Abstract
High-mobility group box 1 (HMGB1) mediates acute lung injury in a mouse model of paraquat poisoning. However, published reports showing a clinically relevant association between HMGB1 and paraquat exposure are lacking. The objective of the present study was to investigate the potential role of serum HMGB1 level as a prognostic marker of mortality in patients with paraquat poisoning in a clinical setting. This retrospective observational cohort study included a convenience sample of 92 patients with acute paraquat poisoning admitted to the emergency room (ER) of The First Hospital of Jilin University between January 2014 and December 2016. Baseline serum HMGB1 levels and other laboratory parameters were measured on admission. Cumulative incidence of mortality during the first 30 days after admission was 50% (n = 46/92). Serum HMGB1 levels were higher in fatalities than survivors (P = 0.015), 30-day mortality increased with increasing baseline serum HMGB1 level (P < 0.001), and higher serum HMGB1 levels were associated with an increase in 30-day mortality on Kaplan-Meier analysis. Multivariate Cox regression analysis identified baseline serum HMGB1 levels, white blood cell count, and serum lactic acid levels as independent prognostic markers of 30-day mortality. These data suggest that serum HMGB1 levels measured on admission to the ER are an independent predictor of 30-day mortality in patients with acute paraquat poisoning.
Subject terms: Biophysical chemistry, Prognostic markers
Introduction
Paraquat is an organic compound containing the active ingredient 1,1′-dimethyl-4,4′-bipyridinium dichloride that is widely used as a herbicide. Globally, paraquat is a leading cause of lethal poisoning, particularly in developing countries1–6. In fact, oral ingestion of paraquat for deliberate self-harm has become a severe public health problem in several countries1–4.
Currently, oxidative stress is implicated as the mechanism underlying the toxic effects produced by paraquat. Biotransformation of paraquat in cells results in the generation of reactive oxygen species (ROS) and apoptosis-related molecules, which cause multiple organ dysfunction in humans and animals, mainly involving the lung, kidney, heart, liver, and central nervous system.
Recent evidence suggests that the molecular mechanisms underlying paraquat toxicity include the release of damage-associated molecular patterns, including high-mobility group box 1 (HMGB1), a chromatin-binding protein that can be secreted by necrotic or inflammatory cells. Previously, we reported that a signaling cascade involving HMGB1, toll-like receptor 4 (TLR4), interleukin 23 (IL23), and IL17A mediated acute lung injury (ALI) in a mouse model of paraquat poisoning7. These findings suggest that targeting the HMGB1-TLR4-IL23-IL17A axis represents a potential therapeutic strategy for treating paraquat poisoning. However, published reports showing a clinically relevant association between HMGB1 and paraquat exposure are lacking. Therefore, the objective of the present study was to investigate the potential role of serum HMGB1 level as a prognostic marker of mortality in patients with paraquat poisoning in a clinical setting.
Methods
Study subjects
This study was approved by the ethics committee of The First Hospital of Jilin University and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients on admission to the emergency room (ER).
This retrospective observational cohort study included a convenience sample of patients presenting to the ER of The First Hospital of Jilin University between January 2014 and December 2016 with acute paraquat poisoning, defined as an oral intake of 2–200 mL paraquat during the prior 24 hours. Exclusion criteria were: (1) diabetes mellitus, hypertension, renal failure, or malignancy; (2) >24 hours since oral intake of paraquat; or (3) incomplete follow-up data. Hospitalized patients were followed up to death. Discharged patients were followed up to death or for 30 days. Twenty healthy subjects were enrolled to investigate baseline HMGBI levels in a group of healthy controls.
Sample collection and biochemical analyses
Each patient provided a peripheral venous blood sample (5 mL) during the first 24 hours after admission to the ER. Sera were isolated, frozen, and stored at −80 °C until use. Serum HMGB1 levels were measured using an enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions (Yanhui Biotech, Shanghai, China).
Baseline hematological and laboratory parameters, including white blood cell (WBC), neutrophil, lymphocyte, and platelet counts, hemoglobin, prothrombin time, total bilirubin, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, serum amylase, troponin I (cTnI), pH, partial pressure of carbon dioxide (PaCO2), and partial pressure of oxygen (PaO2) were measured using a Hitachi 7600 Clinical Analyzer (Hitachi, Tokyo, Japan), Sysmex CA-7000 System (Sysmex, Kobe, Japan), and Sysmex XE-5000 (Sysmex)8, according to standard protocols.
Statistical analysis
Statistical analyses were conducted using SPSS, v24 (SPSS, Chicago, IL, USA). Values are expressed as mean ± standard error of the mean (SEM) for normally distributed data or median and interquartile ranges for non-normally distributed data. One-way analysis of variance (ANOVA) and the Kruskal–Wallis H test or Mann–Whitney U test were performed to evaluate between-group differences. Chi-squared test was conducted to evaluate between-group differences stratified by gender and in 30-day mortality. A Kaplan-Meier curve was constructed to describe survival. Univariate and multivariate Cox regression analyses were performed to identify predictors of 30-day mortality; results are summarized as hazard ratios with corresponding 95% confidence intervals (CIs). Variables with a P value < 0.1 on univariate analysis were included in the multivariate analysis. P < 0.05 was considered statistically significant.
Results
Study population
This study enrolled 304 patients with an oral intake of 2–200 ml paraquat. A total of 212 patients were excluded. Of these, 21 patients had diabetes mellitus, 29 patients had hypertension. 19 patients had renal failure, 22 patients had malignancy, 104 patients had inadequate follow up data, and >24 hours had passed since the oral intake of paraquat in 17 patients. The remaining 92 patients were included in the final analysis. These patients had a median age of 33.5 years (range: 13–77 years) and 47 were female and 45 were male.
Baseline demographic and clinical characteristics
Patients were divided into tertiles according to their baseline serum HMGB1 levels: tertile 1, HMG1:0.41–6.67 μg/mL; tertile 2, 7.12–11.36 μg/mL; tertile 3, 11.56–15.34 μg/mL. Baseline demographic and clinical characteristics of included patients stratified by HMGB1 tertile are summarized in Table 1. Baseline WBC and neutrophil counts and serum lactic acid levels significantly increased across tertiles from lowest to highest serum HMGB1 levels, while arterial pH decreased.
Table 1.
Baseline characteristics of patients stratified by serum HMGB1 tertile (n = 92).
| Variable | Tertile 1 (n = 30) | Tertile 2 (n = 30) | Tertile 3 (n = 32) | P value |
|---|---|---|---|---|
| Age | 37.5 (27.5–48.0) | 30.0 (23.75–39.25) | 33.5 (25.0–48.25) | 0.162 |
| Gender(male/female), n | 11:19 | 15:15 | 19:13 | 0.2 |
| Time from ingestion to ED (hrs.) | 6.0 (3.75–11.25) | 6.5 (4.0–18.25) | 6.0 (4.0–8.75) | 0.592 |
| MAP (mmHg) | 89.67 (86.25–96.75) | 86.67 (82.17–96.17) | 90.17 (86.67–100.33) | 0.227 |
| Respiratory rate (breaths/min) | 20 (16–20) | 20 (18–20) | 20 (18–23) | 0.184 |
| pH | 7.41 (7.39–7.45) | 7.41 (7.39–7.44) | 7.39 (7.32–7.41) | 0.006 |
| PaCO2 (mmHg) | 37.5 (31.0–41.0) | 39.5 (34.5–40.3) | 36.0 (21.341.0) | 0.265 |
| PaO2 (mmHg) | 88.0 (75.0–101.0) | 93.0 (77.3–103.0) | 89.0 (71.3–105.3) | 0.642 |
| lactic acid (mmol/L) | 1.0 (0.7–2.0) | 1.35 (0.6–3.4) | 4.1 (2.5–11.7) | <0.001 |
| WBC (×109/L) | 10.94 (7.5–15.4) | 10.6 (8.5–17.4) | 17.0 (9.3–22.0) | 0.042 |
| Neutrophil (×109/L) | 9.2 (5.9–13.9) | 9.1 (6.7–15.1) | 14.3 (7.5–20.5) | 0.034 |
| Lymphocyte (×109/L) | 0.9 (0.6–1.8) | 1.1 (0.7–1.8) | 1.3 (0.8–1.6) | 0.947 |
| Hg (g/L) | 141.5 (117.8–149.8) | 145.0 (129.8–155.3) | 152.0 (134.0–158.8) | 0.077 |
| Platelet (×109/L) | 254.4 ± 87.56 | 212.2 ± 81.75 | 227.5 ± 67.64 | 0.119 |
| BUN | 5.2 (3.9–6.6) | 4.8 (3.2–6.2) | 5.1 (4.1–7.3) | 0.411 |
| Cr | 62.0 (48.6–117.6) | 62.4 (53.8–112.7) | 87.3 (59.3–133.5) | 0.101 |
| Albumin (g/dL) | 41.5 (39.7–46.2) | 43.6 (41.0–6.1) | 4.4 (40.3–47.6) | 0.473 |
| total bilirubin | 13.0 (9.5–15.2) | 15.6 (13.0–24.6) | 17.5 (10.5–23.4) | 0.058 |
| ALT (U/L) | 24.5 (17.3–34.3) | 26.0 (19.7–36.3) | 26.2 (20.9–45.0) | 0.164 |
| AST (U/L) | 19.8 (12.6–27.0) | 19.1 (13.0–34.4) | 23.0 (19.4–43.2) | 0.128 |
| cTnI | 0.012 (0.01–0.02) | 0.012 (0.01–0.02) | 0.012 (0.012–0.028) | 0.285 |
| serum amylase | 72.0 (52.0–105.8) | 71.5 (38.5–100.8) | 79.4 (55.5–120.0) | 0.651 |
MAP, mean arterial pressure; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; WBC, white blood cell; Hg, hemoglobin; CK, creatine kinase; BUN, blood urea nitrogen; AST, aspartate transaminase; ALT, alanine transaminase.
Comparison of the serum HMGB1 level between fatalities and survivors
Mean serum HMGB1 levels in healthy controls, fatalities, and survivors are shown in Fig. 1. Mean serum HMGB1 level in healthy controls was 0.53 [range, 0.00–1.30] μg/L, and significantly lower than for fatalities or survivors (P < 0.001). Mean baseline serum HMGB1 level was significantly higher in fatalities vs. survivors (11.79 [range, 0.41–15.34] μg/L) vs. 7.51 [range, 0.49–15.20] μg/L, P = 0.015) (Fig. 1).
Figure 1.
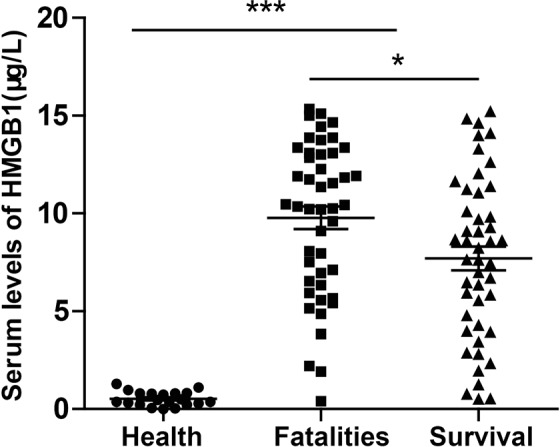
Comparison of serum HMGB1 levels in healthy controls, survivors and fatalities; F = 45.05, P < 0.001; survivors and fatalities, t = 2.47, P = 0.015; data are presented as mean ± SEM. ***P < 0.001, *P < 0.05.
Comparison of the serum HMGB1 level in females and males
Mean serum HMGB1 levels in females and males are shown in Fig. 2. There was no significant difference in mean serum HMGB1 level between females and males (t = 1.60, P = 0.11) (Fig. 2).
Figure 2.
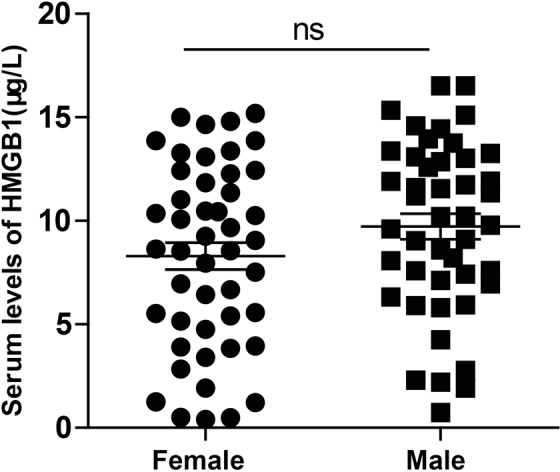
Comparison of serum HMGB1 levels in females and males; t = 1.60, P = 0.11. Data are presented as mean ± SEM; ns = not significant.
Association of serum HGB1 level with mortality rate
All patients were treated according to standard procedures in the ER. Cumulative incidence of mortality during the first 30 days after admission was 50% (n = 46/92). The ratio of survivors to fatalities in each HMGB1 tertile is shown in Table 2. The 30-day mortality rate was significantly increased across tertiles from lowest (tertile 1, 26.7%; tertile 2, 43.3%) to highest (tertile 3, 78.1%) serum HMGB1 levels. A Kaplan-Meier curve was constructed to describe survival. The log rank test of a difference in survival between tertiles was significant (P < 0.001) (Fig. 3).
Table 2.
30-day mortality stratified by serum HMGB1 tertile.
| HMGB1 Tertile | Survivors | Fatalities | 30-days mortality (%) | χ2 | P |
|---|---|---|---|---|---|
| Tertile 1 | 22 | 8 | 26.7 | ||
| Tertile 2 | 17 | 13 | 43.3 | 5.4 | 0.02* |
| Tertile 3 | 7 | 25 | 78.1 | 17.2 | <0.001** |
*tertile 1 vs. tertile 2.
**tertile 1 vs. tertile 2 vs. tertile 3.
Figure 3.
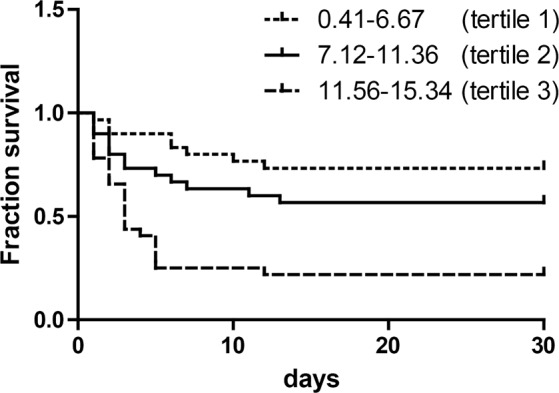
Kaplan-Meyer survival curve stratified by serum HMGB1 tertile (μg/L); χ2 = 21.86, P < 0.001 by the log-rank test.
Risk factor analysis for 30-day mortality
Multivariate Cox proportional hazards regression analyses identified baseline serum HMGB1 level, WBC count, and serum lactic acid levels as independent prognostic markers of 30-day mortality in patients with acute paraquat poisoning (Table 3).
Table 3.
Risk factors for 30-day mortality.
| Hazard ratio | 95% confidence interval | P | |
|---|---|---|---|
| HMGB1 | 1.635 | 1.086–2.460 | 0.018 |
| lactic acid | 1.726 | 1.086–2.743 | 0.021 |
| WBC | 1.508 | 1.024–2.223 | 0.038 |
HMGB1, high-mobility group box 1; WBC, white blood cell.
Discussion
The results of the current study investigating acute paraquat poisoning in humans showed that 30-day mortality was increased in patients with higher baseline serum HMGB1 levels, and baseline serum HMGB1 level was an independent prognostic marker of 30-day mortality in this patient population.
Paraquat is a poisonous herbicide that has toxic effects due to its redox activity. Paraquat induces oxidative damage and cell death by generating ROS, including hydrogen peroxide, superoxide anion, and hydroxyl radicals.
HMGB1 in involved in infection, organ dysfunction and immune responses in acute injury and chronic disease, including myocardial infarction, stroke, acute lung injury, acute liver injury7,9, ischemia–reperfusion injury, sepsis, fibrotic disorders, and cancer10. HMGB1 is an alarmin, with distinct intracellular and extracellular effects. It is present in the cell nucleus and cytoplasm and can be translocated to the extracellular space11. HMGB1can be actively released from cells, including macrophages, monocytes, natural killer cells, dendritic cells, endothelial cells and platelets, or passively produced by necrotic and damaged cells12,13. In the extracellular space, HMGB1 mediates inflammation and chemotaxis12 and has far reaching effects causing remote organ dysfunction in sterile injury14.
Death in paraquat poisoning is usually caused by ALI15,16. The development and severity of ALI is controlled by pulmonary neutrophil infiltration17–19. Findings from our previous study revealed that the HMGB1-TLR4-I-L23-IL-17A axis regulated neutrophil infiltration and ALI in a mouse model of paraquat poisoning7. Conversely, administration of HMGB1-specific neutralizing antibodies or the HMGB1-binding antagonist thrombomodulin can rescue mice and rats20–22 from lethal sepsis. Taken together, data from our studies and previous animal experiments suggest that neutralization of HMGB1 represents a potential therapeutic option for the treatment of paraquat poisoning. However, the results should be interpreted with caution, as the specific mechanism of action of HMGB1 in humans remains to be elucidated.
The previous reports and findings from the current study suggest an association between HMGB1 and the mechanism of paraquat toxicity, in which HMGB1 may function as a regulator of cytokine, chemokine, and growth factor activity, coordinating the inflammatory and immune response7,23. Further investigations regarding this association are warranted to expand understanding of paraquat toxicity and inform the development of targeted therapeutics.
Some studies have determined that initial leucocyte level and arterial lactate are predictors of mortality in patients with paraquat poisoning17,24–26. This is consistent with our findings. Reliable predictors of prognosis are important to inform clinical treatment decision making in this patient population.
This study was associated with several limitations. First, this was a cross-sectional study and cause and effect of HMGB1 in the etiology of paraquat poisoning could not be elucidated. Second, oral paraquat poisoning was based on patient self- report. Evidence suggests that plasma paraquat levels have prognostic value in patients with acute paraquat poisoning27. However, plasma paraquat levels were not measured in the current study as access to testing is limited by time and cost, and most ER physicians do not rely on plasma paraquat measurements for clinical decision making.
In summary, the results of the current study suggest that serum HMGB1 level measured on admission to the ER is a feasible index for assessing the outcome of patients with acute paraquat poisoning, and strategies that decrease serum HMGB1 levels may have role in the management of these patients. Further studies elucidating the role of HMGB1 in paraquat poisoning are required to guide the development of effective clinical interventions.
Supplementary information
serum HMGB-1 values for individual patients between fatalities and survivors
Acknowledgements
The authors thank Dahai Xu for helpful discussions and suggestions.
Author Contributions
Bailing Yan designed the study. Zuolong Liu and Wei Li collected the data. Feng Chen, Bailing Yan and Dan Li analyzed the data and drafted the manuscript. All authors have read and approved the final manuscript.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dan Li and Bailing Yan contributed equally.
Contributor Information
Dan Li, Email: 81945935@qq.com.
Bailing Yan, Email: yanbailing@163.com.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43877-1.
References
- 1.Hsu CW, et al. Early hemoperfusion may improve survival of severely paraquat-poisoned patients. PloS one. 2012;7:e48397. doi: 10.1371/journal.pone.0048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng, J., Huo, D., Wu, Q., Zhu, L. & Liao, Y. Xuebijing for paraquat poisoning. The Cochrane database of systematic reviews, Cd010109, 10.1002/14651858.CD010109.pub2 (2013). [DOI] [PubMed]
- 3.Chen HW, Tseng TK, Ding LW. Intravenous paraquat poisoning. J Chin Med Assoc. 2009;72:547–550. doi: 10.1016/s1726-4901(09)70426-5. [DOI] [PubMed] [Google Scholar]
- 4.Kim SJ, Gil HW, Yang JO, Lee EY, Hong SY. The clinical features of acute kidney injury in patients with acute paraquat intoxication. Nephrol. Dial. Transplant. 2009;24:1226–1232. doi: 10.1093/ndt/gfn615. [DOI] [PubMed] [Google Scholar]
- 5.Bromilow RH. Paraquat and sustainable agriculture. Pest Manag Sci. 2004;60:340–349. doi: 10.1002/ps.823. [DOI] [PubMed] [Google Scholar]
- 6.Li H, et al. Neuroprotective effects of tert-butylhydroquinone on paraquat-induced dopaminergic cell degeneration in C57BL/6 mice and in PC12 cells. Arch. Toxicol. 2012;86:1729–1740. doi: 10.1007/s00204-012-0935-y. [DOI] [PubMed] [Google Scholar]
- 7.Yan B, Chen F, Xu L, Xing J, Wang X. HMGB1-TLR4-IL23-IL17A axis promotes paraquat-induced acute lung injury by mediating neutrophil infiltration in mice. Scientific reports. 2017;7:597. doi: 10.1038/s41598-017-00721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambayya A, et al. Haematological reference intervals in a multiethnic population. PloS one. 2014;9:e91968. doi: 10.1371/journal.pone.0091968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Sun R, Wei H, Tian Z. High-mobility group box 1 (HMGB1)-Toll-like receptor (TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: Interaction of gammadelta T cells with macrophages. Hepatology. 2013;57:373–384. doi: 10.1002/hep.25982. [DOI] [PubMed] [Google Scholar]
- 10.Bertheloot D, Latz E. HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cellular & Molecular Immunology. 2017;14:43–64. doi: 10.1038/cmi.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pisetsky DS, Erlandsson-Harris H, Andersson U. High-mobility group box protein 1 (HMGB1): an alarmin mediating the pathogenesis of rheumatic disease. Arthritis Res Ther. 2008;10:209. doi: 10.1186/ar2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nature Reviews Immunology. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 13.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 14.Sodhi CP, et al. Intestinal Epithelial TLR-4 Activation Is Required for the Development of Acute Lung Injury after Trauma/Hemorrhagic Shock via the Release of HMGB1 from the Gut. J. Immunol. 2015;194:4931. doi: 10.4049/jimmunol.1402490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eddleston M, Phillips MR. Self poisoning with pesticides. BMJ (Clinical research ed.) 2004;328:42–44. doi: 10.1136/bmj.328.7430.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeyaratnam J. Acute pesticide poisoning: a major global health problem. World health statistics quarterly. Rapport trimestriel de statistiques sanitaires mondiales. 1990;43:139–144. [PubMed] [Google Scholar]
- 17.Kang C, et al. Absolute lymphocyte count as a predictor of mortality in emergency department patients with paraquat poisoning. PloS one. 2013;8:e78160. doi: 10.1371/journal.pone.0078160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butt Y, Kurdowska A, Allen TC. Acute Lung Injury: A Clinical and Molecular Review. Arch Pathol Lab Med. 2016;140:345–350. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]
- 19.Koike M, et al. Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration. Scientific reports. 2015;5:11722. doi: 10.1038/srep11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin S, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J. Exp. Med. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suda K, et al. Anti-high-mobility group box chromosomal protein 1 antibodies improve survival of rats with sepsis. World J Surg. 2006;30:1755–1762. doi: 10.1007/s00268-005-0369-2. [DOI] [PubMed] [Google Scholar]
- 22.Yang H, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang R, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Zhao D, Li Y, Gao J, Feng S. Arterial lactate in predicting mortality after paraquat poisoning: A meta-analysis. Medicine. 2018;97:e11751. doi: 10.1097/md.0000000000011751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng S, Gao J, Li Y. A retrospective analysis of leucocyte count as a strong predictor of survival for patients with acute paraquat poisoning. PloS one. 2018;13:e0201200. doi: 10.1371/journal.pone.0201200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, et al. The significance of serum uric acid level in humans with acute paraquat poisoning. Scientific reports. 2015;5:9168. doi: 10.1038/srep09168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gil HW, Kang MS, Yang JO, Lee EY, Hong SY. Association between plasma paraquat level and outcome of paraquat poisoning in 375 paraquat poisoning patients. Clin. Toxicol. 2008;46:515. doi: 10.1080/15563650701549403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
serum HMGB-1 values for individual patients between fatalities and survivors
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


