Abstract
AIM
To assess the inflammatory cytokines expression in aqueous humor in diabetic primary open angle glaucoma (POAG) patients.
METHODS
A cross-sectional study on 87 eyes, distributed as following: 26 eyes from diabetic patients, 16 eyes with POAG and 21 eyes from diabetic POAG patients; healthy controls (24 eyes) were recruited from patients undergoing conventional cataract surgery. A volume of 100 µL of aqueous humor (AH) was collected during phacoemulsification and 21 inflammatory markers were quantified using a Luminex® cytometric bead assay: IL-1Ra, IL-1α, IL-1β, IL-5, IL-6, IL-10, IL-17, GM-CSF, IFNγ, CCL2, CCL3, CCL4, CXCL5, CXCL8, bFGF, VEGF, TNFα. Main changes in cytokine profile were analyzed and compared between groups. Data on demographics, duration of glaucoma, intraocular pressure (IOP), number of anti-glaucoma substances were recorded for correlation analysis and prediction models.
RESULTS
Significant differences in cytokine expression between groups were detected for CXCL5 (P<0.001), CXCL8 (P=0.004), IL-1α (P<0.001), IL-2 (P<0.001), CCL4 (P=0.003), CCL5 (P<0.001) and TNFα (P=0.05). Post-hoc analysis identified IL-2 (P=0.009) and CXCL5 (P<0.001) as “separation markers” between POAG and diabetic POAG eyes. In POAG patients, the “separation markers” could highly predict the TNFα levels F(1, 16)=14.639, P<0.001, whereas in diabetic patients F(1, 24)=4.844, P=0.006 and diabetic POAG patients F(1, 19)=2.358, P=0.05 the level of prediction was inferior.
CONCLUSION
Our results reveal an inflammatory model based on increased TNFα levels in POAG eyes. Simultaneous co-stimulatory molecules and additional inflammatory pathways need to be further explored in diabetic POAG cases, since the prediction model could only partially explain the increased TNFα level in this category of patients.
Keywords: primary open angle glaucoma, cytokines, inflammation, diabetes
INTRODUCTION
Glaucoma represents the main cause of irrevesible blindness worldwide[1]–[2]. The elevated intraocular pressure (IOP) remains the main risk factor[3], but recent theories refer to other pathogenic pathways[4] contributing to progressive loss of ganglion cells and vision decay[1]. These theories implicate aberrant immunity[5] enhanced inflammatory response[6], vascular dysregulation[7]–[9] ischemia[10], hypoxia[11] or increased oxidative stress[6],[12].
For ocular or systemic pathologies with inflammatory compound, the analysis of cytokines in biological fluids has become extremely attractive for research[13]–[17]. In glaucoma, previous studies measured inflammatory cytokines in aqueous humor (AH) and reported elevated levels of different interleukins[17]–[18].
Methods such as the enzyme-linked immunosorbent assay (ELISA) or polymerase chain reaction (PCR) are extremely useful when assessing these types of molecules, despite their great disadvantage of impossible multi-parameter measurements from small volumes. Cytometric bead assay techniques offer this possibility, in a fast, reproducible and highly accurate manner, allowing simultaneous detection of multiple cytokines from small volume samples[4]–[5].
The aim of our study was to investigate the inflammatory profile in primary open angle glaucoma (POAG) patients and assess the changes induced in the cytokines expression in the AH, when another systemic pro-inflammatory disease, such as diabetes mellitus (DM), was additionally present.
SUBJECTS AND METHODS
Ethical Approval
Study protocol respected the declaration of Helsinki, as revised in 2013, and was approved by the local university Ethics Committee. Informed consent was obtained from all participants, prior to their inclusion in the study.
This cross-sectional study included 87 eyes from 87 patients recruited during September 2015-September 2016. Patients were distributed in four groups: 26 eyes from 26 diabetic patients, 16 eyes from 16 POAG patients and 21 eyes from 21 diabetic POAG patients; healthy controls (24 eyes) were recruited from 24 patients undergoing conventional cataract surgery.
The AH was obtained under sterile conditions before the cataract surgery. At the beginning of the surgery, through an anterior chamber (AC) paracentesis, a 30G needle attached to a tuberculin syringe (1 mL) was inserted to extract a total volume of 100 µL AH. The samples were immediately transferred to polyethylene sterile tubes (Eppendorf 3810X®), stored at -80°C, within two hours from harvesting, until further analysis. The needle was not in contact with iris, lens or corneal endothelium, nor the AC contained any viscoelastic substance. No anti-inflammatory medication was administered preoperatory to the patients.
A Luminex® cytokine polystyrene color bead based multiplex assay (Human Cytokine Premixed Kit A FCST03 LHSC000e, RnD Systems®) was used to measure the concentrations of the following cytokines: interleukin-1 receptor antagonist (IL-1Ra), interleukin-1α (IL-1α), interleukin-1β (IL-1β), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 10 (IL-10), interleukin 17 (IL-17), granulocyte macrophage colony stimulating factor (GM-CSF), interferon-gamma (IFN-γ), monocyte chemotactic protein-1 (CCL2), macrophage inflammatory protein 1-alpha (CCL3), macrophage inflammatory protein 1-beta (CCL4), CXC motif chemokine 5 (CXCL5), interleukin 8 (CXCL8/IL-8), basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), α tumor necrosis factor (TNFα). The assay was conducted according to manufacturer's indications.
For the glaucoma groups, we have included only POAG cases, where the diagnosis had been established prior to the study initiation, based on the European Glaucoma Society criteria[19]. For all patients, demographic data (age, sex), duration of glaucoma/diabetes, glaucoma treatment, best corrected visual acuity in decimals by Snellen charts, and preoperative IOP (mm Hg) by Goldmann tonometer were recorded. Automated standard perimetry (central 24-2, Humphrey® Carl Zeiss Meditech) quantified the mean deviation (MD) and pattern standard deviation (PSD) in decibels (dB). Classification of glaucoma damage was based on Hodapp criteria, described elsewhere[20]. More than 85% of patients used more than one type of IOP lowering substance, all containing preservatives.
Diabetic patients had only mild forms of diabetic retinopathy (DR) or none at all; blood sugar level (mg/dL) and glycosylated hemoglobin (HbA1c, %) were measured in all these patients. Cataract surgery was indicated by the ophthalmologist when lens opacification became visually significant and prevented proper fundus monitoring. Only uncomplicated cases of cataract surgery were selected. Patients with history of ocular trauma, uveitis, previous eye surgeries, including laser therapy, age-related macular degeneration, retinal vascular occlusions or systemic autoimmune/inflammatory diseases that could have influenced the ocular local and systemic inflammation status, were excluded.
Statistical Analysis
Data were analyzed both qualitatively and quantitatively. Qualitative analysis was based on “heat map” graphs (Excel®, Microsoft, Office) showing minimum to maximum values; this processing rapidly identified dispersion, extreme values and allowed for Gaussian or non-Gaussian data categorizing in the study groups.
Quantitative analysis was performed using the SPSS® 20.0 (IBM Corp., New York, NY, USA) software. For categorical variables, the frequency distribution and percentages were calculated and compared by χ2 test. For numerical variables in parametric distribution, two-sample independent t-test was performed. Statistical significance was accepted at P<0.05. For differences in IOP, duration of POAG/DM and cytokine concentrations, the Mann-Whitney U test was used. Correlations between cytokine concentrations and patients' demographic data, including age and IOP, were calculated by Spearman correlation test. One-way analysis of variance (ANOVAs) were used to test for differences in cytokine levels among multiple groups, then a post-hoc analysis was used (Tukey honest significant difference test) to identify the specific differences between groups. Interaction tests and multiple regression analysis were further employed for prediction calculations.
RESULTS
A total of 87 AH samples were collected from 87 eyes in 87 patients. Groups characteristics, clinical and demographical, could be followed in Table 1.
Table 1. Clinical and demographic data in the study groups.
| Parametera | Control group (n=24) | DM group (n=26) | POAG group (n=16) | POAG+DM group (n=21) | Pa |
| Age (y) | 72.33±11.26 | 69.04±9.46 | 75.69±5.54 | 59.95±3.89a | <0.05 |
| Sex ratio (M:F) | 1:2 | 1:1.16 | 2:1 | 2:1 | 0.873 |
| IOP (mm Hg) | 14.21±2.68 | 15.50±1.9 | 18.19±4.3 | 20.33±2.3a | 0.05 |
| Anti-glaucoma substances (number) | - | - | 3±0.87 | 2.85±1.02 | 0.48 |
| Glaucoma therapy | |||||
| Treated eyes | 16 (100) | 21 (100) | |||
| Active substance | |||||
| BB | 11 (68) | 16 (76) | 0.129 | ||
| PGA | 16 (100) | 21 (100) | - | ||
| AA | 3 (18.75) | 4 (19) | 0.891 | ||
| CAI | 5 (31) | 9 (42) | 0.093 | ||
| Number of drops | |||||
| 1 | 2 (12.5) | 3 (14.2) | 0.681 | ||
| ≥2 | 14 (87.5) | 18 (85.8) | 0.462 | ||
| MD (dB) | - | - | -10.59±8.35 | -8.13±7.9 | 0.083 |
| PSD (dB) | - | - | 3.25±4.22 | 2.05±0.90a | 0.260 |
| Duration of glaucoma (mo) | - | - | 45.63±26.48 | 36.86±15.86 | 0.581 |
| Duration of diabetes (mo) | - | 133.56±77.82 | - | 115.62±81.24 | 0.119 |
| Blood sugar level (mg/dL) | - | 177.12±19.54 | - | 155.05±21.83 | 0.127 |
| HbA1c (%) | - | 8.12±1.81 | - | 7.09±1.35 | 0.095 |
IOP: Intraocular pressure; BB: Beta blocker; PGA: Prostaglandin analogue; AA: Alpha agonists; CAI: Carbonic anhydrase inhibitors; MD: Mean deviation; PSD: Pattern standard deviation; HbA1c: Glycosylated hemoglobin; SD: Standard deviation. aSignificance of differences between groups: χ2 test, two samples independent t-test or Mann-Whitney U test.
mean±SD, n (%)
The eyes with combined pathology (POAG+DM) had the youngest age when compared to healthy controls (P<0.001), POAG patients (P=0.000) or DM eyes (P=0.000), respectively. Also there has been noted a larger proportion of males among glaucoma patients compared to healthy controls or diabetic patients. Mean IOP was significantly higher in the diabetic POAG group, when compared to POAG patients (P=0.04), DM eyes (P<0.001) or healthy subjects (P<0.001), respectively. Similar number of topical anti-glaucoma substances was used to control the IOP in the glaucoma groups. Functional parameters (MD, PSD) were comparable between glaucoma patients (P>0.05).
Cytokine Analysis
From the 21 molecules, six could not be accurately dosed (IL-1β, IL-5, IL-6, IL-10, IL-17, IFNγ), since results were obtained by extrapolation; as such they were excluded from further analysis. Table 2 illustrates all means, medians and range variation for the remaining molecules, as quantitatively measured in each study group.
Table 2. Aqueous cytokine concentrations in the study groups.
| Cytokines (pg/mL) | CTRa eyes | DMb eyes | POAGc eyes | POAG+DMd eyes | Pe |
| IL-1α | 9.51±4.83 | 11.71±8.99 | 14.73±5.02 | 18.60±2.69 | 0.000 |
| Median | 8.7 | 10.93 | 16.55 | 18.18 | |
| Range | 1.05-22.40 | 3.34-36.01 | 5.34-21.99 | 13.03-23.12 | |
| IL-1Ra | 656.35-518.31 | 1319.75±832.76 | 2276.10±1301.65 | 2960.02+/2675.07 | 0.000 |
| Median | 538 | 801.245 | 1374 | 2397 | |
| Range | 126.94-2516.07 | 179.45-5431.78 | 243.97-12729 | 1084.14-12729 | |
| IL-2 | 107.29+/26.74 | 87.55±29.32 | 98.08±33.20 | 68.56±20.24 | 0.000 |
| Median | 113.70 | 86.24 | 94.40 | 74.23 | |
| Range | 13.49-138.49 | 30.63-142.96 | 49.39-165.28 | 30.63-98.82 | |
| IL-4 | 39.37±12.62 | 37.53±11.62 | 41.95±13.76 | 46.44-7.23 | 0.06 |
| Median | 40 | 37.29 | 39.58 | 45.42 | |
| Range | 8.84-55.10 | 14.05-57.55 | 19.13-72.40 | 32.87-59.99 | |
| G-CSF | 43.51±70.31 | 42.55±28.83 | 74.60+/168.74 | 62.64+/66.75 | 0.636 |
| Median | 26.81 | 33.37 | 2.89 | 33.38 | |
| Range | 1.33-277.24 | 3.30-119.94 | 1.15-652.44 | 11.35-290.64 | |
| GM-CSF | 2.82±0.85 | 2.84±1.00 | 3.01±0.73 | 3.18±0.63 | 0.442 |
| Median | 2.3 | 2.7 | 3.04 | 3.2 | |
| Range | 1.18-4.66 | 1.77-5.43 | 1.72-4.05 | 1.99-4.05 | |
| CXCL8 (IL-8) | 12.13±3.68 | 19.25±8.51 | 22.02±13.61 | 29.51±11.92 | 0.004 |
| Median | 5.09 | 15.83 | 20.46 | 25.62 | |
| Range | 1.04-97.75 | 12.39-44.51 | 4.93-43.30 | 13.76-46.25 | |
| bFGF | 39.46±31.71 | 36.98±10.26 | 52.71-32.62 | 44.42±26.56 | 0.259 |
| Median | 33.04 | 32.72 | 37.50 | 37.37 | |
| Range | 11.48-74.56 | 2.86-69.74 | 23.96-114.05 | 21.06-134.09 | |
| VEGF | 50.02±19.89 | 96.42±60.17 | 78.66±55.53 | 84.11±72.05 | 0.162 |
| Median | 52.39 | 64.84 | 50.38 | 62.29 | |
| Range | 7.23-91.12 | 23.83-505.15 | 16.47-236.49 | 30.81-356.65 | |
| CCL2 (MCP-1) | 449±260.81 | 464.24±276.32 | 383.59±249.32 | 505.12±397.33 | 0.376 |
| Median | 395.64 | 407.16 | 363.12 | 439.62 | |
| Range | 29.58-1054.44 | 71.27±1224.13 | 177.18-913.69 | 249.92-1913.00 | |
| CCL3 (MIP-1α) | 138.26±54.08 | 141.50±54.39 | 158.26±29.01 | 167.04±16.11 | 0.1 |
| Median | 126.08 | 133.32 | 167.48 | 166.21 | |
| Range | 43.96-315.92 | 52.75-338.24 | 99.46-205.12 | 135.16-202.17 | |
| CCL4 (MIP-1β) | 9.78±8.80 | 11.32±5.22 | 25.59±29.41 | 19.73±13.63 | 0.003 |
| Median | 7.85 | 10.59 | 15.00 | 14.27 | |
| Range | 2.21-44.43 | 1.54±21.74 | 4.51-119.22 | 5.81-61.73 | |
| CCL5 (RANTES) | 2.49±1.07 | 3.49±2.32 | 4.30+/1.61 | 4.81±1.39 | 0.000 |
| Median | 2.36 | 3.22 | 3.93 | 4.96 | |
| Range | 1.05-5.65 | 1.87-11.04 | 1.48-6.82 | 2.34-6.83 | |
| CXCL5 (ENA-78) | 19.34±9.22 | 27.19±10.06 | 24.79±10.48 | 40.61-3.16 | 0.000 |
| Median | 20.86 | 24.73 | 20.36 | 40.11 | |
| Range | 2.99-34.13 | 14.09-43.22 | 10.37-52.50 | 34.19-46.55 | |
| TNFα | 2.41±0.95 | 2.68±0.83 | 2.84±0.60 | 3.27±0.68 | 0.05 |
| Median | 2.31 | 2.52 | 2.76 | 3.17 | |
| Range | 1.21-20.96 | 1.67-5.31 | 1.95-3.94 | 2.55-5.31 |
IL-1Ra: Interleukin-1 receptor antagonist; IL-1α: Interleukin-1α; IL-2: Interleukin 2; IL-4: Interleukin 4; GM-CSF: Granulocyte macrophage colony stimulating factor; CCL2: Monocyte chemotactic protein-1; CCL3: Macrophage inflammatory protein 1-alpha; CCL4: Macrophage inflammatory protein 1-beta; CCL5(RANTES): Chemokine (C-C motif) ligand 5; CXCL5: CXC Motif chemokine 5; CXCL8/IL-8: Interleukin 8; bFGF: Basic fibroblast growth factor; VEGF: Vascular endothelial growth factor; TNFα: α tumor necrosis factor. a Control group; bDiabetes group; cPrimary open angle glaucoma group; dPrimary open angle glaucoma+diabetes group; eOne way ANOVA analysis was performed to compare the four groups; P<0.05 for statistically significant difference.
mean±SD
One way ANOVA analysis identified the significantly different concentrations of cytokines between the study groups: CCL4 (P=0.003), CCL5 (P<0.001), CXCL5 (P<0.001), CXCL8 (P=0.004), IL-1α (P<0.001), IL-1Ra (P<0.001) and TNFα (P=0.05). A post-hoc analysis identified the specific differences in cytokine concentrations between POAG and diabetic POAG eyes. As such, IL-2 (P=0.009) and CXCL5 (P<0.001) were significantly different in diabetic POAG eyes vs POAG eyes.
The influence of IOP (≤/>18 mm Hg), age (≤/>60 years old) and sex (males vs females) was studied upon the levels of the separation markers. Table 3 resumes the changes in cytokine expression when these limits were tested.
Table 3. Influence of IOP, age and sex upon the cytokine concentrations.
| Cytokine (pg/mL) | Age (y) |
Pa | IOP (mm Hg) |
Pa | Sex |
Pa | ||||
| ≤60 | >60 | ≤18 | >18 | M | F | |||||
| CXCL5 | 34.27±10.94 | 23.59±10.27 | 0.012 | 24.17±10.40 | 35.34±10.85 | 0.002 | 30.78±10.45 | 24.28±12.14 | 0.04 | |
| CXCL8 | 26.48±18.86 | 16.48±13.98 | 0.006 | 16.21±16.54 | 29.30±13.08 | 0.000 | 23.23±16.18 | 17.11±16.71 | 0.08 | |
| IL-1α | 15.62±6.42 | 11.92±5.54 | 0.000 | 11.64±6.18 | 17.06±4.06 | 0.000 | 14.31±4.53 | 12.27±7.38 | 0.112 | |
| TNFα | 3.04±0.78 | 3.02±2.67 | 0.619 | 2.68±0.98 | 3.80±3.49 | 0.024 | 3.18±3.03 | 2.88±0.66 | 0.09 | |
| CCL4 | 16.99+/12.12 | 14.97+/18.40 | 0.577 | 12.04±8.03 | 23.96±25.03 | 0.029 | 18.66±19.54 | 12.61±11.19 | 0.07 | |
| CCL5 | 4.23±1.98 | 3.35±1.77 | 0.04 | 3.36±1.97 | 4.40±1.48 | 0.008 | 3.09±1.6 | 3.45±2.15 | 0.276 | |
| IL-2 | 82.28±32.05 | 95.28±28.93 | 0.05 | 92.73±30.35 | 85.04±31.15 | 0.281 | 97.73±30.35 | 85.04±31.15 | 0.375 | |
| IL-1Ra | 2232.43±2398 | 1388.53±1976 | 0.07 | 1458±2002.17 | 2263.37±2548.92 | 0.111 | 1574.94±1239.51 | 185.86±2864.83 | 0.555 | |
CXCL5: CXC motif chemokine 5; CXCL8/IL-8: Interleukin 8; IL-1α: Interleukin-1α; TNFα: α tumor necrosis factor; CCL4: Macrophage inflammatory protein 1-beta; CCL5 (RANTES): Chemokine (C-C motif) ligand 5; IL-2: Interleukin 2; IL-1Ra: Interleukin-1 receptor antagonist. aStatistical difference, t-test, P<0.05.
mean±SD
Results showed that the mean concentrations of CXCL5, CXCL8, IL-1α, CCL5 and CCL4 increased in a significant manner, if the IOP exceeded 18 mm Hg (P<0.05). Alltogether, younger patients exhibited a stronger inflammatory response (CXCL5, CXCL8, IL-1α, CCL5), since all analyzed cases with an age below 60y produced higher concentrations of cytokines as compared to older age (P<0.05). IL-2, normally expressed as anti-inflammatory molecule, exhibited lower levels if patients had higher IOPs or younger age, suggesting a failure of the compensatory mechanisms once the IOP increases and more inflammation is triggered.
Correlation analysis showed that in diabetic patients the IOP correlated significantly with the levels of CCL4 (ρ=0.535, P=0.005) and CXCL5 (ρ=0.537, P=0.005). An inverse negative correlation was found for CXCL8 with the age of the subjects (ρ=-0.424, P=0.03) suggesting a more prominent inflammatory response in younger patients. A similar age-related finding was detected in the POAG group regarding age of the subjects and CCL5 level (ρ=-0.497, P=0.05). Also the IOP was correlated with the CXCL8 level (ρ=0.600, P=0.014). Related to the functional damage in glaucoma patients, the MD values in the POAG group were inversely correlated with TNFα (ρ=-0.561, P=0.024), yet localized glaucoma damage (PSD) was not correlated with any inflammatory molecule.
For diabetic POAG patients, the age was correlated with CXCL8 (ρ=-0.536, P=0.04) and CCL5 (ρ=0.592, P=0.06) levels. IOP was correlated with a larger number of molecules from the “separation markers” category, such as CXCL5 (ρ=0.650, P=0.005), TNFα (ρ=0.539, P=0.001), IL-1α (ρ=0.544, P=0.04) or IL-1Ra (ρ=0.513, P=0.02). Also in these patients, glaucoma duration was correlated both with the TNFα level (ρ=0.561, P=0.024) and the number of IOP lowering substances (ρ=0.438, P=0.04).
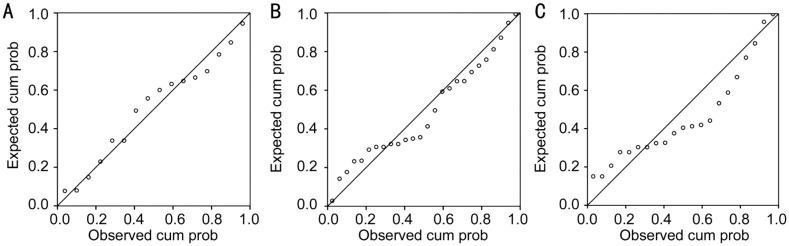
Further statistical analysis allowed for creation of inflammatory mathematical models for each study group. The “separation” markers elicited either direct pro-inflammatory effect (CXCL5, CXCL8) or they could indirectly modulate the Th1 cells activity (IL-1α, IL-1Ra, IL-2, CCL4, CCL5, TNFα) to increase inflammation. Still, regardless of the activated inflammatory pathway, all molecules increased TNFα's expression. As such, a step wise multiple regression analysis studied the influence of the “separation” markers upon TNFα level in each category of patients (Figure 1).
Figure 1. Normal probability plot (P-P) expected vs observed cumulated probability for TNFα throughout the study.
A: POAG group (R2=0.842); B: DM group (R2=0.580); C: POAG+DM group (R2=0.281).
In the POAG group, the highest level of statistical relevance was found: R2=0.842, F(1,16)=14.639, P≤0.001; thus in POAG eyes, TNFα level was largely explained by the levels of IL-2, IL-1α, CXCL8 and CXCL5 all together. For diabetic patients, the same inflammatory molecules explained less of the TNFα concentration, R2=0.580, F(1,24)=4.844, P=0.006, while in the diabetic POAG eyes, IL-2, IL-1α, CXCL8 explained the least of TNFα concentration compared to all the other study groups (R2=0.281), with borderline statistical significance F(1,19)=2.358, P=0.05. In the latter model, CXCL5 was eliminated from the predictors, to avoid statistical redundancy due to its high level of correlation with IL-2 (ρ=0.847, P<0.001). Glaucoma treatment influenced very little the level of TNFα in our study: R2=0.06, F(1,36)=4.663, P=0.017, similar to the IOP influence upon the level of this cytokine: R2=0.052, F(1,36)=5.974, P=0.034. Other parameters did not reach statistical significance as predictors for our TNFα inflammatory model.
DISCUSSION
Altered cytokines composition in AH have been identified in multiple ocular diseases: glaucoma, age related macular degeneration, diabetic retinopathy or uveitis[13]. Our study assessed the changes in cytokine expression in diabetic POAG patients, based on the hypothesis that additional pro-inflammatory pathology (DM) could induce identifiable changes in AH composition in POAG eyes.
The global analysis of all cytokines that were significantly different between groups (CXCL5, CXCL8, IL-1α, IL-1, IL-2) point out that all molecules belong to the early “acute inflammatory” phase reactants, capable of increasing the expression of one another. Additionally, these cytokines have been reported as direct or indirect regulators of TNFα expression[21]–[22].
TNFα as pivotal inflammatory marker in POAG or diabetes is not new[21]–[22]. However, the original element of this study resides in the attempt to create a model for the inflammation in diabetic POAG eyes based on some cytokines that were differently expressed in this category and the end-point inflammatory pathway they trigger (TNFα over-expression). Thus, authors aimed to explain how much of the TNFα's level could be predicted based on the indentified “separation markers”. As such in simple POAG cases, 80% of TNFα's level could be explained by an increase in IL-2, IL-1α, CXCL8 and CXCL5 expression, while in diabetic eyes, the model predicted less (<60%) from the TNFα's level. In diabetic POAG patients, the same molecules could not explain the TNFα's increased expression in a relevant manner (<30%). This finding could be only explained by the high proportion of cytokines that exhibit a polymorphic role and influence the production or release of other cytokine types, through multiple pathways that cannot be assessed and fully identified in a cross-sectional study. Our results show that the inflammation induced additionally by diabetes in POAG eyes changed the cytokine inflammatory profile in AH, but the mechanism was not similar to non-diabetic POAG eyes, at least for the model we tested. Collateral inflammatory pathways, co-stimulatory signals and cytokines should be further investigated.
The TNFα molecule can normally be found in healthy eyes. Depending on microenvironment changes, it can exhibit both neuroprotective and neurodegenerative properties[23]. In normal conditions, retinal tissue expresses receptors for TNFα; unless hypoxia develops, the increased TNFα levels do not trigger apoptotic signals for the ganglion cells[24]. This could support the results we obtained in the inflammatory model in POAG eyes. Still, Sawada et al[22] reported increased TNFα levels in hypertensive glaucoma patients compared to healthy subjects, through ischemic mechanical mechanism. Moreover, Huang et al[17] reported increased expression of TNFα associated with the increase in IOP in glaucoma patients; in this direction, our study found similar results for all glaucoma patients with an IOP over 18 mm Hg.
We have chosen 18 mm Hg as cut-off value, because according to Hodapp criteria[20], our glaucoma patients were categorized as moderate to advanced glaucoma. In consequence, similar to Advaned Glaucoma Interventional Study (AGIS)[24], we have chosen this limit to investigate the cytokines levels in our study in a dychotomic manner. Since Fingeret et al[25] reported an increased glaucoma risk after the age of 60y, we studied this cut-off values also in our patients. Our results showed that mean concentrations of cytokines (CXCL5, CXCL8, IL-1α, CCL5 and CCL4) increase in a significant manner when IOP increases, alltogether with a stronger inflammatory response ellicited at younger ages. Mean IOP and the intensity of treatment (number of anti-glaucoma medications) influenced the TNFα inflammatory model very little in this study, yet our results are similar to other published data, which found little or no correlation between the intensity of treatment and the cytokine expression[13],[26]. Nevertheless, the authors acknowledge other studies[27], where multiple therapy generated additive inflammation in the anterior segment in glaucoma patients. Still, in our study, the intensity of treatment upon the TNFα inflammatory profile in POAG patients was not statistically significant; this group homogeneity could be explained by the fact that all POAG patients received prostaglandin analogue (PGA), and in both glaucoma subgroups, over 85% of subjects received more than two topical substances. The very little number of patients with a single type of medication (PGA) in each glaucoma group (2 eyes with POAG eyes vs 3 eyes with diabetic POAG) and the administration of fixed combinations (PGA+β blocker, CAI+β blocker), might have made the statistical calculations very hazardous and, for the authors, impossible to assess correctly which substance or combination of substances influenced the TNFα model in a significant manner, if the case in this study.
Regarding topical treatment in our POAG patients, all medication used during the study contained preservatives, which are known to increase the ocular inflammation[27]. Very few patients received only one IOP lowering substance. Therefore, corruption of our data due to these two major confounders (preservatives and multiple therapy) has been diminished and homogeneity among subjects was achieved. Authors are aware that the best profiles might have been obtained from naïve glaucoma patients, before starting any treatment, or leaving the patients without treatment after a proper washout period. Still, for ethical reasons this was neither possible, nor applicable in our patients. Anti-glaucoma medication with preservatives reflects the clinical reality of the local national medical system, since public health insurance, at the time of the study, covered only the costs for these types of anti-glaucoma medications. The present research was conducted in a cross-sectional manner. The concept of predictive models was imported from other research branches[28], but usually involves dynamic processes. Authors would like to acknowledge that mathematical validity cannot reproduce the dynamic homeostatic and adaptive changes that occur spontaneously in biological systems[29]. Further investigations in longitudinal studies and better standardizations on a wider number of subjects are needed for proper validation of our results and in order to obtain a better understanding of such complex in vivo interactions.
In conclusion, our study revealed that diabetes induced a significant change in the cytokine expression in primary open angle glaucoma patients and modified both the signaling molecules and the inflammatory pathways from the initial TNFα model. Further studies that could identify collateral molecules and co-stimulatory pathways in combined ocular neurodegenerative diseases are needed.
Acknowledgments
Authors' contributions: Concept: Pantalon A; Design: Pantalon A, Feraru C, Chiseliţă D; Supervision: Feraru C, Chiseliţă D; Resource: Pantalon A; Materials: Pantalon A, Constantinescu D; Data collection and/or processing: Pantalon A, Obadă O; Analysis and/or interpretation: Pantalon A; Literature research: Pantalon A, Obadă O; Critical reviews: Chiseliţă D, Feraru C, Constantinescu D.
Conflicts of Interest: Pantalon A, None; Obadă O, None; Constantinescu D, None; Feraru C, None; Chiseliţă D, None.
REFERENCES
- 1.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Ederer F, Gaasterland DE, Sullivan EK, AGIS Investigators The Advanced Glaucoma Intervention Study (AGIS): 1. Study design and methods and baseline characteristics of study patients. Control Clin Trials. 1994;15:299–325. doi: 10.1016/0197-2456(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 4.Tong Y, Zhou YL, Zheng Y, Biswal M, Zhao PQ, Wang ZY. Analyzing cytokines as biomarkers to evaluate severity of glaucoma. Int J Ophthalmol. 2017;10(6):925–930. doi: 10.18240/ijo.2017.06.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong M, Huang P, Li W, Li Y, Zhang SS, Zhang C. T-helper1/T-helper2 cytokine imbalance in the iris of patients with glaucoma. PLoS One. 2015;10(3):e0122184. doi: 10.1371/journal.pone.0122184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li G, Luna C, Liton PB, Navarro I, Epstein DL, Gonzalez P. Sustained stress response after oxidative stress in trabecular meshwork cells. Mol Vis. 2007;13:2282–2288. [PMC free article] [PubMed] [Google Scholar]
- 7.Flammer J, Konieczka K, Bruno RM, Virdis A, Flammer AJ, Taddei S. The eye and the heart. Eur Heart J. 2013;34(17):1270–1278. doi: 10.1093/eurheartj/eht023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mozaffarieh M, Grieshaber MC, Flammer J. Oxygen and blood flow: players in the pathogenesis of glaucoma. Mol Vis. 2008;14:224–233. [PMC free article] [PubMed] [Google Scholar]
- 9.McMonnies CW. Glaucoma history and risk factors. J Optom. 2017;10(2):71–78. doi: 10.1016/j.optom.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakabayashi M. Review of the ischemia hypothesis for ocular hypertension other than congenital glaucoma and closed-angle glaucoma. Ophthalmologica. 2004;218(5):344–349. doi: 10.1159/000079477. [DOI] [PubMed] [Google Scholar]
- 11.Helbig H, Schlötzer-Schrehardt U, Noske W, Kellner U, Foerster MH, Naumann GO. Anterior-chamber hypoxia and iris vasculopathy in pseudoexfoliation syndrome. Ger J Ophthalmol. 1994;3(3):148–153. [PubMed] [Google Scholar]
- 12.Agarwal R, Gupta SK, Agarwal P, Saxena R, Agrawal SS. Current concepts in the pathophysiology of glaucoma. Indian J Ophthalmol. 2009;57(4):257–266. doi: 10.4103/0301-4738.53049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chua J, Vania M, Cheung CM, Ang M, Chee SP, Yang H, Li J, Wong TT. Expression profile of inflammatory cytokines in aqueous from glaucomatous eyes. Mol Vis. 2012;18:431–438. [PMC free article] [PubMed] [Google Scholar]
- 14.Borkenstein A, Faschinger C, Maier R, Weger M, Theisl A, Demel U, Graninger W, Irene H, Mossböck G. Measurement of tumor necrosis factor-alpha, interleukin-6, Fas ligand, interleukin-1α, and interleukin-1β in the aqueous humor of patients with open angle glaucoma using multiplex bead analysis. Mol Vis. 2013;19:2306–2311. [PMC free article] [PubMed] [Google Scholar]
- 15.Hautala N, Glumoff V, Hautala T, Vainio O. IL-2 may possess neuroprotective properties in glaucomatous optic neuropathy. Acta Ophthalmol. 2012;90(3):e246–e247. doi: 10.1111/j.1755-3768.2011.02219.x. [DOI] [PubMed] [Google Scholar]
- 16.Gramlich OW, Beck S, von Thun Und Hohenstein-Blaul N, Boehm N, Ziegler A, Vetter JM, Pfeiffer N, Grus FH. Enhanced insight into the autoimmune component of glaucoma: IgG autoantibody accumulation and pro-inflammatory conditions in human glaucomatous retina. PLoS One. 2013;8(2):e57557. doi: 10.1371/journal.pone.0057557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang P, Qi Y, Xu YS, Liu JH, Liao DP, Zhang SS, Zhang C. Serum cytokine alteration is associated with optic neuropathy in human primary open angle glaucoma. J Glaucoma. 2010;19(5):324–330. doi: 10.1097/IJG.0b013e3181b4cac7. [DOI] [PubMed] [Google Scholar]
- 18.Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, Campos R, Ernst D, Gaur A. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol. 2004;110(3):252–266. doi: 10.1016/j.clim.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 19.European Glaucoma Society Guidelines. 4th edition. 2014. p. pp11. Available at: http://bjo.bmj.com/content/101/5/73.
- 20.Hodapp E, Parrish RK, Anderson DR. Clinical decision in glaucoma. St Louis: The CV Mosby; 1993. [Google Scholar]
- 21.Seet LF, Finger SN, Chu SW, Toh LZ, Wong TT. Novel insight into the inflammatory and cellular responses following experimental glaucoma surgery: a roadmap for inhibiting fibrosis. Curr Mol Med. 2013;13(6):911–928. doi: 10.2174/15665240113139990021. [DOI] [PubMed] [Google Scholar]
- 22.Sawada H, Fukuchi T, Tanaka T, Abe H. Tumor necrosis factor-alpha concentrations in the aqueous humor of patients with glaucoma. Invest Ophthalmol Vis Sci. 2010;51(2):903–906. doi: 10.1167/iovs.09-4247. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs C, Forster V, Balse E, Sahel JA, Picaud S, Tessier LH. Retinal-cell-conditioned medium prevents TNF-alpha-induced apoptosis of purified ganglion cells. Invest Ophthalmol Vis Sci. 2005;46(8):2983–2991. doi: 10.1167/iovs.04-1177. [DOI] [PubMed] [Google Scholar]
- 24.AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 12. Baseline risk factors for sustained loss of visual field and visual acuity in patients with advanced glaucoma. Am J Ophthalmol. 2002;134(4):499–512. doi: 10.1016/s0002-9394(02)01659-8. [DOI] [PubMed] [Google Scholar]
- 25.Fingert JH, Stone EM, Sheffield VC, Alward WL. Myocilin glaucoma. Surv Ophthalmol. 2002;47(6):547–561. doi: 10.1016/s0039-6257(02)00353-3. [DOI] [PubMed] [Google Scholar]
- 26.Bagnis A, Papadia M, Scotto R, Traverso CE. Antiglaucoma drugs: the role of preservative-free formulations. Saudi J Ophthalmol. 2011;25(4):389–394. doi: 10.1016/j.sjopt.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byles DB, Frith P, Salmon JF. Anterior uveitis as a side effect of topical brimonidine. Am J Ophthalmol. 2000;130(3):287–291. doi: 10.1016/s0002-9394(00)00491-8. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Boyd S. Fast model predictive control using online optimization. IEEE Trans Contr Syst Technol. 2010;18(2):267–278. [Google Scholar]
- 29.Bhattacharya SK, Lee RK, Grus FH, Seventh ARVO/Pfizer Ophthalmics Research Institute Conference Working Group. Molecular biomarkers in glaucoma. Invest Ophthalmol Vis Sci. 2013;54(1):121–131. doi: 10.1167/iovs.12-11067. [DOI] [PMC free article] [PubMed] [Google Scholar]