Abstract
Objective
To evaluate whether administration of antenatal late-preterm betamethasone is cost-effective in the immediate neonatal period.
Study Design
Cost-effectiveness analysis of late-preterm betamethasone administration with a time horizon of 7.5 days was conducted using a health-system perspective. Data for neonatal outcomes, including respiratory distress syndrome (RDS), transient tachypnea of the newborn (TTN), and hypoglycemia, were from the Antenatal Betamethasone for Women at Risk for Late Preterm Delivery trial. Cost data were derived from the Healthcare Cost and Utilization Project from the Agency for Healthcare Research and Quality, and utilities of neonatal outcomes were from the literature. Outcomes were total costs in 2017 United States dollars and quality-adjusted life-years (QALYs) for each individual infant as well as for a theoretical cohort of the 270,000 late-preterm infants born in 2015 in the United States.
Results
For an individual patient, compared to withholding betamethasone, administering betamethasone incurred a higher total cost ($6,592 versus $6,265) and marginally lower QALYs (0.02002 QALYS versus 0.02006 QALYs) within the studied time horizon. For the theoretical cohort of 270,000 patients, administration of betamethasone was $88 million more expensive ($1,780 million versus $1,692 million) with lower QALYs (5,402 QALYs versus 5,416 QALYs), compared to withholding betamethasone. For administration of betamethasone to be cost-effective, the rate of hypoglycemia, RDS, or TTN among late-preterm infants receiving betamethasone would need to be less than 20.0%, 4.5%, and 2.4%, respectively.
Conclusion
Administration of betamethasone in the late-preterm period is likely not cost-effective in the short-term.
Keywords: Late-preterm, betamethasone, hypoglycemia, respiratory distress syndrome, transient tachypnea of the newborn
INTRODUCTION
Neonates born in the late-preterm (34&0/7 to 36&6/7 weeks gestation) period comprise the majority of preterm births, and these neonates are at significant risk of respiratory and other morbidities [1]. Recently, it was found that betamethasone decreases the rate of immediate neonatal respiratory morbidity but increases the rate of neonatal hypoglycemia [2, 3]. Therefore, betamethasone administration in late-preterm pregnancies at risk of preterm delivery is now standard of care in the United States but not in the United Kingdom [4, 5]. To date, there has been no cost-effectiveness analysis evaluating betamethasone administration comparing the benefit of decreased respiratory complications against the risk of increased hypoglycemia for late-term pregnancies that receive betamethasone. We conducted a cost-effectiveness analysis comparing the costs, the risks (hypoglycemia), and the benefits (reduced respiratory morbidity) of late-preterm betamethasone administration. We hypothesized that in late-preterm pregnancies, in the short-term, administering betamethasone would be cost-effective compared to withholding betamethasone.
METHODS
This cost-effectiveness analysis used a health-systems perspective to compare betamethasone administration versus no betamethasone administration to patients at risk of delivery in the late-preterm period. We used neonatal quality-adjusted life years (QALY) for effectiveness, with the willingness-to-pay (WTP) threshold defined as $100,000/QALY [6]. We followed the recent recommendations in the Journal of the American Medical Association regarding conduct and reporting of cost-effectiveness analyses [7].
We created a decision analytic model based on neonatal outcomes from the Antenatal Betamethasone for Women at Risk for Late Preterm Delivery (ALPS) trial with a time horizon of 7.5 days (median duration of neonatal admission in the trial) [2]. For neonatal outcomes, we considered short-term respiratory morbidity defined as transient tachypnea of the newborn (TTN), respiratory distress syndrome (RDS), and/or hypoglycemia (glucose level less than 40 mg/dL at any time during the initial hospitalization after birth). We also analyzed the cost-effectiveness of betamethasone administration using the ALPS trial’s primary outcome (a composite of respiratory morbidity in the first 72 hours of life including continuous positive airway pressure or high-flow nasal cannula for ≥ 2 hours continuously, oxygen with a fraction of inspired oxygen of at least 0.30 for ≥ continuous 4 hours continuously, mechanical ventilation, extracorporeal membrane oxygenation, stillbirth, or neonatal death) and severe respiratory morbidity composite (in the first 72 hours, continuous positive airway pressure or high-flow nasal cannula for ≥ 12 hours continuously or oxygen with a fraction of inspired oxygen of at least 0.30 for ≥ 24 hours continuously, mechanical ventilation, extracorporeal membrane oxygenation, stillbirth, or neonatal death) [2]. Our model considered outcomes on the individual-patient level and on a population-level, using a theoretical cohort of 270,000 infants, which is the approximate number of late preterm births in 2015 in the United States [8].
TreeAge Pro 2017 (TreeAge Software Inc, Williamstown, MA) was used to create our decision analytic model. This study involved use of only published data and was therefore exempt from Institutional Review board approval. Due to the short time horizon and the fact that neonates were hospitalized regardless of whether or not they received antenatal corticosteroids, we considered only the health care sector perspective and not a societal perspective in the reference case analysis [7].
Probabilities, Costs, Utilities, and Effectiveness
Probabilities, costs, utilities, and effectiveness are shown Table 1. Probabilities were derived from the ALPS trial [2]. We assumed women at risk of late preterm delivery would be admitted for inpatient management of preterm labor, so we considered only the costs of betamethasone ($169 for 24 mg) and neonatal medical care [9]. Cost data were derived from the Healthcare Cost and Utilization Project from the Agency for Healthcare Research and Quality and were based on a combination of median hospital costs from Medicare-Severity Diagnosis Groups (DRG) and principal diagnoses from the 9th edition of the International Classification of Diseases (ICD-9) (Table 1) [10]. We converted all costs to 2017 price level using the medical component of the Consumer Price Index [11]. For a premature infant with neither respiratory morbidity nor hypoglycemia, diagnosis-related group (DRG) 792 “prematurity without major problems” was used to approximate cost. For neonates with hypoglycemia only, the cost of neonates discharged with ICD- 9 775.6 “neonatal hypoglycemia” was used. For infants with RDS, the cost associated with ICD-9 code 769 “respiratory distress syndrome” was used. For TTN, ICD-9 code 770.6 (newborn transient tachypnea) was used. Costs were added for the dual outcomes (RDS/hypoglycemia and TTN/hypoglycemia) via a micro-costing technique.
Table 1:
Model Inputs
| Outcome | Probabilitya | Cost (2017$)b | Utilityc | QALYd | |
|---|---|---|---|---|---|
| With BMZh | Without BMZ | ||||
| Overall RDSe | 0.0552 | 0.064 | ---- | ---- | ---- |
| Overall TTNf | 0.067 | 0.099 | ---- | ---- | ---- |
| Overall Hypoglycemiag | 0.24 | 0.15 | ---- | ---- | ---- |
| RDS without hypoglycemiae | 0.042 | 0.054 | 35,813 | 0.87 | 0.018 |
| TTN without hypoglycemia f | 0.051 | 0.084 | 9,292 | 0.93 | 0.019 |
| Hypoglycemia without respiratory morbidityg | 0.21 | 0.13 | 9,733 | 0.94 | 0.019 |
| RDS and hypoglycemia | 0.013 | 0.0096 | 45,545 | 0.87 | 0.018 |
| TTN and hypoglycemia | 0.016 | 0.015 | 19,025 | 0.90 | 0.018 |
| No adverse outcome | 0.67 | 0.71 | 2,240 | 1 | 0.021 |
source 2
source 10. For patients who received BMZ $169 was added to the cost to account for the cost of the medication.
source 12
quality-adjusted life-year source
respiratory distress syndrome
transient tachypnea of the newborn
defined as blood glucose measurement of <40 mg/dL at any time during the initial hospitalization after birth
betamethasone
Due to the impossibility of obtaining utilities from the neonatal perspective, we used parental utilities derived from a well-validated study of over 4,000 parents as proxy measures using the standard gamble method and assumed that parental utilities approximate neonatal utilities [12]. For RDS we used the utility of a 10-day intensive care unit hospitalization (0.87), for TTN we used a moderate allergic reaction (0.93), and for hypoglycemia we used the utility of a 10-day hospitalization (0.94). For RDS with hypoglycemia, we assumed the utility of the combined outcome would not be worse than RDS alone and thus used the same utility value as RDS. For TTN with hypoglycemia, we used the utility of a severe case of gastroenteritis (0.90) to account for the more intensive care required for infants with both conditions. For a healthy infant we used a utility of a healthy child (1.0) [12]. For all outcomes, we multiplied the utility value by our time horizon of 7.5 days, as the median length of stay in the trial was 7 days in the betamethasone group and 8 days in the placebo group with no statistically significant difference between them (p=0.20) [2]. To date there are no demonstrated differences in effectiveness in late-preterm steroids at different gestational ages, so we did not stratify our model by gestational age [2].
Analysis
We calculated the cost-effectiveness of betamethasone administration in the late-preterm period on an individual level as well as based on a hypothetical cohort of 270,000 late-preterm births. When applicable, the incremental cost-effectiveness ratio (ICER) was calculated. ICER is a ratio of the difference in costs between two interventions and the difference in effectiveness as measured by QALYs. We conducted several one-way sensitivity analyses varying the costs and probabilities. A tornado diagram included multiple such sensitivity analyses to assess which variables were most sensitive in our model. We also conducted threshold analyses of costs and probabilities using net monetary benefit, defined as (WTP × QALYs) - cost.
To ensure comparability with the outcomes of the ALPS trial, we also considered the primary outcome of the ALPS trial itself, a composite of respiratory morbidity [2]. Because the costs and utilities associated with this composite outcome are unknown, we conducted a sensitivity analysis with a range of costs from that of TTN to RDS and a range of utilities from that of RDS to TTN. In a similar manner, we analyzed the cost-effectiveness of betamethasone administration using the severe respiratory complication composite from the ALPS trial [2].
RESULTS
We found that betamethasone administration had slightly higher cost and marginally less effectiveness for short-term outcomes than withholding betamethasone. For an individual patient, compared to withholding betamethasone, betamethasone administration was more costly ($6,592 versus $6,266) but slightly less effective [7.31 quality-adjusted days (0.02002 QALY) versus 7.32 quality-adjusted days (0.02006 QALY)]. Table 2 describes the prevalence of adverse neonatal outcomes as well as cost and QALYs for the theoretical population of 270,000 late-preterm infants. In this cohort, betamethasone administration would decrease the incidence of RDS by 3,420 and TTN by 8,973 but increase the incidence of neonatal hypoglycemia by 22,995 cases annually. Compared to withholding betamethasone, betamethasone administration was associated with a higher cost ($1,780 million versus $1,692 million) and less effectiveness (5,405 QALYs versus 5,416 QALYs). Thus, withholding betamethasone dominated betamethasone administration and was cost-saving, i.e. less costly and more effective. Of note, even if betamethasone were provided free-of-charge (i.e., $0 cost for medication administration), withholding administration was still more effective and less costly.
Table 2:
Results per 270,000 Births
| Betamethasone Administration | No Betamethasone Administration | Difference | |
|---|---|---|---|
| Outcome (n of cases) | |||
| RDS alonea | 11,286 | 14,688 | −3,420 |
| TTN aloneb | 13,748 | 22,721 | −8,973 |
| Hypoglycemia alonec | 56,894 | 33,899 | 22,995 |
| RDS and hypoglycemia | 3,564 | 2,592 | 972 |
| TTN and hypoglycemia | 4,342 | 4,009 | 333 |
| No adverse outcome | 180,166 | 192,091 | −11,925 |
| Cost (in millions of 2017$) | $1,780 | $1,692 | $88 |
| Effectiveness (QALY)d | 5,405 | 5,416 | −11 |
respiratory distress syndrome
transient tachypnea of the newborn
defined as blood glucose measurement of <40 mg/dL at any time during the initial hospitalization after birth
quality-adjusted life-years
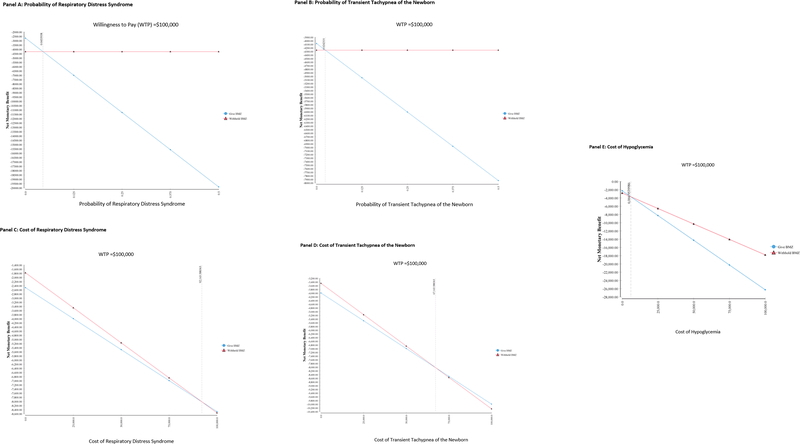
The cost of treatment of hypoglycemia, TTN, and RDS had the biggest impact on the cost-effectiveness outcomes. For the threshold analyses, betamethasone administration became cost-effective if the probability of RDS, TTN, or hypoglycemia with betamethasone administration declined to 4.5%, 2.4%, or 20%, respectively (Figure 1, Panel A and B, respectively). Further threshold analyses suggested that betamethasone administration became cost-effective if the cost of treating RDS exceeded $92,144, if the cost of treating TTN exceeded $67,144, or if the cost of treating hypoglycemia was below $6,067 (Figure 1, Panel C, D, and E, respectively).
Figure 1:
Threshold Analyses
Panel A: Probability of Respiratory Distress Syndrome
Panel B: Probability of Transient Tachypnea of the Newborn
Panel D: Cost of Transient Tachypnea of the Newborn
Panel E: Cost of Hypoglycemia
When we considered the primary composite outcome of the ALPS trial, withholding betamethasone became less costly until the cost of the composite outcome reached $32,860. Withholding betamethasone became more effective if the QALY of the composite outcome was lower than 0.017; given the QALY used in our model for RDS was 0.018, it is unlikely that the composite outcome would have a lower QALY than RDS alone. Finally, in the model considering the severe respiratory morbidity composite outcome of ALPS trial, withholding betamethasone became less costly until the cost of the composite outcome exceeded $23,396. Withholding betamethasone was more effective until the QALY of the composite outcome was below 0.018 QALY, which is the QALY utilized in our model for RDS.
DISCUSSION
Although betamethasone administration has become routine for late-preterm pregnancies at risk of imminent delivery in the United States, our analysis suggests this practice may not be cost-effective in the immediate neonatal period due to the increased rate of hypoglycemia seen with betamethasone administration and the relatively low rate of respiratory morbidity at this gestational age.
There has been only one published paper on the cost-effectiveness analysis of late-preterm betamethasone, which found betamethasone administration to be cost-effective in the long-term [13]. However, the prior study was limited in that there is no evidence that betamethasone-administration changes childhood outcomes in the long-term [14]. In particular, antenatal corticosteroid exposure has not been shown to reduce the incidence of chronic lung disease, which was one of the main outcomes of the previous cost-effectiveness analysis [13, 14]. Although late-prematurity is a risk factor for later pulmonary disease including asthma, wheeze, and chronic obstructive pulmonary disease, this risk appears to be independent of neonatal respiratory outcomes other than bronchopulmonary dysplasia and has not been shown to be affected by antenatal steroid administration [15–17]. Notably in the ALPS trial the rate of bronchopulmonary dysplasia was significantly lower in neonates who received corticosteroids (0.1% vs. 0.6%) but the absolute risk was very small in either case [2]. In fact, although there is no evidence that antenatal corticosteroids affect long term outcomes in children born late-preterm, there is evidence that hypoglycemia does [18]. For instance, hypoglycemia is the only independent risk factor for neurodevelopmental delay in childhood for children born moderately preterm [18]. Therefore, since the benefit of antenatal corticosteroids is in the short-term, this represents the most appropriate time-horizon for a cost-effectiveness analysis. Of note, a recently published conference abstract provided cost data from the ALPS trial and concluded that betamethasone administration was less costly than withholding it [19]. However, the cost of hypoglycemia treatment was not included [19].
Our threshold analyses suggest that the probabilities of RDS and hypoglycemia after betamethasone administration could, based on published studies, realistically cross the thresholds for making betamethasone administration cost-effective, but that the model is relatively insensitive to the probability of TTN [2, 20, 21]. Betamethasone would only become cost-effective if the probability of hypoglycemia in betamethasone-treated infants was lower than 20%, compared to the 24% seen in the ALPS trial. The only study reporting rates of hypoglycemia after betamethasone in the late-preterm period reported a non-statistically significant hypoglycemia rate of 7% in infants exposed to placebo compared to 11% in infants exposed to betamethasone, which was much lower than the threshold required to make betamethasone administration the more cost-effective option in our model [20].
Our study has multiple strengths. First, the probability data used in our model were derived from a recent multi-center randomized trial [2]. Second, our cost estimates are based on robust national data, and are supported by other studies [22, 23]. Third, instead of utilizing a composite primary outcome of varying clinical significance, our model utilized specific clinical diagnoses, which increases the clinical significance of our model [24]. Finally, we included the outcome of hypoglycemia, which was not included in the previous analyses [13, 19]. Although the ALPS trial authors note that hypoglycemia was not associated with any “adverse events,” there is still a cost for hypoglycemia monitoring and treatment that must be considered. We acknowledge that hypoglycemia was a secondary outcome in the trial, and therefore our results should be interpreted with some caution. However, much like respiratory distress, neonatal hypoglycemia may not be a benign condition—for treatment, infants require a higher-level of pediatric care with frequent blood glucose measurements, and many need intravenous therapy [25]. Furthermore, even if treated, neonatal hypoglycemia may be associated with an increased risk of long-term neonatal neurologic sequelae [26–28].
Our study does have some limitations to consider. First, our effectiveness outcome was QALYs, but there are no neonatal utility data available. Though other cost-effectiveness studies on neonatal outcomes rely on assumed utilities based on clinical opinion, we chose to use parental utilities as a surrogate measure for clinically similar neonatal outcomes [12, 29]. Second, we used a time period of 7.5 days based on the ALPS trial data suggesting a median length of stay between 7 and 8 days; however, this time horizon in our initial model may not have accounted for the risk of prolonged hospitalizations for some infants [2]. However, the costs are global costs for the diagnoses, incorporating the average lengths of stay. Third, although there may theoretically be differential effects of betamethasone at different gestational ages across the late-preterm period, this has not been demonstrated, and so we did not account for this possibility [2]. Because respiratory morbidity is known to decrease with increasing gestational age over the late preterm period [1], further analyses should consider specific gestational age ranges. Fourth, our cost data are based on principal discharge diagnoses of RDS, TTN, or hypoglycemia. It is possible that our cost data overestimates or underestimates the actual cost of treatment of late-preterm infants because infants who did not have the principal discharge diagnoses of these conditions would not have been included in cost estimates. Fifth, we did not consider the impact of a partial course of betamethasone administration on neonatal outcomes. Finally, we considered the maternal cost only as the cost of the medication without accounting for administration cost. Notably, 76.5% of patients in ALPS had diagnoses that are routinely managed with inpatient admission (preterm labor with intact membranes, ruptured membranes, or expected delivery for hypertensive disorder) [2]. Patients who received betamethasone in the outpatient setting would occur additional costs of a specific outpatient visit, which would further decrease the cost-effectiveness of this intervention.
There is no doubt that infants born in the late-preterm period suffer more morbidity than infants born at term, and that betamethasone administration is associated with decreased rates of neonatal morbidity when given prior to 33 weeks and 6/7 days gestation [30–32]. Though ACOG now recommends betamethasone administration to all eligible late-preterm pregnancies at risk of imminent delivery, the economic advantage of betamethasone at this gestational age is still unclear [4]. Indeed some experts have urged caution before widespread adoption of this intervention [33, 34, 35]. Further research is needed.
FUNDING
National Institutes of Health grant T32-HD-55172–9 to AKL. Agency for Healthcare Research and Quality Grant K01 HS022330 and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grant R21 DK110530 to SHC.
Footnotes
DISCLOSURE OF INTERESTS
The authors have no disclosures.
DETAILS OF ETHICS APPROVAL
Not required; no human or animal subjects were involved in the study
REFERENCES
- 1.Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, Gregory K, Haberman S, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304(4):419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gyamfi-Bannerman C, Thom EA, Blackwell SC, Tita AT, Reddy UM, Saade GR, et al. Antenatal Betamethasone for Women at Risk for Late Preterm Delivery. N Engl J Med. 2016;374(14):1311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Obstetrics. Committee Opinion No. 713: Antenatal Corticosteroid Therapy for Fetal Maturation. Obstet Gynecol. 2017;130(2):e102–e9. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence. Preterm labour and birth 2015. [Available from: https://www.nice.org.uk/guidance/ng25/chapter/Recommendations#maternal-corticosteroids. [PubMed]
- 6.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7. [DOI] [PubMed] [Google Scholar]
- 7.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093–103. [DOI] [PubMed] [Google Scholar]
- 8.Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: Final Data for 2015. Natl Vital Stat Rep. 2017;66(1):1. [PubMed] [Google Scholar]
- 9.Lexicomp Online. Pediatric & Neonatal Lexi-Drugs Hudson, Ohio: Lexi-Comp, Inc; [cited 2017 November 29]. Available from: http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/5004822?hl=718330#. [Google Scholar]
- 10.Agency for Healthcare Research and Quality. HCUPnet 2017. [cited 2017 November 22]. Available from: https://hcupnet.ahrq.gov/#setup. [DOI] [PubMed]
- 11.United States Department of Labor Bureau of Labor Statistics. Consumer Price Indices 2017. [cited 2017 November 27]. Available from: https://www.bls.gov/.
- 12.Carroll A, Downs S. Improving decision analyses: parent preferences (utility values) for pediatric health outcomes. J Pediatr. 2009;155(1):21–5, 5 e1–5. [DOI] [PubMed] [Google Scholar]
- 13.Bastek JA, Langmuir H, Kondapalli LA, Pare E, Adamczak JE, Srinivas SK. Antenatal corticosteroids for late-preterm infants: a decision-analytic and economic analysis. ISRN Obstet Gynecol. 2012;2012:491595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotecha SJ, Dunstan FD, Kotecha S. Long term respiratory outcomes of late preterm-born infants. Semin Fetal Neonatal Med. 2012;17(2):77–81. [DOI] [PubMed] [Google Scholar]
- 16.Collaco JM, McGrath-Morrow SA. Respiratory Phenotypes for Preterm Infants, Children, and Adults: Bronchopulmonary Dysplasia and More. Ann Am Thorac Soc. 2018;15(5):530–8. [DOI] [PubMed] [Google Scholar]
- 17.Pike KC, Lucas JS. Respiratory consequences of late preterm birth. Paediatr Respir Rev. 2015;16(3):182–8. [DOI] [PubMed] [Google Scholar]
- 18.Kerstjens JM, Bocca-Tjeertes IF, de Winter AF, Reijneveld SA, Bos AF. Neonatal morbidities and developmental delay in moderately preterm-born children. Pediatrics. 2012;130(2):e265–72. [DOI] [PubMed] [Google Scholar]
- 19.Gyamfi-Bannerman C Antenatal late preterm steroids: a cost analysis. Am J Obstet Gynecol. 2018;218(1):S14. [Google Scholar]
- 20.Porto AM, Coutinho IC, Correia JB, Amorim MM. Effectiveness of antenatal corticosteroids in reducing respiratory disorders in late preterm infants: randomised clinical trial. BMJ. 2011;342:d1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balci O, Ozdemir S, Mahmoud AS, Acar A, Colakoglu MC. The effect of antenatal steroids on fetal lung maturation between the 34th and 36th week of pregnancy. Gynecol Obstet Invest. 2010;70(2):95–9. [DOI] [PubMed] [Google Scholar]
- 22.Rawat M, Chandrasekharan P, Turkovich S, Barclay N, Perry K, Schroeder E, et al. Oral Dextrose Gel Reduces the Need for Intravenous Dextrose Therapy in Neonatal Hypoglycemia. Biomed Hub. 2016;1(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrou S, Khan K. Economic costs associated with moderate and late preterm birth: primary and secondary evidence. Semin Fetal Neonatal Med. 2012;17(3):170–8. [DOI] [PubMed] [Google Scholar]
- 24.Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health. 2015;18(2):161–72. [DOI] [PubMed] [Google Scholar]
- 25.Thompson-Branch A, Havranek T. Neonatal Hypoglycemia. Pediatr Rev. 2017;38(4):147–57. [DOI] [PubMed] [Google Scholar]
- 26.McKinlay CJD, Alsweiler JM, Anstice NS, Burakevych N, Chakraborty A, Chase JG, et al. Association of Neonatal Glycemia With Neurodevelopmental Outcomes at 4.5 Years. JAMA Pediatr. 2017;171(10):972–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKinlay CJ, Alsweiler JM, Ansell JM, Anstice NS, Chase JG, Gamble GD, et al. Neonatal Glycemia and Neurodevelopmental Outcomes at 2 Years. N Engl J Med. 2015;373(16):1507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucas A, Morley R, Cole TJ. Adverse neurodevelopmental outcome of moderate neonatal hypoglycaemia. BMJ. 1988;297(6659):1304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chatroux LR, Savitsky LM, Zwerling B, Williams J, Cahill AG, Caughey AB. Standardizing the Response to Category II Tracings during Induction with Oxytocin: A Cost-Effectiveness Analysis. Am J Perinatol. 2017;34(12):1255–63. [DOI] [PubMed] [Google Scholar]
- 30.McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111(1):35–41. [DOI] [PubMed] [Google Scholar]
- 31.Teune MJ, Bakhuizen S, Gyamfi Bannerman C, Opmeer BC, van Kaam AH, van Wassenaer AG, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205(4):374 e1–9. [DOI] [PubMed] [Google Scholar]
- 32.Melamed N, Klinger G, Tenenbaum-Gavish K, Herscovici T, Linder N, Hod M, et al. Short-term neonatal outcome in low-risk, spontaneous, singleton, late preterm deliveries. Obstet Gynecol. 2009;114(2 Pt 1):253–60. [DOI] [PubMed] [Google Scholar]
- 33.Kaempf JW, Suresh G. Antenatal corticosteroids for the late preterm infant and agnotology. J Perinatol. 2017;37(12):1265–7. [DOI] [PubMed] [Google Scholar]
- 34.Kamath-Rayne BD, Rozance PJ, Goldenberg RL, Jobe AH. Antenatal corticosteroids beyond 34 weeks gestation: What do we do now? Am J Obstet Gynecol. 2016;215(4):423–30. [DOI] [PubMed] [Google Scholar]
- 35.Jobe AH, Goldenberg RL. Antenatal corticosteroids: an assessment of anticipated benefits and potential risks. Am J Obstet Gynecol. 2018;219(1):62–74. [DOI] [PubMed] [Google Scholar]