. Res 28 (Suppl. 1), LB–SU03 (2013). [Google Scholar]
- 334.Mogil RJ et al. Effect of low-magnitude, high-frequency mechanical stimulation on BMD among young childhood cancer survivors: a randomized clinical trial. JAMA Oncol 2, 908–914 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Leonard MB et al. Effect of low magnitude mechanical stimuli on bone density and structure in pediatric Crohn’s disease: a randomized placebo controlled trial. J. Bone Miner. Res 31, 1177–1188 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.DiVasta AD et al. The ability of low-magnitude mechanical signals to normalize bone turnover in adolescents hospitalized for anorexia nervosa. Osteoporosis Int 28, 1255–1263 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Fritton JC, Rubin CT, Qin YX & McLeod KJ Whole-body vibration in the skeleton: development of a resonance-based testing device. Ann. Biomed. Eng 25, 831–839 (1997). [DOI] [PubMed] [Google Scholar]
- 338.Muir J, Kiel DP & Rubin CT Safety and severity of accelerations delivered from whole body vibration exercise devices to standing adults. J. Sci. Med. Sport 16, 526–531 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Kiel DP et al. Insights from the conduct of a device trial in older persons: low magnitude mechanical stimulation for musculoskeletal health. Clin. Trials 7, 354–367 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kiel DP et al. Low-magnitude mechanical stimulation to improve bone density in persons of advanced age: a randomized, placebo-controlled trial. J. Bone Miner. Res 30, 1319–1328 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Asselin P, Spungen AM, Muir JW, Rubin CT & Bauman WA Transmission of low-intensity vibration through the axial skeleton of persons with spinal cord injury as a potential intervention for preservation of bone quantity and quality. J. Spinal Cord Med 34, 52–59 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.International Organization for Standardization. Evaluation of human exposure to whole-body vibration [ISO 2631–1:1985] (ISO, 1985).
- 343.Kiiski J, Heinonen A, Jarvinen TL, Kannus P & Sievanen H Transmission of vertical whole body vibration to the human body. J. Bone Miner. Res 23, 1318–1325 (2008). [DOI] [PubMed] [Google Scholar]
- 344.Ozcivici E et al. Mechanical signals as anabolic agents in bone. Nat. Rev. Rheumatol 6, 50–59 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Martinac B Mechanosensitive ion channels: molecules of mechanotransduction. J. Cell Sci 117, 2449–2460 (2004). [DOI] [PubMed] [Google Scholar]
- 346.Kung C, Martinac B & Sukharev S Mechanosensitive channels in microbes. Annu. Rev. Microbiol 64, 313–329 (2010). [DOI] [PubMed] [Google Scholar]


