Abstract
Aim
This nonsystematic review examined differences in the composition of raw maternal breastmilk and pasteurised donor milk and possible health effects on preterm infants.
Methods
We searched PubMed up to July 2018 for studies published in English that focused on four comparisons as follows: raw maternal milk versus donor milk, human milk before and after Holder pasteurisation, milk from mothers who delivered preterm and at term and milk collected during early and late lactation. We also searched for possible effects of the milk components, as well as the effects of maternal and donor milk on preterm infants’ health.
Results
Raw maternal milk contained factors involved in antioxidant and anti‐inflammatory defence, gut microbiome establishment and the maturation of immune defences, food tolerability and metabolism. Many of these factors were reduced or abolished in processed donor milk. Both maternal milk and donor milk have been associated with a reduced incidence of necrotising enterocolitis. High‐dose feeding with maternal milk during the neonatal period reportedly reduced the risk of other morbidities and promoted growth and neurodevelopment.
Conclusion
Many of the components in raw maternal breastmilk were lacking in pasteurised donor milk, which was inferior in promoting the growth and development of very preterm infants.
Keywords: Donor milk, Maternal milk, Morbidity, Pasteurisation, Preterm
Abbreviations
- BDNF
Brain‐derived neurotrophic factor
- EGF
Epidermal growth factor
- HMO
Human milk oligosaccharides
- IGF
Insulin‐like growth factor
- NEC
Necrotising enterocolitis
- ROP
Retinopathy of prematurity
- sIgA
Secretory immunoglobulin‐A
- TGF
Transforming growth factor
Key notes.
This review focused on differences in the composition of raw maternal breastmilk and pasteurised donor milk and the possible health effects on preterm infants.
Maternal milk prevented necrotising enterocolitis and other morbidities and promoted growth and neurodevelopment.
Donor milk was associated with reduced necrotising enterocolitis compared to preterm formula, but it was inferior to maternal milk in promoting the growth and development of very preterm infants.
Introduction
Despite efforts to provide optimal nutrition, most extremely preterm infants suffer from postnatal growth retardation. This has been associated with retinopathy of prematurity (ROP) 1, bronchopulmonary dysplasia, late‐onset sepsis, necrotising enterocolitis (NEC) 2, 3 and neurodevelopmental impairment 4. Strategies to reduce prematurity‐related complications are urgently needed, and the increased use of maternal milk may have preventative effects 5, 6. Therefore, current guidelines recommend early enteral feeding, with expressed maternal milk and pasteurised human donor milk as a second choice.
In Sweden, extremely preterm infants always receive human milk during their hospital stay. Mothers are encouraged to start expressing milk for their infants soon after delivery. If maternal milk production is insufficient, or the mother is unable to express milk, or decides not to, infants are fed donor milk from a milk bank until a postmenstrual age of approximately 34 weeks. After that age, they receive preterm formula based on cows’ milk. Both expressed maternal milk and donor milk are regularly analysed for their respective macronutrient and energy contents, and the milk is then individually fortified with bovine‐derived fortifiers to meet current nutritional recommendations. Notably, donor milk is only provided until several weeks before term‐equivalent age. This means that infants who receive donor milk miss human milk intake during the critical developmental period before term, as well as the six months of exclusive breastfeeding and subsequent partial breastfeeding recommended by the World Health Organization 7. In Sweden, the percentage of extremely preterm infants who were exclusively receiving maternal milk at discharge decreased from 55% in 2004 to 16% in 2013 (Fig. 1) 8. A mother's motivation to express milk and then breastfeed is influenced by the information that she receives. It may be insufficient to tell a mother that her milk is the best choice, without presenting the specific advantages and disadvantages of the alternatives. There is a need for a comprehensive summary of the evidence that maternal milk is superior to donor milk in preventing the complications of prematurity.
Figure 1.
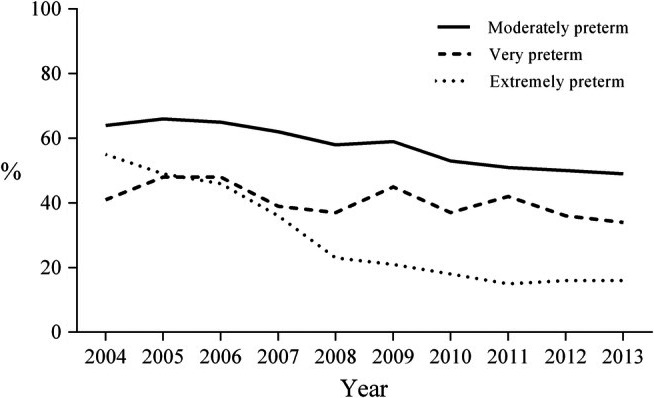
Exclusive maternal breastmilk feeding at discharge from neonatal units, among infants of three gestational age groups: extremely preterm (n=550), very preterm (n=1848) and moderately preterm (n=12 958). Data from the Swedish Neonatal Quality register (2004–2013) 8.
During the third trimester, a foetus swallows approximately 200–250 mL of amniotic fluid each day for each kilogram of foetal weight 9. Amniotic fluid, like maternal milk, contains nutrients and bioactive factors that promote gut maturation, protect against infection and facilitate growth 9, 10. Very preterm infants miss a large portion of the immunoglobulin G transfer from the mother during late gestation, and their immune system is still immature at birth 11. Extremely preterm newborn infants have five to 10 times more microbial infections than infants born at term 12. Breastmilk contains factors that protect against infection and modulate the infants’ immunological development 13. These components can also reduce the infants’ future incidence of diseases that involve dysregulated immunity, such as allergies 14, inflammatory bowel disease 15, insulin‐dependent diabetes mellitus 16 and some lymphomas 17.
The nutrient content of breastmilk varies greatly between mothers and depends on her diet, the gestational age at delivery, the lactation stage, the time of day, the size of the milk portion and whether it is foremilk or hindmilk 18. During the first three to five days after delivery, colostrum is produced, which is particularly rich in the nutrients and factors that are important for gut maturation and defence against infections. Transitional milk is then produced for the next two weeks’ post‐partum, after which the milk is considered mature.
Expressed maternal milk is usually consumed raw and sometimes after refrigeration and freezing and thawing. Bank milk is typically mature milk from the mothers of term infants and has usually been frozen and thawed before Holder pasteurisation, which implies that it has been heated to 62.5°C for 30 min. Thereafter, the milk is frozen again and reheated before it is fed to the infant. Studies with different designs have compared maternal milk and donor milk and examined the effects of storage and pasteurisation, with highly variable outcomes. In this review, pasteurisation always refers to Holder pasteurisation, since this is the method currently recommended for human milk banks. Table 1 presents several important bioactive factors that are altered after pasteurisation.
Table 1.
Some human milk bioactive components/functions shown to be affected by Holder pasteurization
| Component concentrations/functions | Change with pasteurization | Reference |
|---|---|---|
| Secretory IgA | 51% lower | Christen et al. 32 |
| Lactoferrin | 91% lower | Christen et al. 32 |
| Lysozyme | 59% lower | Christen et al. 32 |
| BSSL activity | Inactivated | Baro et al. (S62) |
| Insulin | 46% lower | Ley et al. (S110) |
| Adiponectin | 33% lower | Ley et al. (S110) |
| IGF‐1 | 39% lower | Goelz et al. (S115) |
| TGF‐β | 14% lower | Reeves et al. (S136) |
| Maternal leucocytes | Destroyed | Goldman (S154) |
| Antioxidant capacity | 67% lower | Silvestre et al. (S160) |
| Bacterial growth rate | Doubled | Christen et al. 32 |
This paper reviews the differences between expressed raw maternal breastmilk and pasteurised donor milk in terms of composition and in relation to growth and morbidities. Our aim was to review the relevant scientific reports, so that adequate information could be provided to healthcare professionals and parents, to promote well‐informed decisions about breastmilk expression and later breastfeeding. We also briefly address the long‐term effects of maternal milk.
Methods
PubMed was searched up to July 2018 for papers in English. We initially searched for papers that addressed the composition of human milk and differences in the composition between maternal milk and donor milk and raw and pasteurised milk. This review also explored differences in milk composition after term and preterm delivery, as well as in early and mature human milk. After that, we looked for papers that described functions of milk components, especially bioactive compounds, and how they were affected by Holder pasteurisation. Finally, we searched for comparisons between feeding infants fortified raw maternal breastmilk or fortified pasteurised donor milk with regard to growth, morbidity and neurodevelopment.
This was not a systematic review of all the factors present in maternal and donor milk and their possible effects on very preterm infants. No other database than PubMed was used. Our aim was to review information that may be of value for healthcare professionals when they inform parents about the advantages and disadvantages of feeding very preterm infants maternal milk or donor milk.
Results
Human milk composition
Macronutrients
Human milk shows wide variations in fat, protein and carbohydrate contents 19. Donor milk contains significantly less protein, fat and energy than the milk, provided by the mothers of preterm infants 20. Compared to term milk, preterm milk is richer in cholesterol, phospholipids and long chain polyunsaturated fatty acids, which are integral cellular membrane components and essential for brain cell growth and myelin development in newborn infants 21. These components decrease in milk as lactation progresses. A review of the effects of pasteurisation on human milk concluded that Holder pasteurisation did not significantly affect its composition with regard to saccharides, protein, fat, energy or fatty acids 22.
Bioactive components
In addition to macronutrients, breastmilk is rich in bioactive components, such as oligosaccharides, immunoglobulins, lactoferrin, cytokines, enzymes, growth factors, hormones, anti‐inflammatory agents, microbial factors and fatty acids, particularly in the first months after birth. These factors appear to play a multitude of overlapping roles in gut and general development 23, although little is presently known about their functions in very preterm neonates.
While milk proteins mainly serve as a source of amino acids for infant growth, peptides may also possess bioactive capacities, either directly or after digestion 24. Bioactive milk proteins can provide protection from infections, enhance nutrient absorption and promote immune system development and neurodevelopment. Some of these proteins are destroyed or inactivated by pasteurisation, leading to different properties in raw maternal milk and pasteurised donor milk 22. In preterm formula, the proteins are derived from bovine milk, which differs from human milk in many respects.
The functions of milk proteins and peptides have mainly been investigated in experimental studies. In vitro models have revealed that human milk proteins generate a wide variety of bioactive peptides. Beta‐casein 25 is the most abundant casein in milk and the greatest source of bioactive peptides 24. In vitro studies have shown that kappa‐casein from breastmilk inhibits Helicobacter pylori binding to human gastric mucosa 26. Pasteurisation did not appear to affect the bioactive peptides derived from the digestion of major human milk proteins 25.
The most prevalent whey protein in human milk is alpha‐lactalbumin, which accounts for 20–25% of total milk protein. It plays several physiological roles during the neonatal period, including providing a balanced supply of essential amino acids. Moreover, alpha‐lactalbumin digestion results in the transient formation of peptides with immune‐stimulatory and bactericidal properties, which may be protective against infection 24, 27. One study reported that pasteurisation did not alter the alpha‐lactalbumin and serum albumin concentrations in breastmilk 28.
Lymphocytes migrate from the mother's intestine to the mammary gland, where they are transformed to immunoglobulin‐A‐producing cells and produce the secretory immunoglobulin‐A (sIgA) found in human milk 29. Through this process, the milk contains antibodies that are directed against microbial antigens present in the mother's gut. The milk sIgA blocks the mucosal adherence of bacterial, parasitic and viral pathogens and is critical for maintaining a diversified microbiotic environment 30, 31. Thus, sIgA in breastmilk transfers maternal immunity to infectious agents and other antigens in the mother's and hence the infant's environment to the infant.
One study showed that pasteurisation of previously frozen milk reduced sIgA by 51% 32, while another reported that sIgA was 60% lower in pasteurised term donor milk compared to fresh term milk 28. Research also showed that stool samples from breastfed term infants contained large amounts of intact sIgA, with the highest concentrations during the first weeks of life 33.
The iron‐binding protein lactoferrin is a major whey protein in human milk. In the gut, it binds to lactoferrin receptors that are expressed in the small intestine. Human lactoferrin receptors have also been found in monocytes, lymphocytes, platelets, fibroblasts and bone 34. Stool samples of full‐term exclusively breastfed infants contain intact lactoferrin and the concentration decreases with age and is naturally associated with the decreasing concentrations in milk 33. It has been suggested that absorption of lactoferrin‐bound iron in milk is the main route for iron uptake during the neonatal period 35. However, neonatal lactoferrin knockout mice exhibited no evidence of reduced intestinal iron uptake 36. Lactoferrin has been reported to protect newborn infants from infection by withholding iron from bacteria 37 and by destabilising the bacterial cell surface 38. Lactoferrin has also been found to enter cell nuclei and affect the expression of genes, modulate cell proliferation and differentiation 39 and interact with the immune system 34, 40.
Studies have shown that lactoferrin has high structural homology between species and bovine lactoferrin exerts biological effects on human enteral cells 41. In addition, bovine milk‐based formulas contain very small amounts of lactoferrin without supplementation 42.
The process of freezing, thawing, pasteurising and freezing and thawing again reportedly decreases the lactoferrin concentration in human milk by 70% 43. Other studies have demonstrated that 91% of lactoferrin was lost after the pasteurisation of previously frozen milk 32 and that pasteurised donor milk had a 44% lower lactoferrin concentration than fresh milk 28. In preterm infants, enteral supplementation with bovine lactoferrin decreased invasive fungal infections 44, late‐onset sepsis 45, 46, 47 and NEC 48. In some countries, bovine lactoferrin is added to commercial formulas, and the US Food and Drug Administration has classified bovine lactoferrin as generally recognised as safe 49.
A randomised controlled trial investigated enteral administration of human recombinant lactoferrin (talactoferrin) to infants with birthweights of 750–1500 g It reduced hospital‐acquired infections by nearly 50%, along with a significant reduction in coagulase‐negative Staphylococcus and, or, line infections. However, the authors stated that talactoferrin was no longer available in the United States 50, 51. A Cochrane review from 2017 concluded that low‐quality evidence suggested that supplementing oral feeds with lactoferrin, bovine or talactoferrin, with or without probiotics, decreased late‐onset sepsis and NEC stage II or III in preterm infants, without adverse effects. Ongoing trials will provide evidence from over 6000 infants 52.
Lysozyme is a host defence protein that is secreted in high concentrations in human milk. It is part of the innate immune system and causes lysis of bacteria via degradation of their outer wall. The presence of lysozyme may stimulate overgrowth of intestinal bifidobacteria, which are apparently resistant to lysozyme 53. Lysozyme is excreted in the faeces of preterm infants with a gestational age of 28–30 weeks 54 but not in healthy term infants 33. Pasteurisation reportedly reduces lysozyme by 59% 32 and pasteurised donor milk showed 60% lower lysozyme activity than raw milk 28. Similarly, concentrations of another host defence protein, lactoperoxidase, were 82% lower in donor milk than in the breastmilk of mothers delivering at term 28.
Osteopontin is a multifunctional protein that is present in most tissues and body fluids. It is found in high concentrations in human milk 55, 56 and the highest levels have been found three to seven days’ post‐partum 57. Experimental studies have revealed that milk osteopontin plays essential roles in immunological, intestinal and early cognitive development 55. It can bind to various receptors of enterocytes or be absorbed into systemic circulation. Osteopontin concentrations have been found at lower levels in bovine milk than in human milk and even lower levels have been found in bovine milk‐based formula 42, 56. Infants born at term who received formula with added bovine osteopontin had lower tumour necrosis factor‐alpha serum concentrations than infants on nonsupplemented formula and similar concentrations to breastfed infants. They also had fewer fevers, increased proportions of T cells and upregulated pathways of cell proliferation and cell to cell adhesion. The effects of osteopontin supplements appeared to be dose dependent in one study, with some functions promoted by lower osteopontin concentrations and others by higher concentrations 58. Osteopontin has been found to have high affinity for lactoferrin and may act as a carrier protein for lactoferrin in milk and as a modulator of lactoferrin's anti‐microbial and immune‐stimulatory activities 59. Little is known about the biological functions of milk osteopontin in preterm infants and we were unable to find any studies on the effect of pasteurisation on osteopontin concentrations. In a preterm animal model that used piglets, formula supplemented with osteopontin reduced the severity, but not the incidence, of NEC 60.
Bile salt‐stimulated lipase in milk facilitates lipid digestion and absorption, especially in immature infants with low endogenous capacity to digest fat early in life (S61 in Appendix S1). Bile salt‐stimulated lipase is inactivated by pasteurisation (S62) and one study showed that feeding infants with pasteurised maternal milk reduced fat absorption and growth (S63). When recombinant human bile salt‐stimulated lipase was used to supplement pasteurised human milk or formula, the absorption of docosahexaenoic acid and arachidonic acid was increased, but not the total fat absorption (S64). Treatment with recombinant human bile salt‐stimulated lipase significantly improved growth in infants born small for gestational age, but not in infants born appropriate for gestational age and was associated with increased risk of infections and gastrointestinal intolerability (S65).
Milk fat globules comprise a core of mainly triglycerides, surrounded by a membrane composed of phospholipids, cholesterol, proteins and glycoproteins, including several proteins with known or suggested bio‐activities. Free fatty acids and monoglycerides, which are digestion products of the milk fat globule triglyceride core, have been shown to act as detergents and have lytic functions against viruses, bacteria and protozoa (S66). Human milk fat globule membranes contain a multitude of proteins: in one study, 20% were shown to be related to immune responses and 19% to cell communication and signal transduction (S67). In addition, milk fat globule membranes also contain compounds involved in central nervous system development, such as choline and sphingomyelin. Filaments on the surface of human milk fat globules have been reported to contain mucin‐1, which inhibits bacterial adhesion to other cells (S68), and lactadherin, which protects against the rotavirus infection (S69). Both mucin‐1 and lactadherin survive and maintain their integrity in the stomachs of preterm infants. Their concentrations in human milk have been found to be highest during the first weeks of lactation and to decline thereafter (S70). The average milk fat globule size in mature milk increases with the lactation progress, and thereby, the proportion of milk fat globule membrane decreases. One study showed that, human milk fat globules significantly decreased in size during pasteurisation and showed a greater globule size distribution (S71). Another study reported that storage and cooling affected the stability and membrane composition of bovine milk fat globules and that pasteurisation drastically modified milk fat globule membrane composition and functionality (S72).
Choline is an essential nutrient in breastmilk and it is critical for nervous system development. It is a precursor of membrane constituents such as phosphatidylcholine and sphingomyelin and of the neurotransmitter acetylcholine. A study in rats showed that brain function was positively impacted by oral choline supplementation during neurogenesis and synaptogenesis, corresponding to a period from gestation to four years of age in humans (S73). Notably, phosphatidylcholine is a main component of pulmonary surfactant and the major transporter for arachidonic acid and docosahexaenoic acid (S74). During pregnancy, choline has been shown to selectively transfer from the mother to the foetus and plasma choline concentrations after preterm birth have been reported to decrease to approximately 50% of those in cord blood within 48 h. It has been suggested that choline undernourishment may contribute to adverse neurodevelopmental outcomes after preterm birth (S75). While some authors have reported that preterm human milk contained less choline than term milk during the first weeks of life (S76), others found more phosphocholine in preterm than term milk (S77). Bovine and human milk have a nearly identical phospholipid content, which was not altered by the pasteurisation of bovine milk (S78). An ongoing trial is investigating the effects of choline and docosahexaenoic acid supplementation in preterm infants (NCT02509728).
Human milk oligosaccharides (HMOs) are a group of complex sugars that are highly abundant in human milk. More than 120 different HMO structures have been identified, and it has been shown that each mother produces a unique composition (S79,S80). Most HMOs contain the sugar fucose and a mother's ability to fucosylate HMOs is genetically determined (S81). Some fucosylated HMOs prevent adhesion of enteric pathogens to the intestinal mucosa (S81). In vitro digestion studies have demonstrated that HMOs were relatively resistant to human digestion (S82,S83), with a small fraction absorbed into the infants’ systemic circulation and excreted intact in the urine (S84). Another study reported that most HMOs were either metabolised by intestinal microorganisms or excreted with faeces (S85). Within the gastrointestinal tract, different HMO categories apparently serve as metabolic substrates for specific bacteria, selectively promoting bifidobacterial growth in particular (S86,S87).
Experimental studies have shown that HMOs prevented adhesion to epithelial cells of pathogens, such as bacteria (S88), viruses (S89) and parasites (S90). In addition, HMOs inhibited proliferation of Group B Streptococcus, a leading cause of invasive infection in newborn infants (S91) and dose‐dependently reduced Candida albicans invasion of human premature intestinal cells (S92). A study of formula‐fed piglets reported that HMOs increased the abundance of various immune cells in both infected and noninfected animals (S93). Interestingly, some HMOs made urogenital tract epithelial cells more resistant to uropathogenic Escherichia coli (S94). Human milk oligosaccharides contain large amounts of sialic acid. A review summarised the evidence that supported a role for dietary sialic acid as an essential nutrient for optimal brain development (S95).
Similar HMO concentrations have been found in preterm and term milk (S96,S97), but preterm milk contained a much wider variation in the percentage of HMOs that contained fucose or sialic acid than term milk. This may have been due to the immaturity of the fucosylation process, which may influence the developing microbiota (S98). It has been suggested that preterm infants may benefit from supplementation with fucosylated HMOs, for example, from pooled donor human milk (S99). Human milk has been shown to contain 100‐fold to 1,000‐fold higher concentrations of oligosaccharides than bovine milk (S100). Pasteurisation or freeze drying of donor milk reportedly did not influence HMO content (S101).
Glycosaminoglycans are long un‐branched polysaccharides that are found on cell surfaces and cell–extracellular matrix interfaces. They regulate many processes, including cell growth and differentiation, cell to cell and cell to matrix interactions and anti‐infective and anti‐inflammatory processes (S102). Studies have found that, approximately 55% of the glycosaminoglycans in human milk are chondroitin sulphate and 40% are heparin. The concentrations of these glycosaminoglycans were several times higher in human milk than in bovine milk, which showed a different glycosaminoglycan profile. During the first month of lactation, preterm milk was reported to contain significantly more glycosaminoglycans than term milk (S103) and they were not affected by pasteurisation (S104).
Growth factors, adipokines, hormones and cytokines
Insulin in human milk is thought to play important roles in the development of gut function and general metabolism in infants (S105,S106). Insulin concentrations have been reported to be higher in milk (60±41 μU/mL) than in maternal serum. Insulin levels in mature breastmilk were similar after term and preterm deliveries and remained unchanged after more than one year of lactation (S107–S109). Pasteurisation has been reported to reduce milk insulin by 46.1% (S110).
Serum adiponectin improves insulin sensitivity and lipid metabolism (S111). Higher levels of adiponectin have been found in preterm than term breastmilk (S112). One study demonstrated that pasteurisation reduced donor human milk adiponectin by 32.8% (S110).
Insulin‐like growth factors (IGFs) possess growth‐promoting and anti‐apoptotic effects, and their presence in milk is thought to stimulate gut maturation and growth. One study of early human milk found that IGF‐2, and its binding protein IGF‐binding protein 2, was present in higher concentrations than IGF‐1 and IGF‐binding protein 3. Preterm milk contained higher IGF‐binding protein 2 and IGF‐binding protein 3 concentrations than term milk and IGF‐binding protein 3 levels declined with time (S113). Enteral administration of IGF‐1 to preterm infants supplemented with formula reduced gut permeability, but did not otherwise improve intestinal maturation (S114). Another study found that pasteurisation of human milk significantly reduced IGF‐1 by 39.4% (S115).
Epidermal growth factor (EGF) plays important roles in intestinal development, starting during gestation when the foetus swallows amniotic fluid, which contains concentrations of EGF that are correlated with gestational age (S116). After birth, intestinal EGF is mainly obtained from breastmilk and the highest EGF concentrations have been found in colostrum (S117). One study found that EGF levels were 50–80% higher in preterm milk than term milk (S118). Another reported that Holder pasteurisation did not affect EGF concentrations (S115). Infant formulas do not contain EGF. In a rat model, supplementing bovine‐based formula with EGF dramatically reduced the occurrence of NEC (S119).
Brain‐derived neurotrophic factor (BDNF) has been shown to be involved in neuron growth and differentiation, synaptic plasticity and memory formation (S120). Variants of the BDNF gene have been associated with severe ROP (S121) and infants who developed severe ROP had lower serum BDNF levels at 10‐14 days after birth than infants who did not develop ROP (S122). BDNF serum levels were higher at four to six months of age in breastfed term infants than in term infants fed formula (S123). Human milk contains BDNF, which is thought to be mainly produced by BDNF‐expressing milk leucocytes (S124). Since cellular milk components are inactivated by pasteurisation, it can be speculated that BDNF concentrations would be lower in donor milk than maternal milk.
Cytokines are pluripotent polypeptides, with multiple and often overlapping effects, which act as messengers among cells and contribute to immune system development and function. Human milk contains various cytokines that can cross the intestinal barrier and the cytokine profile in breastmilk has been shown to vary with gestational age at delivery (S125). Milk cytokines can be divided into two categories: those that enhance inflammation or defend against infection and those that reduce inflammation. One study found that banked pasteurised donor milk contained cytokine concentrations that were similar to those in maternal milk after six weeks of lactation. The concentrations were lower than in milk that had been produced earlier. This provides evidence that donor milk provides less of these factors during the first weeks of life (S126).
The most abundant cytokines in human milk are from the transforming growth factor‐beta (TGF‐beta) family (S127). These cytokines have immune‐regulatory properties and seem to be necessary for healthy immune maturation in mammals (S128) and for breastmilk induction of immune tolerance (S129). TGF‐beta has been shown to suppress inflammatory responses in the developing intestine and protect against mucosal injury (S130). Of the three TGF‐beta isoforms, TGF‐beta 2 is the most abundant in breastmilk (S130). TGF‐beta 1 and TGF‐beta 2 concentrations in breastmilk have reportedly been associated with Ig‐A concentrations and TGF‐beta in colostrum may initiate IgA production in newborn infants (S131). In cultured immature human intestinal epithelial cells, TGF‐beta 2 attenuated interleukin‐1 beta‐induced pro‐inflammatory cytokine production (S132). Only a minor proportion of TGF‐beta in human milk is bioactive, whereas the major part is latent and requires stimulation for bioactivity. Although preterm milk contains more TGF‐beta than term milk, preterm milk shows minimal TGF‐beta bioactivity in the native state. It has been demonstrated that latent TGF‐beta could be activated by neuraminidase, which existed in lower quantities in the immature gut than the mature gut (S133). Preterm baboons’ intestines, especially those that developed NEC, expressed less TGF‐beta 2 than term intestines (S134) and enteral administration of recombinant TGF‐beta 2 protected mice from experimental NEC‐like injuries (S135). Pasteurisation modestly reduced TGF‐beta 2 by 14% in one study, but concentrations were fivefold higher in preterm maternal milk than in donor milk (S136).
Activin A is a member of the TGF‐beta family that shows trophic and neuroprotective effects in the central nervous system. Human milk contains Activin A and one study found that the concentration did not differ significantly with respect to gestational age or whether or not it was pasteurised (S137).
Living cellular components
Human milk contains various types of maternal living cells derived from the mother's breast and blood, as well as a wide variety of microbes. Some of these cells are thought to protect infants from infections and promote immune development (S138). Modern technology has enabled researchers to characterise the cellular composition of human milk and one study reported large variations in cellular content, ranging from 10 000 to 13 000 000 cells/mL (S139). The cellular composition has also been shown to be highly variable. These variations have been associated with the lactation stage, the health status of the mother and the infant and other factors indicating that human milk is a dynamic fluid that adjusts to the infant's needs.
Breastmilk leucocytes include granulocytes and mononuclear cells, such as lymphocytes, monocytes and macrophages. One study reported that colostrum had a higher leucocyte content (13–70%) than mature milk (0–2%) (S140). An interesting finding was that maternal infection, or infection in just the infant, was associated with an increased milk leucocyte population, which decreased when the infection resolved (S140). After newborn baboons ingested human milk with labelled leucocytes, these cells showed high activity in the gastrointestinal tract and were present in various organs, including the liver and spleen (S141).
Breastmilk stem cells were first discovered in 2007 (S142) and have since been investigated using in vitro differentiation assays. Some human breastmilk stem cells can differentiate into milk‐producing cells and also into cells with properties of neurons, cardiomyocytes, osteoblasts, chondrocytes, adipocytes, hepatocytes and pancreatic beta cells (S143). However, the roles of breastmilk stem cells for the breastfed infant remain unknown. The long‐term impact of breastfeeding has been illustrated by better one‐year graft function rates among breastfed recipients (82%) than among nonbreastfed recipients (57%) who received kidney transplants from their mothers later in life. (S144).
Micro ribonucleic acids (micro RNAs) are small noncoding RNA molecules that act on messenger RNA and serve as crucial regulators of gene expression in many biological processes. Human milk cells are one of the richest sources of micro RNAs. The majority of the most abundant micro RNAs are involved in immune responses, development, growth, metabolism, reproduction and enzyme regulatory activity (S145). Interestingly, most of the micro RNAs that are highly expressed in human milk cells regulate the IGF‐1 receptor and some play critical roles in insulin receptor signalling (S145). Food‐derived micro RNAs can survive the gastrointestinal tract, enter the systemic circulation and be transferred to various organs, where they regulate gene expression (S146,S147). Preterm delivery results in a unique profile of breast milk micro RNAs with metabolic targets (S148), but the roles of these milk micro RNAs remain unexplored in preterm infants. One study reported that pasteurising human milk did not alter the distribution or expression of four micro RNAs involved in the immune response (S149).
Human milk also contains bacteria, viruses and fungi. These include both beneficial bacteria, which develop into healthy gut flora, and pathogens such as the cytomegalovirus and human immunodeficiency virus that may be transferred to the infant. Breastmilk is a natural lactic acid bacterial source for colonisation of the infant's intestine (S150), and the transfer of maternal gut bacteria to the breast has been demonstrated (S151). Studies have shown that preterm infants who were fed maternal milk had increased gut microbial diversity (S152) and different gut microbial profiles, with a higher abundance of Bifidobacteriaceae, than those fed donor milk (S153). Pasteurisation has been demonstrated to inactivate the cellular components of milk (S154) and reduce the bactericidal effects of human breastmilk (S155). Pasteurised human milk showed increased bacterial growth after incubation compared to raw human milk 32.
Human milk has antioxidant properties and contains vitamin C, vitamin E, enzymes and other factors that protect against oxidative stress (S156,S157). Extremely preterm infants have low antioxidant capacity and are especially vulnerable to the oxidative stress associated with infections, mechanical ventilation, parenteral nutrition and blood transfusions (S158). It has been suggested that bronchopulmonary dysplasia, ROP, NEC and patent ductus arteriosus are all facets of an oxygen radical disease (S159). Pasteurisation has been shown to significantly reduce the total antioxidant capacity of breastmilk (S160).
Effects of maternal milk versus donor milk on growth and morbidities
Ethical concerns have prevented randomised controlled trials comparing exclusive feeding with maternal milk and donor milk. However, one study compared fortified maternal milk with fortified donor milk in infants with a gestational age of less than 32 weeks. This covered a period of exclusive feeding with human milk, from the discontinuation of parenteral nutrition until the transition to formula. Infants who received at least 80% maternal milk gained weight more rapidly than those receiving less than 20% maternal milk and weight gain correlated with the proportion of maternal milk intake (S161). In a study of 243 infants with a gestational age of less than 30 weeks, infants who were exclusively fed maternal milk (maternal milk group) were compared to infants who received maternal milk plus pasteurised donor milk from women who had delivered preterm infants (donor milk group) or maternal milk plus preterm formula (formula group) (S162). A human milk fortifier was added when the milk intake reached 100 mL/kg/d. In the donor milk group, 17% of infants were switched to preterm formula due to poor growth, compared to none in the maternal milk group. Proliferative ROP stage 3 was found in 5.6% in the maternal milk group compared to 19% in the donor milk group (p=0.05). The maternal milk group also showed fewer episodes of late‐onset sepsis and, or, NEC and fewer infection‐related events than the other groups. These morbidities were negatively correlated with the cumulative maternal milk intake, indicating dose‐dependent effects. Moreover, infants in the maternal milk group stayed in hospital one week less than the infants in the other groups. The authors concluded that donor milk offered no short‐term advantages over preterm formula as a substitute for maternal milk.
Two studies since 2017 have focused on reduced weight gain in preterm infants supplemented with fortified donor milk, compared to infants exclusively fed with fortified maternal milk. The first reported that infants with birthweights of less than 1000 g had a slower rate of weight gain during the first month of life and lower cognitive scores at one and two years of age if they were supplemented with more than 50% donor milk compared to infants who were exclusively fed maternal milk (S163). The second study found a dose–response relationship between maternal milk and growth in infants with a gestational age of 32 weeks or less or a birthweight of 1800 g or less. The mean weight gain velocity before 36 weeks of postmenstrual age, or discharge from the neonatal intensive care unit, decreased by 0.17 g per kg per day and the mean head circumference velocity by 0.01 cm per week for every 10% increase in donor milk intake compared to maternal milk (S164).
Discussion
This paper presents a selection of the most studied elements of breastmilk. We still have very limited knowledge about the roles of these compounds in very preterm infants, despite the wealth of information about various milk components, their effects and their interactions with each other and other factors. Raw maternal milk contains bioactive factors that are involved in antioxidant and anti‐inflammatory defence, gut microbiome establishment and the maturation of functions, such as immune defence, food tolerability and metabolism. Exposure to the protective immunological factors and microbiome in maternal milk (S125) counteracts the deficiencies of the infant's innate immune system and supports overall immune function.
It is clear that many of the elements that are present in the raw breastmilk of mothers who have delivered preterm infants are reduced or abolished in processed mature banked milk from mothers of term‐born infants and absent or found in other forms in bovine‐derived formula. It has been pointed out that the most profound difference between maternal milk and donor milk after preterm deliveries was observed when donor milk was substituted for maternal milk during the early critical postbirth window. It is thought that components in maternal milk promote nutritional and immunologic programming and the growth of certain organs during this period (S165).
Several studies have shown a dose‐dependent relationship between maternal milk intake during the neonatal period and a reduced frequency of ROP (S166,S167), NEC (S168,S169), infections (S170,S171), bronchopulmonary dysplasia (S172) and improved neurodevelopment (S173, Compared to preterm formula, donor milk was found to prevent NEC (S174), which has been reported to affects 5% to 22% of infants with a birthweight of less than 1000 g in high‐income countries (S176). However, donor milk has also been reported to result in lower rates of weight gain, linear growth and head growth (S174). Other authors stated that no evidence had been put forward to indicate that donor milk reduced ROP or other morbidities or promoted neurodevelopment (S175).
The main limitation of this review was that it was not a systematic review of all the factors present in maternal and donor milk and their possible effects on very preterm infants. No other database than PubMed was used. We set out to review information of importance for healthcare professionals and parents about advantages and disadvantages of feeding very preterm infants maternal milk or donor milk.
It is well known that maternal milk is the best choice for all infants. The World Health Organization recommends exclusive breastfeeding up to six months of age and continued breastfeeding, together with other foods, up to two years of age and beyond 7. In 2018, a European report on 6592 infants born with a gestational age of less than 32 weeks from 11 countries reported large regional variations in breastmilk feeding. At hospital discharge, the rate of any breastmilk feeding ranged from 36% to 80% (S177). Intake of maternal milk was more important during the first weeks of life than later, with regard to preventing ROP and other prematurity‐related morbidities 6. In the Extremely Preterm Infants in Sweden study of infants born before a gestational age of 27 weeks from April 2002 to March 2007, 70% of infants were fed exclusively with maternal milk at four weeks of life. At discharge, 50% received maternal milk, partly or exclusively 20. No later reports of maternal milk intake in the neonatal period of extremely preterm infants in Sweden have been published, but from 2004 to 2013, the rates of exclusively feeding maternal milk to extremely preterm infants at discharge declined from 55% to 16% in Sweden 8.
The World Health Organization and the United Nations Children's Fund launched the Baby‐friendly Hospital Initiative to implement practices that promote breastfeeding and defined 10 steps to successful breastfeeding (S178). In 2012, an expanded version was published to meet the needs of preterm and sick infants, emphasising the need for antenatal information that was specifically designed for neonatal intensive care (S179). According to the parents of very preterm infants in one study, the main factors that facilitated maternal milk feeding were the contribution of maternal milk to infant growth and well‐being and the parents’ knowledge of the benefits of breastfeeding (S180). Other essential factors were the availability of education about breastfeeding and support to mothers who were motivated to provide their own milk.
Conclusion
Maternal milk contains many bioactive factors which are adjusted for the specific needs of her very preterm infant. Pasteurised donor milk contains less nutrients and bioactive factors than maternal milk and does not promote growth, health and development as well. Providing maternal milk can be viewed as an example of personalised medicine. Healthcare professionals and parents need comprehensive and up‐to‐date information about the benefits of maternal milk for very and extremely preterm infants. We believe that this will facilitate well‐informed decisions about maternal milk feeding that may improve outcomes.
Conflicts of interest
The authors have no conflicts of interest to declare.
Supporting information
Appendix S1 References.
References
- 1. Hellstrom A, Ley D, Hansen‐Pupp I, Niklasson A, Smith L, Lofqvist C, et al. New insights into the development of retinopathy of prematurity–importance of early weight gain. Acta Paediatr 2010; 99: 502–8. [DOI] [PubMed] [Google Scholar]
- 2. Klevebro S, Lundgren P, Hammar U, Smith LE, Bottai M, Domellof M, et al. Cohort study of growth patterns by gestational age in preterm infants developing morbidity. BMJ Open 2016; 6: e012872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin CR. Preventing bioenergetic failure in the preterm infant. Arch Dis Child Fetal Neonatal Ed 2016; 101: F99–101. [DOI] [PubMed] [Google Scholar]
- 4. Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006; 117: 1253–61. [DOI] [PubMed] [Google Scholar]
- 5. Cortez J, Makker K, Kraemer DF, Neu J, Sharma R, Hudak ML. Maternal milk feedings reduce sepsis, necrotizing enterocolitis and improve outcomes of premature infants. J Perinatol 2018; 38: 71–4. [DOI] [PubMed] [Google Scholar]
- 6. Corpeleijn WE, Kouwenhoven SM, Paap MC, van Vliet I, Scheerder I, Muizer Y, et al. Intake of own mother's milk during the first days of life is associated with decreased morbidity and mortality in very low birth weight infants during the first 60 days of life. Neonatology 2012; 102: 276–81. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization . Global strategy for infant and young child feeding. Geneva, Switzerland: World Health Organization, 2003. [Google Scholar]
- 8. Ericson J, Flacking R, Hellstrom‐Westas L, Eriksson M. Changes in the prevalence of breast feeding in preterm infants discharged from neonatal units: a register study over 10 years. BMJ Open 2016; 6: e012900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol 2005; 25: 341–8. [DOI] [PubMed] [Google Scholar]
- 10. Dasgupta S, Arya S, Choudhary S, Jain SK. Amniotic fluid: Source of trophic factors for the developing intestine. World J Gastrointest Pathophysiol 2016; 7: 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Englund JA. The influence of maternal immunization on infant immune responses. J Comp Pathol 2007; 137(Suppl 1): S16–9. [DOI] [PubMed] [Google Scholar]
- 12. Clapp DW, Kliegman RM, Baley JE, Shenker N, Kyllonen K, Fanaroff AA, et al. Use of intravenously administered immune globulin to prevent nosocomial sepsis in low birth weight infants: report of a pilot study. J Pediatr 1989; 115: 973–8. [DOI] [PubMed] [Google Scholar]
- 13. Lewis ED, Richard C, Larsen BM, Field CJ. The importance of human milk for immunity in preterm infants. Clin Perinatol 2017; 44: 23–47. [DOI] [PubMed] [Google Scholar]
- 14. Saarinen UM, Kajosaari M. Breastfeeding as prophylaxis against atopic disease: prospective follow‐up study until 17 years old. Lancet 1995; 346: 1065–9. [DOI] [PubMed] [Google Scholar]
- 15. Koletzko S, Sherman P, Corey M, Griffiths A, Smith C. Role of infant feeding practices in development of Crohn's disease in childhood. BMJ 1989; 298: 1617–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mayer EJ, Hamman RF, Gay EC, Lezotte DC, Savitz DA, Klingensmith GJ. Reduced risk of IDDM among breast‐fed children. The Colorado IDDM Registry. Diabetes 1988; 37: 1625–32. [DOI] [PubMed] [Google Scholar]
- 17. Davis MK, Savitz DA, Graubard BI. Infant feeding and childhood cancer. Lancet 1988; 2: 365–8. [DOI] [PubMed] [Google Scholar]
- 18. Neville MC, Keller RP, Seacat J, Casey CE, Allen JC, Archer P. Studies on human lactation I. Within‐feed and between‐breast variation in selected components of human milk. Am J Clin Nutr 1984; 40: 635–46. [DOI] [PubMed] [Google Scholar]
- 19. Weber A, Loui A, Jochum F, Buhrer C, Obladen M. Breast milk from mothers of very low birthweight infants: variability in fat and protein content. Acta Paediatr 2001; 90: 772–5. [PubMed] [Google Scholar]
- 20. Stoltz Sjöström E, Öhlund I, Tornevi A, Domellöf M. Intake and macronutrient content of human milk given to extremely preterm infants. J Hum Lact 2014; 30: 442–9. [DOI] [PubMed] [Google Scholar]
- 21. Bitman J, Wood L, Hamosh M, Hamosh P, Mehta NR. Comparison of the lipid composition of breast milk from mothers of term and preterm infants. Am J Clin Nutr 1983; 38: 300–12. [DOI] [PubMed] [Google Scholar]
- 22. Peila C, Moro GE, Bertino E, Cavallarin L, Giribaldi M, Giuliani F, et al. The effect of Holder pasteurization on nutrients and biologically‐active components in donor human milk: A review. Nutrients 2016; 8: 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lonnerdal B. Bioactive proteins in human milk: mechanisms of action. J Pediatr 2010; 156(Suppl 2): S26–30. [DOI] [PubMed] [Google Scholar]
- 24. Wada Y, Lonnerdal B. Bioactive peptides derived from human milk proteins–mechanisms of action. J Nutr Biochem 2014; 25: 503–14. [DOI] [PubMed] [Google Scholar]
- 25. Wada Y, Lonnerdal B. Bioactive peptides released from in vitro digestion of human milk with or without pasteurization. Pediatr Res 2015; 77: 546–53. [DOI] [PubMed] [Google Scholar]
- 26. Stromqvist M, Falk P, Bergstrom S, Hansson L, Lonnerdal B, Normark S, et al. Human milk kappa‐casein and inhibition of Helicobacter pylori adhesion to human gastric mucosa. J Pediatr Gastroenterol Nutr 1995; 21: 288–96. [DOI] [PubMed] [Google Scholar]
- 27. Lonnerdal B, Lien EL. Nutritional and physiologic significance of alpha‐lactalbumin in infants. Nutr Rev 2003; 61: 295–305. [DOI] [PubMed] [Google Scholar]
- 28. Akinbi H, Meinzen‐Derr J, Auer C, Ma Y, Pullum D, Kusano R, et al. Alterations in the host defense properties of human milk following prolonged storage or pasteurization. J Pediatr Gastroenterol Nutr 2010; 51: 347–52. [DOI] [PubMed] [Google Scholar]
- 29. Goldman AS. The immune system of human milk: antimicrobial, antiinflammatory and immunomodulating properties. Pediatr Infect Dis J 1993; 12: 664–71. [DOI] [PubMed] [Google Scholar]
- 30. Suzuki K, Nakajima A. New aspects of IgA synthesis in the gut. Int Immunol 2014; 26: 489–94. [DOI] [PubMed] [Google Scholar]
- 31. Hanson LA, Korotkova M, Telemo E. Breast‐feeding, infant formulas, and the immune system. Ann Allergy Asthma Immunol 2003; 90(Suppl 3): 59–63. [DOI] [PubMed] [Google Scholar]
- 32. Christen L, Lai CT, Hartmann B, Hartmann PE, Geddes DT. The effect of UV‐C pasteurization on bacteriostatic properties and immunological proteins of donor human milk. PLoS ONE 2013; 8: e85867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davidson LA, Lonnerdal B. Persistence of human milk proteins in the breast‐fed infant. Acta Paediatr Scand 1987; 6: 733–40. [DOI] [PubMed] [Google Scholar]
- 34. Suzuki YA, Lopez V, Lonnerdal B. Mammalian lactoferrin receptors: structure and function. Cell Mol Life Sci 2005; 62: 2560–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lopez V, Suzuki YA, Lonnerdal B. Ontogenic changes in lactoferrin receptor and DMT1 in mouse small intestine: implications for iron absorption during early life. Biochem Cell Biol 2006; 84: 337–44. [DOI] [PubMed] [Google Scholar]
- 36. Ward PP, Mendoza‐Meneses M, Cunningham GA, Conneely OM. Iron status in mice carrying a targeted disruption of lactoferrin. Mol Cell Biol 2003; 23: 178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bullen JJ. Iron‐binding proteins in milk and resistance to Escherichia coli infection in infants. Proc R Soc Med 1972; 65: 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ellison RT 3rd, Giehl TJ, LaForce FM. Damage of the outer membrane of enteric gram‐negative bacteria by lactoferrin and transferrin. Infect Immun 1988; 56: 2774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liao Y, Jiang R, Lonnerdal B. Biochemical and molecular impacts of lactoferrin on small intestinal growth and development during early life. Biochem Cell Biol 2012; 90: 476–84. [DOI] [PubMed] [Google Scholar]
- 40. Spadaro M, Caorsi C, Ceruti P, Varadhachary A, Forni G, Pericle F, et al. Lactoferrin, a major defense protein of innate immunity, is a novel maturation factor for human dendritic cells. FASEB J 2008; 22: 2747–57. [DOI] [PubMed] [Google Scholar]
- 41. Lonnerdal B, Jiang R, Du X. Bovine lactoferrin can be taken up by the human intestinal lactoferrin receptor and exert bioactivities. J Pediatr Gastroenterol Nutr 2011; 53: 606–14. [DOI] [PubMed] [Google Scholar]
- 42. Chatterton DE, Nguyen DN, Bering SB, Sangild PT. Anti‐inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int J Biochem Cell Biol 2013; 45: 1730–47. [DOI] [PubMed] [Google Scholar]
- 43. Arroyo G, Ortiz Barrientos KA, Lange K, Nave F, Miss Mas G, Lam Aguilar P, et al. Effect of the various steps in the processing of human milk in the concentrations of IgA, IgM, and lactoferrin. Breastfeed Med 2017; 12: 443–5. [DOI] [PubMed] [Google Scholar]
- 44. Manzoni P, Stolfi I, Messner H, Cattani S, Laforgia N, Romeo MG, et al. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: a randomized controlled trial. Pediatrics 2012; 129: 116–23. [DOI] [PubMed] [Google Scholar]
- 45. Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H, et al. Bovine lactoferrin supplementation for prevention of late‐onset sepsis in very low‐birth‐weight neonates: a randomized trial. JAMA 2009; 302: 1421–8. [DOI] [PubMed] [Google Scholar]
- 46. Akin IM, Atasay B, Dogu F, Okulu E, Arsan S, Karatas HD, et al. Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T‐regulatory cells. Am J Perinatol 2014; 31: 1111–20. [DOI] [PubMed] [Google Scholar]
- 47. Ochoa TJ, Zegarra J, Cam L, Llanos R, Pezo A, Cruz K, et al. Randomized controlled trial of lactoferrin for prevention of sepsis in peruvian neonates less than 2500 g. Pediatr Infect Dis J 2015; 34: 571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Manzoni P, Meyer M, Stolfi I, Rinaldi M, Cattani S, Pugni L, et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very‐low‐birth‐weight neonates: a randomized clinical trial. Early Hum Dev 2014; 90(Suppl 1): S60–5. [DOI] [PubMed] [Google Scholar]
- 49. Manzoni P. Clinical benefits of lactoferrin for infants and children. J Pediatr 2016; 173(Suppl): S43–52. [DOI] [PubMed] [Google Scholar]
- 50. Sherman MP, Sherman J, Arcinue R, Niklas V. Randomized control trial of human recombinant lactoferrin: A substudy reveals effects on the fecal microbiome of very low birth weight infants. J Pediatr 2016; 173(Suppl): S37–42. [DOI] [PubMed] [Google Scholar]
- 51. Sherman MP, Adamkin DH, Niklas V, Radmacher P, Sherman J, Wertheimer F, et al. Randomized controlled trial of Talactoferrin oral solution in preterm infants. J Pediatr 2016; 175: 68–73 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pammi M, Suresh G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2017; 6: CD007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sakurai T, Hashikura N, Minami J, Yamada A, Odamaki T, Xiao JZ. Tolerance mechanisms of human‐residential bifidobacteria against lysozyme. Anaerobe 2017; 47: 104–10. [DOI] [PubMed] [Google Scholar]
- 54. Schanler RJ, Goldblum RM, Garza C, Goldman AS. Enhanced fecal excretion of selected immune factors in very low birth weight infants fed fortified human milk. Pediatr Res 1986; 20: 711–5. [DOI] [PubMed] [Google Scholar]
- 55. Jiang R, Lonnerdal B. Biological roles of milk osteopontin. Curr Opin Clin Nutr Metab Care 2016; 19: 214–9. [PubMed] [Google Scholar]
- 56. Schack L, Lange A, Kelsen J, Agnholt J, Christensen B, Petersen TE, et al. Considerable variation in the concentration of osteopontin in human milk, bovine milk, and infant formulas. J Dairy Sci 2009; 92: 5378–85. [DOI] [PubMed] [Google Scholar]
- 57. Nagatomo T, Ohga S, Takada H, Nomura A, Hikino S, Imura M, et al. Microarray analysis of human milk cells: persistent high expression of osteopontin during the lactation period. Clin Exp Immunol 2004; 138: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lonnerdal B, Kvistgaard AS, Peerson JM, Donovan SM, Peng YM. Growth, nutrition, and cytokine response of breast‐fed infants and infants fed formula with added bovine osteopontin. J Pediatr Gastroenterol Nutr 2016; 62: 650–7. [DOI] [PubMed] [Google Scholar]
- 59. Yamniuk AP, Burling H, Vogel HJ. Thermodynamic characterization of the interactions between the immunoregulatory proteins osteopontin and lactoferrin. Mol Immunol 2009; 46: 2395–402. [DOI] [PubMed] [Google Scholar]
- 60. Moller HK, Thymann T, Fink LN, Frokiaer H, Kvistgaard AS, Sangild PT. Bovine colostrum is superior to enriched formulas in stimulating intestinal function and necrotising enterocolitis resistance in preterm pigs. Br J Nutr 2011; 105: 44–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 References.


