Abstract
Obtaining reliable longitudinal information about everyday functioning from individuals with Parkinson’s disease (PD) in natural environments is critical for clinical care and research. Despite advances in mobile health technologies, implementation of digital outcome measures is hindered by a lack of consensus on the type and scope of measures, the most appropriate approach for data capture (e.g. in clinic or at home), and the extraction of timely information that meets the needs of patients, clinicians, caregivers, and healthcare regulators. The MDS Task Force on Technology proposes the following objectives to facilitate the adoption of mobile health technologies: (1) identification of patient-centered and clinically-relevant digital outcomes; (2) selection criteria for device combinations that offer an acceptable benefit-to-burden ratio to patients and that deliver reliable, clinically relevant insights; (3) development of an accessible, scalable, and secure platform for data integration and data analytics; and (4) agreement on a pathway for approval by regulators, adoption into e-health systems and implementation by healthcare organizations. We have developed a tentative roadmap that addresses these needs by providing the following deliverables: (1) results and interpretation of an online survey to define patient-relevant endpoints; (2) agreement on the selection criteria for use of device combinations; (3) an example of an open-source platform for integrating mobile health technology output; and (4) recommendations for assessing readiness for deployment of promising devices and algorithms suitable for regulatory approval. This concrete implementation guidance, harmonizing the collaborative endeavor among stakeholders, can improve assessments of individuals with PD, tailor symptomatic therapy, and enhance healthcare outcomes.
Keywords: Mobile health technologies, wearable technology, Parkinson disease, remote monitoring
INTRODUCTION
In 2016, the International Parkinson and Movement Disorders Society Task Force on Technology published a summary of the challenges and opportunities related to the integration of technologies into the clinical management of PD.1 Despite the increasing miniaturization and portability of mobile health technologies, and despite the worldwide increase in deployment of commercially available devices,2 there remains a large gap in their adoption and wide-scale implementation in both care and research.3 The mission of this Task Force is to develop a framework for the development, accessibility, and long-term adherence of mobile health technologies to enhance care and research objectives related to PD. For this purpose, mobile health technologies are defined here as “wearable” or portable devices that can provide objective measures and that include mobile and digital applications, as well as body-worn (adhered to a body surface) or frequently used (e.g., smartphone) patient-centered devices. This inclusion of mobile and digital interfaces captures the spectrum of devices used by patients irrespective of how they are worn. The final product, as outlined below, is meant to integrate the needs of all stakeholders but be flexible enough to adapt to individual patient needs.
Mobile health technologies have to date integrated only partially into clinical practice and clinical trials, which continue to rely on clinical scales and episodic assessments made in somewhat artificial environments, for primary and secondary endpoints.4,5 People with PD are particularly prone to performance bias,6 as exemplified by the improved performance of their movements when observed in clinical practice. In order to develop digital outcomes with functional relevance to patients, further proliferation of independent devices that merely target narrow, disconnected aspects of behavior (e.g., number of steps) need to be reconsidered in favor of comprehensive longitudinal tracking of patient-centered motor and non-motor data, in home and community settings. Interoperable platforms with communication standards that integrate different devices by providing application programming interfaces (APIs)7 stand to facilitate regulatory approval and adoption by health care organizations.
The purpose of this position paper is to propose a roadmap for the development of patient-centered digital outcomes and their integration into both clinical care8 and research that is sensitive to the needs of all relevant stakeholders, most critically patients. The final product aims to facilitate patient self-monitoring, clinician-based tailoring of symptomatic therapy, and also to serve as objective endpoints in clinical research, initially as surrogate and exploratory outcomes, but with time perhaps even as primary outcomes. We envision that the roadmap proposed here will likely influence these important areas of research and clinical management. Important related areas that are outside the purview of this roadmap are the use of mobile health technologies for supporting the clinical diagnosis (or aim at serving as a diagnostic test), measuring the underlying neurodegenerative progression (as disentangled from fluctuations in motor or non-motor behaviors),9 detecting prodromal symptoms at a population level,10,11 integrating digital outcomes into closed-loop treatment systems to assist in timing and dosage of therapy, or selecting patient subgroups for testing of future disease-modifying treatments.
Current gaps in the use of mobile health technologies
Digital measures derived from wearables/mobile technologies and applications are slowly starting to emerge as secondary or exploratory outcome measures in the context of clinical trials4,12 and, to a lesser extent, as treatment targets in clinical care. Most often, digital outcomes have been developed: (1) to capture constructs of interest in isolation (e.g., tremor or bradykinesia)13 without “painting a global picture” and also without focusing on patient-centered outcomes; (2) by developers working in isolation from patients, clinicians or scientific societies; and (3) excluding the wide range of non-motor features, which are prominent sources of disability for many patients. Adoption of mobile health technologies has been hindered by the presentation and interpretation of the data, often in relation to a population mean rather than to a patient’s own baseline, and disconnected from patients’ functional disability levels. Patient compliance and technology illiteracy have been poorly addressed, particularly when it comes to wearing multiple sensors for long periods of time. An exception was a recent study that addressed the long-term compliance of patients to wear a smartwatch, a body-worn sensor, and a smartphone; in this study, a helpdesk to support patients proved a critical strategy to improve adherence.14 Another pitfall is the aspirational development of mobile health technologies as fulfilling diagnostic needs. While technologies can be harnessed for validating patient-centered outcomes and for supporting a clinical diagnosis, they remain inadequate as stand-alone measures for “diagnostic accuracy”. Aiming at fulfilling this goal perpetuates the concept that the many molecular subtypes subsumed within the clinical diagnosis of PD can be unified by an “ideal” set of behavioral features.
Validation.
A separate challenge exists for the process of validation of mobile health technologies since we would expect them to be more discriminative or sensitive than previously developed clinical scales for motor and non-motor symptoms. A classic validation paradigm would require the outcomes of mobile technology to “correlate” with these “gold standard” clinical scales, but this should not necessarily be the case. Currently available scales may function well to capture differences at the group level but may be less suitable to capture changes within an individual. This is where mobile technologies have the potential to excel, perhaps justifying imperfect correlations with clinical scales simply because the objective measurements outperform the more subjective clinical assessments, which are prone to substantial inter- and intra-rater variability. In other words, large differences or big detection gaps between digital outcomes and existing scales are in fact desirable because both capture different and perhaps even complementary domains (as, for example, the “Mobile Parkinson Disease Score” obtained with smartphones and data analyzed with machine learning).17 To be validated, nevertheless, mobile health technologies will require such aspects as accuracy (laboratory validity), reliability (test-retest within and between sensors), sensitivity, and minimal clinically significant difference for any endpoint of interest when tested against direct patient input or any robust measure of clinical meaningfulness (e.g., a pull test to compare a new digital biomarker for balance). For clinical studies, the greater precision will allow greater signal-to-noise ratio for endpoints of interest and a subsequent reduction in the number of subjects required for enrolling into clinical trials.
Integration and standardization.
Currently, to comprehensively capture several motor and non-motor measures, clinicians need to combine multiple mobile health technologies from different, non-compatible manufacturers, operating on separate platforms. Moreover, the unsupervised, unstructured setting in which wearable-derived measures are obtained introduces confounding variables that cannot be as easily controlled as in the well-structured, more “repeatable” environment of a clinic or research laboratory. These challenges to interpretation and scoring are believed to be outweighed by the ostensible superiority of continuous monitoring and the increasing reliance on big data, according to which sophisticated analytic systems can extract signals of presumed relevance from background noise, and as such supplement careful history and neurological examination. A word of caution here is that “big data” do not necessarily equate to “good data”, and that spurious and irrelevant conclusions can be reached from big data analytics designed to “uncover hidden patterns”.16
Unmet needs and the required levels of development
This Task Force consensus paper addresses two major unmet needs regarding the interface between technology and clinical evaluation for care and research. One is the ability to use multiple devices to capture data collected in “free-living” conditions that are relevant to the patient’s functioning. The other is the data integration into open-source systems designed to generate individualized feedback to patients, clinicians, researchers, and caregivers. Such developments should be responsive to the needs of all stakeholders caring for PD and should include a pathway for regulatory approval, adequate licensing protection, appropriate reimbursement, and wide patient access using a model that allows for sustainability and growth.
In particular, we address four specific needs:
(1). Defining relevant patient-centered digital targets and outcomes to be captured with mobile health technologies (What to measure)
Behavioral measurements should be relevant to the patient. This patient-centered scope maximizes the likelihood of acquiring data that facilitate clinically important decision-making by the clinician and promote long-term adherence by the patient. Furthermore, it defines a major boundary: not anything that can be measured should be measured. Patient-centered measurement of the and presence severity of motor and non-motor symptoms should focus on how they impair activities of daily living (ADLs).18 Instrumental ADLs can include symptoms (“impairments”) and activities (“disabilities”), the latter being typically more reliable for self-reporting measures.19 Domains deemed patient-relevant cannot be identified by clinicians or researchers alone. Nonetheless, selected non-motor endpoints, such as sleep and heart rhythm monitors – both included in most common smartwatches – can be made available for shared decision making because of their clear relevance, even when patients do not prioritize these domains themselves.
(2). Selection criteria to guide the choice of mobile health technology (How to measure)
Mobile health technologies should be unobtrusive to patients and capable of capturing the phenomena of interest at intervals that balance patient burden and accuracy. Besides validation issues (this is covered by point 4), a critical component of developing useful mobile health technologies is the standardization of sensor measurements to create an established, broadly accepted common set of metrics. Addressing the critical questions of minimal precision and uniformity of instruments (e.g., is 1m/s captured with device A the same as with device B?) as well as the reliance on one versus multiple sensors are vital and could help establish minimal guidelines for adequacy of sensors. In addition, optimal outcomes of algorithms will depend on the adequate balance between prescribed, action-dependent tasks (such as finger tapping, spiral drawing, digital diaries) and natural, action-independent behaviors (i.e. passive tracking) and the contextual environment where some measurements occur (specifically, in supervised versus unsupervised settings).
(3). Web-based, open-source, modular, scalable and secure platforms for data analysis, integration, and visualization (What to display)
Patient-centered and clinically relevant digital outcomes collected through mobile health technologies would ideally be analyzed to anticipate periods of impairment in ADL or instrumental ADLs before they occur, helping to individualize both reactive and proactive/preemptive clinical decisions. Such analysis should be integrated and summarized in a display format that is individualized, palatable, and visually intuitive in the context of real-life conditions so that it serves the needs of the end users (i.e., patients and the professional team that treats them). Moreover, by not affecting proprietary algorithms from a source device, an open source/open access concept could stimulate developmental aspects of sensors, software, algorithms, visualization and communication tools, while promoting licensing as well as protecting intellectual property.
(4). Establish a roadmap for regulatory approval and adoption into health care systems (How to disseminate)
Demonstration of the utility to providers and patients, and efficiencies in data processing will facilitate the integration of mobile health technologies into digital health models with subsequent approval by regulators and adoption by healthcare delivery organizations. Its integration with digital applications will be critical given the increasing relevance of telemedicine and other types of medicine that use data from the home environment, in replacing or supplementing traditional models of patient visits with health professionals.
Proposed roadmap
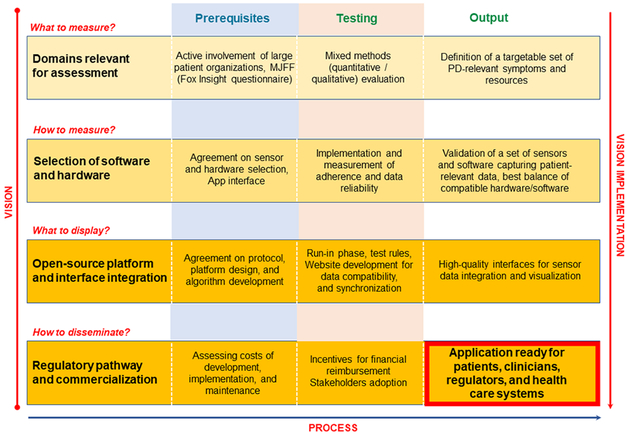
The MDS Task Force on Technology has developed a tentative roadmap that addresses these four defined needs by providing the following milestones and deliverables. Both the “vision” and “process” for this roadmap were conceptualized by consensus among Task Force members, with milestones representing the vision and developmental steps representing the process (Figure 1).
Figure 1. Levels and phases of development of patient-relevant mobile health technologies.
Early to late milestones (“vision”) are organized vertically, from top to bottom, and tentative high-level technical steps horizontally, from left to right (“process”).
1). Relevant patient-centered digital targets and outcomes
To determine patient-defined targets and outcomes, adaptable to individual patient needs, data derived from surveys of over 10,000 patients through the Michael J Fox Foundation’s Fox Insight project has been identified to extract patient-relevant targets. Pioneer work in this cohort used free text responses to the questions “What bothers you the most about your PD?” and “In what way does this problem bother you by affecting your daily functioning?” The answers were analyzed with natural language processing and machine learning approaches and identified four motor (tremor, rigidity, bradykinesia, postural instability) and six non-motor (sleep, fatigue, cognition, mood, pain, constipation) symptoms as patient-relevant.20 Additional patient cohorts should also be considered to account for geographical and social diversity.21 Importantly, the standardization of patient-relevant outcomes poses the risk of losing individualization and will require safeguard mechanisms to ensure that outcomes are tailored to individual patient needs. Some domains may benefit from a clinician’s perspective to avoid overlooking important phenomena patients may not recognize when they are just emerging (e.g., freezing of gait, eye motility disturbances, postural deficits with deviations of the center of mass, impulsivity, multitasking deficits and reduced attention contributing to postural instability and falls, and nocturnal events/dream enactment behaviors), progressively subtle changes (e.g., gradual changes in physical activity), potentially severe events (e.g., complex dyskinesias, and behavioral fluctuations), and non-motor manifestations (e.g., nocturnal monitoring for sleep-related movements, skin impedance or heart-rate variability for autonomic impairment). Personalization through digital diaries (e-diaries) could serve to expand the range of the latter category. The MDS Task Force on Technology will invite patients, caregivers and representatives of advocacy organizations to further refine these patient-centered digital targets/outcomes.
2). Proposal for selection criteria for useful sensors/hardware combinations
Selection criteria for data collection in real life and real time must address the needs of the user (e.g., comfort and user requirements to achieve good compliance), other stakeholders (e.g., provide research-grade sensitive and specific outcomes) and technical aspects (e.g., battery life, data storage, compatibility with other systems). Also, if the monitoring period is to be over years and/or patients have to wear more than one device, periodic monitoring may be more successful than continuous assessment. While continuous monitoring appears attractive, systematic evaluation of the benefits and trade-offs of longer (e.g., 1 month, 6 months) versus shorter (e.g., 1 day, 1 week) durations have not yet been determined. A set of criteria (Table 1) is proposed as the basis for the evaluation of potentially meaningful and relevant devices for use by various stakeholders. Mobile health technology developers will be asked to provide detailed information about these aspects when a system is considered for use by the community, and to satisfy platform compliance and assist regulatory needs.
Table 1.
User-based considerations for choosing data collection methods with mobile health technologies
| Patient/Caregiver | Healthcare provider | Researcher | |
|---|---|---|---|
| Number of sensors | Minimal number of sensors easy-to-access location/s | Number of sensors locations based on clinical purpose | Number location of sensors based on targeted accuracy |
| Sensor burden | Minimal patient caregiver burden over a long time | Minimal clinician burden over a long time | Potentially greater burden in patients clinicians over a short time |
| Frequency | Less frequent use to enhance adherence during data capture | Frequency depending on use of data | More frequent use to ensure, e.g., high signal to noise ratio |
| Targets | 1–2 domains at low- frequency intervals, based on identified problems | Possibly 1–2 domains at periodic intervals, according to patient’s clinician’s goals | Likely multiple domains at frequent intervals, according to research objectives |
| User friendliness | Easy to use, ready (ideally 24/7) access to helpdesk to facilitate compliance minimal manual skill level required to operate the system. | Easy to use in clinical practice; helpdesk to troubleshoot range of potential problems. Facilitate patient compliance by reviewing data. | Usability compliance – less of an issue for fully supervised sessions; will have to ensure ease of use to facilitate patient compliance for unsupervised monitoring |
| Supervised vs. unsupervised | Unsupervised data collection ensured by friendly, acceptable device to user | Reliance only on unsupervised data collection | Reliance on supervised unsupervised data collection |
| Desirable technical aspects | Long battery life, low charging, easy or automatic uploading downloading, small size, low weight, water-proof. Low level of expertise to use underst output. | Battery life, need for charging, size, weight less critical for supervised /short duration sessions. High level of expertise to analyze. | |
| Validation | Must show correlation with global patient-centered scales for the appropriate domains. | Monitoring of motor fluctuations medication titration. | May not strongly correlate with the total or even specific items of such gold stards as the UPDRS. Observation or video analysis may be needed. |
3). Open-source platform standards for mobile health technologies
Innovative mobile health technologies have data platforms that transfer measured outcomes or targets to the user, which can be, depending on the application, the patient (via, e.g., the electronic health record)7,8 or caregiver, members of the healthcare provider team, or insurance or public organizations. Unfortunately, a joint and interoperable platform standard does not exist, neither for technical features nor for adaptability to various clinical or health administration users. In collaboration with method experts from academy and industry, we will elaborate recommendations for a platform standard that could enable a centralized, open source web-based structure where mobile health technologies with different technology standards and requirements can be integrated. These recommendations will satisfy the following criteria: (1) user-friendly accessibility; (2) support of scalability and exchangeability; (3) data storage meeting regulatory and security standards; (4) periodic evaluation of calibration, with validation strategies supporting evolving technologies and algorithms; and (5) data access and sharing to include governance and right management regarding privacy and ownership/licensing of medical data. Such a process for data platform standardization will ensure adequate data management for self-management (of the patient), care and research applications, support regulatory bodies by providing testable quality standards for licensing, and certification and approval procedures of new healthcare technologies.
The MDS Task Force on Technology in collaboration with the Rating Scales Electronic Development Ad Hoc Committee is developing an early, proof of concept “integration” effort through the development of an e-Diary/Tracker for PD. Such an initiative could be an example for future efforts and serve to answer two critical questions: (1) Can a central body such as MDS invest the necessary resources for the development, maintenance, and administration (including dynamic decision-making on which elements to include or exclude) of a needed interoperable open source security-compliant platform into which mobile health technologies integrate? (2) Should MDS develop criteria for existing and emerging applications as “clinically meaningful” and “sufficiently accurate” about which sensitivity, specificity, and diagnostic accuracy cannot be ascertained given the problems with using current clinician-rated rating scales as the gold standard?
4). Regulatory pathways and commercialization
We expect that standards for integrated healthcare technology platforms will increase the adoption of mobile health technologies into clinical care and clinical trials. Unifying open source platforms are expected to encourage commercial developers to further innovate both the hardware and proprietary algorithms since they can read and synchronize data from multiple devices. Furthermore, such platforms will need to be accessible and user-friendly by all users to allow clinicians and patients to shop for any combination of devices, from any company, in the hope of finding whichever meets their individual needs. Importantly, they should also be attractive to companies as a “marketplace” in which a range of hardware solutions competes for patients’ and clinicians’ interests, without concerns for inter-device compatibility or ability to synchronize data from other devices. Future research studies will have to show the cost-effectiveness of mobile health technologies to justify that any investments in hardware or IT connections are offset by lower healthcare expenditures and better outcomes. For example, it will be helpful to demonstrate that more personalized care leads to improved health of patients and reductions in the number of planned or unplanned hospital admissions. Such research should also provide a benchmark for discussion between payers and commercial companies, ascertaining reasonable pricing that will help with its implementation and long-term sustainability.
Anticipated Challenges
An obvious and enormous challenge ahead is the change in behavior required of both patients and professionals to ensure widespread adoption and long-term use and of regulators and health care systems to maintain such platforms. The financial model must be sustainable and the full extent of regulatory hurdles fully understood and overcome. To this end, the ownership of running, managing, and administrating open source platforms would be ideal for an organization such as MDS to take on. A pilot use of competing platforms might be considered as an exploratory step to inform the one providing the best clinical value and integration with developers as well as technology and healthcare industries. The final major challenge is that of seamlessly coupling the outcome of mobile health technologies to the existing IT infrastructure at hospitals such as in electronic medical records as well as determining the financial costs associated with using and maintaining (updating) periodic technological improvements.
CONCLUSIONS AND NEXT STEPS
The time has come to further develop and integrate mobile health technologies into the routine assessment and care of patients with PD. The improvements in sophistication, versatility, and wearability of these technologies have reached a state of maturity that is adequate for the collection of patient-relevant data. To harness these opportunities, the MDS Task Force on Technology recommends that mobile health technologies will need to: (1) target deficits confirmed to be relevant to patients; (2) be derived from ideally a single or else a combination of devices that deliver an acceptable benefit-to-burden ratio to patients and, at the same time, yield clinically useful information; (3) be integrated and synchronized into patient management platform standards, delivering individualized data to patients, caregivers, and clinicians; and (4) be approved by regulators, weaved into digital health systems, and uniformly adopted by health care delivery organizations. Added value and appropriate cost-benefit ratios still need to be determined before MDS, or any other PD-focused organization in its place can assume the ownership of hiring expert technology services to run a platform and coordinate its maintenance and administration (including dynamic decision-making on which elements to include or exclude). This collaborative endeavor will encourage the development of integrated, multi-channel systems that can achieve more sophisticated characterization of patients’ function, better tailoring of symptomatic therapy, greater patient engagement and self-assessment by patients, and overall improved health care outcomes.
Financial disclosures
Dr. Espay has received grant support from the NIH, Great Lakes Neurotechnologies and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, Adamas, Acadia, Acorda, Neuroderm, Impax, Sunovion, Lundbeck, Osmotica Pharmaceutical, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from USWorldMeds, Lundbeck, Acadia, Sunovion, the American Academy of Neurology, and the Movement Disorders Society. He serves as Chair of the MDS Task Force on Technology.
Dr. Hausdorff has received grant support from the NIH, the Michael J Fox Foundation for Parkinson’s Research, the EU (H2020), the BSF, the Israeli Science Foundation and the National Multiple Sclerosis Society. He has or currently serves on the Movement Disorders Society Technology Task Force and on Michael J Fox Foundation task force on gait, on the board of the the International Society for the Measurement of Physical Behavior, and on advisory boards for Sanofi and Biogen. He has submitted a patent application on the use of body-fixed sensors for assessing PD symptoms, the intellectual property rights for which are held by the Tel Aviv Medical Center
Dr. Sanchez-Ferro research is sponsored by the “Consejería de Educación, Juventud y Deporte of Comunidad de Madrid” and the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007–2013) under REA agreeement n 291820. He holds a patent on a Method and Apparatus for Motor Function Characterization and has received spaker honoraria from Teva, Zambon, Abbvie, Alter and Novartis pharmaceutica. Dr. Sánchez-Ferro is the current Chair of the Rating Scales Electronic Development Ad Hoc Committee.
Dr. Klucken has received institutional research grants from Bavarian Research Foundation; Emerging Field Initiative, FAU; EIT-Health; EIT-Digital; EU (H2020), German Research Foundation (DFG); BMBF. Industry sponsored institutional IITs and grants from Teva GmbH; Licher MT GmbH; Astrum IT GmbH; Alpha-Telemed AG. He works on advisory boards in the field of healthcare technologies and digital health of different associations of medical professionals, industries, and political authorities. He holds shares of Portabiles HealthCare Technologies GmbH, Portabiles GmbH, Alpha-Telemed AG, and received compensation and honoraria from serving on scientific advisory boards for LicherMT GmbH, Abbvie GmbH, UCB Pharma GmbH, Athenion GmbH, and Thomashilfen GmbH; as well as lecturing from UCB Pharma GmbH, TEVA Pharma GmbH, Licher MT GmbH, Desitin GmbH, Abbvie GmbH, Solvay Pharmaceuticals, and Ever Neuro Pharma GmbH; Dr. Klucken has a patent related to gait assessments pending.
Dr. Merola is supported by NIH (KL2 TR001426) and has received speaker honoraria from CSL Behring, Cynapsus Therapeutics, Lundbeck, AbbVie, and Abbott. He has received grant support from Lundbeck and Abbott.
Dr. Bonato has received grant support from the American Heart Association, the Department of Defense, the Michael J Fox Foundation, the National Institutes of Health, the National Science Foundation, and the Peabody Foundation including sub-awards on SBIR grants to Barrett Technology (Newton MA), BioSensics (Watertown MA) and Veristride (Salt Lake City UT). He has also received grant support from Emerge Diagnostics (Carlsbad CA), MC10 (Lexington MA), Mitsui Chemicals (Tokyo Japan), Pfizer (New York City NY), Shimmer Research (Dublin Ireland), and SynPhNe (Singapore). He serves in an advisory role the Michael J Fox Foundation, the NIH-funded Center for Translation of Rehabilitation Engineering Advances and Technology, and the NIH-funded New England Pediatric Device Consortium. He also serves on the Scientific Advisory Boards of Hocoma AG (Zurich Switzerland) and Trexo (Toronto Canada) in an uncompensated role.
Dr. Paul has received grant funding from The University of Sydney.
Dr. Horak has financial interest in ADPM, a company with commercial interests in technology. This conflict has been reviewed and managed by OHSU.
Dr. Vizcarra has nothing to disclose.
Dr. Mestre receives grants from University of Ottawa Medical Associates, Parkinson Canada, Parkinson Research Consortium, LesLois Foundation, PSI Foundation, U Ottawa Brain and Mind Institute and Michael J Fox Foundation, he provided consulting services to Abbvie, CHDI Foundation/Management, and has received honoraria for speaker engagement by U of Ottawa, Abbvie, International Parkinson and Movement Disorders Society. He received a salary from University of Ottawa Medical Associates.
Dr. Reilmann is founding director and owner of the George-Huntington-Institute, a private research institute focused on clinical and preclinical research in Huntington’s disease, and QuantiMedis, a clinical research organization providing Q-Motor (quantitative motor) services in clinical trials and research. He holds appointments at the Dept. of Radiology of the University of Muenster and at the Department of Neurodegenerative Diseases and Hertie-Institute for Clinical Brain Research, University of Tuebingen. Dr. Reilmann serves as elected member of the Steering Committees of the European Huntington Disease Network (EHDN) and the Huntington Study Group (HSG), co-chair of the Task Force on Huntington’s disease and member of the Task Force on Technology of the International Parkinson and Movement Disorder Society (IPMDS). He has provided consulting services, advisory board functions, clinical trial services, quantitative motor analyses, and/or lectures for Actelion Pharmaceuticals, Amarin Neuroscience, AOP Orphan Pharmaceuticals, Cure Huntington Disease Initiative Foundation (CHDI), Desitin, IONIS Pharmaceuticals, Ipsen, Lundbeck, Link Medicine, MEDA Pharma, Medivation, Mitoconix, Neurosearch, Novartis AG, Omeros, Pfizer, Prana Biotechnology, Prilenia, Raptor Pharmaceuticals, Roche, Siena Biotech, Temmler, Teva, uniQure, Vaccinex, Wave Life Sciences, and Wyeth Pharmaceuticals. He has received grant support from the Bundesministerium für Bildung und Forschung (BMBF), the Cure Huntington Disease Initiative Foundation (CHDI), the Deutsche Forschungsgemeinschaft (DFG), the Deutsches Zentrum für Neurodegeneration und Entzündung (DZNE), the European Union 7th Framework Program (EU-FP7), the European Huntington Disease Network (EHDN), the High-Q-Foundation, and the National Science Foundation (NSF).
Dr. Nieuwboer has received grant support from the European Union, Research Funds Flanders, KU Leuven BOF Research Funds, Jacques and Gloria Gossweiler Foundation, Kind Baudouin Foundation and the Michael J Fox Foundation.
Dr. Dorsey has received honoraria for speaking at American Academy of Neurology courses, American Neurological Association, and University of Michigan; received compensation for consulting services from 23andMe, Abbott, Abbvie, American Well, Biogen, Clintrex, DeciBio, Denali Therapeutics, GlaxoSmithKline, Grand Rounds, Karger, Lundbeck, MC10, MedAvante, Medical-legal services, Mednick Associates, National Institute of Neurological Disorders and Stroke, Olson Research Group, Optio, Prilenia, Putnam Associates, Roche, Sanofi, Shire, Sunovion Pharma, Teva, UCB and Voyager Therapeutics; research support from Abbvie, Acadia Pharmaceuticals, AMC Health, Biosensics, Burroughs Wellcome Fund, Davis Phinney Foundation, Duke University, Food and Drug Administration, GlaxoSmithKline, Greater Rochester Health Foundation, Huntington Study Group, Michael J. Fox Foundation, National Institutes of Health/National Institute of Neurological Disorders and Stroke, National Science Foundation, Nuredis Pharmaceuticals, Patient-Centered Outcomes Research Institute, Pfizer, Prana Biotechnology, Raptor Pharmaceuticals, Roche, Safra Foundation, Teva Pharmaceuticals, University of California Irvine; editorial services for Karger Publications; and ownership interests with Blackfynn (data integration company) and Grand Rounds (second opinion service).
Dr. Rochester receives funding from the European Union; Medical Research Council, UK; Engineering and Physical Sciences Research Council, UK; National Institute of Health Research, UK; Parkinson’s UK, Stroke Association; Alzheimers Society, UK; and has participated in Advisory Boards of Biogen.
Prof. Bloem currently serves as Associate Editor for the Journal of Parkinson’s disease, serves on the editorial of Practical Neurology, has received honoraria from serving on the scientific advisory board for Abbvie, Biogen, UCB and Walk with Path, has received fees for speaking at conferences from AbbVie, Zambon and Bial, and has received research support from the Netherlands Organization for Scientific Research, the Michael J Fox Foundation, UCB, Abbvie, the Stichting Parkinson Fonds, the Hersenstichting Nederland, the Parkinson’s Foundation, Verily Life Sciences, Horizon 2020, the Topsector Life Sciences and Health, and the Parkinson Vereniging. He is currently an officer (secretary-elect) for the MDS.
Dr. Maetzler receives or received funding from the European Union, the Michael J. Fox Foundation, Robert Bosch Foundation, Abbvie, Neuroalliance, Lundbeck and Janssen, and holds part of a patent for the assessment of dyskinesias (German patent office, 102015220741.2). He received speaker honoraria from GlaxoSmithKline, Abbvie, Bayer, UCB, Licher MT and Rölke Pharma, and was invited to Advisory Boards of Lundbeck, Market Access & Pricing Strategy GmbH, Abbvie and Biogen. He serves as co-chair of the MDS Technology Task Force.
Footnotes
Financial disclosures related to research covered in this article: A workshop for completion of this article took place in Hong Kong, on October 9, 2019, after the MDS Annual Meeting and Room charges and room accommodations were covered for the participants.
REFERENCES
- 1.Espay AJ, Bonato P, Nahab FB, et al. Technology in Parkinson’s disease: Challenges and opportunities. Mov Disord. 2016;31(9):1272–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godinho C, Domingos J, Cunha G, et al. A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson’s disease. J Neuroeng Rehabil. 2016;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Uem JM, Isaacs T, Lewin A, et al. A Viewpoint on Wearable Technology-Enabled Measurement of Wellbeing and Health-Related Quality of Life in Parkinson’s Disease. J Parkinsons Dis. 2016;6(2):279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artusi CA, Mishra M, Latimer P, et al. Integration of technology-based outcome measures in clinical trials of Parkinson and other neurodegenerative diseases. Parkinsonism Relat Disord. 2018;46 Suppl 1:S53–s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merola A, Sturchio A, Hacker S, et al. Technology-based assessment of motor and nonmotor phenomena in Parkinson disease. Expert Rev Neurother. 2018;18(11):825–845. [DOI] [PubMed] [Google Scholar]
- 6.Lord S, Godfrey A, Galna B, Mhiripiri D, Burn D, Rochester L. Ambulatory activity in incident Parkinson’s: more than meets the eye? J Neurol. 2013;260(12):2964–2972. [DOI] [PubMed] [Google Scholar]
- 7.Mandl KD, Kohane IS. A 21st-Century Health IT System - Creating a Real-World Information Economy. N Engl J Med. 2017;376(20):1905–1907. [DOI] [PubMed] [Google Scholar]
- 8.Mandl KD, Kohane IS. Time for a Patient-Driven Health Information Economy? N Engl J Med. 2016;374(3):205–208. [DOI] [PubMed] [Google Scholar]
- 9.Merchant KM, Cedarbaum JM, Brundin P, et al. A Proposed Roadmap for Parkinson’s Disease Proof of Concept Clinical Trials Investigating Compounds Targeting Alpha-Synuclein. J Parkinsons Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirelman A, Gurevich T, Giladi N, Bar-Shira A, Orr-Urtreger A, Hausdorff JM. Gait alterations in healthy carriers of the LRRK2 G2019S mutation. Ann Neurol. 2011;69(1):193–197. [DOI] [PubMed] [Google Scholar]
- 11.Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1600–1611. [DOI] [PubMed] [Google Scholar]
- 12.Lipsmeier F, Taylor KI, Kilchenmann T, et al. Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial. Mov Disord. 2018;33(8):1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heldman DA, Espay AJ, LeWitt PA, Giuffrida JP. Clinician versus machine: reliability and responsiveness of motor endpoints in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(6):590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva de Lima AL, Hahn T, Evers LJW, et al. Feasibility of large-scale deployment of multiple wearable sensors in Parkinson’s disease. PLoS One. 2017;12(12):e0189161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Fauw J, Ledsam JR, Romera-Paredes B, et al. Clinically applicable deep learning for diagnosis and referral in retinal disease. Nat Med. 2018;24(9):1342–1350. [DOI] [PubMed] [Google Scholar]
- 16.Frohlich H, Balling R, Beerenwinkel N, et al. From hype to reality: data science enabling personalized medicine. BMC Med. 2018;16(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhan A, Mohan S, Tarolli C, et al. Using Smartphones and Machine Learning to Quantify Parkinson Disease Severity: The Mobile Parkinson Disease Score. JAMA neurology. 2018;75(7):876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Organization WH. International Classification of Functioning, Disability and Health ICF Geneva. 2001;World Health Organization. [Google Scholar]
- 19.Bravell ME, Zarit SH, Johansson B. Self-reported activities of daily living and performance-based functional ability: a study of congruence among the oldest old. European journal of ageing. 2011;8(3):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arbatti L, Marras C, Standaert DG, Tanner CM, Nguyen A, Shoulson I. Problems That Bother Parkinson Patients: The Basis for a Patient-Reported Natural History. Ann Neurol. 2018;84:S1–S280. [Google Scholar]
- 21.Serrano JA, Larsen F, Isaacs T, et al. Participatory design in Parkinson’s research with focus on the symptomatic domains to be measured. J Parkinsons Dis. 2015;5(1):187–196. [DOI] [PubMed] [Google Scholar]
- 22.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]