Abstract
Epigenetics, the study of the processes that control gene expression without a change in DNA sequence, highlights the importance of environmental factors in gene regulation. This paper maps the terrain of epigenetics and identifies four main research subfields: gene expression; molecular epigenetics; clinical epigenetics and epigenetic epidemiology. Within and across these fields, we analyse of what is conceptualised as environment and demonstrate the variable ways authors understand epigenetics environments. Then, following an analysis of the discursive strategies employed by epigenetics researchers, we demonstrate how authors portray the interactions between genes, epigenetics, and environment as relationships linking the outside (where the environment is located) with the inside (where the genes are located). We argue that authors assign specific roles to each actor: the environment as the active player initiating the relationship, the genes as recipients, and epigenetics as mediators between environment and genes. Framed as mediators, epigenetic markers can be understood as enablers of communication between environment and genome, capable of processing and organising signals so as to regulate the interactions between the actors of epigenetic relationships. This finding complicates the observation by social science scholars that the interactions between environment and genes can be understood through the concept of signal.
Keywords: epigenetics, environment, review, narrative
Introduction
Epigenetics is a rapidly expanding field in the world of bioscience. This growth is visible in the exponential rise of publications (Haig, 2012), as well as in the increasing number of research centres, national and international consortia1 and journals2 specifically dedicated to epigenetics research.
One frequently used definition of epigenetics is “the study of changes in gene function that are mitotically and/or meiotically heritable and that do not entail a change in the sequence of DNA” (Armstrong, 2014: 2), that is, the study of heritable signals that allow a cell to ‘remember’ past events and which are not part of DNA. According to this definition, epigenetics is the study of processes that regulate gene expression and do not entail a change in DNA sequence. This molecular understanding of epigenetics contrasts with the first historical definition of the term established by developmental biologist Conrad Waddington in 1940. He described epigenetics as “the interactions of genes with their environment, which bring the phenotype into being” (Waddington, 2012: 11). Waddington defined epigenetics in a broad, non-molecular sense and was particularly interested in the processes by which environmental stimuli may interact with genotypes in both individual development and natural selection, through what he called the ‘epigenetic landscape’. Throughout the 20th and early 21st centuries, the meaning of the word epigenetics has become more elaborated, changing in light of new discoveries and developments (Dolinoy et al., 2007).
However diverse the multiple definitions of epigenetics, all suggest that the point of focus in epigenetics is not genes per se, but what surrounds the genes - the ‘epi’ to the genes. This comes as a challenge to the gene-centric approach in biology which dominated 20th century genomics. It was built on the assumption that life is detached from its environment, with genes at the centre of biological explanation (Nicolosi and Ruivenkamp, 2012). In this approach, everything outside of the gene was by definition ‘environment’. Research in the 20th century, termed the ‘century of the gene’, viewed the gene as the core explanatory concept of biological structure and function (Keller, 2000). The century of the gene reached its climax and conclusion with the Human Genome Project (HGP) – an international research effort that aimed to sequence and map a full human genome. The HGP was hoped to bring substantial improvements in health through the detection and modification of genes. While the HGP was completed in 2003, it showed that the deterministic view of the gene as an autonomous agent controlling traits and developmental processes did not hold true. Instead, the completion of the HGP led to the conception of a “vast reactive genome” (Keller, 2011) embedded in a complex regulatory network. As some argue, the completion of the HGP marked the beginning of the ‘postgenomic age’ and the new ‘postgenomic view’ of the gene, which underlines the reactivity of the genome to environmental signals (Meloni, 2014, Meloni, 2015); other authors have highlighted the important role of systemic and multi-omics approaches as constitutive of the field of postgenomics - if there is such a field (Stevens, 2015, Schnittker, 2016).
The renewed attention to epigenetics in the 21st century in particular is linked to the hope that epigenetics will provide an opportunity “to anchor the environment to the genome” (Meloni and Testa, 2014: 436), challenging the gene-centric view of biology. Epigenetics is postgenomic by definition for its focus on genes in relation to their ‘epi’, that is, their environment. With epigenetics, the gene turns into an embedded and plastic entity responsive to its environment. Research in epigenetics deflates the role of genes as the privileged cause of phenotypes, by highlighting that environmental factors can impact gene regulation by leaving marks on the epigenome (Jablonka and Lamb, 2015). Therefore, the field of epigenetics challenges the gene-centric approach to biology by placing the environment at the centre of its attention (Nicolosi and Ruivenkamp, 2012, Pickersgill et al., 2013).
Anthropologists and sociologists have paid particular attention to the field of environmental epigenetics, which studies the molecular mechanisms linking environmental factors such as nutrition or stress to changes in gene regulation. Authors have explored how researchers in environmental epigenetics articulate environmental factors outside the body to epigenetic changes occurring molecularly inside the body (Landecker and Panofsky, 2013, Lock, 2015, Lock and Palsson, 2016, Niewöhner, 2011, Niewöhner, 2015). For example, Landecker’s (2011) study of nutritional epigenetics (an area of environmental epigenetics) showed how, in such studies, food is treated as a set of molecules capable of entering the body and alter bodily functions through epigenetics mechanisms. She argues that the conceptualisation of nutrition as an environmental factor in epigenetics has changed the way we think about food: food is understood and analysed as molecules “which exist in a cloud around us, and over which we often have limited individual control” (Landecker, 2011: 190). Niewöhner (Niewöhner, 2011) discussed the ‘molecularization of biography and milieu’ to describe how traumatic events in people’s biographies, such as child abuse, are operationalized by epigenetics researchers as ‘social environment’ and turned into standardised representations of forms of social change that can be molecularised and correlated to changes in the material body. Also other authors have drawn attention to how personal and social practice is operationalised as ‘environment’ in such specific ways, diagnosing reductionism: because epigenetics researchers seek to make the environment measurable, they tend to look for proxies that can stand in for the complexity of the social and material environment (Kenney and Müller, 2017, Lock and Palsson, 2016). This comes at the cost of those aspects of social and personal practices that have no evident material substrate. Other authors have highlighted social and political issues related to this problem: Kenney and Müller (Kenney and Müller, 2017), for example, in their study of the epigenetics of ‘maternal care’, discussed the underlying and unexamined assumptions about sex, gender and sexuality that shape this area of epigenetics research. Mansfield (Mansfield, 2012) analysed the way mothers and the pregnant body are conceptualized as environments for developing fetal genomes. These authors have argued that research on maternal effects in epigenetics resonate with current narratives of individualization, which tend to shift responsibility from collective to individuals, and especially rendering mothers responsible for the health of their children (Kenney and Müller, 2017, Mansfield, 2012, Pickersgill et al., 2013).
This literature provides valuable insights, by drawing attention to the political, phenomenological and epistemological assumptions that underpin epigenetics research. However, this body of work has mostly been focused on the specific field of environmental epigenetics, and to date, little research has looked in detail at how the environment is understood conceptually in the context of epigenetics more broadly. This paper starts to fill this gap by taking a closer look at the ‘epi’ in epigenetics, by first providing an analysis of what is conceptualised as the environment in the broad field of epigenetics, and then examining how the interactions between the genes and environment are conceptualised and articulated in the literature.
We carried out a broad review and synthesis of the epigenetics literature. This work complements scholarship in the social sciences that has so far focused on rather narrow areas of epigenetics research – often that of ‘environmental epigenetics’ or ‘transgenerational epigenetics’, which focus on the passing on of epigenetic changes from one generation to the next. This means that other areas of epigenetics research have been neglected, particularly the epigenetic approach that views epigenetics as a pathological process. This review and synthesis is the first to examine epigenetics acknowledging the diversity of research being carried out under the umbrella term ‘epigenetics’. Our aim is not to propose an exact and definitive definition of epigenetics, but to characterise the research being done and discussed under the label of epigenetics, by first analysing how authors define the notion of environment in relation to their research, and then exploring how the authors discuss the interactions between environment and genes.
Literature review and synthesis methods
Inclusion criteria and search strategy
In this paper, we discuss and analyse the diversity of research being done in the field by reviewing and synthesising the literature published under the umbrella term epigenetics. The literature on epigenetics is vast. The database Web of Science, for the year 2016 alone, counts more than 2,300 papers with epigenetic(s) in their title. We adopted some of the methods of a systematic review to locate and delineate relevant literature.
Specific inclusion criteria were applied to restrict the results to papers most relevant to our focus on how the environment is operationalised in epigenetic research. Using the electronic database Medline, we searched for reviews in the field of epigenetics research, which included the term ‘environment’ (or a synonym) in their title or abstract and focused on cancer. Because there is already a large number of review papers available in the field of epigenetics, in our Medline search, we only included these. We also restricted this systematic search to cancer, because it is the most commonly studied disease in epigenetics (approximatively 70% of publications in the field) (Martin, 2015a).
In addition to the systematic search and to gain an insight on epigenetics more broadly (not only focusing on cancer and reviews), we conducted manual searches of key journals publishing in the field (e.g. Clinical Epigenetics; Epigenomics) and identified key authors in the literature. Most cited papers using Scopus were also included. Finally, we consulted reading lists for courses in epigenetics.
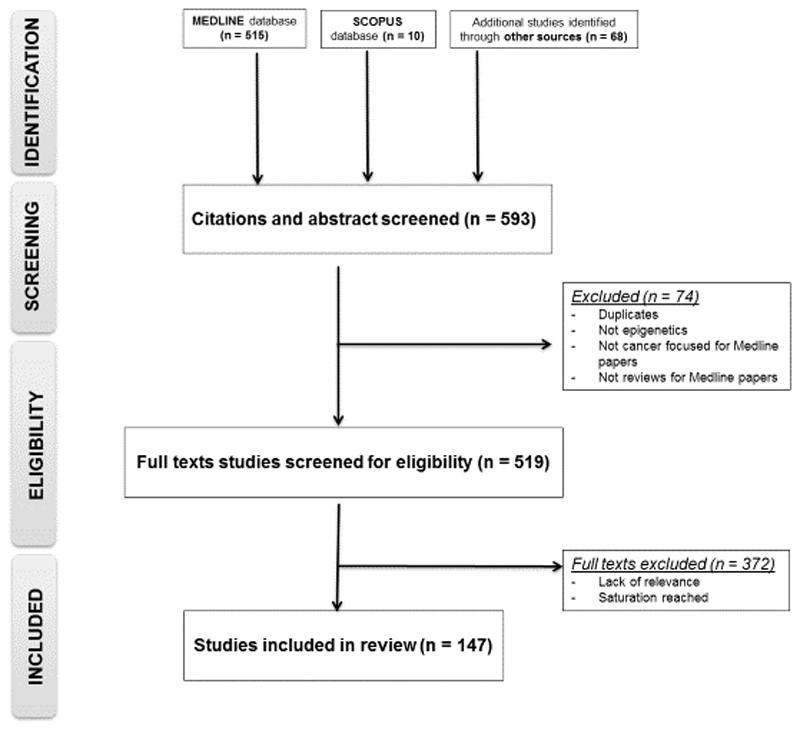
Initially, article titles and abstracts were screened, followed by full text screening. We stopped including further papers when content analysis no longer yielded new insights. 147 papers in total were analysed at this step of the review. Details of the paper selection are documented in a flowchart (Figure 1).
Figure 1. Flow chart describing the selection process of papers included in this review.
Data extraction
Data extraction sheets were completed for each paper included in the review. We examined each paper according to four key ordering principles that were used to extract data:
-
1)
The research questions – what are the research questions explored in this paper?
-
2)
The disciplines – what are the authors’ disciplines?
-
3)
The technology – what technologies were used to carry out epigenetics research?
-
4)
The notion of ‘environment’ – how did the authors of this paper make use of the notion of environment in the epigenetics research they carried out?
Primary synthesis
As part of our primary synthesis, using the literature selected, we characterised the research being done and discussed under the umbrella term epigenetics, to then find ways to meaningfully organise the field in different categories. Using the data extraction sheets for a first subset of papers (67 papers), we grouped together papers that used similar conceptual and methodological approaches. Within this first exercise, we identified four main groups (‘categories’) of epigenetic research, each group representing a subfield in epigenetics. The categories we identified correspond to existing classifications of epigenetics subfields that were sometimes used in papers. We then reviewed the second subset of papers (80 papers) and coded each of them along the four categories identified.
To assess the validity of our analysis, we used a mapping exercise with three researchers carrying out research in epigenetic epidemiology in the UK. In this exercise, we asked researchers to draw a map of the epigenetics field as they understand it, specifically asking them to draw lines between areas of research. The discussions that followed helped establish links between the categories.
Secondary synthesis
As a secondary synthesis, we explored relationships in the data and identified overarching themes that are present throughout the epigenetics literature. We analysed the discursive strategies authors use to discuss how environment and genome(s) interact, drawing on Myers’ approach to discourse analysis and his concept of narrative (Myers, 1990b).
Primary synthesis: Literature review and synthesis results
Our review and synthesis identified four main subfields of epigenetics research: gene expression; molecular epigenetics; clinical epigenetics and epigenetic epidemiology. Each epigenetic subfield is defined by its own set of research questions and disciplinary approaches. We now describe the four subfields in order to portray the epigenetic landscape. Each subfield is discussed along four ordering principles: (1) the research questions – what are the main research questions explored in this subfield?; (2) the disciplines – what are the dominant disciplinary approaches in this area of research?; (3) the technology – what technologies were discussed in this epigenetics subfield?; (4) the notion of ‘environment’ – how is the notion of environment defined in this epigenetics subfield?
1. Gene expression
Gene expression research considers factors that influence gene expression during cellular programming and shape the epigenome. The subfield of gene expression can also be referred to as research exploring the mechanisms of gene regulation. This subfield has a long history: molecular geneticists traditionally engaged with research in gene expression to explore the mechanisms by which genes are regulated. The increased availability of next-generation sequencing technologies has brought new insights onto the molecular mechanisms of gene expression and has led researchers to focus their attention on the structure of the epigenome and its influence on gene expression. Some argue that epigenetics research is one aspect of research on gene expression (Niewöhner, 2011).
More specifically, gene expression is the process by which genetic instructions are used to synthesize gene products. The regulation of gene expression is the critical link between the genome and cellular morphology. Researchers carrying out gene expression analysis examine the molecular mechanisms by which transcription factors can influence chromatin structure, programme the epigenome and play an important role on gene expression (Drouin, 2014, Zaret and Carroll, 2011). In cancer research, gene expression analysis aims to explore genes that are activated or repressed during tumour development, and ultimately identify the molecular events at the basis of the generation and maintenance of the cancer cell (Bashyam, 2002, Bertucci et al., 2003).
Similar to research in the subfield of molecular epigenetics (see next section), research in the subfield of gene expression operates at a molecular level and explores the molecular basis for gene regulation. However, these two subfields are distinct in their approach to epigenetic changes: whereas molecular epigenetics examines the impact of epigenetic changes on gene expression and ultimately the development of pathology, gene expression research takes a wider approach and considers other molecular elements at the source of pathology.
Disciplines
Researchers in this subfield originate from several closely related disciplines, such as molecular genetics, molecular biology and developmental biology (Drouin, 2014, Zaret and Carroll, 2011). Molecular genetics explores the chemical structure, functions, replication and mutations of the DNA and RNA which are involved in the transmission of genetic information (Mosby's Medical Dictionary, 2009). Molecular genetics explores the role of chemical interactions in the replication of DNA, its transcription into RNA and translation into proteins (Farlex Partner Medical Dictionary, 2012). Finally, developmental biology examines the life processes occurring during the stages of prenatal life, between growth and maturation (Mosby's Dental Dictionary, 2008).
Technologies used in this subfield
Scientific papers in gene expression recurrently mention the crucial role of technological advances in the development of the field. In particular, authors emphasise the multiple benefits of next-generation sequencing technologies, which have made techniques such as genome-wide analysis, and high-density microarray analysis possible (Bashyam, 2002, Bertucci et al., 2003, Wei et al., 2004, Zaret and Carroll, 2011). Those techniques have brought molecular details to the genome structure.
Notion of environment
Overall, little explicit reference to the notion of environment is made in the subfield of gene expression. In a few instances, authors mention the importance of understanding the micro-level environment to gene expression, encompassing the environment inside the cell and surrounding the cell. Some authors discuss the chromatin environment to cell programming (Drouin, 2014), others investigate the intra and intercellular signals which influence interactions among organs (Alberghina et al., 2004). In other cases, authors are interested in the influence of macro-level environmental factors on gene expression. For example, Wei et al. (2004) investigate the biological consequences of metals such as chromium on gene expression.
2. Molecular epigenetics
The subfield of molecular epigenetics is concerned with understanding the molecular mechanisms underlying the actions of epigenetic changes. It mostly focuses on the study of three different epigenetic changes: DNA methylation, histone modifications, and non-coding RNAs (Portela and Esteller, 2010). Researchers sometimes refer to this subfield as ‘basic research’ in epigenetics (Bohacek and Mansuy, 2013) or pre-clinical epigenetics research (Claes et al., 2010).
DNA methylation is the most commonly studied epigenetic change, with studies investigating the general principles and mechanisms in its functioning, or exploring the roles of this epigenetic change in gene regulation (Jaenisch and Bird, 2003, Jones, 2012, Smith and Meissner, 2013). Studies have also been conducted to explore the molecular mechanisms and functions of other epigenetic changes, such as histone modifications (Cohen et al., 2011, Kouzarides, 2007), while increasingly, research has focused on the study of non-coding RNA as important regulators of gene expression that could help improve the understanding of the molecular underpinnings of cancer (Nana-Sinkam and Croce, 2011, Tang et al., 2014).
Cancer is the most commonly studied pathology in molecular epigenetics (and other epigenetic subfields) (Martin, 2015a). Molecular epigenetics research concerned with cancer considers epigenetic changes as common drivers of cancer initiation and progression (Jones and Baylin, 2007, Timp and Feinberg, 2013). Extensive research is carried out to identify the DNA methylation patterns involved in various types of cancer, including lung cancer (Herceg and Vaissiere, 2011), ovarian cancer (Barton et al., 2008), bladder cancer (Besaratinia et al., 2013, Enokida and Nakagawa, 2008), breast cancer (Reynolds et al., 2006) and prostate cancer (Damaschke et al., 2013).
Disciplines
A large part of the research carried out in molecular epigenetics is based on the disciplines of molecular genetics and molecular biology (Bhattacharjee et al., 2013, Poirier and Vlasova, 2002). With an important part of molecular epigenetics focusing on cancer, the more specialised disciplines of cancer biology or tumour biology also appear to be particularly influential in this subfield (Caren et al., 2013, Timp and Feinberg, 2013). Epigenetic research inspired by these disciplines aims to identify epigenetic changes which are responsible for the development of tumours.
Technology
The molecular epigenetics literature reviewed highlights the value of novel technologies. Studies report the benefits of genome-wide microarray based technologies and next-generation sequencing platforms coupled with cutting-edge bioinformatics for molecular epigenetics (Boehm and Hahn, 2011, Butcher and Beck, 2008, Smith and Meissner, 2013, Yamane et al., 2007, Zaret and Carroll, 2011). Next generation sequencing technologies allow the survey of genome-wide epigenetic variation at high resolution. The review shows that one of the common approaches used to detect DNA methylation is methylated DNA immunoprecipitation sequencing (MeDIP-seq), while chromatin immunoprecipitation followed by sequencing (ChIP-seq) has now become the standard approach to detect histone-tail modifications (Bell and Spector, 2011, Kouzarides, 2007, Weaver et al., 2004a).
Notion of environment
References to the notion of environment are made throughout the molecular epigenetics literature. There are a few instances where the notion of environment is discussed at a micro level. For example, some authors discuss the role of the extracellular environment in tumour development (Ahmed, 2007, Pistollato et al., 2015). However, in most cases, authors discuss environmental factors at a macro-level and aim to examine molecularly the mechanistic links between environmental cues (external to the body) and epigenetic alterations (Feil and Fraga, 2012). The review suggests that research in molecular epigenetics focuses on three types of environmental factors: the social environment, lifestyle factors and metals/chemicals. These three categories, traditionally used in public health, are used in the epigenetic literature to represent environmental factors. In this review, we combine these categories to describe the breath of environmental factors discussed in the literature.
The ‘social environment’ is the focus of numerous studies in molecular epigenetics. Authors discuss the effect of child abuse or maternal care on the epigenome. In a study in rodents, Weaver et al. (Weaver et al., 2004a) demonstrated that maternal behaviour produces stable effects on the DNA methylation patterns of the offspring. Lifestyle factors are also studied for their effect on epigenetic changes. Studies have explored the early influences of nutrition on the establishment and maintenance of epigenetic mechanisms. Other lifestyle factors examined include cigarette smoking or alcohol. Finally, a number of papers in molecular epigenetics report on studies exploring the impact of metals such as cadmium, arsenic, or chromium on the epigenome. For example, Fragou et al. (Fragou et al., 2011) discuss heavy-metal induced DNA methylation and histone modifications.
For a detailed description of the environmental factors discussed in molecular epigenetics, including a full list of references, please refer to Appendix 1.
Importance is also given to the timing of the environmental exposure. Authors are not only concerned with the nature of the environment but also with the timing of the exposure to a particular environment. Often the emphasis is placed on measuring the impact of early-life environmental exposure on epigenetic changes (Jimenez-Chillaron et al., 2012, Waterland and Jirtle, 2003, Weaver et al., 2006). For example, Monk et al. study the fetal environment which they believe is influenced by maternal prenatal stress and adversity (Monk et al., 2012).
3. Clinical epigenetics
Clinical epigenetics seeks to translate epigenetics knowledge into clinical care and treatment. Research in clinical epigenetics is based on the discovery of the importance of epigenetic changes in the development of pathology. It examines how epigenetic changes can be exploited for both diagnosis and prognosis through the development of biomarkers, and for therapy though the development of epigenetic drugs.
Epigenetic drugs are prominently discussed in the clinical epigenetics literature. Research on epigenetic drugs consists of identifying certain epigenetic changes as potential pharmacological target. It is based on research highlighting the reversibility of epigenetic changes (Baylin and Jones, 2011, Besaratinia et al., 2013, Popovic et al., 2013). Literature discusses compounds capable of inhibiting or reversing epigenetic processes (Anestopoulos et al., 2015, Lu et al., 2012). Authors also report that there are now FDA approved drugs for epigenetic therapy in cancer (Besaratinia et al., 2013, Duvic et al., 2007, Falahi et al., 2014, Popovic et al., 2013). Some authors have referred to research concerned with the development of cancer epigenetic drugs as ‘pharmacoepigenomics’ (Anestopoulos et al., 2015, Claes et al., 2010, Mai and Altucci, 2009). Claes et al. discuss pharmacoepigenomics as what is capable of “bringing epigenetics to the bedside” (Claes et al., 2010: 153).
Research on epigenetic biomarkers forms another significant part of the clinical epigenetics literature. This is linked to findings in molecular epigenetics suggesting that epigenetic changes play a role in the development of cancer. Epigenetic changes in this subfield are viewed as epigenetic biomarkers (Ahmed, 2007), for the information it can provide on the human body. In cancer research, authors discuss the use of epigenetic biomarkers for diagnosis and early detection strategies (Ahmed, 2007, Claes et al., 2010, Timp and Feinberg, 2013, Baylin and Jones, 2011); others explore how epigenetic profiles could be used as biomarkers for prognosis and molecular classification of cancer patients (Ahmed, 2007, Anestopoulos et al., 2015, Barton et al., 2008, Baylin and Jones, 2011, Claes et al., 2010); finally some examine how epigenetic biomarkers could operate as predictive markers to assess therapeutic responsiveness to cancer therapy.
The literature on epigenetic biomarkers is infused with discourses on personalised medicine. Epigenetics research on biomarkers responds to the long-standing ambition to elaborate a patient stratification strategy based on molecular signatures, which would lead to tailored therapy to the needs and specific conditions of each patient (Hojfeldt et al., 2013). For example, some existing research in clinical epigenetics aims to identify epigenetic differences which can help explain inter-individual variation in therapy response (Claes et al., 2010).
Disciplines
Research in clinical epigenetics is highly influenced by the disciplines of molecular biology and medicinal chemistry (Hojfeldt et al., 2013). Molecular biology contributes to defining the disease-relevant epigenetic changes that are reversible and can serve as potential drug target, while the discipline of medicinal chemistry is used to discover and develop improved inhibitors active in drug therapy.
Technology used in clinical epigenetics
Similar to the other epigenetic subfields, literature in clinical epigenetics highlights the value of novel technologies such as the next-generation sequencing. Authors emphasise how the recent technological developments in genetics have provided the tools to understand the molecular mechanisms of epigenetic regulation, hence contributing to the advancements in the clinical applications of epigenetics (Berdasco and Esteller, 2010, Boultwood and Wainscoat, 2007, Hojfeldt et al., 2013, Popovic et al., 2013).
Notion of environment
In this subfield, little explicit reference is made to the notion of environment. In cases where authors discuss the notion of environment, a distinction is made between macro-level and micro-level environment. Some authors discuss the environment at a micro level, such as the extracellular microenvironment of cancer cells (Ahmed, 2007, Claes et al., 2010, Esteller, 2005) and its importance in cancer initiation and progression. Other papers highlight the role of macro-level environmental factors, such as alcohol, tobacco or diet, in cancer development (Feinberg, 2007, Lima et al., 2010).
4. Epigenetic epidemiology
Barrow and Michels define epigenetic epidemiology as “the study of variation in epigenetic traits and the risk of disease in population” (Barrow and Michels, 2014: 7). This research studies epigenetic changes at a population level in order to understand the risks of diseases for a specific population. Traditionally, this subfield aims to gain a molecular understanding of correlations between environmental factors and human disease phenotypes (Heijmans and Mill, 2012). Epigenetic epidemiology is also concerned with identifying epigenetic markers of environmental disease risk, which can help tease out gene-environment interactions and understand the mechanisms underpinning environmentally-driven diseases (Castillo-Fernandez et al., 2014).
In relation to cancer, research in epigenetic epidemiology is concerned with the study of epigenetic marks which are associated with cancer. It explores how epigenetic marks can be used as biomarkers, and investigates whether these epigenetic marks may explain the link between certain exposures and cancer. For example, research in epigenetics has demonstrated that smoking and air pollution, which are the leading risk factors for lung cancer, can influence DNA methylation patterns, hence impacting the epigenome (Barrow and Michels, 2014).
Disciplines
The discipline of epidemiology (or genetic epidemiology) has been increasingly used in epigenetics (Bell and Saffery, 2012, Bell and Spector, 2011, Castillo-Fernandez et al., 2014). In epigenetic epidemiology, researchers explore epigenetic traits using a population-level approach in order to understand the risks of diseases for a specific population.
Technology
The literature in epigenetic epidemiology reports the benefits of next-generation sequencing technologies, which have allowed researchers to perform genome-wide analysis of epigenetic variation at a high resolution (Barrow and Michels, 2014, Bell and Saffery, 2012, Bell and Spector, 2011, Breitling et al., 2011). Microarrays are also frequently discussed in the epigenetic epidemiology literature (Barrow and Michels, 2014, Borghol et al., 2011). Research in epigenetic epidemiology commonly builds on Epigenome-Wide Association Studies (EWAS), which are based on the use of microarrays or next generation sequencing technology. Such studies are genome-wide which means that they can analyse epigenetic variation on a large scale.
The notion of environment
The notion of environment is heavily referred to in the epigenetic epidemiology literature. Papers in this subfield describe correlations between environmental factors and epigenetic changes which are linked to human disease phenotypes (Baccarelli and Bollati, 2009). The review reveals different understandings of what constitutes the environment in epigenetic epidemiology. Similar to the subfield of molecular epigenetics, epigenetic epidemiology has focused on the study of three types of environmental factors: the social environment, lifestyle factors and metals/chemicals.
Studies in epigenetic epidemiology have explored the influence of social environments on the epigenome at a population level. The social environment is operationalised in a variety of ways: some studies understand child abuse as a form of social environment, others examine prenatal stress, or parental care, while some explore the effects of socio-economic positions on the epigenome.
Our review also shows that research on lifestyle factors, including smoking, alcohol consumption, nutrition or physical activity, is an important part of the epigenetic epidemiology literature. Lifestyle factors are conceptualised as a form of environment to the individual.
The third category of environmental factor most researched in epigenetic epidemiology is environmental chemicals or metals. Research in this area is concerned with the study of heavy metals such as lead, nickel, arsenic or cadmium or air pollution.
For a detailed description of the environmental factors discussed in epigenetic epidemiology, including a full list of references, please refer to Appendix 2.
Authors are not only concerned with the magnitude of the exposure to environmental factors, but also with the timing of the exposure (Barua and Junaid, 2015, Dolinoy et al., 2007, Thornburg et al., 2010, Bell and Spector, 2011, Burdge et al., 2009). The in-utero impacts of environmental factors on the epigenome are an important area of study in epigenetics epidemiology. For example, Burdge et al. discuss the extent to which the intra-uterine environment, including nutrition, play a role in the epigenetic regulation of specific genes (Burdge et al., 2009).
Summary of the primary synthesis
The primary synthesis shows that research published under the label of ‘epigenetics’ comprises a wide range of approaches coming from different disciplines, including molecular biology or epidemiology, and with them come a number of different ways of approaching pathology. A large body of research explores epigenetics as a pathway to diseases, that is, it operates based on the understanding that epigenetics play a role in the development of pathology. Drawing from this approach, research explores the role of epigenetics in the development of diseases (i.e. molecular epigenetics focused on cancer), or examines how epigenetic changes can aide the development of biomarkers for clinical diagnosis, and the development of epigenetic drugs (i.e. clinical epigenetics). Another approach explores epigenetics at a population level in order to understand the risks of diseases for a population; this approach views epigenetics as adaptive mechanisms to changes in the environment (i.e. epigenetic epidemiology).
This finding suggests the flexible nature of the concept of epigenetics, which means that it can be used in different ways. It corroborates findings from other social science studies, namely the flexibility of the concept of epigenetics and the versatile nature of this research field (Meloni and Testa, 2014, Niewöhner, 2011, Pickersgill, 2016, Waggoner and Uller, 2015). Meloni and Testa have also highlighted the multiple understandings of what constitutes epigenetics, and argued that this multiplicity is essential to understand the growth of the epigenetics field in the life sciences. We corroborate this analysis, and we also provide a map of the diverse approaches to epigenetics research.
Our primary synthesis then suggests a number of different ways of understanding the environment, and various approaches to exploring its role on gene expression. We first underline the flexible nature of the notion of environment in epigenetics. Epigenetics environments are multiple, and authors mean different things by environment in epigenetics. For example, some conceptualise the ‘epi’ to the genes as the “extracellular microenvironment” which is studied for its role on cancer cell development (Ahmed, 2007: 104), while others see heavy metals such as toxins outside the body (Fragou et al., 2011) or social practices such as maternal care (Weaver et al., 2004a) as environmental factors influencing the epigenome. Our primary synthesis also shows that not all areas of epigenetics study the environment in the same way. Publications in the subfields of molecular epigenetics and epigenetic epidemiology define the notion of environment and operationalise it in a specific way: the environment is often understood on a macro level, that is, as external to individual bodies, exemplified by factors such as nutrition or smoking. In contrast, in the subfields of clinical epigenetics or gene expression, little explicit reference to environment is made. In the latter fields, the environment is mostly examined at a micro-level, such as in studies exploring the role of a liver tumours’ environment in the development of liver cancer (Berasain et al., 2010). This finding complements current social science literature around epigenetics which has mostly looked at epigenetics research that operationalises the environment with factors external to the body (Landecker, 2011, Niewöhner, 2011). Our primary synthesis therefore adds nuance to the social science literature that has, so far, not looked systematically into how the environment is operationalised in epigenetics. It underlines the versatile nature of the research carried out in epigenetics. At the same time, as will be shown in the secondary synthesis, the various approaches to epigenetics have in common the ways they conceptualise the interactions between genes and the environment – as complex relationships where the environment is framed as the active actor initiating the relationship, the genes are the invariant in the relationship, receiving signals, while epigenetics are framed as the mediators enabling communication between environment and genes.
Secondary synthesis
After having explored what constitutes the environment in epigenetics, in our secondary synthesis, we examine the common ways in which authors across all subfields discuss the interactions between the environment and genes. We start by analysing the discursive strategies authors use to discuss how environment and genome are seen to interact. We then draw conclusions as to what this means for the concept of epigenetics.
To carry out this secondary synthesis, we selected 23 articles from the total number of papers included in the review. These are most highly cited papers or articles well-known in the field for their contribution.
We draw on Myers’ concept of narrative (Myers, 1990b, Myers, 1990a, Myers, 1991) to examine the discursive strategies used by epigenetics authors to frame their research. Myers argues that the production of scientific knowledge takes place when scientific writing occurs (Myers, 1990b, Myers, 1991), with reviews as key spaces within which scientific knowledge is organised and constructed towards building a narrative. Reviews consolidate what a field is about, as they entail processes of selecting, interpreting, arranging and recontextualising scientific statements into a new paper, all this with a view of the scientific field and beyond. Myers sees review articles as narratives composed of recurrent patterns used to persuade readers of the veracity of the authors’ claims. He identifies two main features in scientific narratives: (1) the identification of new actors; (2) the construction of a chronological sequence. In the first one, Myers argues that the building of a scientific narrative relies on the identification of a new set of actors, discussed for their role in scientific work. Those actors are not necessarily individuals involved in science, but they can also be the objects studied, the techniques and methods or the disciplines. The second feature of scientific narratives consists of the construction of a chronology in which scientific events are discussed in relation to past events. According to Myers, a scientific narrative reorders the past, situates scientific events in relation to this past, and shapes the future by suggesting what can be done next. In our review, we specifically focus on exploring the actors of the epigenetics narrative and their interactions, rather than historical tendencies.
Drawing on Myers’ approach, we explore the ways authors across all subfields discuss the notion of environment in relation to genes and construct epigenetics knowledge. We show how authors recurrently frame the interactions between environment and genes as a matter of complex relationships between environment, epigenetic markers, genes and pathologies. The environment is framed as the active player initiating the relationship, while genes are conceptualised as the invariant receiving signals. Epigenetic markers are constructed as mediators between environment and genes capable of regulating and organising their complex interactions. We term this the epigenetics narrative of relationships. We now examine the rhetorical tools that form this narrative. They include: introducing the actors of the epigenetic relationships; assigning roles to the actors; framing epigenetic markers as mediators of environment within the genome.
Introducing the actors of the epigenetic relationships
The notion of the environment is not discussed on its own right in the papers reviewed, but always in relation to its other, namely the genetic. By being defined as ‘the other’ of the genetic level, environment obtains its meaning in reference to what it is not. We use Myers’ concept of actors to discuss the various notions, such as the genes, authors bring up in the literature to consider the environment. Who these actors are varies from study to study: for example, some papers identify nutrition (a specific environmental factor) and epigenetic changes as the main actors of their epigenetic ‘story’ (Pistollato et al., 2015), others define the environment, epigenetic changes, genes and cancer (a specific phenotype) as the actors involved in their study (Berdasco and Esteller, 2010). These actors are the subjects of most sentences in papers reviewed, which suggests they are the centre of the epigenetic narrative.
The actors identified for their role in epigenetics phenomena may vary, but the way they are discussed together remains the same and follows specific modes. The first mode introduces the actors of the epigenetics narrative and suggests a relationship between them. In the following, Borgol et al. (2011) introduce their hypothesis and study aim:
A working hypothesis is that socio-economic circumstances leave their mark on the epigenome leading to stable changes in expression of genes critical for human health, such as those involved in cardiovascular, immune, stress response and behavioural pathologies. (…) Specifically, we aimed to establish whether childhood SEP [socio-economic position] was associated with differential DNA methylation. (Borghol et al., 2011: 63)
The authors define environment (operationalised as socio-economic circumstances), epigenetic changes, genes and pathologies as the actors of their epigenetic story. The authors introduce the actors and establish links between them: in a first step, they make the assumption of an association between environment and epigenetic changes; in a second step, they suggest that epigenetic changes lead to changes in gene expression mechanisms involved in the development of pathologies. Finally, the authors introduce the aim of their study, which is to explore whether environment is associated with changes in epigenetic markers. Epigenetic studies aim to find associations between the actors, in particular between the environment, epigenetic changes and the genes. The epigenetic narrative is therefore built on the assumption that actors are related – we term this the epigenetic narrative of relationships.
Assigning roles to the actors of epigenetic relationships
Below we explore how authors in the literature construct these relations by looking at the role given to each actor in the relationship. We start by looking at the role of environment in epigenetic relationships. The way the environment is discussed follows specific patterns. The first one consists in framing the environment as “environmental signals” (environment and environmental signals are used synonymously in the literature). Di Stefano and Dyson (2013) examine the role of histone demethylases (regulators of chromatin) in environment/genes interactions:
studies suggest that demethylase activities are a component of the critical connections that enable environmental signals to modulate the epigenetic landscape of a cell. (Di Stefano and Dyson, 2013: 13)
In the above, cells, epigenetic changes (referred to as “demethylase activities”) and environmental signals are the actors of the epigenetic ‘story’. The concept of signal is not specific to the field of epigenetics but has become widely used in 21st century biological research (Landecker, 2016). Landecker suggests that the environment outside of individual bodies is increasingly seen as a set of signals. They can be understood as theoretical tools which enable research in the life sciences to investigate the world external to an organism as a set of factors or exposures, which are transduced into molecular form as “signal cascades” causing changes in the organism’s biology. As Landecker points out, epigenetics research builds on the concept of signal to partly explain the interactions between environment and genes.
Another discursive strategy is the construction of environment as an active player in epigenetic relationships, using active verbs to connect environment to other actors. In their highly cited paper, Weaver et al. (2004) describe the relation between maternal care on the one hand, and changes in DNA methylation and chromatin structure in the offspring on the other hand:
our findings provide the first evidence that maternal behavior produces stable alterations of DNA methylation and chromatin structure, providing a mechanism for the long-term effects of maternal care on gene expression in the offspring. (Weaver et al., 2004a: 852) (italics added)
In this quote, environment (operationalised as maternal care), DNA methylation and chromatin structure are positioned in a relationship, where the first is considered to act on the second two, which in turn are involved in gene expression. Authors use the verb “produce” to link environment to the other actors and suggest cause and effect between them. This active verb suggests agency of the environmental factor, which is seen to drive the relationship between the actors. This situates the environmental actor at the top of epigenetic relationships, whose role is to send signals to the other actors.
The relationships constructed by authors resemble that of causal chains, as the quote below suggests:
Different environmental cues influence epigenetic modification of histones or DNA and alter access of transcription factors (TFs) to the DNA sequence, thereby affecting gene expression. (Claes et al., 2010: 153) (italics added)
In this account, the environment is positioned at the top of the relationship and constructed as the stronger actor. The authors use active verbs to connect the environmental factor to the other actors and underline how it impacts the others. The adverb “thereby” denotes the idea of relation: authors argue that through the connection between environment and epigenetics, environment can also affect genes. The authors suggest a causal relationship between the actors. They imply the active role of environment that can initiate changes in the epigenome, changes that are involved in gene regulation.
In contrast, the genes are repeatedly situated at the end of the relationship and framed as constant. For example, Weaver et al. discuss the relationship between environment and “the fixed genome” (Weaver et al., 2004a: 852). This echoes Lappé and Landecker (Lappé and Landecker, 2015: 158) who observed that scientists recurrently conceptualise the epigenome as plastic, while they see the genome as static. This observation is substantiated by our analysis: authors portray the epigenome as a dynamic actor that can change in time according to environmental influences, while they conceptualise the genome as invariant, located at the end of the causal chain.
Throughout the literature, the relationship between actors has a specific direction of travel: it moves from the outside to the inside, from the peripheral to the central, the most central actor being the genes. In this model, the environment is framed as the starting point of the relationship, while genes are the end point. The following quote illustrates this:
Environmental factors, including xenobiotic chemicals, behavior, and even low dose radiation, can also directly affect methylation and chromatin remodelling factors to alter the fetal epigenome and subsequent gene expression patterns. (Dolinoy et al., 2007: 302) (italics added)
As the quote demonstrates, relationships between actors transcend distinct spaces: between the environment, located at the outside of the relationship (either outside of individual bodies or at the periphery of the rest of the actors), and the genes located inside individual bodies. The discursive strategies employed to discuss the environment and its interactions with genes construct active relationships between the actors. Each actor is given a specific role: the environment, situated at the top of the relationship, is constructed as the driving force in the relationships who sends signals to the other actors; the genes, located at the end of the relationship, are framed as an invariant actor who receives signals from the other actors; finally epigenetics are situated in between the two previous actors and framed as a link between environment and genes. In the next section, we explore in more details the role given to epigenetics in the relationship.
Epigenetics as mediators regulating the relationship between environment and genome
In order to articulate the molecular interactions between genes and environment, the epigenetics narrative builds on a series of concepts. Landecker argues that the interactions between genes and environment in epigenetics can be understood through the concept of signal (Landecker, 2016). A signal can be defined as anything that serves to indicate, warn or direct. The metaphor of signal evokes communication over long distances when face-to-face communication is impossible. One example is the smoke signal, which has been commonly used to transmit news, alert of danger or gather people in a common area. The key characteristic of signals is therefore that they enable communication over long distances, between actors located in distinct spaces where a deeper form of communication is impossible. In the case of epigenetics, the relationships between environment and genes take place across distinct spaces. Landecker showed masterfully how, by conceptualising the environment as signals, authors can examine the environment molecularly for its action inside the body and in interaction with other entities, without the environment itself having to travel inside the body. The use of the concept of signal in epigenetics relates to the transduction model in biology. This model supposes that an entity at the surface of a cell functions as both receptor and relay: it receives external input, and relays it down the line inside the cell. This happens without external entities actually having to enter the cell. Although the concept of signal is useful to understand epigenetic relationships between actors located in distinct spaces, we argue that it oversimplifies the interactions between environment and genes. With the concept of signal, the communication between the actors appears linear, and epigenetics are portrayed as mere transmitters of information between the environment and the genome. Our analysis suggests a more complex type of communication between the actors of the epigenetics relationships, which is captured by the concept of ‘mediation’. We now discuss how researchers construct epigenetic relationships through the concept of mediation, and we outline what the idea of mediation tells us about the nature of epigenetics generally.
Our analysis suggested that epigenetic mechanisms are framed as mediating processes between environment and genome. Lutz and Tureki (2014) review evidence exploring the epigenetic mechanisms of DNA methylation involved in childhood maltreatment. In the following quote, they introduce epigenetics, define the actors of the epigenetic relationships and specify the actors’ role in these relationships:
Epigenetics refers to the collective chemical and physical processes that program the genome to express its genes in a time- and cell-dependent manner. These mechanisms are capable of conveying information through meiotic and mitotic divisions in the absence of a change in the DNA sequence. The epigenome is responsive to developmental, physiological and environmental cues. As such, epigenetics explains how the environment regulates the genome, and are well suited to mediate the effects of early environmental factors (Lutz and Turecki, 2014: 143)
These authors position environment, epigenetic changes and genome in a relationship together and explain their role: they describe how epigenetics are processes that regulate the genome; they then add that epigenetics respond to environmental influences. This leads the authors to portray epigenetics as an intermediary between environment and genome, with the idea that epigenetics can mediate the influence of environment within the genome. Positioned in between environment and genome, epigenetics are understood to work simultaneously with each of the actors: epigenetics can be modified by environmental factors, while they also regulate the genome. As such, epigenetic are framed as mediators that enable the communication between the genome and environment, which cannot communicate on their own.
In a highly cited paper, Weaver et al. (2004) explicitly use the metaphor of the ‘mediation’ to describe the role of epigenetics:
Epigenetic modifications of targeted regulatory sequences in response to even reasonably subtle variations in environmental conditions might then serve as a major source of epigenetic variation in gene expression and function, and ultimately as a process mediating such maternal effects. We propose that effects on chromatin structure such as those described here serve as an intermediate process that imprints dynamic environmental experiences on the fixed genome, resulting in stable alterations in phenotype. (Weaver et al., 2004a: 852)
Here, the authors suggest that epigenetic changes act as a mediator between a “dynamic” environment and a “fixed” genome. With these two adjectives, they imply that the environment can change in time, while the genes are constructed as constant. Communication between environment and genes is made possible because epigenetic mediators are mutable actors in the relationship: on the one hand, epigenetics can be modified by subtle changes in the environment – epigenetics mutate for the environment inside the body; on the other hand, epigenetics bear modifications in its structure to influence gene expression, while the genome remains fixed – epigenetics mutates in place of the immovable genome.
Epigenetic mediators are therefore portrayed as enablers of communication between a dynamic environment and a constant genome, thanks to their ability to mutate. But as the quote below suggests, epigenetic mediators do more than this – they also organise the conversation between the actors. Below, the authors summarise the role of chromatin modifications (which are epigenetic modifications) in epigenetic relationships:
Chromatin modifications integrate and process external signals and relay them in order to influence transcriptional regulation and the utilization of the genome. (Brookes and Shi, 2014: 249) (italics added)
The authors point out that chromatin modifications have the important role of receiving, integrating and processing external input (from then environment) and relaying it inside the cell. At the same time, chromatin modifications regulate the genome. The verbs ‘integrate’ and ‘process’ (in italics) suggest the smart nature of epigenetics, which can make sense of signals and incorporate them, to then articulate the interactions between environment and genome. As such, epigenetics are not understood as a simple relay of information between environment and genome, but as the actors responsible for receiving information from the environment, processing it and using it to articulate interactions.
The transgenerational epigenetics literature provides another interesting space to explore the role given to epigenetics mediators in epigenetic relationships. Champagne and Curley (2008) highly cited paper examines the long-term consequences of early-environmental experiences, such as the disruption of the mother-infant relationship. They discuss the effects of maternal care (operationalised as licking and grooming in rodents) on the epigenome and its consequences across generations:
Thus licking/grooming is associated with epigenetic effects in female offspring that mediate long-term changes in the expression of a gene involved in maternal behavior and as such mediates the transmission of maternal care across generations. (Champagne and Curley, 2008: 596) (italics added)
Temporality is added to the epigenetic relationship, with temporal phrases situating in time the interactions between the actors (italics in the text). This suggests that communication between environment and genome not only takes place across distinct spaces but also across different times. Authors describe epigenetics as actors which can mediate early-life environmental experiences of an offspring into a long-term gene expression change in this animal, but also into behavioural changes in later generations. Framed as mediators, epigenetics can receive signals from the environment, organise and store them over time, to then relay them at specific time points in the long-term.
Our paper challenges the view of epigenetic relationships as signals. We argue that the interactions between genome and environment should be seen through the lens of the concept of mediation. Conceptualised as mediators, epigenetics enable complex communication between the environment and genome. This is made possible because epigenetics can mutate in light of the environment and in place of the genome. Then, epigenetics organises and processes information from the environment to distribute its effects to the genome and across time. As a mediator in real life would do, epigenetics take on information and demands from both sides – it receives signals from the environment, and regulates the expression of a fixed genome – to then suggest a solution that fits both environment and genome. Authors conceptualise epigenetics as mediators to portray them as the key actors capable of integrating signals to regulate the epigenetics relationships.
Conclusion
In the last decade epigenetics has grown substantially and earned the reputation of ‘the next big thing in the life sciences’ (Ebrahim, 2012). The rise of epigenetics has been accompanied by a mix of anticipations and promises. Expectations in epigenetics are partly linked to the hope that epigenetics will provide the opportunity to explore the influence of the environment on the genome and more generally on the development of diseases. The way the environment is framed in epigenetics is an important question because of its implications for society. For example, if cigarette smoking is conceptualised as an environmental exposure, could that mean that women of reproductive age are banned from smoking? The notion of environment in epigenetics raises questions in relation to individuals’ responsibility for their own health. Some epigenetics researchers are interested in how individuals can ‘change’ their genes through epigenetic changes induced by individual behaviours such as diet (Spector, 2012). This is particularly relevant to contemporary public health policies based on behaviour change programmes, which encourage individuals to change their lifestyles.
Our review and synthesis was carried out to explore the ways in which research being discussed under the label of epigenetics understands the environment. By examining the ways in which authors write about epigenetics, we also consider how they produce epigenetic knowledge by writing it. In our primary synthesis, we first explored how authors define the notion of environment. We showed the fluid nature of environment in epigenetics. Some conceptualise the environment at a micro level with for example the “extracellular microenvironment” studied for its role on cancer cell development (Ahmed, 2007: 104), while others see heavy metals such as toxins outside the body (Fragou et al., 2011) or social practices such as maternal care (Weaver et al., 2004a) as environmental factors influencing the epigenome.
In our secondary synthesis, we discussed the common ways in which authors portray the interactions between environment and genes as relations. The discursive strategies discussed all form part of the epigenetic narrative of relationships. Taken together, these strategies construct epigenetics research as the study of complex relationships between environment and genes, which transcend distinct spaces and time periods. When authors portray the interactions between genes, the epigenome, and environment as relationships linking the outside (where the environment is located) with the inside (where the genes are located), they assign specific roles to each actor: the environment is framed as the active player initiating the relationship, genes are constructed as recipients, while epigenetics are constructed as mediators of the environment within the genome. As part of the epigenetic narrative of relationships, we showed that authors rely on the concept of mediation to make sense of the interactions between the epigenetic actors. This finding complicates the observation by other social science scholars that the interactions between environment and genes in epigenetics can be understood through the concept of signal. Our review is the first to discuss the uses of the concept of mediation in epigenetics literature. We argue that the epigenetics are predominantly framed as mediators. In other words, epigenetics are understood as enablers of communication between environment and genes, capable of organising, processing and redistributing signals in order to regulate the actors of the epigenetic relationships.
Supplementary Material
Acknowledgements
This work was funded by the Wellcome Trust (grant reference number: WT108574MA). The authors would like to thank David Armstrong, David Wyatt and Marion Fahey for their helpful contributions to this project.
Footnotes
The International Human Epigenome Consortium (IHEC), the NIH Roadmap Epigenomics Mapping Consortium, the BLUEPRINT project, the Genetics of DNA Methylation Consortium (GoDMC), the Italian Epigenetics Consortium (EPIGEN), etc.
Clinical epigenetics, Epigenetics, etc.
References
- Ahmed FE. Colorectal cancer epigenetics: the role of environmental factors and the search for molecular biomarkers. Journal of Environmental Science & Health Part C Environmental Carcinogenesis & Ecotoxicology Reviews. 2007;25:101–54. doi: 10.1080/10590500701399184. [DOI] [PubMed] [Google Scholar]
- Alberghina L, Chiaradonna F, Vanoni M. Systems biology and the molecular circuits of cancer. Chembiochem. 2004;5:1322–33. doi: 10.1002/cbic.200400170. [DOI] [PubMed] [Google Scholar]
- Anestopoulos I, Voulgaridou GP, Georgakilas AG, Franco R, Pappa A, Panayiotidis MI. Epigenetic therapy as a novel approach in hepatocellular carcinoma. Pharmacology & Therapeutics. 2015;145:103–19. doi: 10.1016/j.pharmthera.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Armstrong L. Epigenetics. London: Garland Science; 2014. [Google Scholar]
- Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Current opinion in pediatrics. 2009;21:243–251. doi: 10.1097/mop.0b013e32832925cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A, Wright RO, Bollati V, Tarantini L, Litonjua AA, Suh HH, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. Rapid DNA Methylation Changes after Exposure to Traffic Particles. American Journal of Respiratory and Critical Care Medicine. 2009;179:572–578. doi: 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow TM, Michels KB. Epigenetic epidemiology of cancer. Biochemical and Biophysical Research Communications. 2014;455:70–83. doi: 10.1016/j.bbrc.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Barton CA, Hacker NF, Clark SJ, O'brien PM. DNA methylation changes in ovarian cancer: implications for early diagnosis, prognosis and treatment. Gynecologic Oncology. 2008;109:129–39. doi: 10.1016/j.ygyno.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Barua S, Junaid MA. Lifestyle, pregnancy and epigenetic effects. Epigenomics. 2015;7:85–102. doi: 10.2217/epi.14.71. [DOI] [PubMed] [Google Scholar]
- Bashyam MD. Understanding cancer metastasis: an urgent need for using differential gene expression analysis. Cancer. 2002;94:1821–9. doi: 10.1002/cncr.10362. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Jones PA. A decade of exploring the cancer epigenome — biological and translational implications. Nature Reviews Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JT, Saffery R. The value of twins in epigenetic epidemiology. International Journal of Epidemiology. 2012;41:140–150. doi: 10.1093/ije/dyr179. [DOI] [PubMed] [Google Scholar]
- Bell JT, Spector TD. A twin approach to unraveling epigenetics. Trends in Genetics. 2011;27:116–125. doi: 10.1016/j.tig.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waterland RA, Styblo M, Achanzar WE, Webber MM, Waalkes MP. Molecular events associated with arsenic-induced malignant transformation of human prostatic epithelial cells: aberrant genomic DNA methylation and K-ras oncogene activation. Toxicology and Applied Pharmacology. 2005;206:288–298. doi: 10.1016/j.taap.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Berasain C, Goni S, Castillo J, Latasa MU, Prieto J, Avila MA. Impairment of pre-mRNA splicing in liver disease: mechanisms and consequences. World Journal of Gastroenterology. 2010;16:3091–102. doi: 10.3748/wjg.v16.i25.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdasco M, Esteller M. Aberrant Epigenetic Landscape in Cancer: How Cellular Identity Goes Awry. Developmental Cell. 2010;19:698–711. doi: 10.1016/j.devcel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Bertucci F, Viens P, Hingamp P, Nasser V, Houlgatte R, Birnbaum D. Breast cancer revisited using DNA array-based gene expression profiling. International Journal of Cancer. 2003;103:565–71. doi: 10.1002/ijc.10867. [DOI] [PubMed] [Google Scholar]
- Besaratinia A, Cockburn M, Tommasi S. Alterations of DNA methylome in human bladder cancer. Epigenetics: Official Journal of the DNA Methylation Society. 2013;8:1013–22. doi: 10.4161/epi.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee P, Banerjee M, Giri AK. Role of genomic instability in arsenic-induced carcinogenicity. A review. Environment International. 2013;53:29–40. doi: 10.1016/j.envint.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Boehm JS, Hahn WC. Towards systematic functional characterization of cancer genomes. Nature Reviews Genetics. 2011;12:487–98. doi: 10.1038/nrg3013. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Mansuy IM. Epigenetic inheritance of disease and disease risk. Neuropsychopharmacology. 2013;38:220–36. doi: 10.1038/npp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghol N, Suderman M, Mcardle W, Racine A, Hallett M, Pembrey M, Hertzman C, Power C, Szyf M. Associations with early-life socio-economic position in adult DNA methylation. International Journal of Epidemiology. 2011 doi: 10.1093/ije/dyr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boultwood J, Wainscoat JS. Gene silencing by DNA methylation in haematological malignancies. British Journal of Haematology. 2007;138:3–11. doi: 10.1111/j.1365-2141.2007.06604.x. [DOI] [PubMed] [Google Scholar]
- Breitling Lutz p, Yang R, Korn B, Burwinkel B, Brenner H. Tobacco-Smoking-Related Differential DNA Methylation: 27K Discovery and Replication. American Journal of Human Genetics. 2011;88:450–457. doi: 10.1016/j.ajhg.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton CV, Byun H-M, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal Tobacco Smoke Exposure Affects Global and Gene-specific DNA Methylation. American Journal of Respiratory and Critical Care Medicine. 2009;180:462–467. doi: 10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes E, Shi Y. Diverse epigenetic mechanisms of human disease. Annual Review of Genetics. 2014;48:237–68. doi: 10.1146/annurev-genet-120213-092518. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Lillycrop KA, Jackson AA. Nutrition in early life, and risk of cancer and metabolic disease: alternative endings in an epigenetic tale? British Journal of Nutrition. 2009;101:619–30. doi: 10.1017/S0007114508145883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher LM, Beck S. Future impact of integrated high-throughput methylome analyses on human health and disease. Journal of Genetics & Genomics. 2008;35:391–401. doi: 10.1016/S1673-8527(08)60057-0. [DOI] [PubMed] [Google Scholar]
- Caren H, Pollard SM, Beck S. The good, the bad and the ugly: epigenetic mechanisms in glioblastoma. Molecular Aspects of Medicine. 2013;34:849–62. doi: 10.1016/j.mam.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Fernandez J, Spector T, Bell J. Epigenetics of discordant monozygotic twins: implications for disease. Genome Medicine. 2014;6:60. doi: 10.1186/s13073-014-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. Maternal regulation of estrogen receptor alpha methylation. Current Opinion in Pharmacology. 2008;8:735–9. doi: 10.1016/j.coph.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neuroscience & Biobehavioral Reviews. 2009;33:593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Choi S-W, Friso S. Epigenetics: A New Bridge between Nutrition and Health. Advances in Nutrition: An International Review Journal. 2010;1:8–16. doi: 10.3945/an.110.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhuri S, Cui Y, Klaassen CD. Molecular targets of epigenetic regulation and effectors of environmental influences. Toxicology & Applied Pharmacology. 2010;245:378–93. doi: 10.1016/j.taap.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes B, Buysschaert I, Lambrechts D. Pharmaco-epigenomics: discovering therapeutic approaches and biomarkers for cancer therapy. Heredity. 2010;105:152–160. doi: 10.1038/hdy.2010.42. [DOI] [PubMed] [Google Scholar]
- Cohen I, Poręba E, Kamieniarz K, Schneider R. Histone Modifiers in Cancer: Friends or Foes? Genes & Cancer. 2011;2:631–647. doi: 10.1177/1947601911417176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collotta M, Bertazzi PA, Bollati V. Epigenetics and pesticides. Toxicology. 2013;307:35–41. doi: 10.1016/j.tox.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Cortessis VK, Thomas DC, Levine AJ, Breton CV, Mack TM, Siegmund KD, Haile RW, Laird PW. Environmental epigenetics: prospects for studying epigenetic mediation of exposure–response relationships. Human Genetics. 2012;131:1565–1589. doi: 10.1007/s00439-012-1189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaschke NA, Yang B, Bhusari S, Svaren JP, Jarrard DF. Epigenetic susceptibility factors for prostate cancer with aging. Prostate. 2013;73:1721–30. doi: 10.1002/pros.22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stefano L, Dyson NJ. The emerging roles for histone demethylases in the modulation of signaling pathways. Biomolecular Concepts. 2013;4:13–27. doi: 10.1515/bmc-2012-0031. [DOI] [PubMed] [Google Scholar]
- Dik S, Scheepers PT, Godderis L. Effects of environmental stressors on histone modifications and their relevance to carcinogenesis: a systematic review. Critical Reviews in Toxicology. 2012;42:491–500. doi: 10.3109/10408444.2012.684146. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Jirtle RL. Environmental epigenomics in human health and disease. Environmental and Molecular Mutagenesis. 2008;49:4–8. doi: 10.1002/em.20366. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: Linking early developmental environment to adult disease. Reproductive Toxicology. 2007;23:297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Drouin J. Minireview: Pioneer Transcription Factors in Cell Fate Specification. Molecular Endocrinology. 2014;28:989–998. doi: 10.1210/me.2014-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM, Frankel SR. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim S. Epigenetics: the next big thing. International Journal of Epidemiology. 2012;41:1–3. doi: 10.1093/ije/dys015. [DOI] [PubMed] [Google Scholar]
- Enokida H, Nakagawa M. Epigenetics in bladder cancer. International Journal of Clinical Oncology. 2008;13:298–307. doi: 10.1007/s10147-008-0811-1. [DOI] [PubMed] [Google Scholar]
- Esteller M. Dormant hypermethylated tumour suppressor genes: questions and answers. Journal of Pathology. 2005;205:172–80. doi: 10.1002/path.1707. [DOI] [PubMed] [Google Scholar]
- Falahi F, Van Kruchten M, Martinet N, Hospers GA, Rots MG. Current and upcoming approaches to exploit the reversibility of epigenetic mutations in breast cancer. Breast Cancer Research. 2014;16:412. doi: 10.1186/s13058-014-0412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlex Partner Medical Dictionary. [Accessed March 10 2016];2012 Available: http://medical-dictionary.thefreedictionary.com/molecular+biology.
- Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nature Review Genetics. 2012;13:97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- Feinberg AP. An epigenetic approach to cancer etiology. Cancer Journal. 2007;13:70–4. doi: 10.1097/PPO.0b013e31803c6e3b. [DOI] [PubMed] [Google Scholar]
- Forrester TE, Badaloo AV, Boyne MS, Osmond C, Thompson D, Green C, Taylor-Bryan C, Barnett A, Soares-Wynter S, Hanson MA, Beedle AS, et al. Prenatal Factors Contribute to the Emergence of Kwashiorkor or Marasmus in Severe Undernutrition: Evidence for the Predictive Adaptation Model. PLoS ONE. 2012;7:e35907. doi: 10.1371/journal.pone.0035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragou D, Fragou A, Kouidou S, Njau S, Kovatsi L. Epigenetic mechanisms in metal toxicity. Toxicology Mechanisms & Methods. 2011;21:343–52. doi: 10.3109/15376516.2011.557878. [DOI] [PubMed] [Google Scholar]
- Gomes MV, Pelosi GG. Epigenetic vulnerability and the environmental influence on health. Experimental Biology & Medicine. 2013;238:859–65. doi: 10.1177/1535370213490630. [DOI] [PubMed] [Google Scholar]
- Haig D. Commentary: The epidemiology of epigenetics. International Journal of Epidemiology. 2012;41:13–16. doi: 10.1093/ije/dyr183. [DOI] [PubMed] [Google Scholar]
- Heijmans BT, Kremer D, Tobi EW, Boomsma DI, Slagboom PE. Heritable rather than age-related environmental and stochastic factors dominate variation in DNA methylation of the human IGF2/H19 locus. Human Molecular Genetics. 2007;16:547–554. doi: 10.1093/hmg/ddm010. [DOI] [PubMed] [Google Scholar]
- Heijmans BT, Mill J. Commentary: The seven plagues of epigenetic epidemiology. International Journal of Epidemiology. 2012;41:74–78. doi: 10.1093/ije/dyr225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herceg Z, Vaissiere T. Epigenetic mechanisms and cancer: an interface between the environment and the genome. Epigenetics: Official Journal of the DNA Methylation Society. 2011;6:804–19. doi: 10.4161/epi.6.7.16262. [DOI] [PubMed] [Google Scholar]
- Hicks SD, Middleton FA, Miller MW. Ethanol-induced methylation of cell cycle genes in neural stem cells. Journal of Neurochemistry. 2010;114:1767–1780. doi: 10.1111/j.1471-4159.2010.06886.x. [DOI] [PubMed] [Google Scholar]
- Hojfeldt JW, Agger K, Helin K. Histone lysine demethylases as targets for anticancer therapy. Nature Reviews Drug Discovery. 2013;12:917–930. doi: 10.1038/nrd4154. [DOI] [PubMed] [Google Scholar]
- Hou L, Wang H, Sartori S, Gawron A, Lissowska J, Bollati V, Tarantini L, Zhang FF, Zatonski W, Chow W-H, Baccarelli A. Blood leukocyte DNA hypomethylation and gastric cancer risk in a high-risk Polish population. International journal of cancer. Journal international du cancer. 2010;127:1866–1874. doi: 10.1002/ijc.25190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Zhang X, Wang D, Baccarelli A. Environmental chemical exposures and human epigenetics. International Journal of Epidemiology. 2011 doi: 10.1093/ije/dyr154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonka E, Lamb MJ. Epigenetic Inheritance. In: Wright JD, editor. International Encyclopedia of the Social & Behavioral Sciences. Second Edition. Oxford: Elsevier; 2015. [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics. 2003;33(Suppl):245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jimenez-Chillaron JC, Diaz R, Martinez D, Pentinat T, Ramon-Krauel M, Ribo S, Plosch T. The role of nutrition on epigenetic modifications and their implications on health. Biochimie. 2012;94:2242–63. doi: 10.1016/j.biochi.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nature Reviews Genetics. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The Epigenomics of Cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller EF. The Century of the Gene. Cambridge: Harvard University Press; 2000. [Google Scholar]
- Keller EF. Genes, genomes and genomics. Biological Theory. 2011;6:132–140. [Google Scholar]
- Kenney M, Müller R. Of rats and women: Narratives of motherhood in environmental epigenetics. BioSocieties. 2017;12:23–46. [Google Scholar]
- Kouzarides T. Chromatin Modifications and Their Function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Labonté B, Suderman M, Maussion G, et al. Genome-wide epigenetic regulation by early-life trauma. Archives of General Psychiatry. 2012;69:722–731. doi: 10.1001/archgenpsychiatry.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landecker H. Food as exposure: Nutritional epigenetics and the new metabolism. BioSocieties. 2011;6:167–194. doi: 10.1057/biosoc.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landecker H. The social as signal in the body of chromatin. The Sociological Review Monographs. 2016;64:79–99. [Google Scholar]
- Landecker H, Panofsky A. From Social Structure to Gene Regulation, and Back: A Critical Introduction to Environmental Epigenetics for Sociology. Annual Review of Sociology. 2013;39:333–357. [Google Scholar]
- Lappé M, Landecker H. How the genome got a life span. New Genetics and Society. 2015;34:152–176. doi: 10.1080/14636778.2015.1034851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop KA, Phillips ES, Torrens C, Hanson MA, Jackson AA, Burdge GC. Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPARα promoter of the offspring. The British journal of nutrition. 2008;100:278–282. doi: 10.1017/S0007114507894438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SC, Hernandez-Vargas H, Herceg Z. Epigenetic signatures in cancer: Implications for the control of cancer in the clinic. Current Opinion in Molecular Therapeutics. 2010;12:316–24. [PubMed] [Google Scholar]
- Lock M. Comprehending the body in the era of the epigenome1. Current Anthropology. 2015;56:151–177. [Google Scholar]
- Lock M, Palsson G. Can Science Resolve the Nature/Nurture Debate? Cambridge UK: Polity; 2016. [Google Scholar]
- Lu Q, Quinn AM, Patel MP, Semus SF, Graves AP, Bandyopadhyay D, Pope AJ, Thrall SH. Perspectives on the Discovery of Small-Molecule Modulators for Epigenetic Processes. Journal of Biomolecular Screening. 2012;17:555–571. doi: 10.1177/1087057112437763. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Turecki G. DNA methylation and childhood maltreatment: From animal models to human studies. Neuroscience. 2014;264:142–156. doi: 10.1016/j.neuroscience.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai A, Altucci L. Epi-drugs to fight cancer: from chemistry to cancer treatment, the road ahead. International Journal of Biochemistry & Cell Biology. 2009;41:199–213. doi: 10.1016/j.biocel.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Mansfield B. Race and the new epigenetic biopolitics of environmental health. BioSocieties. 2012;7:352–372. [Google Scholar]
- Martin P. Comprehending the body in the era of the epigenome. Current Anthropology. 2015a;56:151–177. [Google Scholar]
- Martin TC, Bell JT, Spector TD. Twin Studies and Epigenetics. In: Wright JD, editor. International Encyclopedia of the Social & Behavioral Sciences. 2nd edition. Oxford: Elsevier; 2015b. [Google Scholar]
- Martinez-Zamudio R, Ha HC. Environmental epigenetics in metal exposure. Epigenetics: Official Journal of the DNA Methylation Society. 2011;6:820–7. doi: 10.4161/epi.6.7.16250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgowan PO, Sasaki A, D’alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature neuroscience. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcgowan PO, Sasaki A, Huang TCT, Unterberger A, Suderman M, Ernst C, Meaney MJ, Turecki G, Szyf M. Promoter-Wide Hypermethylation of the Ribosomal RNA Gene Promoter in the Suicide Brain. PLoS ONE. 2008;3:e2085. doi: 10.1371/journal.pone.0002085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcguinness D, Mcglynn LM, Johnson PC, Macintyre A, Batty GD, Burns H, Cavanagh J, Deans KA, Ford I, Mcconnachie A, Mcginty A, et al. Socio-economic status is associated with epigenetic differences in the pSoBid cohort. International Journal of Epidemiology. 2012 doi: 10.1093/ije/dyr215. [DOI] [PubMed] [Google Scholar]
- Meloni M. The social brain meets the reactive genome: Neuroscience, epigenetics and the new social biology. Frontiers in Human Neuroscience. 2014;8 doi: 10.3389/fnhum.2014.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni M. Epigenetics for the social sciences: justice, embodiment, and inheritance in the postgenomic age. New Genetics and Society. 2015;34:125–151. [Google Scholar]
- Meloni M, Testa G. Scrutinizing the epigenetics revolution. Biosocieties. 2014;9:431–456. doi: 10.1057/biosoc.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Spicer J, Champagne FA. Linking Prenatal Maternal Adversity to Developmental Outcomes in Infants: The Role of Epigenetic Pathways. Development and psychopathology. 2012;24:1361–1376. doi: 10.1017/S0954579412000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LE, Huang WY, Chung J, Hayes RB. Epidemiologic considerations to assess altered DNA methylation from environmental exposures in cancer. Annals of the New York Academy of Sciences. 2003;983:181–96. doi: 10.1111/j.1749-6632.2003.tb05973.x. [DOI] [PubMed] [Google Scholar]
- Mosby's Dental Dictionary. [Accessed March 10 2016];2008 Available: http://medical-dictionary.thefreedictionary.com/developmental+biology.
- Mosby's Medical Dictionary. [Accessed March 10 2016];2009 Available: http://medical-dictionary.thefreedictionary.com/molecular+genetics.
- Myers G. Making a discovery: Narratives of split genes. In: NASH C, editor. Narrative in cul-ture: The uses of storytelling in the sciences, philosophy, and literature. London: Routledge; 1990a. [Google Scholar]
- Myers G. Writing Biology: Texts in the Social Construction of Scientific Knowledge. Madison,WI: University of Wisconsin Press; 1990b. [Google Scholar]
- Myers G. Stories and Styles in Two Molecular Biology Review Articles. In: PARADIS CBJ, editor. Textual dynamics of the professions. Madison: University of Wisconsin Press; 1991. [Google Scholar]
- Nana-Sinkam SP, Croce CM. Non-coding RNAs in cancer initiation and progression and as novel biomarkers. Molecular Oncology. 2011;5:483–91. doi: 10.1016/j.molonc.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolosi G, Ruivenkamp G. The epigenetic turn: Some notes about the epistemological change of perspective in biosciences. Medicine, Health Care and Philosophy. 2012;15:309–319. doi: 10.1007/s11019-011-9342-z. [DOI] [PubMed] [Google Scholar]
- Niewöhner J. Epigenetics: Embedded bodies and the molecularisation of biography and milieu. BioSocieties. 2011;6:279–298. [Google Scholar]
- Niewöhner J. Epigenetics: localizing biology through co-laboration. New Genetics and Society. 2015;34:219–242. [Google Scholar]
- Pickersgill M. Epistemic modesty, ostentatiousness and the uncertainties of epigenetics: on the knowledge machinery of (social) science. The Sociological Review Monographs. 2016;64:186–202. doi: 10.1002/2059-7932.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickersgill M, Niewöhner J, Müller R, Martin P, Cunningham-Burley S. Mapping the new molecular landscape: social dimensions of epigenetics. New Genetics and Society. 2013;32:429–447. doi: 10.1080/14636778.2013.861739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsner JR, Hu H, Ettinger A, Sánchez BN, Wright RO, Cantonwine D, Lazarus A, Lamadrid-Figueroa H, Mercado-García A, Téllez-Rojo MM, Hernández-Avila M. Influence of Prenatal Lead Exposure on Genomic Methylation of Cord Blood DNA. Environmental Health Perspectives. 2009;117:1466–1471. doi: 10.1289/ehp.0800497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperi C, Vlastos F, Farmaki E, Martinet N, Papavassiliou AG. Epigenetic effects of lung cancer predisposing factors impact on clinical diagnosis and prognosis. Journal of Cellular & Molecular Medicine. 2008;12:1495–501. doi: 10.1111/j.1582-4934.2008.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistollato F, Giampieri F, Battino M. The use of plant-derived bioactive compounds to target cancer stem cells and modulate tumor microenvironment. Food & Chemical Toxicology. 2015;75:58–70. doi: 10.1016/j.fct.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Poirier LA, Vlasova TI. The prospective role of abnormal methyl metabolism in cadmium toxicity. Environmental Health Perspectives. 2002;110:793–795. doi: 10.1289/ehp.02110s5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic R, Shah MY, Licht JD. Epigenetic therapy of hematological malignancies: where are we now? Therapeutic Advances in Hematology. 2013;4:81–91. doi: 10.1177/2040620712466864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portela A, Esteller M. Epigenetic modifications and human disease. Nature Biotechnology. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- Powledge TM. Behavioral Epigenetics: How Nurture Shapes Nature. BioScience. 2011;61:588–592. [Google Scholar]
- Reynolds PA, Sigaroudinia M, Zardo G, Wilson MB, Benton GM, Miller CJ, Hong C, Fridlyand J, Costello JF, Tlsty TD. Tumor Suppressor p16INK4A Regulates Polycomb-mediated DNA Hypermethylation in Human Mammary Epithelial Cells. Journal of Biological Chemistry. 2006;281:24790–24802. doi: 10.1074/jbc.M604175200. [DOI] [PubMed] [Google Scholar]
- Ruiz-Hernandez A, Kuo C-C, Rentero-Garrido P, Tang W-Y, Redon J, Ordovas JM, Navas-Acien A, Tellez-Plaza M. Environmental chemicals and DNA methylation in adults: a systematic review of the epidemiologic evidence. Clinical Epigenetics. 2015;7:55. doi: 10.1186/s13148-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabunciyan S, Aryee MJ, Irizarry RA, Rongione M, Webster MJ, Kaufman WE, Murakami P, Lessard A, Yolken RH, Feinberg AP, Potash JB, et al. Genome-Wide DNA Methylation Scan in Major Depressive Disorder. PLoS ONE. 2012;7:e34451. doi: 10.1371/journal.pone.0034451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salnikow K, Costa M. Epigenetic mechanisms of nickel carcinogenesis. Journal of environmental pathology, toxicology and oncology : official organ of the International Society for Environmental Toxicology and Cancer. 2000;19:307–318. [PubMed] [Google Scholar]
- Schnittker J. Postgenomics: Perspectives on Biology after the Genome. Contemporary Sociology. 2016;45:497–498. [Google Scholar]
- Senut M-C, Cingolani P, Sen A, Kruger A, Shaik A, Hirsch H, Suhr ST, Ruden D. Epigenetics of early-life lead exposure and effects on brain development. Epigenomics. 2012;4:665–674. doi: 10.2217/epi.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nature Reviews Genetics. 2013;14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- Spector T. Identically Different. Why you can change your genes. London: Weidenfield and Nicolson; 2012. [Google Scholar]
- R SS, Stevens H, editors. Postgenomics: Perspectives on Biology after the Genome. Durham: Duke University Press; 2015. [Google Scholar]
- Supic G, Jagodic M, Magic Z. Epigenetics: a new link between nutrition and cancer. Nutrition & Cancer. 2013;65:781–92. doi: 10.1080/01635581.2013.805794. [DOI] [PubMed] [Google Scholar]
- Szyf M. The early-life social environment and DNA methylation. Clinical Genetics. 2012;81:341–349. doi: 10.1111/j.1399-0004.2012.01843.x. [DOI] [PubMed] [Google Scholar]
- Talikka M, Sierro N, Ivanov NV, Chaudhary N, Peck MJ, Hoeng J, Coggins CR, Peitsch MC. Genomic impact of cigarette smoke, with application to three smoking-related diseases. Critical Reviews in Toxicology. 2012;42:877–89. doi: 10.3109/10408444.2012.725244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YT, Xu XH, Yang XD, Hao J, Cao H, Zhu W, Zhang SY, Cao JP. Role of non-coding RNAs in pancreatic cancer: the bane of the microworld. World Journal of Gastroenterology. 2014;20:9405–17. doi: 10.3748/wjg.v20.i28.9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg KL, Shannon J, Thuillier P, Turker MS. In Utero Life and Epigenetic Predisposition for Disease. Advances in genetics. 2010;71:57–78. doi: 10.1016/B978-0-12-380864-6.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timp W, Feinberg AP. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nature reviews. Cancer. 2013;13:497–510. doi: 10.1038/nrc3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecki G, Ernst C, Jollant F, Labonté B, Mechawar N. The neurodevelopmental origins of suicidal behavior. Trends in Neurosciences. 2012;35:14–23. doi: 10.1016/j.tins.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Vaiserman A. Epidemiologic evidence for association between adverse environmental exposures in early life and epigenetic variation: a potential link to disease susceptibility? Clinical Epigenetics. 2015;7:96. doi: 10.1186/s13148-015-0130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaissiere T, Sawan C, Herceg Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutation Research. 2008;659:40–8. doi: 10.1016/j.mrrev.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Virani S, Colacino JA, Kim JH, Rozek LS. Cancer epigenetics: a brief review. Ilar Journal. 2012;53:359–69. doi: 10.1093/ilar.53.3-4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington CH. The epigenotype. 1942. International Journal of Epidemiology. 2012;41:10–13. doi: 10.1093/ije/dyr184. [DOI] [PubMed] [Google Scholar]
- Waggoner MR, Uller T. Epigenetic determinism in science and society. New Genetics and Society. 2015;34:177–195. doi: 10.1080/14636778.2015.1033052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland RA, Jirtle RL. Transposable Elements: Targets for Early Nutritional Effects on Epigenetic Gene Regulation. Molecular and Cellular Biology. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition. 2004;20:63–68. doi: 10.1016/j.nut.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Lin J-R, Smith CA, Jirtle RL. Post-weaning diet affects genomic imprinting at the insulin-like growth factor 2 (Igf2) locus. Human Molecular Genetics. 2006;15:705–716. doi: 10.1093/hmg/ddi484. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Travisano M, Tahiliani KG. Diet-induced hypermethylation at agouti viable yellow is not inherited transgenerationally through the female. The FASEB Journal. 2007;21:3380–3385. doi: 10.1096/fj.07-8229com. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D'alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004a;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Diorio J, Seckl JR, Szyf M, Meaney MJ. Early Environmental Regulation of Hippocampal Glucocorticoid Receptor Gene Expression: Characterization of Intracellular Mediators and Potential Genomic Target Sites. Annals of the New York Academy of Sciences. 2004b;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y-D, Tepperman K, Huang M-Y, Sartor MA, Puga A. Chromium Inhibits Transcription from Polycyclic Aromatic Hydrocarbon-inducible Promoters by Blocking the Release of Histone Deacetylase and Preventing the Binding of p300 to Chromatin. Journal of Biological Chemistry. 2004;279:4110–4119. doi: 10.1074/jbc.M310800200. [DOI] [PubMed] [Google Scholar]
- Wild CP, Scalbert A, Herceg Z. Measuring the exposome: a powerful basis for evaluating environmental exposures and cancer risk. Environmental & Molecular Mutagenesis. 2013;54:480–99. doi: 10.1002/em.21777. [DOI] [PubMed] [Google Scholar]
- Yamane K, Tateishi K, Klose RJ, Fang J, Fabrizio LA, Erdjument-Bromage H, Taylor-Papadimitriou J, Tempst P, Zhang Y. PLU-1 Is an H3K4 Demethylase Involved in Transcriptional Repression and Breast Cancer Cell Proliferation. Molecular Cell. 2007;25:801–812. doi: 10.1016/j.molcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes & Development. 2011;25:2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilinger S, Kühnel B, Klopp N, Baurecht H, Kleinschmidt A, Gieger C, Weidinger S, Lattka E, Adamski J, Peters A, Strauch K, et al. Tobacco Smoking Leads to Extensive Genome-Wide Changes in DNA Methylation. PLoS ONE. 2013;8:e63812. doi: 10.1371/journal.pone.0063812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FF, Cardarelli R, Carroll J, Zhang S, Fulda KG, Gonzalez K, Vishwanatha JK, Morabia A, Santella RM. Physical activity and global genomic DNA methylation in a cancer-free population. Epigenetics. 2011;6:293–299. doi: 10.4161/epi.6.3.14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.