Abstract
Feeling of knowing (FOK) is a metacognitive process which allows individuals to predict the likelihood that they will be able to remember, in the future, information which they currently cannot recall. Although FOK provides evidence for the mechanisms of metacognitive systems, the neurobiological basis of FOK is still unclear. We investigated the neural correlates of FOK induced by an episodic memory task in 77 younger adult participants. Data were gathered using event-related potentials (ERPs). ERP components during high, low, extremely high and extremely low FOK judgments were analyzed. Stimulus-locked ERP analyses indicated that FOK judgment was associated with greater positivity for P200 component at frontal, central, and parietal electrode zones and greater negativity for the N200 component at parietal electrode zones. Furthermore, results revealed that amplitude of the ERP components for FOK judgments were affected by the level of FOK judgment. Results suggest that ERP components of FOK judgment observed within a 200 ms time window support the perceptual fluency-based model.
Keywords: Feeling-of-knowing, Episodic memory, Metacognition, Event-related potentials
Introduction
Metacognition refers to the ability of an individual to monitor and control his or her own cognitive system and includes both prospective and retrospective monitoring activated at different stages during acquisition, retention, and retrieval of information. Feeling of knowing (FOK) is one such metacognitive process which allows individuals to make predictions about the likelihood of their remembering, in future, information which they currently cannot recall, based on whether they feel like the information is stored in memory (Hertzog et al. 2010; Modirrousta and Fellows 2008; Souchay et al. 2002).
First proposed by Hart (1965), the FOK paradigm is conducted by asking participants to evaluate the likelihood of recognizing information which they had previously failed to recall, either from long-term memory (e.g. semantic memory) or from the recent past (e.g. episodic memory). While semantic FOK is defined by attempts to recall world knowledge or facts that were learned before the experiment, episodic FOK is the attempt to recall newly learned information in a particular context (Hertzog et al. 2010). In FOK studies, people are asked to recall either general knowledge information (e.g. ‘What is the highest mountain in the world?’) or recently learned verbal material such as word pairs. For the items they fail to recall, they are asked to provide a FOK judgment based on the following question: ‘Although I cannot recall the answer right now, would I be able to find it among a number of alternatives?’ Participants are generally asked to provide a Likert-type rating. Following this judgment phase, participants are given a recognition test in which each cue word is presented along with different alternatives. FOK performance is calculated as the ratio between FOK judgments and correct and incorrect recognition performance of an individual (Pannu and Kaszniak 2005).
Two main theoretical explanations have been proposed to explain how FOK judgments are made when predicting future memory performance (Perrotin et al. 2007). According to the domain and cue familiarity views, FOK judgments increase in relation to the familiarity of the cue (Reder and Ritter 1992; Thomas et al. 2012). Several studies showed that the advance familiarity of a question enhanced the speed (Reder 1987), increased the probability of recall or recognition (Reder 1987; Schwartz and Metcalfe 1992) and enhanced FOK judgments elicited following recall failure, and increased the latency of “don’t know” responses as well as the tendency to make a “know” response (Glucksberg and McCloskey 1981; Klin et al. 1997). Alternatively, according to Koriat’s (1993) accessibility model, FOK is based on an automatic inspection of the amount and accessibility of partial information retrieved in relation to the target. Koriat (1993) proposed that FOK judgments are made by using mnemonic cues to identify whether the target is in memory and will be recognized in the future. Studies which supported the accessibility model indicated that accessibility would seem to be a global, unrefined heuristic that responds to the mere amount of information irrespective of its correctness (Koriat and Lieblich 1977). In addition, it was mentioned that FOK judgments are sensitive to the mere accessibility of information due to FOK judgments following a commission error are higher than following an omission error (Koriat 1995; Krinsky and Nelson 1985; Nelson and Narens 1990). These two explanations can be integrated into a two-step model: first, cue familiarity is assessed; then, if the cue seems familiar, accessibility of the by-products of the retrieval process is assessed (Koriat and Levy-Sadot 2001). A third account still assumes a combined operation of the familiarity and accessibility heuristics (Koriat 2007). Both familiarity and accessibility enhanced FOK judgments, but when familiarity was high, the effects of accessibility were found. This significant pattern was reported several times and results support the proposed cascaded model of FOK (e.g. Koriat and Levy-Sadot 2001; Schreiber and Nelson 1998; Vernon and Usher 2003).
Behavioral and neural differences between different metacognitive judgments are still interesting research question. Metacognitive judgments are made either retrospectively based on current memory performance such as retrospective confidence judgments (RCJ) and judgment of learning (JOL) or prospectively with respect to previous memory performance like FOK judgments. Behavioral (Koriat 1993; Metcalfe et al. 1993; Yonelinas 1994) and neuroimaging studies (Chua et al. 2009; Modirrousta and Fellows 2008) emphasized the difference between the source of retrospective and prospective judgments, despite their collapse mechanisms. To illustrate, Le Berre et al. (2016) investigated the episodic memory monitoring performance of abstinent alcoholics and they found impaired FOK but intact RCJ.
One of the critical questions is whether FOK judgments are dissociable from memory. Many neuropsychological studies (Modirrousta and Fellows 2008; Pannu and Kaszniak 2005; Perrotin et al. 2007) showed that memory and metamemory processes are dissociable and that the medial prefrontal cortex (mPFC) is critical for accurate FOK judgments. Despite the large body of behavioral research, the neurobiological basis of FOK remains unclear. The aim of the present study was to investigate neural correlates of FOK judgments by measuring event-related potentials (ERP) during an episodic memory task based on the recall-FOK judgment-recognition paradigm.
Neuropsychology and neurobiology of episodic FOK
FOK judgments have been used to investigate metamemory processes in healthy adults (Souchay et al. 2007) and patient populations including obsessive compulsive disorder (Tuna et al. 2005), Korsakoff’s syndrome (Shimamura and Squire 1986), Alzheimer’s disease (Souchay et al. 2002), and Parkinson’s disease (Ivory et al. 1999) patients for whom memory and metamemory complaints are integral elements of the symptomatology. Results suggest that patients with prefrontal cortex (PFC) damage show low FOK accuracy (Schnyer et al. 2004) whereas temporal lobe regions do not have an effect on FOK accuracy (Shimamura and Squire 1986). These findings lend support to the specific role of prefrontal regions during FOK judgments (Funnell et al. 1996). Other studies (Perrotin et al. 2007; Schnyer et al. 2005) have revealed that FOK accuracy is associated with the ventro-medial part of the PFC, which is thought to play a role in the assessment of partial information retrieved from the hippocampus and the medial temporal lobe (MTL).
Neuroimaging studies indicate that several brain regions are differentially associated with semantic and episodic FOK judgments. Reggev et al. (2011) found that the posterior midline network was activated during episodic retrieval and episodic monitoring, but not semantic monitoring. Elsewhere, greater right inferior frontal cortex activation was observed during semantic FOK judgments than during episodic monitoring and ‘don’t know’ responses (Kikyo and Miyashita 2004; Maril et al. 2003, 2005). Moreover, Maril et al. (2005) showed that the PFC and parietal regions were activated during FOK judgments and tip of the tongue (TOT) state.
Studies investigating neural correlates of FOK have mostly analyzed the ERP correlates of familiarity and recollection-based recognition rather than classical recall—FOK judgment—recognition paradigm. In a study by Paynter et al. (2009), subjects were shown a series of math problems and were instructed to decide whether the answer to a given problem could be quickly retrieved from memory or had to be calculated on paper. Stimulus-locked ERP analysis showed that accurate retrieval trials were associated with early frontal P200 component (epoched from 180 to 280 ms) and a frontal-central P300 component (epoched from 300 to 550 ms), however these electrophysiological components were not obtained for subjects who never obtained a successful on-time retrieval. The authors concluded that initial FOK relies on a rapid assessment of the ease with which perceptual processing (perceptual fluency) of the stimulus occurs (within 200 ms after the stimulus onset). Also, of interest, ERP components were greater in amplitude in the right hemisphere possibly suggesting right hemispheric involvement in FOK processes.
Episodic memory, defined as conscious memory for life events, has been suggested to be related to the MTL and parietal contributions to encoding and retrieval. Also, activation of lateral and medial parietal regions has shown to correlate with the phenomenological experience of remembering (Wagner et al. 2005). Maril et al. (2003) found retrieval-related activations in the left inferior frontal gyrus, the left superior parietal cortex, and the anterior cingulate and suggested that FOK is associated with partial access to highly-frequent targets, supporting the cue-familiarity hypothesis. Modirrousta and Fellows (2008) investigated the role of the mPFC in memory monitoring in patients with mPFC damage using three types of metamemory judgments: judgment-of-learning (JOL), FOK, and recall confidence. Whereas JOL performance was not affected by damage to ventral mPFC, both recall confidence and FOK judgments were negatively affected, suggesting that mPFC is important for FOK judgments.
Task performance is determined by strategy selection and adaptation. According to Reder (1987), individuals use the familiarity of a question’s features to decide whether the answer is known and retrievable. Reder and Ritter (1992) investigated the FOK heuristic using a task that consisted of a series of unfamiliar math problems in which some of the problems were shown to the participants several times during the experiment. Results showed that participants were highly accurate in making FOK judgments and showed successful FOK performance for problems that looked similar. It was concluded that individuals are good at deciding whether they know the answer and that the initial assessment is made as a result of a partial match of the previously seen problem with the new problem.
Few studies have measured ERPs during the classical recall-FOK judgment-recognition paradigm to investigate the temporal dynamics of FOK. We anticipate that ERP components of FOK judgments will reflect rapid assessment of cue familiarity, and accessibility of the item in memory. Previous recognition memory literature (e.g. Curran 2004; see for a review, Rugg and Curran 2007) indicates that familiarity-based memory processes are associated with frontal activity within a 300–500 ms time window, while recollection-based memory processes are related to late parietal component (400–800 ms time window). Therefore, based on previous literature, it can be hypothesized that ERP components of FOK observed at an early time window will support the familiarity-based effect (Koriat 1993). However, if late ERP components (after 400 ms) are obtained during FOK judgments this will support the accessibility effect (Metcalfe and Dunlosky 2008). On the other hand, FOK judgments may depend on both familiarity and accessibility as evidence for each effect has been observed during recognition judgments, but at differing latencies and topographies. That is, recollected items will usually also seem familiar, for obvious reasons. There are also additional potential contributors to, and ERP effects of FOK responses. For example, ‘context familiarity’ (Addante et al. 2012b; Montaldi and Mayes 2010) could play a role in FOK since it may be that the context of the prior occurrence is retrieved but the specific item is not, hence the failure of recall but the contextual sense that the memory is retrievable later. This phenomenon has also been linked with specific ERP effects that are dissociable from the FN400 and late positive component (LPC) of item familiarity and recollection, respectively, occurring later in time (800–1200 ms) and in different topographies (Addante et al. 2012a, b).
Behavioral models (e.g. Nelson and Narens 1980) in cognitive psychology have provided useful information regarding the structure of metamemory processes. However, it remains unclear which brain regions are used during metacognitive processes, and which brain regions modulate the subjective level of FOK judgment. Although previous studies (e.g. Kikyo and Miyashita 2004; Kikyo et al. 2002; Maril et al. 2003; Schnyer et al. 2005) indicated that the frontal lobe plays a significant role during FOK judgments, the role of other regions during FOK judgments are still unclear. Recently, Wokke et al. (2017) investigated relationships between first and second-order decision task performance and metacognition. Results supported hierarchical model of metacognition which proposed that prefrontal lobe activation (e.g. prefrontal theta oscillation) is crucial for metacognition. On the other hand, the ERP correlates of FOK judgments is currently unknown. The primary objective of the present study was to explore the electrophysiological correlates of FOK judgments using a recall-FOK judgment-recognition paradigm. Following previous studies (e.g. Maril et al. 2003; Paynter et al. 2009) which showed association between FOK judgment and perceptual fluency, we hypothesized that FOK judgment associated with early time window ERP components which occurs within 200–300 ms after the stimulus onset. Confidence judgments have also been shown to affect choice and behavior and do so irrespective of their accuracy. People are often overconfident in their knowledge, making choices based on their level of confidence even when confidence is unrelated to actual performance. Koriat and Goldsmith (1996) argued that high and low levels of FOK are related to retrieved content, making metamemory judgment and objective accuracy difficult to disentangle. Prior ERP studies have shown that confidence level, rather than awareness, modulates the different ERP components, such as P300 (Eimer and Mazza 2005), FN400, and LPC (Addante et al. 2012a; Curran 2004). Damasio (1996) also suggested that, during situations of uncertainty, cognitive processes may become insufficient leading to affective states playing a greater role in decision making, and subjects’ confidence levels about their responses or decisions may complicate interpretation of the findings. In addition, fMRI studies have provided some evidence that brain regions involved in high versus low confidence are at least partially separate from each other (e.g. Chua et al. 2006; Henson et al. 2000; Moritz et al. 2006). Thus, ERP correlates of FOK judgment may be affected by level of FOK judgment (e.g. high vs. low FOK judgment). In sum, the second objective of the study is to investigate temporal dynamics of degree of FOK judgment. We expected that that ERP characteristics of high FOK judgments will be different compared to low FOK judgments (differences between overall, high and low FOK judgments are explained in Data analysis section), and characteristics of these ERP components will vary in different electrode regions. Specifically, following the previous studies (Bonini et al. 2014; Carter et al. 1998; Fleming et al. 2014; Hilgenstock et al. 2014; Shimamura and Squire 1986; Wokke et al. 2017), we hypothesized that the high FOK judgment associated with frontal electrodes, on the other hand, low FOK judgment is linked to distributed neural substrates rather than strictly to frontal lobe function. Although ERP is not the best localization technique, this study will provide novel insight into the temporal dynamics of FOK.
Method
Participants
These were 81 undergraduate students from several departments. All of whom were right-handed and reported normal or corrected-to-normal vision. Participants were advised to abstain from lack of sleep, alcohol, and caffeine on the evening before the study. Participants with neurological and/or psychiatric disorders and those who were taking or had recently stopped taking antidepressants, psychotherapeutic drugs, or drugs that could affect their cognitive processes were not admitted to the study. The study conformed to the Declaration of Helsinki and was approved by the host University of Research Ethics Committee. Participants provided informed consent and they were given financial compensation and/or course credits where applicable. Four participants were excluded due to equipment failure or excessive electroencephalographic (EEG) artifacts. The remaining 77 participants (48 female) had a mean age of 21.12 (range 18–26).
Stimuli
A total of 176 words were selected from a word-frequency database in Turkish (Göz 2003). The study list consisted of 44-word pairs (total 88 words). The remaining words were used for the recognition phase of the task. All words were high frequency nouns, five or six letters long. The word-pairs were designed by randomly matching words. Four word-pairs (two at the beginning and two at the end of each list) were not included in the statistical analyses in order to minimize primacy and recency effects.
Experimental tasks
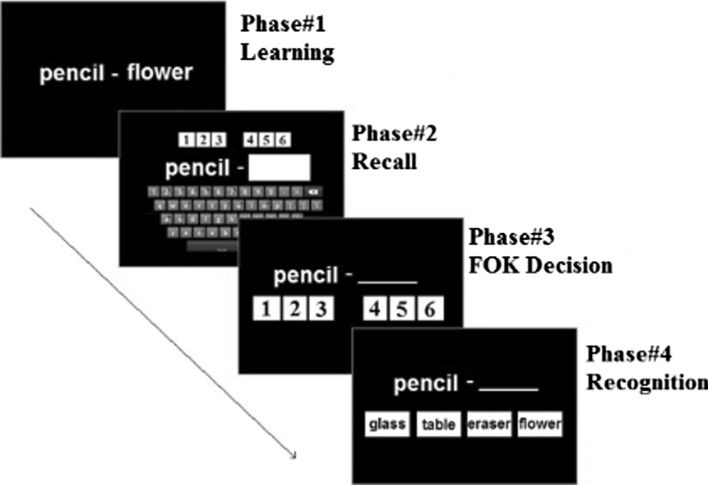
A classical FOK paradigm (Hart 1965) was used to measure participants’ FOK performance (see Fig. 1). The task consisted of four consecutive phases. In the first (learning) phase, each word-pair was initially presented, and participants were instructed to memorize the words. At the end of the learning phase participants were informed about the upcoming cued-recall test and the FOK judgments. In the second phase (cued-recall), the first word of each word-pair was presented and the participants were asked to recall which word it was paired with and indicate their level of confidence for each answer using a 6-point Likert-type rating scale (1: definitely not sure, 6: definitely sure). In the third phase, for the words they failed to remember, they were asked to give a FOK judgment. They were given instructions about what FOK judgments referred to and were asked to make a FOK judgment based on the following question: “Even though I don’t remember the answer now, do I know the answer to the extent that I am able to pick the correct answer from among several choices in the future?” Participants were asked to use a 6-point Likert-type rating scale for their FOK judgment (1: definitely will not be able to find the correct answer, 6: definitely will be able find the correct answer). Participants then completed a recognition test, comprising the fourth phase, in which each cue word was presented along with four alternatives. The alternatives consisted of one correct answer, one word from the study list, and two words from the initial pool. Participants’ FOK accuracy, which refers to how well people are able to predict their future memory performance, was calculated by using the Goodman–Kruskal’s Gamma correlation, a commonly used measure of FOK accuracy (Nelson 1984). In addition, da (the distance between the means of the evidence distributions in SD units which have unequal variance, see Macmillan and Creelman 2005) and meta-da (measure of metacognitive sensitivity, see Fleming and Lau 2014; Maniscalco and Lau 2012; Wokke et al. 2017) were also calculated because it has been claiming that correlation efficiencies have poor resolution and calibration standards to measure metacognitive judgments (Higham et al. 2016).
Fig. 1.
Experimental task was used in the study
Procedure
Participants gave informed consent to participate in the study after the purpose and the nature of the experiment was fully explained. Participants were informed that they are free to withdraw the study whenever they want. The experiment was programmed using Visual Studio 2010 Ultimate. There was no time limit during any phase of the experiment. However, participants were instructed to perform each phase of the task as fast and accurately as possible. Duration between each stimulus (after a decision was made) was 500 ms in all phases of the experiment. The program was run on a Windows XP computer with a 21-in. monitor. Each word-pair and alternatives were typed in black, Arial 24-point, uppercase letters on a white background.
EEG recording and preprocessing
ERPs were recorded during FOK judgment. EEG/EOG signals were recorded for 1200 ms after the stimulus onset using 32 Ag/AgCl electrodes mounted in elastic Quick-caps (Neuromedical Supplies, Compumedics, Inc., Charlotte). EOG signal was measured from two bipolar channels: one was formed by two electrodes placed at the outer canthus of each eye; another, by two electrodes below and above the left eye. EEG signal was recorded from 30 (FP1, FP2, F7, F8, F3, F4, Fz, FT7, FT8, FC3, FC4, FCz, T7, T8, C3, C4, Cz, TP7, TP8, CP3, CP4, CPz, P7, P8, P3, P4, Pz, O1, O2, Oz) electrodes arranged according to the standard 10–20 system, with additional electrodes placed at BP1/BP2 and also on the left and right mastoids (M1/M2). All EEG electrodes were referenced on-line to an electrode at vertex and re-referenced off-line to linked mastoids. EEG and EOG signals were amplified and recorded at a 1000 Hz sampling rate using Synamp2 amplifier at AC mode (Neuroscan, Compumedics, Inc., Charlotte) with high- and low-pass filter set at 0.15 and 100 Hz, respectively. EEG electrode impedance was kept below 5 kΩ.
EEG data pre-processing was conducted using Edit 4.5 (Neuroscan, Compumedics, Inc. Charlotte) and applied to each participant’s dataset. Data were down-sampled to 250 Hz to reduce computational demands and then low-pass filtered at 30 Hz and high-pass filtered at 0.15 Hz. EEG segments were extracted with an interval of 200 ms preceding and 1000 ms following the stimulus onset. Artifact rejection was performed in two steps. First, trials containing activity exceeding a threshold of ± 100 µV at vertical and horizontal EOG and EEG channels were automatically detected and rejected. Second, we manually removed trials with saccades identified over the horizontal EOG channel. For the computation of ERPs, artifact-free segments were baseline corrected using 100 ms pre-stimulus period and then averaged for the experimental conditions (FOK decision). ERPs were obtained by stimulus-locked (stimulus onset) averaging of the EEG recorded in FOK judgment condition.
Data analysis
In the present study, the average of ERPs was determined in temporal direction. In order to obtain overall average, acquired and filtered epochs from 77 participants were derived in compliance with phases of task and/or type of response. Accordingly, low FOK judgment ratings (1, 2, 3) and high FOK judgment ratings (4, 5, 6) were calculated as overall low and overall high ERP means. The Likert Scale is a uni-dimensional scale giving limited options, and the space between each choice cannot possibly be equidistant. Additionally, individuals may opt for the mid-point on a scale due to a ‘social desirability bias’ (Garland 1991; Matell and Jacoby 1972). Therefore, it might fail to measure participants’ true responses. To eliminate the issue, only the extreme FOK judgment ratings (extremely low FOK: 1–2; and extremely high FOK: 5–6) were included in a second analysis (e.g. FOK ratings of 3 or 4 were not included). Thus, ERP averages of 56 participants who had extremely high and extremely low FOK judgment ratings were included in the calculation of ERP averages. As mentioned earlier ERP correlates of FOK judgment is still unknown. Thus, we analyzed grand average ERP for eight different time windows, namely 50–100, 100–200. 200–300, 300–400, 400–500, 500–600, 600–700, and 700–800 ms. Also, based upon previous neuroimaging literature of FOK mentioned above, we focused on three main electrode zones, namely frontal (F3, Fz, F4), fronto-central (FC3/Cz/FC4) and parietal (P3/Pz/P4). We detected extreme noise the on FCz electrode, thus FCz electrode was excluded from statistical analysis and Cz electrode was included. Finally, we analyzed ERPs in eight different intervals at three electrode regions. These time ranges sufficiently included the latency variations of the ERP peaks that were obtained under all conditions of the experimental paradigms. Thus, ERP grand averages were calculated for four conditions; overall high FOK, overall low FOK, extremely high FOK and extremely low FOK judgments (see Figs. 2, 3). ERPs were exported as mean amplitudes per electrode within specific time window for statistical analyses, as explained below section.
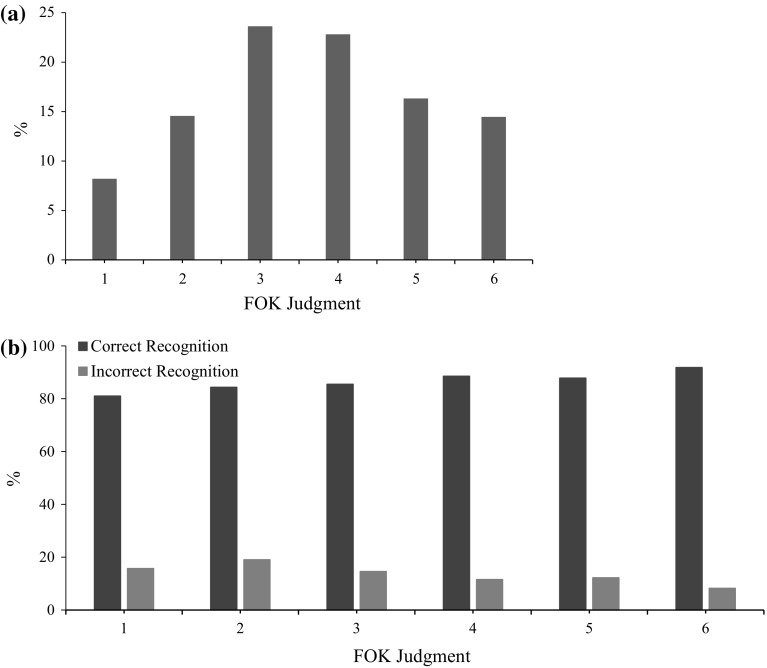
Fig. 2.
Performance measures of participants. a Bars represent the percentage of division of responses across FOK level. b Bars represent the percentage of correctly and incorrectly recognized items across FOK level
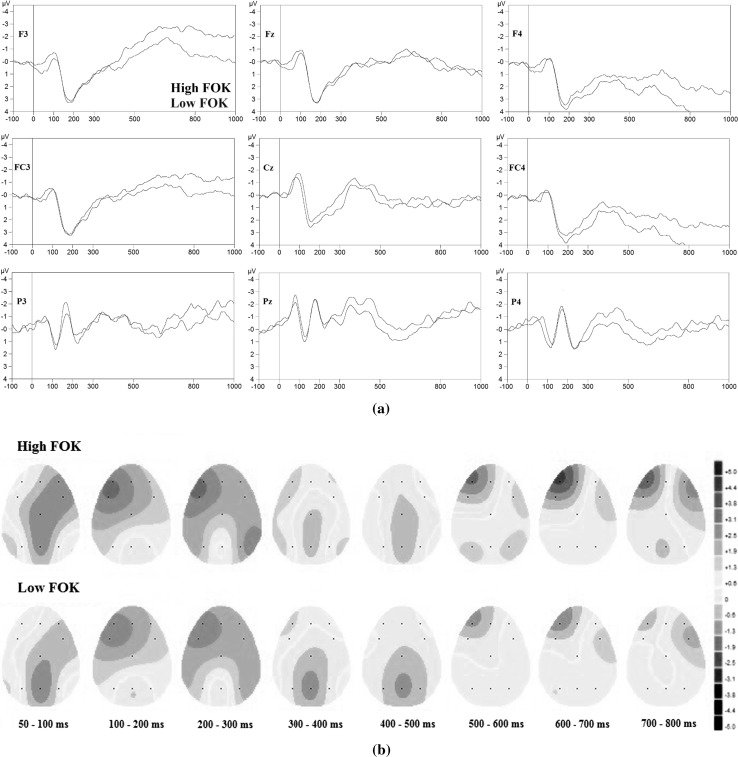
Fig. 3.
Stimulus-locked ERP grand average (n = 77) in overall high (black line) and overall low (red line) FOK decisions a at F3, Fz, F4, FC3, Cz, FC4, P3, Pz, and P4 electrode sites and b Headplots in overall high (top) and overall low (bottom) FOK decisions. Stimulation applied at ‘0.0 ms’ time point (stimulus onset). (Color figure online)
All statistical analyses were carried out using IBM SPSS Statistics 20 package program. Mean and amplitude values were recorded for 9 electrodes: F3, Fz, F4, FC3, Cz, FC4, P3, Pz, and P4 representing three brain regions: frontal, central/fronto-central, and parietal. ERP topographies for the 9 electrodes are presented for each experimental condition. Statistical analyses were performed with mean voltage amplitudes of ERP wave forms in designated time windows, using ANOVA that included Greenhouse–Geisser corrections in cases where factors had more than two levels. The analyses were conducted on all nine electrode sites unless stated otherwise. All reported differences are significant at least at a level of p < .05 and were followed by Bonferroni post hoc comparison tests if necessary. For post hoc comparison, α was assigned the value of .05 for each p among a set of p values, such that for a set of p values did not exceed a critical value. The data for each ERP time windows were analyzed with condition (high vs. low), electrode site (frontal, central/fronto-central and parietal) separately as within-subjects factors. All participants who had at least 10 artifact free trials per condition were included in analyses. After artifact rejection the average number of useable trials for different conditions were comparable in high FOK (M = 19.47, SD = 3.93), low FOK (M = 16.58, SD = 4.98), extremely high FOK (M = 14, SD = 3.7), extremely low FOK (M = 13.18, SD = 3.27).
Results
Behavioral data
The mean (n = 77) numbers of the recalled trials were 14.65 (SD = 7.07) and mean numbers of trials (unrecalled items) during FOK decision were 25.35 (SD = 7.11; Min/Max: 17/37). Median for FOK rating was 3.69 (SE = 0.09). Average Gamma correlation was for overall FOK 0.19 (SE = .06), for high FOK 0.25 (SE = .06), for low FOK 0.22 (SE = .06), for extremely high FOK (n = 56) 0.28 (SE = .06) and for extremely low FOK judgment 0.21 (SE = .07). The mean number of confidence ratings for recall phase was 2.74 (SD = .42).
Pearson correlation showed that mean response latencies during overall FOK decision negatively correlated with overall FOK rating (r = − .38, p < .01) which is consistent with previous findings (Nelson and Narens 1980).
Paired sample t test results showed that participants’ RT (ms) during extremely high FOK judgments (M = 2732.05) was faster than RT during extremely low FOK judgments (M = 3136.67) (t = 3.45, p < .05). However, there was no significant difference in RT between overall high (M = 3693.25) and overall low (M = 3858.19) FOK judgments. In addition, the correlation between mean of confidence rating during recall phase and FOK judgment was significant (r = .31, p < .01). This correlation was higher for high FOK judgments (r = .32, p < .01) than low FOK judgements (r = .07, p > .05).
To test the hypothesis of Signal Detection Theory (SDT) which claimed there should be positive correlation between da and meta-da. Pearson’s correlation was calculated and there was a significant positive relationship between da and meta-da (r = .37, p < .001). The mean level of meta-da (M = 0.42) was significantly higher than the mean da (M = 0.01), t(74) = − 2.72, p < .01. Metacognitive sensitivity was evaluated by the mean value of meta-da/da ratio (M = 0.24), suggesting that 24% of FOK judgments can predict future recognition performance. Log likelihood of meta-da fits did not correlate with meta-da and meta-da − da (subtraction da from meta-da) indicating that measured variation in absolute and relative metacognitive sensitivity do not be explained with variation in the quality of data fitting (for detail, see Maniscalco and Lau 2012).
Figure 2a showed that concentration of FOK judgment responses towards the middle of scale (option 3 and 4) and response rates for FOK judgments revealed significant FOK judgment main effect, F(6, 73) = 155.59, p < .001. Also, frequency of correct and incorrect recognition across FOK judgment level was presented at Fig. 2b. Numbers of correctly recognized items were higher than incorrectly recognized items across all FOK judgment level, t(77) = 26.27, p < .001.
ERP results
Figure 3 shows the grand average (Fig. 3a) and the scalp topographies (Fig. 3b) filtered waveforms during overall high and overall low FOK judgments for nine recording sites. The grand average (Fig. 4a) and the scalp topographies (Fig. 4b) filtered waveforms during extremely high and extremely low FOK judgments for nine recording sites are shown in Fig. 4. All figures show both the pre-stimulus EEG that serves as the baseline in the present study and the post-stimulus ERP; abbreviated to EEG/ERP. All scalp topographies were presented for eight different time windows.
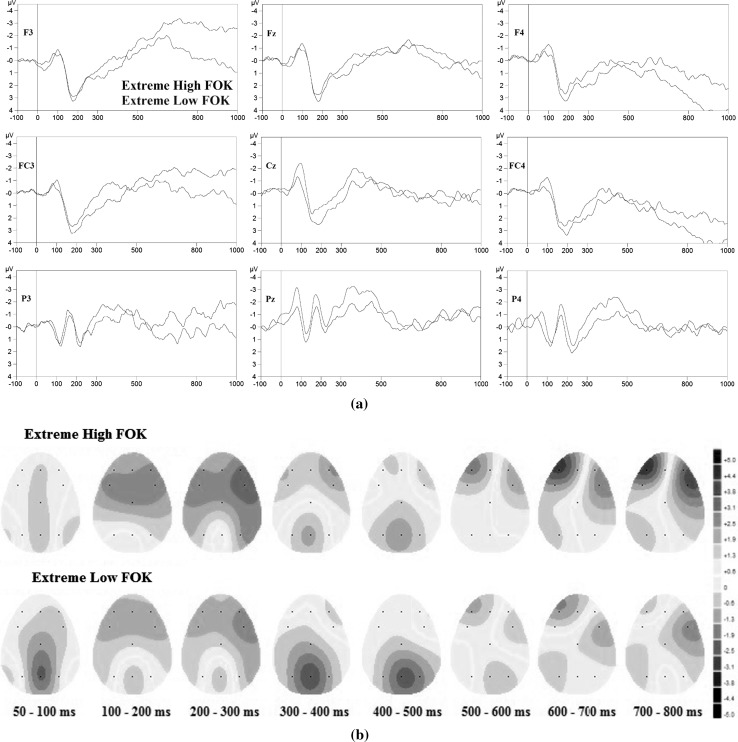
Fig. 4.
Stimulus-locked ERP grand average (n = 48) extremely high (black line) and extremely low (red line) FOK decisions a at F3, Fz, F4, FC3, Cz, FC4, P3, Pz, and P4 electrode sites and b Headplots in extremely high (top) and extremely low (bottom) FOK decisions. Stimulation applied at ‘0.0 ms’ time point (stimulus onset). (Color figure online)
Visual analysis
Figure 3a shows ERPs triggered in response to overall high and overall low FOK judgments, and Fig. 3b shows headplots following stimulus onset in response to different FOK decisions. In general, during FOK judgment, two significant components at approximately 100 ms (N100) and at approximately 200 ms (P200) following stimulus onset were observed at all electrode locations and significant ERP component at approximately 200 ms (N200) following stimulus onset components were recorded at parietal electrodes. In details, FOK judgment produced bigger N100 amplitude at midline electrodes (Fz, Cz and Pz) than lateral electrodes. During FOK judgment, a significant and characteristic positivity was recorded at approximately 170 ms (P200 time window) at all electrodes. In general, P200 at frontal and central/fronto-central electrodes was higher in amplitude than that at parietal electrodes, respectively. In addition, at parietal electrodes, an enhanced negativity for FOK judgment (for all of overall high, and low conditions) started at approximately 180 ms (N200 time window), and this negativity was not observed at frontal and central/fronto-central electrodes.
Figure 4a shows ERPs triggered in response to extremely high and extremely low FOK judgments and Fig. 4b shows headplots following stimulus onset in response to extremely low and extremely high FOK judgments. Similarly, extremely high and extremely low FOK judgment produced significant N100 and P200 components at all electrode locations and significant N200 components at parietal electrodes. In details, extremely low FOK judgment produced bigger N100 amplitude compared to extremely high FOK judgment. Extremely low FOK judgment also leads to larger parietal N200 but smaller P200 amplitudes than extremely high FOK judgment.
There is also a negative late component was recorded at approximately 400–550 ms time window (N400) especially at fronto-central and parietal electrodes. Extremely low FOK judgment produced bigger N400 amplitudes than extremely high FOK judgment at Cz, Pz and P4 electrodes. Lastly, another negative (at F3 and FC3) and positive (at F4 and FC4) slow wave was observed at approximately 600–800 ms, and high FOK judgment produced bigger amplitude than low FOK judgment (See Figs. 3a, 4a).
Statistical results
Statistical analyses were conducted in three steps. Firstly (n = 77) to compare overall low and overall high FOK judgments, separate Greenhouse–Geisser corrected 2 (condition: overall high and overall low FOK judgments) × 3 (electrode location: frontal, central/fronto-central, and parietal) repeated measures ANOVAs were conducted for each time window separately. In the second step (n = 56) to compare extremely high and extremely low FOK judgments, similar 2 (condition: extremely high and extremely low FOK judgments) × 3 (locations: frontal, central/fronto-central, and parietal) repeated measures ANOVA were conducted ANOVA results were summarized at Table 1. Significant results and follow-up comparisons were presented at below section.
Table 1.
2 (Condition) × 3 (Electrode location) repeated measure ANOVA results
| Overall FOK judgments | Extreme FOK judgments | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Electrode Site | Interaction | Condition | Electrode Site | Interaction | ||||||||
| Time window | Region | F | η 2 | F | η 2 | F | η 2 | F | η 2 | F | η 2 | F | η 2 |
| 50–100 | Frontal | 2.36 | .04 | 36.79*** | .38 | 1.55 | .03 | .01 | .00 | 11.93*** | .33 | .49 | .02 |
| Fronto-central | 2.70 | .11 | 39.62*** | .40 | .09 | .00 | .88 | .04 | 13.10*** | .36 | 6.56** | .22 | |
| Parietal | .09 | .00 | 22.05*** | .36 | .14 | .00 | 1.19 | .07 | 11.00*** | .41 | .55 | .03 | |
| 100–200 | Frontal | .06 | .00 | 26.78*** | .30 | 1.47 | .02 | .01 | .00 | 6.00** | .20 | 1.41 | .06 |
| Fronto-central | .04 | .00 | 18.16*** | .23 | .03 | .00 | 7.26* | .24 | 4.32* | .16 | 3.56* | .13 | |
| Parietal | .13 | .00 | 1.76 | .04 | .79 | .02 | 1.71 | .10 | .19 | .01 | .98 | .06 | |
| 200–300 | Frontal | .02 | .00 | 8.68*** | .12 | 1.33 | .02 | .06 | .00 | 4.07 | .15 | .60 | .02 |
| Fronto-central | .16 | .00 | 11.57*** | .16 | .65 | .01 | 3.46 | .13 | 3.84 | .14 | .51 | .02 | |
| Parietal | 1.41 | .03 | 14.50*** | .27 | .43 | .01 | 3.85 | .19 | 5.64** | .26 | .44 | .03 | |
| 300–400 | Frontal | .23 | .00 | 7.33** | .11 | .68 | .01 | .68 | .03 | 4.32 | .15 | .16 | .01 |
| Fronto-central | .16 | .00 | 14.64*** | .20 | .65 | .01 | 2.60 | .10 | 12.84*** | .36 | .07 | .00 | |
| Parietal | 2.89 | .07 | 15.42*** | .29 | .53 | .01 | 3.40 | .18 | 11.08*** | .41 | .41 | .03 | |
| 400–500 | Frontal | .18 | .00 | 2.05 | .03 | .10 | .00 | .96 | .04 | .36 | .02 | .24 | .01 |
| Fronto-central | .08 | .00 | 8.69** | .13 | .98 | .02 | .17 | .01 | 5.98** | .21 | .57 | .02 | |
| Parietal | .70 | .02 | 20.95*** | .34 | 1.83 | .04 | 1.48 | .09 | 9.71** | .38 | .34 | .02 | |
| 500–600 | Frontal | .04 | .00 | 26.00*** | .30 | .44 | .01 | .50 | .02 | 5.07* | .17 | .28 | .01 |
| Fronto-central | .00 | .00 | 11.08*** | .16 | .14 | .00 | .52 | .02 | 2.33 | .10 | 3.38 | .13 | |
| Parietal | .11 | .00 | 4.27* | .10 | 1.09 | .03 | .03 | .00 | 2.43 | .14 | .11 | .01 | |
| 600–700 | Frontal | 1.79 | .03 | 48.92*** | .44 | 2.12 | .03 | .40 | .02 | 22.74*** | .48 | 3.99 | .14 |
| Fronto-central | .99 | .02 | 19.42*** | .25 | .67 | .01 | .35 | .02 | 13.38*** | .37 | 10.88** | .32 | |
| Parietal | 1.43 | .03 | 2.67 | .06 | 1.93 | .05 | .03 | .00 | 1.48 | .09 | 3.77 | .19 | |
| 700–800 | Frontal | 1.28 | .02 | 63.15*** | .51 | 8.67** | .13 | .22 | .01 | 35.85*** | .60 | 8.56*** | .26 |
| Fronto-central | .65 | .01 | 24.93*** | .29 | 1.70 | .03 | 1.16 | .05 | 13.58*** | .37 | 20.12*** | .47 | |
| Parietal | 7.09** | .15 | 3.10 | .07 | 1.82 | .05 | .09 | .01 | 4.85* | .23 | 1.66 | .09 | |
*p <.05; **p <.01; ***p <.001
Comparisons between overall high and low FOK judgments on ERPs
50–100 ms time window
Electrode location main effect was significant for three regions (F ≥ 22.05, p ≤ .001). For frontal region, F3 electrode (M = 0.19) was significantly lower in amplitude when compared to F4 (M = − 1.04) and Fz (M = − 0.47) electrodes. For fronto-central region, results indicated that FC3 electrode (M = − 0.09) was lower in amplitude when compared to FC4 (M = − 1.31) and Cz (M = − 1.36) electrodes. For parietal region, Pz electrode site (M = − 1.96) was higher in amplitude when compared to P4 (M = − 0.81) and P3 (M = − 0.13) electrodes.
100–200 ms time window
Electrode location main effect was significant for frontal and fronto-central regions (F ≥ 18.16, p ≤ .001). For frontal region, results indicated that higher amplitude values were obtained at F3 electrode site (M = 2.23) compared to Fz (M = 1.80) and F4 (1.20) electrode sites. For fronto-central region, higher amplitude values were obtained at FC3 electrode (M = 2.14) compared to FC4 (M = 1.14) and Cz (M = 1.22) electrodes.
200–300 ms time window
Electrode location main effect was significant for three regions (F ≥ 11.57, p ≤ .001). For frontal region, results indicated that F3 electrode (M = 2.30) was significantly higher in amplitude when compared to Fz (M = 1.74) and F4 (M = 1.63) electrodes. For fronto-central region, Cz electrode (M = 1.15) was significantly lower in amplitude when compared to FC4 (M = 1.69) and FC3 (M = 2.19) electrodes. For parietal region, results indicated that Pz electrode site (M = 0.06) was lower in amplitude when compared to P3 (M = 0.84) and P4 (M = 1.64) electrode sites.
300–400 ms time window
Electrode location main effect was significant for three regions (F ≥ 7.33, p ≤ .01). For frontal region, results indicated that higher amplitude values were obtained at F3 electrode site (M = 0.97) compared to Fz (M = 0.24). For fronto-central region, higher amplitude values were obtained at Cz electrode (M = − 0.68) compared to FC3 (M = 0.59) and FC4 (M = 0.03) electrodes. For parietal region, results showed that Pz electrode site (M = − 1.48) was significantly higher in amplitude when compared to P4 (M = − 0.19) and P3 (M = 0.00) electrode.
400–500 ms time window
Results showed that there was a significant main effect of electrode location for fronto-central and parietal regions (F ≥ 8.69, p ≤ .01). For fronto-central region, Cz electrode (M = − 0.82) was significantly higher in amplitude when compared to FC4 (M = − 0.12) and FC3 (M = 0.05) electrodes. For parietal region, Pz electrode (M = − 1.87) was significantly higher in amplitude when compared to P4 (M = − 1.14) and P3 (M = − 0.34) electrodes.
500–600 ms time window
Electrode location main effect was significant for three regions (F ≥ 4.27, p ≤ .05). For frontal region, results indicated that higher amplitude values were obtained at F3 electrode (M = − 1.35) compared to to Fz (M = − 0.75) and F4 (M = 0.11) electrodes. For fronto-central region, FC4 electrode (M = 0.46) was significantly higher than Cz (M = − 0.16) while significantly lower than FC3 (M = − 0.69) electrodes. For parietal region, P3 electrode (M = 0.32) was significantly lower in amplitude than Pz (M = − 0.37) electrode.
600–700 ms time window
Results showed that there was a significant main effect of electrode location for frontal and fronto-central regions (F ≥ 19.42, p ≤ .001). For frontal region, F3 electrode site (M = − 1.92) was significantly higher in amplitude when compared to Fz (M = − 1.03) and F4 (M = .15) electrode sites. For fronto-central region, FC3 electrode (M = − 1.05) was higher in amplitude when compared to FC4 (M = 0.69) and Cz (M = − 0.21) electrodes.
700–800 ms time window
Electrode location main effect was significant for frontal and fronto-central regions (F ≥ 24.93, p ≤ .001). For frontal region, results indicated that higher amplitude values were obtained at F3 electrode (M = − 1.51) compared to Fz (M = − 0.36) and F4 (M = 0.96) electrodes. For fronto-central region, higher amplitude values were obtained at FC4 electrode (M = 1.36) compared to FC3 (M = − 0.77) and Cz (M = − 0.17). Results indicated that there was a significant main effect of condition for parietal region (F = 7.09, p ≤ .01), suggested that overall high FOK judgments (M = − 0.92) was significantly higher in amplitude when compared to overall low FOK judgments (M = 0.01). Also, the interaction effect was significant for frontal region (F = 8.67, p ≤ .01), suggested that overall high FOK judgments were significantly higher in amplitude when compared to overall low FOK judgments at F3 electrode.
Comparisons between extremely high and low FOK judgments on ERPs
50–100 ms time window
Electrode location main effect was significant for three regions (F ≥ 11.00, p ≤ .001). For frontal region, F3 electrode (M = .42) was significantly lower in amplitude when compared to Fz (M = − 0.43) and F4 (M = − 0.97) electrodes. For fronto-central region, results indicated that FC3 electrode (M = − 0.03) was lower in amplitude when compared to FC4 (M = − 0.93) and Cz (M = − 1.23) electrodes. For parietal region, P3 electrode (M = 0.25) was lower in amplitude than Pz (M = − 1.24) electrode. Also, the interaction effect was significant for fronto-central region (F = 6.56, p ≤ .01), suggested that extreme high FOK judgments were significantly lower in amplitude than extreme low FOK judgments at FC4 electrode.
100–200 ms time window
Electrode location main effect was significant for frontal and fronto-central regions (F ≥ 4.32, p ≤ .05). For frontal region, results indicated that higher amplitude values were obtained at F3 electrode (M = 2.32) compared to Fz (M = 1.32) electrode. For fronto-central region, higher amplitude values were obtained at FC3 electrode (M = 1.98) compared to FC4 (M = 1.23) electrode. Condition main effect was significant for fronto-central region, F = 7.26, p ≤ .05, suggested that extreme high FOK judgments (M = 2.00) were higher in amplitude than extreme low FOK judgments (M = 0.89). The interaction effect was also significant for fronto-central region (F = 3.56, p ≤ .05) and extreme high FOK judgments were higher in amplitude than extreme low FOK judgments at FC4 and Cz electrodes.
200–300 ms time window
There was a significant main effect of electrode location was for parietal regions (F = 5.64, p ≤ .01, suggested that Pz electrode site (M = 0.26) was lower in amplitude when compared to P4 (M = 1.69) electrode.
300–400 ms time window
Electrode location main effect was significant for fronto-central and parietal regions (F ≥ 11.08, p ≤ .001). For fronto-central region, higher amplitude values were obtained at FC3 electrode (M = 1.03) compared to FC4 (M = 0.61) and Cz (M = − 0.62) electrodes. For parietal region, results showed that Pz electrode site (M = − 2.24) was significantly higher in amplitude when compared to P4 (M = − 1.12) and P3 (M = − 0.54) electrodes.
400–500 ms time window
Results showed that there was a significant main effect of electrode location for fronto-central and parietal region (F ≥ 5.98, p ≤ .01). For fronto-central region, Cz electrode (M = − 0.40) was significantly lower in amplitude when compared to FC3 (M = 0.53) electrode. For parietal region, Pz electrode (M = − 2.02) was significantly higher in amplitude than P3 (M = − 0.38) electrode.
500–600 ms time window
Electrode location main effect was significant for frontal region (F = 5.07, p ≤ .05) and results indicated that higher amplitude values were obtained at F4 electrode (M = 0.92) compared to F3 (M = − 0.40).
600–700 ms time window
Results showed that there was a significant main effect of electrode location for frontal and fronto-central regions (F ≥ 13.38, p ≤ .001). For frontal region, F3 electrode site (M = − 1.92) was significantly higher in amplitude when compared to Fz (M = − 0.14) and F4 (M = 0.68) electrode sites. For fronto-central region, FC3 electrode (M = − 0.96) was higher in amplitude when compared to FC4 (M = 0.65) and Cz (M = 0.50) electrodes. Also, the interaction effect was significant for fronto-central region (F = 10.88, p ≤ .01), suggested that extreme high FOK judgments were significantly higher in amplitude when compared to extreme low FOK judgments at FC4 electrode.
700–800 ms time window
Electrode location main effect was significant for three regions (F ≥ 4.85, p ≤ .05). For frontal region, results indicated that higher amplitude values were obtained at F3 electrode (M = − 1.61) compared to Fz (M = − 0.03) and F4 (M = 1.46) electrodes. For fronto-central region, higher amplitude values were obtained at FC4 electrode (M = 0.97) compared to FC3 (M = − 0.66) and Cz (M = 0.57). For parietal region, P4 electrode site (M = 0.96) was significantly higher in amplitude than Pz (M = − 0.04) electrode site. The interaction effect of condition and frontal electrode site was significant (F = 8.56, p ≤ .001) and extreme high FOK judgments were higher in amplitude when compared to extreme low FOK judgments at F3 electrode site. The interaction effect was also significant for fronto-central region (F = 20.12, p ≤ .001), suggested that extreme high FOK judgments were significantly higher in amplitude when compared to extreme low FOK judgments at FC4 electrode.
Discussion
Here, we investigated neural ERP components of FOK during an episodic memory task featuring a recall-FOK judgment-recognition paradigm. ERP components during high, low, extremely high and extremely low FOK judgments were analyzed. Our behavioral findings supported the previous literature showing that participants who gave high FOK judgments spent less time on target items and that means response latencies during FOK decision correlated with FOK rating. Stimulus-locked ERP analyses indicated that electrode site had a significant effect on ERPs. Specifically, FOK judgments were associated with greater positivity for P200 component at frontal, central/fronto-central and parietal electrode sites and greater negativity for the N200 component at parietal electrode sites. Furthermore, our results revealed that amplitude of the ERP components during FOK judgments were affected by the level of FOK judgment responses. Specifically, at frontal and central/fronto-central electrodes, higher FOK judgments produced higher amplitudes for P200 component; while, at parietal sites low FOK judgments produced higher amplitudes for N200. The implications of these findings are evaluated below.
Henson et al. (1999, 2000) reported that during episodic retrieval tasks monitoring processes are associated with activations in the right dorsolateral prefrontal region and that these activations are linked to unsuccessful retrieval rather than successful retrieval. In the present study, we found that the neural correlates of FOK judgments at frontal, central/fronto-central, and parietal regions appeared early in processing (within 200 ms following stimulus onset). The frontal distribution of these components is consistent with previous neuropsychological and brain imaging studies (Fernandez-Duque et al. 2000; Pannu and Kaszniak 2005; Simons and Spiers 2003), which show that the frontal lobes are crucial for memory-monitoring processes. It also suggests that FOK may be associated with similar neural networks to other metamemory processes (e.g. JOL, TOT). Although Paynter et al. (2009) reported a specific right-hemispheric involvement in FOK processes during math problems, we used a different experimental task and our results did not support a right hemispheric dominance for FOK judgments. The ERP components recorded during FOK judgments in the present study had similar amplitudes in both the right and the left hemispheres.
Previous studies have shown that the P200 component is associated with various cognitive components such as short-term and working memory (Lefebvre et al. 2005), repetition priming or familiarity effects, and implicit memory (Rugg and Doyle 1994). Differences in P200 amplitude suggest that anterior and posterior (including Pz electrode site) distributional differences are produced during memory processes like encoding and rehearsal. Generally, low recognition performance leads to larger frontal and smaller parietal/occipital amplitudes than high recognition performance (Dunn et al. 1998). P200 amplitude was also higher for words that had been seen before (Evans and Federmeier 2007). Other ERP studies (e.g. Doyle et al. 1996; Rugg and Nieto-Vegas 1999) also report that familiarity-based ERP components (e.g. P200) occur within 300 ms following stimulus onset. This P200 component has generally been associated with perceptual processing of stimuli (or perceptual fluency), suggesting that perceptual fluency is used to guide metamemory judgments or FOK judgments (Paynter et al. 2009). The fronto-central P200 component observed in our study appears to show a similar latency and scalp distribution to previous ERP studies (Diana et al. 2005; Paynter et al. 2009) and in line with these studies, we conclude that the P200 component in our study may be playing a similar role in rapidly assessing familiarity of the stimuli in order to guide a successful search. As suggested by Reder et al. (2000), whether a search should be attempted depends on a quick evaluation of the familiarity of the stimulus, consistent with the preliminary hypothesis of FOK relying on the P200 ERP component.
As previously mentioned, the distinction between metacognitive judgments has been a trend research question. Some of behavioral models claimed that both retrospective and prospective metacognitive judgments are strongly related to strength of memory performance (Dougherty 2001; Jang and Nelson 2005; Modirrousta and Fellows 2008). On the other hand, it was also suggested that the source of metacognitive judgments differs in terms of the way evaluating information (e.g. familiarity). For instance, while FOK judgment is based on familiarity processes, the source of JOL and RCJ is related with accessibility of the cue (Busey and Tunnicliff 2000; Koriat and Levy-Sadot 2001; Metcalfe et al. 1993). Considering results of the present study, it was indicated that neither high nor low FOK judgments did not correlate with the high and low confidence judgments, suggested that underlie different type of memory judgment. Additionally, in the light of neuroimaging studies (for a review see, Metcalfe and Schwartz 2016), although prospective memory tasks were associated with lateral areas of prefrontal and parietal regions (e.g. Do Lam et al. 2012; Kikyo and Miyashita 2004; Kikyo et al. 2002; Maril et al. 2003, 2005), retrospective memory tasks demand a neural activation involvement of medial areas of prefrontal, temporal, parietal regions, and medial and lateral parietal regions (e.g. Chua et al. 2006; Jing et al. 2004; Moritz et al. 2006). Thus, these studies confirmed our ERP findings about higher N200 amplitudes observed at parietal electrodes during FOK judgements and it can be concluded that there was a parietal involvement of prospective metacognitive judgments.
Previous fMRI studies (Kikyo and Miyashita 2004; Kikyo et al. 2002; Maril et al. 2003; Schnyer et al. 2005) have shown graded activation during FOK, with greater activity for higher compared to lower levels of FOK. Comparisons of high versus low FOK judgments showed greater activity in the ventrolateral PFC and dorsolateral PFC (Chua et al. 2009) and also ventral posterior parietal cortex (PPC) (Elman et al. 2012). These areas were also significantly correlated with FOK. These similar patterns of increasing activity for higher compared to lower FOK judgments have been shown using Likert-type rating scales (Kikyo et al. 2002). Thus, these results indicate that, while growing evidence suggests that the PPC modulates subjective level of FOK, the frontal cortices also appear to play a role in subjective evaluation. In our study, for P200 peak, we found that extremely high FOK judgments produced higher amplitudes than extremely low FOK judgment ratings at fronto-central electrodes. The amplitude of P200 component at the frontal electrodes was higher than that at the central/fronto-central and parietal electrode sites, in line with previous findings demonstrating a relationship between frontal areas and level of FOK judgment responses. Thus, the P200 component observed in our study during FOK judgment may reflect the assessment of whether a specific stimulus or cue (e.g. the first word of each pair) seems sufficiently familiar and accessible to be able to guide a decision for future memory performance (either high or low FOK judgment). We hypothesized that if familiarity and accessibility of the cue is high, RT of the FOK judgment would be faster and the level of FOK judgment would be higher (and vice versa). Supporting this, negative significant correlations (r = − .32, p <.05) were found between RT for extremely high FOK judgments and P200 amplitude. Thus, it can be concluded that faster RT and higher FOK judgment ratings may lead to higher P200 amplitudes. Consequently, our ERP results support the idea that FOK judgment is based on processes emerging as rapidly as 200 ms following the appearance of the target stimulus, and that the frontal cortices modulate this cognitive process.
Functional brain imaging studies indicate that the parietal cortex is activated during episodic recall (Wagner et al. 2005) and metamemory (Chua et al. 2009, 2014; Kim and Cabeza 2007; Moritz et al. 2006). Patients with parietal cortex lesions showed lower confidence in their abilities to recollect information, although their memory performance was intact (Simons et al. 2010). McCurdy et al. (2013) also showed that metacognitive accuracy in healthy individuals is related to grey matter volume in the medial parietal cortex. Few studies have shown greater neural activity for low FOK compared to high FOK in any regions. However, Elman et al. (2012) showed greater activity for low FOK in the dorsal PPC. We observed greater amplitude of the N200 component during both extremely high and extremely low FOK judgment ratings at parietal electrodes but not at frontal or central/fronto-central electrodes, with this effect being more pronounced for extremely low FOK judgments. These results confirm previous findings indicating a role for parietal areas in FOK and also support the hypothesis (e.g. Fleming et al. 2014) that metacognition is linked to distributed neural substrates rather than strictly to frontal lobe function. Even though we hypothesized that ERP wave forms of high and low FOK judgments would be different, these produced similar ERP wave forms. We can speculate that although there is some overlap between the neural bases of low and high FOK, low and high FOK judgments are primarily related with different brain regions. Specifically, our results showed that the P200 component at frontal, central, and parietal areas are more likely related to high FOK, but the parietal N200 component more likely relates to low FOK. It should also be noted that FOK judgment is task sensitive, and different regions are responsible for different levels of memory confidence, memory conflict, and memory retrieval. Further studies are needed to address the role of frontal and parietal cortices for high and low FOK across different memory tasks.
A negative ERP component (also called N400 and/or old/new effect) which is within 350–450 ms after the stimulus onset was observed at fronto-central and parietal electrodes and low FOK judgments produced bigger N400 amplitude than high FOK judgments. This effect is supported by retrieval processes known as familiarity and recollection. Current evidence indicates that the earlier N400 component is related to a familiarity process (especially “know” response) while the later parietal old/new component is related to a recollection process (especially “remember” responses) (Curran 2000; Curran and Dien 2003; Düzel et al. 1997; Rugg and Curran 2007, for a review). The familiarity processes between 300 and 500 ms were associated with frontal and prefrontal brain regions. On the other hand, recollection processes were the most prominent between 400 and 800 ms and correlated with activation in different regions including temporal, parietal and occipital regions (e.g. Diana et al. 2005; Herzmann et al. 2012). A number of studies (Kim 2010; Rugg and Curran 2007; Rugg and Nagy 1989) have concluded that familiarity-based recognition is fast and relatively automatic. However, recollection is conceived as a slower, more effortful and conscious process. In our study, FOK judgments elicited this negative late component and this pattern is consistent with the proposal that the mid-frontal effect is linked to familiarity-based recognition (Rugg and Curran 2007; for review, Stenberg et al. 2009; Voss and Paller 2009).
In addition, a negative slow wave at F3 and FC3 electrodes and positive slow wave at F4 and FC4 electrodes (within 500–800 ms after the stimulus onset) were observed during FOK judgments, and high FOK judgments elicited bigger amplitude than low FOK judgments. This component has been also recorded previous episodic memory studies (Rugg and Wilding 2000; Senkfor and Van Petten 1998; Trott et al. 1999; Van Petten et al. 2000) in which subjects were required to retrieve episodic information about the study context of recognized items have that implied an additional old/new effect. This effect starting around 500 ms and often last until the end of the recording period. This effect has been linked to post-retrieval processes (Allan et al. 1998; Mecklinger 1998), monitoring and verification processes of memorized items (Rugg and Wilding 2000). In conclusion, we argued that FOK decision is an ongoing process and different (but interrelated) ERP responses (in here three ERPs) emerged during this process. We also argued that these ERPs related with distinct aspect of FOK decision. Specifically, we found that ERP correlates of FOK judgments emerge as rapidly as 200 ms following presentation of a stimulus and FOK judgment is based on perceptual fluency processes. After the FOK judgment, individuals make second attempt around 400 ms about familiarity of episodic information and lastly, monitor and verify the decision has been made around 700–800 ms. Our results provide direct evidence for distinct neurobiological correlates that may reflect metacognitive processing during episodic FOK judgments. These results should be examined further in future studies using different memory tasks. However, there were some limitations to the present study. Firstly, the procedures used were chosen to reflect standard, frequently-used FOK paradigms. However, the use of a 6-point Likert-type measure to assess FOK may not be the best scale. Although we attempted to minimize this issue by only analyzing extreme scores, a different scale (e.g. yes, no, and don’t know) for assessing FOK may be more appropriate. Secondly, in line with previous studies, there was no time limit for any phase of the experiment. The impact of time-stress on decision making is not unique and the results may not be similar to other causes of psychological stress (i.e., anxiety). Ariely and Zakay (2001) argued that the role of duration should be considered separately in two aspects of behavior: encoding and making choices. In the case of making choices, the role of duration should be very different. When making decisions about future events, decision makers take into account the expected intensity and duration (for detail see, Ariely et al. 2000). Time limitation may have yielded different findings in terms of level of FOK judgments. The use of a different study design may help to address these issues.
Acknowledgements
This research was supported by The Scientific and Technological Research Council of Turkey (Grant No. 112K072). We thank Georg Northoff and two reviewers for their helpful comments on a previous version of the manuscript. We also thank Katie Peterson for proofreading.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Metehan Irak, Phone: +90-212-381 0449, Email: metehan.irak@eas.bau.edu.tr.
Can Soylu, Email: can.soylu@eas.bau.edu.tr.
Gözem Turan, Email: gozem.turan@eas.bau.edu.tr.
Dicle Çapan, Email: diclecapan@gmail.com.
References
- Addante RJ, Ranganath C, Olichney J, Yonelinas AP. Neurophysiological evidence for a recollection impairment in amnesia patients that leaves familiarity intact. Neuropsychologia. 2012;50(13):3004–3014. doi: 10.1016/j.neuropsychologia.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addante RJ, Ranganath C, Yonelinas AP. Examining ERP correlates of recognition memory: evidence of accurate source recognition without recollection. NeuroImage. 2012;62(1):439–450. doi: 10.1016/j.neuroimage.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan K, Wilding EL, Rugg M. Electrophysiological evidence for dissociable processes contributing to recollection. Acta Psychol. 1998 doi: 10.1016/s0001-6918(97)00044-9. [DOI] [PubMed] [Google Scholar]
- Ariely D, Zakay D. A timely account of the role of duration in decision making. Acta Physiol. 2001;108(2):187–207. doi: 10.1016/s0001-6918(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Ariely D, Kahneman D, Loewenstein G. Joint commentary on the importance of, duration in ratings of and choices between, sequences of outcomes. J Exp Psychol Gen. 2000;129:524–529. doi: 10.1037//0096-3445.129.4.524. [DOI] [PubMed] [Google Scholar]
- Bonini F, Burle B, Lieǵeois-Chauvel C, Reǵis J, Chauvel P, Vidal F. Action monitoring and medial frontal cortex: leading role of supplementary motor area. Science. 2014;343(6173):888–891. doi: 10.1126/science.1247412. [DOI] [PubMed] [Google Scholar]
- Busey TA, Tunnicliff J. Accounts of the confidence-accuracy relation in recognition memory. Most. 2000;7(1):26–48. doi: 10.3758/bf03210724. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Understanding metamemory: neural correlates of the cognitive process and subjective level of confidence in recognition memory. NeuroImage. 2006;29(4):1150–1160. doi: 10.1016/j.neuroimage.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Chua E, Schacter DL, Sperling R. Neural correlates of metamemory. J Cognitive Neurosci. 2009;21(9):1751–1765. doi: 10.1162/jocn.2009.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EF, Pergolizzi D, Weintraub RR. The cognitive neuroscience of metamemory monitoring: understanding metamemory processes, subjective levels expressed, and metacognitive accuracy. In: Fleming SM, Frith CD, editors. The cognitive neuroscience of metacognition. Berlin: Springer; 2014. pp. 267–291. [Google Scholar]
- Curran T. Brain potentials of recollection and familiarity. Mem Cognit. 2000;28(6):923–938. doi: 10.3758/bf03209340. [DOI] [PubMed] [Google Scholar]
- Curran T. Effects of attention and confidence on the hypothesized ERP correlates of recollection and familiarity. Neuropsychologia. 2004;42(8):1088–1106. doi: 10.1016/j.neuropsychologia.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Curran T, Dien J. Differentiating amodal familiarity from modality-specific memory processes: an ERP study. Psychophysiology. 2003;40:979–988. doi: 10.1111/1469-8986.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc B Biol Sci. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Diana RA, Vilberg KL, Reder LM. Identifying the ERP correlate of a recognition memory search attempt. Cognitive Brain Res. 2005;24(3):674–684. doi: 10.1016/j.cogbrainres.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Lam ATA, Axmacher N, Fell J, Staresina BP, Gauggel S, Wagner T, et al. Monitoring the mind: the neurocognitive correlates of metamemory. PLoS ONE. 2012 doi: 10.1371/journal.pone.0030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty MRP. Integration of the ecological and error models of overconfidence using a multiple-trace memory model. J Exp Psychol Gen. 2001;130(4):579–599. doi: 10.1037//0096-3445.130.4.579. [DOI] [PubMed] [Google Scholar]
- Doyle MC, Rugg MD, Wells T. A comparison of the electrophysiological effects of formal and repetition priming. Psychophysiology. 1996;33(2):132–147. doi: 10.1111/j.1469-8986.1996.tb02117.x. [DOI] [PubMed] [Google Scholar]
- Dunn BR, Dunn DA, Languis M, Andrews D. The relation of ERP components to complex memory processing. Brain Cognit. 1998;36(3):355–376. doi: 10.1006/brcg.1998.0998. [DOI] [PubMed] [Google Scholar]
- Düzel E, Yonelinas AP, Mangun GR, Heinze HJ, Tulving E. Event-related brain potential correlates of two states of conscious awareness in memory. Proc Natl Acad Sci USA. 1997;94(May):5973–5978. doi: 10.1073/pnas.94.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M, Mazza V. Electrophysiological correlates of change detection. Psychophysiology. 2005;42(3):328–342. doi: 10.1111/j.1469-8986.2005.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Klostermann EC, Marian DE, Verstaen A, Shimamura AP. Neural correlates of metacognitive monitoring during episodic and semantic retrieval. Cognitive Affect Behav Neurosci. 2012;12(3):599–609. doi: 10.3758/s13415-012-0096-8. [DOI] [PubMed] [Google Scholar]
- Evans KM, Federmeier KD. The memory that’s right and the memory that’s left: event-related potentials reveal hemispheric asymmetries in the encoding and retention of verbal information. Neuropsychologia. 2007;45(8):1777–1790. doi: 10.1016/j.neuropsychologia.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque D, Baird JA, Posner MI. Executive attention and metacognitive regulation. Conscious Cognit. 2000;9(2):288–307. doi: 10.1006/ccog.2000.0447. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Lau HC. How to measure metacognition. Front Hum Neurosci. 2014;8:1–3. doi: 10.3389/fnhum.2014.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Ryu J, Golfinos JG, Blackmon KE. Domain-specific impairment in metacognitive accuracy following anterior prefrontal lesions. Brain. 2014;137(10):2811–2822. doi: 10.1093/brain/awu221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell M, Metcalfe J, Tsapkani K. In the mind but not on the tongue: feeling of knowing in an anomic patient. In: Reder LM, editor. Implicit memory and metacognition Erlbaum. Hillsdale, NJ: Erlbaum; 1996. pp. 171–194. [Google Scholar]
- Garland R (1991) The mid-point on a rating scale: is it desirable. Mark Bull 2(1):66–70. https://doi.org/citeulike-article-id:4775464
- Glucksberg S, McCloskey M. Decisions about ignorance: knowing that you don’t know. J Exp Psychol Hum Learn Mem. 1981;7(5):311–325. [Google Scholar]
- Göz I. Yazılı Türkçe’nin kelime sıklığı sözlüğü. Ankara: TDK Yayınları; 2003. [Google Scholar]
- Hart JT. Memory and the feeling-of-knowing experience. J Educ Psychol. 1965;56(4):208–216. doi: 10.1037/h0022263. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: a functional MRI test of the monitoring hypothesis. Brain. 1999;122(7):1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Dolan RJ. Confidence in recognition memory for words: dissociating right prefrontal roles in episodic retrieval. J Cognitive Neurosci. 2000;12(6):913–923. doi: 10.1162/08989290051137468. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Dunlosky J, Sinclair SM. Episodic feeling-of-knowing resolution derives from the quality of original encoding. Mem Cognit. 2010;38(6):771–784. doi: 10.3758/MC.38.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzmann G, Jin M, Cordes D, Curran T. A within-subject ERP and fMRI investigation of orientation-specific recognition memory for pictures. Cognitive Neurosci. 2012;3(3–4):174–192. doi: 10.1080/17588928.2012.669364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham PA, Zawadzka K, Hanczakowski M. Internal mapping and its impact on measures of absolute and relative metacognitive accuracy. In: Dunlosky J, Tauber S, editors. Oxford handbook of metamemory. Oxford: Oxford University Press; 2016. [Google Scholar]
- Hilgenstock R, Weiss T, Witte OW. You’d better think twice: post-decision perceptual confidence. NeuroImage. 2014;99:323–331. doi: 10.1016/j.neuroimage.2014.05.049. [DOI] [PubMed] [Google Scholar]
- Ivory S-J, Knight R, Longmore B, Caradoc-Davies T. Verbal memory in non-demented patients with idiopathic Parkinsons disease. Neuropsychologia. 1999;37(7):817–828. doi: 10.1016/s0028-3932(98)00131-6. [DOI] [PubMed] [Google Scholar]
- Jang Y, Nelson TO. How many dimensions underlie judgments of learning and recall? Evidence from state-trace methodology. J Exp Psychol Gen. 2005;134(3):308–326. doi: 10.1037/0096-3445.134.3.308. [DOI] [PubMed] [Google Scholar]
- Jing L, Niki K, Xiaoping Y, Yue L. Knowing that you know and knowing that you don’t know: a fMRI study on feeling-of-knowing (FOK) Acta Psychol Sin. 2004;36:426–433. [Google Scholar]
- Kikyo H, Miyashita Y. Temporal lobe activations of “feeling-of-knowing” induced by face-name associations. NeuroImage. 2004;23(4):1348–1357. doi: 10.1016/j.neuroimage.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Kikyo H, Ohki K, Miyashita Y. Neural correlates for feeling-of-knowing: an fMRI parametric analysis. Neuron. 2002;36(1):177–186. doi: 10.1016/s0896-6273(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. NeuroImage. 2010;50(4):1648–1657. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- Kim H, Cabeza R. Trusting our memories: dissociating the neural correlates of confidence in veridical versus illusory memories. J Neurosci. 2007;27(45):12190–12197. doi: 10.1523/JNEUROSCI.3408-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin CM, Guzmán AE, Levine WH. Knowing that you don’t know: metamemory and discourse processing. J Exp Psychol Learn Mem Cognit. 1997;23(6):1378–1393. doi: 10.1037//0278-7393.23.6.1378. [DOI] [PubMed] [Google Scholar]
- Koriat A. How do we know that we know? The accessibility model of the feeling of knowing. Psychol Rev. 1993;100(4):609–639. doi: 10.1037/0033-295x.100.4.609. [DOI] [PubMed] [Google Scholar]
- Koriat A. Dissociating knowing and the feeling of knowing: further evidence for the accessibility model. J Exp Psychol Gen. 1995;124(3):311–333. [Google Scholar]
- Koriat A. Metamemory and Consciousness. In: Thompson E, Zelazo PD, editors. The Cambridge handbook of consciousness. Cambridge: Cambridge University Press; 2007. pp. 289–326. [Google Scholar]
- Koriat A, Goldsmith M. Monitoring and control processes in the strategic regulation of memory accuracy. Psychol Rev. 1996;103(3):490–517. doi: 10.1037/0033-295x.103.3.490. [DOI] [PubMed] [Google Scholar]
- Koriat A, Levy-Sadot R. The combined contributions of the cue-familiarity and accessibility heuristics to feelings of knowing. J Exp Psychol Learn Mem Cognit. 2001;27(1):34–53. [PubMed] [Google Scholar]
- Koriat A, Lieblich I. A study of memory pointers. Acta Physiol. 1977;41(2–3):151–164. [Google Scholar]
- Krinsky R, Nelson TO. The feeling of knowing for different types of retrieval failure. Acta Psychol. 1985;58(2):141–158. doi: 10.1016/0001-6918(85)90004-6. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Müller-Oehring EM, Kwon D, Serventi MR, Pfefferbaum A, Sullivan EV. Differential compromise of prospective and retrospective metamemory monitoring and their dissociable structural brain correlates. Cortex. 2016;81:192–202. doi: 10.1016/j.cortex.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre CD, Marchand Y, Eskes GA, Connolly JF. Assessment of working memory abilities using an event—related brain potential (ERP)—compatible digit span backward task. Clin Neurophysiol. 2005;116(7):1665–1680. doi: 10.1016/j.clinph.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: a user’s guide. New York: Lawrence Erlbaum Associates; 2005. [Google Scholar]
- Maniscalco B, Lau H. A signal detection theoretic approach for estimating metacognitive sensitivity from confidence ratings. Conscious Cognit. 2012;21(1):422–430. doi: 10.1016/j.concog.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Maril A, Simons JS, Mitchell JP, Schwartz BL, Schacter DL. Feeling-of-knowing in episodic memory: an event-related fMRI study. NeuroImage. 2003;18(4):827–836. doi: 10.1016/s1053-8119(03)00014-4. [DOI] [PubMed] [Google Scholar]
- Maril A, Simons JS, Weaver JJ, Schacter DL. Graded recall success: an event-related fMRI comparison of tip of the tongue and feeling of knowing. NeuroImage. 2005;24(4):1130–1138. doi: 10.1016/j.neuroimage.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Matell MS, Jacoby J. Is there an optimal number of alternatives for Likert-scale items? Effects of testing time and scale properties. J Appl Psychol. 1972;56(6):506–509. [Google Scholar]
- McCurdy LY, Maniscalco B, Metcalfe J, Liu KY, de Lange FP, Lau H. Anatomical coupling between distinct metacognitive systems for memory and visual perception. J Neurosci. 2013;33(5):1897–1906. doi: 10.1523/JNEUROSCI.1890-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklinger A. On the modularity of recognition memory for object form and spatial location: a topographic ERP analysis. Neuropsychologia. 1998;36(5):441–460. doi: 10.1016/s0028-3932(97)00128-0. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Dunlosky J. Metamemory. In: Roediger HL, editor. Learning and memory: a comprehensive reference. Oxford: Elsevier; 2008. pp. 349–362. [Google Scholar]
- Metcalfe J, Schwartz BL. The ghost in the machine: self-reflective consciousness and the neuroscience of metacognition. In: Dunlosky J, Tauber SK, editors. The Oxford handbook of metamemory. New York: Oxford University Press; 2016. pp. 407–424. [Google Scholar]
- Metcalfe J, Schwartz BL, Joaquim SG. The cue-familiarity heuristic in metacognition. J Exp Psychol Learn Mem Cognit. 1993;19(4):851–861. doi: 10.1037//0278-7393.19.4.851. [DOI] [PubMed] [Google Scholar]
- Modirrousta M, Fellows LK. Medial prefrontal cortex plays a critical and selective role in “feeling of knowing” meta-memory judgments. Neuropsychologia. 2008;46(12):2958–2965. doi: 10.1016/j.neuropsychologia.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Montaldi D, Mayes AR. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus. 2010;20(11):1291–1314. doi: 10.1002/hipo.20853. [DOI] [PubMed] [Google Scholar]
- Moritz S, Gläscher J, Sommer T, Büchel C, Braus DF. Neural correlates of memory confidence. NeuroImage. 2006;33(4):1188–1193. doi: 10.1016/j.neuroimage.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Nelson T. A comparison of current measures of the accuracy of feeling of knowing predictions. Psychol Bull. 1984;95(1):109–133. [PubMed] [Google Scholar]
- Nelson TO, Narens L. Norms of 300 general-information questions: accuracy of recall, latency of recall, and feeling-of-knowing ratings. J Verbal Learn Verbal Behav. 1980;19(3):338–368. [Google Scholar]
- Nelson T, Narens L. Metamemory: a theoretical framework and new findings. Psychol Learn Motiv. 1990;26(July):125–173. [Google Scholar]
- Pannu JK, Kaszniak AW. Metamemory experiments in neurological populations: a review. Neuropsychol Rev. 2005 doi: 10.1007/s11065-005-7091-6. [DOI] [PubMed] [Google Scholar]
- Paynter CA, Reder LM, Kieffaber PD. Knowing we know before we know: ERP correlates of initial feeling-of-knowing. Neuropsychologia. 2009;47(3):796–803. doi: 10.1016/j.neuropsychologia.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotin A, Belleville S, Isingrini M. Metamemory monitoring in mild cognitive impairment: evidence of a less accurate episodic feeling-of-knowing. Neuropsychologia. 2007;45(12):2811–2826. doi: 10.1016/j.neuropsychologia.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Reder LM. Strategy selection in question answering. Cognitive Psychol. 1987;19(1):90–138. [Google Scholar]
- Reder LM, Ritter FE. What determines initial feeling of knowing? Familiarity with question terms, not with the answer. J Exp Psychol Learn Mem Cognit. 1992;18(3):435–451. [Google Scholar]
- Reder LM, Nhouyvanisvong A, Schunn CD, Ayers MS, Angstadt P, Hiraki K. A mechanistic account of the mirror effect for word frequency: a computational model of remember-know judgments in a continuous recognition paradigm. J Exp Psychol Learn Mem Cognit. 2000;26(2):294–320. doi: 10.1037//0278-7393.26.2.294. [DOI] [PubMed] [Google Scholar]
- Reggev N, Zuckerman M, Maril A. Are all judgments created equal? An fMRI study of semantic and episodic metamemory predictions. Neuropsychologia. 2011;49(5):1332–1342. doi: 10.1016/j.neuropsychologia.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cognitive Sci. 2007;11(6):251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Doyle MC. Event-related potentials and stimulus repetition in direct and indirect tests of memory. In: Heinze HJ, Münte TF, Mangun GR, editors. Cognitive electrophysiology. Boston, MA: Birkhäuser; 1994. pp. 124–148. [Google Scholar]
- Rugg MD, Nagy ME. Event-related potentials and recognition memory for words. Electroen Clin Neuro. 1989;72(5):395–406. doi: 10.1016/0013-4694(89)90045-x. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Nieto-Vegas M. Modality-specific effects of immediate word repetition: electrophysiological evidence. NeuroReport. 1999;10(12):2661–2664. doi: 10.1097/00001756-199908200-00041. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Wilding EL. Retrieval processing and episodic memory. Trends Cognitive Sci. 2000 doi: 10.1016/s1364-6613(00)01445-5. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Verfaellie M, Alexander MP, LaFleche G, Nicholls L, Kaszniak AW. A role for right medial prefrontal cortex in accurate feeling-of-knowing judgments: evidence from patients with lesions to frontal cortex. Neuropsychologia. 2004;42(7):957–966. doi: 10.1016/j.neuropsychologia.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Schnyer DM, Nicholls L, Verfaellie M. The role of VMPC in metamemorial judgments of content retrievability. J Cognitive Neurosci. 2005;17(5):832–846. doi: 10.1162/0898929053747694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber TA, Nelson DL. The relation between feelings of knowing and the number of neighboring concepts linked to the test cue. Mem Cognit. 1998;26(5):869–883. doi: 10.3758/bf03201170. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Metcalfe J. Cue familiarity but not target retrievability enhances feeling-of-knowing judgments. J Exp Psychol Learn Mem Cognit. 1992;18(5):1074–1083. doi: 10.1037//0278-7393.18.5.1074. [DOI] [PubMed] [Google Scholar]
- Senkfor AJ, Van Petten C. Who said what? An event-related potential investigation of source and item memory. J Exp Psychol Learn Mem Cognit. 1998;24(4):1005–1025. doi: 10.1037//0278-7393.24.4.1005. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Squire LR. Memory and metamemory. a study of the feeling-of-knowing phenomenon in amnesic patients. J Exp Psychol Learn Mem Cognit. 1986;12(3):452–460. doi: 10.1037//0278-7393.12.3.452. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4(8):637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Mazuz YS, Berryhill ME, Olson IR. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cereb Cortex. 2010;20(2):479–485. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souchay C, Isingrini M, Gil R. Alzheimer’s disease and feeling-of-knowing in episodic memory. Neuropsychologia. 2002;40(13):2386–2396. doi: 10.1016/s0028-3932(02)00075-1. [DOI] [PubMed] [Google Scholar]
- Souchay C, Moulin CJA, Clarys D, Taconnat L, Isingrini M. Diminished episodic memory awareness in older adults: evidence from feeling-of-knowing and recollection. Conscious Cognit. 2007;16(4):769–784. doi: 10.1016/j.concog.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Stenberg G, Hellman J, Johansson M, Rosén I. Familiarity or conceptual priming: event-related potentials in name recognition. J Cognitive Neurosci. 2009;21(3):447–460. doi: 10.1162/jocn.2009.21045. [DOI] [PubMed] [Google Scholar]
- Thomas AK, Bulevich JB, Dubois SJ. An analysis of the determinants of the feeling of knowing. Conscious Cognit. 2012;21(4):1681–1694. doi: 10.1016/j.concog.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Trott CT, Friedman D, Fabiani M, Snodgrass JG, Ritter W, Fabiani M, Snodgrass JG. Episodic priming and memory for temporal source: event-related potentials reveal age-related differences in prefrontal functioning. Psychol Aging. 1999;14(3):390–413. doi: 10.1037//0882-7974.14.3.390. [DOI] [PubMed] [Google Scholar]
- Tuna Ş, Tekcan AI, Topçuoǧlu V. Memory and metamemory in obsessive–compulsive disorder. Behav Res Ther. 2005;43(1):15–27. doi: 10.1016/j.brat.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Senkfor AJ, Newberg WM. Memory for drawings in locations: spatial source memory and event-related potentials. Psychophysiology. 2000;37(4):551–564. [PubMed] [Google Scholar]
- Vernon D, Usher M. Dynamics of metacognitive judgments: pre- and postretrieval mechanisms. J Exp Psychol Learn Mem Cognit. 2003;29(3):339–346. doi: 10.1037/0278-7393.29.3.339. [DOI] [PubMed] [Google Scholar]
- Voss JL, Paller KA. An electrophysiological signature of unconscious recognition memory. Nat Neurosci. 2009;12(3):349–355. doi: 10.1038/nn.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cognitive Sci. 2005 doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wokke M, Cleeremans A, Ridderinkhof KR. Sure I’m sure: prefrontal oscillations support metacognitive monitoring of decision-making. J Neurosci. 2017;37(4):781–789. doi: 10.1523/JNEUROSCI.1612-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J Exp Psychol Learn Mem Cognit. 1994;20(6):1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]