Abstract
The management of patients with shock is extremely challenging because of the myriad of possible clinical presentations in cardiogenic shock, septic shock and hypovolemic shock and the limitations of contemporary therapeutic options. The treatment of shock includes the administration of endogenous catecholamines (epinephrine, norepinephrine, and dopamine) as well as various vaso-pressor agents that have shown efficacy in the treatment of the various types of shock. In addition to the endogenous catecholamines, dobutamine, isoproterenol, phenylephrine, and milrinone have served as the mainstays of shock therapy for several decades. Recently, experimental studies have suggested that newer agents such as vasopressin, selepressin, calcium-sensitizing agents like levosimendan, car-diac-specific myosin activators like omecamtiv mecarbil (OM), istaroxime, and natriuretic peptides like nesiritide can enhance shock therapy, especially when shock presents a more complex clinical picture than normal. However, their ability to improve clinical outcomes remains to be proven. It is the purpose of this review to describe the mechanism of action, dosage requirements, advantages and disadvantages, and specific indications and contraindications for the use of each of these catechola-mines and vasopressors, as well as to elucidate the most important clinical trials that serve as the basis of contemporary shock therapy.
Keywords: Shock, cardiogenic shock, septic shock, shock therapy, endogenous catecholamines, exogenous catecholamines, inotropes, vasopressors
1. Introduction
The treatment of cardiogenic shock, septic shock, and hypovolemic shock include the administration of endogenous catecholamines (epinephrine, norepinephrine, and dopamine) as well as various vasopressor agents that have shown efficacy in the treatment of the various types of shock. In addition to the endogenous catecholamines, exogenous catecholamines like Dobutamine, isoproterenol, phenylephrine, and milrinone have served as the mainstays of shock therapy for several decades. Vasopressin, selepressin, calcium-sensitizing agents like levosimendan, cardiac-specific myosin activators like omecamtiv mecarbil (OM), istaroxime, and natriuretic peptides like nesiritide can enhance therapy when shock is especially complex. It is the purpose of this communication to describe the mechanisms of action, dosage requirements, advantages/disadvantages, and indications/contraindications for the use of each of these catecholamines and vasopressors and to discuss the importance of the major clinical trials that serve as the basis of contemporary shock therapy.
2. Classification of Shock and General Principles of Treating Shock
Shock is defined as inadequate organ and peripheral tissue perfusion and is categorized on the basis of its etiology as being either hypovolemic, cardiogenic, or restrictive (vasodilatory/distributive).
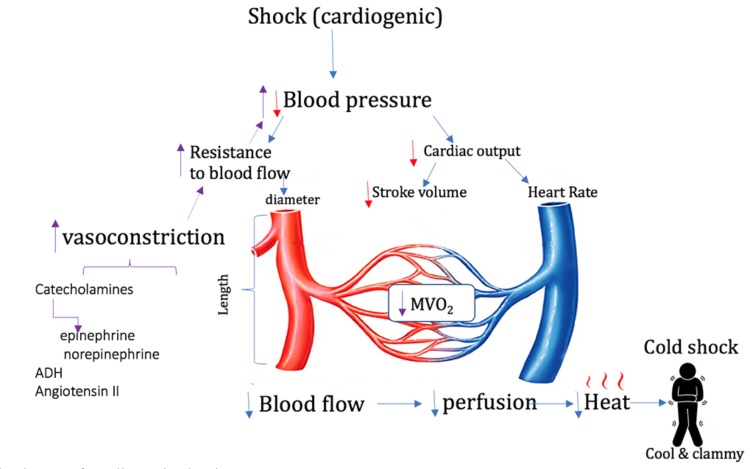
In hypovolemic shock, the addition of intravascular volume (preload) combined with drugs specifically capable of increasing LV contractility and stroke volume (SV) can be used to improve cardiac output (CO). Unfortunately, the degree to which the SV can be enhanced pharmacologically is limited by the fact that these drugs also increase the heart rate.
Cardiogenic shock is most commonly caused by an acute myocardial infarction but it can also result from hindrances to adequate cardiac filling such as pericardial tamponade or valvar stenosis. It is characterized by initial hypotension that triggers a vasoconstrictor release to re-establish normal blood pressure (Fig. 1). However, despite the restoration of normal mean arterial pressure (MAP) in both hypovolemic and cardiogenic shock by these compensatory measures, the MVO2 is often decreased in both of these types of “cold shock”. If cardiogenic shock is due to pericardial tamponade, immediate physical intervention to relieve the tamponade is required. However, if cardiogenic shock is due to acute myocardial infarction, therapy can vary widely depending upon the hemodynamic sequelae of the infarction. Both hypovolemic shock (inadequate preload) and cardiogenic shock (impaired cardiac contractility) are characterized by low left ventricular stroke volume, though unlike hypovolemic shock, cardiogenic shock is often accompanied by an inappropriately slow heart rate.
Fig. (1).
Simplified scheme of cardiogenic shock.
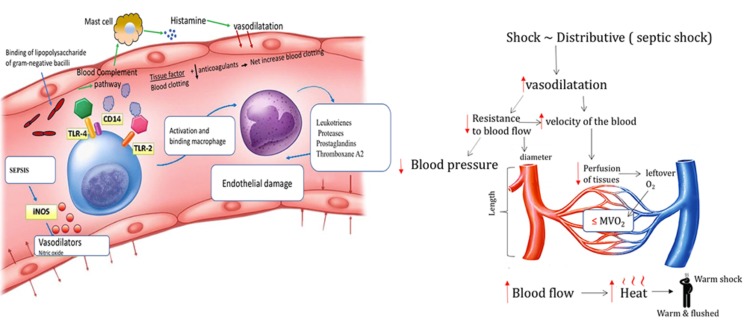
Left Panel: Gram-positive and gram-negative bacteria, viruses, and fungi have unique cell-wall molecules called pathogen-associated molecular patterns that bind to pattern-recognition receptors (toll-like receptors [TLRs]) on the surface of immune cells. The lipopolysaccharide of gram-negative bacilli binds to lipopolysaccharide-binding protein, CD14 complex. The gram-positive bacteria and the lipopolysaccharide of gram-negative bacteria bind to TLR-2 and TLR-4. Those are proinflammatory cytokines that activate the adaptive immune and both direct and indirect host injury. Sepsis increases the activity of inducible nitric oxide synthase (iNOS), which increases the synthesis of nitric oxide (NO), a potent vasodilator. Cytokines activate endothelial cells, injure endothelial cells by inducing neutrophils, monocytes, macrophages, and platelets to bind to endothelial cells and also activate the coagulation cascade.
Right Panel: Simplified scheme of septic shock described in the text above.
Vasodilatory/distributive, shock is characterized by excessive arteriolar vasodilatation that causes a decrease in systemic vascular resistance (SVR) with resultant hypotension that leads to inadequate peripheral perfusion in the presence of warm extremities, hence the term “warm shock”. Septic shock (Fig. 2) is the most common cause of “warm shock” and it is also the most common type of shock overall [1, 2]. Restoration of mean arterial pressure (MAP) is most often achieved by using drugs that increase the SVR. However, initial therapy aimed solely at increasing the SVR may result in only a modest increase in the CO.
Fig. (2).
Simplified scheme of septic shock.
Hypovolemic shock is usually the simplest form of shock to treat but many of its treatment strategies do not apply for the other types of shock. Thus, the therapy of shock, regardless of its etiology, demands a thorough knowledge of cardiovascular physiology and the pharmacology of the drugs that are used to treat its derangements.
3. Endogenous Catecholamines
The endogenous catecholamines epinephrine, norepinephrine, and dopamine all display variable physiologic effects across the dosing range and substantial patient variability in dose–response [1, 3].
4. Epinephrine
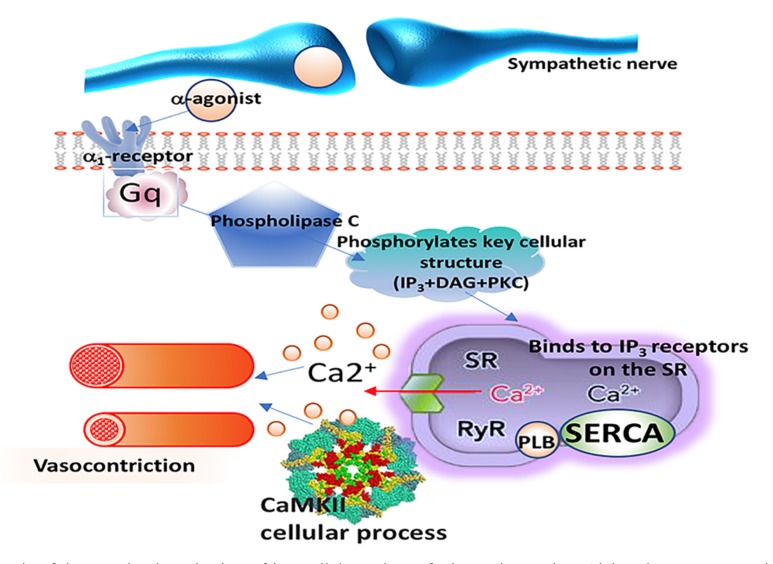
Epinephrine (“adrenalin”) is a nonselective agonist of all adrenergic receptors, including the major subtypes α1, α2, β1, β2, and β3. Epinephrine increases SVR via α1 receptor-dependent vasoconstriction (Fig. 3) and increases cardiac output via its binding to β1 receptors. As a result, epinephrine is especially useful for the treatment of acute LV failure during cardiac surgery because it predictably increases cardiac output. It is most useful as an inotrope in patients who are hypotensive with no myocardial ischemia, especially following cardiac surgery [3-7].
Fig. (3).
Schematic of the postulated mechanism of intracellular actions of adrenergic agonists. Alpha-adrenoceptor agonists (α-agonists) bind to α-receptors on vascular smooth muscle and induce smooth contraction and vasoconstriction, thus mimicking the effects of sympathetic adrenergic nerve activation to the blood vessels. The α-adrenergic receptor, activates a different regulatory G protein (Gq), which acts through the IP3 signal transduction pathway activates the release of calcium from the sarcoplasmic reticulum(SR) which by itself and through the calcium–calmodulin dependent protein kinases(CaMKII) influences cellular processes, which in vascular smooth muscle leads to vasoconstriction.
Epinephrine doses above 0.3-0.5 mcg/kg/min are considered high, but there is no defined maximum epinephrine dose for refractory shock [8, 9]. Unfortunately, the use of epinephrine may be limited because it promotes the development of atrial and ventricular arrhythmias. Another reason for avoiding epinephrine is concern that it may cause elevated lactate levels that could not only be directly harmful but might also confound the serial trending of serum lactate levels [10]. The mechanism of hyperlactemia in sepsis is multifactorial and results from factors beyond hypoxic tissue injury alone [11-13]. Indeed, serum lactic acid levels can be elevated in the presence of adequate systemic perfusion, MAP, and peripheral oxygen delivery [14]. Ven Genderen et al. showed that septic shock behaves differently from other forms of shock in that even when cardiac output and other systemic parameters are optimized, there continues to be a regional microvascular oxygen mismatch [15]. Thus, Rivers et al. warn against using lactate clearance as the only marker of sepsis recovery and state that lactate clearance, central venous oxygen saturation (ScvO2), and other markers represent complementary end points that are not mutually exclusive [16, 17]. However, much like vasopressor-induced sinus tachycardia, elevated lactate may be a beneficial compensatory mechanism [18] by providing a dual action of epinephrine on the heart. Randomized controlled clinical trials have shown that concentrated sodium lactate improves cardiac output among post-CABG and heart failure patients [19, 20].
5. Norepinephrine
Norepinephrine (“noradrenalin”) is an α1-adrenergic receptor agonist with modest β-agonist activity that makes it a vasoconstrictor but a less potent inotrope. Since norepinephrine is virtually a “pure” vasoconstrictor it may actually reduce CO in patients with cardiac dysfunction because of the strong increase in afterload, although many patients with cardiogenic shock can maintain CO during norepinephrine therapy [21, 22]. Because norepinephrine has minimal chronotropic effects, it is useful in settings in which heart rate stimulation may be undesirable. Norepinephrine increases both systolic and diastolic blood pressures so it increases coronary blood flow and thus, may improve cardiac function indirectly [23]. Norepinephrine is the first-line vasopressor for all forms of shock with severe hypotension [1, 3, 24].
6. Dopamine
Dopamine binds weakly to β1-adrenergic receptors but has a high binding affinity at dopamine receptors and at trace amine-associated receptor 1 (TAAR1) [25]. At low doses, dopamine inhibits the release of norepinephrine in peripheral blood vessels, thereby acting as a mild vasodilator. It also inhibits the re-uptake of norepinephrine in presynaptic sympathetic nerve terminals resulting in an indirect increase in cardiac contractility and heart rate. The direct vasodilator effect of dopamine tends to offset the indirect vasoconstriction effect of the secondary increase in norepinephrine so there is usually only a mild increase in SVR. The net effect of the combination of increased contractility, heart rate and only a slight increase in SVR is to improve CO, dramatically in some cases [26]. At higher infusion rates (10-20 mcg/kg/min), α1-adrenergic receptor–mediated vasoconstriction dominates the peripheral response and further increases blood pressure [26, 27] but the CO and peripheral tissue perfusion may not continue to improve.
At low doses, dopamine promotes vasodilation and increased blood flow in the coronary, renal, mesenteric and cerebral vascular beds by acting on D1 postsynaptic dopaminergic receptors and it provides additional blood flow to the kidneys by stimulating their D2 presynaptic receptors. Low doses of dopamine (below 4 mcg/kg/min) cause renal vasodilation and natriuretic effects that increase urine output but the impact on creatinine clearance and renal blood flow varies [28-32] and the clinical significance of “renal-dose” dopamine remains unclear. As a result, dopamine is no longer recommended for vasopressor support in septic shock except in patients with bradycardia who have a low risk of developing tachyarrhythmias [24].
7. Comparison of Endogenous Catecholamines
Epinephrine and norepinephrine have equal affinity at both alpha1 and alpha2 receptors. Norepinephrine is slightly lower in potency than epinephrine and approximately 100-fold more potent than dopamine for raising MAP [21, 27, 33, 34]. Epinephrine is more effective than norepinephrine or dopamine in increasing CO in septic shock [7]. In patients with septic shock and a MAP <70 mm Hg despite norepinephrine infusion, adding epinephrine increases MAP, HR, and cardiac index more than adding dobutamine does. Norepinephrine carries a lower tachyarrhythmia risk than either dopamine or epinephrine when used for vasopressor support [27, 34-37]. However, prolonged norepinephrine infusion can have a direct toxic effect on cardiac myocytes by inducing apoptosis via protein kinase A activation and increased cytosolic calcium influx [38]. Likewise, the use of epinephrine in high doses for long periods of time is toxic to arterial walls and causes focal regions of myocardial contraction-band necrosis and myocyte apoptosis [39].
8. Exogenous Catecholamines
8.1. Dobutamine
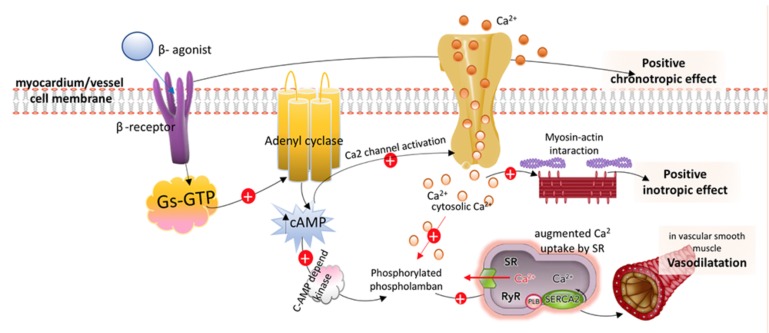
Dobutamine directly stimulates β1-receptors and α1 receptors but has a weak affinity for β2 activity, leading to a substantial increase in SV and CO, a moderate increase in HR, and an inconsistent effect on MAP (Fig. 4) [3]. This means that dobutamine is a potent inotrope whose use is less hampered by induced sinus tachycardia than other inotropes. The α1 receptors in vascular smooth muscle, to which dobutamine binds in a combined agonist and antagonist manner, results in a net effect of mild vasodilation, particularly at doses below 5 mcg/kg/min. Furthermore, dobutamine infusions of up to 15 mcg/kg/min increase cardiac contractility without affecting SVR in most patients. However, at higher doses dobutamine causes more vasoconstriction [40]. These varying dose-related reactions to dobutamine by the peripheral vasculature result from the counterbalancing effects of α1-mediated vasoconstriction and β2-mediated vasodilation.
Fig. (4).
Simplified schematic of postulated intracellular actions of β-adrenergic agonist. β-Receptor stimulation, through a stimulatory Gs-GTP unit activates the adenyl cyclase system, which results in increased concentrations of cAMP. In cardiac myocytes, 1-receptor activation through increased cAMP concentration activates Ca2 channels, which leads to Ca2-mediated enhanced chronotropic responses and positive inotropy by increasing the contractility of the actin-myosin-troponin system. In vascular smooth muscle, Ca2 stimulation and increased cAMP results in stimulation of a cAMP-dependent protein kinase, phosphorylation of phospholamban, and augmented Ca2 uptake by the sarcoplasmic reticulum (SR), which leads to vasodilation.
Clinically, dobutamine increases cardiac output in patients in shock and heart failure by increasing stroke volume and decreasing SVR. It also increases cerebral oxygenation during hypoxia and/or anemia and may be effective in improving neurological outcomes in ischemic cerebral injury via its action on β1-receptors. It may also increase cerebral tolerance to anemia and hypoxia dobutamine [41].
Dobutamine’s effects on MAP can vary considerably because they depend on the relative changes in CO and SVR from baseline values. In cardiogenic shock when the baseline CO is low and SVR is high, dobutamine may raise MAP by increasing the stroke volume (SV) and CO while the SVR declines [3]. However, if the SVR drops too much, the net effect of dobutamine infusion may be hypotension if the CO has not increased proportionately. This can be a particular problem in patients with vasodilatory shock where the baseline CO may already be relatively high in the presence of a low SVR.
The chronotropic response to dobutamine infusion is dose-related and can negate the beneficial effects of increasing the CO in some instances, though as mentioned, this is less of a problem with dobutamine than with other inotropes. At doses up to 5 mcg/kg/min, the SV usually increases without significant tachycardia but above doses of 10 mcg/kg/min the tachycardia worsens without a parallel increase in CO because of the decreased diastolic filling time that limits stroke volume [42]. This problem can often be addressed more effectively by combining low-dose dopamine with low-dose dobutamine rather than by simply increasing the dose of dobutamine [43].
A major advantage of dobutamine in post-cardiotomy patients [44], cardiogenic or septic shock [45] and hypotension following acute MI [46, 47] is that it usually causes a prompt improvement in CO and has a half-life of less than 2 minutes, allowing for the rapid titration and stabilization of optimal infusion rates. Dobutamine should be used with caution in patients with atrial fibrillation because it can increase the velocity of conduction through the atrioventricular (AV) node [48], thereby increasing the likelihood of ventricular fibrillation.
8.2. Isoproterenol
Isoproterenol (“Isuprel”) is an analog of epinephrine and a non-selective β-adrenergic agonist with a low affinity for α-adrenergic receptors [49]. Its potential usefulness as a strong inotrope with both systemic and pulmonary vasodilatory actions is limited primarily by its profound chronotropic effect. Isoproterenol is a more potent vasodilator than dobutamine [50], and can be associated with significant improvement in the microcirculation, especially in septic shock where it has been shown to improve both the mixed venous oxygen saturation (SvO2) and cardiac index [51]. Isoproterenol is useful as adjunctive therapy for cardiac arrest, congestive heart failure and all three types of shock.
8.3. Phenylephrine
Phenylephrine (“neosynephrine”) is a powerful vasoconstrictor due to its potent α-adrenergic activity and its near total lack of affinity for β-adrenergic receptors. It is useful as an adjunct to inotropic agents in situations where the SVR needs to be increased without significant alterations in other cardiac parameters. According to the Surviving Sepsis Guidelines phenylephrine is contraindicated in patients with septic shock “…. except when 1) Septic shock persists despite the use of 2 or more inotrope/vasopressor agents along with low-dose vasopressin, 2) Cardiac output is known to be high, or 3) Norepinephrine is considered to have already caused serious arrhythmias” [24]. However, in cross-over studies, phenylephrine was shown to be as efficacious as norepinephrine in septic shock patients with a high CO [21, 52]. In addition, it may be useful when septic shock is resistant to maximum levels of dopamine [53].
Phenylephrine is invaluable for the treatment of hypertrophic obstructive cardiomyopathy (HOCM) because it increases the afterload of the left ventricle by increasing the SVR. This enhances the cross-sectional area of the LV outflow tract, thereby decreasing its dynamic gradient during ventricular systole caused by the septal hypertrophy.
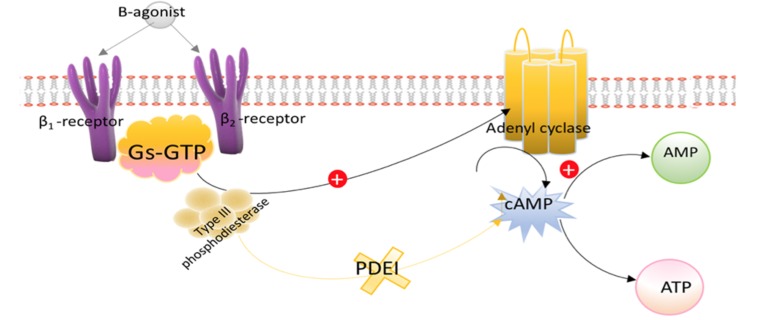
8.4. Milrinone
The primary advantage of Milrinone over other inotropes is that it increases the heart’s contractility while significantly decreasing both SVR and pulmonary vascular resistance. This unique combination makes it perhaps the most useful drug for the treatment of low output syndrome following cardiac surgery. Milrinone acts by inhibiting phosphodiesterase 3 (PDI), thus mimicking β-1 and β-2 activation (Fig. 5) [54].
Fig. (5).
Basic mechanism of action of PDIs. PDIs lead to increased intracellular concentration of cAMP, which increases contractility in the myocardium and leads to vasodilation in vascular smooth muscle.
Milrinone is usually administered either as loading dose of 50 mcg/kg over 10 minutes or it can be initiated at its maintenance dose of 0.5 mcg/kg/min without a loading dose [3, 55]. Milrinone has a longer half-life than most other inotropes and it is quite effective in patients with chronic heart failure who have downregulated or desensitized adrenergic receptors or after long-standing β-agonist administration. Renal impairment significantly increases the half-life of milrinone so its maintenance dose should be adjusted accordingly in patients with renal failure. Since milrinone does not stimulate β-1 receptors, its inotropic action persists in the presence of concurrent β-blockers [56]. Combining milrinone with a direct β-1 agonist may further increase the CO in patients with severely impaired cardiac function, but this combination is accompanied by more frequent adverse events [57, 58].
Milrinone has also been shown to be effective in acute decompensated heart failure. It is the drug of choice in patients with high SVR and low CO, but caution must be exercised in patients with low SVR or hypovolemia, such as those in shock, because Milrinone administration may make them too hypotensive [3].
9. Vasopressors and Other Agents
9.1. Vasopressin
Vasopressin, or antidiuretic hormone (ADH), contains arginine and for that reason, it is also known as arginine vasopressin (AVP) or argipressin [59]. Vasopressin is a V1a, V1b, and V2 receptor agonist and its two primary actions are vasoconstriction and the maintenance of homeostasis through fluid conservation and the regulation of glucose and salt levels in the blood [60, 61]. Because vasopressin can reduce pulmonary vascular resistance (PVR) while simultaneously increasing SVR, it can be very effective in post-cardiac surgery patients, especially those with right ventricular failure [62, 63], particularly when combined with milrinone.
Vasopressin also causes an increase in vascular sensitivity to norepinephrine which can augment its pressor effects that, fortunately, are preserved during the hypoxic and acidotic conditions in shock patients. Low vasopressin doses (0.03-0.04 U/min) can also restore the relative vasopressin deficiency that often develops in shock, resulting in an improvement in MAP and the reduction of catecholamine requirements [3, 64-66]. It is useful in vasodilatory shock following LVAD placement [67] and after cardiac transplantation [68]. On the other hand, higher vasopressin doses can cause mesenteric ischemia and should be used only as salvage therapy in patients with refractory vasodilatory shock [24, 69-71].
9.2. Selepressin
Selepressin, a novel, selective vasopressin V1A receptor agonist, is a potent vasopressor, and it has also been shown to reduce fluid requirements and limit edema formation in animal septic shock models [72-75] and is now in clinical development for the treatment of septic shock. In a phase I first-in-human trial, selepressin infusion in 30 healthy subjects with infusion rates up to 3.0 ng/kg/minute for 6 h showed V1A-agonistic vasopressor properties, was safe and well tolerated, and showed no signs of vasopressin V2 activity. In the first-in-patient pilot phase IIa randomized, placebo-controlled trial, the hypothesis was that selepressin maintains adequate arterial pressure in the absence of norepinephrine and shortens the duration of organ dysfunction in patients with early septic shock. It has been shown that selepressin at an infusion rate of 2.5 ng/kg/minute rapidly replaced norepinephrine while maintaining target MAP and may have improved fluid balance and shortened the time of mechanical ventilation. Further studies of selepressin’s mechanism of action and additional larger randomized controlled trials to investigate its efficacy are needed and ongoing to assess its ability to improve the treatment outcome of patients in septic shock [76].
9.3. Calcium-sensitizing Agents
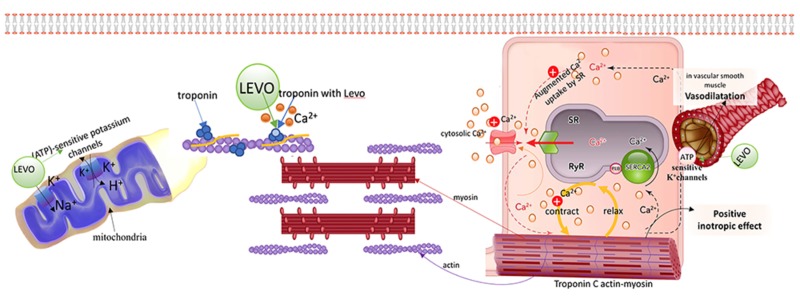
Levosimendan is an inotropic agent with vasodilator properties that is especially effective for the treatment of patients with acutely decompensated heart failure. Its inotropic properties come from its ability to sensitize the myocardium to calcium by binding to cardiac troponin C. Its vasodilatory effect is the result of opening ATP-sensitive potassium channels in vascular smooth muscle to cause smooth muscle relaxation. The combination of increased cardiac contraction and peripheral vasodilation decrease both preload and afterload, thus improving cardiac output. Levosimendan also has a cardioprotective effect because it opens the mitochondrial ATP-sensitive potassium channels in cardiac muscle [77] (Fig. 6). These unique characteristics of levosimendan allow the myocardium to contract more vigorously without a commensurate increase in its oxygen requirements [78] and without impairing diastolic relaxation [79]. It also has anti-inflammatory [80], anti-oxidative [81], and anti-apoptotic [82] properties and decreases ischemic reperfusion injury [83].
Fig. (6).
Levosimendan (LEVO) binds to troponin C during systole, increasing the sensitivity of the myocardium to calcium which increases cardiac contractility during systole, but it does not affect diastolic function. Levo leads to an opening of the active sites of troponin C, increasing its sensitivity to calcium. Levo also has a cardioprotective effect because it opens the mitochondrial ATP-sensitive potassium channels in cardiac muscle.
Levosimendan is also effective in the presence of sepsis and septic shock. It improves microcirculatory flow, renal function, hepatic function and overall hemodynamics better than dobutamine in patients with septic shock [84, 85]. Levosimendan is also used for the treatment of cardiogenic shock due to its profound effect on the CI but is of limited value because of its vasodilatory effects on both the systemic and pulmonary vascular beds [86]. While levosimendan does not increase the risk of ischemic episodes or tachyarrhythmias in patients with cardiogenic shock [87], its effectiveness as sole therapy is limited in patients with a systolic blood pressure <90 mm Hg [87]. Nevertheless, when combined with other adjunctive therapies such as norepinephrine or balloon counterpulsation, levosimendan may be useful in patients with cardiogenic shock [88].
The question of whether using levosimendan improves outcomes in patients undergoing cardiac surgeryrequiring cardiopulmonary bypass (CPB) has no definitive answer because the pertinent study results have been mixed. The 2007 SURVIVE trial, which compared levosimendan to dobutamine in CPB patients, failed to show any mortality advantage of levosimendan despite a reduction in plasma B-type natriuretic peptide [89]. Furthermore, the CHEETAH and LEVO-CTS trials showed no advantage in using levosimendan prophylactically to prevent postoperative low output syndrome [90].
However, in other studies, levosimendan was shown to improve the ability to wean patients from CPB, lower inotrope use, decrease myocardial infarction rates, and lower lactate levels when compared to a placebo [91, 92], dobutamine [93], or milrinone [94]. Its salutary effects are more pronounced in patients undergoing CABG surgery with preoperative LV ejection fractions less than 25% [91]. There are many potential reasons for the mixed results of clinical trials with levosimendan [77-79, 86-89] and interestingly, both the LEVO-CT trial and a previous meta-analysis suggested that levosimendan may be beneficial only when administered as high-dose boluses in patients with severe left ventricular dysfunction [90].
9.4. Cardiac-specific Myosin Agents
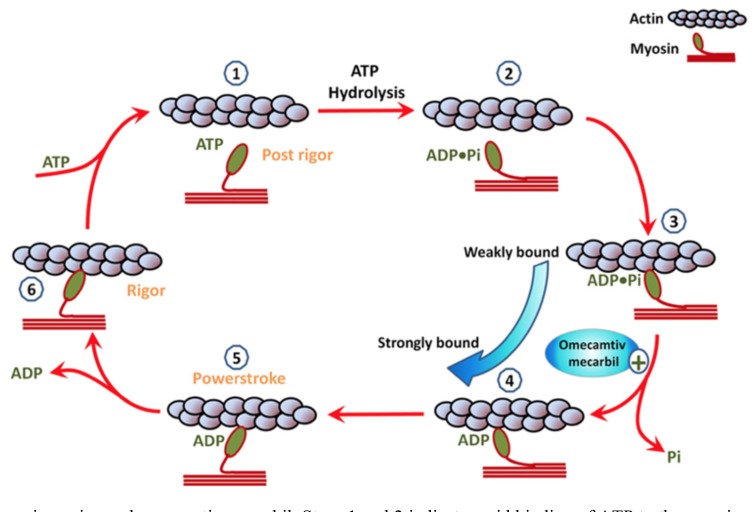
Omecamtiv mecarbil (OM) enhances myosin cross-bridge formation and duration by specifically activating myocardial ATPase and accelerating the transition rate from myosin that is weakly bound to actin into myosin that is strongly bound to actin [95]. This results in a prolongation of systolic ejection time, increased cardiac contractility, and improved energy utilization (steps 3 to 4 in Fig. 7). Delineation of the mechanism of action of OM has provided valuable insights into understanding how the force of cardiac contraction is generated by molecular motors (Fig. 7). OM does not alter myosin morphology per se but rather, it causes an accumulation of cardiac myosin just prior to ventricular systole by binding to the actin filament at more sites and in greater affinity, which in turn, enhances the strength of ventricular contraction. The overall result of OM administration is an increase in left ventricular systolic ejection time, increased sarcomere shortening, and improved stroke volume while leaving the systolic blood pressure unchanged [96]. Importantly, the improvement in cardiac output that results from this combination of OM actions is independent of intracellular calcium and cAMP levels [97, 98]. In order to test the hypothesis that a substance like OM might benefit patients with heart failure who have preserved ejection fraction (HF-rEF), direct activators of cardiac myosin were identified using a reconstituted cardiac sarcomere assay [99]. The initial results of using OM in healthy volunteers and in patients with acute or chronic HF-rEF suggested that OM is safe and that it improves systolic ejection duration and stroke volume without increasing the demand for more ATP energy or oxygen requirements and without altering intracellular calcium levels [99]. However, in the ATOMIC-AHF study, OM did not improve the primary endpoint of dyspnea when compared with placebo and although it appeared to be safe, there seemed to be an improvement in dyspnea only with higher doses of OM [100, 101]. Unfortunately, the OM-treated patients had more episodes of myocardial ischemia, though this difference was not temporally related to OM exposure. There was also a slight increase in plasma troponin in the OM group, though it did not correlate with OM plasma concentrations.
Fig. (7).
The actin–myosin engine and omecamtiv mecarbil. Steps 1 and 2 indicate rapid binding of ATP to the myosin complex allowing the myosin to unbind from actin. Step 3 indicates that, ATP is hydrolyzed into ADP and inorganic phosphate (Pi). This energy allows the myosin head to stretch. Step 4 indicates that, the myosin-ADP-Pi complex bonds to actin in a weakly bound state as it scans for a proper binding site. Step 5 indicates that, once fully attached, the myosin-ADP-Pi strongly bonds to actin, and the release of Pi from the complex causes the myosin head to bend and the actin filament to move. Step 6 indicates that ADP is released and rapidly replaced by ATP, and the cycle is then ready to repeat. Omecamtiv mecarbil accelerates the transition from the weakly bound state to the strongly bound state. (reproduced be permission from Aronson D., et al.) [95].
Despite the promising results of preclinical studies [97, 99, 102, 103] and human phase II studies [101], OM has been shown to increase the oxygen demand of the heart [104] and anginal symptoms have been reported at high OM concentrations. Because of these mixed results, it is suspected that OM may not act exclusively on cardiac β-myosin [105] but that it also opens RyR2 channels causing the release of calcium from the sarcoplasmic reticulum. This could explain the elevated oxygen consumption under baseline conditions reported recently by Bakkehaug et al. [104]. However, it has not been documented that the angina symptoms observed at high OM concentrations [105] are directly related to the known activation of RyR2 receptors by OM. In a recent study, OM was shown to activate ryanodine receptors directly in cardiac muscle but not in skeletal muscle but again, the troponin levels were increased at high doses of OM [106]. Although OM remains promising, further investigations are needed to document that it is safe to use in patients with acute and chronic heart failure and in the presence of reduced ejection fraction. [107]. OM is currently being evaluated in a large Phase 3 trial in patients with HF-rEF (GALACTIC-HF). This trial will compare OM titrated to 50 mg orally twice daily with placebo in patients 18 to 85 years of age with New York Heart Association (NYHA) class II–IV symptoms and an LVEF of 35% or less who are admitted for an HF exacerbation, hospitalized with a prior HF exacerbation, or had an urgent HF admission within the last year. The primary endpoints are cardiovascular death or readmission for HF. While OM has the potential to fill a largely unmet clinical need, the results of Novartis’ PIONEER-HF trial evaluating sacubitril/valsartan in a similar population are expected to be published ahead of GALACTIC-HF. It is important to note that the endpoints for these trials differ, with PIONEER-HF evaluating only N-terminal pro-BNP levels as well as the incidences of hyperkalemia, symptomatic hypotension, and angioedema. This will give OM an advantage if it can show reduced cardiovascular mortality and rehospitalization [108, 109].
9.5. Istaroxime
Istaroxime inhibits sodium/potassium (Na-K) ATPase and stimulates the calcium ATPase isoform 2a (SERCA2a), causing the sarcoplasmic reticulum to re-uptake calcium during ventricular diastole. This combination of Na-K AT-Phase inhibition and SERCA2a stimulation increases the cardiac output without increasing the heart rate or causing cardiac arrhythmias. In patients with acute heart failure, istaoxime increases the systolic BP and reduces the wedge pressure, heart rate and LV end-diastolic volume [110]. Currently, clinical trials are underway to evaluate the safety and efficacy of Istaroxime in patients with acute heart failure and depressed LV function.
9.6. Natriuretic Peptides
Nesiritide is recombinant human brain natriuretic peptide (BNP) that is a vasodilator, enhances sodium excretion, and suppresses both the renin-angiotensin-aldosterone system and the sympathetic nervous system [111]. In a randomized, controlled trial in patients with acute congestive heart failure, Nesiritide improved dyspnea and reduced the wedge pressure, but it conveyed no mortality benefit in comparison to standard vasoactive drug therapy. In the more recent ASCEND-HF Trial (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure), nesiritide again had no effect on mortality or rehospitalization for heart failure within 30 days [112]. Nesiritide should be used with caution in cardiogenic shock because of its propensity to cause hypotension.
10. Specific Drugs for the Treatment of Shock
10.1. Cardiogenic Shock
In the past two decades, the survival rate for patients with cardiogenic shock following an acute MI has increased from 44% in 1995 [113] to over 50% in 2005 [114], to 67% more recently [115]. Much of that improvement is attributable to the more informed use of inotropes, vasopressors, vasodilators and a variety of new drugs that have become available. Since severe vasodilation resulting from receptor desensitization, inflammation, acidemia, hypocalcemia, and the relative deficiency of vasopressin and corticosteroids is present in most cases of refractory shock [2, 9, 54], an immediate fluid challenge should be the first step in its therapy. This should be followed by the initial administration of weaker inotropes like dobutamine or low-dose epinephrine but if a satisfactory response is not elicited, stronger vasopressors like norepinephrine [116] should be administered [117]. The ACC/AHA guidelines for the management of hypotension complicating acute MI recommend dobutamine as the first-line inotropic agent if the systolic blood pressure is between 70 and 100 mm Hg in the absence of signs and symptoms of shock. However, more recent guidelines recommend a combination of norepinephrine and dobutamine over dopamine for cardiogenic shock [27, 46, 47]. The risk of tachyarrhythmias during inotropic therapy is least with milrinone, intermediate with dobutamine or epinephrine, and highest with dopamine [54, 45].
Dobutamine is also recommended for acute cardiogenic shock with hypotension and for septic shock with myocardial dysfunction, as well as in patients with severe renal failure [3, 47]. Although milrinone produces greater vasodilation and cardiac preload in such patients, dobutamine is preferable because it causes a greater increase in myocardial contractility [118]. However, since milrinone reduces PVR more than dobutamine, it is preferable in patients with significant right ventricular dysfunction. It is also the inotrope of choice in patients with chronic HF, especially in the presence of pulmonary hypertension, right ventricular failure, or b-blocker therapy [3, 119, 120]. The safety and efficacy of levosimendan for the treatment of cardiogenic shock could potentially be clarified by a new randomized clinical trial in patients with low baseline ejection fractions in which the levosimendan is administered only as large-dose boluses. Until such clarification, the potential benefit of levosimendan in cardiogenic shock will remain controversial.
10.2. Vasodilatory/Distributive Shock
In septic shock, norepinephrine is more effective than the combined therapy of dopamine and vasopressin with the addition of phenylephrine in non-responders [35] Several studies have shown dopamine to increase the mortality when it is used as the first-line vasopressor [121]. However, the combination of dopamine and dobutamine at a dose of 7.5 mcg/kg/min each improves cardiac hemodynamics while limiting important side effects better than either individual agent administered at 15 mcg/kg/min [118]. Epinephrine is the vasopressor of choice in septic shock refractory to high-dose norepinephrine, especially when the HR is high or the CO is low [3, 24, 9]. Randomized trials have shown similar mortality with epinephrine or norepinephrine in patients with shock [33, 65]. Epinephrine is approximately 100-fold more potent than dobutamine or dopamine. The CATS trial comparing norepinephrine plus dobutamine to epinephrine alone for patients with septic shock showed similar mortality rates and adverse events at 90 days despite more lactic acidosis in the epinephrine group [21, 27, 33, 52].
Hopefully, these clinical trials and others will confirm that a number of promising new drugs are capable of improving on the current status of therapy for all types of shock.
Conclusion
Shock can sometimes be difficult to categorize accurately and is often difficult to treat correctly because of its various etiologies and the multitude of treatment options available. Therapies that are optimal for one type of shock might be harmful in another type, so recognition of the type of shock is critical to successful therapy. In addition, a thorough understanding of the physiology of the various types of shock and of the pharmacology of shock therapy is essential to optimal outcomes.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Vincent J.L., De Backer D. Circulatory shock. N. Engl. J. Med. 2013;369:1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 2.Russell J.A. Management of sepsis. N. Engl. J. Med. 2006;355(16):1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 3.Hollenberg S.M. Vasoactive drugs in circulatory shock. Am. J. Respir. Crit. Care Med. 2011;183(7):847–855. doi: 10.1164/rccm.201006-0972CI. [DOI] [PubMed] [Google Scholar]
- 4.Sato Y., Matsuzawa H., Eguchi S. Comparative study of effects of adrenaline, dobutamine and dopamine on systemic hemodynamics and renal blood flow in patients following open heart surgery. Jpn. Circ. J. 1982;46(10):1059–1072. doi: 10.1253/jcj.46.1059. [DOI] [PubMed] [Google Scholar]
- 5.Butterworth JFt Dobutamine increases heart rate more than epinephrine in patients recovering from aortocoronary bypass surgery. J. Cardiothorac. Vasc. Anesth. 1992;6(5):535–541. doi: 10.1016/1053-0770(92)90095-o. [DOI] [PubMed] [Google Scholar]
- 6.Prielipp R.C., MacGregor D.A., Royster R.L., et al. Dobutamine antagonizes epinephrine’s biochemical and cardiotonic effects: Results of an in vitro model using human lymphocytes and a clinical study in patients recovering from cardiac surgery. Anesthesiology. 1998;89(1):49–57. doi: 10.1097/00000542-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Mahmoud K.M., Ammar A.S. Norepinephrine supplemented with dobutamine or epinephrine for the cardiovascular support of patients with septic shock. Indian J. Crit. Care Med. 2012;16(2):75–80. doi: 10.4103/0972-5229.99110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinman M.E., Goldberger Z.D., Rea T., et al. 2017 American Heart Association focused update on adult basic life support and cardiopulmonary resuscitation quality: An Update to the American Heart Association Guidelines for Cardio pulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2018;137:e7–e13. doi: 10.1161/CIR.0000000000000539. [DOI] [PubMed] [Google Scholar]
- 9.Bassi E., Park M., Azevedo L.C. Therapeutic strategies for highdose vasopressor-dependent shock. Crit. Care Res. Pract. 2013;2013:654708. doi: 10.1155/2013/654708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy B., Perez P., Perny J., et al. Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Crit. Care Med. 2011;39(3):450–455. doi: 10.1097/CCM.0b013e3181ffe0eb. [DOI] [PubMed] [Google Scholar]
- 11.Bolton J.D. Clinical use of lactate testing in shock states. Seminars in Anesthesia. Perioperative Medicine and Pain. 2007;26:35–39. [Google Scholar]
- 12.Kraut J.A., Madias N.E. Lactic acidosis. N. Engl. J. Med. 2014;371(24):2309–2319. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 13.Levraut J., Ciebiera J.P., Chave S., et al. Mild hyperlactatemia in stable septic patients is due to impaired lactate clearance rather than overproduction. Am. J. Respir. Crit. Care Med. 1998;157(4 Pt 1):1021–1026. doi: 10.1164/ajrccm.157.4.9705037. [DOI] [PubMed] [Google Scholar]
- 14.Gibot S. On the origins of lactate during sepsis. Crit. Care. 2012;16:151. doi: 10.1186/cc11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Genderen M.E., Klijn E., Lima A., et al. Microvascular perfusion as a target for fluid resuscitation in experimental circulatory shock. Crit. Care Med. 2014;42(2):e96–e105. doi: 10.1097/CCM.0b013e3182a63fbf. [DOI] [PubMed] [Google Scholar]
- 16.Chertoff J., Chisum M., Garcia B. Lactate kinetics in sepsis and septic shock: A review of the literature and rationale for further research. J. Intensive Care. 2015;3:39. doi: 10.1186/s40560-015-0105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivers EP, Elkin R, Cannon CM. Counterpoint: Should lactate clearance be substituted for central venous oxygen saturation as goals of early severe sepsis and septic shock therapy? No. . Chest. 2011 doi: 10.1378/chest.11-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wutrich Y., Barraud D., Conrad M., et al. Early increase in arterial lactate concentration under epinephrine infusion is associated with a better prognosis during shock. Shock. 2010;34(1):4–9. doi: 10.1097/SHK.0b013e3181ce2d23. [DOI] [PubMed] [Google Scholar]
- 19.Nalos M., Leverve X., Huang S., et al. Half-molar sodium lactate infusion improves cardiac performance in acute heart failure: A pilot randomised controlled clinical trial. Crit. Care. 2014;18(2):R48. doi: 10.1186/cc13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leverve X.M., Boon C., Hakim T., et al. Half-molar sodium-lactate solution has a beneficial effect in patients after coronary artery bypass grafting. Intensive Care Med. 2008;34(10):1796–1803. doi: 10.1007/s00134-008-1165-x. [DOI] [PubMed] [Google Scholar]
- 21.Morelli A., Ertmer C., Rehberg S., et al. Phenylephrine versus norepinephrine for initial hemodynamic support of patients with septic shock: a randomized, controlled trial. Crit. Care. 2008;12(6):R143. doi: 10.1186/cc7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maas J.J., Pinsky M.R., de Wilde R.B., et al. Cardiac output response to norepinephrine in postoperative cardiac surgery patients: interpretation with venous return and cardiac function curves. Crit. Care Med. 2013;41(1):143–150. doi: 10.1097/CCM.0b013e318265ea64. [DOI] [PubMed] [Google Scholar]
- 23.Tune J.D., Richmond K.N., Gorman M.W., et al. Control of coronary blood flow during exercise. Exp. Biol. Med. (Maywood) 2002;227:238–250. doi: 10.1177/153537020222700404. [DOI] [PubMed] [Google Scholar]
- 24.Dellinger R.P., Levy M.M., Rhodes A., et al. Surviving sepsiscampaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 25.Grandy D.K., Miller G.M., Li J.X. “TAARgeting Addiction”-The alamo bears witness to another revolution: An overview of the plenary symposium of the 2015 behavior, biology and chemistry conference. Drug Alcohol Depend. 2016;159:9–16. doi: 10.1016/j.drugalcdep.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bronwen J.B., Knights K.M. Pharmacology for Health Professionals. 2nd ed. Elsevier Australia; 2009. p. 192. [Google Scholar]
- 27.De Backer D., Biston P., Devriendt J., et al. Comparison of dopamine and norepinephrine in the treatment of shock. N. Engl. J. Med. 2010;362(9):779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 28.Ichai C., Soubielle J., Carles M., et al. Comparison of the renal effects of low to high doses of dopamine and dobutamine in critically ill patients: A single-blind randomized study. Crit. Care Med. 2000;28(4):921–928. doi: 10.1097/00003246-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Friedrich J.O., Adhikari N., Herridge M.S., et al. Meta-analysis: Low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann. Intern. Med. 2005;142(7):510–524. doi: 10.7326/0003-4819-142-7-200504050-00010. [DOI] [PubMed] [Google Scholar]
- 30.Ungar A., Fumagalli S., Marini M., et al. Renal, but not systemic, hemodynamic effects of dopamine are influenced by the severity of congestive heart failure. Crit. Care Med. 2004;32(5):1125–1129. doi: 10.1097/01.ccm.0000124871.58281.d1. [DOI] [PubMed] [Google Scholar]
- 31.Juste R.N., Panikkar K., Soni N. The effects of low-dose dopamine infusions on haemodynamic and renal parameters in patients with septic shock requiring treatment with noradrenaline. Intensive Care Med. 1998;24(6):564–568. doi: 10.1007/s001340050616. [DOI] [PubMed] [Google Scholar]
- 32.Girbes A.R., Patten M.T., McCloskey B.V., et al. The renal and neurohumoral effects of the addition of low-dose dopamine in septic critically ill patients. Intensive Care Med. 2000;26(11):1685–1689. doi: 10.1007/s001340000686. [DOI] [PubMed] [Google Scholar]
- 33.Annane D., Vignon P., Renault A., et al. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: A randomised trial. Lancet. 2007;370(9588):676–684. doi: 10.1016/S0140-6736(07)61344-0. [DOI] [PubMed] [Google Scholar]
- 34.Myburgh J.A., Higgins A., Jovanovska A., et al. A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med. 2008;34(12):2226–2234. doi: 10.1007/s00134-008-1219-0. [DOI] [PubMed] [Google Scholar]
- 35.Patel G.P., Grahe J.S., Sperry M., et al. Efficacy and safety of dopamine versus norepinephrine in the management of septic shock. Shock. 2010;33(4):375–380. doi: 10.1097/SHK.0b013e3181c6ba6f. [DOI] [PubMed] [Google Scholar]
- 36.Levy B., Perez P., Perny J., et al. Comparison of norepinephrine-dobutamine to epinephrine for hemodynamics, lactate metabolism, and organ function variables in cardiogenic shock. A prospective, randomized pilot study. Crit. Care Med. 2011;39(3):450–455. doi: 10.1097/CCM.0b013e3181ffe0eb. [DOI] [PubMed] [Google Scholar]
- 37.De Backer D., Creteur J., Silva E., et al. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: Which is best? Crit. Care Med. 2003;31(6):1659–1667. doi: 10.1097/01.CCM.0000063045.77339.B6. [DOI] [PubMed] [Google Scholar]
- 38.Communal C., Singh K., Pimentel D.R., et al. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the β-adrenergic pathway. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 39.Singh K., Xiao L., Remondino A., et al. Adrenergic regulation of cardiac myocyte apoptosis. J. Cell. Physiol. 2001;189:257–265. doi: 10.1002/jcp.10024. [DOI] [PubMed] [Google Scholar]
- 40.Ruffolo R.R., Jr The pharmacology of dobutamine. Am. J. Med. Sci. 1987;294:244–248. doi: 10.1097/00000441-198710000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Kurita T., Kawashima S., Morita K., et al. Dobutamine, a β1 adrenoceptor agonist, increases cerebral oxygenation during acute anemia and apneic hypoxia. Neurocrit. Care. 2017;27(3):420–429. doi: 10.1007/s12028-017-0423-6. [DOI] [PubMed] [Google Scholar]
- 42.Romson J.L., Leung J.M., Bellows W.H., et al. Effects of dobutamineon hemodynamics and left ventricular performance after cardiopulmonary bypass in cardiac surgical patients. Anesthesiology. 1999;91(5):1318–1328. doi: 10.1097/00000542-199911000-00024. [DOI] [PubMed] [Google Scholar]
- 43.Richard C., Ricome J.L., Rimailho A., et al. Combined hemodynamic effects of dopamine and dobutamine in cardiogenic shock. Circulation. 1983;67(3):620–626. doi: 10.1161/01.cir.67.3.620. [DOI] [PubMed] [Google Scholar]
- 44.Butterworth JFt, Prielipp RC, Royster RL, et al. Dobutamine increases heart rate more than epinephrine in patients recovering from aortocoronary bypass surgery. J. Cardiothorac. Vasc. Anesth. 1992;6(5):535–541. doi: 10.1016/1053-0770(92)90095-o. [DOI] [PubMed] [Google Scholar]
- 45.Jentzer J., Coons J., Pharm D., et al. Pharmacotherapy update on the use of vasopressors and inotropes in the intensive care unit. J. Cardiovasc. Pharmacol. Ther. 2015;20(3):249–260. doi: 10.1177/1074248414559838. [DOI] [PubMed] [Google Scholar]
- 46.Antman E.M., Anbe D.T., Armstrong P.W., et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: A report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation. 2004;110(9):e82–e292. [PubMed] [Google Scholar]
- 47.Werdan K., Russ M., Buerke M., et al. Cardiogenic shock due to myocardial infarction: Diagnosis, monitoring and treatment: A German-Austrian S3 Guideline. Dtsch. Arztebl. Int. 2012;109(19):343–351. doi: 10.3238/arztebl.2012.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volkow N.D., Wang G.J., Kollins S.H., et al. Evaluating dopamine reward pathway in ADHD: Clinical implications. JAMA. 2009;302(10):1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mozayani A., Raymon L. Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media; 2003. pp. 541–542. [Google Scholar]
- 50.Rhodes A., Evans L.E., Alhazzani W., et al. Surviving sepsis campaign: International guidelines for the management of sepsis and septic shock. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 51.Leone M., Boyadjiev I., Boulos E., et al. A reappraisal of isoproterenol in goal-directed therapy of septic shock. Shock. 2006;26(4):353–357. doi: 10.1097/01.shk.0000226345.55657.66. [DOI] [PubMed] [Google Scholar]
- 52.Morelli A., Lange M., Ertmer C., et al. Short-term effects of phenylephrine on systemic and regional hemodynamics in patients with septic shock: A crossover pilot study. Shock. 2008;29(4):446–451. doi: 10.1097/shk.0b013e31815810ff. [DOI] [PubMed] [Google Scholar]
- 53.Jain G., Singh D.K. Comparison of phenylephrine and norepinephrine in the management of dopamine-resistant septic shock. Indian J. Crit. Care Med. 2010;14(1):29–34. doi: 10.4103/0972-5229.63033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bangash M.N., Kong M.L., Pearse R.M. Use of inotropes and vasopressor agents in critically ill patients. Br. J. Pharmacol. 2012;165(7):2015–2033. doi: 10.1111/j.1476-5381.2011.01588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baruch L., Patacsil P., Hameed A., et al. Pharmacodynamic effects of milrinone with and without a bolus loading infusion. Am. Heart J. 2001;141(2):266–273. doi: 10.1067/mhj.2001.111404. [DOI] [PubMed] [Google Scholar]
- 56.Carceles M.D., Fuentes T., Aroca V., et al. Effects of milrinone on contractility and cyclic adenosine monophosphate production induced by beta1- and beta2-adrenergic receptor activation in human myocardium. Clin. Ther. 2007;29:1718–1724. doi: 10.1016/j.clinthera.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Rossinen J., Harjola V.P., Siirila-Waris K., et al. The use of more than one inotrope in acute heart failure is associated with increased mortality: A multi-centre observational study. Acute Card. Care. 2008;10(4):209–213. doi: 10.1080/17482940802262376. [DOI] [PubMed] [Google Scholar]
- 58.Royster RL. Combined inotropic effects of amrinone and epinephrine after cardiopulmonary bypass in humans. Anesth. Analg. 1993;77(4):662–672. doi: 10.1213/00000539-199310000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Anderson D.A. Dorland’s Illustrated Medical Dictionary. 32nd ed. New York: Elsevier; 2012. [Google Scholar]
- 60.Caldwell H.K., Young W.S., III . Oxytocin and Vasopressin: Genetics and Behavioral Implications (PDF). In: Lajtha A., Lim R., editors. Handbook of Neurochemistry and Molecular Neurobiology: Neuroactive Proteins and Peptides. 3rd ed. Berlin: Springer; 2006. pp. 573–607. [Google Scholar]
- 61.Babar S.M. SIADH associated with ciprofloxacin. Ann. Pharmacother. 2003;47(10):1359–1363. doi: 10.1177/1060028013502457. [DOI] [PubMed] [Google Scholar]
- 62.Morelli A., Ertmer C., Rehberg S., et al. Continuous terlipressin versus vasopressin infusion in septic shock (TERLIVAP): A randomized, controlled pilot study. Crit. Care. 2009;13(4):R130. doi: 10.1186/cc7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luckner G., Mayr V.D., Jochberger S., et al. Comparison of two dose regimens of arginine vasopressin in advanced vasodilatory shock. Crit. Care Med. 2007;35(10):2280–2285. doi: 10.1097/01.ccm.0000281853.50661.23. [DOI] [PubMed] [Google Scholar]
- 64.Russell J.A., Fjell C., Hsu J.L., et al. Vasopressin compared with norepinephrine augments the decline of plasma cytokine levels in septic shock. Am. J. Respir. Crit. Care Med. 2013;188(3):356–364. doi: 10.1164/rccm.201302-0355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dellinger R.P., Levy M.M., Rhodes A., et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 66.Polito A., Parisini E., Ricci Z., et al. Vasopressin for treatment of vasodilatory shock: An ESICM systematic review and meta-analysis. Intensive Care Med. 2012;38(1):9–19. doi: 10.1007/s00134-011-2407-x. [DOI] [PubMed] [Google Scholar]
- 67.Argenziano M., Chen J.M., Choudri A.F., et al. Management of vasodilatory shock after cardiac surgery: Identification of predisposing factors and use of a novel pressor agent. J. Thorac. Cardiovasc. Surg. 1998;116:973–980. doi: 10.1016/S0022-5223(98)70049-2. [DOI] [PubMed] [Google Scholar]
- 68.Argenziano M., Chen J.M., Cullinane S., et al. Cardiac transplantation for end-stage heart disease. Cardiol. Rev. 1999;7(6):349–355. doi: 10.1097/00045415-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 69.Torgersen C., Dunser M.W., Wenzel V., et al. Comparing two different arginine vasopressin doses in advanced vasodilatory shock: A randomized, controlled, open-label trial. Intensive Care Med. 2010;36(1):57–65. doi: 10.1007/s00134-009-1630-1. [DOI] [PubMed] [Google Scholar]
- 70.Luckner G., Mayr V.D., Jochberger S., et al. Comparison of two dose regimens of arginine vasopressin in advanced vasodilatory shock. Crit. Care Med. 2007;35(10):2280–2285. doi: 10.1097/01.ccm.0000281853.50661.23. [DOI] [PubMed] [Google Scholar]
- 71.Tayama E., Ueda T., Shojima T., et al. Arginine vasopressin is an ideal drug after cardiac surgery for the management of low systemic vascular resistant hypotension concomitant with pulmonary hypertension. Interact. Cardiovasc. Thorac. Surg. 2007;6(6):715–719. doi: 10.1510/icvts.2007.159624. [DOI] [PubMed] [Google Scholar]
- 72.He X., Su F., Taccone F.S., et al. A selective V1A receptor agonist, selepressin, is superior to arginine vasopressin and to norepinephrine in ovine septic shock. Crit. Care Med. 2016;44(1):23–31. doi: 10.1097/CCM.0000000000001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maybauer M.O., Maybauer D.M., Enkhbaatar P., et al. The selective vasopressin type 1a receptor agonist selepressin (FE 202158) blocks vascular leak in ovine severe sepsis. Crit. Care Med. 2014;42(7):e525–e533. doi: 10.1097/CCM.0000000000000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rehberg S., Ertmer C., Vincent J.L., et al. Role of selective V1a receptor agonism in ovine septic shock. Crit. Care Med. 2011;39(1):119–125. doi: 10.1097/CCM.0b013e3181fa3898. [DOI] [PubMed] [Google Scholar]
- 75.Rehberg S., Yamamoto Y., Sousse L., et al. Selective V1a agonism attenuates vascular dysfunction and fluid accumulation in ovine severe sepsis. Am. J. Physiol. Heart Circ. Physiol. 2012;303(10):H1245–H1254. doi: 10.1152/ajpheart.00390.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Russell J., Vincent J., Kjølbye A., et al. Selepressin, a novel selective vasopressin V1A agonist, is an effective substitute for norepinephrine in a phase IIa randomized, placebo-controlled trial in septic shock patients. Crit. Care. 2017;21:213. doi: 10.1186/s13054-017-1798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lehtonen L., Põder P. The utility of levosimendan in the treatment of heart failure. Ann. Med. 2007;39(1):2–17. doi: 10.1080/07853890601073346. [DOI] [PubMed] [Google Scholar]
- 78.Gordon A.C., Perkins G.D., Singer M., et al. Levosimendan for the prevention of acute organ dysfunction in sepsis. N. Engl. J. Med. 2016;375(17):1638–1648. doi: 10.1056/NEJMoa1609409. [DOI] [PubMed] [Google Scholar]
- 79.Ukkonen H., Saraste M., Akkila J., et al. Myocardial efficiency during calcium sensitization with levosimendan: A noninvasive study with positron emission tomography and echocardiography in healthy volunteers. Clin. Pharmacol. Ther. 1997;61:596–607. doi: 10.1016/S0009-9236(97)90139-9. [DOI] [PubMed] [Google Scholar]
- 80.Wang Q., Yokoo H., Takashina M., et al. Anti-inflammatory profile of levosimendan in cecal ligation-induced septic mice and in lipopolysaccharide-stimulated macrophages. Crit. Care Med. 2015;43:e508–e520. doi: 10.1097/CCM.0000000000001269. [DOI] [PubMed] [Google Scholar]
- 81.Hasslacher J., Bijuklic K., Bertocchi C., et al. Levosimendan inhibits release of reactive oxygen species in polymorphonuclear leukocytes in vitro and in patients with acute heart failure and septic shock: A prospective observational study. Crit. Care. 2011;15:R166. doi: 10.1186/cc10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paraskevaidis I.A., Parissis J.T.Th., Kremastinos D. Anti-inflammatory and anti-apoptotic effects of levosimendan in decompensated heart failure: A novel mechanism of drug-induced improvement in contractile performance of the failing heart. Curr. Med. Chem. Cardiovasc. Hematol. Agents. 2005;3(3):243–247. doi: 10.2174/1568016054368232. [DOI] [PubMed] [Google Scholar]
- 83.du Toit E.F., Genis A., Opie L.H., Pollesello P. A role for the RISK pathway and K(ATP) channels in pre- and post-conditioning induced by levosimendan in the isolated guinea pig heart. Br. J. Pharmacol. 2008;154(1):41–50. doi: 10.1038/bjp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morelli A., Donati A., Ertmer C., et al. Levosimendan for resuscitating the microcirculation in patients with septic shock: A randomized controlled study. Crit. Care. 2010;14:R232. doi: 10.1186/cc9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Memiş D., Inal M.T., Sut N. The effects of levosimendan vs dobutamine added to dopamine on liver functions assessed with noninvasive liver function monitoring in patients with septic shock. J. Crit. Care. 2012;27:318.e1–e6. doi: 10.1016/j.jcrc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 86.Buerke M., Lemm H., Krohe K., et al. Levosimendan in the treatment of cardiogenic shock. Minerva Cardioangiol. 2010;58(4):519–530. [PubMed] [Google Scholar]
- 87.Shang G., Yang X., Song D. Effects of levosimendan on patients with heart failure complicating acute coronary syndrome: A meta-analysis of randomized controlled trials. Am. J. Cardiovasc. Drugs. 2017;17:453. doi: 10.1007/s40256-017-0237-0. [DOI] [PubMed] [Google Scholar]
- 88.Russ M.A., Prondzinsky R., Christoph A., et al. Hemodynamic improvement following levosimendan treatment in patients with acute myocardial infarction and cardiogenic shock. Crit. Care Med. 2007;35(12):2732–2739. doi: 10.1097/01.CCM.0000287524.17358.48. [DOI] [PubMed] [Google Scholar]
- 89.Mebazaa A., Nieminen M.S., Packer M., et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: The SURVIVE Randomized Trial. JAMA. 2007;297(17):1883–1891. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 90.Mehta R.H., Leimberger J.D., van Diepen S., et al. Levosimendan in patients with left ventricular dysfunction undergoing cardiac surgery. LEVO-CTS Investigators. N. Engl. J. Med. 2017;376(21):2032–2042. doi: 10.1056/NEJMoa1616218. [DOI] [PubMed] [Google Scholar]
- 91.Levin R., Degrange M., Del Mazo C., et al. Preoperative levosimendan decreases mortality and the development of low cardiac output in high-risk patients with severe left ventricular dysfunction undergoing coronary artery bypass grafting with cardiopulmonary bypass. Exp. Clin. Cardiol. 2012;17(3):125–130. [PMC free article] [PubMed] [Google Scholar]
- 92.Toller W., Heringlake M., Guarracino F., Algotsson L. Preoperative and perioperative use of levosimendan in cardiac surgery: European expert opinion. Int. J. Cardiol. 2015;184:323–336. doi: 10.1016/j.ijcard.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 93.Levin R.L., Degrange M.A., Porcile R., et al. The calcium sensitizer levosimendan gives superior results to dobutamine in postoperative low cardiac output syndrome. Rev. Esp. Cardiol. 2008;61(5):471–479. [PubMed] [Google Scholar]
- 94.De Hert S.G., Lorsomradee S., Cromheecke S., et al. The effects of levosimendan in cardiac surgery patients with poor left ventricular function. Anesth. Analg. 2007;104(4):766–773. doi: 10.1213/01.ane.0000256863.92050.d3. [Erratum in: Anesth Analg 2007; 104]. [6]. [DOI] [PubMed] [Google Scholar]
- 95.Aronson D., Krum H. Novel therapies in acute and chronic heart failure. Pharmacol. Ther. 2012;135(1):1–17. doi: 10.1016/j.pharmthera.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 96.Liu L.C., Dorhout B., van der Meer P., et al. Omecamtiv mecarbil: A new cardiac myosin activator for the treatment of heart failure. Expert Opin. Investig. Drugs. 2016;25(1):117–127. doi: 10.1517/13543784.2016.1123248. [DOI] [PubMed] [Google Scholar]
- 97.Shen Y.T., Malik F.I., Zhao X., et al. Improvement of cardiac function by a cardiac Myosin activator in conscious dogs with systolic heart failure. Circ Heart Fail. 2010;3(4):522–527. doi: 10.1161/CIRCHEARTFAILURE.109.930321. [DOI] [PubMed] [Google Scholar]
- 98.Teerlink J.R., Metra M., Zacà V., et al. Agents with inotropic properties for the management of acute heart failure syndromes. Traditional agents and beyond. Heart Fail. Rev. 2009;14(4):243–253. doi: 10.1007/s10741-009-9153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Malik F.I., Hartman J.J., Elias K.A., et al. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011;331(6023):1439–1443. doi: 10.1126/science.1200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Teerlink J.R., Felker G.M., McMurray J.J., et al. Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure: The ATOMIC-AHF study. J. Am. Coll. Cardiol. 2016;67(12):1444–1455. doi: 10.1016/j.jacc.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 101.Teerlink J.R., Felker G.M., McMurray J.J., et al. Chronic oral study of myosin activation to increase contractility in heart failure (COSMIC-HF): A phase 2, pharmacokinetic, randomised, placebo-controlled trial. Lancet. 2016;388(10062):2895–2903. doi: 10.1016/S0140-6736(16)32049-9. [DOI] [PubMed] [Google Scholar]
- 102.Utter M.S., Ryba D.M., Li B.H., et al. Omecamtiv mecarbil, a cardiac myosin activator, increases Ca2+ sensitivity in myofilaments with a dilated cardiomyopathy mutant tropomyosin E54K. J. Cardiovasc. Pharmacol. 2015;66(4):347–353. doi: 10.1097/FJC.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mamidi R., Gresham K.S., Li A. Molecular effects of the myosin activator omecamtiv mecarbil on contractile properties of skinned myocardium lacking cardiac myosin binding protein-C. J. Mol. Cell. Cardiol. 2015;85:262–272. doi: 10.1016/j.yjmcc.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bakkehaug J.P., Kildal A.B., Engstad E.T., et al. Myosin activator omecamtiv mecarbil increases myocardial oxygen consumption and impairs cardiac efficiency mediated by resting myosin ATPase activity. Circ Heart Fail. 2015;8:766–775. doi: 10.1161/CIRCHEARTFAILURE.114.002152. [DOI] [PubMed] [Google Scholar]
- 105.Cleland J.G.F., Teerlink J.R., Senior R., et al. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: A double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378:676–683. doi: 10.1016/S0140-6736(11)61126-4. [DOI] [PubMed] [Google Scholar]
- 106.Nánási P., Jr, Gaburjakova M., Gaburjakova J., et al. Omecamtiv mecarbil activates ryanodine receptors from canine cardiac but not skeletal muscle. Eur. J. Pharmacol. 2017;809:73–79. doi: 10.1016/j.ejphar.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 107.Planelles-Herrero V.J., Hartman J.J., Robert-Paganin J., et al. Mechanistic and structural basis for activation of cardiac myosin force production by omecamtiv mecarbil. Nat. Commun. 2017;8(1):190. doi: 10.1038/s41467-017-00176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Horváth B., Szentandrássy N., Veress R., et al. Frequency-dependent effects of omecamtiv mecarbil on cell shortening of isolated canine ventricular cardiomyocytes. Naunyn Schmiedebergs Arch. Pharmacol. 2017;390(12):1239–1246. doi: 10.1007/s00210-017-1422-z. [DOI] [PubMed] [Google Scholar]
- 109. PharmaPoint: Heart Failure—Global Drug Forecast and Market Analysis to 2026 New York, New York: Global Data. 2017.
- 110.Gheorghiade M., Ambrosy A.P., Ferrandi M., et al. Combining SERCA2a activation and Na-K ATPase inhibition: A promising new approach to managing acute heart failure syndromes with low cardiac output. Discov. Med. 2011;12(63):141–151. [PubMed] [Google Scholar]
- 111.Colucci W.S., Elkayam U., Horton D.P., et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N. Engl. J. Med. 2000;343(4):246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 112.O’Connor C.M., Starling R.C., Hernandez A.F., et al. Effect of nesiritide in patients with acute decompensated heart failure. N. Engl. J. Med. 2011;365(1):32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 113.Holmes D.R., Jr, Bates E.R., Kleiman N.S., Sadowski Z. Contemporary reperfusion therapy for cardiogenic shock: The GUSTO-I trial experience. The GUSTO-I Investigators. Global utilization of streptokinase and tissue plasminogen activator for occluded coronary arteries. J. Am. Coll. Cardiol. 1995;26(3):668–674. doi: 10.1016/0735-1097(95)00215-p. [DOI] [PubMed] [Google Scholar]
- 114.Babaev A., Frederick P.D., Pasta D.J., et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 2005;294(4):448–454. doi: 10.1001/jama.294.4.448. [DOI] [PubMed] [Google Scholar]
- 115.Anderson M.L., Peterson E.D., Peng S.A., et al. Differences in the profile, treatment, and prognosis of patients with cardiogenic shock by myocardial infarction classification: A report from NCDR. Circ. Cardiovasc. Qual. Outcomes. 2013;6(6):708–715. doi: 10.1161/CIRCOUTCOMES.113.000262. [DOI] [PubMed] [Google Scholar]
- 116.Martin C., Papazian L., Perrin G., et al. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest. 1993;103(6):1826–1831. doi: 10.1378/chest.103.6.1826. [DOI] [PubMed] [Google Scholar]
- 117.Levy B., Dusang B., Annane D., et al. Cardiovascular response to dopamine and early prediction of outcome in septic shock: A prospective multiple-center study. Crit. Care Med. 2005;33(10):2172–2177. doi: 10.1097/01.ccm.0000181297.14319.3c. [DOI] [PubMed] [Google Scholar]
- 118.Mager G., Klocke R.K., Kux A., et al. Phosphodiesterase III inhibition or adrenoreceptor stimulation: milrinone as an alternative to dobutamine in the treatment of severe heart failure. Am. Heart J. 1991;121(6 pt 2):1974–1983. doi: 10.1016/0002-8703(91)90834-5. [DOI] [PubMed] [Google Scholar]
- 119.Metra M., Nodari S., D’Aloia A., et al. Beta-blocker therapy influences the hemodynamic response to inotropic agents in patients with heart failure: A randomized comparison of dobutamine and enoximone before and after chronic treatment with metoprolol or carvedilol. J. Am. Coll. Cardiol. 2002;40(7):1248–1258. doi: 10.1016/s0735-1097(02)02134-4. [DOI] [PubMed] [Google Scholar]
- 120.Tilley D.G., Rockman H.A. Role of beta-adrenergic receptor signaling anddesensitization in heart failure: new concepts and prospects for treatment. Expert Rev. Cardiovasc. Ther. 2006;4:417–432. doi: 10.1586/14779072.4.3.417. [DOI] [PubMed] [Google Scholar]
- 121.Overgaard C., Džavík V. Inotropes and vasopressors review of physiology and clinical use in cardiovascular disease. Circulation. 2008;118:1047–1056. doi: 10.1161/CIRCULATIONAHA.107.728840. [DOI] [PubMed] [Google Scholar]