Abstract
Objectives:
Given that people with higher intelligence have been shown to live longer, enjoy better health, and have more favourable health behaviours, we investigated the association between childhood IQ and a range of important dental health and service-use indicators at age 38.
Methods:
Longstanding prospective study of a complete birth cohort, with childhood IQ (assessed at ages 7, 9, 11, and 13 years) used to allocate participants (N = 891) to one of four ordinal categories of childhood IQ.
Results:
There were distinct and consistent gradients by childhood IQ in almost all of the dental caries experience measures (with the exception of filled teeth) whereby each was most severe in the lowest child IQ category and least severe in the highest; the exception was the mean FT score, for which there was no discernible gradient. Indicators of self-care and periodontal disease experience showed similar gradients, and multivariate modelling using the continuous IQ score confirmed the observed patterns.
Conclusions:
Childhood cognitive function is a key determinant of oral health and dental service-use by midlife, with those with lower cognitive capacity as children likely to have poorer oral health, less favourable oral-health-related beliefs, and more detrimental self-care and dental visiting practices by age 38. There is a need to shape dental clinical services and public health interventions so that people with the poorest cognitive function do not continue to be disadvantaged.
Keywords: dental public health, health services research, psychosocial factors, quality-of-life, epidemiology, behavioral science
Introduction
Dental caries and adult periodontitis are both largely irreversible and cumulative chronic conditions which are highly prevalent, as is their clinical endpoint of tooth loss. Their aetiology involves a complex mix of biological, environmental and social influences1, and the risk accumulation life course model is the most relevant one for considering their occurrence2. Much of the earlier research on oral health used a biomedical perspective, focusing on the person-level biological and dietary influences, while more contemporary models of health and oral health have emphasised the importance of the wider sociocultural and political context in which those personal characteristics are manifest3–5. This has been a positive development, but a missing piece of the puzzle has been the role of cognitive function, given the crucial role that intelligence is understood to play in determining social position6 and the increasing importance to oral health (with age) of social position in adulthood7.
Known as cognitive epidemiology, studying the role of intelligence in the occurrence of morbidity and mortality has attracted considerable attention in recent years, with a number of reports from longstanding prospective cohort studies8. A consistent finding of this work is that people with higher intelligence live longer, enjoy better health, and have more favourable health behaviours9. This has also included oral health. For example, an investigation of the association of IQ measured in adolescence with self-reported health by age 40 in a large US cohort study found that higher cognitive scores predicted better general and mental health, along with lower odds of having a number of conditions, including self-reported “severe tooth or gum trouble”10. Other work with that same cohort has highlighted an important role for intelligence in health behaviours known to be important for oral health, with, for example, better cognitive performance at ages 15–23 years predicting higher rates of dental floss use and lower rates of smoking and consumption of sugary drinks in middle age11. Data from a British cohort study showed that more intelligent children grow up to exercise more and eat more healthily as adults12. Given the key role of sugars exposure in dental caries occurrence13, these findings suggest that intelligence might be a key determinant of oral health through life.
How might cognitive function influence oral health and disease experience? It is useful to consider the mechanisms posited to explain the prediction of longevity by childhood IQ14. First, cognitive function in childhood might reflect adverse exposures in the prenatal, perinatal and early childhood periods; second, it might reflect overall body structure integrity; third, it predicts healthy behaviours (such as avoiding tobacco); fourth, it determines entry into healthy environments (such as less hazardous occupations, better functioning family units in adulthood, or social milieux in which having visibly missing teeth is stigmatising); finally, those with compromised cognitive abilities may encounter barriers to accessing health care or in understanding health messages and advice. Each of these might be testable with oral health measures. The first might be reflected in an inverse association between childhood IQ and developmental defects of the enamel of particular teeth. The second might be apparent with an indicator such as fluctuating asymmetry15, whereby bilateral asymmetry in tooth size or form might be more common in those with lower childhood IQ. The third mechanism is most readily tested in relation to oral health, with oral-health-related behaviours routinely measured in prospective studies of oral health. The fourth can be tested either directly (by examining domains such as occupation or family functioning) or indirectly (with measures such as tooth loss). The fifth would be reflected in differences in self-reported access to oral health care.
The role of cognitive function in determining oral health through the life course has not been investigated to date. Accordingly, the aim of this study was to investigate whether childhood IQ predicts oral health and disease experience by age 38.
Methods
Study participants were members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of the health and behaviour of a complete birth cohort of consecutive births between April 1, 1972, and March 31, 1973, in Dunedin, New Zealand (NZ)16. The cohort of 1,037 children (91% of eligible births; 52% boys) was constituted at age 3 years. Eligibility was based on residence in the province and participation in the first assessment at age 3 years. Cohort families represent the full range of socioeconomic status in the general population of New Zealand’s South Island and are primarily of white European ancestry. Follow-up assessments were conducted with informed consent at 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, and most recently at 38 years of age, when 95·4% of the 1,007 living study members took part.
The Otago Ethics Committee approved each assessment phase of the study. Study members gave informed consent before participating in the phase. They were physically examined, interviewed, and completed self-report questionnaires as appropriate.
Childhood IQ was individually assessed at ages 7, 9, 11, and 13 years by means of the Wechsler Intelligence Scale for Children-revised17. The IQs determined at these four ages were averaged into one measure and standardised. The WISC-R test comprises a series of subtests that yield indices standardised to population norms (with a mean of 100 and a standard deviation of 15). Tests were administered by trained psychometrists who were blind to the study members’ previous IQ data. For our bivariate analyses, we recoded childhood IQ into four ordinal categories representing the four quartiles (41–84, 85–99, 100–114 and 115+).
Childhood socio-economic status (SES) used data collected on parental SES with standard NZ occupationally-based indices with a 6-interval classification18,19. We used the mean SES score from assessments undertaken at birth, 3, 5, 7, 9, 11, 13 and 15 years of age. A measure of adult SES was obtained using the Study member’s occupation, assessed at age 38.
The current investigation uses dental clinical data from age 38. Dental examinations for dental caries experience and missing teeth were conducted by calibrated examiners. Repeat examinations were not possible because of logistical constraints imposed by the tightly scheduled assessment undergone by Study members. An estimation of accumulated tooth loss due to caries was obtained by observing the presence or absence of each tooth, and ascertaining the reason for its absence. Only teeth which had been lost because of caries (determined by asking the Study member at the time) are included in estimations of tooth loss due to caries and in the ‘M’ component of DMF scores. Teeth extracted for reasons other than dental caries (such as impaction or orthodontic treatment) were not included in the computation of tooth loss due to dental caries. Dental examiners were unaware of participants’ IQ.
Other dental clinical data (including root surface caries status) were also collected at age 38. The periodontal examination involved full-mouth recording of gingival recession (distance in mm from the cemento-enamel junction to the gingival margin) and probing depth (distance in mm from the gingival margin to the base of the pocket) at three sites (mesiobuccal, buccal, and distolingual) per tooth (excluding third molars), using the Hu-Friedy PCP-2 probe. Midbuccal measurements for molars were made at the midpoint of the mesial root. All measurements were rounded down to the nearest whole millimeter at the time of recording. One recording of gingival bleeding was made for each examined tooth once it had been probed. Periodontal measurements excluded those who reported a history of cardiac valvular anomalies or rheumatic fever. A Simplified Oral Hygiene Index (OHI-S)20 was recorded for each participant; it was then used in growth trajectory modelling to allocate participants to one of three lifetime plaque trajectory categories21.
The short-form Oral Health Impact Profile (OHIP-14)22 was administered by trained interviewers at age 38; for each of the 14 items, Study members were asked how often they had experienced the problem in the previous 4 weeks. We then calculated a total OHIP-14 score by summing responses over all 14 items, with possible scores ranging from 0 to 56, after which we determined the proportion of people reporting one or more items ‘fairly often’ or ‘very often’.
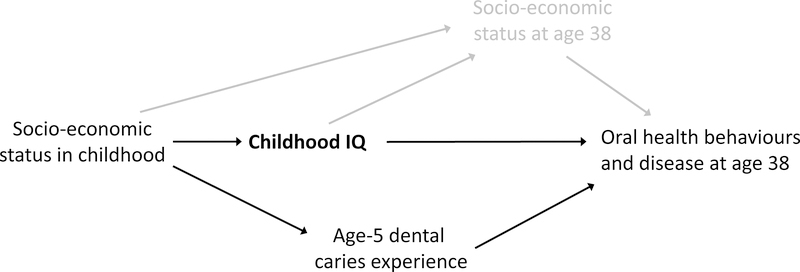
The statistical analysis used cross-tabulations (and Chi-squared statistics) for categorical dependent variables (such as the prevalence of missing teeth), and analysis of variance for continuous dependent variables (such as the number of missing teeth). Multivariate modelling was undertaken using negative binomial regression for the latter, and relative risks for the former were computed using the GLM command in Stata with a modified Poisson approach1 using robust error variances. In the modelling, we adjusted for sex and age-5 dmfs score (the latter as a measure of “baseline” oral disease experience). The modelling was guided by a Directed Acyclic Graph (Figure 1). Reporting of the data complied with the STROBE guidelines.
Figure 1.
Directed Acyclic Graph for childhood IQ and age-38 oral behaviours and disease
Results
At age 38, dental clinical examination data were available for 916 dentate individuals (49.8% females), of whom data on childhood IQ were available for 891 (97.3%), while age-5 dmfs score data were available for 818 (89.3%). Appendix Tables 1 and 2 compare the oral disease and impact of oral conditions among those for whom childhood IQ were available and those for whom it was not. There were no statistically significant differences between the two groups.
The number of teeth present ranged from 6 to 32 (mean 27.5; sd 2.8). Standardised childhood IQ scores ranged from 41 to 141 (mean 100.8; sd 14.1). The mean age-5 dmfs scores in the childhood IQ ordinal categories (41–84, 85–99, 100–114 and 115+) were 4.2, 3.8, 3.5 and 2.6, respectively (P = 0.14).
Summary data on participants’ oral disease and plaque control by age 38 are presented in Table 1 by ordinal categories of childhood IQ. Their cumulative dental caries experience (DMFT) showed a distinct and consistent gradient whereby it was highest in the lowest child IQ category and lowest in the highest, with the absolute difference between the two extreme categories being 2.0 teeth. There were similarly consistent gradients in almost all of the other dental caries experience measures; the exception was the mean FT score, for which there was no discernible gradient. Cumulative periodontal disease experience showed similar gradients, with the prevalence and extent of AL being highest in the lowest child IQ category and lowest in the highest. The same held for bleeding on probing, and for the four plaque control indicators.
Table 1.
Age-38 dental caries, tooth loss and periodontal disease experience and plaque control habits by ordinal categories of standardised childhood IQ score (brackets contain standard deviations unless otherwise indicated)
| Standardised childhood IQ score: | |||||
|---|---|---|---|---|---|
| 41–84 | 85–99 | 100–114 | 115–141 | All | |
| Number (%) | 106 (13.0) | 287 (35.1) | 298 (36.4) | 127 (15.5) | 818 (100.0) |
| Dental caries/tooth loss | |||||
| Mean coronal DMFT | 9.9 (5.7) | 9.1 (5.2) | 8.1 (5.2) | 7.8 (5.3)a | 8.7 (5.3) |
| Mean coronal DT | 2.0 (3.6) | 1.2 (2.2) | 0.8 (1.7) | 0.6 (2.2)a | 1.1 (2.2) |
| Mean coronal MT | 1.7 (2.6) | 1.0 (1.8) | 0.7 (1.7) | 0.6 (2.2)a | 0.9 (2.0) |
| Mean coronal FT | 6.2 (4.2) | 6.9 (4.5) | 6.7 (4.4) | 6.8 (4.7) | 6.7 (4.5) |
| Mean root DS | 1.4 (8.3) | 0.7 (2.7) | 0.3 (1.5) | 0.5 (1.7)a | 0.6 (3.6) |
| Mean root DFS | 1.6 (8.3) | 1.0 (3.1) | 0.5 (1.8) | 0.8 (2.2) | 0.9 (3.8) |
| 1+ missing teeth (%) | 53 (50.0) | 107 (37.3) | 82 (27.5) | 27 (21.3)a | 269 (32.9) |
| 3+ missing teeth (%) | 28 (26.4) | 40 (13.9) | 25 (8.4) | 7 (5.5)a | 100 (12.2) |
| 1+ coronal DT (%) | 55 (51.9) | 126 (43.9) | 96 (32.2) | 26 (20.5)a | 303 (37.0) |
| Periodontal statusb | |||||
| 1+ sites, 5+mm ALc (%) | 35 (33.0) | 70 (24.4) | 54 (18.1) | 18 (14.2)a | 177 (21.6) |
| 1+ sites, 6+mm ALc (%) | 20 (18.9) | 39 (13.6) | 26 (8.7) | 8 (6.3)a | 93 (11.4) |
| Extent of 5+mm AL | 4.0 (10.9) | 3.2 (11.9) | 1.1 (5.0) | 0.6 (2.4)a | 2.1 (8.7) |
| Extent of BOPd | 33.0 (28.4) | 23.3 (22.3) | 19.1 (20.1) | 16.6 (18.1)a | 22.0 (22.3) |
| Plaque control and self-care | |||||
| Mean OHI-S score | 1.0 (0.7) | 0.7 (0.6) | 0.6 (0.5) | 0.5 (0.4)a | 0.7 (0.5) |
| High plaque trajectory (%) | 29 (27.4) | 36 (12.5) | 17 (5.7) | 2 (1.6)a | 84 (10.3) |
| Brush twice daily (%)e | 42 (41.2) | 156 (55.1) | 188 (63.7) | 88 (69.3)a | 474 (58.7) |
| Floss daily (%) | 6 (5.7) | 20 (7.0) | 34 (11.4) | 20 (15.7)a | 88 (9.9) |
| Current smoker (%)f | 43 (41.0) | 88 (30.7) | 54 (18.1) | 21 (16.5)a | 206 (25.2) |
P<0.05
Data missing for 17 individuals who could not be periodontally examined for medical reasons
% of sites with 5+mm attachment loss
% of teeth showing bleeding on probing (BOP) during the periodontal assessment
Data missing for 11 individuals
Data missing for 1 individual
Mean OHIP-14 scores showed a consistent gradient whereby they were highest in the lowest child IQ category and lowest in the highest, with the mean score in the former being more than twice that of the latter (Table 2). There was a similar gradient in the prevalence of one or more OHIP-14 impacts. The proportion who usually visited for dental check-ups was highest among those in the highest IQ category, with a consistent gradient observed, while the opposite gradient was seen for having had more than five years since the last dental visit.
Table 2.
Age-38 OHIP-14 scores and aspects of dental visiting, by ordinal categories of childhood IQ (brackets contain percentages unless otherwise indicated)
| Standardised childhood IQ score: | |||||
|---|---|---|---|---|---|
| 41–84 | 85–99 | 100–114 | 115–141 | All | |
| Mean OHIP-14 score (SD) | 10.7 (9.8) | 9.3 (8.0) | 6.6 (7.2) | 5.0 (6.0)a | 7.8 (7.9) |
| 1+ OHIP-14 impactsb | 33 (31.1) | 68 (23.7) | 59 (19.8) | 17 (13.4)a | 177 (21.6) |
| Visit for check-ups | 30 (28.3) | 113 (39.4) | 141 (47.3) | 74 (58.3)a | 358 (43.8) |
| >5 years since last dental visit | 28 (26.4) | 53 (18.5) | 51 (17.1) | 11 (8.7)a | 143 (17.5) |
P<0.05
‘Fairly often’ or ‘Very often’
The outcome of the multivariate modelling is summarised in Table 3. It used the continuous standardised childhood IQ variable rather than the four categories. Only the model for coronal FT did not show a strong association with childhood IQ.
Table 3.
Summary of multivariate models using childhood IQ score as the predictor (and then adjusting for sex and age-5 dmfs score; data are regression coefficients unless otherwise indicated)
| Dependent variable | Unadjusted regression coefficient or relative risk (95% CI) | Adjusted regression coefficient or relative risk (95% CI) | P value |
|---|---|---|---|
| Dental caries/tooth loss | |||
| Mean coronal DMFT | −0.005 (−0.009, −0.002) | −0.005 (−0.008, −0.002) | <0.05 |
| Mean coronal DT | −0.031 (−0.041, −0.021) | −0.034 (−0.044, −0.024) | <0.0001 |
| Mean coronal MT | −0.023 (−0.033, −0.013) | −0.023 (−0.034, −0.013) | <0.0001 |
| Mean coronal FT | 0.001 (−0.002, 0.005) | 0.002 (−0.001, 0.006) | 0.222 |
| Mean root DS | −0.025 (−0.041, −0.008) | −0.029 (−0.046, −0.011) | <0.05 |
| Mean root DFS | −0.020 (−0.035, −0.006) | −0.020 (−0.035, −0.006) | <0.05 |
| 1+ missing teeth | 0.978 (0.972, 0.984) | 0.980 (0.974, 0.987)a | <0.0001 |
| 3+ missing teeth | 0.967 (0.956, 0.978) | 0.967 (0.956, 0.978)a | <0.0001 |
| 1+ coronal DT | 0.981 (0.975, 0.986) | 0.980 (0.975, 0.986)a | <0.0001 |
| Periodontal status | |||
| 1+ sites, 5+mm AL | 0.983 (0.974, 0.992) | 0.982 (0.973, 0.991)a | <0.0001 |
| 1+ sites, 6+mm AL | 0.977 (0.964, 0.990) | 0.976 (0.964, 0.989)a | <0.0001 |
| Extent of 5+mm AL | −0.042 (−0.061, −0.024) | −0.046 (−0.064, −0.028) | <0.0001 |
| Extent of BOP | −0.014 (−0.019, −0.008) | −0.014 (−0.020, −0.009) | <0.0001 |
| Plaque control | |||
| Mean OHI-S score | −0.015 (−0.021, −0.009) | −0.015 (−0.021, −0.009) | <0.0001 |
| High plaque trajectory | 0.953 (0.941, 0.964) | 0.950 (0.939, 0.961)a | <0.0001 |
| Brush twice daily | 1.009 (1.005, 1.014) | 1.010 (1.006, 1.014)a | <0.0001 |
| Floss daily | 1.022 (1.007, 1.038) | 1.024 (1.008, 1.040)a | <0.05 |
| OHRQoL and dental care | |||
| Mean OHIP-14 score | −0.019 (−0.025, −0.013) | −0.019 (−0.024, −0.013) | <0.0001 |
| 1+ OHIP-14 impacts | 0.985 (0.976, 0.994) | 0.985 (0.976, 0.994)a | <0.05 |
| Visit for check-ups | 1.014 (1.008, 1.020) | 1.014 (1.008, 1.020)a | <0.0001 |
| 5+ years since last visit | 0.985 (0.975, 0.995) | 0.984 (0.974, 0.993)a | <0.05 |
Relative risk; this is interpreted as the relative risk of the event occurring for every “increase” in childhood IQ by 1 IQ point
We repeated the models controlling for SES in childhood (measured as the average household SES from birth through to age 15 years), and the findings were essentially the same (Appendix Table 3). We then replaced childhood SES with age-38 SES (Appendix Table 4), with the result that childhood IQ was not a predictor of DMFT, FT, root DS, root DFS, periodontitis prevalence, floss use or having 1+ OHIP-14 impacts.
There were gradients by IQ category in dental beliefs (Table 4), with those in the highest child IQ category having the highest proportion with favourable beliefs, and those in the lowest child IQ category having the lowest proportion (with the exception of drinking fluoridated water, where the gradient was in the opposite direction). There was a consistent gradient in the mean number of belief items rated as important.
Table 4.
Dental beliefs at age 38 by ordinal categories of childhood IQ (brackets contain row percentages unless otherwise indicated)
| Standardised childhood IQ score: | |||||
|---|---|---|---|---|---|
| 41–84 | 85–99 | 100–114 | 115–141 | All | |
| Believes to be importanta: | |||||
| Avoiding a lot of sweet foods | 70 (68.0) | 226 (80.4) | 232 (78.6) | 110 (87.3)b | 638 (79.3) |
| Using fluoride toothpaste | 88 (86.3) | 251 (89.6) | 280 (94.9) | 120 (94.5)b | 739 (91.9) |
| Visiting dentist regularly | 83 (80.6) | 240 (86.3) | 258 (87.5) | 119 (93.7)b | 700 (87.3) |
| Keeping teeth/gums clean | 96 (94.1) | 273 (97.8) | 290 (98.3) | 127 (100.0)b | 786 (97.9) |
| Drinking fluoridated water | 79 (76.7) | 200 (71.4) | 213 (72.0) | 85 (67.5) | 577 (71.7) |
| Using dental floss | 72 (70.6) | 221 (78.9) | 245 (82.8) | 117 (92.1)b | 655 (81.4) |
| Mean number of important items (SD): | 4.6 (1.7) | 4.9 (1.6) | 5.1 (1.2) | 5.3 (0.9)c | 5.1 (1.4) |
‘Extremely important’ or ‘Fairly important’ (13 missing responses for the first and the last two last items; 14 missing for the second; and 15 missing for the other two)
P<0.05
P<0.05; oneway ANOVA: the 41–84 differs from the highest two groups; the 85–99 and 100–114 groups do not differ; the 115+ group does not differ from the 100–114 group
Discussion
This study has found marked, consistent gradients in age-38 oral health and disease experience and dental care usage by childhood IQ among participants in a longstanding New Zealand birth cohort study. Children with lower IQ ended up with greater dental caries experience (in all its manifestations except experience of filled surfaces), more extensive periodontal attachment loss and gingivitis, and greater impacts on their day-to-day lives. Their plaque control was also poorer, they were less likely to be routine users of dentistry, more likely to not have made a dental visit for at least five years, and they had fewer favourable oral health beliefs.
This study has a number of weaknesses and strengths which should be considered before examining the findings. One weakness was that we did not have complete data for the entire cohort, but a comparison of the age-38 oral health characteristics of those with childhood IQ and age-5 caries experience data with those who did not have that information shows that there were no important differences. Turning to the strengths, we used a broad range of clinical oral disease and self-reported oral health measures, along with information on dental beliefs and dental visiting; this is a far broader set of dental measures than has ever been used in cognitive epidemiology. Thus, our data are unprecedented. Another strength was the robustness of the exposure measure, childhood IQ. It was determined at four ages (7, 9, 11, and 13 years) using the gold standard measure, and averaging those score to get a single childhood score. The use of trained psychometrists who were blind to the study members’ previous IQ data would also have enhanced the validity of the exposure measure. Moreover, using childhood IQ rather than adult IQ means that we do not have the issue of reverse causation to consider23,24, whereby a lifetime of poor health and adverse health behavours might have had the effect of reducing IQ by the time it is measured in adulthood. Finally, our multivariate models controlled for early childhood dental caries experience (age dmfs), in order to partition the variance in age-38 oral disease which could be attributed to poor oral health in childhood.
The findings provide ample support for the third, fourth and fifth of the mechanisms proposed by to explain effects of childhood IQ on health. The gradients observed in Tables 2 and 3 demonstrate the importance of cognitive functioning for the adoption and persistence of healthy behaviours: the proportion of current smokers was highest in the lowest IQ group and highest in the highest IQ group, while twice-daily toothbrushing and daily flossing showed the expected gradients in the opposite direction. These gradients were reflected in the plaque scores and membership of the high plaque trajectory (the latter reflecting lifelong dental plaque control effectiveness). For each of those measures, the differences between the highest and lowest IQ groups were considerable. Our evidence to support the fourth mechanism (entry into healthy environments) is more indirect, but no less compelling. It is most apparent in the gradients in tooth-loss experience and in smoking, supporting the contention that those with poorer cognitive function in childhood are more likely to end up in social environments where the gradual loss of teeth is more common and less likely to be stigmatising. However, a more focused examination of the intraoral patterns of tooth loss—whether the teeth were visibly missing—according to childhood IQ would provide better evidence to confirm or refute such a hypothesis. There is support for the fifth mechanism (barriers to obtaining health care) in the marked gradient observed in the proportion of participants whose last dental visit had been made more than five years previously.
The association between childhood IQ and adult dental health did change slightly when we added controls for childhood SES. This can be seen in comparing the data in Table 3 with those in Appendix Table 3. However, the difference is not great, because childhood IQ and childhood SES are also correlated, and childhood SES is not as strongly related to adult dental health as childhood IQ is. Controlling for “destination SES” (that is, in adulthood) did make a difference, most notably for those oral disease characteristics with either a considerable “filled” component to them (such as DMFT and root DFS) or a more recent pattern of development (such as root DS or periodontitis). The latter is heavily influenced by recent smoking, and that is strongly associated with age-38 SES in this cohort25. Arguably, though, we should not control for SES in adulthood in this study26, given that childhood IQ is itself an important predictor of SES in adulthood (in the Dunedin cohort, the mean standardised childhood IQ scores in the high, medium and low age-38 SES groups were 108.8, 99.2 and 92.2, respectively). As such, SES in adulthood is not so much a confounder as it is a potential mediator of the childhood IQ-adult dental health association.
The most commonly used model of oral health4 includes tiers of influence at the individual, household and community levels, with the latter including characteristics as diverse as the social environment, the health-care system, the physical environment and culture. It has recently been applied to life-course data from the Dunedin Study cohort1, and oral health beliefs were found to play an important role; as observed in the current study, early-life cognitive function is a key shaper of those beliefs. Also included in the Fisher-Owens model’s rationale4—but not examined in the abovementioned analysis—were the notions of vulnerability and resilience, whereby some individuals are better-equipped for dealing with adversity and other challenges, and such people would be likely to have better oral health as they aged than those who were more vulnerable and less resilient. Characteristics which are likely to influence both vulnerability and resilience are personality traits and cognitive function. The role of personality in the oral health of the Dunedin cohort has been reported previously, with negative emotionality particularly strongly associated with greater oral disease experience by age 3227. Our current findings add to that work by underlining the central importance of early-life cognitive function (“intelligence”). Given the central role of cognitive function uncovered here, an important challenge will be to shape dental clinical services and public health interventions so that people with the poorest cognitive function do not continue to be disadvantaged.
In conclusion, childhood cognitive function is a key determinant of oral health and dental service-use by midlife, with those with lower cognitive capacity as children likely to have poorer oral health, less favourable oral-health-related beliefs, and more detrimental self-care and dental visiting practices by age 38.
Supplementary Material
Acknowledgments
The authors thank the study members for their continuing participation in the Dunedin Study, and study founder, Dr. Phil Silva. The age-38 data collection was supported by a program grant from the NZ HRC and grants from the National Institute on Aging (NIA; Grant G032282) and the Medical Research Council (Grant MRK00381X). The Dunedin Multidisciplinary Health and Development Research Unit is supported by the NZ HRC. The authors report no conflicts of interest related to this study.
Footnotes
References
- 1.Broadbent JM, Zeng J, Foster Page LA, Baker SR, Thomson WM. Oral health-related beliefs, behaviours, and outcomes through the life course. J Dent Res. 2016;95:808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicolau B, Thomson WM, Steele JG, Allison PJ. Life-course epidemiology: concepts and theoretical models with particular reference to oral chronic conditions. Community Dent Oral Epidemiol. 2007;35:241–249. [DOI] [PubMed] [Google Scholar]
- 3.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Social Behavior. 1995;36:1–10. [PubMed] [Google Scholar]
- 4.Fisher-Owens SA, Gansky SA, Platt LJ, Weintraub JA, Soobader MJ, Bramlett MD, Newacheck PW. Influences on children’s oral health: a conceptual model. Pediatrics. 2007;120:e510–e520. [DOI] [PubMed] [Google Scholar]
- 5.Solar O, Irwin A. A conceptual framework for action on the social determinants of health Social Determinants of Health Discussion Paper 2 (Policy and Practice). Geneva: World Health Organization, 2010. [Google Scholar]
- 6.Whalley LJ, Deary IJ. Longitudinal cohort study of childhood IQ and survival up to age 76. Brit Med J. 2001;322:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomson WM. Social inequality in oral health. Community Dent Oral Epidemiol. 2012;40 (Suppl. 2):28–32. [DOI] [PubMed] [Google Scholar]
- 8.Deary IJ. Cognitive epidemiology: its rise, its current issues, and its challenges. Pers Indiv Dif. 2010;49:337–343. [Google Scholar]
- 9.Schaefer J, Caspi A, Belsky D, Harrington H, Houts R, Israel S, Levine M, Sugden K, Williams B, Poulton R, Moffitt TE. Early-life intelligence predicts midlife biological age. J Gerontol: Psychol Sci 2015;71:968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Der G, Batty GD, Deary IJ. The association between IQ in adolescence and a range of health outcomes at 40 in the 1979 US National Longitudinal Study of Youth. Intelligence. 2009;37:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wraw C, Der G, Gale CR, Deary IJ. Intelligence in youth and health behaviours in middle age. Intelligence. 2018;69:71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanazawa S Childhood intelligence and adult obesity. Obesity. 2013;21:434–440. [DOI] [PubMed] [Google Scholar]
- 13.Moynihan PJ, Kelly SA. Effect on caries of restricting sugars intake: systematic review to inform WHO guidelines. J Dent Res. 2014;93:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottfredson LS, Deary IJ. Intelligence predicts health and longevity, but why? Curr Dir Psychol Sci. 2004;13:1–4. [Google Scholar]
- 15.Milne BJ, Belsky J, Poulton R, Thomson WM, Caspi A, Kieser JA. Fluctuating asymmetry and physical health outcomes among young adults. Evol Hum Behav. 2003;24:53–63. [Google Scholar]
- 16.Poulton R, Moffitt TE, Silva PA. The Dunedin Multidisciplinary Health and Development Study: Overview of the first 40 years, with an eye to the future. Soc Psychiatry Psychiatr Epidemiol. 2015;50:679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wechsler D Manual for the Wechsler Intelligence Scale for Children – Revised. New Yorik: Psychological Corporation, 1974. [Google Scholar]
- 18.Irving JC, Elley WB. A socio-economic index for the female labour force in New Zealand. N Z J Educ Stud. 1977;12:154–163. [Google Scholar]
- 19.Elley WB, Irving JC. The Elley-Irving socio-economic index 1981 Census revision. N Z J Educ Stud. 1985;20:115–128. [Google Scholar]
- 20.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. [DOI] [PubMed] [Google Scholar]
- 21.Broadbent JM, Thomson WM, Boyens JV, Poulton R. Dental plaque and oral health during the first 30 years of life. J Am Dent Assoc. 2011;142:415–426. [DOI] [PubMed] [Google Scholar]
- 22.Slade GD. Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol. 1997;25:284–90. [DOI] [PubMed] [Google Scholar]
- 23.Belsky DW, Caspi A, Goldman-Mellor S, Meier MH, Poulton R, Moffitt TE. Is obesity associated with a decline in intelligence quotient during the first half of the life course? Amer J Epidemiol. 2013;178:1461–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belsky DW, Caspi A, Israel S, Blumenthal J, Poulton R, Moffitt TE. Cardiorespiratory fitness and cognitive function at midlife: neuroprotection or neuroselection? Annals Neurol. 2015;77:607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng J, Williams SM, Fletcher DJ, Cameron CM, Broadbent JM, Shearer DM, Thomson WM. Re-examining the periodontal effects of smoking and periodontitis in the Dunedin study with an enhanced analytical approach. J Periodontol. 2014;85:1390–1397. [DOI] [PubMed] [Google Scholar]
- 26.Meehl PE. High School yearbooks: a reply to Schwartz. J Abnorm Psychol. 1971;77:143–148. [DOI] [PubMed] [Google Scholar]
- 27.Thomson WM, Caspi A, Poulton R, Moffitt TE, Broadbent JM. Personality and oral health. Eur J Oral Sci. 2011;119: 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.