Abstract
Short biologic half-lives limit the therapeutic utility of many small molecules. One approach to extending the half-life of pharmacologically active small molecules is conjugation to less degradable nanoparticles; here we report the synthesis and activity of six targeted polymeric (PEG-b-PLA) nanoparticles for use as adenosine receptor agonists. Using click chemistry, PLA-b-PEG400-N3 and PLA-b-PEG2000 block copolymers were bound to adenosine at the 3′,4′-OH, 5′-OH, and 6-NH2 positions with an acetylene group. Activity of the conjugates as adenosine receptor ligands was tested by their capacity to stimulate cAMP increases in RAW264.7 murine macrophage cells. Only adenosine-conjugated nanoparticles (A-3′,4′-OH-TPN2), in which PEG2000 was bound to adenosine on the 3′,4′ hydroxyl groups, stimulated cAMP increases and these increases were blocked by selective antagonists of both adenosine A2A and A2B receptors, consistent with ligation of these receptors. Adenosine nanoparticles were tested in vivo in a rat model of post-traumatic osteoarthritis; intra-articular injection of adenosine nanoparticles prevented the development of osteoarthritis in this model. These studies suggest that attachment of adenosine to biodegradable nanoparticles provides a novel approach to achieving prolonged therapeutic effects.
Subject terms: Acute inflammatory arthritis, Drug delivery
Introduction
Biodegradable nanoparticles (NPs) have gained increasing interest for their ability to provide a viable carrier for delivery of vaccines, genes, drugs and other biomolecules. Their enhanced biocompatibility and prolonged release profiles make them useful for a variety of medical applications1. Biodegradable NPs improve solubility of hydrophobic drugs, increase local concentration, provide longer clearance time, increase probability of interactions (e.g. when activation of a receptor is critical), and generally have low toxicity. For example, it has been observed that to avoid clearance by the reticuloendothelial system (RES), the addition of polyethylene glycol (PEG) on the surface of NPs is required2. As a consequence of surface PEG molecules, higher maximum tolerated doses (MTD) of nanoparticles (NPs) are realized3.
Osteoarthritis (OA) is the most common type of arthritis affecting as many as 29 million people in the United States alone. One in every two people will likely be affected by osteoarthritis. OA is a degenerative joint disorder in which the articular cartilage is destroyed and chondrocytes, the cells that synthesize and maintain cartilage, play a central role in cartilage destruction. Age, injury and inflammation reduce the capacity of chondrocytes to synthesize and maintain ATP, a molecule which is important for maintaining chondrocyte and cartilage homeostasis. The pathogenesis of osteoarthritis involves low grade inflammation, destruction of articular cartilage and reactive overgrowth of bone in the affected joints. At the present time, therapy is, for the most part, palliative including use of nonsteroidal anti-inflammatory drugs (e.g. ibuprofen4,5), narcotic analgesics6, exercise, acupuncture7,8, and injections of anti-inflammatory agents (e.g. glucocorticoids9) or other substances (hyaluronic acid10,11) into the joint. Thus, short of total joint replacement, there are few long-term effective therapies available. Notice, that although currently available injectable agents (corticosteroids, hyaluronate) provide symptomatic relief, none of these agents are restorative. Therefore, improved and more effective treatments of osteoarthritis are required.
Recently, Corciulo and coworkers have shown that intracellular and extracellular levels of ATP fall after treatment of mouse chondrocytes and rat tibia explants with IL-1β, an inflammatory mediator that participates in OA pathogenesis12. In the extracellular space ATP is hydrolyzed to adenosine which is a ligand for a family of cell surface G protein coupled receptors (A1R, A2AR, A2BR, A3R). Mice deficient in the A2A adenosine receptor (A2AR) or the ecto-enzyme that mediates the conversion of AMP to adenosine (ecto-5′nucleotidase) develop spontaneous OA12. Adenosine itself is extremely short-lived in biologic fluids; in whole blood adenosine has a half-life of 1–4 seconds and likely has a similar short half-life in the joint13. In a rat model of post-traumatic OA the intra-articular injection of liposomal suspensions containing adenosine prevents development of OA12. Based on these results these authors formulated the hypothesis that extracellular adenosine is an important homeostatic mechanism for chondrocytes and loss of adenosine-A2AR-mediated chondrocyte homeostasis contributes to OA development. It follows that adenosine A2A receptors might be an excellent target for the treatment or prevention of OA.
Whereas liposomes are excellent for microencapsulation and drug delivery, once open and their cargo is released, the clearance time of the drug does not significantly increase relative to its application as a molecular formulation. Hence, the relative advantage of the liposomes is their prolonged release process. Therefore, the next logical step is the attachment of the molecule under study (adenosine) to a carrier by a chemical bond. For these studies we have selected poly(lactic acid)-poly(ethylene glycol) (PLA-PEG) nanoparticles (NPs). While the majority of PLA-PEG NPs have been used for encapsulation of bioactive molecules, surface functionalized PLA-PEG NPs are ideal when activation of the receptor is required14,15. Adenosine A2A receptor inhibits osteoclast differentiation and activates osteoblasts, stimulates chondrocytes and suppresses inflammation, among many other effects16–18. Agents that target A2AR effectively inhibit inflammatory osteolysis in inflamed bone17. Thus, an agent that targets A2AR could be a new therapeutic that promotes bone regeneration and increases bone volume.
One of the concerns in using PLA-PEG nanoparticles is their hydrolysis to lactic acid. However, it has been well established that the damage done during OA is mediated as a result of changes in the chondrocytes, not the acid milieu. The chondrocytes have to be alive to mediate OA damage to the cartilage18. Here we report on the first preparation of adenosine-functionalized PLA-PEG NPs, and their use for treatment in a rat model of post-traumatic osteoarthritis. Our hypothesis is that such NPs will be highly effective, since given the number of surface functionalized adenosine molecules, one NP can potentially activate more than one receptor.
Results and Discussion
Synthesis of adenosine-functionalized pla-b-peg copolymers
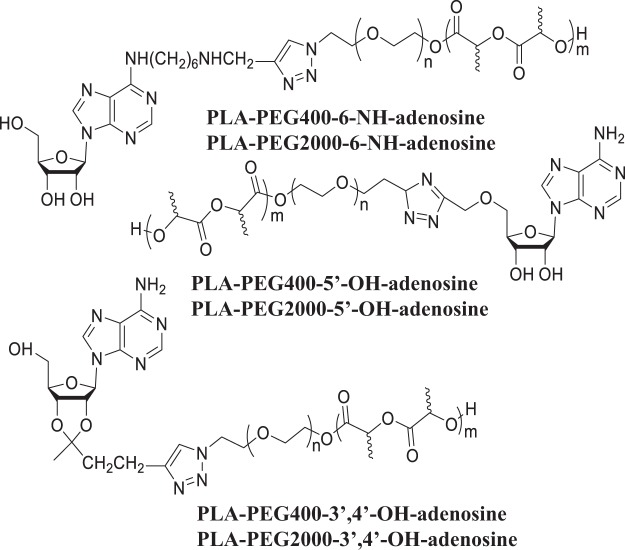
Adenosine has three potential sites for polymer attachment –the 3′,4′-OH groups, the 5′-OH group, and the 6-NH2 group. Our hypothesis has been that these different attachments should result in adenosine, having free NH2 and/or OH groups with different spacing, hence with different H-bonding schemes (molecular recognition), different H-bonding strength, and thus different interaction energies with the receptor, which might result in different bioactivities. Figure 1 shows the six adenosine-terminated PLA-PEG block copolymers prepared in this study. Block copolymers had been prepared using both PEG400 and PEG2000.
Figure 1.
Adenosine-terminated PLA-PEG block copolymers.
While PLA-PEG biodegradable nanoparticles have been made before, the attachments of adenosine using the 3′,4′-OH, 5′-OH, and 6-NH2 groups have raised significant synthetic challenges, and has required the development of new strategies for functionalized adenosine molecules. The overall strategy for the synthesis of adenosine functionalized biodegradable nanoparticles involves four parts: 1. Synthesis of poly[lactic acid]-poly[ethylene glycol] (PLA-PEG) block copolymer terminated with an azide group (PLA-b-PEG-N3); 2. Synthesis of adenosine functionalized with an acetylene group (–C≡C–H); 3. Connection of the adenosine to the block copolymer using click chemistry between the acetylene and the azide; 4. Preparation of nanoparticle from the adenosine-functionalized block copolymer. NPs for control experiments do not carry adenosine, and the PEG is terminated with a methyl group. All syntheses described here were the same for PEG400 and PEG2000.
Synthesis of PLA-b-PEG-N3
The synthesis of HO-PEG-N3 was carried out as described by Mahou and Wandrey19 (Fig. 2). Ring opening polymerization of D,L-lactide was performed using HO-PEG-N3 in dry toluene, with Sn(Oct)2 as the catalyst. The product was purified by precipitation from dichloromethane solution using diethyl ether.
Figure 2.
Adenosine-terminated PLA-PEG block copolymers.
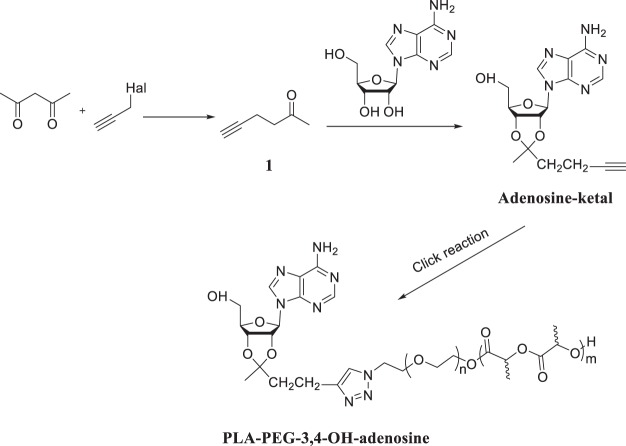
Synthesis of PLA-PEG-3′,4′-OH-adenosine
Figure 2 shows the synthetic strategy. An attachment through the 3′,4′-OH groups is accomplished by reacting the with a ketone, forming a 5-membered ketal ring. The attachment to the azide-terminated PLA-PEG block copolymer, using click chemistry, requires an acetylene group, hence we first prepared 5-Hexyn-2-one (1 in Fig. 3) using a method reported by Görl and Alt20. 2,4-Pentanedione and propargyl chloride were allowed to react in ethyl alcohol containing anhydrous potassium carbonate resulting in the desired compound. This ketone was then reacted with adenosine in anhydrous DMF, using triethyl orthoformate as water scavenger, and HCl in dioxane as a catalyst21, yielding the desired ketal, which could be purified using chromatography. The reaction of alkynated adenosine with PLA-b-PEG-N3 was carried out in anhydrous DMF, using CuBr and the catalyst and N,N,N′,N′′,N′′-pentamethyl-diethylenetriamine (PMDETA) as the base (Copper(I)-catalyzed azide-alkyne cycloaddition –“click chemistry”)22. The product was purified by successive precipitations from THF using water, and was freeze-dried to yield a white powder.
Figure 3.
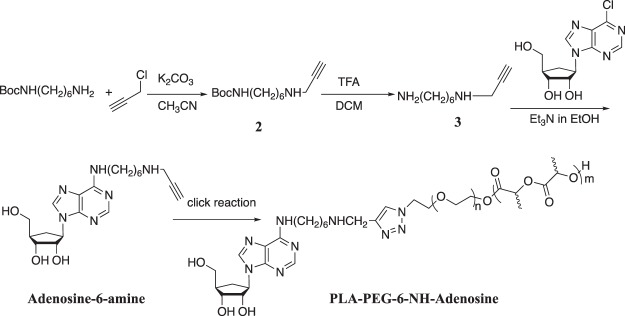
Synthesis of PLA-b-PEG400-6-NH-adenosine, and PLA-b-PEG2000-6-NH-adenosine.
Synthesis of PLA-b-PEG-6-NH-adenosine
Figure 3 presents the synthetic strategy. The attachment of the PLA-b-PEG to adenosine was accomplish by first reacting an acetylene-terminated primary hexamethylene diamine with 6-chloro-9-(β-D-ribofuranosyl)-purine. A reaction of mono Boc-protected hexamethylenediamine with propargyl chloride in anhydrous acetonitrile using K2CO3 as acid scavenger, resulted in tert-butyl(6-(pro-2-yn-1-ylamino)hexyl)carbamate in 68% yield after column chromatography on a basic alumina (Dichloromethane: MeOH = 50:1). The removal of the Boc protecting group was accomplished in 96% yield using trifluoroacetic acid in dichloromethane. The resulting free amine was reacted with 6-chloro-9-(β-D-ribofuranosyl)purine in ethanol, using trimethylamine as the acid scavenger. Purification using silica gel chromatography (CH3OH:Dichloromethane, 1:10) provided the desired product in 50% yield. A click reaction with the azide-terminated PLA-b-PEG - as described above –provides the PLA-b-PEG-6-NH-adenosine.
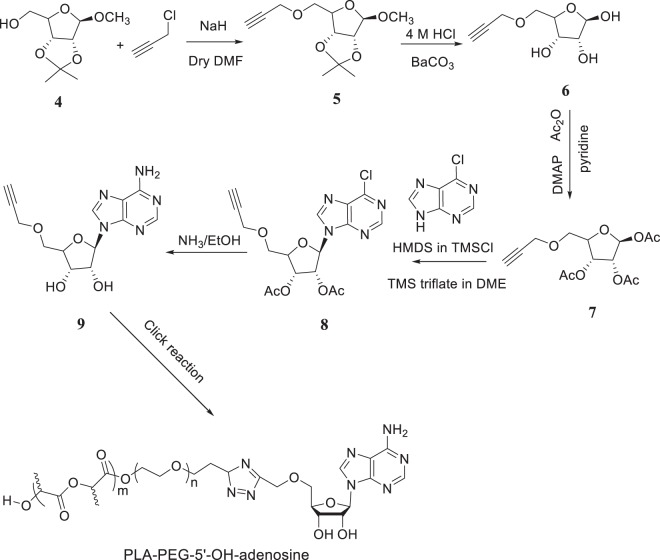
Synthesis of PLA-b-PEG-5′-OH-adenosine
The synthetic strategy for the synthesis of PLA-b-PEG-5′-OH-adenosine is presented in Fig. 4. This synthesis is more involved since the purine and the furanose parts of the adenosine must be connected. Methyl 5-O-propargyl-2,3-O-isopropylidene-β-D-ribofuranose was prepared first deprotonating 2,3-O-isopropylidene-β-D- ribofuranoside by NaH in dry DMF. The alkoxide was allowed to react with propargyl chloride, providing compound 5 in Fig. 4. The methyl group was removed using HCl to yield compound 6, which was then actylated using acetic anhydride in dry pyridine with DMAP as acid scavenger, providing compound 7. Next, 6-Chloropurine was treated with 1,1,1,
Figure 4.
Synthesis of PLA-b-PEG400-5′-OH-adenosine, and PLA-b-PEG200-5′-OH-adenosine.
3,3,3-hexamethyldisilazane and chlorotrimethylsilane, and the silylated compound, which was used without further purification, in Vorbruggen coupling with compound 7 to yield compound 8. Finally, compound 8 was allowed to react with ammonia in ethanol to yield the desired adenosine derivative. A click reaction with the azide-terminated PLA-b-PEG - as described above –provides the PLA-b-PEG-5′-OH-adenosine.
Synthesis of adenosine-functionalized nanoparticles
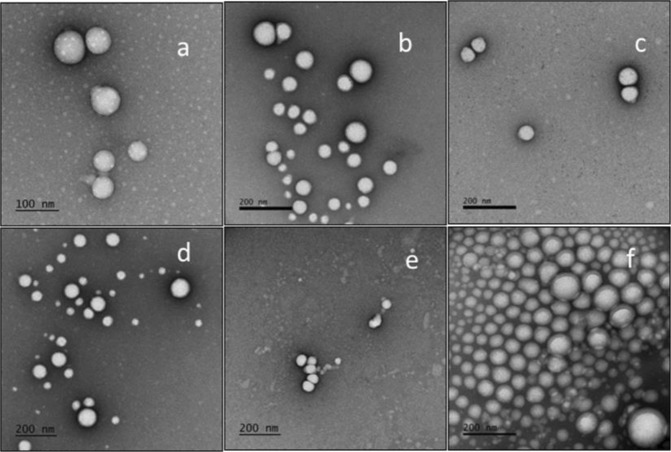
Nanoparticles were made by dissolving the polymer in ethyl acetate and adding it to an aqueous Pluronic F68 solution. An emulsion was formed using a vortex shaker, which was then ultrasonicated, and the organic solvent was removed under reduced pressure. The resulting nanoparticle suspension was ultracentrifuged, and the resulting pellet was re-suspended in pH = 7.4 PBS. The nanoparticles were filtered through a 1 µm glass filter fist and then 0.45 µm and stored at 4 °C until use. The following nanoparticles were prepared: PLA-PEG400-3′,4′-OH-adenosine, PLA-PEG2000-3′,4′-OH-adenosine, PLA-PEG400-5′-OH-adenosine, PEG2000-5′-OH-adenosine, PEG400-NH-adenosine, and PEG2000-NH-adenosine. Nanoparticle size and size distribution (S1-7) were determined by dynamic light scattering (Table 1). Transmission electron microscopy studies of the six adenosine attached copolymer nanoparticles showed an exclusively spherical morphology in Fig. 5. Because the nanoparticles aggregate when the temperature increases and TEM data collection takes three hours, during which time the temperature of the nanoparticles increases from 4 °C (their storage temperature) to room temperature, there is some variability in the apparent size. Moreover, nanoparticles tend to aggregate at high concentrations. Nonetheless, the TEM images in Fig. 5 show both single and aggregated nanoparticle clusters.
Table 1.
Diameter of nanoparticles (light scattering measurements).
| Diameter Size (nm) | PDI | |
|---|---|---|
| PLA-PEG400-3′,4′-OH-adenosine | 131 | 0.156 |
| PLA-PEG400-6-NH-adenosine | 141 | 0.133 |
| PLA-PEG400-5′-OH-adenosine | 137 | 0.145 |
| MeOPEG550-PLA(Control) | 133 | 0.091 |
| PLA-PEG2000-3,4-OH-adenosine | 129 | 0.126 |
| PLA-PEG2000-6-NH-adenosine | 144 | 0.159 |
| PLA-PEG2000-5-OH-adenosine | 140 | 0.181 |
Figure 5.
The shape of adenosine-functionalized nanoparticles. Transmission electron microscopy images for six nanoparticles. (a) PLA-PEG400-3′,4′-OH-adenosine; (b) PLA-PEG400-6-NH-adenosine; (c) PLA-PEG400-5′-OH-adenosine; (d) PLA-PEG2000-3,4-OH-adenosine; (e) PLA-PEG2000-6-NH-adenosine; (f) PLA-PEG2000-5-OH-adenosine.
Stimulation of adenosine receptors in RAW264.7 cells
To determine whether the adenosine-conjugated nanoparticles stimulate adenosine receptors in RAW264.7 cells, we tested the effect of NPs alone (control), adenosine, and the six types of adenosine-conjugated NPs on cAMP accumulation. As shown in Fig. 6A, following incubation of RAW264.7 cells incubation with adenosine alone stimulated a 50% increase in cellular cAMP and only the PEG2000 nanoparticles conjugated to adenosine at the ribose 3′,4′-OH groups stimulated a similar and significant increase in cAMP accumulation. None of the adenosine conjugated to PLA-PEG400 nanoparticles had any effect on cAMP accumulation. Similarly, the 5′-OH- and amine-conjugated PLA-PEG2000 nanoparticles had no significant effect on cAMP content.
Figure 6.
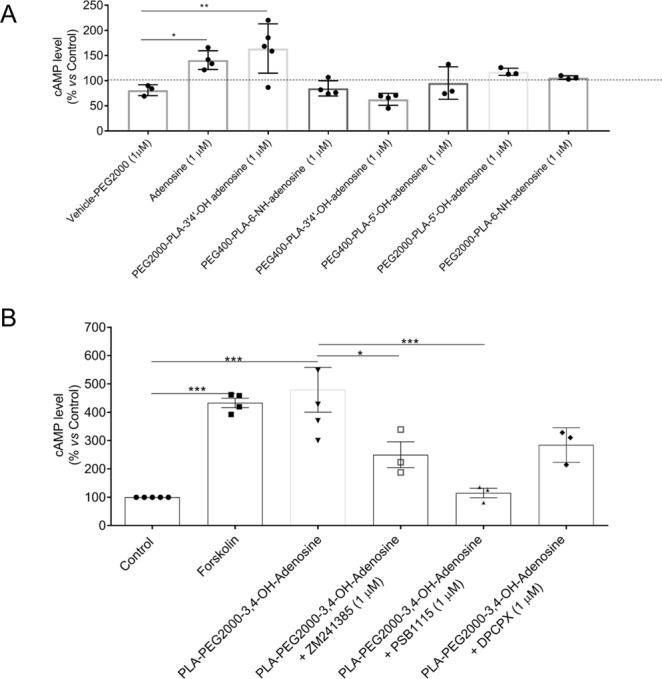
Adenosine and Adenosine functionalized nanoparticles stimulate cAMP accumulation. (A) Adenosine and PLA-PEG2000-3,4-OH-adenosine (PEG2000-Ado) but not PEG400-Ado or PEG2000-PLA-5′-OH-adenosine or PEG2000-PLA-6-NH-adenosine particles stimulate a significant increase in cellular content of cAMP as compared to vehicle in the RAW 264.7 cell line. (B) Adenosine A2A and A2B antagonists reverse the effect of adenosine functionalized nanoparticles on cAMP accumulation. Co-treatment with A2AR antagonist ZM241385, the A2BR antagonist PSB1115 and the poorly selective A1R/A2R antagonist DPCPX significantly reduced the PEG2000-Ado effect on cAMP production. Data are expressed as % control cAMP content and each data point shown represents results of experiments carried out on a single day in duplicate. (*p < 0.05; **p < 0.01; n = 3–5).
To understand the pharmacologic mechanism by which the adenosine-conjugated particles acted on cells we determined whether selective adenosine A2A and A2B receptor antagonists blocked the effect of the particles on cAMP accumulation in primary murine chondrocytes. To determine maximal cAMP increases in these cells we incubated the cells with forskolin, an agent which bypasses cell surface receptors to stimulate accumulation of cAMP in the primary cells (Fig. 6B). Forskolin induced a 4-fold increase in cAMP which was similar to that induced by the PLA-PEG2000-3′,4′-OH-adenosine particles. We found that the adenosine-conjugated nanoparticles stimulated cAMP accumulation via both A2A and A2B adenosine receptors since co-incubation with selective antagonists for either A2A receptor (ZM241385), or A2B receptor (PSB1115) diminished the effect of the adenosine nanoparticles on cAMP accumulation (Fig. 6B).
To further pinpoint which adenosine receptors are activated by the adenosine-conjugated nanoparticles and their effect on chondrocyte function we tested the effects of the particles on expression of message for inflammatory mediators in primary murine chondrocytes. We observed that adenosine-conjugated nanoparticles diminished the IL-1β-stimulated expression of IL-6, MMP13 and Collagen 10, an effect that was partially reversed by A2AR (ZM241385 and SCH58261) and A2BR (PSB1115) antagonists (Fig. 7A). In contrast, as shown in Fig. 7B,C, the A2AR antagonists only partially reversed the effect of the adenosine-conjugated nanoparticles on IL-1β-stimulated MMP13 and Collagen-10 expression whereas the A2BR antagonist completely reversed this effect. These findings suggest that, at the concentrations used, A2AR antagonists are not as potent as the A2BR antagonist for reversing the effect of the conjugated nanoparticles on cells. Both the adenosine-conjugated nanoparticles and adenosine alone diminished IL-1β stimulated activation of NF-kb, a central intracellular signal for inflammation (Fig. 8).
Figure 7.
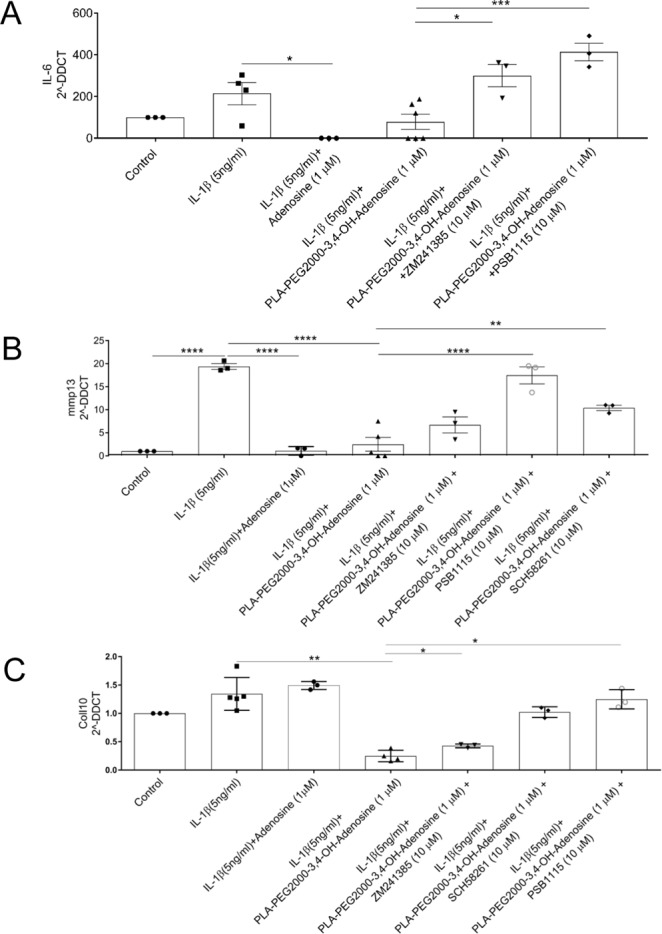
Adenosine functionalized PLA nanoparticles regulate expression of inflammatory mediators and markers. Chondrocytes were incubated with IL-1b, nanoparticles and adenosine A2A receptor antagonists (ZM241385 and SCH58261) or an A2B antagonist (PSB1115), as described. RNA was isolated and subject to reverse transcription and real time-PCR, as described and levels of specific mRNA were calculated and normalized to LDH. The functionalized nanoparticles diminished message for: (A) IL-6; (B). MMP-13, and; (C). Col10a1. Each point represents a separate determination. (*p < 0.05; **p < 0.01; ***p < 0.001; n = 3–5).
Figure 8.
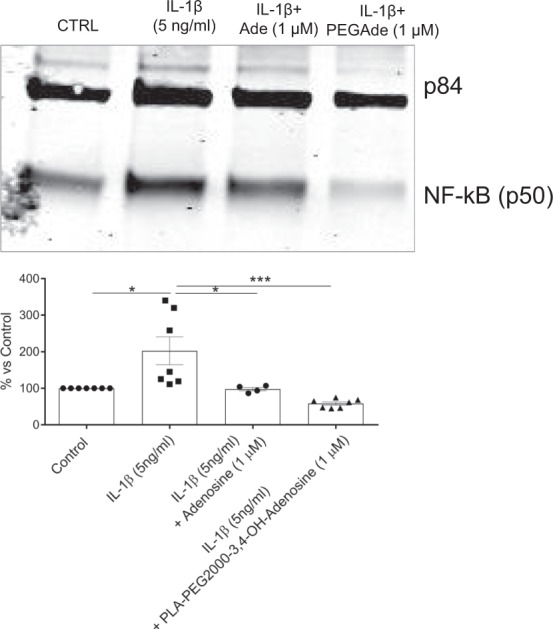
PEG2000-Ado NPs and adenosine decrease IL-1β induced NF-kB nuclear translocation in primary murine chondrocytes. Primary murine chondrocytes were cultured in the absence (Control) or presence of IL-1β and either adenosine or adenosine conjugated nanoparticles for 24 hours at 37 °C. Nuclear fractions were isolated, as described, and proteins isolated. After separation of proteins by electrophoresis through 10% polyacrylamide gels the proteins were then transferred to a nitrocellulose membrane, as described. Nuclear NFkB and P84 (a control nuclear protein) were detected following decoration with appropriate antibodies and quantitated by densitometry of bands on Western Blot. Data are expressed as % control levels. Each point represents results from an individual experiment (*p < 0.05; ***p < 0.001; n = 4–7).
Taken together the results of the in vitro studies clearly demonstrate that the adenosine-functionalized particles bind to and activate adenosine A2A and A2B receptors on murine cells as well as adenosine. Moreover, like adenosine the particles are not selective for A2A or A2B receptors. Because prior experiments had demonstrated that PLA polymers are degradable in vivo we next investigated the effect of these nanoparticles in the prevention of osteoarthritis in a rat model23,24.
Intra-articular injection of nanoparticles prevents progression of osteoarthritis in a rat post-traumatic osteoarthritis model
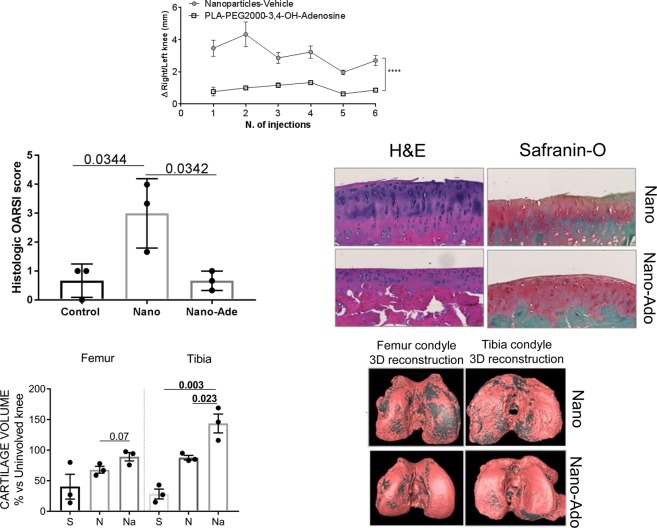
Because we found that the adenosine-conjugated PEG2000 NPs acted at adenosine A2AR and A2BR, we next determined whether these NPs could ameliorate damage in a rat post-traumatic osteoarthritis model. As shown in Fig. 8, intra-articular injection of the adenosine-conjugated nanoparticles diminished swelling in affected knees whereas unconjugated nanoparticles had no effect on knee swelling (Fig. 9A). In rats treated with adenosine conjugated-NP there was markedly reduced fibrillation of the cartilage surface (H&E staining) and less proteoglycan loss (Safranin-O staining) resulting in a significantly decreased OARSI score as compared to the unconjugated nanoparticles (Fig. 9B). More importantly, intra-articular injection of the knees with adenosine-conjugated nanoparticles prevented loss of cartilage (the pink material in these reconstructed microCT images, Fig. 9C), as compared to the unconjugated nanoparticles. These results provide further evidence that intraarticular injection of an adenosine receptor agonist is useful for the treatment of osteoarthritis.
Figure 9.
Adenosine-conjugated NPs preserve articular cartilage in a rat model of post traumatic osteoarthritis. Intra-articular injection of adenosine-conjugated NPs diminishes knee swelling (A). Histologic analysis reveal cartilage protection with less fibrillation, proteoglycan loss (red surface in the Safranin-O stained slides) and decrease of OARSI score in Nano-Ado treated rats compared to the rats treated with the vehicle (B). Moreover, the reconstruction of the µCT data reveals a reduction of the cartilage surface damage (C); cartilage is pink and the bone below is grey). The total volume of the cartilage in the OA knee compare to the uninvolved knee is 68% in the vehicle group and 89% of the Nano-Ado group (****p < 0.0001; n = 3 for each group).
Corciulo and colleagues recently reported that endogenously generated adenosine plays a critical role in maintaining chondrocyte and cartilage homeostasis12. Because injections of adenosine alone, which has an extremely short half-life (1–4 seconds) in biological fluids13, does not affect the course of osteoarthritis12 we have sought to develop alternative approaches to delivery of adenosine or adenosine receptor agonists with a prolonged duration of action to osteoarthritic cartilage we developed adenosine-conjugated nanoparticles. Here we report that intraarticular injection of adenosine-conjugated nanoparticles, which act as adenosine receptor agonists, but not unconjugated nanoparticles prevents progression of post-traumatic osteoarthritis in a rodent model. These nanoparticles may provide an appropriate therapeutic for the treatment of osteoarthritis.
Conclusion
We report the synthesis and activity of three adenosine-functionalized PLA-b-PEG biodegradable NPs. Using click chemistry, PLA-b-PEG-N3 block copolymers were connected to adenosine at the 3′,4′-OH, 5′-OH, and 6-NH2 positions with an acetylene group. Of the three different adenosine-functionalized NPs, one, in which the copolymer was bound to the adenosine on the 3′,4′-hydroxyl groups, induced cellular increases in cAMP, in an adenosine A2A and A2B receptor-dependent fashion. These NPs were then tested in a rat model of post-traumatic osteoarthritis and were found to effectively block the development of osteoarthritis in this model. We conclude that adenosine-functionalized PLA-b-PEG particles are a novel approach to developing long lasting therapies for local treatment of such conditions as osteoarthritis.
Material and Methods
Material
Poly(ethylene glycol) (PEG400), poly(ethylene glycol) (PEG2000), propargyl chloride, methyltrichlorosilane, stannous octoate, adenosine, p-toluene sulfonyl chloride, sodium azide, 2,4-pentanedione, anhydrous potassium carbonate, triethyl orthoformate, sodium bicarbonate, trifluoroacetic acid, triethyl amine, sodium hydride, copper bromide, barium carbonate, concentrated hydrochloric acid, 4-dimethylaminopyridine, acetic anhydride, 1,1,1,3,3,3-hexamethyldisilazane, chlorotrimethylsilane, TMS triflate, 4% NH3/EtOH, Pluronic Methoxypoly(ethylene glycol), potassium iodide. N-boc-1,6-diaminohexane, N,N,N,N,N″ pentamethyldiethylenetriamine, 6-chloro-9-(β-D-ribofuranosyl)purine, silver oxide, 6-chloro-9-(β-D-ribofuranosyl)purine and 6-Chloro-7H-purine, Methyl 2,3-O-isopropylidene-β-D-ribofuranoside were purchased from commercial sources and used as received. D,L-Lactide was recrystallized from toluene then dried overnight before used. Phosphate buffered saline (PBS) was purchased from Boston BioProduct. Glass filter were purchased from Sterlitech Corporation and 0.45 µm filter was purchased from Pall Corporation. All common solvents were used as received without further distillation. Adenosine receptor antagonists 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX, A1/A2 receptor antagonist), 4-(-2-[7-amino-2-{2-furyl}{1,2,4}triazolo{2,3-a} {1,3,5}triazin-5-yl-amino]ethyl)phenol (ZM241385, A2A receptor antagonist), 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine (SCH58261, A2A receptor antagonist), 1-Propyl-8-(4-sulfophenyl)xanthine (PSB1115, A2B receptor antagonist) were purchased from SigmaAldrich.
Use of experimental animals
These experiments were reviewed and approved by the NYU School of Medicine Institutional Animal Care and Use Committee. All procedures were performed in accordance with the guidelines set forth by the Institutional Animal Care and Use Committee.
Synthetic procedures
Synthesis of a-methoxyl-ω-hydroxyl PEG
Glassware was silanized by rinsing with a 5% methyltrichlorosilane solution in toluene, then with acetone and finally dried overnight at 130 °C22. To a mixture of MeO-PEG550 (293 mg, 0.12 mmol) and D,L-lactide(7.01 g, 48.62 mmol) was added, under dry condition, a solution of Sn(Oct)2 (2 drops) in anhydrous toluene (11.2 ml). The reaction mixture was degassed by vacuum and filled by nitrogen and then stirred in a preheated oil bath at 110 °C for 24 h. Toluene was removed under reduced pressure, and the obtained product was dissolved into a minimum volume of dichloromethane and subsequently precipitated in ethyl ether. The precipitate was then dissolved into a minimum amount of THF, further precipitated in water, and subsequently was dried over vacuum overnight. (MeO-PEG550, Mn,NMR = 18725 g/mol). 1H NMR (500 MHz, CDCl3): δ = 5.21–5.15 (m, 300 H, CH PLA), 3.73–3.80 (m, 4 H), 3.63 (m, 58 H, CH2 PEG), 3.38 (s, 3 H), 1.58 (m, 974 H, CH3 PLA) ppm.
Synthesis of PLA-β-PEG-N3
A typical synthesis is as follows. Glassware was silanized by rinsing with a 5% methyltrichlorosilane solution in toluene, then with acetone and finally dried overnight at 130 °C25. To a mixture of HO-PEG400-N3 or (HO-PEG2000-N3) (0.12 mmol) and D,L-lactide(7.01 g, 48.62 mmol) was added, under dry condition, a solution of Sn(Oct)2 (2 drops) in anhydrous toluene(11.2 ml). The reaction mixture was degassed by vacuum and filled by nitrogen and then stirred in a preheated oil bath at 110 °C for 24 h. Toluene was removed under reduced pressure, and the obtained product was dissolved into a minimum volume of dichloromethane and subsequently precipitated in ethyl ether. The precipitate was then dissolved into a minimum amount of THF, further precipitated in water, and subsequently was dried over vacuum overnight. (PLA-PEG400-N3, Mn,NMR = 18975 g/mol. PLA-PEG2000-N3, Mn,NMR = 21890 g/mol) PLA-PEG2000-N3, 1H NMR (500 MHz, CDCl3): δ = 5.21–5.15 (m, 30 H, CH PLA), 3.76 (m, 2 H, CH2 PEG), 3.68 (m, 2 H, PEG), 3.66 (m, 14 H, PEG), 1.58 (m, 974 H, CH3 PLA). IR (Nujol): υ = 2944 (w), 2353 (w), 1750 (s), 1182 (s), 1080 cm−1 (s).
Synthesis of 5-hexyn-2-one (Compound 1 Fig. 2)
2,4-Pentanedione (5 g, 5 ml, 50 mmol), anhydrous potassium carbonate (7.5 g, 55 mol), and propargyl chloride (3.5 g, 3.5 ml, 48 mmol) were dissolved in 25 ml of ethanol19. The reaction mixture was stirred under reflux for 24 h. After cooling to room temperature, 15 ml of water were added. The mixture was then extracted with diethyl ether, and the organic phase was washed with brine and dried over sodium sulfate. Removal of the solvent and subsequent vacuum distillation yielded 5-hexyn-2-one as colorless liquid in 48% yield.
Synthesis of adenosine-ketal
5-hexyn-2-one (0.5 g, 5.21 mmol), adenosine (1.4 g, 5.24 mmol) and triethyl orthoformate (0.776 g, 5.24 mmol) were dissolved in 18 ml of DMF20. Then the 4 M HCl in 1,4-dioxane (4.08 ml) was added to the mixture. The reaction mixture was stirred under room temperature overnight. The reaction mixture was poured into diethyl ether (250 ml). Then the oil residue was dissolved in chloroform (100 ml) and the organic phase was washed with sodium bicarbonate solution (2%) for one time and then water for three times, then dried over anhydrous MgSO4. The solvent was removed via rotary evaporator. The crude product was purified through chromatography (CHCl3:CH3OH = 10:1). 1H NMR (CDCl3) δ 8.32 (s, 1 H), 7.85 (s, 1 H), 6.49 (d, J = 10 Hz, 1 H), 5.92 (d, J = 5 Hz, 2 H), 5.85 (s, NH2, 2 H), 5.24 (d, J = 4 Hz, 1 H), 5.23 (d, J = 4 Hz, 1 H), 4.54 (s, 1 H), 3.99 (d, J = 13 Hz, 1 H), 3.82 (d, J = 13 Hz, 1 H), 2.47 (m, 2 H), 2.15 (t, 2 H), 2.03 (s, CH, 1 H), 1.36 (s, 3 H) ppm. IR (Nujol): υ = 3628 (w), 3312 (s), 3250 (m), 3098 (m), 2703 (m), 2100 (w), 1687 (s), 1608 cm−1 (s)
Synthesis of tert-Butyl(6-(pro-2-yn-1-ylamino)hexyl)carbamate (Compound 2, Fig. 3)
Propargyl chloride (344.4 mg, 4.6 mmol, 1 equiv.), Boc-protected amine (1.00 g, 4.62 mmol, 1 equiv.) and anhydrous K2CO3 were in acetonitrile. The reaction was stirred at room temperature for 16 h. Then, 100 ml of water and 100 ml of diethyl ether were added. The organic phase was separated and the aqueous phase extracted with diethyl ether (2 × 50.0 ml). The solvent was removed under reduced pressure. The crude product was purified by column chromatography on a basic alumina (CH2Cl2:CH3OH = 50:1). After drying on high-vacuum the product was obtained as a yellow oil (0.7991 g, 68%) yield. 1H NMR (CDCl3) δ 4.5 (s, 1 H, NH), 3.45 (s, 2 H), 3.14 (d, 2 H), 2.72 (m, 2 H), 2.23 (s, 1 H), 1.5 (s, 8 H), 1.46 (s, 9 H) ppm.
Synthesis of 6-(pro-2-yn-1-ylamino)hexylamine (Compound 3, Fig. 3 tert-Butyl(6-(pro-2-yn-1-ylamino)hexyl)carbamate
(514 mg, 2.0 mmol) was subjected to a solution of 10% TFA in dichloromethane (15 ml) for 3 h. The water was added and the organic phase was separated. Then the water phase was neutralized with 10% NaHCO3 to pH 8.0. Then removal of water is evaporated under reduced vacuum. Dichloromethane was used to wash the residue and combine organic solvent. The product was obtained through removing dichloromethane and as a yellow oil (300 mg, 96%) The crude product was used directly for next step.
Synthesis of Adenosine-6-amine
A mixture of 6-chloro-9-(β-D-ribofuranosyl)purine (527 mg, 1.84 mmol), compound 3 (300 mg, 1.94 mmol), 1.84 mmol of Et3N and 25 ml of ethanol was refluxed at 60 °C for 18 h26. After completion of the reaction the solvent was evaporated under high vacuum. Purification using silica gel chromatography (CH3OH:dichloromethane = 1:10) and the product was gained as white solid (380 mg, 50%) yield. 1H NMR (DMSO-d6) δ 8.34 (s, 1 H), 8.21 (s, 1 H), 7.9 (s, NH, 1 H), 5.89 (d, J = 8 Hz, 1 H), 5.44 (d, J = 4 Hz, 2 H), 5.19 (d, J = 4 Hz, 1 H), 4.63 (m, 1 H), 4.17 (d, J = 4 Hz, 1 H), 3.97 (t, 1 H), 3.70 (dd, J = 12 HZ, J = 4 Hz, 1 H), 3.58 (dd, J = 12 Hz, J = 4 Hz, 1 H), 3.47 (s, 2 H), 3.12 (m, 2 H) 2.51 (t, 2 H), 1.60 (m, 2 H), 1.40 (s, 2 H), 1.31 (m, 4 H) ppm. IR (Nujol): υ = 3504 (w), 3343 (s), 3264 (s), 3148 (s), 2927 (m), 2855 (s), 2150 (w), 1800 (m), 1625 cm−1 (s).
Synthesis of methyl 2,3-O-(1-methylethylidene)-5-O-2-propyn-1-yl-β-D-ribofuranoside (Compound 5, Fig. 4)
Methyl 2,3-O-isopropylidene-β-D-ribofuranoside (4; 4.0 g, 20 mmol) was dissolved in dry dimethylformamide (DMF; 30 ml)27–30. This was cooled (0 °C), and NaH (60% in mineral oil, 1.78 g, 23 mmol) was slowly added. The mixture was allowed to warm to room temperature and cooled again, and propargyl chloride (0.31 mol) was added very slowly. The mixture was stirred at room temperature overnight. The mixture was treated with methanol (10 ml) and concentrated in vacuum. It was coevaporated with toluene (2 × 10 ml). The (black) mixture was extracted with water and ethyl acetate (25 ml each). The water layer was subsequently extracted with CH2Cl2. The organic layers were combined, dried (MgSO4), and concentrated. The residue was purified by column chromatography (Eluent 10% CH3OH in ethyl acetate): yield 3.0 g (12.5 mmol, 62%).
Synthesis of 5-O-propargayl-R,β-D-ribofuranose (Compound 6, Fig. 4). methyl 2,3-O-(1-methylethylidene)-5-O-2-propyn-1-yl- β-D-Ribofuranoside
(3 g, 12.5 mmol) was dissolved in 60 ml of HCl (0.04 M) and was refluxed for 2 h25. The solution was neutralized with BaCO3, filtered, and concentrated. The mixture was purified by column chromatography (Eluent 10% MeOH in Ethyl acetate): yield 893 mg (4.7 mmol, 38%).
Synthesis of 5-O-propargayl-R,β-D-ribofuranose triacetate (compound 7, Fig. 4)
5-O-propargayl-R,β-D-ribofuranose (4.7 mmol) was dissolved in 50 ml of pyridine25. A catalytic amount of (dimethylamino)pyridine (DMAP) (60 mg, 0.47 mmol) and acetic anhydride (1.2 g, 11.75 mmol) were added. The mixture was stirred for 2 h at room temperature, concentrated in vacuum and coevaporated with toluene. The oil was extracted with water and ethyl acetate (25 ml each). The organic layer was dried (MgSO4), concentrated, and purified by column chromatography (Eluent: ethyl acetate): yield 1.09 g (3.5 mmol, 74%). 1 H NMR (CDCl3) δ 6.16 (s, 1 H), 5.43 (m, 1 H), 5.36 (m, 1 H), 4.39 (m, 1 H), 4.31 (s, 2 H), 3.72 (dd, 2 H), 2.45 (s, 1 H), 2.11 (3 × s, 9 H) ppm.
Synthesis of 6-chloro-9-(5-O-2-propyn-1-yl-β-D-ribofuranosyl)-9H-Purine 3′,4′-diacetate (Compound 8, Fig. 4)
Silylation of 6-Chloropurine was accomplished by treating (770 mg, 5 mmol) of the compound with 1,1,1,3,3,3-hexamethyldisilazane (HMDS; 20 ml, 93.1 mmol) and 50 µL of chlorotrimethylsilane (TMSCl; 0.4 mmol) at 130 °C for 20 h25. The silylated compound was concentrated and used without further purification. Vorbruggen Coupling was carried out by first adding the silylated purine (5 mmol) to 5-O-propargayl-R,β-D-ribofuranose triacetate (3.5 mmol) in 7.5 ml of dry 1,2-dichloroethane. The resulting solution was coevaporated twice with dry 1,2-dichloroethane and subsequently dissolved in 75 ml of dry 1,2-dichloroethane. The solution was gently refluxed, and after 5 min TMS triflate (0.5 ml, 2.58 mmol) was added. The mixture was refluxed for 2 h, cooled to room temperature, and diluted with CH2Cl2. It was extracted with 5% NaHCO3 and water. The organic layer was dried (MgSO4), concentrated, and 6-Chloro-9-(3,4-di-O-acetyl-5-O-propargyl-β-D-ribofuranosyl)-purine was purified by column chromatography (eluent 3% acetone in DMC) to yield 700 mg (49%). 1H NMR (CDCl3) δ 8.80 (d, 1 H), 8.73 (d, 1 H), 5.83 (d, J = 5 Hz, 1 H), 5.62 (d, J = 5 Hz, 1 H), 5.34 (dd, J = 5 Hz, 1 H), 4.46 (s, 1 H), 4.33 (m, 2 H), 3.59 (dd, 2 H), 2.56 (s, 1 H), 2.05 (3 × s, 9 H) ppm.
Synthesis of 5-O-propargyl-adenosine (compound 9, Fig. 4)
Compound 8 (700 mg, 1.71 mmol) was dissolved in 4% NH3/EtOH (30 ml), and the mixture was stirred overnight at room temperature26. The mixture was concentrated and purified by column chromatography (Eluent 10% methanol in dichloromethane) to yield 300 mg (58%). 1H NMR (CDCl3) δ 8.72 (s, 1 H), 8.53 (s, 1 H), 6.16 (d, J = 5 Hz, 1 H), 4.70 (s, NH, 1 H), 4.65 (d, J = 5 Hz, 1 H), 4.50 (d, J = 5 Hz, 1 H), 4.45 (d, J = 5 Hz, 1 H), 4.18 (s, 2 H), 3.90 (d, J = 10 Hz, 1 H), 3.79 (d, J = 10 Hz, 1 H), 2.49 (s, 1 H) ppm. IR (Nujol): υ = 3258 (w), 3150 (m), 3062 (w), 2922 (s), 2852(m), 1670 (s), 1660 (m), 1582 (s), 1100 cm−1 (s);
General procedure for the reaction of acetylene-functionalized adenosine to PLA-β-PEG-N3
The representative synthesis (PLA-β-PEG400-N3) was as follows27. To a degassed solution of PLA-b-PEG-N3(200 mg, 10 µmol, 1 equiv) and alkyne adenosine (18 equiv) in anhydrous DMF (12 ml) was added with a syringe, a degassed solution of CuBr (10 mg, 69 µmol, 6.9 equiv) and PMDETA(37 µL, 0.179 mmol, 17 equiv) in anhydrous DMF(800 µL). The reaction mixture was stirred for 16 h at 65 °C under nitrogen. The solution was concentrated under reduced pressure and the residue was dissolved into chloroform, then washed with saline solution and water until the organic phase become colorless. Then the organic phase was dried by anhydrous sodium sulfate and removed by rotary evaporation. The residue was dissolved in minimum amount of THF, and further precipitate in water for third times. The precipitate was freeze-dried to yield a white powder.
General procedure for nanoparticle preparation
The copolymer attached with adenosine (30 mg in total) was dissolved in ethyl acetate (1.2 ml)26. The above organic phase was added to 3.3 ml of an aqueous phase containing 1% w/v Pluronic F68. The mixture was then vigorously shaken using a vortex shaker for 1 min. The resulting emulsion was ultrasonicated (using an ultrasonic probe) for 3 min and the organic solvent was removed under reduced pressure using a rotary evaporator. The resulting nanoparticle suspension was ultracentrifuged at 1600 g for 45 min and the pellet was resuspended in 3 ml of pH = 7.4 PBS. The nanoparticles were filtered through a 1 µm glass filter fist and then 0.45 µm and stored at 4 °C until use.
Characterization of nanoparticles
NMR spectroscopy
NMR spectroscopy was performed in CDCl3 or DMSO-d6. 1H NMR spectroscopy was performed on a Bruker Avance 500 spectrometer at 500 MHz(1H).
Concentration of nanoparticles
Based on the Beer’s Law, the various standard solution of adenosine is made and being quantified through calorimetric assays. Then the data was graphed based on the concentrations and a Best-Fit line was gained through the points (Fig. 10). From the slope of the best–fit line together with the absorbance, the concentration of nanoparticles can be calculated.
Figure 10.
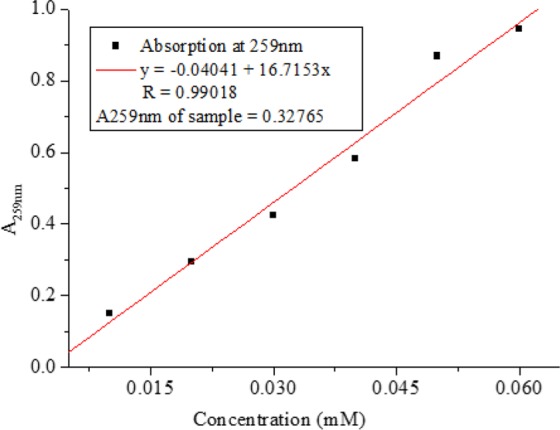
The best-fit line of the standard solution of adenosine.
Dynamic Light Scattering (DLS)
Measurement of the nanoparticle diameter was performed using a Nano ZS from Malvern (90° scattering angle) at 20 °C. The particle size distribution values are given by the DLS apparatus. DLS was run for every new batch of nanoparticles. The sample was diluted until a good PDI value was obtained, showing a narrow size distribution of the nanoparticles.
Transmission Electron Microscopy (TEM)
Place 3 µL of sample onto carbon coated 400 mesh Cu/Rh grid (Ted Pella Inc, Redding, CA) and stain with 1% uranyl acetate in distill water (polysciences, Inc, Warrington, PA). Stained grids were examined under FEI Talos120C transmission electron microscope and photographed with a Gatan OneView digital camera.
Measurement of cyclic AMP in macrophage cell line
RAW264.7 cells were plated in 12 well/plate (500,000 cell/well). The cells were pre-treated for 10 minutes at 37 °C with 4-(3-butoxy- 4-methoxybenzyl)-2-imidazolidinone in order to inhibit phosphodiesterases. Cells were then treated with adenosine (1 µM) and nanoparticles (1 µM) for 10 minutes. A2BR and A2AR antagonists, respectively PSB1115 (1 µM) and ZM241385 (1 µM) were added at the last 5 minutes of the previous incubation. After incubation cells were washed with cold PBS and incubated with 0.1 M of HCl for 10 minutes at RT. Cytoplasmic cell content was collected and centrifuged at 600 g. cAMP amount was evaluated by using a kit following the manufacture instructions (Enzo Life science).
Mouse primary chondrocyte extraction and culture
Articular mouse chondrocytes were obtained from C57BL/6 mice. After sacrifice of 5/7-day-old mice, the femurs were dislocated from the hip. Soft tissue was removed and the femoral head, femoral condyles, and tibial plateau were isolated and placed in culture medium (DMEM supplemented with 2 mM-glutamine, 50 U/mL penicillin, and 0.05 mg/mL streptomycin). Cartilage tissue was incubated in a solution of collagenase D (3 mg/ml) for 45 minutes at 37 °C. After agitation, the tissue was incubated in a new collagenase D solution under the conditions previously described. Cartilage pieces were then incubated overnight at 37 °C in a new solution of collagenase D (0.5 mg/ml). Sheets of cells were detached from the bone, passed through a 72-µm nylon mesh and plated at a density of 8 × 103 cells/cm2.
Primary chondrocytes (80% confluence) were starved overnight and treated for 24 h with mouse recombinant IL-1β (5 ng/ml). For the evaluation of NF-kB nuclear translocation, cells were collected and nuclear and cytosolic protein components were separated using NE-PER kit (Thermo Scientific) following the manufacturer’s protocol.
Total and nuclear protein fractions were quantified using the BCA kit (Thermo Scientific). Western blotting was performed loading 4 µg of protein. Proteins were separated by electrophoresis through 10% polyacrylamide gels by SDS-PAGE and then transferred to a nitrocellulose membrane. Non-specific antibody binding was blocked with 3% BSA in TBS/Tween 20 buffer. Membranes were incubated overnight at 4 °C with the specific primary antibody (1:1000), and after washing, incubated with goat anti-rabbit IRDye 800 CW and goat anti-mouse IRDye 680 RD (1:5000). Membranes were scanned with Li-cor Odyssey equipment and the intensity of the protein bands were quantified by densitometric analysis using Image Studio 2.0.38 software.
Reverse transcription and Real Time PCR
RNA extraction was performed from mouse primary chondrocytes using RNeasy Mini Kit (Qiagen, Invitrogen) and QIAshredder colums (Qiagen, Invitrogen), following the manufacturer’s protocol. RNA reverse transcription was performed using the MuLV Reverse Transcriptase PCR Kit (Applied Biosystems). After RNA reverse transcription to cDNA, real time PCR reactions were performed for a relative quantification of collagen X, MMP-13 and IL-6 performed on a Stratagene Mx3005P (Agilent Technologies, CA, USA) with Brilliant SYBR Green Kit QPCR Master Mix (Stratagene, Agilent Technologies, CA, USA), according to the manufacturer’s protocol.
MTT assay
Cell viability was performed in RAW 164.7 cell line after 24 hours treatment with the compound PLA-PEG2000-3,4-OH-Adenosine (1 µM) or adenosine alone (1 µM). The assay was performed following the protocol provided by the manufacture (CellTiter 96® Non-Radioactive Cell Proliferation Assay-Promega, CA, USA).
Induction of post-traumatic OA (PTOA) in rats and treatment with nanoparticles
The posttraumatic OA (PTOA) model used is a non-invasive method for inducing anterior cruciate ligament (ACL) rupture in rat knees in vivo with a single load of tibial compression. We chose this model in rats because of the technical ease in injecting the knees of these animals and because correction of an induced lesion would better mimic the situation in humans. The procedure was performed under anesthesia (1–3% isoflurane) as previously described12. All experimental groups of rats, as described below, consisted of 3 rats and each experimental group was repeated once (total of 6 rats per experimental group). This number of rats was chosen because larger group sizes led to operator overload and diminished quality of results. Animals were not randomized for these studies.
Rats were treated with intra-articular injections of 100 ml of a liposomal suspension containing a high concentration of adenosine (10 mg kg/1), empty liposomes or with saline for 8 weeks. The animals were separated into two main cohorts, the prevention group received injections commencing immediately after ACL rupture, and the treatment group received the first injection 7 days after ACL rupture. Injections were performed every 10 days thereafter in both groups. Knee swelling and weight in the rats were measured before every injection. At the end of the experiment rats were killed and both legs were collected for immunohistochemistry and microcomputed tomography (μCT) analysis12.
µCT Cartilage examination
After sacrifice both legs were excised and fixed with 4% PFA for 48 hours and then preserved in 70% ethanol. After washing with PBS, rat knees (femoral and tibial surfaces, n = 3 for each group) were incubated in PBS containing the ionic contrast agent Hexabrixr (40% v/v) for 6 hours. All joints were evaluated in a (16 mm) scanning tube providing a volex size of 10.5 µm and scanned at 55 kV, 181 µA, and 110 minutes of acquisition time. During scanning the samples were wrapped in paper soaked in PBS to avoid dehydration12.
After µCT analysis the samples were washed with PBS and decalcified in 10% EDTA for 4 weeks. Paraffin-embedded histological sections (5 µm) were cut, mounted and prepared for analysis with H&E and Safranin O/Fast green staining to assay different cartilage components. Slides were scanned using a Leica microscope equipped with Slidepath Digital Image Hub version 3.0 Software.
Data analysis
µCT analysis of bone and cartilage volume was performed using CTvox software to reconstruct 2D and 3D images and to calculate various bone characteristics. Amira software (FEI, Oregon-USA) was used to reconstruct mice and rat joints from µCT data based on differential density of bone and Hexabrix-treated cartilage. Statistical significance for differences between groups was determined using Student’s T-test, two-way or one-way ANOVA, as appropriate, using GraphPad software (GraphPad, San Diego, CA). If the overall differences were significant (F < 0.05) then differences between groups were analyzed by Bonferroni post-hoc testing.
OARSI Score
Histology and immunohistochemistry were performed as previously described27 and OARSI score determined blindly for each specimen taking into account the severity of cartilage degradation, cartilage calcification, presence of osteophytes and their size.
Ethical Approval and Informed Consent
All experiments with animals were reviewed and approved by the NYU School of Medicine Institutional Animal Care and Use Committee.
Supplementary information
Acknowledgements
This work was supported by grants from the National Institute of Arthritis, Musculoskeletal and Skin Diseases (RO1-AR054897 and RO1-AR056672), the NYU-HHC Clinical and Translational Science Institute (UL1-TR000038 from the National Center for Advancing Translational Sciences) and the Arthritis Foundation.
Author Contributions
A.U. and B.C. wrote the main manuscript text. X.L. synthesized and characterized all the adenosine derivatives, polymers and nanoparticles, and supplied Table 1. C.C. carried out all the biological work and supplied Figures 5, 6, 7, and 8. S.A. was an undergraduate student who had prepared the first nanoparticle sample.
Data Availability
The data that support the findings of this study are available from the corresponding author on request.
Competing Interests
C.C. and B.C. have filed a patent application on the use of A2AR agonists to treat and prevent osteoarthritis. B.C. holds patents numbers 5,932,558; 6,020,321; 6,555,545; 7,795,427; adenosine A1R and A2BR antagonists to treat fatty liver (pending); adenosine A2AR agonists to prevent prosthesis loosening (pending). B.C. is a consultant for AstraZeneca and Horizon Pharmaceuticals. BNC has stock in CanFite Biopharmaceuticals and Regenosine, Inc. C.C. has stock in Regenosine, Inc. A.U. does not have competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abraham Ulman, Email: au323@nyu.edu.
Bruce N. Cronstein, Email: Bruce.Cronstein@nyumc.org
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43834-y.
References
- 1.Bhattacharjee S, et al. Formulation and Application of Biodegradable Nanoparticles Based Biopharmaceutical Delivery - An Efficient Delivery System. Current Pharmaceutical Design. 2016;22:3020–3033. doi: 10.2174/1381612822666160307151241. [DOI] [PubMed] [Google Scholar]
- 2.Storm G, Belliot SO, Daemen T, Lasic DD. Surface Modification of Nanoparticles to Oppose Uptake by the Mononuclear Phagocyte System. Adv. Drug Delivery Rev. 1995;17:31–48. doi: 10.1016/0169-409X(95)00039-A. [DOI] [Google Scholar]
- 3.Alexis F, Pridgen EM, Langer R, Farokhzad OM. Nanoparticle Technologies for Cancer Therapy. Handb. Exp. Pharmacol. 2010;197:55–86. doi: 10.1007/978-3-642-00477-3_2. [DOI] [PubMed] [Google Scholar]
- 4.Haghighat A, Behnia A, Kaviani N, Khorami BJ. Evaluation of Glucosamine Sulfate and Ibuprofen Effects in Patients with Temporomandibular Joint Osteoarthritis Symptom. Res. Pharmacy Practice. 2013;2:34–39. doi: 10.4103/2279-042X.114087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adatia A, Rainsford KD, Kean WF. Osteoarthritis of the Knee and Hip. Part II: Therapy with Ibuprofen and a Review of Clinical Trials. J. Pharmacy and Pharmacology. 2012;64:626–636. doi: 10.1111/j.2042-7158.2012.01456.x. [DOI] [PubMed] [Google Scholar]
- 6.O’Neil CK, Hanlon JT, Marcum ZA. Adverse Effects of Analgesics Commonly Used by Older Adults with Osteoarthritis: Focus on Non-Opioid and Opioid Analgesics. Am. J. Geriatric Pharmacotherapy. 2012;10:331–342. doi: 10.1016/j.amjopharm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Spaeth RB, Retzepi K, Ott D, Kong J. Acupuncture Modulates Cortical Thickness and Connectivity in Knee Osteoarthritis Patients. Scientific Reports. 2014;4:6482–6488. doi: 10.1038/srep06482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo HG, Yoo WH. Acupuncture with Gold Thread for Osteoarthritis of the Knee. New England Journal of Medicine. 2013;369:e37. doi: 10.1056/NEJMicm1202540. [DOI] [PubMed] [Google Scholar]
- 9.Baschant U, Lane NE, Tuckermann J. The Multiple Facets of Glucocorticoid Action in Rheumatoid Arthritis. Nature reviews. Rheumatology. 2012;8:645–55. doi: 10.1038/nrrheum.2012.166. [DOI] [PubMed] [Google Scholar]
- 10.Fakhari A, Berkland C. Applications and Emerging Trends of Hyaluronic Acid in Tissue Engineering, as a Dermal Filler and in Osteoarthritis Treatment. Acta Biomaterialia. 2013;9:7081–7092. doi: 10.1016/j.actbio.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwasek B, Bogdal D. The Use of Hyaluronic Acid in the Treatment of Osteoarthritis of Knee Cartilage. Technical Transactions. 2014;111:57–68. [Google Scholar]
- 12.Corciulo C, et al. Endogenous Adenosine Maintains Cartilage Homeostasis and Exogenous Adenosine Inhibits Osteoarthritis Progression. Nature Communications. 2016;8:15019–15031. doi: 10.1038/ncomms15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Möser GH, Schrader J, Deussen A. Turnover of adenosine in plasma of human and dog blood. Am. J. Physiol. 1989;256:C799–806. doi: 10.1152/ajpcell.1989.256.4.C799. [DOI] [PubMed] [Google Scholar]
- 14.Feng X, et al. Tumor-Homing and Penetrating Peptide-Functionalized Photosensitizer-Conjugated PEG-PLA Nanoparticles for Chemo-Photodynamic Combination Therapy of Drug-Resistant Cancer. ACS Appl. Mater. Interfaces B. 2016;8:17817–17832. doi: 10.1021/acsami.6b04442. [DOI] [PubMed] [Google Scholar]
- 15.Tang X, et al. Anti-Transferrin Receptor-Modified Amphotericin B-Loaded PLA-PEG Nanoparticles Cure Candidal Meningitis and Reduce Drug Toxicity. Int. J. Nanomed. 2015;10:6227–6241. doi: 10.2147/IJN.S84656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mediero A, et al. Adenosine A2A Receptor Activation Prevents Wear Particle-Induced Osteolysis. Sci. Transl. Med. 2012;4:65–74. doi: 10.1126/scitranslmed.3003393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mediero A, Kara FM, Wilder T, Cronstein BN. Adenosine A2A Receptor Ligation Inhibits Osteoclast Formation. Am. J. Pathol. 2012;180:775–786. doi: 10.1016/j.ajpath.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, et al. Induced Superficial Chondrocyte Death Reduces Catabolic Cartilage Damage in Murine Posttraumatic Osteoarthritis. J. Clin. Invest. 2016;126:2893–2902. doi: 10.1172/JCI83676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahou R, Wandrey C. Versatile Route to Synthesize Heterobifunctional Poly(ethylene glycol) of Variable Functionality for Subsequent Pegylation. Polymers. 2012;4:561–589. doi: 10.3390/polym4010561. [DOI] [Google Scholar]
- 20.Görl C, Alt HG. The Combination of Mononuclear Metallocene and Phenoxyimine Complexes to Give Trinuclear Catalysts for the Polymerization of Ethylene. J. Organometallic Chemistry. 2007;692:5727–5753. doi: 10.1016/j.jorganchem.2007.10.001. [DOI] [Google Scholar]
- 21.Ott J, Seela F. R- and S-alkylidene Acetals of Adenosine: Stereochemical Probes for the Active site of Adenosine Deaminase. Bioinorg. Chem. 1981;10:82–89. doi: 10.1016/0045-2068(81)90045-6. [DOI] [Google Scholar]
- 22.Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function From a Few Good Reactions. Angewandte Chemie International Edition. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Serra T, Ortiz-Hernandez M, Engel E, Planell JA, Navarro M. Relevance of PEG in PLA-based blends for tissue engineering 3D-printed scaffolds. Materials Science & Engineering, C: Materials for Biological Applications. 2014;38:55–62. doi: 10.1016/j.msec.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Shen J, Shi D, Shi C, Li X, Chen M. Fabrication of dopamine modified polylactide-poly(ethylene glycol) scaffolds with adjustable properties. Journal of Biomaterials Science, Polymer Edition. 2017;28:2006–2020. doi: 10.1080/09205063.2017.1366250. [DOI] [PubMed] [Google Scholar]
- 25.Bensaid F, et al. Y-Shaped mPEG-PLA Cabazitaxel Conjugates: Well-Controlled Synthesis by Organocatalytic Approach and Self-Assembly into Interface Drug-Loaded Core-Corona Nanoparticles. Biomacromolecules. 2013;14:1189–1198. doi: 10.1021/bm400161g. [DOI] [PubMed] [Google Scholar]
- 26.Zhang XF, et al. Synthesis and Characterization of the Paclitaxel/MPEG-PLA Block Copolymer Conjugate. Biomaterials. 2005;26:2121–2128. doi: 10.1016/j.biomaterials.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 27.Bhattarai S, et al. α,β-Methylene-ADP (AOPCP) Derivatives and Analogues: Development of Potent and Selective ecto-5′-Nucleotidase (CD73) Inhibitors. J. Med. Chem. 2015;58:6248–6263. doi: 10.1021/acs.jmedchem.5b00802. [DOI] [PubMed] [Google Scholar]
- 28.van Tilburg EWP, et al. 5′-O-alkyl Ethers of N,2-substituted Adenosine Derivatives: Partial Agonists for the Adenosine A1 and A3 Receptors. J. Med. Chem. 2001;44:2966–2975. doi: 10.1021/jm001114o. [DOI] [PubMed] [Google Scholar]
- 29.Mackiewicz N, et al. Precise Engineering of Multifunctional PEGylated Polyester Nanoparticles for Cancer Cell Targeting and Imaging. Chem. Mater. 2014;26:1834–1847. doi: 10.1021/cm403822w. [DOI] [Google Scholar]
- 30.Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis and cartilage/OARS. Osteoarthritis Research Society. 2010;18(Suppl 3):S24–34. doi: 10.1016/j.joca.2010.05.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on request.