Abstract
Background / Objectives:
Lichen Planus-like Keratoses (LPLKs) are benign skin lesions that can mimic malignancy; the clinical and dermoscopic features distinguishing LPLKs from skin tumors have not been extensively studied. The objective of this study was to identify dermoscopic features that may prevent unnecessary biopsies of LPLKs.
Methods:
Retrospective, observational study of biopsied skin lesions. We compared 355 LPLKs to 118 non-LPLK lesions with LPLK in the differential diagnosis biopsied from August 1, 2015, to December 31, 2016. The investigators were blinded to the diagnosis of the lesions. A single-centre, observational study in a tertiary centre.
Results:
LPLKs were most frequently non-pigmented (61.7%), truncal (52.1%), and on sun-damaged skin (69.6%); the majority occurred in Whites (95.5%) and females (62.8%). Dermoscopically, LPLKs were more likely than non-LPLKs to have scale (42.5% v. 31.4%, p=0.03) and orange color (8.2% v. 0.9%, p=0.01). Among lesions with peppering (n=76; 63 LPLK and 13 non-LPLK, coarse ± fine peppering (73% v. 38.5%, p=0.02) and peppering as the only feature (34.9% v. 0%, p=0.01) were associated with LPLK.
Conclusions:
LPLKs can be challenging to distinguish from benign and malignant skin tumors. The presence of dermoscopic scale and orange color may aid in the recognition of LPLK. Coarse peppering and the presence of peppering as the only dermoscopic feature may further aid the identification of pigmented LPLKs.
Keywords: Lichenoid Keratosis, Lichen Planus-like Keratosis, Benign Lichenoid Keratosis, LPLK, BLK, Dermoscopy
Introduction:
Lichen planus-like keratosis (LPLK), also known as benign lichenoid keratosis (BLK), is a cutaneous lesion hypothesised to derive from an immunologically-mediated involution of a precursor skin lesions such as solar lentigo and seborrheic keratosis.1–7 LPLK can be confused with a spectrum of amelanotic and pigmented skin tumors and often undergoes biopsy to rule out malignancy.8–11 A large histopathologic series from the United States found that approximately 2% of all skin biopsies are LPLKs, with nearly 90% having a clinical impression of keratinocyte carcinoma.12 Two predominant clinical-histopathological types have been described: (1) a “classic” presentation of a pink or red, papule or plaque with histologic features of epidermal acanthosis and a band-like lichenoid lymphocytic infiltrate, and an (2) “atrophic” variant characterised as a hyperpigmented patch with epidermal atrophy, papillary dermal scarring, patchy lymphocytic infiltrates, and melanin incontinence on histology.12 Despite its common occurrence,12 few studies have examined the clinical and dermoscopic characteristics associated with LPLKs, particularly non-pigmented LPLKs.13–19 Prevalent dermoscopic features described include the presence of SK or SL features along with evidence of gray or blue-gray dots (i.e., peppering/granularity).14,15,17,20 Our primary study objective was to identify clinical or dermoscopic features associated with LPLKs that undergo biopsy. A secondary objective was to characterise the prevalence and association of features by pigmentation status.
Methods:
Institutional Review Board approval was obtained by MSK IRB board for retrospective review and study of existing data without written informed consent. We searched institutional pathology reports from January 1, 2000 through December 31, 2016 for the presence of the terms “LPLK”, “Lichenoid Keratosis”, “Lichen Planus like Keratosis”, “Lichen Planus-Like Keratosis”, “Involuting Lichenoid Plaque”, and “BLK” in either the clinical differential or the final histopathological diagnosis, yielding 5,557 unique reports. To consistently assess clinical and dermoscopy features, we restricted our study to lesions (n=536) taken with the identical camera (VEOS DS3, Canfield Scientific, NJ, USA) and for which both clinical, polarised dermoscopy, and non-polarised dermoscopy images were available; this corresponded to August 1, 2015 through December 31, 2016. We excluded lesions in which the clinical or histopathological diagnosis did not definitively include LPLK (n=24), collision tumors (n=15), and lesions with out-of-focus or inadequate quality images (n=24), yielding 473 unique lesions.
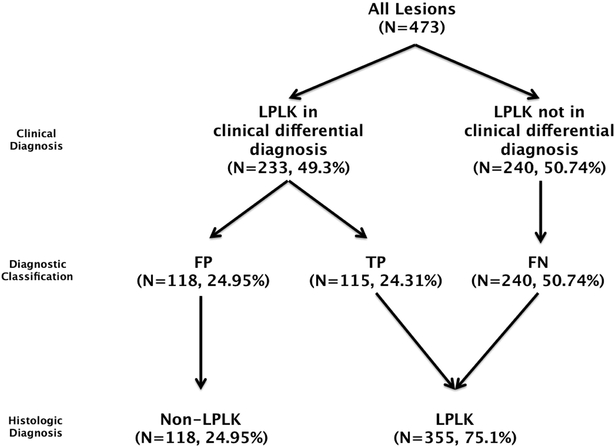
We manually reviewed patients’ electronic medical records and retrieved demographic characteristics at the time of biopsy, as well as the anatomical location of the lesion. We recorded the clinical differential diagnosis, in both the pathology report and physician note, and the final histopathological diagnosis. We classified lesions as: (a) True Positive (TP): LPLK was present in the clinical differential diagnosis and the histopathologic diagnosis was LPLK; (b) False Positive (FP): LPLK was present in the clinical differential diagnosis but the histopathologic diagnosis was not LPLK; and (c) False Negative (FN): LPLK was not present in the clinical differential diagnosis but the histopathologic diagnosis was LPLK. FP lesions served as the comparative control group of lesions. (Figure 1)
Figure 1.
Methodology and data classification.
Image analysis:
We assessed the clinical close-up image, polarised dermoscopy image, and non-polarised dermoscopy image of each lesion. Clinical images were assessed for the presence/absence of chronically sun-damaged background skin (defined as presence of telangiectasias and/or lentigines in the surrounding non-lesional skin) and morphology (raised vs. flat). Dermoscopy analysis was performed by reviewing the polarised and non-polarised dermoscopic images and if a feature was present in either image it was recorded as present. We reviewed images for the presence/absence of features published in the third consensus conference of the International Society of Dermoscopy.21 Additional features analyzed included presence/absence of colors, organisation, sharp borders, and non-specific vessels, defined as subtle, non-classifiable vessels more prominent than background skin vessels of the surrounding normal skin. The rationale to create the term “non-specific vessels” arose from the observation and hypothesis by the authors that non-pigmented, flat LPLKs have subtle, not easily characterised vessels compared to those visualised in superficial pink basal cell carcinomas. Sharp borders were defined as abruptly ending margins of a lesion, irrespective of morphology (i.e., moth-eaten or smooth). When peppering (gray or blue-gray dots) was present, we further classified the size of the dots as fine vs. coarse ± fine and the distribution as generalised (diffusely present) or non-generalised (not diffusely present). Lesion images were randomised and K.L. and C.N-D. performed a blinded, consensus analysis of the clinical and dermoscopic features. M.M. resolved cases of disagreement.
Statistical analysis:
Descriptive and relative frequencies were used to describe the distribution of patient characteristics, and clinical/dermoscopic characteristics of the study lesions. Pearson’s Chi-square and Fisher’s exact test were used to assess differences in distribution of dermoscopic features: (a) between LPLK and non-LPLK lesions, (b) pigmented LPLK and non-pigmented non-LPLK, (c) pigmented LPLK and pigmented non-LPLK lesions, and (d) non-pigmented LPLK and non-pigmented non-LPLK lesions. All analyses were performed with Stata v.14.2, Stata Corporation, College Station, TX.
Results
Over the 16-month period, approximately 55,000 dermatology outpatient visits occurred and ~13,000 skin specimens were submitted to pathology. A total of 473 lesions from 444 patients met criteria for study inclusion. The mean (SD) age of the 444 patients was 65 (12) years and the majority was white (89.4%, n=423) and female (61.2%, n=290) (Supplementary Table 1). Of the 473 lesions, 24.3% (n=115) were true positives, 50.7% (n=240) were false negative, and 24.9% (n=118) were false positives (Figure 1). White race was associated with LPLK diagnosis (95.5% vs. 71.2%, p<0.0001). Age and sex were not associated with LPLK diagnosis.
Differential diagnosis:
Overall, 75.1% (n=355) of lesions were histopathologically LPLKs; of these 32.4% (n=115) had a clinical differential diagnosis that included LPLK at the time of the biopsy. LPLK was included in the differential diagnosis of a total of 233 (49.3%) lesions: 49.4% (n=115) were LPLK and 50.6% (n=118) were not LPLK. Non-LPLK diagnoses included: actinic keratosis (AK) (21.1%, n=25), BCC (18.64%, n=22), SCC (13.6%, n=16), melanoma (8.5%, n=10), seborrheic keratosis (7.6%, n=9), lentigo (5.9%, n=7), nevus (5.1%, n=6), atypical melanocytic proliferation (4.2%, n= 5), verrucoid keratosis (3.4%, n=4) and each of: benign sun-damaged skin, acantholytic acanthoma, clear cell acanthoma, granuloma annulare, verruca plana, spongiotic dermatitis, neurofibroma, ectatic follicle with perifollicular fibrosis and inflammation, scar, ruptured cyst, tinea versicolor, epidermal hyperplasia with parakeratosis, obstruction-related duct ectasia, and perifollicular lymphogranulomatous dermatitis (Supplementary Table 3).
Clinical diagnoses considered for the entire 473 lesion cohort included LPLK (49.3%, n=233), BCC (40.4%, n=191), SCC (20.7%, n=98), nevus (13.3%, n=63), melanoma (9.5%, n=45), SK (5.7%, n=27), lentigo (4.2%, N=20), actinic keratosis (3.4%, n=16) and other (3%, n=14) (Supplementary Table 2). The most common clinical diagnoses included in the differential diagnosis of LPLKs included BCC (42.3%, n=150), LPLK (32.4%, n=115), and SCC (21.4%, n=76). The clinical differential diagnosis of melanoma was more prevalent among non-LPLK lesions compared to LPLKs (18.6% vs. 6.5%, p<0.001); no other differences were found.
Clinical Findings:
LPLKs were most frequently non-pigmented (61.7%, n=219), flat (58.6%, n=208), located on the trunk (52.1%, n=185), and with evidence of chronic sun-damage (69.6%, n=247). No differences between LPLKs and non-LPLKs were identified with regards to size, pigmentation, morphology, or background skin sun-damage (Supplementary Table 1). LPLKs were more frequently located on the trunk compared to non-LPLKs (52.1% vs. 33.9%, p<0.001).
Prevalent Dermoscopic Features of LPLKs
LPLKs had colors pink (87%, n=309) and/or tan (31.6%, n=112) most frequently (Table 1). Scale was present in 42.5% (n=151) and sharp borders were identified in 23.1% (n=82). The majority had vessels (64.5%, n=229); the most frequent vessel morphology was dotted (31.6%, n=112). Thirty-one percent of LPLKs (n=110) had shiny white blotches and strands and 18.6% (n=66) had rosettes. Erosions or ulcers were present in 17.5% (n=62) of cases. Peppering (gray or blue-gray dots) was found in 17.8% (n=63) of LPLKs; when present, its distribution was generalised/diffuse in 55.6% (n=35) and its size was coarse ± fine in 73% (n=46) of lesions.
Table 1.
Association of dermoscopic features and LPLK diagnosis.
| Feature (present) | Total | LPLK | Non-LPLK | P-value |
|---|---|---|---|---|
| N=473(%) | N= 355(%) | N= 118(%) | ||
| Organised | 148 (31.3) | 120 (33.8) | 28 (23.7) | 0.041* |
| Scale | 188 (39.8) | 151 (42.5) | 37 (31.4) | 0.032* |
| Sharp borders | 107 (22.6) | 82 (23.1) | 25 (21.2) | 0.667 |
| Brown color | 166 (35.1) | 112 (31.6) | 54 (45.8) | 0.005* |
| Black color | 1 (0.2) | 1 (0.3) | 0 (0) | 0.564 |
| White color | 47 (9.9) | 30 (8.5) | 17 (14.4) | 0.061 |
| Blue-grey color | 78 (16.5) | 64 (18.0) | 14 (11.9) | 0.118 |
| Pink color | 403 (85.2) | 309 (87) | 94 (79.7) | 0.05* |
| Orange color | 30 (6.3) | 29 (8.2) | 1 (0.9) | 0.005* |
| Atypical pigment network | 8 (1.7) | 4 (1.1) | 4 (3.4) | 0.099 |
| Typical pigment network | 11 (2.3) | 6 (1.7) | 5 (4.2) | 0.112 |
| Angulated lines | 1 (0.2) | 0 (0) | 1 (0.9) | 0.083 |
| Regular dots | 4 (0.9) | 1 (0.3) | 3 (2.5) | 0.02* |
| Irregular dots | 29 (6.1) | 16 (4.5) | 13 (11) | 0.011* |
| Regular globules | 3 (0.6) | 1 (0.3) | 2 (1.7) | 0.094 |
| Irregular globules | 3 (0.6) | 1 (0.3) | 2 (1.7) | 0.094 |
| Tan peripheral structureless areas | 1 (0.2) | 0 (0) | 1 (0.9) | 0.083 |
| Blue-white veil | 0 (0) | 0 (0) | 0 (0) | -- |
| Scar-like depigmentation | 3 (0.6) | 2 (0.6) | 1 (0.9) | 0.736 |
| Peppering / granularity | 76 (16.1) | 63 (17.8) | 13 (11) | 0.085 |
| Diffuse peppering throughout the lesion** | 39 (51.3) | 35 (55.6) | 4 (30.8) | 0.104 |
| Peppering: coarse ± fine granules** | 51 (67.1) | 46 (73) | 5 (38.5) | 0.016* |
| Only peppering present** | 22 (29) | 22 (34.9) | 0 (0) | 0.011* |
| Shiny White blotches & strands | 140 (29.6) | 110 (31) | 30 (25.4) | 0.252 |
| Shiny white streaks | 8 (1.7) | 5 (1.4) | 3 (2.5) | 0.408 |
| Rosettes | 79 (16.7) | 66 (18.6) | 13 (11) | 0.056 |
| Ulceration/Erosion | 79 (16.7) | 62 (17.5) | 17 (14.4) | 0.440 |
| Blue-grey ovoid nests | 1 (0.2) | 1 (0.3) | 0 (0) | 0.564 |
| Blue globules | 4 (0.9) | 4 (1.1) | 0 (0) | 0.247 |
| Moth eaten borders | 28 (5.9) | 15 (4.2) | 13 (11) | 0.007* |
| Cerebriform pattern | 14 (3) | 9 (2.5) | 5 (4.2) | 0.345 |
| Fingerprinting | 13 (2.8) | 8 (2.3) | 5 (4.2) | 0.253 |
| Comedo-like openings | 15 (3.2) | 11 (3.1) | 4 (3.4) | 0.876 |
| Milia-like cysts | 3 (0.6) | 2 (0.6) | 1 (0.9) | 0.736 |
| Keratin plug | 18 (3.8) | 13 (3.7) | 5 (4.2) | 0.777 |
| Moth eaten borders | 28 (5.9) | 15 (4.2) | 13 (11) | 0.007* |
| White circles | 4 (0.9) | 4 (1.1) | 0 (0) | 0.247 |
| Blood spots | 12 (2.5) | 10 (2.8) | 2 (1.7) | 0.502 |
| Milky red globules | 12 (2.5) | 10 (2.8) | 2 (1.7) | 0.502 |
| Any vessels present | 300 (63.4) | 229 (64.5) | 71 (60.2) | 0.397 |
| Polymorphous vessels | 32 (6.8) | 23 (6.5) | 9 (6.5) | 0.67 |
| Arborising vessels | 10 (2.1) | 6 (1.7) | 4 (3.4) | 0.266 |
| Vessels, string of pearls | 2 (0.4) | 0 (0) | 2 (1.7) | 0.014* |
| Vessels, crown vessels | 0 (0) | 0 (0) | 0 (0) | -- |
| Vessels, Multifocal | 30 (6.3) | 23 (6.5) | 7 (5.9) | 0.833 |
| Vessels, Focal | 36 (7.6) | 30 (8.5) | 6 (5.1) | 0.232 |
| Vessels, Diffuse | 125 (26.4) | 98 (27.6) | 27 (22.9) | 0.313 |
| Vessels in the periphery | 21 (4.4) | 18 (5.1) | 3 (2.5) | 0.248 |
| Vessels in the centre | 9 (1.9) | 5 (1.4) | 4 (3.4) | 0.172 |
Statistically significant difference
These features were examined only for the lesions where peppering / granularity was present.
Dermoscopic Features of Pigmented vs. Non-Pigmented LPLKs
Compared to pigmented LPLKs, non-pigmented LPLKs were more likely to have scale (48.9% v. 32.3%, p=0.001) and shiny white blotches and strands (37% v. 21.3%, p=0.005) than pigmented LPLKs. Pigmented LPLKs were more likely to have peppering (gray or blue-gray dots) (46.3% v. 0%, p<0.001) than non-pigmented LPLKs.
Dermoscopic Features Associated with LPLKs
LPLKs were more likely than non-LPLKs to be dermoscopically organised (33.8% v. 23.7%, p=0.04) and to have scale (42.5% v. 31.4%, p=0.03) and orange color (8.2% v. 0.9%, p=0.01). Although the presence of peppering was not statistically significant (17.8% v. 11%, p=0.09), considering only lesions that had peppering (n=76; 63 LPLK and 13 non-LPLK), LPLKs were more likely to have coarse ± fine granules (73% v. 38.5%, p=0.02) and to have peppering as the only feature present (34.9% v. 0%, p=0.01). LPLKs were less likely than non-LPLKs to have moth-eaten borders (4.2% v. 11%, p=0.01) and irregular dots (4.5% v. 11%, p=0.01). Finally, there was a trend toward significance for the presence of rosettes in LPLK (18.6% v. 11%, p=0.056) (Table 1, Figure 2)
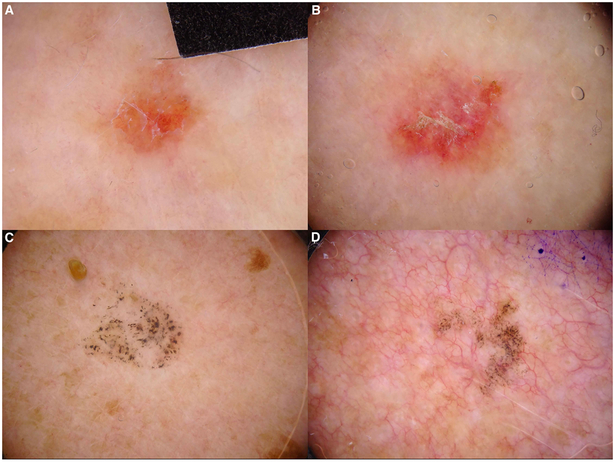
Figure 2. Lichen planus-like keratosis.
Representative dermoscopic images of non-pigmented LPLKs (A-B) that demonstrate scale and orange color and pigmented lichen planus-like keratosis (C-D) that show coarse peppering and peppering as the only dermoscopic feature.
Dermoscopic Features Associated with LPLKs: Pigmented lesions
In a subgroup analysis of pigmented lesions (n=190; 136 LPLK and 54 non-LPLK), LPLKs were more likely than non-LPLKs to have any peppering (46.3% v. 24.1%, p=0.005) and orange color (9.6% v. 0%, p=0.02). Pigmented LPLKs were less likely than pigmented non-LPLKs to have brown color (82.3% v. 98.2%, p=0.03) and moth-eaten borders (7.3% v. 24.1%, p=0.003) (Supplementary Table 4)
Dermoscopic Features Associated with LPLKs: Non-pigmented lesions
In a subgroup analysis of non-pigmented lesions (n=283; 219 LPLKs and 64 non-LPLKs), LPLKs were more likely than non-LPLKs to have scale (48.9% v. 34.4%, p=0.04) and less likely than non-LPLKs to be flat (53.4% v. 68.8%, p=0.02). We found no differences in the prevalence of shiny white blotches and strands (37% v. 39.1%, p=0.71), serpentine vessels (8.7% v. 12.5%, p=0.34), arborising vessels (0.9% v. 3.1%, p=0.12), erosions or ulcers (23.4% v. 23.4%, p=1.0) and non-specific vessels (20.3% v. 18.8%, p=0.79) between non-pigmented LPLK and non-LPLK. (Supplementary Table 4)
Discussion
We examined clinical and dermoscopic features of biopsied LPLKs from a tertiary care hospital in the northeastern United States. Similar to other reports, we found that LPLKs were infrequently included within the clinical differential diagnosis; keratinocyte carcinoma was the most common diagnosis considered by physicians for these lesions, and biopsied LPLKs typically occurred in older white females on sun-exposed areas of the trunk.12,22
To the best of our knowledge, this is the first study to investigate the clinical and dermoscopic features of LPLKs using a comparison group and blinded review. Overall, we found the presence of scale and orange color to be significantly associated with the diagnosis of LPLK, present in 42.5% and 8.2% of LPLKs, respectively. Additionally, we identified a trend towards significance for the presence of rosettes in LPLKs. However, both scale and rosettes can be found in both malignant and benign tumors and these features are unlikely to be of significant utility in clinical practice.23 Orange color may be a valuable clue to the diagnosis of LPLK; however, its low prevalence limits its value. Orange color has also been reported in skin conditions that were not included among our non-LPLK comparator group, such as xanthomas, granulomatous diseases, and pigmented purpuric dermatosis.24–26 Notably, we did not find any significant differences in the prevalence of BCC dermoscopic features between non-pigmented LPLK and non-LPLK; this finding suggests that increased detection pressure for early presentations of non-pigmented superficial basal cell carcinoma is likely to be associated with increased biopsies for LPLK, which is consistent with the authors’ personal experience. The finding that LPLK is frequently misdiagnosed as basal cell carcinoma and the prevalence of BCC-associated dermoscopic features seen in LPLKs from our study supports this hypothesis.22 Finally, it is notable that after step-sectioning consecutive tissue blocks of LPLK, Kulburg et al found that 5% of cases harbored basal cell carcinoma. 27
Although the presence of peppering was not statistically significant for the diagnosis of LPLK, in the subgroup of lesions that had any peppering, the presence of coarse granules was associated with LPLK. Furthermore, all lesions with peppering as the sole dermoscopic feature present were LPLKs, suggesting that this pattern is highly specific to LPLK. However, this finding should be interpreted with caution as melanoma with extensive regression or even a totally regressed primary melanoma can simulate this dermoscopic appearance. Moscarella et al. have reported a case of “lichenoid keratosis-like melanoma” presenting as a patch with peppering as the only dermoscopic feature.8 Lallas et al. suggested that the risk of melanoma is higher for lesions with a high degree of dermoscopic regression features.9 As the presence of scar-like depigmentation was rare among LPLKs in our study, the identification of this component of dermoscopic regression should strongly warrant consideration of melanoma.
Few previous dermoscopic studies have examined the features of non-pigmented LPLKs in detail; in fact, most studies have been restricted to pigmented LPLKs and have included small sample sizes.13–16,18 Bugatti et al and Bassoli et al found regression to be present in 93.8% and 82.1% of their cases, respectively; this contrasts with our finding of regression in 17.8% of biopsied LPLKs.14,16 We identified features suggesting the presence of an seborrheic keratosis or solar lentigo precursor lesion (i.e. moth eaten borders, comedo-like openings, milia-like cysts, fingerprinting and/or cerebriform pattern) in only 11.6% (n=41) of LPLKs whereas other studies have reported a higher incidence (71.4% - 100%).14,15 Finally, the association between coarse peppering and pigmented LPLK is in agreement with previous observations that reported that ‘coarse’ granularity is specific to LPLK and ‘fine’ granularity is more suggestive of melanoma.15
Limitations:
The retrospective, single-centre design of our study limits its validity and generalisability. As identification of false positive lesions relied on clinician documentation in a pathology order, we may not have included lesions that clinically and/or dermoscopically mimicked LPLK. In addition, all the lesions studied were biopsied; thus, our findings are not generalisable to LPLKs that may be clinically recognised. In addition, our comparison group did not include typical examples of keratinocyte carcinoma or benign tumors. As our objective was to identify clues that might prevent unnecessary biopsy of LPLK, we specifically restricted inclusion of non-LPLK lesions to those that mimic the appearance of LPLK. Finally, all feature analyses were performed on images and imaging technique can modify the appearance of clinical and dermoscopic features (i.e., vessel morphology); the use of a single camera type and a standardised imaging protocol at our institution ensured a minimal level of consistency in the image acquisition process.
Conclusion:
LPLKs are challenging to distinguish from benign and malignant skin tumors and are often biopsied to rule out skin cancer (Figure 3). We identified that the presence of dermoscopic scale and orange color may aid in the recognition of LPLK. Coarse peppering and the presence of peppering as the only dermoscopic feature may further aid the identification of pigmented LPLKs. A large, multi-centre prospective study is needed to validate these findings. Finally, non-morphologic clues in the context of the above findings are likely integral to the recognition of LPLK, such as older age, white race, female sex, and location on the sun-exposed areas of the trunk, as described in previous studies and supported by our data.
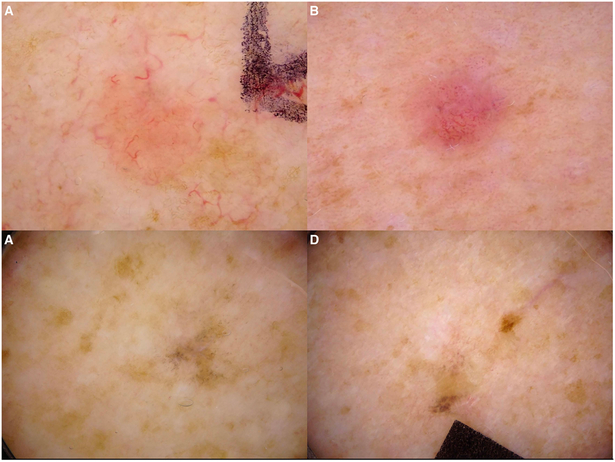
Figure 3. Lichen planus-like keratosis and mimickers.
Non-pigmented LPLK (A) and basal cell carcinoma (B) with a similar dermoscopic appearance. Pigmented LPLK (C) and melanoma (D) with a similar dermoscopic appearance.
Supplementary Material
Learning Points.
The presence of dermoscopic scale and orange color are associated with the diagnosis of LPLK; coarse peppering and the presence of peppering as the only dermoscopic feature are associated with pigmented LPLKs.
Our findings could help avoid unnecessary biopsies of LPLKs that clinically mimic skin cancer.
Acknowledgments
Funding/Support: This study was supported in part through the NIH/NCI Cancer Center Support Grant P30CA008748.
Footnotes
IRB approval status: IRB approval was obtained at Memorial Sloan Kettering Cancer Center for retrospective review and study of existing data without written informed consent.
Conflicts of Interest Disclosure: None declared.
Study concept and design: Drs Liopyris, Navarrete-Dechent and Marchetti
Acquisition, analysis, and interpretation of data: Drs Liopyris, Navarrete-Dechent, Dusza, Marghoob and Marchetti
Drafting of the manuscript: Drs Liopyris, Navarrete-Dechent, Dusza, Marghoob and Marchetti
Critical revision of the manuscript for important intellectual content: Drs Liopyris, Navarrete-Dechent, Dusza, Marghoob, Deng, Wilson and Marchetti
Statistical analysis: Dr. Dusza
Study supervision: Drs. Marchetti and Marghoob
Financial disclosure: None to declare
Funding/Sponsor was involved:
Design and conduct of the study: No
Collection, management, analysis and interpretation of the data: No
Preparation, review, or approval of the manuscript: No
Decision to submit the manuscript for publication: No
References
- 1.Mehregan AH. Lentigo senilis and its evolutions. Journal of Investigative Dermatology. 1975;65(5):429–433. [DOI] [PubMed] [Google Scholar]
- 2.Goldenhersh MA, Barnhill RL, Rosenbaum HM, Stenn KS. Documented evolution of a solar lentigo into a solitary lichen planus-like keratosis. J Cutan Pathol 1986;13(4):308–311. [DOI] [PubMed] [Google Scholar]
- 3.Berger TG, Graham JH, Goette DK. Lichenoid benign keratosis. Journal of American Dermatology. 1984;11(4 Pt 1):635–638. [DOI] [PubMed] [Google Scholar]
- 4.Berman A The Involuting Lichenoid Plaque. Arch Dermatol 1982;118(2):93–96. doi: 10.1001/archderm.1982.01650140025012. [DOI] [PubMed] [Google Scholar]
- 5.Frigy AF, Cooper PH. Benign lichenoid keratosis. Am J Clin Pathol 1985;83(4):439–443. [DOI] [PubMed] [Google Scholar]
- 6.Hafner C, Stoehr R, van Oers JMM, et al. FGFR3and PIK3CAmutations are involved in the molecular pathogenesis of solar lentigo. British Journal of Dermatology. 2009;160(3):546–551. doi: 10.1111/j.1365-2133.2008.08963.x. [DOI] [PubMed] [Google Scholar]
- 7.Groesser L, Herschberger E, Landthaler M, Hafner C. FGFR3, PIK3CA and RAS mutations in benign lichenoid keratosis. British Journal of Dermatology. 2012;166(4):784–788. doi: 10.1111/j.1365-2133.2011.10788.x. [DOI] [PubMed] [Google Scholar]
- 8.MD EM, MD IZ, MD GP, et al. Lichenoid keratosis-like melanomas. Journal of the American Academy of Dermatology. 2011;65(3):e85–e87. doi: 10.1016/j.jaad.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 9.Lallas A, Apalla Z, Moscarella E, et al. Extensive regression in pigmented skin lesions: a dangerous confounding feature. Dermatol Pract Concept. 2012;2(2):1–4. doi: 10.5826/dpc.0202a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RAPTOULIS G, SPENCER R, EINSTEIN B, OLIVIERO M, BRAUN R, Rabinovitz H. Lichen Planus?like Keratosis of the Face: A Simulator of Melanoma In Situ. Dermatol Surg 2007;33(7):854–856. doi: 10.1111/j.1524-4725.2007.33183.x. [DOI] [PubMed] [Google Scholar]
- 11.Buçard AM, MD AMB, MD JM-D-C, et al. Regressive scalp lesions: Dermoscopic and confocal clues. Journal of the American Academy of Dermatology. 2015;72(1):S27–S29. doi: 10.1016/j.jaad.2014.04.061. [DOI] [PubMed] [Google Scholar]
- 12.Morgan MB, Stevens GL, Switlyk S. Benign lichenoid keratosis: a clinical and pathologic reappraisal of 1040 cases. Am J Dermatopathol. 2005;27(5):387–392. [DOI] [PubMed] [Google Scholar]
- 13.Tschandl P, Gambardella A, Boespflug A, et al. Seven Non-melanoma Features to Rule Out Facial Melanoma. Acta Derm Venerol 2017;97(10):1219–1224. doi: 10.2340/00015555-2759. [DOI] [PubMed] [Google Scholar]
- 14.Bassoli S, Rabinovitz HS, Pellacani G, et al. Reflectance confocal microscopy criteria of lichen planus-like keratosis. Journal of the European Academy of Dermatology and Venereology. 2011;26(5):578–590. doi: 10.1111/j.1468-3083.2011.04121.x. [DOI] [PubMed] [Google Scholar]
- 15.Zaballos P, Blazquez S, Puig S, et al. Dermoscopic pattern of intermediate stage in seborrhoeic keratosis regressing to lichenoid keratosis: report of 24 cases. British Journal of Dermatology. 2007;157(2):266–272. doi: 10.1111/j.1365-2133.2007.07963.x. [DOI] [PubMed] [Google Scholar]
- 16.Bugatti L, Filosa G. Dermoscopy of lichen planus-like keratosis: a model of inflammatory regression. Journal of the European Academy of Dermatology and Venereology. 2007;21(10):1392–1397. doi: 10.1111/j.1468-3083.2007.02296.x. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe S, Sawada M, Dekio I, Ishizaki S, Fujibayashi M, Tanaka M. Chronology of lichen planus-like keratosis by dermoscopy: a summary of 17 cases. Dermatol Pract Concept. 2016;6(2):1–7. doi: 10.5826/dpc.0602a06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.BS CRM, med PTC, BS ACM, MD HK. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. Journal of the American Academy of Dermatology. 2011;64(6):1068–1073. doi: 10.1016/j.jaad.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 19.Zaballos P, Martí E, Cuéllar F, Puig S, Malvehy J. Dermoscopy of Lichenoid Regressing Seborrheic Keratosis. Arch Dermatol 2006;142(3):1–1. doi: 10.1001/archderm.142.3.410. [DOI] [PubMed] [Google Scholar]
- 20.Zaballos P, Salsench E, Serrano P, Cuellar F, Puig S, Malvehy J. Studying Regression of Seborrheic Keratosis in Lichenoid Keratosis with Sequential Dermoscopy Imaging. Dermatology. 2010;220(2):103–109. https://www.karger.com/DOI/10.1159/000265556. [DOI] [PubMed] [Google Scholar]
- 21.Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: Results of the third consensus conference of the International Society of Dermoscopy. Journal of the American Academy of Dermatology. 2016;74(6):1093–1106. doi: 10.1016/j.jaad.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maor D, Ondhia C, Yu LL, Chan JJ. Lichenoid keratosis is frequently misdiagnosed as basal cell carcinoma. Clin Exp Dermatol 2017;42(6):663–666. doi: 10.1111/ced.13178. [DOI] [PubMed] [Google Scholar]
- 23.Liebman TN. Rosettes May Be Observed in a Range of Conditions. Arch Dermatol 2011;147(12):1468–1468. doi: 10.1001/archdermatol.2011.312. [DOI] [PubMed] [Google Scholar]
- 24.Palmer A, Bowling J. Dermoscopic appearance of juvenile xanthogranuloma. Dermatology. 2007;215(3):256–259. doi: 10.1159/000106586. [DOI] [PubMed] [Google Scholar]
- 25.Geller S, Pulitzer M, Myskowski PL. Solitary orange papule on the back of a middle-aged man. Dermatol Pract Concept. 2018;8(1):51–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banuls J, Arribas P, Berbegal L, DeLeon FJ, Frances L, Zaballos P. Yellow and orange in cutaneous lesions: clinical and dermoscopic data. Journal of the European Academy of Dermatology and Venereology. 2015;29(12):2317–2325. doi: 10.1111/jdv.13249. [DOI] [PubMed] [Google Scholar]
- 27.Kulburg A, Weyers W. Regressing basal-cell carcinoma masquerading as benign lichenoid keratosis. Dermatol Pract Concept. 2016;6(4):1–6. doi: 10.5826/dpc.0604a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.