Abstract
Children with prenatal tobacco exposure (PTE) exhibit early self-regulatory impairments, reflecting a life-course persistent propensity toward behavioral disinhibition. Previously, we demonstrated the protective role of parental responsiveness for reducing the risk of exposure-related disruptive behavior in adolescence. Here, we expanded this line of inquiry, examining whether responsiveness moderates the relation of PTE to a broader set of behavioral disinhibition features in early childhood and testing alternative explanatory models, i.e., ‘diathesis-stress’ vs. ‘differential vulnerability.’ PTE was assessed prospectively using interviews and bioassays in the Midwestern Infant Development Study (MIDS). Mother-child dyads (N=276) were re-assessed at approximately 5 years of age in a preschool follow-up. We quantified maternal responsiveness and child behavioral disinhibition via a combination of directly-observed activities in the lab and developmentally-sensitive questionnaires. Results supported a diathesis-stress pattern. Children with PTE and less responsive mothers showed increased disruptive behavior and lower effortful control compared to children without PTE. In contrast, exposed children with more responsive mothers had self-regulatory profiles similar to their non-exposed peers. We did not observe sex differences. Findings provide greater specification of the protective role of maternal responsiveness for self-regulation in children with PTE and help to clarify mechanisms that may underscore trajectories of exposure-related behavioral disinhibition.
Keywords: Prenatal tobacco exposure, parenting, executive function, self-regulation, disruptive behavior
Despite widespread recognition of the adverse effects of prenatal tobacco exposure (PTE) on fetal development, smoking during pregnancy remains common. In 2014, 8.4% of US women smoked during pregnancy based on birth certificate data (CDC, 2016). In addition to an increased risk of perinatal complications (Difranza, Aligne, & Weitzman, 2004; Himes, Stroud, Scheidweiler, Niaura, & Huestis, 2013; Windham, Hopkins, Fenster, & Swan, 2000), the offspring of women who smoke during pregnancy are 2 to 4 times more likely than their non-exposed peers to be diagnosed with a disruptive behavior disorder by middle childhood and are more likely to show severe antisocial behavior and substance use disorders in adolescence and adulthood (Cornelius, Goldschmidt, De Genna, & Larkby, 2012; Fergusson, Woodward, & Horwood, 1998; Gaysina et al., 2013; Murray, Irving, Farrington, Colman, & Bloxsom, 2010; Paradis, Fitzmaurice, Koenen, & Buka, 2011; Wakschlag & Hans, 2002; Wakschlag, Leventhal, Cook, & Pickett, 2000; Wakschlag, Pickett, Cook, Benowitz, & Leventhal, 2002). A series of large-scale genetically-informed studies have cast doubt about a causal nature of these associations (D’Onofrio et al., 2010; Maughan, Taylor, Caspi, & Moffitt, 2004; Roza et al., 2009), but have been limited with respect to the measurement of both PTE and child outcomes. More recently, studies using well-controlled, prospective designs and sensitive, multi-informant measures have shown exposure effects that are small, but independent of confounding influences (Estabrook et al., 2016; Gaysina et al., 2013; Knopik et al., 2016). These conflicting findings highlight a need for nuanced, developmentally-informed studies to clarify the mechanisms that underscore differences in outcome amongst children with PTE.
We have suggested that atypical patterns of behavior and cognition found among children with PTE can best be characterized as behavioral disinhibition, a profile of deficient self-regulation that often precedes severe antisocial behavior and substance dependence in adulthood (Clark, Espy, & Wakschlag, 2016; Tarter et al., 2003; Zucker, Heitzeg, & Nigg, 2011). Behavioral disinhibition is a broad behavioral profile, encompassing impulsiveness, aggression, resistance, poor executive control and sensation seeking (Lester et al., 2012; Nigg, 2017; Tarter, 2002). This integrative phenotype meaningfully and efficiently bridges early patterns of dysregulation with later antisocial trajectories in the PTE population. In particular, the use of this higher order construct enables developmental coherence in identification of these patterns. That is, rather than studying precursors in very young children and then frank clinical disorder at older ages, the behavioral disinhibition framework enables identification of component features at very young ages, which can continue to be measured as component features along with clinical symptoms across development (Carter, Gray, Baillargeon, & Wakschlag, 2013). Beginning in infancy, children with PTE show higher levels of temperamental irritability, poorer attention, and altered patterns of arousal (Clark, Espy, et al., 2016; Espy, Fang, Johnson, Stopp, & Wiebe, 2011; Law et al., 2003; Shisler et al., 2016; Wiebe et al., 2014; Willoughby, Greenberg, Blair, & Stifter, 2007). By preschool age, PTE is associated with increased activity levels and disruptive behavior, and deficits in executive control, including lower levels of selective attention and inhibitory control (Huijbregts, Séguin, Zoccolillo, Boivin, & Tremblay, 2007; Hutchinson et al., 2010; Martin, Dombrowski, Mullis, Wisenbaker, & Huttenen, 2006; Mezzacappa, Buckner, & Earls, 2011; Noland et al., 2005; Wakschlag, Leventhal, Pickett, Pine, & Carter, 2006; Wiebe, Clark, de Jong, et al., 2015). These difficulties are particularly prominent in emotionally evocative or motivational contexts (Huijbregts, Warren, De Sonneville, & Swaab-Barneveld, 2008; Wiebe et al., 2016). In middle childhood and adolescence, children with PTE show poorer performance on measures of vigilance and cognitive flexibility and emotion processing (Cornelius, Ryan, Day, Goldschmidt, & Willford, 2001; Kristjansson, Fried, & Watkinson, 1989; Wakschlag et al., 2009), while parents and teachers report increased levels of distractibility, rule-breaking and aggression (Cornelius, Goldschmidt, De Genna, et al., 2012; Wakschlag et al., 2011; Yang, Decker, & Kramer, 2013). Thus, across multiple points in childhood, children with PTE show the deficits in cognitive and behavioral control that characterize behavioral disinhibition (Zucker et al., 2011).
Early childhood is a key inflection point in the behavioral disinhibition trajectory, with deficient self-regulation during this period setting the stage for future psychopathology and adverse functional outcomes (Moffitt, 1993; Moffitt et al., 2011). As children develop an ability to recognize and evaluate their own and others’ emotions and executive control capacities increase, they are expected to coordinate their behavior independently in increasingly complex social and academic environments (Carlson & Moses, 2001; Cole, Zahn-Waxler, & Smith, 1994; Kopp, 1982; Wakschlag, Tolan, & Leventhal, 2010). By the time they transition to formal schooling, most children have developed the rudimentary ability to self-regulate their attention, emotional expressions and behavior (Clark et al., 2013). Thus, delays or deficits in self-regulation are likely to be especially prominent by the end of preschool, a critical period for shaping school readiness and adaptive function (Blair & Raver, 2015).
One of the most robust predictors of children’s self-regulatory competence is maternal sensitive responsiveness, the tendency of a mother to respond with warmth, promptness, and contingency, driven by her sensitivity to her child’s emotional expressions and bids for attention (Bernier, Carlson, & Whipple, 2010; Clark & Woodward, 2015; Eiden & Leonard, 2007; Kochanska, Murray, & Harlan, 2000; Landry, Smith, & Swank, 2006; Wakschlag & Hans, 2002). Predictable, contingent patterns of maternal behavior theoretically provide a consistent feedback loop that facilitates children’s understanding of which behaviors are most appropriate in a given context (von Suchodoletz, Trommsdorff, & Heikamp, 2011; Wakschlag & Hans, 2002). From an attachment perspective, continued signals from a mother that she is available and sensitive to her child’s needs also provide a secure environment for the child, shielding the child from stress and boosting his/her confidence to explore and learn from the environment (Ainsworth, 1979; Wolff & Ijzendoorn, 1997). Through these responsive caregiver cues, the child gradually internalizes strategies for coping with emotional and motivational demands in the environment and is able to assume more autonomous regulation of his/her behavior, learning how this behavior affects environmental responses and resting on the secure knowledge that the environment will respond in predictable ways (Chase-Lansdale, Wakschlag, & Brooks-Gunn, 1995). While studies of maternal responsiveness have predominantly focused on parent-child interactions in infancy and the early preschool period, there is increasing evidence for the impact of maternal responsiveness on children’s self-regulation during the late preschool period and transition to kindergarten (Chazan-Cohen et al., 2009; Pianta, Nimetz, & Bennett, 1997; von Suchodoletz et al., 2011) and studies typically report moderate stability in responsive, sensitive maternal behavior across early childhood (Perry, Mackler, Calkins, & Keane, 2014; Spinrad et al., 2012).
Previously, we found that maternal responsiveness, as observed during parent-child interactions in the first years of life, moderated the relation of PTE to conduct problems in a high-risk sample of 9–11 year olds (Wakschlag & Hans, 2002). Notably, conduct problems were elevated only in boys whose mothers showed low levels of responsiveness during early childhood. Exposed boys with responsive mothers and exposed girls showed levels of conduct similar to their non-exposed peers. In another study, we found that paternal responsiveness during adolescence buffered the relation of PTE to disruptive behavior in adolescents (Wakschlag et al., 2011). To our knowledge, only two other groups have examined responsive parenting as a potential protective factor in this population; both focused on early childhood and reported null effects. In the Quebec Longitudinal Study (Huijbregts, Seguin, Zoccolillo, Boivin, & Tremblay, 2008), observed maternal responsiveness did not moderate the relation of PTE to maternal ratings of toddler’s physical aggression. Likewise, in Godleski, Eiden, Scheutze, Colder, & Heutis (2016), observed maternal responsiveness was not associated with maternally-reported regulatory difficulties in toddlers with PTE, although there also was no direct correlation between PTE and self-regulation in this study. Notably, all of these studies concentrated primarily on clinical outcome measures and did not assess multiple features of behavioral disinhibition.
The scarcity of research examining the impact of parenting on the PTE-behavioral disinhibition link, as well as the discrepant findings reported in studies of different age groups, highlight a need to more deeply explicate the mechanisms by which parent-child interactions may foster adaptive pathways in children with PTE. This is especially true in light of evidence that mothers who smoke during pregnancy are less emotionally responsive, show higher levels of negative affect and parenting stress, and report more harsh, punitive parenting practices relative to mothers who do not smoke (Fergusson et al., 1998; Lynch, Johnson, Kable, Carroll, & Coles, 2011; Schuetze & Eiden, 2007; Tandon et al., 2013). Studies focused on early childhood and the transition to kindergarten are especially relevant for prevention science, given that self-regulation is central to school readiness and interventions tailored to this period are likely to be most cost-effective (Bruner, 2002; Early Childcare Research Network, 2005; Nelson et al., 2017). Moreover, it is critical that these studies employ sensitive, multidimensional measurement approaches in order to capture the variability in self-regulatory capacities that characterizes this age range (Clark, Chevalier, et al., 2016; Wakschlag et al., 2010).
The traditional model for parenting research assumes that children at risk will be more vulnerable to poor parenting practices, a ‘diathesis-stress’ or ‘dual risk’ perspective (Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2007). This perspective is in line with our assumption that sensitive, responsive parenting buffers the relation of PTE to behavioral disinhibition, whereas lower responsiveness compounds the vulnerability of exposed children. In recent years, however, there has been increasing support for a ‘differential susceptibility’ model, whereby some children show greater sensitivity to their rearing environments in a ‘for better or for worse’ fashion (Belsky et al., 2007; Belsky & Pluess, 2009; Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2011). From this latter, evolutionarily-based perspective, exposure to prenatal adversity is theorized to induce adaptive epigenetic changes in utero that render an organism more malleable or plastic in the face of both positive and negative postnatal experiences (Pluess & Belsky, 2011; Rabinowitz & Drabick, 2017). In support of the differential susceptibility model, children with difficult temperament (Pluess & Belsky, 2010), low birth weight and preterm birth (Gueron-sela, Atzaba-poria, Meiri, & Marks, 2015), particular genetic alleles (Van Ijzendoorn, Belsky, & Bakermans-Kranenburg, 2012) and high neuroendocrine reactivity (Obradović, Bush, Stamperdahl, Adler, & Boyce, 2010) have been shown to benefit more from positive rearing experiences than children without these characteristics. As noted above, previous studies have not systematically tested these alternative theoretical models for responsiveness effects in children with PTE.
Consistent with diathesis-stress models, human and animal studies indicate that PTE disrupts the development of neural systems involved in self-regulation. In addition to exerting vasoconstrictive and anorexigenic effects on the fetus, nicotine binds directly to nicotinic acetylcholine receptors in the brain (Wickstrom, 2007). Premature stimulation of these receptors spurs alterations to synaptogenesis, receptor density, presynaptic signaling, and neurotransmitter release across multiple neurotransmitter systems (Dwyer, McQuown, & Leslie, 2009; Mychasiuk, Muhammad, Gibb, & Kolb, 2013; Slikker, Xu, Levin, & Slotkin, 2005; Slotkin, 2004; Slotkin et al., 2015). PTE is also associated with reduced dopamine turnover, changes to dopamine receptor binding in the forebrain, and reduced neuron density in the dorsolateral prefrontal cortex of exposed fetuses (Chatterton et al., 2017; Heath & Picciotto, 2009). Moreover, these neural alterations vary by sex, with male rodents being more vulnerable to PTE-modulated alterations in receptor density and neural signaling than their female counterparts (Slotkin et al., 2007). Neuroimaging studies of children and adolescents with PTE have revealed disruptions to white matter development and cortical thinning in several neural regions, most notably in the orbitofrontal cortex, a key area for emotional and behavioral regulation (Bublitz & Stroud, 2012; El Marroun et al., 2014; Jacobsen et al., 2007; J. Liu, Cohen, Gongvatana, Sheinkopf, & Lester, 2011; Lotfipour et al., 2010). Coupled with evidence from rodent studies that less responsive parenting correlates with reduced hippocampal and prefrontal volumes, while responsive parenting (i.e., licking and grooming) can mitigate the neurodevelopmental impact of prenatal adversity (Kundakovic & Champagne, 2015; D. Liu et al., 1997; Luby et al., 2013), it is possible that exposed children who go on to experience less responsive caregiving are at dual risk for behavioral disinhibition due to vulnerabilities in key neural regions associated with stress modulation and self-regulation.
On the other hand, recent models have conceptualized prenatal substance exposure as a potent stressor, which triggers adaptive changes to stress response systems in the developing fetus (Lester & Padbury, 2009; McDonald et al., 2006). Indeed, a number of studies have suggested deviant neuroendocrine and behavioral responses to stress in children with PTE (Huijbregts, van Berkel, Swaab-Barneveld, & van Goozen, 2011; Park, O’Malley, King, & Picciotto, 2014; Ramsay, Bendersky, & Lewis, 1996; Schuetze, Lopez, & Granger, 2009) and animal studies suggest that PTE leads to hyper-reactivity of the hypothalamic-pituitary-adrenal (HPA) axis (He et al., 2017). These effects appear be modulated by epigenetic changes to the expression of genes involved in the HPA axis and glucocorticoid receptor density in the hippocampus, an important regulator of cortisol release (He et al., 2017; Stroud et al., 2014). Such neurophysiological changes are similar to those reported for animals exposed to other forms of prenatal stress, where male rodents often show greater susceptibility than females (Bale, 2011; Bock, Wainstock, Braun, & Segal, 2015; Li, Gonzalez, & Zhang, 2012; although see Sandman, Glynn, & Davis, 2013 for evidence that females do not escape the effects of prenatal adversity). Thus, increased reactivity and malleability of physiological and neural systems could magnify the impact of both adverse and favorable environmental influences in children with PTE (Pluess & Belsky, 2011). Given calls for the detailed analysis of moderation effects to address both diathesis-stress and differential vulnerability perspectives in parenting research (Roisman et al., 2012), we examined whether hypothesized interactions between PTE and maternal responsiveness were more consistent with a diathesis-stress or a differential susceptibility model.
Against this background, we drew on data from the Midwestern Infant Development Study-Preschool Phase (MIDS-P). This sample is optimized for modeling individual differences in pathways from PTE to behavioral disinhibition due to oversampling of PTE (50%), bio-assay validation of exposure status, high-quality, multi-modal measurement of responsiveness, and behavioral disinhibition, and assessment of several confounding factors. The primary aims of the present study were to:
Determine whether maternal responsiveness moderates the association between PTE and multi-faceted measures of behavioral disinhibition in young children with PTE. Testing alternative diathesis-stress and differential susceptibility models, we hypothesized that the diathesis-stress model would be supported, consistent with the assumption that maternal responsiveness operates as a protective buffer against neurodevelopmental vulnerabilities associated with PTE.
Determine whether associations between PTE, maternal responsiveness and behavioral disinhibition are moderated by sex. Based on our previous findings (Wakschlag & Hans, 2002) and on evidence for enhanced vulnerability to prenatal adversity in males (Bale, 2011), we expected that boys with PTE would be more vulnerable to low maternal responsiveness than their female counterparts.
Method
Participants
Details regarding the MIDS cohort are described elsewhere (Espy et al., 2011; Fang, Johnson, Stopp, & Espy, 2011). Briefly, pregnant mothers were recruited through fliers distributed to obstetrics clinics in a small city in Nebraska and a rural area in southern Illinois. Women who contacted the laboratory to express interest in the study were screened to ensure over-recruitment of smokers, no illegal drug use, and an even representation of household income to needs and ethnicity across smoking and non-smoking study groups. Mothers who reported consuming >4 drinks in a single sitting during pregnancy (n = 8) were excluded from this study, as were children born before 35 weeks (n = 8). All mothers were recruited prior to the 28th gestational week. Of the 369 children who were eligible and participated in the perinatal study phases, 29 subsequently became ineligible for MIDS-P due to a diagnosis of pervasive developmental delay or genetic disorder in the child, international relocation, or parent custody loss. A further 5 sets of twins were excluded from analyses because multiple birth is associated with altered parenting behavior (Beer et al., 2013). Our analytic sample in this study includes 276 children and their mothers, representing 84% of the eligible follow-up sample (32 lost to follow-up, 15 declined participation, 7 missing covariates). Complete data for the laboratory measures was available for 234 (85%) of the children, whereas the remainder were unable to complete the laboratory visit due to relocation and therefore had only questionnaire and interview data.
Table 1 provides descriptive information for the analytic sample. Reflecting the demographics of women who smoke during pregnancy in the US (Mumford, Hair, Yu, & Liu, 2014), sociodemographic disadvantage was prevalent: 8% of mothers had not completed high school, 78% were eligible for Medicaid, and the median household income was $32,664 at the 5- year follow-up. Mean reported prenatal alcohol use (drinks per day) for exposed and non-exposed groups was .04 and 0 drinks/day, respectively [t (274) = 5.6, p <.001]. While self-reported maternal drug use was an exclusion criterion at enrollment, the prevalence of maternal marijuana use indicated by later interviews or by meconium for exposed and unexposed groups was 20% and 4%, respectively [X2(1) = 16.24, p <.001.]
Table 1.
Descriptive characteristics of PTE and non-exposed (NE) groups
| NE (n = 138) |
PTE (n = 138) |
p | |
|---|---|---|---|
| Child characteristics | |||
| n (%) Male | 70 (51.08) | 69 (49.31) | .904 |
| M (SD) age (months) at follow-up | 61.69 (3.81) | 61.04 (3.83) | .159 |
| n (%) White | 92 (66.67) | 89 (64.49) | .229 |
| n (%) Hispanic | 15 (10.86) | 21 (15.21) | .284 |
| M (SD) Gestational age | 39.11 (1.20) | 39.14 (1.10) | .821 |
| M (SD) Birth Weight | 3403.68 (470.19) | 3410.66 (426.73) | .898 |
| Prenatal characteristics | |||
| M (SD) Propensity score a | .32 (.17) | .70 (.17) | <.001 |
| M (SD) LISRES prenatal stress | 49.80 (6.22) | 52.19 (5.51) | .001 |
| Postnatal characteristics b | |||
| M (SD) LISRES postnatal stress | 49.43 (6.12) | 51.09 (6.27) | .027 |
| M (SD) Income to needs | 1.78 (1.44) | 1.58 (1.19) | .235 |
| n (%) Household smoking | 30 (21.74) | 107 (77.53) | <.001 |
| M (SD) Maternal responsiveness | .02 (.68) | −.05 (.67) | .407 |
| Child Behavioral Disinhibition Outcomes | |||
| M (SD) Executive control | 51.87 (8.50) | 48.78 (7.92) | .004 |
| M (SD) CBQ Effortful control | 5.50 (.65) | 5.34 (.85) | .095 |
| M (SD) ECI inattentive/hyperactive symptoms | 51.38 (9.52) | 53.77 (11.07) | .055 |
| M (SD) MAP-DB Disruptive behavior c | 3.85 (.16) | 3.92 (.18) | .002 |
Probability score based on maternal age, education, race, diet, health, alcohol and marijuana use, IQ, mental health and personality characteristics (Fang et al., 2010).
All measured during the 5-year follow-up assessment
Variable log transformed
Procedure
All procedures were approved by human subjects protection boards at the participating institutions and mothers provided written, informed consent to participation. Within 6 months of the child participants’ 5th birthdays, families were contacted and invited to participate in MIDS-P, a follow-up study consisting of a home visit and a laboratory-based assessment. Relevant measures administered during each visit are detailed below.
Measures
Tobacco Exposure
Exposure was determined using a multi-step approach (see Espy et al., 2011). Mothers reported on their smoking status during eligibility screening and in month-by-month timeline follow-back interviews administered at 16 and 28 weeks of pregnancy and at delivery. Mothers who reported smoking at dates past the last menstrual period were classified as pregnancy smokers. Cotinine levels were assayed from maternal urine samples at these prenatal time points and from infant meconium at birth. Two mothers who did not report smoking were reclassified as smokers based on cotinine samples higher than 100ng/mL at these time points. Finally, as a measure of postnatal tobacco exposure, mothers reported the number of smokers residing in the child’s home during the 5-year interview. Children living with any smokers were classified as exposed at this postnatal time point. Based on this measure, 137 (50%) of the sample were living in households with smokers at age 5 years.
Maternal Responsiveness
Mothers were assigned an overall responsiveness score using a multi-method approach combining home-based, lab-based and maternal report measures of responsiveness. The Early Childhood Home Observation for the Measurement of the Environment (EC-HOME; Caldwell & Bradley, 1984) was administered during the home visit at the follow-up assessment. This is an observational and interview-based measure of the quality of the home environment. The 8-item ‘Warmth/Responsiveness’ subscale includes items such as, “During visit, parent answers child’s questions verbally” which are evaluated with a yes/no format. Thirty percent of ratings were completed by two raters and the Spearman’s correlation between these raters for the scale was .93, p <001. Disagreements were resolved through discussion during regular study fidelity meetings.
During the laboratory visit, mothers completed a standardized set of activities with their children during the Disruptive Behavior-Diagnostic Observation Schedule and their behavior was subsequently coded using the Parenting Clinical Observation Schedule (PCOS; Hill, Mascowitz, Danis, & Wakschlag, 2008). Parent-child activities are designed to elicit parenting behaviors across varying contexts, and included a tidying task (putting crayons away), jigsaw puzzle building, and a waiting task, in which the mother instructed the child to occupy him/herself while she completed a questionnaire. Maternal scaffolding, responsiveness to positive child behavior, warmth, engagement, labeling, and intensity and predominance of positive emotion during each task were rated on a 4-point scale from 0 = none to 3 = high and these scores were summed to produce an overall score for responsive involvement. Weighted kappa inter-rater reliability, based on 20% of tapes that were coded by independent raters, ranged from .65 for labeling, to .82 for scaffolding, with an average weighted kappa of .77 across all coded behaviors.
Finally, the 11 – item Warmth and Involvement scale from the Parenting Styles and Dimensions Questionnaire (Robinson, Mandleco, Olsen, & Hart, 1999) provided a self-reported measure of parent responsiveness. Parents rate statements such as, “I give comfort and understanding when my child is upset” on a 1 to 5-point scale. The measure is widely used in parenting research and correlated with parent-child interaction ratings in a previous study (Coolahan, McWayne, Fantuzzo, & Grim, 2002; Olivari, Tagliabue, & Confalonieri, 2013).
Pearson correlations among the parenting measures ranged from .16, p = .008 to .21, p .002. In cases with missing data for any parenting measure, multiple imputation was performed using the Marcov Chain Monte Carlo Method SAS 9.3. Ten imputations were performed based on 100 iterations each. The mean of these imputations was used as the child’s imputed value. In the interests of analytic parsimony and to provide a more robust, multi-contextual measure of maternal responsiveness, the mean of the standardized scores for the three parenting measures (i.e., scores were equally weighted) was used as the responsiveness score for each child.
Measures of Child Behavioral Disinhibition
Behavioral disinhibition incorporates deficits in executive control, attention, and inhibitory control, as well as aggression and behavioral undercontrol (Tarter et al., 2003; Zucker et al., 2011). A combination of neuropsychological assessment, clinical checklist and dimensional assessments was used to capture this phenotypic profile.
Executive Control
During the laboratory-based assessment, children completed measures of executive control, including the Nebraska Barnyard working memory task (adapted from the Noisy Book task; Hughes, Dunn, & White, 1998) and a Go/No-go inhibitory control task (Simpson & Riggs, 2006; Wiebe, Sheffield, & Espy, 2012). Both measures were programmed and administered using E-Prime software (Psychology Software Tools, Pittsburgh, PA). The Nebraska Barnyard is a span-type task. Children are presented with a 2 × 2 grid of colored buttons depicting barnyard animals (cat on orange button, horse on black button, pig on pink button, cow on brown button). As children press the buttons on a touch screen, they emit noises associated with the animals. After a training phase to familiarize children with the buttons, the animal pictures are removed and children are asked to press the buttons that correspond to sequences of animal names in the order verbalized by the examiner. The task proceeds from sequences of 2 to 8 animal names, with 3 trials possible at each level. If the child fails to replicate the sequence for all three trials at a given level, the task is discontinued. The task advances automatically if children pass the first two trials at a given span level, in which case credit is automatically granted for the third trial. The dependent variable was the total number of correct trials.
In the go/no-go task, children are instructed to press on a button in response to cartoon images of fish while avoiding making a response to shark images. After a short training phase, children complete 40 trials (75% fish, 25% sharks). Each stimulus is presented for a maximum of 1500ms after an inter-stimulus interval of 750ms. Children are provided with a 500ms feedback stimulus of a bubbling fishing net when they correctly respond to a fish and a buzzing, breaking net when they incorrectly respond to a shark. A d’prime score was calculated using the standardized probability of responding to a shark subtracted from the standardized probability of responding to a fish. In the interests of parsimony, dependent variables for these executive control tasks were standardized to a mean of 50 and the mean of these scores was used as a total executive control score (see Clark, Wakschlag, & Espy, 2016 for further details).
Effortful Control
Parents completed the 12-item Effortful Control scale from the Children’s Behavior Questionnaire (CBQ) – Short Form (Putnam & Rothbart, 2006). Effortful control is considered a constitutionally-based ability to manage affect and physiological reactivity through the regulation of attention and the scale is linked theoretically to the adult personality dimension of constraint/conscientiousness (Rothbart, Sheese, Rueda, & Posner, 2011). Items such as, “when drawing or coloring in a book, shows strong concentration,” which are rated from 1 – extremely untrue to 7 – extremely true. Scales from the very short form of the CBQ correlate well with the longer version of the CBQ, which has demonstrated reliability across international samples (Rothbart, Ahadi, Hershey, & Fisher, 2001).
Disruptive Behavior
During the laboratory visit, mothers completed the Multidimensional Assessment Profile of Disruptive Behavior (MAP-DB; Wakschlag et al., 2014), a 118-item questionnaire designed to differentiate normative misbehavior from clinically concerning disruptive behavior within the developmental context of early childhood. The MAP-DB assesses quality (e.g., dysregulation), context (e.g., during daily routines vs. out of the blue) and frequency (on a 6-item scale from “never” to “many times each day) of child disruptive behavior as a means of developmentally-sensitive differentiation. Item response theory analysis was used to identify items with maximum differentiation and factor coherence for MAP-DB scale (Wakschlag et al., 2014, 2018). For this study, a disruptive behavior dimensional composite score was generated by summing scores across all of these items.
Inattentive/hyperactive symptoms
Mothers completed the DSM-based Stonybrook Early Childhood Symptom Inventory ADHD scale (Gadow & Sprafkin, 2000). Items such as, “fidgets with hands or feet or squirms in seat” are rated on a 4-point scale from “never” to “very often.” The measure discriminates effectively between children with and without clinical diagnoses of ADHD and correlates with scores from similar instruments (Sprafkin, Volpe, Gadow, Nolan, & Kelly, 2002). In this study, we used the t-score from the scale to obtain a continuous measure of difficulties with inattention and hyperactivity/impulsivity.
Confounding Factors
Maternal smoking propensity score
A propensity score was used to minimize the effects of selection bias associated with PTE on study outcomes (see Fang et al., 2010 for a complete description). This score was calculated using generalized bootstrap modeling in the R “Twang” package based on 42 confounding variables, including several maternal characteristics such age, education, race, diet, health and pregnancy history, alcohol, marijuana and prescription medicine consumption, IQ, depression, anxiety, hyperactivity, attention and impulsivity symptoms, and personality characteristics such as hostility and psychoticism.
Household stress
Women who smoke during pregnancy are more likely to live in stressful environments (Clark, Espy, et al., 2016; Pickett, Wilkinson, & Wakschlag, 2009; Weaver, Campbell, Mermelstein, & Wakschlag, 2008) and family and household stress was measured both prenatally (at 16 or 28 weeks) and at the 5-year follow-up time point using the Life Stressors and Social Resources Inventory (LISRES; Moos & Moos, 1994). This scale assesses perceived stress associated with extended family, friends, negative life events, children, a spouse or partner, work, home and neighborhood, and health. The scale shows adequate internal (α = .83–.84) and inter-rater reliability (r = .67 – .70; Moos, 1995). Total prenatal and 5- year LISRES stress scores were created by averaging the standard scores for all stressors at each time point. Given that stress associated with children is likely to be confounded with measures of parent responsiveness, we excluded LISRES child-related stress from our indicators of pre– and postnatal stress.
Household income to needs
Household income-to-needs was used as a marker for the level of poverty in the home and was calculated by dividing maternal-reported household income by the federal poverty threshold for the year the interview was conducted (Brooks-Gunn & Klebanov, 1996). Thus, a score of 1 would indicate an income equivalent to the federal poverty threshold, whereas a score below 1 would indicate an income below this threshold.
Statistical methods
Analyses were conducted in two major phases. First, descriptive statistics and correlations were examined for all predictors. These analyses showed that the MAP-DB scores were skewed (skewness = 1.46; kurtosis = 2.73) and this variable was therefore log transformed (skewness = .9; kurtosis = .67) for all reported analyses. Second, hierarchical linear regression models were constructed for each of the four behavioral disinhibition outcomes: executive control, effortful control, disruptive behavior, and inattentive/hyperactive symptoms. In the first step of the regression equations, control variables, including child age in months at the 5-year follow-up, child sex (1 = male, 0 = female), the maternal smoking propensity score, the LISRES prenatal and postnatal stress scores, household income to needs, and household tobacco use, were entered in a single block. The primary predictors of interest, which included PTE (1 = yes, 0 = no) and maternal responsiveness, were then entered. In the final step, two and three way interactions between sex, PTE, and parent responsiveness, were entered. A backward trimming approach was used for these interactions, such that those that did not contribute significantly to the model (p > 0.05) were iteratively removed.
Significant interactions between PTE and responsiveness were probed based on Roisman et al.’s (2012) recommendations. First, we graphed and estimated the slopes for maternal responsiveness values 2 SDs above/below the sample mean. Next, we conducted a simple slopes analysis using the ‘process’ macro (Hayes, 2016) to quantify the relation between parenting and the behavioral disinhibition outcome in each study group. Thereafter, we used tools developed by Roisman and colleagues (Fraley, 2012; Roisman et al., 2012) to conduct a region of significance (ROS) analysis, which determined the range of values of maternal responsiveness for which the association between PTE and the self-regulatory outcome was significant. A diathesis-stress effect is supported when the interaction is significant primarily at lower levels of maternal responsiveness. Conversely, a differential susceptibility effect is supported when the interaction is significant at both the low and high levels of maternal responsiveness (i.e., the effect is ‘for better’ and ‘for worse’). Finally, we examined the Proportion of Interaction index (POI), which measures the area between the two regression lines to the left of the cross-over point on the interaction plot. POI values closer to 0 or 100 favor a diathesis stress effect, indicating that the area on one side of the cross-over point is much larger than the other. Values close to .5 indicate that the areas on either side of the crossover point are relatively even. This is evidence for a differential susceptibility effect because the likelihood of benefitting from high maternal responsiveness is generally equivalent to the likelihood of being adversely affected by low maternal responsiveness.
Results
Univariate relations between predictors and covariates
Table 2 provides the univariate correlations between the variables considered in subsequent analyses. Contrary to previous research, mean levels of maternal responsiveness did not differ between the PTE and non-exposed groups. However, maternal responsiveness correlated with all of measures of behavioral disinhibition.
Table 2.
Correlations between study predictors and outcomes
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. PTE | ||||||||||||
| 2. Maternal responsiveness | −.05 | |||||||||||
| 3. Child age (months) | −.09 | −.05 | ||||||||||
| 4. Male sex | −.01 | −.07 | .01 | |||||||||
| 5. Propensity score | .75** | −.05 | −.10 | .05 | ||||||||
| 6. LISRES prenatal stress | .20** | −.18** | −.16** | −.00 | .25** | |||||||
| 7. LISRES postnatal stressa | .13* | −.16** | −.04 | −.01 | .22** | .55** | ||||||
| 8. Household income to needsa | −.08 | .05 | −.02 | .08 | −.16** | −.27** | −.30** | |||||
| 9. Household smokinga | .56*** | −.10 | −.10 | −.04 | .45** | .20** | .20** | −.25** | ||||
| 10. Executive control | − 19** | .21** | .26** | −.05 | −.16** | −.16** | −.16* | .21** | −.22** | |||
| 11. CBQ Effortful control | −.11 | .26** | −.01 − | .31** | −.09 | −.12* | −.07 | .05 | −.13* | .17* | ||
| 12. MAP-DB Disruptive behavior | 19** | −.27** | −.07 | .11 | .14* | .26** | .35** | −.10 | 17** | − 17** | −.32** | |
| 13. ECI inattentive/hyperactive symptoms | .12 | −.16** | .05 | .02 | .11 | .23** | .29** | −.08 | .16** | −.12 | −.26** | .63** |
Measured at the 5-year follow-up assessment
p <.05;
p <.01
Relation of PTE and maternal responsiveness to behavior disinhibition in early childhood
Table 3 describes a series of regression models predicting each of the child behavioral disinhibition measures. Contrary to our hypothesis, PTE and responsiveness did not interact to predict child executive control task performance. There was a main effect of maternal responsiveness, however, where each increase in the responsiveness score predicted a 1.86 point increase in performance in executive control performance (p = .02). Notably, PTE was not significant in this model with all other predictors included. PTE and maternal responsiveness did not interact with child sex to predict executive control. Overall, the model explained approximately 17% of the variance in executive control performance.
Table 3.
Predictors of behavioral disinhibition
| Predictors |
Executive Control F(9, 224) = 4.92, p < .001, R2 = .17 |
CBO Effortful Control F(10, 265) =6.58, p < .001, R2 = .20 |
MAP-DB Disruptive Behavior F(10, 265) = 7.88, p < .001, R2 = .23 |
ECI Inattentive/hyperactive Symptoms F(9, 265) = 3.73, p < .001, R2 = .12 |
|---|---|---|---|---|
| Intercept | 17.99 (11.91) | 6.38 (.87) | 3.53 (.20) | 11.10 (12.46) |
| Child age (months) | .59*** (.17) | −.01 (.01) | −.01 (.01) | .21 (.16) |
| Male sex | −.80 (1.03) | −.45*** (.08) | .04 (.02) | .42 (1.20) |
| Propensity score | 2.02 (3.13) | .11 (.26) | −.08 (.06) | −1.37 (3.67) |
| LISRES Prenatal stress | −.02 (.10) | −.01 (.01) | .01 (.01) | .15 (.12) |
| LISRES postnatal stress | −.07 (.10) | .01 (.01) | .01*** (.02) | .39** (.12) |
| Household income to needs | 1.04* (.46) | −.01 (.04) | .01 (.01) | .34 (.49) |
| Household smoking | −1.31 (1.28) | −.13 (.10) | .01 (.01) | 1.93 (1.49) |
| PTE | −2.31 (1.64) | −.08 (.14) | .07* (.03) | .93 (1.96) |
| Maternal responsiveness | 1.86* (.81) | .08 (.09) | −.02 (.02) | −1.52 (.91) |
| PTE × maternal responsiveness | .35** (.13) | −.08** (.03) |
Measured at the 5-year follow-up assessment
p <.05;
p <.05
In terms of CBQ Effortful Control scale scores, there was a significant interaction between PTE and maternal responsiveness (p = .007; R2 Δ = .02; see Figure 1). A simple slopes analysis to probe the nature of this interaction indicated that higher maternal responsiveness predicted increased effortful control for children with PTE [B (SE) = .42 (.09), p < .001], whereas it did not correlate with effortful control in the non-exposed group, B (SE) = .08 (.09), p = .26. A ROS analysis revealed that the association between responsiveness and effortful control in the PTE group was significant for maternal responsiveness values below −.68 and above 1.77. Given that scores for maternal responsiveness in the sample ranged from −2.50 to 1.48 and the value of 1.77 is outside of this range, this finding suggests that PTE children in the low range of maternal responsiveness (< −.68) were affected, whereas those in the high range were not– a diathesis-stress effect. As further support for a diathesis-stress effect, the POI index indicated that only 38% of the area for the interaction plot was above the crossover point on the graph, i.e., most of the interaction effect was attributable to lower scores for exposed children who had less responsive mothers. There were no significant interactions among PTE, sex and maternal responsiveness, although male children were rated almost half a point lower in effortful control than their female counterparts (p < .001). Collectively, predictors accounted for 21% of the variance in child effortful control scores.
Figure 1.
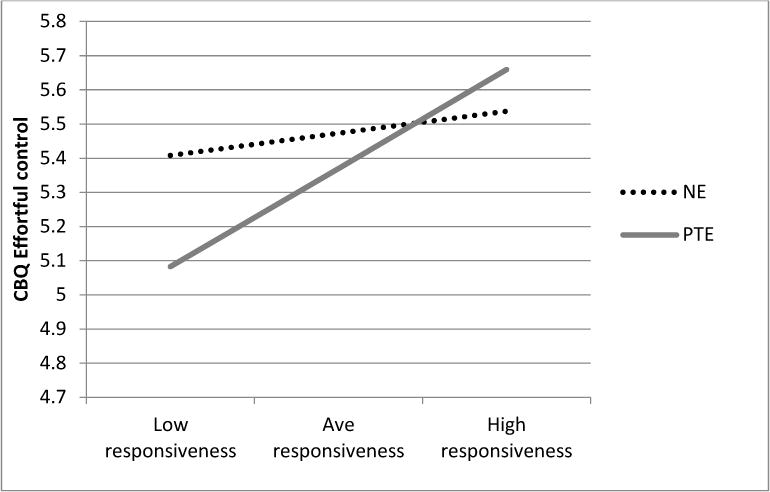
Predicted CBQ Effortful Control scores for exposed (PTE) and non-exposed (NE) children with low (-2 SDs of mean), average (sample mean), and high (+ 2SDs of mean) levels of maternal responsiveness. Estimates are based on a female, 5-year old child with continuous covariates centered at the sample mean.
The interaction between PTE and responsiveness also emerged as significant for MAP-DB disruptive behavior scores, p = .008, R2 Δ = .02 (See Figure 2). Again, there was a significant association between maternal responsiveness and disruptive behavior in the PTE group [B (SE) = −.09 (.02), p < .001], whereas the relation was not significant in the non-exposed group, B (SE) = −.02 (.02), p = .46. The ROS analysis showed that the relation of maternal responsiveness to MAP-DB scores in the PTE group was significant for values below .07 and above 3.71, supporting a diathesis-stress effect. The POI index was .13, indicating that the majority of the interaction effect was carried by the adverse effects of low maternal responsiveness for children with PTE. The main effect of PTE was also significant in the model (p = .03). There were no other interactions. The model accounted for 23% of the variance in MAP-DB scores.
Figure 2.
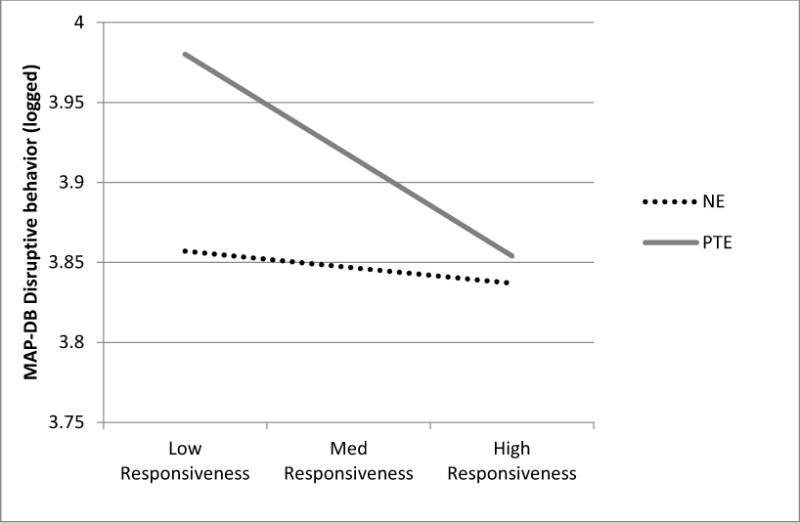
Predicted MAP-DB scores for exposed and non-exposed children with low (−2 SDs of mean), average (sample mean), and high (+ 2SDs of mean). Estimates are based on a female, 5- year old child with continuous covariates centered at the sample mean.
Finally, neither PTE nor responsiveness predicted children’s inattentive/hyperactive symptoms, as measured by the ECI. There were no significant interactions in this model.
Discussion
Children with PTE are characterized by self-regulatory impairments that increase their risk for psychopathology across the lifespan (Cornelius, Goldschmidt, & Day, 2012; Monshouwer & Huizink, 2011; Wakschlag et al., 2006). Less is known regarding the postnatal factors that may compound or mitigate this risk. Over and above several environmental stressors and risk factors that co-occur with PTE, this study shows that exposed children whose mothers are sensitive, warm, and responsive are at reduced risk for impairments in areas associated with behavioral disinhibition. Collectively, our findings highlight parent-child interactions as a promising target for early intervention to support self-regulation and reduce the risk of externalizing trajectories among children with PTE.
Consistent with previous findings in this and other cohorts (Clark, Espy, et al., 2016; Cornelius, Goldschmidt, De Genna, et al., 2012; Mezzacappa et al., 2011; Wakschlag et al., 2006; Wiebe et al., 2016), young children with PTE were vulnerable to deficits in executive control and increased disruptive behavior relative to their non-exposed peers. Scores from the various measures were moderately correlated, suggesting that difficulties cluster into a broader profile of behavioral disinhibition. Nonetheless, distinct predictors emerged as important for the various outcome measures. Our primary hypothesis was supported with respect to child effortful control and disruptive behavior: children with PTE were at higher risk than their non-exposed peers when their mothers showed low levels of responsiveness. Conversely, exposed children whose mothers were responsive showed similar scores to their non-exposed peers. Importantly, this buffering effect of maternal responsiveness was significant even after accounting for several pre- and postnatal stressors that correlate with PTE and with children’s self-regulation (Betts, Williams, Najman, & Alati, 2015; Evans & Rosenbaum, 2008; Merz et al., 2014). Overall, our findings for these measures are consistent with a diathesis-stress, as opposed to a differential susceptibility model (Roisman et al., 2012).
These findings underscore the importance of considering the type of prenatal adversity in models of differential susceptibility, dual risk, and prenatal programming. The nature of intrauterine adversity associated with epigenetic and other changes that putatively confer plasticity may be quite specific. While PTE has been linked to fetal HPA axis reactivity, decreased fetal growth, and difficult infant temperament, all factors implicated in differential susceptibility (Belsky et al., 2007; Pluess & Belsky, 2011), PTE alone, when separated from commonly associated prenatal stressors, may restrict fetal growth and neural development, but may not influence plasticity. Indeed, the sheer viability of the fetus may come at the expense of neural development. In this way, optimal rearing may attenuate or help to compensate for the effects of PTE on child self-regulation, but may not yield better-than-average outcomes. Further investigations that characterize the timing and dosage of varying forms of prenatal adversity will be necessary to build a more complete picture of the circumstances under which differential susceptibility vs. diathesis stress effects hold.
Contrary to hypotheses, there was no interaction between PTE and responsiveness when considering executive control as an outcome. The lack of an interaction may reflect the fact that these executive control tasks did not incorporate strong emotional or motivational elements, a factor that has been shown to be important in previous studies of children with PTE (Wiebe, Clark, De Jong, et al., 2015). The central role of postnatal environmental factors in accounting for executive outcomes accords with the protracted development of cognitive capacities and neural systems that support and are modulated by executive control (Gogtay et al., 2004; Wolfe & Bell, 2004). Similarly, only postnatal stress exposure was associated with elevated inattentive/hyperactive symptoms. It is possible that the DSM-based checklist used to measure hyperactive/inattentive symptoms in this study, in contrast to the dimensional approach of other measures, obscured subtle, subclinical effects of PTE on attention regulation. It is also possible that PTE interacts with specific risk alleles to place children at stronger risk for ADHD-type symptoms and that these more nuanced associations were not captured in this study (Becker, El-Faddagh, Schmidt, Esser, & Laucht, 2008; O’Brien, Mustanski, Skol, Cook, & Wakschlag, 2013).
The buffering effect of maternal responsiveness on different facets of behavioral disinhibition is consistent with our previous findings in an adolescent boys (Wakschlag & Hans, 2002), but inconsistent with studies that have examined interactions between PTE and maternal responsiveness in toddlers (Godleski et al., 2016; Huijbregts et al., 2007). Note that, for both of the latter studies, maternal responsiveness was measured in infancy. It is possible that moderation effects for maternal responsiveness in relation to disinhibited behavior are more evident in preschool and school-aged children because parent-child dyadic behavior is more complex and entrenched by this stage of development. For instance, maternal responsiveness is likely to incorporate more diverse linguistic strategies at this age point, whereas these types of responses will be less clear at younger ages. Similarly, because self-regulation is an important developmental milestone for this stage of development, inappropriate or disruptive behavior may be more noticeable to mothers. Finally, former studies have tended to rely on single measures of parenting responsiveness and relatively coarse outcome measures. For instance, Huijbregts et al., (2007) used a relatively brief checklist measure of aggressive/hyperactive behavior administered at 17 – 42 months. A multi-method approach, incorporating detailed assessments of parenting and of self-regulatory outcomes, may be necessary to capture these interactions between pre- and postnatal experiences.
Findings did not support our secondary hypotheses regarding sex-specific effects of PTE and maternal responsiveness. Previous studies have indicated that PTE boys may be at greater risk for self-regulatory deficits and elevated conduct problems than girls (Wiebe, Clark, de Jong, et al., 2015; Willoughby et al., 2007) and that PTE boys may benefit to a greater extent than girls from high levels of maternal responsiveness (Eiden et al., 2015; Wakschlag & Hans, 2002). Variable findings with respect to sex are perhaps attributable to sample size limitations in a number of studies, but may also reflect variation across development. For instance, Wiebe et al. (2015) reported that developmental differences in self-regulation were more pronounced in exposed boys at 3 years of age, a period of particularly rapid growth and inter-individual variability in self-regulation. While intensive longitudinal studies will be necessary to test these nuanced developmental effects, our findings indicate that high levels of maternal responsiveness are equally beneficial for young boys and girls with PTE and therefore that interventions targeting parent-child interactions are likely to benefit both genders.
This study included rare, multi-method, prospective measurement of PTE combined with detailed assessment of maternal responsiveness and various dimensions of self-regulation, which typically are absent from large epidemiological studies. The use of a propensity score to account for multiple psychological and social factors that predispose a mother to smoke during pregnancy is an important strength, highlighting the robust nature of the links from parenting to behavioral outcomes in this high-risk cohort. However, the sample size was comparatively small, which limited our capacity to control for the multitude of hereditary influences known to confound PTE (D’onofrio et al., 2010). Our measurement of postnatal tobacco exposure did not incorporate bioassays. Assessment of paternal responsiveness would also have provided a more complete picture of individual children’s experiences and remains a critical question for future research, especially in light of our previous finding that paternal responsiveness buffered the association between PTE and disruptive behavior in adolescents (Wakschlag et al., 2011) and considering that children PTE are less likely to grow up in households with a stable father presence (Gilman, Breslau, Subramanian, Hitsman, & Koenen, 2008). Finally, it is important to acknowledge the transactional nature of parent-child interactions (Sameroff, 2010). Children who are less regulated are likely to be more difficult to parent and lower ratings of responsiveness are likely to reflect, at least in part, a response to the child’s characteristics.
The study demonstrates the importance of sensitive responsiveness in maternal-child interactions for fostering resilience among children with PTE and highlights the utility of a developmental approach that captures the dynamic influences of pre- and postnatal experiences on children’s behavioral regulation. Given this interplay of pre- and postnatal influences, prevention efforts will ideally be multi-tiered, targeted both at the reduction of tobacco consumption in pregnant mothers, as well as the enhancement of parent-child interactions during this critical early childhood period.
Acknowledgments
This research was supported by NIDA grants RO1DA023653 (PIs: Kimberly Andrews Espy and Lauren Wakschlag); RO1DA014661 (PI: Kimberly Andrews Espy); and K23DA037913 (PI: Suena Massey). We wish to acknowledge Desiree M. de Jong and all of the members of the Developmental Cognitive Neuroscience Laboratory for their work on data collection, coding and entry. A special thank you to the families who generously shared their time and personal experiences to make this work possible.
References
- Ainsworth MDS. Infant-mother attachment. The American Psychologist. 1979;34(10):932–937. doi: 10.1037/0003-066X.34.10.932. [DOI] [PubMed] [Google Scholar]
- Bale TL. Sex differences in prenatal epigenetic programing of stress pathways. Stress. 2011;14(4):348–356. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- Becker K, El-Faddagh M, Schmidt MH, Esser G, Laucht M. Interaction of Dopamine Transporter Genotype with Prenatal Smoke Exposure on ADHD Symptoms. web of Pediatrics. 2008;152(2):263–270. doi: 10.1016/jjpeds.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Beer C, Israel C, Johnson S, Marlow N, Whitelaw A, Glazebrook C. Twin birth: An additional risk factor for poorer quality maternal interactions with very preterm infants? 2013 doi: 10.1016/j.earlhumdev.2013.02.006. https://doi.org/10.10167j.earlhumdev.2013.02.006. [DOI] [PubMed]
- Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn M. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16(6):300–304. doi: 10.1111/j.1467-8721.2007.00525.x. [DOI] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bernier A, Carlson SM, Whipple N. From external regulation to self-regulation: early parenting precursors of young children’s executive functioning. Child Development. 2010;81(1):326–339. doi: 10.1111/j.1467-8624.2009.01397.x. [DOI] [PubMed] [Google Scholar]
- Betts KS, Williams GM, Najman JM, Alati R. the Relationship Between Maternal Depressive, Anxious, and Stress Symptoms During Pregnancy and Adult Offspring Behavioral and Emotional Problems. Depression and Anxiety. 2015 Jan;22:82–90. doi: 10.1002/da.22272. [DOI] [PubMed] [Google Scholar]
- Blair C, Raver CC. School Readiness and Self-Regulation: A Developmental Psychobiological Approach. Annual Review of Psychology. 2015;66(1):711–731. doi: 10.1146/annurev-psych-010814-015221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Wainstock T, Braun K, Segal M. Stress In Utero: Prenatal Programming of Brain Plasticity and Cognition. Biological Psychiatry. 2015;78(5):315–326. doi: 10.1016/j.biopsych.2015.02.036. https://doi.org/10.1016Zj.biopsych.2015.02.036. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Klebanov PK. Ethnic Differences in Children’s Intelligence Test Scores: Role of Economic Deprivation, Home Environment, and Maternal Characteristics. Child Development. 1996;67(2):396–408. doi: 10.1111/1467-8624.ep9605280317. [DOI] [PubMed] [Google Scholar]
- Bruner C. A stitch in time: Calculating the costs of school unreadiness. Washington D.C: 2002. [Google Scholar]
- Bublitz MH, Stroud LR. Maternal Smoking During Pregnancy and Offspring Brain Structure and Function: Review and Agenda for Future Research. Nicotine & Tobacco Research. 2012;14(4):388–397. doi: 10.1093/ntr/ntr191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell B, Bradley R. HOME Observation for the Measurement of the Environment. Little Rock, AR: University of Arkansas at Little Rock; 1984. [Google Scholar]
- Carlson SM, Moses LJ. Individual Differences in Inhibitory Control and Children’s Theory of Mind. 2001;72(4):1032–1053. doi: 10.1111/1467-8624.00333. https://doi.org/0009-3920/2001/7204-0007. [DOI] [PubMed] [Google Scholar]
- Carter AS, Gray SAO, Baillargeon RH, Wakschlag LS. A multidimensional approach to disruptive behaviors: Informing life span research from an early childhood perspective. In: Tolan PH, Leventhal B, editors. Disruptive Behavior Disorders. New York, NY: Springer; 2013. pp. 103–135. [Google Scholar]
- CDC. Smoking prevalence and cessation before and during pregnancy: Data from the birth certificate, 2014. 2016 Retrieved from https://www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_01.pdf. [PubMed]
- Chase-Lansdale L, Wakschlag LS, Brooks-Gunn J. A psychological perspective on the development of caring in children and youth: the role of the family. Journal of Adolescence. 1995;18:515–556. [Google Scholar]
- Chatterton Z, Hartley BJ, Seok MH, Mendelev N, Chen S, Milekic M, Haghighi F. In utero exposure to maternal smoking is associated with DNA methylation alterations and reduced neuronal content in the developing fetal brain. Epigenetics & Chromatin. 2017;10(1):4. doi: 10.1186/s13072-017-0111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazan-Cohen R, Raikes H, Brooks-Gunn J, Ayoub C, Pan BA, Kisker EE, Fuligni AS. Low-income children’s school readiness: Parent contributions over the first five years. Early Education and Development. 2009;20(6):958–977. doi: 10.1080/10409280903362402. [DOI] [Google Scholar]
- Clark CAC, Chevalier N, Nelson JM, James TD, Garza JP, Choi H-J, Espy KA. Executive control in early childhood. Monographs of the Society for Research in Child Development. 2016 doi: 10.1111/MONO.12268. [DOI] [PubMed]
- Clark CAC, Espy KA, Wakschlag LS. Developmental pathways from prenatal tobacco and stress exposure to behavioral disinhibition. Neurotoxicology and Teratology. 2016;53:64–74. doi: 10.1016/j.ntt.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CAC, Sheffield TD, Chevalier N, Nelson JM, Wiebe SA, Espy KA. Charting early trajectories of executive control with the shape school. Developmental Psychology. 2013;49(8) doi: 10.1037/a0030578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CAC, Woodward LJ. Relation of perinatal risk and early parenting to executive control at the transition to school. Developmental Science. 2015;18(4) doi: 10.1111/desc.12232. [DOI] [PubMed] [Google Scholar]
- Cole PM, Zahn-Waxler C, Smith KD. Expressive control during a disappointment: Variations related to preschoolers’ behavior problems. Developmental Psychology. 1994;30(6):835–846. doi: 10.1037/0012-1649.30.6.835. [DOI] [Google Scholar]
- Coolahan K, McWayne C, Fantuzzo J, Grim S. Validation of a multidimensional assessment of parenting styles for low-income African-American families with preschool children. Early Childhood Research Quarterly. 2002;17(3):356–373. doi: 10.1016/S0885-2006(02)00169-2. [DOI] [Google Scholar]
- Cornelius MD, Goldschmidt L, Day NL. Neurotoxicology and Teratology Prenatal cigarette smoking: Long-term effects on young adult behavior problems and smoking behavior. Neurotoxicology and Teratology. 2012;34(6):554–559. doi: 10.1016/j.ntt.2012.09.003. https://doi.org/10.1016Zj.ntt.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Goldschmidt L, De Genna NM, Larkby C. Long-term effects of prenatal cigarette smoke exposure on behavior dysregulation among 14-year-old offspring of teenage mothers. Maternal and Child Health web. 2012;16:694–705. doi: 10.1007/s10995-011-0766-0. https://doi.Org/10.1007/s10995-011-0766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Ryan CM, Day NL, Goldschmidt L, Willford JA. Prenatal tobacco effects on neuropsychological outcomes among preadolescents. web of Developmental and Behavioral Pediatrics: JDBP. 2001;22(4):217–225. doi: 10.1097/00004703-200108000-00002. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11530894. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Singh A, Iliadou A, Lambe MP, Hultman CM, Grann M, Lichtenstein P. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: a population-based study in Sweden. Archives of General Psychiatry. 2010;67(5):529–538. doi: 10.1001/archgenpsychiatry.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco exposure and children’s health. Pediatrics. 2004;113(4):1007–1015. [PubMed] [Google Scholar]
- Dwyer J, McQuown S, Leslie F. The dynamic effects of nicotine on the developing brain. Pharmacology & Therapeutics. 2009;122(2):125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Early Childcare Research Network, N. Characteristics and quality of child care for toddlers and preschoolers. In: NICHD, editor. Child care and child development: Results from the NICHD study of early child care and youth development. New York, NY: Guildford Press; 2005. pp. 91–102. [Google Scholar]
- Eiden RD, Leonard KE. A conceptual model for the development of externalizing behavior among kindergarten children of alcoholic families: Role of parenting and children’s self-regulation. Developmental Psychology. 2007;43(5):1187–1201. doi: 10.1037/0012-1649.43.5.1187.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Molnar DS, Granger DA, Colder CR, Schuetze P, Huestis MA. Prenatal tobacco exposure and infant stress reactivity: Role of child sex and maternal behavior. Developmental Psychobiology. 2015;57(2):212–225. doi: 10.1002/dev.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H, Schmidt MN, Franken IHA, Jaddoe VWV, Hofman A, van der Lugt A, White T. Prenatal tobacco exposure and brain morphology: a prospective study in young children. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2014;39(4):792–800. doi: 10.1038/npp.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: an evolutionary-neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Espy KA, Fang H, Johnson C, Stopp C, Wiebe SA. Prenatal tobacco exposure: developmental outcomes in the neonatal period. Developmental Psychology. 2011;47:153–156. doi: 10.1037/a0020724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrook R, Massey SH, Clark CAC, Burns JL, Mustanski BS, Cook EH, Wakschlag LS. Separating Family-Level and Direct Exposure Effects of Smoking During Pregnancy on Offspring Externalizing Symptoms: Bridging the Behavior Genetic and Behavior Teratologic Divide. Behavior Genetics. 2016;46(3):389–402. doi: 10.1007/s10519-015-9762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Rosenbaum J. Self-regulation and the income-achievement gap. Early Childhood Research Quarterly. 2008;23(4):504–514. https://doi.Org/10.1016/j.ecresq.2008.07.002. [Google Scholar]
- Fang H, Johnson C, Chevalier N, Stopp C, Wiebe S, Wakschlag LS, Espy KA. Using propensity score modeling to minimize the influence of confounding risks related to prenatal tobacco exposure. Nicotine and Tobacco Research. 2010;12(12):1211–1219. doi: 10.1093/ntr/ntq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Johnson C, Stopp C, Espy KA. A new look at quantifying tobacco exposure during pregnancy using fuzzy clustering. Neurotoxicology and Teratology. 2011;33(1):155–165. doi: 10.1016/j.ntt.2010.08.003. https://doi.org/10.1016Zj.ntt.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Archives of General Psychiatry. 1998;55(8):721–727. doi: 10.1001/archpsyc.55.8721. [DOI] [PubMed] [Google Scholar]
- Fraley RC. Probing interactions in differential susceptibility research. 2012 Retrieved January 1 2017, from http://www.yourpersonality.net/interaction/
- Gadow KD, Sprafkin J. The Early Childhood Inventory - 4 Screening Manual. Stoney Brook, NY: Checkmate Plus; 2000. [Google Scholar]
- Gaysina D, Fergusson DM, Leve LD, Horwood J, Reiss D, Shaw DS, Harold GT. Maternal Smoking During Pregnancy and Offspring Conduct Problems Evidence From 3 Independent Genetically Sensitive Research Designs. 2013:1–8. doi: 10.1001/jamapsychiatry.2013.127. [DOI] [PMC free article] [PubMed]
- Gilman SE, Breslau J, Subramanian SV, Hitsman B, Koenen KC. Social factors, psychopathology, and maternal smoking during pregnancy. American web of Public Health. 2008;95(3):448–453. doi: 10.2105/AJPH.2006.102772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godleski SA, Eiden RD, Schuetze P, Colder CR, Huestis MA. Tobacco exposure and maternal psychopathology: Impact on toddler problem behavior. Neurotoxicology and Teratology. 2016;57:87–94. doi: 10.1016/j.ntt.2016.07.003. https://doi.org/10.10167j.ntt.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueron-sela N, Atzaba-poria N, Meiri G, Marks K. The Caregiving Environment and Developmental Outcomes of Preterm Infants: Diathesis Stress or Differential Susceptibility Effects? 2015;86(4):1014–1030. doi: 10.1111/cdev.12359. [DOI] [PubMed] [Google Scholar]
- He X, Lu J, Dong W, Jiao Z, Zhang C, Yu Y, Xu D. Prenatal nicotine exposure induces HPA axis-hypersensitivity in offspring rats via the intrauterine programming of up-regulation of hippocampal GAD67. Archives of Toxicology. 2017;91(12):1–17. doi: 10.1007/s00204-017-1996-8. [DOI] [PubMed] [Google Scholar]
- Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: Modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56(SUPPL 1):254–262. doi: 10.1016/j.neuropharm.2008.07.020. https://doi.org/10.1016Zj.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C, Mascowitz K, Danis B, Wakschlag LS. Validation of a clinically sensitive observational coding system for parenting behaviors: The Parenting Clinical Observation Schedule. Parenting: Science and Practice. 2008;8(2):153–185. doi: 10.1080/15295190802045469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes SK, Stroud LR, Scheidweiler KB, Niaura R, Huestis M. Prenatal tobacco exposure, biomarkers for tobacco in meconium and neonatal grwoth outcomes. web of Pediatrics. 2013;162(5):970–975. doi: 10.1016/jjpeds.2012.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C, Dunn J, White A. Trick or treat?: uneven understanding of mind and emotion and executive dysfunction in “hard-to-manage” preschoolers. web of Child Psychology and Psychiatry, and Allied Disciplines. 1998;39(7):981–994. https://doi.org/9804031. [PubMed] [Google Scholar]
- Huijbregts SCJ, Séguin JR, Zoccolillo M, Boivin M, Tremblay RE. Associations of maternal prenatal smoking with early childhood physical aggression, hyperactivity-impulsivity, and their co-occurrence. web of Abnormal Child Psychology. 2007;35:203–215. doi: 10.1007/s10802-006-9073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts SCJ, Seguin J, Zoccolillo M, Boivin M, Tremblay RE. Maternal prenatal smoking, parental antisocial behavior, and early childhood physical aggression. Development and Psychopathology. 2008;20:437–453. doi: 10.1017/S0954579408000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts SCJ, van Berkel SR, Swaab-Barneveld H, van Goozen SHM. Neurobiological and behavioral stress reactivity in children prenatally exposed to tobacco. Psychoneuroendocrinology. 2011;36(6):913–918. doi: 10.1016/j.psyneuen.2010.12.008. https://doi.org/10.1016Zj.psyneuen.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Huijbregts SCJ, Warren AJ, De Sonneville LMJ, Swaab-Barneveld H. Hot and cool forms of inhibitory control and externalizing behavior in children of mothers who smoked during pregnancy: An exploratory study. web of Abnormal Child Psychology. 2008;36:323–333. doi: 10.1007/s10802-007-9180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson AJ, Pickett KE, Green J, Wakschlag LS, Hutchinson J, Pickett E, Wakschlag LS. Smoking in pregnancy and disruptive behaviour in 3-year-old boys and girls : an analysis of the UK Millennium Cohort Study. web of Epidemiology and Community Health. 2010;64(1):82–88. doi: 10.1136/jech.2009.089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, Mencl WE. Prenatal and Adolescent Exposure to Tobacco Smoke Modulates the Development of White Matter Microstructure. web of Neuroscience. 2007;27(49):13491–13498. doi: 10.1523/JNEUROSCI.2402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Marceau K, Bidwell LC, Palmer RHC, Smith TF, Todorov A, Heath AC. Smoking During Pregnancy and ADHD Risk: A Genetically Informed, Multiple-Rater Approach. 2016 Jan; doi: 10.1002/ajmg.b.32421. [DOI] [PMC free article] [PubMed]
- Kochanska G, Murray KT, Harlan ET. Effortful control in early childhood: continuity and change, antecedents, and implications for social development. Developmental Psychology. 2000;36(2):220–232. doi: 10.1037/0012-1649.36.2.220. [DOI] [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18(2):199–214. doi: 10.1037/0012-1649.18.2.199. [DOI] [Google Scholar]
- Kristjansson EA, Fried PA, Watkinson B. Maternal smoking during pregnancy affects children’s vigilance performance. Drug and Alcohol Dependence. 1989;24:11–19. doi: 10.1016/0376-8716(89)90003-3. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Champagne FA. Early-life experience, Epigenetics, and the developing brain. Neuropsychopharmacology. 2015;40(1):141–153. doi: 10.1038/npp.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Swank PR. Responsive parenting: establishing early foundations for social, communication, and independent problem-solving skills. Developmental Psychology. 2006;42(4):627–642. doi: 10.1037/0012-1649.42A627. [DOI] [PubMed] [Google Scholar]
- Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111(6):1318–1323. doi: 10.1542/peds.11L6.1318. [DOI] [PubMed] [Google Scholar]
- Lester BM, Lin H, DeGarmo DS, Fisher Pa, Lagasse LL, Levine TP, Higgins RD. Neurobehavioral disinhibition predicts initiation of substance use in children with prenatal cocaine exposure. Drug Alcohol Depend. 2012;126(1–2):80–86. doi: 10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Padbury JF. Third pathophysiology of prenatal cocaine exposure. Developmental Neuroscience. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- Li Y, Gonzalez P, Zhang L. Fetal stress and programming of hypoxic/ischemic-sensitive phenotype in the neonatal brain: Mechanisms and possible interventions. Progress in Neurobiology. 2012;98(2):145–165. doi: 10.1016/j.pneurobio.2012.05.010. https://doi.org/10.10167j.pneurobio.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(5332):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Liu J, Cohen RJ, Gongvatana A, Sheinkopf SJ, Lester BM. The impact of prenatal exposure to cocaine and tobacco on diffusion tensor imaging and sensation seeking in adolescents. web of Pediatrics. 2011;159(5):771–775. doi: 10.1016/jjpeds.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfipour S, Ferguson E, Leonard G, Perron M, Pike B, Richer L, Se JR. Orbitofrontal Cortex and Drug Use During Adolescence. Archives of General Psychiatry. 2010;66(11):1244–1252. doi: 10.1001/archgenpsychiatry.2009.124. [DOI] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Barch D. The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatrics. 2013;167(12):1135–1142. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch ME, Johnson KC, Kable JA, Carroll J, Coles CD. Smoking in pregnancy and parenting stress: Maternal psychological symptoms and socioeconomic status as potential mediating variables. Nicotine and Tobacco Research. 2011;13(7):532–539. doi: 10.1093/ntr/ntr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RP, Dombrowski S, Mullis C, Wisenbaker J, Huttenen M. Smoking during pregnancy: Association with childhood temperament, behavior, and academic performance. Journal of Pediatric Psychology. 2006;31(5):490–500. doi: 10.1093/jpepsy/jsj041. [DOI] [PubMed] [Google Scholar]
- Maughan B, Taylor A, Caspi A, Moffitt TE. Prenatal Smoking and Early Childhood Conduct Problems. Archives of General Psychiatry. 2004;61:836–843. doi: 10.1001/archpsyc.6L8.836. [DOI] [PubMed] [Google Scholar]
- McDonald SD, Walker M, Perkins SL, Beyene J, Murphy K, Gibb W, Ohlsson A. The effect of tobacco exposure on the fetal hypothalamic-pituitary-adrenal axis. BJOG: An International web of Obstetrics and Gynaecology. 2006;113(11):1289–1295. doi: 10.1111/j.1471-0528.2006.01089.x. https://doi.Org/10.1111/j.1471-0528.2006.01089.x. [DOI] [PubMed] [Google Scholar]
- Merz EC, Landry SH, Williams JM, Barnes MA, Eisenberg N, Spinrad TL, Clancy-Menchetti J. Associations among parental education, home environment quality, effortful control, and preacademic knowledge. web of Applied Developmental Psychology. 2014;35(4):304–315. doi: 10.1016/j.appdev.2014.04.002. https://doi.org/10.10167j.appdev.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzacappa E, Buckner JC, Earls F. Prenatal cigarette exposure and infant learning stimulation as predictors of cognitive control in childhood. Developmental Science. 2011;14(4):881–891. doi: 10.1111/j.1467-7687.2011.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychological Review. 1993;100(4):674–701. doi: 10.1037/0033-295X.100A674. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshouwer K, Huizink C. Prenatal Smoking Exposure and the Risk of Behavioral Problems and Substance Use in Adolescence : the TRAILS Study. 2011:342–350. doi: 10.1159/000334507. [DOI] [PubMed]
- Moos RH. Development and Applications of New Measures of Life Stressors, Social Resources, and Coping Responses. European web of Psychological Assessment. 1995;11(1):1–13. doi: 10.1027/1015-575911.1.1. [DOI] [Google Scholar]
- Moos RH, Moos BS. The Life Stressors and Social Resources Inventory. Odessa, FL: Psychological Assessment Resources; 1994. [Google Scholar]
- Mumford EA, Hair E, Yu T, Liu W. Women’s longitudinal smoking patterns from preconception through child’s kindergarten entry: Profiles of biological mothers of a 2001 birth cohort. Maternal Child Health web. 2014;18(4):810–820. doi: 10.1016/j.neuron.2009.10.017.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J, Irving B, Farrington DP, Colman I, Bloxsom AJ. Very early predictors of conduct problems and crime : results from a national cohort study. 2010;11:1198–1207. doi: 10.1111/j.1469-7610.2010.02287.x. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Muhammad A, Gibb R, Kolb B. Long-term alterations to dendritic morphology and spine density associated with prenatal exposure to nicotine. Brain Research. 2013;1499:53–60. doi: 10.1016/j.brainres.2012.12.021. https://doi.org/10.10167j.brainres.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Nelson T, Nelson JM, James TD, Clark CAC, Kidwell KM, Espy KA. Executive control goes to school: Implications of preschool executive control for observed elementary classroom behavior. Developmental Psychology. 2017;53(5):836–844. doi: 10.1037/dev0000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. web of Child Psychology and Psychiatry and Allied Disciplines. 2017 doi: 10.1111/jcpp.12675. [DOI] [PMC free article] [PubMed]
- Noland JS, Singer LT, Short EJ, Minnes S, Arendt RE, Kirchner HL, Bearer C. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicology and Teratology. 2005;27(3):429–438. doi: 10.1016/j.ntt.2005.02.001. https://doi.org/10.1016Zj.ntt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- O’Brien TC, Mustanski BS, Skol A, Cook EH, Wakschlag LS. Do dopamine gene variants and prenatal smoking interactively predict youth externalizing behavior? Neurotoxicology and Teratology. 2013;40:67–73. doi: 10.1016/j.ntt.2013.09.002. https://doi.org/10.1016Zj.ntt.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradovic J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: the interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development. 2010;81(1):270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivari MG, Tagliabue S, Confalonieri E. Parenting Style and Dimensions Questionnaire: A Review of Reliability and Validity. Marriage and Family Review. 2013;49(6):465–490. doi: 10.1080/01494929.2013.770812. [DOI] [Google Scholar]
- Paradis AD, Fitzmaurice G, Koenen KC, Buka SL. Maternal smoking during pregnancy and criminal offending among adult offspring. web of Epidemiology and Community Health. 2011;65(12):1145–1150. doi: 10.1136/jech.2009.095802.Maternal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A, O’Malley SS, King SL, Picciotto MR. Mediating Role of Stress Reactivity in the Effects of Prenatal Tobacco Exposure on Childhood Mental Health Outcomes. Nicotine & Tobacco Research. 2014;16(2):174–185. doi: 10.1093/ntr/ntt131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry NB, Mackler JS, Calkins SD, Keane SP. A transactional analysis of the relation between maternal sensitivity and child vagal regulation. Developmental Psychology. 2014;50(3):784–793. doi: 10.1037/a0033819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianta RC, Nimetz SL, Bennett E. Mother-child relationships, teacher-child relationships, and school outcomes in preschool and kindergarten. Early Childhood Research Quarterly. 1997;72(3):263–280. doi: 10.1016/S0885-2006(97)90003-X. [DOI] [Google Scholar]
- Pickett KE, Wilkinson RG, Wakschlag LS. The psychosocial context of pregnancy smoking and quitting in the Millennium Cohort Study. web of Epidemiology and Community Health. 2009;63:474–480. doi: 10.1136/jech.2008.082594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M, Belsky J. Differential Susceptibility to Parenting and Quality Child Care. Developmental Psychology. 2010;46(2):379–390. doi: 10.1037/a0015203. [DOI] [PubMed] [Google Scholar]
- Pluess M, Belsky J. Prenatal programming of postnatal plasticity. Development and Psychopathology. 2011:29–38. doi: 10.1017/S094579410000623. [DOI] [PubMed]
- Putnam SP, Rothbart MK. Development of Short and Very Short Forms of the Children’s Behavior Questionnaire. 2006;57(1):103–113. doi: 10.1207/s15327752jpa8701_09. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JA, Drabick DAG. Do children fare for better and for worse ? Associations among child features and parenting with child competence and symptoms q. Developmental Review. 2017;45:1–30. https://doi.org/10.1016Zj.dr.2017.03.001. [Google Scholar]
- Ramsay DS, Bendersky M, Lewis M. Effect of prenatal alcohol and cigarette exposure on two and six-month old infants’ adrenocortical reactivity to stress. web of Pediatric Psychology. 1996;27(6):833–840. doi: 10.1002/nbm.3066.Non-invasive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CC, Mandleco B, Olsen SF, Hart CH. Authoritative, authoritatian, and permissive parenting practices: Development of a new measure. Psychological Reports 1999 [Google Scholar]
- Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, Haydon KC. Distinguishing differential susceptibility from diathesis–stress: Recommendations for evaluating interaction effects. Development and Psychopathology. 2012;24(2):389–409. doi: 10.1017/S0954579412000065. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, Fisher P. Investigations of Temperament at Three to Seven Years: The Children’s Behavior Questionnaire. Child Development. 2001;72(5):1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Sheese BE, Rueda MR, Posner MI. Developing Mechanisms of Self-Regulation in Early Life. Emotion Review. 2011;3(2):207–213. doi: 10.1177/1754073910387943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza SJ, Verhulst FC, Jaddoe VWV, Steegers EAP, Mackenbach JP, Hofman A, Tiemeier H. Maternal smoking during pregnancy and child behaviour problems: The generation R study. International web of Epidemiology. 2009;35(3):680–689. doi: 10.1093/ije/dyn163. [DOI] [PubMed] [Google Scholar]
- Sameroff A. A unified theory of development: A dialectic integration of naure and nurture. Child Development. 2010;51(1):6–22. doi: 10.1111/j.1467-8624.2009.01378.x. Retrieved from http://onlinelibrary.wiley.com/doi/10.1111/j.1467-8624.2009.01378.x/full. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn LM, Davis EP. Is there a viability-vulnerability tradeoff? Sex differences in fetal programming. web of Psychosomatic Research. 2013;75(4):327–335. doi: 10.1016/jjpsychores.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Eiden R. The association between prenatal exposure to cigarettes and infant and maternal negative affect. Infant Behavior and Development. 2007;30(3):387–398. doi: 10.1016/j.biotechadv.2011.08.021.Secreted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Lopez F, Granger DA. The association between prenatal exposure to cigarettes and cortisol reactivity in 7-month old infants. Developmental Psychobiology. 2009;50(8):819–834. doi: 10.1002/dev.20334.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shisler S, Eiden RD, Molnar DS, Schuetze P, Coles CD, Huestis M, Colder CR. Effects of fetal tobacco exposure on focused attention in infancy. Infant Behavior and Development. 2016;45:8–10. doi: 10.1016/j.infbeh.2016.07.008. https://doi.org/10.10167j.infbeh.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A, Riggs KJ. Conditions under which children experience inhibitory difficulty with a “button-press” go/no-go task. web of Experimental Child Psychology. 2006;94:18–26. doi: 10.1016/jjecp.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Slikker W, Xu ZA, Levin ED, Slotkin TA. Mode of action: Disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction - Developmental neurotoxicity of nicotine. Critical Reviews in Toxicology. 2005;35(8–9):703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: Nicotine, environmental tobacco smoke, organophosphates. Toxicology and Applied Pharmacology. 2004;198(2):132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: Indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at 6 months of age. Neuropsychopharmacology. 2007;32(5):1082–1097. doi: 10.1038/sj.npp.1301231. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Skavicus S, Card J, Stadler A, Levin ED, Seidler FJ. Developmental neurotoxicity of tobacco smoke directed toward cholinergic and serotonergic systems: More than just nicotine. Toxicological Sciences. 2015;147(1):178–189. doi: 10.1093/toxsci/kfv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinrad TL, Eisenberg N, Silva KM, Eggum ND, Reiser M, Edwards A, Gaertner BM. Longitudinal relations among maternal behaviors, effortful control and young children’s committed compliance. Developmental Psychology. 2012;48(2):552–566. doi: 10.1037/a0025898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprafkin J, Volpe RJ, Gadow KD, Nolan EE, Kelly K. A DSM-IV-referenced screening instrument for preschool children: the Early Childhood Inventory-4. web of the American Academy of Child and Adolescent Psychiatry. 2002;41(5):604–612. doi: 10.1097/00004583-200205000-00018. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Rodriguez D, Mccallum M, Salisbury AL, Phipps MG, Marsit CJ. Maternal smoking during pregnancy and infant stress response: Test of a prenatal programming hypothesis. Psychoneuroendocrinology. 2014;48:29–40. doi: 10.1016/j.psyneuen.2014.05.017. https://doi.org/10.10167j.psyneuen.2014.05.017.Maternal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon M, Si X, Belden A, Spitznagel E, Wakschlag LS, Lubya J. Parenting Practices in Pregnancy Smokers Compared to Non Smokers. J Clin Med Res. 2013;5(2):84–91. doi: 10.4021/jocmr1283w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE. Etiology of adolescent substance abuse: a developmental perspective. The American web on Addictions. 2002;11:171–191. doi: 10.1080/1055049029008796. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius J, Pajer KA, Vanyukov M, Clark DC. Neurobehavior disinhibition in childhood predicts early age onset substance use disorder. American Journal of Psychiatry. 2003 Jun;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. 2003. [DOI] [PubMed] [Google Scholar]
- Van Ijzendoorn MH, Belsky J, Bakermans-Kranenburg MJ. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility A meta-analysis of child and adolescent gene-by-environment studies. Translational Psychiatry. 2012;2(8):e147–6. doi: 10.1038/tp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Suchodoletz A, Trommsdorff G, Heikamp T. Linking maternal warmth and responsiveness to children’s self-regulation. Social Development. 2011;20(3):486–503. doi: 10.1111/j.1467-9507.2010.00588.x. [DOI] [Google Scholar]
- Wakschlag LS, Briggs-Gowan MJ, Choi SW, Nichols SR, Kestler J, Burns JL, Henry D. Advancing a multidimensional, developmental spectrum approach to preschool disruptive behavior. web of the American Academy of Child and Adolescent Psychiatry. 2014;52(1):82–96.e3. doi: 10.1016/jjaac.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Hans SL. Maternal smoking during pregnancy and conduct problems in high-risk youth: a developmental framework. Development and Psychopathology. 2002 Oct;14:351–369. doi: 10.1017/S0954579402002092. 2000. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Henry DB, Blair RJR, Dukic V, Burns J, Pickett KE. Unpacking the association: Individual differences in the relation of prenatal exposure to cigarettes and disruptive behavior phenotypes. Neurotoxicology and Teratology. 2011;33(1):145–154. doi: 10.1016/j.ntt.2010.07.002. https://doi.org/10.1016Zj.ntt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Kistner EO, Pine DS, Biesecker G, Pickett KE, Skol AD, Cook E. WakshlagMAOA.pdf. Molecular Psychiatry. 2009:1–10. doi: 10.1038/mp.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Leventhal BL, Cook E, Pickett KE. Intergenerational health consequences of Maternal smoking. TEN. 2000;2(1):47–54. [Google Scholar]
- Wakschlag LS, Leventhal BL, Pickett KE, Pine DS, Carter AS. Elucidating early mechanisms of developmental psychopathology: The case of prenatal smoking and disruptive behavior. Child Development. 2006;77(4):893–906. doi: 10.1111/j.1467-8624.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Perlman S, Blair RJR, Leibenluft E, Briggs-Gowan MJ, Pine DS. The neurodevelopmental basis of early childhood disruptive behavior. Irritable and callous phenotypes as exemplars. American Journal of Psychiatry. 2018;175:114–130. doi: 10.1176/appi.ajp.2017.17010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Cook E, Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: A review. American web of Public Health. 2002;92(6):966–974. doi: 10.2105/AJPH.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Tolan PH, Leventhal BL. Research Review: “Ain”t misbehavin’: Towards a developmentally-specified nosology for preschool disruptive behavior. web of Child Psychology and Psychiatry and Allied Disciplines. 2010;51(1):3–22. doi: 10.1111/j.1469-7610.2009.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver K, Campbell R, Mermelstein R, Wakschlag LS. Pregnancy smoking in context: the influence of multiple levels of stress. Nicotine & Tobacco Research : Official web of the Society for Research on Nicotine and Tobacco. 2008;10(6):1065–1073. doi: 10.1080/14622200802087564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom R. Effects of Nicotine During Pregnancy: Human and Experimental Evidence. Current Neuropharmacology. 2007;5(3):213–222. doi: 10.2174/157015907781695955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe SA, Clark CAC, De Jong DM, Chevalier N, Espy KA, Wakschlag L. Prenatal tobacco exposure and self-regulation in early childhood: Implications for developmental psychopathology. Development and Psychopathology. 2015;27(2) doi: 10.1017/S095457941500005X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe SA, Clark CAC, de Jong DM, Chevalier N, Espy KA, Wakschlag LS. Prenatal tobacco exposure and self-regulation in early childhood : Implications for developmental psychopathology. 2015;27:397–409. doi: 10.1017/S095457941500005X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe SA, Clark CAC, de Jong DM, Chevalier N, Espy KA, Wakschlag LS. Prenatal tobacco exposure and self-regulation in early childhood : Implications for developmental psychopathology. 2016;27:397–409. doi: 10.1017/S095457941500005X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe SA, Fang H, Johnson C, James KE, Espy KA, Wiebe SA, James KE. Determining the impact of prenatal tobacco exposure on self-regulation at 6 months. Developmental Psychology. 2014 doi: 10.1037/a0035904. [DOI] [PMC free article] [PubMed]
- Wiebe SA, Sheffield TD, Espy KA. Separating the Fish From the Sharks: A Longitudinal Study of Preschool Response Inhibition. Child Development. 2012;83(4):1245–1261. doi: 10.1111/j.1467-8624.2012.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby MT, Greenberg M, Blair CB, Stifter C. Neurobehavioral consequences of prenatal exposure to smoking at 6 to 8 months of age. Infancy. 2007:273–301. [Google Scholar]
- Windham GC, Hopkins B, Fenster L, Swan SH. Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiology. 2000;11(4):427–433. doi: 10.1097/00001648-200007000-00011. [DOI] [PubMed] [Google Scholar]
- Wolfe CD, Bell MA. Working memory and inhibitory control in early childhood: Contributions from physiology, temperament, and language. Developmental Psychobiology. 2004;44(1):68–83. doi: 10.1002/dev.10152. [DOI] [PubMed] [Google Scholar]
- De Wolff MS, Van Ijzendoorn MH. Sensitivity and Attachment: A Meta-Analysis on Parental Antecedents of Infant Attachment Author (s): Marianne S. de Wolff and Marinus H van IJzendoorn Published by : Wiley on behalf of the Society for Research in Child Development Stable URL. Child Development. 1997;68(4):571–591. doi: 10.1111/j.1467-8624.1997.tb04218.x. [DOI] [PubMed] [Google Scholar]
- Yang S, Decker A, Kramer MS. Exposure to parental smoking and child growth and development: a cohort study. BMC Pediatrics. 2013;13(1):104. doi: 10.1186/1471-2431-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Heitzeg MM, Nigg JT. Parsing the undercontrol-disinhibition pathway to substance use disorders: A multilevel developmental problem. Child Development Perspectives. 2011;5(4):248–255. doi: 10.1111/j.1750-8606.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


