Abstract
Thermal disinfection is commonly used to prevent the proliferation of culturable Legionella in engineered water systems (EWS). In response to such stress, culturable Legionella populations can switch into a viable but nonculturable (VBNC) state. The importance of such VBNC Legionella cells is currently hotly debated. Here, we investigated the stress response patterns and transitions of the bacteria to the VBNC state at 55°C, 60°C and 70°C on two L. pneumophila strains for >80 days using a combination of cell-based viability indicators. Complete loss of culturability at 55°C, 60°C and 70°C occurred after 3-8 hours, 60 min and <2 min, respectively. In contrast, L. pneumophila strains required 9 days at 55°C, 8 hours at 60°C and 20 min at 70°C to achieve a 2 log reduction in cells with intact membranes and high esterase activity; a 4 log reduction was achieved only after 150, 8-15 and 1-4 days, respectively. In parallel, the presence of diagnostic outer-membrane epitopes (OMEs) and changes in the infectivity patterns of the two strains towards amoebae and THP-1 cells were assessed. OMEs were more persistent than viability indicators, showing their potential as targets for VBNC Legionella detection. L. pneumophila strains infected amoebae and THP-1 cells for at least 85 days at 55°C and 60°C and for up to 8 days at 70°C. However, they did so with reduced efficiency, requiring prolonged co-incubation times with the hosts and higher Legionella cell numbers in comparison to culturable cells. Consequently, infection of amoebae by thermally induced VBNC L. pneumophila with lowered virulence can be expected in EWS. Although the gold standard method cannot detect VBNC Legionella, it provides important information about the most virulent bacterial subpopulations. Our results indicate that a prolonged thermal regime ≥60°C at the central parts of warm water systems is not only effective against culturable L. pneumophila but in the long run even against VBNC cells.
Keywords: Legionella, VBNC, Acanthamoeba, macrophages, thermal disinfection, viability
1. Introduction
Temperature is one of the key factors that influences the proliferation of Legionella in natural and man-made water systems. This has been observed for temperatures ranging between 12°C and 50°C (ESGLI, 2017; Lesnik et al., 2016); however, optimal replication temperatures are between 32°C and 40°C (ESGLI, 2017; Lesnik et al., 2016). These temperatures are normally encountered in cooling towers and spas which are common sources of large outbreaks of Legionnaires’ disease (LD). LD cases also occur sporadically from cold and specifically from hot water systems colonized with this bacterium (Bartram et al., 2007; ECDC, 2017).
To prevent and control the proliferation of Legionella in engineered water systems (EWS), several disinfection strategies are applied (ESGLI, 2017). Among them, a temperature control regime is recommended for the EWS of large buildings (e.g. hospitals). In European hot water systems, for example, water leaving the heaters should have a minimum temperature of 60°C and should return at a minimum of 55°C (ESGLI, 2017). In cold water systems, the well-characterised range of temperatures in which Legionella replicates (>20°C) should be avoided. Although such measures have proven efficient to reduce culturable Legionella concentrations from EWS, they are not successful in eradicating the bacterium (Allegra et al., 2011; Mouchtouri et al., 2007; Rhoads et al., 2015). Thermal shocks at higher temperatures such as 70°C and 80°C are therefore also suggested as disinfection measures (reviewed in Whiley et al., 2017). However, Legionella may not only survive (Farhat et al., 2010) but also recolonise the water system weeks after thermal treatment (Steinert et al., 1998; Vervaeren et al., 2006).
A number of factors may be responsible for hampering the effectiveness of thermal treatments: (i) within water pipes Legionella lives in biofilms in a free-living form or intracellularly within free-living amoebae (FLA), which not only protect the bacteria from external stressors but also provide nutritional sources to favour their replication (Declerck, 2010); (ii) different Legionella species and strains have different thermal susceptibilities (Cervero-Aragó et al., 2015; Sharaby et al., 2017); (iii) Legionella and/or other thermophilic bacteria can adapt and transfer genes (Lesnik et al., 2016; Sharaby et al., 2017); (iv) improperly managed or designed system hydraulics (Bédard et al., 2015) and (v) the presence of viable but nonculturable (VBNC) cells not detectable by culture-based methods, which could overestimate the performance of the thermal treatments applied (Allegra et al., 2011; Kirschner, 2016).
Legionella, as other gram-negative bacteria, may enter the VBNC state under conditions of environmental stress such as starvation (Schrammel et al., 2018a), high temperatures (Allegra et al., 2008; Chang et al., 2007) and oxidising agents (Türetgen, 2008). In this state, the bacterium does not grow on standard artificial media, the gold-standard method for the enumeration of the bacteria and risk assessment, but is still active, maintaining low metabolic activity, membrane integrity, virulence-related protein expression (Alleron et al., 2013; Schrammel et al., 2018a) and a low level of virulence (Al-Bana et al., 2014; Dietersdorfer et al., 2018). The importance and potential health relevance of such VBNC Legionella is intensively discussed in the current literature. Moreover, the presence of nonculturable Legionella and the higher numbers of Legionella detected by cultivation-independent methods such as qPCR or direct detection with antibody based assays (Füchslin et al., 2010) in comparison to the standard culture-based method, may confuse the operators of water systems (Kirschner, 2016). For example, the diversity on the lipopolysaccharide (LPS) and specifically the outer-membrane epitopes (OMEs) of L. pneumophila have been used in the past years for serotyping L. pneumophila strains for diagnostic purposes (Helbig et al., 1997). However, little is known about the persistence of such OMEs under different environmental conditions and their relation to Legionella viability and infectivity (Schrammel et al., 2018b).
In the present study, we assessed the impact of three temperatures currently used to control culturable Legionella in EWS — 55°C, 60°C and 70°C — on the viability of two L. pneumophila strains in ultrapure water microcosms for >80 days. The microcosms were monitored by analysing the total cell count (TCC) and using viability indicators that comprise culturability, cellular integrity, DNA content, membrane integrity, esterase activity of the cells, and the presence of OMEs. Notably, we also semi-quantitatively assessed changes in the infectivity pattern of the two L. pneumophila strains using an Acanthamoeba host as an amoeba model and a macrophage-like cell model (differentiated from THP-1 cells).
By comprehensively studying the viability and infectivity of culturable and specifically VBNC L. pneumophila in a multiparametric way we intended to elucidate the impact that elevated temperatures have on the two states of these important opportunistic waterborne pathogens.
2. Material and Methods
2.1. Selected L. pneumophila strains
A L. pneumophila SG1 subtype mAb Benidorm strain isolated from a clinical source (LpClin) and a L. pneumophila SG1 mAb subtype Philadelphia strain (LpParis) were used for all experiments (Schrammel et al., 2018a, 2018b). The two strains belong to the most virulent mAb 3/1 subtype according to the Dresden panel (Helbig et al., 1997). Strains were stored at -80°C in cryotubes (Rotistore, Carl Roth, Karlsruhe, Germany).
2.2. Amoeba culture
The Acanthamoeba castellanii genotype T4 strain (SPA08) isolated from a keratitis patient (Dietersdorfer et al., 2018) was chosen as a host amoeba model system. The strain was cultivated and prepared as explained elsewhere (Dietersdorfer et al., 2018). First, 0.4 mL axenic trophozoite suspensions of 1×105 amoeba cells in peptone-yeast-glucose (PYG) medium were transferred to 12-well cell culture plates. The plates were incubated at 30°C for 1 h to allow amoeba adherence to the bottom of the wells. After that, the PYG medium was removed by aspiration and replaced by 1 mL of 1:10 PYG medium diluted in Page's amoeba saline (PAS, ATCC1323).
2.3. Generation of THP-1 macrophages
THP-1 cells, acquired from V. Mündler (Medical University of Vienna, Austria), constitute a human monocytic cell line derived from an acute monocytic leukaemia patient. Cells were maintained and prepared as explained elsewhere (Dietersdorfer et al., 2018). First, monocytes were counted and adjusted to a final concentration of 1×105 cells/mL using a haemocytometer. One millilitre of monocyte suspension was transferred to a 12-well cell culture plate. Then, 50 ng/mL of phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich) was added for 72 h to differentiate the cells into macrophage-like cells. After 3 days, the cells were washed twice with pre-warmed phosphate-buffered saline (PBS). Finally, 1 mL of fresh RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich) without antibiotics was added to each well.
2.4. Generation of microcosms, heat treatments and sample monitoring
All Legionella microcosms were prepared as detailed in Schrammel et al. (2018a) and in the SI. First, three independent cultures of the two L. pneumophila strains were grown in liquid buffered yeast extract (liBYE, modified from Edelstein, 1981) until they reached the stationary phase. In this phase L. pneumophila expresses similar traits to its transmissive state in the environment (Al-Bana et al., 2014). Cultures were then washed twice by centrifugation at 3,500 × g at 20°C. Supernatants were discarded, and cells were resuspended in ultrapure water (Simplicity® Ultrapure water (Merck, Darmstadt, Germany)) by vortexing. After checking the OD600 value, the volume required to achieve a cell concentration of 1×108 cells/mL in 350 mL of autoclaved ultrapure water pre-heated at 55°C, 60°C or 70°C was inoculated (T0). Triplicate bottles of the Legionella microcosms were incubated without shaking at the selected temperatures and monitored over time (Tmin, Th, Tdays). For each temperature, different sampling time points were chosen. Bottles were shaken 20 times and from each microcosm, samples of 15-20 mL were taken and cooled by placing the tube in cold water (approximately 18°C) for 2 min and vortexed for 20 s. Samples were then prepared for further analyses: culturability, analysis of cellular integrity and DNA content, viability indicators, presence of OMEs and infection assays (Dietersdorfer et al., 2018; Schrammel et al., 2018a, 2018b).
2.5. Analysis of Legionella culturability
Tenfold dilution series of Legionella microcosm samples were transferred to buffered charcoal-yeast extract (BCYE) agar plates without antibiotics (BioMerieux, Vienna, Austria). Complete loss of culturability was confirmed when no colonies were observed after plating 0.5 mL of direct microcosm samples after 10 days of incubation at 37°C in triplicate. When a positive Legionella infection in amoebae and THP-1 macrophages was confirmed by microscopy (see below), 1 mL of the co-culture suspension was harvested and directly plated on BCYE agar. All plates were incubated at 37°C for up to 10 days and checked every 3-4 days for L. pneumophila colony growth.
2.6. Characterisation of L. pneumophila cells by flow cytometric (FCM) analyses
The details of all staining procedures, the FCM protocol analysis, the gating strategy and the FCM results calculation are described extensively in our previous publications (Schrammel et al., 2018a, 2018b) and can be found in the Supplemental Information (SI). Legionella microcosms were analysed using an Attune NxT Flow Cytometer (Life Technologies, Darmstadt, Germany) equipped with a 488 nm flat-top laser at 50 mW. The flow cytometric assays had a limit of quantification (LOQ) of 1.3 × 104 cells/mL (Schrammel et al., 2018a, 2018b).
2.6.1. Viability indicators
Total cell counts (TCC), cell integrity and DNA content of the bacterial cells were analysed using SYBR Green I (SGI) staining. The membrane integrity of the cells was assessed using a combination of SYBRGreen I/propidium iodide (SGI/PI) staining. The esterase activity of the cells was analysed by staining with 5(6)-carboxyfluorescein-diacetate (CFDA).
2.6.2. Quantification of the presence of specific OMEs
Four monoclonal antibodies (mAbs) from the Dresden panel used in typing assays for the lipopolysaccharide (LPS) of L. pneumophila SG1 and a mAb targeting major outer-membrane protein (MOMP) were used to assess the presence of the respective OMEs (Helbig et al., 1997). MAb 3/1, 8/5 and MOMP, specific for both test strains; mAb 20/1, specific for LpClin; and mAb 8/4, specific for LpParis were assessed by immunofluorescence staining (Schrammel et al., 2018b). The number of stained cells and the staining intensity were analysed by FCM.
2.7. Infection of amoebae and THP-1 macrophages
For each temperature and strain, three L. pneumophila microcosms were analysed in parallel. Each bacterial suspension was directly added to amoeba and macrophage cultures at different multiplicities of infection (MOI): 1, 100 or 400 legionellae per amoeba; and 50 or 100 legionellae per THP-1 macrophage. To enhance the interaction between legionellae and host cells, amoeba plates were centrifuged at 500 × g for 5 min and THP-1 macrophages at 300 × g for 5 min. Amoeba co-cultures were then incubated at 30°C, whereas co-cultures with human cells were incubated at 37°C with 5% CO2 (Dietersdorfer et al., 2018).
2.8. Monitoring of L. pneumophila infections
Co-cultures were observed daily using an inverted microscope (Olympus CK 2, Vienna, Austria). When host cells showed any sign of infection (intracellular replication of Legionella cells) the respective well was harvested, fixed and stained using the Monofluo L. pneumophila IFA Test Kit (Bio-Rad Laboratories Diagnostics Group, Redmond, WA, USA) as previously explained (Dietersdorfer et al., 2016). All slides were observed using a Nikon Eclipse 8000 epifluorescence microscope and pictures were taken using the Nikon DS-QiMc camera and NIS Elements BR 2.3 software (Nikon, Surrey, UK). Infection was considered positive when vesicles of more than five bright Legionella cells were observed within one host cell. Cultures of hosts without bacteria were used as negative controls.
2.9. Statistics
The data set obtained was analysed using Spearman’s rank correlation (SPSS vers. 24, IBM International), as most of the data did not follow a normal distribution. Correlations between viability indicators were accepted as significant for p-values <0.05 and as highly significant for p <0.01 (Tables SI 4-6).
3. Results
We monitored the impact that temperature had on L. pneumophila in a multiparametric way, using viability indicators that comprised culturability, an assessment of cell integrity and DNA content, membrane integrity and esterase activity of the cells as well as the presence of virulence-related epitopes. Most importantly, we also semi-quantitatively assessed changes in the infectivity pattern of the bacteria using amoeba and a macrophage-like host model.
3.1. Loss of L. pneumophila culturability
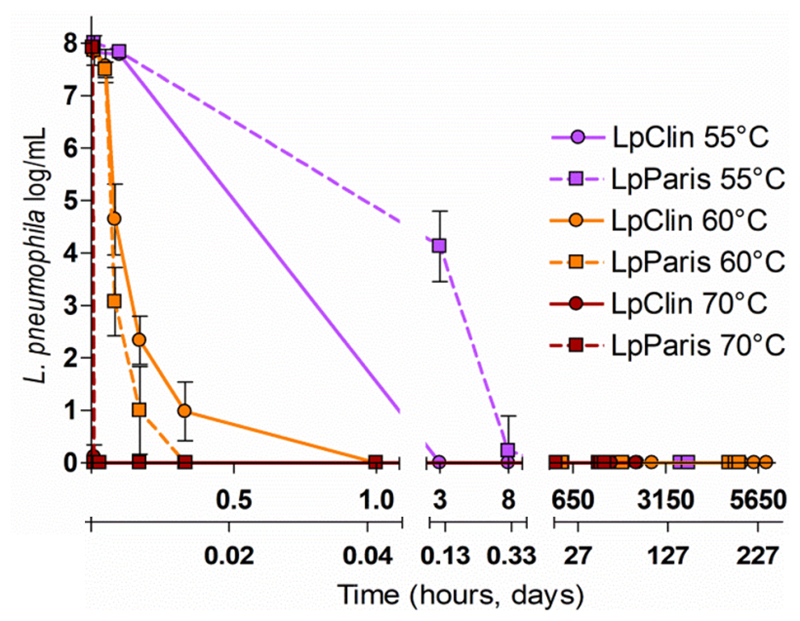
At 55°C, LpClin and LpParis completely lost culturability on plates (8 log reduction, <1 CFU/mL) at T3h and T8h, respectively. At 60°C, the total loss of culturability was observed already at T30min for LpParis and at T1h for LpClin. At 70°C, neither of the two strains were culturable at T2min. Once nonculturable, none of the L. pneumophila strains formed colonies on standard media plated directly from the water microcosms (Figure 1).
Figure 1.
Culturability of the two L. pneumophila strains at 55°C, 60°C and 70°C on BCYE agar plates. Results are shown as triplicate average values ± Standard deviation.
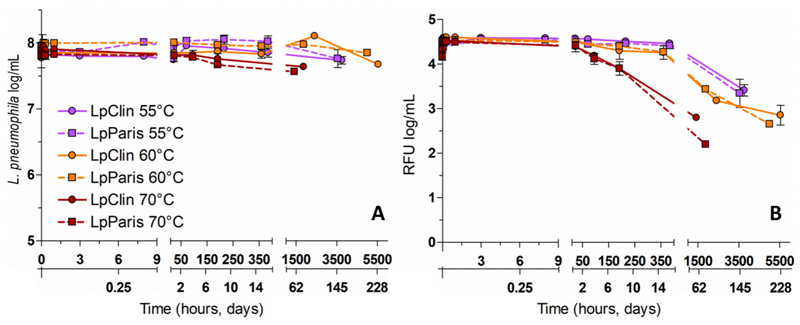
3.2. Total cell counts, cell integrity and DNA content
Despite the rapid reduction in culturability observed at different temperatures, the concentration of intact cells (TCC) remained constant throughout the different experiments (~ 8 log cells/mL, Figure 2A). However, variations in the mean fluorescence intensity (MFI) of SG1-stained cells were observed (Figure 2B). After an initial constant phase of 15 days, the MFI of L. pneumophila strains incubated at 55°C and 60°C was reduced by 1 log at T152days (55°C) and 1.5 log at T200days (60°C). At 70°C, after an initial constant phase of 24 h, the MFI decreased by 1.5-2 log at T80days indicating a degradation of the DNA content.
Figure 2.
Total cell counts, cell integrity (A) and DNA content represented by the MFI of cells positively stained with SGI (B) of the two L. pneumophila strains at 55°C, 60°C and 70°C. Results are shown as triplicate average values ± Standard deviation. RFU (relative fluorescence intensity).
3.3. Membrane integrity
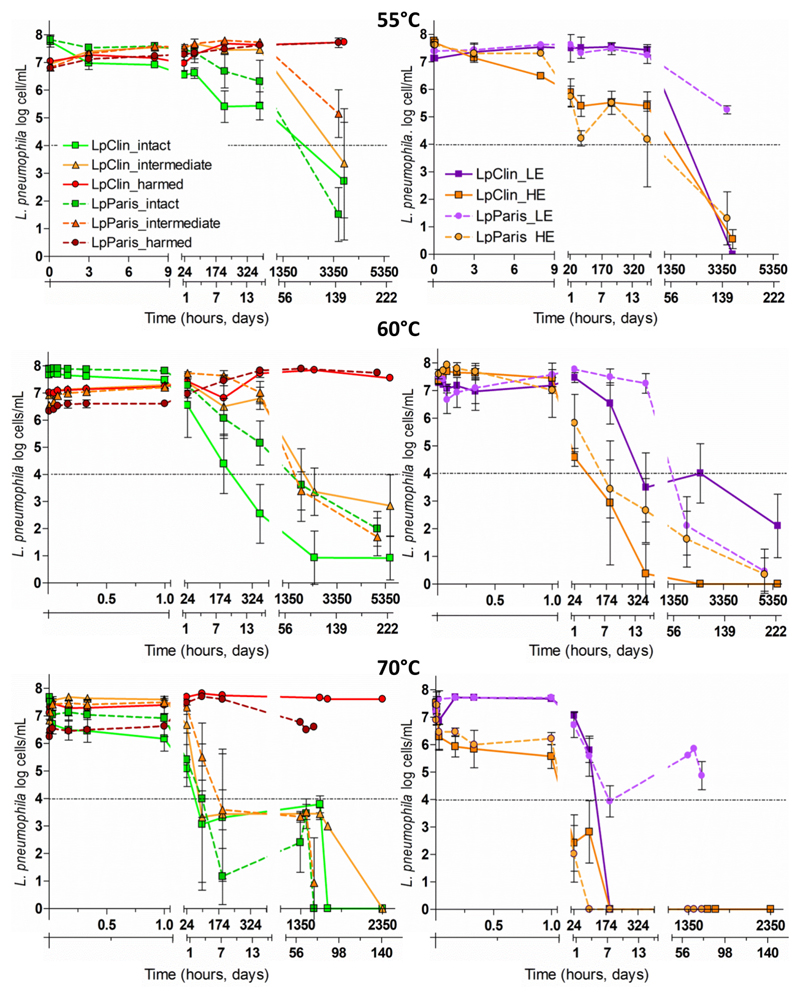
Although all L. pneumophila microcosms were generated from bacterial liquid cultures to promote homogeneous bacterial populations (SI), three different subpopulations were observed for all Legionella strains and temperatures analysed immediately after the inoculation into ultrapure water (SI Table 2). One subpopulation of the cells comprised cells with intact membranes, a second subpopulation comprised cells with harmed membranes and a third subpopulation comprised cells in the so-called intermediate-membrane state, located between the previous subpopulations in the flow cytometry plots (Figure 3).
Figure 3.
Membrane integrity (left column) and esterase activity (right column) of the two L. pneumophila strains at 55°C, 60°C and 55 60 70°C. For membrane integrity, three different subpopulations are displayed: cells with intact membranes (green), cells with membranes in an intermediate state (orange) and cells with harmed membranes (red). For esterase activity two subpopulations per strain are displayed: cells with high activity (HE, regular lines), and cells with low activity (LE, dashed lines). The results are shown as triplicate average values ± standard deviation. Dashed black lines show the LOQ, 1.3× 104 cells/mL (Schrammel et al., 2018a).
At 55°C, the number of cells with intact membranes (approximately 70% of the TCC, SI Table 2) gradually decreased over time, following a different pattern depending on the strain analysed. LpClin, which was nonculturable at T3h, showed a simultaneous 1 log reduction in cells with intact membranes (Figure 3). After that, it continued decreasing by up to 5 log at T152days. Despite the loss of culturability, the concentration of LpParis cells with intact membranes remained stable during the first 72 h (approximately 80% of the TCC, SI Table 2). After that, it started decreasing by more than 6 log at T152days (Figure 3). Cells of both test strains shifted from the intact-membrane subpopulation gate to the subpopulation of cells with intermediate-state membranes over time. Then cells shifted to the subpopulation of cells with harmed membranes. Thus, at T152days, intermediate-state cells were 1 log (LpParis) and 3.6 log (LpClin) lower (Figure 3). Consistently, the concentration of cells with harmed membranes (dead cells) was proportionally higher.
At 60°C, the number of intact cells decreased faster for LpClin than for LpParis (Figure 3). Intact cells decreased below the LOQ (>4 log reduction) at T15days for LpClin and at T88days for LpParis. Although the concentration of cells with intermediate membranes increased between 0.5 log (LpClin) and 1.2 log (LpParis) during the first 24 h, after longer incubation times (T88days and T217days), the same subpopulations were reduced by ~3 and 4.5 log, respectively.
At 70°C, the subpopulations with intact membranes decreased between 0.5 (LpParis) and 1 log (LpClin) already at T10min (Figure 3) and at T4days concentrations were below the LOQ (>4 log reduction). After a slight (0.8-0.9 log) initial increase the cells with intermediate membranes state were reduced by >3 log at T8days and by >5 log at T98days.
3.4. Esterase activity
According to the mean fluorescence intensity of the CFDA staining, two different subpopulations per L. pneumophila strain were distinguished in the flow cytometry histograms: a subpopulation with high esterase activity (HE) and one with low esterase activity (LE). At 55°C, LE cells showed a similar pattern for LpClin and LpParis. LE cell numbers (approximately 30% of the TCC, SI Table 2) were stable over 16 days. However, at T152days they were reduced by 7 log (LpClin) and 2.7 log (LpParis) (Figure 3). In the case of HE cells (approximately 70% of the TCC, SI Table 2), the LpClin subpopulation decreased gradually immediately after inoculation, reaching a 4 log reduction (below the LOQ) at T152days. In contrast, the number of HE cells of LpParis was stable for the first 8 h and then followed the same trend as LpClin, decreasing >4 log at T152days (Figure 3).
At 60°C, the LE subpopulations of both L. pneumophila strains remained stable for 24 h. At T88days LE cells were reduced by >4 log. The HE subpopulations of the two strains remained stable for the first hour. A >4 log reduction was already observed at T8days (Figure 3).
At 70°C, the LE subpopulations of both strains remained relatively stable only for the first hour. A ~4 log reduction was already observed at T8days. The concentration of HE cells immediately decreased after inoculation and was reduced by 1 log at T2min of thermal exposure. Already at T1day, both HE subpopulations were below the LOQ (Figure 3).
3.5. Presence of specific OMEs
Four monoclonal antibodies (mAbs) from the Dresden panel were used to assess the presence of OMEs. MAb 3/1, 8/5 and MOMP, specific for both test strains; mAb 20/1, specific for LpClin; and mAb 8/4, specific for LpParis. Two different subpopulations of cells were distinguished for all mAbs analysed, according to their mean fluorescence intensity (MFI): low fluorescent (LF) and high fluorescent (HF) cells. In line with our previous study, the MFI accounts to the average number of epitopes on the surface of the cells available for antibody binding (Schrammel et al., 2018b).
Our results showed no differences between strains or temperatures during the first 8 days with MOMP staining, during which stable numbers of LF and HF cells were observed (approximately 70% and 30% of the TCC, respectively). At 55°C, the LF subpopulation of LpClin and LpParis remained stable for 194 days (Figure 4). At 60°C, LF cells decreased between 4 log (LpParis) and 5 log (LpClin) after T200days, and at 70°C, a 3 log reduction was observed for both strains at T60days. The HF subpopulation had a dramatic 7 log reduction at incubation times >T15days, for both strains and the three temperatures analysed, with the exception of the HF subpopulation of LpClin at 55°C, which was reduced by only 1 log at T194days. Thus, at increasing temperatures the number of epitopes per cell strongly decreased (HF) and subsequently, at longer exposure times the number of cells expressing the MOMP epitope also decreased.
Figure 4.
Concentration of mAbs-stained cells of the two L. pneumophila strains at 55°C, 60°C and 70°C. For each mAb tested two different subpopulations are displayed: highly fluorescent cells (HF, dashed lines), low fluorescent cells (LF, solid lines). Results are shown as triplicate average values ± Standard error. Dashed black lines show the LOQ, 1.3× 104 cells/mL.
The mAb 3/1 binds to a specific LPS epitope of L. pneumophila SG1 strains that belong to the most virulent Pontiac group; the two strains used in the current study belonged to that group. At 55°C, the number of cells expressing the 3/1 epitope (LF) remained stable throughout the experiment (37% of the TCC). However, at higher temperatures, a reduction of 0.5 log (60°C) and 0.5-1 log (70°C) was observed for both, LpClin and LpParis, at T200days and T59days, respectively (Figure 4). Regarding, the HF subpopulation (approximately 63% of the TCC), at 55°C a reduction of <1 log occurred at T194days. At higher temperatures, this subpopulation was reduced by >7 log at T8days and T15days at 70°C and 60°C, respectively (Figure 4).
The isotype-IgM antibody 8/5 binds to a specific LPS epitope of all L. pneumophila SG1 strains. The number of cells expressing the 8/5 epitope (LF subpopulations) remained stable (60% of the TCC) for both strains and all temperatures analysed. A similar trend was observed for the cells highly expressing the 8/5 epitope (HF subpopulations) except for LpParis at 70°C which was 5 log reduced at T59days (Figure 4).
The mAb 20/1 binds specifically to certain mAb subtypes of L. pneumophila SG1, such as OLDA and Benidorm (LpClin) and the mAb 8/4 binds specifically to certain mAb subtypes of L. pneumophila SG1, such as OLDA, Oxford and Philadelphia (LpParis). The results obtained show that temperature did not impact the number of cells expressing these epitopes (LF) neither the number of epitopes expressed by cell (HF) (data not shown).
3.6. Infectivity of L. pneumophila strains after incubation at 55°C
In parallel to the viability parameters, we analysed the infectivity patterns of the two L. pneumophila strains. The methodology used enabled us to semi-quantitatively assess changes in the efficiency of L. pneumophila to cause infection in amoebae and THP-1 macrophages. The infectivity efficiency was represented by the different MOIs tested, the days in co-culture required to observe an infection and the number of replicates in which such infections were observed (Figure 5). In general, at all temperatures tested and for both host models, the two strains required longer co-culture times to observe an infection in comparison to T0 (with culturable cells). Neither LpClin nor LpParis recovered culturability after passage through amoebae or THP-1 macrophages.
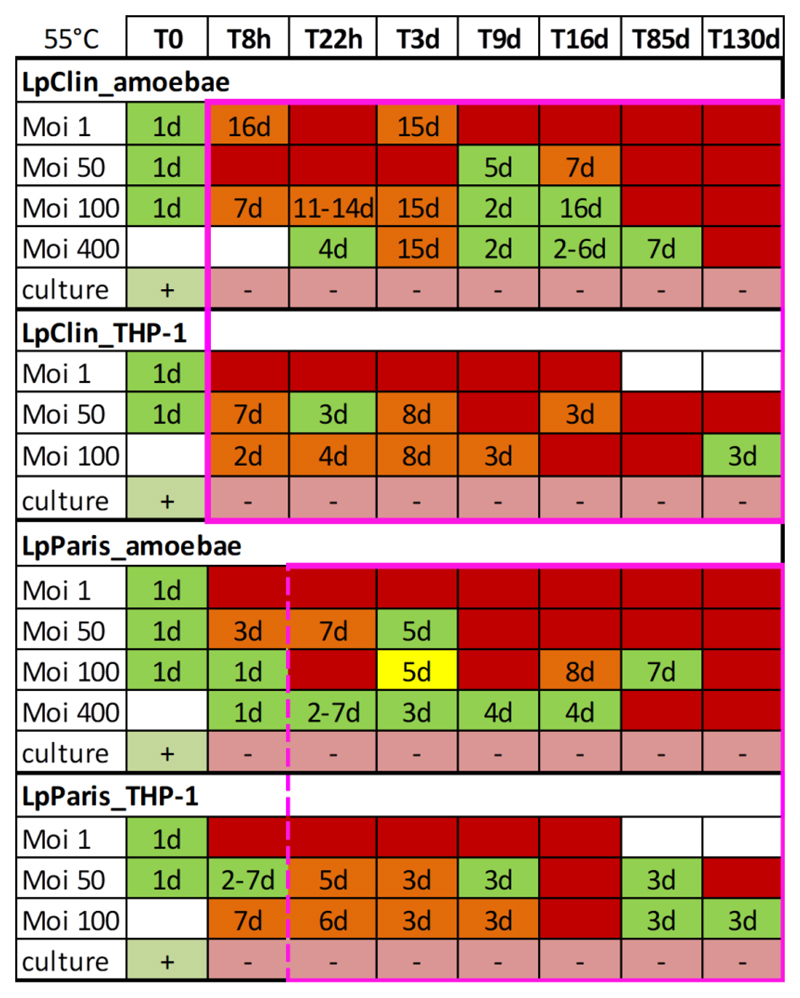
Figure 5.
Culturability and infectivity patterns of the two L. pneumophila strains incubated at 55°C. Legionella microcosms were analysed at different times. Nonculturable cells (8 log reduction) are plotted within the pink square box. The dashed line accounts for differences between replicates. Infectivity was analysed by co-culture with Acanthamoeba and THP-1 cells at different MOIs. Culturability after passage through hosts is plotted below the MOIs; (+) culturable and (-) nonculturable. The numbers within the wells represent the days required to observe an infection in co-culture with the hosts. Green wells: all replicates infective; yellow wells: two replicates infective; orange wells: one replicate infective; red wells: no infection observed. White wells without number: not analysed; h=hours; d=days.
At 55°C, the two L. pneumophila strains were able to replicate in amoebae and THP-1 macrophages for as long as they were culturable for all MOIs tested. Once nonculturable, the two strains infected amoebae for up to 85 days with high variability between replicates, especially for the lower MOIs. Moreover, the time required to observe infection in amoeba changed from 1 day at T0 for up to 16 days in some cases (e.g., LpClin at T3days or T16days) (Figure 5). No infections of amoebae were observed at T130days. A similar pattern was observed for THP-1 macrophages. Once nonculturable, LpClin and LpParis continued to infect THP-1 macrophages at the highest MOIs (50 and 100), with high variability between replicates. Although both strains did not infect THP-1 macrophages at single time points (LpClin at T85days, and LpParis at T16days), the two L. pneumophila strains were infective at T130days, the latest time point tested using MOI 100 (Figure 5, SI Figure 2).
3.7. Infectivity of L. pneumophila strains after incubation at 60°C
At 60°C, the infection patterns were comparable to those at 55°C. With the reduction in the number of culturable cells, the MOIs and incubation times needed to observe infection were extended. Once nonculturable, LpClin and LpParis infected amoebae at MOIs 100 and 400 for up to 15 days with a high variability between replicates specifically for the smallest MOI (Figure 6). At T15days, L. pneumophila microcosms needed prolonged incubation times (11-17 days) for a detectable infection. LpParis infected amoebae until T43days only at the highest MOI, 400 (Figure 6). LpClin also infected amoebae also at later time points until T86days at MOI 400 and occasionally at MOI 100, always requiring longer co-culture times in comparison to T0.
Figure 6.
Culturability and infectivity patterns of the two L. pneumophila strains incubated at 60°C. Legionella microcosms were analysed at different times. Nonculturable cells (8 log reduction) are plotted within the pink square box. The dashed line accounts for differences between replicates. Infectivity was analysed by co-culture with Acanthamoeba and THP-1 cells at different MOIs. Culturability after passage through hosts is plotted below the MOIs; (+) culturable and (-) nonculturable. The numbers within the wells represent the days required to observe an infection in co-culture with the hosts. Green wells: all replicates infective; yellow wells: two replicates infective; orange wells: one replicate infective; red wells: no infection observed. White wells without number: not analysed; h=hours; d=days.
Regarding co-cultures with THP-1 macrophages, on-going infections were observed for the two L. pneumophila strains for up to 15 days with a slight variability between replicates and requiring longer co-culture times in comparison to T0. In the long exposure period, the two strains continued to infect THP-1 macrophages until the latest time point tested (T83days for LpParis and T114days for LpClin) for at least one of the two MOIs tested (Figure 6).
3.8. Infectivity of L. pneumophila strains after incubation at 70°C
LpClin and LpParis infected amoebae for up to 8 days for at least one of the MOIs tested (mostly at MOI 400), and a high variability between replicates was observed (Figure 7). At least two of the triplicate microcosms of the two strains infected the THP-1 macrophages at all times analysed for up to 8 days at 70°C (Figure 7, SI). Thereafter, no more infections were observed in amoebae or in THP-1 macrophages (Figure 7).
Figure 7.
Culturability and infectivity patterns of the two L. pneumophila strains incubated at 70°C. Legionella microcosms were analysed at different times. Nonculturable cells (8 log reduction) are plotted within the pink square box. The dashed line accounts for differences between replicates. Infectivity was analysed by co-culture with Acanthamoeba and THP-1 cells at different MOIs. Culturability after passage through hosts is plotted below the MOIs; (+) culturable and (-) nonculturable. The numbers within the wells represent the days required to observe an infection in co-culture with the hosts. Green wells: all replicates infective; yellow wells: two replicates infective; orange wells: one replicate infective; red wells: no infection observed. White wells without number: not analysed; m=minutes; h=hours; d=days.
3.9. Correlation between the different viability indicators
In a comprehensive statistical approach, we analysed how the different parameters are related to each other. Overall, the correlation pattern between parameters was strongly influenced by the temperature applied. At 55°C, we observed a significantly positive correlation between the concentrations of culturable cells and the number of cells with intact membranes and high esterase activity (p<0.01). At this temperature both subpopulations were >4 log reduced after 150 days of incubation.
In comparison to 55°C, at 60°C a steeper decreasing trend of the concentration of viable cells was observed. It took 15 days to observe a >4 log reduction in cells with intact membranes and low esterase activity. However, HE cells showed the same reduction already at T8days of heat exposure. At 70°C, again the most sensitive viability indicators, such as the cells with intact membranes and HE activity, were reduced by >4 log at T4days and T24h, respectively; it took between 4-8 days to observe the same reduction in the number of LE cells. At higher temperatures, the aforementioned parameters also significantly correlated with the concentration of cells with intermediate-state membranes (p<0.01), low esterase activity (p<0.01) and degradation of DNA (p<0.05), represented by the MFI of cells stained with SGI. Thus, higher temperatures synchronised the L. pneumophila subpopulation dynamics, showing a common decreasing trend over time.
Conversely, cells with membranes in an intermediate state and low esterase activity withstood 55°C for a longer time than the more active subpopulations (Tables SI 4-6).
Among the OMEs, several correlations were observed. In most cases, the concentration of cells with a high number of epitopes (HF subpopulations) of mAb 3/1 and mAb 8/5 were positively inter-correlated (p<0.01). The concentration of HF and LF subpopulations also correlated with some viability indicators, specifically with cells with intact and intermediate-state membranes, high esterase activity and the MFI of cells stained with SGI (p<0.05 and p<0.01 depending on HF/LF mAb subpopulation and strain) (Tables SI 4-6).
4. Discussion
LD is an atypical pulmonary infection that causes substantial morbidity and mortality worldwide (Phin et al., 2014). Approximately 90% of LD infections are caused by Legionella pneumophila, the transmission of which is generally associated with exposure to aerosols produced from colonised water systems (Buse et al., 2012; Phin et al., 2014). Within such systems, Legionella survives in existing biofilms, replicating inside FLA (Declerck, 2010). Once inhaled, L. pneumophila encounters alveolar macrophages in the human body and uses mechanisms similar to those utilised in the environment, eventually causing human pulmonary infection (Buse et al., 2012). To control Legionella proliferation in EWS, preventive measures and disinfection strategies based on specific thermal regimes are currently used. However, the bacteria are often not successfully eliminated from the system (Farhat et al., 2010; Steinert et al., 1998; Whiley et al., 2017). The ability of Legionella to switch into a VBNC state as a survival strategy against stressors and the implications that this may have on the disinfection strategies is currently hotly debated.
In the present study, we monitored the impact of three temperatures currently used to control culturable Legionella in EWS (55°C, 60°C and 70°C) on the viability of two L. pneumophila microcosms in ultrapure water for >80 days, one of the longest and most comprehensive studies performed for culturable and specifically for VBNC L. pneumophila thermal exposure.
For each temperature tested, we observed distinct phases of VBNC cell formation and, depending on the parameters analysed, how temperature impacted the differential development of L. pneumophila subpopulations. The results obtained showed that culturability, among the viability indicators used, was the most sensitive to temperature as previously reported by Hoefel et al. (2003). At 55°C, a complete loss of culturability (8 log reduction) was observed at T3-8h for both L. pneumophila strains. Although differences between strains were observed, both strains were more resistant to temperature than the strains used by Cervero-Aragó et al. (2015) which were reduced by 8 log in approximately 18 min at 55°C. At 60°C, the two L. pneumophila strains stopped growing on plates at T0.5h and T1h. The results are in agreement with a study by Stout et al. (1986), who observed the same reduction after approximately 28 min. Differences between strains could be explained by a distinct composition of the bacterial membranes (Shevchuk et al., 2011) and, as suggested in Sharaby et al., (2017), by a distinct isolation source (clinical vs environmental) that might have exerted a selective pressure on the strains. At 70°C, the two strains were nonculturable at T2min of incubation, as also observed by Cervero-Aragó et al. (2015) despite the different strains used to perform the heat treatments. Thus, at 70°C differences between strains do not longer play a role.
Following culturability, the subpopulations of cells with HE activity and cells with intact membranes were consecutively the most sensitive. At 55°C, it took 150 days to observe a >4 log reduction in the concentration of cells with intact membranes and HE activity. In comparison to 55°C, at 60°C a steeper decreasing trend of the concentration of viable cells was observed. It took 8 days to observe a >4 log reduction in cells with HE activity and 15 days for cells with intact membranes and LE activity. At 70°C cells with intact membranes and HE activity, were reduced by >4 log at T4days and T24h, respectively; it took between 4-8 days to observe the same reduction in the number of LE cells. These data suggest that at temperatures above 55°C, L. pneumophila reduces its metabolic activity before the thermal stress harms the bacterial membranes. Once membranes are harmed, degradation of the DNA takes place as observed by the reduction in the MFI of SGI positively stained cells (McDougald et al., 1998). Strong correlations between viability indicators were observed; particularly at the highest temperatures in which cells could not withstand the stressor and viable cells were uniformly reduced following similar subpopulation dynamics.
The two L. pneumophila strains remained infective for Acanthamoeba and THP-1 cells depending on the temperature used. At 55°C the two L. pneumophila strains infected amoebae and THP-1 cells after heat treatments for 85 and 130 days and after 114 days at 60°C. At 70°C, the two L. pneumophila strains infected amoebae and THP-1 cells for a maximum of 8 days. However, after the total loss of culturability, they infected the host cells with reduced efficiency, requiring higher bacterial concentrations (higher MOIs) and longer co-incubation times as only part of the cells remained infective. Moreover, a high variability between replicates was observed. Similar results were also observed for the same strains under starvation conditions (Dietersdorfer et al., 2018).
The LPS of L. pneumophila is one of the virulence factors associated to the pathogenesis of the bacteria as, as part of the bacterial envelope, it mediates the contact between pathogens and host cells (Shevchuk et al., 2011). Moreover, the high diversity in the LPS composition has been used as serotyping tool for L. pneumophila strains and serogroups, e.g. the Dresden panel. Specifically, the LPS provides several epitopes/binding sites for monoclonal antibodies (mAbs). Among them, the epitope recognized by the mAbs 3/1 from the Dresden panel has been detected in 78% of community acquired and travel-associated LD cases (Reichardt et al., 2010). In their study Reichardt et al., (2010) showed a phase-variable expression of L. pneumophila mAb 3/1 that regulates the hydrophobicity of the bacterial membranes and suggested that such modulation ability maybe linked to the high virulence observed for the mAb 3/1 strains. The major outer-membrane protein (MOMP) is one of the most abundant proteins of L. pneumophila’s envelope and it has been described as an enhancer of bacterial phagocytosis by human monocytic cells (Shevchuk et al., 2011).
In the present study we studied for the first time the impact of thermal stress on the expression of several OMEs likely to correlate with the infectivity of thermally produced VBNC L. pneumophila cells. Our results showed that although in some cases the number of epitopes per cell was reduced at increasing temperatures (e.g. MOMP, 3/1); the total number of cells expressing such epitopes remained stable over time even when neither infection nor viable cells were observed. This supports the idea that their presence per se is not necessarily associated with viability or infectivity (Schrammel et al., 2018b). Hence, we considered VBNC L. pneumophila subpopulations viable when they showed high metabolic activity, an intact membrane with a cytoplasmic space containing DNA (SGI-positive) and, ultimately, were infective. Unfortunately, comparisons between viability indicators and the number of thermally induced VBNC L. pneumophila cells that remained infective at the latest sampling points were not possible because some of the viable subpopulations analysed had a very low cell number (close or below the LOQ). In our previous study on the infectivity of starved VBNC Legionella, we estimated that between 0.7-6.3% of 1×105 cells remained infective at T221days under starvation conditions (Dietersdorfer et al., 2018). Moreover, Al-Bana et al. 2014 calculated that 1-3 out of 100,000 starved VBNC L. pneumophila cells resuscitated in amoebae.
Epalle et al. (2015) monitored the membrane integrity of three L. pneumophila SG1 strains exposed to 50°C, 56°C, 60°C, 65°C and 70°C over 1 h and checked whether thermally induced VBNC L. pneumophila cells at 60°C and 70°C were able to infect macrophage-like cell lines U937, HL-60 and the epithelial cell line A549, as well as the amoeba A. polyphaga. Major differences in the methodology used, such as different preparation of initial cell suspensions, concentrations of cells analysed and a different gating strategy complicate the comparison between studies regarding membrane integrity (e.g. when comparing Allegra et al., 2011, 2008), emphasising the need for standardised protocols in cell-based analyses by flow cytometry (Schrammel et al., 2018a). Overall, it seems that the two L. pneumophila strains in the present study maintained the integrity of the membranes longer than the strains used by Epalle et al. (2015), specifically at temperatures <70°C. That could explain why, in addition to the lower MOIs tested, they did not observe direct infection of human cells after 30 min at 60°C or 70°C. After 30 min at 70°C, 2 out of 3 L. pneumophila strains used in their study infected A. polyphaga using lower MOIs and recovered culturability on BCYE agar plates. After infecting amoebae, the two resuscitated strains were able to infect the U937 and HL-60 cell lines. Although in our study heat-induced infective VBNC L. pneumophila cells were observed in co-culture with the two hosts, they never recovered culturability. In a previous study from our group, the same L. pneumophila strains in a VBNC state induced by starvation conditions recovered culturability only after infecting primary monocyte-derived macrophages (Dietersdorfer et al., 2018) proving again that resuscitation on agar plates is a complex mechanism that requires further research (Pinto et al., 2015).
4.1. Implications of the results obtained on the actual prevention strategies against Legionella
By maintaining the water heater set point temperature at 55°C or 60°C, parts of the L. pneumophila populations — most likely nonculturable — may still infect amoebae for up to 85 days, whereas at 70°C, they may remain infective for up to 8 days. Assuming that VBNC L. pneumophila cells are characterized by an intact membrane and high esterase activity (Schrammel et al., 2018a) at least 1% of the cells were viable after 9 days at 55°C, 8 h at 60°C and 20 min at 70°C. These values represent minimum values as it cannot be ruled out at the moment that cells with intermediate membranes and low esterase activity are viable as well. Although 70°C proved to be effective in reducing the viability of L. pneumophila by >4 log during continuous long-term treatment, the maintenance of an EWS at 70°C for prolonged periods of time through the entire system is neither realistic nor affordable. At the same time, our results showed that a short thermal treatment at 70°C (<1 h) is less effective in reducing viability of culturable and nonculturable L. pneumophila; therefore, a recolonisation of the system cannot be excluded (reviewed in Whiley et al., 2017). Relatively recent studies have shown not only that short thermal shocks at high temperatures can potentially favour the development of thermophilic Legionella strains (Allegra et al., 2011) due to the intrinsic potential of the genus to adapt to a wide range of temperatures (Lesnik et al., 2016; Sharaby et al., 2017) but also that they could enhance a faster recolonisation of the EWS by eliminating competitive bacteria (Ji et al., 2018; Vervaeren et al., 2006). Moreover, among all Legionella species, L. pneumophila is the most commonly detected species in hot water systems, suggesting a better adaptation of the bacteria to high temperatures (Lesnik et al., 2016; Mouchtouri et al., 2007). Such adaptation is likely to be related to a species- or even strain-specific membrane composition (Shevchuk et al., 2011).
Chang et al. (2007) observed that starved VBNC Legionella cells showed a higher resistance towards temperature in terms of membrane integrity than the culturable cells. Thus, the activation of the stringent response due to nutrient scarcity probably activates physiological or structural changes that protect the cells against temperature as well (Chang et al., 2007). Here, we used ultrapure water to produce the bacterial microcosms because it was a more standardisable water matrix. Therefore, an impact of starvation on the results obtained in the present study cannot be ruled out.
In EWS, Legionella lives associated with FLA. Cervero-Aragó et al. (2013) reported that trophozoites of Acanthamoeba and Vermamoeba, two of the most prevalent amoebae in EWS (Thomas and Ashbolt, 2011), were sensitive to temperatures >50°C, whereas their cystic forms withstood higher temperatures. Thus, maintaining a temperature of 60°C would inactivate amoebic trophozoites and promote their encystment, blocking their availability as natural replicators of Legionella. Moreover, as noted by Bédard et al. (2015), the thermal regime must be combined with good system hydraulics that ensure a delivery of high temperature to the terminal ends (e.g. taps, showers) the common areas likely to be colonised by Legionella due to the lower temperatures and chlorine concentrations. Additionally, more control on the water use frequency might help managing the microbial community in these terminals (Ji et al., 2018). Finally, the effectiveness of the thermal regime implemented should be monitored over time. According to legislation, in many countries, such surveillance is made using standard culture-based methods. Although these methods are not useful for detecting bacteria in the VBNC state, here we observed that they provide useful information about the concentration of the most virulent bacterial subpopulations.
Conclusions
Currently, there is a big debate about the significance and health relevance of the VBNC state of Legionella. In this study, we could show that thermally-induced VBNC L. pneumophila may still be infective on amoebae and THP-1. However, they do so with reduced efficiency, most likely due to a decreasing number of infective cells and to a lower infectivity of VBNC compared to culturable cells. Whether they would be infective for humans needs to be further studied. In any case, infection of amoebae could lead to a recolonisation of the system.
An EWS that maintains a constant thermal regime at 60°C in the water heater tank and recirculating lines and has good hydraulics that ensure that a high temperature reaches all parts of the system may not only avoid the proliferation of culturable but also of VBNC L. pneumophila according to our results. This temperature would also reduce the availability of amoebae as natural replicators of the bacteria. Our results also showed that a short (<1 h) exposure to 70°C is less effective in reducing viability of culturable and nonculturable L. pneumophila. Further research is needed to see how different thermal treatments in simulating plumbing conditions impact the viability of L. pneumophila within mixed microbial communities.
To provide effective system surveillance, culture-based methods provide information about the concentration of the most virulent Legionella. Cell-based methods and the use of OMEs might be potential complementary tools to study and detect Legionella VBNC cells. However, before their application in risk assessment, further development is needed to use them in mixed environmental samples with low Legionella concentrations, with similar efficiency as culture based methods.
Supplementary Material
Acknowledgements
This study was supported by the Austrian Science Fund (FWF) [Project P24535-B22] and the Austrian Federal Ministry for Science and Research (HSRSM) [Project LE103HS001]. We thank the staff of the Water Hygiene Unit and the Molecular Parasitology Unit at the Centre for Pathophysiology, Infectiology and Immunology, Medical University of Vienna. We also thank A. Ohradanova-Repic, H. Stockinger and specifically V. Mündler from our institute for helping us with the THP-1 cell line.
References
- Al-Bana BH, Haddad MT, Garduño RA. Stationary phase and mature infectious forms of Legionella pneumophila produce distinct viable but non-culturable cells. Environ Microbiol. 2014;16:382–395. doi: 10.1111/1462-2920.12219. [DOI] [PubMed] [Google Scholar]
- Allegra S, Berger F, Berthelot P, Grattard F, Pozzetto B, Riffard S. Use of flow cytometry to monitor Legionella viability. Appl Environ Microbiol. 2008;74:7813–7816. doi: 10.1128/AEM.01364-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegra S, Grattard F, Girardot F, Riffard S, Pozzetto B, Berthelot P. Longitudinal evaluation of the efficacy of heat treatment procedures against Legionella spp. in hospital water systems by using a flow cytometric assay. Appl Environ Microbiol. 2011;77:1268–1275. doi: 10.1128/AEM.02225-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alleron L, Khemiri A, Koubar M, Lacombe C, Coquet L, Cosette P, Jouenne T, Frere J. VBNC Legionella pneumophila cells are still able to produce virulence proteins. Water Res. 2013;47:6606–17. doi: 10.1016/j.watres.2013.08.032. [DOI] [PubMed] [Google Scholar]
- Bartram J, Chartier Y, Lee JV, Bond K, Surman-Lee S. Legionella and the Prevention of Legionellosis. 2007 [Google Scholar]
- Bédard E, Fey S, Charron D, Lalancette C, Cantin P, Dolcé P, Laferrière C, Déziel E, Prévost MM, Laferriére C, Déziel E, Prévost MM. Temperature diagnostic to identify high risk areas and optimize Legionella pneumophila surveillance in hot water distribution systems. Water Res. 2015;71:244–56. doi: 10.1016/j.watres.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Buse HY, Schoen ME, Ashbolt NJ. Legionellae in engineered systems and use of quantitative microbial risk assessment to predict exposure. Water Res. 2012;46:921–33. doi: 10.1016/j.watres.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Cervero-Aragó S, Rodríguez-Martínez S, Canals O, Salvadó H, Araujo RM. Effect of thermal treatment on free-living amoeba inactivation. J Appl Microbiol. 2013;116:1–9. doi: 10.1111/jam.12379. [DOI] [PubMed] [Google Scholar]
- Cervero-Aragó S, Rodríguez-Martínez S, Puertas-Bennasar A, Araujo RMRM. Effect of Common Drinking Water Disinfectants, Chlorine and Heat, on Free Legionella and Amoebae-Associated Legionella. PLoS One. 2015;10:e0134726. doi: 10.1371/journal.pone.0134726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-W, Hwang Y-H, Cheng W-Y, Chang C-P. Effects of chlorination and heat disinfection on long-term starved Legionella pneumophila in warm water. J Appl Microbiol. 2007;102:1636–1644. doi: 10.1111/j.1365-2672.2006.03195.x. [DOI] [PubMed] [Google Scholar]
- Declerck P. Biofilms: the environmental playground of Legionella pneumophila. Environ Microbiol. 2010;12:557–66. doi: 10.1111/j.1462-2920.2009.02025.x. [DOI] [PubMed] [Google Scholar]
- Dietersdorfer E, Cervero-Aragó S, Sommer R, Kirschner AK, Walochnik J. Optimized methods for Legionella pneumophila release from its Acanthamoeba hosts. BMC Microbiol. 2016;16:74. doi: 10.1186/s12866-016-0691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietersdorfer E, Kirschner A, Schrammel B, Ohradanova-Repic A, Stockinger H, Sommer R, Walochnik J, Cervero-Aragó S. Starved viable but non-culturable (VBNC) Legionella strains can infect and replicate in amoebae and human macrophages. Water Res. 2018;141 doi: 10.1016/j.watres.2018.01.058. [DOI] [PubMed] [Google Scholar]
- ECDC. Legionnaires’ disease - Annual Epidemiological Report for 2015. 2017 https://ecdc.europa.eu/en/publications-data/legionnaires-disease-annual-epidemiological-report-2015.
- Edelstein PH. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J Clin Microbiol. 1981;14:298–303. doi: 10.1128/jcm.14.3.298-303.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epalle T, Girardot FF, Allegra SS, Maurice-Blanc CC, Garraud O, Riffard S. Viable but Not Culturable Forms of Legionella pneumophila Generated After Heat Shock Treatment Are Infectious for Macrophage-Like and Alveolar Epithelial Cells After Resuscitation on Acanthamoeba polyphaga. Microb Ecol. 2015;69:215–224. doi: 10.1007/s00248-014-0470-x. [DOI] [PubMed] [Google Scholar]
- European Study Group for Legionella Infections (ESGLI) European Technical Guidelines for the Prevention, Control and Investigation, of Infections Caused by Legionella species. 2017 [Google Scholar]
- Farhat M, Trouilhé M-C, Briand E, Moletta-Denat M, Robine E, Frère J. Development of a pilot-scale 1 for Legionella elimination in biofilm in hot water network: heat shock treatment evaluation. J Appl Microbiol. 2010;108:1073–82. doi: 10.1111/j.1365-2672.2009.04541.x. [DOI] [PubMed] [Google Scholar]
- Füchslin HP, Kötzsch S, Keserue HA, Egli T. Rapid and quantitative detection of Legionella pneumophila applying immunomagnetic separation and flow cytometry. Cytom Part A. 2010;77:264–274. doi: 10.1002/cyto.a.20858. [DOI] [PubMed] [Google Scholar]
- Helbig JH, Kurtz JB, Pastoris MC, Pelaz C, Lück PC. Antigenic lipopolysaccharide components of Legionella pneumophila recognized by monoclonal antibodies: Possibilities and limitations for division of the species into serogroups. J Clin Microbiol. 1997;35:2841–2845. doi: 10.1128/jcm.35.11.2841-2845.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefel D, Grooby WL, Monis PT, Andrews S, Saint CP. Enumeration of water-borne bacteria using viability assays and flow cytometry: A comparison to culture-based techniques. J Microbiol Methods. 2003;55:585–597. doi: 10.1016/S0167-7012(03)00201-X. [DOI] [PubMed] [Google Scholar]
- Ji P, Rhoads WJ, Edwards MA, Pruden A. Effect of heat shock on hot water plumbing microbiota and Legionella pneumophila control. Microbiome. 2018;6:1–14. doi: 10.1186/s40168-018-0406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner AKT. Determination of viable legionellae in engineered water systems: Do we find what we are looking for? Water Res. 2016;93:276–88. doi: 10.1016/j.watres.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnik R, Brettar I, Höfle MG. Legionella species diversity and dynamics from surface reservoir to tap water: from cold adaptation to thermophily. ISME J. 2016;10:1064–1080. doi: 10.1038/ismej.2015.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougald D, Rice SA, Weichart D, Kjelleberg S. Nonculturability: Adaptation or debilitation? FEMS Microbiol Ecol. 1998;25:1–9. doi: 10.1016/S0168-6496(97)00063-9. [DOI] [Google Scholar]
- Mouchtouri V, Velonakis E, Hadjichristodoulou C. Thermal disinfection of hotels, hospitals, and athletic venues hot water distribution systems contaminated by Legionella species. Am J Infect Control. 2007;35:623–7. doi: 10.1016/j.ajic.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Phin N, Parry-Ford F, Harrison T, Stagg HR, Zhang N, Kumar K, Lortholary O, Zumla A, Abubakar I. Epidemiology and clinical management of Legionnaires’ disease. Lancet Infect Dis. 2014;14:1011–1021. doi: 10.1016/S1473-3099(14)70713-3. [DOI] [PubMed] [Google Scholar]
- Pinto D, Santos MA, Chambel L. Thirty years of viable but nonculturable state research: Unsolved molecular mechanisms. Crit Rev Microbiol. 2015;41:61–76. doi: 10.3109/1040841X.2013.794127. [DOI] [PubMed] [Google Scholar]
- Reichardt K, Jacobs E, Röske I, Helbig JH. Legionella pneumophila carrying the virulence-associated lipopolysaccharide epitope possesses two functionally different LPS components. Microbiology. 2010;156:2953–2961. doi: 10.1099/mic.0.039933-0. [DOI] [PubMed] [Google Scholar]
- Rhoads WJ, Ji P, Pruden A, Edwards MA. Water heater temperature set point and water use patterns influence Legionella pneumophila and associated microorganisms at the tap. Microbiome. 2015 doi: 10.1186/s40168-015-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrammel B, Cervero-Aragó S, Dietersdorfer E, Walochnik J, Lück C, Sommer R, Kirschner A. Differential development of Legionella sub-populations during short- and long-term starvation. Water Res. 2018a;141:417–427. doi: 10.1016/j.watres.2018.04.027. [DOI] [PubMed] [Google Scholar]
- Schrammel B, Petzold M, Cervero-Aragó S, Sommer R, Lück C, Kirschner A. Persistent presence of outer membrane epitopes during short- and long-term starvation of five Legionella pneumophila strains. BMC Microbiol. 2018b;18:75. doi: 10.1186/s12866-018-1220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharaby Y, Rodríguez-Martínez S, Oks O, Pecellin M, Mizrahi H, Peretz A, Brettar I, Höfle MG, Halpern M. Temperature-Dependent Growth Modeling of Environmental and Clinical Legionella pneumophila Multilocus Variable-Number Tandem-Repeat Analysis (MLVA) Genotypes. Appl Environ Microbiol. 2017;83:1–13. doi: 10.1128/aem.03295-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchuk O, Jäger J, Steinert M. Virulence properties of the Legionella pneumophila cell envelope. Front Microbiol. 2011;2:1–12. doi: 10.3389/fmicb.2011.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert M, Ockert G, Lück C, Hacker J. Regrowth of Legionella pneumophila in a heat-disinfected plumbing system. Zentralblatt fur Bakteriol. 1998;288:331–342. doi: 10.1016/S0934-8840(98)80005-4. [DOI] [PubMed] [Google Scholar]
- Stout JE, Best MG, Yu VL. Susceptibility of members of the family Legionellaceae to thermal stress: Implications for heat eradication methods in water distribution systems. Appl Environ Microbiol. 1986;52:396–399. doi: 10.1128/aem.52.2.396-399.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JM, Ashbolt NJ. Do free-living amoebae in treated drinking water systems present an emerging health risk? Environ Sci Technol. 2011;45:860–9. doi: 10.1021/es102876y. [DOI] [PubMed] [Google Scholar]
- Türetgen I. Induction of viable but nonculturable (VBNC) state and the effect of multiple subculturing on the survival of Legionella pneumophila strains in the presence of monochloramine. 2008;58:153–156. [Google Scholar]
- Vervaeren H, Temmerman R, Devos L, Boon N, Verstraete W. Introduction of a boost of Legionella pneumophila into a stagnant-water model by heat treatment. FEMS Microbiol Ecol. 2006;58:583–592. doi: 10.1111/j.1574-6941.2006.00181.x. [DOI] [PubMed] [Google Scholar]
- Whiley H, Bentham R, Brown MH. Legionella persistence in manufactured water systems: Pasteurization potentially selecting for thermal tolerance. Front Microbiol. 2017;8 doi: 10.3389/fmicb.2017.01330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.