Abstract
Fluoride ingestion has been linked to changes in behavior in mice and rats, related to dose, sex of the animal, and the timing of exposure. Previous studies have shown the behavior of female rats to be most affected by postnatal fluoride exposure, and in this study we determined the effects of postnatal fluoride exposure on anxiety related behavior and serotonin. Mice given 50 ppm fluoride in drinking water had increased entries in the open arms of the elevated plus maze, suggesting reduced anxiety. Both peripheral and central serotonin was increased in the fluoride treated mice. In a cohort of children drinking water containing 2.5 ppm fluoride, serum serotonin was also increased as compared to controls. The mechanisms by which fluoride results in an increase peripheral and central serotonin are not well understood, but warrant further study, as these effects may also be relevant to prenatal fluoride related changes in behavior in both mice and humans.
Introduction
Fluoride is a highly electronegative anion, known for its ability to increase the rate of hydroxyapatite formation and remineralization of carious lesions in teeth (1). This property of fluoride has led to its highly effective use in the prevention of dental caries. However, fluoride is reported to have other effects in cells and tissues that are evidenced by changes in behavior in animals (2–4), and IQ in humans (5–7).
The effects of fluoride are influenced by experimental animal strain, dose, timing and sex (2,8,9). Mullinex et al. showed that the behavior of male Sprague Dawley rats was more affected by prenatal fluoride exposure (0.13 mg/kg), whereas female rat behavior was more affected by postnatal fluoride exposure (100 mg/l in drinking water) (2). At low concentrations of fluoride in water (10 mg/l,) Bartos et al. showed that female Wistar rats exposed to fluoride during gestation and lactation, had reduced anxiety related behavior beginning at postnatal day 45, whereas similar reductions in anxiety in male rats were not evident before postnatal day 90 (4). McPherson showed no anxiety related behaviors in Long-Evans hooded male rats given 10 or 20 mg/l fluoride in drinking water beginning at gestation through postnatal day 60 (10). However, it is not known whether prolonged fluoride exposure beyond day 60 could have resulted in anxiety related changes in the male Long-Evans rats similar to that found in Bartos’ study (4).
Human epidemiological studies also indicate that fluoride can have neurotoxic effects (5,7,11). However, the mechanisms for the cellular and behavioral effects of fluoride remain poorly understood. High levels of fluoride can increase ER stress and oxidative stress (12) possibly related to reduced oxidative stress response (13,14). Male Wistar rats given 100 mg/L fluoride in drinking water for 30 days have increased serotonin in the hippocampus and cortex (15). Bartos et. al showed reduced synthesis of nAChR in brains of female Wistar rats exposed to from 10 mg/L to 30 mg/L fluoride in drinking water through pregnancy, lactation and continuing postnatally for 90 days (13). Similarly, Reddy et al. reported that male Wistar rats given 0.18, 20, 60 and 100 mg/L fluoride in drinking water for 90 days showed dose-dependent increases in the levels of epinephrine, histamine, serotonin and glutamate and a corresponding decrease in the levels of norepinephrine, acetylcholine and dopamine in brain tissues (14).
These studies suggest the possibility that the effects of fluoride on behavior are related to alterations in neurochemicals. In this study, we used female C57BL/6J mice to determine the effect of postnatal fluoride in drinking water on anxiety related behaviors, and serotonin in serum and brain. We used C57BL/6J mice, because this strain is well characterized and does not have known behavioral alterations, and used females, because previous studies in rats show that females are most sensitive to the postnatal neurotoxic effects of fluoride (2).
The fluoride treated mice were given a single fluoride dose, 50mg/L in drinking water ad libitum. This dose is high enough to result in obvious dental fluorosis without overt signs of acute toxicity. To determine the relevance of our finding that serum serotonin concentrations were increased in fluoride treated mice, we measured serum serotonin levels in a cohort of children who drank water containing an average of 2.5 mg/L fluoride, as compared to children drinking the same water supplemented with fluoride free bottled water. Similar to mice, increased fluoride exposure correlated to increased levels of serum serotonin in children.
Materials and Methods
Animals
Three-week-old C57BL/6J female mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were randomly assigned to either experimental (treatment) or control groups and housed 5 mice per cage. All animals were maintained in the UCSF animal care facility, with a light cycle of 12 hrs. (light (7am-7pm) and 12 hrs. dark (7pm-next day 7am)) and were fed a standard rodent diet. The animal care facility is a barrier facility, accredited by Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All procedures are conducted under the approval of UCSF Animal Care and Use Committee, and animals are checked daily for signs of distress and discomfort, with necessary care administered by a veterinarian.
Water was given via plastic water bottles to avoid a loss of fluoride through absorption onto glass surfaces. Control mice were given deionized water and treatment groups were given deionized drinking water supplemented with 50 mg/L sodium fluoride (50ppmF, Sigma-Aldrich, St. Louis, MO) beginning at 21 days of age, and continuing for up to 42 weeks. The volume of water consumed was measured once per week.
One group of 30 mice (group 1), including 15 control and 15 fluoride treated mice were maintained for 7 weeks and then tested for anxiety related behaviors using the open field and elevated plus maze. A second group of 40 mice (20 control and 20 fluoride treated), were also tested in the open field and elevated plus maze after 7 weeks, retested at 23 weeks, and maintained for up to 42 weeks. At 12 week and 42 weeks brain fluoride was measured in fluoride treated and control mice. Serum serotonin was measured at 12, 20, and 42 weeks, and brain serotonin was compared by immunohistochemistry at after 42 weeks.
Behavioral testing
All behavior tests were done at the Neurobehavioral Core for Rehabilitation Research at the University of California, San Francisco (UCSF). One day prior to the behavior tests the cages were transferred to the test room, to allow the mice to adapt to the testing facility environment.
Open Field (OF)
The behavior of control and fluoride exposed mice was compared in the groups 1 (15 mice per treatment) and 2 (20 mice per treatment) in the open field test after 7 weeks. Each mouse was placed in the center of a square arena made of plastic (40 cm X 40 cm, Kinder Scientific Company, Poway, CA) and was allowed to explore the arena freely for 10 mins under lighted conditions. The mouse’s behavior was recorded using MMKS Motor Monitor tracking system (Kinder Scientific Company, Poway, CA). The distance covered during the test session was analyzed as a parameter for general activity levels, and the time spent in the center of the open field (a rectangle 20 cm from sidewalls) was determined to assess anxiety levels. Increases in center time with concomitant decreases in periphery time are considered an index of reductions in anxiety.
Elevated Plus Maze (EPM)
Following the open field test, control and fluoride exposed mice were tested in the EPM (PM2000, Kinder scientific company, Poway, CA). The EPM is a plus-shaped maze constructed of black Plexiglas with two opposing open arms and two closed arms (Mouse arena dimensions: Each arm is 5 cm wide and 40 cm long, intersection is 5 cm by 5 cm, closed wall is 15 cm high). The maze was set at an elevation of 80 cm above the floor. In lighted conditions, the mouse was placed at the central platform and allowed to explore the maze for 10 mins. Time spent, distance traveled, and number entries into the open arm were recorded. All data were recorded using MMKS Motor Monitor Ver1.0 tracking system (Kinder scientific company, Poway, CA), with the assumption that a more anxious mouse would spend less time in the open arms of the arena. After 23 weeks, 10 control and 10 fluoride treated mice from group 2 were retested in the EPM.
Social behaviors
As part of our normal colony management, we checked for wounds, missing fur, whisker status, etc. in all mice. Group 2 mice were photographed after 25 weeks of treatment to document increased wounding and hair loss in the control as compared to the fluoride treated mice. The wounding and hair loss of these mice was monitored daily by a veterinarian, who tested for parasites, fugus and bacteria. Pathogens were not identified, and wounded areas were treated first with 0.2% Nolvasan solution, followed by BNP triple antibiotics ointment (ointment containing acitracin, neomycin, and polymyxin B).
Brain fluoride concentrations.
Whole brains were isolated from 3 control and 3 fluoride treated mice after 12 weeks treatment and from 5 control and 5 fluoride treated mice after 42 weeks of treatment. Following sacrifice, the brain tissue was immediately removed, sectioned in half, weighed, and homogenized in 500μl deionized water. Fluoride concentrations in serum and mouse brain homogenates were determined following acid microdiffusion, based on the method previously described by Taves (16). Briefly, 2 mls of hexamethyldisiloxane presaturated 6 N HCl was placed in a plastic petri dish with a test tube cap glued on it. The test tube cap was filled with 100 μl of 1.65 M NaOH. The petri dish was sealed with a lid with a small hole. Serum or homogenized brain tissue was added to the HCl through the hole, which was then rapidly sealed with Vaseline and paraffin. Fluoride was allowed to diffuse from the acidified samples as form of HF and then trap into NaOH for 22 hrs. The NaOH and trapped fluoride was dried in 65°C oven, and then was reconstituted to neutral pH with 500 μl of 0.66 M acetic acid. A fluoride ion-specific electrode (Mettler Toledo, Columbus, OH) was used to measure the fluoride concentration of the buffered solution, based on the measurements of known standards.
Serum serotonin
Mice:
Blood was collected from control and fluoride treated mice after 12, 20, and 42 wks of treatment. Mice were anesthetized with 240 mg/kg tribromoethanol (Sigma-Aldrich, St. Louis, MO). Blood was collected by cardiac puncture and transferred to serum separator tube (BD microtainer Cat#365967). After 15 mins on ice, the samples were centrifuged for 5 mins at 12000 rpm speed and the serum was stored at −80°C until assayed for serotonin.
Human:
Following IRB approval by the ethics committee of Trakya University and Marmara University, consent was obtained and serum was collected from 31 children ages 7–13 year of age. Twenty nine children lived in the village of Hanliyenice, Turkey, and drank well water containing 2.5 mg/L fluoride. Twenty six children exclusively drank well water containing, and 3 children substituted their drinking water with fluoride free bottled water. Serum was obtained from an additional 2 children who drank from a low fluoride water source (<0.5 ppm fluoride) in the town where all of the children attended school. All samples were collected in the morning, and following collection, the serum was separated from whole blood, and stored at −80° until assayed for serotonin
Serum serotonin concentrations were measured using a serotonin ELISA Kit (ab133053, Abcam, CA, USA) according to the manufacturer’s instructions.
Serotonin immunostaining of mouse brain
After 42 wks treatment the remaining Group 2 mice were anesthetized with 240 mg/kg tribromoethanol, and perfuse-fixed with PBS and 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO). Brains were dissected and post-fixed in 4% paraformaldehyde for 24 hrs, followed by dehydration in 30% sucrose for 3 days. Dehydrated brains were embedded in O.C.T Compound (Sakura Finetek USA, Ins, Torrance, CA) and then stored at −80°C. Embedded and frozen brains were sectioned coronally at a thickness at 16 μm with Leica CM3050 cryostat microtome (Leica Microsystems Inc, Buffalo Grove, IL) at −14°C.
To compare the gross brain morphology, cryosections were defrosted and air-dried at room temperature for 2 hrs. After rehydration with 100%, 95% and 70 % ethanol for 3 mins individually, the sections were equilibrated with PBS and stained with 0.2% cresyl violet/sodium acetate solution for 3 mins. Afterward, the sections were rinsed twice with water for 3 mins and dehydrated with 70%, 95% and 100% ethanol. After hyalinization with Xylene and mounting, the sections were photographed with a Nikon Eclipse 300 microscope (Compix Inc, Sewickley, PA).
Cryosections from 2 control and 2 fluoride treated mice (6 sections from each animal) were immunostained for serotonin by first blocking with 3% BSA for 2 hrs, followed by overnight incubation at 4°C with rat monoclonal anti-serotonin antibody (Abcam, Cambridge, MA). The following day, after one wash with 1.6% NaCl/0.04% Tween/PBS, and then three washes with 0.2% Tween/PBS, sections were incubated with AlexaFluor 595 goat anti-rat antibody (Santa Cruz Biotechnology). Nuclei were stained with Hoechst (Life technologies, Grand Island, NY) for 5 mins and sections mounted with ProLong Diamond Antifade Mounting medium (Life technologies, Grand Island, NY). The sections were photographed with Leica TCS SP5 Spectral Confocal Microscope (Leica Microsystems Inc, Buffalo Grove, IL).
Statistical Analysis:
Weight and water consumption, fluoride concentrations in the brain tissues, and serum serotonin levels in control and fluoride treated mice were compared by ANOVA followed by Bonferroni post-tests. Individual behaviors were compared by Student’s t test.
Results:
Prolonged fluoride treatment resulted in reduced weight gain over time.
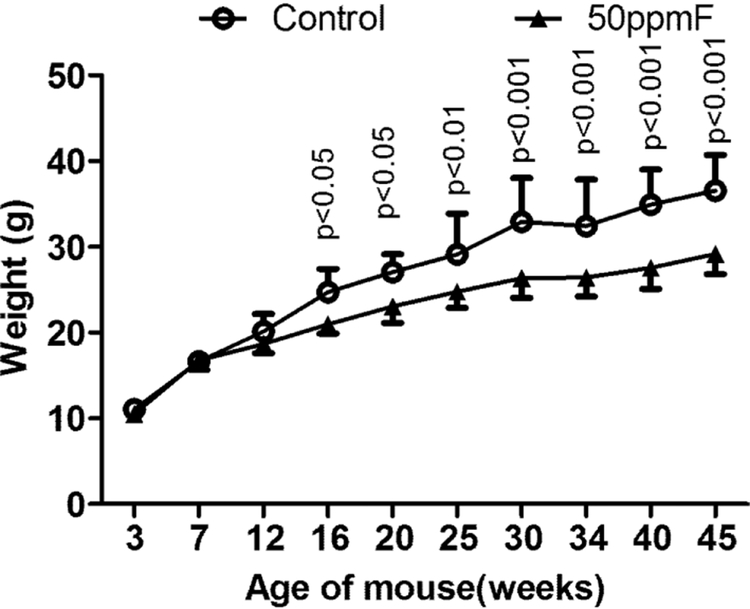
Mice exposed to 50 mg/L fluoride in drinking water weighed significantly less beginning at 16 weeks (wks) of age (13 wks of fluoride exposure) (Fig 1) A reduction in the rate of weight gain of the fluoride-exposed mice continued up until the end of the study when the animals were 45 wks of age.
Fig 1.
After 12 weeks, fluoride treated mice weighed significantly less than the control mice. Value=Mean ± SD; n=10 per group P values determined by two-way ANOVA.
Water consumption of control and fluoride groups was monitored in a subset of mice. In wk 1, the fluoride treated mice drank significantly less water, with no significant differences in subsequent weeks.
Activity of mice in the open field test was unchanged at 7 weeks post fluoride treatment.
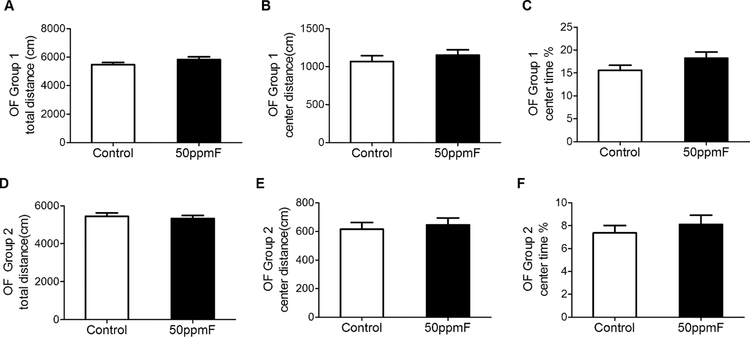
Open field testing was done after 7 wks in both groups. It is interesting to note that though the total distance traveled in both groups was the same, mice in group 1 spent more time in the center than group 2 mice. However in both groups, there were no significant differences between control and fluoride treated mice (Fig 2).
Fig 2.
The open field test shows no differences between control and fluoride treated mice after 7 wks treatment in duplicate tests for A) in the total distance traveled; B) the distance traveled in the center; and C) the % time spent in the center. n=15 for group 1; n=20 for group 2 mice; Value= Mean ± SEM; Student’s t-test showed no significant differences between groups.
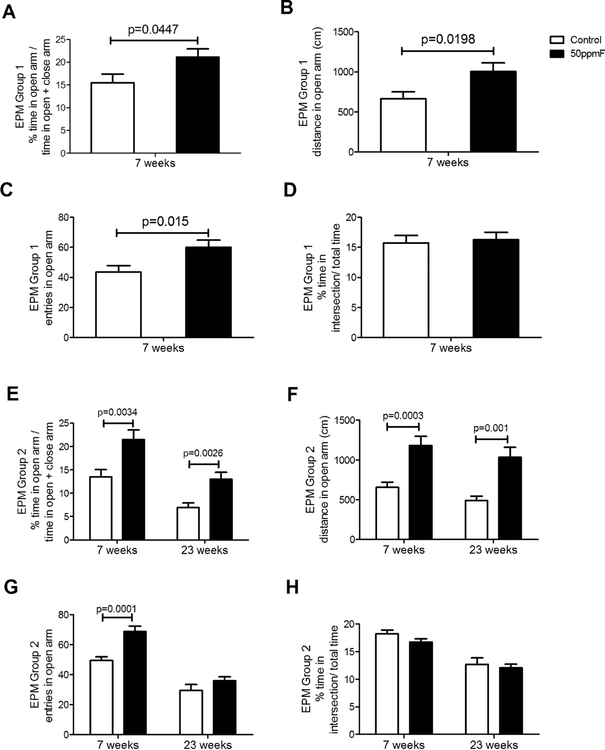
Fluoride treated mice spent more time in the open arms of the EPM.
After 7 wks of treatment, fluoride exposed mice in both group 1 and group 2 spent significantly more time in the open arms, traveled a greater distance in the open arms, and entered the open arms at higher frequency as compared to closed arms (see Fig 3). There were no differences in the time spent at intersection in both the treatment groups at both time points, suggesting that decision making ability was not altered by fluoride treatment. When group 2 mice were retested after an additional 16 weeks fluoride exposure (total of 23 weeks, the results were similar with significantly more time and distance spent in the open arms by the fluoride treated as compared to control mice.
Fig 3.
In the elevated plus maze (EPM) both groups of fluoride treated mice spent significantly more time in the open arms (A,E) and traveled a greater distance in the open arms (B,F). After 7 wks fluoride exposure, fluoride treated mice had more open arm entries (C, G); while mice retested after 23 wks fluoride exposure did not show differences in the percent time spend in the intersection of the open arms). Groups 1 (n=15); Group 2 (n=20) at 7 wks; Group 2 (n= 10) at 23 wks; Value=Mean+ SEM; comparisons done using Student’s t-test.
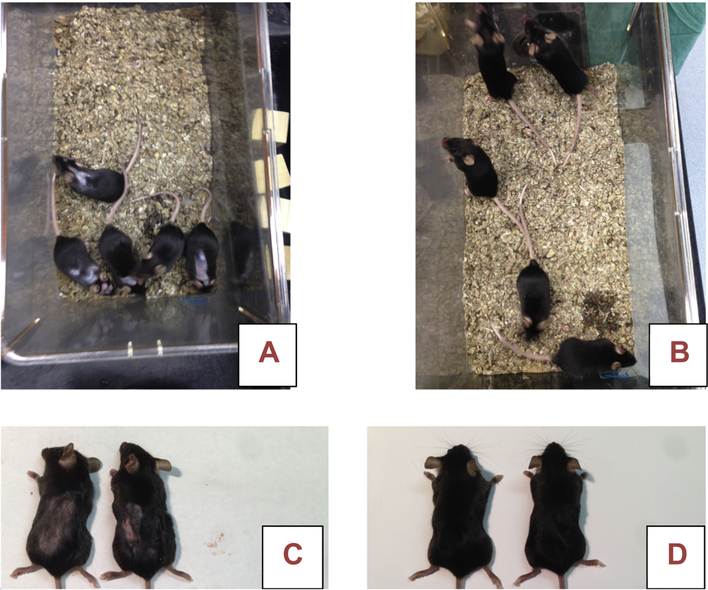
Control mice had increased hair loss, and wounds, as compared to fluoride treated mice
Hair loss and increased evidence of wounding was observed in the control mice, generally beginning after 10 weeks of fluoride exposure (see Fig 4).
Fig 4.
Photos of 5 mice from one cage each of Group 2 control (A,C) and fluoride treated (B,D) mice after 25 weeks of treatment. Note signs of localized wounding and hair loss in control mice as compared to fluoride treated mice
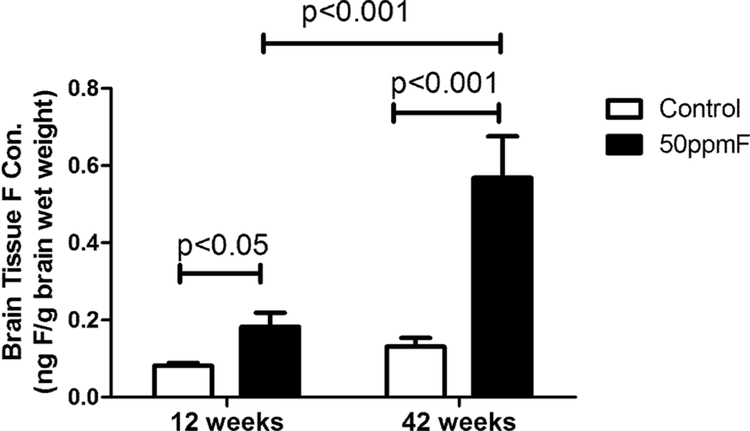
Brain fluoride concentrations increased with time of exposure.
Fluoride concentrations in brain were significantly higher in the fluoride exposed animals as compared to 12 wk controls (see Fig 5). After treatment, brain fluoride levels in the treated mice (0.18 ± 0.02 ng/g wet weight) were 2-fold that of controls (0.08 ± 0.003 ng/g wet weight), and after 42 wks, fluoride levels in the treated group (0.57 ± 0.09 ng/g wet weight) increased to 4-fold as compared to controls (0.13 ± 0.02 ng/g wet weight).
Fig 5.
Fluoride exposure increased the fluoride concentration in brain tissues. Fluoride levels of the treatment group were significantly higher than controls after both 12 and 42 weeks. Value= Mean ± SD, n=6 per group; two-way ANOVA
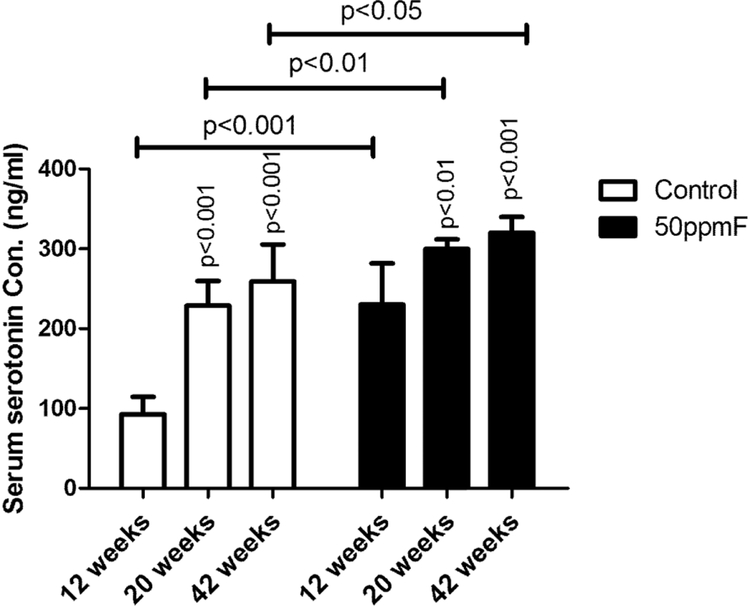
Serotonin was increased significantly with higher levels of fluoride exposure.
Serum serotonin levels in fluoride treated mice were significantly increased as compared to controls after 12, 20 and 42 wks of treatment. In controls, serum serotonin also increased significantly at 20 and 42 wks, as compared to 12 wks (see Fig 6). The greatest difference in serum serotonin levels in fluoride treated as compared to control mice, was in the youngest group of animal measured, after 12 weeks fluoride treatment.
Fig 6.
Serum serotonin concentrations were significantly increased as compare to controls at 12, 20, and 42 weeks. However there was not a significant increase in serum serotonin at 42 weeks as compared to 20 weeks. Value=Mean ± SD; n=6 per group; two-way ANOVA
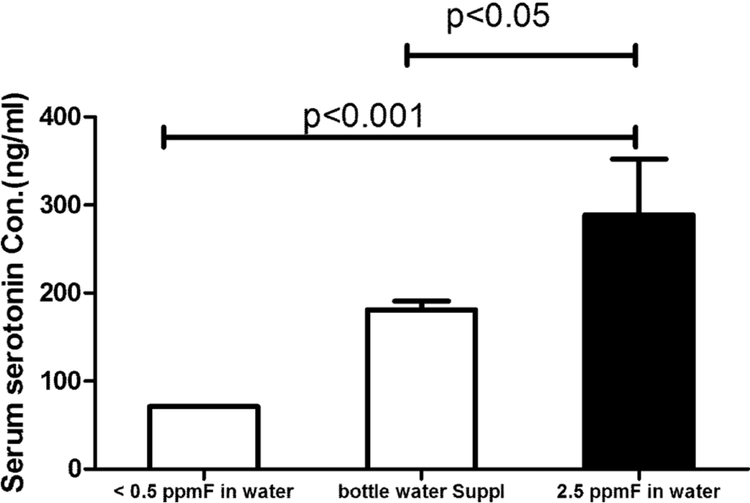
Serum serotonin levels in children drinking fluoride containing water were significantly higher than that of children who substituted their drinking water with low fluoride containing bottled water.
Serum serotonin levels from the 26 children who drank only well water averaged 267 ± 45 ng/ml (mean ± SD), with no statistically significant difference in boys as compared to girls. The 3 children who substituted their drinking water with fluoride-free bottled water had significantly reduced serum serotonin levels of 187 ± 9 ng/ml (p<0.01), as compared to the children who drank only well water. The average serum serotonin levels for the 2 children outside the village, drinking non fluoridated water, was even lower at 84 ng/ml (see Fig 7).
Fig 7.
Serum serotonin levels in children from Hanliyenice, Turkey who exclusively drank water containing 2.5 ppm fluoride had significantly increased serum serotonin as compared to controls who supplemented with fluoride free bottled water; Value= Mean ± SD; One-way ANOVA
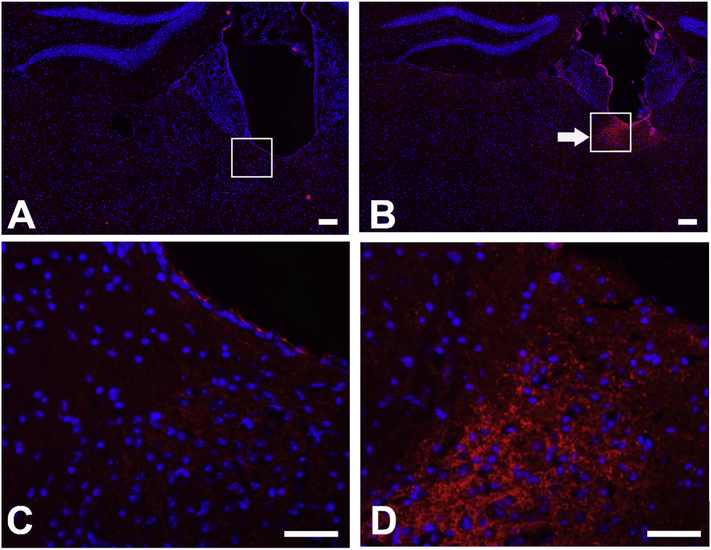
Serotonin immunostaining was increased in brains of fluoride exposed as compared to control mice
When we compared 6 serial immunostained sections from 2 brains from each group (control and fluoride exposed, we observed no obvious changes in brain morphology in the fluoride treated as compared to control mice (data not shown). Though there was no systematic quantification of the relative intensity of immunostaining, the differences in staining intensity between sections from control and fluoride treated brains showed that consistent with an increase in serum serotonin in fluoride treated mice, there was increased serotonin immunostaining in the brain, in particular in the area of the paraventricular nucleus (see boxed area in Fig 8B, and enlarged in 8D). There was no positive staining when consecutive sections were stained without primary antibodies.
Fig.8.
Serotonin immunostaining in brain of fluoride treated mice for 42 wks, showed increased serotonin immunostaining, in particular, in the region of the paraventricular thalamic nucleus (PVN). A) control mice, with a magnified view of the region of the PVT shown in C. B) fluoride treated mice with magnified view of the PVT shown D.
Scale bars A,B = 100 μM; C,D = 50 μM
Discussion:
The open field test measures spontaneous motor activity and exploratory behavior as measures of hyperactivity in animals (17). We found no differences in the behavior of the control and fluoride exposed female mice after 7 weeks (49 days) in the open field test, These results are similar to those of Pereira et al, who found no differences in locomotion in the open field test in adult male Wistar rats given 100 ppm postnatal fluoride for 30 days (15), and McPherson et al, who found that male Long Evans rats exposed to either 20 or 10 mg/L fluoride beginning in gestation, showed no differences in locomotor activity as compared to controls when tested at 40 days of age (10). However, with a longer duration of fluoride exposure, Bartos et al., found that both male and female Wistar rats given a lower fluoride concentration of 5 and 10 mg/L in drinking water beginning during gestation and continuing through lactation, had significantly decreased locomotion in the open field test at 90 days of age, as compared to control rats (4). Taken together, these studies suggest that the effects of fluoride on locomotor activity as measured in the open field test, may be dependent on the age or length of time of exposure fluoride exposure.
In the elevated plus maze (EPM), in which anxiety is assessed by the willingness of animals to enter and explore the open arms, we found that fluoride treated mice showed less anxiety in exploring the open arms after 7 weeks (49 days), and after 23 weeks (161 days) fluoride exposure. These findings are similar to those of Bartos et al., who found that female Wistar rats exposed to 10mg/L fluoride beginning during gestation spent significantly more time in the open arms as compared to controls after 45 days of age and male rats who spent significantly more time in the open arms of the EPM after 90 days of age (4). Consistent with Bartos’ studies, which found changes in male rats only after 90 days of age, McPherson et al, found that male Long-Evans rats exposed to 10 or 20 mg/L fluoride during gestation, also did not show change in behavior the EPM at 40 days of age (10). Taken together, these findings show that fluoride exposure can result in decreased anxiety related behaviors, and that females may be affected after less time of exposure as compared to males.
When group 2 mice were retested at 23 weeks, fluoride exposed mice spent a significantly greater amount of time in the open arms and traveled a greater distance in the open arms as compared to controls given 0 ppm fluoride in drinking water, though unlike after 7 weeks, there was no difference between the group in the number of entries into the open arms. Serotonin levels increased in 23 wk mice exposed to fluoride as compared to control mice. It is noteworthy that serotonin serum concentrations increased in control mice at 23 wks as compared to 7 wks. It is not clear whether increased serotonin levels in 23 week old control mice could have influenced these differences.
In one of the few reports of the effects of fluoride on behavior in mice, Liu and co-workers found that though there was no effect of 10mg/L fluoride in drinking water in postnatal BalB/C male mice for 4 weeks (28 days) on locomotor activity in the open field test, but that male BalB/C mice spent significantly reduced time in the open arms. These findings are opposite to our findings of increased time spent in open arms of the EPM in C57BL/6J female mice exposed to 50 mg/L fluoride for 7 weeks. The differences between our findings and those of Liu et al, may be related to the strain of mice used. BALB/c is an inbred stress-sensitive mouse strain exhibiting low brain serotonin content due to a loss-of-function single nucleotide polymorphism (SNP; C1473 G) in Tph2, with an approximate 50% reduction in tryptophan hydroxylase (Tph2), the enzyme that converts tryptophan into serotonin (5-HT) (18–20). This suggests the possibility that stress related differences in the effects of fluoride on mouse behavior in different strains may be related to serotonin levels.
Serotonin is a monoaminergic neurotransmitter produced by several cell types including serotonergic neurons, enterochromatin cells and renal proximal tubule cells. In the periphery, a large amount of serotonin is stored in platelets, and it is thought that peripheral serotonin cannot pass the blood brain barrier. Therefore, there are two independent systems of organization for serotonin: one in the central nervous system and the other in the periphery (21,22). The action of serotonin on pre- and post-serotoninergic receptors is limited by the recapture of serotonin, mediated by phosphorylation by the plasma membrane serotonin transporter (23,24), whose activity depends on ionic membrane gradients of Na+,K+, and Cl (25). The second mechanism is the degradation of serotonin by monoamine oxidase (MOA) (26), a process that has been shown to result in the increase in ROS in cardiomyoctes (27).
To investigate the possibility that serotonin also increased in the brain in fluoride treated female mice, we immunostained for serotonin in fixed brain sections. We found increased immunostaining for serotonin, in particular, in the area of paraventricular thalamic nucleus (PVN). The PVN is heavily innervated by neuropeptides, including serotonin, and is the only thalamic nucleus connected to the group of structures comprising the amygdala, bed nucleus of the stria terminalis, nucleus accumbens and infralimbic/subgenual anterior cingulate cortex (28). These neurotransmitter systems and structures are involved in regulating motivation and mood, The PVT is involved in fear processing in the amygdala (29), and therefore it is possible that fluoride related effects in decreasing anxiety related behaviors are initiated in the PVN.
We found that serum serotonin levels in fluoride treated mice were significantly increased as compared to control mice at all three time points (20, 20 and 42 weeks of fluoride exposure). Similar to McPherson et. al, we also found that brain fluoride concentrations increased with time of fluoride exposure (10), suggesting that fluoride increases serotonin both peripherally and centrally. It is interesting to note that serum serotonin did not continue to increase in either control or fluoride exposed mice at 42 week as compared to 20 weeks. This effect may be related to relatively decreasing serotonin levels with age, or alternatively may represent a change in the regulation of serotonin uptake into platelets (30,31).
Fluoride has been shown to decrease expression of the nicotinic acetyl choline receptor nAChR (13). Activation of nAChRs, has been shown to increase activity of the serotonin transporter (SERT) in the prefrontal cortex (32), and therefore downregulation of nAChR would decrease cellular uptake of serotonin. This possibility is consistent with our findings of increased extracellular serotonin in the presence of fluoride. Serotonin can also be removed through degradation by MOA. This process results increased production of reactive oxygen species in cardiomycytes and hepatocytes. Increased ROS has been correlated with increased anxiety related behaviors (33), and aggressiveness (34). It is therefore possible the effects of fluoride in reducing anxiety could be related to a relative reduction in the production of ROS (13,14). However, mechanisms by how fluoride influences serotonin concentrations remain to be investigated.
To determine the potential relevance of these studies to humans, we identified a well-controlled cohort of children exposed drinking water containing 2.5 mg/l fluoride. The 26 children who exclusively drank well water containing 2.5 ppm fluoride had significantly increased serum serotonin as compare to the 3 children who substituted their drinking water with fluoride free bottled water. Serum serotonin levels in children who went to the same school, but were not exposed to fluoride in food or water, had even lower serum serotonin levels. These findings of a correlation between water fluoridation and serum serotonin in a well-controlled cohort of children, may be important when considering the effects of fluoride on the developing brain. Lack of serotonin reuptake during brain development has been linked to changes in the prefrontal cortex and behavioral outcomes (35)
In summary, our findings show an inverse relation between fluoride ingestion and anxiety related behavior, and increased extracellular serotonin. We are the first to show that similar effects in humans. Our findings underlie the need for additional studies, including those focused on the mechanism of action by which fluoride modulates neurotransmitters such as serotonin, and how sex and the timing of exposure contribute to these effects.
Supplementary Material
Highlights.
Fluoride added to drinking water postnatally, resulted in reduced anxiety in mice
Increased fluoride was associated with significantly increased serum serotonin in mice and in children
Fluoride concentrations in brain increased with increased time of exposure
Serotonin immunolocalization was increased in long term fluoride exposed brain
Acknowledgements
This project was supported by NIH/NIEHS R21ES017813 to PDB, and Bridge funding from the UCSF School of Dentistry, and the UCSF Center for Children’s Oral Health Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fejerskov O, Manji F, and Baelum V (1990) The nature and mechanisms of dental fluorosis in man. Journal of dental research 69 Spec No, 692–700; discussion 721 [DOI] [PubMed] [Google Scholar]
- 2.Mullenix PJ, Denbesten PK, Schunior A, and Kernan WJ (1995) Neurotoxicity of sodium fluoride in rats. Neurotoxicol Teratol 17, 169–177 [DOI] [PubMed] [Google Scholar]
- 3.Basha PM, and Sujitha NS (2012) Combined impact of exercise and temperature in learning and memory performance of fluoride toxicated rats. Biological trace element research 150, 306–313 [DOI] [PubMed] [Google Scholar]
- 4.Bartos M, Gumilar F, Bras C, Gallegos CE, Giannuzzi L, Cancela LM, and Minetti A (2015) Neurobehavioural effects of exposure to fluoride in the earliest stages of rat development. Physiol Behav 147, 205–212 [DOI] [PubMed] [Google Scholar]
- 5.Choi AL, Sun G, Zhang Y, and Grandjean P (2012) Developmental fluoride neurotoxicity: a systematic review and meta-analysis. Environ Health Perspect 120, 1362–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang QQ, Du J, Ma HH, Jiang SJ, and Zhou XJ (2008) Fluoride and children’s intelligence: a meta-analysis. Biological trace element research 126, 115–120 [DOI] [PubMed] [Google Scholar]
- 7.Bashash M, Thomas D, Hu H, Martinez-Mier EA, Sanchez BN, Basu N, Peterson KE, Ettinger AS, Wright R, Zhang Z, Liu Y, Schnaas L, Mercado-Garcia A, Tellez-Rojo MM, and Hernandez-Avila M (2017) Prenatal Fluoride Exposure and Cognitive Outcomes in Children at 4 and 6–12 Years of Age in Mexico. Environ Health Perspect 125, 097017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bera I, Sabatini R, Auteri P, Flace P, Sisto G, Montagnani M, Potenza MA, Marasciulo FL, Carratu MR, Coluccia A, Borracci P, Tarullo A, and Cagiano R (2007) Neurofunctional effects of developmental sodium fluoride exposure in rats. Eur Rev Med Pharmacol Sci 11, 211–224 [PubMed] [Google Scholar]
- 9.Everett ET (2011) Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. Journal of dental research 90, 552–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McPherson CA, Zhang G, Gilliam R, Brar SS, Wilson R, Brix A, Picut C, and Harry GJ (2018) An Evaluation of Neurotoxicity Following Fluoride Exposure from Gestational Through Adult Ages in Long-Evans Hooded Rats. Neurotox Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malin AJ, and Till C (2015) Exposure to fluoridated water and attention deficit hyperactivity disorder prevalence among children and adolescents in the United States: an ecological association. Environ Health 14, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki M, Sierant ML, Antone JV, Everett ET, Whitford GM, and Bartlett JD (2014) Uncoupling protein-2 is an antioxidant that is up-regulated in the enamel organ of fluoride-treated rats. Connect Tissue Res 55 Suppl 1, 25–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartos M, Gumilar F, Gallegos CE, Bras C, Dominguez S, Monaco N, Esandi MDC, Bouzat C, Cancela LM, and Minetti A (2018) Alterations in the memory of rat offspring exposed to low levels of fluoride during gestation and lactation: Involvement of the alpha7 nicotinic receptor and oxidative stress. Reprod Toxicol 81, 108–114 [DOI] [PubMed] [Google Scholar]
- 14.Reddy YP, Tiwari SK, Shaik AP, Alsaeed A, Sultana A, and Reddy PK (2014) Effect of sodium fluoride on neuroimmunological parameters, oxidative stress and antioxidative defenses. Toxicol Mech Methods 24, 31–36 [DOI] [PubMed] [Google Scholar]
- 15.Pereira M, Dombrowski PA, Losso EM, Chioca LR, Da Cunha C, and Andreatini R (2011) Memory impairment induced by sodium fluoride is associated with changes in brain monoamine levels. Neurotox Res 19, 55–62 [DOI] [PubMed] [Google Scholar]
- 16.Taves DR (1968) Determination of submicromolar concentrations of fluoride in biological samples. Talanta 15, 1015–1023 [DOI] [PubMed] [Google Scholar]
- 17.Langford-Smith A, Malinowska M, Langford-Smith KJ, Wegrzyn G, Jones S, Wynn R, Wraith JE, Wilkinson FL, and Bigger BW (2011) Hyperactive behaviour in the mouse model of mucopolysaccharidosis IIIB in the open field and home cage environments. Genes Brain Behav 10, 673–682 [DOI] [PubMed] [Google Scholar]
- 18.Razzoli M, Carboni L, Andreoli M, Ballottari A, and Arban R (2011) Different susceptibility to social defeat stress of BalbC and C57BL6/J mice. Behav Brain Res 216, 100–108 [DOI] [PubMed] [Google Scholar]
- 19.Heinla I, Ahlgren J, Vasar E, and Voikar V (2018) Behavioural characterization of C57BL/6N and BALB/c female mice in social home cage - Effect of mixed housing in complex environment. Physiol Behav 188, 32–41 [DOI] [PubMed] [Google Scholar]
- 20.Bach H, Arango V, Huang YY, Leong S, Mann JJ, and Underwood MD (2011) Neuronal tryptophan hydroxylase expression in BALB/cJ and C57Bl/6J mice. J Neurochem 118, 1067–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kranz GS, Wadsak W, Kaufmann U, Savli M, Baldinger P, Gryglewski G, Haeusler D, Spies M, Mitterhauser M, Kasper S, and Lanzenberger R (2015) High-Dose Testosterone Treatment Increases Serotonin Transporter Binding in Transgender People. Biol Psychiatry 78, 525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Merahbi R, Loffler M, Mayer A, and Sumara G (2015) The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett 589, 1728–1734 [DOI] [PubMed] [Google Scholar]
- 23.Jayanthi LD, Samuvel DJ, Blakely RD, and Ramamoorthy S (2005) Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol Pharmacol 67, 2077–2087 [DOI] [PubMed] [Google Scholar]
- 24.Annamalai B, Mannangatti P, Arapulisamy O, Shippenberg TS, Jayanthi LD, and Ramamoorthy S (2012) Tyrosine phosphorylation of the human serotonin transporter: a role in the transporter stability and function. Mol Pharmacol 81, 73–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schanberg SM (1963) A study of the transport of 5-hydroxytryptophan and 5-hydroxytryptamine (serotonin) into brain. J Pharmacol Exp Ther 139, 191–200 [PubMed] [Google Scholar]
- 26.Shore PA, Mead JA, Kuntzman RG, Spector S, and Brodie BB (1957) On the physiologic significance of monoamine oxidase in brain. Science 126, 1063–1064 [DOI] [PubMed] [Google Scholar]
- 27.Bianchi P, Pimentel DR, Murphy MP, Colucci WS, and Parini A (2005) A new hypertrophic mechanism of serotonin in cardiac myocytes: receptor-independent ROS generation. FASEB J 19, 641–643 [DOI] [PubMed] [Google Scholar]
- 28.Hsu DT, Kirouac GJ, Zubieta JK, and Bhatnagar S (2014) Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front Behav Neurosci 8, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, Darvas M, Parada LF, Palmiter RD, He M, Huang ZJ, and Li B (2015) The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenner B, Harney JT, Ahmed BA, Jeffus BC, Unal R, Mehta JL, and Kilic F (2007) Plasma serotonin levels and the platelet serotonin transporter. J Neurochem 102, 206–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taborskaya KI, Frolova MY, and Kuleva NV (2016) [Comparative Analysis of Serotonin Levels in Rat Platelets, Serum and Brain on the Aging]. Tsitologiia 58, 115–119 [PubMed] [Google Scholar]
- 32.Awtry TL, Frank JG, and Werling LL (2006) In vitro regulation of serotonin transporter activity by protein kinase A and nicotinic acetylcholine receptors in the prefrontal cortex of rats. Synapse 59, 342–349 [DOI] [PubMed] [Google Scholar]
- 33.Rammal H, Bouayed J, and Soulimani R (2010) A direct relationship between aggressive behavior in the resident/intruder test and cell oxidative status in adult male mice. Eur J Pharmacol 627, 173–176 [DOI] [PubMed] [Google Scholar]
- 34.Bouayed J, Rammal H, and Soulimani R (2009) Oxidative stress and anxiety: relationship and cellular pathways. Oxid Med Cell Longev 2, 63–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witteveen JS, Middelman A, van Hulten JA, Martens GJ, Homberg JR, and Kolk SM (2013) Lack of serotonin reuptake during brain development alters rostral raphe-prefrontal network formation. Front Cell Neurosci 7, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.