Summary
The neuropathic pain phenotype is the consequence of functional and morphological reorganization of the PNS and CNS. This reorganization includes DRGs and the spinal cord, and extends to multiple supraspinal areas including the limbic and reward systems. Several recent papers show that acute manipulation of cortical and subcortical brain areas causally correlates with the cognitive, emotional and sensory components of neuropathic pain, yet mechanisms responsible for pain chronification remain largely unknown. Here we show that nucleus accumbens expression of ΔFos-B, a transcription factor that plays a critical role in addiction and in the brain response to stress, is reduced long term following peripheral neuropathic injury. Conversely, boosting ΔFos-B expression in the nucleus accumbens by viral transfection causes a significant and long-lasting improvement of the neuropathic allodynia. We suggest that ΔFos-B in the nucleus accumbens is a key modulator of long term gene expression leading to pain chronification.
Keywords: Limbic system, chronic pain, gene expression, immediate early genes
Chronic pain is a major public health concern with enormous socio-economic costs, estimated to reach up to $635 billion annually (Institute of Medicine Committee on Advancing Pain Research. Washington, DC: National Academies Press (US); 2011). Over the past 50 years, work on several animal models has importantly advanced our understanding of the pathophysiology of chronic pain, revealing chronic pain-associated cellular and molecular alterations at the level of the DRG neurons, the spinal cord and the brainstem 1,2,3. However, it is only over the last 10-15 years that work in human patients as well as in animal models has shown that the CNS re-arrangement in chronic pain also includes the forebrain 4,5,6,7. Strong evidence now shows that supraspinal areas not only undergo important reorganization in chronic pain, but also have a causal role in the multiple aspects of the chronic pain phenotype 8,9,10,11. Accordingly, numerous papers show functional and morphological alterations in several non-somatosensory brain areas across various pain models 12,6,13,14,15,16. The nucleus accumbens (NAc) is a key structure in the reorganization of the brain networks in neuropathic pain condition. Inactivation of the nucleus accumbens attenuates pain in different neuropathic pain models,17,18. Decreased motivation in the chronic pain state was shown to be associated with long term depression of synapses on D2-expressing neurons in the NAc core 8. Importantly, both direct optogenetic activation of the mPFC terminals within the NAc 9,19 and chemogenetic manipulation of D2-expressing spiny neurons in the NAc shell 11 affect pain perception in neuropathic pain animals. Thus, NAc activity is critical for the pain phenotype. The transition of pain from an acute, adaptive condition to a chronic, maladaptive one continues for months after injury and is associated with widespread long term changes in brain gene expression 20. Among the several transcription factors that are known modulators of gene expression in the NAc, ΔFos-B is particularly intriguing. Contrary to other members of the Fos family, ΔFos-B is very stable with some isoforms having half-lives of more than 200 hours 21. Additionally, ΔFos-B promotes spine growth 22, a common mechanism underlying synaptic plasticity 23 and it is strongly induced in the striatum in response to several different chronic stimulations including drug abuse and stress 24. Thus, we hypothesized that ΔFos-B is implicated in the long-term regulation of gene expression in the NAc in response to peripheral neuropathic injury. Here we show that ΔFos-B expression in the nucleus accumbens is decreased over the long-term following neuropathic injury and that virally-driven ΔFos-B overexpression in the NAc causes a significant long-term improvement in tactile allodynia.
Materials and Methods
Animals.
Adult male Sprague Dawley rats (Harlan, Indianapolis, IN; 200 – 250g) were used throughout the experiments. Animals were housed on soft bedding in groups of three per cage on a 12-h light/dark cycle in a temperature-controlled environment (21 ± 2°C) with food and water available ad libitum. For all animals, handling and testing were performed during the light period. To minimize stress, they were handled regularly before injury and before behavioral testing. All experimental procedures were approved by the Northwestern University Institutional Animal Care and Use Committee and all efforts were made to minimize the number of animals used and their suffering.
Spared Nerve Injury (SNI).
SNI was used as an animal model of persistent peripheral neuropathic pain 25. Animals were anesthetized with isoflurane (1.5 −2%) and a mixture of 30% N2O and 70% O2. The sciatic nerve of the left hind leg was exposed at the level of trifurcation into the sural, tibial, and common peroneal nerves. The tibial and common peroneal nerves were tightly ligated and severed, leaving the sural nerve intact. Animals in the sham injury group served as the control as their sciatic nerves were exposed, as in the SNI procedure, but they received no further manipulations.
Behavioral testing.
Tactile sensitivity of the hind paw was measured using withdrawal responses to a series of von Frey filaments as previously described 26. Animals were placed in a Plexiglass box with a wire grid floor and allowed to habituate to the environment for 10 – 15 minutes. Von Frey filaments of varying forces (Stoelting Co, USA) were applied to the lateral part of the plantar surface of the hind paw. Filaments were applied in either ascending or descending strengths to determine the filament strength closest to the hind paw withdrawal threshold. Each filament was applied for a maximum of 2 seconds at each trial; paw withdrawal during the stimulation was considered a positive response. Given the response pattern and the force of the final filament, 50% response threshold (in grams) was calculated.
Cold allodynia was tested by applying 1 drop (about 0.1 ml) of acetone solution on the injured hind paw. Rats were then observed for 5 min and we measured the duration of their withdrawal reaction, which was then converted to a 0–4 scale.
Behavioral tests were conducted on three different animal groups. The first group included the same animals described in Chang et al. (2014) 18. This experimental group included SNI and Sham-operated rats. These animals were tested for tactile allodynia right before being sacrificed, just to ensure the effectiveness of the SNI surgery and were only used to collect tissue for qPCR analysis.
Two other groups of animals were tested to measure the behavioral effects of gene replacement therapy in SNI. The first group included 2 cohorts (AAVGFP/AAVFosB), only the contralateral NAc was injected, and the animals were tested up to 63 days after peripheral surgery. The second group also included 2 cohorts: SNI/FosB and SNI/empty vector, but the animals were injected bilaterally and were followed for only 14 days after surgery.
Gene Expression Analysis.
Gene expression was performed on NAc from SNI and sham operated rats at Day5 and Day28 post-surgery. Rats were deeply anesthetized with isoflurane and rapidly decapitated; brains were removed while immersed in frozen TBS. Brains were sliced coronally from Bregma 2.20 to 1.00, allowing easy visualization of the NAc. The contra- and ipsi-lateral NAc were removed separately, snap frozen in liquid nitrogen, and stored at −80°C. RNA was extracted using a Qiagen RNeasy RNA extraction kit; DNA contamination was prevented by first using a column that binds DNA while allowing RNA to flow through. RNA was reverse transcribed into cDNA using Roche’s First Strand cDNA Synthesis kit and oligo dT primers. RNA yield and quality was assessed measuring the 260/280nm and 260/230nm absorbance ratios.
The samples used for the measurements in this paper were obtained from the same rats used in Chang et al. (2014)18. Thus, we could calculate the correlation between the relative abundance of the Fos family transcripts (described here) and the transcript for DR1, DR2 and KOR whose expression was described previously 18.
RT-qPCR was performed using a Roche Lightcycler 480 (LC480) with either Roche probes master mix or SYBR green master mix, primers (0.4 μM), hydrolysis probes and NAc cDNA (primers are listed in Table 1). All genes of interest were normalized to the GAPDH reference gene. For hydrolysis probe reactions, a single gene of interest was multiplexed with the reference gene GAPDH in the same well. Reactions consisted of a 5-minute hot start incubation at 95°C, followed by 45 cycles of 10-second at 95°C, 10-second at 60°C, and 1-second at 72°C. Primers were initially validated using a SYBR green RT-qPCR assay, which allowed for melting temperature analysis, demonstrating a single peak for each gene product. RT-qPCR samples were also run on 1.8% agarose gels to verify single gene products. Although primers were intron spanning, cDNA negative and reverse transcriptase negative controls were performed for all targets and did not give a signal. As recommended in published guidelines for RT-qPCR methods, all data were efficiency corrected. Reaction efficiency for each gene product was assessed using standard curves obtained with progressive dilutions of NAc cDNA. All data were efficiency corrected using Roche LC480 software and the Delta Delta Ct method 27. For multiplex reactions, efficiency curves were run using multiplex parameters, while for SYBR green reactions efficiency curves were done with SYBR green reaction mixes. Within the recommendations of the MIQE RT-qPCR guidelines 28, a reference gene can be validated by running another reference gene against it. Thus, to validate GADPH as a reference gene, tubulin was run relative to GAPDH for all samples and no differences in tubulin were detected when run against GAPDH (data not shown). Statistical analyses were done using a two-way ANOVA test, with Fisher LSD for post-hoc analysis.
Table 1. Primer sequences for genes of interest.
Abbreviations: KOR = kappa-opioid receptor, DR1a = dopamine type 1a receptor, DR2 = dopamine type 2 receptor, Tubulin = alpha tubulin, GAPDH = glyceraldehyde-3-phosphate dehydrogenase.
| cFos | L gggagtgaagaccatgt | R cttcggattctccgtttctct |
| ΔFosB | L aggcagagctggagtcggaga | R gccgaggacttgaacttcactcg |
| FosB | L gtgagagatttgccagggtc | R agagagaagccgtcaggttg |
| DR1a | L cgaactgtatggtgcccttc | R gatggaatcgatgcagaatg |
| DR2 | L aacaccaagcgcagcagt | R tcctcagggtgggtacagtt |
| KOR | L aagcggtgttttagggacttc | R tttctaactctgtttgtgctctgg |
| Tubulin (alpha1b) | L cttctaacccgtagctatcatgc | R gccatgttccaggcagtag |
| GAPDH | L ctgcaccaccaactgcttag | R tgatggcatggactgtgg |
Virus Injections.
Adeno associated virus (AAV) has the ability to efficiently transfect neurons and expresses for long periods of time 29. AAV serotype 2 containing plasmids expressing ΔFosB and GFP, and AAV GFP empty vector were kindly provided by Dr. Eric Nestler (Icahn School of Medicine at Mount Sinai, New York). The ΔFosB viral constructs selectively infect neurons and have shown no toxicity compared with empty vector (carrying GFP only) viruses. Additional information regarding these viruses can be found 30,31. Viral vectors were injected three weeks before SNI surgery to allow for maximal expression to occur and for animals to fully recover from injection surgery. Previous studies have shown maximal viral expression by 10 days, persisting at least 6 months 30,31. Either empty vector (n=8) or ΔFosB (n=12) viruses were injected into the nucleus of accumbens (NAc) on the right hemisphere (contralateral to the peripheral surgery) only, at 1.7-1.8 mm from Bregma, 1.0-1.2 lateral to midline, and 6.6-6.8 ventral from brain surface. Post-hoc imaging of the injection sites showed that viral expression was by and large limited to the NAc and was particularly strong in the NAc core. Briefly, the animals were anesthetized using isoflurane (5% induction, 2.5 – 3% maintenance). Adequacy of anesthesia was evaluated by assessing any withdrawal reflex in response to a noxious paw pinch; a craniotomy was then performed to expose the surface of the brain for viral injection. An incision was made with a sterile scalpel and any bleeding cauterized. The location of the injection entry site on the skull was determined using a stereotaxic frame, and a small portion of the skull (<3 mm diameter) removed at this location using a dental drill. A fine tipped glass pipette pulled on a vertical puller was filled with the viral suspension. 0.5 μL of viral suspension was delivered by pressure-injection. Following the injection, the pipette was left in place for five minutes and then slowly backed out of the brain. Three weeks following AAV injections, when the rats had fully recovered, SNI surgeries were carried out on the paw contralateral to the AAV injections.
A second cohort of animals was injected bilaterally in the NAc. The procedures were the same as the unilateral injections, except that both hemispheres were targeted.
Immunohistochemistry.
SNI rats were anesthetized using ketamine/xylazine (80 and 10 mg/0.1 kg, respectively), and transcardially perfused with saline (0.9%) followed by 4% paraformaldehyde. Brains and spinal cords were removed and post-fixed in paraformaldehyde for 2 hours. Tissue was cryoprotected stepwise to 30% sucrose (brains) and 20% sucrose (spinal cords). 40 micron slices of brain tissue were cut using a microtome. Primary antibodies were incubated overnight in 1% normal goat serum (NGS), 0.2% Triton-X 100, in TBS. Sections were then washed in TBS, followed by secondary antibody (in TBS with 0.1% BSA) for 1 hour at room temperature, and further washing and mounting. Primary antibodies: Abcam ab4674 GFAP 1:1200, Millipore MAB377 NeuN 1:500, Invitrogen G10362 GFP 1:2,000.
Results
Fos-family gene expression is reduced in the NAc of SNI rats.
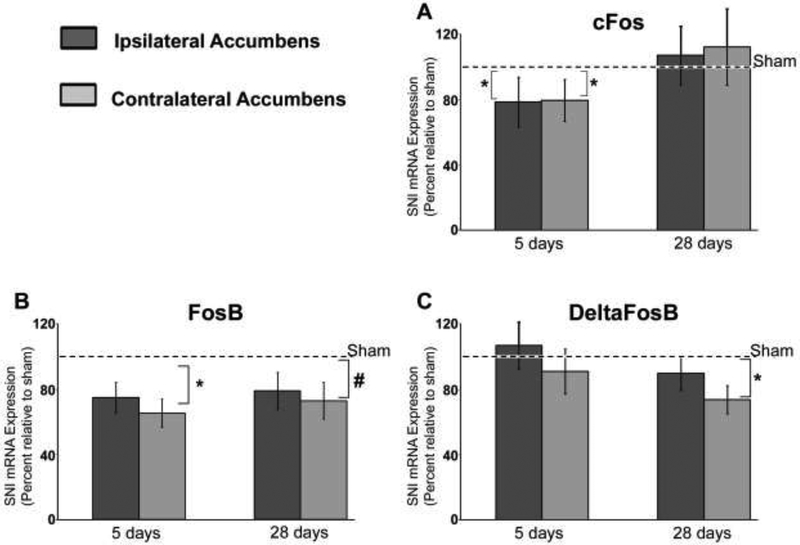
We took advantage of the spared nerve injury (SNI) model of neuropathic pain to test the hypothesis that ΔFosB expression in the NAc is altered in neuropathic pain. NAc tissue samples were collected 5 and 28 days post SNI/sham surgery and analyzed via quantitative RT-PCR (RT-qPCR). Tissue samples from the NAc, ipsilateral and contralateral to nerve injury, were processed separately in order to test for possible lateralization of the observed effects. The expression level in the SNI NAc ipsilateral to the peripheral lesion was normalized to the ipsilateral sham NAc, and the expression in the SNI NAc contralateral to lesion was normalized to the contralateral sham NAc. We have previously shown that in the same cDNA samples a significant downregulation of NAc dopamine receptor (DR1a and DR2) and Kappa opioid receptor transcript is detectable in the NAc contralateral to nerve injury 28 days after SNI surgery 18. We had also found that at early post SNI stage (5 days) the expression level of these diverse receptors showed a high covariance, suggesting the possible activation of a common transcriptional plan. To build upon these studies, we measured the expression level of transcripts of the Fos family of early genes. We focused our analysis on Fos family early genes because of their involvement in NAc remodeling in response to a number of factors, including drugs of abuse and stress 32,33,34. In SNI rats, we detected a significant reduction of c-Fos transcript in both the ipsi- and contra-lateral NAc 5 days post-surgery (Fig. 1a). However, 28 days post SNI surgery c-Fos transcript levels on both sides had returned to control values. Analysis of FosB, another Fos family member, also revealed a significant reduction in SNI rats at the 5 day time-point. In contrast with the c-Fos reduction, the decrease in FosB transcript was maintained through the 28 day time-point (Fig. 1b). Although the difference appeared bilateral, statistical analysis showed significance was only reached in the NAc contralateral to nerve injury. Finally, we measured ΔFosB, which is a stable spliced product of FosB and mediates some of the chronic changes in the NAc in response to addiction and antidepressant drugs 35. Analysis of ΔFosB transcript in the NAc of SNI rats revealed no significant changes 5 days post SNI, but 28 days post SNI a significant reduction was detected in the NAc contra-lateral to nerve injury (Fig. 1c).
Figure 1. FosB and ΔFosB transcript are reduced in the NAc of SNI rats.
Transcript levels were measured via qRT-PCR at day 5 and 28 post surgery. Relative transcript abundance in ipsilateral SNI was normalized the ipsilateral sham, while the contralateral SNI was normalized to the contralateral sham. Dotted line represents levels in sham. Each gene of interest was normalized to GAPDH. A, cFos transcript is reduced both in the ipsi (35%) and contra-lateral (30%) NAc at day 5, but reverts to normal by day 28. B, FosB transcript shows a significant reduction in the SNI NAc contra-lateral to nerve injury at 5 days post SNI (35%); at day 28 the reduction was 28%, p=0.087). C, ΔFosB demonstrated no significant changes at day 5 post SNI on either the ipsi or contra-lateral SNI NAc. However, a significant (27%) reduction of ΔFosB was detected at 28 days post SNI only on the SNI contra-lateral to nerve injury. * P < 0.05, # P = 0.087.
AAV-mediated overexpression of ΔFosB in the NAc reduces allodynia in SNI rats.
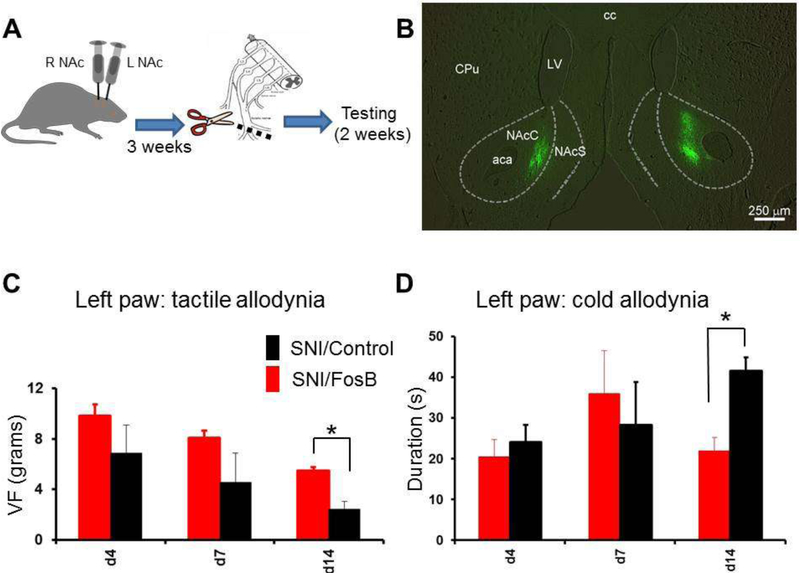
If ΔFosB reduction has a causal role for the pain phenotype, then normalizing its expression in the NAc of SNI rats may ameliorate the phenotype. To test this hypothesis, we used viral vectors to overexpress ΔFosB in the NAc of SNI rats. AAVs containing GFP labeled ΔFosB or empty vector were injected into the right NAc of naive rats and three weeks were allowed for virus expression and recovery. Three weeks later, SNI surgery was carried out on the left paw of all rats (SNI AAV/ΔFosB, n=12; SNI AAV/GFP, n=9) and von-Frey measurements were performed twice a week for 10 weeks to determine pain behavior. Tactile allodynia was significantly reduced in the ΔFosB injected SNI rats for the duration of the experiment (Fig. 2), while no change in cold allodynia was detected. At the conclusion of the behavioral testings, rats were perfused and brains sectioned to verify virus expression. Sections from several ΔFosB and control empty vector rats were co-stained with GFAP and no overlap was detected between GFAP and GFP (data not shown), demonstrating AAV expression is not in glia, consistent with previous reports that ΔFosB AAV expression is found only in neurons. Surprisingly, we could identify GFP in only 14 of the 21 injected rats, while in the remaining 7 rats (4 empty vector, 3 ΔFosB) the GFP could not be detected. Because the ΔFosB treated rats in which AAV expression wasn’t detected did show behavioral improvements we hypothesized that in these rats the immune system may have cleared out the virus, as it was expressing for over three months. If this was the case, it is interesting that the improvement in pain behavior outlasted the AAV ΔFosB expression. We then hypothesized that GFP expression would be more consistent at an earlier time from injection. Thus, we injected another cohort of SNI rats and we measured the pain phenotype for only 2 weeks. We also used this opportunity to inject the animals bilaterally in the NAc to test whether the behavioral effect is stronger. In this cohort of animals, GFP expression was detected in all 22 rats, on both ipsilateral and contralateral sides (Fig. 3B), supporting the idea that the lack of detection in some animals at a later time point might have been caused by an immune reaction. Again, ΔFosB expression significantly ameliorated the pain phenotype (Fig. 3C), reproducing the earlier experiment. Interestingly, in these rats with bilateral ΔFosB injection, cold allodynia was also ameliorated (Fig. 3D).
Figure 2. Virus-driven overexpression of FosB in the NAc contralateral to the peripheral lesion reduces tactile allodynia in SNI rats.
A, schematic representing the experimental design and timeline. B, tactile allodynia was significantly reduced for up to 2 months after surgery in SNI rats overexpressing ΔFosB in the NAc contralateral to the peripheral injury. The numbers on the abscissa represent the 3 baseline measurements (before surgery), and the day after SNI surgery. C, Cold allodynia (measured using acetone test), however, was unaffected by this treatment. Data from 11 ΔFosB-injected and 9 control-injected rats. *p<0.05.
Figure 3. Virus-driven bilateral overexpression of FosB in the NAc reduces both tactile and cold allodynia in SNI rats.
A, schematic representing the experimental design with bilateral viral injections, and the timeline. B, representative photograph of a rat brain section shows bilateral GFP expression in the virus injection sites. C, tactile allodynia was significantly reduced 14 days after surgery in SNI rats overexpressing ΔFosB/GFP bilaterally in the NAc. D, in contrast with data from mono-laterally injected SNI rats, bilateral ΔFosB overexpression significantly reduced also cold allodynia at the same time point. Data from 13 ΔFosB-injected and 8 control-injected rats. * P < 0.05.
Discussion
ΔFosB level in the NAc correlates with neuropathic allodynia.
The nucleus accumbens has a central role in the CNS reorganization in neuropathic pain. In line with its well-known physiological roles, the nucleus accumbens mediates chronic pain-associated impairments in motivation 8 and social ability11. Additionally, several reports demonstrate that the nucleus accumbens also modulates the sensory component of chronic pain, including tactile allodynia 17,18,9,19,11. Here we show that AAV-driven overexpression of the transcription factor ΔFosB in the nucleus accumbens causes a long-term improvement in the sensory pain component in SNI rats. This effect appears selective for the neuropathic pain phenotype, and not a general effect on tactile perception, because ΔFosB overexpression did not change the baseline pain threshold, in line with results obtained in the Nestler lab evaluating hot plate responses 31.
Interestingly, mono-lateral ΔFosB overexpression (in the NAc contralateral to the SNI surgery) selectively improved the tactile allodynia, without detectable effects on thermal allodynia. Bilateral overexpression, however, ameliorated both conditions. The PCR data show that although the reduction in Fos family genes expression only reached statistical significance in the contralateral nucleus accumbens, a clear bilateral trend was present for all 3 Fos transcripts investigated. This partial lateralization of the SNI effects on gene expression, on the other hand, reminds of our previous results obtained analyzing DR1a, DR2, and KOR transcript expression in the very same tissue samples 18. At functional level, however, NAc reorganization in SNI rodents appears largely bilateral, as intrinsic excitability and morphology of indirect pathway spiny neurons are altered in both hemispheres 11. Thus, although this is a point that may warrant future investigations, it is likely that the differential effects on pain behavior of the bilateral and the unilateral FosB overexpression are mostly due to a total dose effect. Finally, no overt correltaion was detectable between the location of the injections in different animals (fluorescence located mostly in the core, the shell or equal) and the behaviors investigated (not shown).
Expression of ΔFosB in the NAc correlates with DR1,DR2 and KOR.
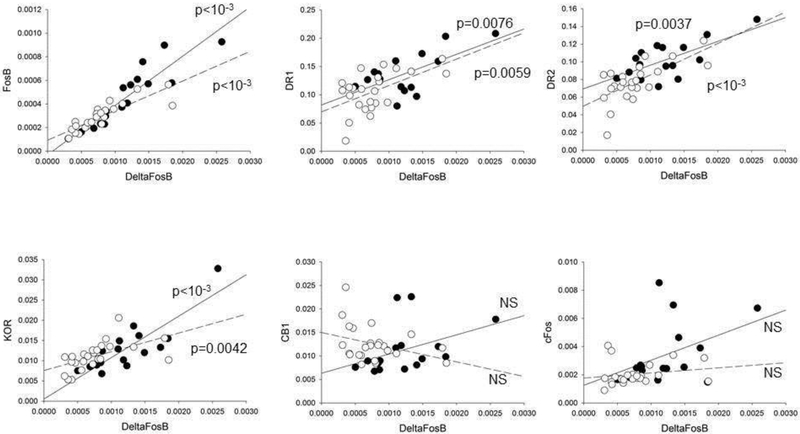
We had previously found covariance between the expression levels of DR1, DR2, KOR and cannabinoid receptor 1 (CB1) transcripts in the NAc of SNI animals 18. We wondered whether the Fos family immediate early genes may serve as regulators of gene expression for chronic pain persistence. Thus, we investigated the potential correlation between the changes in NAc expression of Fos family transcription factors and the transcripts of these receptors in SNI rats. This was possible because Fos genes transcripts quantification was performed on cDNA obtained from the very same samples previously used for DR1, DR2, KOR and CB1 transcript quantification. As expected, ΔFosB was highly correlated with FosB both 5 and 28 days after surgery (p<0.0001 in both cases, Fig. 4). Interestingly, we found that ΔFosB was also significantly correlated with KOR, DR1a, and DR2 (Fig. 4). cFos and CB1 transcript levels, on the contrary, did not significantly correlate with ΔFosB. A similar pattern of correlations was also found in sham rats (not shown). The correlation of FosB/ΔFosB with KOR, DR1a, and DR2 transcript levels suggests that FosB/ΔFosB works as a master regulator of cellular rearrangement of the NAc in SNI.
Figure 4. ΔFosB transcript levels correlate with expression of FosB, DR1a, DR2, and KOR in the NAc of SNI rats.
Regression analysis of ΔFosB, FosB, cFos, DR1a, DR2, KOR and CB1 transcript in the NAc of SNI rats 5 and 28 days after injury. ΔFosB is correlated with FosB, DR1a, DR2, and KOR, but not with CB1 or cFos (p values of linear regressions are indicated in the plots). Filled symbols and solid regression lines indicate data at 28 days post SNI; hollow symbols and dashed lines indicate data at 5 days post SNI. For this analysis, data obtained from the right and the left NAc were plotted together.
Potential cellular mechanisms affected by FosB dysregulation.
Numerous papers show that although altered activity of the NAc in chronic pain is the consequence, at least in part, of changes in the properties of synaptic inputs to the NAc from other brain areas such as the basolateral amygdala 8, the prefrontal cortex 9 and the VTA 11, intrinsic functional and morphological properties of NAc neurons are necessary and sufficient to modulate the pain phenotype 11. In order for these alterations to persist long-term, they require local regulation of gene expression. Accordingly, we previously found that the expression level of different genes in the NAc of SNI animals appears the result of a coordinated gene expression response that starts within a few days after injury and settles toward a new steady state about a month later. Alterations in the expression levels of transcription factors are ideal mediators of long-lasting processes and our present results showing that FosB/ΔFosB transcripts are significantly reduced in the NAc of SNI rats support the idea of a concerted gene expression modulation within the NAc network. ΔFos-B in particular appears ideally suited to mediate long-term regulation of gene expression because contrary to the other members of this family, which are transiently expressed and undergo rapid degradation, it is stable 36 and accumulates in the NAc in response to chronic stimuli, such as chronic stress (reviewed by Nestler, 2015) 35. Given that FosB/ΔFosB is a major transcription factor with numerous targets 37, our finding suggests that many additional transcripts are likely also dysregulated as consequence of this reduction. In line with this idea, recent RNA-sequencing work has shown that 691 genes are downregulated and 135 upregulated in the NAc of SNI mice 38.
ΔFosB expression in the NAc mediates several processes that are relevant for the reorganization observed in chronic pain. For example, ΔFosB expression has been associated with increased spine number in NAc SPNs 39. Since both the dendritic length and the number of synaptic contacts are reduced in NAc iSPNs of SNI animals 1 week after injury 11, the reduction in NAc ΔFosB described here may mediate these structural effects. ΔFosB was also found to be co-regulated and part of a positive feedforward loop with CaMKII, at least in response to drugs of abuse 40. Because CaMKII upregulates voltage gated potassium current and decreases firing in NAc shell neurons 41, if this correlation is maintained in SNI animals, the decreased ΔFosB level in SNI may contribute to the decrease in iSPNs rheobase measured in slices from SNI animals 11. Additionally, ΔFosB overexpression in the NAc promotes resilience to chronic stress 35. Similarly, when we overexpressed ΔFosB in the NAc of SNI rats, these animals showed a prolonged reduction in allodynia. Because chronic pain is a stressor, it is not surprising that similar mechanisms contribute to the brain response to both these conditions. In this context it is also notable that ΔFosB expression in the NAc is promoted by activation of the ventral tegmental area (VTA)24; as VTA activity is decreased in SNI11 it is possible that the reduced ΔFosB expression is the consequence, at least in part, of the VTA deactivation. Additionally, it has been shown that environmental enrichment causes strong ΔFosB expression in all striatal regions, including the NAc 24 and has analgesic effects in SNI 42. Thus it may be tempting to hypothesize that ΔFosB regulation may contribute to the analgesic effects of environmental enrichment. This hypothesis however is weakened by the observation that environmental enrichment does not improve the depression-like symptoms of neuropathic pain 42.
Conclusions
1- Fos-family gene expression is reduced in the NAc of SNI rats.
2- Restoring ΔFosB levels in the NAc, has a long lasting analgesic effect in neuropathic pain rats.
3- We suggest that FosB/ΔFosB levels serve as master regulator of gene expression in the in the NAc network reorganization that underlies neuropathic pain.
Acknowledgments:
This work was supported by NIH grants: NS064091 (MM) and NS057704 (AVA). The authors thank Dr. Eric Nestler (Icahn School of Medicine at Mount Sinai, New York) for providing the ΔFosB-GFP viral constructs.
List of abbreviations
- aca
anterior commissure
- BSA
bovine serum albumin
- CB1
cannabinoid receptor type 1
- CNS
central nervous system
- CPu
caudate putamen
- DR1
dopamine receptor type 1
- DR2
dopamine receptor type 2
- DRG
dorsal root ganglion
- GFAP
glial fibrillary acidic protein
- GFP
green fluorescent protein
- iSPN
indirect pathway spiny neuron
- KOR
kappa opioid receptor
- LV
lateral ventricle
- NAc
nucleus Accumbens
- mPFC
medial prefrontal cortex
- PNS
peripheral nervous system
- qPCR
quantitative polymerase chain reaction
- SNI
spared nerve injury
- TBS
Tris-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Authors declare no competing financial interest
References
- 1.Basbaum AI Spinal mechanisms of acute and persistent pain. Regional anesthesia and pain medicine 24, 59–67 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Woolf CJ & Salter MW Neuronal plasticity: increasing the gain in pain. Science 288, 1765–1769 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Devor M Centralization, central sensitization and neuropathic pain. Focus on "sciatic chronic constriction injury produces cell-type-specific changes in the electrophysiological properties of rat substantia gelatinosa neurons". Journal of neurophysiology 96, 522–523, doi: 10.1152/jn.00365.2006 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Apkarian AV et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 10410–10415, doi: 10.1523/JNEUROSCI.2541-04.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H et al. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 7445–7453, doi: 10.1523/JNEUROSCI.1812-08.2008 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metz AE, Yau HJ, Centeno MV, Apkarian AV & Martina M Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proceedings of the National Academy of Sciences of the United States of America 106, 2423–2428, doi: 10.1073/pnas.0809897106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji G et al. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 5451–5464, doi: 10.1523/JNEUROSCI.0225-10.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz N et al. Chronic pain. Decreased motivation during chronic pain requires long-term depression in the nucleus accumbens. Science 345, 535–542, doi: 10.1126/science.1253994 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee M et al. Activation of corticostriatal circuitry relieves chronic neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 5247–5259, doi: 10.1523/JNEUROSCI.3494-14.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z et al. Role of Prelimbic GABAergic Circuits in Sensory and Emotional Aspects of Neuropathic Pain. Cell reports 12, 752–759, doi: 10.1016/j.celrep.2015.07.001 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Ren W et al. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat Neurosci 19, 220–222, doi: 10.1038/nn.4199 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao MG et al. Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience 26, 8923–8930, doi: 10.1523/JNEUROSCI.2103-06.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XY et al. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science 330, 1400–1404, doi: 10.1126/science.1191792 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Mutso AA et al. Abnormalities in hippocampal functioning with persistent pain. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 5747–5756, doi: 10.1523/JNEUROSCI.0587-12.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly CJ, Huang M, Meltzer H & Martina M Reduced Glutamatergic Currents and Dendritic Branching of Layer 5 Pyramidal Cells Contribute to Medial Prefrontal Cortex Deactivation in a Rat Model of Neuropathic Pain. Front Cell Neurosci 10, 133, doi: 10.3389/fncel.2016.00133 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radzicki D, Pollema-Mays SL, Sanz-Clemente A & Martina M Loss of M1 Receptor Dependent Cholinergic Excitation Contributes to mPFC Deactivation in Neuropathic Pain. The Journal of neuroscience : the official journal of the Society for Neuroscience 37, 2292–2304, doi: 10.1523/JNEUROSCI.1553-16.2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarkis R, Saade N, Atweh S, Jabbur S & Al-Amin H Chronic dizocilpine or apomorphine and development of neuropathy in two rat models I: behavioral effects and role of nucleus accumbens. Experimental neurology 228, 19–29, doi: 10.1016/j.expneurol.2010.12.004 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Chang PC et al. Role of nucleus accumbens in neuropathic pain: linked multiscale evidence in the rat transitioning to neuropathic pain. Pain 155, 1128–1139, doi: 10.1016/j.pain.2014.02.019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez E et al. Corticostriatal Regulation of Acute Pain. Front Cell Neurosci 11, 146, doi: 10.3389/fncel.2017.00146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarado S et al. Peripheral nerve injury is accompanied by chronic transcriptome-wide changes in the mouse prefrontal cortex. Molecular pain 9, 21, doi: 10.1186/1744-8069-9-21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Kelz MB, Hope BT, Nakabeppu Y & Nestler EJ Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. The Journal of neuroscience : the official journal of the Society for Neuroscience 17, 4933–4941 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maze I et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science 327, 213–216, doi: 10.1126/science.1179438 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holtmaat A & Svoboda K Experience-dependent structural synaptic plasticity in the mammalian brain. Nature reviews. Neuroscience 10, 647–658, doi: 10.1038/nrn2699 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Lobo MK et al. DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 18381–18395, doi: 10.1523/JNEUROSCI.1875-13.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decosterd I & Woolf CJ Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87, 149–158 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Chaplan SR, Bach FW, Pogrel JW, Chung JM & Yaksh TL Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods 53, 55–63 (1994). [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ & Schmittgen TD Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408, doi: 10.1006/meth.2001.1262 (2001). [DOI] [PubMed] [Google Scholar]
- 28.Bustin SA et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical chemistry 55, 611–622, doi: 10.1373/clinchem.2008.112797 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Chamberlin NL, Du B, de Lacalle S & Saper CB Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain research 793, 169–175 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winstanley CA et al. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 10497–10507, doi: 10.1523/JNEUROSCI.2566-07.2007 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zachariou V et al. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci 9, 205–211, doi: 10.1038/nn1636 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Perrotti LI et al. Induction of DeltaFosB in reward-related brain structures after chronic stress. The Journal of neuroscience : the official journal of the Society for Neuroscience 24, 10594–10602, doi: 10.1523/JNEUROSCI.2542-04.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larson EB et al. Striatal regulation of DeltaFosB, FosB, and cFos during cocaine self-administration and withdrawal. Journal of neurochemistry 115, 112–122, doi: 10.1111/j.1471-4159.2010.06907.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vialou V et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci 13, 745–752, doi: 10.1038/nn.2551 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nestler EJ FosB: a transcriptional regulator of stress and antidepressant responses. European journal of pharmacology 753, 66–72, doi: 10.1016/j.ejphar.2014.10.034 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carle TL et al. Proteasome-dependent and -independent mechanisms for FosB destabilization: identification of FosB degron domains and implications for DeltaFosB stability. The European journal of neuroscience 25, 3009–3019, doi: 10.1111/j.1460-9568.2007.05575.x (2007). [DOI] [PubMed] [Google Scholar]
- 37.Kelz MB et al. Expression of the transcription factor DeltaFosB in the brain controls sensitivity to cocaine. Nature 401, 272–276, doi: 10.1038/45790 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Descalzi G et al. Neuropathic pain promotes adaptive changes in gene expression in brain networks involved in stress and depression. Science signaling 10, doi: 10.1126/scisignal.aaj1549 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee KW et al. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America 103, 3399–3404, doi: 10.1073/pnas.0511244103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robison AJ et al. Fluoxetine epigenetically alters the CaMKIIalpha promoter in nucleus accumbens to regulate DeltaFosB binding and antidepressant effects. Neuropsychopharmacology 39, 1178–1186, doi: 10.1038/npp.2013.319 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kourrich S, Klug JR, Mayford M & Thomas MJ AMPAR-independent effect of striatal alphaCaMKII promotes the sensitization of cocaine reward. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 6578–6586, doi: 10.1523/JNEUROSCI.6391-11.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vachon P et al. Alleviation of chronic neuropathic pain by environmental enrichment in mice well after the establishment of chronic pain. Behavioral and brain functions : BBF 9, 22, doi: 10.1186/1744-9081-9-22 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]