Abstract
The meninges are membranous layers surrounding the central nervous system. In the head, the meninges lie between the brain and the skull, and interact closely with both during development. The cranial meninges originate from a mesenchymal sheath on the surface of the developing brain, called primary meninx, and undergo differentiation into three layers with distinct histological characteristics: the dura mater, the arachnoid mater, and the pia mater. While genetic regulation of meningeal development is still poorly understood, mouse mutants and other models with meningeal defects have demonstrated the importance of the meninges to normal development of the calvaria and the brain. For the calvaria, the interactions with the meninges are necessary for the progression of calvarial osteogenesis during early development. In later stages, the meninges control the patterning of the skull and the fate of the sutures. For the brain, the meninges regulate diverse processes including cell survival, cell migration, generation of neurons from progenitors, and vascularization. Also, the meninges serve as a stem cell niche for the brain in the postnatal life. Given these important roles of the meninges, further investigation into the molecular mechanisms underlying meningeal development can provide novel insights into the coordinated development of the head.
Keywords: meninges, head mesenchyme, calvaria, brain development, craniofacial development
1. Introduction
The vertebrate central nervous system (CNS) is encased with three layers of membranes called the meninges (Greek: meninx-membrane). The meninges provide a protective cover to the underlying soft neural tissue of the brain and the spinal cord. Furthermore, they attach the CNS parenchyma to the bony skull or the vertebral column. The meninges also contain space through which cerebrospinal fluid (CSF) travels around CNS. In addition to these structural roles, a growing body of evidence has indicated that the meninges are actively involved in development of the brain and the calvaria (the top part of the skull), and even serve as a stem cell niche postnatally (Adeeb et al., 2012; Decimo et al., 2012; Gagan et al., 2007; Siegenthaler and Pleasure, 2011; Richtsmeier and Flaherty, 2013). From a medical perspective, defects in the meninges are thought to underlie two neurodevelopmental disorders in humans, Dandy-Walker malformation and cobblestone lissencephaly (Siegenthaler and Pleasure, 2011). Both conditions impair the function of the brain.
Despite the profound importance of the meninges, we have very limited information about the formation, differentiation, and the molecular characteristics of this tissue. In this review, we summarize the current understanding of the process of meningeal development, interactions between the meninges and the developing calvaria, and interactions between the meninges and the developing brain. As such, the focus is on the cranial meninges covering the brain. Discussions of the spinal meninges can be found in other reviews (Lopes, 2009; Nagel et al., 2018; Sakka et al., 2016).
2. Organization of the meninges
In adult mammals, the meninges are made of three distinct layers (Fig. 1): the outermost dura mater (dura- tough, mater-mother), the middle arachnoid mater (arachne-spider), and the innermost pia mater (pia- tender), each named for their histological appearances. The dura mater is also referred to as pachymeninx (pachy-thick), while the arachnoid mater and the pia mater are together called leptomeninx (lepto- thin) (Adeeb et al., 2012; Vanderah and Gould, 2015).
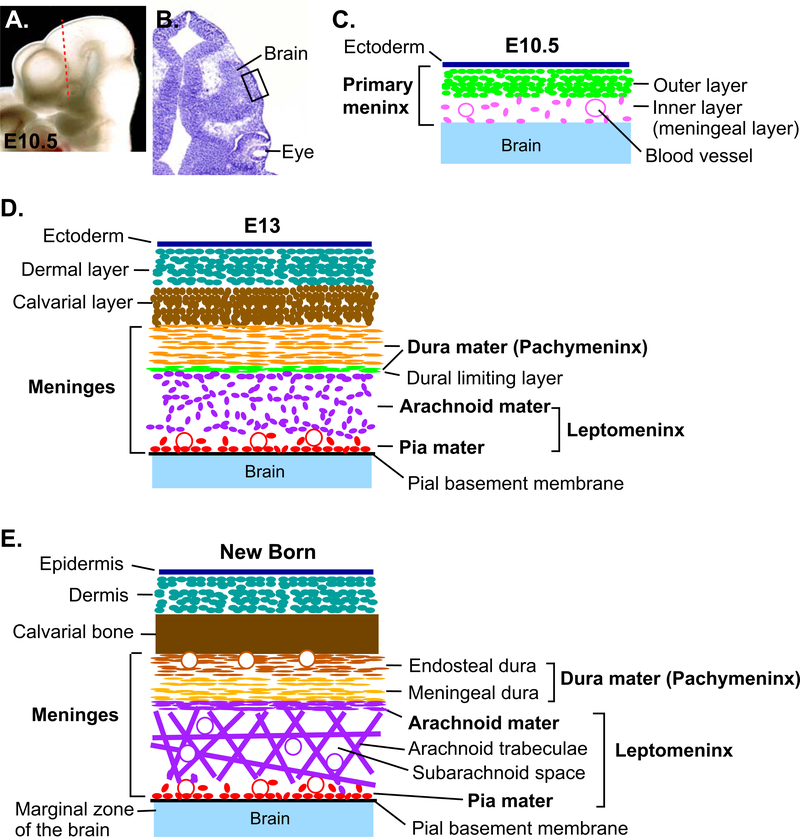
Figure 1. Development of the meninges.
(A) Lateral view of the head of E10.5 mouse embryo. (B) A coronal section through the head of E10.5 mouse embryo at a position shown in A (dotted line). The section was stained with cresyl violet to show the morphology. (C) A schematic of the tissue layers from the boxed area in B. After the primary meninx is established around the brain at ~E9.5, the mesenchyme becomes divided into an outer dense layer and an inner reticular layer (meningeal layer) at ~E10.5. (D) Around E13, the meninges begin to differentiate into the pachymeninx (dura mater) and the leptomeninx (arachnoid mater and pia mater) separated by dural limiting layer. (E) Later, the dura mater appears as two tightly attached layers, the endosteal dura and the meningeal dura. The endosteal dura serves as periosteum of the inner surface of the calvarial bone. The cavitation of the leptomeninges generates arachnoid trabeculae made of collagen fibers and the fibroblasts, and the subarachnoid space inside the trabeculae.
The dura mater is a thick and dense collagenous membrane attached to the inner surface of the skull. It is made of two layers - the outer layer (called endosteal layer/dura or periosteal layer/dura) serves as the periosteum of the internal surface of the skull bone. The inner layer (called meningeal layer/dura or dura mater proper) is fused to the endosteal layer in most regions, and they are separated only at the sites of dural venous sinuses for the drainage of venous blood from the brain. The dura mater folds and invaginates into the cranial cavity at major boundaries between the sub-regions of the brain. These folds are called dural reflections. For example, falx cerebri separates the two cerebral hemispheres along the midline, and tentorium cerebelli separates the cerebral hemispheres posteriorly from the cerebellum. The dura mater, especially the endosteal layer, contains numerous blood vessels, which are mainly involved in supplying blood to the calvaria (Adeeb et al., 2012; Vanderah and Gould, 2015). In addition, the lymphatic vessels within the dura mater serve as a drain for CSF from the CNS (Aspelund et al., 2015; DiNuoscio and Atit, 2019; Louveau et al., 2015).
The arachnoid mater is a thin, translucent membrane containing a few layers of flattened cells. Underneath this membrane is arachnoid trabeculae, which is spongy connective tissue made of collagen fibers and fibroblasts. Openings in this meshwork constitute the subarachnoid space, and blood vessels and CSF travel through this space (Decimo et al., 2012; Lopes, 2009; Vanderah and Gould, 2015).
The pia mater has a delicate, single cell-layer membrane that closely adheres to the surface of the brain. In addition, blood vessels branch out from the subarachnoid space through the pia mater into the brain. These blood vessels, as well as their perivascular connective tissue and perivascular space, are all considered a continuation of the pia mater (Decimo et al., 2012; Lopes, 2009; O’Rahilly and Muller, 1986; Vanderah and Gould, 2015). The pial basement membrane is a sheet of extracellular matrix (ECM) immediately internal to the pia mater. It contains diverse ECM proteins including laminins, collagen IV, heparan sulfate proteoglycans, and nidogen (also known as entactin) (Halfter et al., 2002). The pial basement membrane forms the border between the meninges and the brain parenchyma, and it is closely involved in brain development (discussed below).
3. Development of the meninges
3.1. Histological description of the process
Embryonic development of the meninges has been described in multiple species by many researchers, with some works dating back over 200 years (Adeeb et al., 2012). Here, we will focus on the description of the process in mammals based on studies of human, mouse, and rat (Adeeb et al., 2012; Angelov and Vasilev, 1989; Decimo et al., 2012; Lopes, 2009; McLone and Bondareff, 1975; O’Rahilly and Muller, 1986).
During early embryogenesis, mesenchyme cells begin to surround the hindbrain at the time of neural tube closure, and continue to spread to the midbrain and the forebrain levels (O’Rahilly and Muller, 1986). As a result, a mesenchymal sheath is established over the brain by Carnegie stage 15 in humans (5th week of gestation) and embryonic day (E) ~E9.5 in mice (Angelov and Vasilev, 1989; McLone and Bondareff, 1975; O’Rahilly and Muller, 1986). This sheath is the primary meninx (also known as primitive meninx or meninx primitiva), and it is the primordium for the meninges, skull, and the scalp (O’Rahilly and Muller, 1986). The primary meninx also contains a vascular plexus along the surface of the brain (perineural vascular plexus), which will develop into the blood vessels embedded in the meninges and those entering the brain (Decimo et al., 2012). The nascent pia mater is already histologically discernable over some parts of the brain as cells intervening the vascular plexus and the wall of the brain (O’Rahilly and Muller, 1986). Fibroblasts in the pia mater produce ECM proteins to lay down the pial basement membrane that will separate the meninges and the brain.
Subsequently, the primary meninx is divided into an outer dense layer and an inner reticular layer at ~E10.5 in mice (Fig. 1). The inner layer is considered to be the meningeal mesenchyme although it has not been proven whether this layer gives rise to all three meninges including the dura mater. By stage 17 in humans (6th week of gestation) and ~E13 in mice, the mesenchyme around the brain is organized into multiple distinct layers (Fig. 1): the most external layer is the dermal layer. The next layer is what has been called a ‘skeletogenic’ layer, for giving rise to the skull (O’Rahilly and Muller, 1986). However, more recent studies have found that the mesenchyme cells in this layer on the apical side of the head may contribute to the sutures (soft tissue joints) but not the bone plates of the calvaria (Roybal et al., 2010; Yoshida et al., 2008). Therefore, the term ‘calvarial’ layer (Siegenthaler and Pleasure, 2011) is more appropriate in that it includes the precursors of the sutures as well as the bone. Internal to the calvarial layer, the meningeal primordium begins to be separated into pachymeninx (dura mater) and leptomeninx (arachnoid mater and pia mater) by the dural limiting layer. Pachymeninx contains longitudinally arranged fibroblasts, while leptomeninx is a meshwork of loosely organized cells. Dural limiting layer itself is a sheet of condensed cells, and it is thought to contribute to both the dura mater and the arachnoid mater ultimately. Thus, the external portion of the dural limiting layer is usually included in the definition of pachymeninx (Lopes, 2009; O’Rahilly and Muller, 1986). Differentiation of the meningeal layers progresses from basal to apical direction (Vivatbutsiri et al., 2008). During this process, leptomeninx undergoes cavitation to make the arachnoid trabeculae and the subarachnoid space. The dura mater gets filled with accumulating collagen fibers (Angelov and Vasilev, 1989), and the lymphatic vessels in the dura mater develop in the early postnatal life (Antila et al., 2017).
3.2. Origins of the meningeal cells
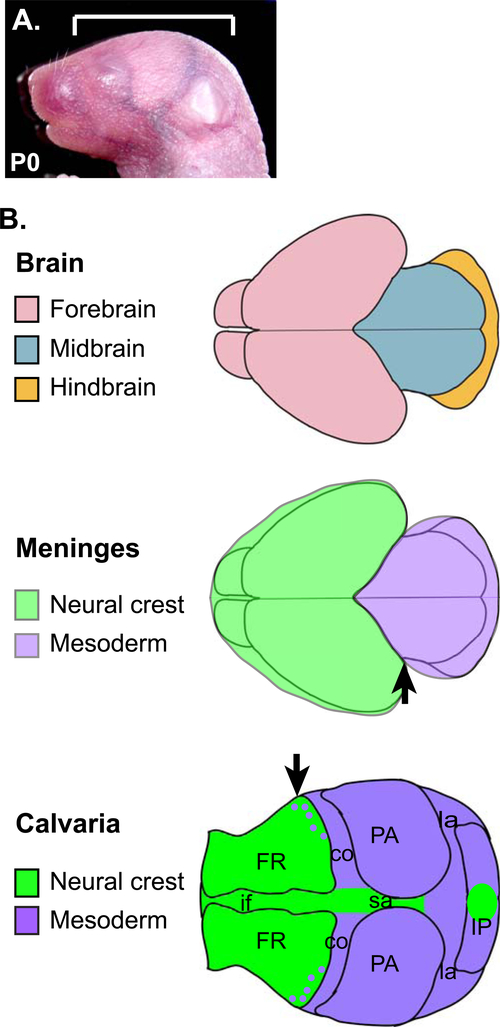
Early experiments on quail and chick chimeras showed that neural crest-derived cells, generated from the caudal forebrain and the midbrain levels, contributed to the meninges associated with the forebrain. In contrast, mesoderm-derived cells gave rise to the meninges of the midbrain and the hindbrain (Fig. 2) (Couly and Le Douarin, 1987; Couly et al., 1992; Le Douarin and Kalcheim 1999). However, in all areas of the meninges, the endothelial cells of the blood vessels were strictly of mesoderm origin (Le Douarin and Kalcheim 1999). Histological observations in human fetuses also suggested that the cranial meninges originated from both the neural crest (which is derived from the ectoderm) and the mesoderm. Furthermore, the prechordal plate and the paraxial mesoderm were named as the source of the mesodermal cells (O’Rahilly and Muller, 1986).
Figure 2. Contribution of the cells from the neural crest and the mesoderm to the meninges and the calvaria.
A) Lateral view of the head of a newborn mouse. The bracket indicates the area depicted in B. B) Dorsal views of the head showing the three components of the head. Note the difference in the rostro-caudal positions of the neural crest-mesoderm boundary in the meninges and in the calvaria (arrows). co: coronal suture, FR: frontal bone, if: interfrontal suture, IP: interparietal bone, la: lambdoidal suture, PA: parietal bone, sa: sagittal suture.
In mice, Cre-loxP technology and Cre-dependent reporter lines have enabled long-term lineage tracing of specific groups of cells (Soriano, 1999). Using this tool, several studies have examined the contribution of neural crest versus mesoderm cells to various tissues of the craniofacial area. The results in the meninges were consistent with those from the avian studies. Using Wnt1-Cre, which is active in the neural crest (Danielian et al., 1998), Jiang et al (Jiang et al., 2002) found neural crest-derived cells in all 3 layers of the meninges associated with the forebrain cerebral hemispheres, but not in the meninges covering the midbrain or the hindbrain. Another study (Yoshida et al., 2008) used mesoderm-specific Mesp1-Cre (Saga et al., 1999) in conjunction with Wnt1-Cre, to provide positive evidence that the meninges of the midbrain and the hindbrain were of mesoderm origin whereas the meninges of the forebrain were of neural crest origin (except for the endothelial cells).
3.3. Regulation of meningeal development
Molecular genetic regulation of meningeal development is poorly understood. Table 1 lists genes expressed in the developing meninges of mouse embryos during early stages (E10.5-E13.5) based on our literature search and Gene Expression Database query (C. M. Smith et al., 2014). Among these, a few genes with functional importance have been identified from studies of mice exhibiting meningeal defects. Other candidates for regulators of meningeal development would be the genes associated with cobblestone lissencephaly and Dandy-Walker malformation in humans (discussed below), and they are listed in Table 2 and Table 3.
Table 1.
Genes expressed in the developing meninges in mice at early stages (E10 - E13.5)
| Gene Symbol | Gene Name | References |
|---|---|---|
| Aldh1a2 | aldehyde dehydrogenase family 1, subfamily A2 | Siegenthaler et al., 2009 |
| Alx4 | aristaless-like homeobox 4 | Rice et al., 2003; Vivatbutsiri et al., 2008 |
| Ambp | alpha 1 microglobulin/bikunin precursor | Sanchez, Martinez, et al., 2002 |
| Ano1 | anoctamin 1, calcium activated chloride channel | Gritli-Linde et al., 2009 |
| Ano6 | anoctamin 6, calcium activated chloride channel | Gritli-Linde et al., 2009 |
| Apod | apolipoprotein D | Sanchez, Ganfornina, et al., 2002 |
| Bmp4 | bone morphogenetic protein 4 | Thompson et al., 2014 |
| Bmp7 | bone morphogenetic protein 7 | Vivatbutsiri et al., 2008 |
| Cxcl12 | chemokine (C-X-C motif) ligand 12 | D. Daniel et al., 2005; Borrell and Marin, 2006 |
| Cxcr2 | chemokine (C-X-C motif) receptor 2 | Luan et al., 2001 |
| Fli 1 | friend leukemia integration 1 | Hart et al., 2000 |
| Foxc1 | forkhead box C1 | Kume et al., 1998; Rice et al., 2003; Mishra et al., 2016 |
| Foxc2 | forkhead box C2 | Rice et al., 2003 |
| Foxd2 | forkhead box D2 | Wu et al., 1998 |
| Fras1 | fraser extracellular matrix complex subunit 1 | Makrygiannis et al., 2013 |
| Gja1 | gap junction protein, alpha 1 | Vivatbutsiri et al., 2008 |
| Grn | granulin | R. Daniel et al., 2003 |
| Hlf | hepatic leukemia factor | Hitzler et al., 1999 |
| Id1 | inhibitor of DNA binding 1 | Evans and O’Brien, 1993 |
| Lamc1 | laminin gamma 1 | Makrygiannis et al., 2013 |
| Lamc3 | laminin gamma 3 | Barak et al., 2011 |
| Msx1 | msh homeobox 1 | MacKenzie et al., 1992; Rice et al., 2003; Thompson et al., 2014 |
| Msx2 | msh homeobox 2 | MacKenzie et al., 1992 |
| Nedd4 | neural precursor cell expressed, developmentally down- regulated 4 | Liu et al., 2009 |
| Rbp1 | retinol binding protein 1, cellular | Ruberte et al., 1993 |
| Rdh10 | retinol dehydrogenase 10 | Romand et al., 2008 |
| Sorcs2 | sortilin-related VPS10 domain containing receptor 2 | Boggild et al., 2018 |
| Sparc | secreted acidic cysteine rich glycoprotein | Vincent et al., 2008 |
| Tgfb1 | transforming growth factor, beta 1 | Heine et al., 1987; Thompson et al., 2014 |
| Tgfbr2 | transforming growth factor, beta receptor 2 | Wang et al., 1995 |
| Vtn | vitronectin | Seiffert et al., 1995 |
| Wls | wntless WNT ligand secretion mediator | Yeung et al., 2014 |
| Zic1 | zinc finger protein of the cerebellum 1 | Thompson et al., 2014 |
| Zic2 | zinc finger protein of the cerebellum 2 | Thompson et al., 2014 |
| Zic3 | zinc finger protein of the cerebellum 2 | Thompson et al., 2014 |
Table 2.
Genes associated with cobblestone lissencephaly in humans
| Gene Symbol | Gene Name | References |
|---|---|---|
| LAMB1 | Laminin, beta 1 | Radmanesh et al., 2013 |
| TMTC3 | Transmembrane and tetratricopeptide repeat domains- containing protein 3 | Jerber et al., 2016 |
| ISPD | Isoprenoid synthase domain-containing protein | Vuillaumier-Barrot et al., 2012 |
| POMGNT2 | Protein o-mannose beta-1,4-n- acetylglucosaminyltransferase 2 | Manzini et al., 2012 |
| POMT1 | Protein o-mannosyltransferase 1 | Beltran-Valero de Bernabe et al., 2002 |
| RXYLT1 | Ribitol xylosyltransferase 1 | Vuillaumier-Barrot et al., 2012 |
| B3GALNT2 | Beta-1,3-n-acetylgalactosaminyltransferase 2 | Stevens et al., 2013 |
| B4GAT1 | Beta-1,4-glucuronyltransferase 1 | Buysse et al., 2013 |
Table 3.
Genes associated with Dandy-Walker malformation in humans
| Gene Symbol | Gene Name | References |
|---|---|---|
| ALG3 | Alg3, S.cerevisiae, homolog of | Sun et al., 2005 |
| AP1S2 | Adaptor-related protein complex 1, sigma 2 subunit | Cacciagli et al., 2014 |
| EBP | Emopamil-binding protein | Derry et al., 1999 |
| FOXC1 | Forkhead box c1 | Aldinger et al., 2009 |
| LAMC1 | Laminin, gamma 1 | Darbro et al., 2013 |
| LARGE1 | Acetylglucosaminyltransferase-like protein | van Reeuwijk et al., 2007 |
| NID1 | Nidogen 1 | Darbro et al., 2013 |
| NPHP3 | Nephrocystin 3 | Bergmann et al., 2008 |
| PIEZO2 | Piezo-type mechanosensitive ion channel component 2 | McMillin et al., 2014 |
| POMT1 | Protein o-mannosyltransferase 1 | Beltran-Valero de Bernabe et al., 2002 |
| SLC45A1 | Solute carrier family 45, member 1 | Anazi et al., 2017 |
| TMEM138 | Transmembrane protein 138 | Lee et al., 2012 |
| TMEM67 | Transmembrane protein 67 | U. M. Smith et al., 2006 |
| ZIC1 | Zic family, member 1 | Grinberg et al., 2004 |
| ZIC4 | Zic family, member 4 | Grinberg et al., 2004 |
Foxc1 (formerly known as Mf1), encoding a forkhead/winged helix transcription factor, is the most extensively studied gene in the context of meningeal development. Mouse Foxc1 is expressed throughout the primary meninx from its first appearance (Kume et al., 1998; Mishra et al., 2016), and continues to be expressed in all three layers of the meninges (Zarbalis et al., 2007). Foxc1 was first linked to the meninges through a spontaneous mutation in mice called congenital hydrocephalous (ch) (Gruneberg, 1943), which turned out to be a point mutation in Foxc1 creating a truncated protein (Kume et al., 1998). ch homozygote pups (Foxc1ch/ch) died at birth, suffering from an enlarged and hemorrhaging brain, loss of the calvaria, and other defects throughout the body. Severe meningeal defects were noted in Foxc1ch/ch mutants, and the hydrocephaly was attributed to the failure in development of the subarachnoid space (Green, 1970). Based on later histological examination and transmission electron microscopy, the defects were evident in Foxc1ch/ch mutants from E13.5, when the meningeal mesenchyme appeared abnormally compact. The three layers of the meninges initially differentiated on the baso-lateral side of the brain, but only the pia mater continued to develop. Expression of several genes were lost or reduced in the apical meningeal mesenchyme at E13.5-E14.5, and the arachnoid mater and the dura mater failed to form here (Vivatbutsiri et al., 2008).
A similar phenotype was found in a mutant with targeted null alleles of Foxc1 (Foxc1lacZ/lacZ), namely, lack of the arachnoid mater and the dura mater over the cerebral hemispheres (Siegenthaler et al., 2009). From E12.5, the apical side of the mutant forebrain was deficient in meningeal fibroblasts, though the pia mater seemed to be present judging from the staining for the laminin in the pial basement membrane (Hecht et al., 2010). The pial basement membrane was eventually disintegrated in the mutants at late fetal stages, but it was thought to be a secondary consequence of the early meningeal defects (Hecht et al., 2010). In the hindbrain area of the Foxe1lacZ/lacZ mutant, the prospective arachnoid mater was thin and dense, and the prospective dura mater was disorganized at E12.5-E13.5 (Kume et al., 1998). An additional insight into the role of Foxc1 was provided by a hypomorphic mutation in mice named hole in the head (hith), recovered from a chemical mutagenesis screen (Zarbalis et al., 2007). Foxclhith/hith mutants showed a highly localized meningeal deficiency at the apex of the head at E18.5, together with defects in the skull and the brain at this location. The fact that the meninges were preferentially affected on the apical side suggested that Foxc1 was particularly important for the apical progression of meningeal differentiation. Combined, the findings from various Foxc1 mutants have established that this gene is a crucial, intrinsic regulator of early development of the meninges. However, the molecular and cellular mechanism of its function in the process remains unknown.
Zic family genes encode zinc finger domain transcription factors important for diverse events of embryonic development (Diamand et al., 2018). In mice, Zic1, Zic2, and Zic3 are expressed in the meninges (Inoue et al., 2008), and Zic1/3 double mutation caused a decrease in proliferation of the meningeal fibroblasts at E15.5. Expression of several genes was also reduced in the mutant meninges including Lamc1 (encoding laminin subunit gamma 1), and the pial basement membrane was disrupted by E17.5 (Inoue et al., 2008). Interestingly, Foxc1 was down-regulated in the meninges of Zic1/3 mutants, and thus Foxc1 may be a downstream mediator of ZIC function in the meningeal development. However, it is unknown whether Zic1/3 mutants have early meningeal defects as Foxc1 mutants do. Twist1 (twist basic helix-loop-helix transcription factor 1) is another transcription factor gene implicated in meningeal development. It is expressed broadly in the head mesenchyme including the meningeal layer (Tischfield et al. 2017). Deletion of Twist1 in the meninges led to hypoplasia of the dura mater and the arachnoid mater, and compaction of the subarachnoid space (Tischfield et al. 2017).
Among intercellular signaling pathways, there is direct evidence that transforming growth factor β (TGFβ) signaling is required for normal development of the meninges. Tgfbr2, which encodes a receptor component, is expressed in the meningeal mesenchyme from early stages (Wang et al., 1995). In mouse embryos with neural crest-specific deletion of Tgfbr2, the forebrain meninges failed to develop; at E14.5, it was replaced with only a single layer of cells that did not proliferate (Ito et al., 2003). In addition, wingless-integrated (WNT)/β-catenin signaling and retinoic acid (RA) signaling were shown to influence meningeal development. Constitutive activation of WNT/β-catenin pathway in the head ectoderm induced the expression of Wnt6, which in turn activated WNT/β-catenin pathway in the neural crest-derived meninges over the forebrain. This led to increased cell proliferation and expansion of the meningeal layer at E14.5 (Choe et al., 2012). In a later study, β-catenin was directly activated or removed in the neural crest-derived cells, and the results suggested that WNT/β-catenin signaling promoted the general expansion of the head mesenchyme cells rather than regulating the specification of the meningeal cells (Choe, Zarbalis, et al., 2014). Interestingly, removing β-catenin in the non-meningeal head mesenchyme also caused hypoplasia of the meninges on the baso-lateral side of the brain, indicating that WNT/β-catenin pathway uses a non-cell autonomous mechanism here (DiNuoscio and Atit, 2019). Contrary to the phenotype from excess WNT, treating mouse embryos with exogenous RA at E10 led to thin and discontinuous meninges (Jiang et al., 2002). However, removing endogenous RA from ~E11.5 did not significantly affect the meninges (Haushalter et al., 2017). Therefore, RA signaling may be dispensable for normal development of the meninges although its over-activation is deleterious.
Disruption of the pial basement membrane has been reported in mouse mutants of several genes for ECM or cell adhesion complex components, including Apbb1 and Apbb2 (amyloid beta (A4) precursor protein binding, family B, member 1 and member 2) (Guenette et al., 2006), Col4a1 (collagen type IV alpha 1) (Labelle-Dumais et al., 2011), Lama1 (laminin alpha 1) (Ichikawa- Tomikawa et al., 2012), Lamc1 (laminin gamma 1) (Halfter et al., 2002), and Ptk2 (protein tyrosine kinase 2, previously called focal adhesion kinase or FAK) (Beggs et al., 2003) (reviewed in Siegenthaler and Pleasure, 2011). However, these genes are likely required for maintaining the structural integrity of the meninges and the basement membrane, rather than regulating development of the meninges.
4. Influence of the meninges on calvarial development
The mammalian calvaria comprises five pieces of bone, namely, a pair of frontal bones, a pair of parietal bones, and a piece of interparietal bone, joined by soft connective tissue of the sutures (Fig. 2) (Ferguson and Atit, 2018; Ishii et al., 2015). Like the meninges, the calvaria develops from the embryonic head mesenchyme surrounding the brain, and thus its progenitors are presumably included in the primary meninx described earlier. The calvaria is also of dual origin (Fig. 2) (Jiang et al., 2002; Yoshida et al., 2008): the frontal bone is mostly made of neural crest-derived cells with a minor contribution from mesodermal cells, while the parietal bone is entirely made of mesodermal cells. The interparietal bone contains both groups of cells. At the end of development, the calvaria and the meninges are almost continuous in that the outer layer of the dura mater makes the periosteum on the inner surface of the calvarial bone.
Since the calvaria and the meninges form in close apposition, it is expected that there would be extensive regulatory interactions between the two structures during development (Fig. 3). This topic was first studied through surgical manipulations (removals and transplantations) of the meninges in late fetal, neonatal, or young postnatal rats and rabbits (reviewed in Grova et al., 2012; Lenton et al., 2005). In these experiments, the dura mater was found necessary and sufficient for re-ossification after the calvarial bone was removed or injured (Hobar et al., 1996; Mabbutt and Kokich, 1979), which indicated that the dura mater was a source of osteogenic signals. On the other hand, the dura mater from underneath a normally patent suture was necessary and sufficient to maintain the patency (Levine et al., 1998; Opperman et al., 1993). A similar result was obtained in mouse embryos in late gestation (E16.5) (Kim et al., 1998). Various secreted molecules, including members of TGFβ, FGF (fibroblast growth factor), and BMP (bone morphogenetic protein) family, were expressed in the dura mater, and thought to mediate the interaction with the calvaria (Kim et al., 1998; Lenton et al., 2005; Levi et al., 2012; Spector et al., 2002; Warren et al., 2003). Together, these studies have shown that the dura mater has an instructive role toward calvarial patterning and morphogenesis late in development.
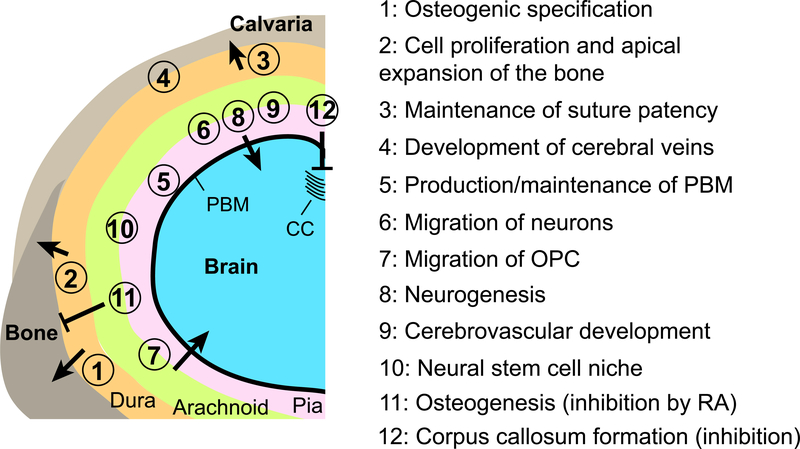
Figure 3. Influences of the meninges to development of the calvaria and the brain.
A schematic representation of a coronal section of the head of mouse embryos at late gestation stages (>E12.5). The left-dorsal quadrant of the head is shown. The positions of the circled numbers indicate the meningeal component thought to be responsible for each interaction. For the numbers straddling the arachnoid mater and the pia mater, there is not enough evidence to assign the function to only one layer or the other, and both layers are likely involved. CC: corpus callosum, OPC: oligodendrocyte precursor cells, PBM: pial basement membrane, RA: retinoic acid.
In comparison to the late stages, interactions between the meninges and the calvaria during early development have received much less attention. The current evidence for such interaction is mostly based on correlation of normal developmental events or mutant phenotypes between the two structures. Around stage 17 (6th week of gestation) in humans and ~E13 in mice, the dural limiting layer appears on the baso-lateral side of the brain, marking the differentiation of the meninges into pachymeninx and leptomeninx (O’Rahilly and Muller, 1986; Vivatbutsiri et al., 2008). Subsequently, the meningeal differentiation progresses toward the apex. Interestingly, the frontal bone and the parietal bone of the calvaria also arise from the mesenchyme on the baso-lateral side of the brain (called supra-orbital mesenchyme for its location above the eyes) at ~E12.5 in mice, and undergo apical expansion over the following days (Fig. 3) (Deckelbaum et al., 2012; Ferguson et al., 2018; Ishii et al., 2015; Twigg and Wilkie, 2015). Furthermore, the growth of the skull bone correlated closely with the extent of the dural limiting layer during the expansion (O’Rahilly and Muller, 1986). This observation raised an intriguing possibility that the nascent dura mater guides calvarial bone growth.
More support for the above idea came from Foxc1 mutant mice. As described earlier, Foxc1ch/ch and Foxc1lacZ/lacZ mutants had severe meningeal defects, in which the arachnoid mater and the dura mater failed to form on the apical side of the brain. In addition, the calvarial bone was missing from the apical side at birth (Kume et al., 1998; Rice et al., 2003). In these mutants, the calvarial bone development was arrested at E13.5, shortly after initiation of osteogenesis in the supraorbital mesenchyme (Rice et al., 2003; Vivatbutsiri et al., 2008). The bone rudiments showed decreased cell proliferation and apical growth (Machida et al., 2014; Rice et al., 2003). Importantly, during normal development, Foxc1 is not expressed in the bone rudiments at this age, but instead it is strongly expressed in the underlying meninges (Rice et al., 2003). Moreover, the onset of the calvarial defect in Foxc1 mutants was preceded by the meningeal defect (E12.5 according to Hecht et al., 2010). These data indicated that Foxc1 regulated the early growth of the calvarial bone indirectly, through an interaction between the meninges and the calvaria. The molecular identity of this interaction is still unknown.
Similar to Foxc1 mutants, neural crest-specific Tgfbr2 knockout mutants had severe meningeal defects (discussed above), which were accompanied by calvarial defects (Ito et al., 2003). Both the frontal bone and the parietal bone failed to develop in these mutants. Because the parietal bone is of mesoderm origin, this phenotype indicated that Tgfbr2 regulated development of the parietal bone in a cell non-autonomous manner. During normal development, the parietal bone is in contact with the neural crest-derived meninges covering the cerebral hemispheres (Fig. 2). Therefore, the parietal bone defect in neural crest-specific Tgfbr2 mutants was attributed to the loss of the underlying meninges, which implied that signals from the meninges were essential to development of the parietal bone (Ito et al., 2003).
In the aforementioned experiment where RA treatment of mouse embryos led to meningeal defects, the embryos exhibited a partial to complete loss of the parietal bone and the interparietal bone at E17.5 (Jiang et al., 2002). Because the degree of the calvarial defects correlated with the severity of the meningeal defects in different animals, the calvarial phenotype was interpreted as a consequence of the loss of the meninges (Jiang et al., 2002). However, a recent study has suggested a more direct effect of RA on calvarial development (Ferguson et al., 2018). They found that RA treatment up-regulated expression of anti-osteogenic genes HoxA1 (homeobox A1), HoxC8 (homeobox C8), and Hand2 (heart and neural crest derivatives expressed 2) in the calvarial mesenchyme to inhibit osteogenic specification. Notably, the meninges produce RA from ~E12.5 (Siegenthaler et al., 2009), which plays an important role in brain development (discussed below). Therefore, the adverse effect of RA on calvarial osteogenesis would suggest that a mechanism to curb RA signaling is crucial to normal development of the calvaria. Indeed, calvarial mesenchyme expresses an RA-degrading enzyme Cyp26b1 (cytochrome P450, family 26, subfamily b, polypeptide 1) at E14.5 (Visel et al., 2004), and inactivation of this gene resulted in severe hypoplasia of the calvarial bone (Maclean et al., 2009).
5. Influence of the meninges on brain development
The meninges play multiple crucial roles in development of the brain, and the list continues to grow (Fig. 3).
First, the meninges are thought to provide trophic factors necessary to the survival of the cells in the brain. When the neural crest was ablated in early chick embryos to preclude development of the forebrain meninges, the forebrain neuroepithelium experienced massive apoptosis and subsequent degeneration (Etchevers et al., 1999). However, it is unknown which factor(s) from the meninges mediate this function.
Second, the meninges regulate the migration and positioning of neurons by secreting molecules that can attract or repel cells. The meninges express a chemoattractant CXCL12 (chemokine (C-X-C motif) ligand 12, also known as SDF-1), through direct transcriptional activation by FOXC1 (Borrell and Marin, 2006; Zarbalis et al., 2012). Thus, neurons and neural progenitors expressing the receptors, CXCR4 and CXCR7, are guided to and retained in the marginal zone of the brain immediately underneath the meninges. Examples of the cells subject to this control include Cajal-Retzius cells and interneurons of the cerebral cortex, neural progenitors in the dentate gyrus of the forebrain, and neural progenitors in the cerebellum (Bagri et al., 2002; Borrell and Marin, 2006; Klein et al., 2001; Li et al., 2009; Ma et al., 1998; Paredes et al., 2006; Stumm et al., 2003; Zhu et al., 2002). On the other hand, BMP4, BMP7, and TGFβ1 from the meninges repel oligodendocyte precursor cells from the ventral forebrain to direct them into the cerebral cortex (Choe, Huynh, et al., 2014). RA signaling also regulates the migration of cortical neurons. Upon deletion of an RA-synthesizing enzyme expressed in the meninges, altered migration of the neurons led to abnormal layering in the cerebral cortex (Haushalter et al., 2017).
Third, the pial basement membrane plays a structural role to control the migration and positioning of neurons (Decimo et al., 2012; Siegenthaler and Pleasure, 2011). During normal development of the brain, a special population of cells called radial glia send out processes from the ventricular zone toward the surface of the brain (Chou et al., 2018). These processes serve as a scaffold for radial migration of the neurons. The endfeet of the radial glial processes attach to the pial basement membrane to obtain physical stability, and the migrating neurons are stopped in the marginal zone by the pial basement membrane. In mouse mutants with defects in the pial basement membrane (discussed above), the radial endfeet became detached prematurely, and the distribution of the neurons became abnormal in the cerebral cortex and the cerebellum (Beggs et al., 2003; Guenette et al., 2006; Halfter et al., 2002; Ichikawa-Tomikawa et al., 2012; Inoue et al., 2008; Labelle-Dumais et al., 2011; Zarbalis et al., 2007).
Fourth, the meninges regulate generation of neurons from neural progenitors (neurogenesis), which occurs through asymmetric cell divisions of the progenitors. This function has been elucidated mainly from the studies of Foxc1 mutants lacking most of the meninges (Siegenthaler et al., 2009). In the cerebral cortex of E14.5 Foxc1lacZ/lacZ mutants, symmetric divisions increased and asymmetric divisions decreased compared with control animals. As a consequence, the cerebral cortex became elongated but contained fewer differentiated neurons than normal. Importantly, the cortical phenotype was partially rescued by in utero RA treatment at E10.5-E13.5, while the meningeal defects were unchanged. This result led to a model that the meninges-derived RA is important for cortical neurogenesis (Siegenthaler et al., 2009). The rescue of Foxc1 cortical defects clearly indicates that RA is sufficient to promote neurogenesis in the absence of the meninges. Whether the meningeal RA is necessary for cortical neurogenesis during normal development, in the presence of the meninges, has been debated (Chatzi et al., 2013; Haushalter et al., 2017). In addition to the cerebral cortex, the meninges regulate the neurogenesis in the embryonic dentate gyrus. Here, BMP7 from the meninges has been identified as a key factor (Choe et al., 2013). For the cerebellum, CXCL12 from the meninges suppresses neuronal differentiation while promoting proliferation (Haldipur et al., 2014).
Fifth, the meninges regulate development of the blood vessels of the brain. In Foxc1ch/ch and Foxc1lacZ/lacZ mutants, blood vessels in the cerebral cortex were increased in diameter but decreased in density at E14.5 (Mishra et al., 2016). Once again, this phenotype was attributed to the meninges deficiency and the consequent loss of RA. Consistently, inactivation of an RA synthesis enzyme caused similar cerebrovascular defects as Foxc1 mutation (Bonney et al., 2016). In this context, RA signaling had dual effects on the activity of WNT/β-catenin pathway in the endothelial cells, to allow normal growth of the vasculature while preventing inappropriate growth (Bonney et al., 2016). A recent study investigated development of the cerebral veins specifically. They found that TWIST1-controlled production of BMP2 and BMP4 from the endosteal dura mater and the calvarial bone were important for the growth and remodeling of the cerebral veins (Tischfield et al., 2017).
Sixth, the meninges affect formation of the corpus callosum, a thick bundle of axons in the dorsal midline of the brain connecting the two cerebral hemispheres. BMP7 produced by the meninges inhibited outgrowth of the callosal axons, and WNT3 from the neurons antagonized the effect of BMP7 to allow development of the corpus callosum (Choe et al., 2012).
Finally, the meninges form a niche for neural stem cells (reviewed in Decimo et al., 2012). A group of cells expressing Nestin, a marker of stem/progenitor cells, were found in the leptomeninges of rats from a late fetal stage to adulthood (Bifari et al., 2009; Bifari et al., 2015). A lineage-tracing experiment confirmed that they were indeed stem cells for cortical neurons (Bifari et al., 2017). The role of the meninges as a stem cell niche is thought to be related to the presence of fractones, which are specialized ECM structures enriched with growth factors (Bifari et al., 2015).
As expected from the profound influence of the meninges on brain development, defects in the meninges have been linked to neurodevelopmental disorders in humans. Cobblestone lissencephaly is a condition where the surface of the cerebral cortex lacks the normal ridges and grooves but has small bumps (Devisme et al., 2012; Verrotti et al., 2010). This phenotype occurs when the integrity of the pial basement membrane is compromised, allowing over-migration of the neurons into the meningeal layers. Mutations underlying cobblestone lissencephaly have mostly been identified in the genes associated with ECM (Table 2) (Devisme et al., 2012; Verrotti et al., 2010). Dandy-Walker malformation is characterized by hypoplasia of the cerebellum and hydrocephaly (accumulation of CSF inside the brain) (Imataka et al., 2007), and FOXC1 is one of the genes associated with this condition in humans (Table 3) (Aldinger et al., 2009). Because studies in mice have shown that Foxc1 is expressed in the meninges but not in the brain (Aldinger et al., 2009; Kume et al., 1998), Dandy-Walker malformation is thought to result from meningeal defects. Although the meningeal phenotype is unknown for most of the human patients, imaging studies on those with FOXC1 mutations have found evidence of meningeal deficiency (Aldinger et al., 2009).
6. Conclusion
In this review, we have discussed the current understanding of the process of meningeal development, and the role of the meninges in regulating development of the neighboring structures. The pervasive defects in mouse Foxc1 mutants have clearly illustrated the critical role of the meninges in development of the head as a whole. The presence of the meninges is essential to normal development of the calvaria and the brain. At the same time, there are examples where the meninges have adverse effects (inhibition of calvarial osteogenesis by RA, and inhibition of corpus callosum development by BMP7), which need to be antagonized. Thus, the interactions with the meninges should be closely considered when the molecular regulation of calvarial development or brain development is studied.
This review has also highlighted numerous questions that are yet to be answered regarding meningeal development. How do the head mesenchyme cells of the primary meninx become specified into the meningeal, calvarial, or dermal fate? Among the cells to become the meninges, how are they divided into the components of the dura mater, the arachnoid mater, or the pia mater? Do the meninges regulate similar aspects of calvarial development as they do for the brain, including cell migration, vascularization, and differentiation from progenitor cells? What factor(s) mediate the influence of the meninges on calvarial development? Are the interactions between the meninges and the surrounding tissues reciprocal - in other words, does the calvaria or the brain influence development of the meninges? To address the issue of specification and differentiation of meningeal cells, it would be important to identify distinct populations of cells from the primary meninx and follow their segregation in terms of the molecular characteristics over the course of development. This can be achieved by single cell RNA sequencing and clustering (Griffiths et al., 2018). Elucidating the signaling interactions between the meninges and the calvaria would be facilitated by tools to dissect tissue-specific roles of a gene, such as mouse lines expressing Cre recombinase in one tissue but not the other. To our knowledge, there is no published Cre line fitting this description. Applying these and other modern investigative techniques will greatly improve our knowledge of meningeal development, which could also advance our understanding of calvarial development and brain development.
Acknowledgements
Work in Jeong laboratory is funded by NIH/NIDCR (R01 DE026798).
Funding
National Institute of Dental and Craniofacial Research (R01 DE026798 to JJ)
References
- Adeeb N, Mortazavi MM, Tubbs RS, & Cohen-Gadol AA (2012). The cranial dura mater: a review of its history, embryology, and anatomy. Childs Nerv Syst, 28(6), 827–837. doi: 10.1007/s00381-012-1744-6 [DOI] [PubMed] [Google Scholar]
- Aldinger KA, Lehmann OJ, Hudgins L, Chizhikov VV, Bassuk AG, Ades LC, … Millen KJ (2009). FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformation. Nature Genetics, 41(9), 1037–U1116. doi: 10.1038/ng.422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anazi S, Maddirevula S, Faqeih E, Alsedairy H, Alzahrani F, Shamseldin HE, … Alkuraya FS (2017). Clinical genomics expands the morbid genome of intellectual disability and offers a high diagnostic yield. Mol Psychiatry, 22(4), 615–624. doi: 10.1038/mp.2016.113 [DOI] [PubMed] [Google Scholar]
- Angelov DN, & Vasilev VA (1989). Morphogenesis of rat cranial meninges. A light- and electron-microscopic study. Cell Tissue Res, 257(1), 207–216. [DOI] [PubMed] [Google Scholar]
- Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T, … Alitalo K (2017). Development and plasticity of meningeal lymphatic vessels. Journal of Experimental Medicine, 214(12), 3645–3667. doi: 10.1084/jem.20170391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, … Alitalo K (2015). A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. Journal of Experimental Medicine, 212(7), 991–999. doi: 10.1084/jem.20142290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, & Pleasure SJ (2002). The chemokine SDF1 regulates migration of dentate granule cells. Development, 129(18), 4249–4260. [DOI] [PubMed] [Google Scholar]
- Barak T, Kwan KY, Louvi A, Demirbilek V, Saygi S, Tuysuz B, … Gunel M (2011). Recessive LAMC3 mutations cause malformations of occipital cortical development. Nat Genet, 43(6), 590–594. doi: 10.1038/ng.836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, … Reichardt LF (2003). FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron, 40(3), 501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Valero de Bernabe D, Currier S, Steinbrecher A, Celli J, van Beusekom E, van der Zwaag B, … Brunner HG (2002). Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet, 71(5), 1033–1043. doi: 10.1086/342975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann C, Fliegauf M, Bruchle NO, Frank V, Olbrich H, Kirschner J, … Omran H (2008). Loss of nephrocystin-3 function can cause embryonic lethality, meckel-gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. American Journal of Human Genetics, 82(4), 959–970. doi: 10.1016/j.ajhg.2008.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifari F, Berton V, Pino A, Kusalo M, Malpeli G, Di Chio M, … Decimo I (2015). Meninges harbor cells expressing neural precursor markers during development and adulthood. Front Cell Neurosci, 9, 383. doi: 10.3389/fncel.2015.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifari F, Decimo I, Chiamulera C, Bersan E, Malpeli G, Johansson J, … Krampera M (2009). Novel stem/progenitor cells with neuronal differentiation potential reside in the leptomeningeal niche. J Cell Mol Med, 13(9B), 3195–3208. doi: 10.1111/j.1582-4934.2009.00706.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifari F, Decimo I, Pino A, Llorens-Bobadilla E, Zhao S, Lange C, … Carmeliet P (2017). Neurogenic Radial Glia-like Cells in Meninges Migrate and Differentiate into Functionally Integrated Neurons in the Neonatal Cortex. Cell Stem Cell, 20(3), 360–+. doi: 10.1016/j.stem.2016.10.020 [DOI] [PubMed] [Google Scholar]
- Boggild S, Molgaard S, Glerup S, & Nyengaard JR (2018). Highly segregated localization of the functionally related vps10p receptors sortilin and SorCS2 during neurodevelopment. J Comp Neurol, 526(8), 1267–1286. doi: 10.1002/cne.24403 [DOI] [PubMed] [Google Scholar]
- Bonney S, Harrison-Uy S, Mishra S, MacPherson AM, Choe Y, Li D, … Siegenthaler JA (2016). Diverse Functions of Retinoic Acid in Brain Vascular Development. J Neurosci, 36(29), 7786–7801. doi: 10.1523/JNEUROSCI.3952-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell V, & Marin O (2006). Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci, 9(10), 1284–1293. doi: 10.1038/nn1764 [DOI] [PubMed] [Google Scholar]
- Buysse K, Riemersma M, Powell G, van Reeuwijk J, Chitayat D, Roscioli T, … van Bokhoven H (2013). Missense mutations in beta-1,3-N-acetylglucosaminyltransferase 1 (B3GNT1) cause Walker-Warburg syndrome. Hum Mol Genet, 22(9), 1746–1754. doi: 10.1093/hmg/ddt021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciagli P, Desvignes JP, Girard N, Delepine M, Zelenika D, Lathrop M, … Villard L (2014). AP1S2 is mutated in X-linked Dandy-Walker malformation with intellectual disability, basal ganglia disease and seizures (Pettigrew syndrome). European Journal of Human Genetics, 22(3), 363–368. doi: 10.1038/ejhg.2013.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzi C, Cunningham TJ, & Duester G (2013). Investigation of retinoic acid function during embryonic brain development using retinaldehyde-rescued Rdh10 knockout mice. Developmental Dynamics, 242(9), 1056–1065. doi: 10.1002/dvdy.23999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe Y, Huynh T, & Pleasure SJ (2014). Migration of Oligodendrocyte Progenitor Cells Is Controlled by Transforming Growth Factor beta Family Proteins during Corticogenesis. Journal of Neuroscience, 34(45), 14973–14983. doi: 10.1523/Jneurosci.1156-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe Y, Kozlova A, Graf D, & Pleasure SJ (2013). Bone Morphogenic Protein Signaling Is a Major Determinant of Dentate Development. Journal of Neuroscience, 33(16), 6766–6775. doi: 10.1523/Jneurosci.0128-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe Y, Siegenthaler JA, & Pleasure SJ (2012). A cascade of morphogenic signaling initiated by the meninges controls corpus callosum formation. Neuron, 73(4), 698–712. doi: 10.1016/j.neuron.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe Y, Zarbalis KS, & Pleasure SJ (2014). Neural Crest-Derived Mesenchymal Cells Require Wnt Signaling for Their Development and Drive Invagination of the Telencephalic Midline. Plos One, 9(2). doi:ARTNe8602510.1371/journal.pone.0086025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou FS, Li R, & Wang PS (2018). Molecular components and polarity of radial glial cells during cerebral cortex development. Cell Mol Life Sci, 75(6), 1027–1041. doi: 10.1007/s00018-017-2680-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, & Le Douarin NM (1992). The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development, 114(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Couly GF, & Le Douarin NM (1987). Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev Biol, 120(1), 198–214. [DOI] [PubMed] [Google Scholar]
- Daniel D, Rossel M, Seki T, & Konig N (2005). Stromal cell-derived factor-1 (SDF-1) expression in embryonic mouse cerebral cortex starts in the intermediate zone close to the pallial-subpallial boundary and extends progressively towards the cortical hem. Gene Expr Patterns, 5(3), 317–322. doi: 10.1016/j.modgep.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Daniel R, Daniels E, He ZH, & Bateman A (2003). Progranulin (acrogranin/PC cell-derived growth factor/granulin-epithelin precursor) is expressed in the placenta, epidermis, microvasculature, and brain during murine development. Developmental Dynamics, 227(4), 593–599. doi: 10.1002/dvdy.10341 [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, & McMahon AP (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol, 8(24), 1323–1326. [DOI] [PubMed] [Google Scholar]
- Darbro BW, Mahajan VB, Gakhar L, Skeie JM, Campbell E, Wu S, … Bassuk AG (2013). Mutations in extracellular matrix genes NID1 and LAMC1 cause autosomal dominant Dandy-Walker malformation and occipital cephaloceles. Hum Mutat, 34(8), 1075–1079. doi: 10.1002/humu.22351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decimo I, Fumagalli G, Berton V, Krampera M, & Bifari F (2012). Meninges: from protective membrane to stem cell niche. Am J Stem Cells, 1(2), 92–105. [PMC free article] [PubMed] [Google Scholar]
- Deckelbaum RA, Holmes G, Zhao ZC, Tong CX, Basilico C, & Loomis CA (2012). Regulation of cranial morphogenesis and cell fate at the neural crest-mesoderm boundary by engrailed 1. Development, 139(7), 1346–1358. doi: 10.1242/dev.076729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry JM, Gormally E, Means GD, Zhao W, Meindl A, Kelley RI, … Herman GE (1999). Mutations in a delta 8-delta 7 sterol isomerase in the tattered mouse and X-linked dominant chondrodysplasia punctata. jderry@immunex.com. Nat Genet, 22(3), 286–290. doi: 10.1038/10350 [DOI] [PubMed] [Google Scholar]
- Devisme L, Bouchet C, Gonzales M, Alanio E, Bazin A, Bessieres B, … Encha-Razavi F (2012). Cobblestone lissencephaly: neuropathological subtypes and correlations with genes of dystroglycanopathies. Brain, 135(Pt 2), 469–482. doi: 10.1093/brain/awr357 [DOI] [PubMed] [Google Scholar]
- Diamand KEM, Barratt KS, & Arkell RM (2018). Overview of Rodent Zic Genes. Adv Exp Med Biol, 1046, 179–207. doi: 10.1007/978-981-10-7311-3_10 [DOI] [PubMed] [Google Scholar]
- DiNuoscio G, & Atit RP (2019). Wnt/beta-catenin signaling in the mouse embryonic cranial mesenchyme is required to sustain the emerging differentiated meningeal layers. Genesis, 57(1), e23279. doi: 10.1002/dvg.23279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchevers HC, Couly G, Vincent C, & Le Douarin NM (1999). Anterior cephalic neural crest is required for forebrain viability. Development, 126(16), 3533–3543. [DOI] [PubMed] [Google Scholar]
- Evans SM, & O’Brien TX (1993). Expression of the helix-loop-helix factor Id during mouse embryonic development. Dev Biol, 159(2), 485–499. doi: 10.1006/dbio.1993.1258 [DOI] [PubMed] [Google Scholar]
- Ferguson JW, & Atit RP (2018). A tale of two cities: The genetic mechanisms governing calvarial bone development. Genesis, e23246. doi: 10.1002/dvg.23248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JW, Devarajan M, & Atit RP (2018). Stage-specific roles of Ezh2 and Retinoic acid signaling ensure calvarial bone lineage commitment. Dev Biol, 443(2), 173–187. doi: 10.1016/j.ydbio.2018.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagan JR, Tholpady SS, & Ogle RC (2007). Cellular dynamics and tissue interactions of the dura mater during head development. Birth Defects Res C Embryo Today, 81(4), 297–304. doi: 10.1002/bdrc.20104 [DOI] [PubMed] [Google Scholar]
- Green MC (1970). The developmental effects of congenital hydrocephalus (ch) in the mouse. Dev Biol, 23(4), 585–608. [DOI] [PubMed] [Google Scholar]
- Griffiths JA, Scialdone A, & Marioni JC (2018). Using single-cell genomics to understand developmental processes and cell fate decisions. Molecular Systems Biology, 14(4). doi:ARTNe804610.15252/msb.20178046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg I, Northrup H, Ardinger H, Prasad C, Dobyns WB, & Millen KJ (2004). Heterozygous deletion of the linked genes ZIC1 and ZIC4 is involved in Dandy-Walker malformation. Nature Genetics, 36(10), 1053–1055. doi: 10.1038/ng1420 [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A, Vaziri Sani F, Rock JR, Hallberg K, Iribarne D, Harfe BD, & Linde A (2009). Expression patterns of the Tmem16 gene family during cephalic development in the mouse. Gene Expr Patterns, 9(3), 178–191. doi: 10.1016/j.gep.2008.11.002 [DOI] [PubMed] [Google Scholar]
- Grova M, Lo DD, Montoro D, Hyun JS, Chung MT, Wan DC, & Longaker MT (2012). Models of cranial suture biology. J Craniofac Surg, 23(7 Suppl 1), 1954–1958. doi: 10.1097/SCS.0b013e318258ba53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneberg H (1943). The development of some external features in mouse embryos. Journal of Heredity, 34(3), 89–+. doi:DOI 10.1093/oxfordjournals.jhered.a105255 [DOI] [Google Scholar]
- Guenette S, Chang Y, Hiesberger T, Richardson JA, Eckman CB, Eckman EA, … Herz J (2006). Essential roles for the FE65 amyloid precursor protein-interacting proteins in brain development. EMBO J, 25(2), 420–431. doi: 10.1038/sj.emboj.7600926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldipur P, Gillies GS, Janson OK, Chizhikov VV, Mithal DS, Miller RJ, & Millen KJ (2014). Foxc1 dependent mesenchymal signalling drives embryonic cerebellar growth. Elife, 3. doi: 10.7554/eLife.03962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfter W, Dong S, Yip YP, Willem M, & Mayer U (2002). A critical function of the pial basement membrane in cortical histogenesis. J Neurosci, 22(14), 6029–6040. doi:20026580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A, Melet F, Grossfeld P, Chien K, Jones C, Tunnacliffe A, … Bernstein A (2000). Fli-1 is required for murine vascular and megakaryocytic development and is hemizygously deleted in patients with thrombocytopenia. Immunity, 13(2), 167–177. doi:Doi 10.1016/S1074-7613(00)00017-0 [DOI] [PubMed] [Google Scholar]
- Haushalter C, Schuhbaur B, Dolle P, & Rhinn M (2017). Meningeal retinoic acid contributes to neocortical lamination and radial migration during mouse brain development. Biol Open, 6(2), 148–160. doi: 10.1242/bio.021063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht JH, Siegenthaler JA, Patterson KP, & Pleasure SJ (2010). Primary cellular meningeal defects cause neocortical dysplasia and dyslamination. Ann Neurol, 68(4), 454–464. doi: 10.1002/ana.22103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine U, Munoz EF, Flanders KC, Ellingsworth LR, Lam HY, Thompson NL, … Sporn MB (1987). Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol, 105(6 Pt 2), 2861–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitzler JK, Soares HD, Drolet DW, Inaba T, O’Connel S, Rosenfeld MG, … Look AT (1999). Expression patterns of the hepatic leukemia factor gene in the nervous system of developing and adult mice. Brain Res, 820(1–2), 1–11. [DOI] [PubMed] [Google Scholar]
- Hobar PC, Masson JA, Wilson R, & Zerwekh J (1996). The importance of the dura in craniofacial surgery. Plast Reconstr Surg, 98(2), 217–225. [DOI] [PubMed] [Google Scholar]
- Ichikawa-Tomikawa N, Ogawa J, Douet V, Xu Z, Kamikubo Y, Sakurai T, … Arikawa-Hirasawa E (2012). Laminin alpha1 is essential for mouse cerebellar development. Matrix Biol, 31(1), 17–28. doi: 10.1016/j.matbio.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imataka G, Yamanouchi H, & Arisaka O (2007). Dandy-Walker syndrome and chromosomal abnormalities. Congenit Anom (Kyoto), 47(4), 113–118. doi: 10.1111/j.1741-4520.2007.00158.x [DOI] [PubMed] [Google Scholar]
- Inoue T, Ogawa M, Mikoshiba K, & Aruga J (2008). Zic deficiency in the cortical marginal zone and meninges results in cortical lamination defects resembling those in type II lissencephaly. J Neurosci, 28(18), 4712–4725. doi: 10.1523/JNEUROSCI.5735-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Sun J, Ting MC, & Maxson RE (2015). The Development of the Calvarial Bones and Sutures and the Pathophysiology of Craniosynostosis. Curr Top Dev Biol, 115, 131–156. doi: 10.1016/bs.ctdb.2015.07.004 [DOI] [PubMed] [Google Scholar]
- Ito Y, Yeo JY, Chytil A, Han J, Bringas P Jr., Nakajima A, … Chai Y (2003). Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development, 130(21), 5269–5280. doi: 10.1242/dev.00708 [DOI] [PubMed] [Google Scholar]
- Jerber J, Zaki MS, Al-Aama JY, Rosti RO, Ben-Omran T, Dikoglu E, … Gleeson JG (2016). Biallelic Mutations in TMTC3, Encoding a Transmembrane and TPR-Containing Protein, Lead to Cobblestone Lissencephaly. Am J Hum Genet, 99(5), 1181–1189. doi: 10.1016/j.ajhg.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, & Morriss-Kay GM (2002). Tissue origins and interactions in the mammalian skull vault. Dev Biol, 241(1), 106–116. doi: 10.1006/dbio.2001.0487 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Rice DP, Kettunen PJ, & Thesleff I (1998). FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development, 125(7), 1241–1251. [DOI] [PubMed] [Google Scholar]
- Klein RS, Rubin JB, Gibson HD, DeHaan EN, Alvarez-Hernandez X, Segal RA, & Luster AD (2001). SDF-1 alpha induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development, 128(11), 1971–1981. [DOI] [PubMed] [Google Scholar]
- Kume T, Deng KY, Winfrey V, Gould DB, Walter MA, & Hogan BLM (1998). The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell, 93(6), 985–996. doi:Doi 10.1016/S0092-8674(00)81204-0 [DOI] [PubMed] [Google Scholar]
- Labelle-Dumais C, Dilworth DJ, Harrington EP, de Leau M, Lyons D, Kabaeva Z, … Gould DB (2011). COL4A1 mutations cause ocular dysgenesis, neuronal localization defects, and myopathy in mice and Walker-Warburg syndrome in humans. PLoS Genet, 7(5), e1002062. doi: 10.1371/journal.pgen.1002062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Silhavy JL, Lee JE, Al-Gazali L, Thomas S, Davis EE, … Gleeson JG (2012). Evolutionarily Assembled cis-Regulatory Module at a Human Ciliopathy Locus. Science, 335(6071), 966–969. doi: 10.1126/science.1213506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenton KA, Nacamuli RP, Wan DC, Helms JA, & Longaker MT (2005). Cranial suture biology. Curr Top Dev Biol, 66, 287–328. doi: 10.1016/s0070-2153(05)66009-7 [DOI] [PubMed] [Google Scholar]
- Levi B, Wan DC, Wong VW, Nelson E, Hyun J, & Longaker MT (2012). Cranial suture biology: from pathways to patient care. J Craniofac Surg, 23(1), 13–19. doi: 10.1097/SCS.0b013e318240c6c0 [DOI] [PubMed] [Google Scholar]
- Levine JP, Bradley JP, Roth DA, McCarthy JG, & Longaker MT (1998). Studies in cranial suture biology: regional dura mater determines overlying suture biology. Plast Reconstr Surg, 101(6), 1441–1447. [DOI] [PubMed] [Google Scholar]
- Li G, Kataoka H, Coughlin SR, & Pleasure SJ (2009). Identification of a transient subpial neurogenic zone in the developing dentate gyrus and its regulation by Cxcl12 and reelin signaling. Development, 136(2), 327–335. doi: 10.1242/dev.025742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Oppenheim RW, Sugiura Y, & Lin W (2009). Abnormal development of the neuromuscular junction in Nedd4-deficient mice. Dev Biol, 330(1), 153–166. doi: 10.1016/j.ydbio.2009.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes MBS (2009). Meninges: Embryology In Lee JH (Ed.), Meningiomas (pp. 25–29). London: Springer London. [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, … Kipnis J (2015). Structural and functional features of central nervous system lymphatic vessels. Nature, 523(7560), 337–341. doi: 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J, Furuta Y, Du JG, & Richmond A (2001). Developmental expression of two CXC chemokines, MIP-2 and KC, and their receptors. Cytokine, 14(5), 253–263. doi:DOI 10.1006/cyto.2001.0882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, … Springer TA (1998). Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A, 95(16), 9448–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbutt LW, & Kokich VG (1979). Calvarial and sutural re-development following craniectomy in the neonatal rabbit. J Anat, 129(Pt 2), 413–422. [PMC free article] [PubMed] [Google Scholar]
- Machida A, Okuhara S, Harada K, & Iseki S (2014). Difference in apical and basal growth of the frontal bone primordium in Foxc1ch/ch mice. Congenit Anom (Kyoto), 54(3), 172–177. doi: 10.1111/cga.12053 [DOI] [PubMed] [Google Scholar]
- MacKenzie A, Ferguson MW, & Sharpe PT (1992). Expression patterns of the homeobox gene, Hox-8, in the mouse embryo suggest a role in specifying tooth initiation and shape. Development, 115(2), 403–420. [DOI] [PubMed] [Google Scholar]
- Maclean G, Dolle P, & Petkovich M (2009). Genetic disruption of CYP26B1 severely affects development of neural crest derived head structures, but does not compromise hindbrain patterning. Dev Dyn, 238(3), 732–745. doi: 10.1002/dvdy.21878 [DOI] [PubMed] [Google Scholar]
- Makrygiannis AK, Pavlakis E, Petrou P, Kalogeraki E, & Chalepakis G (2013). Segmental and restricted localization pattern of Fras1 in the developing meningeal basement membrane in mouse. Histochem Cell Biol, 140(5), 595–601. doi: 10.1007/s00418-013-1150-5 [DOI] [PubMed] [Google Scholar]
- Manzini MC, Tambunan DE, Hill RS, Yu TW, Maynard TM, Heinzen EL, … Walsh CA (2012). Exome Sequencing and Functional Validation in Zebrafish Identify GTDC2 Mutations as a Cause of Walker-Warburg Syndrome. American Journal of Human Genetics, 91(3), 541–547. doi: 10.1016/j.ajhg.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLone DG, & Bondareff W (1975). Developmental morphology of the subarachnoid space and contiguous structures in the mouse. Am J Anat, 142(3), 273–293. doi: 10.1002/aja.1001420302 [DOI] [PubMed] [Google Scholar]
- McMillin MJ, Beck AE, Chong JX, Shively KM, Buckingham KJ, Gildersleeve HI, … Bamshad MJ (2014). Mutations in PIEZO2 cause Gordon syndrome, Marden-Walker syndrome, and distal arthrogryposis type 5. Am J Hum Genet, 94(5), 734–744. doi: 10.1016/j.ajhg.2014.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Choe Y, Pleasure SJ, & Siegenthaler JA (2016). Cerebrovascular defects in Foxc1 mutants correlate with aberrant WNT and VEGF-A pathways downstream of retinoic acid from the meninges. Dev Biol, 420(1), 148–165. doi: 10.1016/j.ydbio.2016.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel SJ, Reddy CG, Frizon LA, Chardon MK, Holland M, Machado AG, … Wilson S (2018). Spinal dura mater: biophysical characteristics relevant to medical device development. J Med Eng Technol, 42(2), 128–139. doi: 10.1080/03091902.2018.1435745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rahilly R, & Muller F (1986). The meninges in human development. J Neuropathol Exp Neurol, 45(5), 588–608. [PubMed] [Google Scholar]
- Opperman LA, Sweeney TM, Redmon J, Persing JA, & Ogle RC (1993). Tissue interactions with underlying dura mater inhibit osseous obliteration of developing cranial sutures. Dev Dyn, 198(4), 312–322. doi: 10.1002/aja.1001980408 [DOI] [PubMed] [Google Scholar]
- Paredes MF, Li G, Berger O, Baraban SC, & Pleasure SJ (2006). Stromal-derived factor-1 (CXCL12) regulates laminar position of Cajal-Retzius cells in normal and dysplastic brains. J Neurosci, 26(37), 9404–9412. doi: 10.1523/JNEUROSCI.2575-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmanesh F, Caglayan AO, Silhavy JL, Yilmaz C, Cantagrel V, Omar T, … Gleeson JG (2013). Mutations in LAMB1 cause cobblestone brain malformation without muscular or ocular abnormalities. Am J Hum Genet, 92(3), 468–474. doi: 10.1016/j.ajhg.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R, Rice DP, Olsen BR, & Thesleff I (2003). Progression of calvarial bone development requires Foxc1 regulation of Msx2 and Alx4. Dev Biol, 262(1), 75–87. [DOI] [PubMed] [Google Scholar]
- Richtsmeier JT, & Flaherty K (2013). Hand in glove: brain and skull in development and dysmorphogenesis. Acta Neuropathol, 125(4), 469–489. doi: 10.1007/s00401-013-1104-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romand R, Kondo T, Cammas L, Hashino E, & Dolle P (2008). Dynamic expression of the retinoic acid-synthesizing enzyme retinol dehydrogenase 10 (rdh10) in the developing mouse brain and sensory organs. J Comp Neurol, 508(6), 879–892. doi: 10.1002/cne.21707 [DOI] [PubMed] [Google Scholar]
- Roybal PG, Wu NL, Sun J, Ting MC, Schafer CA, & Maxson RE (2010). Inactivation of Msx1 and Msx2 in neural crest reveals an unexpected role in suppressing heterotopic bone formation in the head. Dev Biol, 343(1–2), 28–39. doi: 10.1016/j.ydbio.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberte E, Friederich V, Chambon P, & Morriss-Kay G (1993). Retinoic acid receptors and cellular retinoid binding proteins. III. Their differential transcript distribution during mouse nervous system development. Development, 118(1), 267–282. [DOI] [PubMed] [Google Scholar]
- Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, & Inoue T (1999). MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development, 126(15), 3437–3447. [DOI] [PubMed] [Google Scholar]
- Sakka L, Gabrillargues J, & Coll G (2016). Anatomy of the Spinal Meninges. Oper Neurosurg (Hagerstown), 12(2), 168–188. doi: 10.1227/NEU.0000000000001048 [DOI] [PubMed] [Google Scholar]
- Sanchez D, Ganfornina MD, & Martinez S (2002). Expression pattern of the lipocalin apolipoprotein D during mouse embryogenesis. Mech Dev, 110(1–2), 225–229. [DOI] [PubMed] [Google Scholar]
- Sanchez D, Martinez S, Lindqvist A, Akerstrom B, & Falkenberg C (2002). Expression of the AMBP gene transcript and its two protein products, alpha(1)-microglobulin and bikunin, in mouse embryogenesis. Mech Dev, 117(1–2), 293–298. [DOI] [PubMed] [Google Scholar]
- Seiffert D, Iruela-Arispe ML, Sage EH, & Loskutoff DJ (1995). Distribution of vitronectin mRNA during murine development. Dev Dyn, 203(1), 71–79. doi: 10.1002/aja.1002030108 [DOI] [PubMed] [Google Scholar]
- Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, … Pleasure SJ (2009). Retinoic acid from the meninges regulates cortical neuron generation. Cell, 139(3), 597–609. doi: 10.1016/j.cell.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler JA, & Pleasure SJ (2011). We have got you ‘covered’: how the meninges control brain development. Current Opinion in Genetics & Development, 21(3), 249–255. doi: 10.1016/j.gde.2010.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM, Finger JH, Kadin JA, Richardson JE, & Ringwald M (2014). The Gene Expression Database for Mouse Development (GXD): Putting Developmental Expression Information at Your Fingertips. Developmental Dynamics, 243(10), 1176–1186. doi: 10.1002/Dvdy.24155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith UM, Consugar M, Tee LJ, McKee BM, Maina EN, Whelan S, … Johnson CA (2006). The transmembrane protein meckelin (MKS3) is mutated in Meckel-Gruber syndrome and the wpk rat. Nature Genetics, 38(2), 191–196. doi: 10.1038/ng1713 [DOI] [PubMed] [Google Scholar]
- Soriano P (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet, 21(1), 70–71. doi: 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Spector JA, Greenwald JA, Warren SM, Bouletreau PJ, Detch RC, Fagenholz PJ, … Longaker MT (2002). Dura mater biology: autocrine and paracrine effects of fibroblast growth factor 2. Plast Reconstr Surg, 109(2), 645–654. [DOI] [PubMed] [Google Scholar]
- Stevens E, Carss KJ, Cirak S, Foley AR, Torelli S, Willer T, … Muntoni F (2013). Mutations in B3GALNT2 cause congenital muscular dystrophy and hypoglycosylation of alpha-dystroglycan. Am J Hum Genet, 92(3), 354–365. doi: 10.1016/j.ajhg.2013.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, … Schulz S (2003). CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci, 23(12), 5123–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Eklund EA, Chung WK, Wang C, Cohen J, & Freeze HH (2005). Congenital disorder of glycosylation id presenting with hyperinsulinemic hypoglycemia and islet cell hyperplasia. J Clin Endocrinol Metab, 90(7), 4371–4375. doi: 10.1210/jc.2005-0250 [DOI] [PubMed] [Google Scholar]
- Thompson CL, Ng L, Menon V, Martinez S, Lee CK, Glattfelder K, … Jones AR (2014). A high-resolution spatiotemporal atlas of gene expression of the developing mouse brain. Neuron, 83(2), 309–323. doi: 10.1016/j.neuron.2014.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischfield MA, Robson CD, Gilette NM, Chim SM, Sofela FA, DeLisle MM, … Engle EC (2017). Cerebral Vein Malformations Result from Loss of Twist1 Expression and BMP Signaling from Skull Progenitor Cells and Dura. Dev Cell, 42(5), 445–461 e445. doi: 10.1016/j.devcel.2017.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg SR, & Wilkie AO (2015). A Genetic-Pathophysiological Framework for Craniosynostosis. Am J Hum Genet, 97(3), 359–377. doi: 10.1016/j.ajhg.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reeuwijk J, Grewal PK, Salih MA, Beltran-Valero de Bernabe D, McLaughlan JM, Michielse CB, … Voit T (2007). Intragenic deletion in the LARGE gene causes Walker-Warburg syndrome. Hum Genet, 121(6), 685–690. doi: 10.1007/s00439-007-0362-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrotti A, Spalice A, Ursitti F, Papetti L, Mariani R, Castronovo A, … Iannetti P (2010). New trends in neuronal migration disorders. Eur J Paediatr Neurol, 14(1), 1–12. doi: 10.1016/j.ejpn.2009.01.005 [DOI] [PubMed] [Google Scholar]
- Vincent AJ, Lau PW, & Roskams AJ (2008). SPARC is expressed by macroglia and microglia in the developing and mature nervous system. Developmental Dynamics, 237(5), 1449–1462. doi: 10.1002/dvdy.21495 [DOI] [PubMed] [Google Scholar]
- Visel A, Thaller C, & Eichele G (2004). GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res, 32(Database issue), D552–556. doi: 10.1093/nar/gkh029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivatbutsiri P, Ichinose S, Hytonen M, Sainio K, Eto K, & Iseki S (2008). Impaired meningeal development in association with apical expansion of calvarial bone osteogenesis in the Foxc1 mutant. J Anat, 212(5), 603–611. doi: 10.1111/j.1469-7580.2008.00893.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillaumier-Barrot S, Bouchet-Seraphin C, Chelbi M, Devisme L, Quentin S, Gazal S, … Seta N (2012). Identification of Mutations in TMEM5 and ISPD as a Cause of Severe Cobblestone Lissencephaly. American Journal of Human Genetics, 91(6), 1135–1143. doi: 10.1016/j.ajhg.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YQ, Sizeland A, Wang XF, & Sassoon D (1995). Restricted expression of type-II TGF beta receptor in murine embryonic development suggests a central role in tissue modeling and CNS patterning. Mech Dev, 52(2–3), 275–289. [DOI] [PubMed] [Google Scholar]
- Warren SM, Brunet LJ, Harland RM, Economides AN, & Longaker MT (2003). The BMP antagonist noggin regulates cranial suture fusion. Nature, 422(6932), 625–629. doi: 10.1038/nature01545 [DOI] [PubMed] [Google Scholar]
- Wu SC, Grindley J, Winnier GE, Hargett L, & Hogan BL (1998). Mouse Mesenchyme forkhead 2 (Mf2): expression, DNA binding and induction by sonic hedgehog during somitogenesis. Mech Dev, 70(1–2), 3–13. [DOI] [PubMed] [Google Scholar]
- Yeung J, Ha TJ, Swanson DJ, Choi K, Tong Y, & Goldowitz D (2014). Wls provides a new compartmental view of the rhombic lip in mouse cerebellar development. J Neurosci, 34(37), 12527–12537. doi: 10.1523/JNEUROSCI.1330-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, & Iseki S (2008). Cell lineage in mammalian craniofacial mesenchyme. Mech Dev, 125(9–10), 797–808. doi: 10.1016/j.mod.2008.06.007 [DOI] [PubMed] [Google Scholar]
- Zarbalis K, Choe Y, Siegenthaler JA, Orosco LA, & Pleasure SJ (2012). Meningeal defects alter the tangential migration of cortical interneurons in Foxc1hith/hith mice. Neural Dev, 7, 2. doi: 10.1186/1749-8104-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbalis K, Siegenthaler JA, Choe Y, May SR, Peterson AS, & Pleasure SJ (2007). Cortical dysplasia and skull defects in mice with a Foxc1 allele reveal the role of meningeal differentiation in regulating cortical development. Proc Natl Acad Sci U S A, 104(35), 14002–14007. doi: 10.1073/pnas.0702618104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yu T, Zhang XC, Nagasawa T, Wu JY, & Rao Y (2002). Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nature Neuroscience, 5(8), 719–720. doi: 10.1038/nn881 [DOI] [PMC free article] [PubMed] [Google Scholar]