Abstract
Hearing loss is one of the most prevalent sensory deficits worldwide and can result from the death of mechanosensory hair cells that transduce auditory signals in the cochlea. The mammalian cochlea lacks the capacity to regenerate these hair cells once damaged, and currently there are no biological therapies for hearing loss. Understanding the signaling pathways responsible for hair cell development can inform regenerative strategies and identify targets for treating hearing loss. The canonical Wnt and Notch pathways are critical for cochlear development; they converge on several key molecules, such as Atoh1, to regulate prosensory specification, proliferation, hair cell differentiation, and cellular organization. Much work has focused on Wnt and Notch modulation in the neonatal mouse cochlea, where they can promote hair cell regeneration. However, this regenerative response is limited in the adult cochlea and this might be attributed to age-dependent epigenetic modifications. Indeed, the epigenetic status at key gene loci undergoes dynamic changes during cochlear development, maturation, and aging. Therefore, strategies to improve regenerative success in the adult cochlea might require the modulation of Wnt, Notch, or other pathways, as well as targeted epigenetic modifications to alter the activity of key genes critical for supporting cell proliferation or transdifferentiation.
Keywords: mouse cochlea, development, regeneration, clinical trials, epigenetics, CRISPR-Cas9, regenerative medicine
Hearing loss is the most prevalent sensory disorder worldwide. Here we review the progress, challenges, and potential of Wnt and Notch signaling in hearing regeneration. We also discuss ongoing clinical trials and the promise of epigenome editing for the amelioration of hearing loss.
Main Text
Hearing loss is the most common sensory disorder worldwide and is especially prevalent among seniors. A substantial contributor to this loss is likely age-related degeneration of mechanosensory hair cells in the cochlea.1 The organ of Corti, the functional unit responsible for transducing auditory signals in the cochlea, is a highly organized epithelial mosaic comprised of one row of inner hair cells (which act as the primary receivers), three rows of outer hair cells (that serve to amplify and sharpen the tuning of sound), and adjoining non-sensory supporting cells. In non-mammalian vertebrates, lost hair cells are replaced via a two-step mechanism involving the direct transdifferentiation of supporting cells into hair cells coupled with the proliferation of supporting cells. In adult mammals, however, cochlear hair cell replacement is not observed and any resulting hearing loss is permanent.2 Recent studies in the inner ear have largely focused on the development of the organ of Corti in mammalian models and understanding the molecular signals underpinning its development. This information could be utilized to regenerate lost hair cells in the mature organ.
While hearing loss can be caused by damage to the hair cells, it can also be caused by damage to or loss of the synapses of the primary auditory neurons (also known as spiral ganglion neurons) that innervate the hair cells or the neurons themselves. Additionally, it can be caused by damage to the stria vascularis that contributes to cochlear blood supply and ionic balance, as well as genetic hearing loss.3, 4, 5
Under the control of several molecular pathways, the organ of Corti and all other inner ear epithelia derive from the otic placode in the head ectoderm, which invaginates to form the otocyst. The otocyst is subsequently organized into ducts and chambers, including the cochlea, saccule, utricle, semicircular canals, and endolymphatic duct. In mice, outgrowth of the cochlear duct begins in the ventral otocyst and the prosensory domain is specified in the floor of the cochlear duct. As the duct extends and coils in the developing embryo, prosensory cells undergo proliferation, terminal mitosis, and differentiation into hair cells or supporting cells (Figure 1), and these continue to mature until the onset of hearing approximately 2 weeks after birth.2, 6, 7, 8, 9
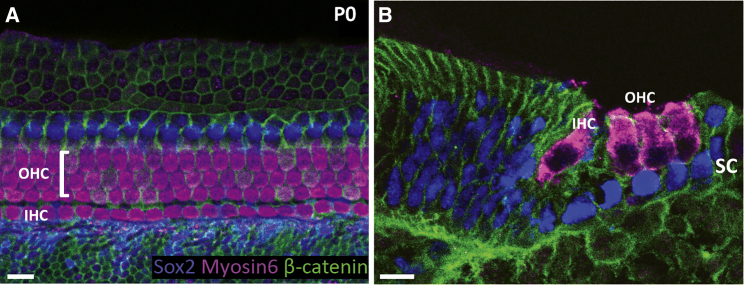
Figure 1.
Cellular Organization of the Mouse Organ of Corti at Birth
The organ of Corti is organized into one row of inner hair cells (IHCs), three rows of outer hair cells (OHCs), and surrounding non-sensory supporting cells (SCs). (A) Whole-mount and (B) transverse sections through a P0 mouse cochlea shows Sox2-positive nuclei of SCs (blue) and MyosinVI-positive hair cells (magenta). Cell membranes are labeled with β-catenin (green). Scale bars: 10 μm.
Proper spatiotemporal activation of key signaling pathways is integral to the development of the cochlear sensory epithelium. Two molecular pathways that play significant roles in the development of vertebrate hair cells, as well as their regeneration, are the canonical Wnt and Notch signaling pathways. Here we provide a concise review of the functions of the Wnt and Notch signaling pathways in mouse cochlear development and we discuss how their modulation can promote hair cell regeneration during neonatal and adult stages. We also explore the growing area of research into age-dependent epigenetic changes in the mammalian cochlea and how they might be targeted to improve regenerative success in adults. In addition, we discuss some of the clinical applications of this research. For the purpose of this review, we focus only on the mammalian auditory system. For a discussion of hair cell regeneration in the mammalian vestibular system, see Burns and Stone10 and Li et al.11, and for non-mammalian vertebrates, see Kniss et al.12
Overview of the Canonical Wnt and Notch Signaling Pathways
The canonical Wnt pathway is known to direct cell proliferation, differentiation, and fate determination during embryonic development, and it regulates stem cell control and homeostasis in adult tissues. Wnts are a family of secreted glycoproteins that bind Frizzled receptors and low-density lipoprotein receptor-related protein 5/6 (LRP5/6) co-receptors. Formation of the Wnt-Frizzled-LRP5/6 trimeric complex leads to a downregulation of glycogen synthase kinase 3β (GSK3β) activity and a resultant stabilization of β-catenin, the key effector molecule of the canonical Wnt pathway. Stabilized β-catenin translocates to the nucleus and interacts with T cell factor/lymphoid enhancer factor (TCF/Lef) transcription factors to initiate target gene expression. In the absence of Wnt ligands, however, cytoplasmic β-catenin is phosphorylated by GSK3β and subjected to proteasome-mediated degradation.13 The specific Wnt(s) and Frizzled receptor(s) responsible for activating this pathway in the cochlea remain to be identified.
The Notch signaling pathway also regulates cell proliferation, differentiation, and fate decisions during embryonic development, and promotes the maintenance of adult tissues. Notch receptors are a family of single-pass transmembrane proteins that interact with membrane-bound Notch ligands Jagged (Jag) 1-2 and Delta-like (Dll) 1, 3, and 4 on adjacent cells. Ligand-receptor interaction results in sequential cleavage of the receptor by the ADAM family of metalloproteases and γ-secretase, forming a Notch receptor intracellular domain (NICD). NICD interacts with the recombination signal binding protein for immunoglobulin kappa J region (RBPJκ) transcription factor in the nucleus to affect target gene expression.14
The Roles of Wnt and Notch Signaling in Mammalian Cochlear Hair Cell Development
In early inner ear development, Wnt-responsive cells from the dorsal otocyst migrate ventrally to contribute to the future cochlear sensory epithelium.15 Prosensory specification in the elongating cochlear duct is mediated by Jag1-Notch1 signaling via lateral induction.16 Ectopic expression of NICD in non-sensory regions of the mouse cochlea leads to the formation of prosensory patches expressing Jag1 and the transcription factor Sox2, another early prosensory cell marker.17 Sox2 expression is required for prosensory competence and Jag1 mutants exhibit a downregulation of Sox2, suggesting that Sox2 expression is downstream of Jag1-mediated Notch signaling.17, 18 Once specified, the proliferation of Sox2-positive prosensory cells is regulated by canonical Wnt signaling.19
Using TCF/Lef:H2B-GFP mice, we previously showed Wnt reporter activity in early prosensory cells.19 This Wnt reporter activity overlaps with the expression of stabilized, or active, β-catenin in the developing cochlear duct (Figure 2). Inhibition of Wnt signaling during this early mitotic phase blocks the proliferation of prosensory cells, and, conversely, activation of the pathway further promotes proliferation.19 Adequate proliferation of prosensory cells is, in part, regulated by Notch signaling through transcriptional downregulation of the cell-cycle inhibitor p27Kip1, which plays a pivotal role in the correct spatiotemporal pattern of cell-cycle exit in the organ of Corti from the apex to the base.20
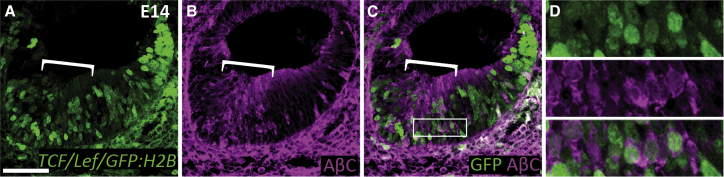
Figure 2.
Wnt Reporter Activity Overlaps with Active β-Catenin Expression in the Embryonic Cochlear Duct
(A) Transverse section through an embryonic day (E)14 cochlea of a TCF/Lef:H2B-GFP reporter mouse. (B) Same E14 section is stained for active β-catenin (AβC). (C) Merged view of GFP and active β-catenin shows overlapped expression. (D) Enlarged image of box in (C). Scale bar: 50 μm (A–C). The white brackets in (A)–(C) indicate the area of the developing organ of Corti.
Following terminal mitosis, prosensory cells differentiate into hair cells or supporting cells. The basic helix-loop-helix (bHLH) transcription factor Atoh1 is both necessary and sufficient for hair cell differentiation, and its expression is regulated by Sox2 and canonical Wnt and Notch pathways. Sox2 activates Atoh1 through interaction with the Atoh1 3′ enhancer, however, subsequent downregulation of Sox2 is required for Atoh1 expression.18, 21 Canonical Wnt is also required for Atoh1 expression and the loss of β-catenin inhibits hair cell differentiation.19, 22 Atoh1, however, is initially upregulated in a larger group of prosensory cells than those that eventually acquire a hair cell fate.23, 24 Generating the proper mosaic of hair cells and supporting cells is mediated by Notch signaling via lateral inhibition. Nascent hair cells downregulate Notch activity, but they express Jag2 and Dll1 to activate the Notch pathway in adjacent cells destined to acquire a supporting cell fate. Notch inhibition upregulates Atoh1, but it also downregulates Sox2, and this might play a role in maintaining Atoh1 expression in differentiating hair cells.25 Conversely, Notch activation in adjacent cells stimulates expression of the Hes/Hey family of transcription factors, which appears to inhibit Atoh1 expression by recruiting the transcriptional co-repressor Groucho to the Atoh1 promoter.23 Once differentiated, supporting cells retain their expression of Sox2, although Atoh1 is downregulated in mature hair cells26 (which can be identified by their expression of MyosinVI; Figure 1).
Fine-tuning of the Wnt and Notch pathways is also crucial for the development and patterning of the cochlea. Binding of R-spondins to their Lgr4/5/6 receptors typically enhances Wnt signaling.27 However, in the mammalian cochlea, the loss of Lgr5/R-spondin signaling increases canonical Wnt activity, and Lgr4/5 and R-spondin2 mutants exhibit an overproduction of hair cells.28, 29 Furthermore, loss of Kremen1, a receptor for the dickkopf (Dkk) family of Wnt antagonists, biases prosensory cells toward a hair cell fate.30 The Notch pathway modifiers, Lfng and Mfng, also play a role in patterning of the cochlea and Lfng and Mfng mutants exhibit duplications in inner hair cells.31 While Wnt and Notch pathways play key roles in the development and patterning of the cochlea, components of both of these pathways continue to be expressed in the neonatal mouse cochlea.32, 33
Hair Cell Regeneration in the Postnatal Cochlea through Notch Inhibition
Given its roles in hair cell development, the Notch pathway has been a key target for hair cell regeneration. Inhibition of Notch signaling in the neonatal cochlea with pharmacologic agents or through genetic manipulation induces the direct transdifferentiation of supporting cells into hair cells, albeit at the cost of supporting cells.34, 35, 36 Notch inhibition in supporting cells promotes histone H3K9 acetylation (H3K9ac) at the Atoh1 promoter and a downregulation of the Hes/Hey family of transcription factors, both of which appear to contribute to an increase in Atoh1 expression.23, 34
While Notch inhibition promotes the transdifferentiation of supporting cells into hair cells in the neonatal cochlea, it has been reported that supporting cells lose their ability to respond to Notch inhibition rapidly within the first postnatal week.37 Indeed, Notch inhibition in the adult mouse cochlea has shown variable results on hair cell regeneration, which might be due to differences in the damage paradigms used or the limited specificity of γ-secretase inhibitors on the Notch signaling pathway. Nevertheless, an earlier study demonstrated that, following acoustic injury in adult mice, administration of the γ-secretase inhibitor LY411575 results in the formation of new hair cells in the outer hair cell region and a mild reduction in noise-induced auditory brainstem response (ABR) threshold shifts, although the mice were still functionally deaf.34 Similarly, knockdown of the Hes family members Hes1 and Hes5 with small interfering RNA (siRNA) is also sufficient for the upregulation of Atoh1 mRNA and an increase in hair cell numbers in both neonatal and adult cochleae. Although, the newly generated hair cells exhibit an immature phenotype and lineage-tracing studies are still needed to confirm the origins of these new hair cells.38, 39
Clinical Applications of Notch Signaling Inhibitors
The potential use of Notch inhibitors for hair cell regeneration in humans is being assessed by the REGAIN (regeneration of inner ear hair cells with gamma-secretase inhibitors) consortium in conjunction with Audion Therapeutics. In February 2019, they announced positive results from their first-in-human phase I clinical trial to evaluate the safety of local delivery of the Notch signaling inhibitor LY3056480 in patients with mild-to-moderate sensorineural hearing loss. Results showed that the drug was well tolerated and no local or systemic adverse events were reported. The phase II efficacy trial was initiated in January 2019, with primary endpoints including pure tone hearing levels and speech in noise performance.40
Clinical Applications of Ectopic Atoh1 Expression
While knockdown of Notch pathway components is sufficient for Atoh1 upregulation, direct and ectopic expression of Atoh1 in vivo was also shown to induce new hair cells in young mammalian cochleae.2, 10, 41, 42, 43 Novartis Pharmaceuticals is currently conducting a clinical trial on the use of CGF166, a recombinant adenovirus 5 (Ad5) vector encoding the human Atonal transcription factor, in patients with severe-to-profound bilateral hearing loss for safety, tolerability, and changes in vestibular and auditory functions.44 This is the first attempt by any group to clinically evaluate genetic modulation for hearing loss. However, many studies have shown a decrease in the ability of Atoh1 to induce hair cells in the adult organ of Corti;45 thus, the efficacy and robustness of Atoh1 overexpression alone for hair cell regeneration in the mammalian cochlea might be limited.
Hair Cell Regeneration in the Postnatal Cochlea through Wnt Activation
Stabilization of β-catenin in the neonatal cochlea promotes the proliferation of typically quiescent supporting cells and, to a limited degree, the induction of new hair cells.46, 47, 48 A subset of supporting cells expressing Lgr5, a component of the Wnt pathway, is especially responsive to Wnt stimulation.46, 47, 48, 49, 50 When isolated and grown in single-cell suspension, Lgr5-positive cells proliferate to form colonies and differentiate into hair cells; this effect is further enhanced by Wnt stimulation but lost when exposed to Wnt inhibitors.46, 50 β-catenin stabilization in Lgr5-positive cells of neonatal mice in vivo also results in a transient expansion of Lgr5-positive cells, as well as the induction of new hair cells.46, 47 In our previous studies, we used in vivo and in vitro mouse models to elucidate the temporal patterns as well as the mechanism of the regenerative response to Wnt activation in embryonic and neonatal cochleae.19, 42 We showed that the decline in regenerative capacity at older neonatal stages is correlated with changes in the downstream transcriptional response to Wnt activation, thus suggesting that Wnt activation alone may be insufficient to promote adult hair cell regeneration.42
Combinational Strategies for Hair Cell Regeneration
There is a growing consensus that combinational strategies may be able to synergistically enhance the regenerative capacity in neonatal and adult cochleae. For example, the in vitro hair cell yield from Lgr5-positive cells isolated from neonatal mice and grown as organoids can be further improved by treatment with the Wnt activator CHIR99021 (CHIR) and the histone deacetylase (HDAC) inhibitor valproic acid (VPA; which is thought to enhance histone H3K9 acetylation),51 along with a cocktail of growth factors.52 These findings were successfully reproduced in Lgr5-positive cells isolated from adult mice, as well as non-human primates and healthy human inner ear tissue.52 It is important to note, however, that cells and organoids grown from dissociated single-cell preparations demonstrate a higher capacity for proliferation and differentiation in comparison to Lgr5+ cells within intact cochlear tissue.
In addition to VPA, the combination of Wnt activation with other forms of pharmacological and genetic manipulation can also produce a more robust regenerative response in the neonatal cochlea. For instance, the combination of β-catenin stabilization with ectopic Atoh1 expression in vivo in Lgr5-positive cells of the neonatal cochlea promotes a synergistic increase in supporting cell proliferation and hair cell induction.53 These newly generated hair cells were shown to survive to adulthood and were innervated by neurons.53 Furthermore, the combination of Wnt activation with Notch inhibition in the neonatal cochlea was also shown to promote extensive supporting cell proliferation and hair cell regeneration, with partial restoration of supporting cell numbers.54, 55 Similarly, β-catenin gain-of-function combined with Sox2 haploinsufficiency can enhance cochlear proliferation and hair cell formation during juvenile stages, although this effect is not observed in adult mice.56
Clinical Applications for Combinatorial Therapeutics
To date, only one combinatorial therapy for hair cell regeneration is being evaluated in the clinic. Frequency Therapeutics developed FX-322, a proprietary combination of CHIR and VPA, to regenerate hair cells.52 They successfully completed a first-in-human randomized, double-blind, placebo-controlled safety study for local delivery of a single dose of FX-322 in adults undergoing cochlear implantation surgery. Results confirmed the safety and tolerability of FX-322 in awake patients; the phase II portion of this trial is currently underway.57
Epigenetic Modifications and Improving Regenerative Success in the Adult Mammalian Cochlea
Although modulation of signaling pathways can promote hair cell regeneration in the neonatal cochlea, this regenerative response is lost or limited in adult stages (for a review on the potential role of epigenetic regulation on hair cell development and regeneration, see Layman and Zuo58). For instance, while Notch inhibition in early neonatal stages promotes the transdifferentiation of supporting cells to hair cells, it has been reported that this capacity for transdifferentiation is lost by postnatal day (P)3.37 Similarly, stabilization of β-catenin as late as P4 promotes supporting cell proliferation and the induction of new hair cells; however, this effect is no longer observed in the mature cochlea.47 This decline in the regenerative response as the cochlea matures over the postnatal period appears to occur with most forms of manipulation tested to date. Moreover, many Wnt signaling components are still expressed in the P30 mouse cochlea, suggesting that some other factors are preventing this pathway from promoting proliferation and/or hair cell differentiation.32 Use of Wnt and Notch modulators in combination with drugs targeted against chromatin-remodeling enzymes, such as HDAC inhibitors used by McLean and colleagues,52 highlights the positive effects that altering access to target genes can have on the regenerative response of mature inner ear tissue. These findings suggest that epigenetic modifications during maturation of the hearing organ might explain our limited ability to generate new hair cells to replace lost or damaged hair cells in the adult.
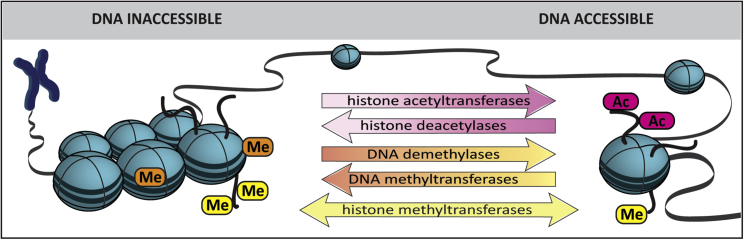
Chromatin structure and access to target genes is regulated by modifications on both DNA and histone proteins (Figure 3). Histone modifications include histone acetylation or methylation on lysine (K) residues in H3 and H4 tails. Histone acetylation via histone acetyltransferases (HATs) is predominantly associated with an open chromatin structure and transcriptional activation. Conversely, acetyl groups can be removed by HDACs, resulting in more compact chromatin structure and transcriptional repression. Lysine residues on H3 and H4 proteins can also be mono-, di-, or tri-methylated (me, me2, or me3); methylation can promote transcriptional activation or repression, depending on the target site. DNA methylation via DNA methyltransferase enzymes (DNMTs) is generally associated with transcriptional repression.59
Figure 3.
Chromatin Structure and Chromatin Accessibility
Acetylation (Ac) of histone H3 and H4 tails by histone acetyltransferases is predominantly associated with an open chromatin structure. Removal of acetyl groups by histone deacetylases is associated with chromatin condensation. Methylation (Me) of H3 and H4 tails by histone methyltransferases can promote an open or closed chromatin structure, depending on the target site. DNA methylation by DNA methyltransferases is generally associated with limited chromatin accessibility, while the removal of methyl groups by DNA demethylases is associated with increased DNA accessibility.
The overall epigenetic status of the mouse organ of Corti switches from high levels of H3K9 acetylation (H3K9ac) in young adults to H3K9 methylation (H3K9me) in the aging cochlea.60 H3K9ac is associated with open chromatin structure and H3K9me is associated with chromatin condensation.59, 61, 62 The Atoh1 gene locus is also subject to epigenetic modifications at different stages of development. Prior to hair cell differentiation, prosensory cells are in a bivalent or poised state with H3K4me3 and H3K27me3 modifications at the Atoh1 locus. At the onset of hair cell differentiation and concomitant with Atoh1 upregulation, inhibitory H3K27me3 is reduced and H3K9ac is increased. Downregulation of Atoh1 mRNA during hair cell maturation is accompanied by a reduction in H3K9ac and acquisition of H3K9me3. Intriguingly, the H3K4me3/H3K27me3 bivalent state persists in supporting cells of the neonatal mouse cochlea from P1 to at least P6, and this might, in part, explain the transient capacity for hair cell induction in neonatal stages.63 Furthermore, the transient capacity for supporting cell proliferation appears to be correlated with increased methylation on Sox2 enhancers NOP1 and NOP2, although the promoter region of Sox2 remains demethylated.64 In addition to modifications on DNA and histone proteins, gene expression is also regulated by non-coding RNAs and they play a significant role in the capacity for hair cell regeneration.65
Age-dependent changes in chromatin structure affect the transcriptional response to modulation of signaling pathways and, consequently, they can regulate the regenerative response of the cochlea. To improve regenerative success in the adult stages, targeting epigenetic enzymes such as HATs, HDACs, and DNMTs, in addition to Wnt and Notch modulation, might prove to be a useful approach. Indeed, some small molecule inhibitors against DNMTs and HDACs are approved for use in patients, and they may be adopted for the mammalian auditory system.66 However, a major question that remains is which gene loci should be targeted to stimulate hair cell regeneration.
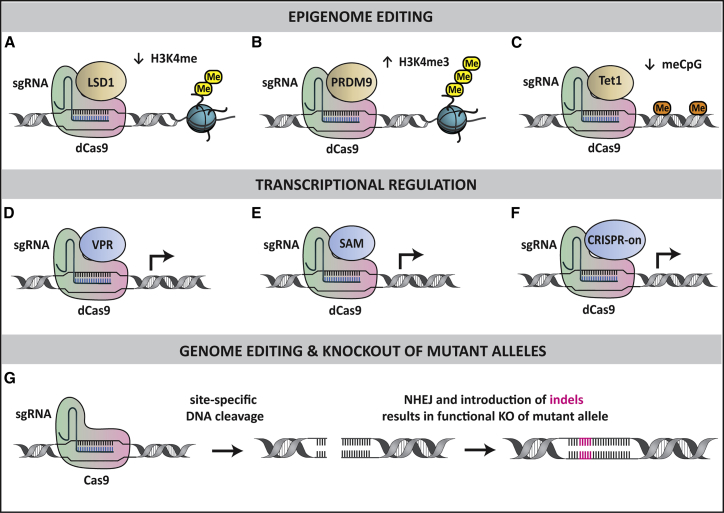
If such target genes are identified, epigenetic editing and transcriptional regulation with CRISPR-Cas9 might represent a feasible approach to hearing regeneration. Catalytically inactive dead Cas9 (dCas9) and single-guide RNA (sgRNA) complexes can be fused to engineered epigenetic (Figures 4A–4C) and transcriptional regulatory complexes (Figures 4D–4F) to activate or repress target genes in a site-specific manner.67, 68, 69, 70, 71, 72 Nuclease-active Cas9 can also be used to make permanent modifications to genomic DNA and this might be useful for some hereditary forms of hearing loss (Figure 4G).73 In fact, a recent study demonstrated that in vivo delivery of Cas9-sgRNA to the postnatal cochlea, which targeted a specific mutant allele, could ameliorate the progression of hearing loss in mice.74 Although it is yet to be tested in adult mice and optimized for efficient delivery to target cells, the advent of genome- and epigenome-editing techniques such as CRISPR-cas9 has the potential to treat both hereditary and acquired forms of hearing loss in humans.
Figure 4.
Epigenome Editing, Transcriptional Regulation, and Genome Editing with CRISPR-Cas9
Epigenome editing. dCas9-sgRNA complexes can be fused to epigenetic enzymes, such as (A) lysine-specific histone demethylase 1 (LSD1), (B) histone-lysine N-methyltransferase PRDM9, and (C) methylcytosine dioxygenase Tet1, for targeted epigenome editing at specific DNA loci. Transcriptional regulation. dCas9-sgRNA complexes can also be fused to engineered transcriptional regulatory complexes, such as (D) VP64-p65-Rta (VPR), (E) Synergistic Activation Mediator (SAM), and (F) CRISPR-on, to activate gene expression at specific loci. Genome editing and knockout of mutant alleles. (G) Cas9-sgRNA complexes generate a double-stranded break at the targeted allele. In mitotically quiescent cells, such as SCs of the mature mammalian cochlea, breaks can be repaired by the non-homologous end joining (NHEJ) process that leaves small insertions and deletions (indels) at the cut site. This produces frameshift and nonsense mutations, resulting in functional knockout (KO) of the mutant allele.
Animals
Mice were maintained and euthanized in accordance with Institutional Animal Care and Use Committee regulations at Sunnybrook Research Institute.
Conclusions
The Wnt and Notch signaling pathways play significant roles in the development of the mammalian cochlea, including prosensory specification, proliferation, cell fate determination, and hair cell differentiation.2, 6, 7, 8, 9, 17, 18, 19, 20, 23, 25 Both pathways appear to converge on key molecules such as Sox2 and Atoh1, and fine-tuning of the two pathways is important for patterning within the cochlea.19, 22, 23, 25, 27, 28, 29, 30, 31 Given their roles in development, activation of canonical Wnt signaling following birth can promote supporting cell proliferation and limited hair cell induction, and inhibition of the Notch pathway can promote the transdifferentiation of supporting cells to hair cells.34, 35, 36, 37, 38, 39, 46, 47, 49, 50, 52, 53, 54, 55 There has been some success in hair cell regeneration following Wnt and Notch modulation in the mature mammalian cochlea.34, 52 However, it is important to note that many of these newly generated hair cells show limited survival and do not appear to fully differentiate, lacking mature stereocilia, and they may fail to form proper synaptic connections with auditory neurons.75
Even with the limited efficacy demonstrated to date, this line of research has led to several human clinical trials aimed at evaluating the safety and efficacy of therapeutic modalities for hair cell regeneration.40, 44, 57 The limited regenerative capacity of the mature cochlea might be attributed to age-dependent epigenetic modifications. Therefore, improving regenerative success during adult stages might require targeting epigenetic enzymes along with Wnt and Notch modulators, among others, as well as utilizing epigenome-editing techniques such as CRISPR-dCas9.59, 60, 61, 62, 63, 64, 67, 68, 69, 70, 71, 72, 73, 74
Conflicts of Interest
The authors declare no competing financial interests. B.E.J. is an employee and stockholder of Otonomy Inc.
Acknowledgments
We thank Dr. T. Piotrowski for providing valuable comments on the manuscript. Funding was provided by the Koerner Foundation (to A.D.); the Canadian Institutes of Health Research Canada Graduate Scholarship-Master’s Award, the Raymond H.W. Ng Graduate Scholarship, and a University of Toronto Fellowship Award (to A.S.); and the Sunnybrook Hearing Regeneration Initiative.
References
- 1.Müller U., Barr-Gillespie P.G. New treatment options for hearing loss. Nat. Rev. Drug Discov. 2015;14:346–365. doi: 10.1038/nrd4533. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson P.J., Huarcaya Najarro E., Sayyid Z.N., Cheng A.G. Sensory hair cell development and regeneration: similarities and differences. Development. 2015;142:1561–1571. doi: 10.1242/dev.114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heeringa A.N., Köppl C. The aging cochlea: Towards unraveling the functional contributions of strial dysfunction and synaptopathy. Hear. Res. 2019 doi: 10.1016/j.heares.2019.02.015. Published online March 2, 2019. [DOI] [PubMed] [Google Scholar]
- 4.Meas S.J., Zhang C.L., Dabdoub A. Reprogramming Glia Into Neurons in the Peripheral Auditory System as a Solution for Sensorineural Hearing Loss: Lessons From the Central Nervous System. Front. Mol. Neurosci. 2018;11:77. doi: 10.3389/fnmol.2018.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kremer H. Hereditary hearing loss; about the known and the unknown. Hear. Res. 2019 doi: 10.1016/j.heares.2019.01.003. Published online January 10, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Groves A.K., Fekete D.M. Shaping sound in space: the regulation of inner ear patterning. Development. 2012;139:245–257. doi: 10.1242/dev.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Żak M., Klis S.F., Grolman W. The Wnt and Notch signalling pathways in the developing cochlea: Formation of hair cells and induction of regenerative potential. Int. J. Dev. Neurosci. 2015;47(Pt B):247–258. doi: 10.1016/j.ijdevneu.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Ohyama T., Groves A.K., Martin K. The first steps towards hearing: mechanisms of otic placode induction. Int. J. Dev. Biol. 2007;51:463–472. doi: 10.1387/ijdb.072320to. [DOI] [PubMed] [Google Scholar]
- 9.Jansson L., Kim G.S., Cheng A.G. Making sense of Wnt signaling-linking hair cell regeneration to development. Front. Cell. Neurosci. 2015;9:66. doi: 10.3389/fncel.2015.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns J.C., Stone J.S. Development and regeneration of vestibular hair cells in mammals. Semin. Cell Dev. Biol. 2017;65:96–105. doi: 10.1016/j.semcdb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W., You D., Chen Y., Chai R., Li H. Regeneration of hair cells in the mammalian vestibular system. Front. Med. 2016;10:143–151. doi: 10.1007/s11684-016-0451-1. [DOI] [PubMed] [Google Scholar]
- 12.Kniss J.S., Jiang L., Piotrowski T. Insights into sensory hair cell regeneration from the zebrafish lateral line. Curr. Opin. Genet. Dev. 2016;40:32–40. doi: 10.1016/j.gde.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Nusse R., Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Nowell C.S., Radtke F. Notch as a tumour suppressor. Nat. Rev. Cancer. 2017;17:145–159. doi: 10.1038/nrc.2016.145. [DOI] [PubMed] [Google Scholar]
- 15.Brown A.S., Rakowiecki S.M., Li J.Y.H., Epstein D.J. The cochlear sensory epithelium derives from Wnt responsive cells in the dorsomedial otic cup. Dev. Biol. 2015;399:177–187. doi: 10.1016/j.ydbio.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiernan A.E. Notch signaling during cell fate determination in the inner ear. Semin. Cell Dev. Biol. 2013;24:470–479. doi: 10.1016/j.semcdb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan W., Jin Y., Stanger B., Kiernan A.E. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc. Natl. Acad. Sci. USA. 2010;107:15798–15803. doi: 10.1073/pnas.1003089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puligilla C., Kelley M.W. Dual role for Sox2 in specification of sensory competence and regulation of Atoh1 function. Dev. Neurobiol. 2017;77:3–13. doi: 10.1002/dneu.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacques B.E., Puligilla C., Weichert R.M., Ferrer-Vaquer A., Hadjantonakis A.K., Kelley M.W., Dabdoub A. A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea. Development. 2012;139:4395–4404. doi: 10.1242/dev.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murata J., Ohtsuka T., Tokunaga A., Nishiike S., Inohara H., Okano H., Kageyama R. Notch-Hes1 pathway contributes to the cochlear prosensory formation potentially through the transcriptional down-regulation of p27Kip1. J. Neurosci. Res. 2009;87:3521–3534. doi: 10.1002/jnr.22169. [DOI] [PubMed] [Google Scholar]
- 21.Kempfle J.S., Turban J.L., Edge A.S.B. Sox2 in the differentiation of cochlear progenitor cells. Sci. Rep. 2016;6:23293. doi: 10.1038/srep23293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi F., Hu L., Jacques B.E., Mulvaney J.F., Dabdoub A., Edge A.S.B. β-Catenin is required for hair-cell differentiation in the cochlea. J. Neurosci. 2014;34:6470–6479. doi: 10.1523/JNEUROSCI.4305-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdolazimi Y., Stojanova Z., Segil N. Selection of cell fate in the organ of Corti involves the integration of Hes/Hey signaling at the Atoh1 promoter. Development. 2016;143:841–850. doi: 10.1242/dev.129320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Driver E.C., Sillers L., Coate T.M., Rose M.F., Kelley M.W. The Atoh1-lineage gives rise to hair cells and supporting cells within the mammalian cochlea. Dev. Biol. 2013;376:86–98. doi: 10.1016/j.ydbio.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell D.P., Chrysostomou E., Doetzlhofer A. Canonical Notch signaling plays an instructive role in auditory supporting cell development. Sci. Rep. 2016;6:19484. doi: 10.1038/srep19484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dabdoub A., Puligilla C., Jones J.M., Fritzsch B., Cheah K.S.E., Pevny L.H., Kelley M.W. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc. Natl. Acad. Sci. USA. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Lau W., Peng W.C., Gros P., Clevers H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Genes Dev. 2014;28:305–316. doi: 10.1101/gad.235473.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulvaney J.F., Yatteau A., Sun W.W., Jacques B., Takubo K., Suda T., Yamada W., Dabdoub A. Secreted factor R-Spondin 2 is involved in refinement of patterning of the mammalian cochlea. Dev. Dyn. 2013;242:179–188. doi: 10.1002/dvdy.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Żak M., van Oort T., Hendriksen F.G., Garcia M.I., Vassart G., Grolman W. LGR4 and LGR5 regulate hair cell differentiation in the sensory epithelium of the developing mouse cochlea. Front. Cell. Neurosci. 2016;10:186. doi: 10.3389/fncel.2016.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulvaney J.F., Thompkins C., Noda T., Nishimura K., Sun W.W., Lin S.Y., Coffin A., Dabdoub A. Kremen1 regulates mechanosensory hair cell development in the mammalian cochlea and the zebrafish lateral line. Sci. Rep. 2016;6:31668. doi: 10.1038/srep31668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basch M.L., Brown R.M., 2nd, Jen H.I., Semerci F., Depreux F., Edlund R.K., Zhang H., Norton C.R., Gridley T., Cole S.E. Fine-tuning of Notch signaling sets the boundary of the organ of Corti and establishes sensory cell fates. eLife. 2016;5:19921. doi: 10.7554/eLife.19921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geng R., Noda T., Mulvaney J.F., Lin V.Y.W., Edge A.S.B., Dabdoub A. Comprehensive expression of Wnt signaling pathway genes during development and maturation of the mouse cochlea. PLoS ONE. 2016;11:e0148339. doi: 10.1371/journal.pone.0148339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maass J.C., Gu R., Cai T., Wan Y.W., Cantellano S.C., Asprer J.S.T., Zhang H., Jen H.I., Edlund R.K., Liu Z., Groves A.K. Transcriptomic analysis of mouse cochlear supporting cell maturation reveals large-scale changes in Notch responsiveness prior to the onset of hearing. PLoS ONE. 2016;11:e0167286. doi: 10.1371/journal.pone.0167286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizutari K., Fujioka M., Hosoya M., Bramhall N., Okano H.J., Okano H., Edge A.S. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 2013;77:58–69. doi: 10.1016/j.neuron.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto N., Tanigaki K., Tsuji M., Yabe D., Ito J., Honjo T. Inhibition of Notch/RBP-J signaling induces hair cell formation in neonate mouse cochleas. J. Mol. Med. (Berl.) 2006;84:37–45. doi: 10.1007/s00109-005-0706-9. [DOI] [PubMed] [Google Scholar]
- 36.Bramhall N.F., Shi F., Arnold K., Hochedlinger K., Edge A.S. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Reports. 2014;2:311–322. doi: 10.1016/j.stemcr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maass J.C., Gu R., Basch M.L., Waldhaus J., Lopez E.M., Xia A., Oghalai J.S., Heller S., Groves A.K. Changes in the regulation of the Notch signaling pathway are temporally correlated with regenerative failure in the mouse cochlea. Front. Cell. Neurosci. 2015;9:110. doi: 10.3389/fncel.2015.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du X., Li W., Gao X., West M.B., Saltzman W.M., Cheng C.J., Stewart C., Zheng J., Cheng W., Kopke R.D. Regeneration of mammalian cochlear and vestibular hair cells through Hes1/Hes5 modulation with siRNA. Hear. Res. 2013;304:91–110. doi: 10.1016/j.heares.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du X., Cai Q., West M.B., Youm I., Huang X., Li W., Cheng W., Nakmali D., Ewert D.L., Kopke R.D. Regeneration of cochlear hair cells and hearing recovery through Hes1 modulation with siRNA nanoparticles in adult guinea pigs. Mol. Ther. 2018;26:1313–1326. doi: 10.1016/j.ymthe.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.International Standard Randomised Controlled Trial Number (ISRCTN) registry A first-in-human study of the safety and efficacy of a new drug, a gamma secretase inhibitor, to treat people with sensorineural hearing loss. 2017. http://www.isrctn.com/ISRCTN59733689
- 41.Liu Z., Dearman J.A., Cox B.C., Walters B.J., Zhang L., Ayrault O., Zindy F., Gan L., Roussel M.F., Zuo J. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J. Neurosci. 2012;32:6600–6610. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samarajeewa A., Lenz D.R., Xie L., Chiang H., Kirchner R., Mulvaney J.F., Edge A.S.B., Dabdoub A. Transcriptional response to Wnt activation regulates the regenerative capacity of the mammalian cochlea. Development. 2018;145:dev166579. doi: 10.1242/dev.166579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su G., Morris J.H., Demchak B., Bader G.D. Biological network exploration with Cytoscape 3. Curr. Protoc. Bioinformatics. 2014;47 doi: 10.1002/0471250953.bi0813s47. 8.13.1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ClinicalTrials.gov. Safety, Tolerability and Efficacy for CGF166 in Patients With Unilateral or Bilateral Severe-to-profound Hearing Loss. 2018. https://clinicaltrials.gov/ct2/show/NCT02132130
- 45.Kelly M.C., Chang Q., Pan A., Lin X., Chen P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J. Neurosci. 2012;32:6699–6710. doi: 10.1523/JNEUROSCI.5420-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chai R., Kuo B., Wang T., Liaw E.J., Xia A., Jan T.A., Liu Z., Taketo M.M., Oghalai J.S., Nusse R. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc. Natl. Acad. Sci. USA. 2012;109:8167–8172. doi: 10.1073/pnas.1202774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi F., Hu L., Edge A.S. Generation of hair cells in neonatal mice by β-catenin overexpression in Lgr5-positive cochlear progenitors. Proc. Natl. Acad. Sci. USA. 2013;110:13851–13856. doi: 10.1073/pnas.1219952110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skene P.J., Henikoff S. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. eLife. 2017;6:e21856. doi: 10.7554/eLife.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chai R., Xia A., Wang T., Jan T.A., Hayashi T., Bermingham-McDonogh O., Cheng A.G. Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J. Assoc. Res. Otolaryngol. 2011;12:455–469. doi: 10.1007/s10162-011-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi F., Kempfle J.S., Edge A.S.B. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J. Neurosci. 2012;32:9639–9648. doi: 10.1523/JNEUROSCI.1064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hezroni H., Sailaja B.S., Meshorer E. Pluripotency-related, valproic acid (VPA)-induced genome-wide histone H3 lysine 9 (H3K9) acetylation patterns in embryonic stem cells. J. Biol. Chem. 2011;286:35977–35988. doi: 10.1074/jbc.M111.266254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLean W.J., Yin X., Lu L., Lenz D.R., McLean D., Langer R., Karp J.M., Edge A.S.B. Clonal expansion of Lgr5-positive cells from mammalian cochlea and high-purity generation of sensory hair cells. Cell Rep. 2017;18:1917–1929. doi: 10.1016/j.celrep.2017.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuo B.R., Baldwin E.M., Layman W.S., Taketo M.M., Zuo J. In vivo cochlear hair cell generation and survival by coactivation of β-catenin and Atoh1. J. Neurosci. 2015;35:10786–10798. doi: 10.1523/JNEUROSCI.0967-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ni W., Lin C., Guo L., Wu J., Chen Y., Chai R., Li W., Li H. Extensive supporting cell proliferation and mitotic hair cell generation by in vivo genetic reprogramming in the neonatal mouse cochlea. J. Neurosci. 2016;36:8734–8745. doi: 10.1523/JNEUROSCI.0060-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ni W., Zeng S., Li W., Chen Y., Zhang S., Tang M., Sun S., Chai R., Li H. Wnt activation followed by Notch inhibition promotes mitotic hair cell regeneration in the postnatal mouse cochlea. Oncotarget. 2016;7:66754–66768. doi: 10.18632/oncotarget.11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atkinson P.J., Dong Y., Gu S., Liu W., Najarro E.H., Udagawa T., Cheng A.G. Sox2 haploinsufficiency primes regeneration and Wnt responsiveness in the mouse cochlea. J. Clin. Invest. 2018;128:1641–1656. doi: 10.1172/JCI97248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ClinicalTrials.gov. First in Human safety study of FX-322 in adults undergoing cochlear implantation. 2018. https://clinicaltrials.gov/ct2/show/NCT03300687
- 58.Layman W.S., Zuo J. Epigenetic regulation in the inner ear and its potential roles in development, protection, and regeneration. Front. Cell. Neurosci. 2015;8:446. doi: 10.3389/fncel.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Handy D.E., Castro R., Loscalzo J. Epigenetic modifications: basic mechanisms and role in cardiovascular disease. Circulation. 2011;123:2145–2156. doi: 10.1161/CIRCULATIONAHA.110.956839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watanabe K., Bloch W. Histone methylation and acetylation indicates epigenetic change in the aged cochlea of mice. Eur. Arch. Otorhinolaryngol. 2013;270:1823–1830. doi: 10.1007/s00405-012-2222-1. [DOI] [PubMed] [Google Scholar]
- 61.Yan C., Boyd D.D. Histone H3 acetylation and H3 K4 methylation define distinct chromatin regions permissive for transgene expression. Mol. Cell. Biol. 2006;26:6357–6371. doi: 10.1128/MCB.00311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bannister A.J., Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stojanova Z.P., Kwan T., Segil N. Epigenetic regulation of Atoh1 guides hair cell development in the mammalian cochlea. Development. 2015;142:3529–3536. doi: 10.1242/dev.126763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waldhaus J., Cimerman J., Gohlke H., Ehrich M., Müller M., Löwenheim H. Stemness of the organ of Corti relates to the epigenetic status of Sox2 enhancers. PLoS ONE. 2012;7:e36066. doi: 10.1371/journal.pone.0036066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doetzlhofer A., Avraham K.B. Insights into inner ear-specific gene regulation: Epigenetics and non-coding RNAs in inner ear development and regeneration. Semin. Cell Dev. Biol. 2017;65:69–79. doi: 10.1016/j.semcdb.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Copeland R.A., Olhava E.J., Scott M.P. Targeting epigenetic enzymes for drug discovery. Curr. Opin. Chem. Biol. 2010;14:505–510. doi: 10.1016/j.cbpa.2010.06.174. [DOI] [PubMed] [Google Scholar]
- 67.Kearns N.A., Pham H., Tabak B., Genga R.M., Silverstein N.J., Garber M., Maehr R. Functional annotation of native enhancers with a Cas9-histone demethylase fusion. Nat. Methods. 2015;12:401–403. doi: 10.1038/nmeth.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cano-Rodriguez D., Gjaltema R.A., Jilderda L.J., Jellema P., Dokter-Fokkens J., Ruiters M.H., Rots M.G. Writing of H3K4Me3 overcomes epigenetic silencing in a sustained but context-dependent manner. Nat. Commun. 2016;7:12284. doi: 10.1038/ncomms12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X.S., Wu H., Ji X., Stelzer Y., Wu X., Czauderna S., Shu J., Dadon D., Young R.A., Jaenisch R. Editing DNA methylation in the mammalian genome. Cell. 2016;167:233–247.e17. doi: 10.1016/j.cell.2016.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chavez A., Scheiman J., Vora S., Pruitt B.W., Tuttle M., P R Iyer E., Lin S., Kiani S., Guzman C.D., Wiegand D.J. Highly efficient Cas9-mediated transcriptional programming. Nat. Methods. 2015;12:326–328. doi: 10.1038/nmeth.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Konermann S., Brigham M.D., Trevino A.E., Joung J., Abudayyeh O.O., Barcena C., Hsu P.D., Habib N., Gootenberg J.S., Nishimasu H. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517:583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng A.W., Wang H., Yang H., Shi L., Katz Y., Theunissen T.W., Rangarajan S., Shivalila C.S., Dadon D.B., Jaenisch R. Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res. 2013;23:1163–1171. doi: 10.1038/cr.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki K., Tsunekawa Y., Hernandez-Benitez R., Wu J., Zhu J., Kim E.J., Hatanaka F., Yamamoto M., Araoka T., Li Z. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature. 2016;540:144–149. doi: 10.1038/nature20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao X., Tao Y., Lamas V., Huang M., Yeh W.H., Pan B., Hu Y.J., Hu J.H., Thompson D.B., Shu Y. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature. 2018;553:217–221. doi: 10.1038/nature25164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Z., Fang J., Dearman J., Zhang L., Zuo J. In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic Atoh1 expression. PLoS ONE. 2014;9:e89377. doi: 10.1371/journal.pone.0089377. [DOI] [PMC free article] [PubMed] [Google Scholar]