Abstract
Apparent diffusion coefficient (ADC) of cerebral water, as measured by diffusion MRI, rapidly decreases in ischaemia highlighting a lesion in acute stroke patients. The MRI T2 relaxation time changes in ischaemic brain such that T2 in ADC lesions may be informative of the extent of tissue damage, potentially aiding in stratification for treatment. We have developed a novel user-unbiased method of determining the changes in T2 in ADC lesions as a function of clinical symptom duration based on voxel-wise referencing to a contralateral brain volume. The spherical reference method calculates the most probable pre-ischaemic T2 on a voxel-wise basis, making use of features of the contralateral hemisphere presumed to be largely unaffected. We studied whether T2 changes in the two main cerebral tissue types, i.e. in grey matter (GM) and white matter (WM), would differ in stroke.
Thirty-eight acute stroke patients were accrued within 9 hours of symptom onset and scanned at 3T for 3D T1-weighted, multi b-value diffusion and multi-echo spin echo MRI for tissue type segmentation, quantitative ADC and absolute T2 images, respectively. T2 changes measured by the spherical reference method were 1.94 ± 0.61, 1.50 ± 0.52 and 1.40 ± 0.54 ms/hour in the whole, GM and WM lesions, respectively. Thus, T2 time courses were comparable between GM and WM independent of brain tissue type involved. We demonstrate that T2 changes in ADC-delineated lesions can be quantified in the clinical setting in a user unbiased manner and that T2 change correlated with symptom onset time, opening the possibility of using the approach as a tool to assess severity of tissue damage in the clinical setting.
Keywords: T2 relaxation time, grey matter, white matter, diffusion MRI, acute ischaemic stroke
1. Introduction
Ischaemia resulting in catastrophic energy failure followed by anoxic depolarization, causes a dramatic drop in the apparent diffusion coefficient (ADC) of water, which enables detection of ischaemic stroke in the brain by diffusion MRI (Moseley et al., 1990; Busza et al., 1992). In acute strokes, brain parenchyma with an ADC lowered by more than 20% is commonly considered as the ischaemic core (Hossmann, 1994; Burgess and Kidwell, 2011). MRI relaxation time changes in the ischaemic core are very small in magnitude in the early moments of ischaemia (Kauppinen, 2014). However, recent evidence points to a potential value of relaxation times in stroke onset time evaluation (Siemonsen et al., 2009; Jokivarsi et al., 2010; McGarry et al., 2016). Combined diffusion and relaxometric MRI would therefore bear an impact on stroke patient stratification, keeping in mind that up to 30% of acute patients present with unknown onset (Thomalla et al., 2011). While diffusion MRI contrast is conspicuous to the naked eye in acute stroke, T2-weighted or quantitative T2 MRI signals become brighter only many hours after symptom onset which is often outside the time window for treatment with thrombolysis. The so-called diffusion/FLAIR MRI mismatch, which exploits qualitative signal intensities of diffusion and T2-weighted images, has been successfully used to stratify stroke patients within the thrombolysis time window of 4.5 hours (Thomalla et al., 2011; Thomalla et al., 2018). Similarly to T2-weighted signal, the quantitative T2 relaxation time image also shows weak contrast in early hours of stroke, thus requiring special means to measure the effects of ischaemia on quantitative T2. The mirror reference approach uses an anatomically homologous contralateral brain volume as a non-ischaemic reference to measure a difference in quantitative T2 in the ADC-defined ischaemic core (Siemonsen et al., 2009). Using the mirror reference procedure, it has been shown that quantitative T2 at 1.5T increases by ~10% by 4 hours after symptom onset in brain parenchyma, providing a means to estimate lesion age within the thrombolysis time window (Siemonsen et al., 2009). The mirror reference approach requires precise positioning of non-ischaemic reference into intact brain parenchyma (Norton et al., 2017), which is not often straightforward in aged human brain.
The brain parenchyma consists of two main tissue types, grey matter (GM) and white matter (WM), having greatly different cell and biochemical compositions, receptor distributions, electrophysiology, metabolic rates of oxygen and glucose, and haemodynamics. Because of these fundamental neurobiological differences, these two brain tissue types show inherently different T1 and T2 relaxation time values resulting in contrast in respective MR images. ADC values are quantitively comparable in GM and WM and, consequently, orientation-unbiased ADC images show little contrast between these tissue types. Whether the above-mentioned quantitative MRI parameters have different time courses in GM and WM following stroke remains to be studied. Diffusion MRI of rat brain shows a dramatic drop in ADC when cerebral blood flow (CBF) reduces by approximately 80% (Hossmann, 1994; Grohn et al., 2000). Recent studies on human stroke patients using either perfusion MRI (Bristow et al., 2005) or perfusion computerized tomography (CT) (Chen et al., 2017) in combination with diffusion MRI reported that CBF thresholds for the ischaemic core in GM is higher than in WM. In addition to different haemodynamic thresholds to ischaemia, anoxic depolarizations show different kinetics in the two tissue types. Anoxic depolarisation in ischaemic feline GM attains the lowest values in 5 – 10 minutes, whereas in WM it reaches the minimum by ~30 – 40 minutes (Kumura et al., 1999). Anoxic depolarization is associated with a shift of water from the extracellular into the intracellular space, i.e. cytotoxic oedema, which is one of key factors leading to a decrease both in ADC (Sotak, 2002) and T2 relaxation rate (Knight et al., 2015). In global ischaemia in cats, ADC in the thalamus (representing GM) decreases with a time constant that is three-fold faster than in the internal capsule (representing WM) (Pierpaoli et al., 1996) in accordance with the anoxic depolarization data (Kumura et al., 1999). In the light of these observations it would be of clinical interest to determine stroke onset time-dependent quantitative T2 changes in GM and WM to deduce the best use of this MRI indicator for brain tissue state assessment in stroke patient management.
In the present paper we present a novel method to measure T2 relaxation time change as a function of stroke onset time in mixed tissue type lesions, as well as in GM and WM separately, in the ADC-defined ischaemic core. The approach is called the spherical reference method which, with the aid of T2-weighted MR images, is based on a voxel-level search for contralateral, non-ischaemic, reference sphere voxels that are optimally matched to the parenchyma that is affected by stroke. We have used both the spherical and mirror reference approaches (Siemonsen et al., 2009) to quantify effects of acute stroke on quantitative T2 in the ADC-defined stroke lesion.
2. Methods
2.1. Human data acquisition and processing
The study received ethical approval to accrue acute stroke patients from South West Frenchay Research Ethics Committee for participants in Bristol (ref 13/SW/0256), Scotland A REC for participants in Glasgow (ref 16/SS/0223) and UK National Research Ethics Service committees (ref: 12/SC/0292 and 13/SC/0362) for participants in Oxford. Informed consent was received either from participants or their legal representative prior to enrolment to the study. Stroke patients were recruited if they presented in the emergency department with witnessed symptom onset so that MRI scans could be completed within 9 hours of onset. Details of the human acute stroke cohort is given in Table 1. The project was carried out in accordance with the Declaration of Helsinki.
Table 1.
Patient data presented for the whole cohort in the hyperacute phase (all hyperacute patients) and for the subset who also received a follow-up MRI scan. Ranges presented in the table in parentheses are in the format: median (minimum – maximum).
| Variable | All Hyperacute Data | Subset with Both Hyperacute and Follow-up Scans |
|---|---|---|
| Patients, n (% overall cohort) | 38 (100%) | 16 (42.1%) |
| Female, n (% group) | 12 (31.6%) | 5 (31.3%) |
| Scan site, n (% group) | ||
| Bristol | 19 (50.0%) | 3 (18.8%) |
| Glasgow | 15 (39.5%) | 11 (68.8%) |
| Oxford | 4 (10.5%) | 2 (12.5%) |
| Age | 68 (31 – 87) | 68 (45 – 87) |
| Received r-tPA before MRI, n(% group) | 25 (65.8%) | 12 (75.0%) |
| Median time from rtPA to MRI (h:min) | 3:17 (0:29 – 7:18) | 4:12 (0:38 – 7:18) |
| Median time from onset to MRI (h:min) | 5:38 (2:25 – 9:29) | 6:52 (3:04 – 9:29) |
| Days from onset to follow-up MRI | - | 1 (1 – 4) |
| NIHSS† on admission | 6 (1 – 28) | 8.5 (1 – 21) |
| Stroke subtype‡, n (% group) | ||
| Lacunar (LACS) | 15 (39.5%) | 6 (37.5%) |
| Partial Anterior Circulation (PACS) | 11 (28.9%) | 5 (31.3%) |
| Posterior Circulation (POCS) | 4 (10.5%) | 1 (6.3%) |
| Total Anterior Circulation (TACS) | 8 (21.1%) | 4 (25.0%) |
| Left hemisphere stroke | 15 (39.5%) | 6 (37.5%) |
| ADC lesion volume [cm3] | ||
| Hyperacute | 1.95 (0.04 – 97.51) | 3.57 (0.12 – 20.50) |
| Follow-up | - | 4.01 (0 – 44.53) |
Scores on the National Institutes of Health Stroke Scale (NIHSS) range 0 – 42, with higher scores indicating greater severity.
Strokes classified according to the Oxford Stroke Classification Scale (Bamford et al., 1991)
All MRI scans on acute stroke patients were acquired using 3T scanners: Philips Achieva (Bristol); Siemens Magnetom Prisma (Glasgow); Siemens Magnetom Verio (Oxford). At each of the three sites, a 32-channel head-coil was used. The details of the T1, diffusion, and T2 pulse sequences and acquisition parameters are given in Table 2.
Table 2.
MRI sequence details and acquisition parameters.
| Site | T2 Sequence | TR [ms] | TE [ms] | Resolution [mm3] | ||
| Bristol | GRASE | 3000 | 20, 40, 60, 80, 100 | 0.6×0.6×2.3 | ||
| Glasgow | TSE | 12500 | 9.5, 66, 123 | 1.7×1.7×2.0 | ||
| Oxford | FSE | 12000 | 7.7, 77, 177 | 1.8×1.7×2.0 | ||
| Site | T1 Sequence | TR [ms] | TE [ms] | Resolution [mm3] | TI [ms] | Flip Angle |
| Bristol | 3D FFE | 6.84 | 3.18 | 1.0×1.0×1.1 | N/A | 8° |
| Glasgow | MP-RAGE | 2200 | 2.28 | 0.9×0.9×0.9 | 900 | 9° |
| Oxford | MP-RAGE | 1800 | 4.55 | 1.5×1.5×1.0 | 900 | 8° |
| Site | Diffusion Sequence | TR [ms] | TE [ms] | Resolution [mm3] | b-value [s/mm2] (Multiplicity) | Independent Gradient Directions |
| Bristol | SE-EPI | 3009 | 60.5 | 1.2×1.2×4.4 | 0 (1), 1000 (3) | 3 |
| Glasgow | SE-EPI | 8000 | 90 | 0.9×0.9×2.0 | 0 (3), 1000 (20) | 20 |
| Oxford | SE-EPI | 5300 | 91 | 1.8×1.8×5.0 | 0 (1), 1000 (1) | 3 |
Quantitative T2 maps were obtained by fitting a mono-exponential decay on a voxel-wise basis. All T2-weighted images of the echo-series were summed to produce a sum-over-echoes image, which was subject to bias-field correction (Zhang et al., 2001) using FSL FAST (FMRIB, Oxford, UK).
For diffusion MRI data using three orthogonal diffusion-sensitising gradients at a common b-value, orientation-independent apparent diffusion coefficient (ADC) maps were obtained from
where S1,2,3 are the signal intensities at the three orthogonal directions, S and S0 are the signal intensities with (b = 1000 s/mm2) and without (b = 0) diffusion weighting respectively and b is the b-value.
Diffusion-weighted data with twenty independent diffusion-gradient directions (b=1000 mm2/s) and three b=0 images were processed using FSL DTIFIT from which the resulting mean-diffusivity maps provided the ADC values.
2.2. Image registration
Firstly, brain-extracted ADC images were non-linearly registered to the T2-weighted image space using FSL FNIRT via the diffusion S0 image and the T2 sum-over-echoes image. If T1-weighted images were available, the T2-weighted image space was then linearly (six degrees of freedom) registered to the T1-weighted image space and then the latter space was linearly registered to the MNI frame at 1 mm3 isotropic resolution, using FSL FLIRT (Jenkinson and Smith, 2001). If T1-weighted images were not available, instead, the T2-weighted image space was linearly registered directly to the MNI frame at 1 mm3 isotropic resolution. All relevant images were then linearly registered to the MNI frame using the registration maps (or combinations of maps) generated by the previous stages.
2.3. Lesion identification and mask creation
A reference tissue mask was first created by the application of thresholds on T2 and ADC to reduce the contribution from CSF. These thresholds were typically 30 ms < T2 < 200 ms and 0.1 µm2 ms-1 < ADC < 1.5 µm2 ms-1, respectively. A lesion tissue mask was then created in two stages. First, the thresholds (which could vary in individual cases) 30 ms < T2 < 200 ms, 0.2 – 0.4 µm2 ms-1 < ADC < 0.55 – 0.60 µm2 ms-1 were applied and, secondly, the additional criterion that the ADC should be less than one half-width half-maximum from the median ADC of the reference tissue was applied. The lesion mask was then further refined by removing all but the largest contiguous cluster (or clusters, in cases with more than one lesion). Subsequently, the lesion mask was then removed from the reference tissue mask to create a non-lesion tissue mask.
In order to examine GM and WM tissue only, further masks were created. GM and WM masks were obtained using FSL FAST with the T2-weighted, sum-over-echoes image as input, and specifying four tissue-types to be segmented. If available, T1-weighted images were used to generate a subcortical-structures mask via FSL FIRST. This mask was subtracted from the GM and WM masks to produce purely cortical GM and WM masks. These resulting masks were logically combined with the lesion and non-lesion tissue masks when investigating the tissue types separately. All masks were inspected visually. Note that when total lesions (‘all tissue’) were investigated, these masks were not employed.
2.4. Algorithm for T2 change due to ischaemia: Spherical Reference method
To calculate the change in T2 due to ischaemia for each voxel in the lesion, it is necessary to provide reference, T2R, values to which each voxel’s ischaemic T2 can be compared. That is, an estimate is needed for the pre-ischaemic T2 within each voxel of the lesion. Reference T2 values have previously been obtained (Siemonsen et al., 2009; Jokivarsi et al., 2010) from single contralateral voxels in the unaffected hemisphere, the so-called mirror reference method. However, such a method is prone to error due to the imperfect symmetry and inherent heterogeneity of the human brain, particularly in older patients and patients with cerebrovascular disease where T2 hyperintense WM lesions are common. To circumvent this issue, pre-ischaemic (reference) T2R values are calculated (via T2-weighted MR images) from a spherical volume of a given radius, centred on the contralateral voxel in the unaffected hemisphere.
To achieve this, the algorithm adopts the premise that, in the hyperacute phase of ischaemia, the T2-weighted images are relatively insensitive to ischaemia and therefore the intensity values within each voxel of the lesion are sufficiently close to their pre-ischaemic values to be taken as estimates for their pre-ischaemic values (Welch et al., 1995; Moseley et al., 1990; McGarry et al., 2016). With this premise, the intensity IL of a lesion voxel can be used to condition the reference T2 for that voxel.
Despite the relative insensitivity to ischaemia of the T2-weighted intensities, there may still be a dependency between the intensities and T2 values in normal brain tissue within any given region. Therefore, it is possible to obtain an estimate of pre-ischaemic T2 by using the intensity estimate of the pre-ischaemic intensity (i.e., the ischaemic intensity value IL) to calculate a type of conditional expectation of the T2 values within the reference sphere of a given lesion voxel. That is, an estimate of the pre-ischaemic T2 is obtained from the mean T2 of normal brain tissue within a reference sphere, conditioned by the intensity value IL of the ischaemic lesion voxel.
To effect the conditioning, we employ a penalty function FL(I) which weights the sum over the T2 values associated with voxels, indexed by ρ, in the reference sphere SL. Therefore, let the T2R be given by
where NSL is the number of voxels in the reference sphere SL, and where the penalty function is normalised by This may be equivalently expressed as
where the integrals are over the values contained in the reference sphere, P(I) is the probability density for the intensities, P(T2|I) is the conditional probability density for T2 given the intensity I, and 〈T2|I〉 is the mean T2 conditioned on I.
In the limit that FL(I) is constant, is the unweighted mean T2 of all the voxels in the reference sphere. Conversely, in the limit that FL(I) becomes a delta-function positioned at I = IL, then However, in the present context, the use of a delta-function is impractical. Instead, a penalty function of finite width is employed, such that
where AL is a normalisation constant which depends on the reference sphere SL associated with each lesion voxel. With this penalty function, T2R can be regarded as the mean T2 within the reference sphere, conditioned on a Gaussian neighbourhood of IL.
Thus, for a given lesion voxel with intensity IL and T2-value , from its reference sphere (centred on the contralateral voxel) we can obtain a reference T2-value . We can therefore compute a measure of the estimated change in T2 due to ischaemia as a difference or a ratio for each lesion voxel, and use the median of these changes as an overall measure of the change in the ischaemic lesion. This method can also be used to estimate changes in other MRI parameters, such as the ADC value, provided that the distributions of this parameter and the intensity values are not statistically independent.
In our implementation of this algorithm, the T2-weighted intensities were obtained from the sum-over-echoes image after bias-field correction. The reference sphere radius and penalty function width, σ, were obtained by optimisation on each patient data set by using the unaffected tissue in the hemisphere of the lesion (via the non-lesion tissue mask) to minimise the function f(β) = MAD(ΔT2 + β|med(ΔT2)|, with β = 5, where ΔT2 is the calculated change in T2, med(ΔT2) is the median, and MAD(ΔT2) is the median absolute deviation. Median values for the reference sphere radii and penalty function width are provided in the Supplementary Material, Tables S1 and S2.
A work-flow for image post-processing, the creation of various masks and use of these masks and images as inputs to the spherical reference algorithm, is shown in Figure 1.
Figure 1.
Flowchart showing the overall process of obtaining med(ΔT2) for the lesion, and a calculated ΔT2 image via the spherical reference method.
The illustrated process begins with the four MNI-registered, brain-extracted, input images: ADC, T2-weighted (echo-summed), quantitative T2, and T1-weighted. Stage 1) Lesion Identification. ADC and T2 images are used to identify the ischaemic lesion and create three masks: the lesion mask; the non-lesion mask (normal tissue without CSF); the non-lesion, lesion-hemisphere mask (normal tissue in the hemisphere of the lesion). Stage 2) Segmentation. GM and WM segmentations are produced from the T2-weighted image, and a subcortical structure segmentation is produced from the T1-weighted image. Stage 3) Mask Combination. If GM and WM tissue is to be studied separately, the subcortical segmentation masks are logically removed from the GM and WM segmentation masks, and the resulting masks are logically combined with the three masks of stage (1) to produced cortical GM and WM versions (see centre grey square of the flowchart). Stage 4) Penalty Function Optimisation. The spherical reference algorithm (inputs are T2 and T2-weighted images) is executed many times in order optimise the penalty function radius and width. This is achieved by minimising MAD(ΔT2) + 5|med(ΔT2)|, where ΔT2 is calculated between the voxels of the non-lesion, lesion-hemisphere mask and the contralateral hemisphere voxels of the non-lesion mask. Stage 5) Spherical Reference Algorithm. Using the optimised penalty function radius and width from stage (4), and via the T2 and T2-weighted images, med(ΔT2) is calculated for the lesion, using the lesion and non-lesion masks (or their segmented versions). A ΔT2 image (shown in the top-right of the figure) can be produced by replacing the lesion mask with a lesion-hemisphere mask (not shown in the figure).
2.5. Mirror Reference method
We used the method essentially as proposed by Siemonsen et al. (Siemonsen et al., 2009) to determine a reference volume from the contralateral, non-ischaemic hemisphere, but without the need for manual adjustments. Since all image processing was performed in the MNI frame, the midline of the brain was consistently defined. Therefore, the lesion mask (see section above) could simply be reflected about the midline and then logically combined with the non-lesion tissue mask to ensure that this contralateral (mirror reference) mask also contains valid tissue. To investigate GM and WM separately, the same operations were performed on these masks after logically combining with the GM and WM segmentation masks (see section 2.3). T2 statistics could then be easily compared between the lesion and the contralateral mask regions.
2.6. Weighted regression analysis and statistics
Weighted, least-squares, linear regressions were used to analyse the results obtained from the spherical and mirror reference methods, and the lesion ADC (median) values. The weights used for the regressions were the inverse-square errors of the medians (or combinations or medians). The errors for the medians were determined using the method of McKean and Schrader (McKean and Schrader, 1984; Price and Bonett, 2001) which derives the errors from the 95% confidence intervals of the distributions.
The order of calculations for quantities of interest is slightly different when comparing the spherical and mirror reference methods. In the former method, differences or ratios are formed for each lesion voxel, producing a distribution from which measures can be obtained, namely, median values, MADs, and errors in the medians. We note here that the errors in the medians tend to be close to where N is the number of voxels in the lesion, which is the relationship expected when the distribution is Gaussian. In contrast, in the mirror reference method, quantities of interest are first calculated from the two regions; the lesion and contralateral reference. The medians are then combined as a difference or ratio. MADs and errors in medians, however, are propagated (in the usual manner for independent measures of error) for the difference or ratio of medians from the two regions. If the same order of calculations was followed for the spherical reference method, a second set of optimised penalty function parameters would have been required for this alternative procedure.
Using a bootstrap method, it was found that of the order of at least 20 voxels were required to determine reasonably accurate estimates for the errors. Therefore, prior to performing the weighted regression, data were rejected if their region voxel count (whether connected or not) was less than 20 (which corresponds to a volume of 20 mm3). For the mirror reference method, this test was applied to both the lesion and contralateral reference region.
All weighted regressions were performed within MATLAB (Release 2016b, The MathWorks, Inc., Natick, MA, United States) using fitlm, which returns all regression parameters, confidence intervals, and significance tests. In order to compare regressions between GM and WM, the two data sets were concatenated and weighted regressions were performed with four parameters {a, b, c, d} such that the dependent variable y was fitted to y = a + bT + γ(c + dT), where T is the onset time, and γ = 1 for the GM data, and γ = 0 for the WM data. The fitted parameters c and d are the differences between the GM and WM intercepts and gradients, respectively, and thus can be subjected to a significance test for the null hypothesis, which fitlm provides.
3. Results
ADC and quantitative T2 images from four stroke patients with lesions involving different vascular territories are shown in Figure 2. Stroke lesions present as hypointense volumes in ADC images compared to the surrounding tissue. The stroke lesions’ boundaries are shown on the ADC images [Figure 2(a), (c), (e), and (g)] from which they were derived. Superimposed on the T2 images [Figure 2(b), (d), (f), and (h)] are the lesions segmented into GM and WM tissue types.
Figure 2.
Examples of ADC images (a, c, e, g) with lesions demarcated, and quantitative T2 images (b, d, f, h) with lesions segmented into WM (blue) and GM (red).
Panels (a, b): NIHSS at admission, 18; patient age, 45; stroke classification, TACS; onset time, 415 min. Panels (c, d): NIHSS at admission, 10; patient age, 57; stroke classification, LACS; onset time, 569 min. Panels (e, f): NIHSS at admission, 3; patient age, 86; stroke classification, POCS; onset time, 408 min. Panels (g, h): NIHSS at admission, 5; patient age, 80; stroke classification, POCS; onset time, 338 min.
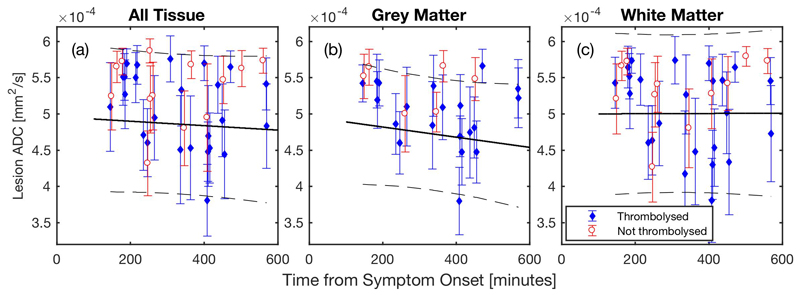
Low ADC is a well-established MRI biomarker for ischaemia in the human brain (Welch et al., 1995; Hjort et al., 2005; Burgess and Kidwell, 2011). The median ADC in the total lesions [Figure 3(a)], segmented GM [Figure 3(b)], and WM [Figure 3(c)] are shown as a function of onset time (see Table 3 for regression data). The ADC values are within the range reported for an ischaemic core in all volumes at the time of MR scans in the hyperacute phase of stroke, and the low ADC was the primary MRI criterion for assigning the lesion to ischaemic core (both in thrombolysed and not thrombolysed patients, Table S3). However, a low diffusivity lesion may be transient, and therefore it may not be a genuine core (Kranz and Eastwood, 2009). To address this issue, a subcohort of patients consented for a follow-up MRI 1 – 4 days after symptom onset. ADC lesion volumes for both hyperacute and follow-up MRI are shown in Figure 4. It is evident that on average the ADC lesions expanded from 1st to 2nd scan (Table 1). However, a tendency towards volume decline was measured in 5 cases (31% of patients with follow-up scans), 4 of which were thrombolysed. Based on these data from the follow-up MRI, the ADC lesion in the hyperacute phase was regarded to represent the ischaemic core in 69% of all patients.
Figure 3.
Lesion ADC values. The solid black line is the linear regression with observational 95% confidence intervals displayed by dashed black lines. The displayed error bars are the median absolute deviations. Regression results are given in Table 3.
Table 3.
Weighted linear regression results for the ADC within the lesion [see Figure 3(a) – (c)]. r2 is the square of the correlation coefficient, RMSE is the root-mean-square error of the residuals, p is the two-tailed p-value, n is the number of data points. Errors in the intercept and gradient are the 95% confidence intervals.
| Tissue | Intercept [mm2/s] | Gradient [mm2/s/h] | r2 | RMSE | p | n |
|---|---|---|---|---|---|---|
| All | 4.96×10-4 ± 0.44×10-4 | -1.8×10-6 ± 7.7×10-6 | 0.0065 | 4.69×10-5 | 0.629 | 38 |
| GM | 4.96×10-4 ± 0.54×10-4 | -4.2×10-6 ± 9.1×10-6 | 0.0369 | 3.68×10-5 | 0.347 | 26 |
| WM | 5.00×10-4 ± 0.46×10-4 | 0.1×10-6 ± 8.3×10-6 | 2.1×10-5 | 5.27×10-5 | 0.979 | 35 |
Figure 4.
ADC lesion volumes (in mm3) for the subset of patients with both a hyperacute scan (scan 1) and a follow-up scan (scan 2).
Three quantities for quantitative T2 were examined for the total lesion (All Tissue in Figure 5), GM, and WM using the spherical reference method: med(ΔT2) [Figure 5(a) – (c)]; med(rT2) [Figure 5(d) – (f)]; and med(rI) [Figure 5(g) – (i)]. It can be seen that both med(ΔT2) and med(rT2) showed an increasing trend as a function of onset time, whereas the slope of med(rI) was not significantly different to zero. Table 4 summarizes the quantified parameters for the three MRI measures in the total lesion, GM, and WM.
Figure 5.
Spherical Reference method regressions for All Tissue, GM and WM lesions.
The solid black line is the linear regression with observational 95% confidence intervals displayed by dashed black lines. The ‘error’ bars displayed in the plots are the median absolute deviations (MADs) and represent the spread of values within the lesion or, alternatively, can be regarded as representing the tissue-state heterogeneity of the lesion. Regression results are given in Table 4.
Table 4.
Weighted linear regression results for spherical reference [Figure 5(a) – (i)] and mirror reference [Figure 6(a) – (i)] approaches. The data are displayed in the same order as the Figure panels. *Units for median ∆T2 (spherical reference) and the difference of median T2 are ms. Ratios are dimensionless. Units for the gradients are quoted in the appropriate units per hour. r2 is the square of the correlation coefficient, RMSE is the root-mean-square error of the residuals, p is the two-tailed p-value (testing the gradient against the null hypothesis), pGM-WM is the two-tailed p-value testing the hypothesis that the gradients for GM and WM are identical), n is the number of data points. Errors in the intercept and gradient are the 95% confidence intervals.
| Method | Measure | Tissue | Intercept* | Gradient* | r2 | RMSE | p | pGM-WM | n |
|---|---|---|---|---|---|---|---|---|---|
| Spherical Reference | Median ∆T2 |
All | -4.8 ± 3.3 | 1.94 ± 0.61 | 0.534 | 2.96 | 1.91×10-7 | 38 | |
| GM | -2.8 ± 2.6 | 1.50 ± 0.52 | 0.583 | 1.68 | 3.62×10-6 | 0.810 | 27 | ||
| WM | -1.2 ± 2.8 | 1.40 ± 0.54 | 0.460 | 2.21 | 5.71×10-6 | 35 | |||
| Median T2 ratio |
All | 0.955 ± 0.032 | 0.0202 ± 0.0058 | 0.583 | 0.0296 | 2.43×10-8 | 38 | ||
| GM | 0.992 ± 0.024 | 0.0112 ± 0.0045 | 0.514 | 0.0165 | 2.60×10-5 | 0.438 | 27 | ||
| WM | 1.001 ± 0.029 | 0.0142 ± 0.0056 | 0.445 | 0.0241 | 1.07×10-5 | 35 | |||
| Median intensity ratio |
All | 0.9991 ± 0.0011 | 2.2×10-4 ± 2.2×10-4 | 0.103 | 8.08×10-4 | 0.049 | 38 | ||
| GM | 1.0008 ± 0.0007 | -9.2×10-5 ± 9.4×10-5 | 0.139 | 3.16×10-4 | 0.055 | 0.0689 | 27 | ||
| WM | 0.9999 ± 0.0005 | 0.5×10-4 ± 1.2×10-4 | 0.023 | 5.32×10-4 | 0.383 | 35 | |||
| Mirror Reference | Difference of medianT2 |
All | -3.4 ± 4.2 | 2.30 ± 0.83 | 0.465 | 3.73 | 2.42×10-6 | 38 | |
| GM | -10.1 ± 6.1 | 3.2 ± 1.1 | 0.592 | 4.21 | 4.30×10-6 | 0.0311 | 26 | ||
| WM | -0.6 ± 2.9 | 1.60 ± 0.57 | 0.498 | 2.36 | 2.18×10-6 | 35 | |||
| Ratio of median T2 |
All | 0.986 ± 0.041 | 0.0214 ± 0.0079 | 0.453 | 0.0403 | 3.65×10-6 | 38 | ||
| GM | 0.917 ± 0.073 | 0.028 ± 0.013 | 0.449 | 0.0531 | 1.80×10-4 | 0.102 | 26 | ||
| WM | 1.013 ± 0.033 | 0.0158 ± 0.0062 | 0.447 | 0.0284 | 1.13×10-5 | 35 | |||
| Ratio of median intensities |
All | 0.964 ± 0.037 | 0.0125 ± 0.0076 | 0.238 | 0.0395 | 1.89×10-3 | 38 | ||
| GM | 0.959 ± 0.031 | 0.0104 ± 0.0061 | 0.344 | 0.0216 | 1.65×10-3 | 0.612 | 26 | ||
| WM | 0.976 ± 0.022 | 0.0080 ± 0.0046 | 0.279 | 0.0196 | 1.12×10-3 | 35 | |||
For the two measures of quantitative T2 change, GM and WM values correlated well with onset time, with gradients 1.4 – 2.0 ms h-1 for med(ΔT2), and 0.011 – 0.020 h-1 for med(rT2). Trends and statistics were similar for both of these measures. T2 changes as a function of stroke onset time (median ± 95% confidence bounds) measured by the spherical reference method were 1.94 ± 0.61, 1.50 ± 0.52 (p = 0.38 vs. the whole lesion) and 1.40 ± 0.54 (p = 0.18 vs the whole lesion) ms/hour in the whole, GM and WM lesions, respectively. For med(rI), the gradient was not statistically different to zero. For all quantities investigated with the spherical reference method, no significant differences in intercept or gradient were found between WM and GM.
Figure 6 shows the T2 data obtained by the mirror reference method as follows: Figure 6(a) – (c), (the difference in median T2); Figure 6(d) – (f), (the ratio of median T2), and Figure 6(g) – (i), (the ratio of median T2-weighted intensities). Positive slopes were evident for all volumes studied both for and A tendency to a positive slope can be seen also for Quantitative indices for fitted regression lines are given in Table 4.
Figure 6.
Mirror Reference method regressions for All Tissue, GM and WM lesions.
The solid black line is the linear regression with observational 95% confidence intervals displayed by dashed black lines. The ‘error’ bars depicted in the plots are the propagated MADs (see Methods section) from the lesion and contralateral regions. Regression results are given in Table 4.
For the two measures of quantitative T2 change that were used in the mirror reference method, GM and WM data correlated well with onset time, with gradients 1.6 – 3.2 ms h-1 for the difference of medians, and 0.016 – 0.028 h-1 for the ratio of medians. Trends and statistics were similar for both of these measures. For the intensity ratio, the gradient was statistically significant with values 0.008 – 0.013 h-1. For all quantities investigated with the mirror reference method, no significant differences in intercept or gradient were found between WM and GM.
For analogous measures of quantitative T2 change between the spherical and mirror reference methods, the general trends were similar but the 95% confidence intervals, p-values, and RMSE tended to be smaller for the spherical reference method. For the intensity ratios, however, while the mirror reference method produced significant trends, the spherical reference method did not. The 95% confidence intervals and RMSE were also much smaller in the latter method.
The patients recruited to the study were treated according to the NHS standard-of-care protocols, thus recombinant plasminogen activator was administered to eligible patients often prior to MRI. Linear regression characteristics for quantitative T2 data are also presented separately for thrombolysed and not thrombolysed patients (Table S4). It is evident that while ΔT2 as determined by both methods showed highly significant correlation with onset time in thrombolysed patients, it was not different from zero in not thrombolysed cases (Table S4). Thrombolysis is not given to patients with mild and small strokes and we believe that non-significant T2 change within the time frame up to 9 hours reflected mild disease states.
4. Discussion
We have developed a robust and user-unbiased procedure to quantify effects of stroke on T2 relaxation time in the ADC-defined ischaemic core and have applied it to MRI data from a cohort of acute ischaemic stroke patients. The quantitative T2 changes measured by the spherical reference method, as a function of symptom onset time, agree well with those determined by the recently-introduced mirror reference method, which was the first method to objectively measure the effect of ischaemia on T2 relaxation time both in preclinical (Jokivarsi et al., 2010; McGarry et al., 2016) and clinical (Siemonsen et al., 2009) data sets. Our results show that increase in T2 relaxation time in quantitative terms is similar both in GM and WM to ischaemia with symptom onset time, an observation that may have potential value in interpreting MRI scans of stroke patients.
While the reduction of ADC due to ischaemia has a large dynamic range, making it conspicuous by the bare eye, effects of ischaemia on T2 are small, requiring special referencing to non-ischaemic brain parenchyma to become detectable. The mirror reference method, wherein a homologous contralateral brain volume is chosen, utilises anatomical criteria for selection of a non-ischaemic reference region to determine the difference of average T2s. Using the mirror reference in rodent models, it was reported that T2 initially shortens both in the ischaemic core and parenchyma surrounding the core, the so-called ischaemia expansion tissue, followed by a time-dependent prolongation (Jokivarsi et al., 2010). The temporal behaviour of T2 during the first hours has been attributed to cytotoxic edema (linked to anoxic depolarisation) followed by disintegration of intracellular macromolecules by proteases and lipases; the so-called cytotoxic edema dissociation model (Knight et al., 2015). The ΔT2 data as a function of symptom onset obtained by both the spherical and mirror reference methods here indicate that ΔT2 may be negative also in human brain in the early hours of stroke. Interestingly, Siemonsen and coworkers (Siemonsen et al., 2009) reported only positive ΔT2 by the mirror reference approach in acute stroke patients scanned at 1.5T. Reasons for the discrepancy of ΔT2 data between the two studies remains unknown to us,, however, that the mirror reference method is critically dependent on accurate delineation of the reference volume (Norton et al., 2017), which may not be always obtainable in the human brain, as well as on the absence of any pathology in the reference that may affect T2. The latter factor becomes a serious concern in stroke patients, who often are aged with neurological co-morbidities that may influence MRI parameters.
The spherical reference method was designed to minimise the effects of potential confounds caused by brain asymmetry and tissue pathology in the reference volume, and also to make the reference selection user-unbiased. Reference volume delineation is based on T2-weighted signal intensity in the voxels of the ADC-defined lesion by seeking matching voxels in the contralateral hemisphere. Experimentally, T2-weighted signal shows no time-dependency in the ischaemic core of rat stroke for approximately the first 5 hours (Knight et al., 2015). In stroke patients, T2-weighted hyperintensity is commonly present only hours or days after symptom onset. Therefore, it is reasonable to use the T2-weighted signal in the ADC lesion during the first hours of stroke as a starting point in the search of a non-ischaemic reference.
In the spherical reference method, there are two parameters associated with the penalty function that are optimised for each patient data set: the radius of the reference sphere and the penalty function width (Tables S1 and S2 for optimised parameter values). The optimisation procedure assumes that if the spherical reference method is used to calculate med(ΔT2) between two hemispheres of normal brain tissue the result should be approximately zero. However, there are at least two reasons why this assumption is not straightforward, and one or more of these could be the cause of the large range of optimised parameter values (Table S1). Firstly, normal tissue needs to be properly identified. In the current method, this is achieved by creating a non-lesion mask by removing the lesion mask from the brain mask (with CSF areas removed by applying an upper T2 limit). This simple method has obvious weaknesses: for example, in assuming that all the ischaemic lesions have been identified, or in assuming that the region affected by ischaemia is entirely within the ADC-defined region, or by assuming that there are no other pathologies present. In patients with white matter T2 hyperintensities there is some suggestion that their presence in images might affect the optimised parameters (Table S2). Secondly, presumably due to imperfect bias-field correction (but perhaps also other issues or tissue characteristics), a zero-crossing for med(ΔT2) (as a function of reference sphere radius and penalty function width) is not always observed. For this reason, and also in the case where more than one zero-crossing is observed, the function MAD(ΔT2) + 5|med(ΔT2)| is minimised instead. A further point of concern with the current implementation of the optimisation is its lengthy execution time which, in addition to the other stages of the spherical reference method, would render the method of limited value in a clinical setting. Nonetheless, there is hitherto unexplored scope for improved efficiency, for example in reducing the number of normal-brain voxels required, or in employing smarter optimal-parameter search algorithms.
ΔT2 data as a function of symptom onset do not obviously differ when analysed by the two methods (Table 4). However, the spherical reference method shows smaller variation (95% confidence intervals on fitting parameters and regression lines) and RMSEs that are, throughout, substantially lower than by the mirror reference approach, indicating that the former is more robust in selecting non-ischaemic tissue that is similar to that affected by stroke. Median absolute deviations (displayed as ‘error’ bars in Figures 5 and 6) also tend to be smaller in the spherical reference method, as do the errors in the median values (used for the weighted regressions), which also imply that the voxel-selectivity at the centre of this method improves precision while maintaining accuracy.
The order of (final) calculations for quantities of interest is different between the mirror and spherical reference methods. In the mirror reference method, median values are obtained from the lesion and mirror regions before combining these two values into a ratio or difference. The spherical reference method, however, is designed to place more importance on calculations at a voxel-wise level. In doing so, images of T2 change (such as the ΔT2 image in Figure 1) or histograms of T2 change in lesions or surrounding tissue (not shown) can be produced and analysed in terms of magnitude and spatial extent with regard to the ADC-defined lesion (or other images). This requires calculating the ratio or difference for each voxel in the lesion (or entire ipsilateral hemisphere) before any summarising median value is obtained. Thus, the order of final calculations is different for the proposed spherical reference method (ratio or difference, then median) to the mirror reference method (median values, then ratio or difference). It should be noted, nonetheless, that both methods can, in principle, be modified so that the final calculations have the same order. The mirror reference method can be changed so that each lesion voxel is combined with its contralateral voxel (assuming it exists) before taking the median of the combined values. The spherical reference method can be altered such that medians can be calculated from the lesion region and from the calculated reference values before combining them. The disadvantage of the modified mirror reference method is that it becomes more sensitive to mismatches in tissue types between ipsilateral and contralateral voxels. The disadvantage of the modified spherical reference method is that the ability to produce images or histograms of T2 change is lost (note that it is likely that the optimised penalty function parameters will be different in the modified method).
Comparing the measures of T2 change (Table 4) between GM and WM in the mirror reference method, it is observed that there is more variability in the figures for GM than for WM. Unambiguously establishing which voxels are those primarily containing GM tissue can be problematic, particularly when analysing images which have undergone one or more spatial (registration) transformations. This, in addition to the known variability associated with the exact placement of the contralateral reference region, could be the main reasons for the larger uncertainty in the GM results. In this regard, it is interesting that the results of the spherical reference method do not appear to exhibit this tendency. This could be attributable to the reduced reliance of this method on the contralateral voxel positions and its voxel-matching feature. Nonetheless, both methods could potentially be improved by incorporating the partial-volume information obtained from the segmentation procedure (FSL FAST).
Our data show that time courses of ΔT2 in GM and WM are similar as a function of stroke symptom onset. At first glance, this is unexpected in the light of animal studies which have demonstrated different time dependencies in anoxic depolarisations (Kumura et al., 1999) and ADC (Pierpaoli et al., 1996) in the two brain tissue types. It should be realised, however, that there are several fundamental experimental differences between our clinical data and those from animal studies. Firstly, in the current study the earliest time points for MRI were obtained around 140 minutes after symptom onset, thus, no very early MRI data of stroke were available to us. Secondly, CBF thresholds for neurological symptoms and ischaemic energy failure (which triggers the drop in ADC) are different (Hossmann, 1994). Preclinical studies have shown that spontaneous electrical activity becomes disrupted at CBF levels that are 50 – 60% below the physiological level (Hossmann, 1994), whereas the ADC drop has a flow threshold that is approximately 80% below the normal flow (Busza et al., 1992). These CBF thresholds are time-dependent so that the ADC may decrease at a higher CBF level over a period of time than if the occlusion is instantaneous. Thus, the preclinical studies have reported electrophysiological and ADC data as a function of ischaemia, whereas in our clinical cohort the exact onset of ischaemia remains unknown, notwithstanding the ostensible symptom onset time.
Limitations
Prior to drawing conclusions regarding potential clinical implications of the current methodology and data in stroke medicine, several limitations should be considered. Firstly, the sample size is modest, comprising of a heterogeneous distribution of involved vascular territories. It is impossible to conclude, from the current data set, whether ΔT2 as a function of onset time is quantitatively uniform in different vascular territories. Secondly, the majority of patients in the current cohort (66%) were thrombolysed prior to MRI. Recanalization may or may not normalise ADC (Li et al., 1999) or T2 (Kettunen et al., 2002) depending on the duration of ischaemia. Preclinical data demonstrate that if recanalization is successful, ADC recovers and T2 overshoots transiently (Kettunen et al., 2002), but if ADC remains low, T2 increases with time producing a prolonged T2 (Li et al., 1999). We have used the same ADC criterion for all patients to delineate the lesion, and furthermore, as the follow-up MRI data from thrombolysed patients indicate that the size of the ADC lesions increased in 11 out of 16 cases, we assumed that thrombolysis prior to first MRI had minimal or no effect on lesion T2. Thirdly, the three hospitals that were involved in this study used two types of MRI pulse sequences to quantify T2 in humans, which yield different absolute T2 values from brain parenchyma (this was partly the reason for considering ratio measures as well as difference measures of T2 change). The critical parameter used in determining the effects of stroke is ΔT2. However, in the current data set it is impossible to separate ΔT2s in ADC lesions measured by either of the two MRI methods.
In spite of the limitations above, the current data have potential clinical implications warranting further investigation. Firstly, quantitative ADC and T2 in combination may offer an objective way to evaluate effects of stroke on brain tissue, aiding in patient stratification. Recent clinical studies have used DWI/T2-FLAIR signals to evaluate lesion age (Thomalla et al., 2009; Thomalla et al., 2018). The approach exploits a higher signal intensity arising due to the lengthened T2 and increased proton density as a proxy of lesion age. However, this signal increase may be counteracted by a T1 contribution, which increases with time after onset in the first hours in animal models (Kauppinen, 2014; McGarry et al., 2016), decreasing the FLAIR signal. “DWI/T2-FLAIR” mismatch as a proxy of lesion age is commonly determined visually, being classified by a binary value of whether or not a FLAIR and DWI lesion can both be seen (Thomalla et al., 2011). In a trial of 643 patients, it was shown that in the time range 181 – 270 minutes, 53% of patients present a mismatch and 47% do not, and that patients with no mismatch, who would otherwise be ineligible for thrombolysis, greatly benefited from treatment (Thomalla et al., 2018). The spherical reference approach may provide even more quantitative information on the age of stroke lesions than visually-determined DWI/T2-FLAIR mismatch in an unambiguous manner, but the latter approach is pending a clinical trial. Secondly, stroke onset time-dependent T2 changes in GM and WM are comparable within the time window studied here. Therefore, T2 relaxation time serves as a proxy of tissue state independently of the brain tissue type in the ADC lesion volume. It should be noted that the situation may be different when T2-weighted intensities are used, due to potential contribution by T1 in scan acquisitions and the inherently distinct T1s in GM and WM.
5. Conclusions
We have introduced a novel user-independent spherical reference method to quantify T2 relaxation time changes in acute ischaemic stroke lesions with low ADC, both in GM and WM volumes. Using this technique, ΔT2 increases by 1.4 – 1.5 ms/h in both tissue types. We believe that quantitative MRI, exploiting T2 and ADC, will have potential clinical impact in the management of acute stroke patients, allowing an objective tissue-state evaluation of ischaemic parenchyma. The use of both quantitative T2 and DWI/FLAIR mismatch would represent a shift towards the use of tissue-based MRI indices to assess tissue state in stroke patients.
Supplementary Material
Acknowledgements
The study is funded by the Dunhill Medical Trust (grants R385/1114 and OSRP1/1006). Support by the National Institute for Health Research Oxford Biomedical Centre Programme, the National Institute for Health Research Clinical Research Network, and the Wellcome Trust Institutional Strategic Support Fund (2015-2015) are acknowledged. We acknowledge the support of the National Institute for Health Research Clinical Research Network (NIHR CRN). We wish to acknowledge the facilities provided by Oxford Acute Vascular Imaging Centre.
References
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–6. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- Bristow MS, Simon JE, Brown RA, Eliasziw M, Hill MD, Coutts SB, Frayne R, Demchuk AM, Mitchell JR. MR perfusion and diffusion in acute ischemic stroke: human gray and white matter have different thresholds for infarction. J Cereb Blood Flow Metab. 2005;25:1280–7. doi: 10.1038/sj.jcbfm.9600135. [DOI] [PubMed] [Google Scholar]
- Burgess RE, Kidwell CS. Use of MRI in the assessment of patients with stroke. Curr Neurol Neurosci Rep. 2011;11:28–34. doi: 10.1007/s11910-010-0150-2. [DOI] [PubMed] [Google Scholar]
- Busza AL, Allen KL, King MD, van Bruggen N, Williams SR, Gadian DG. Diffusion-weighted imaging studies of cerebral ischemia in gerbils. Potential relevance to energy failure. Stroke. 1992;23:1602–12. doi: 10.1161/01.str.23.11.1602. [DOI] [PubMed] [Google Scholar]
- Chen C, Bivard A, Lin L, Levi CR, Spratt NJ, Parsons MW. Thresholds for infarction vary between gray matter and white matter in acute ischemic stroke: A CT perfusion study. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17744453. in press. 271678X17744453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohn OH, Kettunen MI, Penttonen M, Oja JM, van Zijl PC, Kauppinen RA. Graded reduction of cerebral blood flow in rat as detected by the nuclear magnetic resonance relaxation time T2: a theoretical and experimental approach. J Cereb Blood Flow Metab. 2000;20:316–26. doi: 10.1097/00004647-200002000-00013. [DOI] [PubMed] [Google Scholar]
- Hjort N, Butcher K, Davis SM, Kidwell CS, Koroshetz WJ, Röther J, Schellinger PD, Warach S, Østergaard L. Magnetic resonance imaging criteria for thrombolysis in acute cerebral infarct. Stroke. 2005;36:388–97. doi: 10.1161/01.STR.0000152268.47919.be. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–65. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jokivarsi KT, Hiltunen Y, Grohn H, Tuunanen P, Grohn OH, Kauppinen RA. Estimation of the onset time of cerebral ischemia using T1ρ and T2 MRI in rats. Stroke. 2010;41:2335–40. doi: 10.1161/STROKEAHA.110.587394. [DOI] [PubMed] [Google Scholar]
- Kauppinen RA. Multiparametric magnetic resonance imaging of acute experimental brain ischaemia. Prog NMR Spectr. 2014;80:12–25. doi: 10.1016/j.pnmrs.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Kettunen MI, Grohn OH, Silvennoinen MJ, Penttonen M, Kauppinen RA. Quantitative assessment of the balance between oxygen delivery and consumption in the rat brain after transient ischemia with T2 -BOLD magnetic resonance imaging. J Cereb Blood Flow Metab. 2002;22:262–70. doi: 10.1097/00004647-200203000-00003. [DOI] [PubMed] [Google Scholar]
- Knight MJ, McGarry BL, Rogers HJ, Jokivarsi KT, Grohn OH, Kauppinen RA. A spatiotemporal theory for MRI T2 relaxation time and apparent diffusion coefficient in the brain during acute ischaemia: Application and validation in a rat acute stroke model. J Cereb Blood Flow Metab. 2015;36:1232–43. doi: 10.1177/0271678X15608394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranz PG, Eastwood JD. Does diffusion-weighted imaging represent the ischemic core? An evidence-based systematic review. AJNR Am J Neuroradiol. 2009;30:1206–12. doi: 10.3174/ajnr.A1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumura E, Graf R, Dohmen C, Rosner G, Heiss WD. Breakdown of calcium homeostasis in relation to tissue depolarization: comparison between gray and white matter ischemia. J Cereb Blood Flow Metab. 1999;19:788–93. doi: 10.1097/00004647-199907000-00009. [DOI] [PubMed] [Google Scholar]
- Li F, Han SS, Tatlisumak T, Liu KF, Garcia JH, Sotak CH, Fisher M. Reversal of acute apparent diffusion coefficient abnormalities and delayed neuronal death following transient focal cerebral ischemia in rats. Ann Neurol. 1999;46:333–42. doi: 10.1002/1531-8249(199909)46:3<333::aid-ana9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- McGarry BL, Rogers HJ, Knight MJ, Jokivarsi KT, Sierra A, Grohn OH, Kauppinen RA. Stroke onset time estimation from multispectral quantitative magnetic resonance imaging in a rat model of focal permanent cerebral ischemia. Int J Stroke. 2016;11:677–82. doi: 10.1177/1747493016641124. [DOI] [PubMed] [Google Scholar]
- McKean JW, Schrader RM. A comparison of methods for studenttizing the samle median. Comm Stats Simul Comput. 1984;13:751–73. [Google Scholar]
- Moseley ME, Cohen Y, Mintorovitch J, Chileuitt L, Shimizu H, Kucharczyk J, Wendland MF, Weinstein PR. Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Reson Med. 1990;14:330–46. doi: 10.1002/mrm.1910140218. [DOI] [PubMed] [Google Scholar]
- Norton TJT, Pereyra M, Knight MJ, McGarry BM, Jokivarsi KT, Grohn OHJ, Kauppinen RA. Stroke onset time determination using MRI relaxation times without non-ischaemic reference in a rat stroke model. Biomed Spectrosc Imaging. 2017;6:25–35. doi: 10.3233/BSI-160155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Alger JR, Righini A, Mattiello J, Dickerson R, Des Pres D, Barnett A, Di Chiro G. High temporal resolution diffusion MRI of global cerebral ischemia and reperfusion. J Cereb Blood Flow Metab. 1996;16:892–905. doi: 10.1097/00004647-199609000-00013. [DOI] [PubMed] [Google Scholar]
- Price RM, Bonett DG. Estimating the variance of sample median. J Stats Comput Simulat. 2001;68:295–305. [Google Scholar]
- Siemonsen S, Mouridsen K, Holst B, Ries T, Finsterbusch J, Thomalla G, Ostergaard L, Fiehler J. Quantitative T2 values predict time from symptom onset in acute stroke patients. Stroke. 2009;40:1612–6. doi: 10.1161/STROKEAHA.108.542548. [DOI] [PubMed] [Google Scholar]
- Sotak CH. The role of diffusion tensor imaging in the evaluation of ischemic brain injury: A review. NMR Biomed. 2002;15:561–9. doi: 10.1002/nbm.786. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Cheng B, Ebinger M, Hao Q, Tourdias T, Wu O, Kim JS, Breuer L, Singer OC, Warach S, Christensen S, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol. 2011;10:978–86. doi: 10.1016/S1474-4422(11)70192-2. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Rossbach P, Rosenkranz M, Siemonsen S, Krützelmann A, Fiehler J, Gerloff C. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol. 2009;65:724–32. doi: 10.1002/ana.21651. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, Cheripelli B, Cho TH, Fazekas F, Fiehler J, Ford I, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611–22. doi: 10.1056/NEJMoa1804355. [DOI] [PubMed] [Google Scholar]
- Welch KM, Windham J, Knight RA, Nagesh V, Hugg JW, Jacobs M, Peck D, Booker P, Dereski MO, Levine SR. A model to predict the histopathology of human stroke using diffusion and T2-weighted magnetic resonance imaging. Stroke. 1995;26:1983–9. doi: 10.1161/01.str.26.11.1983. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.