Abstract
The regulatory light chain (RLC) of myosin is commonly tagged to monitor myosin behavior in vitro, in muscle fibers, and in cells. The goal of this study was to prepare smooth muscle myosin (SMM) filaments containing a single head labeled with a quantum dot (QD) on the RLC. We show that when the RLC is coupled to a QD at Cys-108 and exchanged into SMM, subsequent filament assembly is severely disrupted. To address this, we used a novel approach for myosin by implementing the SpyTag002 SpyCatcher002 system to prepare SMM incorporated with RLC constructs fused to SpyTag or SpyCatcher. We show that filament assembly, actin-activated steady-state ATPase activities, ability to be phosphorylated, and selected enzymatic and mechanical properties were essentially unaffected if either SpyTag or SpyCatcher were fused to the C-terminus of the RLC. Crucially for our application, we also show that a QD coupled to SpyCatcher can be covalently attached to a RLC-Spy incorporated into a SMM filament without disrupting the filament, and that the filaments can move along actin in vitro.
Keywords: Myosin filaments, regulatory light chain, total internal reflection microscopy, electron microscopy, quantum dots
Introduction
Myosin II is a class of molecular motors found in all three types of muscle, cardiac, skeletal, and smooth (SMM) as well as in nonmuscle (NMM) cells. Myosin II has two N-terminal head domains that contain the ATP and actin binding sites as well as an extended α-helix to which the regulatory light chains (RLC) and essential light chains (ELC) bind to form the lever arms. C-terminal to the two heads, the heavy chains merge into the coiled-coil rod domain consisting of the subfragment 2 (S2) and light meromyosin (LMM) domains. Under physiological conditions, myosin assembles into filaments stabilized by intermolecular ionic interactions between LMM domains.
The function of all myosin IIs can be regulated by phosphorylation of the RLC by myosin light chain kinase (MLCK). There are two general effects of phosphorylation. Depending upon the type of myosin, RLC phosphorylation activates the ATPase activity and stabilizes the filamentous versus monomeric state to various extents. Other important functions of myosin II are attributed to the RLC such as, folding to the 10S conformation (Trybus and Lowey 1988), stiffness of the lever arm (Matthew et al. 1998), coupling of ATPase and motility (Trybus et al. 1994), and intermolecular interactions in the dephosphorylated state (Wendt et al. 2001; Ni et al. 2012; Liu et al. 2006). It is therefore not surprising that modifications throughout the RLC sequence can affect myosin function in general (Ikebe et al. 1994; Ikebe et al. 1999; Kambara et al. 1999; Trybus and Chatman 1993; Ni et al. 2009; Rowe and Kendrick-Jones 1992; Ni et al. 2012; Rowe and Kendrick-Jones 1993; Sweeney et al. 1994; Szczesna et al. 2001; Kazmierczak et al. 2018).
Despite the sensitivity of myosin function to RLC modifications, the RLC is commonly tagged to monitor myosin interactions, localization, and dynamics (Heissler and Sellers 2015; Brack et al. 2004; Liu et al. 2006; Olney et al. 1996; Gollub et al. 1999; Wu et al. 1999). Unlike full length myosin, which is difficult to express and purify in sufficient quantity, the relatively small (~20 kDa) RLC is easy to express in E. coli and purify, and can be exchanged onto myosin by several methods with little effect on ATPase activity. The smooth muscle RLC has a single native cysteine residue (C108) that can be labeled with small extrinsic probes without a large effect on myosin function. Importantly, the RLC can be exchanged with good efficiency into many types of muscle fibers (Allen et al. 1996; Gollub et al. 1999; Kazmierczak et al. 2018). GFP-tagged RLC has been used to study the localization and mechanics of myosin in cells (Komatsu et al. 2000; Peterson et al. 2004; Beach et al. 2011), as well as the mechanical properties of NMM expressed in insect cells (Nagy et al. 2013; Melli et al. 2018).
This report contains results from two general types of experiments where we extended the approaches outlined above to label the RLC to study SMM function. First, we labeled the RLC at Cys-108 with a QD, which is a much larger tag than previously used. Interestingly, this severely interfered with SMM filament assembly in vitro. Since proper filaments are required for our experiments, we switched to the SpyTag002 (Spy) SpyCatcher002 (SpyC) system (Keeble et al. 2017) to attach a quantum dot (QD) to the RLC after filaments had already been formed. The 14 amino acid peptide, Spy, spontaneously forms an isopeptide bond with SpyC (15.5 kDa IgG domain), covalently linking the two and anything attached to them. For our application, we engineered either Spy or SpyC into the RLC at the N or C terminus and show that Spy on the N-terminus of the RLC severely interferes with SMM filament assembly, but the Spy and SpyC on the C-terminus do not. Additionally, we found that these C-terminal RLC constructs do not significantly affect selected indicators of kinetic and mechanical behaviors of these filaments. To our knowledge this is the first report of using the Spy-SpyC system in myosin.
Materials and Methods
Buffers and Proteins.
Filament buffer: 10 mM NaPi, pH 7.0, 5 mM MgCl2, 125 mM NaCl, 0.1 mM EGTA, 1 mM DTT, and 30 nM NaN3. Conjugation buffer: 20 mM HEPES, pH 7.2, 0.5 M NaCl, 0.1 mM EGTA, 5 mM DTT, 30 nM NaN3. Exchange Buffer: 20 mM NaPi, pH 7.5, 0.5 M NaCl, 5 mM EGTA, 2 mM EDTA, 1 mM DTT, 30 nM NaN3. Actin, Alexa Fluor-488-labeled (Thermo Fisher Scientific), tetramethylrhodamine isothiocyanate;-labeled and biotin-labeled actin (5%) were prepared as described (Haldeman et al. 2014; Brizendine et al. 2015). Chicken gizzard SMM was prepared and rhodamine labeled as described. Native RLC was purified from SMM (Facemyer and Cremo 1992). The following extinction coefficients (0.1% w/v) were used to determine protein concentrations at 280 nm: SMM, 0.56; RLC (277 nm), 0.337; Spy-RLC/RLC-Spy, 0.341; RLC-SpyC, 0.547; His-Cys-SpyC, 1.02. Protein molecular weights were: SMM, 480 kDa; RLC, 19.7 kDa; Spy-RLC/RLC-Spy, 21.85 kDa; RLC-SpyC, 34.59 kDa; His-Cys-SpyC, 15.9 kDa.
Protein expression and purification.
His6-Cys-SpyCatcher002 protein expression was as described in (Keeble et al. 2017). pDEST14-Cys-SpyCatcher002 was a gift from M. Howarth (Addgene plasmid # 102829). Briefly, the plasmid was amplified in DH5α E. coli by overnight LB culture with 50 µg m−1 ampicillin and purified using a Monarch Plasmid Miniprep kit (NEB). The plasmid was then transformed into BL21 DE3 pLysS competent E. coli. (Invitrogen). For protein expression, transformed bacteria were grown in LB with 50 µg m−1 ampicillin and chloramphenicol to OD600 0.5–0.7 at 37 °C while shaking at 250 rpm. To induce protein expression, IPTG was added to a final concentration of 0.4 mM and incubated for 4 hr at 37 °C and 225 rpm. His6-Cys-SpyCatcher002 was purified using an AKTA explorer and a 5 ml HiTrap Chelating HP column (GE Healthcare) charged with 0.1M NiSO4. The buffer was exchanged by dialysis into conjugation buffer and the protein was stored at −80 °C.
Chicken smooth muscle RLC (UniProtKB - P02612) and SpyTag002/SpyCatcher002 constructs were synthesized by GenScript Biotech Corp ((New Jersey, USA) with the necessary complementary overhangs for ligation-independent cloning using the aLICator expression system (Thermo Scientific). The vector used was pLATE11 for generation of untagged protein. After the construct DNA was ligated into pLATE11 according to the manufacturer’s protocol, the vector was transformed into chemically competent E. coli NEB5α cells (NEB), amplified, and purified as above. The plasmid was transformed into BL21 Star (DE3) competent E. coli (Invitrogen). For protein expression, transformed bacteria were grown in LB with 100 µg/ml ampicillin to OD600 0.6–0.8 at 37 °C and 250 rpm. Protein expression was induced as above. RLC-SpyTag002/SpyCatcher002 constructs (Fig. 1B-D) were purified by anion exchange using an AKTA FPLC and a 6 ml Resource Q column (GE Healthcare). Proteins were dialyzed into 50 mM NH4CO3 pH 7.9, 0.1 mM EDTA, 0.1 mM EGTA, and 1 mM DTT then concentrated to ~6–8 mg/ml with a Savant Speed-Vac System (Thermo Scientific) and stored at −80 °C.
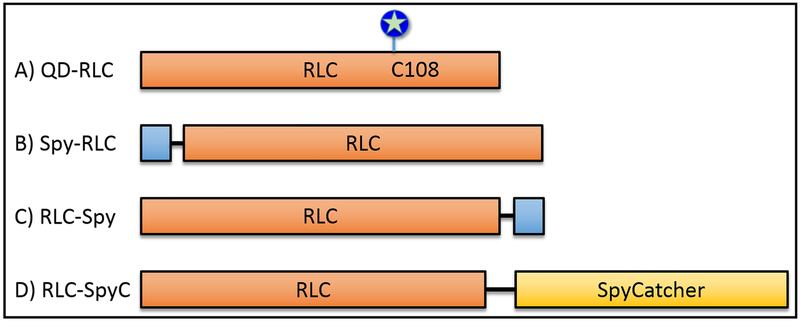
Figure 1. RLC constructs.
Orange boxes represents the RLC sequence (UniProtKB, P02612). Blue boxes represent the SpyTag002 sequence (VPTIVMVDAYKRYK, (Keeble et al. 2017)), black line represents GSGESG linker, and the yellow box represents the SpyCatcher002 sequence (Keeble et al. 2017). Lengths of boxes reflect approximate relative sequence lengths. A) QD-RLC (RLC = 20 kDa), QD (blue circle/star) attached to RLC at Cys-108. B) Spy-RLC, SpyTag on N-terminus of RLC. C) RLC-Spy, SpyTag on C-terminus of RLC. D) RLC-SpyC, SpyCatcher (15.5 kDa) fused to the C-terminus of RLC via a 2X length linker (34.6 kDa).
Functionalization of QDs.
Amine QDs (50–100 µl of 525, 585, or 655 nm, 8 µM, Invitrogen) were centrifuged for 3 min at 2,400 x g, and washed twice in a 100 kDa cutoff centrifugal filter unit (Millipore) in DTT-free conjugation buffer. A 2 fold molar excess of maleimide-PEG6-N-hydroxysuccinimide (NHS), (SM(PEG)6, Thermo Scientific) was added to the QDs and allowed to react for 30 min at RT. Unreacted amines on the QD were reacted with 2 mM NHS-acetate (Thermo Scientific) for 30 min at RT. The NHS reactions were quenched with 4 mM hydroxylamine (Sigma). Excess reactants and side-products were removed with a NAP-5 desalting column (GE Healthcare) equilibrated in DTT-free conjugation buffer.
Coupling QDs to RLC or SpyC.
Protein cysteines were reduced with addition of fresh DTT (5 mM final) and allowed to incubate on ice for at least 1 hr. Excess DTT was then removed with a PD minitrap G-10 column (GE Healthcare) equilibrated in DTT-free conjugation buffer. The reduced protein was added to the QDs in a 2-fold molar excess, and reacted for 2 hr at RT, then overnight on ice. The reaction was quenched with 5 mM DTT. Unreacted protein was removed through a 100 kDa cutoff centrifugal filter unit (Millipore) by suspending 5 times in filament buffer and stored on ice until use. QDs coupled to the RLC at Cys-108 since it is the only Cys present in the RLC. QDs were assumed to be coupled to SpyCatcher at the sole Cys engineered near the N-terminus.
RLC-exchange.
RLC constructs (Fig. 1) were exchanged onto rhodamine-labeled SMM as described (Ellison et al. 2000). Briefly, the exchange reaction was carried out in exchange buffer at 2 mg ml−1 SMM with a 10 fold molar excess of RLC to SMM heads with additional freshly added 10 mM DTT and 1 mM ATP. To prepare SMM with QD-RLC, a mixture of QD-RLC and native RLC in a 1:30 ratio was added to an equimolar amount of SMM heads. For both the QD-RLC and the RLC construct exchange reactions, the mixture was heated to 42 °C for 30 min. The sample was then allowed to cool at RT for 10 min, followed by addition of MgCl2 to 20 mM and placed on ice for 2 hr. Any precipitated protein was removed by centrifugation for 5 min at 16,100 x g at 4 °C in a refrigerated micro centrifuge (Eppendorf 5415R). Excess RLC was removed from SMM by applying the sample to a pre-spun (150 x g, 2 min) 10 ml Sephacryl S-400 HR (GE Healthcare) spin column equilibrated in exchange buffer. SMM exchanged with RLC was separated from free RLC by centrifugation at 250 x g for 10 min at 4 °C. The exchange efficiency of Spy or SpyC RLC constructs (Fig. 1B-D) was determined by SDS-PAGE and gel densitometry after staining with Coomassie blue. Gels were imaged with a Bio-Rad ChemiDoc XRS Imaging system and analyzed with ImageLab 3.0 software (Bio-Rad).
Preparation of SMM-QD filaments.
After the QD-RLC was exchanged into SMM, the product was mixed with rhodamine labeled SMM in a 1:2 to 1:3 molar ratio and then filaments were formed by dialysis into filament buffer and cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) as described (Haldeman et al. 2014; Brizendine et al. 2015). This was assumed to yield a final QD to SMM ratio of ~1:60–120.
Preparation of SMM filaments incorporated with Spy-RLC, RLC-Spy, and RLC-SpyC.
After the exchange reaction, filaments were formed by dialysis and cross-linked with EDC as previously described (Brizendine et al. 2017; Brizendine et al. 2015; Haldeman et al. 2014).
SMM filament phosphorylation.
Filaments were phosphorylated by myosin light chain kinase (MLCK) as previously described (Brizendine et al. 2015; Haldeman et al. 2014). Phosphorylation of the Spy RLC constructs were detected by urea gel electrophoresis. Phosphorylated RLC-SpyC was not resolved from the unphosphorylated form by urea gel electrophoresis so Pro-Q Diamond stain (Thermo Scientific) was used to confirm phosphorylation.
Motility assays and TIRF imaging.
A/Mf motility assays were performed as described (Brizendine et al. 2017; Brizendine et al. 2015). Actin was attached to a PEG surface and rhodamine-labeled SMM filaments were observed moving along the actin. The rhodamine was imaged using a 532 nm excitation laser and a 585/65 nm emission filter (Chroma). 585 nm QDs were imaged with a 405 nm excitation laser and a 585/65 nm emission filter (Chroma). See (Brizendine et al. 2015) for other details about the TIRF microscope.
Electron microscopy.
Performed as described (Haldeman et al. 2014) at the Multiscale Microscopy Core (MMC) with technical support from the Oregon Health & Science University (OHSU)-FEI Living Lab and the OHSU Center for Spatial Systems Biomedicine (OCSSB).
Results and Discussion
QD-RLC incorporated into SMM interferes with filament assembly.
The goal of this study was to prepare SMM filaments that contain a QD on only one myosin head out of the hundreds of heads in a filament. QDs, due to their brightness and photostability, are required to monitor head dynamics while the myosin filament interacts with and moves relative to actin (results to be published elsewhere).
Our first approach was to attach the QD to purified native RLC at Cys-108, then exchange the QD-RLC (Fig. 1A) at high ionic strength into monomeric SMM before lowering the ionic strength to promote filament formation. Amine QDs were reacted with maleimide-PEG-NHS to couple the QD to the RLC (see Materials and Methods) under conditions that minimized more than one RLC per QD. The QD-RLC was diluted with a 30 fold excess of unlabeled RLC and the mixture was added to rhodamine-labeled SMM (at high ionic strength) so that the total [RLC] was equimolar to the head concentration. Prior experience showed that under this condition the exchange protocol (42 °C, 30 min) leads to ~50% exchange. Samples were exposed to filament forming conditions by dialysis to lower ionic strength. A portion of the uniform cloudy suspension was reserved, and the remainder centrifuged to pellet the filaments, leaving a clear supernatant. Filament pellets were resuspended in filament buffer. Total internal reflection fluorescence (TIRF) microscopy detecting rhodamine was used to examine the filament ultrastructure. Fig 2A shows unexchanged rhodamine-labeled SMM filament suspension pre-spin, which was indistinguishable from images of filaments post-spin and from SMM filaments exchanged with a 10-fold excess native RLC (data not shown). Filaments appear as bright oblong shapes that we have previously characterized (Brizendine et al. 2015; Brizendine et al. 2017; Haldeman et al. 2014). The majority were single filaments (Fig. 2A, carets), but some filaments in small aggregates were also evident (arrows).
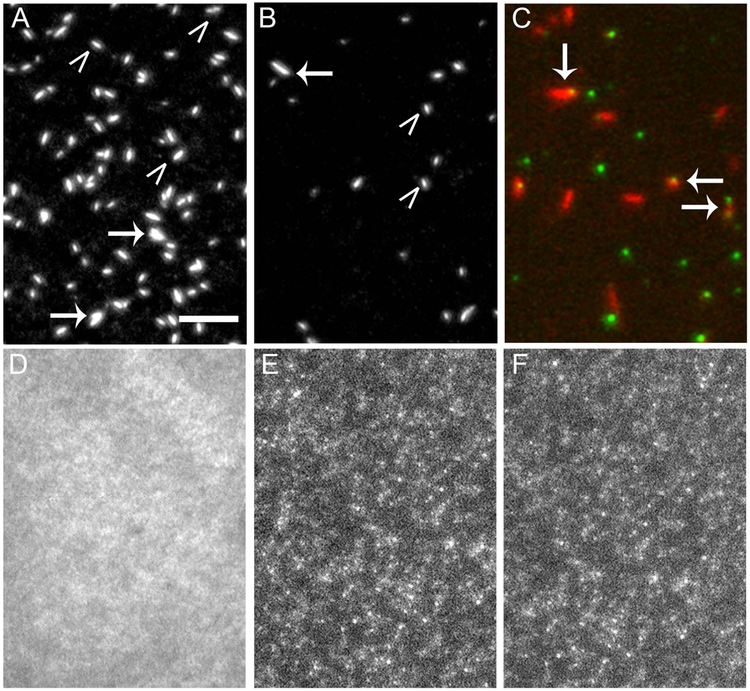
Figure 2. QD labeled RLC interferes with SMM filament formation.
TIRF microscopy images of rhodamine labeled SMM filaments in filament buffer on a glass coverslip. Images show how various modifications to the RLC interfere with filament formation. Scale bar = 5 µm and applies to all images. A) Control SMM. B) SMM with a 525 nm QD- RLC. In A and B, SMM is 20 µg ml−1, white carets point to single filaments, arrows point to aggregates. C) Overlaid image of 20 µg ml−1 SMM filaments (red) with 525 nm QD (green) chemically coupled to the RLC at Cys-108. Arrows point to co-localized filaments and QDs. D) SMM with a 585 nm QD-RLC. E) SMM with a 655 nm QD-RLC. In C and D, SMM is 0.5 µg ml−1.
Similar to control SMM, filaments prepared in the same manner with SMM exchanged with 525 nm QD-RLC appeared essentially the same pre-and post-spin. Fig. 2B shows a representative TIRF image of the filaments post-spin. Fig 2C shows a composite image of the filaments (red) and the 525 nm QDs (green). Filaments localized with QDs (arrows) were observed along with unlabeled filaments and free QDs. In a representative field (not shown), approximately ~50% of the filaments appeared to be co-localized with a single QD and ~5–10% co-localized with two QDs. On average, a filament contains 360 heads or 180 myosins (Haldeman et al. 2014). Therefore, we can estimate that the ratio of QD/myosin is ~1/300. As expected, QD-labeled filaments moved along surface-attached actin (Mf/A motility assay) in the presence of ATP (data not shown, see below).
In contrast to control and 525 nm QD SMM, SMM prepared using the same protocol but with 585 and 655 nm QD-RLC did not form normal filaments. Fig. 2D is a representative TIRF image of the 585 nm QD preparation pre-spin, which shows that most of the myosin is not assembled into filaments. Post-spin, pellets were present but did not smoothly resuspend and could not be mechanically resuspended. Large aggregates fell to the bottom of the tube quickly, and therefore could not be imaged. Figures 2E and 2F show TIRF images of the pellet material remaining suspended for the 585 and 655 nm QD-RLC samples, respectively. For both samples, regions of higher fluorescence perhaps representing small filaments or filament aggregates were observed within a cloud of background fluorescence, but normal filaments did not appear to be present. This result was reproducible across multiple (>6) SMM preparations and coupling reactions.
It is surprising that such a drastic effect on filament formation occurred at the low estimated ratio of QD-RLC to RLC. Since QDs are present in excess to the myosin in our protocol, we tested whether the presence of these free QDs were disrupting filament formation. Normal filaments formed even in the presence of excess QDs if the QDs were not attached to the RLC (data not shown). Adding 1 mg ml−1 BSA to discourage non-specific QD-SMM interactions did not alter the results. These data suggest that the lack of normal filaments was not due to non-specific adsorption of the QD onto SMM.
The mechanism for how 585 and 655 nm QDs on Cys-108 of the RLC interferes with filament formation remains unknown. However, because small amounts of QD-RLC cause essentially total interference, it suggests that not only is filament assembly disrupted, but also that the disrupted assembly must cause aggregation to filaments that did not happen to contain a QD-RLC. This leads to a total lack of normal filaments and also large aggregates. Although exact specifications of the QDs used here such as composition, size, and surface coating are proprietary, the quoted diameter range for functionalized QDs in general is given as 15–21 nm. In the range of 525 nm to 655 nm, it is well established that the nanocrystal core/shell size increases from 2.5 to 8 nm diameter (Michalet et al. 2005), so it is reasonable to assume that the longer wavelength QDs are larger in diameter than the shorter wavelength QDs. Since there could be other differences it is hard to speculate why the larger QDs cause the aggregation observed. In summary, coupling of the QD to the RLC through Cys-108 was not a robust method to prepare SMM filaments with incorporated QDs because QDs emitting at longer wavelengths could not be used.
Effects of incorporating Spy-RLC, RLC-Spy, or RLC-SpyC constructs into SMM.
To completely avoid QD interference with filament formation, we concluded that it was necessary to attach the QD to the RLC after filaments had already been formed. Our approach was to use the SpyTag002 (Spy) and SpyCatcher002 (SpyC) system (Keeble et al. 2017). We expressed RLC constructs with N- or C-terminal Spy or with C-terminal SpyC (Fig. 1B-D) and exchanged them into SMM prior to filament assembly. We used a 10-fold excess of RLC construct over SMM heads during exchange, giving exchange efficiencies > 50% (Table 1). After removing the free excess RLC construct, the ionic strength was lowered by dialysis to promote filament formation. Interestingly, the Spy-RLC construct severely interfered with SMM filament formation (Fig. 3A). These preparations, although not exposed to QDs, looked similar to the 585 and 655 QD-RLC SMM preparations with punctate dots surrounded by background fluorescence. Attempts to phosphorylate SMM filaments incorporated with Spy-RLC with MLCK were not successful (data not shown), even though in a separate experiment, Spy peptide up to 25 µM showed no measurable inhibition of MLCK activity with RLC as a substrate. These effects may be due to the specific sequence of the Spy because in comparison, NMM filaments with a GFP-RLC appeared normal (Nagy et al. 2013), with merely slowed phosphorylation rates by MLCK (Kengyel et al. 2010).
Table 1.
Summary of effects of RLC modifications incorporated into SMM filaments.
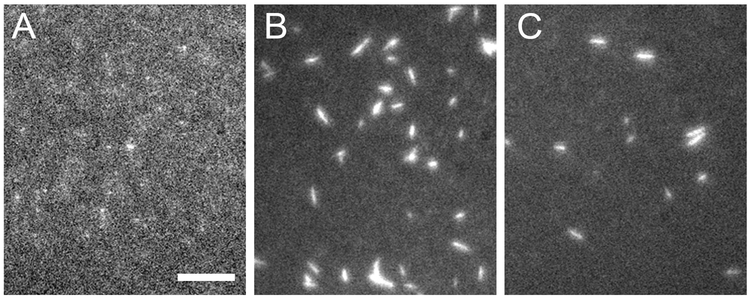
Figure 3. TIRF microscopy images of SMM filaments incorporated with Spy-RLC, RLC-Spy, and RLC-SpyC.
Scale bars = 5 µm, filaments were on glass coverslips in filament buffer. A) 5 µg ml−1 SMM Spy-RLC filaments with 80% Spy-RLC and 20% RLC. B) 20 µg ml−1 SMM RLC-Spy filaments with 75% RLC-Spy and 25% RLC. C) 20 µg ml−1 SMM RLC-SpyC filaments with 63% RLC-SpyC and 37% RLC.
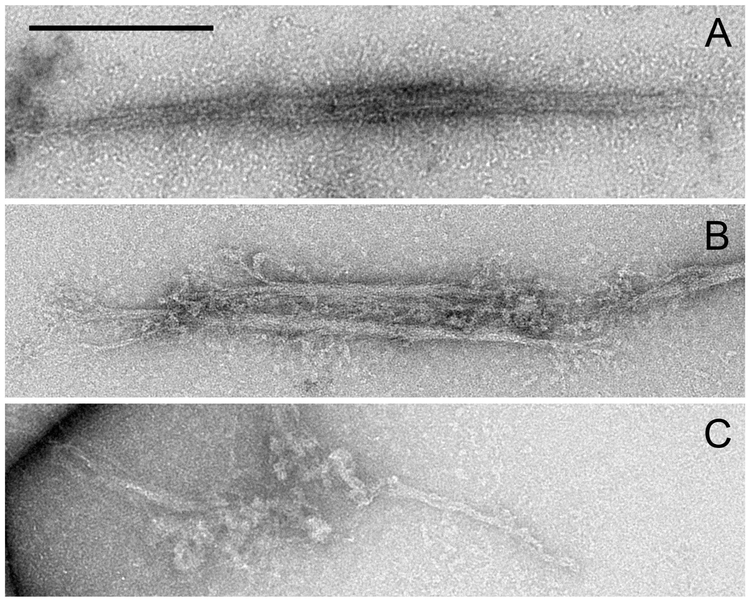
A similar preparation to that shown in Fig. 3A was centrifuged to pellet any filaments or filament aggregates. Approximately 50% of the SMM remained in the supernatant. In contrast, ~10–20% of control SMM remains in the supernatant under these conditions. This suggests that the presence of the Spy-RLC inhibits filament assembly. Electron microscopy was performed on the pellet suspension to further characterize the filaments. Control SMM filaments showed side polar filament structure (Fig. 4A) with an average length of 0.62 ± 0.18 µm (S.D.) (n= 18), which closely matches our previous observations (Haldeman et al. 2014). Of the few filaments observed in the SMM with incorporated Spy-RLC preparation, most were aggregated with other filaments, either end to end or side to side as observed in Fig. 4B, were notably frayed throughout, and were shorter than control filaments (0.32 ± 0.18 µm (S.D.), n = 13). Also present were small amorphous aggregates that were not observed in the control preparations (Fig. 4C). The Spy-RLC appears to cause non-physiological interactions between SMM molecules that compete with normal filament assembly.
Figure 4. Electron micrographs of SMM filaments.
Scale bars = 0.2 µm. A) Control SMM filaments. B) SMM Spy-RLC filaments. C) Improperly formed SMM Spy-RLC filaments.
SMM incorporated with RLC-Spy or RLC-SpyC forms normal filaments that have normal functional properties.
In contrast to the Spy-RLC, the two C-terminal constructs, RLC-Spy and RLC-SpyC (Fig. 1C, D), did not appreciably interfere with filament formation (Fig. 3B, C, respectively). Additionally, for both preparations, essentially 100% of the RLC present in the filaments was phosphorylated by MLCK (data not shown).
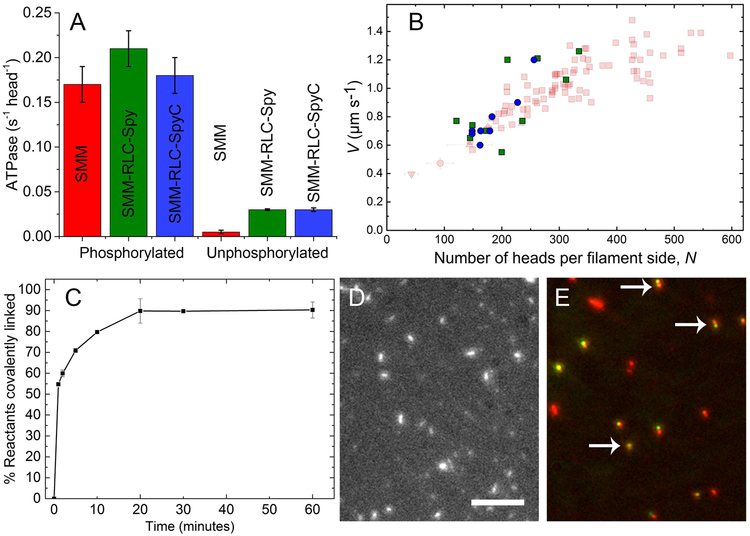
For our experiments, filaments incorporated with RLC-Spy and RLC-SpyC must have normal functional characteristics under conditions we use to image the movement/dynamics of the QD (see below). This requires filament crosslinking with EDC to prevent ATP-induced filament disassembly. We have previously shown that EDC crosslinking does not alter filament ATPase activity (Haldeman et al. 2014). In the phosphorylated state, the actin-activated ATPase activities at 50 µM actin were similar to control SMM (Fig. 5A, left). However, in the unphosphorylated state, both the RLC-Spy and RLC-SpyC filaments showed a 6-fold higher actin-activated ATPase than control (Fig. 5A, right). This indicates that the presence of the RLC-Spy and RLC-SpyC in excess over RLC in these filaments leads to some loss of the normal level of down-regulation. While this is not a significant concern for our experiments, it suggests that some of the structural interactions that stabilize the interacting heads motif may be altered (Wendt et al. 2001; Salzameda et al. 2006). This is surprising especially for the RLC-Spy, which is only a 20 amino acid C-terminal extension.
Figure 5. Characterization of SMM filaments incorporated with RLC-Spy and RLC-SpyC.
A) Actin-activated ATPase of phosphorylated and unphosphorylated control SMM (red), SMM RLC-Spy (green) and SMM RLC-SpyC (blue). Phosphorylated, 0.17 ± 0.02, 0.21 ± 0.02, and 0.18 ± 0.02 s−1 head −1 (S.D. n = 3), respectively. Unphosphorylated, 0.005 ± 0.002, 0.03 ± 0.001, and 0.03 ± 0.002 s−1 head −1 (S.D. n = 3), respectively. Error bars are S.D., n=3. Control SMM shown is unlabeled, uncrosslinked SMM exchanged with native RLC. Activities of other controls with and without crosslinking were similar, as previously shown in (Haldeman et al. 2014). Assays for actin-activated ATPase were at 30 °C as previously described (Brizendine et al. 2017) except the buffer contained 50 mM NaCl and [Actin] = 50 µM. Phosphate was determined as described (Brizendine et al. 2017). B) Velocity (V) vs N plot showing control SMM data (faded red) from (Brizendine et al. 2015). SMM RLC-Spy (green) and SMM RLC-SpyC filament data (blue) are plotted for comparison. C) Reaction timecourse of 0.5 µM SMM RLC-Spy filaments mixed with 1 µM SpyC in filament buffer. % Reactants covalently linked was determined by SDS-PAGE and gel densitometry. Error bars are S.D., n=2. D) TIRF microscopy image of 20 µg ml−1 rhodamine labeled SMM RLC-Spy filaments that were reacted with equimolar SpyC for 1 hr at RT in filament buffer. Scale bar = 5 µm. E) Overlaid image of 20 µg ml−1 SMM RLC-Spy filaments (red) reacted at 1 µM with 12.5 nm 585 nm QD-SpyC (green) for 1 hr at RT in filament buffer. Arrows point to co-localized filaments and QDs.
To characterize mechanical function, we used the Mf/A motility assay (phosphorylated myosin filaments move over surface-attached actin) to determine the relationship between velocity (V) and the number of myosin heads per filament side (N) Fig. 5B (see (Brizendine et al. 2015)). The control SMM data (red) are from (Brizendine et al. 2015). The V for SMM with RLC-Spy (green squares) and SMM with RLC-SpyC (blue circles) show a similar increasing V with N. The simplest hypothesis from this limited data (n = 10 and 8, respectively) is that the kinetic and mechanical parameters that underlie V vs. N are not appreciably altered by the presence of RLC-Spy or RLC-SpyC.
SMM filaments incorporated with RLC-Spy react with SpyC.
To test whether SpyC could access and covalently react with the RLC-Spy incorporated into filaments, SpyC (1 µM) was mixed with SMM RLC-Spy filaments (0.5 µM, uncrosslinked) and the reaction time course was monitored by the appearance of a ~35 kDa band by SDS gel electrophoresis (Fig. 5C). As expected (Zakeri et al. 2012; Keeble et al. 2017), the reaction reached ~90% completion within 20 min. Fig. 5D shows that the filaments remained intact after this reaction occurred.
SMM filaments incorporated with RLC-Spy can be labeled with a QD.
Fig. 5E shows an overlaid image of the filament (rhodamine, red) and QD (green) fluorescence from a 1 hr reaction of 12.5 nM 585 nm QD-SpyC with 1 µM SMM RLC-Spy filaments. The arrows indicate filaments that co-localized with a QD. Importantly, the filaments appear similar to control (Fig. 2A), in stark contrast to the QD-RLC filaments where the QD was attached at Cys-108 of the RLC (Fig. 2C, D). These data suggest that even if SpyC is coupled to a QD, it can still access and covalently react with RLC-Spy incorporated into filaments. Additionally, QD-SpyC labeled SMM RLC-Spy filaments move normally in the Mf/A assay as seen in Movie 1.
Summary.
Our interests are to understand the mechanochemistry of myosin filaments interacting with actin in vitro. For some of this work we require QDs specifically and stably attached to the myosin head domain. To our knowledge this has not been previously accomplished with myosin filaments from any muscle type. Using previously published methods to tag the SMM RLC with small extrinsic probes and exchange the RLC into SMM, we have shown that SMM with 585 and 655 nm QDs coupled to the RLC completely lacks the ability to form normal filaments, while the presence of 525 nm QDs permits nearly normal filament formation. We also report a new method to tag myosin filaments with QDs that we found was successful even for longer wavelength QDs. We prepared functional SMM filaments containing the RLC fused to a Spy or SpyC. By systematically testing both N- and C-terminal constructs, we found a solution that allows for the attachment of QDs to the RLC incorporated into filaments without significant interference with the structural and enzymatic properties of filaments. This approach may be useful as a general method to attach a wide variety of extrinsic probes to myosin filaments in vitro and possibly in cells.
Supplementary Material
Movie 1. Example movie of SMM RLC-Spy filaments labeled with 585 nm QD-SpyC moving in Mf/A assay. 585 nm QD-SpyC (green) labeled SMM RLC-Spy filaments are seen moving on surface attached actin filaments (red) at 20 µM ATP. The myosin filaments consisted of 3% SMM RLC-Spy and 97% SMM. The SMM filaments (0.5 mg ml−1) were reacted with 2 nM 585 nm QD-SpyC for 2 hr before the assay was performed. Field is 512 × 512 pixels, 200 frames were collected at 0.1 s exposure time and played at 150 frames per second. Each pixel is 87 × 87 nm.
Acknowledgments
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number 1R01AR071405 (to C.R.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional funding support was provided by an American Heart Association Predoctoral Fellowship award number 18PRE34030372 and by the Mick Hitchcock Scholarship (both to R.K.B.). The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Allen TS, Ling N, Irving M and Goldman YE. 1996. Orientation changes in myosin regulatory light chains following photorelease of ATP in skinned muscle fibers. Biophys J 70(4):1847–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach JR, Licate LS, Crish JF and Egelhoff TT. 2011. Analysis of the role of Ser1/Ser2/Thr9 phosphorylation on myosin II assembly and function in live cells. BMC Cell Biol 12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Brandmeier BD, Ferguson RE, Criddle S, Dale RE and Irving M. 2004. Bifunctional rhodamine probes of Myosin regulatory light chain orientation in relaxed skeletal muscle fibers. Biophys J 86(4):2329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizendine RK, Sheehy GG, Alcala DB, Novenschi SI, Baker JE and Cremo CR. 2017. A mixed-kinetic model describes unloaded velocities of smooth, skeletal, and cardiac muscle myosin filaments in vitro. Sci Adv 3(12):eaao2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizendine RK, Alcala DB, Carter MS, Haldeman BD, Facemyer KC, Baker JE and Cremo CR. 2015. Velocities of unloaded muscle filaments are not limited by drag forces imposed by myosin cross-bridges. Proc Natl Acad Sci U S A 112(36):11235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison PA, Sellers JR and Cremo CR. 2000. Kinetics of smooth muscle heavy meromyosin with one thiophosphorylated head. J Biol Chem 275(20):15142–51. [DOI] [PubMed] [Google Scholar]
- Facemyer KC and Cremo CR. 1992. A New Method to Specifically Label Thiophosphorylatable Proteins with Extrinsic Probes. Labeling of serine-19 of the regulatory light chain of smooth muscle myosin. Bioconj Chem 3(5):408–13. [DOI] [PubMed] [Google Scholar]
- Gollub J, Cremo CR and Cooke R. 1999. Phosphorylation regulates the ADP-induced rotation of the light chain domain of smooth muscle myosin. Biochemistry 38(31):10107–18. [DOI] [PubMed] [Google Scholar]
- Haldeman BD, Brizendine RK, Facemyer KC, Baker JE and Cremo CR. 2014. The Kinetics Underlying the Velocity of Smooth Muscle Myosin Filament Sliding on Actin Filaments in Vitro. J Biol Chem 289(30):21055–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissler SM and Sellers JR. 2015. Four things to know about myosin light chains as reporters for non-muscle myosin-2 dynamics in live cells. Cytoskeleton (Hoboken) 72(2):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebe M, Reardon S, Mitani Y, Kamisoyama H, Matsuura M and Ikebe R. 1994. Involvement of the C-terminal residues of the 20,000-dalton light chain of myosin in the regulation of smooth muscle actomyosin. Proc Natl Acad Sci U S A 91(19):9096–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebe R, Reardon S, Mitsui T and Ikebe M. 1999. Role of the N-terminal region of the regulatory light chain in the dephosphorylation of myosin by myosin light chain phosphatase. J Biol Chem 274(42):30122–6. [DOI] [PubMed] [Google Scholar]
- Kambara T, Rhodes TE, Ikebe R, Yamada M, White HD and Ikebe M. 1999. Functional significance of the conserved residues in the flexible hinge region of the myosin motor domain. J Biol Chem 274(23):16400–6. [DOI] [PubMed] [Google Scholar]
- Kazmierczak K, Liang J, Yuan CC, Yadav S, Sitbon YH, Walz K, Ma W, Irving TC, Cheah JX, Gomes AV et al. 2018. Slow-twitch skeletal muscle defects accompany cardiac dysfunction in transgenic mice with a mutation in the myosin regulatory light chain. FASEB J doi: 10.1096/fj.201801402R:fj201801402R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeble AH, Banerjee A, Ferla MP, Reddington SC, Anuar I and Howarth M. 2017. Evolving Accelerated Amidation by SpyTag/SpyCatcher to Analyze Membrane Dynamics. Angew Chem Int Ed Engl 56(52):16521–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kengyel A, Wolf WA, Chisholm RL and Sellers JR. 2010. Nonmuscle myosin IIA with a GFP fused to the N-terminus of the regulatory light chain is regulated normally. J Muscle Res Cell Motil 31(3):163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S, Yano T, Shibata M, Tuft RA and Ikebe M. 2000. Effects of the regulatory light chain phosphorylation of myosin II on mitosis and cytokinesis of mammalian cells. J Biol Chem 275(44):34512–20. [DOI] [PubMed] [Google Scholar]
- Liu X, Shu S, Hong MS, Levine RL and Korn ED. 2006. Phosphorylation of actin Tyr-53 inhibits filament nucleation and elongation and destabilizes filaments. Proc Natl Acad Sci U S A 103(37):13694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew JD, Khromov AS, Trybus KM, Somlyo AP and Somlyo AV. 1998. Myosin essential light chain isoforms modulate the velocity of shortening propelled by nonphosphorylated cross-bridges. J Biol Chem 273(47):31289–96. [DOI] [PubMed] [Google Scholar]
- Melli L, Billington N, Sun SA, Bird JE, Nagy A, Friedman TB, Takagi Y and Sellers JR. 2018. Bipolar filaments of human nonmuscle myosin 2-A and 2-B have distinct motile and mechanical properties. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS and Weiss S. 2005. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307(5709):538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Takagi Y, Billington N, Sun SA, Hong DK, Homsher E, Wang A and Sellers JR. 2013. Kinetic characterization of nonmuscle myosin IIb at the single molecule level. J Biol Chem 288(1):709–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni S, Hong F, Haldeman BD, Baker JE, Facemyer KC and Cremo CR. 2012. Modification of interface between regulatory and essential light chains hampers phosphorylation-dependent activation of smooth muscle myosin. J Biol Chem 287(26):22068–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni S, Hong F, Brewer PD, Ikebe M, Onishi H, Baker JE, Facemyer KC and Cremo CR. 2009. Kinetic and motor functions mediated by distinct regions of the regulatory light chain of smooth muscle myosin. Biochim Biophys Acta 1794(11):1599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Sellers JR and Cremo CR. 1996. Structure and Function of the 10 S Conformation of Smooth Muscle Myosin. J Biol Chem 271(34):20375–84. [DOI] [PubMed] [Google Scholar]
- Peterson LJ, Rajfur Z, Maddox AS, Freel CD, Chen Y, Edlund M, Otey C and Burridge K. 2004. Simultaneous stretching and contraction of stress fibers in vivo. Mol Biol Cell 15(7):3497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe T and Kendrick-Jones J. 1992. Chimeric myosin regulatory light chains identify the subdomain responsible for regulatory function. EMBO J 11(13):4715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe T and Kendrick-Jones J. 1993. The C-terminal helix in subdomain 4 of the regulatory light chain is essential for myosin regulation. EMBO J 12(12):4877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzameda B, Facemyer KC, Beck BW and Cremo CR. 2006. The N-terminal lobes of both regulatory light chains interact with the tail domain in the 10 S-inhibited conformation of smooth muscle myosin. J Biol Chem 281(50):38801–11. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Yang Z, Zhi G, Stull JT and Trybus KM. 1994. Charge replacement near the phosphorylatable serine of the myosin regulatory light chain mimics aspects of phosphorylation. Proc Natl Acad Sci U S A 91(4):1490–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesna D, Ghosh D, Li Q, Gomes AV, Guzman G, Arana C, Zhi G, Stull JT and Potter JD. 2001. Familial hypertrophic cardiomyopathy mutations in the regulatory light chains of myosin affect their structure, Ca2+ binding, and phosphorylation. J Biol Chem. p 7086–92. [DOI] [PubMed] [Google Scholar]
- Trybus KM and Lowey S. 1988. The Regulatory Light Chain is Required for Folding of Smooth Muscle Myosin. J Biol Chem 263(31):16485–92. [PubMed] [Google Scholar]
- Trybus KM and Chatman TA. 1993. Chimeric Regulatory Light Chains as Probes of Smooth Muscle Myosin Function. J Biol Chem 268(6):4412–19. [PubMed] [Google Scholar]
- Trybus KM, Waller GS and Chatman TA. 1994. Coupling of ATPase activity and motility in smooth muscle myosin is mediated by the regulatory light chain. J Cell Biol 124(6):963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt T, Taylor D, Trybus KM and Taylor K. 2001. Three-dimensional image reconstruction of dephosphorylated smooth muscle heavy meromyosin reveals asymmetry in the interaction between myosin heads and placement of subfragment 2. Proc Natl Acad Sci U S A 98(8):4361–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Clack BA, Zhi G, Stull JT and Cremo CR. 1999. Phosphorylation-dependent structural changes in the regulatory light chain domain of smooth muscle heavy meromyosin. J Biol Chem 274(29):20328–35. [DOI] [PubMed] [Google Scholar]
- Zakeri B, Fierer JO, Celik E, Chittock EC, Schwarz-Linek U, Moy VT and Howarth M. 2012. Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc Natl Acad Sci U S A 109(12):E690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1. Example movie of SMM RLC-Spy filaments labeled with 585 nm QD-SpyC moving in Mf/A assay. 585 nm QD-SpyC (green) labeled SMM RLC-Spy filaments are seen moving on surface attached actin filaments (red) at 20 µM ATP. The myosin filaments consisted of 3% SMM RLC-Spy and 97% SMM. The SMM filaments (0.5 mg ml−1) were reacted with 2 nM 585 nm QD-SpyC for 2 hr before the assay was performed. Field is 512 × 512 pixels, 200 frames were collected at 0.1 s exposure time and played at 150 frames per second. Each pixel is 87 × 87 nm.