INTRODUCTION.
Rectal squamous cell carcinoma (SCC) is a rare tumor with unresolved etiology. Human immunodeficiency virus (HIV)-infected individuals and solid organ transplant recipients experience >30-fold and approximately 3-fold elevated rates of rectal SCC, respectively, suggesting immunosuppression plays a role.1 HIV-infected homosexual men have >60-fold higher rates of rectal SCC, similar to anal SCC. These patterns, which differ from the more common rectal adenocarcinoma, raise the possibility of shared etiology between rectal and anal SCC, with human papillomavirus type 16 (HPV16) a likely candidate.2
METHODS.
We obtained rectal SCC specimens identified from the Iowa and Hawaii cancer registries (1992-2015) for pathology review and HPV16 testing. Cancer registry clinical notes and pathology reports for 92 cases were reviewed. We selected 37 cases with the strongest supporting evidence for a rectal SCC diagnosis based on anatomic location (e.g., >4cm above the anal verge or >2cm above dentate line) and treatment data (e.g., radiotherapy directed at rectum). Formalin-fixed paraffin-embedded tumor blocks were available for 24 cases. For comparison, we also selected 11 anal SCCs and 11 rectal adenocarcinomas [AdCAs] as control tumors.
Two gastrointestinal pathologists independently reviewed slides stained for hematoxylin and eosin (H&E); p40, which stains positive in SCCs; and CAM5.2, which identifies low molecular weight cytokeratins present in rectal but not anal epithelial cells3 (see Figure 1 for details). Confirming rectal location was important since it is possible that diagnostic errors resulting from anatomic proximity could result in misclassification between anal vs. rectal SCC. We tested tumors for HPV16 (as well as oncogenic HPV types 18, 33, and 45) using both DNA and RNA amplification methods targeting the E6 gene.4 Finally, tumors were evaluated for full-length HPV16 using in situ hybridization (ISH).5
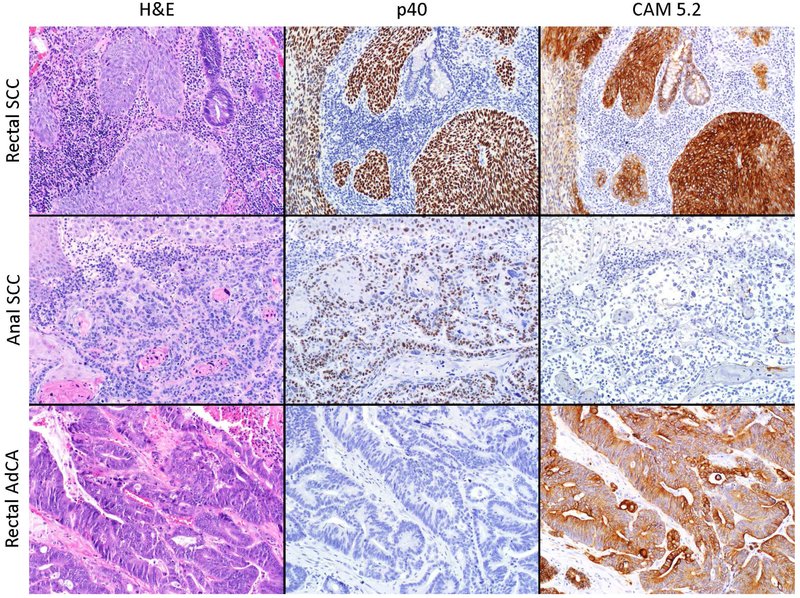
Figure 1.
Example cases of anal SCC, rectal SCC, and rectal AdCa evaluated in this study. Hematoxylin and eosin (H&E) and p40 immunohistochemistry (clone BC28, Biocare, Pacheco CA) were used to confirm squamous vs. non-squamous histology. CAM5.2 immunohistochemistry (clone Cam5.2, Ventana Medical Systems, Tucson AZ) marks low molecular weight cytokeratins present in epithelial cells of the rectum but not the anus and was used to confirm tumor location.
RESULTS.
All SCC tumors selected from cancer registries were confirmed as SCCs based on H&E and p40 staining. In contrast, no rectal AdCAs stained for p40. All rectal AdCAs and 20 (83%) rectal SCCs stained CAM5.2-positive, supporting rectal origin (Figure 1). In contrast, 4 (36%) anal SCCs stained for CAM5.2, significantly lower than rectal SCC (chi-square P=0.02). Review of adjacent normal tissue for 7 rectal SCCs, 2 anal SCCs, and 2 rectal AdCAs supported use of CAM5.2 to differentiate rectal from anal location. Normal columnar tissue adjacent to rectal SCC and AdCA stained CAM5.2-positive. Anal transition zone epithelium in one normal tissue section stained CAM5.2-positive, but other normal tissue adjacent to anal SCCs did not. CAM5.2 staining resulted in reclassification of 4 rectal SCCs as anal tumors and 4 anal SCCs as rectal tumors.
HPV16 E6 DNA was present (>1 copy/cell) in rectal SCCs and anal SCCs at similar frequency (63% [95%CI 41-81%] vs. 63% [24-91%]) and level (geometric mean 0.31 vs. 0.14 copies/cell, P=0.77). HPV16 E6 RNA status was also similar between rectal SCCs and anal SCCs (78% [95%CI 56-93%] vs. 56% [21-86%] positive; geometric mean 0.0087 vs. 0.00021 copies/housekeeping gene RPLP0, P=0.20). In contrast DNA and RNA levels were undetectable (<1 copy/cell) in rectal AdCAs. HPV16 ISH staining was present in 71% (95%CI 49-87%), 60% (26-88%), and 0% of rectal SCCs, anal SCCs, and rectal AdCAs, respectively. Two HPV16-positive rectal SCCs tested positive for additional HPV types: one for HPV45 DNA, and another for HPV33 DNA and RNA. Additional HPV types were not detected in anal SCCs.
DISCUSSION.
Herein we characterized both pathology markers and HPV16 infection in two dozen putative rectal SCC tumors. CAM5.2 immunohistochemistry corroborated clinical records, supporting the hypothesis that SCCs can arise in the rectum and are not simply misclassified anal cancers. It is possible that CAM5.2 imperfectly distinguishes rectal from anal epithelium, particularly for tumors arising in the transitional zone. Overall, however, CAM5.2 positivity clearly differed between tumors for which clinical information supported a diagnosis of rectal SCC (83%) and anal SCC (36%).
Importantly, oncogenic HPV16 infection was present in similarly high proportions of rectal and anal SCCs, assessed using three different methods. Prior studies have been small case series with mixed results, but some have also shown detection of HPV16 in rectal SCCs.6,7 In summary, we demonstrated that rectal SCC is a distinct clinical entity from anal SCC, and that HPV16 could play an etiologic role in this rare tumor. A viral etiology is further supported by the increased risk in immunosuppressed populations, a hallmark of virus-associated cancers.8 Given demonstrated vaccine efficacy against persistent HPV16 infection at multiple anatomic sites, rectal SCC might be a preventable disease, although its rarity limits the potential public health impact of measures targeted specifically at this tumor.
Acknowledgments
Funding: This work was funded through the Intramural Research Program at the National Cancer Institute.
Abbreviations
- SCC
squamous cell carcinoma
- HPV16
human papillomavirus type 16
- AdCA
adenocarcinoma
- H&E
Hematoxylin and eosin
Footnotes
Disclosure: No authors report any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coghill AE, Shiels MS, Rycroft RK, et al. Rectal squamous cell carcinoma in immunosuppressed populations: is this a distinct entity from anal cancer? AIDS 2016; 30(1): 105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frisch M, Glimelius B, van den Brule AJ, et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med 1997; 337(19): 1350–8. [DOI] [PubMed] [Google Scholar]
- 3.Nahas CS, Shia J, Joseph R, et al. Squamous-cell carcinoma of the rectum: a rare but curable tumor. Dis Colon Rectum 2007; 50(9): 1393–400. [DOI] [PubMed] [Google Scholar]
- 4.Taberna M, Resteghini C, Swanson B, et al. Low etiologic fraction for human papillomavirus in larynx squamous cell carcinoma. Oral Oncol 2016; 61: 55–61. [DOI] [PubMed] [Google Scholar]
- 5.Huang CC, Qiu JT, Kashima ML, Kurman RJ, Wu TC. Generation of type-specific probes for the detection of single-copy human papillomavirus by a novel in situ hybridization method. Mod Pathol 1998; 11(10): 971–7. [PubMed] [Google Scholar]
- 6.Sotlar K, Koveker G, Aepinus C, Selinka HC, Kandolf R, Bultmann B. Human papillomavirus type 16-associated primary squamous cell carcinoma of the rectum. Gastroenterology 2001; 120(4): 988–94. [DOI] [PubMed] [Google Scholar]
- 7.Kong CS, Welton ML, Longacre TA. Role of human papillomavirus in squamous cell metaplasia-dysplasia-carcinoma of the rectum. Am J Surg Pathol 2007; 31(6): 919–25. [DOI] [PubMed] [Google Scholar]
- 8.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007; 370(9581): 59–67. [DOI] [PubMed] [Google Scholar]