Abstract
The paper aims to provide a state-of-the-art review of methods for evaluating the effectiveness and effect of unloader knee braces on the knee joint and discuss their limitations and future directions. Unloader braces are prescribed as a non-pharmacological conservative treatment option for patients with medial knee osteoarthritis to provide relief in terms of pain reduction, returning to regular physical activities, and enhancing the quality of life. Methods used to evaluate and monitor the effectiveness of these devices on patients’ health are categorized into three broad categories (perception-, biochemical-, and morphology-based), depending upon the process and tools used. The main focus of these methods is on the short-term clinical outcome (pain or unloading efficiency). There is a significant technical, research, and clinical literature gap in understanding the short- and long-term consequences of these braces on the tissues in the knee joint, including the cartilage and ligaments. Future research directions may complement existing methods with advanced quantitative imaging (morphological, biochemical, and molecular) and numerical simulation are discussed as they offer potential in assessing long-term and post-bracing effects on the knee joint.
Keywords: Unloader knee brace, Knee osteoarthritis, Quantitative imaging, Finite element modelling, Post-bracing effects
Background
Osteoarthritis (OA) is one of the leading causes of physical disability in the world, affecting a growing portion of the elderly and has no cure. The healthcare costs associated with this disability are rising at an exponential rate, especially in the ageing community, and has become a significant public health issue [1, 2]. While there is no consensus on where, when, and how OA begins in the knee, it is understood that it involves soft tissues (articular and meniscal cartilage and cruciate and collateral ligaments), hard tissues (femur and tibia bones), loading characteristics, and stability of the joint [3]. The complex interplay of structural and biochemical changes in the knee joint results in common complaints of joint pain and instability in addition to other clinical symptoms of joint space narrowing (JSN), joint stiffness, and limited range of motion [4]. Biomechanical studies have shown that OA leads to approximately 60–80% of the total knee load passes through the medial compartment due to the loss of mechanical capabilities of the cartilage [5]. It also alters gait characteristics by decreasing external knee extension moments in terminal stance [6], and increases external knee adduction moments (KAM) in stance phase [7, 8]. This leads to a sedentary lifestyle, which starts a process of deterioration of these tissues.
Only biomechanical interventions or surgical replacement of the knee joint can reduce or control the pain, improve function, enhance the quality of life, and in turn, stops or slows down the cycle. Clinicians do not recommend surgical replacement or realignment of the joint as the primary treatment option for young and physically active persons and patients with significant confounding clinical factors. These factors are high blood pressure, diabetics, excessive body weight, heart disease, cartilage damage due to trauma, and documented treatment failures, including nonsteroidal anti-inflammatory medications, steroid injections, and viscoelastic supplementation. There are several non-surgical and biomechanical intervention treatment options available to improve the function of the knee and physical mobility, as well as reduce pain by unloading the affected knee compartment, while avoiding negative effects of them.
Researchers have used various techniques to shift the weight away from the diseased knee compartment, such as, altered gait kinematics, adopting novel gait patterns through gait retraining [9, 10], employing assistive and gait altering devices [11–13], and reducing body mass at the external knee adduction moment [14]. Unloader knee braces, wedge shoes, insoles, and joint strengthening physical exercises are the most often prescribed methods by clinicians as conservative treatment options for such patients [9, 13, 14]. Among these treatments, unloader braces are the most often studied and effective treatment option for patients with medial OA, since they offer a reasonable alternative to surgical realignment or replacement procedures [15–17].
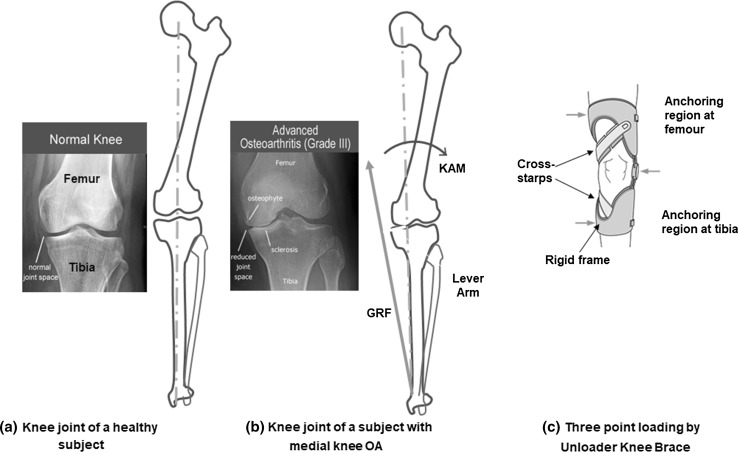
The unloader braces reduce the KAM by applying an opposing external valgus moment about the knee joint based on the three-point loading principle (shown in Fig. 1c), which improves the distribution of the compressive load and corrects alignment [18–20]. Clinicians and researchers have employed several methods to measure the effectiveness of the unloader braces. These methods are designed in terms of reduced pain, improved function, and indirectly quantifying the amount of unloading that happens due to bracing by quantifying the counteracting valgus moment and monitoring treatment in clinical settings. These methods can be broadly classified into three main categories, namely: (1) morphology-based, (2) biomechanics-based, and (3) perception-based methods.
Fig. 1.
Effect of OA on Knee Joint. a Healthy knee joint; b narrow joint space along the medial; c force acting by the unloader brace using three-point loading mechanism
Several systematic reviews have been published on quantifying the effectiveness of the unloader braces in improving clinical outcomes and mechanical leverage. However, to the best of our knowledge, there is no reported research, which surveyed and categorized evaluation methods for quantifying the long-term effects of unloader braces on the health of the knee joint tissues. In this paper, a state-of-the-art review of the current evaluation methods is presented, as well as potential techniques to develop new evaluation methods and quantitative measures, and directions for future research.
Change in joint loading characteristics: mechanobiological perspective
Normal physiological loading is essential for facilitating and maintaining the health of the soft tissues and bones [24]. The soft tissues are the primary load-bearing zone of the knee joint during gait that allows smooth joint movement and distributes the forces across the tibio-femoral joint. They contain cells (chondrocytes) and extracellular matrix (ECM) with water, collagen fibers, and protein molecules [21, 22]. Normal physiological loading creates dynamic compression in the musculoskeletal system, cartilage, and meniscus. The loading at the foot, transmitted via knee joint cartilage. This loading transfer mechanism involves sliding and rigid-body displacement of the cartilage at the tibio-femoral interface. There is also a constrained and unconstrained movements of the cartilage in the direction and opposite direction of the load transfer, respectively [23]. This loading generates compression strain along the loading direction and shear strain in the anterior-posterior plane of the femur and tibia. Subsequently, it leads to complex mechanical and chemical compositional changes within the cartilage tissue (matrix and cell deformation, hydrostatic and osmotic pressure, fluid flow, altered water content, ion concentration, and fixed charge density). These changes are detected by mechanoreceptors, which initiate intracellular signaling, leading to the tissue remodeling process, and restoring its original shape upon load removal [24].
The coarse and circumferential Type I collagen fiber bundles of the meniscus provide compressive strength and dynamic shear modulus that are one-half and one-fourth to one-sixth times greater than the compressive strength and dynamic shear modulus of articular cartilage, respectively [25]. Authors have reported that the posterior horn of the medial meniscus had the least movement due to its attachment to the tibial plateau by the menisci-tibial portion of the posterior oblique ligament, and the anterior horn of the lateral meniscus had the most significant movement [26, 27]. During daily physical activity, the menisci experience high levels of axial tibio-femoral forces that compress the menisci at the articulating surface of the joint. The horn attachments and wedge shape of the meniscus convert the vertical compressive forces to horizontal hoop stresses along the circumferential collagen fibers of the meniscus [22]. As a result, the meniscus deforms radially and the generated shear forces between the collagen fibers changes the morphology of the collagen. This change in morphology of collagen fibers transduces the applied compressive and shear forces into tensile hoop forces [28]. However, the firm attachments by the anterior and posterior ligaments prevent the meniscus from extruding peripherally during loading [29].
Osteoarthritis alters the biomechanical characteristics and bio-chemical composition of the knee joint. These changes are cyclic in nature and progressively damages the joint with day-to-day physical activities. Apart from soft tissues, OA also affects the structural, biochemical, and load-bearing ability of the subchondral bone [30]. During the initial stages of OA, elevated bone remodeling and subchondral bone loss were observed due to microdamage in the subchondral region [30]. These damages induce porosity in the subchondral region and gradually decrease the thickness of the subchondral plate. In the late stage of OA, loading and cartilage thinning increases bone density, bone volume, thickens the subchondral bone plate, increases trabecular thickness and thickness of the calcified region, and decreases trabecular separation and bone marrow spacing [31, 32]. The meniscal damages also lead to pathological changes in subchondral bone (increased bone mineral density, bone cysts, and bone marrow lesions) [33, 34].
Current qualitative and quantitative measures
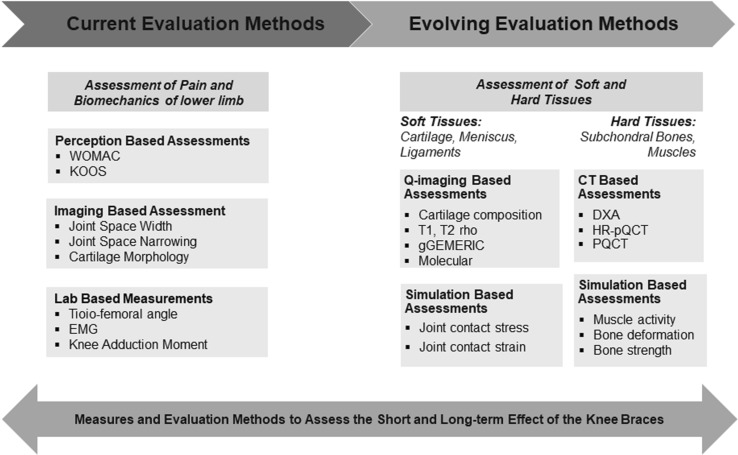
This section outlines the categorization of existing methods, measurable variables, techniques, tools, and survey instruments to diagnose knee health during post-bracing condition. The most often used surrogate biomarkers to study the efficacy of the braces are (i) semi-quantitative measures, including knee pain, joint function, and quality of life based on self-reported questionnaires, and (ii) quantitative measures, including morphological changes and biomechanical loading effects in the tibio-femoral joint. Choice of these measures primarily depends upon the purpose of the evaluation, which can be either treatment monitoring, functional performance, mechanical effectiveness, or energy expenditure, such as, clinical symptoms, strength, and gait, as shown in Table 1.
Table 1.
Qualitative and quantitative measures to evaluate the effectiveness of braces on overall knee health
| Symptoms | Measures outcomes | Evaluation methods and instruments |
|---|---|---|
| Clinical symptoms | Knee pain, joint stiffness and function, quality of life, physical activity | VAS, WOMAC, QoL, KOOS-QoL, IPAQ, UCLA Scale, IKDC |
| Functional effects | Knee proprioception, stability, posture control | KOOS, KSS, IPAQ, VAS, Lequesne index, Safety and observance |
| Morphological effect | JSW, JSN, tibio-femoral Angle, hip–knee–ankle angle, condylar separation, angle condylar separation distance | Weight-bearing lower limb radiographs, ultrasound, fluoroscopy, gait analysis, loaded MRI, morphological MRI, WORMS, BLOCKS grading |
| Gait effects | Frontal plane kinematics, sagittal plane kinematics, lower limb malalignment, knee adduction flexion angles, knee forces and moments | 3D motion analysis, dynamic simulations, gait analysis |
| Muscle strength | Muscle co-contraction, medial/lateral, flexors/extensors co-contraction ratios | Electromyograms (EMG), muscle MRI |
Perception-based evaluation methods
The perception-based evaluation methods are mainly self-reported outcomes based on different types of survey questionnaires and visual scales to define the state of disease progression at the point of monitoring the treatment. The most frequently used questionnaires in assessing the effectiveness of valgus unloader braces in clinical settings are the Visual Analogue Scale (VAS), Knee Osteoarthritis Outcome Score (KOOS), Knee Society Score (KSS), Quality of Life Scale (QOLS), Western Ontario and McMaster Universities’ Arthritis Index (WOMAC), and the International Physical Activity Questionnaire (IPAQ).
The WOMAC score has been used extensively in both observational and treatment monitoring studies to examine the changes due to bracing treatment during walking and stair-climbing tests [35–40]. The authors found that a positive relationship existed between brace wear usage and activity level, and improvements in WOMAC pain, stiffness, and function. Rezaeian et al. [41] studied the unloading effect of a valgus brace by comparing the patients’ pain and function from WOMAC scores with the alignment angle and reported that the WOMAC scores (pain and function) were better biomarkers than the alignment angle.
Kirane et al. (2010) used KOOS scores to study the effectiveness of the unloader brace on knee pain, structure, and function in medial various knee OA and observed a significant reduction in knee pain and functional improvement [11–13]. The VAS, has been used to measure the efficacy of the unloader braces in subjects with medial knee OA and reported a significant reduction of pain throughout the day and improvement in engaging in various activities as well (stair ascending and descending, and comfortable while speed walking) [11, 13, 38]. Thoumie et al. [42] assessed the effect of unloading knee brace after 6-week treatment in terms of 24 h-pain relief (100-mm VAS) as the primary outcome at 6 weeks, the secondary outcomes including pain on motion (100-mm VAS), function (Lequesne index), safety and observance. Hjartarson et al. [43] studied the effect of the unloader braces by using two different scores (KSS and KOSS) and reported that there was a significant improvement in both scores over 52 weeks of the bracing treatment.
There are a few of other instruments, including the IPAQ [44], multi-item physical activity questionnaires [University of California at Los Angeles (UCLA) activity rating scale] [45, 46], and the International Knee Documentation Committee (IKDC). All are used to study knee health and monitor the level of the physical activity [47, 48]. However, these self-reported evaluation methods based on physical activity have not been implemented in evaluating knee health during bracing treatment and can be potential biomarkers to assess the duration and doses of the bracing treatment and monitor the knee health during the post-bracing condition based on the level of physical activity.
All of these perception-based methods assess pain, function, and quality of life, which are the qualitative biomarkers of the disease progression. The quality of these studies entirely depends on the disease condition, mood, sleep, and physical activities, and the score provided by the patients. Even though these methods are well accepted by clinicians, they are unable to quantify the morphological, biochemical, and biomechanical changes in the soft tissue regions, are not sensitive enough to capture the improvement of the knee health during the bracing treatment in patients with mild OA, and are incapable of predicting the long- and short-term effects on the overall physical activity of the subject.
Morphology-based evaluation methods
The metabolic process of OA initiates with the thinning of the superficial layer of the articular cartilage that gradually disrupts the calcified cartilage and leads to secondary changes in the subchondral bone region [49, 50]. This degradation process affects the morphological changes (osteophytes, subchondral sclerosis, cysts, bone plate thinning, and narrowing down of the joint space) of the bone [51], thickness, surface area, and volume of the weight-bearing region of the articular and meniscal cartilage. Currently, several imaging modalities, including the standard standing radiography, MRI, CT scans, and ultrasound have been used to capture the morphological changes mentioned in Table 1. As per the complexity and computational requirement of the imaging techniques, these methods are broadly classified into semi-quantitative and quantitative morphological measurement.
Direct morphological measurements help in diagnosing the disease concerning one dimensional measure. The standing weight-bearing antero-posterior lower limb radiograph is the most traditional method to capture measures, including JSN angle, joint space width (JSW), minimum JSW (MJSW), hip–knee–ankle angle, condylar separation distance, and condylar separation angle [52]. The standing radiographs also capture the structural changes (osteophytes, subchondral sclerosis, cyst) during the later stages of OA. The Kellgren–Lawrence (K–L) grading method from the standard radiographic images of the lower limb is another way to differentiate the stages of OA [53]. Studies based on radiography and fluoroscopy-based gait analysis quantify the morphological change in the knee joint in terms of the tibio-femoral angle, joint space, K–L grade, compartmental separation, and condylar separation angle. Studies concluded that the unloader braces significantly correct these morphological changes [11, 54–56]. While direct radiographic measures are the less expensive way to measure OA progression with fewer measurement steps [57], they fail to visualize the morphological feature of the soft tissues [58]. Its accuracy and precision depend on the reproducibility of radiographic images and adequate positioning of the knee [59]. Due to the lack of references in the radiographic images, clinicians face difficulties validating MJSW with other imaging modalities. As the medial and lateral tibio-femoral joint compartments are physically connected, the narrowing of one compartment can result in the widening of the other, adding further complications to appropriate interpretation [60].
Due to advanced image processing techniques, MR-based semi-quantitative morphological imaging techniques, including Whole-Organ Magnetic Resonance Imaging Score (WORMS), Boston Leeds Osteoarthritis Knee Score (BLOKS), and MRI Osteoarthritis Knee Score (MOAK) examine morphological abnormalities at various anatomical sub-region locations of soft tissue and bones [61–65]. The magnetic resonance observation of the cartilage repair tissue (MOCART) scoring system is very popular in studying the health of articular cartilage and the surrounding structures during graft repair, chondrocyte transplantation, and autologous matrix-induced chondrogenesis [66–69].
The semi-quantitative scoring methods, WORM and BLOKS, provide an indirect regional and sub-regional assessment of cartilage, meniscal morphology, and subchondral plate degeneration in tibio-femoral joint MR-based measurements and are sensitive enough to capture the degenerative changes in soft tissues and other compartments, capable of determining the spatial distribution of femorotibial cartilage loss. In early OA, cartilage swelling is visible under the unloaded MRI image, but it is in a compressed state in radiographs [70]. The JSN obtained from the MR was more sensitive than the fixed-flexion radiographs, and the location-specific measures of JSW obtained from the MR show greater responsiveness than minimum JSW obtained from radiographs [71].
Biomechanics-based evaluation methods
Mechanical loading plays a key role in the progression of OA, and the medial tibio-femoral compartment of the knee undergoes 60 to 80 percentage of the total knee load resulting in higher KAM and various deformity [8, 72]. For equilibrium and stability of the knee during gait, this external KAM must be balanced by the contact force between the tibia and femur along the medial compartment and the magnitude of the KAM has been used as a measure of loading distribution [73, 74]. Several studies have reported that due to the increased load and accelerated cartilage damage in the medial compartment of the knee, subjects with OA have greater muscle co-contraction [75–77]. Therefore, the strength and co-contraction of the muscles around the knee joint and the lower limb are recently considered the indirect measure of the loading in the medial compartment. To study the efficacy of the unloader knee brace, biomechanical methods, including 3-D motion analysis, force plate experiments, and electromyographic patterns have become popular in research settings. Therefore, the strength and co-contraction of the muscles around the knee joint and the lower limb are recently considered the indirect measure of the loading in the medial compartment. To study the efficacy of the unloader knee brace, biomechanical methods, including 3-D motion analysis, force plate experiments, and electromyographic patterns have become popular in research settings.
Three-dimensional motion analysis
Gait analysis uses a motion capture systems and force plates to estimate the kinematics and kinetics of the patient during bracing treatment. Several studies reported the positive effect of braces on the frontal and sagittal plane kinetics [78–81], medial compartment load in vivo condition [82, 83], tibio-femoral contact force, and lower limb alignment [84–88]. Kutzner et al. investigated the effect of three-point loading exerted by valgus braces on the medial compartment load in vivo by using strain gauges and reported that the medial load reduces during walking and stair ascension/descension [83, 89]. The unloader braces with different valgus adjustments have a significant effect on the first and second peaks of KAM, the knee adduction angular impulse, improvement in knee pain and joint function [81, 85–87, 90, 91], and the knee adduction and flexion angle [87, 88]. However, this method is not promising in the study of the kinematic and kinetic of the lower limb for various physical activities with high-flexion, such as squatting and kneeling, due to the limitations of the available measuring devices, and it fails to detect the contact pressure and internal stress distribution in the soft tissues and bone region.
Strength measurement tests
Electromyogram is another assessment technique used to diagnose knee OA by measuring the electrical activity of the lower limb muscles to show whether the pain is due to injury of a nerve or muscle tissue and locate the damaged nerve [92]. Studies have reported that OA subjects showed high KAM, reduced knee flexion, quadriceps weakness, and knee stiffening during weight-bearing [93, 94]. These in turn increase the co-contraction of muscles [95, 96] and joint contact pressures around the knee [97], and accelerate the cartilage damage [98]. Hence, the lost strength of the quadriceps and hamstring and co-contraction ratios of the quadriceps/hamstring and quadriceps/gastrocnemius, represent the indirect measure of the mediolateral distribution of load across the knee, and are useful in studying the effectiveness of the braces [18, 99–102].
Pagani et al. reported a significant decrease in muscle activity and co-contraction level with valgus knee bracing treatment [84, 103]. The authors studied the response for valgus unloader brace on the strength of hamstrings [39], quadriceps maximum voluntary contraction, and arthrogenous muscle [104] and reported that only arthrogenous muscle had significant changes. Electromyography (EMG) technique lacks precise control in large muscles, such as quadriceps and those engaged during knee movement, due to the interweaving arrangement of muscle and difficulties in distinguishing between the neural and muscular contributions to force generation during various physical activities.
Emerging and potential evaluation methods
Even though existing methods are routinely used by clinicians and researchers in studying knee joint health during OA progression, clinicians face challenges in quantifying the effects of the braces on soft and hard tissues, especially in long-term uses. The long-term effects of the biomechanical and physiological changes on the tissues in terms of the shift in contact pressure, contact area, loading characteristics, and response to changes in physiological loading has not yet been studied. Therefore, there is a need for new evaluation methods that can address the consequences of braces on soft and hard tissues during the post-bracing phase of the treatment. Based on these measures, the clinicians would determine the optimal dose, duration, and frequency of brace usage as per the patient’s knee condition to avoid worsening knee health.
Recent advances in imaging techniques, equipment, wearable technologies, computing resources, and computational methods offer several non-ionizing radiation image-based measures, such as T1rho, T2, dGEMRIC, and Sodium Magnetic resonance imaging, which can assess the soft tissue’s health in vivo conditions. The MR-based T1rho and T2 relaxation times describe the changes in the amount of proteoglycan and the water content in the extracellular matrix of the soft tissues and have been used as potential measures to assess early OA [105].
Luke et al. studied the health of meniscus and articular cartilages in marathon runners and showed that long-distance running significantly affects both cartilages biochemically (measured with T1rho and T2), but with no significant morphological changes [106, 107]. Moreover, the results showed that after 48–72 h of the competition, runners had significantly higher water content and depleted proteoglycans. Water content came back to pre-competition level, while the ratio of proteoglycan to water content did not, which indicates more persistent changes in the ECM after a marathon. Souza et al. [108] evaluated the effects of mechanical loading on the knee, using T1rho and T2 relaxation times in patients with and without OA and observed that acute loading significantly decreases T1rho relaxation times in both medial and lateral compartments. Calixto et al. [109] showed that an acute loading affects relaxation times of the menisci in individuals with and without knee OA in a non-uniform manner from the inner periphery to the outer periphery of the tissue.
Subburaj et al. studied the effect of running and acute loading on articular cartilage and meniscus in healthy subjects, using T1rho and T2 as surrogate biomarkers [110, 111]. After 30 min of running exercise, there was a significant reduction in the relaxation times in the weight-bearing region of the articular cartilage and anterior and posterior horns of the medial meniscus due to structural and compositional changes of the collagen matrix and water content. Even though these advanced quantitative techniques have been used in research settings to capture the biochemical and morphological changes in healthy and OA subjects, there still has not been any attempt to apply these techniques to study, understand, and evaluate the effects of unloader braces on the overall health of the knee joint tissues.
The delayed gadolinium enhanced MRI of cartilage (dGEMRIC) is an indirect non-invasive technique for quantifying the cartilage structural composition (glycosaminoglycans (GAGs) ) from T1rho because there is an inverse relationship between the negatively charged contrast agent and the GAGs [112, 113]. The authors reported that proteoglycan content in menisci with meniscal tears increased during meniscal degeneration [22] and identified pre-radiographic degenerative changes in the meniscus [114, 115]. In addition, sodium MRI measures the fixed charged density, which is directly related to the sodium concentration in the cartilage, and have been established to be a sensitive marker for estimating proteoglycan loss and cartilage degeneration [116].
Newbould et al. [117] compared subjects with OA and healthy ones using sodium MRI and observed that T1-weighted sodium MRI relaxation time for OA subjects was significantly different from healthy subjects. They reported stability and reproducibility of the sodium signal in both healthy and diseased cartilage using T1rho. These techniques capture the complex degradation process in the cellular and macromolecular level. MR-based quantitative and molecular imaging modalities have been popular to analyze the morphology (shape, structure, and spatial distribution) and biochemical composition of soft tissue during OA progression. While these advanced imaging modalities offer additional measures (morphological and biochemical) to study the knee, they have not yet been systematically studied or explored in clinical settings to evaluate the efficacy of the braces on the knee joint during OA.
One of the new promising behavioral adaptational methods is Gait Retraining [10]. This approach is directly addresses the patient’s underlying motor pattern through real-time feedback. Cheung et al. [10] evaluated the effectiveness gait retraining in patients with early knee osteoarthritis by measuring WOMAC score, KAM and KFM, during pre-training, post-training, after six-month follow-up. WOMAC score and KAM were found to be significantly lower than the pre-training and the effect was maintained during 6-month follow-up. However, there was no significant changes in KFM during the walking cycle which indicates gait retraining could be useful in reducing knee pain in patients with early knee osteoarthritis [10]. The Biomechanical study shows that Ankle-foot orthosis (AFO) and wedged shoes are equally effective in unloading the medial compartment compared to the unloader brace [16]. The author examined the unloading effects of AFO, knee brace and wedged shoes in subjects with KOA and reported that all devices significantly decrease the first peak of KAM. Therefore, both gait retraining and other orthosis devices could be combined with the bracing treatment to enhance the effectiveness of the unloader braces.
Future directions
The global knee brace market growth is driven by an increasingly ageing population, the number of patients with primary OA, orthopedic knee surgeries, and a rising number of leg injuries in athletics that has led to secondary OA. With the increase in awareness about preventive care for knee health during OA and managing the disease without surgery or joint replacement, there is growing demand for unloader braces among the ageing population and young generations affected by secondary knee OA.
Unloader braces are more effective at reducing pain as compared to other braces (neutral braces or neoprene sleeves) [118]. They are effective treatment for improving pain secondary to medial compartment knee osteoarthritis. Majority of clinical studies on unloader braces reported improvement in pain outcomes using unloader braces. However, variable results were reported as to whether unloader braces significantly improved functional outcomes and stiffness. The clinical community is still unclear about the functional and stiffness effects of the valgus unloader braces even though they still prescribe [118]. As of now, clinicians determine the optimal dose, duration, and frequency of brace usage as per the patient‘s knee condition. When there is a deviation from the protocol, the overall knee health may worsen and lead to irreversible changes to the tissues. The optimal dose of the bracing treatment depends on the complete assessment of joint health and day-to-day physical activities.
Unloader braces act directly on the joint space of the tibio-femoral joint during medial OA and also affect the hip joint [81]. Studies have shown that the gait of knee OA patients with and without bracing affects the involved and contralateral lower limbs [81]. Decreased external KAM of the affected joint and altered ipsilateral hip kinematics and kinetics and contralateral knee and hip kinetics during the gait were observed in patients with braces. Even though the changes in these joints have a minimal clinical impact, they may cause degenerative point stress due to the change in the gait pattern with bracing during routine day-to-day activities [81, 119, 120].
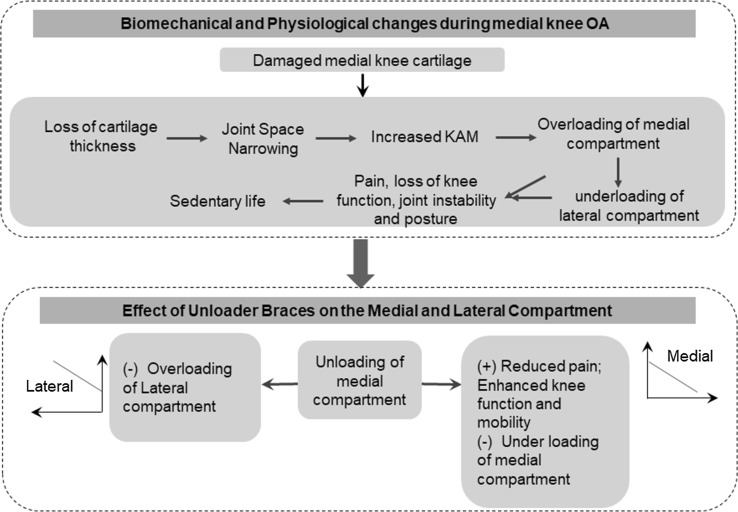
Studies have demonstrated that patients with severe knee OA have an increased hip abduction angle and lean their trunks during the stance phase to reduce the external KAM. This suggests that this compensation would positively affect the progression of the ipsilateral hip OA [121]. These findings also suggest that patients with medial knee OA suffer varying degrees of adverse effects on other joints by wearing unloading braces, as shown in Fig. 2. Therefore, it is necessary to consider not only the involved knee but also the kinematics and kinetics of the contralateral hip and ankle joints, and the health of the soft and hard tissues as additional measures during the evaluation of the bracing effect [122].
Fig. 2.
Knee physiology. Effects of unloader braces on knee health
Although mechanical loading is important for cartilage health [24], altered cyclic loading due to malalignment and joint injury can directly damage the cartilage and subchondral bone. Any abnormal loading (over- or under-loading) of the joint increases the contact stresses and contact kinematics in the weight-bearing regions of the cartilage [123, 124] and in turn modifies the gait by altering the KAM and rotational kinematics of the joint. As a result, both tibio-femoral cartilages fail to adapt the loading during physical activities [125, 126]. The increase in the number of loading cycles and frequency greatly affects the stiffness and thickness and recovery time of the cartilage [118, 127].
During the bracing treatment, the change in the knee alignment and physical activity gradually introduce additional complex dynamic loading along the lateral compartments [122]. Cusin et al., incorporated a measuring device into the brace, measured the horizontal forces at the leg-orthosis interface in the frontal plane and the resulting valgus moment based on the kinematic data. The author estimated the valgus moment produced during the stance phase of gait and proposed that the unloader knee brace must be designed to apply dynamic force (no moment during swing phase of gait) [128].
Several MR studies reported that the altered physiological loading experienced by the cartilage changes the content and orientation of the collagen network, hydration level, content, and distribution of proteoglycan in the long-term [129]. It also alters the quantitative imbalance between anabolic and catabolic activity, resulting in depletion of ECM components [21, 24, 130]. This depletion affects the outer surface and causes substantial damage to the collagen networks, reducing the proteoglycan level over time, resulting in permanent loss of the mechanical function of the cartilage that, in turn, affects the overall knee function.
Similarly, the bone remodeling process is sensitive to the magnitude of the mechanical loading. Animal studies showed that running enhances bone remodeling in subchondral bone regions, resulting in a thicker subchondral bone plate and higher trabecular volume [131]. Stress exercise increases bone formation and bone density and thereby increases the strength of the subchondral bone [132]. Unloading during bed rest and weightlessness in spaceflight significantly reduces its mass and strength [133, 134]. Significant joint unloading disturbs the delicate balance of the homeostasis of weight-bearing bone by uncoupling the bone resorption (osteocytes) cells from bone formation (osteoblast) cells, which leads to decreased bone formation and bone mass [133]. This cyclic pattern of loading and unloading happens spatially and temporally between the medial and lateral compartments, which specifically worsens the health of the cartilages (articular and meniscal) and subchondral bone. Therefore, the biochemical, biomechanical, and structural changes of these tissues during the bracing treatment could be used as potential biomarkers to assess the effectiveness of the braces.
Currently, there is no reported quantitative evidence in the literature on the effectiveness of bracing treatment (i.e., Is it suppressing the disease, progressing the disease in other compartments, or affecting the ligaments, cartilages, and bones? And how does it affect these tissues in the short- and long-term?). Based on the literature, it is speculated that the change in physiological loading due to the braces adversely affect the degree of irreversibility associated with the morphological and biochemical characteristics of the soft tissues, bones, and muscles. If the post-treatment effects associated with the bracing treatment are not entirely reversible, then the efficacy and duration of the treatment should be questioned and regularly monitored. Moreover, prolonged usage of the brace may trigger the lower limb muscles, increasing the intramuscular pressure beneath the brace, reducing the muscle blood flow, and subsequently induce premature muscle fatigue and cause biochemical changes in the cartilages of the tibio-femoral and patella-femoral compartments.
During bracing treatment, clinicians must consider the morphological and biochemical characteristics of soft- and hard tissues while evaluating the short- and long-term effects of the knee braces. The effect of the braces on the other joints must be evaluated by studying several parameters, including morphological changes to the tissues in the joint, contact pressure, the contact area of various tissues, structural misalignment of the tibio-femoral and patella-femoral joints, and the amount of reduction in joint pain. In the literature, all the reported studies on bracing treatment have focused either on the clinical outcomes in terms of pain, function, and quality of life, or effectiveness of joint unloading in terms of quantitative biomechanics, yet, have neglected the quantifying parameters affecting the tissues in the joint in both the short-and long-term.
There is no systematic study on the effect of braces on biochemical and morphological characteristics of the soft tissues, the degree of reversibility of these physiological characteristics associated with unloading and misalignment, the biomechanical effect on other joints and compartments in the joint itself, and the necessity for the advanced quantitative biomarkers for evaluating the bracing treatment. The internal joint contact stress and distribution of forces in the knee joint are directly related to its functional activities, which can help in better diagnosing the disease and health of the soft tissue during the bracing treatment. Therefore, there is a need for developing a detailed assessment of the effectiveness of the bracing treatment. Thus, it is recommended that additional research and clinical studies further investigate the short- and long-term consequences of the braces on the cartilages and bones. These studies may help in improving patient health, understanding the effect of braces, and setting up comprehensive evaluation strategies of the braces that physicians can follow in evaluating the knee joint health at the post-bracing period of knee OA. Controversy still exists as to whether the brace has a real effect on tibiofemoral joint space in the medial compartment during functional activity in OA patients. Previous studies have determined the effect of OA unloader braces using techniques including force sensors, torque analysis, and two-dimensional fluoroscopy with mixed results regarding the effect on knee joint space. These results are limited by a lack of dynamic in vivo quantitative data for joint space because of the difficulty of performing in vivo analysis during movement.
Assessment of the effectiveness of the bracing treatment demand three independent aspects: (i) establishment of additional independent measures, (ii) developing diagnostic techniques to assess these measures, and (iii) studying the short- and long-term effects of the braces on the joint degeneration process. These measures must capture the patient’s perception of pain reduction, changes in knee alignment, improved knee function, changes in muscle activation, and a morphological and biomechanical assessment of the soft and hard tissues. Due consideration of both short- and long-term structural, biomechanical, and biochemical consequences of using these braces must be given when prescribing bracing treatment. There is a need to develop an integrated methodology to assess the short-term and long-term effects of bracing on soft and hard tissues, as well as design, develop, and validate clinically obtainable quantitative measures and establish evaluation techniques and tools to measure patient compliance.
An integrated assessment methodology
In order to evaluate the effectiveness of the braces on knee joint health, this study proposes an integrated methodology, combining very distinct but advanced quantitative techniques: quantitative magnetic resonance imaging (qMRI) and patient-specific finite element analysis of 3D anatomical models reconstructed from MR images (Fig. 3). In addition to the existing pain-score and other functional measures, qMRI techniques, including T1rho and T2 MR relaxation times, sodium, dGEMRIC, and bone measures would capture the biochemical and morphological health of the tissues, (i) during pre- and post-treatment periods to assess the lasting effect of a brace, and (ii) during the bracing treatment to assess and monitor the acute effect of a brace. Patient-specific finite element analysis of 3D anatomical models reconstructed from MR images and other computational models can provide the duration of bracing treatment, the health of tissues, and knee kinematics under low- and high-flexion angles and under strenuous activities and become an efficient tool for the analysis of the knee brace design. This methodology can also be a mechanism to study the dosage required to alleviate pain and to avoid damage to the lateral compartment in the process of unloading the medial compartment during medial knee OA.
Fig. 3.
Evaluation methodology. Schematic representation clinical procedures to measure the effectiveness of unloader braces on the joint
Quantitative imaging should be included in routine clinical monitoring protocol and capture the images from the patients over the bracing treatment, study the quantitative features of soft tissues (estimation of cartilage volume and thickness and relaxation times) along the longitudinal and transverse directions, and analyze the computational models. As of now, these advanced quantitative imaging techniques have only been used in research settings to capture the biomolecular and structural health of the soft tissues. While evaluating the efficacy of the bracing treatment, clinicians could use T1rho and T2 measures, sodium, and dGEMRIC measures in addition to existing measures. These measures may help in predicting the bimolecular and structural degradation of articular cartilages and meniscus. As discussed, abnormal mechanical loading affects the peri-articular bone and bone health influences OA progression by altering the bone mineral density (BMD) [135, 136] that cannot be predicted by radiographs. However, CT imaging modules are very popular in measuring bone mineral density and the functionality of muscle-bone interaction. A dual-energy x-ray absorptiometry (DXA) is typically used to evaluate bone mineral density. High-resolution peripheral quantitative computed tomography (HR-pQCT) and peripheral quantitative computed tomography (PQCT) provide bone mineral density, geometrical parameters (marrow and cross-sectional cortical area, cortical thickness, biomechanical parameters, cross-sectional and moment of inertia, and interaction between muscle and bone morphology) [137–139]. These measures associated with bones have not yet been utilized for studying the effect of bracing treatment. The computational models can address the duration of bracing treatment, the health of tissues, and knee kinematics under low- and high-flexion angles and under strenuous activities and become an efficient tool for the analysis of the knee brace design. The commercial software, such as OpenSim [140, 141], SimTK [142], and AnyBody [143] also provide a platform to develop, analyze, and visualize models of the musculoskeletal system. These software offer numerous inbuilt experimentations tools, such as inverse kinematics, inverse dynamics, static optimization, forward dynamics, computed muscle control. These tools help in analyzing the knee joint function under various loading conditions, studying the effects of musculoskeletal geometry, joint kinematics, and muscle-tendon properties on the muscle, electromyogram-driven neuromusculoskeletal models. The patient-specific models of the knee can be reconstructed from MR images and a dynamic simulation of the knee joint during weight-bearing conditions can be performed. A significant advantage lies in investigating the contact pressure distribution across the knee joint, stresses in the limbs, the amount of unloading needed during bracing by simulating movements (flexion during squatting, kneeling), which are difficult to visualize in real-life experiments due to the limitations of obtaining ethical clearance on such measurements. Therefore, the computational models based on the images provide a better approach to study the knee joint model and predict the internal stress, force transmission, and joint contact stress distribution under dynamic conditions.
Conclusions
In summary, knee OA significantly affects the tissues in the joint, the braces affect the gait and loading patterns in the affected and contralateral compartments of the knee and also the distant hip joint to cause degeneration in these joints. The existing assessment methods in clinical and research settings are limited to study the efficacy of knee braces with respect to the temporal changes in the morphology of bones and joint spaces, kinematics and kinetics of the affected limb, and muscle activity that focuses only on the effects of bracing and after-effects of OA. This review recommends that, (1) in addition to the clinical outcomes, a holistic understanding of the effects of braces to assess the complex interplay of change in loading characteristics is needed, (2) short- and long-term effects of braces on the biochemical and biomechanical behaviors of these soft and hard tissues during this reorganized physiological loading must be understood and studied to improve the efficacy of the bracing treatment, and (3) holistic understanding needed an integrated assessment methodology that combines existing and evolving methods that use advanced techniques to provide a direction for further research and clinical trials in the domain.
Acknowledgments
KS and YHDL would like to acknowledge the funding support by the Changi General Hospital - Singapore University of Technology and Design under the Health Tech Innovation Fund, award no: CGH-SUTD-2015-003. The funding agency had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Abbreviations
- OA
Osteoarthritis
- JSN
Joint space narrowing
- KAM
Knee adduction moment
- ECM
Extracellular matrix
- EMG
Electromyograms
- VAS
Visual Analogue Scale
- KOOS
Knee Osteoarthritis Outcome Score
- QOLS
Quality of Life Scale
- WOMAC
Western Ontario and McMaster Universities’ Arthritis Index
- IPAQ
International Physical Activity Questionnaire
- IKDC
International Knee Documentation Committee
- JSW
Joint space width
- MJSW
Minimum JSW
- K–L
Kellgren–Lawrence
- WORMS
Whole-Organ Magnetic Resonance Imaging Score
- BLOKS
Boston Leeds Osteoarthritis Knee Score
- MOAK
Osteoarthritis Knee Score
- MOCART
Magnetic resonance observation of the cartilage repair tissue
- dGEMRIC
Delayed gadolinium enhanced MRI of cartilage
Authors’ contributions
All the authors participated in the design of the study, drafted the manuscript, and read and approved the final manuscript.
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Rizuwana Parween, Email: rizuwana_parween@sutd.edu.sg.
Duraisamy Shriram, Email: shriram_duraisamy@mymail.sutd.edu.sg.
Rajesh Elara Mohan, Email: rajeshelara@sutd.edu.sg.
Yee Han Dave Lee, Email: dave_lee@cgh.com.sg.
Karupppasamy Subburaj, Email: subburaj@sutd.edu.sg.
References
- 1.Helmick CG, Lawrence RC, Pollard RA, Lloyd E, Heyse SP. Arthritis and other rheumatic conditions: who is affected now, who will be affected later? Arthritis Rheum. 1995;8(4):203–211. doi: 10.1002/art.1790080403. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence RC, Helmick CG, Arnett FC. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778–99. 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed]
- 3.Ahmed AM, Burke DL. In-vitro measurement of static pressure distribution in synovial joints—part I: tibial surface of the knee. J Biomech Eng. 1983;105(3):216–225. doi: 10.1115/1.3138409. [DOI] [PubMed] [Google Scholar]
- 4.Kellgren JH, Lawrence JS. Radiological assessment of rheumatoid arthritis. Ann Rheum Dis. 1957;16(4):485–93. doi: 10.1136/ard.16.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson F, Leitl S, Waugh W. The distribution of load across the knee. A comparison of static and dynamic measurements. J Bone Joint Surg Br. 1980;62(3):346–349. doi: 10.1302/0301-620X.62B3.7410467. [DOI] [PubMed] [Google Scholar]
- 6.Smith AJ, Lloyd DG, Wood DJ. Pre-surgery knee joint loading patterns during walking predict the presence and severity of anterior knee pain after total knee arthroplasty. J Orthop Res. 2004;22(2):260–266. doi: 10.1016/S0736-0266(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 7.Hunt MA, Birmingham TB, Giffin JR, Jenkyn TR. Associations among knee adduction moment, frontal plane ground reaction force, and lever arm during walking in patients with knee osteoarthritis. J Biomech. 2006;39(12):2213–2220. doi: 10.1016/j.jbiomech.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Ellermann A, Zantop T, Rembitzki IV, Hartmut S, Liebau C, Best R. Biomechanical effect of unloader braces for medial osteoarthritis of the knee: a systematic review. Arch Orthop Trauma Surg. 2016;136:649–656. doi: 10.1007/s00402-015-2388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shull PB, Shultz R, Silder A. Toe-in gait reduces the first peak knee adduction moment in patients with medial compartment knee osteoarthritis. J Biomech. 2013;46(1):122–128. doi: 10.1016/j.jbiomech.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 10.Cheung RTH, Ho KKW, Au IPH, An WW, Zhang JHW, Chan ZYS, Deluzio K, Rainbow MJ. Immediate and short-term effects of gait retraining on the knee joint moments and symptoms in patients with early tibiofemoral joint osteoarthritis: a randomized controlled trial. Osteoarthr Cartil. 2018;26(11):1479–1486. doi: 10.1016/j.joca.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Kirane Y, Zifchock R, Gulotta L, Garrison G, Hillstrom H. Knee pain structure and function after one year of unloader bracing for medial knee osteoarthritis. Osteoarthr Cartil. 2010;18:S248–S249. [Google Scholar]
- 12.Ramsey Dan K, Briem Kristin, Axe LS-M Michael J. A mechanical hypothesis for the effectiveness of knee bracing for medial compartment knee osteoarthritis. J Bone Joint Surg Am. 2007;89(11):2398–2407. doi: 10.2106/JBJS.F.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostrander RV, Leddon CE, Hackel JG, O’Grady CP, Roth CA. Efficacy of unloader bracing in reducing symptoms of knee osteoarthritis. Am J Orthop (Belle Mead NJ) 2016;45(5):306–311. [PubMed] [Google Scholar]
- 14.Ramsey DK, Briem K, Axe MJ, Snyder-Mackler L. A mechanical theory for the effectiveness of bracing for medial compartment osteoarthritis of the knee. J Bone Joint Surg Am. 2007;89(11):2398–2407. doi: 10.2106/JBJS.F.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286(2):188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 16.Mauricio E, Sliepen M, Rosenbaum S. Acute effects of different orthotic interventions on knee loading parameters in knee osteoarthritis patients with varus malalignment. Knee. 2018;25(5):825–833. doi: 10.1016/j.knee.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Mistry DA, Chandratreya A, Lee PYF. An update on unloading knee braces in the treatment of unicompartmental knee osteoarthritis from the last 10 years: a literature review. Surg J (N Y) 2018;4(3):e110–e118. doi: 10.1055/s-0038-1661382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moyer RF, Birmingham TB, Bryant DM, Giffin JR, Marriott KA, Leitch KM. Biomechanical effects of valgus knee bracing: a systematic review and meta-analysis. Osteoarthr Cartil. 2015;23(2):178–188. doi: 10.1016/j.joca.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Segal NA, Anderson DD, Iyer KS. Baseline articular contact stress levels predict incident symptomatic knee osteoarthritis development in the MOST cohort. J Orthop Res. 2009;27(12):1562–1568. doi: 10.1002/jor.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodwin JS, Creighton RA, Pietrosimone BG, Spang JT, Blackburn JT. Medial unloader braces and lateral heel wedges do not alter gait biomechanics in healthy young adults. J Sport Rehabil. 10.1123/jsr.2017-0106. [DOI] [PubMed]
- 21.Musumeci G. The effect of mechanical loading on articular cartilage. J Funct Morphol Kinesiol. 2016;1(2):154–161. [Google Scholar]
- 22.Herwig J, Egner E, Buddecke E. Chemical changes of human knee joint menisci in various stages of degeneration. Ann Rheum Dis. 1984;43(4):635–640. doi: 10.1136/ard.43.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan DD, Cai L, Butz KD, Trippel SB, Nauman EA, Neu CP. In vivo articular cartilage deformation: noninvasive quantification of intratissue strain during joint contact in the human knee. Sci Rep. 2016;6(1):19220. doi: 10.1038/srep19220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckstein F, Hudelmaier M, Putz R. The effects of exercise on human articular cartilage. J Anat. 2006;208(4):491–512. doi: 10.1111/j.1469-7580.2006.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fithian DC, Ma Kelly, Mow VC. Material properties and structure–function relationships in the menisci. Clin Orthop Relat Res. 1990;252:19–31. [PubMed] [Google Scholar]
- 26.Woo SL-Y, Buckwalter JA. Injury and repair of the musculoskeletal soft tissues. Savannah, Georgia, June 18–20, 1987. J Orthop Res. 1988;6(6):907–931. doi: 10.1002/jor.1100060615. [DOI] [PubMed] [Google Scholar]
- 27.Thompson WO, Thaete FL, Fu FH, Dye SF. Tibial meniscal dynamics using three-dimensional reconstruction of magnetic resonance images. Am J Sports Med. 1991;19(3):210–215. doi: 10.1177/036354659101900302. [DOI] [PubMed] [Google Scholar]
- 28.Voloshin AS, Wosk J. Shock absorption of meniscectomized and painful knees: a comparative in vivo study. J Biomed Eng. 1983;5(2):157–161. doi: 10.1016/0141-5425(83)90036-5. [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni VV, Chand K. Pathological anatomy of the aging meniscus. Acta Orthop Scand. 1975;46(1):135–140. doi: 10.3109/17453677508989201. [DOI] [PubMed] [Google Scholar]
- 30.Goldring SR. Role of bone in osteoarthritis pathogenesis. Med Clin North Am. 2009;93(1):25–35. doi: 10.1016/j.mcna.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Ding M. Microarchitectural adaptations in aging and osteoarthrotic subchondral bone issues. Acta Orthop. 2010;81(sup340):1–53. doi: 10.3109/17453671003619037. [DOI] [PubMed] [Google Scholar]
- 32.Li G, Yin J, Gao J. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15(6):223. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo GH, Niu J, McLennan CE. Meniscal damage associated with increased local subchondral bone mineral density: a Framingham study. Osteoarthr Cartil. 2008;16(2):261–267. doi: 10.1016/j.joca.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Wluka AE, Pelletier JP. Meniscal extrusion predicts increases in subchondral bone marrow lesions and bone cysts and expansion of subchondral bone in osteoarthritic knees. Rheumatology. 2010;49(5):997–1004. doi: 10.1093/rheumatology/keq034. [DOI] [PubMed] [Google Scholar]
- 35.Kirkley a, Webster-Bogaert S, Litchfield R. The effect of bracing on varus gonarthrosis. J Bone Joint Surg Am. 1999;81(4):539–548. doi: 10.2106/00004623-199904000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Brouwer RW, van Raaij TM, Verhaar JAN, Coene LNJEM, Bierma-Zeinstra SMA. Brace treatment for osteoarthritis of the knee: a prospective randomized multi-centre trial. Osteoarthr Cartil. 2006;14(8):777–783. doi: 10.1016/j.joca.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Van Raaij TM, Reijman M, Brouwer RW, Bierma-Zeinstra SMA, Verhaar JAN. Medial knee osteoarthritis treated by insoles or braces a randomized trial. Clin Orthop Relat Res. 2010;468(7):1926–1932. doi: 10.1007/s11999-010-1274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duivenvoorden T, Brouwer RW, van Raaij TM, Verhagen AP, Verhaar JAN, Bierma-Zeinstra SMA. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;3:CD004020. doi: 10.1002/14651858.CD004020.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hurley ST, Murdock GLH, Stanish WD, Hubley-Kozey CL. Is there a dose response for valgus unloader brace usage on knee pain, function, and muscle strength? Arch Phys Med Rehabil. 2012;93(3):496–502. doi: 10.1016/j.apmr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Briggs K, Matheny L, Steadman J. Improvement in quality of life with use of an unloader knee brace in active patients with OA: a prospective cohort study. J Knee Surg. 2012;25(5):417–421. doi: 10.1055/s-0032-1313748. [DOI] [PubMed] [Google Scholar]
- 41.Rezaeian ZS, Smith MM, Skaife TL, Harvey WF, Gross KD, Hunter DJ. Does knee malalignment predict the efficacy of realignment therapy for patients with knee osteoarthritis? Int J Rheum Dis. 2015;20:1403–1412. doi: 10.1111/1756-185X.12696. [DOI] [PubMed] [Google Scholar]
- 42.Thoumie P, Marty M, Avouac B, Pallez A, Vaumousse A, Pipet LPT, Monroche A, Graveleau N, Bonnin A, Amor CB, Coudeyre E. Effect of unloading brace treatment on pain and function in patients with symptomatic knee osteoarthritis: the ROTOR randomized clinical trial. Sci Rep. 2018;8, Article number: 10519. [DOI] [PMC free article] [PubMed]
- 43.Hjartarson HF, Larsen ST. The clinical effect of an unloader brace on patients with osteoarthritis of the knee, a randomized placebo controlled trial with one year follow up. BMC Musculoskelet Disord. 2018;19(1):341. doi: 10.1186/s12891-018-2256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terwee CB, Bouwmeester W, van Elsland SL, de Vet HCW, Dekker J. Instruments to assess physical activity in patients with osteoarthritis of the hip or knee: a systematic review of measurement properties. Osteoarthr Cartil. 2011;19(6):620–633. doi: 10.1016/j.joca.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 45.van Poppel MNM, Chinapaw MJM, Mokkink LB. Physical activity questionnaires for adults: a systematic review of measurement properties. Sports Med. 2010;40(7):565–600. doi: 10.2165/11531930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 46.Forsén L, Loland NW, Vuillemin A. Self-administered physical activity questionnaires for the elderly: a systematic review of measurement properties. Sports Med. 2010;40(7):601–623. doi: 10.2165/11531350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 47.Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sport Traumatol Arthrosc. 1993;1(3–4):226–234. doi: 10.1007/BF01560215. [DOI] [PubMed] [Google Scholar]
- 48.Jacobi M, Reischl N, Rönn K, Magnusson RA, Gautier E, Jakob RP. Healing of the acutely injured anterior cruciate ligament: Functional treatment with the ACL-Jack, a dynamic posterior drawer brace. Adv Orthop. 2016. 10.1155/2016/1609067. [DOI] [PMC free article] [PubMed]
- 49.Martin J a, Buckwalter J a. Roles of articular cartilage aging and chondrocyte senescence in the pathogenesis of osteoarthritis. Iowa Orthop J. 2001;21(319):1–7. [PMC free article] [PubMed] [Google Scholar]
- 50.Newberry WN, Zukosky DK, Haut RC. Subfracture insult to a knee joint causes alterations in the bone and in the functional stiffness of overlying cartilage. J Orthop Res. 1997;15(3):450–455. doi: 10.1002/jor.1100150319. [DOI] [PubMed] [Google Scholar]
- 51.McErlain DD, Ulici V, Darling M. An in vivo investigation of the initiation and progression of subchondral cysts in a rodent model of secondary osteoarthritis. Arthritis Res Ther. 2012;14(1):R26. doi: 10.1186/ar3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duryea J, Zaim S, Genant HK. New radiographic-based surrogate outcome measures for osteoarthritis of the knee. Osteoarthr Cartil. 2003;11(2):102–110. doi: 10.1053/joca.2002.0866. [DOI] [PubMed] [Google Scholar]
- 53.Kellgren J, Lawrence J. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16(4):494. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horlick SG, Loomer R. Valgus knee bracing for medial gonarthrosis. Clin J Sport Med. 1993;3(4):251–255. [Google Scholar]
- 55.Komistek RD, Dennis DA, Northcut EJ, Wood A, Parker AW, Traina SM. An in vivo analysis of the effectiveness of the osteoarthritic knee brace during heel-strike of gait. J Arthroplasty. 1999;14(6):738–742. doi: 10.1016/s0883-5403(99)90230-9. [DOI] [PubMed] [Google Scholar]
- 56.Dennis AD, Komistek RD. An in vivo analysis of the effectiveness of the osteoarthritic knee brace during heel strike and midstance of gait. Acta Chir Orthop Traumatol Cech. 1999;66(6):323–327. [PubMed] [Google Scholar]
- 57.Oak SR, Ghodadra A, Winalski CS, Miniaci A, Jones MH. Radiographic joint space width is correlated with 4-year clinical outcomes in patients with knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthr Cartil. 2013;21(9):1185–1190. doi: 10.1016/j.joca.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 58.Buckland-Wright JC, Macfarlane DG, Lynch JA, Jasani MK, Bradshaw CR. Joint space width measures cartilage thickness in osteoarthritis of the knee: high resolution plain film and double contrast macroradiographic investigation. Ann Rheum Dis. 1995;54(4):263–268. doi: 10.1136/ard.54.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eckstein F, Le Graverand MPH. Plain radiography or magnetic resonance imaging (MRI): which is better in assessing outcome in clinical trials of disease-modifying osteoarthritis drugs? Summary of a debate held at the World Congress of Osteoarthritis 2014. Semin Arthritis Rheum. 2015;45(3):251–256. doi: 10.1016/j.semarthrit.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Gale DR, Chaisson CE, Totterman SMS, Schwartz RK, Gale ME, Felson D. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthr Cartil. 1999;7(6):526–532. doi: 10.1053/joca.1999.0256. [DOI] [PubMed] [Google Scholar]
- 61.Peterfy CG. Role of MR imaging in clinical research studies. Semin Musculoskelet Radiol. 2001;5(4):365–378. doi: 10.1055/s-2001-19045. [DOI] [PubMed] [Google Scholar]
- 62.Eckstein F, Glaser C. Measuring cartilage morphology with quantitative magnetic resonance imaging. Semin Musculoskelet Radiol. 2004;8(212):329–353. doi: 10.1055/s-2004-861579. [DOI] [PubMed] [Google Scholar]
- 63.Jones G, Glisson M, Hynes K, Cicuttini F. Sex and site differences in cartilage development: A possible explanation for variations in knee osteoarthritis in later life. Arthritis Rheum. 2000;43(11):2543–9. 10.1002/1529-0131(200011)43:11<2543::AID-ANR23>3.0.CO;2-K. [DOI] [PubMed]
- 64.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67(2):206–211. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 65.Alizai H, Virayavanich W, Joseph GB. Cartilage lesion score: comparison of a quantitative assessment score with established semiquantitative MR scoring systems. Radiology. 2014;271(2):479–487. doi: 10.1148/radiol.13122056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marlovits S, Striessnig G, Resinger CT. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52(3):310–319. doi: 10.1016/j.ejrad.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 67.Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: Determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16–23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 68.Welsch GH, Zak L, Mamisch TC, Resinger C, Marlovits S, Trattnig S. Three-dimensional magnetic resonance observation of cartilage repair tissue (MOCART) score assessed with an isotropic three-dimensional true fast imaging with steady-state precession sequence at 3.0 Tesla. Investig Radiol. 2009;44(9):603–612. doi: 10.1097/RLI.0b013e3181b5333c. [DOI] [PubMed] [Google Scholar]
- 69.Dhollander A, Moens K, Van Der Maas J, Verdonk P, Almqvist KF, Victor J. Treatment of patellofemoral cartilage defects in the knee by autologous matrix-induced chondrogenesis (amic) Acta Orthop Belg. 2014;80(2):251–259. [PubMed] [Google Scholar]
- 70.Cotofana S, Eckstein F, Wirth W. In vivo measures of cartilage deformation: patterns in healthy and osteoarthritic female knees using 3T MR imaging. Eur Radiol. 2011;21(6):1127–1135. doi: 10.1007/s00330-011-2057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wirth W, Duryea J, Hellio Le Graverand MP. Direct comparison of fixed flexion, radiography and MRI in knee osteoarthritis: responsiveness data from the osteoarthritis initiative. Osteoarthr Cartil. 2013;21(1):117–125. doi: 10.1016/j.joca.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Felson DT, Radin EL. What causes knee osteoarthrosis: are different compartments susceptible to different risk factors? J Rheumatol. 1994;21(2):181–182. [PubMed] [Google Scholar]
- 73.Zhao D, Banks SA, apos D, Lima DD, Colwell CW, Fregly BJ. In vivo medial and lateral tibial loads during dynamic and high flexion activities. J Orthop Res. 2007;25(5):593–602. doi: 10.1002/jor.20362. [DOI] [PubMed] [Google Scholar]
- 74.Andriacchi TP, Mündermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18(5):514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- 75.Hubley-Kozey CL, Hill NA, Rutherford DJ, Dunbar MJ, Stanish WD. Co-activation differences in lower limb muscles between asymptomatic controls and those with varying degrees of knee osteoarthritis during walking. Clin Biomech. 2009;24(5):407–414. doi: 10.1016/j.clinbiomech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 76.Rudolph KS, Schmitt LC, Lewek MD. Age-related changes in strength, joint laxity, and walking patterns: are they related to knee osteoarthritis? Phys Ther. 2007;87(11):1422–1432. doi: 10.2522/ptj.20060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rutherford DJ, Hubley-Kozey CL, Stanish WD, Dunbar MJ. Neuromuscular alterations exist with knee osteoarthritis presence and severity despite walking velocity similarities. Clin Biomech. 2011;26(4):377–383. doi: 10.1016/j.clinbiomech.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 78.Lindenfeld TN, Hewett TE, Andriacchi TP. Joint loading with valgus bracing in patients with varus gonarthrosis. Clin Orthop Relat Res. 1997;344:290–297. [PubMed] [Google Scholar]
- 79.Hewett TE, Noyes FR, Barber-Westin SD, Heckmann TP. Decrease in knee joint pain and increase in function in patients with medial compartment arthrosis: a prospective analysis of valgus bracing. Orthopedics. 1998;21(2):131–138. doi: 10.3928/0147-7447-19980201-06. [DOI] [PubMed] [Google Scholar]
- 80.Schmalz T, Knopf E, Drewitz H, Blumentritt S. Analysis of biomechanical effectiveness of valgus-inducing knee brace for osteoarthritis of knee. J Rehabil Res Dev. 2010;47(5):419–429. doi: 10.1682/jrrd.2009.05.0067. [DOI] [PubMed] [Google Scholar]
- 81.Toriyama M, Deie M, Shimada N. Effects of unloading bracing on knee and hip joints for patients with medial compartment knee osteoarthritis. Clin Biomech. 2011;26(5):497–503. doi: 10.1016/j.clinbiomech.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 82.Anderson IA, MacDiarmid AA, Harris ML, Gillies RM, Phelps R, Walsh WR. A novel method for measuring medial compartment pressures within the knee joint in-vivo. J Biomech. 2003;36(9):1391–1395. doi: 10.1016/s0021-9290(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 83.Kutzner I, Küther S, Heinlein B. The effect of valgus braces on medial compartment load of the knee joint—in vivo load measurements in three subjects. J Biomech. 2011;44(7):1354–1360. doi: 10.1016/j.jbiomech.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 84.Ramsey DK. A mechanical theory for the effectiveness of bracing for medial compartment osteoarthritis of the knee. J Bone Joint Surg. 2007;89(11):2398. doi: 10.2106/JBJS.F.01136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pagani CHF, Hinrichs M, Brüggemann GP. Kinetic and kinematic changes with the use of valgus knee brace and lateral wedge insoles in patients with medial knee osteoarthritis. J Orthop Res. 2012;30(7):1125–1132. doi: 10.1002/jor.22032. [DOI] [PubMed] [Google Scholar]
- 86.Esrafilian A, Karimi MT, Eshraghi A. Design and evaluation of a new type of knee orthosis to align the mediolateral angle of the knee joint with osteoarthritis. Adv Orthop. 2012;2012:1–6. doi: 10.1155/2012/104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jones RK, Nester CJ, Richards JD. A comparison of the biomechanical effects of valgus knee braces and lateral wedged insoles in patients with knee osteoarthritis. Gait Posture. 2013;37(3):368–372. doi: 10.1016/j.gaitpost.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 88.Larsen BL, Jacofsky MC, Brown JA, Jacofsky DJ. Valgus bracing affords short-term treatment solution across walking and sit-to-stand activities. J Arthroplasty. 2013;28(5):792–797. doi: 10.1016/j.arth.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 89.Pollo FE, Otis JC, Backus SI, Warren RF, Wickiewicz TL. Reduction of medial compartment loads with valgus bracing of the osteoarthritic knee. Am J Sports Med. 2002;30(3):414–421. doi: 10.1177/03635465020300031801. [DOI] [PubMed] [Google Scholar]
- 90.Laroche D, Morisset C, Fortunet C, Gremeaux V, Maillefert JF, Ornetti P. Biomechanical effectiveness of a distraction-rotation knee brace in medial knee osteoarthritis: preliminary results. Knee. 2014;21(3):710–716. doi: 10.1016/j.knee.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 91.Dessery Y, Belzile ÉL, Turmel S, Corbeil P. Comparison of three knee braces in the treatment of medial knee osteoarthritis. Knee. 2014;21(6):1107–1114. doi: 10.1016/j.knee.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 92.Heiden TL, Lloyd DG, Ackland TR. Knee joint kinematics, kinetics and muscle co-contraction in knee osteoarthritis patient gait. Clin Biomech. 2009;24(10):833–841. doi: 10.1016/j.clinbiomech.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 93.Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthr Cartil. 2004;12(9):745–751. doi: 10.1016/j.joca.2004.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Messier SP, Loeser RF, Hoover JL, Semble EL, Wise CM. Osteoarthritis of the knee: effects on gait, strength, and flexibility. Arch Phys Med Rehabil. 1992;73:29–36. [PubMed] [Google Scholar]
- 95.Childs JD, Sparto PJ, Fitzgerald GK, Bizzini M, Irrgang JJ. Alterations in lower extremity movement and muscle activation patterns in individuals with knee osteoarthritis. Clin Biomech. 2004;19(1):44–49. doi: 10.1016/j.clinbiomech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 96.Rudolph KS, Axe MJ, Buchanan TS, Scholz JP, Snyder-Mackler L. Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surg Sport Traumatol Arthrosc. 2001;9(2):62–71. doi: 10.1007/s001670000166. [DOI] [PubMed] [Google Scholar]
- 97.Hodge WA, Carlson KL, Fijan RS. Contact pressures from an instrumented hip endoprosthesis. J Bone Joint Surg. 1989;71(9):1378–1386. [PubMed] [Google Scholar]
- 98.Schmitt LC, Rudolph KS. Influences on knee movement strategies during walking in persons with medial knee osteoarthritis. Arthritis Care Res. 2007;57(6):1018–1026. doi: 10.1002/art.22889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Segal NA. Bracing and orthoses: a review of efficacy and mechanical effects for tibiofemoral osteoarthritis. PM R. 2012;4(5 SUPPL.):S89–S96. doi: 10.1016/j.pmrj.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 100.Ramsey DK, Russell ME. Unloader braces for medial compartment knee osteoarthritis: implications on mediating progression. Sports Health. 2009;1(5):416–426. doi: 10.1177/1941738109343157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Briem K, Ramsey DK. The role of bracing. Sports Med Arthrosc. 2013;21(1):11–17. doi: 10.1097/JSA.0b013e31827562b5. [DOI] [PubMed] [Google Scholar]
- 102.Pollo FE, Jackson RW. Knee bracing for unicompartmental osteoarthritis. J Am Acad Orthop Surg. 2006;14(1):5–11. doi: 10.5435/00124635-200601000-00003. [DOI] [PubMed] [Google Scholar]
- 103.Fantini Pagani CH, Willwacher S, Kleis B, Brüggemann GP. Influence of a valgus knee brace on muscle activation and co-contraction in patients with medial knee osteoarthritis. J Electromyogr Kinesiol. 2013;23(2):490–500. doi: 10.1016/j.jelekin.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 104.Callaghan MJ, Parkes MJ, Felson DT. The effect of knee braces on quadriceps strength and inhibition in subjects with patellofemoral osteoarthritis. J Orthop Sport Phys Ther. 2016;46(1):19–25. doi: 10.2519/jospt.2016.5093. [DOI] [PubMed] [Google Scholar]
- 105.Rauscher I, Stahl R, Cheng J. Meniscal measurements of T1rho and T2 at MR imaging in healthy subjects and patients with osteoarthritis. Radiology. 2008;249(2):591–600. doi: 10.1148/radiol.2492071870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Luke AC, Stehling C, Stahl R. High-field magnetic resonance imaging assessment of articular cartilage before and after marathon running: does long-distance running lead to cartilage damage? Am J Sports Med. 2010;38(11):2273–2280. doi: 10.1177/0363546510372799. [DOI] [PubMed] [Google Scholar]
- 107.Stehling C, Luke A, Stahl R. Meniscal T1rho and T2 measured with 3.0T MRI increases directly after running a marathon. Skelet Radiol. 2011;40(6):725–735. doi: 10.1007/s00256-010-1058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Souza RB, Kumar D, Calixto N. Response of knee cartilage T1rho and T2 relaxation times to in vivo mechanical loading in individuals with and without knee osteoarthritis. Osteoarthr Cartil. 2014;22(10):1367–1376. doi: 10.1016/j.joca.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Calixto NE, Kumar D, Subburaj K. Zonal differences in meniscus MR relaxation times in response to in vivo static loading in knee osteoarthritis. J Orthop Res. 2016;34(2):249–261. doi: 10.1002/jor.23004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Subburaj K, Kumar D, Souza RB. The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. Am J Sports Med. 2012;40(9):2134–2141. doi: 10.1177/0363546512449816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Subburaj K, Souza RB, Wyman BT. Changes in MR relaxation times of the meniscus with acute loading: an in vivo pilot study in knee osteoarthritis. J Magn Reson Imaging. 2015;41(2):536–543. doi: 10.1002/jmri.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Burstein D, Velyvis J, Scott KT. Protocol issues for delayed Gd(DTPA)2-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45(1):36–41. 10.1002/1522-2594(200101)45:1<36::AID-MRM1006>3.0.CO;2-W. [DOI] [PubMed]
- 113.Bashir A, Gray ML, Burstein D. Gd-DTPA2− as a measure of cartilage degradation. Magn Reson Med. 1996;36(5):665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 114.Tiderius CJ, Jessel R, Kim YJ, Burstein D. Hip dGEMRIC in asymptomatic volunteers and patients with early osteoarthritis: the influence of timing after contrast injection. Magn Reson Med. 2007;57(4):803–805. doi: 10.1002/mrm.21190. [DOI] [PubMed] [Google Scholar]
- 115.Sigurdsson U, Siversson C, Lammentausta E, Svensson J, Tiderius C-J, Dahlberg LE. In vivo transport of Gd-DTPA2- into human meniscus and cartilage assessed with delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) BMC Musculoskelet Disord. 2014;15(1):226. doi: 10.1186/1471-2474-15-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shapiro EM, Borthakur A, Gougoutas A, Reddy R. 23NA MRI accurately measures fixed charge density in articular cartilage. Magn Reson Med. 2002;47(2):284–291. doi: 10.1002/mrm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Newbould RD, Miller SR, Tielbeek JAW. Reproducibility of sodium MRI measures of articular cartilage of the knee in osteoarthritis. Osteoarthr Cartil. 2012;20(1):29–35. doi: 10.1016/j.joca.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gohal C, Shanmugaraj A, Tate P, Horner NS, Bedi A, Adili A, Khan M. Effectiveness of valgus offloading knee braces in the treatment of medial compartment knee osteoarthritis: a systematic review. Sports Health. 2018;10(6):500–514. doi: 10.1177/1941738118763913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andriacchi TP. Dynamics of knee malalignment. Orthop Clin North Am. 1994;25(3):395–403. [PubMed] [Google Scholar]
- 120.Bergmann G, Graichen F, Rohlmann A. Hip joint loading during walking and running, measured in two patients. J Biomech. 1993;26(8):969–990. doi: 10.1016/0021-9290(93)90058-m. [DOI] [PubMed] [Google Scholar]
- 121.Briem K, Snyder-Mackler L. Proximal gait adaptations in medial knee OA. J Orthop Res. 2009;27(1):78–83. doi: 10.1002/jor.20718. [DOI] [PubMed] [Google Scholar]
- 122.Neville SR, Brandon SCE, Brown MJ, Deluzio KJ. Validation of method for analysing mechanics of unloader brace for medial knee osteoarthritis. J Biomech. 2018;25(76):253–258. doi: 10.1016/j.jbiomech.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 123.Brand R a. Joint contact stress: a reasonable surrogate for biological processes? Iowa Orthop J. 2005;25:82–94. [PMC free article] [PubMed] [Google Scholar]
- 124.Harrison MH, Schajowicz F, Trueta J. Osteoarthritis of the hip: a study of the nature and evolution of the disease. J Bone Joint Surg Br. 1953;35–B:598–626. doi: 10.1302/0301-620X.35B4.598. [DOI] [PubMed] [Google Scholar]
- 125.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91(Suppl 1):95–101. doi: 10.2106/JBJS.H.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vincent KR, Conrad BP, Fregly BJ, Vincent HK. The pathophysiology of osteoarthritis: a mechanical perspective on the knee joint. PM R. 2012;4(5 SUPPL.):S3–S9. doi: 10.1016/j.pmrj.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaplan JT, Neu CP, Drissi H, Emery NC, Pierce DM. Cyclic loading of human articular cartilage: the transition from compaction to fatigue. J Mech Behav Biomed Mater. 2017;65:734–742. doi: 10.1016/j.jmbbm.2016.09.040. [DOI] [PubMed] [Google Scholar]
- 128.Cusin E, Honeine JL, Schieppati M, Rougier PR. A simple method for measuring the changeable mechanical action of unloader knee braces for osteoarthritis. IRBM. 2018;39(2):136–142. [Google Scholar]
- 129.Kiviranta P, Rieppo J, Korhonen RK, Julkunen P, Töyräs J, Jurvelin JS. Collagen network primarily controls Poisson’s ratio of bovine articular cartilage in compression. J Orthop Res. 2006;24(4):690–699. doi: 10.1002/jor.20107. [DOI] [PubMed] [Google Scholar]
- 130.Musumeci G, Loreto C, Leonardi R. The effects of physical activity on apoptosis and lubricin expression in articular cartilage in rats with glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2013;31(3):274–284. doi: 10.1007/s00774-012-0414-9. [DOI] [PubMed] [Google Scholar]
- 131.Oettmeier R, Arokoski J, Roth AJ, Helminen HJ, Tammi M, Abendroth K. Quantitative study of articular cartilage and subchondral bone remodeling in the knee joint of dogs after strenuous running training. J Bone Miner Res. 1992;7(2 S):S419–S424. doi: 10.1002/jbmr.5650071410. [DOI] [PubMed] [Google Scholar]
- 132.Young DR, Richardson DW, Markel MD, Nunamaker DM. Mechanical and morphometric analysis of the third carpal bone of Thoroughbreds. Am J Vet Res. 1991;52(3):402–409. [PubMed] [Google Scholar]
- 133.Rucci N, Rufo A, Alamanou M, Teti A. Modeled microgravity stimulates osteoclastogenesis and bone resorption by increasing osteoblast RANKL/OPG ratio. J Cell Biochem. 2007;100(2):464–473. doi: 10.1002/jcb.21059. [DOI] [PubMed] [Google Scholar]
- 134.Carmeliet G, Vico L, Bouillon R. Space flight: a challenge for normal bone homeostasis. Crit Rev Eukaryot Gene Expr. 2001;11(1–3):131–144. [PubMed] [Google Scholar]
- 135.Lee JY, Harvey WF, Price LL, Paulus JK, Dawson-Hughes B, McAlindon TE. Relationship of bone mineral density to progression of knee osteoarthritis. Arthritis Rheum. 2013;65(6):1541–1546. doi: 10.1002/art.37926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang Y, Hannan MT, Chaisson CE. Bone mineral density and risk of incident and progressive radiographic knee osteoarthritis in women: the Framingham study. J Rheumatol. 2000;27(4):1032–1037. [PubMed] [Google Scholar]
- 137.MacNeil JA, Boyd SK. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2007;29(10):1096–1105. doi: 10.1016/j.medengphy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 138.Stagi S, Cavalli L, Cavalli T, de Martino M, Brandi ML. Peripheral quantitative computed tomography (pQCT) for the assessment of bone strength in most of bone affecting conditions in developmental age: a review. Ital J Pediatr. 2016;42(1):88. doi: 10.1186/s13052-016-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muhit AA, Arora S, Ogawa M. Peripheral quantitative CT (pQCT) using a dedicated extremity cone-beam CT scanner. Proc SPIE. 2013;8672:867203–867207. doi: 10.1117/12.2006939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Symeonidis I, Kavadarli G, Schuller E, Peldschus S. Simulation of biomechanical experiments in opensim. In: IFMBE Proceedings, vol. 29. 2010; p. 107–110.
- 141.Hall M, Laura E, Diamond LE, Lenton GK, Pizzolato C, Saxby JD. Immediate effects of valgus knee bracing on tibiofemoral contact forces and knee muscle forces. Gait Posture. 2019;68:55–62. doi: 10.1016/j.gaitpost.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 142.Delp SL, Anderson FC, Arnold AS. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng. 2007;54(11):1940–1950. doi: 10.1109/TBME.2007.901024. [DOI] [PubMed] [Google Scholar]
- 143.Ji Z, Wang H, Jiang G, Li L. Analysis of muscle activity utilizing bench presses in the AnyBody simulation modelling system. Model Simul Eng. 2016. 10.1155/2016/3649478.