Abstract
A systematic review and meta-analysis was conducted to determine the efficacy of women-focused ART adherence interventions. Included studies (a) reported on a behavioral ART adherence intervention for cis-women living with HIV, (b) measured ART adherence as an outcome, and (c) employed a randomized controlled trial design. Thirteen studies were included in the meta-analysis. Overall, interventions significantly improved ART adherence compared to control conditions (random-effects d=0.82, 95% CI [0.18, 1.45], p=0.01), however, this was largely driven by two studies that had effect sizes greater than 3 standard errors above the mean effect size. Key moderators were location, recruitment method, group-based intervention, and alteration of the healthcare system. Innovative behavioral interventions that focus on young women and adolescents, target the critical periods of pregnancy and postpartum and test the integration of multiple levels of intervention to create lasting effects on ART adherence are needed.
Keywords: meta-analysis, ART adherence, women, randomized controlled trials
Resumen
Se llevó a cabo una revisión sistemática y un metaanálisis para determinar la eficacia de las intervenciones de adherencia al tratamiento antirretroviral (TAR) centradas en las mujeres. Los estudios incluidos (a) informaron sobre una intervención conductual de adherencia a TAR para mujeres cisgénero que viven con VIH, (b) midieron la adherencia a TAR como resultado y (c) emplearon un diseño de ensayo controlado aleatorio. Se incluyeron trece estudios en el metaanálisis. En general, las intervenciones mejoraron significativamente la adherencia al tratamiento de TAR en comparación con las condiciones de control (efectos aleatorios d = 0,84, IC del 95% [0,21, 1,47], p = 0,01), sin embargo, esto se debió principalmente a dos estudios que tuvieron tamaños de efectos superiores a 3 errores estándares por encima del tamaño medio del efecto. Los moderadores clave fueron la ubicación, el método de reclutamiento, la intervención grupal y la alteración del sistema de salud. Se necesitan intervenciones innovadoras de comportamiento que se enfoquen en mujeres jóvenes y adolescentes, que se centren en los períodos críticos del embarazo y el posparto y que prueben la integración de múltiples niveles de intervención para crear efectos duraderos en la adherencia al tratamiento antirretroviral.
Introduction
Globally, there are an estimated 17.8 million women living with HIV and young women and adolescent girls are disproportionately impacted.(1, 2) HIV/AIDS is also the leading cause of death among women of reproductive age, globally.(3) High adherence to antiretroviral therapy (ART) has significant health benefits for women living with HIV including improved immune functioning and increased life expectancy.(4, 5) Adherence to ART is defined as a participant’s ability to follow prescribed medication orders including time and frequency. Measures of adherence can be expressed as percentages, indicating the number of pills taken divided by the number of pills prescribed for a given period, or as a qualitative assessment. Adherence is the essential behavior to reach HIV viral load suppression, the last step of the HIV treatment cascade. ART adherence among women living with HIV also confers significant public health benefits as it reduces infectiousness to their sexual partners and/or infants during pregnancy and/or breastfeeding. (6–9) Given the global scale and gendered nature of the epidemic, women and girls represent a key population to receive targeted efforts that improve adherence to ART.
Overall, cis-gender (individuals whose gender identities align with their sex-assigned at-birth) differences in ART adherence and HIV treatment outcomes are complex and largely dependent on contextual factors including access to care, institutional prioritization of health for sub-populations, and differing levels of stigma. In a meta-analysis of 56 observational studies conducted globally that reported adherence by gender, Ortega and colleagues found that 62% of women achieved ≥90% adherence compared to 67% of men (p<0.10).(10) Other individual studies in sub-Saharan Africa have found contrary results, particularly that women are more adherent and have stronger immunologic responses. (11, 12) Furthermore, reviews of ART adherence reveal factors such as emotional distress, stigma, and the negative impacts of poor social relationships may be intensified among women living with HIV, which can impede optimal ART adherence. (13, 14) Thus, the factors that influence adherence may be very different between men and women.
Women may face additional mental health barriers in comparison to their male counterparts. The prevalence of depression among women is almost universally higher than that of men globally.(15, 16) Cognitive depressive symptoms and severe depression have both been shown to significantly impact adequate ART adherence in people living with HIV.(17) Specifically, among women living with HIV, depression is a robust predictor of ART non-adherence.(18, 19) Given the high prevalence of depression among women and its impact on ART adherence, mental health factors warrant consideration in investigating the efficacy of HIV adherence interventions addressing women’s needs.
Women also experience key life events, such as pregnancy, the postpartum period, and motherhood, which can have a large impact on a woman’s ability to prioritize her own health. In a review of 51 studies conducted in high, middle, and low-income countries, Nachega and colleagues found that 73.5% of pregnant women had optimal ART adherence while only 53.0% of women postpartum had adequate adherence (i.e. >80% by study cut-off).(19) Among pregnant and postpartum women living with HIV, being younger, having lower levels of education, non-disclosure of their status to partners or family, and having inadequate family support were all associated with lower ART adherence.(20) Moreover, pregnant and postpartum mothers living with HIV face unique circumstances that can impact ART adherence, such as vertical transmission risks, stigma associated with childbearing among HIV positive mothers, change in familial roles, possible postpartum complications (e.g. postpartum depression) and balancing infant care with self-care. (21)
In addition to addressing common behavioral strategies and individual motivations for treatment adherence, utilizing gender empowerment principles and addressing the impact of the systemic oppression of women and gender dynamics may have significant impacts on women’s ability to adhere to ART. Gender empowerment and gender dynamics have been shown to significantly impact women’s health outcomes, including unplanned pregnancy, HIV testing, new STI acquisition, and contraception use.(22) Furthermore, reproductive and maternal-child health interventions that seek to empower women to address their own health needs and behaviors have strong evidence of efficacy.(23, 24) The concepts of gender and empowerment and gender dynamics may be particularly relevant for ART adherence in cases where partner disclosure of status may be difficult, a seroconcordant partner does not prioritize their own treatment, areas where women’s rights are restricted, or situations of poverty where women have less access to education. The extent to which ART adherence interventions for women address issues of gender empowerment or dynamics and the impact this has on ART adherence outcomes, however, is unknown.
With women facing unique barriers to adherence, women-focused behavioral ART adherence interventions appear warranted. However, little is known about the efficacy of these types of interventions. Previous systematic reviews and meta-analyses have focused on the use of ART to treat HIV infection in women (25), the provision antiretrovirals to women to reduce vertical transmission (26, 27) integration of prevention of mother-to-child transmission (PMTCT) interventions into antenatal care (28), and improving retention in HIV care for women. (29) However, no studies have specifically reviewed the evidence on behavioral ART adherence interventions for women living with HIV.
The current systematic review and meta-analysis aims to fill the gaps in knowledge with the following objectives: 1) survey the literature to identify and categorize behavioral interventions that have been conducted to improve ART adherence specifically for cis-gender women living with HIV, 2) determine overall efficacy of randomized controlled trials testing ART adherence interventions for this population, and 3) identify key moderators of the efficacy of these interventions to pinpoint potential avenues for future intervention development.
Methods
Systematic review and search strategy
We carried out a systematic review of the literature and completed a descriptive review of interventions that met our inclusion criteria along with a meta-analysis of intervention ART adherence outcomes. We adhered to PRISMA guidelines (30) (Checklist is included as supplemental material). Relevant studies were located using several search strategies. Electronic databases (PubMed, PyscINFO, CINAHL, and ProQuest Dissertations and Theses Global) were searched on May 23, 2017 using Boolean operators: (ART OR “antiretroviral therapy” OR HAART OR “Highly active antiretroviral therapy”) AND (adherence OR compliance OR nonadherence OR noncompliance) AND (women OR female* OR girl* OR mother*). Conference abstracts were also reviewed from the past 3 years of the International Conference on HIV Treatment and Prevention Adherence (2015, 2016, 2017) and the Inter-CFAR Joint Symposium on HIV Research in Women (2016). Finally, reference sections of relevant review articles were searched for any studies potentially meeting inclusion criteria.
Inclusion criteria
All identified study titles and abstracts were screened for eligibility criteria and studies potentially meeting inclusion criteria underwent full text review. Studies were included in the descriptive review if they: 1) reported on a behavioral/counseling ART adherence intervention for cis-women living with HIV, 2) reported ART adherence for at least one follow-up, and 3) employed a randomized controlled trial (RCT) design. Multi-level interventions that included a behavioral counseling component could have been included, although we did not find any in our search. Studies were not restricted by sample age inclusion criteria. Studies were excluded if they 1) did not report ART adherence outcomes, 2) included an intervention that was not behavioral (for example, the provision of ART with no associated ART adherence counseling), 3) did not focus specifically on cis-gender women living with HIV (for example, interventions that included men and women [such as couples-based interventions] and reported disaggregated results by gender were excluded), and/or 4) only included a description or protocol of an intervention. Furthermore, for studies that met the above inclusion/exclusion criteria but did not provide sufficient statistics to calculate effect sizes, study authors were contacted (number of studies k=5). In the instance where additional information from authors was not available, the study was included in the descriptive review but not the meta-analysis.
Study characteristic coding and inter-rater reliability
Two trained independent raters (JP, DP) coded all eligible studies utilizing a custom coding form. Information on a variety of characteristics was collected including general study information (e.g. year of publication, location), inclusion and exclusion criteria for individual studies, baseline participant characteristics (e.g. age, education, marital status), intervention design (e.g. intervention format, number of sessions, session length) and intervention content (e.g. skills building, goal setting, gender empowerment). A complete list of extracted variables can be found in Supplementary Material.
Inter-rater reliability was calculated for the study characteristic coding. Categorical variables were calculated using Cohen’s k (mean k=0.71, mean agreement was 87.30%). Spearman correlations were calculated for continuous variables (mean r=0.91). Discrepancies between raters were reconciled through discussion.
Coding of methodological quality of included studies
Methodological quality was also assessed using an adapted version of the Downs and Black methodological quality scale.(31) The original scale includes 27 items with sub-scales that cover reporting bias, external validity, internal validity, selection bias, and power. All items were used except one item that addressed power because it was deemed irrelevant, as the purpose of the meta-analysis is to accommodate studies that may be underpowered due to small sample sizes. Methodological quality scores could range from 0 to 26. A score of 24 to 26 was considered excellent, 20 to 23 good, 15 to 19 fair, and a score of 14 or below was considered poor.(32)
Calculation of effect sizes (ESs) and analytic approach
Effect sizes (ESs) were calculated using standardized mean differences (Cohen’s d) comparing the intervention and control condition divided by the pooled standard deviation, to adjust for within study measurement and sample size biases.(33, 34) Positive ESs indicate higher ART adherence in the intervention condition whereas negative ESs indicate higher ART adherence in the control condition at follow-up. Means and standard deviations were the preferred statistical information for calculating ESs but when they were not available other statistical tests were used (e.g. F test statistics). Test statistics that did not control for other variables, such as age or education, were preferred. When baseline ART adherence measures were available, they were utilized in calculating the ESs. When studies reported ART adherence outcomes using multiple measures of adherence (e.g. multiple measures of self-report, pill count, MEMScap; number of studies k=3), ESs were calculated for each individual measure and then these values were averaged. (35–37) Similarly, when studies reported outcomes for multiple follow-up time points (k=4), ESs were calculated for each time point and then collapsed. (38–41) For studies that reported HIV viral load and/or CD4 T-cell count outcomes (40–42), standardized mean difference ESs were also calculated. Two researchers (JP, DP) independently calculated all ESs and any discrepancies were resolved through discussion.
Analyses were conducted following fixed-effects and random-effects assumptions using Stata 15.0. (43–46) The presence of heterogeneity was assessed using the Q statistic and magnitude of heterogeneity across ESs was assessed using the I2 statistic.(47) Higher values of the I2 statistic indicate increased heterogeneity (i.e. more variation in study outcomes than would be expected by sampling error alone). A priori moderators of ES magnitude were examined using modified weighted least squares regression analysis with the inverse variance of the ES as the weights. To test for possible reporting bias toward findings where the intervention condition significantly improved adherence over the control condition, we graphed a funnel plot and implemented the trim and fill technique (48), as well as calculated the Begg’s and Egger’s tests. (49, 50)
Results
A total of 5966 titles and abstracts were reviewed (Figure 1). Of these, 5900 were excluded as information provided in the title and abstract did not meet one or more of the inclusion criteria. Sixty-six articles were deemed potentially relevant and the full text was reviewed to further assess eligibility. Of these, 52 did not meet all inclusion criteria: 6 articles reported on outcomes that did not include adherence, 1 article did not report on a behavioral intervention, 12 articles presented an intervention that was not specifically for women, 27 articles did not employ a randomized controlled trial design, and 1 was found to not focus on an HIV-positive population. An additional 5 were descriptions or protocols of interventions that did not report on outcome results. For these 5 articles, 2 studies were already included in the search with reported outcomes.(51, 52) For the other 3, the literature was searched to see if outcomes were published but all 3 of the protocols were recent and trial results are pending. (53–55)
Figure 1:
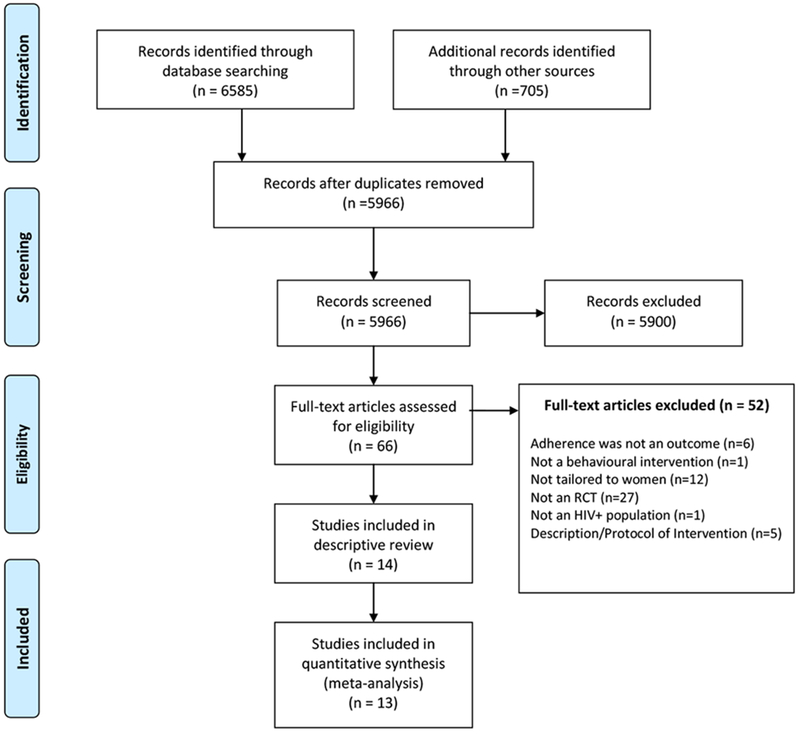
PRISMA flow chart of literature search for randomized controlled trials testing ART behavioral adherence interventions tailored for women living with HIV
Description of studies
Fourteen studies (from 14 published articles) are included in the qualitative synthesis (Table 1). (35–42, 51, 52, 56–59) The majority of studies were conducted in North America (k=7) and Africa (k=4), with two additional studies conducted in Asia and one in South America, contributing a total of 1806 participants. The mean age was 35.8 (SD=7.3) years and 63.2% of participants completed high school/secondary school. Of the studies that reported marital status (k=12), 51.2% of participants were married (SD=27.7, range 23%-93% of samples). The methodological quality of these studies ranged from 15 to 21 and all fell into either the “good” or “fair” categories of methodological quality.
Table 1:
Characteristics of studies included in the qualitative synthesis
| Author and Year | Journal/Conference | Location | Baseline Sample Characteristics | Intervention Design | Adherence Measurement | Baseline Adherence | Methodological Quality |
|---|---|---|---|---|---|---|---|
| da Costa et al., 2012 (35) | International Journal of Medical Informatics | Sao Paulo, Brazil | 29 women; mean age: 34.6; 67% married; 52% completed secondary education | SMS-based intervention tested over 4 months, messages were tied to dose time and delivered 4 times per week vs. control that did not receive SMS messages | SR (number missed in last 30 days), pill count (%, monthly), Medication Event Monitoring System (%, MEMS) caps (monthly); 1, 2, 3, 4 months follow-up | Intervention = 75% (MEMS) Control = 61.54% |
Total Score = 18 Rating = Fair |
| Holstad et al., 2011 (51) | AIDS and Behavior | Atlanta, GA | 207 women; mean age: 43.5; 27.2% married; 80.8 % completed secondary education | Motivation interviewing group based intervention using Social Cognitive Theory to promote medication adherence and risk reduction behaviors (8 sessions) vs. a health promotion group based control (8 sessions) |
MEMS caps (%, 2 weeks), 3, 6, and 9 months follow-up | Intervention = 73.5% (SD=33.5) Control = 74.9% (SD=31.8) |
Total Score = 19 Rating = Fair |
| Holstad et al., 2012 (36) | African Journal of Reproductive Health | Lagos, Nigeria | 60 women; mean age: 30.7; 46% married; 90% completed secondary education | Motivation interviewing group based intervention using Social Cognitive Theory to promote medication adherence and risk reduction behaviors (8 sessions) vs. a health promotion group based control (8 sessions) | SR (VAS [%, 30 days], AGAS [score on scale, 30 days], ACTG [score on scale, up to 3 months ago]); 6 month follow-up | Baseline data not reported | Total Score = 20 Rating = Good |
| Jones et al., 2003 (56) | AIDS Care | Miami, New York City, and New Jersey, USA | 174 women with AIDS; mean age: 37; 48% single; 52% completed secondary education | Cognitive-behavioral stress management/expressive supportive therapy (CBSM+) group intervention (10 sessions) vs. time-matched individual based informational/educational control | SR (AMS [%, 7 days]); baseline, 10 week follow-up; * effect size could only be calculated for low adherers at baseline | * low adherers at baseline; Intervention = 46% (SD = 28.4) Control = 51% (SD=25.7) |
Total Score = 16 Rating = Fair |
| Jones et al., 2007 (57) | AIDS and Behavior | Miami, New York City, and New Jersey, USA | 177 women; mean age: 41; 53% completed secondary education | 2×2 factorial design-Phase 1: CBSM+ group vs. individual (both 10 sessions); Phase 2: Healthier Lifestyles groups vs. individual (both 6 sessions) | SR (ACTG [%, 4 days]); baseline, 3 and 6 month follow-ups *effect size any group vs. individual only | Baseline data not reported | Total Score = 16 Rating = Fair |
| Kempf et al., 2016 (38) | 11th International Conference on HIV Treatment and Prevention Adherence | Rural Alabama, USA | 22 women with depression; mean age: 45.8; 32% married; 82% completed secondary education | Cognitive Behavioral Therapy for Adherence and Depression (CBT-AD) in HIV (10 sessions) vs. time-matched supportive psychotherapy (both via telemedicine) | Wisepill [%, past week]; baseline, 3 and 6 month follow-ups | Intervention = 94.8% (SD=13.2) Control = 84.4% (SD=26.7) |
Total Scoreb = 18 Rating = Fair |
| Kiweewa et al., 2013 (42) | JAIDS: Journal of Acquired Immune Deficiency Syndromes | Kampala, Uganda | 92 women who were WHO stage III/IV or had CD4 cell counts less than 200 attending PMTCT program (pregnancy/postpartum was not an inclusion criteria for entry into the study); mean age: 27.3; 78% married; 52% completed secondary education |
Intervention delivered by nurses trained in ART management and peer counselors (7 sessions across 1 year) with home visits by peer counselors for missed appointments vs. standard clinic-based model | Pill count [%, study period]; 6-12 months after ART initiation | Baseline data not reported | Total Score = 21 Rating = Good |
| Mann 2001 (58) | Annals of Behavioral Medicine | NR | 44 women; mean age: 38.5; 23% married | Expressive future writing group (10 minutes, twice a week for 4 weeks) vs. no writing group | SR (RAND Medical Outcomes Study, [6 point Likert Scale, past month]); baseline, 1 month follow-up | Intervention = 4.54 (SD=0.28; items averaged from a 6 point Likert scale) Control = 4.93 (SD=0.22) |
Total Score = 17 Rating = Fair |
| Messersmith et al., 2017 (37) | 12th International Conference on HIV Treatment and Prevention Adherence | Mityana, Uganda | 133 pregnant women (12-28 weeks) on once day regimens; mean age: 25.4; 73% married; 94% completed secondary education | SMS reminder if Wisepill was not opened within 2 hours of dose time + monthly Information Motivation Behavioral Skills (IMB)-based counseling sessions using Wisepill data for feedback (up to 9 months) vs. no intervention | Wisepill [% and threshold, intervention period]; Intervention period | Intervention = 78.55% (SD=23.9) Control = 75.89% (SD=24.5) |
Total Scoreb = 16 Rating = Fair |
| Nyamathi et al., 2012 (52) | Nursing Research | Andra Pradesh, India | 68 women living with AIDS and on ART for at least 3 months; mean age: 31.2; 52% married; 22% completed secondary education | Asha-Life intervention consisting of 6 group classes and home visits and assistance in overcoming barrier to care by Accredited Social Health Activists (Ashas) vs. 6 group classes time matched to intervention with Asha visits that were less supportive; both lasted 6 months | Pill count [%, past month]; monthly for 6 months | Intervention = 41.7% (SD=9.5) Control = 54.9% (SD=16.9) |
Total Score = 18 Rating = Fair |
| Sarna et al., 2017 (39) | 12th International Conference on HIV Treatment and Prevention Adherence | Kisumu, Kenya | 404 pregnant women (14-36 weeks); mean age: 25; 82% married | Individual self-regulation theory based counseling delivered via cell-phone (maximum of 24 antenatal calls and 16 postnatal calls) vs. standard care | Pharmacy refills [proportion above 90%, time frame unclear]; delivery, 6 weeks postpartum, 14 weeks postpartum | Baseline data not reported | Total Scoreb = 15 Rating = Fair |
| Surilena et al., 2014 (40) | Acta Medica Indonesiana: The Indonesian Journal of Internal Medicine | Jakarta, Indonesia | 160 women infected through their husband/partner; mean age: 33.51; 93% married | Rational emotive behavior therapy (REBT) 6 sessions of individual therapy/week and 2 session of group therapy/week (8 weeks) vs. no intervention | SR (scale not specified [%, past week]); pill count [%, past week]; weekly for 8 weeks | Intervention = 74% (95% CI: [69.0, 75.3]), (Self-report) Control = 77% (95% CI: [71.8, 80.3]) |
Total Score = 17 Rating = Fair |
| Webel, 2010 (41) | AIDS Care | San Francisco, USA | 89 women; mean age: 46.9; 3% married; 62% completed secondary education | Peer-based HIV symptom management intervention using the Positive Self-Management Program (PSMP) delivered to groups (7 weekly sessions) vs. control condition received a copy of HIV Symptom Management: A Manual for People Living with HIV | SR (revised ACTG [% of missed doses, past 7 days]); baseline, weeks 2, 6, 10 and 14 | Intervention = 6.98 (SD=17.53)c Control = 7.92 (SD=18.81) |
Total Score = 18 Rating = Fair |
| Wyatt et al., 2004 (59) | AIDS and Behavior | Los Angeles, USA | 147 women with a history of sexual abuse; mean age: 41; 38% married; 56% completed secondary education | Enhanced Sexual Health Intervention (ESHI) risk reduction group counseling and weekly calls (11 weeks) vs. attention wait-list control (1 group session and 11 weekly calls) | SR (How many days in the past 2 weeks had they taken their HIV medications “exactly as prescribed (on schedule and the correct dose)” [%, 2 weeks]; post-test (after 11 weeks) | Baseline data not reported | Total Score = 20 Rating = Good |
Notes: SR=self-report; MEMS=medication event monitoring system; VAS=visual-analog scale; AGAS=Antiretroviral General Adherence Scale; ACTG=AIDS Clinic Trials Group; AMS=Adherence to Medication Scale; SMS=short message service; NR=not reported; PMTCT=prevention of mother to child transmission; ART=antiretroviral therapy;
Note= Only one measure is reported per study. In studies that reported multiple measures of adherence, the referenced measure is in parentheses and measures with percentages was preferred.
Note = the methodological coding for these studies may be biased due to the fact that coding was based on conference abstracts and slide presentations rather than full manuscripts.
Note = when calculating the effect sizes for this study, these values were transformed to represent % adherence rather than % missed
The majority of samples were recruited from clinics (k=13). Flyers (k=2) and recruiting from community based organizations/support groups (k=3) were additional methods of recruitment. No intervention recruited participants based on level of non-adherence or ART naivety, however, for studies that reported baseline adherence using percentages, the majority had adherence below 90% (k=6). Two studies (37, 39) included only pregnant women and 2 studies (38, 59) focused on women with mental health issues (i.e. depression, history of child sexual abuse).
Behavioral interventions were delivered to individuals (k=6) or in groups (k=6), and two interventions combined these delivery types. All of the interventions included multiple sessions (median number of sessions = 9; M=16.3, SD=17.7), spread over a considerable amount of time (median number of weeks = 15, M=18.8, SD=12.9, range 4-39 weeks). Additionally, most of these interventions addressed issues in addition to ART adherence including sexual risk reduction (k=4), mental health (k=2), and substance use (k=3).
There was considerable variety in the method of delivery of interventions included: 4 used a form of technology (e.g. SMS/text message reminders, cell phone counseling), and 2 altered the healthcare system in some way (e.g. task-shifting to peer counselors and nurses compared to physicians). Furthermore, 8 used a variety of behavioral counseling techniques including cognitive-behavioral therapy (k=8), psycho-education (k=7), and motivational interviewing (k=4) with 5 interventions using two or more of these techniques. The content of the interventions was also variable: 7 incorporated role playing or skills building into the sessions, 4 used goal-setting, 5 included coping strategies, and 9 included discussions of barriers/facilitators to ART adherence and strategies to address them.
Although all of the interventions were for women, very few addressed issues specifically related to gender. Only 3 studies integrated gender empowerment principles or discussed intervention content in terms of gender-specific concepts such as interconnectedness or collectivism. Furthermore, 4 studies utilized theory to guide intervention content, however, none of the theories used were gender-specific or acknowledged gender differences (theories used were: Social Cognitive Theory, Information-Motivation-Behavioral Skills, Self-Regulation Theory).
To measure ART adherence as an outcome, self-report was the most common method (k=8) using a variety of validated scales including the Visual Analog Scale (VAS), the Antiretroviral General Adherence Scale (AGAS), the AIDS Clinical Trial Group scale (ACTG), and the Adherence to Medication Scale (AMS). Several studies used pill counts (k=4) or electronic monitoring (e.g. MEMS cap, Wisepill; k=3). Three studies used multiple methods to measure adherence. Four studies used a reference period for adherence of 30 days, 4 used a 7-day reference period, and 2 used a 2 week reference period. The duration of time between the end of the intervention and the follow-up assessment ranged from immediate post-test to 6 months.
Overall efficacy of the interventions
Of the 14 included studies, 13 reported sufficient ART adherence data (either within the report or when the authors were contacted) and were included in the meta-analysis (Figure 2). (35–42, 52, 56–59) Overall, interventions significantly improved ART adherence compared to control conditions (random-effects d = 0.82, 95% CI [0.18, 1.45], p=0.01). Two studies, Nyamathi et al.(52) and Surilena et al. (40) had ESs greater than 3 standard errors above the mean ES. These two studies were both conducted in Asia (India and Indonesia), were tested against a standard of care control condition, and had a group delivery component. When sensitivity analyses were run without these two studies, the mean ES for interventions on ART adherence was no longer significant (random effects d = 0.20, 95% CI [−0.08, 0.48], p = 0.16). For the full model including all studies, the hypothesis of homogeneity was rejected (Q = 300.79, p<0.001) and the I2 showed high levels of heterogeneity within study effects (I2 = 96.01%). Tests for publication/reporting bias showed relatively low levels of bias: trim and fill results showed no asymmetry for ART adherence and no missing studies were estimated. Furthermore, Begg’s and Egger’s tests were non-significant (Begg’s z = 1.28, p = 0.20, Egger’s bias = 9.36 [SE = 6.18], t = 1.51, p = 0.16).
Figure 2:
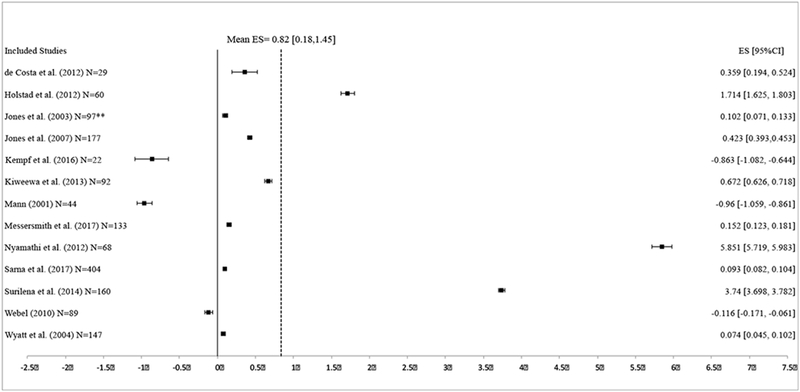
Effect size estimates for ART adherence at follow-up. Effect sizes are in alphabetical order. Positive effect sizes indicate increased ART adherence in the intervention condition at follow-up compared to control condition.
Very few studies reported on HIV viral load (k=3) and CD4 T-cell counts (k=2) as outcomes in addition to ART adherence. In the studies that reported HIV viral load, interventions did not significantly decrease HIV viral load compared to control conditions (random effects d = −0.16, 95% CI −0.40, 0.07, p = 0.16). The hypothesis of homogeneity was not rejected (Q = 0.50, p=0.78), thus moderator analyses were not conducted for HIV viral load outcomes. Additionally, in studies that reported CD4 T-cell count results, interventions did not significantly increase CD4 T-cell counts compared to control conditions (random effects d = 0.14, 95% CI −0.19, 0.47, p = 0.40). Additionally, the hypothesis of homogeneity was not rejected (Q = 0.03, p = 0.87), thus moderator analyses were not conducted for CD4 T-cell count outcomes.
Moderators of ART adherence ES magnitude
Given the high levels of heterogeneity found in the ART adherence analyses, tests for moderators of ES magnitude were warranted. Several study characteristics were related to ES magnitude (Table 2). Studies that were conducted in developing countries had larger ESs (β=0.50, p=0.043). Studies that recruited participants in clinics had smaller ESs (β= −0.76, p<0.001) whereas studies that recruited through flyers had larger ESs (β= 0.66, p=0.001; note: some studies used both methods). Studies with higher attrition rates had larger ESs as did studies that had less educated samples (β=0.55, p=0.02, β=−0.55, p=0.03, respectively). Studies that recruited based on mental health issue or pregnant women did not have larger ESs than studies that did not recruit these specific populations (β=−0.29, p=0.27, β= 0.17, p=0.52, respectively).
Table 2:
Moderators of effect size magnitude for ART adherence outcomes
| Moderator | Beta | p-value | k | mean |
|---|---|---|---|---|
| Publication Year | 0.24 | 0.36 | 2010 | |
| Developing Country | 0.5 | 0.043 | 7 | |
| Sample Size | −0.01 | 0.97 | 104.23 | |
| Methodological Quality | 0.11 | 0.68 | 17.79 | |
| Recruitment | ||||
| By Flyer | 0.66 | 0.001 | 2 | |
| In Clinic | −0.76 | <0.001 | 12 | |
| Through Community Based Organizations/Support Groups | −0.25 | 0.34 | 3 | |
| Inclusion Criteria | ||||
| Pregnant | −0.17 | 0.52 | 2 | |
| Mental Health Issues | −0.29 | 0.27 | 2 | |
| Acceptance Rate | 0.04 | 0.91 | 62.58% | |
| Attrition Rate | 0.55 | 0.02 | 79.32% | |
| Baseline Characteristics | ||||
| Age | −0.33 | 0.2 | 35.23 | |
| Completed Secondary Education | −0.55 | 0.03 | 61.46% | |
| Single | −0.5 | 0.1 | 34.38% | |
| Theory-Based | −0.15 | 0.59 | 4 | |
| Group Component | 0.47 | 0.049 | 7 | |
| Control Group | ||||
| Standard of Care Control/No intervention | 0.32 | 0.22 | 7 | |
| Intervention Not Related to Adherence | −0.29 | 0.27 | 5 | |
| Intervention Length (Days) | 0.05 | 0.85 | 130 | |
| Number of Intervention Sessions | 0.28 | 0.31 | 17 | |
| Length of Follow-up (weeks) | 0.3 | 0.25 | 13.7 | |
| Type of Intervention | ||||
| Technology-based | −0.33 | 0.2 | 4 | |
| Information/education | 0.23 | 0.39 | 6 | |
| Motivational | −0.06 | 0.82 | 3 | |
| Cognitive-behavioral | −0.13 | 0.63 | 8 | |
| Alteration of the healthcare system | 0.53 | 0.02 | 2 | |
| Intervention Content | ||||
| Gender Empowerment | 0.01 | 0.97 | 2 | |
| Cultural Relevance/Tailoring | 0.01 | 0.97 | 2 | |
| Role Playing/Skills building | −0.32 | 0.22 | 6 | |
| Feedback | −0.11 | 0.68 | 1 | |
| Goal Setting | −0.09 | 0.74 | 3 | |
| Discussion of barriers/facilitators/strategies | 0.34 | 0.18 | 8 | |
| Coping | 0.08 | 0.77 | 5 | |
| Symptom management | −0.13 | 0.62 | 2 | |
| Adherence Measure Characteristics | ||||
| Self report | −0.46 | 0.64 | 8 | |
| Wisepill | −1.05 | 0.29 | 2 | |
| Pill count | 0.65 | 0.002 | 4 | |
| 30 day reference period | 0.31 | 0.24 | 4 | |
| 7 day reference period | −0.04 | 0.87 | 4 |
With regards to intervention design and content, interventions that contained a group component had larger effect sizes (β=0.47, p=0.049). Finally, interventions that incorporated an alteration to the healthcare system (e.g. task-shifting from physician centered care to nurse and peer counselor care) had larger effect sizes (β=0.53, p=0.02). Specific intervention content (e.g. gender empowerment, skills building, goal setting) was not significantly related to ES magnitude. Methodological quality also was not significantly related to ES magnitude (β=0.11, p=0.68). Control condition type was also not a significant moderator of ES magnitude (standard of care: β=0.32, p=0.22; Intervention not related to adherence: β= −0.29, p=0.27).
Characteristics of the measures used to assess adherence were also assessed as moderators. The use of self-report and Wisepill devices to measure adherence were not significantly related to ES magnitude, however, the use of pill counts were associated larger effect sizes (β=0.65, p=0.002). Measures that used a reference period of 30 days were not significantly related to ES magnitude nor were those that used a 7-day reference period.
Discussion
This is the first known meta-analysis to assess the efficacy of behavioral ART adherence interventions specifically for women living with HIV and to identify moderators of intervention efficacy. Fourteen ART adherence trials targeting women were identified, and there was significant heterogeneity in the specific types/modalities of adherence interventions, incorporation of gender-focused content or considerations of gender in intervention delivery, geographic context, sampling approaches, and assessment of ART measures. Despite the heavy burden of HIV among women in sub-Saharan Africa, we identified only 4 studies meeting inclusion criteria conducted in this region.
On average, ART adherence interventions identified in this review significantly improved ART adherence compared to control conditions; however, this finding is mainly driven by two interventions with very large effect sizes. (40, 52) Nyamathi and colleagues conducted an intervention in rural India in which women received 6 group classes on HIV/AIDS knowledge, ART adherence, coping, reducing stigma, and nutrition. In addition, HIV-trained village women (“Ashas”) visited women in the intervention condition weekly to monitor barriers to ART adherence and to assist them with accessing healthcare and their prescriptions.(52) The control condition was a usual care program and the Ashas visited with the women in the control condition but did not provide the same supportive role. Surilena and colleagues conducted the other intervention that had a relatively large effect size in Jarkarta, Indonesia.(40) This intervention consisted of 8 weekly sessions utilizing rational-emotive-behavior-based interventional therapy, a type of psychotherapy, and sessions were mixed in format between individual and group sessions. The common features between these two interventions are that they were conducted in Asia, were tested against a standard of care control condition, and had a group delivery component; however, only group delivery component was a significant moderator of effect size magnitude in our analyses. It is unclear if cultural context played a role in effect size magnitude, however, this should be explored in future research.
In general, the other 11 interventions show modest but significant effect sizes with two interventions showing large negative effects. These 11 studies are more consistent with the larger ART adherence intervention literature; previous meta-analyses of ART adherence interventions for general populations living with HIV have revealed significant but small effects (d = 0.35) on ART adherence over time.(60, 61) Furthermore, previous research has shown ART adherence interventions that do not specifically target those who were known to be non-adherent (such as the case with all the studies in this meta-analysis) have even smaller effects (d = 0.19)(60). Thus, the magnitude of the effects sizes found in this systematic review and meta-analysis are consistent with the overall literature of ART adherence interventions.
Two studies included in this review had large negative effect sizes, such that participants in the control condition had better ART adherence at follow-up compared to those in the intervention condition. Of these, one study (38) had substantial differences in baseline adherence between the intervention and control conditions. The intervention condition, utilizing telemedicine-delivered cognitive behavioral therapy (CBT) for HIV positive women with depression, had a baseline adherence of 94.8% (versus 84.4% in the control), which left little room for improvement at follow-up for those in the intervention condition. This study highlights the necessity of recruiting those actually in need of adherence interventions (i.e. women with poor adherence at baseline).
The other study with a large negative effect (58) attempted to increase optimism, and thus increase positive health behaviors like adherence, with an expressive writing intervention in which those randomized to the intervention condition were given a journal and asked to write about the future for 10 minutes a day, twice a week for 4 weeks. Participants randomized to the control condition were not asked to write. HIV positive women who engaged in the writing activity had significantly worse adherence by the end of the study compared to the control condition. This study suggests that simple interventions may not be as effective as interventions that target adherence as a dynamic health behavior with multi-faceted influences.
Only 3 of the studies included in the meta-analysis reported on clinical outcomes (i.e. HIV viral load and CD4 T-cell counts). Of those that reported on clinical outcomes, no significant decreases in HIV viral load or significant increases in CD4 T-cell counts were found. Furthermore, there was not demonstrable heterogeneity in these effect sizes so moderator analyses were not justified leading to a limitation of this meta-analysis. Key clinical markers such as HIV viral load, CD4 T-cell counts, and drug concentrations, should be incorporated into future behavioral ART adherence interventions to parse out the best interventions for clinically meaningful improvements in ART adherence and reinforce the importance of bio-behavioral approaches.
A surprising finding of this meta-analysis was that although all of these interventions recruited only women, the extent of tailoring for women’s needs was lacking. Only 3 studies included in the systematic review specifically stated that they integrated gender empowerment principles or discussed the intervention content in terms of gender-specific concepts such as interconnectedness or collectivism. For interventions delivered in a group format, discussions about difficulties with ART adherence as collectively experienced by women may have occurred but this was largely missing from discussions of intervention results and, thus, cannot be assumed. Gender-specific tailoring of behavioral ART adherence interventions is a clear area of improvement for future behavioral ART adherence interventions given the literature on gender empowerment and other health outcomes.
Furthermore, there were only 2 ART adherence interventions that specifically addressed the critical periods of pregnancy and postpartum and these interventions did not improve medication adherence (mean ES = 0.06; 95% CI = −0.12, 0.25).(37, 39) Targeting interventions specific to the sub-populations of pregnant and postpartum mothers are crucial for achieving “90-90-90” goals of many national and international health organizations (e.g., UNAIDS, WHO, CDC). (62) Although women in these sub-populations generally share common motivations associated with childbirth and motherhood and tend to be already engaged within the health system, this review found that the few behavioral ART adherence interventions that specifically target this time period have limited efficacy. Innovative interventions are urgently needed to increase HIV treatment adherence in this critical population to improve maternal health and limit mother-to-child transmission, particularly during the postpartum period when mothers are more likely to be inadequately adherent. (19)
This meta-analysis found several significant moderators of the magnitude of effect size. Notably, interventions that included a group component were shown to have larger effect sizes compared to interventions that were only delivered individually. Group-based interventions may increase feeling of social support, which has been shown to positively influence ART adherence. (63, 64) There are often organizational barriers to conducting interventions within a group setting, including needing to recruit a large number of women at the same time, physical space requirements, and group session attendance rates. However, the impact of this format on increasing ART adherence may warrant more effort in developing creative ways to incorporate a “group-like” feel, such as through social media or other group chat technologies. (65)
Due to the heterogeneity in specific intervention content, we were unable to quantify through meta-analysis whether specific intervention components were associated with effect size. The number of sessions conducted and the length of intervention time frame did not seem to matter, indicating that a “more is better approach” may not be warranted or necessary. Furthermore, no specific intervention content (e.g. skills-building, discussion of barriers, etc.) was shown to be a panacea to unlocking better ART adherence for women. Overall, standard counseling and psycho/educational approaches are important as a foundation for interventions, however, in order to move beyond modest effects, different, innovative approaches are urgently needed.
ART adherence is dynamic and multifaceted with many driving factors. Previous literature has suggested that in order to increase the impact of ART adherence interventions, there needs to be a focus on structural factors in addition to individual-level behavior change. (66–68) The results of our moderator analyses support this suggestion: interventions that altered the healthcare system in some way had significantly larger ESs. Specifically, task shifting from physician centered care to nurse and peer counselor care (42) and utilizing additional HIV-trained clinic support staff to follow-up with women in the clinic and at home (52) significantly improved ART adherence outcomes. Future intervention development work should focus on the integration of structural and individual levels of behavior change to create dynamic interventions to simultaneously address some of the major factors influencing ART adherence, including clinic level barriers and support.
This meta-analysis has several limitations. This review was limited to English only articles and potentially relevant articles in other languages may have been excluded. Additionally, while we feel that the search string we used balanced being comprehensive while eliminating irrelevant articles, it is possible that the search string was not comprehensive enough. Searching the reference sections of relevant review articles likely helped mitigate this, however, it is possible that there are articles that meet our inclusion criteria that were not picked up in our search. With all systematic reviews and meta-analyses, publication bias is a concern. We feel confident that this was not an issue for this meta-analysis due to the non-significant Begg’s and Egger’s tests and the fact that we were able to capture some of the grey literature through conference abstracts.
Additionally, we operationalized women-focused interventions to be behavioral ART adherence interventions that only recruited and enrolled women. There is likely a larger literature of interventions that focus on gender dynamics and empowerment issues in ART adherence within the context of mixed gender or dyadic interventions However, none of the articles that were reviewed during the full text screening were excluded because they were dyadic interventions, indicating that there are likely very few heterosexual couples-based RCTs focused on ART adherence. Furthermore, this meta-analysis does not answer the question of “are interventions that only recruit women better than interventions that are targeted for all people living with HIV?” This review was also limited to cis-gender women and does not address the intricate issues that impact ART adherence for trans-women.
The majority of studies included in this meta-analysis utilized self-report as their sole measure of adherence. While there is no gold standard for measuring adherence (69), combining different measures of adherence, such as biomarkers with self-report data may yield more accurate trial results.(70) This meta-analysis was also limited by the relatively small sample of studies that met inclusion criteria (k=13). A larger portion of potentially relevant studies was excluded because they did not employ a randomized controlled design. Randomized controlled trials were chosen as the inclusion criteria for this meta-analysis because they are the most rigorous way to determine efficacy of interventions. By using this stringent criterion, we may have excluded important and effective ART adherence interventions, however, these interventions, by nature of their design, would have included more biased results than those tested through RCTs. Notably, 3 of the studies that were included in this meta-analysis were conference abstracts supplemented by further information from authors when contacted. (37–39) These conference abstracts, along with the non-significant reporting bias tests, increase our confidence in the results of this meta-analysis.
Globally, issues of adherence and engagement in care among young women are of particular concern. However, the average age of participants was 35.8 years and no study specifically targeted adolescent girls or older adult women. This limits the conclusions that can be drawn for either age range, both of which may have individual concerns/circumstances that can impede adherence. Adolescent girls who are HIV positive must deal with the transition into adulthood, which means taking on the responsibility of her healthcare from her caregiver and contending with disclosure conversations with sexual partners.(71) Additionally, given that many perinatally-infected children are now aging into adulthood, there may be increased concerns about HIV associated cognitive-development issues that could impact adherence behaviors.(72, 73) Older adult women may have issues associated with menopause or increased co-morbidities that could complicate their healthcare needs and ability to adhere to HIV treatment. (74, 75)
Conclusions
Innovative behavioral interventions are urgently needed in order to achieve UNAIDS 90-90-90 targets globally. (62) Subsequent interventions for women should focus on specific gender-relevant contexts and periods in women’s lives where research has shown that there are challenges of HIV treatment adherence, such as during the postpartum period.(19, 21) Future research should focus on integrating multiple levels of intervention, such as individual counseling and alterations to the healthcare system and through considering cultural contexts to create lasting, dynamic effects on ART adherence. Finally, new behavioral interventions also need to measure the clinical consequences of adherence including HIV viral load and CD4 T-cell counts.
Supplementary Material
Figure 3:
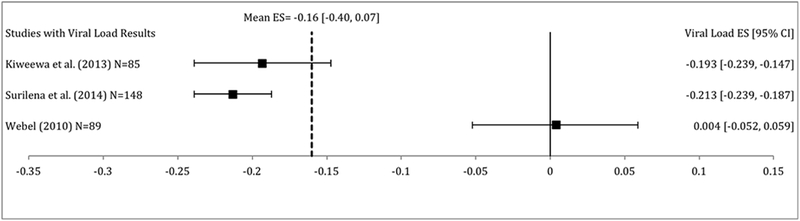
Effect size estimates for HIV viral load at follow-up. Effect sizes are in alphabetical order. Negative effect sizes indicate decreased HIV viral load in the intervention condition at follow-up compared to control condition.
Figure 4:
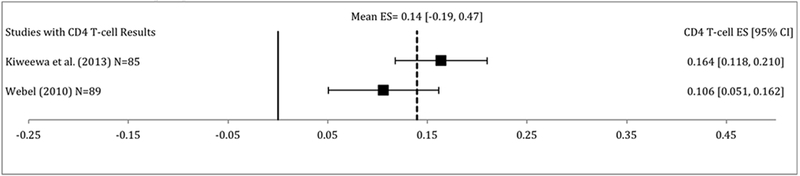
Effect size estimates for CD4 T-cell count at follow-up. Effect sizes are in alphabetical order. Positive effect sizes indicate increased CD4 T-cell counts in the intervention condition at follow-up compared to control condition.
Acknowledgements:
The authors would like to thank Elizabeth Waisel for her assistance with this project and Alison Z. Weber for her translation of the abstract. This manuscript was presented in part at the 13th International Conference of AIDS Impact in Cape Town South Africa, November 13-15, 2017.
Funding: This study was funded by the National Institute of Mental Health (K01MH112443 and T32MH078788) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant R24HD077976). The funders did not have any role in the development, execution, or writing of this manuscript.
Footnotes
Conflict of Interest: Jennifer Pellowski declares that she has no conflict of interest. Devon Price declares that she has no conflict of interest. Abigail Harrison declares that she has no conflict of interest. Emily Tuthill declares that she has no conflict of interest. Landon Myer declares that he has no conflict of interest. Don Operario declares that he has no conflict of interest. Mark Lurie declares that he has no conflict of interest.
Ethical Approval: This article does not contain any studies with human participants performed by any of the authors.
Informed Consent: Not applicable
References
- 1.Harrison A, Colvin CJ, Kuo C, Swartz A, Lurie M. Sustained High HIV Incidence in Young Women in Southern Africa: Social, Behavioral, and Structural Factors and Emerging Intervention Approaches. Curr HIV/AIDS Rep. 2015;12(2):207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. Fact sheet- Latest statistics on the status of the AIDS epidemic.2017. December 11, 2017.
- 3.amFAR. Statistics: Women and HIV/AIDS2017 December 11, 21017.
- 4.Poorolajal J, Hooshmand E, Mahjub H, Esmailnasab N, Jenabi E. Survival rate of AIDS disease and mortality in HIV-infected patients: a meta-analysis . Public Health. 2016;139(3–12). [DOI] [PubMed] [Google Scholar]
- 5.Stickler SM, Fox KA, Baggaley R, Negussie E, de Pee S, Grede N, et al. Retention in care and adherence to ART are critical elements of HIV care interventions. AIDS and Behavior. 2014;18(Supplement 5):S465–75. [DOI] [PubMed] [Google Scholar]
- 6.Adetokunboh OO, Oluwasanu M. Eliminating mother-to-child transmission of the human immunodeficiency virus in sub-Saharan Africa: The journey so far and what remains to be done. Journal of Infection and Public Health. 2016;9(4):394–407. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giuliano M, Andreotti M, Liotta G, Jere H, Sagno JB, Maulidi M, et al. Maternal antiretroviral therapy for the prevention of mother-to-child transmission of HIV in Malawi: maternal and infant outcomes two years after delivery. PLoS One. 2013;8(7):e68950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM. Relation between HIV viral load and infectiousness: a model-based analysis. The Lancet. 2008;372 9635(314–320). [DOI] [PubMed] [Google Scholar]
- 10.Ortega C, Huedo-Medina TB, Santos P, Rodriguez E, Sevilla L, Warren M, et al. Sex differences in adherence to highly active antiretroviral therapy: A meta-analysis. AIDS Care. 2012;24(12):1519–34. [DOI] [PubMed] [Google Scholar]
- 11.Boulle C, Kouanfack C, Laborde-Balen G, Boyer S, Aghokeng AF, Carrieri MP, et al. Gender differences in adherence and response to antiretroviral treatment in the Stratall Trial in rural district hospitals in Cameroon. Journal of acquired immune deficiency syndromes (1999). 2015;69(3):355–64. [DOI] [PubMed] [Google Scholar]
- 12.Maskew M, Brennan AT, Westreich D, McNamara L, MacPhail AP, Fox MP. Gender differences in mortality and CD4 count response among virally suppressed HIV-positive patients. J Womens Health (Larchmt). 2013;22(2):113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monforte AD, Anderson J, Olczak A. What do we know about antiretroviral treatment of HIV in women? Antiviral Therapy. 2013;18(Supplement 2):27–34. [DOI] [PubMed] [Google Scholar]
- 14.Puskas CM, Forrest JI, Parashar S, Salters KA, Cescon AM, Kaida A, et al. Women and the vulnerability to HAART non-adherence: A literature review of treatment adherence by gender from 2000 to 2011. Curr HIV/AIDS Rep. 2011;8:277–87. [DOI] [PubMed] [Google Scholar]
- 15.Grigoriadis S, Robinson GE. Gender issues in depression. Ann Clin Psychiatry. 2007;19(4):247–55. [DOI] [PubMed] [Google Scholar]
- 16.Piccinellie M, Wilkinson G. Gender differences in depression Critical Review. Br J Psychiatry. 2000;177:486–92. [DOI] [PubMed] [Google Scholar]
- 17.Wagner GJ, Goggin K, Remien RH, Rosen MI, Simoni JM, Bangsberg DR, et al. A closer look at depression and its relationship to HIV antiretroviral adherence. Ann Behav Med. 2011;42(3):351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Psaros C, Geller PA, Aaron E. The importance of identifying and treating depression in HIV infected, pregnant women: a review. J Psychosom Obstet Gynaecol. 2009;30(4):275–81. [DOI] [PubMed] [Google Scholar]
- 19.Nachega JB, Uthman OA, Anderson J, Peltzer K, Wampold S, Cotton MF, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS. 2012;26(16):2039–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodgson I, Plummer ML, Konopka SN, Colvin CJ, Jonas E, Albertini J, et al. A systematic review of individual and contextual factors affecting ART initiations, adherence, and retention for HIV-infected pregnant and postpartum women. . PLoS One. 2014;9(11):e111421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Psaros C, Remmert JE, Bangsberg DR, Safren SA, Smit JA. Adherence to HIV care after pregnancy among women in sub-Saharan Africa: Falling off the cliff of the treatment cascade. Curr HIV/AIDS Rep. 2015;12:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghanotakis E, Peacock D, Wilcher R. The importance of addressing gender inequality in efforts to end vertical transmission of HIV. J Int AIDS Soc. 2012;15(Suppl 2):17385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraft JM, Wilkins KG, Morales GJ, Widyono M, Middlestadt SE. An evidence review of gender-integrated interventions in reproductive and maternal-child health. J Health Commun. 2014;19:122–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haberland NA. The case for addressing gender and power in sexuality and HIV education: a comprehensive review of evaluation studies. Int Perspect Sex Reprod Health. 2015;41(1):31–42. [DOI] [PubMed] [Google Scholar]
- 25.Stuart AS, Dokubo EK, Sint TT. Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women. Cochrane Database of Systematic Reviews. 2010;3:CD008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White AB, Mirjahangir JF, Horvath H, Anglemyer A, Read JS. Antiretroviral interventions for preventing breast milk transmission of HIV. Cochrane Database of Systematic Reviews. 2014;10:CD011323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database of Systematic Reviews. 2011;7:CD003510. [DOI] [PubMed] [Google Scholar]
- 28.Tudor Car L, van-Velthoven MH, Brusamento S, Elmoniry H, Car J, Majeed, et al. Integrating prevention of mother-to-child HIV transmission (PMTCT) programmes with other health services for preventing HIV infection and improving HIV outcomes in developing countries. Cochrane Database of Systematic Reviews. 2011;6:CD008741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geldsetzer P, Yapa HM, Vaikath M, Ogbuoji O, Fox MP, Essajee SM, et al. A systematic review of interventions to improve postpartum retention of women in PMTCT and ART care. J Int AIDS Soc. 2016;19(1):20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hooper P, Jutai JW, Strong G, Russel-Minda E. Age-related macular degeneration and low-vision rehabilitation: a systematic review. Can J Ophthalmol. 2008;43(2):180–7. [DOI] [PubMed] [Google Scholar]
- 33.Faraone SV. Interpreting estimates of treatment effects: Implications for managed care. Pharmacy and Therapeutics. 2008;33(12):700–3. [PMC free article] [PubMed] [Google Scholar]
- 34.Huedo-Medina TB, Johnson BT. Estimating the standardized mean difference effect size and its variance from different data sources: A spreadsheet. Storrs, CT, USA2011. [Google Scholar]
- 35.da Costa TM, Barbosa BJ, Gomes e Costa DA, Sigulem D, de Fatima Marin H, Filho AC, et al. Results of a randomized controlled trial to assess the effects of a mobile SMS-based intervention on treatment adherence in HIV/AIDS-infected Brazilian women and impressions and satisfaction with respect to incoming messages. International journal of medical informatics. 2012;81(4):257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holstad MM, Essien JE, Ekong E, Higgins M, Teplinskiy I, Adewuyi MF. Motivational groups support adherence to antiretroviral therapy and use of risk reduction behaviors in HIV positive Nigerian women: a pilot study. African journal of reproductive health. 2012;16(3):14–27. [PMC free article] [PubMed] [Google Scholar]
- 37.Messersmith LJ, Halim N, Simmons E, Bachman DeSilva M, Chemusto H, Gasuza J, … Sabin L The Uganda WiseMama Study: a randomized controlled trial assessing real-time feedback to improve ART adherence among HIV-positive pregnant and postpartum women. 12th International Conference on HIV Treatment and Prevention Adherence; Miami, FL2017. [Google Scholar]
- 38.Kempf M-C, Ott C, Azuero A, Stringer K, Jagielski C, Savage R, Cropsey K, Haberer J, Psaros C, & Safren S A telemedicine-delivered cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV positive women in the deep south. 11th International Conference on HIV Treatment and Prevention Adherence; Fort Lauderdale, FL2016. [Google Scholar]
- 39.Sarna A, Okal J, Matheka J, Saraswati LR, Reynolds N, & Kalibala S Effectiveness of cellphone counseling on PMTCT retention and uptake of early infant diagnosis in Kisumu, Kenya. 12th International Conference on HIV Treatment and Prevention Adherence; Miami, FL2017. [Google Scholar]
- 40.Surilena, Ismail RI, Irwanto, Djoerban Z, Utomo B, Sabarinah, et al. The effect of rational emotive behavior therapy (REBT) on antiretroviral therapeutic adherence and mental health in women infected with HIV/AIDS. Acta medica Indonesiana. 2014;46(4):283–91. [PubMed] [Google Scholar]
- 41.Webel AR. Testing a peer-based symptom management intervention for women living with HIV/AIDS. AIDS Care. 2010;22(9):1029–40 12p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiweewa FM, Wabwire D, Nakibuuka J, Mubiru M, Bagenda D, Musoke P, et al. Noninferiority of a task-shifting HIV care and treatment model using peer counselors and nurses among Ugandan women initiated on ART: evidence from a randomized trial. Journal of acquired immune deficiency syndromes (1999). 2013;63(4):e125–32. [DOI] [PubMed] [Google Scholar]
- 43.StataCorp. Stata Statistical Software: Release 15. College Station, TX: StataCorp, LLC; 2017. [Google Scholar]
- 44.Wilson DB. Meta-analysis macros for SAS, SPSS, and Stata.1998. December 12, 2017.
- 45.Steichen TJ. METATRIM: Stata module to perform nonparametric analysis of publication bias. Boston: College Department of Economics; 2010. [Google Scholar]
- 46.Harbord RM, Harris RJ, Sterne JAC, Steichen TJ. METABIAS: Stata module to test for small-study effects in meta-analysis. Boston: College Department of Economics; 2009. [Google Scholar]
- 47.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index. CHIP Documents. 2006;19. [DOI] [PubMed] [Google Scholar]
- 48.Duval S, Tweddie R. Trim and fill: a simple funnel-plot based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(455–463). [DOI] [PubMed] [Google Scholar]
- 49.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 50.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holstad M, DiIorio C, Kelley M, Resnicow K, Sharma S. Group Motivational Interviewing to Promote Adherence to Antiretroviral Medications and Risk Reduction Behaviors in HIV Infected Women. AIDS & Behavior. 2011;15(5):885–96 12p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nyamathi A, Hanson AY, Salem BE, Sinha S, Ganguly KK, Leake B, et al. Impact of a rural village women (Asha) intervention on adherence to antiretroviral therapy in southern India. Nursing research. 2012;61(5):353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cowan JF, Micek M, Cowan JF, Napua M, Hoek R, Gimbel S, et al. Early ART initiation among HIV-positive pregnant women in central Mozambique: a stepped wedge randomized controlled trial of an optimized Option B+ approach. Implementation science : IS. 2015;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drake AL, Unger JA, Ronen K, Matemo D, Perrier T, DeRenzi B, et al. Evaluation of mHealth strategies to optimize adherence and efficacy of Option B+ prevention of mother-to-child HIV transmission: Rationale, design and methods of a 3-armed randomized controlled trial. Contemporary clinical trials. 2017;57:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenberg NE, van Lettow M, Tweya H, Kapito-Tembo A, Bourdon CM, Cataldo F, et al. Improving PMTCT uptake and retention services through novel approaches in peer-based family-supported care in the clinic and community: a 3-arm cluster randomized trial (PURE Malawi). Journal of acquired immune deficiency syndromes (1999). 2014;67 Suppl 2:S114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones DL, Ishii M, LaPerriere A, Stanley H, Antoni M, Ironson G, et al. Influencing medication adherence among women with AIDS. AIDS Care. 2003;15(4):463–74. [DOI] [PubMed] [Google Scholar]
- 57.Jones DL, McPherson-Baker S, Lydston D, Camille J, Brondolo E, Tobin JN, et al. Efficacy of a group medication adherence intervention among HIV positive women: the SMART/EST Women’s Project. AIDS Behav. 2007;11(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mann T Effects of future writing and optimism on health behaviors in HIV-infected women. Ann Behav Med. 2001;23(1):26–33. [DOI] [PubMed] [Google Scholar]
- 59.Wyatt GE, Longshore D, Chin D, Carmona JV, Loeb TB, Myers HF, et al. The efficacy of an integrated risk reduction intervention for HIV-positive women with child sexual abuse histories. AIDS Behav. 2004;8(4):453–62. [DOI] [PubMed] [Google Scholar]
- 60.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: A research synthesis of trials, 1996-2004. Journal of acquired immune deficiency syndromes (1999). 2006;41(3):285–97. [DOI] [PubMed] [Google Scholar]
- 61.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of intervention in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. Journal of acquired immune deficiency syndromes (1999). 2006;43:S23–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic2014; (JC2684).
- 63.Gonzalez JS, Penedo FJ, Antoni MH, Duran RE, McPherson-Baker S, Ironson G, et al. Social support, positive states of mind, and HIV treatment adherence in men and women living with HIV/AIDS. Health Psychol. 2004;23(4):413–8. [DOI] [PubMed] [Google Scholar]
- 64.Simoni JM, Frick PA, Huang B. A longitudinal evaluation of a social support model of medication adherence among HIV-positive men and women on antiretroviral therapy. Health Psychol. 2006;25(1):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blackstock OJ, Shah PA, Haughton LJ, Horvath HJ, Cunningham CO. HIV-infected women’s perspectives on the use of the internet for social support: a potential role for online group-based interventions. J Assoc Nurses AIDS Care. 2015;26(4):411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dewing S, Matthews C, Lurie M, Kagee A, Padayachee T, Lombard C. Predictors of poor adherence among people on antiretroviral treatment in Cape Town, South Africa: a case-control study. AIDS Care. 2015;27(3):342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simoni JM, Amico KR, Pearson CR, Malow R. Strategies for promoting adherence to antiretroviral therapy: a review of the literature. Curr Infectious Disease Reports. 2008;10(6):515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: Translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glass T, Cavassini M. Asking about adherence - from flipping the coin to strong evidence. Swiss Medical Weekly. 2014;144:w14016. [DOI] [PubMed] [Google Scholar]
- 70.Rhead R, Masimirembwa C, Cooke G, Takaruza A, Nyamukapa C, Mutsimhi C, et al. Might ART adherence estimates be improved by combining biomarker and self-report data. PLoS One. 2016;11(12):e0167852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cervia JS. Easing the transition of HIV-infected adolescents to adult care. AIDS Patient Care STDS. 2013;27(12):692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin SC, Wolters PL, Toledo-Tamula MA, Zeichner SL, Hazra R, Civitello L. Cognitive functioning in school-aged children with vertically acquired HIV infection being treated with highly active antiretroviral therapy (HAART). Dev Neuropsychol. 2006;30(2):633–57. [DOI] [PubMed] [Google Scholar]
- 73.Nichols SL, Chernoff MC, Malee KM, Sirois PA, Woods SP, Williams PL, et al. Executive functioning in children and adolescents with perinatal HIV infection and perinatal HIV exposure. J Pediatric Infect Dis Soc. 2016;5:S15–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duff PF, Money DM, Ogilvie GS, Ranville F, Kestler M, Braschel MC, et al. Severe menopausal symptoms associated with reduced adherence to antiretroviral therapy among perimenopausal and menopausal women living with HIV in Metro Vancouver. Menopause. 2017;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Warren-Jeanpiere L, Dillaway H, Hamilton P, Young M, Goparaju L. Taking it one day at a time: African-American women aging with HIV and co-morbidities. AIDS Patient Care STDS. 2014;28(7):372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


