Abstract
Background & Aims:
Patients with Crohn’s disease (CD) often have bile acid diarrhea (BAD), due to bile acid malabsorption following ileal resection (IR). Bile acid malabsorption increases production of 7α-hydroxy-4-cholesten-3-one (C4), a bile acid precursor. We investigated relationships between serum concentrations of C4 and BAD in patients with CD.
Methods:
We collected demographic data, serum samples, and information on the presence of diarrhea (>3 liquid bowel movements/day), as well as clinical, endoscopic, and histologic scores from 26 patients with CD and IR, 21 patients with CD without IR, and 37 patients with ulcerative colitis (UC). We compared serum concentrations of C4 and fibroblast growth factor 19 (FGF19) between groups. We performed area under the receiver operating characteristic curve (AUROC) analysis to identify the optimal cutoff C4 concentrations for the diagnosis of diarrhea attributable to bile acid malabsorption (BAD), defined as diarrhea and a serum concentration of FGF19 <60 pg/mL.
Results:
Patients with UC had a median serum C4 concentration of 11.8 ng/mL, whereas patients with CD and IR with ileitis (documented endoscopically) had a median concentration of 100.0 ng/mL (P compared to UC<.0001) and patients with CD and IR without ileitis had a median concentration of 51.6 ng/mL (P compared to UC<.001). Patients with CD without IR did not have a significantly higher median concentration of C4 than patients with UC (P=.71), regardless of ileitis (P=.34). When endoscopic findings were confirmed histologically, similar results were found to analyses using endoscopic findings alone. A higher proportion of patients with active UC had diarrhea (72.0% vs 0 patients with inactive UC; P<.001), but their median concentrations of C4 did not differ significantly from that of patients with inactive UC (12.1 ng/mL vs 9.7 ng/mL; P=.3). A cutoff concentration of C4 of 48.3 ng/mL or greater identified patients with diarrhea attributable to bile acid malabsorption with 90.9% sensitivity, 84.4% specificity, and an AUROC 0.94. A significantly higher proportion of patients with concentrations of C4 above this cutoff had BAD (50.0%) than below this cutoff (1.8%) (P<.001). When we analyzed only patients with diarrhea, a C4 cutoff of 48.3 ng/mL identified those with low FGF19 concentrations (<60 pg/mL) with 91% sensitivity and 95.5% specificity (AUROC, 0.99). Above this cutoff, 83.3% of patients had a serum concentration of FGF19 <60 pg/mL compared to 4.5% below this threshold (P<.0001). C4 concentrations correlated with the number of daily bowel movements (r=0.41; P=.004) and correlated inversely with FGF19 concentrations (r= −0.72; P<.0001).
Conclusion:
We observed significantly increased serum concentrations of C4 in patients with CD with IR, compared to patients with UC. A cutoff concentration of C4 above 48.3 ng/mL identifies patients with diarrhea likely attributable to bile acid malabsorption (BAD) with an AUROC value of 0.94. Increased serum levels of bile acid precursors identify patients with diarrhea and a low serum concentration of FGF19, and concentrations of C4 correlate with daily liquid bowel movements and correlate inversely with FGF19 concentrations. C4 may be a biomarker to identify patients with diarrhea attributable to bile acid malabsorption.
Keywords: non-invasive test; monitoring, surgical complications; inflammatory bowel diseases
INTRODUCTION
Ulcerative colitis (UC) and Crohn’s disease (CD) comprise chronic inflammatory bowel diseases (IBD). While UC is isolated to the colon, approximately 25–30% of CD patients have isolated ileal disease and 30–55% have ileocolonic disease. Since ileal disease or resection mediates bile acid malabsorption (BAM), CD patients are at significant risk for bile acid diarrhea (BAD)1.
Ileal dysfunction or resection causes BAM with subsequent secretory diarrhea (BAD) due to the effects of bile acids (BA) on adenylate cyclase in the colonic epithelium compounded by an increase in intestinal permeability1. Length of ileal resection (IR) less than 100 cm causes BAD, while IR greater than 100cm produces steatorrhea, due to depletion of the BA pool. Furthermore, active ileal CD has been shown to have increased prevalence of BAM with a decrease in BA absorptive transporters1. Diarrhea resulting from ileal disease is referred to as type 1 BAD. Type 2 BAD is idiopathic, and type 3 results from abnormal BA absorption from other causes.1,2
BAD often coexists in CD patients with diarrhea, but the optimal method of diagnosing BAD is unclear. An empiric therapeutic trial with bile acid sequestrants (BAS) is often used. However, gastrointestinal side effects, suboptimal dose titration and patient compliance hinder it’s use1,3.75Se-homocholic acid-taurine (SeHCAT) testing uses non-invasive imaging to detect BA retention of radiolabeled HCAT. SeHCAT is available in eight European countries4, but is less available elsewhere. Furthermore, it is unavailable in the USA5. SeHCAT involves radiation exposure and requires two visits. While not affected by intestinal transit, SeHCAT may possibly not discriminate BAM from other conditions that cause rapid intestinal transit.1,5 Patients with BAM develop compensatory increases in the synthesis of BA precursors. 7α-hydroxy-4-cholesten-3-one (C4) is a stable BA precursor that can be measured in the serum. C4 has been demonstrated to correlate well with BAD diagnosed with SeHCAT.6 Furthermore, C4 has been compared to SeHCAT and has been found to correlate appropriately and have similar test characteristics.1,7,8 However, the major impediment to the use of C4 has been availability1.
The use of C4 as a valuable diagnostic test in the irritable bowel syndrome with diarrhea (IBS-D) population is well-described, but detailed data is lacking in CD.7 In a pediatric cross-sectional study using clinical scoring and biomarkers without endoscopic evaluation, C4 was elevated in CD patients (n=44) as compared to UC (n=14) and in CD patients with diarrhea (n=12) as compared to those without.9 Other studies have demonstrated either elevated C4 in patients with type 1 BAD compared to other BAD types or in CD patients with historical categorizations of IR or ileitis compared to controls, however, detailed clinical data was not available to evaluate for confounders.10–12
Another serum diagnostic test, Fibroblast Growth Factor 19 (FGF19), has been described for BAD.12 FGF19 is produced in the ileum in response to BA absorption and suppresses BA production. Studies have shown it to be inversely related to C4 and reduced in patients with BAD compared to controls. FGF19 is also lower in type 1 BAD compared to other types of BAD. Although IR has been shown to produce higher C4 and lower FGF19, limited detailed clinical or endoscopic data has been available to evaluate confounders.11,12 In CD patients with clinical and biomarker, but not endoscopic, disease activity assessments, FGF19 was inversely correlated with IR length and was lower with suspected ileitis.13
In order to clarify the utility of C4 concentrations for the diagnosis of BAD in CD, assessment of the presence of diarrhea with other markers of BAM (FGF19) is critical. Furthermore, evaluation of active CD as the cause of diarrhea is also necessary, since symptoms are neither sensitive nor specific for objective endoscopic inflammation in CD.14 This discordance between symptoms and inflammation underscores the importance of accurately diagnosing alternative causes of diarrhea in CD, such as BAD, to explain this discrepancy. Previous studies have not analyzed C4 concentrations in CD patients with well characterized clinical and endoscopic disease activity. This study aims to investigate the relationship between C4 concentrations and BAD in well-characterized CD patients and appropriate controls.
METHODS
Patients and study design
Patients with CD were selected from the prospectively established University of California, San Diego (UCSD)-Prometheus IBD biobank. This consists of archived specimens and prospectively scored disease activity assessments from a convenience sample of IBD patients. Serum samples are collected at each clinical or endoscopic encounter. Patients were selected from the biobank based on availability of serum samples. In individual patients from the biobank with multiple visits linked with serum collections, individual patient visits–and corresponding serum samples analyzed–were selected using a pre-specified algorithm (Supplementary Appendix). It prioritized temporal proximity of serum collections to endoscopies and availability of stool frequency data. Serum samples, clinical and endoscopic scores were collected at the same timepoint in 40 patients. If serum samples were not collected at the same time as disease activity assessments, medical chart review was performed to ensure changes in clinical or endoscopic status did not occur in the intervening time. Serum samples were collected at clinical visits (with clinical assessments) within 6 weeks of the endoscopy visit in 35 patients. Two patients had serum sample collections at clinic visits at 4 and 8 months apart from an endoscopy, respectively. Seven CD-IR patients did not have available endoscopic activity assessments, but had serum samples collected at clinical visits. Thus, these patients were only included in analyses comparing C4 concentrations in the entire CD-IR group to UC. Histologic assessments of biopsies are from the same endoscopies with activity assessments. CD patients were grouped into those with IR and those without. Patients with UC (without ileal disease), were included as controls. Clinical disease activity indices (CD: Patient-reported Outcomes-2 [PRO2], UC: partial Mayo score), and serum were collected at routine clinical and endoscopy visits. Endoscopic procedures were scored using the simplified endoscopic score for CD (SES-CD) and the Mayo endoscopic sub-score (ESS) for UC. For CD patients, ileal biopsies were scored using the Global Histologic Disease Activity score (GHAS) by a blinded gastrointestinal pathologist (MV). Demographics, serum samples, clinical, endoscopic and histologic scores were prospectively collected. The number of daily liquid bowel movements (BM) was recorded prospectively in 37 clinical visits linked with serum collections and was abstracted from medical record review by 2 reviewers (RB, MD) when prospective data was unavailable (n=39). Eight patients had missing stool frequency data and were excluded from BAD outcome analyses (and only included in analyses comparing C4 concentrations between groups). Patients were excluded if they had a colectomy, an ileostomy or a colostomy. This is due to the lack of interaction of BA’s with the colon, thus BAD would not physiologically be possible and interpretation of BM frequency would be confounded. IR, BAS use, or parenteral nutrition (PN) use was abstracted from medical record review by 2 reviewers (RB, MD). IR lengths were obtained from pathology reports when available, and clinical documentation otherwise. One patient had anti-diarrheal use and had persistent diarrhea (>10 BM/d). Thus, this was not felt to affect the analysis.
Serum C4 concentrations were measured by liquid chromatography and mass spectrometry (LC-MS, Prometheus Laboratories Inc.). This assay has been shown to have a reportable range of 1.5 to 500 ng/mL, and high intra- and inter-assay run accuracy and precision with recoveries of 90–110% and coefficient of variation of less than 10 % spanning the reportable range. The assay is specific for C4 and does not cross-react with cholesterol, 7-alpha cholesterol, 7-beta cholesterol, 7-Keto cholesterol, cholic acid or deoxycholic acid. FGF19 concentrations were measured using a quantitative sandwich enzyme immunoassay using a monoclonal antibody specific for human FGF19 (Quantikine ELISA Human FGF19 kit, R&D Systems, MN).
Definitions:
Ileal and colonic endoscopic remission (ER) were defined as an ileal or colonic SES-CD <3 and <2 in each segment for CD patients. In UC, ER was defined as a UCESS <2. Active colitis was defined as a UC-ESS ≥2 in UC. Diarrhea attributable to BAM (BAD) was defined as having both diarrhea (>3 daily liquid BM’s15,16) with objective evidence of bile acid malabsorption (FGF19 concentration <60pg/mL10). Patients were defined as being in ER for visits within 8 months of endoscopy demonstrating ER without any interval endoscopy demonstrating activity within that period (and in the absence of a change in clinical symptoms). Furthermore, for patient samples collected at visits not within 8 months of endoscopy or if a change in symptoms occurred without endoscopic documentation, categorization of active ileitis or ER could not be performed. Ileal histologic remission was defined as a GHAS ≤2 on ileal biopsies.17 Patients with inactive IBD comprised of inactive UC patients and CD patients with neither colonic nor ileal endoscopic disease activity.
Endpoints:
Primary analysis was performed to determine whether serum C4 concentrations were different in CD-IR patients and CD patients without IR compared to UC patients. In these groups, further analyses were performed in CD patients with and without ileitis. These analyses were repeated for FGF19 concentrations. Other analyses of C4 concentrations included (A) CD IR patients with IR<50cm compared to IR >50cm18,19 and (B) CD IR patients receiving BAS, compared to those not receiving BAS.
Characteristics of test procedure (sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), receiver–operating characteristic (ROC) curve and the area under the curve (AUC)) were used to evaluate optimal cutoff values (Youden’s index) for C4 concentration for diarrhea attributable to BAM.
Statistical analysis
Data analysis included descriptive statistics computed for continuous variables (means and standard deviations analyzed for normally distributed data, medians with interquartile rages (IQR) analyzed for non-normally distributed data). Percentages were used for categorical variables. Between-group comparisons were performed using, Fishers exact test, or Wilcoxon rank sums test, as appropriate. P-values ≤0.05 were considered significant. Due to multiple statistical comparisons, post-hoc Bonferroni corrections were performed (adjusted p-value). All statistical analyses were done using JMP13 (SAS Institute, NC, USA). Analyses included one serum collection per patient. Spearman’s correlation coefficient was used for two comparisons A) C4 concentrations and the number of daily bowel movements as a continuous variable, B) C4 and FGF19 concentrations.
Ethics
All authors had access to study data, reviewed and approved the final manuscript. Study protocol and materials were approved by the institutional review board at UCSD. All patients provided written informed consent.
RESULTS
Patients
Eighty-four patients (CD:47, UC: 37) with serum sample collections were included (Table 1). Twenty-six CD-IR patients were included. Of these, 9 patients had ileitis documented endoscopically (CD, +IR, +ileitis), 10 patients had endoscopic documentation of absence of ileitis (CD, +IR, -ileitis) and categorization of endoscopic activity could not be performed in 7 patients. Twenty-one CD patients without IR were included. Of these, 9 patients had ileitis (CD, -IR, +ileitis) and 12 patients did not have ileitis (CD, -IR, -ileitis). One CD-IR patient required intermittent PN. Thirty-seven UC patients without ileal disease were included. Of the UC patients, 28 patients had active endoscopic disease and 9 patients did not. Stool frequency was recorded in 76 patients. BAS use was recorded in 81 patients. Only CD-IR patients had BAS use (4/23, three patients with missing data).
Table 1:
Patient Characteristics
| Crohn’s Disease (n=47) |
Ulcerative Colitis (n=37) |
p value | |
|---|---|---|---|
| Mean age, years (SD) | 41.3 (15.7) | 44.2 (17.7) | 0.43 |
| Female, n (%) | 29 (61.7) | 18 (48.6) | 0.23 |
| Behavior, n (%) | |||
| B1 | 23 (48.9) | n/a | n/a |
| B2 | 14 (29.8) | n/a | n/a |
| B3 | 10 (21.3) | n/a | n/a |
| Location, n (%) | |||
| CD: L1 | 11 (23.4) | n/a | n/a |
| CD:L2 | 6 (12.8) | n/a | n/a |
| CD:L3 | 30 (63.8) | n/a | n/a |
| UC: Proctitis | n/a | 6 (16.2) | n/a |
| UC: Left Sided | n/a | 11 (29.7) | n/a |
| UC: Extensive | n/a | 20 (54.1) | n/a |
| Perianal Disease, n (%) | 6 (12.8) | n/a | n/a |
| Age at diagnosis (years), n (%) | |||
| <16 | 7 (14.9) | 4 (10.8) | 0.58 |
| 16–40 | 31 (66) | 21 (56.8) | 0.39 |
| >40 | 9 (19.1) | 12 (32.4) | 0.16 |
| Biologic Medication Use | 34 (72.3) | 26 (70.3) | 0.84 |
Ileal resection, ileitis and C4 concentrations:
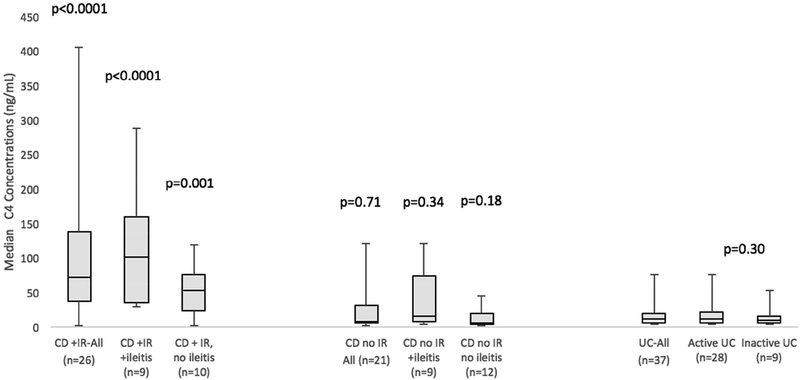
Compared to UC patients (11.8ng/mL, IQR 25–75: 5.5–19.6), significantly higher median C4 concentrations existed in all CD-IR patients (70.8 ng/mL, IQR 25–75: 35.9–138.8, p<0.0001, adjusted p-value <0.001), CD-IR patients with documented endoscopic ileitis (CD, +IR, +ileitis: 100.0 ng/mL, IQR 25–75: 34.5–159.0, p<0.0001, adjusted p-value <0.001) and CD-IR patients with documentation of absence of ileitis (CD, +IR, -ileitis: 51.6ng/mL, IQR 25–75: 23.5–75.7, p=0.001, adjusted p-value =0.006, Figure 1). CD-IR patients with active ileitis (CD, +IR, +ileitis) did not have significantly higher C4 than CD-IR patients without ileitis (CD, +IR, -ileitis, p=0.2). CD patients without IR (7.7 ng/mL, IQR 25–75: 4.8–31.4, p=0.71) did not have higher C4 concentrations than UC. In this group, those with documentation of active ileitis (CD, -IR, +ileitis: 15.2ng/mL, IQR 25–75: 6.8–72.7) did not have higher C4 concentrations compared to UC (p=0.34). Notably, CD patients with neither IR, nor ileitis (CD, -IR, -ileitis: 5.8 ng/mL, IQR 25–75: 3.0–19.5) had similar C4 concentrations to UC patients (p=0.18).
Figure 1: Ileal Resection, Endoscopic Activity and C4 Concentrations.
Compared with UC, higher C4 concentrations existed in CD patients with ileal resections (IR), with or without endoscopically confirmed ileitis. Similar C4 existed between inactive UC and active UC patients (p=0.30).
Compared to inactive IBD patients (11.1%), the only groups to have a greater proportion of patients with diarrhea were CD-IR patients (50.0%, p=0.02, adjusted p-value =0.04) and CD-IR patients with active ileitis (57.1%, p=0.03, adjusted p-value =0.06).
Similar C4 concentrations were seen in UC patients with and without endoscopically active colitis (p=0.3) even though a greater proportion of patients with active UC had diarrhea (72.0%) as compared to inactive IBD patients (11.1%, p<0.001, adjusted p-value <0.003, Supplementary Table 1) or inactive UC patients (0%, p<0.001, adjusted p-value <0.003).
Histologic confirmation of active ileitis or ileal histologic remission yielded similar results to endoscopy alone (Supplementary Figure 1). CD-IR patients, regardless of ileitis on histology, had higher median C4 concentrations compared to UC, while other groups did not. Four CD patients with ileal endoscopic remission did not have histologic remission. Three had IR (C4: 38.5–70.9 ng/mL) and one did not (C4=31.4 ng/mL).
CD patients with IR length >50cm (n=4, IQR 25–75: 92.7–189.5) had numerically higher median C4 concentrations (109.0 ng/mL vs. 45.2 ng/mL, p=0.19) as compared to CD patients with shorter IR length (n=5, IQR 25–75: 35.0–89.9). However, IR length was not associated with the presence of diarrhea (IR≤ 50cm: 20% IR>50cm: 50%, p=0.52).
Diagnostic accuracy of C4 concentrations for bile acid diarrhea
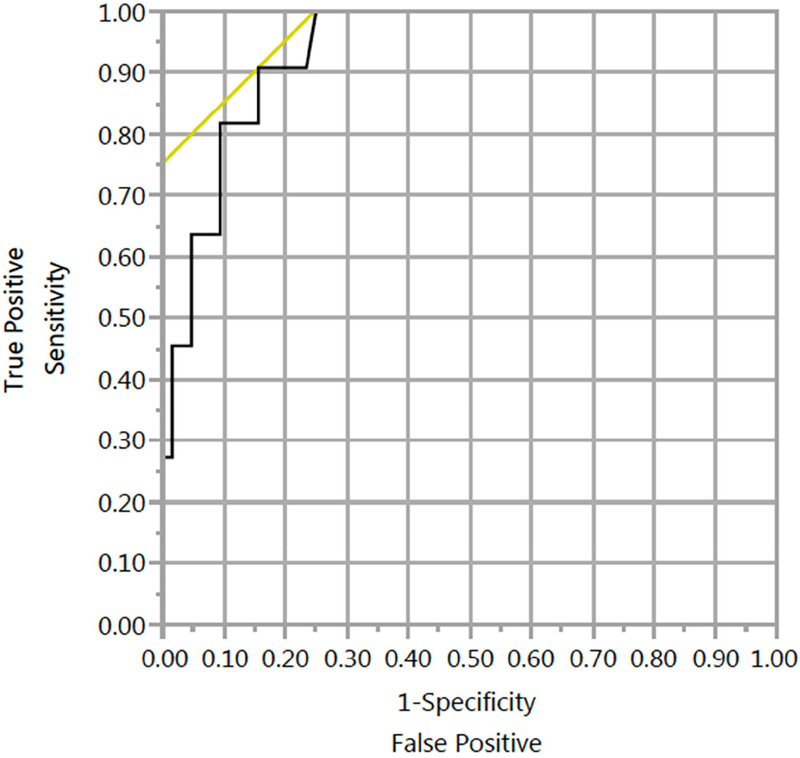
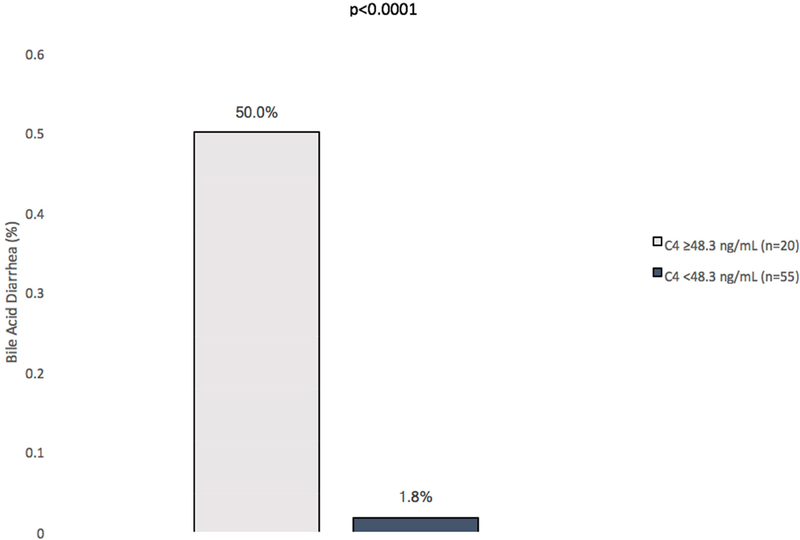
An ROC curve yielded a threshold C4 concentration of 48.3 ng/mL (Figure 2, AUC 0.94, 90.9% sensitivity, 84.4% specificity, PPV: 50%, NPV: 98.2%, n=75) to diagnose BAD (diarrhea with FGF19<60pg/mL). BAD was present in 50.0% of patients with C4 concentrations above 48.3 ng/mL (n=20) compared to 1.8% of patients below this cutoff (n=55, p<0.0001, Figure 3, Supplementary Table 2). In those with BAM (C4 >48.3 ng/mL, n=20) without BAD, median C4 concentrations were 93.6 ng/mL (IQR 25–75: 56.7–120.7, n=10), while concentrations were 131 ng/mL (IQR 25–75: 74.5–196.5, n=10) for those with both BAM and BAD (p=0.14).
Figure 2: ROC analysis for bile acid diarrhea based on C4 concentrations.
ROC analysis indicated an optimal threshold C4 concentration associated with diarrhea for CD and UC patients without colitis (90.9% sensitivity, 84.4% specificity, AUC=0.94). The diagonal line indicates an ideal threshold based on optimal sensitivity and specificity.
Figure 3: Outcomes for Bile Acid Diarrhea based on C4 concentrations.
Concentrations of C4 ≥48.3/mL were associated with higher rates of bile acid diarrhea (>3 liquid BM daily and FGF19 concentration<60pg/mL) compared to those below this concentration.
The proportion of patients with C4 >48.3 ng/mL were, 17/26 (65.4%) CD-IR patients, 2/21 (9.5%) CD patients without IR and 3/37 (8.1%) UC patients. The proportion of patients with BAD were, 45.5% (10/22) CD-IR patients, 0/20 CD patients without IR (0%) and 1/33 UC patients (3.0%).
A sensitivity analysis was performed exclusively in patients with diarrhea for C4 concentrations to detect patients with an FGF19 concentration <60pg/mL. An ROC curve yielded an AUC of 0.99 (Supplementary Figure 2). Above a threshold C4 concentration of 48.3 ng/mL (90.9% sensitivity, 95.5% specificity, PPV: 90.9, NPV:95.5%), higher proportions of patients had FGF19 concentrations <60pg/mL (>48.3ng/mL: 83.3%, 10/12 vs. <48.3ng/mL: 4.5%, 1/22, p<0.0001).
Correlations between bowel movement frequency and C4 concentrations
Significant correlations were found between the number of daily bowel movements as a continuous variable and C4 concentrations (r=0.41, p=0.004, Supplementary Figure 3).
FGF19 Concentrations
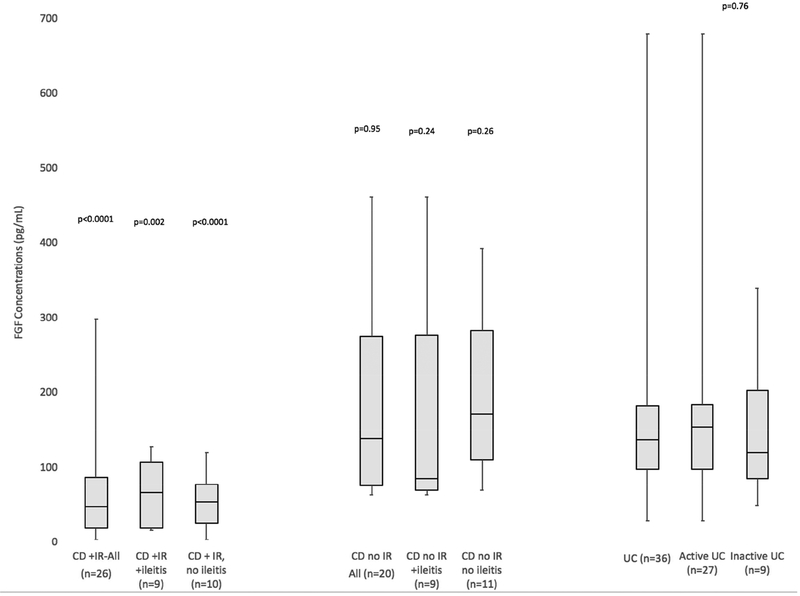
Compared to UC patients, CD-IR patients, CD-IR patients with documented endoscopic ileitis (CD, +IR, +ileitis) and CD-IR patients with documentation of absence of ileitis (CD, +IR, -ileitis) had lower FGF19 concentrations (Figure 4). Other groups did not have significantly different concentrations. A strong inverse correlation existed between C4 and FGF19 concentrations (r= −0.72, p<0.0001, Supplementary Figure 4).
Figure 4: Ileal Resection, Endoscopic Activity and FGF19 Concentrations.
Compared with UC, lower FGF19 existed in CD patients with ileal resections (IR), with or without endoscopically confirmed ileitis. Similar FGF19 existed between inactive UC and active UC patients (p=0.72).
Bile-Acid Sequestrant Use
Only CD-IR patients had BAS use. In this group, those with BAS use (n=4, IQR 25–75: 190.5–317.0), had higher median C4 concentrations (247.0 ng/mL vs. 49.3 ng/mL, p=0.002) than patients without BAS use (n=19, IQR 25–75: 35.0–102.0). To avoid selection bias for more severe BAD in patients with BAS prescriptions, an analysis of only patients with diarrhea was performed. In these patients, C4 concentrations remained associated with BAS use (BAS use: 288.0 ng/mL, n=3, no BAS use: 87.5 ng/mL, n=8, p<0.05). Given this finding and that all patients using BAS were CD-IR patients, a comparison excluding patients using BAS was performed for median C4 concentration between CD-IR patients (49.3 ng/mL, IQR 35.0–102.0) and UC patients and still found a significant difference (p<0.0001).
DISCUSSION
BAD is a common comorbidity in CD patients. Simple BAD diagnostic testing is needed to improve clinical care. To date, there is limited data on C4 concentrations in the setting of BAD in CD patients.
Our study demonstrates higher C4 concentrations in CD-IR patients. C4 concentrations were not associated with CD ileitis in the absence of IR. Furthermore, ileitis in the presence of IR was not associated with more pronounced C4 elevations. Higher C4 concentrations are found in patients with BAS use, suggesting BA sequestration increases excretion and loss of negative-feedback. Although consistent with current literature7,11, the use of prospectively scored endoscopy and histology, as well as appropriate controls, better characterizes C4 concentrations in CD patients with suspected BAD.
C4 threshold concentrations of 48.3ng/mL had a high sensitivity and specificity for detecting diarrhea in the presence of objective evidence of BAM (FGF19<60pg/mL). This entity is likely BAD. Furthermore, in patients with diarrhea, a C4 cutoff above 48.3 ng/mL identified a high proportion of patients with an FGF19 <60pg/mL and was highly sensitive and specific for this outcome. Thus, C4 concentrations can be used to suggest the presence or absence of BAD. This cutoff is similar to previously described thresholds in the IBS-D population.5,7 This is the first study to describe these findings in patients with endoscopically documented disease activity, systematically controlling for ileal and colonic inflammation. Furthermore, we validated these findings with prospective histologic scoring and FGF19 measurements.
The most common clinical scenario for considering BAD in IBD patients is in CDIR patients. This study confirms that CD-IR patients have more BAM with clinically significant diarrhea (BAD). Findings also suggest ileitis is insufficient to either cause BAM biochemically or subsequent BAD. This is similar to the effects of ileal resection and ileitis on vitamin B12 metabolism, in which only IR is associated with deficiency.20
BAS use was associated with higher C4 in the few patients receiving BAS, and this was independent of the presence of diarrhea. Although the sample size is small, this is consistent with previous literature using older assays.21
Study limitations include sample size and missing stool frequency and BAS use data. Retrospective stool frequency assessments were performed in a proportion of patients. However, explicit clinical documentation of frequency of liquid stools was required, and considered missing otherwise. Patients were also assumed to have inactive small bowel disease based on physician assessment and ileo-colonoscopy. Three patients received BAS at the time of serum collections (C4: 144ng/mL-404ng/mL), and all had persistent diarrhea. However, conclusions were unaltered when excluding these patients from analyses. Analyses for IR lengths had small sample sizes, which may predispose to a type 2 error.
C4 was not compared to either SeHCAT or a therapeutic trial, and we assumed similar test characteristics of C4 as compared with SeHCAT.1,7,21,22 This may limit conclusions. However, measured FGF19 was consistent with C4 concentrations in each patient group. Previously studies have demonstrated lower FGF19 with ileitis.13 Literature is conflicting on whether ileitis causes higher C4 concentrations9–12. However, several studies do show elevated C4 levels in this context11,12. Although higher C4 concentrations were seen in CD-IR patients, ileitis in the absence of IR yielded numerically higher C4 and lower FGF19 concentrations. However, statistical significance may have been less evident due to sample size. Additionally, this study used prospective endoscopic scoring with a specific SES-CD cutoff, which may yield different results than previous studies which used historical categorizations of disease location.
One CD-IR patient using PN for short bowel syndrome was included; however, PN is not known to affect C4 concentrations. Ultimately, missing stool frequency data excluded this patient from analyses for BAD outcomes. Serum samples were collected at clinic visits at various times in the day, statin use was not recorded and lipids were not routinely measured. Older studies suggest diurnal variations and possible effects of ethanol, triglyceride and cholesterol levels on C4 measured by LC1,23. However, recent studies contradict this.7,24 Liver disease may affect C4. Two active UC patients had primary sclerosing cholangitis with cholestatic liver enzyme elevations of 1.2 and 3.5 times the upper limit of normal at the time of sampling; however, they did not have elevated C4 (26.1 and 19.0 ng/mL, respectively). A third had a remote liver transplantation due to Wilson’s disease without clinical recurrence, normal liver enzymes and normal C4 (4.4 ng/mL). One patient had non-alcoholic fatty liver disease without elevated transaminases. The remaining patients had neither hepatobiliary abnormalities, nor cholestatic liver enzyme elevations.
This study suggests C4 is a potential biomarker to diagnose diarrhea attributable to bile acid malabsorption, particularly in CD-IR patients. In addition to elevated bile acid precursors accurately identifying patients with both diarrhea and a low FGF19 concentration, strong appropriate correlations of C4 existed with daily liquid bowel movements and FGF19 concentrations. In all patients, C4 concentrations above 48.3 ng/mL identified BAD with an AUC of 0.94, was highly sensitive and specific for this diagnosis and significantly higher proportions of patients had BAD above this cutoff (50% vs. 1.8% p<0.0001). Furthermore, when analyzing only in patients presenting with diarrhea, C4 concentrations above 48.3 ng/mL identified a higher proportion (83.3% vs. 4.5%, p<0.0001) of patients having a low FGF19 concentration with excellent test characteristics (AUC: 0.99, 90.9% sensitivity, 95.5% specificity, PPV: 90.9, NPV:95.5%). At present, no studies demonstrate that BAS initiation based on symptoms combined with C4 concentrations is effective in CD patients with suspected BAD. Although further validation is required, this study suggests that a threshold C4 concentration of 48.3ng/mL can be used to guide clinicians to suggest the presence or absence of diarrhea likely attributable to BAM (BAD). Furthermore, CD-IR patients are the group most likely to have BAD. This avoids treatment of patients without BAD with unnecessary therapy, avoiding confusion related to a possible placebo response. A prospective randomized trial should be conducted to confirm that initiating treatment at this concentration is associated with reduction in diarrhea and to evaluate the optimal rise in C4 concentrations to indicate adequate bile-acid sequestration. This may provide supporting information on the need for dose escalation of therapy in patients with likely BAD who do not respond to initial BAS therapy. C4 testing is available to clinicians and could be used to guide treatment.
Supplementary Material
Editors Notes- What You Need to Know.
1. Background
Crohn’s disease (CD) predisposes to bile acid malabsorption, which increases 7α-hydroxy-4-cholesten-3-one (C4) production.
C4 concentrations are elevated in patients with bile acid diarrhea (BAD).
2. 2. Findings
In CD patients with an ileal resection, C4 concentrations are elevated.
C4 concentrations of 48.3 ng/mL have a 90.9% sensitivity and 84.4% specificity to suggest likely BAD.
In all patients, a C4 threshold of 48.3ng/mL has a 50% positive predictive value and 98.2% negative predictive value for BAD.
Amongst patients with diarrhea, a C4 threshold of 48.3ng/mL has a 90.9% positive predictive value and 95.5% negative predictive value for bile acid malabsorption confirmed with low (<60pg/mL) FGF19 concentrations.
3. Implications for patient care
In CD patients with an ileal resection, the risk of bile acid diarrhea is elevated.
In CD patients, C4 concentrations can be used to suggest the presence or absence of bile acid diarrhea.
Acknowledgments
All authors have approved the final draft submitted. Financial or grant support: C4 concentrations were analyzed by an assay provided by Prometheus Laboratories Inc., San Diego, CA. Assistance with statistical analysis was provided by Prometheus Laboratories Inc., San Diego, CA. NVC holds a Career Development Award (545474) from the Crohn’s and Colitis Foundation (CCF). SS is supported by the American College of Gastroenterology Junior Faculty Development Award and Crohn’s and Colitis Foundation Career Development Award, has received research grants from Pfizer and AbbVie. BSB has support from a Career Development Award from the Crohn’s and Colitis Foundation (CCF) and UCSD KL2 (1KL2TR001444). No other financial support was provided for this study. Writing Assistance: none.
Abbreviations:
- C4
7α-hydroxy-4-cholesten-3-one
- AUC
area under the curve
- BA
bile acid
- BM
bowel movements
- BAM
bile acid malabsorption
- BAD
bile acid diarrhea
- BAS
bile acid sequestrant
- CD
Crohn’s disease
- FGF19
Fibroblast Growth Factor 19
- GHAS
global Histologic Disease Activity score
- IBD
inflammatory bowel diseases
- IQR
interquartile rages
- IR
ileal resection
- LC-MS
liquid chromatography and mass spectrometry
- ESS
Mayo endoscopic sub-score
- NPV
Negative predictive value
- PN
parenteral nutrition
- PRO2
Patient-reported Outcomes-2
- PPV
Positive predictive value
- ROC
receiver–operating characteristic
- SeHCAT
Se-homocholic acid-taurine
- SES-CD
Simplified endoscopic score for CD
- UC
Ulcerative colitis
- UCSD
University of California, San Diego
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential competing interests and disclosures: RB: No conflicts of interest; MD: reports non-financial support from Dr. Falk Pharma, personal fees from Janssen, Merck & Co., Inc., Pfizer and Tillotts Pharma, outside the submitted work. NVC received consultancy fees from Janssen and Takeda, outside of the submitted work. SS has received consulting fees from AbbVie, Pfizer, Takeda and AMAG Pharmaceuticals. PSD: has received research support, consulting fees, and honorariums from Takeda; research support from Pfizer; consulting fees from Janssen; research support from Prometheus. LM, JM, MR and AJ are employees of Prometheus Laboratories Inc., WJS consultant, research support from Prometheus; BSB received consultancy fees from Abbvie, Prometheus Laboratories outside of the submitted work.
REFERENCES
- 1.Mottacki N, Simren M, Bajor A. Review article: bile acid diarrhoea - pathogenesis, diagnosis and management. Aliment Pharmacol Ther. 2016;43(8):884–898. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M Bile Acid diarrhea: prevalence, pathogenesis, and therapy. Gut and liver. 2015;9(3):332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halilbasic E, Claudel T, Trauner M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J Hepatol. 2013;58(1):155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalid U, Lalji A, Stafferton R, Andreyev J. Bile acid malabsoption: a forgotten diagnosis? Clinical medicine (London, England). 2010;10(2):124–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayvargiya P, Camilleri M, Shin A, Saenger A. Methods for diagnosis of bile acid malabsorption in clinical practice. Clin Gastroenterol Hepatol. 2013;11(10):1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajor A, Kilander A, Fae A, et al. Normal or increased bile acid uptake in isolated mucosa from patients with bile acid malabsorption. Eur J Gastroenterol Hepatol. 2006;18(4):397–403. [DOI] [PubMed] [Google Scholar]
- 7.Brydon WG, Culbert P, Kingstone K, et al. An evaluation of the use of serum 7-alpha-hydroxycholestenone as a diagnostic test of bile acid malabsorption causing watery diarrhea. Can J Gastroenterol. 2011;25(6):319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajor A, Kilander A, Fae A, et al. Normal or increased bile acid uptake in isolated mucosa from patients with bile acid malabsorption. Eur J Gastroenterol Hepatol. 2006;18(4):397–403. [DOI] [PubMed] [Google Scholar]
- 9.Gothe F, Beigel F, Rust C, Hajji M, Koletzko S, Freudenberg F. Bile acid malabsorption assessed by 7 alpha-hydroxy-4-cholesten-3-one in pediatric inflammatory bowel disease: correlation to clinical and laboratory findings. J Crohns Colitis. 2014;8(9):1072–1078. [DOI] [PubMed] [Google Scholar]
- 10.Lenicek M, Duricova D, Komarek V, et al. Bile acid malabsorption in inflammatory bowel disease: assessment by serum markers. Inflamm Bowel Dis. 2011;17(6):1322–1327. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M, Nadeau A, Tremaine WJ, et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009;21(7):734–e743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pattni SS, Brydon WG, Dew T, Walters JRF. Fibroblast Growth Factor 19 and 7α-Hydroxy-4-Cholesten-3-one in the Diagnosis of Patients With Possible Bile Acid Diarrhea. Clinical and translational gastroenterology. 2012;3:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolan JD, Johnston IM, Pattni SS, Dew T, Orchard TR, Walters JR. Diarrhea in Crohn’s disease: investigating the role of the ileal hormone fibroblast growth factor 19. J Crohns Colitis. 2015;9(2):125–131. [DOI] [PubMed] [Google Scholar]
- 14.Modigliani R, Mary JY, Simon JF, et al. Clinical, biological, and endoscopic picture of attacks of Crohn’s disease. Evolution on prednisolone. Groupe d’Etude Therapeutique des Affections Inflammatoires Digestives. Gastroenterology. 1990;98(4):811–818. [DOI] [PubMed] [Google Scholar]
- 15.Sandborn W, Feagan B, Lewis J, et al. P533 Correlation of endoscopic and clinical endpoints during induction therapy in patients with moderate-to-severe Crohn’s disease: Analysis from CELEST study. Journal of Crohn’s and Colitis. 2018;12(supplement_1):S375–S376. [Google Scholar]
- 16.McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clinical Infectious Diseases. 2018;66(7):e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinisch W, Colombel J- F, D’Haens G, et al. Characterisation of mucosal healing with adalimumab treatment in patients with moderately to severely active Crohn’s disease: results from the EXTEND Trial. Journal of Crohn’s and Colitis. 2016;11(4):425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersson H, Bosaeus I, Brummer RJ, et al. Nutritional and Metabolic Consequences of Extensive Bowel Resection. Digestive Diseases. 1986;4(4):193–202. [DOI] [PubMed] [Google Scholar]
- 19.Walters JR, Johnston IM, Nolan JD, Vassie C, Pruzanski ME, Shapiro DA. The response of patients with bile acid diarrhoea to the farnesoid X receptor agonist obeticholic acid. Aliment Pharmacol Ther. 2015;41(1):54–64. [DOI] [PubMed] [Google Scholar]
- 20.Battat R, Kopylov U, Szilagyi A, et al. Vitamin B12 deficiency in inflammatory bowel disease: prevalence, risk factors, evaluation, and management. Inflammatory bowel diseases. 2014;20(6):1120–1128. [DOI] [PubMed] [Google Scholar]
- 21.Eusufzai S, Axelson M, Angelin B, Einarsson K. Serum 7 alpha-hydroxy-4-cholesten-3-one concentrations in the evaluation of bile acid malabsorption in patients with diarrhoea: correlation to SeHCAT test. Gut. 1993;34(5):698–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brydon WG, Nyhlin H, Eastwood MA, Merrick MV. Serum 7 alpha-hydroxy-4-cholesten-3-one and selenohomocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoea. Eur J Gastroenterol Hepatol. 1996;8(2):117–123. [DOI] [PubMed] [Google Scholar]
- 23.Galman C, Angelin B, Rudling M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology. 2005;129(5):1445–1453. [DOI] [PubMed] [Google Scholar]
- 24.Sauter GH, Munzing W, von Ritter C, Paumgartner G. Bile acid malabsorption as a cause of chronic diarrhea: diagnostic value of 7alpha-hydroxy-4-cholesten-3-one in serum. Dig Dis Sci. 1999;44(1):14–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.