Abstract
AIM
To investigate dose-dependent effects of N-methyl-D-aspartate (NMDA) on retinal and optic nerve morphology in rats.
METHODS
Sprague Dawley rats, 180-250 g in weight were divided into four groups. Groups 1, 2, 3 and 4 were intravitreally administered with vehicle and NMDA at the doses 80, 160 and 320 nmol respectively. Seven days after injection, rats were euthanized, and their eyes were taken for optic nerve toluidine blue and retinal hematoxylin and eosin stainings. The TUNEL assay was done for detecting apoptotic cells.
RESULTS
All groups treated with NMDA showed significantly reduced ganglion cell layer (GCL) thickness within inner retina, as compared to control group. Group NMDA 160 nmol showed a significantly greater GCL thickness than the group NMDA 320 nmol. Administration of NMDA also resulted in a dose-dependent decrease in the number of nuclei both per 100 µm GCL length and per 100 µm2 of GCL. Intravitreal NMDA injection caused dose-dependent damage to the optic nerve. The degeneration of nerve fibres with increased clearing of cytoplasm was observed more prominently as the NMDA dose increased. In accordance with the results of retinal morphometry analysis and optic nerve grading, TUNEL staining demonstrated NMDA-induced excitotoxic retinal injury in a dose-dependent manner.
CONCLUSION
Our results demonstrate dose-dependent effects of NMDA on retinal and optic nerve morphology in rats that may be attributed to differences in the severity of excitotoxicity and oxidative stress. Our results also suggest that care should be taken while making dose selections experimentally so that the choice might best uphold study objectives.
Keywords: glaucoma, excitotoxicity, N-methyl-D-aspartate, retina, optic nerve, dose-dependent effects
INTRODUCTION
Glaucoma is defined as a chronic eye disease with progressive optic neuropathy, and can result in permanent vision loss. According to the World Health Organization, it is one of the main cause of blindness and visual disability worldwide. The number of individuals with glaucoma related blindness will increase approximately to 111.8 million in 2040[1]–[2]. Affected patients are commonly asymptomatic, and the condition is often only detected at its later stages. The pathology of of glaucoma remains poorly understood[3]–[5]. As elevated intraocular pressure (IOP) has been recognised as one of the main risk factors for glaucomatous optic nerve damage, the current medical therapies are limited to the IOP lowering[6]–[7]. Recent study indicates that reduction in IOP does not always prevent retinal ganglion cells (RGC) loss and optic neuropathy.
N-methyl-D-aspartate (NMDA)-mediated excitotoxicity represents a common pathway for glaucomatous neuropathy and it may therefore be considered as one of the targets in neuroprotective strategies[3],[8]–[11]. To better understand involvement of excitotoxicity in the pathogenesis of glaucomatous neuropathy, investigators in the past have intravitreally injected rats with excitatory neurotransmitters, or their analogues [e.g. kainite, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and NMDA], to induce glaucomatous-like RGC injury[12]–[26]. In particular, NMDA has been considered as a potential agent to serve as an instrument for studying excitotoxicity-related RGC death. Evidence from previous studies exploring excitotoxicity following NMDA exposure is shown in Table 1. Accordingly the excitotoxic effects of NMDA have been studied up to a maximum dose of 200 nmol. Since, the degree of injury caused by NMDA might vary in a dose-dependent manner due to differences in the cellular and molecular targets, it is important to study the effects of NMDA at a dose range over 200 nmol. Moreover, it would be interesting to see if at this higher dose range NMDA induced changes in retinal and optic nerve morphology are dose-dependent. Therefore, the aim of this paper was to elucidate the effect of different doses of NMDA on optic nerve and inner retinal layer morphology in rats at the dose range of 80-320 nmol.
Table 1. Previous studies exploring NMDA-induced retinal excitotoxicity.
| Dose | Evaluated parameters | References |
| Single 2 µL intravitreal injection of 3 different NMDA concentrations (10, 20 and 40 nmol) | H&E staining, TEM, TB and morphometry analysis | 17 |
| Single 2 µL intravitreal injection of 10 mmol NMDA solution | Labelling with retrograde tracer hydroxystilbamidine and RGC counting in flat-mounts of retina | 18 |
| Single 2 µL intravitreal injection of 0.2, 1, 4, 10, or 40 mmol NMDA (corresponding to 0.8, 2, 8, 20 and 80 nmol, respectively) | TUNEL, TEM and morphometry analysis | 19 |
| Single intravitreal injection of 200 nmol NMDA | H&E staining, TUNEL and immunohistochemistry staining | 20, 21 |
| Single 2 µL intravitreal injection of 20 mmol NMDA | Labelling with retrograde tracer Fast Blue and RGC counting in flat-mounts of retina | 22 |
| Single 2 µL intravitreal injection of 20 mmol NMDA | Retrograde labelling with fluorescent tracer FluoroGold and RGC counting in flat-mounts of retina, and immunohistochemistry staining | 23 |
| Single 5 µL intravitreal injection of 0.4 to 40 mmol/L NMDA (corresponding to 2 to 200 nmol, respectively) | Cresyl violet stain with morphometry and choline acetyltransferase assay | 12 |
| Single 5 µL intravitreal injection of 200 nmol NMDA solution | H&E staining | 24 |
| Single 5 µL intravitreal injection of 200 nmol NMDA solution | H&E staining, retrograde labelling with fluorescent tracer FluoroGold and RGC counting in flat-mounts of retina | 25 |
| Single 5 µL intravitreal injection of 2, 10, 20 and 200 nmol NMDA | TUNEL, retrograde labelling with fluorescent tracer FluoroGold and RGC counting in flat-mounts of retina, immunohistochemistry staining | 26 |
H&E: Haematoxylin and eosin stain; TEM: Transmission electron microscopy; TB: Toluidine blue; RGC: Retinal ganglion cells.
MATERIALS AND METHODS
Ethical Approval
All experimental procedures were carried out in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and UiTM animal ethics guidelines (UiTM Care: 118/2015).
Animals
Adult Sprague Dawley rats (180-250 g body weight) were acquired from the Laboratory Animal Care Unit (LACU) of Universiti Teknologi MARA (UiTM), Faculty of Medicine, Sungai Buloh Campus. The rats were maintained under standard laboratory conditions of a 12:12 light-dark cycle at 22°C-23°C, with ad libitum access to food and tap water. All animals were subjected to general and ophthalmic examinations, and only healthy rats were later taken into the study.
Study Design
Forty rats were randomly divided into 4 groups of 10 each: Group 1: control (PBS); group 2: 80 nmol (NMDA); group 3: 160 nmol (NMDA); group 4: 320 nmol (NMDA).
Intravitreal injections were administered in both eyes. Tropicamide at a 1% concentration was used to dilate pupils 10min before the injection. For anaesthesia, a mixture of xylazine (12 mg/kg) and ketamine (80 mg/kg; Troy Laboratories Australia Pty Ltd., Australia) was given through intraperitoneal injection. Powdered NMDA (≥98%, Sigma-Aldrich) was dissolved in 0.1 mol/L of phosphate buffered saline (PBS) to obtain solutions of 80, 160, and 320 nmol. Injections were carried out with a 30-gauge needle mounted on a 10-µL Hamilton syringe. A dissecting microscope was used to insert the needle at the dorsal limbus of the eye. Injection volume was 2 µL. The procedure was performed slowly, over two minutes, to avoid reflux. Enucleation of the eyes was done one week after injection and optic nerve was then isolated. A suture was applied on the globe to mark the orientation, and the enucleated eyes were fixed using 10% formaldehyde for 24h at room temperature (24°C)[14],[27]–[28].
Assessment of Retinal Morphology Using Haematoxylin and Eosin Staining
The eyes were bisected at the equator, and then transferred through increasing concentrations of alcohol, followed by paraffin embedding. Next, section series were cut at 3 µm thickness and stained with H&E. Images were taken using Nikon light microscope (at 20× magnification) and a digital camera and analysed by ImageJ software (NIH, Bethesda, MA, USA). The following parameters were observed and evaluated were independently by two researchers on three randomly selected fields of view: thickness of ganglion cell layer (GCL), thickness of inner retina, area of GCL, area of inner retina, length of GCL. These parameters were used for measuring the thickness of GCL within inner retina, number of nuclei per 100 µm GCL length, and number of nuclei per 100 µm2 of GCL and inner retina[14],[16],[28]–[34]. The two observer's measurements were averaged, and the mean figures used for statistical analysis.
Assessment of Optic Nerve Morphology Using Toluidine Blue Staining
Optic nerve was dissected with scissors at a distance of 1 mm from the eyeball, and overnight fixated using 10% formaldehyde. Then, the tissue was transferred through increasing concentrations of alcohol, followed by paraffin embedding. Section series were cut at 1 µm thickness and then were stained with 1% toluidine blue (Sigma-Aldrich, USA). Examination of the optic nerve was carried out by two independent masked investigators at full cross-sectional view. Extent of injury was quantified according to the following grades[30]–[34]: grade 1: normal axon; grade 2: early, mild lesions in one area with presence of moderate axon degeneration; grade 3: axon degeneration spread to the other nerve area; grade 4: severe damage to axons, with percentage of normal axons equal to that of degenerated axons; grade 5: majority of axons are degenerating across all regions of tissue. The data was analysed by using the mean grades.
TUNEL Assay of Retinal Sections
A TUNEL assay was done for detecting apoptotic DNA fragmentation. The technique was done according to manufacturer's protocol for the ApoBrdU-IHCTM DNA Fragmentation Assay Kit (BioVision, Inc., USA) as it was previously described by Nor Arfuzir et al[30],[32] and Lambuk et al[31],[34]. Serial 3-µm-thick retinal sections were deparaffinised, and then proteinase K antigen retrieval was performed for 20min. This was followed by rinsing in PBS for three minutes. Then, the sections were covered with 100 µL of 3% H2O2 (1:10), diluted in methanol for five minutes, and rinsed again. This was followed by incubation in BrdUTP overnight at room temperature (37°C). Sections were then washed in PBS. Next, sections were incubated for 1.5h with anti-BrdU biotin in a dark, humid chamber. The 3′-Diaminobenzidine (DAB) chromogen solution was used to cover the entire tissue section and to incubate for 15min. This was followed by rinsing them three times, two minutes per rinse. Then, sections were counterstained with methyl green for three minutes. Slides were mounted and air-dried prior to observation under a light microscope at 20×magnification. Apoptotic signal measurements in the GCL were carried out using ImageJ software (NIH, Bethesda, MA, USA). Assessments were completed by two masked independent investigators, and the data were averaged for each eye.
Statistical Analysis
Analysed data were presented as mean difference (averaged between the two investigators)±SD. Statistical analyses were performed using one-way ANOVA with Bonferroni's adjustment; P<0.05 was considered significant.
RESULTS
Effect of Various Doses of NMDA on Retinal Morphology
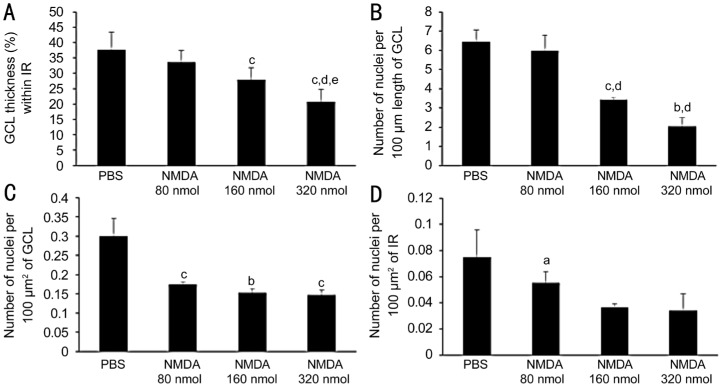
H&E-stained sections were used for morphometric analysis of retinal layers. Microscopic examination showed relatively thinner inner retina and GCL, and reduced number of nuclei within GCL and inner retina, in all NMDA-injected groups, as compared to the vehicle-injected group. Both layers showed greater thinning with increasing NMDA doses (Figure 1). Quantitative evaluation of retinal morphometric changes in response to NMDA exposure, is presented in Figure 2. The parameters measured included, thickness of GCL within inner retina (%), and number of nuclei within GCL and inner retina. All groups treated with NMDA (80, 160 and 320 nmol) showed significantly reduced GCL thickness, with mean values of 33.61±3.82 (P>0.05), 27.98±3.76 (P<0.001), and 20.64±4.09 (P<0.001), as compared to the control group with mean value of 37.75±5.61 (all values %; Figure 2A). Group NMDA 160 nmol showed a significantly greater GCL thickness than the group NMDA 320 nmol (P<0.01). Administration of NMDA also resulted in a dose-dependent decrease in the number of nuclei per 100 µm GCL length. Number of nuclei per 100 µm GCL length in NMDA 160 and NMDA 320 nmol groups was decreased by 1.89-fold, a significant difference as compared to the control group (P<0.01, P<0.001, respectively). No significant difference was observed between the NMDA 80 nmol and PBS groups (P>0.05; Figure 2B). The same parameter in the NMDA 160 nmol and NMDA 320 nmol groups was 1.75- and 1.68-fold lower (P<0.01, P<0.05, respectively) than that in the NMDA 80 nmol group. Number of nuclei per 100 µm2 of GCL was 1.72-, 1.96- and 2.03-fold lower in the NMDA 80, 160 and 320 nmol groups, respectively, than in the control group (P<0.001, P<0.01, P<0.001; Figure 2C). However, the observed effect was not dose-dependent, and no significant difference was observed among the NMDA 80, 160, and 320 nmol groups. The number of retinal cell nuclei per 100 µm2 of inner retina, which includes the GCL and the inner plexiform layer (IPL), is shown in Figure 2D. Group NMDA 320 nmol showed the lowest retinal cell nuclei density, with a mean value of 0.34±0.01 nuclei per 100 µm2; the control group showed a mean value of 0.07±0.02 (P<0.01). However, there were no significant differences between groups NMDA 160 and 320 nmol, as shown in Figure 2D. Groups NMDA 80 and 160 nmol, with mean retinal cell densities of 0.06±0.01, 0.04±0.02 nuclei per 100 µm2, respectively, showed no significant differences when compared to the control group (P>0.05).
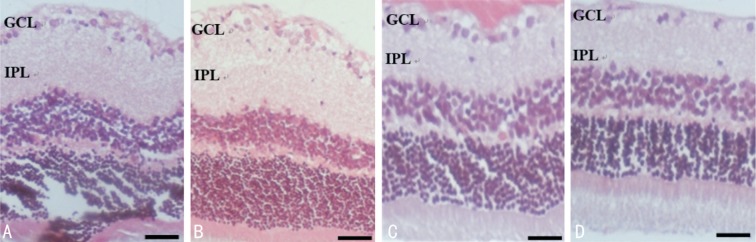
Figure 1. Representative microphotographs of rat retinal sections stained with H&E after administering different doses of NMDA.
A: The PBS group, normal retinal histology; B-D: The NMDA 80, 160 and 320 nmol groups, respectively. NMDA injection, particularly at higher doses, caused severe degenerative changes, with an initial stage of massive cellular swelling, and subsequently, retinal cells loss. Scale bar represents 100 µm. GCL: Ganglion cell layer; IPL: Inner plexiform layer.
Figure 2. Quantitative morphometric analysis of inner retinal changes in response to various doses of NMDA.
A: Thickness of GCL within inner retina (%); B: No. of ganglion cells nuclei per 100 µm GCL length; C: No. of ganglion cells nuclei per 100 µm2 of GCL; D: No. of nuclei per 100 µm2 of inner retina. Group 1 was injected with PBS (vehicle), group 2 was injected with 80 nmol NMDA, group 3 was injected with 160 nmol NMDA, and group 4 was injected with 320 nmol NMDA. Inner retina=GCL+IPL. n=10, aP<0.05 vs PBS, bP<0.01 vs PBS, cP<0.001 vs PBS, dP<0.001 vs NMDA 80 nmol, and eP<0.01 vs NMDA 160 nmol.
Effect of Various Doses of NMDA on Optic Nerve Morphology
Toluidine blue staining was used to grade morphological degenerative changes of the optic nerve. Intravitreal NMDA injection caused dose-dependent damage to the optic nerve. The PBS group featured healthy, intact optic nerve morphology, with no injury to axons with the lowest mean grade (Figure 3). Groups NMDA 160 nmol and 320 nmol showed 1.5- and 1.65-fold higher mean values than did the PBS group (P<0.05 and P<0.001, respectively; Figure 4). NMDA 320 nmol group had a 1.52-fold higher mean grade than did group NMDA 80 nmol (P<0.05). No significant differences were found between between the NMDA 80 nmol and PBS groups. The degeneration of nerve fibres, with increased clearing of cytoplasm, was observed more prominently as the NMDA dose increased.
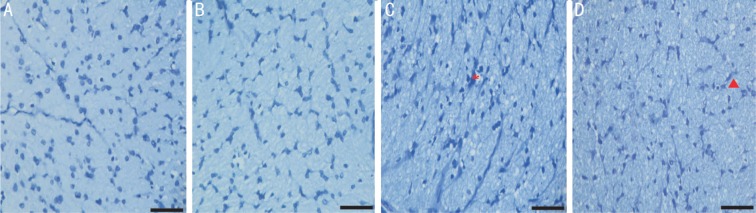
Figure 3. Representative microphotographs of optic nerve semithin sections stained with toluidine blue.
A: The PBS group, normal optic nerve histology; B-D: Groups of NMDA 80, 160 and 320 nmol, respectively; B: Comparable distribution of glial cells to the control group with slight degeneration of nerve fibers; C: Denser glial cells with degeneration of nerve fibers; D: Presence of glial cells and extensive vacuolation indicates progressive degenerative changes occupying the entire section. Scale bar represents 50 µm. Asterisk indicates nerve fiber degeneration; Triangle symbol indicates vacuolation.
Figure 4. Quantitative estimation of the effect of intravitreal administration of different doses of NMDA on optic nerve morphology.
PBS group served as the control. n=6, aP<0.05 vs PBS, bP<0.001 vs PBS, cP<0.05 vs NMDA 80 nmol.
Effect of Various Doses of NMDA on Retinal Cell Survival
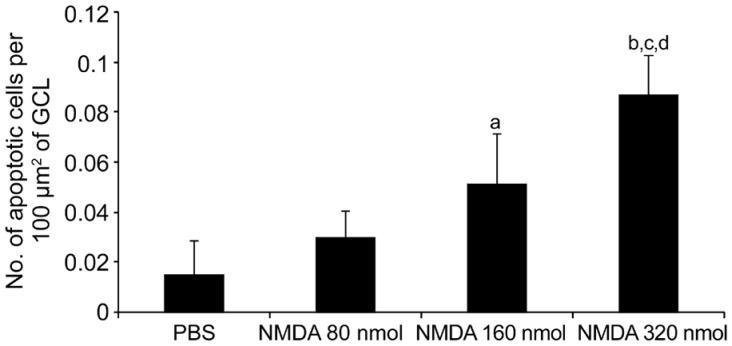
In order to detect the presence of apoptotic cells in GCL, a TUNEL assay was performed on retinal sections. In accordance with the results of H&E staining, TUNEL assay further demonstrated a dose-dependent effect on retinal cell apoptosis by NMDA. As seen in Figure 5, there is a clear trend of increase in the number of apoptotic cells with increasing NMDA dose. Number of TUNEL-positive signals was most abundant in the NMDA 320 nmol group, with 5.7-, 2.89-, and 1.69-fold higher values than those in the PBS, NMDA 80 nmol and NMDA 160 nmol groups, respectively (Figure 6). Despite the lower number of apoptotic cells in the NMDA 80 nmol group, 1.97-fold higher than the PBS group, no significant difference among the groups was evident. NMDA 160 nmol group showed a significantly higher number of apoptotic cells than did the PBS group, a 3.37-fold increase (P<0.01), but this figure was comparable with that of the NMDA 80 nmol group. Intravitreal NMDA injection, particularly at 320 nmol, caused massive degeneration of retinal cells.
Figure 5. Representative TUNEL stained microphotographs of retinal sections.
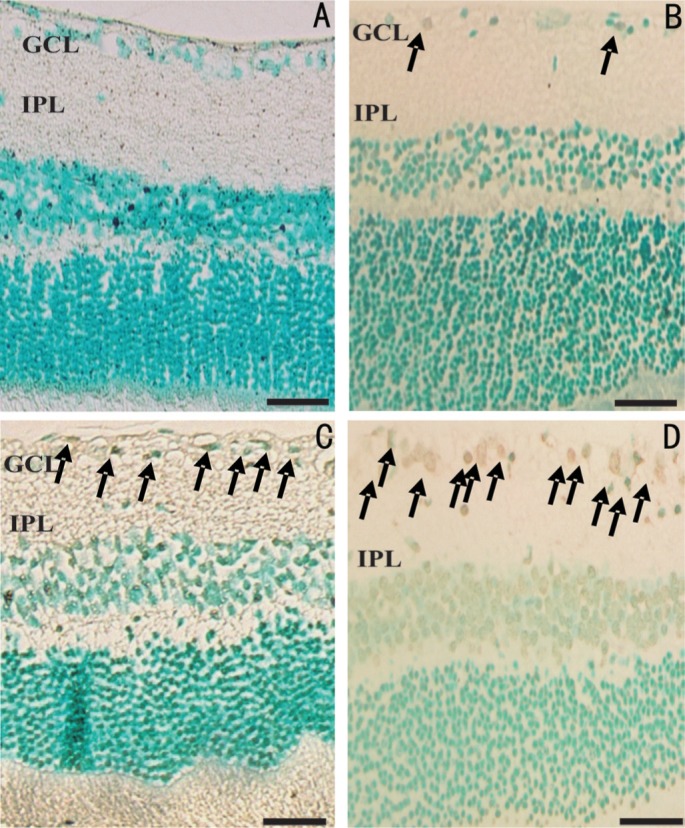
A: PBS group, no apoptotic signal was detected in control group; B-D: Groups of NMDA 80, 160 and 320 nmol, respectively; B: Few detection of apoptosis ganglion cells; C: Several apoptotic signals were detected; D: A multiple presence of apoptotic signals was observed. Scale bar represents 100 µm; arrows indicate the presence of apoptotic cells. GCL: Ganglion cell layer; IPL: Inner plexiform layer.
Figure 6. Quantitative estimation of the retinal cell apoptosis in response to various doses of NMDA, as measured by TUNEL staining.
PBS served as the control. n=6, aP<0.01 vs PBS, bP<0.001 vs PBS, cP<0.01 vs NMDA 80 nmol, dP<0.01 vs NMDA 160 nmol.
DISCUSSION
The deleterious effect of glutamate on RGCs is mediated by NMDA receptors. In excitotoxicity, glutamate triggers the elevation in intracellular Ca2+, followed by the upregulation of neuronal nitric oxide synthases, reactive oxygen species production by mitochondria, dysfunction of mitochondria, and activation of endoplasmic reticulum stress signalling pathways[4],[35]–[38]. Accordingly, the therapeutic potential of NMDA-receptor antagonists has been investigated as tools to inhibit the RGCs loss and to delay sight loss from glaucoma[39]–[41].
Since use of glutamate in experimental procedures produces effects involving all glutamate subtype of receptors, NMDA is used as an instrument to study RGC death due to NMDA-specific excitotoxicity. Hence, previous studies have explored retinal excitotoxicity following exposure to NMDA. At lower doses, acute intravitreal injection of NMDA can injure the GCL and IPL, without prominent effect on other retinal layers[42]. Importantly, it appears likely that intravitreal glutamate injection induced excitotoxic effects in the adult rat retina leading to degenerative changes may be dose-dependent. In one of the studies using a low dose of NMDA (1 µmol) over prolonged period of 2mo, it was observed that degenerative changes in retina progressed in two degenerative stages: 1) the first stage is characterised by massive cellular swelling, and 2) the second stage is characterised by necrotic cell death. Degenerative changes observed in the GCL and inner nuclear layer cells and decreased inner retina thickness appeared concurrently. Two months after injection, degenerative changes in rod cells and some loss of cone photoreceptors nuclei were also observed[43]. However, in order to use NMDA to induce acute degenerative insult, it is important to determine the optimal dose of NMDA that can induce intended severity of retinal degeneration. In one of the studies, intravitreal NMDA in the dose range of 0.1-40 mmol led to dose-dependent cleavage of nuclear DNA into internucleosomal fragments that appeared as TUNEL-positive nuclei in the inner retina. Ultrastructural features consistent with apoptotic changes were described in degenerating RGCs and the cells of inner nuclear layers. Finally a conclusion was drawn that NMDA-induced excitotoxicity involved caspase-involved apoptosis[19].
In the current study, we used the dose range of 80-320 nmol of NMDA and studied the morphological evidence of dose-dependent NMDA-induced inner retina cell loss, and optic nerve damage. We also studied if morphological changes mirrored dose-dependent increases in the number of TUNEL-positive cells. It was clearly demonstrated that NMDA induces retinal and optic nerve morphological changes in a dose-dependent manner and in accordance with changes in morphology we observed increasing number of TUNEL+ cells with increasing dose of NMDA. The findings in the current study are in accordance with previous studies that also demonstrated dose-dependent loss of RGCs and damage to IPL in a dose-dependent manner with NMDA 20-200 nmol[12],[44]. Siliprandi et al[12] showed that the sensitivity of RGCs to NMDA is affected by the size of RGCs and moreover amacrine cells may have dose-dependent sensitivity to NMDA-induced excitotoxicity. In cultured neonatal rat cardiomyocytes, NMDA in the dose range of 10−2 to 10−6 mol/L was shown to cause dose-dependent increase in intracellular Ca2+ and cell death[45]. In hippocampal neuronal cultures, NMDA has been shown to induce production of reactive oxygen species in a dose-dependent manner[46]. It also causes activation of caspase-3 in a dose-dependent manner[47]. Hence, the findings of the current study may be attributed to dose-dependent effects of NMDA on several intracellular events. It is, however, likely that the proportion of NMDA-induced morphological changes may not match the proportional change in the dose. Hence, it is important to optimize the NMDA dose based on the study objectives. Our results suggest that care should be taken while making dose selections experimentally, so that the choice might best uphold study objectives.
Acknowledgments
Foundations: Supported by Universiti Teknologi MARA [No.600-IRMI/MYRA5/3/BESTARI (004/2017); No.600-IRMI/DANA5/3/LESTARI (0076/2016); No.600-IRMI/MyRA5/3/LESTARI (0088/2016)].
Conflicts of Interest: Lambuk L, None; Jafri AJA, None; Iezhitsa I, None; Agarwal R, None; Bakar NS, None; Agarwal P, None; Abdullah A, None; Ismail NM, None.
REFERENCES
- 1.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Chiu SL, Chu CL, Muo CH, Chen CL, Lan SJ. The prevalence and the incidence of diagnosed open-angle glaucoma and diagnosed angle-closure glaucoma: changes from 2001 to 2010. J Glaucoma. 2016;25(5):e514–e519. doi: 10.1097/IJG.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R. Recent advances in pathophysiology and pharmacotherapy of glaucoma. Med Heal Rev. 2009;1(2):75–94. [Google Scholar]
- 4.Ebneter A, Chidlow G, Wood JP, Casson RJ. Protection of retinal ganglion cells and the optic nerve during short-term hyperglycemia in experimental glaucoma. Arch Ophthalmol. 2011;129(10):1337–1344. doi: 10.1001/archophthalmol.2011.269. [DOI] [PubMed] [Google Scholar]
- 5.Casson RJ, Chidlow G, Wood JP, Crowston JG, Goldberg I. Definition of glaucoma: clinical and experimental concepts. Clin Exp Ophthalmol. 2012;40(4):341–349. doi: 10.1111/j.1442-9071.2012.02773.x. [DOI] [PubMed] [Google Scholar]
- 6.Goldblum D, Mittag T. Prospects for relevant glaucoma models with retinal ganglion cell damage in the rodent eye. Vision Res. 2002;42(4):471–478. doi: 10.1016/s0042-6989(01)00194-8. [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarieh M, Flammer J. New insights in the pathogenesis and treatment of normal tension glaucoma. Curr Opin Pharmacol. 2013;13(1):43–49. doi: 10.1016/j.coph.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Mitori H, Izawa T, Kuwamura M, Matsumoto M, Yamate J. Developing stage-dependent retinal toxicity induced by l-glutamate in neonatal rats. Toxicol Pathol. 2016;44(8):1137–1145. doi: 10.1177/0192623316676424. [DOI] [PubMed] [Google Scholar]
- 9.Fahrenthold BK, Fernandes KA, Libby RT. Assessment of intrinsic and extrinsic signaling pathway in excitotoxic retinal ganglion cell death. Sci Rep. 2018;8(1):4641. doi: 10.1038/s41598-018-22848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa M, Yoshitomi T, Izumi Y. (September 3rd 2014). Excitotoxicity and Glaucoma, Ophthalmology-Current Clinical and Research Updates, Pinakin Davey, IntechOpen. http://dx.doi.org/10.5772/57605. Available at: https://www.intechopen.com/books/ophthalmology-current-clinical-and-research-updates/excitotoxicity-and-glaucoma.
- 11.Sarikcioglu L, Demir N, Demirtop A. A standardized method to create optic nerve crush: Yasargil aneurysm clip. Exp Eye Res. 2007;84(2):373–377. doi: 10.1016/j.exer.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Siliprandi R, Canella R, Carmignoto G, Schiavo N, Zanellato A, Zanoni R, Vantini G. N-methyl-D-aspartate-induced neurotoxicity in the adult rat retina. Vis Neurosci. 1992;8(6):567–573. doi: 10.1017/s0952523800005666. [DOI] [PubMed] [Google Scholar]
- 13.Vorwerk CK, Lipton SA, Zurakowski D, Hyman BT, Sabel BA, Dreyer EB. Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Invest Ophthalmol Vis Sci. 1996;37(8):1618–1624. [PubMed] [Google Scholar]
- 14.Chen F, Jiang LB, Shen CY, Wan HF, Xu L, Wang NL, Jonas JB. Neuroprotective effect of epigallocatechin-3-gallate against N-methyl-D-aspartate-induced excitotoxicity in the adult rat retina. Acta Ophthalmol. 2012;90(8):e609–e615. doi: 10.1111/j.1755-3768.2012.02502.x. [DOI] [PubMed] [Google Scholar]
- 15.Gao LX, Chen X, Tang YP, Zhao JH, Li QY, Fan XT, Xu HW, Yin ZQ. Neuroprotective effect of memantine on the retinal ganglion cells of APPswe/PS1ΔE9 mice and its immunomodulatory mechanisms. Exp Eye Res. 2015;135:47–58. doi: 10.1016/j.exer.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Jafri AJA, Agarwal R, Iezhitsa I, Agarwal P, Spasov A, Ozerov A, Ismail NM. Protective effect of magnesium acetyltaurate and taurine against NMDA-induced retinal damage involves reduced nitrosative stress. Mol Vis. 2018;24:495–508. [PMC free article] [PubMed] [Google Scholar]
- 17.Shi JM, Jiang YQ, Liu XY. Morphological changes of retina after N-methyl-D-aspartate induced damage in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2004;29(3):287–291. [PubMed] [Google Scholar]
- 18.Fiedorowicz M, Rejdak R, Schuettauf F, Wozniak M, Grieb P, Thaler S. Age-dependent neuroprotection of retinal ganglion cells by tempol-C8 acyl ester in a rat NMDA toxicity model. Folia Neuropathol. 2014;52(3):291–297. doi: 10.5114/fn.2014.45570. [DOI] [PubMed] [Google Scholar]
- 19.Lam TT, Abler AS, Kwong JM, Tso MO. N-methyl-D-aspartate (NMDA)-induced apoptosis in rat retina. Invest Ophthalmol Vis Sci. 1999;40(10):2391–2397. [PubMed] [Google Scholar]
- 20.Sakamoto K, Suzuki Y, Kurauchi Y, Mori A, Nakahara T, Ishii K. Hydrogen sulfide attenuates NMDA-induced neuronal injury via its anti-oxidative activity in the rat retina. Exp Eye Res. 2014;120:90–96. doi: 10.1016/j.exer.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto K, Okuwaki T, Ushikubo H, Mori A, Nakahara T, Ishii K. Activation inhibitors of nuclear factor kappa B protect neurons against the NMDA-induced damage in the rat retina. J Pharmacol Sci. 2017;pii:S1347-8613(17)30162-7. doi: 10.1016/j.jphs.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi M, Hirooka K, Ono A, Nakano Y, Nishiyama A, Tsujikawa A. The relationship between the renin-Angiotensin-Aldosterone system and NMDA receptor-mediated signal and the prevention of retinal ganglion cell death. Invest Ophthalmol Vis Sci. 2017;58(3):1397–1403. doi: 10.1167/iovs.16-21001. [DOI] [PubMed] [Google Scholar]
- 23.Tsutsumi T, Iwao K, Hayashi H, Kirihara T, Kawaji T, Inoue T, Hino S, Nakao M, Tanihara H. Potential neuroprotective effects of an LSD1 inhibitor in retinal ganglion cells via p38 MAPK activity. Invest Ophthalmol Vis Sci. 2016;57(14):6461–6473. doi: 10.1167/iovs.16-19494. [DOI] [PubMed] [Google Scholar]
- 24.Adachi K, Kashii S, Masai H, Ueda M, Morizane C, Kaneda K, Kume T, Akaike A, Honda Y. Mechanism of the pathogenesis of glutamate neurotoxicity in retinal ischemia. Graefes Arch Clin Exp Ophthalmol. 1998;236(10):766–774. doi: 10.1007/s004170050156. [DOI] [PubMed] [Google Scholar]
- 25.Hama Y, Katsuki H, Tochikawa Y, Suminaka C, Kume T, Akaike A. Contribution of endogenous glycine site NMDA agonists to excitotoxic retinal damage in vivo. Neurosci Res. 2006;56(3):279–285. doi: 10.1016/j.neures.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Manabe S, Lipton SA. Divergent NMDA signals leading to proapoptotic and antiapoptotic pathways in the rat retina. Invest Ophthalmol Vis Sci. 2003;44(1):385–392. doi: 10.1167/iovs.02-0187. [DOI] [PubMed] [Google Scholar]
- 27.Ohzeki T, Machida S, Takahashi T, Ohtaka K, Kurosaka D. The Effect of intravitreal N-methyl-DL-aspartic acid on the electroretinogram in Royal College of surgeons rats. Jpn J Ophthalmol. 2007;51(3):165–174. doi: 10.1007/s10384-007-0420-y. [DOI] [PubMed] [Google Scholar]
- 28.Takahata K, Katsuki H, Kume T, Nakata D, Ito K, Muraoka S, Yoneda F, Kashii S, Honda Y, Akaike A. Retinal neuronal death induced by intraocular administration of a nitric oxide donor and its rescue by neurotrophic factors in rats. Invest Ophthalmol Vis Sci. 2003;44(4):1760–1766. doi: 10.1167/iovs.02-0471. [DOI] [PubMed] [Google Scholar]
- 29.Razali N, Agarwal R, Agarwal P, Kapitonova MY, Kannan Kutty M, Smirnov A, Salmah Bakar N, Ismail NM. Anterior and posterior segment changes in rat eyes with chronic steroid administration and their responsiveness to antiglaucoma drugs. European Journal of Pharmacology. 2015;749:73–80. doi: 10.1016/j.ejphar.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 30.Nor Arfuzir NN, Lambuk L, Jafri AJ, Agarwal R, Iezhitsa I, Sidek S, Agarwal P, Bakar NS, Kutty MK, Yusof AP, Krasilnikova A, Spasov A, Ozerov A, Mohd Ismail N. Protective effect of magnesium acetyltaurate against endothelin-induced retinal and optic nerve injury. Neuroscience. 2016;325:153–164. doi: 10.1016/j.neuroscience.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 31.Lambuk L, Jafri AJA, Arfuzir NNN, Iezhitsa I, Agarwal R, Rozali KNB, Agarwal P, Bakar NS, Kutty MK, Yusof APM, Krasilnikova A, Spasov A, Ozerov A, Ismail NM. Neuroprotective effect of magnesium acetyltaurate against NMDA-induced excitotoxicity in rat retina. Neurotox Res. 2017;31(1):31–45. doi: 10.1007/s12640-016-9658-9. [DOI] [PubMed] [Google Scholar]
- 32.Nor Arfuzir NN, Agarwal R, Iezhitsa I, Agarwal P, Sidek S, Spasov A, Ozerov A, Mohd Ismail N. Effect of magnesium acetyltaurate and taurine on endothelin1-induced retinal nitrosative stress in rats. Curr Eye Res. 2018;43(8):1032–1040. doi: 10.1080/02713683.2018.1467933. [DOI] [PubMed] [Google Scholar]
- 33.Mohd Lazaldin MA, Iezhitsa I, Agarwal R, Bakar NS, Agarwal P, Mohd Ismail N. Time- and dose-related effects of amyloid beta1-40 on retina and optic nerve morphology in rats. Int J Neurosci. 2018;128(10):952–965. doi: 10.1080/00207454.2018.1446953. [DOI] [PubMed] [Google Scholar]
- 34.Lambuk L, Iezhitsa I, Agarwal R, Bakar NS, Agarwal P, Ismail NM. Antiapoptotic effect of taurine against NMDA-induced retinal excitotoxicity in rats. Neurotoxicology. 2019;70:62–71. doi: 10.1016/j.neuro.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Joo CK, Choi JS, Ko HW, Park KY, Sohn S, Chun MH, Oh YJ, Gwag BJ. Necrosis and apoptosis after retinal ischemia: involvement of NMDA-mediated excitotoxicity and p53. Invest Ophthalmol Vis Sci. 1999;40(3):713–720. [PubMed] [Google Scholar]
- 36.Naskar R, Vorwerk CK, Dreyer EB. Concurrent downregulation of a glutamate transporter and receptor in glaucoma. Invest Ophthalmol Vis Sci. 2000;41(7):1940–1944. [PubMed] [Google Scholar]
- 37.Kuehn MH, Fingert JH, Kwon YH. Retinal ganglion cell death in glaucoma: mechanisms and neuroprotective strategies. Ophthalmol Clin North Am. 2005;18(3):383–395. vi. doi: 10.1016/j.ohc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Ebneter A, Casson RJ, Wood JP, Chidlow G. Microglial activation in the visual pathway in experimental glaucoma: spatiotemporal characterization and correlation with axonal injury. Invest Ophthalmol Vis Sci. 2010;51(12):6448–6460. doi: 10.1167/iovs.10-5284. [DOI] [PubMed] [Google Scholar]
- 39.Vasudevan S, Gupta V, Crowston J. Neuroprotection in glaucoma. Indian J Ophthalmol. 2011;59(Suppl1):S102–S113. doi: 10.4103/0301-4738.73700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jafri AJA, Arfuzir NNN, Lambuk L, Iezhitsa I, Agarwal R, Agarwal P, Razali N, Krasilnikova A, Kharitonova M, Demidov V, Serebryansky E, Skalny A, Spasov A, Yusof APM, Ismail NM. Protective effect of magnesium acetyltaurate against NMDA-induced retinal damage involves restoration of minerals and trace elements homeostasis. J Trace Elem Med Biol. 2017;39:147–154. doi: 10.1016/j.jtemb.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Iezhitsa I, Agarwal R. Magnesium acethyltaurate as a potential agent for retinal and optic nerve protection in glaucoma. Neural Regen Res. 2018;13(5):807–808. doi: 10.4103/1673-5374.232470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akaike A, Adachi K, Kaneda K. Techniques for evaluating neuronal death of the retina in vitro and in vivo. Nihon Yakurigaku Zasshi. 1998;111(2):97–104. doi: 10.1254/fpj.111.97. [DOI] [PubMed] [Google Scholar]
- 43.Sisk DR, Kuwabara T. Histologic changes in the inner retina of albino rats following intravitreal injection of monosodium L-glutamate. Graefes Arch Clin Exp Ophthalmol. 1985;223(5):250–258. doi: 10.1007/BF02153655. [DOI] [PubMed] [Google Scholar]
- 44.Sabel BA, Sautter J, Stoehr T, Siliprandi R. A behavioral model of excitotoxicity: retinal degeneration, loss of vision, and subsequent recovery after intraocular NMDA administration in adult rats. Exp Brain Res. 1995;106(1):93–105. doi: 10.1007/BF00241359. [DOI] [PubMed] [Google Scholar]
- 45.Gao X, Xu X, Pang J, Zhang C, Ding JM, Peng X, Liu Y, Cao JM. NMDA receptor activation induces mitochondrial dysfunction, oxidative stress and apoptosis in cultured neonatal rat cardiomyocytes. Physiol Res. 2007;56(5):559–569. doi: 10.33549/physiolres.931053. [DOI] [PubMed] [Google Scholar]
- 46.de Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Aβ oligomers induce neuronal oxidative stress through N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282(15):11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 47.Zhou X, Hollern D, Liao J, Andrechek E, Wang H. NMDA receptor-mediated excitotoxicity depends on the coactivation of synaptic and extrasynaptic receptors. Cell Death Dis. 2013;4:e560. doi: 10.1038/cddis.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]