Abstract
Background:
Delirium is a frequent complication of critical illness, but its diagnosis is more difficult in brain-injured patients due to language impairment and disorders of consciousness. We conducted a prospective cohort study to determine whether Richmond Agitation and Sedation Scale (RASS) scores could be used to reliably diagnose delirium in the setting of brain injury. We also examined clinical factors associated with delirium in patients with subdural hematomas (SDH) and assessed its impact on functional outcome at discharge.
Methods:
We prospectively enrolled 55 patients with the primary diagnosis of SDH admitted to the neurological intensive care unit (ICU) and screened them for delirium with the Confusion Assessment Method-ICU (CAM-ICU). As our primary outcome, we examined whether the standard deviation of RASS scores (RASS dispersion) could be used to diagnose delirium. We also looked at trends in RASS scores as a way to distinguish different causes of delirium. Then, using logistic regression, we identified factors associated with delirium in patients with SDH and quantified the impact of delirium on the modified Rankin Scale at discharge.
Results:
Delirium as defined by the CAM-ICU was present in 35% (N = 19) of patients. RASS dispersion correlated well with the CAM-ICU (AUC of the ROC was 0.84). Analyzing the temporal trend of changes in the RASS was helpful in identifying new brain injuries as the underlying etiology of CAM-ICU positivity. Age, APACHE II scores on admission, baseline functional impairment, midline shift on initial imaging, and infections were associated with an increased risk of delirium. Delirium was associated with a worse functional outcome.
Conclusions:
RASS dispersion correlates highly with CAM-ICU positivity, and monitoring trends in RASS scores can identify delirium caused by new brain injuries. Delirium as defined by the CAM-ICU is common in patients with SDH and portends worse outcomes.
Keywords: Delirium, Subdural hematomas, Traumatic brain injury
Introduction
Delirium is defined as an abrupt change in a patient’s awareness, attention, and cognition, that fluctuates throughout the course of a day [1]. Delirium is thought to be the result of a complex interplay between baseline patient characteristics, like dementia, and precipitating factors, like systemic inflammation, primary brain injury, sleep deprivation, medication effects, and metabolic derangements [2–6]. While many studies have examined the risk factors and clinical significance of delirium in medically critically ill patients, less attention has been paid to its importance and diagnosis in neurologically critically ill patients.
The study of delirium in neurologically critically ill patients has been limited in part because some studies have suggested a high rate of false positives in this population, particularly from new neurological injuries being misclassified as delirium [7]. This is unsurprising as many of the features of delirium can be seen with neurological injury; for example, prominent inattentiveness has been described with both thalamic injury [8] and frontal lobe injury [9], and non-convulsive seizures have been reported to present with delirium [10]. Further complicating the study of delirium in neurologically critically ill patients is that the most common screens, including the Confusion Assessment Method for the intensive care unit (CAM-ICU), perform worse when language comprehension is impaired [11].
Due to the problems with conventional delirium screens in the setting of brain injury, there is a need to identify screening methods more tailored to this population. Better screening would not only be valuable as a way to help study the unique characteristics of delirium in the setting of brain injury, but will also be crucial for patient care as guidelines increasingly call for routine delirium screening in all critically ill patients [12]. To that end, we wondered whether delirium screening could be performed by monitoring for dispersion in scores on the Richmond Agitation and Sedation Scale (RASS) over time.
The RASS was originally developed as a method of monitoring sedation in the ICU [13] and does not depend on language. Recently, its use has been expanded beyond sedation management and has been explored as a method of assessing the degree of neurological dysfunction in critically ill patients [14] and also has been used as a method of evaluating agitated delirium in patients with subarachnoid hemorrhage [15]. We conducted a prospective study in patients with subdural hematomas (SDH), in which we compared RASS dispersion with the CAM-ICU. In a secondary analysis, we then explored factors associated with delirium as defined by the CAM-ICU and studied the effect of delirium on outcomes in patients with SDH.
Methods
Study Design
Patients with a diagnosis of SDH admitted to the Columbia neurological ICU were prospectively enrolled in this study. Patients were included if the primary indication for ICU admission was an SDH, regardless of the acuity of blood within the hematoma. Patients who were expected to stay in the ICU for less than 24 h were excluded [5]. Patients were screened daily for delirium while in the ICU by performing the CAM-ICU [16]. This was administered by one trained research assistant, and typically done in the late morning or early afternoon, to ensure the patient was maximally wakeful. RASS scores were collected daily by the same research assistant in conjunction with the nurse taking care of the patient, regardless of whether the patient was on sedation or not, and again were obtained every day in the late morning or early afternoon [13]. We also collected demographic, clinical, radiographic, and outcome data on all enrolled patients. We interviewed the patient and/or family members to determine a baseline functional status along with a history of alcohol abuse (defined as three or more drinks per day [5]), tobacco use, or dementia. This study was approved by the hospital’s institutional review board, and written informed consent was obtained from the patient or a surrogate in all cases.
Factors associated with delirium were divided into baseline factors (factors present at hospital admission) and precipitating factors (factors that occur during hospitalization), and the precipitators were further categorized into sources of immobilization, medications, iatrogenic events/surgeries, and intercurrent illness [3]. To ensure that the factors preceded the onset of delirium, medications and procedures/interventions were only considered risk factors if administered or performed within the 24 h prior to onset of delirium (initial positive delirium screen); similarly, infections and other ICU complications were considered relevant only when they developed within 24 h prior to the onset of delirium [3].
Management
All patients were prophylactically placed on leveti-racetam when admitted to the neuro-ICU, regardless of acuity of blood. Levetiracetam was continued for 7 days unless the patient developed clinical seizures, at which time it was continued for the duration of the hospital course. For the patients receiving sedation, specific target RASS scores were used for titration. Operative decisions for acute SDH followed the 2006 guidelines from the Brain Trauma Foundation [17]; surgical decisions for the patients with chronic SDH were made at the discretion of the neurosurgical attending in conjunction with the attending neurointensivist. Workup of positive delirium screens, including electroencephalogram and repeat imaging, was determined by the treating team.
Imaging
All patients had initial head imaging, typically a noncontrast head computed tomography scan although in some cases an magnetic resonance imaging study of the brain without contrast was performed. These images were interpreted by trained neuroradiologists, who determined the chronicity of the bleed and identified any other associated findings, including contusions, herniation, and midline shift. Volumes of subdural collections were calculated in the ABC/2 method [18].
Outcomes
The primary outcome measure was correlation between the standard deviation of RASS scores and CAM-ICU positivity. As a secondary outcome, we looked at whether delirium was associated with a worsening in functional status as determined by contrasting the premorbid modified Rankin Scale (mRS) (determined by patient and/or family interview at time of enrollment) and the discharge mRS [19]. An increase in the mRS of two points or more was considered a poor functional outcome.
Statistics
All statistical analyses were performed in R. We used univariable logistic regression to determine factors associated with delirium and with poor outcome. Significance was judged at < 0.05. Stepwise forward logistic regression was used to develop an adjusted odds ratio (OR) for the effect of delirium on functional outcome on discharge. For each individual patient, all RASS scores were collected; we then calculated a mean and standard deviation of RASS scores in each individual patient. The standard deviation of the RASS scores was then used to quantify the degree of RASS dispersion. For patients enrolled in the study for 3 days or more, we analyzed the size and direction of RASS changes from day-to-day. These data were then analyzed with a standard box plot.
Results
Patient Characteristics
Baseline characteristics are shown in Table 1. A total of 55 patients with the primary diagnosis of SDH were enrolled over the 10-month period of the study. Thirty-eight percent of patients (N = 21) had acute SDH. Four patients had small associated contusions, none of which required additional management. Two patients had associated arteriovenous malformations which were thought to be the cause of their SDH. For patients that were not comatose on admission, enrollment and initial delirium screen occurred at a median of 1 day after ICU admission (IQR 1, 2 days); patients who were initially comatose had their initial screen a median of 2 days after admission (IQR 2, 7 days).
Table 1.
Characteristics of overall cohort (N = 55)
| Age, mean (SD) | 69.4 (13.6) |
| Admission APACHE II score, mean (SD) | 9.7 (6) |
| Admission GCS, mean (SD) | 14.1 (2.35) |
| Functional impairment at baseline, N (%) | 16 (29%) |
| Premorbid anticoagulant use, N (%) | 12 (22%) |
| Premorbid antiplatelet use, N (%) | 15 (27%) |
| Pure acute subdural hematoma, N (%) | 21 (38%) |
| Associated contusions, N (%) | 4 (7%) |
| Aphasia, N (%) | 3 (5%) |
| Underwent operation, N (%) | 41 (75%) |
| Developed seizures, N (%) | 7 (13%) |
| Developed delirium, N (%) | 19 (35%) |
| Delirium subtypea | |
| Hypoactive, N (%) | 14 (74%) |
| Hyperactive, N (%) | 1 (5%) |
| Mixed, N (%) | 4 (21%) |
APACHE II acute physiology and chronic health evaluation II; GCS glasgow coma score; SD standard deviation
Hypoactive delirium is defined as having RASS scores that were ≤ 0, hyperactive delirium is defined as having RASS scores > 0, and mixed is defined as patients with both positive and negative RASS scores
Overall, 19 patients (34%) developed delirium according to the CAM-ICU during their course. Of these patients, 10 (53%) were already delirious at time of enrollment. Seven patients (37%) were comatose at time of enrollment and then screened positive for delirium upon awakening. Two patients (10%) developed delirium after initially screening negative at time of enrollment.
Factors Associated with Delirium
Univariate analysis of factors associated with delirium as defined by the CAM-ICU is shown in Table 2. Among baseline factors, older age, higher admission acute physiology and chronic health evaluation II (APACHE II) scores, baseline disability (defined as premorbid mRS higher than 0), and presence of midline shift on initial head imaging were associated with delirium. SDH volume was not associated with delirium. Burr hole surgery was associated with a lower risk of delirium. Development of infections was associated with an increased risk of delirium.
Table 2.
Factors associated with development of delirium
| No delirium (N = 36) | Delirium (N = 19) | p value | OR (95%CI) | |
|---|---|---|---|---|
| Baseline factors | ||||
| Age, mean (SD) | 65.9 (12.8) | 76.1 (12.6) | 0.01 | 1.1 (1.02–1.1) |
| Gender, N female (%) | 12 (33%) | 3 (16%) | 0.2 | 0.4 (0.1–1.4) |
| Dementia, N (%) | 3 (8%) | 3 (15%) | 0.4 | 2 (0.4–12.3) |
| Smoker, N (%) | 1 (3%) | 0 | 0.99 | |
| Alcohol abuse, N (%) | 6 (17%) | 4 (21%) | 0.69 | 1.3 (0.3–5) |
| Baseline functional impairment, N (%) | 6 (17%) | 10 (53%) | 0.007 | 5.6 (1.6–21) |
| APACHE II, mean (SD) | 7.9 (4.5) | 13.1 (7.1) | 0.006 | 1.2 (1.1–1.3) |
| GCS, mean (SD) | 14.6 (1.5) | 13.3 (3.3) | 0.09 | 0.8 (0.6-l.0) |
| Imaging features | ||||
| Acute hemorrhage, N (%)a | 25 (69%) | 16 (84%) | 0.23 | 2.4 (0.6–11.6) |
| Bilateral, N (%) | 13 (36%) | 3 (16%) | 0.28 | 0.4 (0.1–1.9) |
| Midline shift, N (%) | 19 (53%) | 16 (84%) | 0.03 | 4.8 (1.3–23) |
| Volume, mean (SD) | 69.9 (43.9) | 76.9 (48) | 0.6 | 1 (0.99–1.02) |
| Factors affecting mobilization | ||||
| Foley catheter, N (%) | 11 (31%) | 9 (47%) | 0.22 | 2.1 (0.7–6.6) |
| Restraints (2Pt), N (%) | 6 (17%) | 2 (11%) | 0.5 | 0.6 (0.1–2.9) |
| Subdural drain, N (%) | 4 (11%) | 6 (32%) | 0.07 | 3.7 (0.9–17.6) |
| Medicationsb | ||||
| IV narcotic, N (%) | 19 (53%) | 6 (32%) | 0.1 | 0.4 (0.1–1.3) |
| Benzodiazepines, N (%) | 8 (22%) | 4 (21%) | 0.9 | 0.9 (0.2–3.5) |
| Antipsychotics, N (%) | 2 (6%) | 3 (16%) | 0.2 | 3.2 (0.5–26) |
| Propofol, N (%) | 1 (3%) | 2 (11%) | 0.3 | 4.1 (0.1–92) |
| Dexmedetomidine, N (%) | 1 (3%) | 5 (26%) | 0.03 | 12.5 (1.8–251) |
| Iatrogenic factors | ||||
| Post-op pneumocephalus, N (%) | 9 (25%) | 6 (32%) | 0.6 | 1.4 (0.4–4.7) |
| Operation, N (%) | 25 (69%) | 16 (84%) | 0.2 | 2.4 (0.6–12) |
| Burr hole, N (%) | 22 (61%) | 5 (26%) | 0.02 | 0.2 (0.06–0.7) |
| Craniotomy, N (%) | 5 (14%) | 3 (16%) | 0.08 | 3.9 (0.9–16.6) |
| Intercurrent illness | ||||
| Infection, N (%) | 2 (6%) | 7 (37%) | 0.008 | 9.9 (2.1–73.1) |
| Acute kidney injury, N (%) | 1 (3%) | 3 (15%) | 0.11 | 6.6 (0.8–138) |
| Seizure, N (%) | 1 (3%) | 2 (11%) | 0.3 | 4.1 (0.4–92) |
APACHEII acute physiology and chronic health evaluation II; GCS glasgow coma score; IV intravenous; OR odds ratio; SD standard deviation
Defined as either acute hemorrhage or acute on chronic hemorrhage
Medications were only considered to be a risk factor for developing delirium if they were administered within 24 h of developing delirium
Delirium and Dispersion in RASS Scores
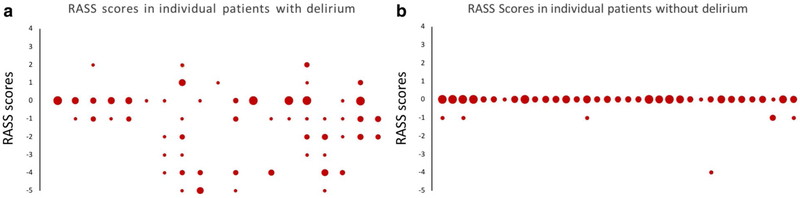
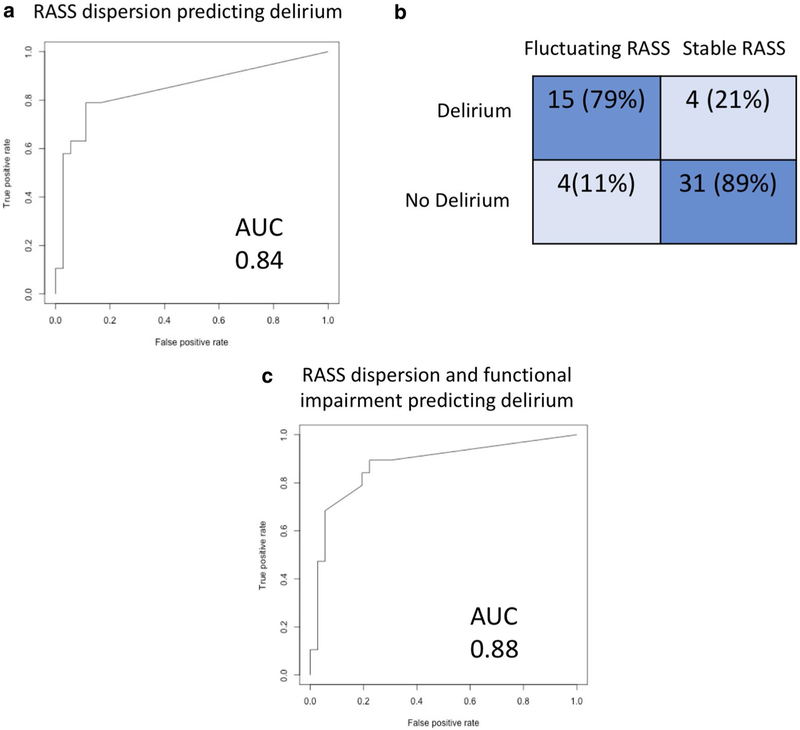
When examining our patient population, RASS scores were noted to be more widely dispersed in patients that screened positive with the CAM-ICU (Fig. 1). We examined whether RASS dispersion (as assessed by the SD of the RASS) reliably diagnosed delirium; a receiver operating characteristic (ROC) curve comparing RASS dispersion to the CAM-ICU is shown in Fig. 2, with an area under the curve (AUC) of 0.84. We then looked at whether a specific cutoff for SD of RASS scores could be defined, such that any patient with a SD larger than that cutoff would be highly likely to have delirium. When the cutoff was set at an SD > 0.4, RASS dispersion had a sensitivity of 81% and a specificity of 89% in terms of predicting CAM-ICU positivity (Fig. 2b). We then combined the baseline factors associated with delirium shown in Table 1 into a model with RASS dispersion, to see whether we could improve the discriminative ability of the test any further; only baseline functional impairment improved the performance, increasing the AUC of the ROC to 0.88 (Fig. 2c).
Fig. 1.
Distribution of RASS scores in each individual patient, comparing patients with (a) and without delirium (b). Along the X axis, each vertical series of circles represents all RASS scores for a single patient. The Y axis refers to scores on the RASS scale. Larger circles mean that the patient had more measurements at a given RASS
Fig. 2.
Reliability of RASS dispersion for identifying delirium. a ROC plot of RASS dispersion for delirium prediction. b 2 × 2 confusion matrix comparing RASS fluctuation to CAM-ICU. Cutoff of SD > 0.4 used for this matrix. c ROC plot showing predictive ability of RASS dispersion and functional impairment combined for delirium prediction
Temporal Pattern of RASS Scores
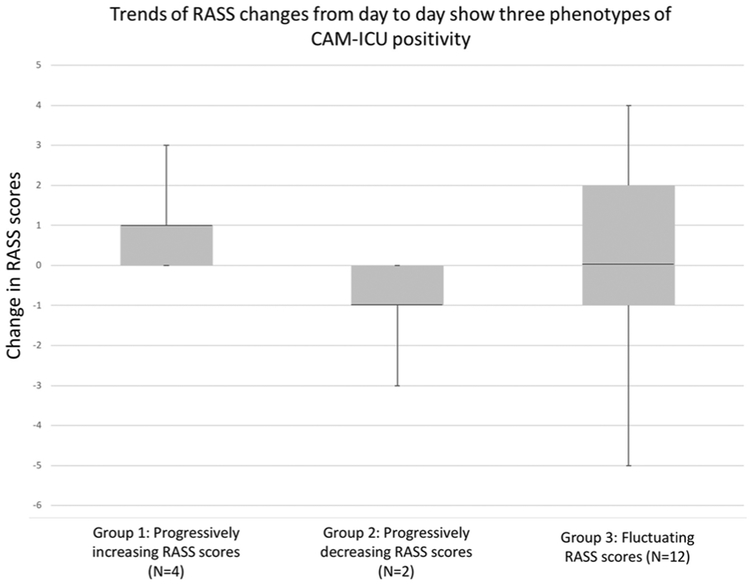
Temporal pattern of RASS changes over time was reviewed for all CAM-ICU-positive patients enrolled in the study for at least 3 days (N = 18), revealing three groups (Fig. 3). In group 1 (N = 4), RASS scores steadily increased over time, in all cases starting with a negative RASS and always plateauing at a score of 0 after several days, at which time CAM-ICU screens were no longer positive. Two of these patients developed uremic encephalopathy prior to screening positive for delirium via the CAM-ICU, and their rising RASS scores correlated with initiation of hemodialysis. The other two patients developed seizures (convulsive in one and non-convulsive in the other) during the 24 h prior to screening positive for delirium, and the rising RASS scores represented slow improvement in mental status after control of the seizures. In group 2 (N = 2), RASS scores steadily declined over time. Both of these patients were eventually found to have worsening SDH with increasing midline shift. The first two groups thus had specific etiologies of their delirium that required a specific intervention. In group 3 (N = 12), there was no clear pattern to the RASS scores, and they fluctuated up and down over the course of the study. None of these patients had clearly identified precipitants for their fluctuations on extensive chart review.
Fig. 3.
Box plots of delta RASS scores show three different populations of CAM-ICU-positive patients. Group 1 represents patients whose RASS scores only increased over time. In two of these patients, this resulted from neurological recovery after effective treatment of seizures; the other two patients steadily improved after initiation of hemodialysis for uremia. Group 2 represents patients whose RASS scores only decreased over time, and both patients were found to have worsening SDH that eventually required surgical intervention. Group 3 represents the rest of the CAM-ICU-positive patients, whose RASS scores fluctuated up and down without clear cause
Delirium and Outcome
Factors associated with poor functional outcome at discharge are shown in Table 3. Age, presence of acute blood on head imaging (both acute and acute on chronic), craniotomy, and development of infections were associated with an increased risk of poor functional outcome. Development of seizures trended toward being associated with a worse outcome but did not reach statistical significance. Delirium as defined by the CAM-ICU was associated with a worse outcome, and this association persisted even when corrected for age and presence of acute blood, with an adjusted OR of 5.8 (95%CI 1.4–30.6).
Table 3.
Factors associated with functional outcome at discharge
| Good outcome (N = 25)a | Bad outcome (N = 30)b | p value | OR (95%CI) | |
|---|---|---|---|---|
| Premorbid and admission factors | ||||
| Gender, N female (%) | 9 (36%) | 6 (20%) | 0.2 | 0.4 (0.1–1.5) |
| Age, mean (SD) | 64.8 (12.1) | 73.2 (13.8) | 0.03 | 1.05 (1.01–1.1) |
| APACHE II, mean (SD) | 8 (5.1) | 11 (6.4) | 0.07 | 1.1 (1–1.2) |
| GCS on admission, mean (SD) | 14.6 (1.5) | 13.8 (2.8) | 0.2 | 0.8 (0.5–1.1) |
| Anticoagulant use, N (%) | 4 (16%) | 8 (27%) | 0.34 | 1.9 (0.5–8} |
| Antiplatelet, N (%) | 6 (24%) | 9 (30%) | 0.62 | 1.4 (0.4–4.7) |
| Imaging characteristics | ||||
| Acute hemorrhage, N (%)c | 15 (60%) | 26 (87%) | 0.03 | 4.3 (1.2–18.1) |
| Midline shift, N (%) | 15 (60%) | 20 (67%) | 0.6 | 1.3 (0.4–4.1) |
| Volume, mean (SD) | 73.7 (47) | 71.3 (44.3) | 0.85 | 1.0 (0.99–1.01) |
| Neurological symptoms | ||||
| Delirium, N (%) | 3 (12%) | 16 (53%) | 0.003 | 8.4 (2.3–41) |
| Hemiparesis, N (%) | 10 (40%) | 11 (37%) | 0.8 | 0.9 (0.3–2.6) |
| Clinical management | ||||
| Operation, N (%) | 18 (72%) | 23 (83%) | 0.7 | 1.3 (0.4–4) |
| Burr hole (overall), N (%) | 17 (68%) | 15 (50%) | 0.18 | 0.5 (0.2–1.4) |
| Craniotomy, N (%) | 2 (8%) | 9 (30%) | 0.06 | 4.9 (1.1–34.8) |
| Hemicraniectomy, N (%) | 0 | 2 (7%) | 0.99 | |
| Post-op subdural drain, N (%) | 3 (12%) | 7 (23%) | 0.3 | 2.2 (0.5–11) |
| Hospital complications | ||||
| Seizure, N (%) | 3 (12%) | 10 (33%) | 0.07 | 3.7 (0.96–18.1) |
| Infection, N (%) | 2 (8%) | 10 (33%) | 0.04 | 5.8 (1.3–40.4) |
APACHEII acute physiology and chronic health evaluation II; GCS glasgow coma score; SD standard deviation
Good outcome is defined as either no increase or a 1-point increase in the modified Rankin Scale at discharge when compared with the premorbid modified Rankin Scale
Bad outcome is defined as an increase in the modified Rankin Scale of 2 or more points when compared to the premorbid modified Rankin Scale
Defined as either acute hemorrhage or acute on chronic hemorrhage
Discussion
Our data suggest that delirium as defined by the CAM-ICU is common in patients with SDH and is associated with a worse functional outcome. Almost half of the patients were positive on the CAM-ICU on admission in our cohort; this suggests that the clinical features used to diagnose delirium are an early development in the setting of SDH and may be a result of the SDH itself. An alternative explanation is that delirium may precede the hematomas in some cases, perhaps putting patients at increased risk of falls and thus causing the SDH itself. Regardless, the presence of delirium early in the hospital course has been demonstrated in other studies in critically ill patients, with 50–75% of patients delirious within the first 24 h of admission [5, 20, 21]. The relatively rapid onset of delirium in critically ill patients may reflect the high severity of illness when compared to non-ICU patients, who tend to develop delirium later [3].
RASS dispersion was strongly associated with CAM-ICU positivity in our study, producing an ROC curve with an AUC of 0.84. The only factor we identified that improved the correlation between RASS dispersion and the CAM-ICU was baseline functional impairment, which was also strongly associated with CAM-ICU positivity in our study. As a delirium screen, RASS dispersion could be used alone or in combination with standard screens; it may be particularly helpful in patients with impaired language comprehension. In order to use RASS dispersion as a screen, scores on the RASS would have to be determined regularly, although the ideal frequency is unknown. Our study obtained RASS scores daily; however, obtaining scores more frequently may make it easier to identify significant measurement-to-measurement fluctuation in a given patient, thereby making it easier to rapidly identify delirium. If scoring multiple times per day, standardization of the timing will be critical to avoid scoring patients when they normally would be asleep. Future studies should identify the ideal frequency of obtaining RASS scores for this purpose.
We found that examining the temporal trend of RASS scores could help identify patients with new or worsening brain injuries that screened positive for delirium in our cohort. These patients have been referred to as false positives in some prior studies of delirium screening; [2] others have argued that the definition of delirium does not connote a specific etiology, and thus do not consider them false positives [11]. Regardless of whether to classify these patients as false positives or not, they require specific diagnostic tests and interventions and may have different prognoses. The inability of standard delirium screens to identify these patients has been cited as a reason why delirium screening is less valuable in the neuro-ICU [2]. Monitoring temporal trends in RASS scores may be added to standard delirium screening to help identify these patients, although more study about the effect of brain injuries on RASS trends will certainly be needed, as the RASS has not been formally studied in the setting of primary brain injury.
Our small sample size prevented us from conducting a multivariable analysis of either secondary outcome (development of delirium and functional outcome at discharge) and limits the strength of conclusions we can draw from our analysis. Nevertheless, a few important findings emerged from our analysis of factors associated with delirium as defined by CAM-ICU positivity. Similar to prior ICU-based cohorts [22], we found age, higher acuity of illness (based on APACHE II scores), infectious complications, and poor baseline functional status to be associated with delirium. This suggests that delirium in brain-injured patients may share some mechanisms in common with the general ICU population. On the other hand, the association between delirium and midline shift is unique to brain-injured patients. Lateral displacement of midline structures is known to correlate closely with symptoms of altered consciousness in the setting of mass lesions [23, 24], which likely explains the effect of midline shift on delirium in our cohort. Among the medications we examined, only dexmedetomidine was associated with delirium in our study; these results should be interpreted with caution considering our small sample size and the relatively infrequent use of psychoactive medications in our cohort. While dexmedetomidine is a sedating medication and could cause many of the clinical symptoms of hypoactive delirium, it is more likely that the association seen in our study is the result of dexmedetomidine being preferentially used for emerging agitation prior to a patient’s first positive delirium screen.
There are several limitations to this study. First, we only obtained scores on the CAM-ICU and RASS once daily, which might have missed some cases of delirium and/or fluctuation in the RASS. Second, the total number of patients was small, and data were collected only at one institution. As discussed before, the small cohort size prevented us from running a multivariable analysis of either secondary outcome. The small cohort also limits the strength of our analysis of RASS score trends, which will need to be studied on a larger scale. A third concern is whether these results will be generalizable, as RASS fluctuation may not correlate as highly with delirium in other brain-injured patient populations, particularly in cohorts where sedating medications are used more commonly. SDH patients tend to represent a very heterogeneous group, with considerable differences existing between acute and chronic hematomas in terms of their management, clinical manifestations, and outcomes; this supports the idea that RASS fluctuation may be more generalizable for patients with different types of brain injury. Finally, in order to have a comparison for RASS fluctuation, we used the CAM-ICU as a gold-standard delirium screen; this is a commonly used screen in neurologically ill patients, but has not been fully validated [7], and utilizes RASS scores for some of its criteria. A definitive study would need to compare RASS fluctuation to DSM-V criteria as the gold standard.
Conclusions
We found that RASS dispersion correlated strongly with delirium as defined by the CAM-ICU, suggesting that it could be used as a delirium screen. Further, monitoring temporal trends in RASS scores may yield insight into the underlying etiology of delirium. We also found that delirium as defined by the CAM-ICU is common in SDH patients admitted to the neurological ICU and shares several factors associated with delirium in other critically ill patient populations. Our data further suggest that delirium may be a poor prognostic sign in SDH patients.
Acknowledgements
Drs. Robinson and Claassen had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Source of support
This study was funded by Dana Foundation, James S. McDonnell Foundation, and the National Library of Medicine (Grant #R01LM011826).
Footnotes
Conflict of interest
The authors declare that they have no relevant conflict of interest, but JC does declare that he is a minority shareholder at iCE Neurosystems.
Ethical Approval
The study was approved by the CUMC Institutional Review Board (IRB).
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Frontera JA. Delirium and sedation in the ICU. Neurocrit Care. 2011;14:463–74. [DOI] [PubMed] [Google Scholar]
- 3.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852–7. [PubMed] [Google Scholar]
- 4.Reznik ME, Schmidt JM, Mahta A, et al. Agitation after subarachnoid hemorrhage: a frequent omen of hospital complications associated with worse outcomes. Neurocrit Care. 2017;26:428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Rompaey B, Elseviers MM, Schuurmans MJ, Shortridge-Baggett LM, Truijen S, Bossaert L. Risk factors for delirium in intensive care patients: a prospective cohort study. Crit Care. 2009;13:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caeiro L, Ferro JM, Albuquerque R, Figueira ML. Delirium in the first days of acute stroke. J Neurol. 2004;251:171–8. [DOI] [PubMed] [Google Scholar]
- 7.Patel MB, Bednarik J, Lee P, et al. Delirium monitoring in neurocritically ill patients: a systematic review. Crit Care Med. 2018;46:1832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmahmann JD. Vascular syndromes of the thalamus. Stroke. 2003;34:2264–78. [DOI] [PubMed] [Google Scholar]
- 9.Alexander MP, Stuss DT, Shallice T, Picton TW, Gillingham S. Impaired concentration due to frontal lobe damage from two distinct lesion sites. Neurology. 2005;65:572–9. [DOI] [PubMed] [Google Scholar]
- 10.Drislane FW. Presentation, evaluation, and treatment of nonconvulsive status epilepticus. Epilepsy Behav. 2000;1:301–14. [DOI] [PubMed] [Google Scholar]
- 11.Mitasova A, Kostalova M, Bednarik J, et al. Poststroke delirium incidence and outcomes: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2012;40:484–90. [DOI] [PubMed] [Google Scholar]
- 12.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. [DOI] [PubMed] [Google Scholar]
- 13.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. [DOI] [PubMed] [Google Scholar]
- 14.Vasilevskis EE, Pandharipande PP, Graves AJ, et al. Validity of a modified sequential organ failure assessment score using the Richmond Agitation-Sedation Scale. Crit Care Med. 2016;44:138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reznik ME, Mahta A, Schmidt JM, et al. Duration of agitation, fluctuations of consciousness, and associations with outcome in patients with subarachnoid hemorrhage. Neurocrit Care. 2018;29:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29:1370–9. [DOI] [PubMed] [Google Scholar]
- 17.Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of acute subdural hematomas. Neurosurgery. 2006;58:S16–24 (discussion Si-iv). [PubMed] [Google Scholar]
- 18.Gebel JM, Sila CA, Sloan MA, et al. Comparison of the ABC/2 estimation technique to computer-assisted volumetric analysis of intraparenchymal and subdural hematomas complicating the GUSTO-1 trial. Stroke. 1998;29:1799–801. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen RS, Ostergaard A, Kjaer P et al. Stroke rehabilitation at home before and after discharge reduced disability and improved quality of life: a randomised controlled trial. Clin Rehabilit. 2016;30:225–36. [DOI] [PubMed] [Google Scholar]
- 20.Mehta S, Cook D, Devlin JW, et al. Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Crit Care Med. 2015;43:557–66. [DOI] [PubMed] [Google Scholar]
- 21.Dubois MJ, Bergeron N, Dumont M, Dial S, Skrobik Y. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27:1297–304. [DOI] [PubMed] [Google Scholar]
- 22.Zaal IJ, Devlin JW, Peelen LM, Slooter AJ. A systematic review of risk factors for delirium in the ICU. Crit Care Med. 2015;43:40–7. [DOI] [PubMed] [Google Scholar]
- 23.Ropper AH. A preliminary MRI study of the geometry of brain displacement and level of consciousness with acute intracranial masses. Neurology. 1989;39:622–7. [DOI] [PubMed] [Google Scholar]
- 24.Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med. 1986;314:953–8. [DOI] [PubMed] [Google Scholar]