Abstract
Stress-induced major depression and mood disorders are characterized by behavioural abnormalities and psychiatric illness, leading to disability and immature mortality worldwide. Neurobiological mechanisms of stress and mood disorders are discussed considering recent findings, and challenges to enhance pharmacological effects of antidepressant, and mood stabilizers. Pharmacological enhancement of ketamine and scopolamine regulates depression at the molecular level, increasing synaptic plasticity in prefrontal regions. Blood-derived neurotrophic factors facilitate mood-deficit symptoms. Epigenetic factors maintain stress-resilience in hippocampal region. Regulation of neurotrophic factors blockades stress, and enhances neuronal survival though it paralyzes limbic regions. Molecular agents and neurotrophic factors also control behavioral and synaptic plasticity in addiction and stress disorders. Future research on neuronal dynamics and cellular actions can be directed to obtain the etiology of synaptic dysregulation in mood disorder and stress. For the first time, the current review contributes to the literature of synaptic plasticity representing the role of epigenetic mechanisms and glucocorticoid receptors to predict depression and anxiety in clinical conditions.
Keywords: Neural plasticity, Mood disorder, Ketamines, Chronic stress, Neurotrophic factor, Epigenetic
Introduction
Stress-induced major depressive (SMD) and major mood disorder (MMD) are leading cause of illness among children and adults worldwide. These prevail lifetime of 17% of the population across the globe (Kessler et al. 2005). World Health Organization estimates that MMD will be the main cause of disability by 2020. MMD also exacts a very large economic burden on society, with an estimated cost of $210.5 billion annually for the treatment expenses (Greenberg et al. 2015). In addition, 23% of suicide victims are due to lack of antidepressant treatment during acute phase of depression (Karch et al. 2009). Developments of depressions, anxiety and mood disorders are prevalent among children who are victims of negligence and maltreatment. Stressful experience in early life has a long-lasting effect on brain development and capacity to respond on later stressful challenges, as the brain adversely regulates neuroendocrine, autonomic, metabolic and immune system functions (Anda et al. 2010). These vulnerabilities indicate the limitation of antidepressant treatment, long response time, and rate of drug efficacy (Gerhard et al. 2016). Most of the patients with mood disorders show the behavioural abnormalities and psychiatric illness that are manifested in cognitive dysfunction and memory loss. Development of anti-depressant drugs in combination with a psychotherapeutic solution with response to stress and mood disorders has been a great challenge for neuroscientists from last two decades.
Underlying epigenetic mechanisms considering the DNA methylation in genes are associated with increased vulnerability for the development of stressed-induced depression. Methylation in the serotonin transporter (SLC6A4), brain-derived neurotrophic factor (BDNF), glucocorticoid receptor (NR3C1), and FK506 binding protein (FKBP5) genes are found to predict depression and anxiety in clinical samples (Bagot et al. 2014). These genes act in response to stress, mood disorder and depression following the development of synaptic plasticity (Weder et al. 2014). Currently, the blockage of chemo-neural singling pathways by low-neurotrophic factors has been considered as an underlying factor for depression, and mood disorder (Autry and Monteggia 2012). Thus, the promotional signalling of neurotrophins including nerve growth factor (NGF) and brain derived neurotrophic factor (BDNF) is strongly linked with antidepressant response to TrkB receptors in limbic regions (Castrén and Kojima 2017). Further it is observed that stressed-related atrophy in limbic region of the brain reduces the brain volume, causes loss of neurons and glia, and disrupts synapse functions of patients with depression and mood disorders (Karatsoreos and McEwen 2013; Duman and Aghajanian 2012). Neurogenesis is needed for antidepressant treatment, depression pathogenesis, and hypothalamic–pituitary–adrenal (HPA) axis regulation (David et al. 2009). The synaptic number and functioning of neurons are decreased in the pre-frontal cortex (PFC) and hippocampal regions in patients with depression and mood disorder. BDNF, vascular-endothelial growth factor (VEGF), and insulin-like growth factor-1 (IGF1) are required for the formation and maintenance of synaptic connections and neural plasticity in CNS. Specifically, BDNF couple with glucocorticoids facilitates synaptic plasticity following morphological and molecular changes by which depression and anxiety deficit symptoms are ameliorated (Nestler et al. 2002). Degradation and inhibition of nuclear factor-B (NF-B) are due to the performance of I_55B kinases (IKK) acts to phosphorylate I_B that plays a significant role in synaptic plasticity to regulate neuronal morphology (Gutierrez et al. 2005). In addition, IKK–NF_B signaling pathway enriches hippocampal neuroplasticity, regulating memory consolidation. The availabilities of receptors in the surface of the membrane and the releasable pool (RRP) of synaptic vesicles (SVs) are some of the genetic factors maintaining α-amino-3-hydroxy-5- methyl- 4- isoxazolepropionic acid (AMPAergic) and γ-amino butyric acid type-A (GABAAergic) synaptic strength. BLA-ventral hippocampus (vHPC) circuit is also identified in the context of anxiety-related social interaction, using optogenetic manipulations (Allsop et al. 2014). Social loss induces post-traumatic stress disorders (PSDs), resulting in a functional increase of mini-excitatory post-synaptic current (mEPSCs) in NAc-MSNs. Long-term-potentiation (LTP) during in vitro procedure alters place cell stability and impedes the consolidation of spatial memory in patients with stress and mood disorders. Most of these epigenomic analyses involve molecular and cellular circuits to identify biomarkers for stress and mood disorders considering neuroendocrine, immune and central nervous systems (LaSalle 2011). The function of neural circuits is altered by low concentrations of cocaine, whereas the presence of high concentrations of cocaine directly blocks Na+ and K+. Overall, cocaine increases the levels of extracellular dopamine activating dopamine receptor (DAR). Changes in synaptic and intrinsic excitability interact with the shape of final nucleus accumbens (NAc)-medium spiny neurons (MSN) output, activating the neural circuit and cocaine-seeking behavior. So far, ketamine including non-selective N-methyl-d-aspartate (NMDA) receptors stands out as a potential agent for the treatments of stress and mood disorder resilience, enhancing antidepressant effects in behaviours and synaptic plasticity (Subhani et al. 2018). Treatment of chronic stress and mood disorder with various antidepressant drugs increases BDNF mRNA at various protein levels in the limbic region (Calabrese et al. 2011). Ketamine, as an antidepressant and a biomarker for mood disorders, promotes neuronal plasticity-related proteins, neurogenesis, synaptogenesis, neural survival and axon elongation (Castrén 2013). It is further observed that many genomic regions are involved in the formation of neural circuitry projection, functioning, and plasticity, following the improvement of neuron structures and neuro-developmental processes (Roberson-Nay et al. 2018). For example, the cell–cell adhensions genes (e.g., CDHs, PCDHAs, PCDHA1C/2C) and genes associated with mood and psychiatric disorders (e.g., HDAC4, NRG1) influence the neural structure and plasticity in DNAm regions. Maresin 1 (MAR1) that is extracted from docosahexaenoic acid biosynthesized improves insulin resistance (Jung et al. 2018). In presence of depression, MAR1 ameliorates inflammation and insulin resistance. It also stimulates AMP-activated protein kinase (AMPK), which enhances ER stress.
Exploring these neurobiological and epigenetic mechanisms of stress and mood disorders, present review offers the recent findings, current problems and new challenges for promoting synaptic plasticity and antistrophic effects to minimize mental deficits and disorders. An attempt is made here to underlie pharmacological and pathophysiological effects in order to establish antidepressant agents, mood stabilizers and neuroleptic agents for stress and mood disorders, incorporating balanced neural plasticity.
Behavioural and neural mechanism
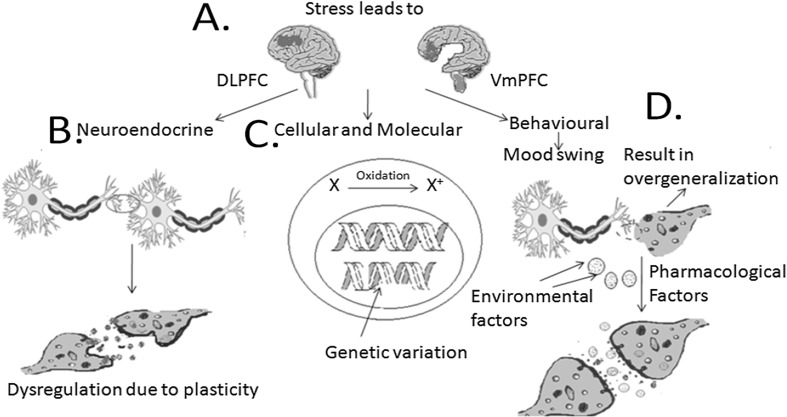
Acute stress is induced during the stress condition considering the alternation of limbic structures and the blockage of neural, molecular and metabolic mechanisms. Stress-related illness alters atrophy of limbic brain structures and reduces the whole brain structures including neural plasticity in patients with depression and mood disorder (Karatsoreos and McEwen 2013). Analysis of CREB and BDNF has shown the decrement of CREB providing evidence with a loss of neural plasticity in patients with depression (Dwivedi et al. 2003). Environmental challenges adopt an organism to exhibit resilience and vulnerability at the level of the nervous system in brain. Neural activity during stress provides neural plasticity for indicating resilient coping (Sinha et al. 2016). A significant level of endocrine increases when subjects are exposed to highly stressful, violent stimuli, and temporally distinct patterns of neural activation. Acute functional neuroplasticity during stress, with distinct and separable brain networks, underlie critical components of the stress response defining a role of VmPFC neuro-flexibility in stress-resilient coping, as shown in Fig. 1. This happens when brain limbi-striatal activation is increased during stress whereas the reduction is noticed in ventromedial prefrontal (VmPFC) and dorsolateral prefrontal cortex (DLPFC). VmPFC is one of the critical loci of neuroplasticity in a resilience-coping network in which signals are increased due to emotional, behavioral control and active coping. Childhood trauma, cumulative adversity, and a history of mood disorders or posttraumatic stress disorder (PTSD) are associated with blunted VmPFC activation during emotion or stress exposure, disrupted VmPFC connectivity with amygdala and poor adaptive coping (Seo et al. 2014; Gee et al. 2013). DLPFC plays a significant role for the interpretation of multiple brain disorders due to its vulnerability to stress and ageing (McEwen and Morrison 2013). It is the region dedicated to high interconnectivity between sensory and motor associated regions of the brain. It directs “formation of coherent behavioral sequences towards the attainment of goals” (Fuster 2008). As DLPFC is exposed to stress, it gets self-modulated along-with other areas of the attentional network. At the same time, larger impairment of attentional shifting can be linked with attenuated functional coupling between DLPFC and areas of premotor and posterior parietal cortex. Mood-related circuitries are disrupted in depression by following the reduction of the prefrontal cortex (PFC) and hippocampus volume. Volume reduction is correlated with length of illness and time of treatment of depression, exposure to stress that causes atrophy, loss of neurons and glia in the PFC and hippocampus regions (Duman and Aghajanian 2012). Number and function of the synapse are disrupted in the case of a patient with depression, as referred in Fig. 1.
Fig. 1.
Induced stress factors deregulate the structural remodelling of brain considering synaptic modifiers such as neurotropins and glucocorticoids disrupt the cognitive ability and decreases the DLPFC and VmPFC region functioning (a). Neural plasticity occurs, b due to dysregulation in the pre- and post-synaptic neural receptor modelling, c due to oxidative stress: in which unwanted oxidation of ions causes malfunctioning of transcription process resulting in genetic variation. d Due to pharmacological and environmental factors:several drugs-abuses (like cocaine) affects synaptic NAc MSN excitability, resulting in the development and expression of addictive plasticity as receptors becomes familiar to these external agents and overgeneralize them as self or natural pathway mediator
Synaptic plasticity that represents the functions of brain including the ability to sense, access and store information, is disrupted due to depression and mood disorder (Holtmaat et al. 2009). Synaptogenesis regulates an interaction between signaling pathways, disrupting key neural pathways in the context of depression (Duman and Aghajanian 2012). Reduction in synaptic plasticity occurs due to HFD-induced diabetes and insulin resistance causing neuronal atrophy in cortical and limbic structures (Arnold et al. 2014; Stranahan et al. 2008; Anisman 2009). Consumption of an HFD causes insulin resistance in cortical and limbic structures decreasing insulin-dependent stimulation of Akt, S6 kinase (S6 K), glycogen synthase kinase (GSK)-3β and mTORC1 signalling (Arnold et al. 2014). The role of functional connectivity of the neurons in hippocampal, cortical, reward, and serotonergic circuits is to maintain stress resilience and vulnerability (Franklin et al. 2012; Arnold and Betsholtz 2013). Epigenomic analyses (LaSalle 2011; Autry et al. 2011) or optogenetic neural activation or silencing (Mei and Zhang 2012) help to identify the involvement of molecular and cellular circuits of stress and mood disorder condition. In the domain of resilience, the normal physiological and behavioural mechanisms are adapted to suppress psychological stress (Pfau and Russo 2015; Bazak et al. 2009); Cohen and Janicki-Deverts 2009). Functional roles of the neuroendocrine, immune, and central nervous systems lead to behavioural resilience to stress. Therefore, resilience is considered an integrated process by which adaptive mechanisms in multiple systems promote and develop personalized science and medicine to combat stress. Indeed this description provides potential targets to develop biomarkers, specific to the type of stress (developmental vs. adulthood), sex, and inflammatory state. It is observed that women are more likely to develop mood disorder than men (Kessler 2003). Continuing identification of sex-based pro-resilience markers enables the development of more effective sex specific treatments, by which women can recover from mood disorders (Hwang et al. 2018). Energy metabolism regulates AMPK, which is a nodal regulator of mTORC1 signalling and synaptic protein synthesis. BDNF has a central role in energy metabolism and cellular respiration (Markham et al. 2012; Marosi and Mattson 2014).
Metabolic disorders are associated with elevated rates of depression and risk factors which contribute to social and traumatic stress (Leone et al. 2012; Luppino et al. 2010; Mansur et al. 2015). Obesity is developed due to the presence of glucocorticoids and inflammatory cytokines. These disorders are linked with the disruption of PFC circuits and neurotransmitter systems involving serotonin, dopamine, endocannabinoids and opioids. Feeding, energy homeostasis, endocrine and neurocrine systems are also influenced by the gut microbiome in the case of healthy behaviour, whereas an imbalanced microbiome-brain interaction is linked to psychiatric illnesses, including depression and anxiety (Mayer et al. 2014; Petra et al. 2015). Excessive caloric intake also increases the risk for uncontrolled excitation of the neuronal activity and stroke, which is mainly due to compromising the integrity of blood–brain-barrier (Kanoski et al. 2010). Changes in synaptic plasticity and neurogenesis enhance with an enriched environment, exercise and learning, while ageing and exposure to drugs effect the neural progenitor cells of the hippocampus. These cells give rise to newborn cells that migrate into the granule cell layer and mature into neurons with the morphological and physiological characteristics of adult granule cells (Van Praag et al. 1999).
Moreover, patients with anxiety have a tone with lost-conditioned, involving the neural modulation of respective stimulus in primary cortices and amygdala. Stimulation representation in sensory organs modulates affective regions. Anxiety patients overgeneralize the stimulus altering the perception in a safe context. Altered neural representations identify neural pathways, participating in affective modulation following the putamen for gain, the ACC for loss, and the amygdala for both gain and loss. Effects are emerged due to fundamental changes in how patients with anxiety and mood disorder perceive the stimulus and to what extent they discriminate it from other stimuli. Affective stimuli induce changes in the representation of the conditional stimulus via amygdala and primary sensory regions, making the subjects more prone to exhibit anxiety symptoms.
Motivation, reward and anxiety are affected by depression (Pasquali 2012; Moulton et al. 2015). Stress, depression and anxiety behaviours are regulated by circulating peptides that include leptin and adiponectin from adipose tissue and ghrelin from the stomach.
Stress and GC-induced sensitization of neuroinflammatory response promote the development of drug addiction, macrophages and microglia. This process undergoes morphological transformation from immature to activated mature with an enlargement of the soma. Hence alterations in neurotransmitters, intracellular signalling, gene transcription, translation and epigenetic changes duly facilitate abnormal functioning of the hypothalamic–pituitary–adrenal (HPA) axis, leading to induced stress (Anacker et al. 2013; Bremner et al. 2003). Glucocorticoid receptor (GR) target gene, serum and glucocorticoid-inducible kinase 1 (SGK1 counteract the cortisol-induced reduction in neurogenesis. Activation of GR gene expression in cortisol increase the power of the SGK1-dependent SGK1 inhibition of the neurogenic Hedgehog pathway by cortisol. Glucocorticoid effects on the brain result in downstream mechanisms causing morphological oligodendrocyte abnormalities by up-regulating SGK1. Decreasing the proliferation of human hippocampal progenitor cells involves GK1 as a key enzyme which reduces the neurogenesis. Glucocorticoid withdrawal leads to the potentiation and maintenance of GR phosphorylation at the serine residues S203 and S211. Neurogenesis is important for antidepressant treatment of SGK1 (David et al. 2009; Chattarji et al. 2015), and HPA axis regulation (Anacker et al. 2013; Bryant 2003); DeRubeis et al. 2008). Neuronal dysfunction and degeneration mechanisms involve lipid peroxidation product 4-hydroxynonenal. These covalently modified proteins result in membrane-associated oxidative stress (Mattson 2009; Calabrese et al. 2009). Ketamine including nonselective NMDA antagonists has been investigated as potential agents for the treatments of stress and mood disorder resilience. Ketamine and other rapid-acting compounds produce rapid-acting effects targeting fast-acting ionotropic glutamate receptors. The metabotropic glutamate receptors (G-protein) have also been examined as potential therapeutic targets for depression.
Role of molecular mechanism for depression
Human brain drops light on the mechanisms of diseases like dysphoria and anguish that uncover several enzymes such as phosphatase to treat depressive behaviours. Mitogen-activated protein kinase (MAPK) upregulates the hippocampal region of a patient diagnosed with depression (Magarin and McEwen 1995).
BDNF has antidepressant effects in behavioural models of depression. Activation of the HPA axis, involving corticotropin-releasing hormone (CRH) receptor-1 (CRHR1) and FK506-binding protein 5 (FKBP5) decreases the synaptic number and functioning of neurons in the PFC and hippocampus regions. GLYX-13 (rapastinel) is a compound that gives a positive effect on hippocampal-dependent learning tasks at the glycine site, containing NMDARs (Zhang et al. 2008; Cohen et al. 1996; Hunter et al. 2015). Postsynaptic endocytosis involves mGluR5- and α1-adrenergic receptor (AR)-dependent LTDs, where α1-AR-initiated LTD mediates modulation of signalling through calcium-permeable AMPA receptors. Stress differentially affects the Gq-receptor-dependent plasticity of excitatory glutamatergic transmission in the BNST, and glutamate-activated Gq-linked signalling pathways. Transfer phosphate groups to serine or threonine residues containing carboxy-terminal proline at one side contribute to the high degree of proline-directed phosphorylation (Huttlin et al. 2010; Lundby et al. 2012). JNKs govern brain morphogenesis and axodendritic architecture during development and regulate important neuron-specific functions such as synaptic plasticity involving NMDA glutamate receptor (NMDAR). This lead to activation, calcium influx and recruitment of AMPARs that lead to long-lasting changes in synaptic transmission and memory formation. The pathophysiological processes of acute brain injuries release signals of brain-derived antigens. Danger-associated molecular patterns (DAMPs) are a set of molecular determinants derived from cellular debris, intracellular proteins, or nuclear DNA/RNA that are released from injured cells following catastrophic events (Hunter et al. 2015). Cytokines are exquisitely sensitive molecules that are produced and released by microglial cells in a context- and cell-dependent fashion. Chemokines are released by Damaged CNS cells, such as macrophage inflammatory protein-1a (MIP-1a or CCL3), monocyte chemotactic protein-1 (MCP-1 or CCL2) (Cousijn et al. 2010; Covington and Miczek 2005), CCL5, or RANTES (Regulated upon Activation, Normal T cell Expressed, and Secreted) (Zaremba et al. 2006; Covington et al. 2011; Dwivedi 2009). These diverse signals trigger peripheral immune cells, causing protective pharmacological and cell-based therapies. The adaptive immune system is exposed to myelin antigens (de Vos et al. 2002; Mecha et al. 2013) which are presented by dendritic cells (DCs) to antigen-specific T cells, resulting in consequent demyelination of the CNS (Dwivedi 2009). Actin dynamics and structural plasticity show the structural remodelling of Cannabinoid Receptor CB1 by regulating the active levels of WAVE1 protein complex in spinal neurons (Njoo et al. 2015). The WAVE proteins act as nucleation promoting factor which further helps in linking upstream signals to the activated actin related protein 2/3 (ARP2/3), and CB1 receptor assembles with GPCR protein in a multiprotein to unfold its biological actions. Synaptic neurotransmission and plasticity are also controlled by membrane-derived bioactive phospholipids triggered at different degrees, loci, and mechanisms of action. Postsynaptic currents induce LPA which is depended on LPA1/Gαi/o-protein/phospholipase C/myosin light chain kinase cascade at the presynaptic site. Receptors present in the surface membrane, and the size of the readily releasable pool (RRP) of synaptic vesicles (SVs) are the important determinants for maintaining afferent AMPAergic and GABAergic synaptic strength (Johnson et al. 2005). Phospholipids act as local messengers in activity-dependent GABAergic STD in a Ca2 + -dependent, spike-independent manner. LPA signalling regulates brain-elemental processing, resulting in MLC phosphorylation, which stimulates the actomyosin contractile apparatus (Moreno-López et al. 2011; Dityatev et al. 2010) and hence underlying STDs due to the reduction in RRP of SVs. Changes in the cytoskeleton of actin are necessary for enabling docking, fusion and exocytosis of SVs with the plasmalemma (Moreno-López et al. 2011; Kourrich et al. 2015; Salazar et al. 2009). Psychological and social factors are responsible for increasing inflammatory cytokines in humans. Introduction of cytokine causes sickness in behaviour with characteristics of depression. Serum levels of the pro-inflammatory cytokines interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF)-α are raised in patients with depression. Antidepressant treatment is used to resume the cytokine levels in a normal manner. Microglia, the brain’s resident innate immune cells produce inflammatory cytokines. Synaptic plasticity via regulation of PI3K-Akt signalling is sustenance by low levels of TNF-α and IL-1β support.
Epigenetic factors associated with stress
Stress-induced behaviours and responses facilitate a higher order relation between the genetic and epigenetic factors. The effects of stress on physiology, brain, and behaviour mediate myriad epigenetic or chromatin remodelling mechanisms, suggesting that one or more HDACs can get dysregulated in the neural circuitries causing repression or silencing of a number of critical genes. Amelioration of the neuropsychiatric diseased condition is done by HDACs which restores the normal histone acetylation level around the affected gene promoters. Psychiatric disorders are involved in the expression of (Cdk5) in the nucleus accumbens (NAc).
Directional modification of Cdk5 promoter can be accomplished by engineering zinc finger protein (ZFP) transcription factors. DNA-binding proteins recognize a specific genetic region and fuse to the p65 transcriptional activation domain of nuclear factor-light-chain-enhancer of activated B cells (NF_B). Rapid and dynamic responses of retrotransposons and brain plasticity aim at maintaining the genomic and transcriptional stability of vulnerable brain regions like hippocampus (Pfau and Russo 2015). Neurological and psychiatric disorders result in genome-wide RNA expression that serves as a transcriptional signature (Kolb and Gibb 2011).
The detail of classification of molecular receptors based on symptoms of depression and mood disorder is specified in Table 1.
Table 1.
Classification of molecular mechanisms and receptors based on depression and mood disorder
| Authors | Molecular and genetic mechanisms | Molecular receptors | Brain regions for stress and mood disorder |
|---|---|---|---|
| Morrison and Baxter (2012) | MAPK1 RNA | Cytokines, cellular stressors | Hippocampus |
| McEwen (1999) | BDNF | Glucocorticoid | ADC, PFC, hippocampus |
| Saarelainen et al. (2003) | BDNF and CREB | TrkB | Hippocampus |
| Zhang et al. (2008) | Cytoplasmic membrane | Glycine-site modulator NMDARs | Hippocampus, PFC |
| Holtmaat et al. (2009) | Cytoplasm, chemokines | Cytoskeleton receptor. Eukaryotic | Hippocampus, PFC |
| Kim et al. (2007) | JNK1 | AMPAR | Hippocampus |
| Lalancette-Hébert et al. (2007) | Cytokines, chemokines and adhesion molecules | Purinergic receptors-P2 receptors, ATP | Hippocampus, visual cortex |
| de Vos et al. (2002) and Mecha et al. (2013) | Myelin antigens | T-cells receptors | Hippocampus |
| Jauch et al. (2006) | Endothelial cells | BBB | CVOs |
| Njoo et al. (2015) | WAVE1 | Cannabinoid receptor CB1; Gq/11 or G12/13 | DRG |
| Moreno-López et al. (2011) | Postsynaptic membrane; GABA | GABAARs; AMPARs | Brainstem; neocortex; hypothalamus |
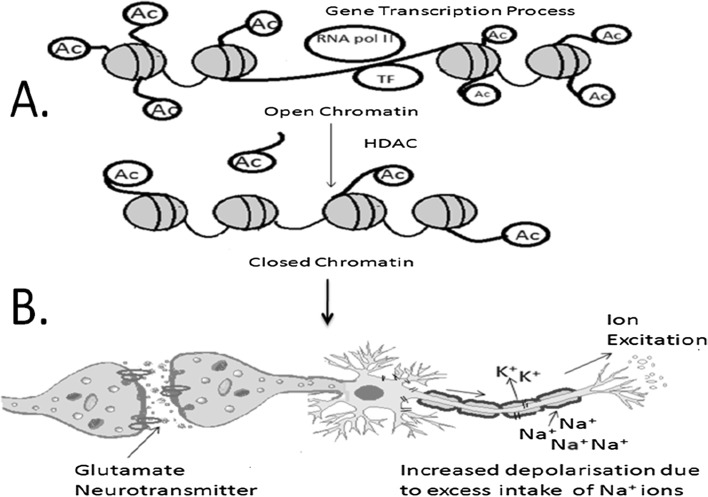
Adaptations to particular environments and experiences are exposed to achieve reproductive success. These are considered as mal-adaptation to another environment, resulting in greater allostatic load/overload. Acute psychological stress increases nor-adrenergic activity. Functional deletion in ADRA2B, and a gene coding for the α2b-adreno receptor, indicating increment in the phasic amygdala responses, and its effect on tonic activity are tested by using MRI techniques. Neural plasticity elucidates the molecular and cellular mechanisms that allow the brain to receive information and make the appropriate adaptive responses to different environmental, social, behavioural, and pharmacological stimuli from other cells. Acute neural plasticity results in the activation of the excitatory neurotransmitter glutamate which causes neuronal depolarization and increased regulation of intracellular Na+ signals. This leads to the subsequent activation of N-methyl-d-aspartate (NMDA) receptors and the influx of Ca2+ ions. A schematic for the above explanation is given in Fig. 2.
Fig. 2.
Acute Neural Plasticity a due to epigenetic or chromosomal dysregulation leading to malfunctioning of neural circuitries which affects the intrinsic cellular excitability. Intrinsic excitability includes factors such as k+ channels alteration by durgs or others agents, increases the depolarization resulting in increase intake of sodium ions and hence changes the nuclueys accumbens
Increasing number of CNS disorders is being shown to be caused by primary abnormalities in chromatin regulatory proteins.
Role of BDNF, mRNA and protein in stress and depression
BDNF, VEGF, and IGF1 play a significant role in depression. Stress and depression decrease the expression and function of BDNF in the blood in PFC and hippocampus. BDNF is required for the formation and maintenance of synaptic connections (Holtmaat et al. 2009; Grandjean and Landrigan 2014). Presence of the BDNFVal66Met allele is sufficient to cause atrophy of neurons in the hippocampus and medial PFC (mPFC) (Du et al. 2009). Individuals with BDNFVal66Met allele have reduced episodic memory and executive function, and also decreased hippocampal volume. BDNF is linked with neural plasticity in CNS (Duman et al. 1997). Remodelling of the hippocampus, prefrontal cortex and amygdala is coincident with stress-induced changes at the level of BDNF. Glucocorticoids couple with other molecules acts in conjunction with BDNF to facilitate the morphological and molecular changes. Further, the interactions between BDNF and other systems facilitate synaptic plasticity within the brain by which depression and anxiety deficit symptoms can be reversed and ameliorated (Nestler et al. 2002). Stress-induced changes are replicated by the chronic administration of GCs, following the elevation of cortisol activation of the hypothalamic/pituitary/adrenal axis in response to stress in the hippocampus and amygdala regions (Mitra and Sapolsky 2008; De Lange et al. 2008). BDNF levels in CA3 return to baseline after recovery from either an acute or chronic stressor (Lakshminarasimhan and Chattarji 2012; Egan et al. 2003; Frank et al. 2011), suggesting that hippocampal BDNF levels are highly dynamic. Neural plasticity in response to stress involves not only the elevation of GCs, but also requires BDNF, other molecules and proteins to induce physiological and morphological changes in neurons for modulating the cellular response to stress.
Neural circuits are altered following prolonged bouts of stress and depression. Met carriers exhibit a reduction in hippocampal volume (Bueller et al. 2006), a decreasing activity in the ventromedial prefrontal cortex and amygdala (Soliman et al. 2010). Acute stress modulates the genotype effects and processing of amygdale in humans. ADRA2B influences brain processes in the case of acute stress in the amygdala (Rasch et al. 2009). These context-dependent genetic characteristics are mediated by the amygdale and PFC. Endocannabinoid (ECB) signalling is affected by normal ageing, neuroinflammatory and neurodegenerative disorders that deteriorate the processing of brain. Different molecular components forming this signaling system change substantially the function of the brain and peripheral tissues. Bioactivity of ECB-based therapeutics varies on the basis of the patient’s age (Velayudhan et al. 2014). Cannabinoid and ECB-related receptors in neurons and glial cells change independently in response to a neurological disease (Lidsky and Schneider 2003). Immune cells that invade the brain parenchyma also express receptors and contribute to cells (Hegde et al. 2010). Deregulation affects neuronal, endocrine and immune cell signalling (Russell and Kahn 2007; Gu and Yakel 2011). The CB1 receptor is the serine kinase mammalian target of rapamycin (mTOR). As a molecular integrator, mTOR enables both cellular-nutrient sensing and energy homeostasis through ERK/MAPK-Akt pathway. mTORC1 has a role in regulating synaptic plasticity and cognitive functions, including memory, through mechanisms that depend on de novo protein synthesis.
Stress induced molecular pathways for cognitive impairment
Symptoms of stress disorders are characterized by impaired cognitive function alongside enhanced emotion through the hypothalamic–pituitary–adrenal (HPA) axis. Although the hippocampus, amygdala, and prefrontal cortex are distinct in their associations with the severity of cognitive and emotional symptoms of stress disorders, there are significant bidirectional connections between these areas. Early investigations into cellular mechanisms underlying these cognitive deficits are focused on the hippocampus (Martin et al. 2000), and reduces the density of dendritic spines (Chen et al. 2010). Stress and mood disorder affects different structural and neurochemical plasticity, and also increases activation in the ventromedial prefrontal cortex (vmPFC), hippocampus and amygdala regions, implicating stress-related vulnerability to chronic health conditions (Liston et al. 2006). Allostatic load enables patients with mood disorder to cope with stressful experiences. Abnormalities constitute a neural vulnerability that combines neural risk factors with maladaptive neural responsiveness to stress. Neural factors establish an association between behavioural and neural measures for the amygdala and hippocampus regions. This association reflects plasticity in brain activation as magnitudes change in stress symptoms over time. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity (Morrison and Baxter 2012) that affects memory by selectively impairing the stability of place cell firing rates (Sandi and Pinelo-Nava 2007). Age and sex-dependent differences are observed in terms of behavioural activity, plasticity and response to stress in the dentate gyrus (Zitman and Richter-Levin 2013; Protopopescu et al. 2005). Stress affects both principal cells and interneurons (Yarom et al. 2008). Pubertal dentate gyrus differs due to basal excitability, suggesting sex-related differences in effects of stress on behaviour. Underlying mechanisms of the longevity and selectivity of the impact of PS are explored considering sex-specific epigenetic, brain structural and behavioral changes (Schmidt and Duman 2010). Mechanisms mediating transgenerational programming of stress responses and pathologies help to explain the generational persistence of human behaviors in families and populations exposed to long-term adversity. Stressful experiences in early life can have long-lasting effects on brain development and capacity to respond to later stressful challenges, as the brain regulates neuroendocrine, autonomic, metabolic and immune system functions in an adverse way (Anda et al. 2010). Adult brain possesses the ability to show reversible structural and functional plasticity to stressful experiences. Social defeat induces PSDs and functional increase in mEPSCs in NAc MSNs. Changes in dendritic spine density and morphology are thought to underlie important aspects of experience-dependent plasticity (Holtmaat et al. 2009). Effects of stress on the structure and function of neurons within the meso corticolimbic brain systems regulate mood and motivation (Christoffel et al. 2011). Recently, there is a focus on drug-induced changes in synaptic excitability and much less attention to intrinsic excitability factors. Intrinsic factors including K+ channels are altered by cocaine forcing neuronal excitability adaptations in the nucleus accumbens (NAc). Brain circuit activity emerges from constant interactions between synaptic and intrinsic cellular excitability factors.
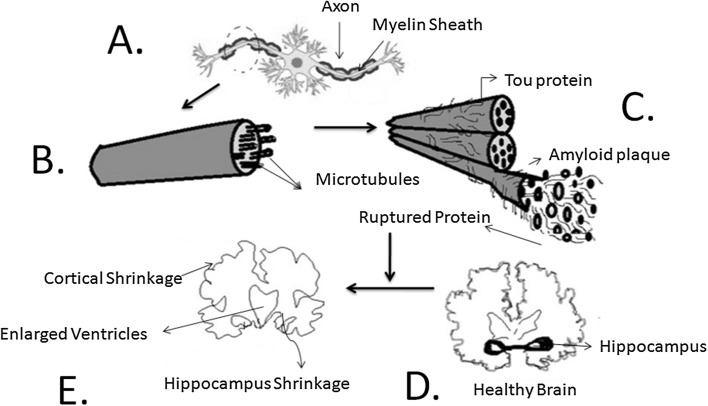
Environmental events and risk factors contribute to depression through the convergence of molecular and cellular mechanisms. This mechanism disrupts neuronal function and morphology including the synaptic deficit, resulting in dysfunction of the circuitry for mood regulation and cognitive function (Duman and Duman 2015). This happens due to the lack of reliable biomarkers of MDD and treatment response. Exposure to stress hormones elicits a complex repertoire of plasticity mechanisms in the different sub-regions of the hippocampus. Acute stress and acute glucocorticoid elevation produce impairment in long-term potentiation (LTP) and primedburst potentiation (Felix-Ortiz et al. 2013). Input from the entorhinal cortex to the dentate gyrus is ramified by the connections between the dentate gyrus and CA3 pyramidal neurons within the hippocampus. Dentate gyrus-CA3 system that adapts structural plasticity plays a role in the memory of sequences of events (Lisman and Otmakhova 2001). This pyramidal cell undergoes a reversible remodeling of their dendrites (McEwen 2010), along with dentate granule neuron dendrites (Gould et al. 1990). The co-expression of MR and GR in the same neurons gives rise to heterodimer formation (Joëls 2006; Karst et al. 2005). Stress reduced LTP in vitro alters place cell stability and impedes the consolidation of spatial memory. Stress selectively impairs the consolidation of spatial memory, without altering the sensory-motor systems. After stress, place cells show an increased tendency to change their firing rate without changing their prefer firing location. Steroid hormones are intercellular mediator and neurotransmitter system participate in structural plasticity. Electrical stimulation of the BLA disrupts the induction of long-term potentiation (LTP), as a measure of synaptic plasticity in the hippocampal CA1 sub-region (Vouimba and Richter-Levin 2005). However, the role of p25 is to generate plasticity in the hippocampus after repeated stress in an amygdala-dependent manner. p25/Cdk5 dependent phosphorylation on Ser211 activates GR (Gräff et al. 2012) while increasing GR phosphorylation expression of histone deacetylase 2 (HDAC2) (Gräff et al. 2012; von Arnim et al. 2010). HDAC2, in turn, suppresses the expression of genes for learning and memory (Gräff et al. 2012), suggesting a mechanism for an elevated p25 generation that leads to cognitive impairment. Blockade of p25 generation protects the hippocampus from the detrimental effects of repeated stress. This pathway activates the excitatory neurotransmitter glutamate and regulates intracellular signalling cascades. Using optogenetic manipulations, a pivotal role of the BLA–ventral hippocampus (vHPC) circuit has been identified in the context of anxiety-related social interaction, precisely behaviours and optogenetic excitation (Allsop et al. 2014; Taliaz et al. 2011). Stress modulates the connection and interaction between BLA and hippocampus (Allsop et al. 2014). Similarly, BLA shows distinctive neural function in fear and reward conditioning. Further, BLA neurons are capable of encoding experiences with both negative and positive emotional valences (Namburi et al. 2015) and reward learning and addiction conditions (Koob 2009; Venero and Borrell (1999); Mercurio et al. 1999). A specific micro-circuits and synaptic mechanisms mediate these effects of stress in the BLA (Anisman and Matheson 2005). Intrinsic factors that are located in the soma, dendrites and axon modulate membrane excitability. This phenomenon is explained in Fig. 3.
Fig. 3.
Optogenetic neural circuit explains the impairment in the structural remodelling of axon myelination a due to induced stress factors. Axon myelin sheath is composed of microtubules (b), and the distortion in the moprphology of microtubules, ruptures the tou protein (binding protein) and forms Amyloid plaque (c). This ruptured protein results in cortical and hippocampus shrinkage, along with the enlargement of ventricles (e), alteration of healthy state of brain (d) into a diseased (Alzheimer disease) brain
Adrenal steroids are important mediators for remodelling of hippocampal neurons during repeated stress (Sousa et al. 2000a, b). Glucocorticoids also synergize with excitatory amino acids to promote excitotoxic damage and impairment of energy generation through inhibition of glucose uptake (Sapolsky 1992). Neural plasticity of antidepressant is observed in molecular and cellular mechanisms (Duman 2014; Christoffel et al. 2011). Nucleus accumbens (NAc) is linked with mood and anxiety disorders and dendritic spine plasticity that underlies neuropsychiatric disorders, such as depression, anxiety, and drug addiction (Holtmaat et al. 2009; Russo et al. 2010). Dysfunction of the mesolimbic dopaminergic reward pathway, including the ventral tegmental area (VTA) dopaminergic cells is linked to neuropsychiatric disorders (Krishnan et al. 2007). Increased firing rates of VTA dopaminergic neurons after social defeat are accompanied by increased release of brain-derived neurotrophic factor (BDNF). NAc shell is considered to be a limbic-motor interface that is responsible for integrating the salience of rewarding and aversive stimuli through its target brain regions (Chen et al. 2010). Glucocorticoids, excitatory and inhibitory neurotransmitters, and neurotrophic factors are some of the mediators of neural plastic. These mediators operate in a nonlinear network and perform biphasic actions and reciprocal interactions leading to disruption and pathophysiology (McEwen 2006). Cellular and molecular mechanisms of anxiety-like behavior have been studied to understand how the brain responds to stressors. Stress hormones modulate function within the brain by changing the structure of neurons. Molecular mechanisms control behavioral and synaptic plasticity in addiction and stress disorders.
Drugs and molecular mechanisms for neural plasticity
Neural plasticity plays an important role in the treatment of mood disorder, depression and stress. Glucocorticoid signalling is essential for neuronal plasticity to stress and antidepressant treatment (Arango-Lievano et al. 2015). In other words, antidepressant treatment increases neural plasticity and structural remodelling of brain considering synaptic modifiers such as neurotrophins and glucocorticoids that deregulate risk factors of stress. BDNF increases glucocorticoid receptors (GR) for suppressing the development of stress. A TrkB-MAPK pathway is sustained GR phosphorylation at BDNF sites, which is transcribed the genetic plasticity. Coordinated actions between BDNF and glucocorticoids promote neuronal plasticity. Glucocorticoids deteriorate the structure and function of brain circuits affecting perception, cognition and mood. Remodelling of neuron disrupts cognitive ability and enhances stress-mediated peaks of glucocorticoids (Pariante 2006). Stress-sensitive pathways influence GR function initiating a cellular pathway to modulate GR activity during stress. Moreover, fluoxetine prevents the neuroplasticity of chronic stress by priming GR phosphorylation at BDNF-sensitive sites. Disruption of GR which is primed by BDNF explains antidepressant resistance through neural plasticity at the molecular and cellular levels. Prolonged medications of antidepressants increase synaptic plasticity in adult hippocampus and enhance expression of neurotrophic factors.
Time-dependent cholinergic induction establishes dynamic hippocampal synaptic plasticity through electrical stimulation. Input activity of septal cholinergic through an optogenetic approach provides different types of hippocampal schaeffer collateral (SC) which is added to CA1 synaptic plasticity. Cholinergic input affords different types of plasticity at different synapses, subject to local glutamatergic activity in each spine with respect to time and context. Input is provided through either its ion channel receptor (α7 nAChR) or the G-protein coupled with another receptor (mAChR). This directly induces hippocampal synaptic plasticity considering time and context in a dependent manner. A non-competitive NMDA antagonist has antidepressant effects in behaviours and synaptic plasticity. As a high-affinity NMDA receptor antagonist, ketamine has a rapid-albeit transitory-antidepressant effect in patients with major depression (Berman et al. 2000; Taliaz et al. 2011). Ketamine, as a nonselective NMDA receptor channel blocker, facilitates antidepressant actions with fewer side effects (Iadarola et al. 2015). Ketamine is dissociative anaesthetic used for surgical procedures in young and old humans. Acute ketamine treatment stimulates a rapid cascade of molecular and cellular events that underlie the long-lasting synaptic and behavioural responses. Contrarily, ketamine’s psychotomimetic effects prevent its use as a chronic antidepressant (Berman et al. 2000). NMDA, as tetrameric membrane inserted protein receptor, comprises both NR1 and NR2 or NR3 subunits (Nishi et al. 2001). NR2 subunits contain NR2B, which is a major subunit in neocortex and hippocampus (Monyer et al. 1994), associated with synaptic plasticity and cognitive functions (von Engelhardt et al. 2008; Van Praag et al. 1999). Expression of NR2B receptor is affected in depression integrating with the cellular basis of learning and memory. Long-term potentiation (LTP) of synaptic transmission is one of the prime candidates for mediating learning and memory, and other forms of experience-dependent plasticity (Malenka and Bear 2004).
Increased proteins are significant for neuronal plasticity. Neuroplasticity molecules modulate in different brain regions after chronic administration of the novel antidepressant agomelatine (Calabrese et al. 2010). Neuronal plasticity is associated with depression as a result of the modified expression of proteins for cellular resiliency. Antidepressant drugs regulate these expressions in order to achieve relevant clinical effects. Chronic treatment with agomelatine MT1/MT2 receptor and 5-HT2C receptor antagonist affects the brain-derived neurotrophic factor (BDNF), fibroblast growth factor (FGF-2), and activity regulated cytoskeleton-associated protein (Arc). Antidepressant action is contributed by neuroplasticity molecules, as it follows a circadian rhythm. mRNA and protein expression of BDNF modulates fibroblast growth factor (FGF-2) and gene expression (Turner et al. 2006; Saal et al. 2003) and enhances antidepressant treatment (Bachis et al. 2008; Leutgeb et al. 2004). Chronic treatment with agomelatine does not affect mRNA, but mRNA is reduced by venlafaxine. Proteins are increased with prolonged treatment of this drug following adaptive changes in hippocampal and cortical areas. Major changes are found in the hippocampus, where agomelatine produces a stable increase of the neurotrophin mRNA after chronic treatment.
Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator (Nestler et al. 2001; Olausson et al. 2006). Drug-abuses induce neuroplasticity following a natural reward pathway through nucleus accumbens (NAc), resulting in the development and expression of addictive behaviour. Drugs activate the mechanisms of plasticity causing a change in the NAc. It’s spinogenesis leads to the initial development of short-term expression of sensitized Amph reward, but it is not critical for continued expression of enhanced drug reward (Balfour et al. 2004). As a common neural pathway, mesolimbic dopamine (DA) system in the NAc plays a central role (Kelley 2004). The role of dopamine D1 receptors (D1R) for sexual experience enhance neural plasticity because NAc ΔFosB induction increases spine density in D1R-containing neurons (Kim et al. 2009; Zhang et al. 2002). Repeated sexual behaviour is highly rewarding (Tenk et al. 2009), and therefore, it causes sensitized drug-related behaviours, including cross-sensitization to amphetamine (Amph)-induce locomotor activity (Bradley and Meisel 2001; Zachariou et al. 2006). Abstinence from sexual experience is found to be critical for enhanced Amph reward, NAc spinogenesis (Pitchers et al. 2010; Nestler 2008), and glutamate receptor trafficking (Colby et al. 2003). Natural and drug rewards are not only converged on the same neural pathway, but also they are united on the same molecular mediators (Nestler et al. 2001).
Therapeutic solution with dual perspective
Major depression is a chronic and debilitating illness that affects approximately 1 in 5 people. Low rates of efficacy, therapeutic time lag, and an undesirable side effect limit the currently available treatments. Behavioural and neuronal deficits of chronic stress and depression are notably reserved by the glutamate NMDA receptor antagonist ketamine (Gerhard et al. 2016). The fundamental philosophy and pathophysiology of depression are still incomplete. Evidence for depression that is associated with atrophy of neurons in cortical and limbic brain regions that control mood and emotion is required for clinical and basic research studies (Duman and Aghajanian 2012). Rapid improvement in depressive symptoms is observed from Antagonists of the N-methyl-d-aspartate (NMDA) receptor, notably ketamine. Ketamine leads to rise in Synaptic connections in the PFC and reverses the deficits caused by chronic stress.
Changes in the neural circuits that serve as controlling influential action are induced by stress. The development of addiction and relapse vulnerability is affected by stress. Stress and stress hormones can be assisted by a switch from goal-directed to habit action. Acute stress factors rehabilitate habitual responding to drug-related cues triggering relapse to addictive behaviour. Mechanism including cognitive process contributes to the effects of stress on addictive behaviour and could have important implications for the treatment of addiction. An acute stressor re-establishes accessible drug-taking habits, hence increasing the risk of relapse to addictive behaviour.
GCs regulate abused behaviour of the drug to a subsequent pro-inflammatory challenge. Glia and its-derived neuroinflammatory mediators play key roles in the development of drug abuse to mediate pro-inflammatory cytokines (Rice and Barone Jr. 2000). Macrophages and microglia undergo a spectrum of activation states, producing varying blends of pro- and anti-inflammatory products. Stressors and pro-inflammatory components contribute identical signature of cytokine in CNS (Maier 2003; Nguyen et al. 1998). Moderate duration of stress elevates different levels of pro-inflammatory cytokines in brain (O’Connor et al. 2003). The neuroimmune microenvironment (NM) exhibits an increase in activation of the glial marker, whereas, delay in activation of NM during stress does not influence tonic induction of increased levels of cytokines.
Medication of drugs for cell proliferation and survival of neurons
A number of newborn neurons, the proliferation of cells and the survival of newborn neuron are influenced by antidepressant treatment (Nakagawa 2002). Downregulation of neurogenesis largely happens due to the block of treatment of antidepressant which is caused by stress. Blockade of intruder stress and maternal separation are tested (Berners-Lee and Fischetti 2001; Nguyen et al. 2000) to find out neurotrophic factors in the development and neuronal survival. Antidepressant treatment upregulates BDNF and decreases its expression bringing a serious consequence for the function of limbic brain structures (Duman et al. 2000). Ketamine and scopolamine emerge as rapid antidepressant actions both at the molecular, cellular and neural levels. Ketamine and other NMDA receptors increase mTORC1 signalling through activation of Akt and ERK which increases syn-aptic number and function in the PFC52 (Zhou et al. 2014). Ketamine acts via blockade of NMDA resulting in disinhibition of glutamate neurons. mTORC1 signalling pathway regulates protein translation following alterations in neuronal activity for synaptic plasticity (Northoff and Panksepp 2008). Molecular signalling underlies change in PFC pyramidal neurons, enhances the number and function of dendritic spines following ketamine treatment. Moreover, treatments are now being designed to increase synaptic plasticity and to oppose the cellular effects of stress and depression through glutamatergic agents. GluN1 that contains glycine-binding sites presents in almost all neurons throughout development, whereas GluN2 that is generated by four different genes (GluN2A, B, C, & D) produces unique physiological, biochemical, pathophysiological properties (Shors 2006) throughout the forebrain.
Drugs, medication and clinical treatment for preventing major depressive disorder
A significant proportion of major depressive disorder (MDD) patients continues to experience cognitive impairment. Erythropoietin (EPO), as an antidepressant drug, alleviates MDD (Lovinger and Kash 2015) related to the impairment of verbal memory and attention. EPO, as a glycoprotein, is synthesized by the kidney and acts as a hormone in the regulation of erythropoiesis. It crosses the blood–brain-barrier and exerts antidepressant and neuroprotective effects while enhancing hippocampus-dependent memory and neuro plasticity (Lovinger and Kash 2015). Lisdexamfetamine dimesylate (LDX) that is a pharmacologically inactive prodrug of d-amphetamine improves executive dysfunction related to Attention Deficit Hyperactivity Disorder (ADHD) treatment in adults after full or partial remission of MDD (Martin and Wang 2010). Clinically, it has been observed that erythropoietin including minocycline, insulin, antidiabetic agents, angiotensin-converting enzyme inhibitors, S-adenosyl methionine, acetyl-l-carnitine, alpha lipoic acid, omega-3 fatty acids, melatonin, modafinil, galantamine, scopolamine, N-acetylcysteine, curcumin, statins, and coenzyme alleviate cognitive dysfunction in MDD. Serotonin [5-hydroxytryptamine (5-HT)] and noradrenaline reuptake inhibitors, and electroconvulsive seizures increase adult neurogenesis for antidepressant treatment (Margineanu et al. 1994). Vortioxetine (LuAA21004) acts as an antagonist of the 5-HT3 and 5-HT7 serotonin receptors, a partial agonist of the 5-HT1B serotonin receptor, agonist of the5-HT1A receptor, and inhibits the serotosnin transporter (Kyzar and Banerjee 2016).
Minocycline is a tetracycline antibiotic commonly used to treat acne, infections of the respiratory trait, and mild rheumatoid arthritis. It promotes hippocampal neurogenesis and exerts anti-apoptotic and anti-inflammatory activities, reducing the expression of pro-inflammatory cytokines (e.g. IL-1β, IL-6, IL-2, TNF-α, INF-γ) and up-regulating anti-inflammatory cytokines (e.g. IL-10). The muscarinic cholinergic system is involved in the pathophysiology of MDD (McEwen and Gianaros 2011). Scopolamine is a potent muscarinic antagonist, which promotes a rapid antidepressant effect (Drevets et al. 2013; Leweke et al. 2012). These clinical procedures play a significant role in the neuro progressive nature of MDD to reduce pro-inflammatory status related to poor neurocognitive performance. Impaired insulin signalling and insulin resistance have been documented to play an important role in the pathogenesis of MDD to enrich synaptic plasticity and cellular survival (Craft et al. 2004; Koay et al. (2014); Rei et al. 2015) in the hippocampus and medial temporal cortex (Hou et al. 2017). These regions are responsible for hypertension, physiological and functional impairment of memory, and depression. Angiotensin-converting enzyme (ACE) inhibitors modulate immune properties and suppress cytokine production (e.g.IL-1, TNF-α), through the interference with NF-κB activation (Andersson et al. 2002; Kettenmann et al. 2011).
Acetyl-L-carnitine (ALC) produces both carbohydrate and lipid metabolism exerting its’ role in the amelioration of the insulin-resistant state. ALC enhances neuronal metabolism in the mitochondria and counteracts against glutamate-mediate neurotoxicity and hypothalamic-adrenocortical hyperactivity. As a pineal gland hormone, melatonin influences the maintenance of circadian rhythms and affects energy metabolism (Tocharus et al. 2014; Hölzel et al. 2009). Modafinil is used as a stimulant-like agent for the treatment of excessive sleepiness in narcolepsy. It has a pleiotropic mechanism for targeting several neuro-transmitters, including serotonin, GABA, glutamate, orexin, and histaminergic systems (Gerrard and Kandlikar 2007; Mitra et al. 2005). Like modafinil, galantamine is a cholinergic agent and a modulator of potent nicotinic receptor. It modulates other neurotransmitter systems, including monoamines, glutamate, and GABA (Colovic et al. 2013; Young et al. 1998). Psychiatric disorders are treated by using N-acetylcysteine (NAC). It acts as a modulator of synaptic glutamate through the cysteine-glutamate exchanger, while increasing glutathione and oxidative defences, enhances neurogenesis and mitochondrial function, decreases pro-inflammatory cytokines, and regulates apoptosis (Samuni et al. 2013). Statins are prescribed to low down the blood cholesterol to prevent cardiovascular illnesses. Simvastatin may have anxiolytic and antidepressant like effects in stress-related murine models (Kapahi et al. 2010) whereas atorvastatin increases hippocampal BDNF levels in rodents (Ludka et al. 2013). Coenzyme Q10 (CoQ10) that protects different cell types, acts as a redox modulator for stabilizing membrane and mitochondrial electron transport chain (Johnson et al. 2003). Adaptations of multiple intracellular pathways and signalling lead to neural plasticity upon antidepressant treatment. cAMP-CREB cascade contributes to actions of chronic antidepressant treatment (Duman et al. 2000), acting as a tricyclic antidepressant (TCA) reuptake inhibitors and monoamine oxidase inhibitors (MAOIs). TCA has side effects that include drowsiness and sedation, memory and cognitive impairments, dry mouth, and increased heart rate (Holtmaat and Svoboda 2009). Blocking of GABA-A receptor increases glutamate transmission (Hao et al. 2018; Holmes and Wellman 2009). Facilitation of AMPA receptor enhances synaptic delivery connecting with a molecular mechanism to treat cognitive impairment (Knafo et al. 2012; Laufer et al. 2016), as cell adhesion molecules and growth factor signalling are critical for brain development and synaptic plasticity. Since synaptic plasticity is essential for learning and memory, any alteration in synaptic plasticity harms synaptogenesis, synaptic maturation, cellular and neural pathways to develop multiple cognitive deficits (Dityatev et al. 2000; Morentin et al. 2014). IFGL enhances LTP in hippocampal regions and facilitates AMPAR delivery at synapses on activation of NMDA receptors (NMDARs). Long-lasting increase in PKC activity provides synaptic and behavioural effects of FGL (Cambon et al. 2004; Govindarajan et al. 2006).
Conclusion and future perspective
Human brain has a considerable degree of plasticity and resilience on reception of increased stress and mood disorders. Allostatic control systems adversely affect brain-body disorder in presence of unabating fatigue. Limbic regions are associated with SMD. Amygdala, which is responsible for neuronal growth, becomes hyperactive during chronic stress. Most of the molecular and cellular mechanisms have also been taken into clinical consideration to extrapolate neural plasticity in response to the deficit of learning and memory, fear, anxiety, mood disorder, stress depression and drug abuse. One of the promising parts of neural plasticity is that the neural alternations that are reversible referring to neuronal atrophy and cell loss. Ketamine which is a drug of abuse causes euphoric and psychotomimetic effects. Persistent use of it causes neuronal damage. It also helps to develop a therapeutic agent and paradigm for drug discovery. One theory states that NMDA receptors on the pyramidal neurons are targeted by ketamine that causes a homeostatic response in the absence of neuronal activity. Stress and mood disorder disrupt memory processing by impairing the ability of the hippocampus to store stable place cell firing. Most of the research highlights the role of amygdala projection neurons and their outputs in modulating stress-induced plasticity mechanisms in the hippocampus and mPFC. Another challenge is to identify the genetic changes that are associated with mood and behavioural disorder. The psychiatric disease shows a disruption in normal plasticity mechanism. The role of epigenetic mechanisms and neurogenesis is to recover the neural disruptions in the limbic regions.
Further molecular investigations including studies on epigenetic regulation need to be performed clinically for developing relevant models to control stress disorder. BDNF and other neurotrophic factors have a subject of potential challenges for neuroscience research to address difficulties in drug delivery, efficacy and side effects. Further studies are required to characterize different mechanisms of NMDA antagonists in antidepressant efficacy. Findings to the study of NMDA antagonists will provide a therapeutic efficacy with minimization of side effects associated with ketamine. Further investigations are to be made on cellular actions of stress and depression considering available interneuron subtypes in the PFC and the influence of ketamine on synaptic plasticity. Many neural mechanisms are affected by cognitive therapy and medication. Brain morphology is altered by cognitive therapy in context to chronic fatigue syndrome. Adaptation of brain to behavioural and pharmaceutical therapies is important for future research. Additional research might be conducted to delineate the pathways by which dimensions of social relationships and networks may affect the brain. Does stress-induced transition from goal-directed drug use to compulsive drug-taking depend on the intensity and type of stress? Poor prediction ability of PTSD based on acute stress symptoms is a clinical challenge for early identification of a stress-induced vulnerability. Cellular mechanism influencing cocaine-induced enhancement of K+ currents is still under investigation. Longitudinal studies of spine and neuronal dynamics may pave the way for clarification of etiologies of synaptic dysregulation during mood disorder.
Acknowledgements
AAR, AB and AP were supported by Program of Competitive Growth of Kazan Federal University and subsidy allocated to Kazan Federal University for the state assignment in the sphere of scientific activities.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Allsop SA, Vander Weele CM, Wichmann R, Tye KM. Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front Behav Neurosci. 2014;8:241. doi: 10.3389/fnbeh.2014.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Cattaneo A, Musaelyan K, Zunszain PA, Horowitz M, Molteni R, Luoni A, Calabrese F, Tansey K, Gennarelli M, Thuret S. Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc Natl Acad Sci. 2013;110(21):8708–8713. doi: 10.1073/pnas.1300886110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Butchart A, Felitti VJ, Brown DW. Building a framework for global surveillance of the public health implications of adverse childhood experiences. Am J Prev Med. 2010;39(1):93–98. doi: 10.1016/j.amepre.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Andersson G. Demographic trends in Sweden: An update of childbearing and nuptiality up to 2002. Demogr Res. 2004;11:95–110. [Google Scholar]
- Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci. 2009;34(1):4–20. [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29(4):525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Arango-Lievano M, Lambert WM, Bath KG, Garabedian MJ, Chao MV, Jeanneteau F. Neurotrophic-priming of glucocorticoid receptor signaling is essential for neuronal plasticity to stress and antidepressant treatment. Proc Natl Acad Sci. 2015;112(51):15737–15742. doi: 10.1073/pnas.1509045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold T, Betsholtz C. Erratum to: the importance of microglia in the development of the vasculature in the central nervous system. Vasc Cell. 2013;5(1):1. doi: 10.1186/2045-824X-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Lucki I, Brookshire BR, Carlson GC, Browne CA, Kazi H, Bang S, Choi BR, Chen Y, McMullen MF, Kim SF. High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis. 2014;67:79–87. doi: 10.1016/j.nbd.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64(2):238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Mallei A, Cruz MI, Wellstein A, Mocchetti I. Chronic antidepressant treatments increase basic fibroblast growth factor and fibroblast growth factor-binding protein in neurons. Neuropharmacology. 2008;55(7):1114–1120. doi: 10.1016/j.neuropharm.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Labonté B, Peña CJ, Nestler EJ. Epigenetic signaling in psychiatric disorders: stress and depression. Dialogues Clin Neurosci. 2014;16(3):281–295. doi: 10.31887/DCNS.2014.16.3/rbagot. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour ME, Yu L, Coolen LM. Sexual behavior and sex-associated environmental cues activate the mesolimbic system in male rats. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2004;29(4):718–730. doi: 10.1038/sj.npp.1300350. [DOI] [PubMed] [Google Scholar]
- Bazak N, Kozlovsky N, Kaplan Z, Matar M, Golan H, Zohar J, Richter-Levin G, Cohen H. Pre-pubertal stress exposure affects adult behavioral response in association with changes in circulating corticosterone and brain-derived neurotrophic factor. Psychoneuroendocrinology. 2009;34(6):844–858. doi: 10.1016/j.psyneuen.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Berners-Lee T, Fischetti M (2001) Weaving the Web: The original design and ultimate destiny of the World Wide Web by its inventor. DIANE Publishing Company
- Bradley KC, Meisel RL. Sexual behavior induction of c-Fos in the nucleus accumbens and amphetamine-stimulated locomotor activity are sensitized by previous sexual experience in female Syrian hamsters. J Neurosci. 2001;21(6):2123–2130. doi: 10.1523/JNEUROSCI.21-06-02123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160(5):924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Bryant RA. Early predictors of posttraumatic stress disorder. Biol Psychiatry. 2003;53(9):789–795. doi: 10.1016/s0006-3223(02)01895-4. [DOI] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59(9):812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Racagni G, Riva MA. Neuronal plasticity: a link between stress and mood disorders. Psychoneuroendocrinology. 2009;34:S208–S216. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Calabrese P, Le Doussal P, Rosso A. Free-energy distribution of the directed polymer at high temperature. EPL (Europhys Lett) 2010;90(2):20002. [Google Scholar]
- Calabrese F, Molteni R, Gabriel C, Mocaer E, Racagni G, Riva MA. Modulation of neuroplastic molecules in selected brain regions after chronic administration of the novel antidepressant agomelatine. Psychopharmacology. 2011;215(2):267–275. doi: 10.1007/s00213-010-2129-8. [DOI] [PubMed] [Google Scholar]
- Cambon K, Hansen SM, Venero C, Herrero AI, Skibo G, Berezin V, Bock E, Sandi C. A synthetic neural cell adhesion molecule mimetic peptide promotes synaptogenesis, enhances presynaptic function, and facilitates memory consolidation. J Neurosci. 2004;24(17):4197–4204. doi: 10.1523/JNEUROSCI.0436-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrén E. Neuronal network plasticity and recovery from depression. JAMA Psychiatry. 2013;70(9):983–989. doi: 10.1001/jamapsychiatry.2013.1. [DOI] [PubMed] [Google Scholar]
- Castrén E, Kojima M. Brain-derived neurotrophic factor in mood disorders and antidepressant treatments. Neurobiol Dis. 2017;97:119–126. doi: 10.1016/j.nbd.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahman MM. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci. 2015;18(10):1364–1375. doi: 10.1038/nn.4115. [DOI] [PubMed] [Google Scholar]
- Chen Y, Rex CS, Rice CJ, Dubé CM, Gall CM, Lynch G, Baram TZ. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci. 2010;107(29):13123–13128. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011;22(5):535–549. doi: 10.1515/RNS.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D. Can we improve our physical health by altering our social networks? Perspect Psychol Sci. 2009;4(4):375–378. doi: 10.1111/j.1745-6924.2009.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ZVI, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol. 1996;50(4):335–362. doi: 10.1016/s0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. Striatal cell type-specific overexpression of ΔFosB enhances incentive for cocaine. J Neurosci. 2003;23(6):2488–2493. doi: 10.1523/JNEUROSCI.23-06-02488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013;11(3):315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn H, Rijpkema M, Qin S, van Marle HJ, Franke B, Hermans EJ, van Wingen G, Fernández G. Acute stress modulates genotype effects on amygdala processing in humans. Proc Natl Acad Sci. 2010;107(21):9867–9872. doi: 10.1073/pnas.1003514107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, III, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology. 2005;183(3):331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Covington HE, Maze I, Sun H, Bomze HM, DeMaio KD, Wu EY, Dietz DM, Lobo MK, Ghose S, Mouzon E, Neve RL. A role for repressive histone methylation in cocaine-induced vulnerability to stress. Neuron. 2011;71(4):656–670. doi: 10.1016/j.neuron.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft JM, Van Eldik LJ, Zasadzki M, Hu W, Watterson DM. Aminopyridazines attenuate hippocampus-dependent behavioral deficits induced by human β-amyloid in a murine model of neuroinflammation. J Mol Neurosci. 2004;24(1):115–122. doi: 10.1385/JMN:24:1:115. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP. Neurogenesis-dependent and-independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lange FP, Koers A, Kalkman JS, Bleijenberg G, Hagoort P, Van der Meer JW, Toni I. Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain. 2008;131(8):2172–2180. doi: 10.1093/brain/awn140. [DOI] [PubMed] [Google Scholar]
- DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9(10):788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Dityateva G, Schachner M. Synaptic strength as a function of post-versus presynaptic expression of the neural cell adhesion molecule NCAM. Neuron. 2000;26(1):207–217. doi: 10.1016/s0896-6273(00)81151-4. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 2010;11(11):735–746. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Zarate CA, Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biol Psychiatry. 2013;73(12):1156–1163. doi: 10.1016/j.biopsych.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, Khairova R, Zhou R, Yuan P, Machado-Vieira R, McEwen BS. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci. 2009;106(9):3543–3548. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clinical Neurosci. 2014;16(1):11. doi: 10.31887/DCNS.2014.16.1/rduman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Duman RS. Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci Lett. 2015;601:20–29. doi: 10.1016/j.neulet.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S, D’Sa C. Neuronal plasticity and survival in mood disorders. Biol Psychiatry. 2000;48(8):732–739. doi: 10.1016/s0006-3223(00)00935-5. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treat. 2009;5:433. doi: 10.2147/ndt.s5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rao JS, Rizavi HS, Kotowski J, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60(3):273–282. doi: 10.1001/archpsyc.60.3.273. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 2013;79(4):658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF. Stress-and glucocorticoid-induced priming of neuroinflammatory responses: potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain Behav Immun. 2011;25:S21–S28. doi: 10.1016/j.bbi.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75(5):747–761. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex. Fourthth. London: Academic; 2008. [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21(3):454–464. doi: 10.1016/j.drudis.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard J, Kandlikar M. Is European end-of-life vehicle legislation living up to expectations? Assessing the impact of the ELV Directive on ‘green’innovation and vehicle recovery. J Clean Prod. 2007;15(1):17–27. [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Short-term glucocorticoid manipulations affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience. 1990;37(2):367–375. doi: 10.1016/0306-4522(90)90407-u. [DOI] [PubMed] [Google Scholar]
- Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci. 2006;103(35):13208–13213. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM, Nieland TJ, Fass DM, Kao PF, Kahn M, Su SC. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483(7388):222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) J Clin Psychiatry. 2015;76(2):155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71(1):155–165. doi: 10.1016/j.neuron.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez H, Hale VA, Dolcet X, Davies A. NF-κB signalling regulates the growth of neural processes in the developing PNS and CNS. Development. 2005;132(7):1713–1726. doi: 10.1242/dev.01702. [DOI] [PubMed] [Google Scholar]
- Hao L, Yang Z, Gong P, Lei J (2018) Maintenance of postsynaptic neuronal excitability by a positive feedback loop of postsynaptic BDNF expression. Cognit Neurodyn 1–14 [DOI] [PMC free article] [PubMed]
- Hegde VL, Nagarkatti M, Nagarkatti PS. Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur J Immunol. 2010;40(12):3358–3371. doi: 10.1002/eji.201040667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 2009;33(6):773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10(9):647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, De Paola V, Hofer SB, Hübener M, Keck T, Knott G, Lee WCA, Mostany R. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nat Protoc. 2009;4(8):1128–1144. doi: 10.1038/nprot.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, Lazar SW. Stress reduction correlates with structural changes in the amygdala. Soc Cognit Affect Neurosci. 2009;5(1):11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D, Wang C, Chen Y, Wang W, Du J. Long-range temporal correlations of broadband EEG oscillations for depressed subjects following different hemispheric cerebral infarction. Cognit Neurodyn. 2017;11(6):529–538. doi: 10.1007/s11571-017-9451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG, Gagnidze K, McEwen BS, Pfaff DW. Stress and the dynamic genome: steroids, epigenetics, and the transposome. Proc Natl Acad Sci. 2015;112(22):6828–6833. doi: 10.1073/pnas.1411260111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttlin EL, Jedrychowski MP, Elias JE, Goswami T, Rad R, Beausoleil SA, Villén J, Haas W, Sowa ME, Gygi SP. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143(7):1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang R-J, Chen H-J, Guo Z-X, Lee Y-S, Liu T-Y (2018) Effects of aerobic exercise on sad emotion regulation in young women: an electroencephalograph study. Cognit Neurodyn 1–11 [DOI] [PMC free article] [PubMed]
- Jauch R, Cho MK, Jäkel S, Netter C, Schreiter K, Aicher B, Zweckstetter M, Jäckle H, Wahl MC. Mitogen-activated protein kinases interacting kinases are autoinhibited by a reprogrammed activation segment. EMBO J. 2006;25(17):4020–4032. doi: 10.1038/sj.emboj.7601285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci. 2006;27(5):244–250. doi: 10.1016/j.tips.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Hansen MK, Watkins LR, Maier SF. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R422–R432. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135(4):1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- Jung TW, Kim HC, El-Aty AA, Jeong JH (2018) Maresin 1 attenuates NAFLD by suppression of endoplasmic reticulum stress via AMPK-SERCA2b pathway. J Biol Chem jbc-RA117 [DOI] [PMC free article] [PubMed]
- Kanoski SE, Zhang Y, Zheng W, Davidson TL. The effects of a high-energy diet on hippocampal function and blood-brain barrier integrity in the rat. J Alzheimers Dis. 2010;21(1):207–219. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PWL, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11(6):453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, McEwen BS. Resilience and vulnerability: a neurobiological perspective. F1000Prime Rep. 2013;5(13):12703. doi: 10.12703/P5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch DL, Dahlberg LL, Patel N, Davis TW, Logan JE, Hill HA, Ortega L. Surveillance for violent deaths—national violent death reporting system, 16 States, 2006. MMWR Surveill Summ. 2009;58(1):1–44. [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schütz G, Joëls M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102(52):19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44(1):161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74(1):5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Welday AC, Song E, Cho J, Sharp PE, Jung MW, Blair HT. Stress-induced alterations in hippocampal plasticity, place cells, and spatial memory. Proc Natl Acad Sci. 2007;104(46):18297–18302. doi: 10.1073/pnas.0708644104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P. Methylphenidate-induced dendritic spine formation and ΔFosB expression in nucleus accumbens. Proc Natl Acad Sci. 2009;106(8):2915–2920. doi: 10.1073/pnas.0813179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knafo S, Venero C, Sánchez-Puelles C, Pereda-Peréz I, Franco A, Sandi C, Suárez LM, Solís JM, Alonso-Nanclares L, Martín ED, Merino-Serrais P. Facilitation of AMPA receptor synaptic delivery as a molecular mechanism for cognitive enhancement. PLoS Biol. 2012;10(2):e1001262. doi: 10.1371/journal.pbio.1001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay LC, Rigby RJ, Wright KL. Cannabinoid-induced autophagy regulates suppressor of cytokine signaling-3 in intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2014;307(2):G140–G148. doi: 10.1152/ajpgi.00317.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gibb R. Brain plasticity and behaviour in the developing brain. J Can Acad Child Adolesc Psychiatry. 2011;20(4):265. [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Calu DJ, Bonci A. Intrinsic plasticity: an emerging player in addiction. Nat Rev Neurosci. 2015;16(3):173–184. doi: 10.1038/nrn3877. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, LaPlant Q, Graham A, Lutter M, Lagace DC, Ghose S. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kyzar EJ, Banerjee R. Targeted epigenetic modulation of gene expression in the brain. J Neurosci. 2016;36(36):9283–9285. doi: 10.1523/JNEUROSCI.1990-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, Nugent AC, Machado-Vieira R, Zarate CA., Jr Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6(3):97–114. doi: 10.1177/2040622315579059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarasimhan H, Chattarji S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS ONE. 2012;7(1):e30481. doi: 10.1371/journal.pone.0030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27(10):2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSalle JM. A genomic point-of-view on environmental factors influencing the human brain methylome. Epigenetics. 2011;6(7):862–869. doi: 10.4161/epi.6.7.16353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer O, Israeli D, Paz R. Behavioral and neural mechanisms of overgeneralization in anxiety. Curr Biol. 2016;26(6):713–722. doi: 10.1016/j.cub.2016.01.023. [DOI] [PubMed] [Google Scholar]
- Leone T, Coast E, Narayanan S, de Graft Aikins A. Diabetes and depression comorbidity and socio-economic status in low and middle income countries (LMICs): a mapping of the evidence. Glob Health. 2012;8(1):1. doi: 10.1186/1744-8603-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305(5688):1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, Klosterkötter J, Hellmich M, Koethe D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2(3):e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126(1):5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11(5):551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26(30):7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Kash TL. Mechanisms of neuroplasticity and ethanol’s effects on plasticity in the striatum and bed nucleus of the stria terminalis. Alcohol Res Curr Rev. 2015;37(1):109. doi: 10.35946/arcr.v37.1.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludka FK, Zomkowski AD, Cunha MP, Dal-Cim T, Zeni ALB, Rodrigues ALS, Tasca CI. Acute atorvastatin treatment exerts antidepressant-like effect in mice via the L-arginine–nitric oxide– cyclic guanosine monophosphate pathway and increases BDNF levels. Eur Neuropsychopharmacol. 2013;23(5):400–412. doi: 10.1016/j.euroneuro.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Lundby A, Secher A, Lage K, Nordsborg NB, Dmytriyev A, Lundby C, Olsen JV. Quantitative maps of protein phosphorylation sites across 14 different rat organs and tissues. Nat Commun. 2012;3:876. doi: 10.1038/ncomms1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Magarin AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69(1):89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Maier SF. Bi-directional immune–brain communication: implications for understanding stress, pain, and cognition. Brain Behav Immun. 2003;17(2):69–85. doi: 10.1016/s0889-1591(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mansur RB, Brietzke E, McIntyre RS. Is there a “metabolic-mood syndrome? A review of the relationship between obesity and mood disorders. Neurosci Biobehav Rev. 2015;52:89–104. doi: 10.1016/j.neubiorev.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Margineanu DG, Gower AJ, Gobert J, Wülfert E. Long-term adrenalectomy reduces hippocampal granule cell excitability in vivo. Brain Res Bull. 1994;33(1):93–98. doi: 10.1016/0361-9230(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Markham A, Cameron I, Bains R, Franklin P, Kiss JP, Schwendimann L, Gressens P, Spedding M. Brain-derived neurotrophic factor-mediated effects on mitochondrial respiratory coupling and neuroprotection share the same molecular signalling pathways. Eur J Neurosci. 2012;35(3):366–374. doi: 10.1111/j.1460-9568.2011.07965.x. [DOI] [PubMed] [Google Scholar]
- Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25(2):89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HG, Wang YT. Blocking the deadly effects of the NMDA receptor in stroke. Cell. 2010;140(2):174–176. doi: 10.1016/j.cell.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RGM. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23(1):649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009;44(10):625–633. doi: 10.1016/j.exger.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci. 2014;34(46):15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22(1):105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8(4):367. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(s1):38–59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress-and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79(1):16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecha M, Feliú A, Carrillo-Salinas FJ, Mestre L, Guaza C. Mobilization of progenitors in the subventricular zone to undergo oligodendrogenesis in the Theiler’s virus model of multiple sclerosis: implications for remyelination at lesions sites. Exp Neurol. 2013;250:348–352. doi: 10.1016/j.expneurol.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Mei Y, Zhang F. Molecular tools and approaches for optogenetics. Biol Psychiat. 2012;71(12):1033–1038. doi: 10.1016/j.biopsych.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio F, Murray BW, Shevchenko A, Bennett BL, Young DB, Li JW, Pascual G, Motiwala A, Zhu H, Mann M, Manning AM. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol Cell Biol. 1999;19(2):1526–1538. doi: 10.1128/mcb.19.2.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci. 2008;105(14):5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Moreno-López C, Santamaría J, Salamero M, Del Sorbo F, Albanese A, Pellecchia MT, Barone P, Overeem S, Bloem B, Aarden W, Canesi M. Excessive daytime sleepiness in multiple system atrophy (SLEEMSA study) Arch Neurol. 2011;68(2):223–230. doi: 10.1001/archneurol.2010.359. [DOI] [PubMed] [Google Scholar]
- Morentin PB, Martinez-Sanchez N, Roa J, Ferno J, Nogueiras R, Tena-Sempere M, Dieguez C, Lopez M. Hypothalamic mTOR: the rookie energy sensor. Curr Mol Med. 2014;14(1):3–21. doi: 10.2174/1566524013666131118103706. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13(4):240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton CD, Pickup JC, Ismail K. The link between depression and diabetes: the search for shared mechanisms. Lancet Diabet Endocrinol. 2015;3(6):461–471. doi: 10.1016/S2213-8587(15)00134-5. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002;22(9):3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Holden SS, Mertens KL, Anahtar M, Felix-Ortiz AC, Wickersham IR. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520(7549):675–678. doi: 10.1038/nature14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Transcriptional mechanisms of addiction: role of ΔFosB. Philos Trans R Soc B Biol Sci. 2008;363(1507):3245–3255. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. ΔFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci. 2001;98(20):11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1β protein in the rat. J Neurosci. 1998;18(6):2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Will MJ, Hansen MK, Hunsaker BN, Fleshner M, Watkins LR, Maier SF. Timecourse and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1β protein response to acute stress. Brain Res. 2000;859(2):193–201. doi: 10.1016/s0006-8993(99)02443-9. [DOI] [PubMed] [Google Scholar]
- Nishi M, Hinds H, Lu HP, Kawata M, Hayashi Y. Motoneuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J Neurosci. 2001;21(23):RC185–RC185. doi: 10.1523/JNEUROSCI.21-23-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njoo C, Agarwal N, Lutz B, Kuner R. The cannabinoid receptor CB1 interacts with the WAVE1 complex and plays a role in actin dynamics and structural plasticity in neurons. PLoS Biol. 2015;13(10):e1002286. doi: 10.1371/journal.pbio.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Panksepp J. The trans-species concept of self and the subcortical–cortical midline system. Trends Cognit Sci. 2008;12(7):259–264. doi: 10.1016/j.tics.2008.04.007. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Johnson JD, Hansen MK, Frank JLW, Maksimova E, Watkins LR, Maier SF. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991(1):123–132. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR. ΔFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci. 2006;26(36):9196–9204. doi: 10.1523/JNEUROSCI.1124-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM. The glucocorticoid receptor: part of the solution or part of the problem? J Psychopharmacol. 2006;20(4_suppl):79–84. doi: 10.1177/1359786806066063. [DOI] [PubMed] [Google Scholar]
- Pasquali R. The hypothalamic–pituitary–adrenal axis and sex hormones in chronic stress and obesity: pathophysiological and clinical aspects. Ann N Y Acad Sci. 2012;1264(1):20–35. doi: 10.1111/j.1749-6632.2012.06569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petra AI, Panagiotidou S, Hatziagelaki E, Stewart JM, Conti P, Theoharides TC. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37(5):984–995. doi: 10.1016/j.clinthera.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfau ML, Russo SJ. Peripheral and central mechanisms of stress resilience. Neurobiol Stress. 2015;1:66–79. doi: 10.1016/j.ynstr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Frohmader KS, Vialou V, Mouzon E, Nestler EJ, Lehman MN, Coolen LM. ΔFosB in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes Brain Behav. 2010;9(7):831–840. doi: 10.1111/j.1601-183X.2010.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, LeDoux J, Silbersweig D. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57(5):464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, Papassotiropoulos A, de Quervain DF. A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proc Natl Acad Sci. 2009;106(45):19191–19196. doi: 10.1073/pnas.0907425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rei D, Mason X, Seo J, Gräff J, Rudenko A, Wang J, Rueda R, Siegert S, Cho S, Canter RG, Mungenast AE. Basolateral amygdala bidirectionally modulates stress-induced hippocampal learning and memory deficits through a p25/Cdk5-dependent pathway. Proc Natl Acad Sci. 2015;112(23):7291–7296. doi: 10.1073/pnas.1415845112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson-Nay R, Wolen AR, Lapato DM, Lancaster EE, Webb BT, Verhulst B, Hettema JM, York TP (2018) Twin study of early-onset major depression finds DNA methylation enrichment for neurodevelopmental genes. bioRxiv 422345
- Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8(9):681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- Russo RM, Gallego A, Comte D, Mocanu VI, Murdie RE, VanDecar JC. Source-side shear wave splitting and upper mantle flow in the Chile Ridge subduction region. Geology. 2010;38(8):707–710. [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37(4):577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23(1):349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar M, Carracedo A, Salanueva ÍJ, Hernández-Tiedra S, Egia A, Lorente M, Vázquez P, Torres S, Iovanna JL, Guzmán M, Boya P. TRB3 links ER stress to autophagy in cannabinoid antitumoral action. Autophagy. 2009;5(7):1048–1049. doi: 10.4161/auto.5.7.9508. [DOI] [PubMed] [Google Scholar]
- Samuni Y, Goldstein S, Dean OM, Berk M. The chemistry and biological activities of Nacetylcysteine. Biochimica et Biophysica Acta (BBA)-General Subjects. 2013;1830(8):4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Sandi C, Pinelo-Nava MT (2007) Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast [DOI] [PMC free article] [PubMed]
- Sapolsky RM. Stress, the aging brain, and the mechanisms of neuron death. Cambridge: MIT Press; 1992. [Google Scholar]
- Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35(12):2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Tsou KA, Ansell EB, Potenza MN, Sinha R. Cumulative adversity sensitizes neural response to acute stress: association with health symptoms. Neuropsychopharmacology. 2014;39(3):670–680. doi: 10.1038/npp.2013.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. Stressful experience and learning across the lifespan. Annu Rev Psychol. 2006;57:55. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Lacadie CM, Constable RT, Seo D. Dynamic neural activity during stress signals resilient coping. Proc Natl Acad Sci. 2016;113(31):8837–8842. doi: 10.1073/pnas.1600965113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327(5967):863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OFX, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97(2):253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OFX, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97(2):253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18(11):1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subhani AR, Kamel N, Saad MNM, Nandagopal N, Kang K, Malik AS. Mitigation of stress: new treatment alternatives. Cognit Neurodyn. 2018;12(1):1–20. doi: 10.1007/s11571-017-9460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J Neurosci. 2011;31(12):4475–4483. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenk CM, Wilson H, Zhang Q, Pitchers KK, Coolen LM. Sexual reward in male rats: effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm Behav. 2009;55(1):93–97. doi: 10.1016/j.yhbeh.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocharus C, Puriboriboon Y, Junmanee T, Tocharus J, Ekthuwapranee K, Govitrapong P. Melatonin enhances adult rat hippocampal progenitor cell proliferation via ERK signaling pathway through melatonin receptor. Neuroscience. 2014;275:314–321. doi: 10.1016/j.neuroscience.2014.06.026. [DOI] [PubMed] [Google Scholar]
- Turner CA, Akil H, Watson SJ, Evans SJ. The fibroblast growth factor system and mood disorders. Biol Psychiatry. 2006;59(12):1128–1135. doi: 10.1016/j.biopsych.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Velayudhan L, Van Diepen E, Marudkar M, Hands O, Suribhatla S, Prettyman R, Murray J, Baillon S, Bhattacharyya S. Therapeutic potential of cannabinoids in neurodegenerative disorders: a selective review. Curr Pharm Des. 2014;20(13):2218–2230. doi: 10.2174/13816128113199990434. [DOI] [PubMed] [Google Scholar]
- Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in the hippocampus: a microdialysis study in freely moving rats. Eur J Neurosci. 1999;11(7):2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- von Arnim CA, Gola U, Biesalski HK. More than the sum of its parts? Nutrition in Alzheimer’s disease. Nutrition. 2010;26(7):694–700. doi: 10.1016/j.nut.2009.11.009. [DOI] [PubMed] [Google Scholar]
- von Engelhardt J, Doganci B, Jensen V, Hvalby Ø, Göngrich C, Taylor A, Barkus C, Sanderson DJ, Rawlins JNP, Seeburg PH, Bannerman DM. Contribution of hippocampal and extra-hippocampal NR2B-containing NMDA receptors toperformance on spatial learning tasks. Neuron. 2008;60(5):846–860. doi: 10.1016/j.neuron.2008.09.039. [DOI] [PubMed] [Google Scholar]
- Vos PE, Battistin L, Birbamer G, Gerstenbrand F, Potapov A, Prevec T, Stepan CA, Traubner P, Twijnstra A, Vecsei L, Von Wild K. EFNS guideline on mild traumatic brain injury: report of an EFNS task force. Eur J Neurol. 2002;9(3):207–219. doi: 10.1046/j.1468-1331.2002.00407.x. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Richter-Levin G. Physiological dissociation in hippocampal subregions in response to amygdala stimulation. Cereb Cortex. 2005;15(11):1815–1821. doi: 10.1093/cercor/bhi058. [DOI] [PubMed] [Google Scholar]
- Weder N, Zhang H, Jensen K, Yang BZ, Simen A, Jackowski A, Lipschitz D, Douglas-Palumberi H, Ge M, Perepletchikova F, O’Loughlin K. Child abuse, depression, and methylation in genes involved with stress, neural plasticity, and brain circuitry. J Am Acad Child Adolesc Psychiatry. 2014;53(4):417–424. doi: 10.1016/j.jaac.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarom O, Maroun M, Richter-Levin G (2008) Exposure to forced swim stress alters local circuit activity and plasticity in the dentate gyrus of the hippocampus. Neural Plast [DOI] [PMC free article] [PubMed]
- Young LT, Dowlatshahi D, MacQueen GM, Wang JF. Increased temporal cortex CREB concentrations and antidepressant treatment in major depression. Lancet. 1998;352(9142):1754–1755. doi: 10.1016/S0140-6736(05)79827-5. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A. An essential role for ΔFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9(2):205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]
- Zaremba PD, Bialek M, Blaszczyk B, Cioczek P, Czuczwar SJ. Non-epilepsy uses of antiepileptic drugs. Pharmacol Rep. 2006;58(1):1. [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. Modelbased analysis of ChIP-Seq (MACS) Genome Biol. 2008;9(9):R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang L, Lou DW, Nakabeppu Y, Zhang J, Xu M. The dopamine D1 receptor is a critical mediator for cocaine-induced gene expression. J Neurochem. 2002;82(6):1453–1464. doi: 10.1046/j.1471-4159.2002.01089.x. [DOI] [PubMed] [Google Scholar]
- Zhou B, Lapedriza A, Xiao J, Torralba A, Oliva A (2014) Learning deep features for scene recognition using places database. In: Advances in neural information processing systems, pp 487–495
- Zitman FMP, Richter-Levin G. Age and sex-dependent differences in activity, plasticity and response to stress in the dentate gyrus. Neuroscience. 2013;249:21–30. doi: 10.1016/j.neuroscience.2013.05.030. [DOI] [PubMed] [Google Scholar]