Abstract
The cost of treating Clostridium difficile infection (CDI) in Spain is substantial. Findings from the randomised, controlled, open-label, phase 3b/4 EXTEND study showed that an extended-pulsed fidaxomicin (EPFX) regimen was associated with improved sustained clinical cure and reduced recurrence of CDI versus vancomycin in patients aged 60 years and older. We assessed the cost-effectiveness of EPFX versus vancomycin for the treatment of CDI in patients aged 60 years and older from the perspective of the National Health System (NHS) in Spain. We used a Markov model with six health states and 1-year time horizon. Health resources, their unit costs and utilities were based on published sources. Key efficacy data and transition probabilities were obtained from the EXTEND study and published sources. A panel of Spanish clinical experts validated all model assumptions. In the analysis, 0.638 and 0.594 quality-adjusted life years (QALYs) per patient were obtained with EPFX and vancomycin, respectively, with a gain of 0.044 QALYs with EPFX. The cost per patient treated with EPFX and vancomycin was estimated to be €10,046 and €10,693, respectively, with a saving of €647 per patient treated with EPFX. For willingness-to-pay thresholds of €20,000, €25,000 and €30,000 per QALY gained, the probability that EPFX was the most cost-effective treatment was 99.3%, 99.5% and 99.9%, respectively. According to our economic model and the assumptions based on the Spanish NHS, EPFX is cost-effective compared with vancomycin for the first-line treatment of CDI in patients aged 60 years and older.
Electronic supplementary material
The online version of this article (10.1007/s10096-019-03503-4) contains supplementary material, which is available to authorized users.
Keywords: Clostridium difficile infection, Cost-effectiveness, Extended-pulsed fidaxomicin, Fidaxomicin, Vancomycin
Introduction
Clostridium difficile is the most frequent bacterial cause of hospital-acquired diarrhoea [1]. The annual incidence of CDI in Spain is estimated at 17.1 cases per 10,000 hospitalised patients [2], ranging from 12.2 to 24.0 cases per 10,000 hospitalisations [3, 4]. A study from the United Kingdom found that the rate of hospital mortality is much higher for patients with hospital-acquired CDI (15.3%) than for those without CDI (1.9%), with infection also substantially increasing the length of stay [5].
One study estimated that 7600 episodes of CDI occurred annually in Spain, with an economic burden of €32,157,093 to the National Health System (NHS) [6]. A study from the USA determined that recurrent CDI was associated with significantly greater likelihood of readmission to hospital (85% vs 41%, respectively; p < 0.001) and longer length of stay when readmitted (mean 18.6 vs 7.6, respectively; p < 0.001) than those patients without recurrent CDI [7]. A recent economic review of published studies reporting CDI-associated burden revealed that in Spain, hospitalisation costs attributable to CDI among all patients were €4265 per patient, rising to €4885 per patient among those aged > 65 years [8]. Furthermore, there was an incremental rise in the cost of treating an initial CDI episode (€3901), first recurrence (€4875) and second recurrence (€5916), with hospitalisation accounting for 96% of costs [8].
Patients with CDI should be managed by discontinuing any antibiotic that might have affected the normal microbial ecology of the large intestine and the use of which favours the proliferation of C. difficile, which releases toxins that induce an inflammatory response [1, 9]. Guideline-recommended antibiotic treatments for initial, non-severe CDI include fidaxomicin, vancomycin [10] or metronidazole [11]. However, recurrent infection is common, occurring in up to 25% of cases treated with vancomycin or metronidazole [12]. Recurrence may be due, among other reasons, to delayed recovery of the intestinal microbiota previously disrupted by CDI-directed treatment [13].
Fidaxomicin is a narrow spectrum macrocyclic antibiotic indicated for the treatment of CDI in adults at a dose of 200 mg twice daily for 10 days [11, 14] and has been associated with greater preservation of the intestinal microbiota than vancomycin [15]. Fidaxomicin treatment also significantly lowers the incidence of recurrent CDI compared with vancomycin [16–18]. A validated in vitro human gut model showed that an extended-pulsed fidaxomicin (EPFX) regimen enables the persistence of fidaxomicin at concentrations inhibitory to C. difficile, facilitating intestinal microbiota recovery [19]. The efficacy and safety of the EPFX regimen (200 mg oral fidaxomicin twice daily on days 1–5, followed by once-daily administration on alternate days on days 7–25), which uses the same number of tablets as the standard fidaxomicin regimen, were compared with standard vancomycin (125 mg orally, four times daily on days 1–10) in the EXTEND randomised, controlled trial of patients 60 years and older with CDI [20]. The primary endpoint of sustained clinical cure rate 30 days after the end of treatment (day 55 for EPFX and day 40 for vancomycin; defined as clinical response at test of cure and no recurrence of CDI) was significantly higher with EPFX (70%) compared with vancomycin (59%; p = 0.030). Until day 90, the rate of sustained clinical cure was significantly higher and recurrence was significantly lower in the EPFX than the vancomycin arm (p ≤ 0.007 and p ≤ 0.001, respectively) [20]. An economic analysis of these data found that the reduced recurrence rate with EPFX made this regimen more cost-effective than vancomycin for first-line treatment of CDI in older patients from the perspective of the United Kingdom NHS [21].
The objective of the present study was to evaluate the cost-effectiveness of EPFX compared with vancomycin for the treatment of CDI in patients aged 60 years and older from the perspective of the NHS in Spain.
Methods
Model design
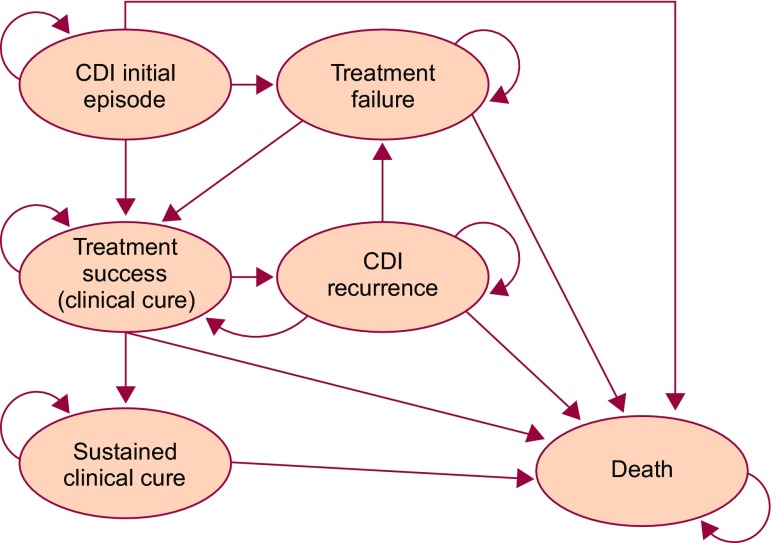
Evaluation of the cost-effectiveness of CDI therapy encompasses appraisal of the initial episode and subsequent treatments for CDI recurrence(s). A cohort-based Markov model [22, 23] consisting of six mutually exclusive health states (Fig. 1) was used, evaluating up to three treatment courses. The characteristics of this model have been described previously in an adaptation to the NHS in England [21]. Briefly, the cohort of patients with CDI moved between health states, according to transition probabilities obtained from clinical studies, in discrete periods of time called cycles (every 5 days in the model). During each cycle patients moved between the health states, generating costs and utilities, which represented quality of life on a standard scale of 0 (dead) to 1 (full health), allowing quality-adjusted life years (QALYs) to be estimated, during a time horizon of 1 year [24].
Fig. 1.
Overview of clinical pathways and health states used in the Markov model. CDI, Clostridium difficile infection. Reproduced with kind permission from Watt M, McCrea C. Cost-effectiveness of extended-pulsed fidaxomicin versus vancomycin in older patients with Clostridium difficile infection in England. Poster presented at the 27th European Congress of Clinical Microbiology and Infectious Diseases 2017, 22–25 April, Vienna, Austria
In accordance with the EXTEND study, our model assumed that patients aged 60 years and older with CDI received treatment with EPFX (200 mg oral fidaxomicin tablets twice daily on days 1–5, followed by once-daily administration on alternate days on days 7–25) or vancomycin (125 mg oral capsules, four-times daily on days 1–10) [20]. In our analysis, the hypothetical patient cohort entered the model with an initial CDI episode and received first-line treatment with EPFX or vancomycin (Fig. 1). Further management assumptions are described in Online Resource 1.
Owing to the paucity of information from clinical practice in Spain regarding the choice of therapy for repeated CDI recurrences and for second- or third-line treatment of CDI, treatment choice was estimated by the panel of clinical experts (authors JMA, BA, JC, SG, MS; Table 1). In this regard, several assumptions were made in the model, which are described in Online Resource 1 [10, 23].
Table 1.
Treatment sequences considered in the model
| First treatment | Treatment received upon first treatment failure | Treatment received upon second treatment failure | |
|---|---|---|---|
| Interventional scenario | |||
| Initial CDI episode | EPFX | Tapered vancomycina | FDX 10 daysb |
| First CDI recurrence | High-dose vancomycinc | EPFX | Tapered vancomycina |
| Second CDI recurrence | FMT | EPFX | FMT |
| Comparator scenario | |||
| Initial CDI episode | Vancomycin 10 daysd | Tapered vancomycin | EPFX |
| First CDI recurrence | EPFX | Tapered vancomycin | FDX 10 days |
| Second CDI recurrence | FMT | EPFX | FMT |
CDI Clostridium difficile infection, EPFX extended-pulsed fidaxomicin, FDX fidaxomicin, FMT faecal microbiota transplantation
aTapered vancomycin regimen: 125 mg four times daily oral vancomycin for 14 days, followed by 125 mg twice-daily oral vancomycin for 7 days, followed by 125 mg once-daily oral vancomycin for 7 days and finally, 125 mg oral vancomycin every 3 days, giving a total of 8 weeks of treatment
bFDX 10 days: standard-regimen fidaxomicin consisting of 200 mg twice daily for 10 days
cHigh-dose vancomycin: dose increased to 250 mg four-times daily for 10 days in 80% of patients and up to 500 mg four times daily in 20% of patients
dVancomycin 10 days: standard-regimen vancomycin consisting of 125 mg four times daily for 10 days
Model inputs
Clinical
Comparative clinical and safety data for the EPFX and vancomycin regimens in the treatment of CDI, as well as the model’s main transition probabilities, including death, were obtained from the EXTEND clinical trial [20, 21] (Table 2). Detailed descriptions of other clinical inputs are in Online Resource 2. All clinical inputs were assumed to be the same for the first, second and third treatment courses [21].
Table 2.
Summary of clinical inputs (cure, recurrence), adverse events and mortality included in the model
| Item | EPFX | Vancomycin |
|---|---|---|
| Clinical cure, % [20] | N = 177 | N = 179 |
| Clinical cure 2 days after EOT | 78.0 | 82.1 |
| Risk of recurrence, % [20] | N = 138 | N = 147 |
| Recurrence at day 40 | 1.4 | 19.7 |
| Recurrence at day 55 | 4.3 | 21.1 |
| Recurrence at day 90 | 7.2 | 22.4 |
| Incidence of adverse events, % [20] | N = 181 | N = 181 |
| Anaemia | 2.8 | 5.5 |
| Heart failure | 2.2 | 5.5 |
| Constipation | 5.5 | 2.8 |
| Diarrhoea | 5.5 | 6.6 |
| Fever | 3.9 | 6.6 |
| CDI | 3.9 | 13.3 |
| Pneumonia | 2.8 | 5.5 |
| Sepsis | 0.6 | 5.0 |
| Urinary tract infection | 3.3 | 6.6 |
| Mortality, % [20] | N = 183 | N = 181 |
| Days 0–10 | 1.4 | |
| Days 11–15 | 1.3 | |
| Days 16–25 | 1.2 | |
| Days 26–30 | 1.0 | |
| Days 31–90 | 0.9 | |
| After day 90 | 0 | |
EPFX extended-pulsed fidaxomicin, EOT end of treatment, CDI Clostridium difficile infection
Costs
Healthcare resources and the corresponding unit costs considered in the model were obtained from Spanish sources (see Online Resource 3; Table ESM 1) [6, 21, 23, 25–27]. Detailed information on cost inputs are available in Online Resource 3. The analysis was performed from the perspective of the NHS in Spain, and only direct healthcare costs expressed in Euros (€) from 2017 were considered.
Utilities
QALYs were calculated from CDI-associated utilities (the quantification of patient-perceived quality of life), obtained from previous studies by Wilcox et al. [28] and Slobogean et al. [29]. The loss of utilities related to adverse events was obtained from several published studies [30–33] (see Online Resource 3, Table ESM 1).
Model outputs and sensitivity analyses
Results are presented as an incremental cost-effectiveness ratio (ICER), i.e., the cost of gaining one QALY with EPFX compared with vancomycin. A base case (deterministic) analysis was performed, incorporating the mean values of all variables. Several sensitivity analyses were also performed: the duration of hospital stay attributable to CDI was modified, taking the 4 days estimated by Monge et al. [34] into consideration; the impact of outlying values of all the model’s variables was assessed; and finally, the cost of an episode of CDI recurrence was considered to be three times greater than that of an initial episode of CDI [21]. The impact of different probabilities for utilities and costs was assessed using 1000 Monte Carlo simulations [35]. Three willingness-to-pay thresholds were considered: €20,000, €25,000 and €30,000 per QALY achieved with EPFX compared with vancomycin [36, 37].
Results
Base case
Applying the EXTEND study outcomes of clinical response to CDI treatment and recurrence (Tables 2 and ESM 1 and Guery et al. [20]) in the model over a time horizon of 1 year, the base case (deterministic) analysis showed 0.638 and 0.594 QALYs per patient treated with EPFX and vancomycin, respectively, a gain of 0.044 QALYs with EPFX (Table 3). Over a 1-year time horizon, the associated treatment cost per patient was €10,046 with EPFX and €10,693 with vancomycin. This would result in a cost saving of €647 per patient treated with EPFX compared with vancomycin. Consequently, EPFX was the dominant treatment associated with higher QALY and lower cost compared with vancomycin (Table 3).
Table 3.
Results (per patient) of the base case (deterministic) and probabilistic analyses
| Base case (deterministic) analysis | |||||
|---|---|---|---|---|---|
| Treatment | Cost | QALY | Cost difference | QALY difference | ICER |
| EPFX | €10,046 | 0.638 | − €647 | 0.044 | EPFX dominates |
| Vancomycin | €10,693 | 0.594 | |||
| Probabilistic analysis | |||||
| Treatment | Cost | QALY | Cost difference | QALY difference | ICER |
| EPFX | €10,051 | 0.635 | − €646 | 0.043 | EPFX dominates |
| Vancomycin | €10,697 | 0.592 | |||
QALY quality-adjusted life-years, EPFX extended-pulsed fidaxomicin, ICER incremental cost-effectiveness ratio (cost per QALY gained with the most effective treatment)
Deterministic sensitivity analysis
Sensitivity analyses (Online Resource 4, Table ESM 2) showed that the variables with the greatest influence on outcome were the relative probability of clinical cure with first-line treatment EPFX compared with vancomycin, hospitalisation costs associated with EPFX treatment (days 5–10), the relative risk of recurrence with EPFX compared with vancomycin after 90 days and the utility associated with sustained clinical cure after first recurrence. In all scenarios, EPFX was the dominant treatment versus vancomycin (Online Resource 4, Table ESM 2). Taking into consideration the CDI-attributable hospital stay of 4 days, the sensitivity analysis showed that EPFX was dominant over vancomycin. Given that the cost of a recurrent CDI episode is three times higher than that of an initial episode, the cost per QALY gained with EPFX was €2747 versus vancomycin; therefore, EPFX would be cost effective.
Probabilistic sensitivity analysis
In the probabilistic analysis, EPFX and vancomycin were associated with costs of €10,051 and €10,697, respectively, with a cost saving of €646 per patient treated with EPFX. EPFX was therefore the dominant treatment (Table 3).
This analysis showed that administration of EPFX had 99.3%, 99.5% and 99.9% respective probabilities of being cost-effective compared with vancomycin at willingness-to-pay thresholds of €20,000, €25,000 and €30,000 per QALY gained. EPFX was therefore dominant over vancomycin.
Discussion
Our analysis of the treatment of CDI in patients aged 60 years and older revealed that the EPFX regimen was associated with a gain of 0.044 QALYs and a cost saving of €647 per patient compared with vancomycin. The EPFX regimen is a cost-effective treatment in most of the comparative analyses with vancomycin using the range of willingness-to-pay thresholds previously suggested for the NHS in Spain [36, 37].
Any evaluation of our results must take into account both the strengths and potential limitations. Regarding the limitations, it should be borne in mind that this is a theoretical model which is, by definition, a simplified simulation of reality. Also, assumptions had to be made in the model with respect to second- and third-line treatment sequences, and with regard to recurrences, as there was no follow-up of patients who failed to respond to the initial treatment in the EXTEND study [20]. In our model, FMT was the third-line treatment, although this practice is not widespread in Spain, highlighting the differences between clinical practice and recommendations in local, national and international treatment guidelines. However, this was regarded as the superior option as it is recommended by ESCMID and IDSA in the case of multi-recurrent CDI [10, 11]. Owing to the need to complete the model in a way that was fair to both treatment options, two assumptions were applied with regard to third-line treatment: (i) clinical cure would occur in all cases and (ii) there would be no further recurrences. These assumptions were validated by the five clinical experts (authors JMA, BA, JC, SG, MS). Moreover, the same mortality rate was assumed regardless of whether patients received EPFX or vancomycin. In the EXTEND study, one treatment-related death occurred in a patient in the vancomycin treatment arm [20]. The costs associated with recurrent episodes of CDI were assumed to be the same as those for the initial episode. This assumption was conservative, as recurrent CDI episodes can incur higher hospitalisation costs compared with initial episodes in clinical practice [38], although a recent study that estimated resource utilisation for the treatment of initial and recurrent CDI was contradictory, finding higher treatment costs for initial compared with recurrent CDI episodes [39].
The state utilities were not obtained from the EXTEND study, but rather from two published studies: one in patients with CDI, including recurrent CDI [28], and the other in patients with CDI who developed infected wounds following surgery for bone fractures [29]. Regarding the validity of performing our model from a Spanish healthcare perspective and sourcing utility data from other countries, it is notable that in a study based on 83,000 assessments of 44 EQ-5D health states from six European countries, including Spain, there was greater variability between individuals than between countries [40]. All the costs used in our model were taken from Spanish sources [6, 25–27].
The conservative nature of our model did not allow for the inclusion of potential costs associated with reducing the risk of transmission of infection, patient isolation and infection control measures, such as the use of disposable gloves and gowns. As has previously been suggested for standard-regimen fidaxomicin [23], had these costs been included in our model, the results are likely to have been even more favourable for EPFX owing to the lower recurrence rate compared with vancomycin.
The structure of the current model is the same as the one used in a recently published study from the United Kingdom [21]. As in our analysis, the study conducted in the United Kingdom concluded that the EPFX regimen would be the dominant treatment—both cost-saving and more effective than vancomycin [21]—with the cost of a treatment cycle of vancomycin in the United Kingdom being considerably higher than the current regimen in Spain (€214 and €34.50, respectively) [21, 25]. The probability that first-line EPFX was cost-effective at a willingness-to-pay threshold of £30,000/QALY was 76% for the patients in the United Kingdom [21].
The EXTEND study demonstrated sustained clinical cure of CDI and significantly lower recurrence rates with EPFX than with vancomycin in a population of patients aged 60 years and older [20]. The results of this economic model suggest that first-line treatment with EPFX would be cost-effective compared with vancomycin according to the willingness-to-pay thresholds normally considered in Spain.
Electronic supplementary material
(DOC 240 kb)
Authors’ contributions
E. González Antona Sánchez, C. López Gutiérrez, C. Rubio-Terrés and D. Rubio-Rodríguez adapted the economic model for this analysis. C. Rubio-Terrés and D. Rubio-Rodríguez wrote the first and subsequent versions of the manuscript. All the authors contributed to fruitful discussion of the results and to the review of the manuscript. C. Rubio-Terrés is the guarantor of the overall content of the paper.
Funding
Financial support for this manuscript was provided by Astellas Pharma Spain. Editorial support was provided by Rhian Harper Owen PhD for Cello Health MedErgy (Europe), and was funded by Astellas Pharma Global Development. Astellas Pharma is the holder of marketing authorisation for fidaxomicin in Spain.
Data availability
Access to anonymised individual patient level data will not be provided for this trial as it meets one or more of the exceptions described under the Sponsor Specific Information for Astellas on www.clinicalstudydatarequest.com.
Conflict of interest
E. González Antona Sánchez and C. López Gutiérrez were employees of Astellas Pharma Spain at the time of preparing this manuscript. C. Rubio-Terrés and D. Rubio-Rodríguez received an honorarium from Astellas Pharma Spain in connection with the development of this manuscript. The remaining authors have no competing interests to declare.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
This was a retrospective study and all patient data were anonymised; informed consent was therefore not required under country-specific regulations. No direct access to source data by the clinical research organisation or the sponsor was allowed.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bassetti M, Villa G, Pecori D, Arzese A, Wilcox M. Epidemiology, diagnosis and treatment of Clostridium difficile infection. Expert Rev Anti-Infect Ther. 2012;10:1405–1423. doi: 10.1586/eri.12.135. [DOI] [PubMed] [Google Scholar]
- 2.Alcala L, Marin M, Martin A, Sanchez-Somolinos M, Catalan P, Pelaez MT, et al. Laboratory diagnosis of Clostridium difficile infection in Spain: a population-based survey. J Hosp Infect. 2011;79:13–17. doi: 10.1016/j.jhin.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Alcala L, Martin A, Marin M, Sanchez-Somolinos M, Catalan P, Pelaez T, et al. The undiagnosed cases of Clostridium difficile infection in a whole nation: where is the problem? Clin Microbiol Infect. 2012;18:E204–E213. doi: 10.1111/j.1469-0691.2012.03883.x. [DOI] [PubMed] [Google Scholar]
- 4.Asensio A, Vaque-Rafart J, Calbo-Torrecillas F, Gestal-Otero JJ, Lopez-Fernandez F, Trilla-Garcia A, et al. (2008) Increasing rates in Clostridium difficile infection (CDI) among hospitalised patients, Spain 1999-2007. Euro Surveill;13. 10.2807/ese.13.31.18943-en [PubMed]
- 5.van Kleef E, Green N, Goldenberg SD, Robotham JV, Cookson B, Jit M, et al. Excess length of stay and mortality due to Clostridium difficile infection: a multi-state modelling approach. J Hosp Infect. 2014;88:213–217. doi: 10.1016/j.jhin.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Asensio A, Bouza E, Grau S, Rubio-Rodriguez D, Rubio-Terres C. Cost of Clostridium difficile associated diarrhea in Spain. Rev Esp Salud Publica. 2013;87:25–33. doi: 10.4321/S1135-57272013000100004. [DOI] [PubMed] [Google Scholar]
- 7.Olsen MA, Yan Y, Reske KA, Zilberberg M, Dubberke ER. Impact of Clostridium difficile recurrence on hospital readmissions. Am J Infect Control. 2015;43:318–322. doi: 10.1016/j.ajic.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Reigadas Ramirez E, Bouza ES. Economic burden of Clostridium difficile infection in European countries. Adv Exp Med Biol. 2018;1050:1–12. doi: 10.1007/978-3-319-72799-8_1. [DOI] [PubMed] [Google Scholar]
- 9.Johnson AP, Wilcox MH. Fidaxomicin: a new option for the treatment of Clostridium difficile infection. J Antimicrob Chemother. 2012;67:2788–2792. doi: 10.1093/jac/dks302. [DOI] [PubMed] [Google Scholar]
- 10.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE et al (2018) Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 31:431–455. 10.1093/cid/cix1085 [DOI] [PMC free article] [PubMed]
- 11.Debast S, Bauer M, Kuijper E. European society of clinical microbiology and infectious diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20:1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 12.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18:21–27. doi: 10.1111/1469-0691.12046. [DOI] [PubMed] [Google Scholar]
- 13.Britton RA, Young VB. Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends Microbiol. 2012;20:313–319. doi: 10.1016/j.tim.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford T, Huesgen E, Danziger L. Fidaxomicin: a novel macrocyclic antibiotic for the treatment of Clostridium difficile infection. Am J Health Syst Pharm. 2012;69:933–943. doi: 10.2146/ajhp110371. [DOI] [PubMed] [Google Scholar]
- 15.Louie TJ, Cannon K, Byrne B, Emery J, Ward L, Eyben M, et al. Fidaxomicin preserves the intestinal microbiome during and after treatment of Clostridium difficile infection (CDI) and reduces both toxin re-expression and recurrence of CDI. Clin Infect Dis. 2012;55(Suppl 2):S132–S142. doi: 10.1093/cid/cis338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louie TJ, Miller MA, Mullane K, Weiss K, Lentnek A, Golan Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 17.Cornely OA, Crook DW, Esposito R, Poirier A, Somero MS, Weiss K, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281–289. doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- 18.Crook DW, Walker AS, Kean Y, Weiss K, Cornely OA, Miller MA, et al. (2012) Fidaxomicin versus vancomycin for Clostridium difficile infection: meta-analysis of pivotal randomized controlled trials. Clin Infect Dis;55 Suppl 2:S93–103. 10.1093/cid/cis499 [DOI] [PMC free article] [PubMed]
- 19.Chilton CH, Crowther GS, Todhunter SL, Ashwin H, Longshaw CM, Karas A, et al. Efficacy of alternative fidaxomicin dosing regimens for treatment of simulated Clostridium difficile infection in an in vitro human gut model. J Antimicrob Chemother. 2015;70:2598–2607. doi: 10.1093/jac/dkv156. [DOI] [PubMed] [Google Scholar]
- 20.Guery B, Menichetti F, Anttila V, Adomakoh N, Aguado JM, Bisnauthsing K, et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect Dis. 2017;18:296–307. doi: 10.1016/S1473-3099(17)30751-X. [DOI] [PubMed] [Google Scholar]
- 21.Cornely OA, Watt M, McCrea C, Goldenberg SD, De Nigris E. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients aged ≥60 years (EXTEND): analysis of cost-effectiveness. J Antimicrob Chemother. 2018;73:2529–2539. doi: 10.1093/jac/dky184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubio-Terrés C, Echevarría A (2006) Modelos de Markov: Una herramienta útil para el análisis farmacoeconómico. PharmacoEconomics Spanish Research Articles (Suppl 2) 3:71–78. http://www.healthvalue.org/pdfs/pharmacoeconomics%20sra%202006d.pdf
- 23.Rubio-Terrés C, Cobo Reinoso J, Grau Cerrato S, Mensa Pueyo J, Salavert Lleti M, Toledo A et al (2015) Economic assessment of fidaxomicin for the treatment of Clostridium difficile infection (CDI) in special populations (patients with cancer, concomitant antibiotic treatment or renal impairment) in Spain. Eur J Clin Microbiol Infect Dis 34:2213–2223. 10.1007/s10096-015-2472-0 [DOI] [PubMed]
- 24.Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME (1997) Recurrent Clostridium difficile diarrhea: characteristics of and risk factors for patients enrolled in a prospective, randomized, double-blinded trial. Clin Infect Dis 24:324–333. 10.1093/clinids/24.3.324 [DOI] [PubMed]
- 25.Consejo General de Colegios Oficiales de Farmacéuticos. BOT Plus 2.0 [Internet]. https://botplusweb.portalfarma.com/
- 26.Isla D, De Castro J, Juan O, Grau S, Orofino J, Gordo R, et al. Costs of adverse events associated with erlotinib or afatinib in first-line treatment of advanced EGFR-positive non-small cell lung cancer. Clinicoecon. Outcomes Res. 2017;9:31–38. doi: 10.2147/CEOR.S121093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Servicio Vasco de Salud (2017) Tarifas para facturación de servicios sanitarios y docentes de osakidetza para el año 2017 [Internet]. https://www.osakidetza.euskadi.eus/contenidos/informacion/libro_tarifas/eu_libro/adjuntos/Libro_de_Tarifas_2017.pdf
- 28.Wilcox MH, Ahir H, Coia JE, Dodgson A, Hopkins S, Llewelyn MJ, et al. Impact of recurrent Clostridium difficile infection: hospitalization and patient quality of life. J Antimicrob Chemother. 2017;72:2647–2656. doi: 10.1093/jac/dkx174. [DOI] [PubMed] [Google Scholar]
- 29.Slobogean GP, O’Brien PJ, Brauer CA. Single-dose versus multiple-dose antibiotic prophylaxis for the surgical treatment of closed fractures. Acta Orthop. 2010;81:256–262. doi: 10.3109/17453671003587119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Institute for Health and Care Excellence (2011) Pazopanib for the first-line treatment of advanced renal cell carcinoma. Technology appraisal guidance [TA215] [Internet]. https://www.nice.org.uk/guidance/ta215/chapter/1-Guidance
- 31.Shyangdan D, Jacob RP, Connock M, Johnston R, Cummins E, Waugh N (2014) Empagliflozin for the treatment of type 2 diabetes: a single technology assessment.. Warwick Evidence [Internet]. https://njl-admin.nihr.ac.uk/document/download/2022671
- 32.Marti SG, Colantonio L, Bardach A, Galante J, Lopez A, Caporale J, et al. A cost-effectiveness analysis of a 10-valent pneumococcal conjugate vaccine in children in six Latin American countries. Cost Eff Resour Alloc. 2013;11:21. doi: 10.1186/1478-7547-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Institute for Health and Care Excellence (2015) Bortezomib for previously untreated mantle cell lymphoma. Technology appraisal guidance [TA370] [Internet]. https://www.nice.org.uk/guidance/ta370/resources/bortezomib-for-previously-untreated-mantle-cell-lymphoma-pdf-82602782522053
- 34.Monge D, Millan I, Gonzalez-Escalada A, Asensio A (2013) The effect of Clostridium difficile infection on length of hospital stay. A cohort study. [In Spanish] Enferm Infecc Microbiol Clin 31:660–664. 10.1016/j.eimc.2012.11.007 [DOI] [PubMed]
- 35.Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Handbooks in health economic evaluation. New York: Oxford University Press; 2006. [Google Scholar]
- 36.Vallejo-Torres L, García-Lorenzo B, Castilla I, Valcárcel Nazco C, García-Pérez L, Linertová R, et al. Valor Monetario de un Año de Vida Ajustado por Calidad: Estimación empírica del coste de oportunidad en el Sistema Nacional de Salud. Informes de Evaluación de Tecnologías Sanitarias SESCS. 2015 [Internet]. https://www3.gobiernodecanarias.org/sanidad/scs/content/3382aaa2-cb58-11e5-a9c5-a398589805dc/SESCS%202015_Umbral%20C.O.%20AVAC.pdf
- 37.Sacristan JA, Oliva J, Del Llano J, Prieto L, Pinto JL (2002) What is an efficient health technology in Spain? [In Spanish] Gac Sanit 16:334–343. [Internet]. http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0213-91112002000400008 [DOI] [PubMed]
- 38.Dubberke ER, Schaefer E, Reske KA, Zilberberg M, Hollenbeak CS, Olsen MA. Attributable inpatient costs of recurrent Clostridium difficile infections. Infect Control Hosp Epidemiol. 2014;35:1400–1407. doi: 10.1086/678428. [DOI] [PubMed] [Google Scholar]
- 39.Zhang D, Prabhu VS, Marcella SW. Attributable healthcare resource utilization and costs for patients with primary and recurrent Clostridium difficile infection in the United States. Clin Infect Dis. 2018;66:1326–1332. doi: 10.1093/cid/cix1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greiner W, Weijnen T, Nieuwenhuizen M, Oppe S, Badia X, Busschbach J, et al. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ. 2003;4:222–231. doi: 10.1007/s10198-003-0182-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 240 kb)
Data Availability Statement
Access to anonymised individual patient level data will not be provided for this trial as it meets one or more of the exceptions described under the Sponsor Specific Information for Astellas on www.clinicalstudydatarequest.com.