Abstract
Role of gut microbiome in obesity and type 2 diabetes (T2D) became apparent from several independent studies, indicating that gut microbiome modulators like prebiotics may improve microbiome perturbations (dysbiosis) to ameliorate metabolic derangements. We herein isolate water soluble, non-digestible polysaccharides from five plant based foods (acorn, quinoa, sunflower, pumpkin and sago seeds) and assess their impact on human fecal microbiome and amelioration of high fat diet (HFD)-induced obesity/T2D in mice. During polysaccharide isolation, purification, biochemical and digestion resistance characterization, and fermentation pattern by human fecal microbiome, we select acorn- and sago- derived prebiotics (on the basis of relatively higher purity and yield and lower protein contamination) and examine their effects in comparison to inulin. Prebiotics treatments in human fecal microbiome culture system not only preserve microbial diversity but also appear to foster beneficial bacteria and short-chain fatty acids (SCFAs). Feeding of acorn- and sago-derived prebiotics ameliorates HFD-induced glucose intolerance and insulin resistance in mice, with effects comparatively superior to those seen in inulin-fed mice. Feeding of both of novel prebiotics as well as inulin increases SCFAs levels in the mouse gut. Interestingly, gut hyperpermeability and mucosal inflammatory markers were significantly reduced upon prebiotics feeding in HFD-fed mice. Hypothalamic energy signaling in terms of increased expression of pro-opiomelanocortin, was also modulated by prebiotics administration. Results demonstrate that these (and/or such) novel prebiotics can ameliorate HFD-induced defects in glucose metabolism via positive modulation of gut-microbiome-brain axis and hence could be useful in preventing/ treating diet-induced obesity/T2D.
Keywords: Prebiotic; Polysaccharides; Fibers; Microbiome; Metabolites; obesity,; diabetes
INTRODUCTION
The relationship between gut microbiome and human health became one of the most significant scientific discoveries of the past decade [1, 2]. The complex composition of gut microbial community influences host health and vice-versa [3]. Gut microbiome perturbations (dysbiosis) have been associated with several human diseases including obesity and diabetes [4]. Therefore, manipulation of gut microbiome can ameliorate host health and disease pathology, remain of great interest to develop therapeutic strategies against such maladies [5]. However, diet is one of the major manipulator of microbial species living in the gut and their metabolic activities via involving fermentation of non-digestible dietary fibers [6]. Produced secondary metabolites after microbial fermentation remain major denominators to define interactions between microbiome and host cellular systems [7]. Dietary fibers and several non-digestible oligo- or poly-saccharides are known to be one of the important microbiome modulators that are fermented by gut microbes [8]. These polysaccharides are neither digestible nor absorbable by mammalian gut, hence reach to lower part of intestine wherein these serve as food/substrate for microbial fermentation and promote the growth and activities of certain health beneficial bacterial communities [9]. Such polysaccharides are generally termed as ‘prebiotics’, however according to recent definition, they must follow three criteria; 1) resistant to mammalian digestive enzymes, acidity and intestinal absorption, 2) fermentable by gut microbiota and 3) growth stimulator of selective gut microbial communities associated with health benefits [10]. Consumption of prebiotics in early and adult life lead to increase production of certain fermentative metabolites like short chain fatty acids (SCFAs i.e. acetate, propionate and butyrate) that exert health beneficial effects to the host [11, 12]. Prebiotics are heterogeneous and complex polysaccharides and are thereby defined either by the origin and/or chemical composition of their single sugar moieties, both of which can impact the pattern of their microbial fermentation as well as microbial consumers of these complex carbohydrates, thereby imparting unique effects on the gut microbiome [13, 14]. Although, the most commonly studied prebiotics are inulin-type (e.g., fructo-oligosaccharides, galacto-oligosaccharides, etc.) [9, 15–17], however newly developed prebiotics are highly warranted to devise strategies specific for increasing demand of prebiotics use in different sectors, as well as trailer according to different pathological conditions to manipulate gut microbiome and benefit host.
Polysaccharides from diverse natural sources have been isolated and studied for health beneficial impact in animal and human studies [18]. Prebiotics such as inulin are known to exhibit anti-carcinogenic effects against colon cancer [19–21]. Prebiotic pectic-oligosachairdes have been found to modulate the gut microbiome (increased Bacteroides-Prevotella groups) with enhanced short-chain fatty acid (SCFAs) production (especially acetate and propionate) in human feces [22]. Oligofructose (Orafti, Tienen, Belgium) prebiotics feeding to obese and diabetic mice has been shown to augment beneficial gut microbiome signature along with improvements in glucose homeostasis, leptin sensitivity, inflammatory status, intestinal turnover and target enteroendocrine cell activity [23]. Short-term feeding of Promitor™ Soluble Corn Fibre (a maize-derived source of dietary fiber) boosted Bifidobacteria population in the gut [24]. Altogether, these studies evidence potent beneficial roles of prebiotics in wide-spectrum health conditions. However, comprehensive effects of prebiotic formulations on host metabolism and the mechanisms underlying these effects remain largely unclear. Therefore, exploration of novel and more defined prebiotics remains requisite and could facilitate the development of more efficient remedies to ameliorate obesity/ diabetes and other human health problems.
In-vitro fermentation systems are becoming popular models to define the direct impact of prebiotics interventions on gut microbiome by saving time, efforts and money, in prior and/or parallel to next level physiological relevance. Recently, Long et al.[25] defined the effects of media conditions and prebiotic fermentation on human gut microbiome using in-vitro culture. Vigsnæs et al. [26] showed that sugar beet arabino-oligosaccharides stimulated dramatic changes in gut microbiome by stimulating the growth of Bifidobacterium spp. and Lactobacillus spp. in an in-vitro fermentation system of ulcerative colitis patient’s feces. Takagi et al. [27] validated high-throughput screening of prebiotics using single-batch fermentation system to analyze the effects on gut microbiome. Recently, we also validated this system to demonstrate the effect of a human-origin probiotic cocktail on the microbiome composition and organic acids levels in human feces [28]. In the present study, we isolate and characterize novel prebiotics from common plant-based food ingredients and analyze their influence on human fecal microbiome and its metabolic activities in comparison to one of the most-widely studied prebiotics namely, inulin. In addition, we demonstrate several beneficial effects of selected prebiotics in the prevention of high-fat diet (HFD)-induced obesity and diabetes in mice via modulating microbiome-gut-brain axis.
RESULTS
Yield and purification of water-soluble polysaccharides.
We extracted total polysaccharides from acorns, pumpkin seeds, quinoa, sunflower seeds and sago, and found that the total polysaccharide yield was highest in sago (17.5% of dry weight) followed by acorns (5%), quinoa (3%), sunflower seeds (0.2%) and pumpkin seeds (0.1%) (Supplementary Figure S1). In terms of the total sugar content in the dry weight of total extracted polysaccharides, the sago extracts were found to possess highest purified sugars (99.8%) followed by quinoa (99.1%), acorns (98.7%), sunflower seeds (69%) and pumpkin seeds (51.2%). Protein content was found to be highest in sunflower seeds (7.5%), followed by pumpkin seeds (6%), acorns (3%) with no detectable protein contamination in quinoa and sago extracts. However, total polyphenolic compounds were lowest in sago extract (0.09%) with increasing trend in quinoa (0.17%), sunflower seeds (0.27%), acorns (0.3%) and pumpkin seeds (0.57%). Interestingly, the polyphenolic contents in these extracts directly correlated with the high antioxidant activity of these extracts (Supplementary Table 1).
Prebiotic features of newly isolated polysaccharides.
Upon analyzing the carbohydrate digestion pattern of different polysaccharide extracts using simulated gastric fluid (pH 1.2), simulated intestinal fluid (pH 7.4 with α-amylase) and mixture of simulated gastric and intestinal fluids (pH 4.5) [29], we found that the sago polysaccharide extract was digested at highest rate upon digestion in simulated gastric juice, while the digestion rate in simulated intestinal fluids was highest for acorn extract (Supplementary Table 2). Interestingly, mixture of simulated gastric and intestinal juice did not exhibit any differential effects on digestion of these polysaccharide extracts, suggesting that these differences might be due to different pH of gastric versus intestinal fluids. Enzyme activities viz. α-amylase, however, did not show any significant difference among five polysaccharide extracts (Supplementary Table 2).
Influence of novel prebiotics on fecal microbiome of healthy and diseased human subjects.
On the basis of our preliminary analyses of prebiotic yield, purity, sugar content and digestibility patterns, we selected acorn and sago prebiotics to further investigate their impact on human fecal microbiome using an ex-vivo anaerobic fecal slurry culture system and compared their effects to those of inulin. In addition, we also designed our study in a way to approximately distinguish the effects of selected prebiotics on gut microbiome in healthy versus diseased (heart failure patients) subjects. Principal co-ordinate analysis (PCoA) of microbial β-diversity (weighted and unweighted unifrac) indicated that the prebiotics treatments induced increased β-diversity in diseased fecal microbiome, while no significant change in β-diversity was seen in healthy fecal microbiome (Figure 1a,b). Alpha-diversity in terms of Shannon index was significantly higher in all prebiotics treatments in both healthy and diseased fecal microbiome (Figure 1c); however, other alpha-diversity indices like phylogenetic degree (PD) tree, number of observed OTUs, and Chao1 were significantly increased in diseased fecal microbiome samples, with minor elevation in healthy feces (Supplementary Figure S2a,c), suggesting that prebiotics intervention modulated the gut microbial diversity more prominently in diseased sample as compared to healthy microbial ecosystem. Interestingly, acorn prebiotic significantly increased Bacteriodetes in healthy fecal microbiome, while both acorns and sago prebiotics significantly increased Bacteriodetes and decreased Firmicutes in diseased microbiome (Figure 1d). However, Proteobacteria abundance was significantly decreased upon all the prebiotic treatments compared to control (non-treated) (Figure 1d). Family Prevotellaceae and genus Prevotella were dominant in healthy feces and were increased further upon inulin and sago treatments, while diseased feces were enriched with Bacteroidaceae and Bacteriodes that were increased after prebiotics treatments (Figure 1e,f). Abundance of unclassified genera belonging to family Enterobacteriaceae was significantly higher in diseased feces compared to healthy samples, but decreased upon prebiotics treatments (Figure 1f) in both types of fecal cultures. Linear discrimination analysis (LDA) shows that the inulin treatment induced minor changes including significantly increased Firmicutes (e.g., Dialister and Megasphaera) in healthy fecal microbiome, while this treatment dramatically changed the microbiome signature in diseased feces by increasing Firmicutes groups viz. Dialister, Megasphaera, Phascoarctobacterium, Faecalibacterium, Blautia, Clostridium, Streptococcus and Enterococcus, as well as Actinobacteria viz. Bifidobacterium. Acorns prebiotics treatment increased Bacteriodetes members viz. Bacteroides and Prevotella; Firmicutes member Fecalibacterium, and Proteobacteria groups viz. Sutterella and Succinovibrio in healthy fecal microbiome (Figure 1g), while it increased Firmicutes viz. Oscillospira, Catenibacterium and Sutterella in diseased microbiome (Figure 1h). The most significant changes in microbiome signature were noticed in sago prebiotics treated healthy feces, where Firmicutes including Bulleidia, Clostridia, Ruminococcus Streptococcaceae, and Lactococcus, and Actinobacteria including Bifidobacteria and Collinsella, and Methanobrevibacter were significantly increased, while Firmicutes member Lactobacillus and Bacteriodetes members Paraprevotella, Butyricomonas, Parabacteriodes and S24_7 were increased in diseased feces (Supplementary Figure S3b). In addition, all the prebiotics significantly decreased Enterobacteriales, unclassified Clostridiales, Trabulsiella, Enterobacter and Anaerostipes (Supplementary Figure S3a,b).
Figure 1. Effect of inoculation of prebiotics (inulin, acorn and sago) on the microbiome diversity and composition in the feces of healthy versus diseased (heart attack patients) subjects.
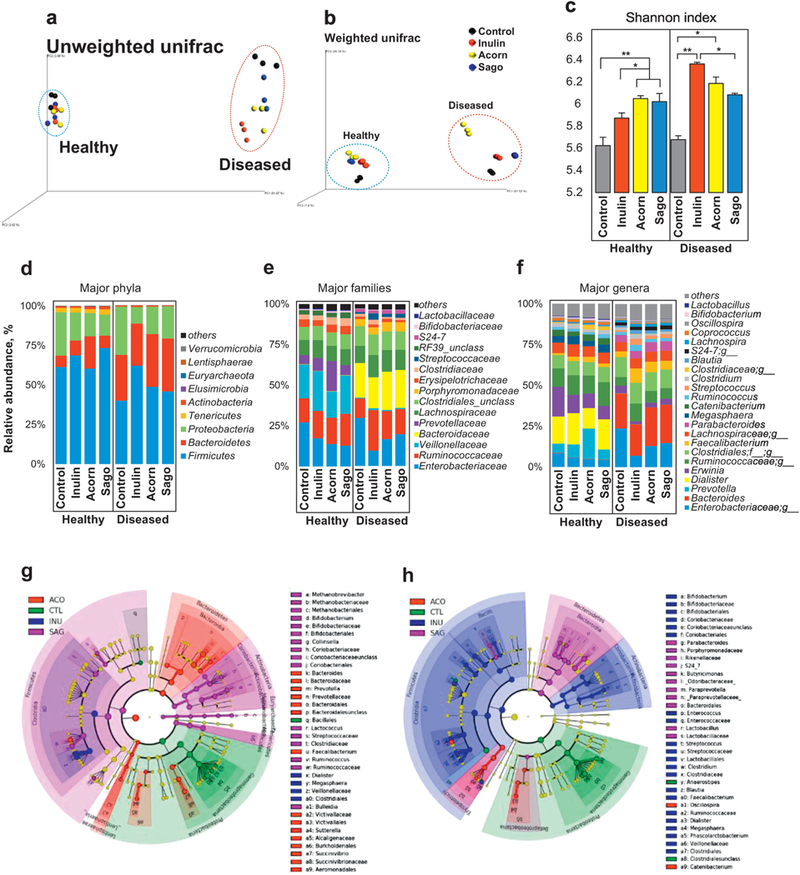
(a,b) PCoA analyses (a: unweighted unifrac; b: weighted unifrac) representing the β-diversity of microbiome; (c) Shannon index representing the α-diversity of microbiome; (d-f) relative abundance of major phyla (d), families (e) and genera (f); and (g,h) Linear discrimination analysis (LDA) using LDA effect size (LefSe) algorithm; in the fecal suspension from healthy and diseased subjects after 9h incubation with (treatment) or without (control) prebiotics. Each dot in PCoA analysis represents two replicates of fecal slurry collected from two different replication experiments of same group. CTL: Control, INU: inulin, ACO: acorn, and SAG: sago. Values are presented as mean ± SD/ SEM. *P<0.05; ** P<0.01; ***P<0.001.
Inoculation of prebiotics decreases pH and enhances SCFAs levels in the feces of healthy and diseased human subjects.
Simple and complex non-digestible polysaccharides primarily go through gut microbiota fermentation wherein gut microbes metabolize these substrates and produce diverse metabolites, mainly organic acids including lactate, acetate, propionate, butyrate, etc. [30, 31]. We assessed the prebiotic fermentative potential of healthy versus diseased human fecal microbiome in fecal culture system (ex-vivo) to produce organic acids and found a significant reduction in the pH of fecal slurry of both healthy and diseased donors after 9h of incubation with each of the prebiotics tested (Figure 2a). Further assessment of SCFAs including lactate, acetate, propionate and butyrate produced upon fermentation of prebiotics by human fecal microbiome revealed that the acorns increased lactate, while sago prebiotics increased acetate production compared non-treated controls in healthy fecal microbiome (Figure 2b). While no significant effect of any of the prebiotics treatments on lactate production was observed in diseased fecal microbiome, acetate production was significantly decreased in these fecal microbiome (Figure 2c). Interestingly, acorns prebiotics increased propionate production in both healthy and diseased fecal microbiome cultures, while sago prebiotics increase propionate only in diseased feces, whereas inulin treatment increased propionate only in healthy fecal microbiome (Figure 2b,c). No significant changes were seen in butyrate production in healthy and diseased microbiome fecal cultures upon prebiotics intervention compared to control (Figure 2b,c). Further correlation analysis of gut microbiome, pH and SCFAs content in healthy fecal specimen suggested that increased lactate production was primarily attributed to sago prebiotics that was also associated with increased abundance of Streptococcus, Actinobacteria and Lactococcus (Figure 2d). Propionate production was significantly increased by inulin and acorns and was correlated with increased abundance of Lactobacillus in inulin; however, Bacteriodetes, Prevotella and Faecalbacterium were positively associated with propionate but insignificantly. In diseased fecal microbiome, acetate production was decreased in all the prebiotics treated groups and was correlated with Rikenellaceae (Figure 2d, lower panel). Increased propionate levels primarily in acorn prebiotics were correlated with Bacteroidetes, Bacteroides, Parabacteroides, S24_7, Rikenellaceae, Paraprevotella, and Sutterella (Figure 2d).
Figure 2. Effects of inoculation of prebiotics (inulin, acorn and sago) on the metabolic activity of microbiome in the feces of healthy versus diseased subjects.
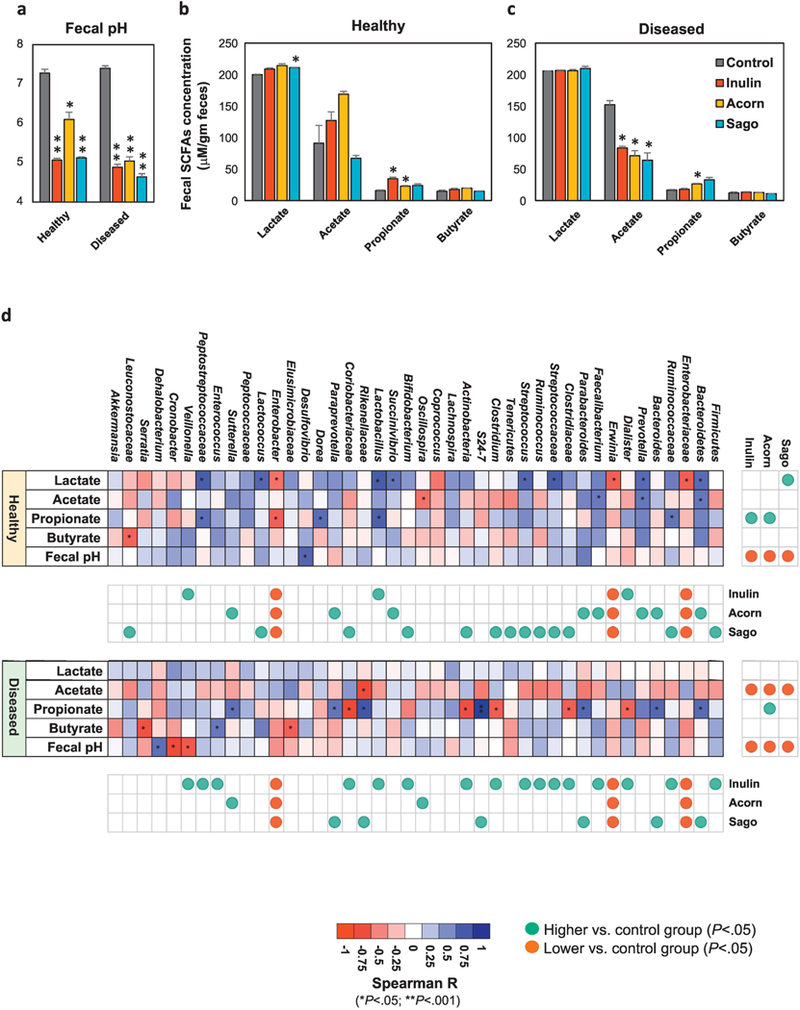
(a) Fecal pH of the fecal suspension from healthy and diseased subjects after 9h incubation with (treatment) or without (control) prebiotics. (b,c) Production of lactate, acetate, propionate and butyrate in the fecal suspension from healthy (b) and diseased (c) subjects after 9h incubation with (treatment) or without (control) prebiotics. (d) Correlation of microbial groups with pH and SCFAs levels in the fecal suspension from healthy and diseased subjects after 9h incubation with (treatment) or without (control) probiotics. Values are presented as mean ± SD/SEM of n=6 replicates per treatment group. P*<0.05; **P<0.01.
Prebiotics prevent development of glucose intolerance and insulin resistance in HFD-fed mice.
To examine the effect of prebiotics on the features of host glucose metabolism, we fed mice with selected prebiotics (acorns and sago) supplemented (5%) in high-fat diet (HFD) for 8 weeks. No notable differences were observed in body weight, total fat depots, and liver and quadriceps muscle weights (Figure 3a; Supplementary Figure S4a–c). However, interestingly, a faster clearance of glucose within 2 hrs during both intraperitoneal (i.p.) and oral glucose tolerance test (IPGTT and OGTT) was seen in both acorn and sago prebiotics fed mice (Figure 3b,c), as also further evident from a significantly decreased area under curve (AUC) of GTTs in acorn and sago fed mice (Supplementary Figure S4d,e), thereby suggesting that both prebiotics prevented the progression of HFD-induced glucose intolerance (a prediabetes condition) in mice. In addition, a significantly faster decline/ clearance of glucose in response to exogenous insulin (Figure 3d), with an increased AUC (Supplementary Figure S4f), was also seen during insulin tolerance test (ITT) in mice fed with prebiotics, especially inulin and acorns, suggesting that prebiotics prevented development of HFD-induced insulin resistance (a hallmark of type 2 diabetes). No significant differences were seen in fasting blood glucose (Supplementary Figure S4g) and food intake (Figure 3e) in prebiotics-fed versus control groups. In addition, calorie intake from HFD were also almost identical among all the groups, and in fact prebiotics fed mice consumed extra calories (Figure 3e,f), suggesting that small differences in calories might not account for differences in body weight and adiposity, however the source of calories and how these get digested may have larger effects. We also noted that white adipose tissue (epididymal/ gonadal) cell size was smaller in prebiotics-fed (especially inulin and acorn) mice compared to control counterparts. In addition, each of the prebiotics significantly decreased fat accumulation in brown adipose tissue (supra-scapular) and liver (Figure 3g). Overall, these results suggest that these prebiotics prevented HFD-induced metabolic derangements i.e., glucose intolerance, insulin resistance and tissue fat accumulation in mice.
Figure 3. Prebiotics prevent high-fat diet (HFD)-induced obesity in mice.
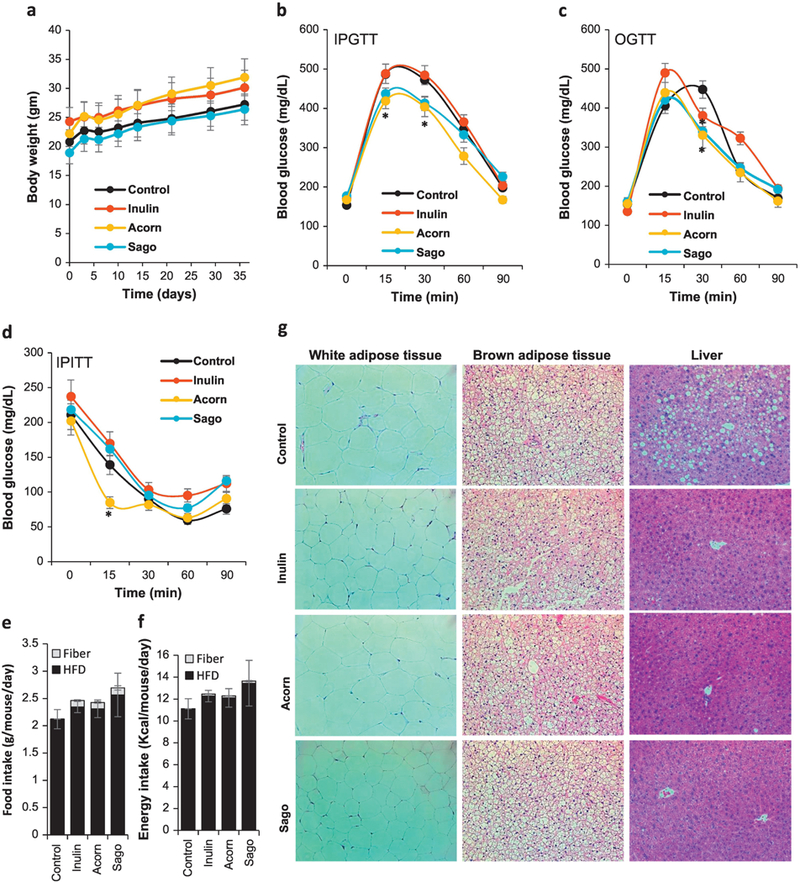
(a) Body weight during HFD feeding with and without prebiotics up to 35 days/ 5 weeks. (b-d) Intraperitoneal glucose tolerance test (IPGTT; b), oral glucose tolerance tests (OGTT; c) and intraperitoneal insulin tolerance test (IPITT; d) in prebiotics-fed and control mice after 5 weeks of intervention. (e) Hematoxylin and eosin (H&E) staining of white adipose tissue (gonadal), brown adipose tissue and liver of prebiotics-fed and control mice. Values are presented as mean ± SD/SEM. P*<0.05; **P<0.01; NS: non-significant.
Prebiotics prevent gut microbiome dysbiosis in diet-induced obese (DIO) mice.
Upon evaluating the impact of these novel prebiotics on HFD-induced gut microbiome dysbiosis in mice, we noted dramatic differences in the β-diversity (a measure of overall gut microbial diversity as revealed by the PCoA analysis) of gut microbiome in all of the prebiotics-fed mice as compared to control (non-treated) group, (Figure 4a; Supplementary Figure S5a), thereby clearly indicating that prebiotics significantly modulated the signatures of gut microbiome in these mice. Furthermore, α-diversity measures including PD whole tree, Chao1, observed OTUs and Shannon index significantly differed between prebiotics versus control groups (Figure 4b, Supplementary Figure S5b–d). Particularly, acorns and sago prebiotics-fed mice appeared to have lower indices of all these measures; however, these differences were associated with the expansion of Bacteroidetes and decline in Firmicutes abundance (Figure 4c, Supplementary Figure S5e). A significantly lower abundance of unclassified Clostridiales group was seen in inulin- and sago-fed groups, with no significant changes in acorns group (Figure 5e, Supplementary Figure S5f, S6). Ruminococcaceae (specifically Oscillospira), Desulfovibrionaceae (genus Desulfovibrio) and Bilophila were lower in all of the prebiotics groups compared to control; however, S24_7 (a major Bacterioidetes family) was significantly higher especially in acorns- and sago-fed mice. Family Erysipelotrichaceae was significantly expanded in all the prebiotics-fed groups (Supplementary Figure S6). In addition, Lactobacilliaceae (Lactobacillus) and Verrucomicrobiaceae (Akkermensia) were higher in inulinfed mice, while no significant changes in the abundance of these clades were seen in acorns-and sago prebiotics-fed mice (Figure 4d). Interestingly, linear discrimination analysis (LDA) using LefSe (LDA Effect Size) demonstrated higher abundance of Bifidobacteria, Mucispirilium, Enterococcus, Lactobacillus, Clostrium, Dorea and Ruminococcus genera are enriched in inulin-fed mice, while Bacteroides, Parabacteroides, S24_7, Lactococcus, Streptococcus and SMB53 abundance were enriched in sago-fed mice (Figure 5e; Supplementary Figure S6). AF12 was only significantly higher in acorns-fed mice (Figure 4e).
Figure 4. Prebiotics modulate the diversity and composition of gut microbiome in HFD-fed mice.
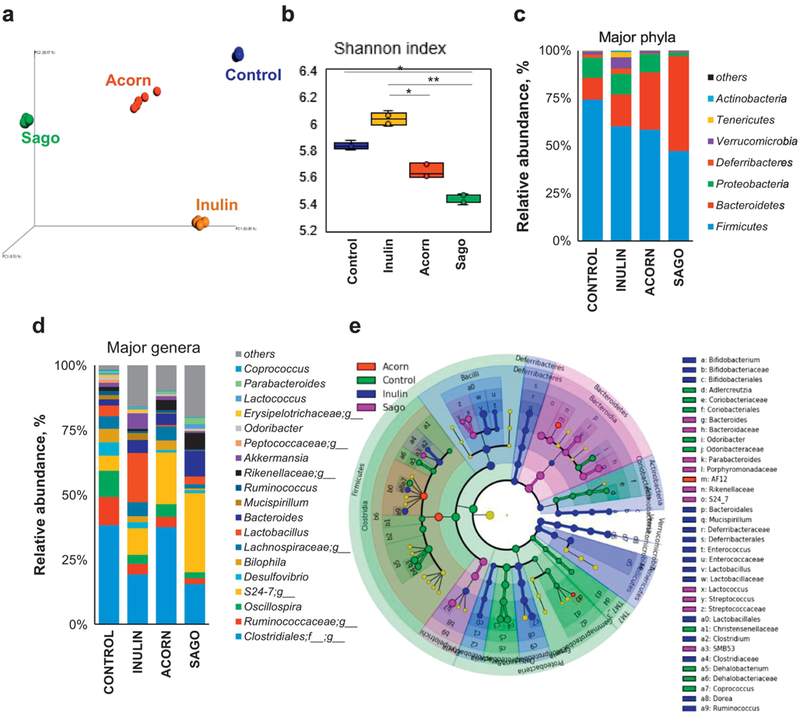
a) PCoA analyses presenting β-diversity (weighted unifrac) of the gut microbiome in prebiotics-fed and control mice after 5 weeks of intervention. (b) Shannon index representing the α-diversity of gut microbiome in prebiotics-fed and control mice after 5 weeks of intervention. (c,d) Relative abundances of major phyla (c) and genera (d) in prebiotics-fed and control mice after 5 weeks of intervention. (d) Linear discrimination analysis (LDA) using LDA effect Size (LefSe) algorithm of gut microbial taxa in prebiotics-fed and control mice after 5 weeks of intervention. Values are presented as mean ± SD/SEM. P*<0.05; **P<0.01.
Figure 5. Prebiotics modulate the metabolic activities of gut microbiome in HFD-fed mice.
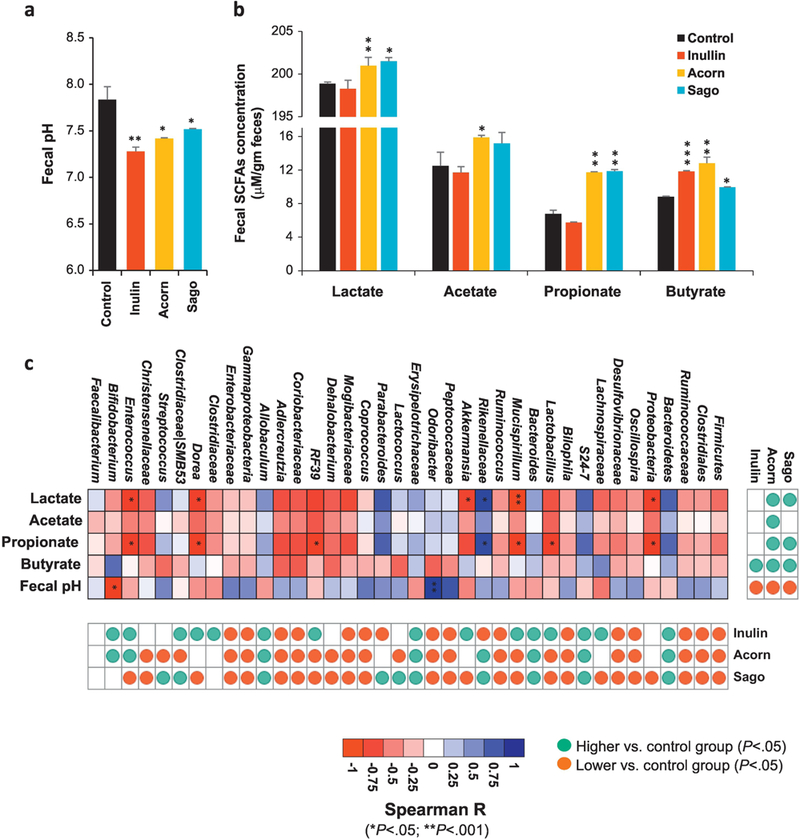
(a,b) Fecal pH (a) and levels of lactate, acetate, propionate and butyrate (b) in the feces of prebiotics-fed and control mice after 5 weeks of intervention. (c) Correlation of microbial groups with pH and SCFAs levels in the feces of prebiotics-fed and control mice after 5 weeks of intervention. Values are presented as mean ± SD/SEM. P*<0.05; **P<0.01; ***P<0.001.
While investigating the impact of prebiotics on metabolic function of gut microbiome, we found that the fecal pH was significantly decreased in all the prebiotics treated mice compared to control (Figure 5a). To find the contribution of prebiotics to produce organic acids or SCFAs via microbial fermentation, we found that acorns and sago prebiotics-fed mice had significantly higher fecal concentration of lactate. Acetate was also higher in acorns-fed mice, while no significant changes in SCFAs were observed in inulin-fed mice. Propionate levels were significantly higher in acorns- and sago-fed mice but not in inulin group. Interestingly, butyrate levels were higher in all the prebiotics-fed groups compared to control, with levels being highest in acorns-fed mice followed by inulin- and sago-fed groups (Figure 5b). Further analysis of correlation between fecal pH, SCFAs levels and microbiome groups indicated that higher lactate and propionate in acorns and sago groups were positively correlated with higher abundance of Bacteroidetes, S24_7, Rikenellaceae and Parabacteroids; lactate levels were negatively correlated with Proteobacteria, Mucispirilium, Akkermansia, Dorea and Enterococcus, while acetate and butyrate levels shared positive _ albeit insignificant _ correlation with Rikenellaceae and Parabacteroides (Figure 5c).
Prebiotics improve gut physiology with decreased intestinal permeability in diet-induced obese (DIO) mice.
Mice fed with prebiotics had higher fecal frequency along with higher stool quantity and lower fecal water content (Supplementary Figure S7a–f), suggesting overall functional improvements in gut physiology. Interestingly, we found that gut permeability (in-terms of the diffusion of 4-kDa-FITC (Fluorescein isothiocyanate)-dextran from gastrointestinal tract into the blood) was dramatically lower in mice fed with prebiotics compared to untreated (control) counterparts (Figure 6a). As anticipated, this was found to be directly associated with higher expression of tight junction proteins including zonulin-1 (ZO1) and Occludin (Ocln) in the intestinal tissues of prebiotics-fed mice versus control group (Figure 6b). In addition, further gene expression analyses revealed lower expression of inflammatory markers including Interleukine-6 (IL-6), tumor necrosis factor-alpha (TNF-α) and chemokine (C-C motif) ligand 2 (Ccl2) and monocyte chemoattractant protein 1 (MCP1) in the intestine of all of the prebiotics-fed mice groups as compared to control. IL-6 was significantly low in acorns-fed mice; TNF-α was significantly low in sago group; while MCP1 was significantly low in all of the three prebiotics groups. Overall, these results hint that prebiotics could help maintain lower/ homeostatic gut permeability (gut ‘leakiness’) with lower intestinal mucosal inflammation.
Figure 6. Prebiotics ameliorate gut permeability, reduce inflammation and modulate gut-brain axis.
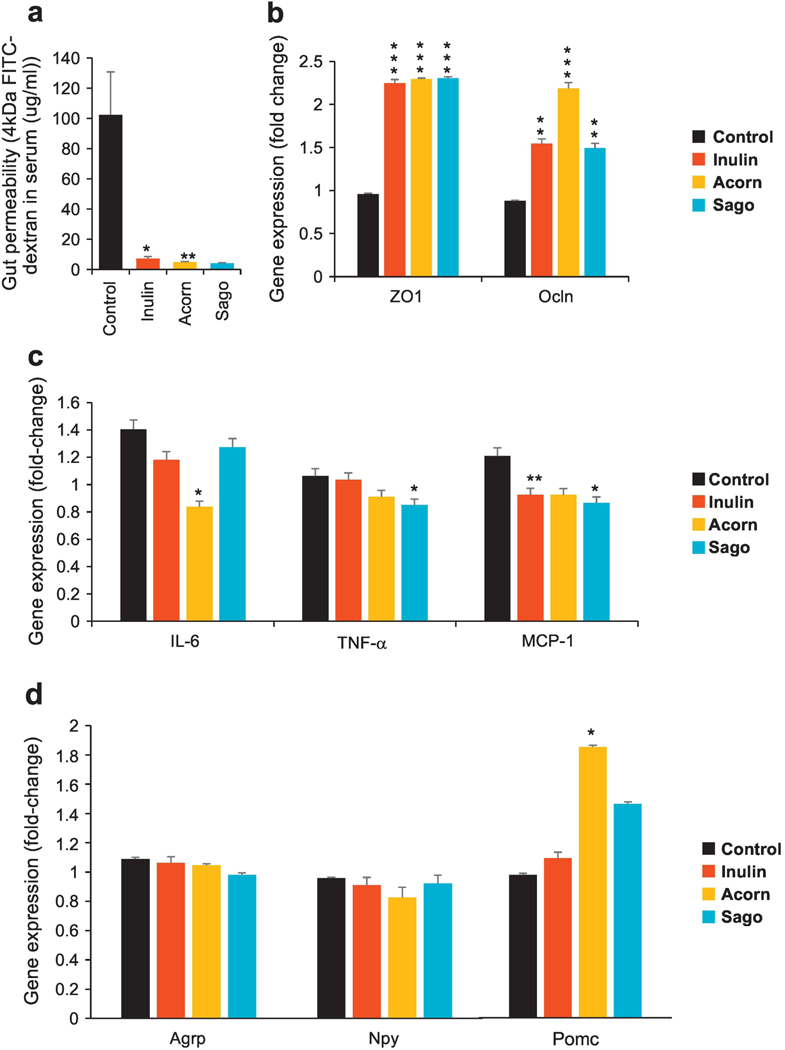
(a) Gut permeability (in terms of the diffusion of 4kDa FITC dextran from gut to blood circulation) in prebiotics-fed and control mice after 5 weeks of intervention. (b) Zonulin-1 (ZO-1) and Occludin (Ocln) mRNA expression in prebiotics-fed and control mice after 5 weeks of intervention. (c) Gene (mRNA) expression of inflammatory markers including Interleukine-6 (IL-6), tumor necrosis factor-alpha (TNF-α) and chemokine (C-C motif) ligand 2/ monocyte chemoattractant protein 1 (Ccl2/ MCP1) in prebiotics-fed and control mice after 5 weeks of intervention. (d) Gene (mRNA) expression of Agouti related protein (AgRP), neuropeptide Y (NPY) and Pro-opiomelanocortin (POMC) in the hypothalamus of (mRNA). Values are presented as mean ± SD/SEM. P*<0.05; **P<0.01; ***P<0.001.
Prebiotics increase hypothalamic energy sensing to regulate/ prevent the hallmarks of obesity.
In an attempt to further estimate whether (and how) prebiotics could interact with gut-brain axis in the hypothalamus region of brain that regulates energy balance via modulating Agouti-related peptide (AgRP), neuropeptide Y (NPY) and Pro-opiomelanocortin (POMC), we found that mice fed with prebiotics–especially acorns–demonstrated significantly higher mRNA expression of POMC and insignificantly (slightly) lower expression of AgRP and NPY. These results hinted that these prebiotics modulated the expression of hypothalamic energy regulating neuronal proteins thereby leading to better energy sensing capacity and whole body energy metabolism regulation.
DISCUSSION
Prevalence of obesity and type 2 diabetes (T2D) is constantly increasing worldwide, raising an urgent need to develop preventive/ therapeutic strategies that can halt this rate. Involvement of gut microbiome in obesity/ T2D pathology has become evident from recent reports [32, 33]; hence, the use of gut microbiome modulators such as prebiotics are seen as vital approaches to prevent/ ameliorate obesity/ T2D epidemic [34, 35]. In addition, the demand of prebiotics is ever-increasing, owing to a plethora of other health-promoting effects [36], again underpinning the need to develop novel health-beneficial prebiotics from natural resources. In this milieu, we herein aimed to isolate and extract some novel prebiotic substrates from unique raw materials and finally selected acorns- and sago-derived prebiotics to examine their influence on human (ex-vivo) and mouse (in-vivo) gut microbiome and SCFAs proportions as well as their impact on the metabolic health of obesity and T2D in mice. We also compared throughout our study the efficacy of these selected prebiotics with the most-widely studied prebiotics i.e., inulin, and interestingly found that our new prebiotics are at least equivalent–or maybe even superior in several effects–to inulin, particularly in terms of several biological outcomes including microbiome-mediated SCFAs production and prevention of features of HFD-induced obesity and T2D via modulation of gut-brain axis.
Typically, prebiotics consist of dietary fibers and oligosaccharides. On the basis of high fiber content, we initially selected five raw materials including acorn, quinoa, sago, and sunflower and pumpkin seeds as potential sources of novel prebiotics. Acorn and sago demonstrated the highest yield of polysaccharides with least protein contamination along with superior amylase resistance, thus exhibiting appropriate prebiotics properties. Interestingly, we found that these selected prebiotics were fermentable by human gut microbiome to produce SCFAs in the fecal microbiome culture system in a way similar to that seen for inulin. Most importantly, we for the first time endeavored to prognosticate the influence of prebiotics on the spectrum of fecal microbiome and its secondary metabolites i.e., SCFAs in healthy versus diseased subjects by exploiting an ex-vivo incubation system established and validated previously by us [28] and other researchers [37]. The results from this trial convincingly show that each of these prebiotics modulated the gut microbiome and SCFAs levels differently in healthy versus diseased fecal cultures, thus suggesting that the health status of the host may influence the effects of prebiotics on gut microbiome and intestinal organic environment. Apparently, diseased conditions generally present with gut microbiome dysbiosis with reduced population of beneficial microbial groups and hence might extend more opportunities for prebiotics to promote the levels of such beneficial microbes and their metabolites that could benefit to combat the disease. Given that the composition of gut microbiome in healthy subjects versus patients with different diseases is generally found to be different, supplementation of prebiotics can be envisaged to favor the growth of specific bacterial groups in these patients that may further cross-feed other/ partner bacteria while suppressing several other bacterial groups via competition for nutrients and adherence sites and/ or by producing inhibitory compounds.
Our results prominently showed that the inoculation of acorn- and sago-prebiotics significantly changed the human fecal microbiome signature, as evident from higher microbial diversity in terms of α-diversity indices including Shannon index, Chao1 and observed OTUs in all of the three prebiotics intervention groups, suggesting that selected prebiotics improved the fecal microbiome composition. Interestingly, albeit not surprisingly, PCoA analyses manifested that all three prebiotics induced more prominent changes in the feces of diseased subjects than of healthy volunteers, suggesting that the beneficial attributes of prebiotics could be more effective in modulating the configuration of gut microbiome in diseased subjects than in healthy microbiome milieus, plausibly because the microbiome in a healthy gut is already diverse, healthy or homeostatic enough. Prebiotics inoculation also appeared to increase the metabolic activity of the human fecal microbiome as evident from higher SCFAs levels and lower fecal pH. Intestinal pH slightly lower than neutral is generally associated with beneficial effects such as constrained growth of various opportunistic pathogens or indigenous pathobionts [38, 39]. For instance, several fruits and resistance starch are known to exhibit beneficial effects via maintaining slightly acidic gut organic environment. Furthermore, we noted that the prebiotics-inoculated fecal specimens had higher levels of SCFAs viz. acetate, propionate and lactate; however, these patterns also differed between specimen from healthy versus diseased subjects. For example, acetate was higher in the feces of healthy donors but lower in diseased counterpart upon prebiotics interventions. All the prebiotics-treated samples had higher propionate in both healthy and diseased fecal cultures, with no significant differences in butyrate levels, suggesting that selected prebiotics including inulin treatments preferentially increased propionate production capacity of the human fecal microbiome. Propionate is known to possess several health benefits including improved serum cholesterol levels and lower lipogenesis and incidences of carcinogenesis in different tissues [40, 41]. Interestingly, propionate levels were higher in inulin- and acorn-inoculated healthy feces but only in acorn-enriched diseased feces. More strikingly, in healthy feces, higher propionate levels were positively correlated with the abundance of Bacteroidetes, Prevotella, Faecalibacterium and Succinivibrio in acorns-inoculated samples and with higher Lactobacillus abundance in inulin-treated feces. Whereas, in diseased feces, Sutterella was the major bacterial genus correlated with higher propionate concentration. These results indicate that the role of different microbial species in producing propionate or the population of propionate-producers per se might differ according to the fecal microbiome in healthy versus diseased subjects. Although, these exciting results established that selected prebiotics are fermentable by human-gut microbiome and produce beneficial SCFAs using ex-vivo human fecal culture system, however, such effects might be different when directly fed to humans, hence further clinical studies are warranted to determine the such effects.
In addition to ex-vivo effects on human fecal microbiome, we also investigated the beneficial impact of selected prebiotics on HFD-induced obesity and T2D in mice, emphasizing mainly on a plausible mechanism i.e., the modulation of gut-brain axis. Prebiotics appeared to prevent HFD-induced glucose intolerance and insulin resistance without affecting body weight and adiposity. Interestingly, such improvements were superior specifically in acorn- than inulin-fed mice. Based on these results, prebiotics appeared to have preserved a better metabolic function to maintain normal glucose homeostasis. This might be through better maintenance of insulin sensitivity (as indicated by better ITT function) in peripheral metabolic organs like adipose tissues and liver [42]. This can be supported by evidences that prebiotics feeding decreases fat accumulation in adipocytes and hepatocytes, since intracellular fat accumulation is detrimental for insulin sensitivity and instigates insulin resistance (a hallmark of T2D) [43]. Low-grade inflammation is another crucial factor that impacts insulin actions; however, the source of this inflammation is not precisely known. It is hypothesized that the gut microbiome may be the primary source of inflammatory signals such as lipopolysaccharide (LPS) and cause endotoxemia due to increased gut permeability (leaky gut). We noted a dramatically lower gut permeability in prebiotics-fed versus control (HFD-fed) mice, which may be attributed to higher expression of tight-junction proteins including ZO-1 and Occludin.
Furthermore, we also noted lower expression of inflammatory genes including IL-6, TNF-α and MCP-1 in the intestinal tissues of prebiotics-fed mice, suggesting that enteric mucosal inflammation remain significantly decreased/ controlled upon prebiotics feeding. Interestingly, SCFAs, especially butyrate and propionate (which were higher in prebiotics-fed mice) are known to reduce gut permeability [44, 45]. Anti-inflammatory actions of SCFAs, mainly via reducing TNF-α production and attenuation of NF-κB activation, have also been reported [46]. Indeed, this decreased gut leakiness along with reduced inflammation may contribute to maintain normal glucose homeostasis even in metabolically stressed conditions such as HFD-feeding.
Gut-brain axis, particularly the hypothalamic signals, are also known to play an important role in the regulation of whole body metabolism [47–49]. We also found slightly increased POMC and decreased AgRP and NPY in the hypothalamus of prebiotics-fed mice, suggesting that the effects of prebiotics in the gut could be signaled into the hypothalamus (a metabolic regulating center). Increased POMC (anorexigenic) and decreased AgRP and NPY (orexigenic) can be involved in food intake behavior; however, we did not see any notable difference in food intake upon prebiotics feeding. Hence, the beneficial effects of these prebiotics on glucose homeostasis could be explained in two possible ways: 1) changes in POMC, AgRP and NPY are too marginal to affect intake behaviors but may impact whole body metabolic rate, as increased POMC enhances whole body metabolic rate; and 2) gut microbiome modulation with increased propionate and decreased gut permeability and inflammation may favor enhanced insulin sensitivity to control glucose homeostasis. Nevertheless, our data concur with other studies reporting that feeding of prebiotics (resistance starch) reduces POMC expression in hypothalamus with no significant changes in AgRP and NPY [50], suggesting that some prebiotic effects might be associated exclusively or more closely with POMC. However, whether and how prebiotic feeding and consequent increased propionate may increase hypothalamic expression of POMC remains unclear and should be an interesting subject for future studies.
Mouse and human gut microbiota are different in terms of both composition as well as function of bacterial strains; however, we noted several effects of these three prebiotics that were common in both mouse and human feces, indicating that (a) the effects of these prebiotics are consistent with regard to specific bacteria independent of the host species and (b) the ex-vivo human fecal slurry system used in here can be exploited as a useful model to predict the effects of such dietary ingredients on fecal microbiota (Supplementary Table S4). For e.g., the relative abundance of the family Enterobacteriacea (and phylum Proteobacteria) was decreased by each of the three prebiotics in all of the three models i.e., healthy and diseased human feces as well as in the mouse gut. This effect might also corroborate the beneficial effects of these prebiotics because Enterobacteriaceae includes, besides several benign gut symbionts, many opportunistic gut pathobionts such as E. coli, Enterobacter, Salmonella, Yersinia, Klebsiella, Shigella, Proteus and Citrobacter. Likewise, the abundance of the genus Erwinia (another opportunistic pathogen belonging to the family Enterobacteriaceae) was decreased by all three prebiotics in both healthy and diseased human feces but not in mouse possibly because its carriage in mouse gut is already too low to be detected. The fecal pH was also decreased by all three prebiotics in all of the three fecal specimens, suggesting increased metabolic activities of fecal bacteria that may have checked the population of these subdominant pathobionts. The abundance of Bacteriodes was increased by both Acorn and Sago prebiotics (but not by inulin) in both mouse and human feces. This effect might be ascribed to differences in the biochemistry of these three polysaccharides. Inulin is a polymer composed mainly of fructose units (fructo-oligasaccharide) [51, 52], while acorn [53] and sago polysaccharides can include amylose and amylopectin and hence can be considered as a kind of resistant starch. Therefore, it is likely that these compounds would favor the growth of Bacteriodes, which are efficient degraders of intact and insoluble plant cell walls and similar complex dietary fibers. This biochemical difference may also underlie the increased abundance of lactic acid bacteria including Lactobacillus, Bifidobacterium and Enterococcus by inulin -but not acorn and sago polysaccharides- in mouse and human feces. Nevertheless, we also observed several effects that were specific to type of host or the prebiotic. For example, the abundance of the phylum Firmicutes, the families Peptococcaceae and Erysipelotrichaceae, and the genera Oscillospira, Desulfovibrio, Bilophila and Muscispirillum was lowered by all three prebiotics only in mice but not in human specimens. Faecalibacterium, which is a beneficial gut bacterium [54–56], was increased by all three prebiotics only in the feces of diseased human subjects but not in healthy humans or in mice. The abundance of the genera Lactococcus and Parabacteroides increased in both humans and mice but only by Sago. Butyrate was increased by all three prebiotics in mice but not in human feces, while lactate and propionate levels were increased variably by acorn and sago in both human and mouse feces (Supplementary Table S4). The data suggest that while many effects of these 3 prebiotics are consistence, some effects are prebiotics and host specific, however further studies are needed to establish such facts.
Our study has few limitations. For example, we clearly demonstrate the beneficial effects of prebiotics in the prevention of HFD-induced obesity and T2D; however, given that such study designs are limited in human clinical scenario, future studies (already being planned by our research group) would address the therapeutic, rather than preventative, importance of prebiotics against diet-induced obesity and T2D. Prebiotics seem to mediate lower gut permeability by enhancing tight junctions and suppressing inflammatory signals along with increased energy sensing molecules (POMC) via modulating gut microbiome-SCFAs interface, thus leading us to propose a physiological mechanism of gut-microbiome-brain axis to prevent obesity and T2D; however, the involvement of specific cell types and underlying mechanism(s) remain to be illustrated. Future studies focusing on how prebiotics mediate gut microbiome metabolites like SCFAs via interactions with signaling molecules (e.g., FFAR2/3) will be of high significance to decipher the molecular mechanism(s). The present study was executed using a translational approach to test the effects of selected prebiotics in human fecal culture system ex-vivo, thereby making a strong foundation for determining (prognosticating) the interaction of prebiotics with human gut microbiome. No doubt, such a culture system may have its own limitations and may not truly mimic the physiological effects happening in human gut in-vivo and hence future more-inclusive molecular and clinical studies remain indispensable to validate and explicate these benefits. We only demonstrate partial chemical characterization of newly isolated prebiotics and hence further analysis of chemical characteristics (e.g., glycomics approaches) are requisite. Nevertheless, these limitations also provide avenues for future studies that will not only fill the gaps in current knowledge but will also provide avenues for potential industrial and clinical applications of novel prebiotics. In conclusion, we herein demonstrate a new set of prebiotics from novel sources that could be exploited to ameliorate obesity and type 2 diabetes via modulating gut-microbiome-brain axis.
MATERIALS AND METHODS
Extraction and preparation of polysaccharides from acorn, quinoa, and sunflower and pumpkin seeds:
Sunflower seeds, pumpkin seeds and quinoa were purchased from Food to Live®, NY, USA. Acorns were purchased from local market of Baghmalek, Iran. After careful cleaning and inspection, the seeds were grounded in a home miller (RRH-A500, China) and passed through a 80-mesh screen. Defatting of powders was done using three volumes of ethanol at 60°C for 12 h; for sunflower and pumpkin seeds, two extra defatting procedures were done with hexane for 12h before ethanol defatting. Defatted and air dried powders were suspended in distilled water (1:10 w/v) and subsequently, hot water extraction of polysaccharides was done at 90°C for 3 h in water bath (Precision®, USA). Following centrifugation of extracts (2000 × g for 10 min, at 20°C), the insoluble residues were removed and the supernatant was then precipitated by adding ethanol (48 h, 4°C) to a final concentration of 80% (v/v). After collecting by centrifugation (8000 × g, 10 min, at 4°C), the precipitate polysaccharides were washed three times with ethanol. For sunflower and pumpkin seed which had high protein contamination, deproteinization was done by the TCA method for three times, as following: pH of crude was adjusted to 3.0 with TCA solution (10%) and kept overnight. After centrifugation for 10 min at 5,000 rpm, the deproteinized supernatant was kept for ethanol participation. Finally, the precipitate was dissolved in deionized water and dried in a freeze-dryer (LABCONE, Freezone 4.5, USA). The yield of obtained polysaccharides was calculated as the weight of obtained powders divided by the initial weight of seeds. The outlines of polysaccharide extraction process from acorn, quinoa, sun flower and pumpkin seed is shown in Supplementary figure S1.
Preparation of resistant starch from sago starch:
Sago (Sabudana) starch was purchased from Jalipur Millers, UK. Preparation of Resistant starch from sago starch was done according to the modified method of Purwani et al. [51], and Siew-Wai et al. [57]. Sago starch was suspended in starch in water (1:20 w/v) and boiled and stirred on plate heater for 10 minutes. After autoclaving at 121°C (1.2 atm) for 1h, the gel was kept at 4°C overnight to develop retro-gradation. Enzymatic hydrolysis of retrograded starch suspension was done by adding 2 units/mL of α-amylase (Sigma-Aldrich, USA) and 1 mL of Pullulanase microbial (Sigma-Aldrich, USA) and incubating for 24h at 70°C in a shaker incubator (150 rpm). This process was repeated twice. Hydrolyzed starch was centrifuged for 10 min at room temperature and the obtained pellets were freeze-dried in a freeze-dryer (LABCONE, Freezone 4.5, USA).
Resistance to acidic and enzymatic digestion:
Resistance of acorn polysaccharide powder samples to acidic and enzymatic digestion was determined and compared based n the method of Tadayoni et al. [58]. Simulated gastric fluid (SGF; pH 1.2) consisted of NaCl (2 g) and HCl (7 ml) while simulated intestinal fluid (SIF; pH 7.4) included KH2PO4 (6.8 g), NaOH (190 ml) and α-amylase (2 unit/ml) (Sigma-Aldrich). Reproduction of a mixture of simulated gastric and intestinal fluid (SMF; pH 4.5) was achieved by mixing SGF and SIF at a ratio of 39:61. Dissolution was carried out in 900 ml of dissolution medium, which was stirred at 100 rpm at 37°C. A sample was taken at 1, 2, 3 h to determine the percentage of hydrolysis, which was calculated based on the reduction of liberated sugar and total sugar content according to 3,5-Dinitrosalicylic acid (DNS) and phenol-sulfuric acid method.
Chemical characterization of polysaccharides:
The total sugar content of the polysaccharides, as an indicator of purity, was measured by phenol sulphuric acid method. Protein quantification was based on the bicinchoninic acid (BCA) method using bovine serum albumin (BSA) as standard, using the Pierce™ BCA Protein Assay Kit (Thermo scientific, USA). Total phenolic composition was determined by the Folin-Ciocalteu colorimetric method, using gallic acid for making the standard curve. The presence of free carbonyl group (C=O), reducing sugars, was determined by DNS method using glucose as a standard. The antioxidant activity of methanol extraction of polysaccharides powders, based on the scavenging activity of the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical, was determined by the method described by Que et al. [59].
Human fecal microbiome fermentation model:
The culture media used for doing ex-vivo human fecal slurry fermentation experiment was prepared according to the recipe devised by Vester-Boler et al. [37]. The composition of media is provided in the supplementary Table S3. Fresh fecal samples were collected from two healthy volunteers and two heart disease patient donors and were immediately stored at −80°C until further use. For the preparation of fecal slurry, the samples were thawed and diluted (1:10 W/V) in anaerobic dilution solution (NaCl 5; glucose 2; Cysteine-HCl 0.3; g/L) and vortexed for 15 minutes or until completely homogenized. The fecal specimens of two healthy and two diseased subjects were pooled, respectively, before the dilution step in order to obtain the two final aliquots, one each of healthy and diseased fecal specimen. The homogenized mixture was filtered through four layers of cheesecloth and was immediately used for inoculation in tubes containing media (control groups) or a combination of media plus fibers (treatment groups). All of the steps involved in inoculum preparation were done inside the anaerobic chamber (Forma anaerobic system, Thermo Scientific, USA). In-vitro anaerobic fermentation using homogenized fecal specimens of healthy and diseased subjects were grouped as follows: a) Control: fermentation media without fiber, b) Control + 1% Inulin, c) Control + 1% Acorn Polysaccharide, and d) Control + 1% Sago resistance starch. Three hundred mg of each fiber was added in a 50 ml tube followed by the addition of 26 mL fermentation media to each tube; in triplicates. These tubes were kept inside the anaerobic chamber for 24h to allow hydration of samples before starting the fermentation experiment. At the beginning of the experiment, 4 mL of freshly prepared fecal inoculums was added in each tube and the tubes were incubated at 37°C for 24 h in the anaerobic chamber with periodic mixing. At 0, 3, 6, 9 and 24h during fermentation, samples were taken out. An aliquot was used for pH measurement using a laboratory pH meter; the remaining sample was centrifuged at 14000g, 10 minutes at 4°C and the supernatant was immediately frozen for SCFAs analysis and the pellet was stored at −80°C for microbiome analysis. The whole anaerobic fermentation experiment was performed twice with triplicates each time and hence the data presented here are the mean of a total of six replicates. However, for cost effectiveness, replicates from each experiment was pooled (i.e. 1st replicate of first experiment was pooled with 1st replicate of second experiment), and resulted pooled replicates were separately analyzed for microbiome sequencing. The microbiome composition analysis was done on 0 hrs (before prebiotics inoculation) and after 9 hrs prebiotics inoculation samples only, as our earlier studies shows that gut microbiome signature after 9hrs in fecal slurry conditions too enriched with Proteobacteria, and start losing diversity in control samples [28], hence the data presented here is only after 9hrs of prebiotics inoculation.
Gut microbiome analysis:
16S rRNA gene amplification and sequencing was executed as per our method described elsewhere [28, 60, 61]. Briefly, approximately 200 mg of fecal slurry pellets or mice feces was used to extract genomic DNA using Qiagen DNA Stool Mini Kit (Qiagen, CA, USA) according to the manufacturer instructions, with a slight modification i.e., using lysis temperature of 95°C instead of 75°C for efficient lysis and DNA yield of gram-positive bacteria. The hypervariable region V4 of bacterial 16S rRNA gene was amplified using the primers 515F (barcoded) and 806R in accordance with the Earth Microbiome Project protocol, with the minor modification as described previously [62]. The resulting amplicons were purified by using AMPure® magnetic purification beads (Agencourt) and the purified PCR products were quantified by using Qubit-3 fluorimeter (InVitrogen). Equal amounts of purified products were pooled; and the pool was quantified again, normalized to 4 nm, denatured and diluted to 8 pM and was sequenced on an Illumina MiSeq sequencer (using Miseq reagent kit v3). The sequences were de-multiplexed, quality filtered, clustered and analyzed using the Quantitative Insights into Microbial Ecology (QIIME, version 1.9.1) software. To avoid bias due to different sequencing depth, the OTU tables were rarefied to the lowest number of sequences per sample (human fecal slurry specimens: 15,358 sequences per sample; mice samples: 20,000 sequences per sample) for computing alpha-diversity metrics within QIIME. Linear discriminant analysis (LDA) analysis and Cladograms were developed on genus level data using LDA effect size (LefSe)[63] on Galaxy platform (https://huttenhower.sph.harvard.edu/galaxy/). OTUs with abundances higher than 1% were included in the subsequent analyses. Taxonomy assignment and diversity analyses were computed within QIIME to compare bacterial species richness between the different experimental groups. Alpha-diversity (rarefaction curve for observed OTUs, Chao1, PD Whole Tree and Shannon) indices were computed with core_diversity_analysis.py script. Beta-diversity was generated within QIIME by using weighted and unweighted Unifrac distance matrices. Principal components analysis (PCoA) was performed (using EMPeror version 0.9.3-dev) to determine the influence of different prebiotics treatments on the overall microbiome composition. The data of bacterial diversity and abundance between the different groups within the same study were compared using non-parametric tests in R statistical software package (version 3.4.3; https://www.r-project.org/). Statistically significant differences in the abundance of microbial groups between different prebiotics groups were calculated by Kruskal-Wallis test followed by Dunn’s post-hoc analysis.
Short-chain fatty acid analysis:
During the fermentation, samples were taken every 3 hours, and supernatants were collected after centrifugation (12,000 g, 10 min). Concentrations of SCFAs (lactate, acetate, propionate and butyrate) were determined using a high-performance liquid chromatography (Waters-2695 Alliance HPLC system, Waters Corporation, Milford, MA, USA) with DAD detector at 210 nm, equipped with a Aminex HPX-87H column (Bio-Rad Laboratories, Hercules, CA). H2SO4 (0.005 N) was used to elute the column with a flow rate of 0.6 ml/min at 25°C.
Mouse HFD-feeding experiment:
Mice:
C57BL/6J mice (n=8–9 in each group; age 8–10 weeks) were randomized into 4 groups; 1) HFD-control; fed with 60% HFD (Research Diets Inc.), 2) HFD-Inulin: fed with HFD supplemented with 5% inulin (Sigma-Aldrich) HFD-Acorn-PS: fed with HFD supplemented with 5% acorn polysaccharides (PS), and 4) HFD-Sago-PS: fed with HFD supplemented sago PS. Food and water were fed ad-libitum for 8 weeks to induce obesity and insulin resistance, and diet intake was measured daily. Body weight was measured and fresh fecal samples were collected once every week. All animal studies and protocols were approved by the Wake Forest Animal Research Program’s Institutional Animal Care and Use Committee.
Glucose and insulin tolerance tests:
Oral and intra-peritoneal (i.p.) glucose tolerance test was performed by glucose (2 mg/g body weight) by oral gavage and intraperitoneal injection, respectively, to overnight fasted mice. For insulin tolerance test, 4–6 hrs fasted mice were given i.p. 0.75U/ kg body weight of insulin (Humulin). Blood glucose was measured at 0 min (before administration of glucose or insulin), 15, 30, 60 and 120 min after glucose/ insulin administration using AccuCheck glucometer kit.
Gut physiology measurements:
The impact of prebiotics on gut physiological measures i.e., fecal frequency per hour, fecal weight dropped per hour, fecal water content and fecal pH was assessed after 8 weeks of starting prebiotics feeding.
In-vivo gut permeability assay:
Four hours after orally administering 4kDa FITC-dextran (60 mg/100 gm body weight) to 4h-fasted mice, the fluorescence intensity in blood collected from the tail vein was measured to determine the gut permeability (intestinal ‘leakiness’)[64, 65].
Organ weight measurements:
After euthanizing mice, fat depots from different sites of body i.e., white adipose tissue (WAT) from mesenteric WAT (mWAT), gonadal WAT (gWAT). retroperitoneal WAT (rWAT), anterior subcutaneous WAT (aWAT) and posterior subcutaneous WAT (pWAT), brown adipose tissue (BAT), and other organs like caecum, liver and skeletal muscles (Quadriceps), were collected and weighted.
Histological analyses:
Mouse gWAT, BAT, ileum and colon were fixed in 10% neutral buffered formalin, processed into paraffin blocks, sectioned at 6 microns, and stained with hematoxylin and eosin. Stained sections were examined by light microscopy (AmScope) and pictures were captured using 9MP digital camera (MU900, AmScope) and images were analyzed using ImageJ software.
Real-time PCR:
Snap-frozen intestine (ileum) and hypothalamus were used for isolating total RNA using RNeasy kit (Qiagen, Gaithersburg, MD), that was reverse transcribed to complementary cDNA using ABI reverse transcription kit. Quantitative gene expression of tight-junction genes including zonulin-1 (ZO-1) and Occludin (Ocln), inflammatory markers including interleukine-6 (IL-6), tumor necrosis factor-alpha (TNF-a) and chemokine (C-C motif) ligand 2/monocyte chemoattractant protein 1 (Ccl2/ MCP1), and hypothalamic energy sensing genes including Agouti related protein (AgRP), neuropeptide Y (NPY) and Pro-opiomelanocortin (POMC) was measured by real-time PCR (ABI 7500; Applied Biosystems). The expression of 18S gene served as an internal control. Three to four independent samples were prepared from 4–6 different mice per treatment group and PCR reactions were carried out in triplicate. The results were expressed as fold difference in gene expression relative to expression in control group normalized with 18S expression, as described in our previous report [66–68].
Statistical analysis:
For mouse studies, data are expressed as mean ±SEM. Statistical significance between groups was determined using unpaired two-tailed Student’s t-test or one-way analysis of variance. P<0.05 was considered statistically significant and all tests were two-sided.
Supplementary Material
FUNDING AND ACKNOWLEDGMENTS
The authors gratefully acknowledge the funding support from the Center for Diabetes, Obesity and Metabolism; the Kermit Glenn Phillips II Chair in Cardiovascular Medicine; the Claude D. Pepper Older Americans Center (funded by P30AG12232); R01AG18915; and the Clinical and Translational Science Center (Clinical Research Unit, funded by UL1TR001420), all at the Wake Forest School of Medicine. We also acknowledge funding support from Department of Defense’s Discovery Award (PR170446).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Marti JM, Martinez-Martinez D, Rubio T, Gracia C, Pena M, Latorre A, et al. Health and Disease Imprinted in the Time Variability of the Human Microbiome. mSystems 2017;2. [DOI] [PMC free article] [PubMed]
- [2].Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med 2016;375:2369–79. [DOI] [PubMed] [Google Scholar]
- [3].Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet 2012;13:260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Integrative HMPRNC. The Integrative Human Microbiome Project: dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe 2014;16:276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kayshap PC, Quigley EM. Therapeutic implications of the gastrointestinal microbiome. Curr Opin Pharmacol 2018;38:90–6. [DOI] [PubMed] [Google Scholar]
- [6].Dahiya DK, Renuka, Puniya M, Shandilya UK, Dhewa T, Kumar N, et al. Gut Microbiota Modulation and Its Relationship with Obesity Using Prebiotic Fibers and Probiotics: A Review. Front Microbiol 2017;8:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yadav H, Jain S, Bissi L, Marotta F Gut Microbiome Derived Metabolites to Regulate Energy Homeostasis: How Microbiome Talks to Host. Metabolomics 2016;6:e150. [Google Scholar]
- [8].Ferrario C, Statello R, Carnevali L, Mancabelli L, Milani C, Mangifesta M, et al. How to Feed the Mammalian Gut Microbiota: Bacterial and Metabolic Modulation by Dietary Fibers. Front Microbiol 2017;8:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wilson B, Whelan K. Prebiotic inulin-type fructans and galacto-oligosaccharides: definition, specificity, function, and application in gastrointestinal disorders. J Gastroenterol Hepatol 2017;32 Suppl 1:64–8. [DOI] [PubMed] [Google Scholar]
- [10].de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol 2008;111:1–66. [DOI] [PubMed] [Google Scholar]
- [11].Slavin J Fiber and prebiotics: mechanisms and health benefits. Nutrients 2013;5:1417–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Balakrishnan M, Floch MH. Prebiotics, probiotics and digestive health. Curr Opin Clin Nutr Metab Care 2012;15:580–5. [DOI] [PubMed] [Google Scholar]
- [13].Louis P, Flint HJ, Michel C. How to Manipulate the Microbiota: Prebiotics. Adv Exp Med Biol 2016;902:119–42. [DOI] [PubMed] [Google Scholar]
- [14].Lin CS, Chang CJ, Lu CC, Martel J, Ojcius DM, Ko YF, et al. Impact of the gut microbiota, prebiotics, and probiotics on human health and disease. Biomed J 2014;37:259–68. [DOI] [PubMed] [Google Scholar]
- [15].Weitkunat K, Schumann S, Petzke KJ, Blaut M, Loh G, Klaus S. Effects of dietary inulin on bacterial growth, short-chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice. J Nutr Biochem 2015;26:929–37. [DOI] [PubMed] [Google Scholar]
- [16].Chen K, Chen H, Faas MM, de Haan BJ, Li J, Xiao P, et al. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol Nutr Food Res 2017;61. [DOI] [PubMed]
- [17].Kelly G Inulin-type prebiotics: a review. (Part 2). Altern Med Rev 2009;14:36–55. [PubMed] [Google Scholar]
- [18].Ahmadi S, Mainali R, Nagpal R, Sheikh-Zeinoddin M, Soleimanian-Zad S, Wang S, Deep G, Mishra SK, Yadav H Dietary Polysaccharides in the Amelioration of Gut Microbiome Dysbiosis and Metabolic Diseases. Obesity & Control Therapies 2017;4:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang D, Sun F, Lu C, Chen P, Wang Z, Qiu Y, et al. Inulin based glutathione-responsive delivery system for colon cancer treatment. Int J Biol Macromol 2018;111:1264–72. [DOI] [PubMed] [Google Scholar]
- [20].Qamar TR, Syed F, Nasir M, Rehman H, Zahid MN, Liu RH, et al. Novel Combination of Prebiotics Galacto-Oligosaccharides and Inulin-Inhibited Aberrant Crypt Foci Formation and Biomarkers of Colon Cancer in Wistar Rats. Nutrients 2016;8. [DOI] [PMC free article] [PubMed]
- [21].Pool-Zobel BL. Inulin-type fructans and reduction in colon cancer risk: review of experimental and human data. Br J Nutr 2005;93 Suppl 1:S73–90. [DOI] [PubMed] [Google Scholar]
- [22].Bindels LB, Neyrinck AM, Salazar N, Taminiau B, Druart C, Muccioli GG, et al. Non Digestible Oligosaccharides Modulate the Gut Microbiota to Control the Development of Leukemia and Associated Cachexia in Mice. PLoS One 2015;10:e0131009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011;60:2775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Costabile A, Deaville ER, Morales AM, Gibson GR. Prebiotic Potential of a Maize-Based Soluble Fibre and Impact of Dose on the Human Gut Microbiota. PLoS One 2016;11:e0144457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Long W, Xue Z, Zhang Q, Feng Z, Bridgewater L, Wang L, et al. Differential responses of gut microbiota to the same prebiotic formula in oligotrophic and eutrophic batch fermentation systems. Sci Rep 2015;5:13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vigsnaes LK, Holck J, Meyer AS, Licht TR. In vitro fermentation of sugar beet arabino-oligosaccharides by fecal microbiota obtained from patients with ulcerative colitis to selectively stimulate the growth of Bifidobacterium spp. and Lactobacillus spp. Appl Environ Microbiol 2011;77:8336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Takagi R, Sasaki K, Sasaki D, Fukuda I, Tanaka K, Yoshida K, et al. A Single-Batch Fermentation System to Simulate Human Colonic Microbiota for High-Throughput Evaluation of Prebiotics. PLoS One 2016;11:e0160533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nagpal R, Wang S, Ahmadi S, Hayes J, Gagliano J, Subashchandrabose S, et al. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci Rep 2018;8:12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jain SK, Jain A, Gupta Y, Ahirwar M. Design and development of hydrogel beads for targeted drug delivery to the colon. AAPS PharmSciTech 2007;8:E56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017;8:172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Viladomiu M, Hontecillas R, Yuan L, Lu P, Bassaganya-Riera J. Nutritional protective mechanisms against gut inflammation. J Nutr Biochem 2013;24:929–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Barlow GM, Yu A, Mathur R. Role of the Gut Microbiome in Obesity and Diabetes Mellitus. Nutr Clin Pract 2015;30:787–97. [DOI] [PubMed] [Google Scholar]
- [33].Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manneras-Holm L, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 2017;23:850–8. [DOI] [PubMed] [Google Scholar]
- [34].Tilg H, Gasbarrini A. Prebiotics for obesity: a small light on the horizon? Gut 2013;62:1096–7. [DOI] [PubMed] [Google Scholar]
- [35].Nicolucci AC, Hume MP, Martinez I, Mayengbam S, Walter J, Reimer RA. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017;153:711–22. [DOI] [PubMed] [Google Scholar]
- [36].Patel S, Goyal A. The current trends and future perspectives of prebiotics research: a review. 3 Biotech 2012;2:115–25. [Google Scholar]
- [37].Vester Boler BM, Rossoni Serao MC, Faber TA, Bauer LL, Chow J, Murphy MR, et al. In vitro fermentation characteristics of select nondigestible oligosaccharides by infant fecal inocula. J Agric Food Chem 2013;61:2109–19. [DOI] [PubMed] [Google Scholar]
- [38].Percy-Robb IW, Collee JG. Bile acids: a pH dependent antibacterial system in the gut? Br Med J 1972;3:813–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kamada N, Chen GY, Inohara N, Nunez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 2013;14:685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hosseini E, Grootaert C, Verstraete W, Van de Wiele T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr Rev 2011;69:245–58. [DOI] [PubMed] [Google Scholar]
- [41].Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, Scott KP, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J 2014;8:1323–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006;444:847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Taylor R Insulin resistance and type 2 diabetes. Diabetes 2012;61:778–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tedelind S, Westberg F, Kjerrulf M, Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol 2007;13:2826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ohata A, Usami M, Miyoshi M. Short-chain fatty acids alter tight junction permeability in intestinal monolayer cells via lipoxygenase activation. Nutrition 2005;21:838–47. [DOI] [PubMed] [Google Scholar]
- [46].Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients 2011;3:858–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].van de Wouw M, Schellekens H, Dinan TG, Cryan JF. Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. J Nutr 2017;147:727–45. [DOI] [PubMed] [Google Scholar]
- [48].Stanley SA, Kelly L, Latcha KN, Schmidt SF, Yu X, Nectow AR, et al. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature 2016;531:647–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Coll AP, Yeo GS. The hypothalamus and metabolism: integrating signals to control energy and glucose homeostasis. Curr Opin Pharmacol 2013;13:970–6. [DOI] [PubMed] [Google Scholar]
- [50].Shen L, Keenan MJ, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, et al. Dietary resistant starch increases hypothalamic POMC expression in rats. Obesity (Silver Spring) 2009;17:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Purwani EY, Purwadaria T, Suhartono MT. Fermentation RS3 derived from sago and rice starch with Clostridium butyricum BCC B2571 or Eubacterium rectale DSM 17629. Anaerobe 2012;18:55–61. [DOI] [PubMed] [Google Scholar]
- [52].Roberfroid MB. Caloric value of inulin and oligofructose. J Nutr 1999;129:1436S–7S. [DOI] [PubMed] [Google Scholar]
- [53].Ahmadi S, Sheikh-Zeinoddin M, Soleimanian-Zad S, Alihosseini F, Yadav H. Effects of different drying methods on the physicochemical properties and antioxidant activities of isolated acorn polysaccharides. Lwt 2019;100:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lopez-Siles M, Duncan SH, Garcia-Gil LJ, Martinez-Medina M. Faecalibacterium prausnitzii: from microbiology to diagnostics and prognostics. ISME J 2017;11:841–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Heinken A, Khan MT, Paglia G, Rodionov DA, Harmsen HJ, Thiele I. Functional metabolic map of Faecalibacterium prausnitzii, a beneficial human gut microbe. J Bacteriol 2014;196:3289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Miquel S, Martin R, Rossi O, Bermudez-Humaran LG, Chatel JM, Sokol H, et al. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol 2013;16:255–61. [DOI] [PubMed] [Google Scholar]
- [57].Siew-Wai L, Zi-Ni T, Karim AA, Hani NM, Rosma A. Fermentation of Metroxylon sagu resistant starch type III by Lactobacillus sp. and Bifidobacterium bifidum. J Agric Food Chem 2010;58:2274–8. [DOI] [PubMed] [Google Scholar]
- [58].Tadayoni M, Sheikh-Zeinoddin M, Soleimanian-Zad S. Isolation of bioactive polysaccharide from acorn and evaluation of its functional properties. Int J Biol Macromol 2015;72:179–84. [DOI] [PubMed] [Google Scholar]
- [59].Que F, Mao L, Zheng X. In vitro and vivo antioxidant activities of daylily flowers and the involvement of phenolic compounds. Asia Pac J Clin Nutr 2007;16 Suppl 1:196–203. [PubMed] [Google Scholar]
- [60].Nagpal R, Newman TM, Wang S, Jain S, Lovato JF, Yadav H. Obesity-Linked Gut Microbiome Dysbiosis Associated with Derangements in Gut Permeability and Intestinal Cellular Homeostasis Independent of Diet. J Diabetes Res 2018;2018:3462092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Nagpal R, Shively CA, Appt SA, Register TC, Michalson KT, Vitolins MZ, et al. Gut Microbiome Composition in Non-human Primates Consuming a Western or Mediterranean Diet. Front Nutr 2018;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Nagpal R, Shively CA, Appt SA,. Register TC, Michalson KT, Vitolins MZ, Yadav H. Gut Microbiome Composition in Non-human Primates Consuming a Western or Mediterranean Diet. Frontiers in Nutrition 2018. [DOI] [PMC free article] [PubMed]
- [63].Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011;12:R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med 2001;193:1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wang L, Llorente C, Hartmann P, Yang AM, Chen P, Schnabl B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods 2015;421:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab 2011;14:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem 2013;288:25088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yadav H, Devalaraja S, Chung ST, Rane SG. TGF-beta1/Smad3 Pathway Targets PP2A-AMPK-FoxO1 Signaling to Regulate Hepatic Gluconeogenesis. J Biol Chem 2017;292:3420–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


