Abstract
Pain has a strong emotional component and is defined by its unpleasantness. Chronic pain represents a complex disorder with anxio-depressive symptoms and cognitive deficits. Underlying mechanisms are still not well understood but an important role for interactions between prefrontal cortical areas and subcortical limbic structures has emerged. Evidence from preclinical studies in the rodent brain suggests that neuroplastic changes in prefrontal (anterior cingulate, prelimbic and infralimbic) cortical and subcortical (amygdala and nucleus accumbens) brain areas and their interactions (corticolimbic circuitry) contribute to the complexity and persistence of pain and may be predetermining factors as has been proposed in recent human neuroimaging studies.
Introduction
Compelling evidence from clinical neuroimaging studies points to an important role of the corticolimbic system in the development, amplification and prediction of chronic pain and its emotional-affective dimension [4, 146]. These corticolimbic brain areas include primarily the medial prefrontal cortex (mPFC), amygdala, nucleus accumbens and hippocampus. Closely related brain areas interacting with limbic circuity such as anterior cingulate cortex (ACC) and insular cortex (IC) have consistently been implicated in acute pain and some forms of chronic pain [164].
Here we review information from preclinical mechanistic studies on neuronal activity and synaptic changes in the (medial) prefrontal cortex and interconnected limbic areas (amygdala, nucleus accumbens/striatum and hippocampus) as pain mechanisms. We also provide clinical context for the preclinical literature by discussing related studies in human subjects.
Prefrontal cortical (PFC) pain mechanisms
The prefrontal cortex is an important neural substrate for executive functions and decision-making; reduced PFC function leads to increased impulsivity, reduced control over social behaviors, and impaired decision making. It is a heterogeneous brain region composed of multiple structures and exhibits species-specific differences between rodents, primates, and humans in connectivity, cytoarchitecture, electrophysiological properties, protein expression, and responses following damage [58, 115, 119]. The rodent nomenclature, including the infra- and pre-limbic and anterior cingulate cortices, will be used in this review. These are thought to correspond to Brodmann areas 25, 32 and 24b, respectively, in primates [151]. Cortical areas other than the PFC, such as the IC, have been implicated in pain processing and pain modulation, including through limbic regions [71, 72, 137], but will not be discussed in detail in this review.
Prelimbic and infralimbic mPFC
The mPFC has emerged as a critical region for top-down cognitive control over emotion-driven behaviors via processes including fear conditioning and extinction. The prelimbic and infralimbic mPFC receive inputs from regions including the thalamus, basolateral amygdala (BLA), hippocampus, and contralateral mPFC [58, 84, 93, 94]. A portion of BLA inputs terminate on GABAergic interneurons, allowing for feedforward inhibition of mPFC output (see Fig. 1) through modulation of mPFC projection neurons [43, 57, 58, 118]. Both the prelimbic and infralimbic cortices send excitatory projections to the amygdala [43, 58, 108, 109, 112, 118, 144]. The prelimbic mPFC predominately targets the BLA, whereas the infralimbic cortex projects to BLA and other amygdala divisions including the lateral amygdala (LA), intercalated cell mass of the amygdala (ITC), and possibly to the lateral central nucleus of the amygdala (CeL). It is the projection to the ITC, directly or indirectly via BLA, that is thought to be important in fear extinction [43, 58, 118]. The ITC sends GABAergic projections to central amygdala (CeA) projection neurons, allowing for feedforward inhibitory control of amygdala output by the mPFC (see Fig. 1). See Figure 2 for a diagrammatic representation of prefrontal cortical neurocircuitry and its interactions with other cortical and subcortical structures.
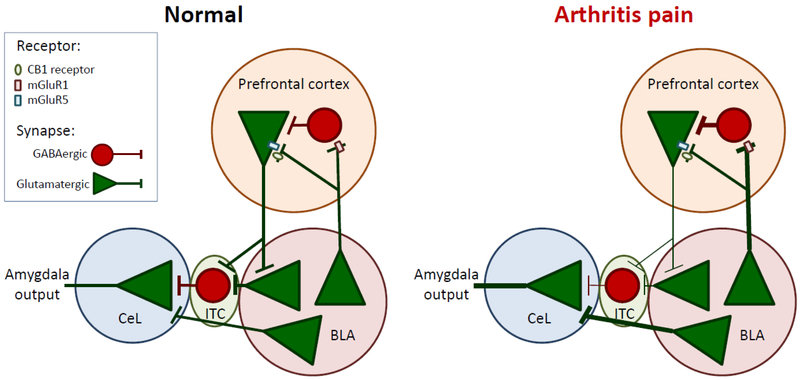
Figure 1. Pain-related changes in cortico-amygdala interactions.
Projection neurons in the BLA synapse on PV-GABAergic interneurons as well as on mPFC projection neurons in the infra- and pre-limbic cortices through a mechanism involving mGluR1, mGluR5, and CB1. PV-GABAergic interneurons in turn project to mPFC projection neurons, resulting in BLA-driven feedforward inhibition of mPFC projection neuron activity. BLA activity is increased in arthritis pain, resulting in pain-related cortical dysfunction. Projection neurons from the infralimbic cortex synapse on BLA projection neurons, as well as GABAergic ITC neurons. GABAergic ITC neurons and BLA projection neurons synapse on CeL projection neurons. Reduced cortical drive onto BLA and ITC neurons in the arthritis pain state results in reduced feedforward inhibition onto CeL projection neurons. This, coupled with increased drive from the BLA, results in pain-related amygdala hyperactivity. mPFC, medial prefrontal cortex; CB1, cannabinoid receptor 1; mGluR, metabotropic glutamate receptor; CeL, lateral division of the central nucleus of the amygdala; ITC, intercalated cell mass of the amygdala.
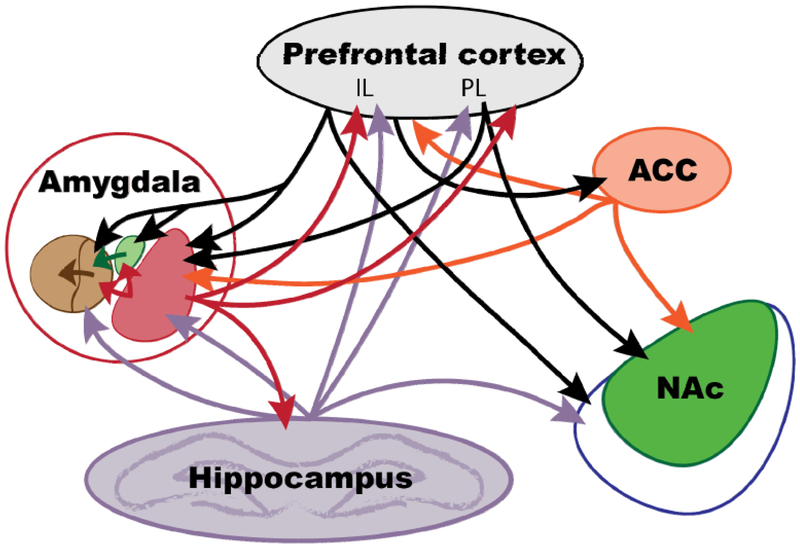
Figure 2. Pain-related cortico-limbic interactions.
Connections between cortical (prefrontal cortex including infralimbic, prelimbic, and anterior cingulate cortices) and limbic (amygdala, hippocampus, and nucleus accumbens) regions. Within the amygdala, the CeA (brown) with its medial (left) and lateral (right) divisions, ITC (green), and LA/BLA are shown. Within the nucleus accumbens, the core (green) and shell (blue) divisions are shown. mPFC, medial prefrontal cortex; IL, infralimbic mPFC; PL, prelimbic mPFC; ACC, anterior cingulate cortex; NAc, nucleus accumbens.
Chronic pain has been proposed to be “persistence of the memory of pain and/or the inability to extinguish the memory of pain evoked by an initial inciting injury” [4]. Given its importance in extinguishing subcortically-driven fear behaviors, it seems reasonable to hypothesize that pain-related loss of mPFC activation could contribute to pain chronification. Indeed, chronic pain is thought to engage similar brain mechanisms as fear extinction [44, 47], and preclinical evidence in acute and chronic pain models has emerged that supports the idea of pain-related cognitive impairment via feedforward inhibition and resultant loss of cortical control over subcortical responses.
Preclinical evidence suggests that mPFC function is impaired in the pain state. Electrophysiology studies in the anesthetized rat demonstrated decreased evoked and background activity in the prelimbic [75] and infralimbic [73] mPFC in acute arthritis pain. In layer V prelimbic mPFC neurons, the spared nerve injury (SNI) model of neuropathic pain resulted in reduced glutamatergic transmission [79] and reduced pyramidal cell firing [163] in brain slice physiology experiments. Accordingly, spontaneous excitatory postsynaptic currents (EPSCs) were reduced in frequency, which corresponded to a reduction in electrically evoked EPSCs [79]. Length and branching of apical dendrites and glutamate concentration in the mPFC were reduced in SNI rats compared to controls. This reduction in neuronal activity was mediated by increased feedforward inhibition from parvalbumin (PV) expressing GABAergic interneurons [79]. In addition, modulatory effects of acetylcholine (ACh) neurotransmission were altered in the SNI model of neuropathic pain [123]. Under normal conditions, ACh increased excitability of layer V prelimbic pyramidal mPFC neurons in brain slices through activation of postsynaptic M1 receptors. In the SNI model, however, this effect was lost due to M1 receptor internalization, suggesting that M1 receptor plasticity and subsequent alterations in cholinergic neurotransmission contribute to pain-related mPFC dysfunction.
Taken together, these data support reduced activity of layer V mPFC neurons in acute and chronic pain states. Reduced mPFC activity has been linked mechanistically to pain behaviors in a series of experiments using manipulations of mPFC activity. Optogenetic activation of layer V prelimbic mPFC neurons in SNI rats significantly inhibited tonic pain responses in the conditioned place preference test and mechanical and thermal sensitivity on the von Frey and Hargreaves tests, respectively [90]. Importantly, pain-related anhedonic and behavioral despair depression-like behaviors were reduced with mPFC activation on the sucrose preference (SPT) and forced swim (FST) tests, respectively. Similar behavioral effects were observed by manipulation of interneurons involved in feedforward inhibition of prelimbic pyramidal cell output. Optogenetic silencing of PV expressing GABAergic interneurons in SNI rats decreased tonic pain responses and mechanical and thermal sensitivity, whereas optogenetic activation exacerbated SNI-associated tonic pain and mechanical and thermal sensitivity [163]. These studies provide a causal link between mPFC activity and pain behaviors, and show the importance of GABAergic interneuron activity for the regulation of mPFC output. Manipulating activity of these interneurons could therefore be a therapeutic strategy for chronic pain.
Mechanisms underlying mPFC deactivation in the pain state are an area of active investigation, but altered inputs from the amygdala appear to play a role. Increased feedforward inhibition driven by glutamatergic BLA projections to the mPFC was observed in the infralimbic cortex in the arthritic pain model [82]. The enhanced BLA-driven synaptic inhibition of layer V pyramidal cells was reduced following pharmacologic restoration of postsynaptic metabotropic glutamate receptor 5 (mGluR5) and presynaptic cannabinoid receptor 1 (CB1) function. This rescue strategy decreased mechanical sensitivity, inhibited nocifensive and averse affective pain behaviors, and restored decision-making on the rodent gambling task [82].
Similar to BLA-driven infralimbic mPFC deactivation, increased activity of BLA neurons projecting to the prelimbic mPFC was found in anesthetized rats after acute arthritis pain induction [75], which corresponded to reduced prelimbic pyramidal cell activity through a mechanism dependent on presynaptic mGluR1 [75, 140]. This effect was due to enhanced feedforward inhibition from the BLA, whereas excitatory inputs did not change. Blockade of BLA hyperactivity with a CRF1 receptor antagonist restored responsiveness and background activity of prelimbic mPFC output neurons to near pre-arthritis levels and inhibited mechanical hypersensitivity, nocifensive and averse affective pain behaviors, as well as restored decision-making on the rodent gambling task [75]. Reduced prelimbic mPFC output was also observed in a neuropathic pain model (SNI) due to feedforward inhibition of layer V pyramidal cells from PV-expressing interneurons [163]. As the BLA projects to these cells [74, 108, 144], this provides further evidence for pain-related BLA-mediated deactivation of prelimbic mPFC output.
These studies support an important role of amygdala-driven deactivation of layer V mPFC output neurons in pain states. Mechanistically, they point to a contribution of CRF, mGluR5, and CB1 to feedforward inhibition of mPFC output by PV-expressing interneurons in the prelimbic, and possibly infralimbic, mPFC. In contrast, there is evidence for layer II/III pyramidal cell hyperexcitability in pain states. In the SNI model, basal dendrite spine density in layer II/III prelimbic mPFC neurons is increased in SNI rats compared to sham controls [101]. The morphological change was accompanied by an increase in N-methyl-D-aspartate (NMDA) receptor-mediated currents and NMDA/AMPA ratio in brain slices, which correlated with increased mechanosensitivity in the von Frey test. Enhanced glutamatergic transmission and excitability of layer II/III prelimbic pyramidal cells were found in the SNI model as the consequence of a hyperpolarizing shift in activation of the cationic hyperpolarization-activated cyclic nucleotide-gated (HCN) channel current, which is indicative of a reduction in open channel probability causing a reduction in the HCN mediated cationic Ih current [38]. Pharmacological HCN channel blockade attenuated cold but not mechanical hypersensitivity in SNI rats [38]. Voltage-dependence of HCN channel activation is regulated by noradrenergic afferents, and intra-mPFC infusion of an α2 receptor agonist had analgesic effects in an SNI model [37], suggesting a role for aberrant pain-related noradrenergic transmission in the prelimbic mPFC. An mGluR1/5 agonist facilitated firing in prelimbic layer V mPFC neurons in the SNI model compared to sham controls [38], and blockade of prelimbic mGluR5 attenuated mechanical hypersensitivity and depression-like behaviors [35], suggesting a role for mGluR5 in these effects. Similarly, increased frequency and amplitude of spontaneous EPSCs were recorded in layer II/III prelimbic pyramidal cells in the complete Freund’s adjuvant (CFA) inflammatory pain model [152]. However, this synaptic change corresponded to a decrease in excitability. In fact, optogenetic activation of these neurons had antinociceptive and anxiolytic-like effects in the CFA model, and optogenetic inhibition of these neurons under normal conditions was anxiogenic [152]. Decreased excitability and behavioral effects were linked mechanistically because both were mitigated by shRNA-induced knockdown of cyclin-dependent kinase 5 (Cdk5).
Anterior cingulate cortex (ACC)
The ACC is a limbic structure that is thought to contribute to affective/motivational rather than sensory/discriminative aspects of pain [18, 105, 108, 109], but see [33]. ACC neurons have bilateral and large receptive fields [160], suggesting they do not provide discriminatory sensory information. The ACC receives nociceptive information from the medial thalamus [105], which allows for ACC contributions to pain processing. Neuroimaging studies demonstrate ACC activation following the offset of a painful thermal stimulus in both rats [18, 85] and humans [18], and these responses were significantly attenuated by intraperitoneal (i.p.) injection of morphine in rats [85, 153]. This nociceptive information is integrated with motivational and affective information received from IC, mPFC and BLA inputs to generate ACC-mediated pain responses [7, 25, 105, 144, 154]. Consistent with this, microinjection of excitatory amino acids into the ACC is sufficient to induce avoidance learning without the need for a noxious stimulus, blockade of excitatory amino acid receptors in the ACC is sufficient to block avoidance learning from a peripheral noxious stimulus, and lesioning of the ACC after conditioning did not affect avoidance learning [77]. The ACC generates these affective and motivational pain responses via projections to the amygdala, NAc, and mPFC [7, 8, 25, 105, 108, 144, 154].
Preclinical studies mechanistically link the ACC to pain processing and pain modulation. Bilateral chemical lesioning of the ACC in rodents abolished supraspinally organized but not spinally organized pain behaviors induced by injection of bee venom into the paw [124]. Optogenetic activation of inhibitory neurocircuitry in the ACC resulting in reduced ACC output inhibited pain responses in the formalin test [59]. Phosphorylation of cAMP response element-binding protein in the ACC, which is thought to be related to pain memory, as well as phosphorylated PKA was increased in rats after two intraplantar injections of carrageenan, which was subsequently reduced by electroacupuncture but not indomethacin [133, 141]. This intervention corresponded to a reduction in pain behaviors. In a chronic neuropathic pain model (spinal nerve ligation model, SNL), bilateral electric lesioning of the ACC significantly decreased escape/avoidance behavior, but did not affect anxiety responses or mechanical sensitivity [56, 89]. In addition, increased levels of c-Fos, a general marker of neuronal activation, after unilateral intraplantar carrageenan injection were directly related to escape/avoidance behavior but not to mechanical sensitivity of the affected paw [56, 145].
Mechanistically, these effects could be due to interactions with pain neurocircuitry in the periaqueductal gray (PAG). The ACC and PAG are among the structures that are activated in anticipation of a noxious stimulus in human subjects [25], and increased negative emotion is associated with activation of ACC-PFC-PAG circuitry [25, 150] and increased activity in the ACC [25]. In a preclinical study, electrical stimulation of the ACC rescued escape/avoidance behavior but not mechanical sensitivity, and this effect was significantly reduced following PAG lesions [56]. Mu opioid receptors are thought to be important for this interaction and for pain modulation by the ACC. In clinical studies of the placebo effect from expectation of pain relief, an opioid antagonist (naloxone) decoupled ACC and PAG activity as well as reduced the effect of placebo analgesia [25, 46]. In a preclinical study, beneficial effects of low dose systemic morphine on affective escape/avoidance behaviors were mimicked by stereotaxic injection into the ACC [56, 86-88, 107].
These studies support an important role of the ACC in mediating affective/motivational aspects of pain, and point to ACC-PAG interactions and mu opioid receptors as important mechanistic contributors. However, a recent study suggests that selective optogenetic activation or silencing of ACC-spinal cord projecting neurons causes mechanical hypersensitivity or antinociception, respectively, through direct activation of excitatory spinal dorsal horn neurons [33].
Amygdala
The amygdala is a limbic brain structure involved in emotions and affective disorders [108, 109, 112, 144, 156]. Identification of a nociceptive input via the spino-parabrachio-amygdaloid pathway, clinical neuroimaging data showing amygdala activation in experimental and clinical pain states, as well as preclinical studies showing maladaptive plasticity and hyperactivity in the pain state have implicated the amygdala as a critical node in emotional affective aspects of pain [47, 108, 109, 135, 136, 144, 146, 147]. Aberrant amygdala function also increases risk of developing chronic pain as increased white matter connectivity within the mPFC-amygdala-hippocampus circuit and reduced amygdala size are independent risk factors for persistence of back pain [147]. The focus of this section is on pain-related amygdala changes and cortico-amygdala interactions. Information about molecular level pain-related amygdala plasticity and therapeutic strategies targeting this plasticity can be found in recent review articles [105, 144, 156].
The amygdala receives cortical (mPFC, ACC, and IC) and thalamic inputs that provide polymodal sensory information to the lateral/basolateral amygdala (LA/BLA) complex [108, 109, 144]. The LA/BLA attaches emotional and affective context to this sensory information, which is transmitted to the CeA for further processing and output to brain centers of behavioral modulation. The BLA also projects to the cortical areas that provide information to the amygdala and exert control functions. Projections to the mPFC in particular are involved in pain-related cognitive dysfunction and in fear conditioning [83, 108, 144], and preferentially target cortico-PAG projection neurons in layer V of the infralimbic cortex and cortico-amygdalar projection neurons in layer II of the prelimbic cortex [34].
The CeA is predominantly composed of GABAergic neurons and serves major output functions for amygdala fear and pain neurocircuitry [108, 109, 144]. Purely nociceptive sensory information is conveyed to the lateral and capsular division(s) of the CeA (CeLC) via the spino-parabrachio-amygdaloid tract from the parabrachial (PB) nucleus (external lateral division). This nociceptive projection also engages feedforward inhibition from interconnected GABAergic interneurons within the CeA [139]. PB input is integrated with polymodal sensory information from the BLA to generate amygdala-mediated pain responses [108, 109, 144]. The medial division of the CeA (CeM) and corticotropin releasing factor (CRF) positive neurons in the lateral CeA serve amygdala output functions, and project to the hypothalamus, other limbic structures, and brainstem regions involved in behavioral expression such as PAG and PB. The CeL can also influence output from the CeM by way of reciprocally connected GABAergic interneurons in the CeA, although this inhibitory neurocircuitry has not yet been well described for amygdala pain mechanisms [36, 43, 49, 66].
The ITC is a region interposed between the CeA and BLA that receives excitatory cortical input from the infralimbic mPFC, as well as input from the BLA [108, 109, 144]. The ITC then sends GABAergic projections to the CeL, allowing for feedforward inhibition of amygdala output though a neuropeptide S (NPS)-dependent mechanism [60, 78, 91, 92, 100, 117, 126, 159]. It is this feedforward inhibition driven by cortical inputs, directly or indirectly via BLA, that has been implicated in fear extinction pathways [43, 108, 109, 118, 144]; dysfunction of this input has been associated with impaired fear extinction [32, 67, 81, 134, 156].
Preclinical in vivo and brain slice physiology studies have identified pain-related dysfunction of this neurocircuitry, resulting in increased neuronal activity and enhanced excitatory synaptic transmission in the pain state. Neuronal excitability is increased in the CeA in acute arthritis [20, 55, 108, 111, 144, 149] and neuropathic [69, 104] pain models, as well as in the BLA [75] in an acute arthritis pain model. In addition, excitatory synaptic transmission at the PB-CeL and BLA-CeL synapses [20, 55, 63, 110, 127], as well as the LA-BLA synapse [75] is enhanced in the acute arthritis pain state. Interestingly, the degree of synaptic potentiation at the PB-CeL synapse occurs through a mechanism involving C-fiber afferents [104] and correlates to the degree of mechanical hypersensitivity in neuropathic pain [69]. It should be noted that the PB input is uniquely characterized by its content of calcitonin gene-related peptide (CGRP), and CGRP receptor activation in the CeLC is critically involved in pain-related plasticity [39, 62-64, 69, 114].
Interactions between the BLA, infralimbic mPFC, and ITC have emerged as important mediators of pain-related dysfunction of cognitive control of amygdala output in the pain state. Synaptic activation of ITC cells [126] and subsequent inhibition of CeL activity [126, 127] is reduced in the acute arthritis pain state. Decreased activity of infralimbic mPFC projection neurons (see Prelimbic and infralimbic mPFC; [75, 144, 156]) accounts for decreased ITC-mediated feedforward inhibitory control of CeL activity, and thus for decreased cognitive control of amygdala output. In line with this, pharmacological activation of infralimbic mPFC CB1 and mGluR5 in the arthritis pain state but not under normal conditions increased evoked and background activity in the anesthetized rat in the infralimbic mPFC and inhibited responses in the CeL [73].
Dysfunction in the interaction between amygdala and cortical control centers such as mPFC has emerged as a key element of acute and chronic pain-related plasticity in the brain, and is thought to be a significant node in the emotional affective dimension of pain and in pain-related cortical dysfunction as well as a predictor of pain vulnerability [146, 147]. Details and pain-related plastic changes in individual elements of the underlying neurocircuitry remain to be determined. Current work in the field focuses on neurochemically and molecularly distinct neurons and synapses in different amygdala regions, their inputs and projection targets, using transgenic, opto- and chemogenetic techniques, which makes the amygdala a model system for the study of brain mechanisms of pain and may help identify new molecular targets and therapeutic strategies for future translational studies.
Nucleus accumbens
The nucleus accumbens (NAc) is a forebrain structure that is involved in reward pathways and integrates cortical and affective information in order to assign motivation and value for selection of appropriate behavioral responses to outside stimuli [2, 4, 7, 8, 52, 70, 102, 129]. Dysfunctions of brain neurocircuitry involved in assigning salience [15, 16, 19, 22, 25] and value [23, 48, 157] are thought to be involved in the transition to chronic pain. The NAc has emerged as an important mediator of this pain-related dysfunction.
The NAc is widely considered to be made up of a shell subregion and a lateral core section [10, 52, 161]. These regions are distinguished by their connectivity to cortical and subcortical regions [10, 52]. In addition, the NAc shell is thought to evaluate impending pain and utilize spatial information from the hippocampus for appetitive learning, and the core activates with expectation of relief of an aversive stimulus and signals the reward value of pain cessation, as well as utilizes information from the BLA for appetitive learning [10, 52, 70, 125]. A diagram of NAc circuitry is included in Figure 2.
Neuroimaging studies have implicated NAc in acute and chronic pain responses. In freely moving rats, decreased NAc activity was observed with thermal [17, 21] and electrical [96] noxious stimuli. Dopamine levels are reduced in the NAc in SNI rats [125] and mechanistically, SNI involves upregulation of GABAergic indirect spiny projection neurons (iSPN), which is related to mechanical hypersensitivity [125]. These deficits were overcome by supplementing dopamine levels with L-DOPA in combination with either naproxen or a D2/D3 receptor agonist, suggesting a role for altered dopamine neurotransmission in these effects and implicating dopamine modulation as a therapeutic strategy for pain [125].
Alterations of NAc circuitry and connectivity have been identified as independent risk factors for development of chronic pain [3, 147]. Connectivity between the NAc and PFC is predictive of progression to chronic pain in patients presenting with low back pain [3, 11, 65, 125, 146, 147]. This was accompanied by decreased gray matter density in the NAc in patients that went on to have persistent back pain [11], and the degree of connectivity was related to underlying spontaneous pain [65]. In addition, fractional anisotropy in the NAc was correlated with that in the mPFC in patients that recovered from subacute back pain, but not in those in whom pain persisted [97].
Several preclinical and clinical studies have implicated reward circuitry and stimulus valuation and salience circuitry, including the NAc, in pain plasticity [2-4, 7, 9, 11, 18, 47, 65, 105, 106, 125, 146, 147, 157]. Pain-related changes in NAc cortical connectivity have emerged as one of the known risk factors for transition from acute to chronic pain [3, 11, 146, 147]. Convergence of pain relief and reward mechanisms is a relatively new and exciting area of pain research. Therapeutic strategies to correct those deficits remain an area for further investigation.
Cortico-hippocampal interactions
The hippocampus is a limbic brain region that is well known for its role in declarative and episodic memory [13, 45]. This is based on findings in humans that hippocampal damage results in amnesic effects [132], as well as findings in rodents that hippocampal lesions result in impaired performance on memory tasks such as the Morris water maze and recognition of sequences of events [13, 53, 80, 98, 99].
The hippocampus has also emerged as a critical node in emotionality, particularly for anxiety and depression [13, 95, 103]. Hippocampal deficits are observed in alcoholism and neuropsychiatric disorders [13, 51, 54, 95]. Hippocampal volume is decreased in human subjects with depression, and smaller volumes could convey increased risk for depressive disorders [31, 95, 113]. Electroconvulsive treatment results in improvement of depressive symptoms and increases hippocampal volume [24, 95]. It has been suggested that anti-depressant drugs exert their effects through increased neurogenesis in the dentate gyrus of the hippocampus [13, 131]. Hippocampal lesions improve anxiety-like behaviors in rats [13, 40]. The hippocampus is also an important brain region in fear contextualization, conditioning, and extinction [1, 12-14, 120, 130, 155, 162] and subjects with larger hippocampal volume show higher fear contingency awareness [26].
The hippocampus is closely interconnected with other limbic and cortical brain regions involved in emotion and cognition. The hippocampus is composed of the Cornu Ammonis (CA1-3) and the dentate gyrus (DG), where hippocampal neurogenesis is thought to occur [148]. It is also organized into dorsal and ventral subregions [138]; the dorsal hippocampus is thought to be involved in cognitive processes, including spatial memory and learning, and the ventral hippocampus in emotion [148]. The ventral hippocampus receives limbic input from the BLA [50, 121]. It also receives indirect input from the mPFC, which is thought to be important for episodic memory and context retrieval [76, 158]. Direct hippocampal projections from the ventral hippocampus to the prelimbic and infralimbic mPFC may be important for expression of anxiety and memory contextualization [68, 116, 122, 142]. The ventral hippocampus also sends excitatory projections to the NAc, which has been shown to drive depression-like behaviors in a chronic stress model [6]. See Figure 2 for a diagram of this cortico-limbic circuitry.
Pain is frequently associated with aversive emotional states and memory deficits. In a murine model of Complex Regional Pain Syndrome (CRPS), mice demonstrated anxiety-like behaviors, mechanical hypersensitivity, and impaired working memory [143]. Rats with SNI-induced neuropathic pain also have short term memory deficits, which corresponded to increased interleukin-1 beta (IL-1β) in the sciatic nerve, serum, PFC, NAc, amygdala, and hippocampus [61]. Interestingly, levels of IL-1β correlated to mechanical withdrawal thresholds in rats with SNI-induced neuropathic pain but not sham controls, implying a direct relationship between enhanced pain-related expression and nociception [41, 42]. Hippocampal plasticity could contribute to both memory and averse affective deficits in the chronic pain state.
In line with this, several studies have demonstrated hippocampal involvement in pain. Spatial encoding by hippocampal place cells is altered in CA1 following SNI in freely moving rats [30]. SNI mice demonstrate learning and memory deficits [103, 128], which corresponds to decreased hippocampal extracellular signal-regulated kinase (ERK) expression and phosphorylation, reduced neurogenesis, and altered synaptic plasticity [103]. Interestingly, increased hippocampal neurogenesis and related hippocampal learning mechanisms may be involved in the development of persistent pain because impairing neurogenesis reduced emergence and/or severity of pain behaviors [5]. However, specific mechanisms of these effects and causal relationships between altered neurogenesis and pain have not yet been established. Mechanistically, short term plasticity and long term potentiation at the CA3-CA1 synapses were impaired, density of presynaptic boutons in CA1 synapses was reduced, and tumor necrosis factor alpha (TNF-α) levels were increased in cerebrospinal fluid, plasma, and the hippocampus in a neuropathic pain mouse model (SNI) [128]. Intrahippocampal injection of TNF-α in naïve rats mimicked the behavioral effects associated with SNI, whereas deletion of the TNF receptor 1 in SNI rats prevented their development, suggesting a role for neuroinflammation in these effects.
Changes in cortico-hippocampal and intrahippocampal interactions have been observed in chronic pain. SNI pain reduces information flow in the mPFC-dorsal CA1 circuit and increases fronto-hippocampal theta entrainment which is inversely related to performance on a spatial working memory task in the freely moving rat [29]. Within the hippocampus, dysregulation of dopamine transmission has been implicated in chronic pain. Freely moving SNI rats lacked the extent of increased dorsal CA1 firing rate present in sham rats when deciding between potential reward locations in a figure-8 maze and had reduced theta phase coherence and dorsoventral connectivity compared to sham animals [28]. This corresponded to increased dopamine D2 receptor and decreased D3 receptor mRNA expression in the dorsal hippocampus, as well as increased D1 receptor, D2 receptor, and tyrosine hydroxylase and decreased D3 receptor mRNA expression in the ventral hippocampus. Systemic application of a D2/D3 receptor agonist reversed electrophysiological and memory but not pain-related behavioral deficits in SNI rats. These deficits were exacerbated by systemic administration of a D2 receptor antagonist, which was related to a selective disruption of hippocampal theta oscillations and decrease in intrahippocampal CA1 connectivity [27]. This set of data suggests that cortico-hippocampal interactions are altered in the pain state, and that modulation of dopamine neurotransmission could be involved in these effects.
Accumulating evidence suggests that the hippocampus is involved in chronic pain. Functional and structural changes in the hippocampus and in hippocampal connectivity to other limbic or cortical structures could contribute to learning and memory deficits, as well as to aversive cognitive and affective states associated with chronic pain.
Conclusions
Chronic pain has significant emotional affective and cognitive components, which contribute to its morbidity. Patients with chronic pain have increased risk for neuropsychiatric conditions such as anxiety and depression, as well as cognitive deficits including impaired decision-making and memory. Mechanisms of persistent pain and its complex emotional affective and cognitive components are not well understood, but it is intuitive to focus on the limbic system and corticolimbic interactions as key players. Convincing evidence suggests that changes in cortical (mPFC and ACC) and subcortical (amygdala and nucleus accumbens) brain regions, as well as the hippocampus, and their interactions contribute to emotional affective and cognitive aspects of pain and pain modulation. Importantly, changes in these circuits are associated with the development of persistent pain and may have predictive value for pain vulnerability. Improving understanding of the neurobiological basis of pain-related changes in these brain regions will provide the knowledge basis required for the development of novel limbic-based therapeutic targets for chronic pain management or even prevention.
Acknowledgements
Work in the authors’ laboratory is supported by National Institutes of Health (NIH) grants NS038261, NS081121, and NS106902.
List of abbreviations
- ACC
Anterior cingulate cortex
- ACh
Acetylcholine
- BLA
Basolateral amygdala
- CB
Cannabinoid receptor
- Cdk5
Cyclin-dependent kinase 5
- CeA
Central nucleus of the amygdala
- CeL
Lateral division of the CeA
- CeM
Medial division of the CeA
- CFA
Complete Freund’s adjuvant
- CA
Cornu Ammonis
- CRF
Corticotropin releasing factor
- CRPS
Complex regional pain syndrome
- DG
Dentate gyrus
- EPSC
Excitatory postsynaptic current
- FST
Forced swim test
- ERK
Extracellular signal-regulated kinase
- HCN
Hyperpolarization-activated cyclic nucleotide–gated channel
- IC
Insular cortex
- IL
Interleukin
- i.p.
Intraperitoneal
- IPSC
Inhibitory postsynaptic current
- ITC
Intercalated cell mass of the amygdala
- iSPN
Indirect spiny projection neuron
- LA
Lateral amygdala
- LA/BLA
Lateral/basolateral amygdala nuclei
- mGluR
Metabotropic glutamate receptor
- mPFC
Medial prefrontal cortex
- NAc
Nucleus accumbens
- NMDA
N-methyl-D-aspartate
- NPS
Neuropeptide S
- PAG
Periaqueductal gray
- PB
Parabrachial nucleus
- PV
Parvalbumin
- SPT
Sucrose preference test
- SNI
Spared nerve injury
- SNL
Spinal nerve ligation
- TNF-α
Tumor necrosis factor alpha
- VTA
Ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
There are no conflicts of interest.
References
- [1].Anagnostaras SG, Gale GD, Fanselow MS, Hippocampus and contextual fear conditioning: recent controversies and advances, Hippocampus 11 (2001) 8–17. [DOI] [PubMed] [Google Scholar]
- [2].Apkarian AV, Pain perception in relation to emotional learning, Curr Opin Neurobiol 18 (2008) 464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Apkarian AV, Baliki MN, Farmer MA, Predicting transition to chronic pain, Curr Opin Neurol 26 (2013) 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Apkarian AV, Baliki MN, Geha PY, Towards a theory of chronic pain, Prog Neurobiol 87 (2009) 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Apkarian AV, Mutso AA, Centeno MV, Kan L, Wu M, Levinstein M, Banisadr G, Gobeske KT, Miller RJ, Radulovic J, Hen R, Kessler JA, Role of adult hippocampal neurogenesis in persistent pain, Pain 157 (2016) 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bagot RC, Parise EM, Pena CJ, Zhang HX, Maze I, Chaudhury D, Persaud B, Cachope R, Bolanos-Guzman CA, Cheer JF, Deisseroth K, Han MH, Nestler EJ, Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression, Nat Commun 6 (2015) 7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Baliki MN, Apkarian AV, Nociception, Pain, Negative Moods, and Behavior Selection, Neuron 87 (2015) 474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV, Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain, J Neurosci 26 (2006) 12165–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Baliki MN, Geha PY, Fields HL, Apkarian AV, Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain, Neuron 66 (2010) 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Baliki MN, Mansour A, Baria AT, Huang L, Berger SE, Fields HL, Apkarian AV, Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain, J Neurosci 33 (2013) 16383–16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV, Corticostriatal functional connectivity predicts transition to chronic back pain, Nat Neurosci 15 (2012) 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ballesteros CI, de Oliveira Galvao B, Maisonette S, Landeira-Fernandez J, Effect of dorsal and ventral hippocampal lesions on contextual fear conditioning and unconditioned defensive behavior induced by electrical stimulation of the dorsal periaqueductal gray, PLoS One 9 (2014) e83342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Barkus C, McHugh SB, Sprengel R, Seeburg PH, Rawlins JN, Bannerman DM, Hippocampal NMDA receptors and anxiety: at the interface between cognition and emotion, Eur J Pharmacol 626 (2010) 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bast T, Zhang WN, Feldon J, The ventral hippocampus and fear conditioning in rats. Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABA(A) agonist muscimol, Exp Brain Res 139 (2001) 39–52. [DOI] [PubMed] [Google Scholar]
- [15].Becerra L, Borsook D, Signal valence in the nucleus accumbens to pain onset and offset, Eur J Pain 12 (2008) 866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D, Reward circuitry activation by noxious thermal stimuli, Neuron 32 (2001) 927–946. [DOI] [PubMed] [Google Scholar]
- [17].Becerra L, Chang PC, Bishop J, Borsook D, CNS activation maps in awake rats exposed to thermal stimuli to the dorsum of the hindpaw, Neuroimage 54 (2011) 1355–1366. [DOI] [PubMed] [Google Scholar]
- [18].Becerra L, Navratilova E, Porreca F, Borsook D, Analogous responses in the nucleus accumbens and cingulate cortex to pain onset (aversion) and offset (relief) in rats and humans, J Neurophysiol 110 (2013) 1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Becerra L, Sava S, Simons LE, Drosos AM, Sethna N, Berde C, Lebel AA, Borsook D, Intrinsic brain networks normalize with treatment in pediatric complex regional pain syndrome, Neuroimage Clin 6 (2014) 347–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bird GC, Lash LL, Han JS, Zou X, Willis WD, Neugebauer V, Protein kinase A-dependent enhanced NMDA receptor function in pain-related synaptic plasticity in rat amygdala neurones, J Physiol 564 (2005) 907–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Borsook D, Becerra L, CNS animal fMRI in pain and analgesia, Neurosci Biobehav Rev 35 (2011) 1125–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Borsook D, Edwards R, Elman I, Becerra L, Levine J, Pain and analgesia: the value of salience circuits, Prog Neurobiol 104 (2013) 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I, Reward deficiency and anti-reward in pain chronification, Neurosci Biobehav Rev 68 (2016) 282–297. [DOI] [PubMed] [Google Scholar]
- [24].Bouckaert F, De Winter FL, Emsell L, Dols A, Rhebergen D, Wampers M, Sunaert S, Stek M, Sienaert P, Vandenbulcke M, Grey matter volume increase following electroconvulsive therapy in patients with late life depression: a longitudinal MRI study, J Psychiatry Neurosci 41 (2016) 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bushnell MC, Ceko M, Low LA, Cognitive and emotional control of pain and its disruption in chronic pain, Nat Rev Neurosci 14 (2013) 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cacciaglia R, Pohlack ST, Flor H, Nees F, Dissociable roles for hippocampal and amygdalar volume in human fear conditioning, Brain Struct Funct 220 (2015) 2575–2586. [DOI] [PubMed] [Google Scholar]
- [27].Cardoso-Cruz H, Dourado M, Monteiro C, Galhardo V, Blockade of dopamine D2 receptors disrupts intrahippocampal connectivity and enhances pain-related working memory deficits in neuropathic pain rats, Eur J Pain (2018). [DOI] [PubMed] [Google Scholar]
- [28].Cardoso-Cruz H, Dourado M, Monteiro C, Matos MR, Galhardo V, Activation of dopaminergic D2/D3 receptors modulates dorsoventral connectivity in the hippocampus and reverses the impairment of working memory after nerve injury, J Neurosci 34 (2014) 5861–5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cardoso-Cruz H, Lima D, Galhardo V, Impaired spatial memory performance in a rat model of neuropathic pain is associated with reduced hippocampus-prefrontal cortex connectivity, J Neurosci 33 (2013) 2465–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cardoso-Cruz H, Lima D, Galhardo V, Instability of spatial encoding by CA1 hippocampal place cells after peripheral nerve injury, Eur J Neurosci 33 (2011) 2255–2264. [DOI] [PubMed] [Google Scholar]
- [31].Chan SW, Harmer CJ, Norbury R, O'Sullivan U, Goodwin GM, Portella MJ, Hippocampal volume in vulnerability and resilience to depression, J Affect Disord 189 (2016) 199–202. [DOI] [PubMed] [Google Scholar]
- [32].Chang CH, Maren S, Strain difference in the effect of infralimbic cortex lesions on fear extinction in rats, Behav Neurosci 124 (2010) 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen T, Taniguchi W, Chen QY, Tozaki-Saitoh H, Song Q, Liu RH, Koga K, Matsuda T, Kaito-Sugimura Y, Wang J, Li ZH, Lu YC, Inoue K, Tsuda M, Li YQ, Nakatsuka T, Zhuo M, Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex, Nat Commun 9 (2018) 1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cheriyan J, Kaushik MK, Ferreira AN, Sheets PL, Specific Targeting of the Basolateral Amygdala to Projectionally Defined Pyramidal Neurons in Prelimbic and Infralimbic Cortex, eNeuro 3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chung G, Kim CY, Yun YC, Yoon SH, Kim MH, Kim YK, Kim SJ, Upregulation of prefrontal metabotropic glutamate receptor 5 mediates neuropathic pain and negative mood symptoms after spinal nerve injury in rats, Sci Rep 7 (2017) 9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A, Encoding of conditioned fear in central amygdala inhibitory circuits, Nature 468 (2010) 277–282. [DOI] [PubMed] [Google Scholar]
- [37].Cordeiro Matos S, Zamfir M, Longo G, Ribeiro-da-Silva A, Seguela P, Noradrenergic fiber sprouting and altered transduction in neuropathic prefrontal cortex, Brain Struct Funct 223 (2018)1149–1164. [DOI] [PubMed] [Google Scholar]
- [38].Cordeiro Matos S, Zhang Z, Seguela P, Peripheral Neuropathy Induces HCN Channel Dysfunction in Pyramidal Neurons of the Medial Prefrontal Cortex, J Neurosci 35 (2015) 13244–13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].D'Hanis W, Linke R, Yilmazer-Hanke DM, Topography of thalamic and parabrachial calcitonin gene-related peptide (CGRP) immunoreactive neurons projecting to subnuclei of the amygdala and extended amygdala, J Comp Neurol 505 (2007) 268–291. [DOI] [PubMed] [Google Scholar]
- [40].Deacon RM, Bannerman DM, Rawlins JN, Anxiolytic effects of cytotoxic hippocampal lesions in rats, Behav Neurosci 116 (2002) 494–497. [DOI] [PubMed] [Google Scholar]
- [41].del Rey A, Apkarian AV, Martina M, Besedovsky HO, Chronic neuropathic pain-like behavior and brain-borne IL-1beta, Ann N Y Acad Sci 1262 (2012) 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].del Rey A, Yau HJ, Randolf A, Centeno MV, Wildmann J, Martina M, Besedovsky HO, Apkarian AV, Chronic neuropathic pain-like behavior correlates with IL-1beta expression and disrupts cytokine interactions in the hippocampus, Pain 152 (2011) 2827–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Duvarci S, Pare D, Amygdala microcircuits controlling learned fear, Neuron 82 (2014) 966–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Egli M, Koob GF, Edwards S, Alcohol dependence as a chronic pain disorder, Neurosci Biobehav Rev 36 (2012) 2179–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Eichenbaum H, Memory: Organization and Control, Annu Rev Psychol 68 (2017) 19–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C, Activation of the opioidergic descending pain control system underlies placebo analgesia, Neuron 63 (2009) 533–543. [DOI] [PubMed] [Google Scholar]
- [47].Elman I, Borsook D, Common Brain Mechanisms of Chronic Pain and Addiction, Neuron 89 (2016) 11–36. [DOI] [PubMed] [Google Scholar]
- [48].Elman I, Borsook D, Volkow ND, Pain and suicidality: insights from reward and addiction neuroscience, Prog Neurobiol 109 (2013) 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, Botta P, Bylund K, Muller C, Kovacevic A, Tovote P, Luthi A, A competitive inhibitory circuit for selection of active and passive fear responses, Nature 542 (2017) 96–100. [DOI] [PubMed] [Google Scholar]
- [50].Felix-Ortiz AC, Tye KM, Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior, J Neurosci 34 (2014) 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Femenia T, Gomez-Galan M, Lindskog M, Magara S, Dysfunctional hippocampal activity affects emotion and cognition in mood disorders, Brain Res 1476 (2012) 58–70. [DOI] [PubMed] [Google Scholar]
- [52].Floresco SB, The nucleus accumbens: an interface between cognition, emotion, and action, Annu Rev Psychol 66 (2015) 25–52. [DOI] [PubMed] [Google Scholar]
- [53].Fortin NJ, Agster KL, Eichenbaum HB, Critical role of the hippocampus in memory for sequences of events, Nat Neurosci 5 (2002) 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Fowler AK, Thompson J, Chen L, Dagda M, Dertien J, Dossou KS, Moaddel R, Bergeson SE, Kruman II, Differential sensitivity of prefrontal cortex and hippocampus to alcohol-induced toxicity, PLoS One 9 (2014) e106945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fu Y, Neugebauer V, Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior, J Neurosci 28 (2008) 3861–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Fuchs PN, Peng YB, Boyette-Davis JA, Uhelski ML, The anterior cingulate cortex and pain processing, Front Integr Neurosci 8 (2014) 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gabbott PL, Warner TA, Busby SJ, Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex, Neuroscience 139 (2006) 1039–1048. [DOI] [PubMed] [Google Scholar]
- [58].Giustino TF, Maren S, The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear, Front Behav Neurosci 9 (2015) 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gu L, Uhelski ML, Anand S, Romero-Ortega M, Kim YT, Fuchs PN, Mohanty SK, Pain inhibition by optogenetic activation of specific anterior cingulate cortical neurons, PLoS One 10 (2015)e0117746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Guerrini R, Salvadori S, Rizzi A, Regoli D, Calo G, Neurobiology, pharmacology, and medicinal chemistry of neuropeptide S and its receptor, Med Res Rev 30 (2010) 751–777. [DOI] [PubMed] [Google Scholar]
- [61].Gui WS, Wei X, Mai CL, Murugan M, Wu LJ, Xin WJ, Zhou LJ, Liu XG, Interleukin-1beta overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents, Mol Pain 12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Han JS, Adwanikar H, Li Z, Ji G, Neugebauer V, Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats, Mol Pain 6 (2010) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Han JS, Li W, Neugebauer V, Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior, J Neurosci 25 (2005) 10717–10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Han S, Soleiman MT, Soden ME, Zweifel LS, Palmiter RD, Elucidating an Affective Pain Circuit that Creates a Threat Memory, Cell 162 (2015) 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, Apkarian AV, Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits, Brain 136 (2013) 2751–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ, Genetic dissection of an amygdala microcircuit that gates conditioned fear, Nature 468 (2010) 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A, Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain, J Neurosci 28 (2008) 8074–8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Hoover WB, Vertes RP, Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat, Brain Struct Funct 212 (2007) 149–179. [DOI] [PubMed] [Google Scholar]
- [69].Ikeda R, Takahashi Y, Inoue K, Kato F, NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain, Pain 127 (2007) 161–172. [DOI] [PubMed] [Google Scholar]
- [70].Ito R, Hayen A, Opposing roles of nucleus accumbens core and shell dopamine in the modulation of limbic information processing, J Neurosci 31 (2011) 6001–6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jasmin L, Burkey AR, Granato A, Ohara PT, Rostral agranular insular cortex and pain areas of the central nervous system: a tract-tracing study in the rat, J Comp Neurol 468 (2004) 425–440. [DOI] [PubMed] [Google Scholar]
- [72].Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT, Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex, Nature 424 (2003) 316–320. [DOI] [PubMed] [Google Scholar]
- [73].Ji G, Neugebauer V, CB1 augments mGluR5 function in medial prefrontal cortical neurons to inhibit amygdala hyperactivity in an arthritis pain model, Eur J Neurosci 39 (2014) 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ji G, Neugebauer V, Modulation of medial prefrontal cortical activity using in vivo recordings and optogenetics, Mol Brain 5 (2012) 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V, Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation, J Neurosci 30 (2010) 5451–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Jin J, Maren S, Prefrontal-Hippocampal Interactions in Memory and Emotion, Front Syst Neurosci 9 (2015) 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Johansen JP, Fields HL, Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal, Nat Neurosci 7 (2004) 398–403. [DOI] [PubMed] [Google Scholar]
- [78].Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC, Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala, Neuron 59 (2008) 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kelly CJ, Huang M, Meltzer H, Martina M, Reduced Glutamatergic Currents and Dendritic Branching of Layer 5 Pyramidal Cells Contribute to Medial Prefrontal Cortex Deactivation in a Rat Model of Neuropathic Pain, Front Cell Neurosci 10 (2016) 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kesner RP, Gilbert PE, Barua LA, The role of the hippocampus in memory for the temporal order of a sequence of odors, Behav Neurosci 116 (2002) 286–290. [DOI] [PubMed] [Google Scholar]
- [81].Kim SC, Jo YS, Kim IH, Kim H, Choi JS, Lack of medial prefrontal cortex activation underlies the immediate extinction deficit, J Neurosci 30 (2010) 832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kiritoshi T, Ji G, Neugebauer V, Rescue of Impaired mGluR5-Driven Endocannabinoid Signaling Restores Prefrontal Cortical Output to Inhibit Pain in Arthritic Rats, J Neurosci 36 (2016) 837–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Klavir O, Prigge M, Sarel A, Paz R, Yizhar O, Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex, Nat Neurosci 20 (2017) 836–844. [DOI] [PubMed] [Google Scholar]
- [84].Krettek JE, Price JL, Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat, J Comp Neurol 172 (1977) 687–722. [DOI] [PubMed] [Google Scholar]
- [85].Kuo CC, Yen CT, Comparison of anterior cingulate and primary somatosensory neuronal responses to noxious laser-heat stimuli in conscious, behaving rats, J Neurophysiol 94 (2005) 1825–1836. [DOI] [PubMed] [Google Scholar]
- [86].LaBuda CJ, Fuchs PN, Low dose aspirin attenuates escape/avoidance behavior, but does not reduce mechanical hyperalgesia in a rodent model of inflammatory pain, Neurosci Lett 304 (2001) 137–140. [DOI] [PubMed] [Google Scholar]
- [87].LaBuda CJ, Fuchs PN, Morphine and gabapentin decrease mechanical hyperalgesia and escape/avoidance behavior in a rat model of neuropathic pain, Neurosci Lett 290 (2000) 137–140. [DOI] [PubMed] [Google Scholar]
- [88].LaGraize SC, Borzan J, Peng YB, Fuchs PN, Selective regulation of pain affect following activation of the opioid anterior cingulate cortex system, Exp Neurol 197 (2006) 22–30. [DOI] [PubMed] [Google Scholar]
- [89].LaGraize SC, Labuda CJ, Rutledge MA, Jackson RL, Fuchs PN, Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain, Exp Neurol 188 (2004) 139–148. [DOI] [PubMed] [Google Scholar]
- [90].Lee M, Manders TR, Eberle SE, Su C, D'Amour J, Yang R, Lin HY, Deisseroth K, Froemke RC, Wang J, Activation of corticostriatal circuitry relieves chronic neuropathic pain, J Neurosci 35 (2015) 5247–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Leonard SK, Ring RH, Immunohistochemical localization of the neuropeptide S receptor in the rat central nervous system, Neuroscience 172 (2011) 153–163. [DOI] [PubMed] [Google Scholar]
- [92].Li W, Chang M, Peng YL, Gao YH, Zhang JN, Han RW, Wang R, Neuropeptide S produces antinociceptive effects at the supraspinal level in mice, Regul Pept 156 (2009) 90–95. [DOI] [PubMed] [Google Scholar]
- [93].Little JP, Carter AG, Subcellular synaptic connectivity of layer 2 pyramidal neurons in the medial prefrontal cortex, J Neurosci 32 (2012) 12808–12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Little JP, Carter AG, Synaptic mechanisms underlying strong reciprocal connectivity between the medial prefrontal cortex and basolateral amygdala, J Neurosci 33 (2013) 15333–15342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W, Cui R, The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex, Neural Plast 2017 (2017) 6871089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lowe AS, Beech JS, Williams SC, Small animal, whole brain fMRI: innocuous and nociceptive forepaw stimulation, Neuroimage 35 (2007) 719–728. [DOI] [PubMed] [Google Scholar]
- [97].Mansour AR, Baliki MN, Huang L, Torbey S, Herrmann KM, Schnitzer TJ, Apkarian AV, Brain white matter structural properties predict transition to chronic pain, Pain 154 (2013) 2160–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mariano TY, Bannerman DM, McHugh SB, Preston TJ, Rudebeck PH, Rudebeck SR, Rawlins JN, Walton ME, Rushworth MF, Baxter MG, Campbell TG, Impulsive choice in hippocampal but not orbitofrontal cortex-lesioned rats on a nonspatial decision-making maze task, Eur J Neurosci 30 (2009) 472–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Meck WH, Church RM, Olton DS, Hippocampus, time, and memory, Behav Neurosci 98 (1984) 3–22. [DOI] [PubMed] [Google Scholar]
- [100].Medina G, Ji G, Gregoire S, Neugebauer V, Nasal application of neuropeptide S inhibits arthritis pain-related behaviors through an action in the amygdala, Mol Pain 10 (2014) 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M, Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain, Proc Natl Acad Sci U S A 106 (2009) 2423–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Mogenson GJ, Jones DL, Yim CY, From motivation to action: functional interface between the limbic system and the motor system, Prog Neurobiol 14 (1980) 69–97. [DOI] [PubMed] [Google Scholar]
- [103].Mutso AA, Radzicki D, Baliki MN, Huang L, Banisadr G, Centeno MV, Radulovic J, Martina M, Miller RJ, Apkarian AV, Abnormalities in hippocampal functioning with persistent pain, J Neurosci 32 (2012) 5747–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Nakao A, Takahashi Y, Nagase M, Ikeda R, Kato F, Role of capsaicin-sensitive C-fiber afferents in neuropathic pain-induced synaptic potentiation in the nociceptive amygdala, Mol Pain 8 (2012) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Navratilova E, Atcherley CW, Porreca F, Brain Circuits Encoding Reward from Pain Relief, Trends Neurosci 38 (2015) 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Navratilova E, Porreca F, Reward and motivation in pain and pain relief, Nat Neurosci 17 (2014) 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Navratilova E, Xie JY, Meske D, Qu C, Morimura K, Okun A, Arakawa N, Ossipov M, Fields HL, Porreca F, Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain, J Neurosci 35 (2015) 7264–7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Neugebauer V, Amygdala pain mechanisms, Handb Exp Pharmacol 227 (2015) 261–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Neugebauer V, Galhardo V, Maione S, Mackey SC, Forebrain pain mechanisms, Brain Res Rev 60 (2009) 226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Neugebauer V, Li W, Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain, J Neurophysiol 89 (2003) 716–727. [DOI] [PubMed] [Google Scholar]
- [111].Neugebauer V, Li W, Bird GC, Bhave G, Gereau R.W.t. , Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5, J Neurosci 23 (2003) 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Neugebauer V, Li W, Bird GC, Han JS, The amygdala and persistent pain, Neuroscientist 10 (2004) 221–234. [DOI] [PubMed] [Google Scholar]
- [113].Nifosi F, Toffanin T, Follador H, Zonta F, Padovan G, Pigato G, Carollo C, Ermani M, Amista P, Perini GI, Reduced right posterior hippocampal volume in women with recurrent familial pure depressive disorder, Psychiatry Res 184 (2010) 23–28. [DOI] [PubMed] [Google Scholar]
- [114].Okutsu Y, Takahashi Y, Nagase M, Shinohara K, Ikeda R, Kato F, Potentiation of NMDA receptor-mediated synaptic transmission at the parabrachial-central amygdala synapses by CGRP in mice, Mol Pain 13 (2017) 1744806917709201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Ongur D, Price JL, The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans, Cereb Cortex 10 (2000) 206–219. [DOI] [PubMed] [Google Scholar]
- [116].Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, Spellman TJ, Gordon JA, Direct Ventral Hippocampal-Prefrontal Input Is Required for Anxiety-Related Neural Activity and Behavior, Neuron 89 (2016) 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Pape HC, Jungling K, Seidenbecher T, Lesting J, Reinscheid RK, Neuropeptide S: a transmitter system in the brain regulating fear and anxiety, Neuropharmacology 58 (2010) 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Pape HC, Pare D, Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear, Physiol Rev 90 (2010) 419–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Huettl P, Dwoskin LP, Bardo MT, Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions, Brain Res Rev 65 (2011) 124–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Phillips RG, LeDoux JE, Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning, Behav Neurosci 106 (1992) 274–285. [DOI] [PubMed] [Google Scholar]
- [121].Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A, Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat, J Comp Neurol 403 (1999) 229–260. [PubMed] [Google Scholar]
- [122].Preston AR, Eichenbaum H, Interplay of hippocampus and prefrontal cortex in memory, Curr Biol 23 (2013) R764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Radzicki D, Pollema-Mays SL, Sanz-Clemente A, Martina M, Loss of M1 Receptor Dependent Cholinergic Excitation Contributes to mPFC Deactivation in Neuropathic Pain, J Neurosci 37 (2017) 2292–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ren LY, Lu ZM, Liu MG, Yu YQ, Li Z, Shang GW, Chen J, Distinct roles of the anterior cingulate cortex in spinal and supraspinal bee venom-induced pain behaviors, Neuroscience 153 (2008) 268–278. [DOI] [PubMed] [Google Scholar]
- [125].Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, Kondapalli J, Apkarian AV, Martina M, Surmeier DJ, The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain, Nat Neurosci 19 (2016) 220–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Ren W, Kiritoshi T, Gregoire S, Ji G, Guerrini R, Calo G, Neugebauer V, Neuropeptide S: a novel regulator of pain-related amygdala plasticity and behaviors, J Neurophysiol 110 (2013) 1765–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Ren W, Neugebauer V, Pain-related increase of excitatory transmission and decrease of inhibitory transmission in the central nucleus of the amygdala are mediated by mGluR1, Mol Pain 6 (2010) 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, Xin WJ, Wei XH, Wang J, Zhu HQ, Wu CY, Qin ZH, Liu G, Liu XG, Peripheral nerve injury leads to working memory deficits and dysfunction of the hippocampus by upregulation of TNF-alpha in rodents, Neuropsychopharmacology 36 (2011) 979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Salgado S, Kaplitt MG, The Nucleus Accumbens: A Comprehensive Review, Stereotact Funct Neurosurg 93 (2015) 75–93. [DOI] [PubMed] [Google Scholar]
- [130].Sanders MJ, Wiltgen BJ, Fanselow MS, The place of the hippocampus in fear conditioning, Eur J Pharmacol 463 (2003) 217–223. [DOI] [PubMed] [Google Scholar]
- [131].Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R, Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants, Science 301 (2003) 805–809. [DOI] [PubMed] [Google Scholar]
- [132].Scoville WB, Milner B, Loss of recent memory after bilateral hippocampal lesions, J Neurol Neurosurg Psychiatry 20 (1957) 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Shao XM, Sun J, Jiang YL, Liu BY, Shen Z, Fang F, Du JY, Wu YY, Wang JL, Fang JQ, Inhibition of the cAMP/PKA/CREB Pathway Contributes to the Analgesic Effects of Electroacupuncture in the Anterior Cingulate Cortex in a Rat Pain Memory Model, Neural Plast 2016 (2016)5320641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Sierra-Mercado D, Padilla-Coreano N, Quirk GJ, Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear, Neuropsychopharmacology 36 (2011) 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D, The human amygdala and pain: evidence from neuroimaging, Hum Brain Mapp 35 (2014) 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Simons LE, Pielech M, Erpelding N, Linnman C, Moulton E, Sava S, Lebel A, Serrano P, Sethna N, Berde C, Becerra L, Borsook D, The responsive amygdala: treatment-induced alterations in functional connectivity in pediatric complex regional pain syndrome, Pain 155 (2014) 1727–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC, Roles of the insular cortex in the modulation of pain: insights from brain lesions, J Neurosci 29 (2009) 2684–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Strange BA, Witter MP, Lein ES, Moser EI, Functional organization of the hippocampal longitudinal axis, Nat Rev Neurosci 15 (2014) 655–669. [DOI] [PubMed] [Google Scholar]
- [139].Sugimura YK, Takahashi Y, Watabe AM, Kato F, Synaptic and network consequences of monosynaptic nociceptive inputs of parabrachial nucleus origin in the central amygdala, J Neurophysiol 115 (2016) 2721–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Sun H, Neugebauer V, mGluR1, but not mGluR5, activates feed-forward inhibition in the medial prefrontal cortex to impair decision making, J Neurophysiol 106 (2011) 960–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Sun J, Shao XM, Fang F, Shen Z, Wu YY, Fang JQ, Electroacupuncture alleviates retrieval of pain memory and its effect on phosphorylation of cAMP response element-binding protein in anterior cingulate cortex in rats, Behav Brain Funct 11 (2015) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Swanson LW, Wyss JM, Cowan WM, An autoradiographic study of the organization of intrahippocampal association pathways in the rat, J Comp Neurol 181 (1978) 681–715. [DOI] [PubMed] [Google Scholar]
- [143].Tajerian M, Leu D, Zou Y, Sahbaie P, Li W, Khan H, Hsu V, Kingery W, Huang TT, Becerra L, Clark JD, Brain neuroplastic changes accompany anxiety and memory deficits in a model of complex regional pain syndrome, Anesthesiology 121 (2014) 852–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Thompson JM, Neugebauer V, Amygdala Plasticity and Pain, Pain Res Manag 2017 (2017) 8296501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Uhelski ML, Morris-Bobzean SA, Dennis TS, Perrotti LI, Fuchs PN, Evaluating underlying neuronal activity associated with escape/avoidance behavior in response to noxious stimulation in adult rats, Brain Res 1433 (2012) 56–61. [DOI] [PubMed] [Google Scholar]
- [146].Vachon-Presseau E, Centeno MV, Ren W, Berger SE, Tetreault P, Ghantous M, Baria A, Farmer M, Baliki MN, Schnitzer TJ, Apkarian AV, The Emotional Brain as a Predictor and Amplifier of Chronic Pain, J Dent Res 95 (2016) 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Vachon-Presseau E, Tetreault P, Petre B, Huang L, Berger SE, Torbey S, Baria AT, Mansour AR, Hashmi JA, Griffith JW, Comasco E, Schnitzer TJ, Baliki MN, Apkarian AV, Corticolimbic anatomical characteristics predetermine risk for chronic pain, Brain 139 (2016) 1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Vasic V, Schmidt MH, Resilience and Vulnerability to Pain and Inflammation in the Hippocampus, Int J Mol Sci 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Veinante P, Yalcin I, Barrot M, The amygdala between sensation and affect: a role in pain, J Mol Psychiatry 1 (2013) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Villemure C, Bushnell MC, Mood influences supraspinal pain processing separately from attention, J Neurosci 29 (2009) 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Vogt BA, Paxinos G, Cytoarchitecture of mouse and rat cingulate cortex with human homologies, Brain Struct Funct 219 (2014) 185–192. [DOI] [PubMed] [Google Scholar]
- [152].Wang GQ, Cen C, Li C, Cao S, Wang N, Zhou Z, Liu XM, Xu Y, Tian NX, Zhang Y, Wang J, Wang LP, Wang Y, Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety, Nat Commun 6 (2015) 7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Wang JY, Huang J, Chang JY, Woodward DJ, Luo F, Morphine modulation of pain processing in medial and lateral pain pathways, Mol Pain 5 (2009) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Williams DJ, Crossman AR, Slater P, The efferent projections of the nucleus accumbens in the rat, Brain Res 130 (1977) 217–227. [DOI] [PubMed] [Google Scholar]
- [155].Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS, Context fear learning in the absence of the hippocampus, J Neurosci 26 (2006) 5484–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Woodhams SG, Chapman V, Finn DP, Hohmann AG, Neugebauer V, The cannabinoid system and pain, Neuropharmacology 124 (2017) 105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Xie JY, Qu C, Patwardhan A, Ossipov MH, Navratilova E, Becerra L, Borsook D, Porreca F, Activation of mesocorticolimbic reward circuits for assessment of relief of ongoing pain: a potential biomarker of efficacy, Pain 155 (2014) 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Xu W, Sudhof TC, A neural circuit for memory specificity and generalization, Science 339 (2013) 1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK, Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain, J Comp Neurol 500 (2007) 84–102. [DOI] [PubMed] [Google Scholar]
- [160].Yamamura H, Iwata K, Tsuboi Y, Toda K, Kitajima K, Shimizu N, Nomura H, Hibiya J, Fujita S, Sumino R, Morphological and electrophysiological properties of ACCx nociceptive neurons in rats, Brain Res 735 (1996) 83–92. [DOI] [PubMed] [Google Scholar]
- [161].Zahm DS, Heimer L, Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell, J Comp Neurol 327 (1993) 220–232. [DOI] [PubMed] [Google Scholar]
- [162].Zhang WN, Bast T, Feldon J, The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus, Behav Brain Res 126 (2001) 159–174. [DOI] [PubMed] [Google Scholar]
- [163].Zhang Z, Gadotti VM, Chen L, Souza IA, Stemkowski PL, Zamponi GW, Role of Prelimbic GABAergic Circuits in Sensory and Emotional Aspects of Neuropathic Pain, Cell Rep 12 (2015) 752–759. [DOI] [PubMed] [Google Scholar]
- [164].Zhuo M, Cortical excitation and chronic pain, Trends Neurosci 31 (2008) 199–207. [DOI] [PubMed] [Google Scholar]