Abstract
Objective
The aim of this study was to present experiences in localization and removal of non-palpable subdermal contraceptive implants with ultrasonography.
Methods
Medical records from January 1, 2016, to April 30, 2018, were retrospectively reviewed for 21 patients who were referred to a single institution and had an impalpable implant despite following the removal instruction. In all the cases, more than one attempt was made to remove the implant before referral. The rod was detected using radiography and ultrasonography. In all the cases, localization of the single implant was achieved with ultrasonography. The distal depth of the rod was measured, and skin marking was made following the echogenicity. The implants were subsequently removed under anesthesia.
Results
In 18 cases, the rods were localized using ultrasonography and successfully removed under local anesthesia. In the other three cases, removal with local anesthesia failed. Although the rod was detected successful with ultrasonography, the implants were removed under general anesthesia in the operating room. The depth from skin to rod, measured with ultrasonography, was >12.0 mm in all the cases and located deep in the muscular layer in the failure cases. The depth of the implants positively correlated with the time spent for removal (r=0.525; P=0.015).
Conclusion
High frequency ultrasonography is a highly accurate tool for localization and measurement of the skin-to-rod depth. It is also useful for removing non-palpable implants. If the depth of the implant is >12.0 mm, removal of the implant in the operating room under general anesthesia is recommended.
Keywords: Contraceptive methods, Female contraception, Device removal, Ultrasound imaging
Introduction
Implanon NXT (Merck & Co. Inc., Whitehouse Station, NJ, USA) is a single-rod subdermal contraceptive implant that consists mainly of selective progestin etonogestrel (ENG) and ethylene vinyl acetated (EVA) copolymer that is surrounded by a rate-controlling EVA membrane [1]. A single implant contains 68 mg of ENG, which is released slowly and steadily in doses of 60–70 μg/day [2]. It has been widely used throughout the world, providing effective contraceptive protection for up to 3 years and does not rely on user compliance [2]. The implant is easily inserted and removed when insertion is performed properly according to the product instructions. It is estimated that only a small proportion of implants is incorrectly inserted and that <0.1% results in difficult removals [3]. Deep or non-palpable implants can occur owing to migration, fibrosis, or weight gain, and often require referrals to experienced clinicians who would use ultrasonography or other imaging tools for removal.
Palpable implants can be easily removed without any imaging guidance through a 2-mm incision near the tip of the rod [4]. However, when inserted improperly or migrated to other places, the implant can be difficult to localize by palpation, and consequently, removal can become complicated. In these cases, follow-up imaging studies should be conducted to establish the strategies for non-palpable implants. Ultrasonography is the most powerful and widely available modality, but it can hardly detect distant migration. Moreover, measurement of implant depth with ultrasonography tends to be underestimated as a result of tissue compression. Two-dimensional radiography can be useful not only for visualizing distant migration but also for revising the discordance between measurement using ultrasonography and the actual depth from the skin since a radiopaque implant has been developed [5].
Previous studies demonstrated different approaches for localization and removal of implants. Singh et al. [6] introduced ultrasonography as a localizing modality to remove non-palpable implants under general anesthesia. James and Trenery [4] introduced the removal of impalpable rods with real-time ultrasonography guidance. However, none of these studies suggested strategies for localizing and removing barely palpable implants under local anesthesia in the outpatient clinic. They did not provide a cutoff point for the need for general anesthesia.
Therefore, the aim of this study was to present experiences in localization and removal of non-palpable subdermal implants with ultrasonography under local anesthesia by a single experienced skillful clinician in a single institution.
Materials and methods
Our retrospective study included a review of the medical records of 21 patients who were referred to the outpatient clinic of the gynecology department of Gangnam Severance Hospital because of an impalpable or barely palpable subdermal contraceptive implant between January 1, 2016, and April 30, 2018. In all the cases, more than one attempt was made to remove the rods before referral, but removal of the implanted device failed despite following the removal instruction. Prior to using ultrasonography, two-dimensional radiography was performed to confirm whether the rod was placed. The radiologist recorded whether the implant was clearly visible or invisible in both lateral and anteroposterior views.
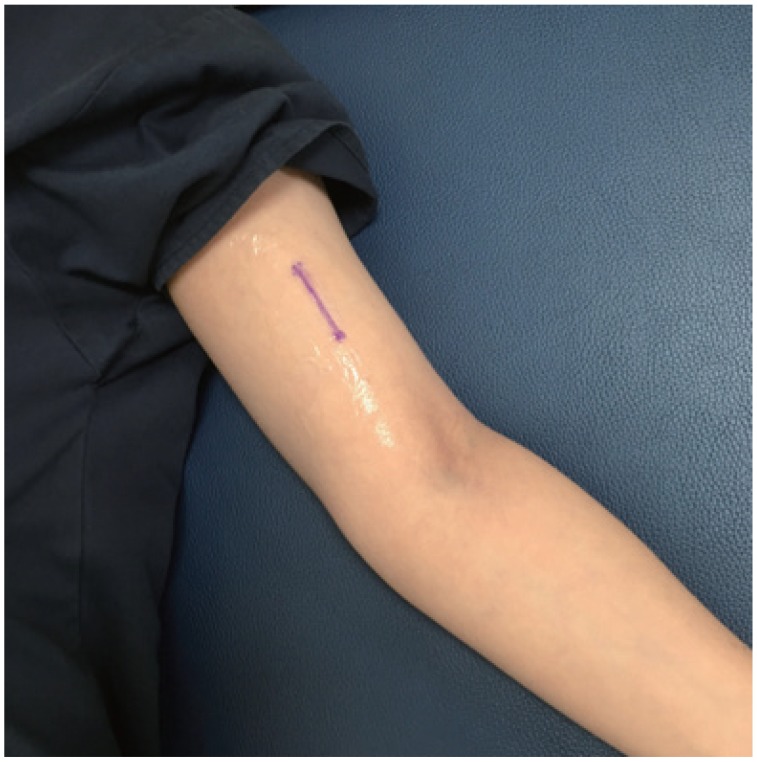
The ultrasonography detection of the rod was performed using a 5- to 12-MHz linear array probe with an iU22 scanner (Philips Medical Systems, Best, The Netherlands) by an experienced radiologist. The patient lied on her back on the examination table, with her arms flexed at the elbow and externally rotated. Ultrasonography scanning was started from the site where the implant was expected to be present, which was assumed through radiography and skin scars associated with insertion or removal. The scanning was performed in the transverse plane, as this was known to be optimal because the implant was visualized as an echogenic spot casting a posterior acoustic shadowing [7]. Tissue harmonic mode was used for the scanning, as this was shown to be the best for the detection of implants because it produces the most prominent posterior acoustic shadow in a previous study [8]. The radiologist measured the depth of the implant from the skin surface. After the full length of the implant was identified, skin marking was made from the proximal to the distal tip (Fig. 1).
Fig. 1. Skin marking made from the proximal to the distal tip with ultrasonography localization.
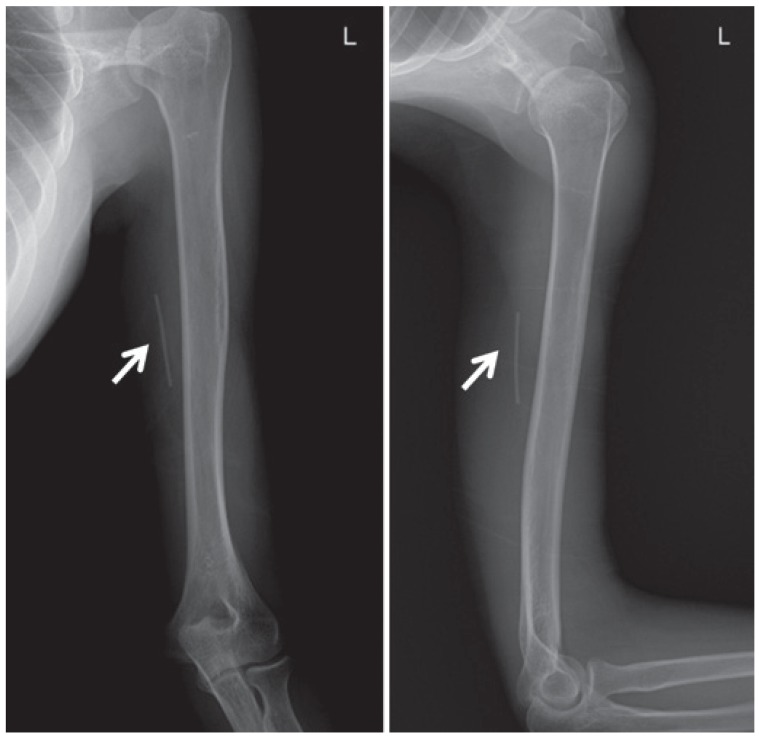
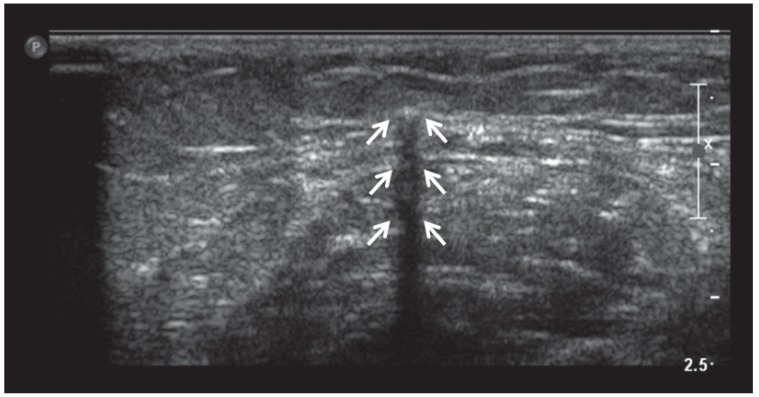
In all the cases, the implants were radiopaque on radiographs (Fig. 2). On ultrasonography, all the implants were depicted as echogenic spots with a posterior acoustic shadow, which is the signature appearance of implants on ultrasonography. The posterior acoustic shadow is a thin and dark wedge shape created by the implant, extending deep to the rod [6] (Fig. 3). This shadow occurs because the implant has a different acoustic impedance compared with the adjacent soft tissue [7].
Fig. 2. A radiopaque implant clearly visible on the radiograph (arrows).
Fig. 3. Implant and posterior acoustic shadow in transverse view (arrow). A posterior acoustic shadow is the signature appearance of implants on ultrasonography, which is a thin and dark wedge shape created by implant, extending deep to the rod.
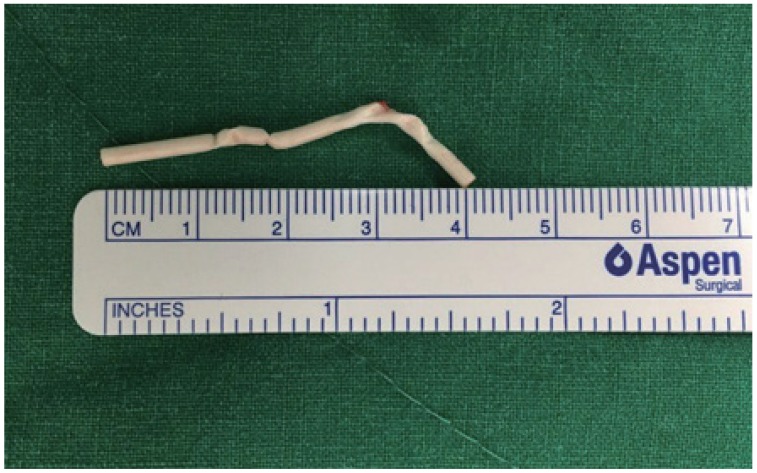
Localized non-palpable implants were subsequently removed under local anesthesia in the outpatient setting. With 2% lidocaine injected along the skin marking, a 5-mm incision, 1 cm from the distal tip, was made perpendicular to the marking. The implant location could be predicted by the depth measured with ultrasonography. By using the tip of sterilized forceps and hemostat, the distal tip of the implant was pulled. During the procedure, the clinician checked for jerkiness or movement abnormality of the patient. The full length of the removed rod was confirmed to be 4 cm to ensure that complete removal was achieved (Fig. 4).
Fig. 4. The full length of the removed rod was confirmed to be 4 cm, ensuring that complete removal was achieved.
In patients who complained of pain while removal was attempted under local anesthesia (cases 19 and 21) or those in whom no implant was found (case 20), exploration for subdermal devices was discontinued and implant removal was attempted in the operating room under general anesthesia. The time spent for successful removal was recorded. In cases of failure of removal, the time spent for exploration without finding the implant was recorded.
Data were presented as mean±standard deviation. The Spearman rho was used to measure the strength of the association between the variables. All the analyses were performed using IBM SPSS Statistics version 23.0 for Windows (IBM Corp., Armonk, NY, USA), and a P-value of <0.05 was considered statistically significant.
Results
Twenty-one patients with non-palpable subdermal implants were referred to the center. Table 1 presents the details of the clinical characteristics of the study participants and the reasons for implant removal. Their ages ranged from 27 and 47 years, with a mean age of 35.8±1.4 years. Body mass index (BMI) ranged from 17.9 to 25.8 kg/cm2, and the mean value was 21.0±0.5 kg/cm2. The interval between the insertion and the request for removal ranged from 2 to 72 months, with a mean duration of 28.4±1.8 months. The most common reason for requesting removal was abnormal uterine bleeding (n=8, 38.1%), followed by arm pain (n=6, 28.6%) and implant exchange (n=3, 14.3%), weight gain (n=2, 0.1%), and pregnancy (n=2, 0.1%).
Table 1. Clinical characteristics of study participants and reasons for removal.
| Case | Age (yr) | BMI | Duration (mon) | Reason for removal |
|---|---|---|---|---|
| 1 | 32 | 18.8 | 36 | Abnormal uterine bleeding |
| 2 | 32 | 20.4 | 12 | Arm pain |
| 3 | 33 | 25.8 | 8 | Arm pain |
| 4 | 41 | 21.4 | 25 | Abnormal uterine bleeding |
| 5 | 45 | 22.7 | 37 | Weight gain |
| 6 | 42 | 19.5 | 36 | Abnormal uterine bleeding |
| 7 | 44 | 21.6 | 2 | Arm pain |
| 8 | 31 | 19.3 | 29 | For pregnancy |
| 9 | 45 | 18.7 | 42 | Abnormal uterine bleeding |
| 10 | 47 | 20.1 | 36 | For exchange |
| 11 | 36 | 23.1 | 28 | Abnormal uterine bleeding |
| 12 | 27 | 20.3 | 36 | For pregnancy |
| 13 | 32 | 25.1 | 26 | Arm pain |
| 14 | 37 | 18.9 | 22 | Abnormal uterine bleeding |
| 15 | 31 | 22.5 | 7 | Arm pain |
| 16 | 42 | 21.3 | 40 | Weight gain |
| 17 | 29 | 17.9 | 20 | Abnormal uterine bleeding |
| 18 | 27 | 20.6 | 19 | Abnormal uterine bleeding |
| 19 | 34 | 21.3 | 72 | For exchange |
| 20 | 33 | 22.7 | 30 | Arm pain |
| 21 | 33 | 18.6 | 34 | For exchange |
BMI, body mass index.
Table 2 shows the details of the measurements during the procedure and the ultrasonography findings. It shows the locations of the implants, whether within the adipose tissue, muscle layer, or overlying fascia. The depth of the rods was measured at the distal tip of the implant. The mean depth of the implants was 7.9 mm.
Table 2. Measurements during the procedure and ultrasound findings.
| Case | Time (min) | Incision (mm) | Depth in US (mm) | Ultrasound findings | Result |
|---|---|---|---|---|---|
| 1 | 5 | 5 | 3.8 | Anteromedial subcutaneous fat layer, intra fascia | S |
| 2 | 5 | 5 | 5.1 | Medial aspect, 5 mm depth from skin | S |
| 3 | 5 | 5 | 9.3 | Median nerve located right under proximal tip | S |
| 4 | 7 | 5 | 6.3 | Superficial to the brachial artery | S |
| 5 | 3 | 5 | 6.8 | Subcutaneous fat layer | S |
| 6 | 3 | 5 | 6.0 | Right overriding brachial artery and vein | S |
| 7 | 10 | 5 | 7.1 | Anteromedial subcutaneous fat layer | S |
| 8 | 15 | 5 | 7.9 | Fascial layer, subcutaneous and muscle junction | S |
| 9 | 3 | 5 | 11.8 | Subcutaneous fat layer | S |
| 10 | 3 | 5 | 9.0 | Not adjacent neuromuscular bundle | S |
| 11 | 5 | 5 | 6.7 | Subcutaneous fat layer | S |
| 12 | 7 | 5 | 7.2 | Subcutaneous fat layer | S |
| 13 | 18 | 10 | 4.1 | 4 mm depth from the skin | S |
| 14 | 5 | 5 | 8.5 | Subcutaneous fat layer | S |
| 15 | 7 | 10 | 10.9 | Fascial layer, subcutaneous and muscle junction | S |
| 16 | 3 | 5 | 9.4 | Anteromedial subcutaneous fat layer | S |
| 17 | 3 | 5 | 3.2 | 3 mm depth from the skin | S |
| 18 | 3 | 5 | 7.5 | Medial aspect, 7.5 mm depth from skin | S |
| 19 | 25 | 10 | 12.7 | Deeper than muscle fascia, probably intramuscle | F |
| 20 | 30 | 10 | 12.0 | Intra-biceps muscle | F |
| 21 | 25 | 10 | 13.5 | Deeper than muscle fascia, probably intramuscle | F |
Time, the time spent for trying to removal with or without success; US, ultrasound; S, success; F, failure.
Table 3 demonstrates the correlation between BMI, age, depth, time spent for removal with local anesthesia, and duration between insertion and removal of the device. Implant depth positively correlated with the time spent for removal (r=0.525; P=0.015). The deeper the implants are located, the longer it takes to remove them. Neither BMI and depth (r=−0.052; P=0.822) nor BMI and time (r=0.158; P=0.495) had a correlation. No significant correlation was found between the time spent for removal and the duration from insertion to removal of the implant (r=0.300; P=0.186) using the Spearman correlation.
Table 3. Mean depth, time and duration.
| Characteristic | Fail (n=3) | Success (n=18) | P-value |
|---|---|---|---|
| Depth in US (mm) | 12.73±0.75 | 7.26±2.74 | 0.003 |
| Time (min) | 26.67±2.89 | 6.12±4.62 | <0.001 |
| Duration (mon) | 45.33±23.18 | 25.61±12.22 | 0.081 |
Data are presented as mean±standard deviation.
Depth in US, the depth from skin to the distal tip of implant measured in ultrasound; Time, the time spent for trying to removal with or without success; Duration, between insertion and removal of implant.
In 18 cases, the rods were localized using ultrasonography, where the skin was marked and successfully removed under local anesthesia in the outpatient setting. The mean removal time was 6.1±4.6 minutes. In cases 19, 20, and 21, the mean time spent for trying to remove but eventually stopping because of pain in the arm or exploring for implants but eventually failing to find an implant was 26.7±2.9 minutes. The mean depth of the implants successfully removed with local anesthesia was 7.2±2.7 mm, while the mean depth in the cases of failed removal was 12.7±0.7 mm (Table 3). The best cutoff depth of the implants was 12.0 mm (sensitivity, 0.935 and 1-specificity, <0.001). All of them were located deeper than the muscle fascia or muscular layer.
Discussion
Following the instructions for insertion, a subdermal contraceptive implant should be placed subdermally at the inner side of the upper non-dominant arm approximately 7 cm above the elbow crease in the groove between the biceps and the triceps [9]. If the implant cannot be visualized, the physician should perform two-dimensional radiography before blunt dissection with forceps to localize the distal end of the implant. In rare cases, the implants can be located at unexpected positions. A case of radiopaque subdermal implant migration into the lung was reported in a 37-year-old woman [10]. In that case, radiography was helpful to localize the migrated rod owing to its radio-opacity.
In some cases, the implants were placed deeper than where they were supposed to be on ultrasonography. In cases 7 and 12, the implants were visualized 7.1 and 7.2 mm deep into the subcutaneous layer on ultrasonography, respectively. However, they were located deeper than the measurement on ultrasonography, so the clinician had to explore the subdermal devices, which took more time than expected. Compression by the probe can artificially reduce the skin-to-implant measurements. The radiologist should be cautious not to press the skin too hard. The underestimated depth of the implants consequently leads to the failure of removal.
If localization by ultrasonography is performed, the removal rate of implants would be high as shown in this study. Therefore, if the rod is not palpable, removal should not be attempted blindly and the patient should be referred to facilities where radiology consultation can be made.
This study suggests that high-frequency ultrasonography is a highly accurate method for localizing impalpable subdermal implants. Measurement of depth from the skin to the implant is worth discussing, describing ultrasonography findings and recording the time spent for removal in each case. All the procedures were performed by a single experienced skillful clinician; therefore, no interobserver variation was observed. At the same time, however, the study is limited by the small number of participants, as this study was performed by a single clinician in a single institution.
The aim of this paper was to show that removal of non-palpable implants is possible without intravenous sedation in most cases. Intravenous sedation is also a burdensome procedure in an outpatient setting because it requires monitoring of the patient's condition and general anesthesia. Intravenous sedation with local anesthesia is insufficient to remove deep implants because of the risk of bleeding or irritation of damaged nerves.
As shown earlier, the depth of the subdermal device is a significant factor that determines the success of implant removal. We presented the cutoff value for removal with local anesthesia in an outpatient clinic setting. Within 12.0 mm of implant depth, all non-palpable implants were successfully removed in the outpatient setting with local anesthesia. However, if the depth of the implant is >12.0 mm, the removal is not likely to succeed in the outpatient setting because patients may complain of pain or the clinician fails to explore the rods. To avoid unnecessary procedures, removal of the implant in the operating room with general anesthesia is recommended when the implant is >12.0 mm deep from the skin. This parameter helps in the removal of non-palpable implants with minimal complications and less invasiveness.
Acknowledgements
This study was supported by a faculty research grant from Yonsei University College of Medicine (6-2015-0095).
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
Ethical approval: The study was approved by the Institutional Review Board of Yonsei University Gangnam Severance Hospital (IRB No. 3-2019-0040) and performed in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained.
Patient consent: The patients provided written informed consent for the publication and the use of their images.
References
- 1.Darney PD. Implantable contraception. Eur J Contracept Reprod Health Care. 2000;5(Suppl 2):2–11. [PubMed] [Google Scholar]
- 2.Mansour D, Mommers E, Teede H, Sollie-Eriksen B, Graesslin O, Ahrendt HJ, et al. Clinician satisfaction and insertion characteristics of a new applicator to insert radiopaque Implanon: an open-label, noncontrolled, multicenter trial. Contraception. 2010;82:243–249. doi: 10.1016/j.contraception.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Mansour D, Fraser IS, Walling M, Glenn D, Graesslin O, Egarter C, et al. Methods of accurate localisation of non-palpable subdermal contraceptive implants. J Fam Plann Reprod Health Care. 2008;34:9–12. doi: 10.1783/147118908783332285. [DOI] [PubMed] [Google Scholar]
- 4.James P, Trenery J. Ultrasound localisation and removal of non-palpable Implanon implants. Aust N Z J Obstet Gynaecol. 2006;46:225–228. doi: 10.1111/j.1479-828X.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 5.Schnabel P, Merki-Feld GS, Malvy A, Duijkers I, Mommers E, van den Heuvel MW. Bioequivalence and x-ray visibility of a radiopaque etonogestrel implant versus a non-radiopaque implant: a 3-year, randomized, double-blind study. Clin Drug Investig. 2012;32:413–422. doi: 10.2165/11631930-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Singh M, Mansour D, Richardson D. Location and removal of non-palpable Implanon® implants with the aid of ultrasound guidance. J Fam Plann Reprod Health Care. 2006;32:153–156. doi: 10.1783/147118906777888549. [DOI] [PubMed] [Google Scholar]
- 7.Shulman LP, Gabriel H. Management and localization strategies for the nonpalpable Implanon rod. Contraception. 2006;73:325–330. doi: 10.1016/j.contraception.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Seo K, Song HT, Suh JS, Yoon CS, Ryu JA, et al. Determination of optimal imaging mode for ultrasonographic detection of subdermal contraceptive rods: comparison of spatial compound, conventional, and tissue harmonic imaging methods. Korean J Radiol. 2012;13:602–609. doi: 10.3348/kjr.2012.13.5.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearson S, Stewart M, Bateson D. Implanon NXT: expert tips for best-practice insertion and removal. Aust Fam Physician. 2017;46:104–108. [PubMed] [Google Scholar]
- 10.Choi JH, Kim HY, Lee SS, Cho S. Migration of a contraceptive subdermal device into the lung. Obstet Gynecol Sci. 2017;60:314–317. doi: 10.5468/ogs.2017.60.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]