Abstract
Psychosis—a cardinal symptom of schizophrenia—has been associated with a failure to appropriately create or use stored regularities about past states of the world to guide the interpretation of incoming information, which leads to abnormal perceptions and beliefs. The visual system provides a test bed for investigating the role of prior experience and prediction, as accumulated knowledge of the world informs our current perception. More specifically, the strength of visual aftereffects, illusory percepts that arise after prolonged viewing of a visual stimulus, can serve as a valuable measure of the influence of prior experience on current visual processing. In this paper, we review findings from a largely older body of work on visual aftereffects in schizophrenia, attempt to reconcile discrepant findings, highlight the role of antipsychotic medication, consider mechanistic interpretations for behavioral effects, and propose directions for future research.
Keywords: schizophrenia, vision, aftereffect, adaptation, predictive coding, excitation/inhibition balance, plasticity
1. Background
Understanding the computational and neural mechanisms that function abnormally in psychosis—a complex constellation of symptoms that reflect a separation from reality—has long been a challenge. This understanding is essential, however, to developing effective and targeted pharmacological and psychological treatments for schizophrenia—the illness in which psychotic symptoms are most enduring, severe, and disabling. Implicit or explicit in many mechanistic accounts of schizophrenia is that psychotic symptoms arise due to inappropriate creation or use of stored regularities to guide the interpretation of incoming information (Gray et al., 1991; Hemsley, 2005; Sterzer et al., 2018). The idea here is that perception is sculpted by a combination of current sensory input, context, and past experience, and that schizophrenia is associated with an abnormality in this integrative, modulatory, and inferential process, which leads to abnormal perceptions and beliefs.
The visual system provides a test bed for investigating the role of prior experience and prediction: accumulated knowledge of the world informs our current perception (Barlow, 1990; Clifford et al., 2000; Knil and Richards, 1996; Rao and Ballard, 1999). Importantly, robust behavioral paradigms have been developed to precisely quantify predictions in the visual system, and parallel work in non-human primates provides a basis for interpretation at the level of single neurons. Accordingly, the visual system holds a unique advantage as a model system for understanding the role of experience in how individuals perceive and interpret the world and holds a clear translational value in understanding abnormalities in psychosis.
One form of altered neural coding and perception due to prior sensory input, in which continuous exposure to a stimulus leads to saturation in the responses of neurons coding the available stimulus features, is called adaptation. In the visual domain, there are well-established methods of quantifying the magnitude of sensory adaptation at the perceptual level and clear indications as to how these perceptual phenomena map onto neural sensitivity changes.
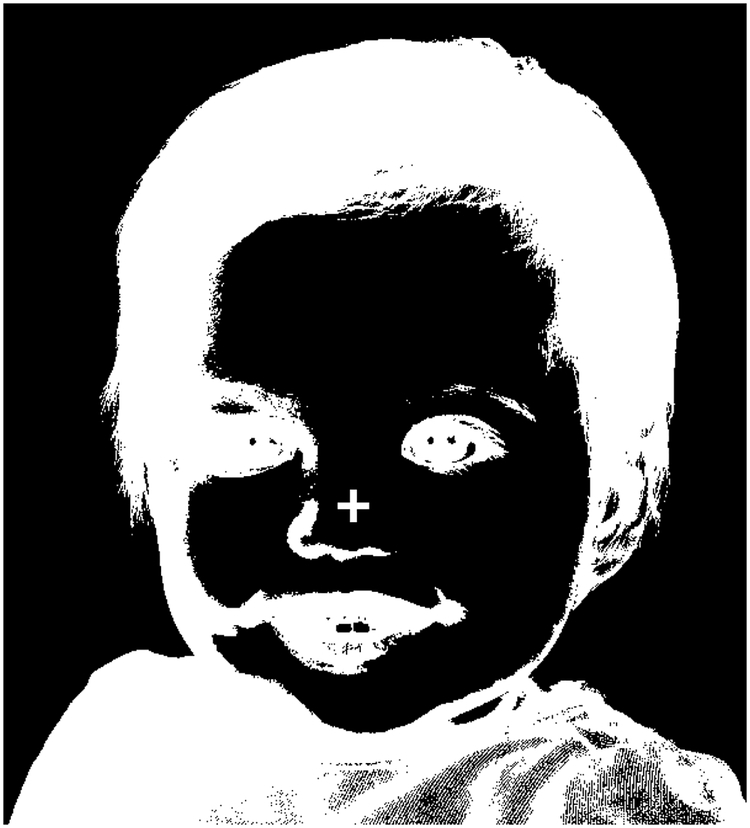
Namely, the magnitude of adaptation can be quantified by measuring the strength of what are known as visual aftereffects: illusory perceptions that follow continuous presentation of a real visual stimulus. Aftereffects are thought to be due to extended firing, during the initial stimulus presentation, of neurons selective to the features of that initial stimulus, leading to inhibition/saturation of the response of those neurons and associated disinhibition of neurons that are selective to complementary features, as part of the brain’s effort to maintain homeostasis with regards to neuronal firing rates (Bednar, 2012). The perceptual result, the aftereffect, is illusory perception of ‘the opposite’ of the initial stimulus (e.g., the complementary color, opposite direction of motion, etc.). For example, stare at the cross at the center of Figure 1 for about one minute, then close your eyes. The image of a face you likely perceive while your eyes are closed is an example of a negative afterimage and arises due to adaptation to luminance. Analogous aftereffects are found for other features, from basic ones such as color and orientation to more derived ones such as facial expression and gender. Importantly, primate neurophysiology work has provided insights into the specific neuronal populations that are adapting for various types of aftereffects to occur—indeed, the notion that different aftereffects relate to adaptation in different and specific neuronal populations has led to the adaptation paradigms’ moniker of ‘the psychophysicists’ microelectrode’ (Frisby, 1979).
Figure 1.
Negative afterimage. Stare at the crosshair for 30–60 seconds, then close your eyes. The inverse of this image should be visible and is an example of a visual aftereffect.
Characterizing abnormalities in the predictions that derive from different types of prior sensory experiences and in their influence on current visual perception, then, has the potential to provide a nuanced and biologically-informed perspective on prediction abnormalities in psychosis generally, but also stands to inform etiological conceptualizations of the subjective alterations in visual perception reported by individuals with schizophrenia more specifically (e.g. Freedman and Chapman, 1973; Silverstein et al., 2017). Experimental evidence for such perceptual alterations comes from a range of tasks (reviewed in Butler et al., 2008; Chen, 2011; Silverstein, 2016; Skottun and Skoyles, 2007; Yoon et al., 2013), and a role of abnormal prediction in these alterations has previously been proposed (Silverstein, 2016). Thus, studying visual aftereffects, along with providing insights into global disease mechanisms, may also shed light on the origins of specific aspects of clinical phenomenology.
Despite this potential for visual aftereffects to inform current mechanistic theories of schizophrenia, the bulk of the studies of visual aftereffects in schizophrenia were conducted prior to the 1970’s. Our aim here is to revive and critically review this literature, reconcile discrepant findings, highlight the role of antipsychotic medication, consider mechanistic interpretations for behavioral effects, and propose directions for future research.
2. Experimental paradigms
Before reviewing findings, we begin by describing the types of visual aftereffects that have been investigated in schizophrenia, which are depicted in Figure 2, and the outcome measures that have been reported. After that, we summarize how aftereffects are quantified experimentally, as well as which factors are known to influence these aftereffects in healthy individuals. We will also briefly highlight what is known about the neuronal bases of these aftereffects. Common to all aftereffect paradigms is an adapter stimulus that the subject views for some period of time, typically followed by a test stimulus on which the subject is instructed to report. The influence of the adapter stimulus on the perception of the test stimulus is the general measure of interest.
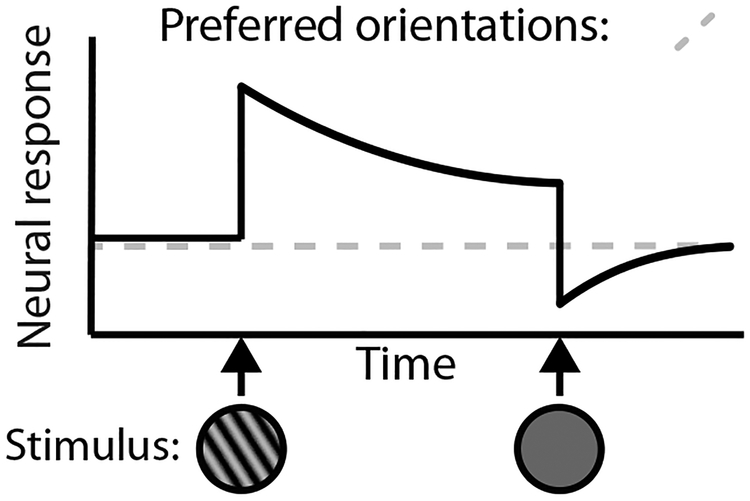
Figure 2.
Schematic depiction of how single neurons implement visual aftereffects, in this case, the tilt aftereffect. The response of a neuron that responds preferentially to leftward orientations is depicted in the solid line, and the response to a neuron that responds preferentially to rightward orientations is depicted in the dotted line. Stimulus presentation is depicted on the x-axis. Upon presentation of a leftward orientation grating, the neuron with a preferred leftward direction begins to respond vigorously, but soon begins to adapt with repeated presentation of this stimulus (i.e. neural firing is attenuated). Upon the offset of this leftward grating and onset of a blank screen, the neuron returns to a below-baseline level of firing. The neuron with a rightward orientation preference remains at baseline, thus resulting in an increase in firing rate of the neuron with a rightward orientation preference relative to the neuron with a leftward orientation preference, which gives rise to the perceptual phenomenon of a rightward bias in the perception of stimulus orientation (i.e. aftereffect). Adaptation is illustrated using orientation as an example here, but it transpires in an analogous fashion with other stimulus properties.
2.1. Negative Afterimages
(Craik, 1940; Kelly and Martinezuriegas, 1993; Virsu and Laurinen, 1977): Upon adapting to a visual stimulus with a given color, subjects will report an illusory image that has the same spatial configuration, i.e. outlines, as the original stimulus but in which each location has a color that is complementary to that shown at the corresponding location in the original stimulus (e.g. green becomes red). Grayscale stimuli can also induce negative afterimages (cf. Figure 1), and in that case each location in the illusory image has a brightness that is the complement of the corresponding location in the original stimulus (i.e. bright becomes dim and vice versa).
2.2. Tilt Aftereffect
(Gibson and Radner, 1937; Greenlee and Magnussen, 1987; Wolfe and O’Connell, 1986): Upon adapting to a grating (or other pattern) that is oriented at a particular angle, subjects are presented with a similar grating that has a slightly different orientation. The original adaptation period results in a perceptual bias so that the angle of the subsequent grating is perceived as farther removed from that of the original grating than it actually is.
2.3. Movement Aftereffect
(Anstis et al., 1998; Mather et al., 2008; Wohlgemuth, 1911): Upon adapting to movement in a particular direction, subjects are asked to report on the movement direction of a test stimulus. The aftereffect consists of the illusory perception that this test stimulus moves in a direction opposite to that of the adapting stimulus. Studies in schizophrenia have, by and large, measured what is known as the Archimedes spiral motion aftereffect. In this paradigm, the adapting stimulus is a spiral rotating in a particular direction. Once the spiral stops rotating, the now-stationary stimulus appears to rotate in the direction opposite to the adapting stimulus.
2.4. Figural Aftereffect:
This term is used in a number of ways in the psychological literature. In a broader sense, the term covers several different aftereffects, including the tilt aftereffect (Day et al., 1959; Wohlgemuth, 1911). In the context of research related to schizophrenia, a series of studies have used this term for an aftereffect that is evident when a set of two vertical lines, one placed straight above the other, is viewed following exposure to an adapting stimulus (Kohler and Wallach, 1944; Prysiazniuk and Kelm, 1965). The adapting stimulus consists of a similar set of two vertical lines but, in addition to being placed above each other, the two are shifted horizontally: one leftward and the other rightward. As a result, the lines in the test stimulus are perceived as shifted horizontally in the opposite directions (i.e. rightward and leftward, respectively).
2.5. McCollough Effect
(McCollough, 1965): The McCollough effect is an aftereffect that is substantially different from the ones discussed so far. It centers not on adaptation to a particular visual feature, but on adaptation to a conjunction of two features. Typically, the subject adapts to a red-colored grating that has one orientation (e.g. horizontal) as well as a green-colored grating that has an orientation orthogonal to the first grating (e.g. vertical). Then, the subject is presented with a test image that is one of the gratings (or, in the classic demonstration, a patchwork of both) but that has no color. The aftereffect consists of the illusory perception that (any part of) a test stimulus that matches one of the adapters in orientation, has a color that is complementary to the color of that adapter.
3. Experimental measures of aftereffects, and factors that influence them
Work on healthy individuals has identified several methods for quantifying the strength of aftereffects. Some studies simply ask subjects to report whether an aftereffect is present or how strong it is, typically by comparing it to some physical reference stimulus that is not, itself, affected by the aftereffect (Georgeson and Turner, 1985; Greenlee and Magnussen, 1987). Another straightforward method involves measuring the duration of the aftereffect rather than its subjective magnitude, by asking subjects to indicate when the aftereffect ends (Leguire and Blake, 1982; Suzuki and Grabowecky, 2003; van de Grind et al., 2001; Verstraten et al., 1998) and sometimes also when it starts (i.e. latency; Suzuki and Grabowecky, 2003). A final, less direct, method involves physically adjusting the test stimulus itself to counteract the aftereffect that affects perception of the test stimulus, in order to quantify the amount of physical change required to exactly cancel the aftereffect. For instance, to measure the strength of an upward movement aftereffect such a nulling, or cancelling, procedure would involve presenting a test stimulus that contains some downward motion rather than being fully stationary, and determining how much downward motion is required for the test stimulus to appear stationary (Blake and Hiris, 1993; Castet et al., 2002). For other aftereffects analogous procedures have been used (Georgeson and Turner, 1985; Kelly and Martinezuriegas, 1993; Thakkar et al., 2018; Wagner, 1971). Each of these methods of measuring aftereffects comes with advantages and disadvantages that matter in specific cases (Brascamp et al., 2010; Castet et al., 2002; Pantle, 1998), but the methods qualitatively agree in the majority of cases.
Across essentially all aftereffects, one factor that influences aftereffect strength is timing: aftereffects get stronger with more prolonged adaptation, and their strength decays over time after the adapter is removed (Greenlee and Magnussen, 1987; Harris and Calvert, 1989; van de Grind et al., 2004; Wolfe and O’Connell, 1986). In addition, different kinds of aftereffects are affected by visual stimulation parameters such as contrast or retinal location. We will briefly address such influences below because they play a role in our later discussions related to schizophrenia. Specifically, for any influence of disease status on aftereffect strength we need to consider whether disease-related alterations in the way a stimulus is represented in early visual processing (e.g., due to impairments in gain control, contrast sensitivity, ability to fixate ones’ gaze, perceptual organization, coherent motion detection) can mediate the strength of aftereffects. One prominent factor that influences effectively all aftereffects is the spatial correspondence between the adapter stimulus and the test stimulus. Some aftereffects have a great deal of such spatial specificity: for negative afterimages, for example, the afterimage appears at the exact retinal location where the adapter stimulus was processed before. For other aftereffects the degree of spatial specificity is reduced, in that aftereffects are still experienced for test stimuli with a location that is somewhat displaced from the adapter location (Afraz and Cavanagh, 2009; Knapen et al., 2009; Wenderoth and Wiese, 2008). Nevertheless, even in those cases aftereffect magnitude is largest at the exact location of the test stimulus.
In addition to these general effects of time and spatial location, which affect aftereffects of all kinds, there are stimulus factors that play a role for specific kinds of aftereffects. As with the factor of spatial correspondence, these factors typically relate to the degree of neural overlap between the adapter stimulus and the test stimulus. For instance, when the movement aftereffect is viewed on a stationary test stimulus, it is strongest after adapting to a slowly moving stimulus; yet for a test stimulus that jitters rapidly (like TV static), faster adapters yield the strongest motion aftereffects (van Boxtel et al., 2006; Verstraten et al., 1998). To give another example: the tilt aftereffect is stronger when the physical orientation of the test stimulus is only slightly different from that of the adapting stimulus (by about 10–20 degrees) than when the orientation difference is larger (Clifford, 2002; Schwartz et al., 2007). In both these examples, and also with regard to location correspondence between adapter and test stimulus, the most plausible explanation is that aftereffects have their origin in neurons that are tuned, i.e. that respond to only a limited range of stimulus features. As a result, the degree to which perception of a given test stimulus draws on neurons that have actually been affected during the adaptation period depends on the degree of correspondence between adapter stimulus and test stimulus in the relevant dimensions. The role of neural tuning in aftereffects will be discussed in more detail in the next section.
A factor of influence that is not captured by the above descriptions in terms of neural correspondence, is stimulus contrast—a measure of the difference in brightness between a stimulus’ darkest and brightest regions. Several studies in healthy populations have investigated the degree to which adapter contrast impacts aftereffect strength. Unsurprisingly, negative afterimages (which reflect adaptation to darker and brighter regions in an image) become stronger when adapter contrast is increased (Gilroy and Blake, 2005; Kelly and Martinezuriegas, 1993). For both movement aftereffects and tilt aftereffects, strength depends on the contrast of both the adapter and the test stimulus. In particular, aftereffect strength increases with increasing adapter contrast but decreases with increasing contrast of the test stimulus (Keck et al., 1976; Nishida et al., 1997; Parker, 1972). These effects, however, may interact with other stimulus parameters, as one study reported the relation between test stimulus contrast and the strength of the tilt aftereffect to reverse as a function of test stimulus duration (Harris and Calvert, 1989).
4. The neural basis of aftereffects
In general terms, aftereffects are thought to arise from a net reduction in responsivity, gradually arising during the adaptation period, of neurons that respond to the adapting stimulus (Figure 2). This reduced responsivity carries over to the neurons’ response to a subsequent test stimulus, resulting in the perceptual effect. This type of change in neural responsivity over time is commonly observed at the single-neuron level (Albrecht et al., 1984; Barlow and Hill, 1963; Kohn and Movshon, 2003), and there is no doubt that it happens during aftereffect paradigms. The perceptual phenomena that define aftereffects, however, are likely the result of a more complex interplay between many neurons that differ in the degree to which their responsivity is affected during the adaptation period (Grunewald and Lankheet, 1996; Mather, 1980; Sutherland, 1961; Tolhurst and Thompson, 1975). For instance, the perception of a contour’s orientation is plausibly rooted in the collective pattern of neural responses among neurons that are tuned to orientation, i.e. that each responds optimally to a given, preferred, orientation and less to different orientations. Individual neurons differ in their preferred orientation, and neurons with different tuning preferences interact through both excitatory and inhibitory connections (Li, 1998). The tilt aftereffect, then, would correspond to an overall imbalance, due to differential sensitivity to the earlier adapter, in response to the test stimulus across this population of orientation-tuned neurons.
One relevant implication that emerges from this broad summary of the neural basis of aftereffects is that different aftereffects arise from different neural populations, depending on the populations’ selectivity to visual input. For instance, negative afterimages emerge, to a substantial degree, due to adaptation at the level of retinal and subcortical neurons (Craik, 1940; Li et al., 2017; Zaidi, 2012), whereas tilt aftereffects are likely related to changes among the orientation-tuned neurons of the primary visual cortex (Clifford, 2002) and motion aftereffects plausibly depend on the responses of movement-tuned neurons in higher visual cortical regions (Huk et al., 2001; Mather, 1980).
5. Critical review of findings in schizophrenia
In the following section, we review findings in schizophrenia. Because existing findings do not paint a coherent picture when comparing across different studies, we also explore clinical and experimental factors that may account for variability across studies. Table 1 summarizes the mixed existing evidence for altered visual aftereffects in schizophrenia, with some studies finding evidence for a greater influence in patients of the adapter stimulus on perception of the test stimulus, some finding less influence, and some finding no difference. Note that null results should be interpreted with caution as sample sizes are small and studies are likely underpowered.
Table 1.
| Study | Aftereffect | Measure | Groups and sample sizes | Medication status | Group effect | Relationship with other measures |
|---|---|---|---|---|---|---|
| Abraham and McCallum (1973) | Spiral aftereffect | Duration | 1) Psychotic inpatients with first- rank (n unknown) 2) Psychotic inpatients without first-rank symptoms (n unknown) 3) Healthy controls (n unknown) |
Not reported | SZP > HC | SAE duration decreased with improvement in clinical status following treatment |
| Barrett and Logue (1974) | Spiral aftereffect | Occurrence | 1) Inpatients with schizophrenia (n=35) 2) Inpatients with organic brain disease (n=35) |
Not reported | SZP > brain injury* | |
| Calver et al. (1991) | Tilt aftereffect | Strength | 1) SZP (n=8; tested before and after medication injection) 2) HC (n=8) |
Medicated | SZP > HC* | Greater tilt aftereffect in SZP before injection than after injection |
| Claridge (1960) | Spiral aftereffect | Duration | 1) Hysteria inpatients (n=16) 2) Dysthymia inpatients (n=16) 3) Schizophrenia inpatients (n=16) 4) Healthy controls (n=16) |
Not reported | SZP > HC Hysteria=HC < Dysthymia |
|
| Day et al (1967) | Tilt aftereffect Figural aftereffect | Strength | 1) SZP inpatients (n=20) 2) non-SZP inpatients (n=20) 3) Healthy controls (n=20) |
Mostly medicated | No group difference | |
| Hersen et al (1972) | Spiral aftereffect | Occurrence Latency Duration | 1) SZP inpatients (n=20) 2) Organic brain disease (n=20) 3) Healthy controls (n=20) |
Not reported | Occurrence: HC > SZP > Brain disease Latency: HC < SZP and brain disease Duration: no difference | |
| Herrington and Claridge (1965) | Spiral aftereffect | Duration | 1) First-episode psychosis inpatients (n=30) 2) Healthy controls (n=18) |
Not reported | No difference | No effect of antipsychotic treatment |
| Kelm (1962) | Figural aftereffect | Strength | Experiment 1 1) SZP inpatients (n=5) 2) HC (n=5) Experiment 2 1) SZP inpatients (n=10) 2) Alcoholic inpatients (n=10) |
Not reported | SZP < HC* | |
| Kelm (1968) | Figural aftereffect | Strength | 1) SZP inpatients (n=12) 2) non-SZP inpatients (n=8) |
Unmedicated for 48 hours | SZP < non-SZP | |
| Krishnamoorti and Shagass (1963) | Spiral aftereffect | Duration | 1) Psychotic inpatients (n=26) 2) Healthy controls (n=20) |
Unmedicated for 7 days | No difference | |
| Rokem et al (2011) | Tilt aftereffect | Strength | 1) Schizophrenia outpatients (n=16) 2) HC (n=20) |
Mostly medicated | No difference | |
| Schein (1960) | Spiral aftereffect | Duration | 1) Psychotic inpatients (n=12) 2) HC (n=23) |
Not reported | No difference | No difference between subjects taking different classes of medications |
| Surguladze et al (2012) | McCollough effect | Occurrence Latency | 1) SZP (n=50, inpatients and outpatients) 2) First-degree relatives (n=61) 2) 50 HC |
All medicated | Frequency: SZP < hC and relatives Latency: SZP > REL > HC | No relationship to medication dose Longer latency correlated with greater positive symptoms in SZP and schizotypy scores in relatives |
| Thakkar et al (2018) | Negative afterimage Tilt aftereffect | Strength | 131 HC | Unmedicated | Negative afterimage: no relationship with schizotypy Tilt aftereffect: negatively related to schizotypy scores |
|
| Tress and Kugler (1979) | Movement aftereffect | Duration | 1) SZP inpatients (n=11) 2) HC (n=15) |
Not reported | No difference | |
| Wertheimer (1954) | Figural aftereffect | Strength | 1) SZP inpatients (n=15) 2) HC (n=15) |
Not reported | SZP < HC* | |
| Wertheimer (1957) | Figural aftereffect | Strength | Experiment 1: 1) SZP inpatients (n=15) 2) HC (n=15) Experiment 2: 1) SZP inpatients (n=17) 2) HC (n=17) |
Not reported | SZP < HC |
One clear source of variability across studies is the type of aftereffect measured. This factor is relevant as different aftereffects involve adaptation occurring in different neuronal populations. All of the studies finding greater aftereffect strengths and durations in schizophrenia measured the movement and tilt aftereffect. On the other hand, studies of the figural aftereffect found, by and large, reduced aftereffect strength in schizophrenia patients. In addition, the one study of the McCollough effect also reported reduced afterimage strength in patients. It is worth noting here that the validity of what has been referred to as the figural aftereffect in clinical studies is debatable. More specifically, the direction of effects in many individuals is in the opposite direction to what you would expect given neural adaptation, thus bringing into question its comparability with other measures of visual aftereffects (Day et al., 1967).
Another source of variability across studies involves the experimental parameters—namely, duration and visual properties of the test and adapter stimuli. Few studies have manipulated these parameters within a single experiment; however, differences in parameter values may explain discrepancies across studies. Of those studies comparing the duration of movement aftereffects between schizophrenia and healthy controls, two studies reported longer aftereffect durations in schizophrenia (Abraham and McCallum, 1973; Claridge, 1960), five studies reported null findings (Herrington and Claridge, 1965; Hersen et al., 1972; Krishnamoorti and Shagass, 1963; Schein, 1960; Tress and Kugler, 1979), and no studies observed longer durations in controls. With the aforementioned caveat regarding null findings in mind, it is nevertheless interesting that those studies reporting greater duration of the movement aftereffect in patients used relatively long adapter durations (1 minute), whereas those that reported null results used shorter adapter durations (15–30 seconds). In this context, it is worth noting that aftereffect strength depends not only on stimulus timing (see above), but also that the physiological events that underlie aftereffects may differ depending on the timescale of adaptation (Kanai and Verstraten, 2005; Wolfe and O’Connell, 1986). Indeed, there is some evidence that results of aftereffect studies involving schizophrenia patients may differ, even qualitatively, depending on stimulus timing as well as other parameters. Specifically, in their investigation of the tilt aftereffect in individuals with schizophrenia (before and after antipsychotic injection), Calvert et al (1991) manipulated the spatial frequency of the adapter stimulus as well as the duration of the test stimulus. For higher contrast adapter stimuli, patients before injection had larger tilt aftereffects than controls at short test durations; this difference was not observed at longer test durations and was, in fact, reversed in sign. That is, for lower contrast stimuli, patients prior to injection had a larger tilt aftereffect only at long test durations. However, these findings should be interpreted with caution given the very small sample sizes (see Table 1). Using the figural aftereffect, Kelm (1962, 1968) also manipulated adapter durations and the duration between adapter and test stimuli. Predictably, aftereffects diminished in strength with increasing duration between adapter and test stimuli; for patients, the direction of the aftereffect actually reversed at long adapter-test intervals. Group differences in aftereffect strength in these studies are difficult to interpret, however, as they only reported differences in the absolute magnitude of aftereffects. In summary, there is some tantalizing, albeit incomplete, evidence that hints at the importance of characterizing the temporal dynamics of visual aftereffects in schizophrenia, and how they vary under different perceptual conditions, for understanding the location of functional abnormalities within the visual processing hierarchy.
A further source of experimental variability across studies is the operational measure of aftereffects used, with different studies reporting occurrence, strength, duration, and/or latency. It is difficult to disentangle the extent to which variability in conclusions across studies is due to differences in outcome measure used as the outcome measure is often confounded with aftereffect type. For motion aftereffects, only the occurrence, latency, and duration of effects have been reported; for the figural aftereffect, only strength has been reported. This lack of overlap between the measures used to quantify the two types of aftereffects should be kept in mind when considering the generally opposite directions of effect that have been reported when comparing patients and controls in terms of these aftereffects. For instance, it is certainly possible that the time course of aftereffects differs in schizophrenia patients, such that duration and strength are differentially affected, but no studies have systematically addressed this. The use of latency and duration measures is also problematic, as it is confounded with general reaction time, which is known to be slowed in schizophrenia across most response domains (reviewed in Nuechterlein, 1977).
Another factor that certainly merits consideration in explaining altered aftereffects in schizophrenia is antipsychotic medication. These drugs achieve therapeutic effects by blocking dopamine (Seeman and Lee, 1975; Seeman et al., 1976), but also act on other neurotransmitter systems. Adaptation at the cellular level is plausibly modulated by these systems (McCormick and Williamson, 1989). Indeed, healthy observers administered antipsychotic medications experience a reduction in the aftereffect strength related to tilt, motion, and color (Harris et al., 1986; Harris et al., 1983; but see Janke and Debus, 1972; Lehmann and Csank, 1957). These findings cannot be accounted for by drug effects on retinal blurring, perceived luminance of the adapter, drowsiness, blinking, or loss of fixation (Harris et al., 1986; Harris et al., 1983). Studies comparing the effect of antipsychotics with other psychotropic medications suggest that drug effects on aftereffects are primarily due to dopaminergic action. The influence of medication in schizophrenia patients is less clear from the literature. As evidenced in Table 1, many studies do not report medication status of patient samples, and there is no clear pattern of findings across those studies that do report medication status. A handful of studies have explicitly reported the effects of medication. Consistent with studies in healthy individuals administered antipsychotic medications, the strength of the tilt aftereffect was greater in patients immediately prior to a neuroleptic injection (when levels of the medication were presumably low) compared to immediately after (Calvert et al., 1991). Additionally, duration of the movement aftereffect decreased following a treatment-related decrease in first-rank symptoms in an inpatient population (Abraham and McCallum, 1973). Although the nature of the treatment is not described, there was most likely a pharmacological component. On the other hand, Lloyd and Newbrough (1964) observed no change in duration of the movement aftereffect before and after antipsychotic administration in schizophrenia patients. Notably, these patients only underwent a 10-day wash-out period, and previous medication use may have had a lingering effect on aftereffects. However, even in a never-medicated group of first-episode patients that improved following antipsychotic treatment, cessation of drugs 8–15 weeks later did not influence the duration of the movement aftereffect (Herrington and Claridge, 1965). Additionally, studies that have reported correlations between aftereffect measures and normalized medication dose have found no evidence for a relationship (Rokem et al., 2011; Surguladze et al., 2012). In summary, there is clear and strong evidence from healthy observers that antipsychotic drugs reduce aftereffect strength and duration. Although some patient studies also support such a relationship between antipsychotic use and reduced aftereffects, other studies report no such relationship. Findings from patient studies are trickier to interpret however given the possibility of receptor changes due to long-term medication use and the likely confounding effect of improvement in clinical status with medication administration. Importantly, because of the direction of the effect of antipsychotic drugs in healthy individuals, findings of increased (rather than decreased) aftereffect strength and duration in schizophrenia patients cannot plausibly be accounted for by neuroleptics.
Another potential factor leading to variability across studies is illness stage and clinical status. Unfortunately, many studies do not report illness stage or clinical status of participants. In his review of movement aftereffect duration in schizophrenia, Harris (1994) made inferences about the clinical status of participants based on age and argued that duration of the movement aftereffect was greatest in acutely ill, untreated schizophrenia patients. These findings are akin to data on contrast sensitivity, with observations of increased contrast sensitivity in unmedicated, first-episode patients (Kelemen et al., 2013; Kiss et al., 2010; Shoshina et al., 2015) and decreased contrast sensitivity in chronic patients, regardless of medication status (Butler et al., 2005; O’Donnell et al., 2006; Skottun and Skoyles, 2007; Slaghuis, 1998). Indeed, Abraham and McCallum (1973) found that duration of the movement aftereffect decreased with improvement in clinical status following treatment. On the other hand, Surguladze (2012) found that more severe positive symptoms were associated with increased latency to report the McCollough effect. It is certainly possible that the influence of clinical status on visual aftereffects is domain- (i.e. motion versus color) and/or measure-specific (i.e. duration versus latency). Alternatively, longer illusion latency and longer illusion duration may both be caused by response slowing, possibly secondary to distraction, which has been found to be more related to positive than negative symptomatology (Green and Walker, 1986).
6. Mechanisms for altered visual aftereffects in schizophrenia
In the following section, we consider possible CNS and non-CNS explanations for altered visual aftereffects in schizophrenia and, following these explanations, we interpret these data in the context of recent comprehensive theoretical accounts of the disorder. Arguably, the most compelling explanation for altered visual aftereffects is that patients with schizophrenia differ from healthy controls in the adaptability of neuronal populations. The plausible difference, discussed above, in exact cortical origin of various aftereffects therefore offers the potential to distinguish visual processing levels in which adaptation is, and is not, affected.
Differences in visual aftereffects may also be secondary to differences in perceived contrast. As noted above, contrast sensitivity is decreased in chronic schizophrenia patients (Butler et al., 2005; O’Donnell et al., 2006; Skottun and Skoyles, 2007; Slaghuis, 1998) and increased in those early in the course of illness (Kelemen et al., 2013; Kiss et al., 2010; Shoshina et al., 2015). Given that aftereffect strength typically increases with increased contrast (see earlier section), it is certainly possible that the observed increases in aftereffect strength and duration in younger, more acutely ill samples may be explained, at least in part, by increases in perceived contrast of the adapter stimulus.
Increased visual processing duration or activity may also contribute to findings of increased aftereffect strength and duration in schizophrenia. For example, visible persistence, which refers to the duration for which a stimulus is visible following termination, has been found to be longer in patients with schizophrenia—as much as twice as long—compared to healthy controls (Schwartz et al., 1994). Longer visible persistence effectively lengthens the adapter stimulus duration, which would be expected to increase aftereffect strength. Whether the relatively short absolute duration by which visible persistence is lengthened in schizophrenia can account for findings of longer and stronger motion and tilt aftereffects is unknown.
Non-CNS explanations for altered visual aftereffects must also be considered. One such factor is eye movements. As noted above, aftereffects all display some degree of spatial selectivity, so breaks in fixation would be expected to yield weaker aftereffects. Indeed, there is a robust literature citing impairments in fixation and gaze control in schizophrenia (e.g. Barton et al., 2007; Hutton and Ettinger, 2006; Thakkar et al., 2011; Thakkar et al., 2015) and fixation losses may explain those studies reporting weaker aftereffects in patients. Only one study to date (Rokem et al., 2011) used an eye tracker to monitor eye movements and exclude trials with large losses of fixation; in this study, there was no difference between patients and controls in the strength of the tilt aftereffect. Two older studies visually inspected eye position during induction of the figural aftereffect. Wertheimer (1954) did not observe any appreciable deviations from fixation, and Kelm (1968) excluded those subjects with large deviations from fixation. In both of these studies, a smaller figural aftereffect was still observed. Although that suggests that smaller figural aftereffects cannot be accounted for by losses of fixation, they do not rule out the possibility of smaller fixational eye movements, that are not easily detected by visual inspection, contributing to reduced figural aftereffects in patients. Notably, increased fixational eye movements certainly cannot account for findings of increased strength and duration of aftereffects. Furthermore, there is no evidence for differences in microsaccade rate in individuals with schizophrenia (unpublished analyses in Demmin et al., 2018; Egana et al., 2013). Nevertheless, considerations related to eye movements could also form a motivation for future work to focus on aftereffects that have a limited degree of spatial selectivity, such as face aftereffects, and that would therefore not be strongly affected by differences in fixation control, and certainly not by small fixational eye movements (Afraz and Cavanagh, 2009).
Group differences in response bias and criterion must also be considered as possible confounding factors. For instance, there may be group differences in the criterion level of visibility of an aftereffect needed to report its offset or onset, or there may be group differences in the cognitive tendency with which subjects report the test stimulus as opposite to the adapter stimulus, particularly as the decision becomes more difficult. To date, these factors have not been appropriately controlled for. One way to address these possible response-level confounds is to use specific variants of the nuller/cancellation method of measuring aftereffect strength, that render an influence of response bias or criterion less likely (Brascamp et al., 2018; Thakkar et al., 2018).
On a broader scale, we can interpret findings of altered visual aftereffects in the context of both computational accounts of visual aftereffects and unified and biologically plausible theoretical frameworks for understanding schizophrenia. One such framework holds that symptoms arise from an imbalance in cortical excitation and inhibition (E/I imbalance) due to NMDA receptor hypofunction (Coyle, 2012; Kantrowitz and Javitt, 2010; Olney et al., 1999) and GABA-ergic dysfunction (Egerton et al., 2017; Phillips et al., 2015; Schmidt and Mirnics, 2015; Thakkar et al., 2017). In this context it is noteworthy that a prominent theory of visual aftereffects postulates that they arise due to short-term changes in lateral interaction strength between feature detectors in a process analogous to the Hebbian learning process by which feature selectivity develops in sensory cortices across development (Bednar and Miikkulainen, 2000). Thus, alterations in visual aftereffects may reflect E/I imbalance and, more specifically, abnormal lateral interactions within visual cortex in schizophrenia. In the early phase of schizophrenia, one possible mechanism for stronger than normal aftereffects is an increase in the rate at which the weights of excitatory connections between inhibitory neurons (Gibson et al., 1999) are updated (or between pyramidal cells and interneurons; Thomson et al., 2002). The increased inhibition for features present in an adapting stimulus would then lead to increases in activity at normally inhibited neurons (i.e., especially those that signal features complementary to those whose processing has been inhibited), as part of the brain’s efforts to maintain homeostasis in firing rates. This hypothesis of increased connection strength during the acute phase of schizophrenia is based on evidence of (a) hyperactivation of the locus coeruleus (LC) in schizophrenia (Alsene & Bakshi, 2011; Yamamoto & Hornykiewicz, 2004) and modeling data indicating that elevated LC firing rate increases both gain and the rate of Hebbian learning (Verguts & Notebaert, 2009); (b) modeling data demonstrating that when cortical activity levels increase, membrane time constants are lowered, causing increases in synchronized firing between cells and aberrant local network formation (Chawla, Lumer, & Friston, 1999); and c) increased baseline synchrony in early schizophrenia (Rivolta et al., 2014; Silverstein et al., 2012; Sun et al., 2013), and increased synchrony being associated with the presence of hallucinations in people with schizophrenia in general (reviewed in Uhlhaas and Singer, 2010). With increasing illness chronicity, many patients would transition to a state of weakened lateral interactions, which we would expect to be associated with weaker aftereffects, consistent with some of the data reviewed above. This change from the acute stage to the chronic stage has been observed in the domain of contrast sensitivity as described above, as well as in the domain of perceptual organization (Silverstein, 2016; Silverstein and Keane, 2011), and it may account for attenuated contextual modulation of vision more broadly (Butler et al., 2008; Phillips et al., 2015), as well as other aspects of disorganization (Olypher et al., 2006; Phillips et al., 2015; Phillips and Silverstein, 2003, 2013) and perception (Butler et al., 2008; Phillips et al., 2015; Rokem et al., 2011; Silverstein, 2016; Silverstein and Keane, 2011) in chronic schizophrenia. While much data fit within this overall framework, longitudinal testing of the same people across different stages of illness are needed to provide strong confirmatory evidence. It would also be helpful, in the short-term, to obtain, in parallel within the same individuals, putative measures of both baseline inhibition strength, for instance as indexed by measures of surround suppression (Anderson et al., 2017; Tibber et al., 2013), and of changes in inhibition strength, as indexed by aftereffects.
A second prominent candidate explanation of schizophrenia is altered predictive coding. Predictive coding accounts of brain function posit that perception is an inferential process by which higher cortical areas generate models of the world based on context and past experience, which are communicated to sensory areas. Mismatches between predictions and sensory information (i.e. prediction errors) lead to either an update in the model or action by the organism to change the sensory input to be more consistent with predictions (Clark, 2013; Friston, 2005). Formalized within a Bayesian framework, sensory information (likelihood) is combined with predictions (priors) to compute the most likely cause of that sensory data (posterior)—which is then experienced as the percept. Psychosis is argued to arise due to perceptual experiences being more influenced by the likelihood distribution (i.e. sensory data) and less influenced by prior beliefs, due either to reduced reliability, or precision, of priors, increased precision of sensory data, or a combination thereof (reviewed in Sterzer et al., 2018; but see Powers et al., 2017). Visual adaptation, and by extension visual aftereffects, have also been explained in the context of prediction (Clifford et al., 2007). In formal Bayesian observer models (Stocker and Simoncelli, 2005), exposure to the adapter stimulus results in an increase in the precision of the likelihood function in the range of values around the adapter’s parameters. Although aftereffect paradigms do not typically measure visual discrimination per se, visual adaptation does regularly result in increased discrimination, depending on the precise relation between the adapter and test stimulus (Clifford, 2002; Clifford et al., 2001; Regan and Beverley, 1985). In this framework, larger aftereffects in individuals with schizophrenia can be interpreted as larger increases in the precision of the likelihood function, which would have the net effect of shifting percepts towards the sensory data, rather than prior beliefs, as has been posited in schizophrenia (Sterzer et al., 2018).
Consistent with the notion that perceptual inference is biased towards sensory data in psychosis, individuals with schizophrenia have been found to be less susceptible to those visual illusions that arise due to the influence of higher-level information on current sensory input (e.g. Dakin et al., 2005; Keane et al., 2013; Schneider et al., 2002; Tibber et al., 2013). A notable example here is the hollow mask illusion, in which a concave face is perceived to be convex. This illusion is argued to arise due to the powerful influence of top-down knowledge that faces are convex (Gregory, 1970). This illusion is weaker in individuals with schizophrenia, particularly those that are acutely psychotic (Keane et al., 2013; Schneider et al., 2002). However, there is also data suggestive of a greater influence of history and knowledge on perception (Corlett et al., 2019). In the visual system, previous exposure to an un-ambiguous image confers better discrimination of that same image presented later in a degraded (i.e. ambiguous) form, and this advantage is greater in individuals prone to psychosis. These apparently contradictory findings have been reconciled by highlighting the potentially critical role of where in a sensory hierarchy predictions are formed, noting that weaker priors from within the visual system, in this case, might lead to greater reliance on top-down information from outside the visual system to help structure the input. Generally, these effects of more or less influence of prior history and context on current processing are cast, in Bayesian terms, as a change in the prior distribution. However, modeling work would suggest that for aftereffects, past history (in this case, exposure to the adapter) exerts an influence on current processing by increasing the precision of the likelihood function. Such an explanation may also warrant consideration for other paradigms and illusions. Finally, it should be noted that a predictive coding failure for aftereffects would be consistent with the hypothesis of excessive updating of weights at excitatory connections onto inhibitory cells in schizophrenia (an aspect of the excitation/inhibition perspective, described above), and so an integration between the two accounts may be possible.
7. Conclusions and future directions
In conclusion, there is tantalizing evidence from a largely older body of work suggesting an abnormal use of prior experience in sculpting perception in schizophrenia in the form of altered visual aftereffects. However, there are clear inadequacies in the current literature. We thus intend this paper to function as much a review of the current literature as a motivation for further investigating visual aftereffects and a call for more rigorous studies. There are clearly significant sources of variability in these findings that prevent conclusive interpretation and that are rooted, at least in part, in differences in relevant stimulus dimensions (i.e. tilt, motion, etc.) and stimulus properties (i.e. duration, spatial frequency, etc.). Indeed, there is evidence for stronger motion and tilt aftereffects in schizophrenia but weaker figural aftereffects, and these findings appear to be moderated by adapter and test durations as well as spatial frequency. One important direction for future work will be to explore what kinds of aftereffects are altered in schizophrenia and to map out the temporal and featural stimulus conditions under which the group differences emerge. In addition, future experimental work should aim to rule out confounds related to response bias and criterion as well as eye movements. To the extent that results can be explained by CNS factors, these types of studies can clarify the extent to which altered visual aftereffects originate in the retina, the subcortical visual pathway, V1, and/or higher visual areas involved in integration and visual cognition. This will further shed light on which neuronal populations are involved in altered adaptation and its dynamics in schizophrenia.
Clinical factors, including duration of psychotic illness, symptom profile, and diagnostic criteria used to define the disorder, are a further source of variability. Because most of the research on aftereffects was done prior to the publication of the DSM-III, it is not clear to what degree older findings apply to DSM-5-defined schizophrenia. Furthermore, aftereffects seem to be stronger in unmedicated individuals in the acute phase of the illness, and antipsychotic medications reduce adaptation duration and strength in healthy observers. Thus, a second important direction of future research will be to gather data using current diagnostic criteria and to account for the effects of medication and illness stage. Future work should also explore the extent to which altered visual adaptation, as measured by visual aftereffects, may explain abnormalities in subjective visual experience as well as psychotic symptoms more broadly. Indeed, prior work has noted a relationship between an altered use of context on visual perception that is strongest in acutely ill patients and is related to disorganization (Keane et al., 2014; Silverstein et al., 2013; Silverstein et al., 2000; Uhlhaas et al., 2006; Uhlhaas et al., 2005; Uhlhaas and Silverstein, 2005).
Another potentially fruitful direction for future research is to investigate the extent to which altered abnormalities in neuronal adaptation, measured using aftereffects, may reflect illness vulnerability. There are two studies that suggest this to be the case. In healthy undergraduates, weaker tilt aftereffects, but not negative afterimages, were related to positive schizotypal traits (Thakkar et al., 2018). In the only study of visual aftereffects in unaffected relatives of schizophrenia patients, relatives tended towards increased latency of the McCollough effect (Surguladze et al., 2012), and longer latencies were associated with greater positive schizotypal traits in these relatives.
Finally, experimental data from visual aftereffect paradigms are amenable to computational modeling approaches, which can aid in dissecting component processes that may be altered in schizophrenia spectrum disorders. Future model-fitting efforts may provide insights into the extent to which putatively altered visual aftereffects in schizophrenia are explained by abnormal lateral interactions within visual cortex (Bednar and Miikkulainen, 2000) and/or abnormal Bayesian inference (Stocker and Simoncelli, 2005).
In conclusion, visual aftereffects may serve as a useful paradigm for understanding both altered visual experience in schizophrenia and more global dysfunction related to predictive processes and short-term synaptic plasticity. Although there is compelling evidence for altered aftereffects in schizophrenia, future work using more rigorous psychophysics and comprehensive and current clinical characterization is necessary to paint a clearer and more complete picture of aftereffects in schizophrenia and to fully realize their potential for understanding the disease.
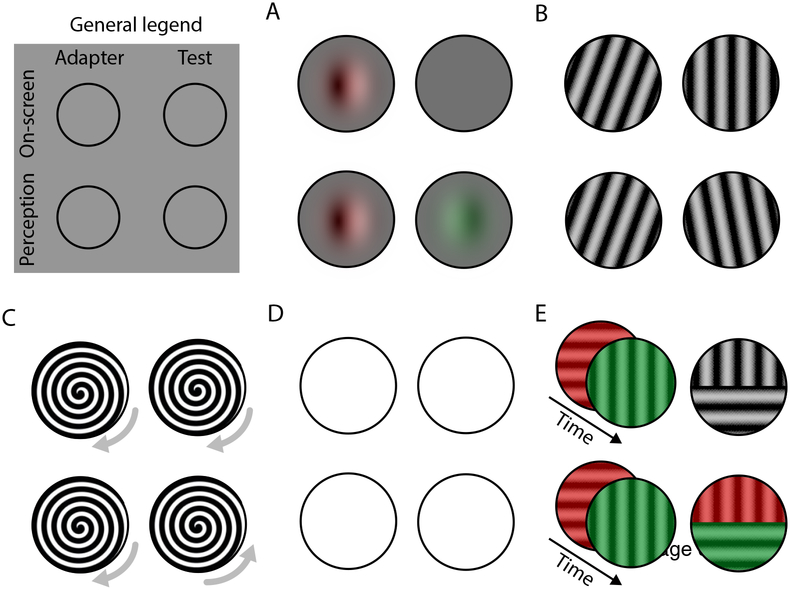
Figure 3.
Illustration of aftereffect paradigms. In the top left is a legend to all panels. Each panel shows one paradigm, all involving an adapter stimulus (left column in each panel) followed by a test stimulus (right columns). Aftereffects constitute altered perception of the test stimulus, so each panel also illustrate subjective perception (bottom rows) in addition to on-screen stimuli (top rows). Aftereffects shown: negative afterimages (A), tilt aftereffect (B), movement aftereffect (C), figural aftereffect (D), and McCollough effect (E). See text for further details.
Highlights.
Abnormal use of prior experience to interpret sensory information in schizophrenia
Visual aftereffects measure influence of prior experience on perception
Evidence for alterations of visual aftereffects in schizophrenia
Significant variability in findings due to type of aftereffect and clinical factors
Findings allow for mechanistic interpretations for altered visual aftereffects
Acknowledgements
The authors would like to thank Rachael Slate and Jessica Fattal for their help with the literature search and for an anonymous reviewer for their thoughtful and helpful reactions. This work was supported by a NARSAD Young Investigator award from the Brain and Behavior Foundation (KNT), NIMH R01 MH112644 (KNT), and a grant from the Michigan State University College of Social Sciences Faculty Initiatives Award (JWB, KNT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Abraham P, McCallum WC, 1973. The CNV and the Spiral After-Effect. Electroencephalogr. Clin. Neurophysiol 33, 205–208. [Google Scholar]
- Afraz A, Cavanagh P, 2009. The gender-specific face aftereffect is based in retinotopic not spatiotopic coordinates across several natural image transformations. J Vis 9(10), 10 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DG, Farrar SB, Hamilton DB, 1984. Spatial contrast adaptation characteristics of neurones recorded in the cat’s visual cortex. J Physiol 347, 713–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Tibber MS, Schwarzkopf DS, Shergill SS, Fernandez-Egea E, Rees G, Dakin SC, 2017. Visual Population Receptive Fields in People with Schizophrenia Have Reduced Inhibitory Surrounds. J. Neurosci 37(6), 1546–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstis S, Verstraten FA, Mather G, 1998. The motion aftereffect. Trends Cogn Sci 2(3), 111–117. [DOI] [PubMed] [Google Scholar]
- Barlow H, 1990. Conditions for versatile learning, Helmholtz’s unconscious inference, and the task of perception. Vision Res 30(11), 1561–1571. [DOI] [PubMed] [Google Scholar]
- Barlow HB, Hill RM, 1963. Evidence for a Physiological Explanation of the Waterfall Phenomenon and Figural after-Effects. Nature 200, 1345–1347. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Pandita M, Thakkar K, Goff DC, Manoach DS, 2007. The relation between antisaccade errors, fixation stability and prosaccade errors in schizophrenia. Exp. Brain Res [DOI] [PubMed] [Google Scholar]
- Bednar JA, 2012. Building a mechanistic model of the development and function of the primary visual cortex. J. Physiol. Paris 106(5–6), 194–211. [DOI] [PubMed] [Google Scholar]
- Bednar JA, Miikkulainen R, 2000. Tilt Aftereffects in a Self-Organizing Model of the Primary Visual Cortex. Neural Comput 12, 1721–1740. [DOI] [PubMed] [Google Scholar]
- Blake R, Hiris E, 1993. Another means for measuring the motion aftereffect. Vision Res 33(11), 1589–1592. [DOI] [PubMed] [Google Scholar]
- Brascamp JW, Becker MW, Hambrick DZ, 2018. Revisiting individual differences in the time course of binocular rivalry. J Vis 18(7), 3. [DOI] [PubMed] [Google Scholar]
- Brascamp JW, van Boxtel JJ, Knapen TH, Blake R, 2010. A dissociation of attention and awareness in phase-sensitive but not phase-insensitive visual channels. J. Cogn. Neurosci 22(10), 2326–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitmeyer BG, Ogmen H, 2000. Recent models and findings in visual backward masking: a comparison, review, and update. Percept. Psychophys 62(8), 1572–1595. [DOI] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC, 2008. Visual perception and its impairment in schizophrenia. Biol. Psychiatry 64(1), 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC, 2005. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch. Gen. Psychiatry 62(5), 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JE, Harris JP, Phillipson OT, 1991. Tilt aftereffect reveals early visual processing deficits in Parkinson’s disease and in chronic schizophrenic patients on depot neuroleptic. Psychopathology 24(6), 375–380. [DOI] [PubMed] [Google Scholar]
- Castet E, Keeble DR, Verstraten FA, 2002. Nulling the motion aftereffect with dynamic random-dot stimuli: limitations and implications. J Vis 2(4), 302–311. [DOI] [PubMed] [Google Scholar]
- Chen Y, 2011. Abnormal visual motion processing in schizophrenia: a review of research progress. Schizophr. Bull 37(4), 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, 2013. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci 36(3), 181–204. [DOI] [PubMed] [Google Scholar]
- Clifford CW, 2002. Perceptual adaptation: motion parallels orientation. Trends Cogn Sci 6(3), 136–143. [DOI] [PubMed] [Google Scholar]
- Clifford CW, Webster MA, Stanley GB, Stocker AA, Kohn A, Sharpee TO, Schwartz O, 2007. Visual adaptation: neural, psychological and computational aspects. Vision Res 47(25), 3125–3131. [DOI] [PubMed] [Google Scholar]
- Clifford CW, Wenderoth P, Spehar B, 2000. A functional angle on some after-effects in cortical vision. Proc Biol Sci 267(1454), 1705–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford CW, Wyatt AM, Arnold DH, Smith ST, Wenderoth P, 2001. Orthogonal adaptation improves orientation discrimination. Vision Res 41(2), 151–159. [DOI] [PubMed] [Google Scholar]
- Corlett PR, Horga G, Fletcher PC, Alderson-Day B, Schmack K, Powers AR 3rd, 2019. Hallucinations and Strong Priors. Trends Cogn Sci 23(2), 114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, 2012. NMDA receptor and schizophrenia: a brief history. Schizophr. Bull 38(5), 920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik KJW, 1940. Origin of visual after-images. Nature 145, 512–512. [Google Scholar]
- Dakin S, Carlin P, Hemsley D, 2005. Weak suppression of visual context in chronic schizophrenia. Curr. Biol 15(20), R822–824. [DOI] [PubMed] [Google Scholar]
- Day RH, Burns R, Singer GHV, Letcher D, 1967. Sensory spatial after-effects in relation to chronological age, mental retardation and schizophrenia. Br. J. Psychol 58(1), 13–27. [DOI] [PubMed] [Google Scholar]
- Day RH, Pollack RH, Seagrim GN, 1959. Figural after-effects: A critical review. Australian Journal of Psychology 11(1), 15–45. [Google Scholar]
- Demmin DL, Davis Q, Roche M, Silverstein SM, 2018. Electroretinographic anomalies in schizophrenia. J. Abnorm. Psychol 127(4), 417–428. [DOI] [PubMed] [Google Scholar]
- Egana JI, Devia C, Mayol R, Parrini J, Orellana G, Ruiz A, Maldonado PE, 2013. Small Saccades and Image Complexity during Free Viewing of Natural Images in Schizophrenia. Front Psychiatry 4, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Modinos G, Ferrera D, McGuire P, 2017. Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis. Transl Psychiatry 7(6), e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman B, Chapman LJ, 1973. Early subjective experience in schizophrenic episodes. J. Abnorm. Psychol 82(1), 46–54. [DOI] [PubMed] [Google Scholar]
- Frisby J, 1979. Seeing: Illusion, Brain and Mind Oxford University Press, Oxford. [Google Scholar]
- Friston K, 2005. A theory of cortical responses. Philos. Trans. R. Soc. Lond. B. Biol. Sci 360(1456), 815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgeson MA, Turner RSE, 1985. Afterimages of Sinusoidal, Square-Wave and Compound Gratings. Vision Res 25(11), 1709–1720. [DOI] [PubMed] [Google Scholar]
- Gibson JJ, Radner M, 1937. Adaptation, after-effect and contrast in the perception of tilted lines. I. Quantitative studies. J. Exp. Psychol 20, 453–467. [Google Scholar]
- Gibson JR, Beierlein M, Connors BW, 1999. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402(6757), 75–79. [DOI] [PubMed] [Google Scholar]
- Gilroy LA, Blake R, 2005. The interaction between binocular rivalry and negative afterimages. Curr. Biol 15(19), 1740–1744. [DOI] [PubMed] [Google Scholar]
- Gray JA, Feldon J, Rawlings JNP, Hemsley DR, Smith AD, 1991. The neuropsychology of schizophrenia. Behav. Brain Sci 14, 1–81. [Google Scholar]
- Green M, Walker E, 1986. Attentional performance in positive- and negative-symptom schizophrenia. J. Nerv. Ment. Dis 174(4), 208–213. [DOI] [PubMed] [Google Scholar]
- Green MF, Lee J, Wynn JK, Mathis KI, 2011. Visual masking in schizophrenia: overview and theoretical implications. Schizophr. Bull 37(4), 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee MW, Magnussen S, 1987. Saturation of the tilt aftereffect. Vision Res 27(6), 1041–1043. [DOI] [PubMed] [Google Scholar]
- Gregory RL, 1970. The Intelligent Eye Weidenfeld and Nicolson, London. [Google Scholar]
- Grunewald A, Lankheet MJ, 1996. Orthogonal motion after-effect illusion predicted by a model of cortical motion processing. Nature 384(6607), 358–360. [DOI] [PubMed] [Google Scholar]
- Harris J, 1994. The duration of the movement aftereffect as an index of psychiatric illness. Perception 23(10), 1145–1153. [DOI] [PubMed] [Google Scholar]
- Harris JP, Calvert JE, 1989. Contrast, spatial frequency and test duration effects on the tilt aftereffect: implications for underlying mechanisms. Vision Res 29(1), 129–135. [DOI] [PubMed] [Google Scholar]
- Harris JP, Gelbtuch MH, Phillipson OT, 1986. Effects of haloperidol and nomifensine on the visual aftereffects of tilt and movement. Psychopharmacology (Berl) 89(2), 177–182. [DOI] [PubMed] [Google Scholar]
- Harris JP, Phillipson OT, Watkins GM, Whelpton R, 1983. Effects of chlorpromazine and promazine on the visual aftereffects of tilt and movement. Psychopharmacology (Berl) 79(1), 49–57. [DOI] [PubMed] [Google Scholar]
- Hemsley DR, 2005. The development of a cognitive model of schizophrenia: placing it in context. Neurosci. Biobehav. Rev 29(6), 977–988. [DOI] [PubMed] [Google Scholar]
- Herrington RN, Claridge GS, 1965. Sedation threshold and Archimedes’ spiral after-effect in early psychosis. J. Psychiatr. Res 3(3), 159–170. [DOI] [PubMed] [Google Scholar]
- Hersen M, Levine J, Church A, 1972. Parameters of the spiral after-effect in organics, schizophrenics, and normals. J. Genet. Psychol 120(2d Half), 177–187. [DOI] [PubMed] [Google Scholar]
- Huk AC, Ress D, Heeger DJ, 2001. Neuronal basis of the motion aftereffect reconsidered. Neuron 32(1), 161–172. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Ettinger U, 2006. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology 43(3), 302–313. [DOI] [PubMed] [Google Scholar]
- Janke W, Debus G, 1972. Double-blind Psychometric Evaluation of Pimozide and Haloperidol versus Placebo in Emotionally labile Volunteers under two Different Work Load Conditions. Pharmacopsychiatry 5, 34–51. [Google Scholar]
- Kanai R, Verstraten FA, 2005. Perceptual manifestations of fast neural plasticity: motion priming, rapid motion aftereffect and perceptual sensitization. Vision Res 45(25–26), 3109–3116. [DOI] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC, 2010. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res. Bull 83(3–4), 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane BP, Erlikhman G, Kastner S, Paterno D, Silverstein SM, 2014. Multiple forms of contour grouping deficits in schizophrenia: what is the role of spatial frequency? Neuropsychologia 65, 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane BP, Silverstein SM, Wang Y, Papathomas TV, 2013. Reduced depth inversion illusions in schizophrenia are state-specific and occur for multiple object types and viewing conditions. J. Abnorm. Psychol 122(2), 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck MJ, Palella TD, Pantle A, 1976. Motion aftereffect as a function of the contrast of sinusoidal gratings. Vision Res 16(2), 187–191. [DOI] [PubMed] [Google Scholar]
- Kelemen O, Kiss I, Benedek G, Keri S, 2013. Perceptual and cognitive effects of antipsychotics in first-episode schizophrenia: the potential impact of GABA concentration in the visual cortex. Prog. Neuropsychopharmacol. Bol. Psychiatry 47, 13–19. [DOI] [PubMed] [Google Scholar]
- Kelly DH, Martinezuriegas E, 1993. Measurements of Chromatic and Achromatic Afterimages. Journal of the Optical Society of America a-Optics Image Science and Vision 10(1), 29–37. [DOI] [PubMed] [Google Scholar]
- Kelm H, 1962. The figural after-effect in schizophrenic patients. J. Nerv. Ment. Dis 135, 338–345. [DOI] [PubMed] [Google Scholar]
- Kelm H, 1968. Visual figural aftereffect in schizophrenic and nonschizophrenic patients. J. Abnorm. Psychol 73(3), 273–275. [DOI] [PubMed] [Google Scholar]
- Kiss I, Fabian A, Benedek G, Keri S, 2010. When doors of perception open: visual contrast sensitivity in never-medicated, first-episode schizophrenia. J. Abnorm. Psychol 119(3), 586–593. [DOI] [PubMed] [Google Scholar]
- Knapen T, Rolfs M, Cavanagh P, 2009. The reference frame of the motion aftereffect is retinotopic. J Vis 9(5), 16 11–17. [DOI] [PubMed] [Google Scholar]
- Knil DC, Richards W, 1996. Perception as Bayesian Inference Cambridge University Press, Cambridge. [Google Scholar]
- Kohler W, Wallach H, 1944. Figural after-effects; An investigation of visual processes. Proceedings of the American Philosophical Society 88, 269–357. [Google Scholar]
- Kohn A, Movshon JA, 2003. Neuronal adaptation to visual motion in area MT of the macaque. Neuron 39(4), 681–691. [DOI] [PubMed] [Google Scholar]
- Krishnamoorti SR, Shagass C, 1963. Some Psychological Test Correlates of Sedation Threshold. Recent Adv. Biol. Psychiatry 6, 256–266. [PubMed] [Google Scholar]
- Leguire LE, Blake R, 1982. Role of threshold in afterimage visibility. J. Opt. Soc. Am 72(9), 1232–1237. [DOI] [PubMed] [Google Scholar]
- Lehmann HE, Csank J, 1957. Differential screening of phrenotropic agents in man: psychophysiologio test data. J Clin Exp Psychopathol 18(3), 222–235. [PubMed] [Google Scholar]
- Li H, Liu X, Andolina IM, Li X, Lu Y, Spillmann L, Wang W, 2017. Asymmetries of Dark and Bright Negative Afterimages Are Paralleled by Subcortical ON and OFF Poststimulus Responses. J. Neurosci 37(8), 1984–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, 1998. A neural model of contour integration in the primary visual cortex. Neural Comput 10(4), 903–940. [DOI] [PubMed] [Google Scholar]
- Lloyd DN, Newbrough JR, 1964. Sensory Changes with Phenothiazine Medication in Schizophrenic Patients. J. Nerv. Ment. Dis 139, 169–175. [DOI] [PubMed] [Google Scholar]
- Mather G, 1980. The movement aftereffect and a distribution-shift model for coding the direction of visual movement. Perception 9(4), 379–392. [DOI] [PubMed] [Google Scholar]
- Mather G, Pavan A, Campana G, Casco C, 2008. The motion aftereffect reloaded. Trends Cogn Sci 12(12), 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollough C, 1965. Color Adaptation of Edge-Detectors in the Human Visual System. Science 149(3688), 1115–1116. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Williamson A, 1989. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc. Natl. Acad. Sci. U. S. A 86(20), 8098–8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida S, Ashida H, Sato T, 1997. Contrast dependencies of two types of motion aftereffect. Vision Res 37(5), 553–563. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, 1977. Reaction time and attention in schizophrenia: a critical evaluation of the data and theories. Schizophr. Bull 3(3), 373–428. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Bismark A, Hetrick WP, Bodkins M, Vohs JL, Shekhar A, 2006. Early stage vision in schizophrenia and schizotypal personality disorder. Schizophr. Res 86(1–3), 89–98. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB, 1999. NMDA receptor hypofunction model of schizophrenia. J. Psychiatr. Res 33(6), 523–533. [DOI] [PubMed] [Google Scholar]
- Olypher AV, Klement D, Fenton AA, 2006. Cognitive disorganization in hippocampus: a physiological model of the disorganization in psychosis. J. Neurosci 26(1), 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantle A, 1998. How do movement aftereffect measures measure up, in: Mather G, Verstraten FA, Anstis S (Eds.), The Motion Aftereffect: A Modern Perspective MIT Press, Cambridge, MA. [Google Scholar]
- Parker DM, 1972. Contrast and size variables and the tilt after-effect. Q. J. Exp. Psychol 24(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Phillips WA, Clark A, Silverstein SM, 2015. On the functions, mechanisms, and malfunctions of intracortical contextual modulation. Neurosci. Biobehav. Rev 52, 1–20. [DOI] [PubMed] [Google Scholar]
- Phillips WA, Silverstein SM, 2003. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav. Brain Sci 26(1), 65–82; discussion 82–137. [DOI] [PubMed] [Google Scholar]
- Phillips WA, Silverstein SM, 2013. The coherent organization of mental life depends on mechanisms for context-sensitive gain-control that are impaired in schizophrenia. Front Psychol 4, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers AR, Mathys C, Corlett PR, 2017. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science 357(6351), 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prysiazniuk AW, Kelm H, 1965. Counter Displacement in the Visual Figural after-Effect. Q. J. Exp. Psychol 17, 69–74. [DOI] [PubMed] [Google Scholar]
- Rao RP, Ballard DH, 1999. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci 2(1), 79–87. [DOI] [PubMed] [Google Scholar]
- Regan D, Beverley KI, 1985. Postadaptation orientation discrimination. J. Opt. Soc. Am. A 2(2), 147–155. [DOI] [PubMed] [Google Scholar]
- Rokem A, Yoon JH, Ooms RE, Maddock RJ, Minzenberg MJ, Silver MA, 2011. Broader visual orientation tuning in patients with schizophrenia. Front Hum Neurosci 5, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein JD, 1960. The duration of the Achimedes spiral afterimage in the diagnosis of brain damage. J. Consult. Psychol 24, 299–306. [DOI] [PubMed] [Google Scholar]
- Schmidt MJ, Mirnics K, 2015. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology 40(1), 190–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider U, Borsutzky M, Seifert J, Leweke FM, Huber TJ, Rollnik JD, Emrich HM, 2002. Reduced binocular depth inversion in schizophrenic patients. Schizophr. Res 53(1–2), 101–108. [DOI] [PubMed] [Google Scholar]
- Schwartz BD, Evans WJ, Pena JM, Winstead DK, 1994. Visible persistence decay rates for schizophrenics and substance abusers. Biol. Psychiatry 36(10), 662–669. [DOI] [PubMed] [Google Scholar]
- Schwartz O, Hsu A, Dayan P, 2007. Space and time in visual context. Nature Reviews Neuroscience 8(7), 522–535. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T, 1975. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science 188(4194), 1217–1219. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K, 1976. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 261(5562), 717–719. [DOI] [PubMed] [Google Scholar]
- Shoshina II, Shelepin YE, Vershinina EA, Novikova KO, 2015. [The Spatial-Frequency Characteristics of the Visual System in Schizophrenia]. Fiziol. Cheloveka 41(3), 29–40. [PubMed] [Google Scholar]
- Silverstein SM, 2016. Visual Perception Disturbances in Schizophrenia: A Unified Model. Nebr. Symp. Motiv 63, 77–132. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Demmin D, Skodlar B, 2017. Space and Objects: On the Phenomenology and Cognitive Neuroscience of Anomalous Perception in Schizophrenia (Ancillary Article to EAWE Domain 1). Psychopathology 50(1), 60–67. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP, 2011. Perceptual organization impairment in schizophrenia and associated brain mechanisms: review of research from 2005 to 2010. Schizophr. Bull 37(4), 690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP, Wang Y, Mikkilineni D, Paterno D, Papathomas TV, Feigenson K, 2013. Effects of short-term inpatient treatment on sensitivity to a size contrast illusion in first-episode psychosis and multiple-episode schizophrenia. Front Psychol 4, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein SM, Kovacs I, Corry R, Valone C, 2000. Perceptual organization, the disorganization syndrome, and context processing in chronic schizophrenia. Schizophr. Res 43(1), 11–20. [DOI] [PubMed] [Google Scholar]
- Skottun BC, Skoyles JR, 2007. Contrast sensitivity and magnocellular functioning in schizophrenia. Vision Res 47(23), 2923–2933. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL, 1998. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J. Abnorm. Psychol 107(1), 49–62. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Adams RA, Fletcher P, Frith C, Lawrie SM, Muckli L, Petrovic P, Uhlhaas P, Voss M, Corlett PR, 2018. The Predictive Coding Account of Psychosis. Biol. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker AA, Simoncelli EP, 2005. Sensory Adaptation within a Bayesian Framework for Perception, in: Weiss Y, Schölkopf B, Platt J (Eds.), Advances in Neural Information Processing Systems MIT Press, Cambridge, MA, pp. 1291–1298. [Google Scholar]
- Surguladze SA, Chkonia ED, Kezeli AR, Roinishvili MO, Stahl D, David AS, 2012. The McCollough effect and facial emotion discrimination in patients with schizophrenia and their unaffected relatives. Schizophr. Bull 38(3), 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland NS, 1961. Figural after-effects and apparent size. Q. J. Exp. Physiol 13, 222–228. [Google Scholar]
- Suzuki S, Grabowecky M, 2003. Attention during adaptation weakens negative afterimages. J. Exp. Psychol. Hum. Percept. Perform 29(4), 793–807. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Antinori A, Carter OL, Brascamp JW, 2018. Altered short-term neural plasticity related to schizotypal traits: Evidence from visual adaptation. Schizophr. Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, Rosler L, Wijnen JP, Boer VO, Klomp DW, Cahn W, Kahn RS, Neggers SF, 2017. 7T Proton Magnetic Resonance Spectroscopy of Gamma-Aminobutyric Acid, Glutamate, and Glutamine Reveals Altered Concentrations in Patients With Schizophrenia and Healthy Siblings. Biol. Psychiatry 81(6), 525–535. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Schall JD, Boucher L, Logan G, Park S, 2011. Response inhibition and response monitoring in a saccadic countermanding task in schizophrenia Biol. Psychiatry 69(1), 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, Schall JD, Logan GD, Park S, 2015. Response inhibition and response monitoring in a saccadic double-step task in schizophrenia. Brain Cogn 95, 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, West DC, Wang Y, Bannister AP, 2002. Synaptic connections and small circuits involving excitatory and inhibitory neurons in layers 2–5 of adult rat and cat neocortex: triple intracellular recordings and biocytin labelling in vitro. Cereb. Cortex 12(9), 936–953. [DOI] [PubMed] [Google Scholar]
- Tibber MS, Anderson EJ, Bobin T, Antonova E, Seabright A, Wright B, Carlin P, Shergill SS, Dakin SC, 2013. Visual surround suppression in schizophrenia. Front Psychol 4, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst DJ, Thompson PG, 1975. Orientation illusions and after-effects: inhibition between channels. Vision Res 15, 967–972. [DOI] [PubMed] [Google Scholar]
- Tress KH, Kugler BT, 1979. Interocular transfer of movement after-effects in schizophrenia. Br. J. Psychol 70(3), 389–392. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Phillips WA, Mitchell G, Silverstein SM, 2006. Perceptual grouping in disorganized schizophrenia. Psychiatry Res 145(2–3), 105–117. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Phillips WA, Silverstein SM, 2005. The course and clinical correlates of dysfunctions in visual perceptual organization in schizophrenia during the remission of psychotic symptoms. Schizophr. Res 75(2–3), 183–192. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Silverstein SM, 2005. Perceptual organization in schizophrenia spectrum disorders: empirical research and theoretical implications. Psychol. Bull 131(4), 618–632. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W, 2010. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11(2), 100–113. [DOI] [PubMed] [Google Scholar]
- van Boxtel JJ, van Ee R, Erkelens CJ, 2006. A single system explains human speed perception. J. Cogn. Neurosci 18(11), 1808–1819. [DOI] [PubMed] [Google Scholar]
- van de Grind WA, van der Smagt MJ, Verstraten FA, 2004. Storage for free: a surprising property of a simple gain-control model of motion aftereffects. Vision Res 44(19), 2269–2284. [DOI] [PubMed] [Google Scholar]
- van de Grind WA, van Hof P, van der Smagt MJ, Verstraten FA, 2001. Slow and fast visual motion channels have independent binocular-rivalry stages. Proc Biol Sci 268(1465), 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraten FA, van der Smagt MJ, van de Grind WA, 1998. Aftereffect of high-speed motion. Perception 27(9), 1055–1066. [DOI] [PubMed] [Google Scholar]
- Virsu V, Laurinen P, 1977. Long-Lasting Afterimages Caused by Neural Adaptation. Vision Res 17(7), 853–860. [DOI] [PubMed] [Google Scholar]
- Wagner H, 1971. The effect of repeated trials on a figural aftereffect. Percept. Psychophys 10(6), 403–407. [Google Scholar]
- Wenderoth P, Wiese M, 2008. Retinotopic encoding of the direction aftereffect. Vision Res 48(19), 1949–1954. [DOI] [PubMed] [Google Scholar]
- Wertheimer M, 1954. The differential satiability of schizophrenic and normal subjects: a test of a deduction from the theory of visual aftereffects. J. Gen. Psychol 51, 291–299. [Google Scholar]
- Wohlgemuth A, 1911. On the after-effect of seen movement. Br. J. Psychol. Monograph Supplement 1, 1–117. [Google Scholar]
- Wolfe JM, O’Connell KM, 1986. Fatigue and structural change: two consequences of visual pattern adaptation. Invest. Ophthalmol. Vis. Sci 27(4), 538–543. [PubMed] [Google Scholar]
- Yoon JH, Sheremata SL, Rokem A, Silver MA, 2013. Windows to the soul: vision science as a tool for studying biological mechanisms of information processing deficits in schizophrenia. Front Psychol 4, 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi Q, 2012. Neural locus of color afterimages. J Ophthalmic Vis Res 7(1), 105–106. [PMC free article] [PubMed] [Google Scholar]