Abstract
Midkine (MDK) and Pleiotrophin (PTN) belong to a group of heparin-binding growth factors that has been shown to have pleiotropic functions in various biological processes during development and disease. Development of the vertebrate eye is a multistep process that involves coordinated interactions between neuronal and non-neuronal cells, but very little is known about the potential function of MDK and PTN in these processes. In this study, we demonstrate by section in situ hybridization, the spatiotemporal expression of MDK and PTN during ocular development in chick and mouse. We show that MDK and PTN are expressed in dynamic patterns that overlap in a few non-neuronal tissues in the anterior eye and in neuronal cell layers of the posterior eye. We show that the expression patterns of MDK and PTN are only conserved in a few tissues in chick and mouse but they overlap with the expression of some of their receptors LRP1, RPTPZ, ALK, NOTCH2,ITGβ1, SDC1, and SDC3. The dynamic expression patterns of MDK, PTN and their receptors suggest that they function together during the multistep process of ocular development and they may play important roles in cell proliferation, adhesion, and migration of neuronal and non-neuronal cells.
Keywords: Midkine, Pleiotrophin, ocular development, cornea, lens, retina
Introduction
Midkine (MDK) and pleiotrophin (PTN) are closely related genes that form a two-member family of heparin binding growth factors. MDK was identified as a retinoic acid-induced gene in carcinoma cells (Kadomatsu et al., 1990). PTN was discovered as a neurite outgrowth-promoting factor in neonatal rat brains (Rauvala and Pihlaskari, 1989), and also as a mitogen for bovine uterus fibroblasts (Li et al., 1990). Since their discovery, several studies have shown that MDK and PTN have pleiotropic functions in various biological processes including cell proliferation, migration, differentiation and survival, and in neural development, angiogenesis, cancer, and inflammation (Choudhuri et al., 1997; Garver et al., 1993; Li et al., 1990; Ohta et al., 1999; Qi et al., 2001; Weckbach et al., 2011; Weckbach et al., 2012), but their function in ocular development remain unclear.
MDK and PTN function by binding various receptors that include receptor protein tyrosine phosphatase beta/zeta (RPTPZ), anaplastic lymphoma kinase (ALK), integrin beta 1 (ITGα1), NOTCH2, lipoprotein receptor-related protein 1 (LRP1), and co-receptors syndecan-1 (SDC1) and syndecan-3 (SDC3) (Huang et al., 2008; Kaspiris et al., 2013; Muramatsu et al., 2000; Muramatsu et al., 2004; Raulo et al., 1994; Reiff et al., 2011; Sakaguchi et al, 2003; Stoica et al., 2002). Through interactions with these receptors, MDK and PTN promote neuronal cell migration and differentiation of neural stem cells (Jung et al., 2004; Maeda et al., 1999), enhance endothelial cell proliferation and angiogenesis (Choudhuri et al., 1997; Laaroubi et al., 1994; Mikelis et al., 2009), act as mitogens for fibroblasts, hepatocytes, and osteoblasts (Asahina et al., 2002; Li et al., 1990; Yang et al., 2003), and play various roles in cancer (see review by Jono and Ando, 2010).
Studies in mice indicate that Mdk is ubiquitously expressed during early embryogenesis and it becomes progressively restricted to the central nervous system, ocular tissues, jaws, somites, and kidneys where it is transiently expressed during organogenesis (Kadomatsu et al., 1990). Also in mice, Ptn is expressed in the central and peripheral nervous system during development (Fan et al., 2000; Nakamoto et al., 1992). Both Mdk and Ptn transcripts and proteins are expressed during branching morphogenesis in salivary glands, lungs, and kidneys (Mitsiadis et al., 1995). Expression of MDK and PTN is conserved between mouse, zebrafish, xenopus, and chick embryos during early development (Cockshutt et al., 1994; Sekiguchi et al., 1995; Winkler and Moon, 2001; Winkler et al., 2014), suggesting that they have similar functions in these model organisms. Studies involving single knockout of either Mdk or Ptn in mice reveal only subtle physical defects with no noticeable malformation in the major organs (Muramatsu et al., 2006). The differences between wild type and Mdk or Ptn single mutants relate to response to neurotoxicity and pharmacological agents in the adults (Herradon and Perez-Garcia, 2014). However, Mdk/Ptn double mutants have low embryonic viability, with only 1/3 of the expected double knockout embryos surviving to birth. The surviving double knockout mutants are smaller than their wild type littermates and they have defects in female reproduction, lower intestinal tract, locomotion, and deficit in auditory response due to loss of β-tectorin (Muramatsu et al., 2006; Zou et al, 2006).
The above studies indicate that MDK and PTN function during early development and organogenesis in tissues that involve epithelial-mesenchymal interactions. Although ocular development involves initial interaction between the neural and cranial ectoderm to form the lens and optic cup, which later interact with the neural crest mesenchyme to form the cornea, iris and other ocular tissues (Beebe and Coats, 2000; Evans and Gage, 2005; Hyer et al, 2003; Lwigale and Bronner-Fraser, 2009), very little is known about the expression and function of MDK and PTN during ocular development. In this study, we examined the spatiotemporal expression of MDK and PTN during ocular development and compare their expression patterns between chick and mice. Our results show that MDK and PTN are expressed in dynamic patterns that partially overlap during ocular development, and that their expression is only conserved in a few ocular tissues between chick and mouse. This work sets the foundation for further investigation of the function of MDK and PTN during ocular development in chick and mouse.
Results
To determine the expression of MDK and PTN, we performed section in situ hybridization on chick and mouse eyes at different stages of development, beginning at the separation of the ectoderm from the lens vesicle through the formation of the cornea, eyelids, and retina. These processes occur between embryonic day (E)3 and E18 in chick (Creuzet et al., 2005; Doh et al., 2010; Lwigale et al., 2005) and between E10.5 and P10 in mice (Pei and Rhodin, 1970; Turner et al., 1990; Young, 1985).
Expression of MDK and PTN during development of the anterior eye in chick
Cornea development begins with the migration of periocular neural crest cells into the presumptive corneal region located between the lens vesicle and the overlying ectoderm. In chick, this process occurs in two successive waves, whereby the initial migration of periocular neural crest cells at E4.5 forms the corneal endothelium, which is followed by a second wave at E6 into the primary stroma that forms the keratocytes (Creuzet et al., 2005; Hay, 1980; Johnston et al., 1979; Lwigale et al., 2005). Our analyses show that in chick, MDK is robustly expressed at E3 in the periocular mesenchyme, optic cup, lens vesicle, and overlying ectoderm (Fig. 1A). At E5, MDK is vividly expressed in the corneal epithelium, but it persists at low levels in the periocular mesenchyme during the formation of the corneal endothelium, in the optic cup region that will later become the ciliary margin, and in the lens epithelium (Fig. 1B). By E7, MDK is localized to the corneal epithelium and endothelium, with the stroma showing weak signals. MDK is also vividly expressed in the iris stroma, ciliary margin, lens epithelium, and in the mesenchyme surrounding the ocular blood vessels (Fig. 1C).
Figure 1. Expression pattern of MDK and PTN during development of the chick anterior eye.
Transverse sections through E3, E5, and E7 eyes showing the spatiotemporal expression of MDK (A-C) and PTN (D-F). Asterisks in B, C, E, and F indicate ocular blood vessels. Arrowheads in F indicate condensed mesenchyme at the boundary between the presumptive drainage angle and trabecular meshwork. Abbreviations: ec, ectoderm; pm, periocular mesenchyme; oc, optic cup; L, lens; ep, corneal epithelium; en, corneal endothelium; st, corneal stroma; ir, iris; cm, ciliary margin. Scale bars represent 100 μm in A, D; B, E; and C, F.
In contrast, expression of PTN is undetectable in the ocular tissues at E3 (Fig. 1D). Its expression is first detected in the periocular mesenchyme, corneal endothelium, and lens epithelium at E5 (Fig. 1E). By E7, PTN is strongly expressed in the corneal stroma and in the condensed mesenchyme that marks the boundary between the presumptive drainage angle and trabecular meshwork (Fig. 1F, arrowheads). PTN is also expressed at low levels in the corneal endothelium, iris stroma, ciliary margin, and lens epithelium at E7.
Expression of Mdk and Ptn during development of the anterior eye in mouse
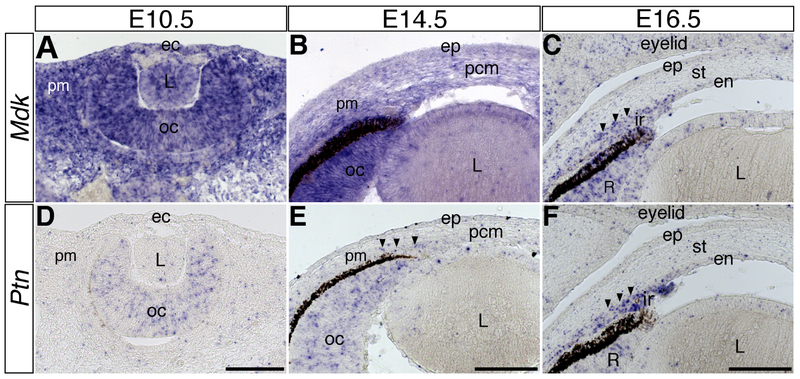
Morphogenesis of the lens and optic cup follow a similar pattern in chick and mice, but corneal development follows a different process. In the mouse, the periocular mesenchyme migrates in a single wave at about E11.5, which becomes the corneal mesenchyme that later differentiates to form the corneal stroma and endothelium (Gage et al., 2005; Pei and Rhodin, 1970). Similar to E3 chick, Mdk is robustly expressed in the periocular mesenchyme, optic cup, lens vesicle, and overlying ectoderm at E10.5 (Fig. 2A). However, by E14.5, vivid expression is only observed in the optic cup and adjacent periocular mesenchyme, whereas the presumptive corneal mesenchyme and lens epithelium show weak staining (Fig. 2B). Similar pattern of Mdk expression is observed at a diminished level at E16.5, with strong staining in the presumptive iris stroma (Fig. 2C).
Figure 2. Expression pattern of Mdk and Ptn during development of the mouse anterior eye.
Transverse sections through E10.5, E14.5, and E16.5 eyes showing the spatiotemporal expression of Mdk (A-C) and Ptn (D-F). Arrowheads in E and F indicate expression of Ptn in mesenchyme of the presumptive iris. Abbreviations: ec, ectoderm; pm, periocular mesenchyme; oc, optic cup; L, lens; ep, corneal epithelium; pcm, presumptive corneal mesenchyme; en, corneal endothelium; st, corneal stroma; ir, iris; pm, periocular mesenchyme; R, neural retina. Scale bars represent 100 μm in A, D; B, E; and C, F.
Expression of Ptn is very low and mostly localized to the optic cup at E10.5 (Fig. 2D), where it persists in the anterior region at E14.5 and E16.5 (Fig. 2E and 2F). Also at E14.5, Ptn is expressed in few cells adjacent to the retinal pigment epithelium that comprise the presumptive iris stroma (Fig. 2E, arrowheads), where it becomes strongly expressed at E16.5 (Fig. 2F, arrowheads). Sporadic staining is also observed in the lens epithelium at E16.5 (Fig. 2F).
Expression of MDK and PTN during development of the chick retina
Development of the retina follows a sequence that is conserved in vertebrates. This process begins with multipotent progenitor cells in the optic cup that undergo temporal differentiation into retinal ganglion cells, horizontal cells, cone photoreceptors, amacrine cells, bipolar cells, rod photoreceptors, and then Müller glia cells (see review by Agathocleous and Harris, 2009). In the chick, development of the neural retina begins with the differentiation of retinal ganglion cells at about E6, and well-defined boundaries of all layers are observed by E18 (Doh et al., 2010). To determine whether MDK and PTN have a potential role during development of the chick neural retina, we examined their expression patterns between E5-E18. Analysis of the posterior eye at E5 shows that the expression of MDK remains evenly robust in the neural retina and the mesenchyme surrounding the retinal pigment epithelium, but it is diminished in the optic nerve (Fig. 3A). Retina sections at subsequent time points show that MDK remains robust at E7 (Fig. 3B), but it becomes restricted to the inner nuclear layer (INL) at E10, where it is maintained at E15 and E18 (Fig. 3C, D, E).
Figure 3. Expression pattern of MDK and PTN during retinal development in chick.
Transverse sections through E5, E7, E10, E15 and E18 eyes showing the spatiotemporal expression of MDK (A-E) and PTN (F-J). All images oriented with the retinal pigmented epithelium (rpe) at the top. Images for A and F represent posterior retina. Rest of the images were acquired from the dorsal retina in the region between the optic nerve and ciliary body. Asterisks in B and G indicate detachment of the rpe (not shown) from the neural retina during tissue processing. Abbreviations: nr, neural retina; mes, mesenchyme; on, optic nerve; gcl, ganglion cell layer; inl, inner nuclear layer; onl, outer nuclear layer. Scale bars represent 100 μm in A,F and B,C,D,E,G,H,I,J.
At E5, PTN is strongly expressed in the optic nerve and by a few cells that line the boundary of the inner retina, prior to the formation of the retinal ganglion cells (Fig. 3F). The strong expression of PTN persists in the optic nerve during subsequent stages of development, and it is still detectable at E18 (data not shown). By E7, expression of PTN is observed throughout the neural retina, with strong staining persisting in the ganglion cell layer (GCL; Fig. 3G). By E10, PTN is localized to the GCL and INL (Fig. 3H), and this pattern remains at E15 with diminished staining of the GCL (Fig. 3I). By E18, PTN is not detectable in the GCL, although the staining remains in the INL and new staining is observed in the outer nuclear layer (ONL; Fig. 3J).
Expression of Mdk and Ptn during development of the mouse retina
In the mouse, retina development occurs between E11 and E18 (Turner et al., 1990). We examined expression patterns of Mdk and Ptn between E13.5-P12 and found that by E13.5, Mdk is expressed in the posterior ocular mesenchyme and the outer neuroblastic layer of the neural retina, with negligible expression in the optic nerve (Fig. 4A). By E16.5, expression of Mdk is maintained in the outer neuroblastic layer and extends at low levels into the inner neuroblastic layer (Fig. 4C). At P0, Mdk is expressed in the INL (Fig. 4D), and it is not detected in the retina by P12 (Fig. 4E).
Figure 4. Expression pattern of Mdk and Ptn during retinal development in mouse.
Transverse sections through E13.5, E14.5, E16.5, P0 and P12 eyes showing the spatiotemporal expression of Mdk (A-E) and Ptn (F-J). All images oriented with the retinal pigmented epithelium (rpe) at the top. Images for A and F represent the posterior retina. Rest of the images were acquired from the dorsal retina in the region between the optic nerve and ciliary body. Abbreviations: mes, mesenchyme; on, optic nerve; onbl, outer neuroblastic layer; inbl, inner neuroblastic layer; inl, inner nuclear layer; gcl, ganglion cell layer. Scale bar represents 100 μm.
Similar to chick, strong expression of Ptn is observed in the mouse optic nerve by E13.5 (Fig. 4F). At this time, Ptn is also expressed in the posterior ocular mesenchyme and neural retina. Between E14.5 and E16.5, Ptn is expressed throughout the neural retina in salt-and-pepper pattern (Fig. 4G and 4H). At P0, expression of Ptn appears to localize in the INL and GCL (Fig. 4I), and it is not detected in the retina by P12 (Fig. 4J).
Expression of MDK and PTN receptors during ocular development
To determine the potential receptors for MDK and PTN during ocular development, we chose the chick eye at E7 because both MDK and PTN are expressed in the anterior and posterior ocular structures at this time. We screened for receptor expression by RTPCR, which indicated that only NOTCH2, ITGβ1, SDC1, and SDC3 were expressed in the cornea, whereas all receptors including LRP1, RPTPZ, and ALKwere expressed in the retina (Fig. 5L). Analysis by in situ hybridization confirmed that NOTCH2 and ITGβ1 are robustly expressed in the cornea, and observed that they are also expressed in the lens epithelium and presumptive iris (Fig. 5A and 5B). In addition, NOTCH2 is expressed in the condensed mesenchyme (Fig. 5A, arrowheads) and in the mesenchyme surrounding the ocular blood vessels. ITGβ1 is also expressed in the lens fiber cells (Fig 5B). SDC1 is strongly expressed in the condensed mesenchyme (Fig. 5C, arrowheads), and at lower levels in the corneal stroma, iris stroma, and lens epithelium (Fig. 5C). SDC3 is sparsely expressed in the cornea and iris stroma (Fig. 5D).
Figure 5. Expression of MDK and PTN receptors during chick ocular development.
(A-D) Transverse sections through E7 anterior eye showing the expression of NOTCH2, ITGβ1, SDC1, and SDC3. (E-K) Transverse sections through E7 retina showing the expression of NOTCH2, ITGβ1, SDC1, SDC3, LRP1, RPTPZ, and ALK. (L) RTPCR results showing amplification of NOTCH2, ITGβ1, LRP1, RPTPZ, ALK, SDC1, SDC3, and GAPDH (control) using cDNA obtained from E7 chick corneas and retinas. All retina images were acquired from the dorsal retina in the region between the optic nerve and ciliary body. Abbreviations: ep, corneal epithelium; st, corneal stroma; en, corneal endothelium; ir, iris; L, lens; rpe, retinal pigmented epithelium; gcl, ganglion cell layer; inl, inner nuclear layer; C, cornea; R, retina. Arrowheads indicate condensed mesenchyme that marks the boundary between the presumptive drainage angle and trabecular meshwork. Asterisks in A and C indicate ocular blood vessels. Scale bar represents 100 μm in A,B,C,D; and 100μm in E,F,G,H,I,J,K.
In the neural retina, NOTCH2 is detected in the progenitor cells located in the mid and peripheral regions (Fig. 5E). SDC3 is strongly expressed in the GCL and in the progenitor cells located in the mid and peripheral regions of the retina (Fig. 5H). RPTPZ is expressed in the progenitor cells located in the mid region of the retina and sparsely in the GCL (Fig. 5J). The remaining receptors ITGβ1 SDC1, LRP1, and ALK are diffusely expressed at low levels throughout the neural retina (Fig. 5F, 5G, 5I, 5K).
Expression of MDK and PTN during late development of the chick anterior eye
Most of the ocular tissues are formed by E12 in chick. At this time, MDK and PTN appear to be exclusively expressed in the epithelial and mesenchymal tissues, respectively (Fig. 6A and 6B). MDK is localized to epithelial layers of the eyelids, nictitating membrane, and cornea (Fig. 6A, arrowheads). It is also detected in the mesenchyme surrounding the blood vessels in the iris and adjacent to the trabecular meshwork, and in the ciliary body and ciliary muscle (Fig. 6A). PTN is expressed in the mesenchyme of the eyelid and nictitating membrane, and at low levels in the mesenchyme adjacent to the iridocorneal angle, in the iris stroma, and in the ciliary body (Fig. 6B).
Figure 6. Expression pattern of MDK and PTN in the anterior eye of E12 chick and receptor expression in the trigeminal ganglion.
Transverse sections through E12 anterior eye showing the expression of (A) MDK in mostly epithelial tissues (arrowheads) and (B) PTN in mesenchymal tissues. (C) RTPCR results showing amplification of NOTCH2, ITGβ1, LRP1, RPTPZ, ALK, SDC1, SDC3, and GAPDH (control) using cDNA obtained from E12 chick trigeminal ganglia. Cross-sections through E12 trigeminal ganglion showing the expression of ITGβ1 (D, D’) and RPTPZ (E, E’), and MDK (F, F’) and PTN (G, G’). Asterisk in A indicates ocular blood vessel..Abbreviations: nm, nictitating membrane; ir, iris; cb, ciliary body; ica, iridocorneal angle; L, lens; m, ciliary muscle; opV, ophthalmic branch of the trigeminal ganglion; mmV, maxillomandibular branch of the trigeminal ganglion. Scale bars represent 250μm in A,B; 250μm in D,E,F,G; and 100μm in D’,E’,F’,G’.
Given that both MDK and PTN are involved in promoting neurite outgrowth (Michikawa et al., 1993; Nakanishi et al., 1997; Raulo et al., 1994) and that ocular tissues are highly innervated by trigeminal sensory nerves at this time (Lwigale, 2001; Lwigale and Bronner-Fraser, 2007), we analyzed the expression of MDK and PTN receptors in the trigeminal ganglion. Initial analysis by RTPCR indicated that only ITGβ1 and RPTPZ are expressed in the trigeminal ganglion (Fig. 6C). This was confirmed by in situ hybridization, which revealed robust expression of both ITGβ1 and RPTPZ by the small support cells, but not the cell bodies of the larger neural crest-derived sensory neurons (Lwigale et al., 2004) (Fig. 6D, 6D’ and 6E, 6E’). To determine whether the receptors were directly involved in cellular interactions within the trigeminal ganglion, we examined the expression of MDK and PTN. Our results indicate that MDK is expressed at low levels in the trigeminal ganglion compared to PTN (Fig. 6F and 6G). Nonetheless, both MDK and PTN are expressed by sensory neurons located in the proximal region of the trigeminal ganglion (Fig. 6E’ and 6F’). Combined, these expression patterns indicate potential MDK/PTN signaling between the neurons and support cells via ITGβ1 and RPTPZ receptors.
Discussion
The cellular and molecular mechanisms regulating the morphogenesis of ocular tissues are not well known. In the present study, we analyzed the expression of MDK and PTN during ocular development in chick and mouse. Our results reveal that MDK and PTN are differentially expressed during ocular development and only overlap in a few areas. Comparison between chick and mouse also show differences in the expression patterns of MDK and PTN. Despite the differences in expression patterns, our results indicate potential roles for MDK and PTN in epithelial-mesenchymal interactions, cell migration and differentiation, and during neurogenesis.
Our results show that MDK is ubiquitously expressed shortly after the formation of the rudimentary eye in both chick and mouse. Given that MDK is induced by retinoic acid (RA) signaling (Kadomatsu et al., 1988), it is likely that its expression mirrors the relatively high levels of RA signaling during early ocular development (Duester, 2009; Matt et al., 2005; Mic et al., 2004; Molotkov et al., 2006). In the anterior eye, expression of MDK becomes localized to the corneal epithelium and endothelium and to the mesenchyme of the presumptive iris in chick, whereas in the mouse, Mdk is downregulated in most of the anterior eye except for the presumptive iris. Conserved expression of MDK in the presumptive iris coupled with the colocalization with NOTCH2, ITGβ1, and SDC1 suggests its potential function in iris development that may involve cell adhesion between the stroma and optic cup. MDK and NOTCH2 are expressed in the mesenchyme surrounding the ocular blood vessels in chick, suggesting a potential role during ocular vasculogenesis. Strong expression of MDK in the corneal epithelium and endothelium may be a result of high RA levels during ocular development. MDK may also play a role in cell adhesion of the chick corneal epithelium and endothelium via NOTCH2 and ITGβ1.
Contrary to MDK, PTN is not detectable in the rudimentary eye of the chick, and it is expressed at low levels in the mouse optic cup. Previously it was shown that Ptn is involved in recruitment of precursor cells during osteogenesis (Imai et al., 1998). Thus, the upregulation of PTN during chick corneal development suggests its potential role in recruiting periocular neural crest cells into the cornea. In the mouse Ptn is not expressed during corneal development, but it is upregulated in the mesenchyme of the presumptive iris similar to Mdk expression. Overlap between Mdk and Ptn during iris development in the mouse suggest that they play a similar role in promoting cell adhesion by signaling through NOTCH2, ITGα1, or SDC1 receptors. We also show that in chick PTN is expressed in the condensed mesenchyme at the boundary between the presumptive drainage angle and trabecular meshwork, where NOTCH2 and SDC1 are also expressed, suggesting that potential signaling between PTN and these two receptors may be involved in promoting condensation of the periocular mesenchyme in this region.
Our results also show differential expression of MDK and PTN in the posterior eye and neural retina. The most striking difference is observed in the optic nerve, where PTN is strongly expressed whereas MDK is expressed at low levels in both chick and mouse, suggesting that PTN plays an important role during optic nerve development, and that it may also be involved in guiding axons from the RGC to the central nervous system. In the chick both MDK and PTN are ubiquitously expressed in the neural retina at E7, and they are both restricted to the INL at subsequent stages of development. PTN is also transiently expressed in the GCL and it localizes to the ONL at E18. The spatiotemporal expression of PTN suggests its potential function during neurogenesis in the various layers of the chick neural retina. In the mouse, Mdk is localized in the outer neuroblastic layer and Ptn is maintained at low levels throughout the neural retina. These expression patterns indicate that both MDK and PTN play important roles during chick retinal development, whereas Mdk is the dominant player in the mouse.
Our data also show that the receptors SDC3 and RPTPZ are expressed in the chick GCL and INL. In the mouse both SDC3 and RPTPZ and is expressed in the GCL and outer neuroblastic layer (Inatani et al., 2002; Klausmeyer et al., 2007). SDC3 is expressed in the developing central nervous system and it is involved in MDK and PTN signaling that induce neurite outgrowth (Raulo et al., 1994; Nakanishi et al., 1997). RPTPZ is expressed in the central nervous system (Canoll et al., 1993; Shintani et al., 1998) and it is also involved in neuron and osteoblast cell migration (Maeda and Noda, 1998; Qi et al., 2001). Based on the expression patterns, it is likely that both MDK and PTN play important roles in neurogenesis, neural migration, and neurite outgrowth during chick retinal development. Mdk is dominant in the mouse, where it may play a role in cell proliferation or maintenance of the progenitor cells in the neuroblastic layer. Although the role of MDK and PTN during avian and mammalian ocular development is yet to be determined, functional studies in the zebrafish indicate that a paralogue of MDK, mdka, is expressed in retinal progenitor cells (Calinescu et al., 2009). Knockout of mdka attenuates cell cycle kinetics, which results in few progenitors whereas its overexpression accelerates the cell cycle and increases their number (Luo et al., 2012).
Our analysis of MDK and PTN expression in the chick anterior eye at E12 show that they are expressed in non-overlapping patterns in non-neural tissues, where they could be involved in epithelial-mesenchymal interactions or cell adhesion. Anterior ocular tissues are highly innervated by trigeminal sensory nerves (Lwigale, 2001; Lwigale and Bronner-Fraser, 2007). Although MDK and PTN have been shown to have neurotrophic properties in cultured sensory neurons and to stimulate neurite outgrowth (Michikawa et al., 1993; Raulo et al., 1994; Nakanishi et al., 1997), it is unlikely that they play these roles during sensory innervation of the anterior ocular tissues. We show that ITGβ1 and RPTPZ, the only receptors present in the trigeminal ganglion, are not expressed by the neurons, but label the support cells. Our results also show that MDK and PTN are expressed by the neural crest-derived neurons located in the proximal region of the trigeminal ganglion (Johnston, 1966; Lwigale, 2001), suggesting that MDK and PTN signal to the support cells through ITGα1 and RPTPZ, and may play a role in their maintenance and adherence to the neurons for proper myelination. Previous studies have shown that RPTPZ is expressed by astrocyte progenitors in the central nervous system and by oligodendrocytes and Schwann cells (Ivanova et al, 2004; Canoll et al., 1996; Shintani et al., 1998). Knockout mice without Rptpz exhibit delayed response to nociception, but it was not clear whether this defect was caused by inadequate myelination of the sensory neurons (Lafont et al., 2009).
In conclusion, our study reveals the spatiotemporal expression patterns of MDK, PTN, and their receptors during ocular development. Despite the differences in expression patterns observed between chick and mouse, our results suggest potential roles for MDK and PTN in both neuronal and non-neuronal processes of ocular development.
Experimental procedures
Embryos
Fertilized white leghorn chicken eggs are obtained from the Department of Poultry Science at Texas A&M University (College Station, TX). Eggs are incubated at 37 °C until the appropriate stages. Timed pregnant C57BL/6J mice were obtained from Jackson Laboratory. All animals were handled in accordance with guidelines from Institutional Animal Care and Use Committee (IACUC) at Rice University.
In situ hybridization
Chick and mouse heads or eyes were collected at time points that correspond with when the corneal layers are forming and neural retinal progenitor cells are differentiating (chick, E3-E18; mouse, E11.5-P12). Freshly isolated tissues were rinsed in Ringer’s solution then fixed overnight at 4°C in modified Carnoy’s fixative (60% ethanol, 30% formaldehyde, 10% glacial acetic acid). Fixed tissues were dehydrated through an ethanol series, cleared with Histosol, and embedded in paraffin. Tissues were sectioned at 10-12 μm.
Section in situ hybridization was performed as previously described (Etchevers et al., 2001). RNA probes were synthesized using cDNA sequences obtained from GenBank (accession numbers in Table 1) and amplified using primers listed in Table 1. The PCR products were cloned into pCRII-TOPO® or pCRIV-TOPO® cloning vectors (ThermoFisher Scientific), and linearized with appropriate restriction enzymes (HindIII, BamHI, SpeI, KpnI, EcoRV, NotI, XbaI, PmeI, or PstI). Digoxigenin-labeled sense and antisense probes were transcribed using Sp6, T3, or T7 polymerases. Sense probes were used in parallel with each gene as controls and they showed no specific signals.
Table 1.
Primers used for in situ hybridization and RT-PCR.
| Species | Gene | NCBI ID | Forward Primer | Reverse Primer |
|---|---|---|---|---|
| chick | MDK | NM_001113289.1 | 5'- CTGCCAAAGCCAAGAAAGGT −3' | 5'- ACCACCTCCTCACATTCAGC −3' |
| PTN | NM_001276362.1 | 5'- ATGCCACAGCAACAACAG −3' | 5'- TTAATCCAGCATCTTCTC −3' | |
| ALK | XM_025148917.1 | 5'- ACTGGCTGTTCACAACATGTGGTG −3' | 5'- GATCTTCCTCCAGTAGCACCTTCCAG - 3' | |
| NOTCH2 | NM_001252033.1 | 5'- GTGTCGAGAAGGCTATCTTG −3' | 5'- GTATCACACAGAGCTCCCTTC −3' | |
| LRP1 | NM_205242.2 | 5'- GTCAAGGCACTGGTAAAACA −3' | 5'- GGAGATTCTGAAGAGAGGGA −3' | |
| ITGβ1 | NM_001039254.2 | 5'- GTTGCTTGATATGGAGTGGA −3' | 5'- GGTCACCTGTACAAGGATTTC −3' | |
| RPTPZ | NM_001199312.1 | 5'- AGCTCGTCTGTATCCTCCGA −3' | 5'- TTCCCACGTAACCAGAAGGC −3' | |
| SDC1 | XM_419972.5 | 5'- GTCCCGCAAACTACAAATCT −3' | 5'- GTTCATCCAGTGAATAGCTTCC −3' | |
| SDC3 | NM_205383.1 | 5'- GATAACAGAAGCACCAGTGATCC −3' | 5'- GACTTCCAGGTCACTGTCGA −3' | |
| GAPDH* | NM_204305.1 | 5'- GATTCTACACACGGACACTTCA −3' | 5'- CTGAGGGAGCTGAGATGATAAC −3' | |
| mouse | Mdk | NM_010784.5 | 5'- AAGATGCAGCACCGAGGCTT −3' | 5'- TTGTACCGCGCCTTCTTCAG −3' |
| Ptn | NM_008973.3 | 5'- ATGTCGTCCCAGCAATATC −3' | 5'- ATCCAGCATCTTCTCCTGTTTC −3' | |
| Alk | NM_007439.2 | 5'- GCTTTGACTTCCCCTGTGAG −3' | 5'- GAAGCGAGGATGTCAGTGGT −3' | |
| Notch2 | D32210.1 | 5'- CCTTATGTGAGGGGTCTGCC −3' | 5'- AATGTACTGCCCGTTCAGGG −3' | |
| Lrp 1 | NM_008512.2 | 5'- TATGAAGGTGGAGAGCCCGA −3' | 5'- TTCCAGGGGTATGCTCGGTA −3' | |
| Itgβ1 | NM_010578.2 | 5'- CTCCGCAAGCCGAGGTC −3' | 5'- TGGAAAACACCAGCAGTCGT −3' | |
| Rptpz | NM_011219.2 | 5'- GACGTTAGCCAGGCCTATCC −3' | 5'- GTGAAGGTCTGCTGGTGGAC −3' | |
| Sdc1 | NM_011519.2 | 5'- CCTAACGCAGAGGAAGGGACC −3' | 5'- GAGGCTGATGGTCAGGTTGA −3' | |
| Sdc3 | NM_011520.3 | 5'- TGGACACCGCCACCCAT −3' | 5'- ACCTCCTTCCGCTCCAGTAT −3' |
Reverse Transcription PCR
RNA was extracted from chick E7 corneas, E7 retinas, and E12 trigeminal ganglia. Corneas were trimmed and included only the epithelium, stroma and endothelium. Retinal tissue included both neural retina and pigmented epithelium. The trigeminal ganglion tissue included the maxillomandibular and ophthalmic branches. Any attached mesenchyme was removed from the retina and trigeminal tissues before extraction. Samples containing three tissues each were collected induplicate. Tissues were collected in TRIzol Reagent (Invitrogen) and RNA was isolated according to manufacturer’s protocol. cDNA was synthesized using SuperScript III First-Strand Synthesis SuperMix (Invitrogen). PCRs were run with GoTaq Polymerase (Promega) and normalized to GAPDH. Primers used for in situ probe synthesis were also used for RT-PCR.
Imaging
Stained sections were imaged using a Zeiss AxioImager Z1 microscope with AxioCam MRc5 camera and AxioVision program (Carl Zeiss AG, Oberkochen, Germany).
KEY RESOURCES TABLE
| Reagent or resource | Source | Identifier |
|---|---|---|
| Experimental Models: Organisms/Strains | ||
| Chicks: White leghorn (Gallus gallus domesticus) | Texas A&M University, Department of Poultry Science, College Station, Texas, USA | |
| Mouse: Mus musculus C57BL/6J | Jackson Labs | JAX: 006494 |
| Oligonucleotides | ||
| Primers for in situ and RT-PCR, see Table 1 | This paper | N/A |
| Recombinant DNA | ||
| pCRII-TOPO cloning kit | Invitrogen | K466001 |
| pCRIV-TOPO cloning kit | Invitrogen | 450030 |
Key findings:
MDK and PTN show unique expression patterns in neuronal and non-neuronal tissues during ocular development.
Expression patterns of MDK and PTN are dynamic and only conserved between chick and mouse in a few ocular tissues.
The receptors RPTPZ, ALK, NOTCH2, ITGβ1, LRP1, SDC1, and SDC3 are expressed various patterns that overlap with MDK and PTN.
The expression profiles of MDK, PTN, and their receptors are consistent with potential functions in cell proliferation, adhesion, migration, and neurite outgrowth during ocular development.
Acknowledgements
We thank members of the Lwigale lab for discussions of the results and editing the early version of the manuscript. This work was supported by NIH grant number EY022158 to PL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agathocleous M, Harris WA, 2009. From Progenitors to Differentiated Cells in the Vertebrate Retina. Annu Rev Cell Dev Biol 25, 45–69. 10.1146/annurev.cellbio.042308.113259 [DOI] [PubMed] [Google Scholar]
- Asahina K, Sato H, Yamasaki C, Kataoka M, Shiokawa M, Katayama S, Tateno C, Yoshizato K, 2002. Pleiotrophin/heparin-binding growth-associated molecule as a mitogen of rat hepatocytes and its role in regeneration and development of liver. Am. J. Pathol. 160, 2191–2205. 10.1016/S0002-9440(10)61167-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DC, Coats JM, 2000. The Lens Organizes the Anterior Segment: Specification of Neural Crest Cell Differentiation in the Avian Eye. Dev. Biol. 220, 424–431. 10.1006/dbio.2000.9638 [DOI] [PubMed] [Google Scholar]
- Calinescu AA, Vihtelic TS, Hyde DR, Hitchcock PF, 2009. Cellular expression of midkine-a and midkine-b during retinal development and photoreceptor regeneration in zebrafish. J. Comp. Neurol. 514, 1–10. 10.1002/cne.21999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoll PD, Barnea G, Levy JB, Sap J, Ehrlich M, Silvennoinen O, Schlessinger J, Musacchio J, 1993. The expression of a novel receptor-type tyrosine phosphatase suggests a role in morphogenesis and plasticity of the nervous system. Dev. Brain Res. 75, 293–298. 10.1016/0165-3806(93)90035-9 [DOI] [PubMed] [Google Scholar]
- Canoll PD, Petanceska S, Schlessinger J, Musacchio JM, 1996. Three forms of RPTP-β are differentially expressed during gliogenesis in the developing rat brain and during glial cell differentiation in culture. J. Neurosci. Res. 44, 199–215. [DOI] [PubMed] [Google Scholar]
- Choudhuri R, Zhang H-T, Donnini S, Ziche M, Bicknell R, 1997. An Angiogenic Role for the Neurokines Midkine and Pleiotrophin in Tumorigenesis. Cancer Res. 57, 1814–1819. [PubMed] [Google Scholar]
- Cockshutt AM, Jonet L, Jeanny J, Vigny M, Raulais D, 1994. Retinoic Acid Induced Heparin-Binding Protein Expression and Localization During Gastrulation, Neurulation, and Organogenesis. Dev. Dyn. 200, 198–211. 10.1002/aja.1002000303 [DOI] [PubMed] [Google Scholar]
- Creuzet S, Couly G, Le Douarin NM, 2005. Patterning the neural crest derivatives during development of the vertebrate head: insights from avian studies. J Anat 207, 447–459. 10.1111/j.1469-7580.2005.00485.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doh ST, Hao H, Loh SC, Patel T, Tawil HY, Chen DK, Pashkova A, Shen A, Wang H, Cai L, 2010. Analysis of retinal cell development in chick embryo by immunohistochemistry and in ovo electroporation techniques. BMC Dev. Biol. 10, 8 10.1186/1471-213X-10-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF, 2001. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 128, 1059–1068. [DOI] [PubMed] [Google Scholar]
- Evans AL, Gage PJ, 2005. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum. Mol. Genet. 14, 3347–3359. 10.1093/hmg/ddi365 [DOI] [PubMed] [Google Scholar]
- Fan Q, Muramatsu T, Kadomatsu K, 2000. Distinct expression of midkine and pleiotrophin in the spinal cord and placental tissues during early mouse development. Dev. Growth Differ. 42, 113–119. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T, 2005. Fate Maps of Neural Crest and Mesoderm in the Mammalian Eye. Invest Ophthalmol Vis Sci 46, 4200–4208. 10.1167/iovs.05-0691 [DOI] [PubMed] [Google Scholar]
- Garver RI, Chan CS, Milner PG, 1993. Reciprocal expression of pleiotrophin and midkine in normal versus malignant lung tissues. Am.J.Respir.Cell Mol.Biol. 9, 463–466. 10.1165/ajrcmb/9.5.463 [DOI] [PubMed] [Google Scholar]
- Golden MG, Dasen JS, 2012. Polycomb repressive complex 1 activities determine the columnar organization of motor neurons. Genes Dev. 26, 2236–2250. 10.1101/gad.199133.112.specifies [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D, 1980. Development of the Vertebrate Cornea, in: International Review of Cytology, Vol. 63 pp. 263–322. [DOI] [PubMed] [Google Scholar]
- Herradón G, Pérez-García C, 2014. Targeting midkine and pleiotrophin signalling pathways in addiction and neurodegenerative disorders: recent progress and perspectives. Br. J. Pharmacol. 171, 837–848. 10.1111/bph.12312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Hoque MO, Wu F, Trink B, Sidransky D, Ratovitski EA, 2008Midkine induces epithelial-mesenchymal transition through Notch2/Jak2-Stat3 signaling in human keratinocytes. Cell Cycle 7, 1613–1622. 10.4161/cc.7.11.5952 [DOI] [PubMed] [Google Scholar]
- Hyer J, Kuhlman J, Afif E, Mikawa T, 2003. Optic cup morphogenesis requires prelens ectoderm but not lens differentiation. Dev. Biol. 259, 351–363. 10.1016/S0012-1606(03)00205-7 [DOI] [PubMed] [Google Scholar]
- Imai S, Kaksonen M, Raulo E, Kinnunen T, Fages C, Meng X, Lakso M, Rauvala H, 1998. Osteoblast Recruitment and Bone Formation Enhanced by Cell Matrix-associated Heparin-binding Growth-associated Molecule (HB-GAM). J. Cell Biol. 143, 1113–1128. 10.1083/jcb.143.4.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inatani M, Tanihara H, 2002. Proteoglycans in retina. Prog. Retin. Eye Res. 21, 429–447. 10.1016/S1350-9462(02)00009-5 [DOI] [PubMed] [Google Scholar]
- Ivanova A, Agochiya M, Amoyel M, Richardson WD, 2004. Receptor tyrosine phosphatase zeta/beta in astrocyte progenitors in the developing chick spinal cord. Gene Expr. Patterns 4, 161–166. 10.1016/j.modgep.2003.09.003 [DOI] [PubMed] [Google Scholar]
- Johnston MC, Noden DM, Hazelton RD, Coulombre JL, Coulombre AJ, 1979. Origins of Avian Ocular and Periocular Tissues. Exp Eye Res 29, 27–43. 10.1016/0014-4835(79)90164-7 [DOI] [PubMed] [Google Scholar]
- Johnston MC, 1966. A Radioautographic Study of the Migration and Fate of Cranial Neural Crest Cells in the Chick Embryo. Anat Rec 156, 143–156. 10.1002/ar.1091560204 [DOI] [PubMed] [Google Scholar]
- Jono H, Ando Y, 2010. Midkine: A novel prognostic biomarker for cancer. Cancers (Basel). 2, 624–641. 10.3390/cancers2020624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C-G, Hida H, Nakahira K, Ikenaka K, Kim H-J, Nishino H, 2004. Pleiotrophin mRNA is highly expressed in neural stem (progenitor) cells of mouse ventral mesencephalon and the product promotes production of dopaminergic neurons from embryonic stem cell-derived nestin-positive cells. FASEB J. 18, 1237–1239. 10.1096/fj.03-0927fje [DOI] [PubMed] [Google Scholar]
- Kadomatsu K, Hung RP, Suganuma T, Murata F, Muramatsu T, 1990. A retinoic acid responsive gene MK found in the teratocarcinoma system is expressed in spatially and temporally controlled manner during mouse embryogenesis. J. Cell Biol. 110, 607–616. 10.1083/jcb.110.3.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadomatsu K, Tomomura M, Muramatsu T, 1988. cDNA cloning and sequencing of new gene intensely expressed in early differentiation stages of embryonal carcinoma cells and in mid-gestation period of mouse embryogenesis. Biochem. Biophys. Res. Commun. 151, 1312–1318. 10.1016/S0006-291X(88)80505-9 [DOI] [PubMed] [Google Scholar]
- Kaspiris A, Mikelis C, Heroult M, Khaldi L, Grivas TB, Kouvaras I, Dangas S, Vasiliadis E, Liote F, Courty J, Papadimitriou E, 2013. Expression of the growth factor pleiotrophin and its receptor protein tyrosine phosphatase beta/zeta in the serum, cartilage and subchondral bone of patients with osteoarthritis. Jt. Bone Spine 80, 407–413. 10.1016/j.jbspin.2012.10.024 [DOI] [PubMed] [Google Scholar]
- Klausmeyer A, Garwood J, Faissner A, 2007. Differential Expression of Phosphacan/RPTPb Isoforms in the Developing Mouse Visual System. J. Comp. Neurol. 504, 659–679. 10.1002/cne.21479 [DOI] [PubMed] [Google Scholar]
- Laaroubi K, Delbé J, Vacherot F, Desgranges P, Tardieu M, Jaye M, Barritault D, Courty J, 1994. Mitogenic and in vitro angiogenic activity of human recombinant heparin affin regulatory peptide. Growth Factors 10, 89–98. 10.3109/08977199409010982 [DOI] [PubMed] [Google Scholar]
- Lafont D, Adage T, Gréco B, Zaratin P, 2009. A novel role for receptor like protein tyrosine phosphatase zeta in modulation of sensorimotor responses to noxious stimuli: Evidences from knockout mice studies. Behav. Brain Res. 201, 29–40. 10.1016/j.bbr.2009.01.025 [DOI] [PubMed] [Google Scholar]
- Li YS, Milner PG, Chauhan a K., Watson M. a, Hoffman RM, Kodner CM, Milbrandt J, Deuel TF, 1990. Cloning and expression of a developmentally regulated protein that induces mitogenic and neurite outgrowth activity. Science 250, 1690–4. [DOI] [PubMed] [Google Scholar]
- Luo J, Uribe RA, Hayton S, Calinescu A, Gross JM, Hitchcock PF, 2012. Midkine-A functions upstream of Id2a to regulate cell cycle kinetics in the developing vertebrate retina Midkine-A functions upstream of Id2a to regulate cell cycle kinetics in the developing vertebrate retina. Neural Dev. 7 10.1186/1749-8104-7-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwigale PY, 2001. Embryonic origin of avian corneal sensory nerves. Dev. Biol. 239, 323–337. 10.1006/dbio.2001.0450 [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Bronner-fraser M, 2009. Semaphorin3A/neuropilin-1 signaling acts as a molecular switch regulating neural crest migration during cornea development. Dev. Biol. 336, 257–265. 10.1016/j.ydbio.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwigale PY, Bronner-Fraser M, 2007. Lens-derived Semaphorin3A regulates sensory innervation of the cornea. Dev. Biol. 306, 750–759. 10.1016/j.ydbio.2007.04.012 [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Conrad GW, Bronner-Fraser M, 2004. Graded potential of neural crest to form cornea, sensory neurons and cartilage along the rostrocaudal axis. Development 131, 1979–1991. 10.1242/dev.01106 [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Cressy PA, Bronner-Fraser M, 2005. Corneal keratocytes retain neural crest progenitor cell properties. Dev. Biol. 288, 284–293. 10.1016/j.ydbio.2005.09.046 [DOI] [PubMed] [Google Scholar]
- Maeda N, Ichihara-Tanaka K, Kimura T, Kadomatsu K, Muramatsu T, Noda M, 1999. A receptor-like protein-tyrosine phosphatase PTPz/RPTPb binds a heparin-binding growth factor midkine. J. Biol. Chem. 274, 12474–12479. [DOI] [PubMed] [Google Scholar]
- Maeda N, Noda M, 1998. Involvement of receptor-like protein tyrosine phosphatase ξ/RPTPβ and its ligand pleiotrophin/HB-GAM in neuronal migration. Neurosci. Res. 31, S301 10.1016/S0168-0102(98)82401-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt N, Dupé V, Garnier J, Dennefeld C, Chambon P, Mark M, Ghyselinck NB, 2005. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development 132, 4789–4800. 10.1242/dev.02031 [DOI] [PubMed] [Google Scholar]
- Michikawa M, Kikuchi S, Muramatsu H, Muramatsu T, Kim SU, 1993. Retinoic Acid Responsive Gene Product, Midkine, Has Neurotrophic Functions for Mouse Spinal Cord and Dorsal Root Ganglion Neurons in Culture. J. Neurosci. Res. 35, 530–539. 10.1002/jnr.490350509 [DOI] [PubMed] [Google Scholar]
- Mikelis C, Sfaelou E, Koutsioumpa M, Kieffer N, Papadimitriou E, 2009. Integrin αvβ3 is a pleiotrophin receptor required for pleiotrophin-induced endothelial cell migration through receptor protein tyrosine phosphatase β/ζ. FASEB J. 23, 1459–1469. 10.1096/fj.08-117564 [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Salmivirta M, Muramatsu T, Muramatsu H, Rauvala H, Lehtonen D, Jalkanen M, Thesleff I, 1995. Expression of the heparin-binding cytokines, midkine (MK) and HB-GAM (pleiotrophin) is associated with epithelial-mesenchymal interactions during fetal development and organogenesis. Development 121, 37–51. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G, 2006. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development 133, 1901–1910. 10.1242/dev.02328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu H, Zou K, Sakaguchi N, Ikematsu S, Sakuma S, Muramatsu T, 1999. LDL receptor-related protein as a component of the midkine receptor. Biochem. Biophys. Res. Commun. 270, 936–941. 10.1006/bbrc.2000.2549 [DOI] [PubMed] [Google Scholar]
- Muramatsu H, 2004. Alpha4 Beta1- and Alpha6 Beta1-Integrins Are Functional Receptors for Midkine, a Heparin-Binding Growth Factor. J. Cell Sci. 117, 5405–5415. 10.1242/jcs.01423 [DOI] [PubMed] [Google Scholar]
- Muramatsu H, Zou P, Kurosawa N, Ichihara-Tanaka K, Maruyama K, Inoh K, Sakai T, Chen L, Sato M, Muramatsu T, 2006. Female infertility in mice deficient in midkine and pleiotrophin, which form a distinct family of growth factors. Genes to Cells 11, 1405–1417. 10.1111/j.1365-2443.2006.01028.x [DOI] [PubMed] [Google Scholar]
- Nakamoto M, Ozawa M, Matsubara T, Miyauchi MT, Obama H, Kagoshima K, 1992. A New Family of Heparin Binding Growth/Differentiation Factors: Differential Expression of the Midkine (MK) and HB-GAM Genes during Mouse Development. J Biochem 349, 346–349. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Kadomatsu K, Okamoto T, Ichihara-Tanaka K, Kojima T, Saito H, Tomoda Y, Muramatsu T, 1997. Expression of syndecan-1 and −3 during embryogenesis of the central nervous system in relation to binding with midkine. J Biochem 121, 197–205. [PubMed] [Google Scholar]
- Ohta S, Muramatsu H, Senda T, Zou K, Iwata H, Muramatsu T, 1999. Midkine Is Expressed During Repair of Bone Fracture and Promotes Chondrogenesis. J. Bone Miner. Res. 14, 1132–1144. 10.1359/jbmr.1999.14.7.1132 [DOI] [PubMed] [Google Scholar]
- Pei YF, Rhodin JAG, 1970. The Prenatal Development of the Mouse Eye. Anat Rec 168, 105–126. 10.1002/ar.1091680109 [DOI] [PubMed] [Google Scholar]
- Qi M, Ikematsu S, Maeda N, Ichihara-Tanaka K, Sakuma S, Noda M, Muramatsu T, Kadomatsu K, 2001. Haptotactic Migration Induced by Midkine. J. Biol. Chem. 276, 15868–15875. 10.1074/jbc.M005911200 [DOI] [PubMed] [Google Scholar]
- Raulo E, Chernousovo MA, Carey DJ, Nolo R, Rauvalaln H, 1994. Isolation of a Neuronal Cell Surface Receptor of Heparin Binding Growth-associated Molecule (HB-GAM). J. Biol. Chem. 269, 12999–13004. [PubMed] [Google Scholar]
- Rauvala H, Pihlaskari R, Laitinen J, Merenmies J, 1989. Extracellular adhesive molecules in neurite growth. Biosci. Rep. 9, 1LP–12. 10.1007/BF01117507 [DOI] [PubMed] [Google Scholar]
- Reiff T, Huber L, Kramer M, Delattre O, Janoueix-Lerosey I, Rohrer H, 2011. Midkine and Alk signaling in sympathetic neuron proliferation and neuroblastoma predisposition. J. Cell Sci. 124, 4699–4708. 10.1242/jcs.101428 [DOI] [PubMed] [Google Scholar]
- Sakaguchi N, Muramatsu H, Ichihara-Tanaka K, Maeda N, Noda M, Yamamoto T, Michikawa M, Ikematsu S, Sakuma S, Muramatsu T, 2003. Receptor-type protein tyrosine phosphatase ζ as a component of the signaling receptor complex for midkine-dependent survival of embryonic neurons. Neurosci. Res. 45, 219–224. 10.1016/S0168-0102(02)00226-2 [DOI] [PubMed] [Google Scholar]
- Sekiguchi K, Yokota C, Asashima M, Kaname T, Fan Q-W, Muramatsu T, Kadomatsu K, 1995. Restricted Expression of Xenopus Midkine Gene during Early Development1. J. Biochem. 118, 94–100. [DOI] [PubMed] [Google Scholar]
- Shintani T, Watanabe E, Maeda N, Noda M, 1998. Neurons as well as astrocytes express proteoglycan-type protein tyrosine phosphatase z/RPTPb: analysis of mice in which the PTPz/RPTPb gene was replaced with the LacZ gene. Neurosci. Lett. 247, 135–138. 10.1016/S0304-3940(98)00295-X [DOI] [PubMed] [Google Scholar]
- Stoica GE, Kuo A, Powers C, Bowden ET, Sale EB, Riegel AT, Wellstein A, 2002. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J. Biol. Chem. 277, 35990–35998. 10.1074/jbc.M205749200 [DOI] [PubMed] [Google Scholar]
- Turner D, Snyder EY, Cepko C, 1990. Lineage-Independent Determination in the Embryonic Mouse Retina of Cell Type. Neuron 4, 833–845. 10.1016/0896-6273(90)90136-4 [DOI] [PubMed] [Google Scholar]
- Weckbach LT, Groesser L, Borgolte J, Pagel J, Pogoda F, Schymeinsky J, Müller-höcker J, Shakibaei M, Muramatsu T, Deindl E, Walzog B, 2012. Midkine acts as proangiogenic cytokine in hypoxia-induced angiogenesis. Am. J. Physiol. Hear. Circ. Physiol. 303, 429–438. 10.1152/ajpheart.00934.2011 [DOI] [PubMed] [Google Scholar]
- Weckbach LT, Muramatsu T, Walzog B, 2011. Midkine in Inflammation. Sci. World J. 11, 2491–2505. 10.1100/2011/517152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler C, Yao S, 2014. The midkine family of growth factors: Diverse roles in nervous system formation and maintenance. Br. J. Pharmacol. 171, 905–912. 10.1111/bph.12462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler C, Moon RT, 2001. Zebrafish mdk2, a Novel Secreted Midkine, Participates in Posterior Neurogenesis. Dev. Biol. 229, 102–118. 10.1006/dbio.2000.9967 [DOI] [PubMed] [Google Scholar]
- Yang X, Tare RS, Partridge KA, Roach HI, Clarke NM, Howdle SM, Shakesheff KM, Oreffo RO, 2003. Induction of Human Osteoprogenitor Chemotaxis, Proliferation, Differentiation, and Bone Formation by Osteoblast Stimulating Factor-1/Pleiotrophin: Osteoconductive Biomimetic Scaffolds for Tissue Engineering. J. Bone Miner. Res. 18, 47–57. 10.1359/jbmr.2003.18.1.47 [DOI] [PubMed] [Google Scholar]
- Young RW, 1985. Cell proliferation during postnatal development of the retina in the mouse. Dev. Brain Res. 21, 229–239. 10.1016/0165-3806(85)90211-1 [DOI] [PubMed] [Google Scholar]
- Zou P, Muramatsu H, Sone M, Hayashi H, Nakashima T, Muramatsu T, 2006. Mice doubly deficient in the midkine and pleiotrophin genes exhibit deficits in the expression of beta-tectorin gene and in auditory response. Lab. Invest. 86, 645–653. 10.1038/labinvest.3700428 [DOI] [PubMed] [Google Scholar]