Abstract
Avoidant behavior is a characteristic feature post-traumatic stress disorder (PTSD) and is modeled in mammals with predator odor. Light avoidance is a hallmark behavioral reaction in planarians. We hypothesized that planarians exposed to frog extract would display enhanced light avoidance that is prevented by fluoxetine. Enhanced light avoidance (i.e., less time spent in light compartment of a dish split into light and dark sides) after a 30-min frog extract exposure (0.0001 – 0.01%) manifested 15 min post-exposure, persisted for at least 24 h, and was counteracted by fluoxetine (10 μM). These results suggest conservation of an anxiety- like behavioral phenotype.
Keywords: planarians, anxiety, PTSD, trauma, invertebrate, fluoxetine
Introduction
Negative phototaxis is the tendency of an organism to travel away from a light stimulus and is a defining behavioral response in aquatic flatworms called planarians (Byrne, 2018; Davidson et al., 2011; Zewde et al., 2018; Mohammed Jawad et al., 2018; Amaning-Kwarteng et al., 2017; Zhang et al., 2013). Planarian light avoidance is highly dependent on external stimuli, and is exacerbated during abstinence from chronic exposure to addictive substances (e.g. cocaine, ethanol, sucrose) but reduced during acute exposure to anxiolytic drugs (e.g. benzodiazepines and ethanol) (Nayak et al., 2016; Ouyang et al., 2017). In assays that mimic conditioned place preference (CPP) in rodents, negative phototaxis is reduced after a conditioning phase in which worms are exposed to addictive substances (e.g. cocaine, ethanol, nicotine, cathinones, sucrose) in the light compartment (i.e., pairing the drug and light compartment during conditioning reduces degree of light avoidance) (Dziedowiec et al., 2018; Mohammed Jawad et al., 2018; Tallarida et al., 2014). Additional mammalian-like responses, such as motility changes, stereotyped behaviors, motor sensitization, drug seeking, and withdrawal, are detectable in planarians during exposure to specific drugs and stimuli (Pagan, 2014, 2017; Pagan et al., 2013; Palladini et al., 1996). Further phenotypic similarities with mammals are also suggested by neurochemical evidence showing that planarians utilize neurotransmitters, including dopamine, serotonin, GABA, glutamate and acetylcholine (Nishimura et al., 2008).
Invertebrate assays that model aspects of post-traumatic stress disorder (PTSD) are lacking. The most useful mammalian models utilize a ‘stressor’ that produces behavioral and biological responses of PTSD that display intensity-dependence, persistence over time, bidirectional expression (enhanced or reduced responsivity), and inter-subject variability (Yehuda and LeDoux, 2007; Daskalakis et al., 2013). Capturing the entire spectrum of the PTSD profile in animal models is challenging, and, even at the mammalian level, there is no universally accepted model. Most paradigms utilize physical, social or psychological stressors that produce at least one of the behavioral and biological features of the PTSD phenotype, such as avoidant behavior, hyperarousal, anxiogenic behavior and alterations in brain stress responses (Whitaker et al., 2014; Knox et al., 2012; Zoladz et al., 2012). A commonly employed paradigm is predatorodor exposure wherein animals exposed to a urine, feces or endogenous chemicals obtained froma predator, display intensity-dependent, enduring increases in avoidant behavior (Whitaker et al., 2014; Corley et al., 2012).
To capitalize on the planarian light-avoidant response, we propose a complementary ‘predator odor’ invertebrate assay – one in which an enduring negative phototaxis is detectable at different time points following direct exposure to frog extract. The hypothesis is that planarians exposed to frog extract will display a long-lasting, concentration-dependent increase in negative phototactic behavior that is counteracted by fluoxetine (Prozac), a selective serotonin reuptake inhibitor (SSRI) and first-line medication for PTSD.
Materials and Methods
Planarians (Dugesia dorotocephala) were purchased from Carolina Biological Supply (Burlington, NC, USA). Frog juice was purchased from Bog Baits (Beaver Dam, WI, USA), and is described as an all-natural frog oil blend derived from frogs that provides a distinctive odor used to lure fish (e.g. used as fish bait). Fluoextine hydrochloride was purchased from Sigma- Aldrich (St. Louis MO, USA). All substances were dissolved in spring water, with stock and working solutions prepared daily, and concentrations were based on prior work (Zewde et al., 2018). Behavioral experiments were conducted between 9 AM and 6 PM. Behavioral responses were quantified by a trained observer with a stopwatch who was blinded to drug treatment in ‘real time’, and no videotaping was used. Because planarians are large enough (3–15 mm in length) so that behavior can be observed and quantified with the naked eye, behavioral responses were quantified without a microscope.
Light/dark experiments were based on previous methodology (Zewde et al., 2018; Dziedowiec et al., 2018). Separate planarians were used for each experimental group (i.e., a single planarian was only tested once). Each planarian was removed from its home jar and placed into a secondary jar (identical to the home jar) containing frog extract (0.0001, 0.001, 0.01%) or water for 30 min. Planarians were next removed and placed into a petri dish containing water. At three different time points following discontinuation of frog extract exposure (0.25, 1 and 24 h), planarians were placed at the midline of a petri dish (5.5 cm diameter) containing spring water. The petri dish was enveloped with a sleeve of black construction paper that covered one half of the dish on the top, bottom and vertical sides to create evenly split dark and ‘ambient’ light environments. Each planarian was given free access to roam the ‘ambient light’ and dark sides of the dish, and time spent in the light compartment was recorded over 10 min. Enhanced negative phototaxis (light avoidance) occurs when a planarian exposed to frog extract spends less time in the light compartment compared to water-exposed planarians. In separate experiments that used identical methodology, planarians were exposed to frog extract (0.01%) or water for 30 min and placed into a petri dish containing water. Twenty-four hours later, planarians were then placed at the midline of a petri dish split into light and dark compartments that contained water or fluoxetine (1, 10 μM), and time spent in the light compartment was quantified for 10 min.
For motility experiments, an individual planarian was removed from its home jar and placed for 30 min into a secondary jar (identical to home jar) containing frog extract (0.01%) or water for 30 min. Planarians were then removed and placed into a transparent petri dish containing water that was placed over graphing paper with gridlines equally spaced 0.5 cm apart. Motility counts were quantified 24 h later as the number of gridlines that planarians crossed in 2.5 min (Raffa and Valdez, 2001).
For statistical analysis, comparisons of group means (± S.E.M.) were evaluated by two-way ANOVA followed by Bonferroni’s post-hoc test to identify group differences or a Student’s t-test (motility data). P < 0.05 was considered significant.
Results
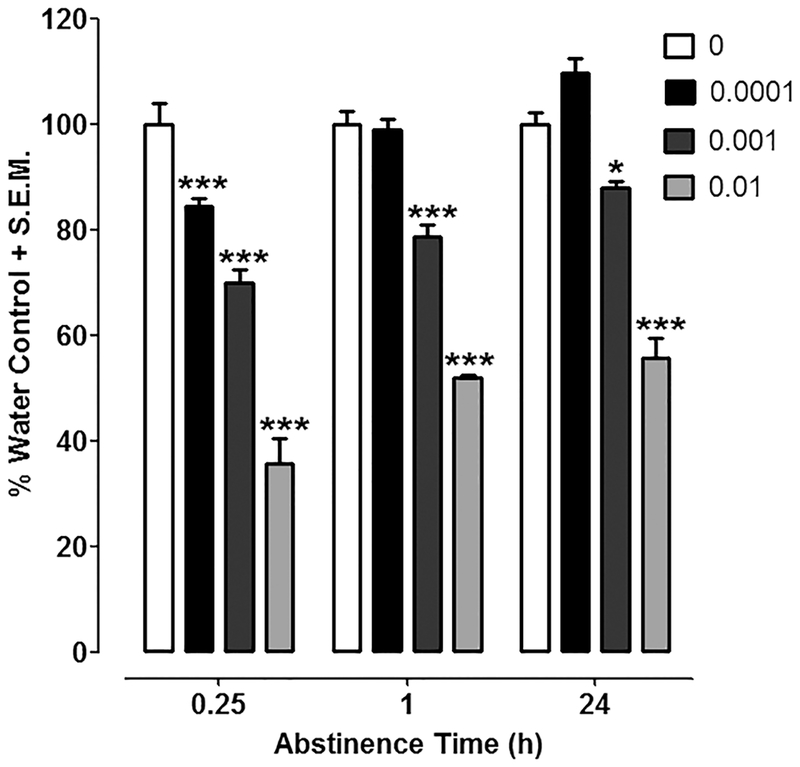
For concentration-effect data with frog extract (Fig. 1), a two-way ANOVA (frog extract exposure x time) revealed effects of frog extract treatment [F(3, 87) = 217.21, P < 0.0001] and time [F(2, 87) = 31.42, P < 0.0001] and a significant interaction [F(6, 87) = 4.08, P < 0.01]. Mean time spent in the light compartment across water-exposed control groups was 43.1 ± 3.5. Planarians exposed to the highest concentration of frog extract (0.01%) displayed enhanced negative phototaxis (i.e., spent less time in light compartment) compared to water-exposed planarians at all 3 post-exposure time points (0.25, 1 and 24 h) (P < 0.001). A concentration of 0.001% frog extract also produced enhanced negative phototaxis at all post-exposure time points [0.25 h (P < 0.001), 1 h (P < 0.001), and 24 h (P < 0.05)]. Compared to water-exposed controls, the lowest concentration of frog extract (0.0001 %) produced enhanced light avoidance at the initial time point [0.25 h (P < 0.001)] but not later time points [1 h and 24 h (P > 0.05)]. In a separate set of experiments conducted 72 h after cessation of frog extract (0.01 %) exposure, the degree of light avoidance in planarians exposed to frog extract was not significantly different from water controls (planarians naïve to frog extract (P >0.05) (data not shown).
Fig. 1. Frog extract enhances light avoidance.
Time spent in the light compartment of a petri dish containing water was quantified 0.25, 1 or 24 h following discontinuation of a 30-min exposure to frog extract (0, 0.0001, 0.001, 0.01 %). Data were presented as percentage of respective water control (i.e., percentage of water control time spent in the light compartment) + S.E.M. N=8–11 planarians/group. ***P < 0.001 or *P < 0.05 compared to water control (0 %).
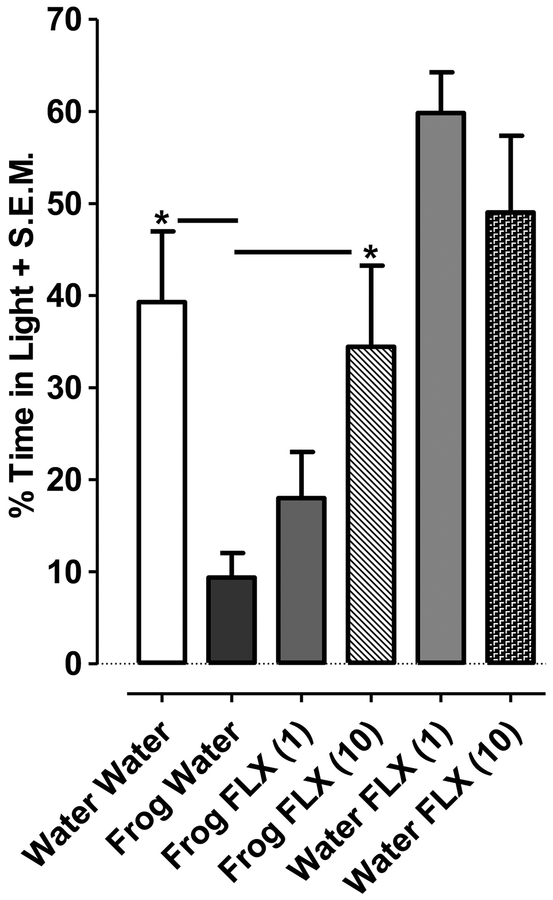
For combination experiments with frog extract and fluoxetine (Fig. 2), a two-way ANOVA (frog extract exposure x fluoxetine treatment) indicated effects of frog extract [F(2, 54) = 3.3, P < 0.05] and fluoxetine [F(1, 54) = 23.72, P < 0.001] (Fig. 2). Planarians exposed to a fixed concentration of frog extract (0.01 %) for 30 min and subsequently tested in water 24 h later displayed enhanced phototaxis compared to frog extract-naïve planarians (i.e., planarians exposed to water and tested in water 24 h later) (P < 0.05). However, this enhanced phototactic response 24 h following exposure to frog extract (0.01 %) was significantly reduced by fluoxetine treatment (10 μM) (P < 0.05). In frog extract-naïve planarians that were exposed to water and tested 24 h later in either fluoxetine (1, 10 μM) or water, time spent in the light compartment was not significantly different (P > 0.05).
Fig. 2. Fluoxetine counteracts the enhancement of light avoidance induced by frog extract.
Time spent in the light compartment of a petri dish that contained fluoxetine (1, 10 μM) or water was quantified 24 following discontinuation of a 30-min exposure to a fixed concentration of frog extract (0.01 %). Data were presented as percentage of time spent in the light + S.E.M. N=9–13 planarians/group. *P < 0.05 compared to frog extract plus water group (Frog Water).
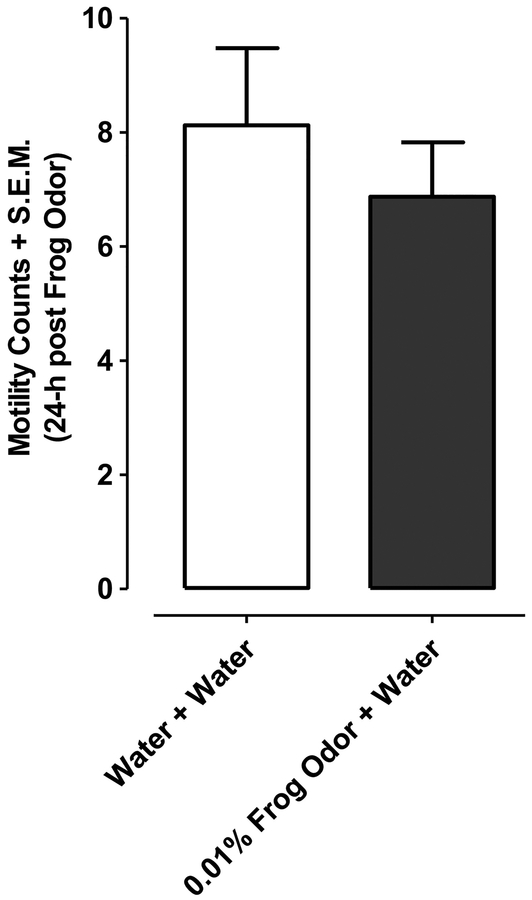
For motility control experiments planarians exposed for 30 min to the highest concentration of frog extract (0.01%) and then tested in water 24 h later did not display motility counts that differed significantly from planarians exposed to water and tested in water (P > 0.05, Student’s t-test) (Fig. 3).
Fig.3. Frog extract does not impact motility 24 h following discontinuation of exposure.
Motility counts (in water over a 2.5-min interval) were quantified 24 h after discontinuation of exposure to frog extract (0.01 %) or water. N=15–16 planarians/group. **P < 0.01 or *P < 0.05 compared to water control (0 μM or 0 %). Data were presented as motility counts + S.E.M. N=8 planarians/group.
Discussion
Planarians exposed to ‘predator odor’ (e.g. frog extract) displayed avoidant behavior that was counteracted by fluoxetine. In mammals predator odor is a psychological stressor that produces a spectrum of behavioral and biological effects that satisfy several diagnostic criteria of PTSD (e.g. avoidant and anxiogenic behaviors that are enduring, intensity-dependent, modulated by putative therapies, and subject to inter-subject variability) (Whitaker et al., 2014). The profile of the planarian avoidant response (enhanced light avoidance) following frog extract exposure shared commonalities with the behavioral phenotype in mammals that ensues after predator odor exposure (Whitaker et al., 2014; Edwards et al., 2013). One commonality was persistence of the avoidant response over time. Enhanced light avoidance in planarians manifested rapidly, presenting 15 min following discontinuation of frog extract exposure, and persisted for at least 24 h before normalizing 72 h after exposure. In humans, the PTSD phenotype can persist indefinitely, and, in mammals, predator odor paradigms produce long- lasting behavioral symptoms that can endure for weeks or months (Whitaker et al., 2014). Because of species- specific differences in pharmacokinetics, metabolism and longevity, comparing quantitative differences in the temporal profile of any biological response across different species is challenging, even when comparing mammals and humans. Given that the enhanced light avoidant response in planarians normalized within a week, it is unlikely that irreversible toxicity resulting from acute frog extract exposure was a major cause of the response. Furthermore, motility deficits were not detected 24 h following discontinuation of frog extract exposure, thereby excluding decreased motility as a potential confound for enhanced light avoidance (i.e., lethargic planarians being less apt to explore the light compartment). Similar to the intensity- dependence of anxiety-like phenotypes in mammalian models of PTSD (Whitaker et al., 2014), the concentration of frog extract to which planarians were initially exposed influenced both the persistence and magnitude of enhanced light avoidance. The highest concentration of frog extract produced the most robust, and most enduring, avoidant response while the lowest concentration produced a modest, and transient, response that was only apparent 15 min after exposure.
Fluoxetine, when administered to planarians 24 h following discontinuation of frog extract exposure, counteracted enhancement of light avoidance in a concentration-dependent manner. The clinical relevance, if any, of the fluoxetine efficacy in the planarian assays is unclear. Fluoxetine, as well as pharmacologically similar SSRIs (sertraline, paroxetine) and SNRIs (venlafaxine), are recognized as first-line medications for major depressive disorder, panic disorder and PTSD (Friedman and Bernardy, 2017; Jeffreys et al., 2012). It was somewhat surprising that fluoxetine did not significantly affect light avoidance in planarians naïve to frog extract, although a trend toward a reduction in light avoidance was observed. Indeed, in a previous study (Zewde et al., 2018), light avoidance was reduced in planarians treated with comparable concentrations of fluoxetine. Reasons for the slight discrepancy between studies is unclear, although differences in experimental factors (e.g. time of year, age of planarians, time of day of testing, magnitude of light avoidance in control planarians) may have contributed.
The neurochemical mechanism underlying fluoxetine efficacy against anxiety-like behavior is not entirely clear. Perhaps the most parsimonious explanation, and one based on both the pharmacodynamic profile of fluoxetine and neurochemical organization in planarians is enhanced 5-HT transmission. Fluoxetine selectively inhibits cellular reuptake of 5-HT while displaying negligible affinity for dopamine and norepinephrine transporters. 5-HT is one of the best-defined neurotransmitter systems in planarians with neurons, receptors, and metabolic pathways all being identified (Saitoh, 1996; Ribeiro et al., 2005, Welsh and Williams, 1970). Levels of 5-HT have been quantified in planarians, with tissue concentrations being greater in head versus tail fragments (Wu et al., 2015; Buttarelli et al., 2008; Ness et al., 1996, Umeda et al., 2005). Also identified in planarians are a monoamine oxidase pathway that facilitates 5-HT catabolism and an ortholog gene of tryptophan hydroxylase (designated DjTPH), the rate- limiting enzyme for 5-HT biosynthesis (Wu et al., 2015). DjTPH mRNA and protein are mainly expressed in the planarian nervous system, and DjTPH immunoreactivity is present at axonal connections in the ventral nerve cords (Nishimura et al., 2007). Several genes of 5-HT receptor- like planarian receptors (5HTLpla 1–4), which are homologous to the human 5-HT1A receptor, have also been identified (Saitoh et al., 1996, Saitoh et al., 1997; Nishimura et al., 2007). The 5- HTpla4 receptor is the most abundantly expressed and displays partial homology to the human 5- HT1A, human 5-HT7 receptor, Xenopus 5-HT7, and Drosophila tyramine receptor. Thus, in our experiments, fluoxetine may have engaged one or more elements of this robust planarian 5-HT system (e.g. blocking cellular uptake, enhancing synthesis, reducing catabolism, altering receptor activity, etc.), leading to an enhancement of 5-HT neurotransmission that reduced anxiety-like behavior induced by frog extract exposure.
Despite the noted similarities between avoidant responses in planarians and mammals following predator odor exposure, multiple limitations are associated with the planarian assays described here. Most apparent is the lack of molecular and neurochemical tools to probe mechanistic underpinnings of the planarian avoidant response, which prevents a more detailed comparison with the biological mechanisms that contribute to the mammalian phenotype. For example, in mammals, exposure to predator odor causes dysregulation of the sympathetic nervous system and hypothalamic–pituitary–adrenal axis, two hallmark characteristics of PTSD patients (Hoffman and Taylor, 2019; Cohen et al., 2006). In addition, the planarian avoidant response itself (i.e., enhanced negative phototaxis) remains somewhat ambiguous in terms of biological meaning and translational significance. Light avoidance is clearly a type of defensive responding, but whether or not it also signifies anxiogenic-like behavior is unclear, although it is notable that negative phototaxis displays bidirectional expression as it is reduced by anxiolytic drugs and enhanced under known anxiogenic conditions (i.e., abstinence from chronic exposure to drugs of abuse and GABA receptor antagonism) (Zewde et al., 2018; Nayak et al., 2016). The planarian assays described here are also limited in phenotypic scope compared to mammalian models, wherein predator odor paradigms are often combined with additional stressors, such as social isolation and defeat, to produce a broader, and more robust, phenotype that better mimics PTSD in humans (Zoladz et al., 2008). Future studies with planarians will investigate effects of frog extract exposure in combination with social isolation to determine if the magnitude and persistence of the planarian avoidant response can be enhanced by a combination of stressors.
In summary, predator odor exposure produced enduring avoidance responses in planarians that resembled anxiety-like behavior and defensive responding. The planarian assays provide a quantifiable avoidant response (light avoidance) following frog extract exposure that is enduring, concentration-dependent, and counteracted by a first-line PTSD medication. Given that planarians are the simplest living animals with bilateral symmetry and a central nervous system with cephalization (Pagán, 2014), the present results indicate that at least some behavioral elements of an anxiety-like phenotype are conserved across species.
Research Highlights.
A anxiety-like phenotype was modeled in planarians with predator odor.
Frog extract produced enhanced light avoidance in planarians.
The planarian avoidant response was enduring and concentration-sensitive.
Fluoxetine counteracted the planarian avoidant response.
Outcomes suggest conservation of an anxiety-like phenotype.
Acknowledgements
This study was funded in part by NIDA grants R25DA033270 and P30 DA013429.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaning-Kwarteng AO, Asif-Malik A, Pei Y, Canales JJ. Relapse to cocaine seeking in an invertebrate. Pharmacol Biochem Behav. 2017. 157:41–46. [DOI] [PubMed] [Google Scholar]
- Byrne T Effects of ethanol on negative phototaxis and motility in brown planarians (Dugesia tigrina). Neurosci Lett. 2018. 685:102–108. [DOI] [PubMed] [Google Scholar]
- Buttarelli FR, Pellicano C, Pontieri FE. Neuropharmacology and behavior in planarians: translations to mammals. Comp Biochem Physiol C Toxicol Pharmacol. 2008. 147:399–408. [DOI] [PubMed] [Google Scholar]
- Cohen H, Matar MA, Richter-Levin G, Zohar J. The contribution of an animal model toward uncovering biological risk factors for PTSD. Ann N Y Acad Sci. 2006. 1071:335–50. [DOI] [PubMed] [Google Scholar]
- Corley MJ, Caruso MJ, Takahashi LK. Stress-induced enhancement of fear conditioning and sensitization facilitates extinction-resistant and habituation-resistant fear behaviors in a novel animal model of posttraumatic stress disorder. Physiol Behav. 2012. 105:408–16. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Yehuda R, Diamond DM. Animal models in translational studies of PTSD. Psychoneuroendocrinology. 2013. 38:1895–911. [DOI] [PubMed] [Google Scholar]
- Davidson C, Prados J, Gibson CL, Young AM, Barnes D, Sherlock R, Hutchinson CV. Shedding light on photosensitive behaviour in brown planaria (Dugesia Tigrina). Perception. 2011. 40:743–6. [DOI] [PubMed] [Google Scholar]
- Dziedowiec E, Nayak SU, Gruver KS, Jennings T, Tallarida CS, Rawls SM. Mu Opioid Receptor Agonist DAMGO Produces Place Conditioning, Abstinence-induced Withdrawal, and Naltrexone-Dependent Locomotor Activation in Planarians. Neuroscience. 2018. 386:214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl Psychiatry. 2013. 3:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MJ, Bernardy NC. Considering future pharmacotherapy for PTSD. Neurosci Lett. 2017. 649:181–185. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Taylor AN. Stress reactivity after traumatic brain injury: implications for comorbid post-traumatic stress disorder. Behav Pharmacol. 2019, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys M, Capehart B, Friedman MJ. Pharmacotherapy for posttraumatic stress disorder: review with clinical applications. J Rehabil Res Dev. 2012. 49:703–15. [DOI] [PubMed] [Google Scholar]
- Knox D, Nault T, Henderson C, Liberzon I. Glucocorticoid receptors and extinction retention deficits in the single prolonged stress model. Neuroscience. 2012. 223:163–73. [DOI] [PubMed] [Google Scholar]
- Mohammed Jawad RA, Hutchinson CV, Prados J. Dissociation of place preference and tolerance responses to sucrose using a dopamine antagonist in the planarian. Psychopharmacology (Berl). 2018. 235:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak S, Roberts A, Bires K, Tallarida CS, Kim E, Wu M, Rawls SM. Benzodiazepine inhibits anxiogenic-like response in cocaine or ethanol withdrawn planarians. Behav Pharmacol. 2016. 27:556–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness DK, Foley GL, Villar D, Hansen LG. Effects of 3-iodo-L-tyrosine, a tyrosine hydroxylase inhibitor, on eye pigmentation and biogenic amines in the planarian, Dugesia dorotocephala. Fundam Appl Toxicol. 1996. 30:153–61. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Kitamura Y, Inoue T, Umesono Y, Yoshimoto K, Takeuchi K, Taniguchi T, Agata K. Identification and distribution of tryptophan hydroxylase (TPH)-positive neurons in the planarian Dugesia japonica. Neurosci Res. 2007. 59:101–6. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Kitamura Y, Umesono Y, Takeuchi K, Takata K, Taniguchi T, Agata K. Identification of glutamic acid decarboxylase gene and distribution of GABAergic nervous system in the planarian Dugesia japonica. Neuroscience. 2008. 153:1103–14. [DOI] [PubMed] [Google Scholar]
- Ouyang K, Nayak S, Lee Y, Kim E, Wu M, Tallarida CS, Rawls SM. Behavioral effects of Splenda, Equal and sucrose: Clues from planarians on sweeteners. Neurosci Lett. 2017. 636:213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán OR, Deats S, Baker D, Montgomery E, Wilk G, Tenaglia M, Semon J. Planarians require an intact brain to behaviorally react to cocaine, but not to react to nicotine. Neuroscience. 2013. 246:265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan OR, The first Brain: The neuroscience of planarians, first ed Oxford University press, New York, NY: (2014). [Google Scholar]
- Pagán OR. Planaria: an animal model that integrates development, regeneration and pharmacology. Int J Dev Biol. 2017. 61:519–529. [DOI] [PubMed] [Google Scholar]
- Palladini G, Ruggeri S, Stocchi F, De Pandis MF, Venturini G, Margotta V. A pharmacological study of cocaine activity in planaria. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996. 115:41–5. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Valdez JM. Cocaine withdrawal in Planaria. Eur J Pharmacol. 2001. 430:143–5. [DOI] [PubMed] [Google Scholar]
- Ribeiro P, El-Shehabi F, Patocka N. Classical transmitters and their receptors in flatworms. Parasitology. 2005. 131: Suppl S19–40. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Yuruzume E, Nakata H. Identification of planarian serotonin receptor by ligand binding and PCR studies. Neuroreport. 1996. 8:173–8. [DOI] [PubMed] [Google Scholar]
- Saitoh O, Yuruzume E, Watanabe K, Nakata H. Molecular identification of a G protein-coupled receptor family which is expressed in planarians. Gene. 1997. 195:55–61. [DOI] [PubMed] [Google Scholar]
- Tallarida CS, Bires K, Avershal J, Tallarida RJ, Seo S, Rawls SM. Ethanol and cocaine: environmental place conditioning, stereotypy, and synergism in planarians. Alcohol. 2014. 48:579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda S, Stagliano GW, Borenstein MR, Raffa RB. A reverse-phase HPLC and fluorescence detection method for measurement of 5-hydroxytryptamine (serotonin) in Planaria. J Pharmacol Toxicol Methods. 2005. 51:73–6. [DOI] [PubMed] [Google Scholar]
- Welsh JH, Williams LD. Monoamine-containing neurons in planaria. J Comp Neurol. 1970. 138:103–15. [DOI] [PubMed] [Google Scholar]
- Whitaker AM, Gilpin NW, Edwards S. Animal models of post-traumatic stress disorder and recent neurobiological insights. Behav Pharmacol. 2014. 25:398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JP, Li MH, Chen JS, Chung SY, Lee HL. Disturbances to neurotransmitter levels and their metabolic enzyme activity in a freshwater planarian exposed to cadmium. Neurotoxicology. 2015. 47:72–81. [DOI] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007. 56:19–32. [DOI] [PubMed] [Google Scholar]
- Zewde AM, Yu F, Nayak S, Tallarida C, Reitz AB, Kirby LG, Rawls SM. PLDT (planarian light/dark test): an invertebrate assay to quantify defensive responding and study anxiety- like effects. J Neurosci Methods. 2018. 293:284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Tallarida CS, Raffa RB, Rawls SM. Sucrose produces withdrawal and dopamine- sensitive reinforcing effects in planarians. Physiol Behav. 2013. 112:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoladz PR, Fleshner M, Diamond DM. Psychosocial animal model of PTSD produces a long- lasting traumatic memory, an increase in general anxiety and PTSD-like glucocorticoid abnormalities. Psychoneuroendocrinology. 2012. 37:1531–45. [DOI] [PubMed] [Google Scholar]