Abstract
BCOR immunoreactivity is a sensitive and highly specific marker for clear cell sarcoma of the kidney (CCSK). However, a subset of adult renal sarcomas which overexpress BCOR are negative for BCOR genetic alterations, including BCOR gene fusions or BCOR-internal tandem duplication, and thus remain unclassified. We report five such undifferentiated renal/perirenal sarcomas which raised the differential diagnosis of CCSK due to their morphologic appearance and strong BCOR immunoreactivity, but which on RNA sequencing (RNA-Seq) proved to be malignant solitary fibrous tumors (SFTs). The neoplasms occurred in patients at an age range of 30–62 years. Three patients were females and two male. Four were primary renal neoplasms while one was perirenal. All five neoplasms were cellular, non-pleomorphic, undifferentiated sarcomas with branching capillary vasculature composed of primitive round to ovoid neoplastic cells with scant cytoplasm and nuclei having fine, evenly-dispersed chromatin. None of the cases demonstrated the typical hyperchromatic fusiform nuclei, prominent collagen deposition, or hemangiopericytomatous vasculature of SFT. All five cases were strongly immunoreactive for BCOR. Three cases were CD34 negative, where the other two were only focally CD34 positive. STAT6 was subsequently found to be positive by immunohistochemistry in all five cases. In summary, we report a previously unrecognized mimic of CCSK: malignant SFTs with an undifferentiated/small round cell phenotype along with branching capillary vasculature, strong immunoreactivity for BCOR, and minimal or no immunoreactivity for CD34. As CCSK is treated with a specific chemotherapy regimen, this distinction has therapeutic implications.
Keywords: Renal Neoplasm, Solitary Fibrous Tumor: BCOR, Translocation
INTRODUCTION
Clear cell sarcoma of the kidney (CCSK) comprises approximately 3% of pediatric renal neoplasms and occurs at a mean patient age of 3 years1,2, though occasional genetically confirmed cases have occurred in teenagers and other putative cases have been reported in adults. The classic morphologic pattern of CCSK is that of nests or cords of neoplastic cells separated by regularly spaced, branching fibrovascular septa. The cord cells can be either round, epithelioid or spindled, and are separated by extracellular mucopolysaccharide that creates a resemblance to clear cytoplasm. The nuclei are round to ovoid with fine chromatin without prominent nucleoli. The septal cells are spindled, fibroblast-like cells that surround thin, regularly-branching capillaries, and quite often form cellular perivascular sheaths. CCSK may also display a variety of variant morphologic patterns that create a broad differential diagnosis, including Wilms tumor, congenital mesoblastic nephroma, rhabdoid tumor, primitive neuroectodermal tumor (PNET), metanephric stromal tumor, synovial sarcoma, and sclerosing epithelioid fibrosarcoma3–6.
Genetic alterations underlying CCSK have been delineated in the past decade. The majority (>90%) of CCSK harbor internal tandem duplications (ITD) in the last exon of the BCOR (Bcl6 interacting co-repressor) gene7–9. A smaller subset of cases diagnosed as CCSK in slightly older patients (ages 8–14 years) harbor a BCOR-CCNB3 gene fusion that is identical to that described in a subset of EWSR1-negative undifferentiated Ewing-like sarcomas of either bone or soft tissue10–12. A third subset of CCSK harbor a YWHAE-NUTM2B gene fusion resulting from a t(10;17)(q22.3;p13.3) translocation which is identical to that seen in high grade endometrial stromal sarcoma13,14. These three genetic alterations appear to be mutually exclusive15,16. All three result in high levels BCOR mRNA expression resulting in BCOR protein overexpression as detected by immunohistochemistry17. The latter has proven to be a sensitive and specific marker of CCSK among pediatric renal neoplasms18. However, we have found that a subset of BCOR-immunoreactive renal neoplasms in adults that resemble CCSK is negative for BCOR or YWHAE genetic alterations, and thus remains unclassified.
We report five such undifferentiated renal/perirenal sarcomas which raised the differential diagnosis of CCSK due to their morphologic appearance and strong nuclear immunolabeling for BCOR. RNA sequencing (RNA-Seq) then demonstrated the characteristic NAB2-STAT6 gene fusion supporting the diagnosis of malignant solitary fibrous tumor (SFT), a previously unrecognized mimic of CCSK.
METHODS
IRB approval
This study was approved by the Institutional Review Boards of the participating institutions.
Cases
The five cases derive from the consultation files of the authors and were originally considered as unclassified, undifferentiated renal/perirenal sarcomas, closely resembling CCSK. All five cases were studied by targeted RNA-seq using the TruSight RNA Fusion Panel (Illumina, San Diego, CA) for further characterization.
Immunohistochemistry
Immunohistochemistry was performed as previously described10 for the following proteins: BCOR, SATB2, CD34, HMB45, TLE1, desmin, and S100.
Scoring of BCOR immunoreactivity in extrarenal solitary fibrous tumors.
BCOR immunoreactivity was scored on the basis of percentage of neoplastic cells showing nuclear labeling (0–100), as well as intensity of labeling, (0=none; 1=weak; 2=moderate; 3=strong). These numbers were multiplied to give a final score or H-index, which ranged to 0–300. For comparison of benign and malignant extrarenal SFTs, we considered cases demonstrating >50% strong nuclear labeling to be positive as previously described17,18. The mean H score of benign and malignant extrarenal SFT also were compared.
Results
Cases
The clinicopathologic features of these cases are summarized in Table 1 (Figures 1–5). Cases 1–4 presented as large renal masses, while case 5 was perirenal. All five cases were considered as undifferentiated sarcomas before RNA sequencing analysis was performed. Unfortunately, clinical follow-up was not available for these cases; three were lost to follow up and two were recent.
Table 1:
Malignant Renal/Perirenal Solitary Fibrous Tumor
| Case | Age/Sex | Location | Tumor Size | IHC Positive | IHC Negative |
|---|---|---|---|---|---|
| 1 | 30/F | Kidney | 10cm | BCOR, STAT6, CD99 (focal), CD34 (focal) |
S100, Desmin, HMB45, Melan A, PAX8 |
| 2 | 40/M | Kidney | 18.5cm | BCOR CD99 (focal) |
CD34, Desmin, HMB45, PAX8, S100 |
| 3 | 62/F | Kidney | 26cm | BCOR, STAT6 CD34 (focal) |
Desmin Actin, S100, CD117, HMB45 |
| 4 | 48/M | Kidney | 10cm | BCOR STAT6 CD99 |
CD34, ERG, HMB45, Melan A, S100, Desmin, Cytokeratin, PAX8 |
| 5 | 46/F | Perirenal | NA | BCOR STAT6 CD99 (focal) |
CD34, CD31, HMB45, Melan A, S100, Desmin, Cytokeratin |
NA=not available; IHC=immunohistochemistry; F=female; M=male
Figure 1:
Malignant SFT of the kidney (Case 1). This is a cellular neoplasm (left) separated from the kidney (right) by a fibrous pseudocapsule (A). The neoplasm is composed of monomorphic round to epithelioid cells with scant pink cytoplasm (B). Multiple areas demonstrate small mucoid cysts, similar to those seen in CCSK (C, D). The neoplasm demonstrated diffuse nuclear labeling for BCOR (E), but also showed diffuse nuclear labeling for STAT6 (F), supporting the molecular finding of a NAB2-STAT6 gene fusion.
Figure 5:
Malignant perirenal SFT (Case 5). This is a cellular neoplasm demonstrating monotonous cells with indistinct cytoplasm and fine open chromatin, along with regular capillary branching vasculature, highly suggestive of CCSK (A, B). The neoplasm demonstrates strong nuclear labeling for BCOR (C), also suggesting CCSK. The neoplasm demonstrates strong diffuse nuclear labeling for STAT6 (D), supporting the RNAseq findings.
The morphology of the cases was similar therefore they are described together. All the cases predominantly contained nests of round to epithelioid neoplastic cells with fine dispersed chromatin separated by a regular branching capillary vasculature, highly reminiscent of the morphology of CCSK. While case 3 did focally demonstrate areas of hemangiopericytomatous vasculature and focal ropy collagen deposition, no case demonstrated the typical bland ovoid fibroblastic cell appearance typical of SFT. Case 1 demonstrated small mucoid cysts associated with increased extracellular mucopolysaccharide material, similar to the myxoid variant pattern of CCSK (Figure 1C, 1D). Cases 2 and 4 were highly cellular, causing overlap with cellular CCSK and other undifferentiated small round blue cell tumors. Four of the five cases demonstrated greater than 4 mitoses per 10 high power fields, supporting their classification as malignant. One case (case 3) had fewer than 4 mitoses per 10 high power fields, but was 26cm in greatest dimension, highly cellular, and showed extensive necrosis, supporting classification as malignant. Using the risk stratification model developed by Demicco et al.19, two of our cases were considered high risk for metastasis whereas two were intermediate risk. Case #5, for which we do not have tumor size, could not be assessed with this model.
Immunohistochemistry
By immunohistochemistry, all five cases were negative for desmin, S100, and HMB45. Two of five cases demonstrated focal immunoreactivity for CD34, with some areas of the tumor demonstrating convincing labeling and others being completely negative. The other three cases were completely negative for CD34. All four cases tested demonstrated immunoreactivity for CD99. All five cases demonstrated strong diffuse nuclear labeling for BCOR (Figure 1E, 2D, 3E, 4E, 5C), furthering the resemblance to CCSK. Following RNA sequencing, all cases were immunolabeled for STAT6 (Figure 1F, 3F, 4F, 5D), and all demonstrated nuclear labeling.
Figure 2:
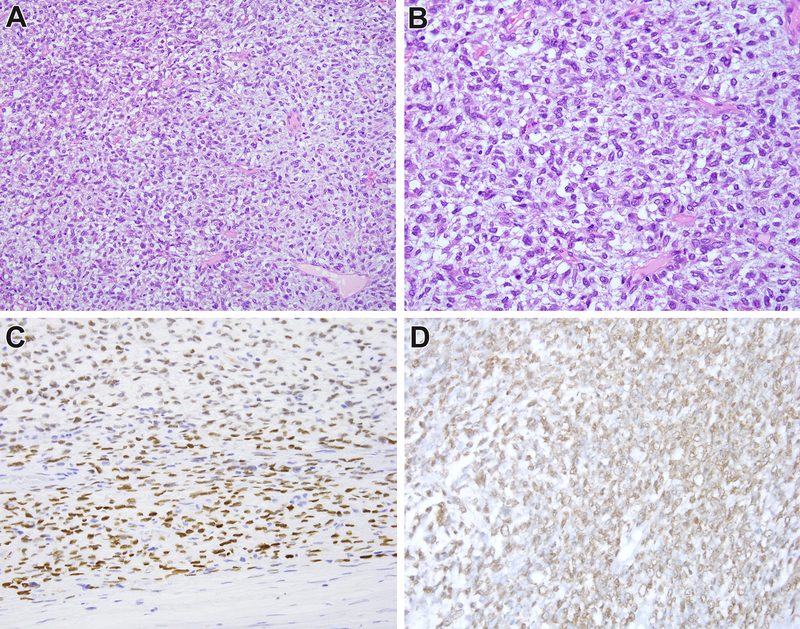
Malignant SFT of the kidney (Case 2). This is a highly cellular renal neoplasm with a primitive small blue round phenotype (A, B, C). There are vague nodular variations in cellularity (B). The neoplasm demonstrated diffuse strong nuclear labeling for BCOR (D) suggesting a diagnosis of cellular CCSK. The neoplasm demonstrated a NAB2-STAT6 gene fusion, supporting the diagnosis of SFT.
Figure 3:
Malignant SFT of the kidney (Case 3). This is a cellular neoplasm composed of bland ovoid to epithelioid cells with thin strands of ropy collagen (A). High power examination reveals a uniform branching capillary vasculature supporting nests and cords of neoplastic cells with indistinct cytoplasm and fine chromatin, suggestive of CCSK (B, C). The neoplasm is negative for CD34 (D), and demonstrates strong diffuse nuclear labeling for BCOR (E). The neoplasm demonstrates strong diffuse nuclear labeling for STAT6 (F), supporting the molecular finding of a NAB2-STAT6 abnormality.
Figure 4:
Malignant SFT of the kidney (Case 4). This an undifferentiated sarcoma centered in the renal pelvis. Note the renal medullary tissue present at the lower left and the renal pelvic urothelium at the upper right (A). The neoplasm permeates the renal sinus fat in a diffuse fashion (B). The neoplasm consists of undifferentiated small round blue cells, with focal clustering (C) but a predominant diffuse sheet-like growth pattern (D). The neoplasm demonstrates strong diffuse nuclear immunoreactivity for BCOR (E) and weaker but tumor cell-specific nuclear labeling for STAT6 (F).
RNA sequencing
All five cases demonstrated a NAB2-STAT6 gene fusion. Four cases demonstrated an NAB2 exon 6-STAT6 exon 16 fusion, while the other (case 3) demonstrated an NAB2 exon 7-STAT6 exon 17 fusion transcript.
Renal and Extrarenal Malignant SFT Overexpress BCOR mRNA
To evaluate BCOR mRNA expression in these neoplasms, we assessed RNA-sequencing data available for four malignant renal/perirenal SFT in this study (cases 1–3 and 5) as compared to over 100 other sarcomas available on the same targeted RNA-sequencing platform previously reported20. This revealed striking BCOR mRNA expression relative to the other sarcomas (Figure 6A), of even greater magnitude than the elevation in STAT6 expression in these neoplasms (Figure 6B). These results parallel the more intense immunoreactivity for BCOR than for STAT6 in these neoplasms. We then evaluated BCOR mRNA expression from a previously published expression profiling study of 20 SFT (14 malignant, 6 benign, across soft tissue, pleura and meningeal sties) using the U133A Affymetrix platform (2,200 transcripts)21. This revealed elevation of BCOR mRNA and STAT6 mRNA relative to 34 common soft tissue sarcomas spanning 7 histologic subtypes (Figure 6C, 6D).
Figure 6. BCOR and STAT6 expression in Solitary Fibrous Tumor (SFT).
(A, B) Targeted RNA sequencing showing marked upregulation of BCOR (A) and STAT6 (B) in 4 SFT study cases (orange) versus other sarcoma types (gray box-plot). (C,D) Expression profiling using the Affymetrix U133A platform showing upregulated BCOR (C) and STAT6 (D) in 23 SFT (various sites and risk of malignancy)(green box-plot) compared to a set of 34 soft tissue sarcomas spanning 7 common histotypes (gray box-plot)21.
Extrarenal solitary fibrous tumors are frequently immunoreactive for BCOR
Given the immunoreactivity identified in these renal neoplasms, we examined a series of benign and malignant extrarenal SFT for BCOR immunoreactivity. The clinicopathologic features of these cases are summarized in Supplemental Table 1. A total of 39 neoplasms were tested, 25 benign and 14 malignant using previously described criteria, such as >4 mitotic figures/10 high power fields22. While 13 of 14 (92%) malignant cases labeled positive (defined as >50% strong labeling) for BCOR, a lower percentage (11 of 25 or 44%) of benign SFT were BCOR immunoreactive (p=.0049, two tailed Fisher exact test). Of note, BCOR labeling was noted to be greater in the cellular areas of three benign SFT than in the hypocellular collagenous areas of these variably cellular neoplasms. The mean H-score of benign cases was 115, while that of the malignant SFT cases was 205. There was no correlation of BCOR labeling with tumor location.
DISCUSSION
We report five cases of renal and perirenal malignant solitary fibrous tumors (SFTs) closely resembling clear cell sarcoma of the kidney (CCSK). These cases demonstrated morphologic features overlapping with CCSK; namely, primitive round to ovoid cells with indistinct cytoplasm and fine nuclear chromatin, separated by branching capillary vasculature. Moreover all the neoplasms displayed strong nuclear labeling for BCOR, a sensitive and specific marker of CCSK in the pediatric kidney. We considered all five cases unclassified sarcomas at the time of diagnosis. None of the cases demonstrated the diffuse CD34 immunoreactivity typical of SFT; two were only focally positive, while three were completely negative. The diagnosis was retrospectively established in each case following RNA sequencing, which demonstrated the characteristic NAB2-STAT6 gene fusion of solitary fibrous tumor. The diagnosis was then corroborated by demonstration of STAT6 nuclear immunoreactivity. Hence, malignant SFT should be added to the differential diagnosis of CCSK. Malignant SFT and synovial sarcoma emerge as two close mimics of CCSK which also frequently demonstrate BCOR immunoreactivity.
We believe that the BCOR immunoreactivity observed in these cases is the result of an intrinsic transcriptional activation rather than an aberrant expression at the protein level for several reasons. First, examination of the RNA sequencing data revealed BCOR mRNA upregulation in all of our cases studied. Second, re-examination of a prior dataset of solitary fibrous tumors tested by the U133A Affymetrix gene expression platform21 demonstrated similar BCOR mRNA overexpression. Finally, we identified frequent BCOR immunoreactivity in a separate cohort of extrarenal SFT. We found that BCOR mRNA expression correlates with risk of malignancy in SFT, including the renal cohort of malignant SFT reported herein; however, occasional benign extrarenal SFTs may demonstrate diffuse nuclear labeling BCOR.
Solitary fibrous tumors typically demonstrate uniform ovoid to spindle cells arranged in a patternless pattern and associated with a stromal component rich in ropy collagen and large branching or staghorn shaped, “hemangiopericytomatous” vessels23. Cellularity is characteristically variable in different areas of the neoplasm. Most solitary fibrous tumors follow a benign clinical course, but 5–10% will metastasize. Malignant solitary fibrous tumors usually retain areas of typical morphology of SFT, but display high cellularity, often organized in a fascicular pattern, increased proliferation defined as >4 mitoses per 10 high power fields, and necrosis24. Less commonly, areas of conventional SFTs show abrupt transition to a high grade sarcomatous and often pleomorphic/anaplastic growth, which has been termed “dedifferentiation”25, 26. The most common histologies for dedifferentiated SFT include undifferentiated pleomorphic sarcoma, but heterologous differentiation, such as rhabdomyosarcomatous, osteosarcomatous, and chondrosarcomatous components, has also been reported27–29. More recently, rare cases of dedifferentiated SFT with a small round cell appearance have been documented24,25, but tumors resembling CCSK have not yet been described.
Approximately 60 cases of renal SFT have been reported in the literature. The clinicopathologic features of renal SFT have generally paralleled their extrarenal counterparts. Most renal SFT have been classified as benign and appear to follow an uneventful clinical course, though rare benign-appearing tumors have metastasized30–33. Approximately 15 cases of malignant renal SFT have been reported, of which approximately half have either recurred or metastasized in limited follow-up. Histologically malignant renal SFT defined either by high cellularity and elevated mitotic activity or by areas of dedifferentiation have also been reported34–40 Malignant SFT with a primitive small round cell appearance as reported in soft tissue25,26 or mimicking CCSK has not previously been reported in the kidney.
By RNA sequencing all five renal/perirenal SFTs demonstrated gene fusions involving NAB2 exon 6/7-STAT6 exons 16/17, which is one of the less common transcript variants, often associated with younger patients, deep seated soft tissue sites, malignant histology and aggressive clinical behavior. In contrast, the most common transcript variant composed of NAB2 exon 4-STAT6 exon 2/3 is found in most benign and pleural SFT41,42.
The differential diagnosis for these neoplasms is broad. CCSK is clearly the neoplasm with greatest overlap, considering the morphology and BCOR immunoreactivity. STAT6 expression has not formally been studied in CCSK; however, analysis of publicly available CCSK RNA Sequencing datasets showed no evidence of STAT6 mRNA overexpression43. Moreover, we have found STAT6 to be negative by immunohistochemistry in 6 CCSK cases tested (not shown). Synovial sarcoma is the other major consideration, given its predilection to involve the kidney, overlapping morphology with SFT, and immunoreactivity for BCOR in approximately half of the cases17. However, these neoplasms are easily distinguished genetically; synovial sarcomas harbor SS18-SSX1/2 fusions, which are not found in SFT, and lack the NAB2-STAT6 gene fusion of SFT. Furthermore, STAT6 nuclear immunoreactivity is present in malignant renal SFT but not in synovial sarcoma44,45.
In summary, we present a group of malignant SFT occurring in the kidney and pararenal area showing a significant morphologic and immunoprofile overlap with CCSK. The tumors had a primitive undifferentiated phenotype of uniform round to ovoid cells, lacking areas of conventional/benign SFT, and showed diffuse nuclear reactivity for BCOR, while being largely negative for CD34. Our report draws attention to this potential diagnostic pitfall for CCSK, adding malignant SFT to the list of tumors frequently showing BCOR overexpression. As CCSK is treated with a specific effective chemotherapy regimen, this distinction has therapeutic implications.
Supplementary Material
Acknowledgement:
We thank Norman Barker MA, MS, RBP for expert photographic assistance.
Disclosures: Supported in part by: P50 CA 140146–01 (CRA), Cycle for Survival (CRA), Kristin Ann Carr Foundation (CRA), Dahan Translocation Carcinoma Fund (PA), Joey’s Wings (PA)
REFERENCES
- 1.Argani P, Perlman EJ, Breslow NE, et al. Clear cell sarcoma of the kidney: a review of 351 cases from the National Wilms Tumor Study Group Pathology Center. Am J Surg Pathol. 2000;24:4–18. [DOI] [PubMed] [Google Scholar]
- 2.Gooskens SL, Furtwangler R, Vujanic GM, et al. Clear cell sarcoma of the kidney: a review. Eur J Cancer. 2012;48:2219–2226. [DOI] [PubMed] [Google Scholar]
- 3.Argani P, Fritsch M, Kadkol SS, et al. Detection of the ETV6-NTRK3 chimeric RNA of infantile fibrosarcoma/cellular congenital mesoblastic nephroma in paraffin-embedded tissue: application to challenging pediatric renal stromal tumors. Mod Pathol 2000; 13:29–36. [DOI] [PubMed] [Google Scholar]
- 4.Argani P, Beckwith JB. Metanephric stromal tumor: report of 31 cases of a distinctive pediatric renal neoplasm. Am J Surg Pathol 2000; 24:917–26. [DOI] [PubMed] [Google Scholar]
- 5.Argani P, Faria PA, Epstein JI, et al. Primary renal synovial sarcoma. Morphologic and molecular delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol 2000; 24:1087–1096. [DOI] [PubMed] [Google Scholar]
- 6.Argani P, Lewin JR, Edmonds P, Netto GJ, Prieto-Granada C, Zhang L, Jungbluth AA, Antonescu CR. Primary renal sclerosing epithelioid fibrosarcoma: report of 2 cases with EWSR1-CREB3L1 gene fusion. Am J Surg Pathol. 2015; 39:365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueno-Yokohata H, Okita H, Nakasato K, et al. Consistent in-frame internal tandem duplications of BCOR characterize clear cell sarcoma of the kidney. Nat Genet. 2015;47:861–863. [DOI] [PubMed] [Google Scholar]
- 8.Astolfi A, Melchionda F, Perotti D, et al. Whole transcriptome sequencing identifies BCOR internal tandem duplication as a common feature of clear cell sarcoma of the kidney. Oncotarget. 2015;6:40934–40939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy A, Kumar V, Zorman B, et al. Recurrent internal tandem duplications of BCOR in clear cell sarcoma of the kidney. Nat Commun. 2015;6:8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Argani P, Kao YC, Zhang L, Bacchi C, Matoso A, Alaggio R, Epstein JI, Antonescu CR. Primary renal sarcomas with BCOR-CCNB3 gene fusion: A report of 2 cases showing histologic overlap with clear cell sarcoma of kidney, suggesting further link between BCOR-related sarcomas of the kidney and soft tissues. Am J Surg Pathol. 2017; 41:1702–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierron G, Tirode F, Lucchesi C et al. A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat Genet 2012; 4; 44:461–6. [DOI] [PubMed] [Google Scholar]
- 12.Kao YC, Owosho AA, Sung YS, Zhang L, Fujisawa Y, Lee JC, Wexler L, Argani P, Swanson D, Dickson BC, Fletcher CDM, Antonescu CR. BCOR-CCNB3 Fusion Positive Sarcomas: A Clinicopathologic and Molecular Analysis of 36 Cases With Comparison to Morphologic Spectrum and Clinical Behavior of Other Round Cell Sarcomas. Am J Surg Pathol. 2018;42:604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Meara E, Stack D, Lee CH, et al. Characterization of the chromosomal translocation t(10;17)(q22;p13) in clear cell sarcoma of kidney. J Pathol. 2012;227:72–80. [DOI] [PubMed] [Google Scholar]
- 14.Lee CH, Ou WB, Mariño-Enriquez A, et al. 14–3-3 fusion oncogenes in high-grade endometrial stromal sarcoma. Proc Natl Acad Sci U S A. 2012;109:929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenny C, Bausenwein S, Lazaro A, et al. Mutually exclusive BCOR internal tandem duplications and YWHAE-NUTM2 fusions in clear cell sarcoma of kidney: not the full story. J Pathol 2016; 238:617–20. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson J, Valind A, Gisselsson D. BCOR internal tandem duplication and YWHAE-NUTM2B/E fusion are mutually exclusive events in clear cell sarcoma of the kidney. Genes Chromosomes Cancer 2016; 55:120–3. [DOI] [PubMed] [Google Scholar]
- 17.Kao YC, Sung YS, Zhang L, Jungbluth AA, Huang SC, Argani P, Agaram NP, Zin A, Alaggio R, Antonescu CR. BCOR overexpression is a highly sensitive marker in round cell sarcomas with BCOR genetic abnormalities. Am J Surg Pathol 2016; 40:1670–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Argani P, Pawel B, Szabo S, Reyes-Múgica M, Timmons C, Antonescu CR. Diffuse strong BCOR immunoreactivity is a sensitive and specific marker for clear cell sarcoma of the kidney (CCSK) in pediatric renal neoplasia. Am J Surg Pathol. 2018; 42:1128–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demicco EG, Wagner MJ, Maki RG, Gupta V, Iofin I, Lazar AJ, Wang WL.. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol. 2017;30:1433–1442 [DOI] [PubMed] [Google Scholar]
- 20.Antonescu CR, Agaram NP, Sung YS, Zhang L, Swanson D, Dickson BC. A distinct malignant epithelioid neoplasm with GLI1 gene rearrangements, frequent S100 protein expression, and metastatic potential: expanding the spectrum of pathologic entities with ACTB/MALAT1/PTCH1-GLI1 fusions. Am J Surg Pathol. 2018;42:553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajdu M, Singer S, Maki RG, Schwartz GK, Keohan ML, Antonescu CR. IGF2 over-expression in solitary fibrous tumours is independent of anatomical location and is related to loss of imprinting. J Pathol. 2010; 221:300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fletcher CDM, Bridge JA, Lee JC. Extrapleural solitary fibrous tumor In Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F (eds): WHO Classification of Tumors of Soft Tissue and Bone. IARC: Lyon 2013, p.80–82 [Google Scholar]
- 23.Thway K, Ng W, Noujaim J, Jones RL, Fisher C. The current status of solitary fibrous tumor: Diagnostic features, variants, genetics. Int J Surg Pathol 2016; 24:281–92. [DOI] [PubMed] [Google Scholar]
- 24.England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13:640–58 [DOI] [PubMed] [Google Scholar]
- 25.Mosquera JM, Fletcher CD. Expanding the spectrum of malignant progression in solitary fibrous tumors: a study of 8 cases with a discrete anaplastic component—is this dedifferentiated SFT? Am J Surg Pathol 2009; 33:1314–21. [DOI] [PubMed] [Google Scholar]
- 26.Collini P, Negri T, Barisella M, Palassini E, Tarantino E, Pastorino U, Gronchi A, Stacchiotti S, Pilotti S. High-grade sarcomatous overgrowth in solitary fibrous tumors: a clinicopathologic study of 10 cases. Am J Surg Pathol. 2012;36:1202–15. [DOI] [PubMed] [Google Scholar]
- 27.Creytens D, Ferdinande l, Van Dorpe J Multifocal cytokeratin expression in a dedifferentiated solitary fibrous tumor with heterologous rhabdomyosarcomatous differentiation: A challenging diagnosis! Int J Surg Pathol 2018; 26:423–427. [DOI] [PubMed] [Google Scholar]
- 28.Masuda Y, Kurisaki-Arakawa A, Hara K, Arakawa A, Oh S, Suzuki K, Yao T, Saito T. A case of dedifferentiated solitary fibrous tumor of the thoracic cavity. Int J Clin Exp Pathol 2013; 15:386–93. [PMC free article] [PubMed] [Google Scholar]
- 29.Thway K, Hayes A, Ieremia E, Fisher C. Heterologous osteosarcomatous and rhabdomyosarcomatous elements in dedifferentiated solitary fibrous tumor: further support for the concept of dedifferentiation in solitary fibrous tumor. Ann Diagn Pathol 2013; 17:457–63. [DOI] [PubMed] [Google Scholar]
- 30.Kuroda N, Ohe C, Sakaida N, Uemura Y, Inoue K, Nagashima Y, Hes O, Michal M. Solitary fibrous tumor of the kidney with focus on clinical and pathobiological aspects. Int J Clin Pathol 2014; 15:2737–42. [PMC free article] [PubMed] [Google Scholar]
- 31.Kouba E, Simper NB, Chen S, Williamson SR, Grignon DJ, Eble JN, MacLennan GT, Montironi R, Lopez-Beltran A, Osunkova AO, Zhang S, Wang M, Wang L, Tran T, Emerson RE, Baldrige LA, Monn MF, Linos K, Cheng L. Solitary fibrous tumour of the genitourinary tract: a clinicopathological study of 11 cases and their association with the NAB2-STAT6 fusion gene. J Clin Pathol 2017; 70 508–513. [DOI] [PubMed] [Google Scholar]
- 32.Khater N, Khauli R, Shahait M, Degheili J, Aoun J. Solitary fibrous tumors of the kidneys: presentation, evaluation, and treatment. Urol Int 2013; 91:373–83. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki H, Kurihara T, Katsuoka Y, Nakano T, Yoshioka M, Miyano S, Sato Y, Uejima I, Hoshikawa M, Takagi M, Chikaraishi T. Distant metastasis from benign solitary fibrous tumor of the kidney. Nephrol Urol 2013; 3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fine SW, McCarthy DM, Chan TY, Epstein JI, Argani P. Malignant solitary fibrous tumor of the kidney. Report of a case and comprehensive review of the literature. Arch Pathol Lab Med 2006; 130:857–861. [DOI] [PubMed] [Google Scholar]
- 35.Creytens D Malignant solitary fibrous tumour of the kidney with lymph node and liver metastases: beware of STAT6 expression in dedifferentiated liposarcoma with a solitary fibrous tumour-like morphology. Pathology 2017; 49:671. [DOI] [PubMed] [Google Scholar]
- 36.Mearini E, Cochetti G, Barillaro F, Fatigoni S, Roila F. Renal malignant solitary fibrous tumor with single lymph node involvement: report of unusual metastasis and review of the literature. Onco Targets Ther 2014; 8:679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao G, Li G, Han R. Two malignant solitary fibrous tumors in one kidney: Case report and review of the literature. Oncol Lett 2012; 4:933–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheung F, Talanki VR, Liu J, Davis JE, Waltzer WC, Corcoran AT. Metachronous malignant solitary fibrous tumor of kidney: Case report and review of literature. Urol Case Rep 2015; 17:45–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Martino M, Bohm M, Klatte T. Malignant solitary fibrous tumour of the kidney: report of a case and cumulative analysis of the literature. Aktuelle Urol 2012; 43:59–62. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh TY, Chang Chien YC, Chen WH, Chen SC, Chang LC, Hwang CC, Chein HP, Chen JR. De novo malignant solitary fibrous tumor of the kidney. Diagn Pathol 2011; 6:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barthelmeß S, Geddert H, Boltze C, Moskalev EA, Bieg M, Sirbu H, Brors B, Wiemann S, Hartmann A, Agaimy A, Haller F. Solitary fibrous tumors/hemangiopericytomas with different variants of the NAB2-STAT6 gene fusion are characterized by specific histomorphology and distinct clinicopathological features. Am J Pathol. 2014. ;184:1209–1218 [DOI] [PubMed] [Google Scholar]
- 42.Huang SC, Li CF, Kao YC, Chuang IC, Tai HC, Tsai JW, Yu SC, Huang HY, Lan J, Yen SL, Lin PC, Chen TC. The clinicopathological significance of NAB2-STAT6 gene fusions in 52 cases of intrathoracic solitary fibrous tumors. Cancer Med. 2016;5:159–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karlsson J, Holmquist Mengelbier L, Ciornei CD, Naranjo A, O’Sullivan MJ, Gisselsson D. Clear cell sarcoma of the kidney demonstrates an embryonic signature indicative of a primitive nephrogenic origin. Genes Chromosomes Cancer. 2014;53:381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida A, Tsuta K, Ohno M, Yoshida M, Narita Y, Kawai A, Asamura H, Kushima R. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol. 2014;38:552–9 [DOI] [PubMed] [Google Scholar]
- 45.Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27:390–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.