Abstract
Background:
Observational studies in Asia show that dietary intake of soy isoflavones had a significant inverse association with coronary heart disease (CHD). A recent randomized controlled trial (RCT) of soy isoflavones on atherosclerosis in the US, however, failed to show their benefit. The discrepancy may be due to the much lower prevalence of S-equol producers in Westerners: Only 20-30% of Westerners produce S-equol in contrast to 50-70% in Asians. S-equol is a metabolite of dietary soy isoflavone daidzein by gut microbiome and possesses the most anti-atherogenic properties among all isoflavones. Several short-duration RCTs documented that soy isoflavones improves arterial stiffness. Accumulating evidence shows that both atherosclerosis and arterial stiffness are positively associated with cognitive decline/dementia. Therefore, potentially, soy isoflavones, especially S-equol, are protective against cognitive decline/dementia.
Methods/Results:
This narrative review of clinical and epidemiological studies provides an overview of the health benefits of soy isoflavones and introduces S-equol. Second, we review recent evidence on the association of soy isoflavones and S-equol with CHD, atherosclerosis, and arterial stiffness as well as the association of atherosclerosis and arterial stiffness with cognitive decline/dementia. Third, we highlight recent studies that report the association of soy isoflavones and S-equol with cognitive decline/dementia. Lastly, we discuss the future directions of clinical and epidemiological research on the relationship of S-equol and CHD and dementia.
Conclusions:
Evidence from observational studies and short-term RCTs suggests that S-equol is anti-atherogenic and improves arterial stiffness and may prevent CHD and cognitive impairment/dementia. Well-designed long-term (≥ 2years) RCTs should be pursued.
Keywords: S-equol, soy isoflavones, atherosclerosis, arterial stiffness, coronary heart disease, cognitive decline, cognitive impairment, dementia
1. Introduction
Soyfoods have commonly been consumed for centuries in northeast Asia of which observational studies show a significant inverse association of dietary intake of soyfoods with coronary heart disease (CHD) [1]. Studies in non-human primates have clearly demonstrated that soy isoflavones, the most bioactive compound in soyfoods, have anti-atherogenic properties [2, 3]. In addition, longitudinal observational studies in northeast Asia have documented that dietary intake of soy isoflavones had a significant inverse association with incident CHD [4, 5]. Accordingly, a randomized clinical trial (RCT) was conducted in the US of the effect of soy isoflavones on intima-media thickness (IMT) of the carotid artery, a surrogate marker of CHD, but the study showed a null result [6]. One possible explanation for this discrepancy may be the difference in S-equol producing capacity between northeast Asians and Westerners. S-equol, a metabolite of dietary source of the isoflavone daidzein by gut microbiome, is considered to possess the most anti-atherogenic properties among all soy isoflavones. However, only 20-30% of Westerners can produce S-equol from daidzein in contrast to 50-70% of northeast Asians. In fact, recent studies from northeast Asia reported that S-equol but not dietary soy isoflavones had significant inverse associations with incident CHD [7] and coronary atherosclerosis [8]. Meanwhile, short-duration RCTs have documented that both dietary source of soy isoflavones and S-equol improved arterial stiffness [9-12], which is an independent and significant predictor of CHD [13]. Accumulating evidence shows that cardiovascular disease (CVD) and its risk factors including atherosclerosis and arterial stiffness are positively associated with cognitive decline and dementia [14]. Therefore, it is possible that soy isoflavones, especially S-equol, are protective against cognitive decline and dementia indirectly through their effects on cardiovascular (CV) risk factors. Furthermore, preclinical and clinical studies suggest that soy isoflavones and S-equol directly ameliorate the pathogenesis of cognitive decline and dementia [15, 16].
In this narrative review of clinical and epidemiological studies, first we provide an overview of the health benefit of soy isoflavones and then introduce S-equol. Second, we review recent evidence on the association of soy isoflavones and S-equol with CHD, atherosclerosis, arterial stiffness and traditional and other CV risk factors. Third, we review the association of atherosclerosis and arterial stiffness with cognitive decline and dementia. Fourth, we highlight several recent studies that report the association of soy isoflavones and S-equol with cognitive decline and dementia. Lastly, we discuss future directions of research on the relationship of S-equol and CVD as well as dementia. Potential mechanisms of isoflavones/S-equol on heart and brain are summarized in the abstract figure.
2. Health benefits of soy isoflavones
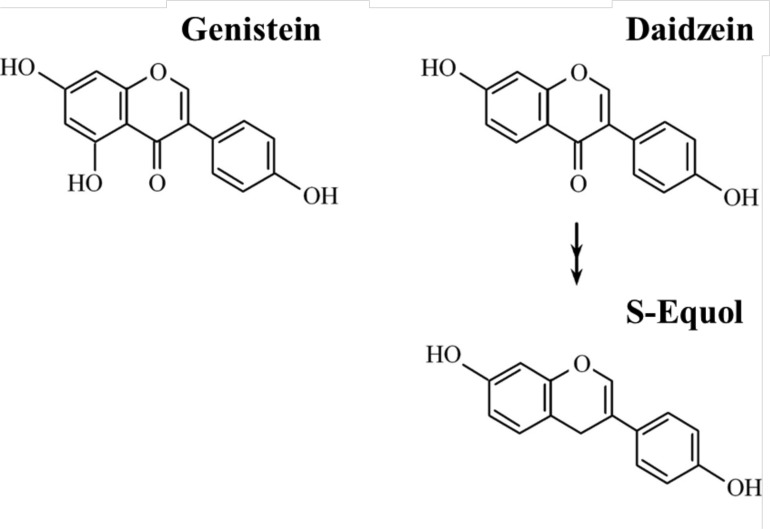
Potential health benefits of soy isoflavones have been investigated extensively for the past three decades [1, 17]. Principal soy isoflavones from dietary sources are genistein and daidzein which are both structurally similar to estradiol (Fig. 1). Estradiol as compared to soy isoflavones has stronger affinity to estrogen receptor alpha (ERα), which is expressed in the breast, bone, endometrium, reproductive tissues (uterus, ovary), kidney, white adipose tissue and liver. In contrast, soy isoflavones as compared to estradiol have stronger affinity to estrogen receptor beta (ERβ), which is expressed in the vasculature, brain, bone, lung, prostate, colon and the immune system [18]. Because a major biological action of soy isoflavones is exerted through ERs, main areas of soy isoflavones research have focused on hormone-dependent cancers (breast and prostate cancers), osteoporosis, and menopausal symptoms [19-23]. Soyfoods are traditionally consumed in northeast Asia where dietary intake of soy isoflavones is 25-50 mg/day in contrast to < 2 mg/day in the US and other Western countries [24-26]. Ecological observations support the notion that soy isoflavones have beneficial effects on these outcomes because morbidity and mortality from breast and prostate cancers as well as prevalence of menopausal vasomotor symptoms are much lower in northeast Asian than in Western countries [27-29], though incidence of hip fracture appears to be similar [30].
The benefits of soyfoods on CVD have received the attention of the public in response to a meta-analysis of soy protein on lipids that was reported in 1995 [31]. This meta-analysis of 38 small RCTs of the effects of soy protein on serum lipid levels showed that an average of 47 g/day of soy protein intake was associated with significant decreases in total cholesterol and low-density-lipoprotein cholesterol (LDL-C) by 23 mg/dL (9%) and 22 mg/dL (13%), respectively. Accordingly, the United States Food and Drug Administration approved a food-labeling health claim for soy protein (25 g/day) for prevention of CHD in 1999 [32]. More recently in 2006, the American Heart Association (AHA) reported a modest effect of soyfoods on LDL-C: a reduction by 3-5%, and stated that the benefit was very small relative to the large amount of soy protein tested, which averaged 50 g/day, more than a half of daily protein intake [33]. In fact, average intake of soy protein in northeast Asia ranges from 6 to 11 g/day [34, 35]. The CV benefit of soyfoods is attributed not only to soy protein and other ingredients of soy products including polyunsaturated fatty acids and dietary fiber as well as the effect of it replacing animal protein and saturated fats in the diet but also to soy isoflavones, which are the most bioactive component of soyfoods [33].
Soy isoflavones are one of the phenolic compounds produced by soy and a class of phytoestrogens [36]. A series of evidence supports that soy isoflavones possess anti-atherogenic properties. First, a seminal work by Clarkson et al. in post-menopausal monkeys showed that soy isoflavones significantly reduced the progression of atherosclerosis [37]. They conducted an RCT of soy protein only (control), soy protein plus soy isoflavones, and soy protein plus estrogen on atherosclerosis in cynomologus monkeys with ovariectomy for a 36-month intervention and showed that intervention groups had significantly slower progression of atherosclerosis as compared to the control group. The anti-atherogenic effect of soy isoflavones in male monkeys (2) was also noted in this RCT. They conducted an RCT of casein/lactalbumin (control), soy protein with and without isoflavones in male cynomolugus monkeys for 14-month intervention and showed that the intervention groups of soy protein with soy isoflavones and without soy isoflavones had 90% and 50% less coronary atherosclerosis, respectively, as compared to the control group. These results indicate that soy isoflavones possess anti-atherogenic properties independent of soy protein among both male and female non-human primates. Second, isoflavones are strong anti-oxidants [38]. Supplementation of soy isoflavones significantly reduced levels of oxidized LDL [39-42] which plays an essential role in the pathogenesis of atherosclerosis [43]. Likewise supplementation of soy isoflavones significantly reduced F2-isoprostanes [40, 44-46], the gold standard biomarker of oxidative stress in vivo [47, 48]. Finally, meta-analyses of RCTs showed that soy isoflavones modestly improved a profile of CV risk factors including blood pressure (BP) [49] and lipids [50].
However, a recent RCT in the US did not show any benefit of dietary soy isoflavones on carotid IMT [6]. This may be due to low prevalence of S-equol producers in Westerners. Monkeys and many other animal species produce S-equol from daidzein thus the anti-atherogenic properties of soy isoflavones observed in animal studies can be attributed to S-equol. However, only 20-30% of Westerners can produce S-equol from daidzein [51]. To support this notion, a recent nested-case-control study of incident CHD conducted in Shanghai, China showed that among dietary sources of soy isoflavones and their various metabolites examined in the urine, only S-equol but not dietary sources or other metabolites had a significant inverse association with incident CHD [7].
2.1. S-equol
S-equol was first isolated from mare urine in 1932 [52]. Since then equol has been reported to be present in the urine or plasma of many animal species [53]. In the 1980’s, S-equol was first identified in human urine [54]. Equol is part of the general class of compounds as nonsteroidal estrogen. Equol can exist in two mirror-image forms known as enantiomers, S-equol and R-equol. However, only S-equol is produced in humans and animals after the consumption of daidzein [54]. S-equol has the highest anti-oxidant properties, the highest bioavailability, and the slowest clearance rate among all soy isoflavones [55-59]. Anti-oxidant properties of S-equol are greater than vitamins C and E in in vitro studies [60, 61]. Affinity of S-equol to ERβ is much higher than its precursor, daidzein, and similar to genistein, another major dietary source of soy isoflavone [62]. S-equol does not undergo biotransformation unlike dietary sources of soy isoflavones and thus possesses the highest bioavailability among all isoflavones [63]. Moreover, the biological activity of S-equol is enhanced by its reduced biding to serum proteins and greater availability for receptor biding [64]. Endogenous estrogens circulate predominantly bound to proteins (albumin, sex-hormone-binding globulin and alpha-fetoprotein) and <5% of estradiol is present in the free form, which is the fraction that is available for receptor occupancy. For S-equol it has been reported that 49.7% circulates in the free form while that of daidzein is 18.7% [64]. S-equol also possesses anti-androgen capacity which may be related to the inhibition of prostate cancer [65].
Biotransformation of dietary sources of soy isoflavone daidzein into S-equol by gut microbiome has been reviewed in recent papers [53, 66-68]. A number of bacteria that transform daidzein into S-equol have been isolated from the feces of animals and humans. These bacteria are anaerobes, rod shaped, and gram positive. In vitro experiments have succeeded in producing S-equol using several bacteria [66]. For example, Uchiyama et al. isolated Lactococcus 20-92 as S-equol-producing bacteria from human feces and succeeded in yielding S-equol from daidzein-rich soy germ [69]. S-equol producing status appears to be determined by the presence of these specific equol-producing bacteria in the intestine [70-72] but not genetics [70-72]. Additionally, S-equol producing status is reported to be relatively stable [71, 73-76].
We summarized studies that reported the prevalence of S-equol producers after the year 2000, of which more than 100 subjects were examined including 15 case-control studies and three RCTs (Table 1). The definition of an S-equol producer varied across studies. Many observational studies in Western countries administered soy products with daidzein before S-equol was measured [75, 77-79]. In contrast, many observational studies in Asia reported prevalence of S-equol producers without using soy challenge test [26, 70, 76, 80-84]. It should be noted that two studies in China reported the prevalence of S-equol producers in the regular diet and after 3-day soy challenge [85, 86]. Prevalence of S-equol producers was 13 to 27% in the regular diet whereas the prevalence jumped up to 50 to 60% after the soy challenge test, suggesting that reported prevalence in Asian countries without soy challenge test potentially underestimates the prevalence. There does not appear to be a sex difference in the prevalence of S-equol producers. Asian Americans appear to have higher prevalence of S-equol producer compared to white Americans [77, 79, 87, 88]. Overall, prevalence of S-equol producers is similar as previously reported: 26-32% in Western countries and up to 75% in Asian countries [53]. However, interpretation of these results is somewhat limited because participants of many studies were volunteers, highly selected groups for RCT or from case-control studies, and the prevalence may not necessarily reflect that of the general adult population.
Table 1.
Reported prevalence of S-equol producers by region.
| Region | Number of Subjects Examined and Study Design | Characteristics of Subjects Examined |
Definition of
S-equol Producer |
Prevalence of S-equol Producers | Reported Year | Ref. |
|---|---|---|---|---|---|---|
| Studies in Asian countries | ||||||
| Japan | 106 Observational |
Japanese female volunteers who participated in health check-up program aged 58 ±10 | 0.4 µM/day in 24-hour urine | 50% | 2000 | [80] |
| Japan | 253 Case control |
Nested Case-control of prostate cancer (141 cases aged 69 ± 7and 112 controls aged 67 ± 9) | >0.5 ng/mL in serum (LOD) | 40% in cases and 50% in controls | 2002 | [229] |
| Japan | 227 Observational |
227 healthy Japanese volunteers (102 men and 125 women) aged >40 | >20 nM/L in serum (non-fasting) | 47% (58% in men and 38% in women) |
2002 | [81] |
| Japan | 276 Case control |
Case control study of prostate cancer (122 cases and 154 age-matched controls), mean age of 68 | >0.5 ng/mL in serum (LOD) | 29% in cases and 45% in controls | 2003 | [89] |
| Japan | 203 Case control |
Case-control study of prostate cancer (52 cases and 151 controls), mean age of 69 | ≥1.9 nM/L in serum | 67.3% in cases and 75.3% in controls | 2004 | [230] |
| Japan, Korea, and the US | 462 Case control |
Case control study of prostate cancer in 295 Japanese (133 cases and 162 controls: median age of 68), 122 Koreans (61 cases and 61 controls: median age of 67) and 45 Americans (24 cases and 21 controls: median age of 62) | 2.1 nM/L in serum | 29% in cases and 46% in controls in Japanese; 30% in cases and 59% in controls in Koreans, and 17% in cases and 14% in controls in Americans | 2004 | [87] |
| Japan | 419 Observational |
Women recruited from a breast cancer screening: mean age of 52 ± 9 for S-equol producers and 50 ± 10 for non-producer | ≥40 nM/L in spot urine when urinary daidzein:10 nM/mg creatinine | 20% | 2008 | [70] |
| Japan and Korea | 202 Observational |
Healthy male volunteers of 102 Japanese and 100 Koreans Age range of 10 to 59 years |
≥2.1 nM/L in serum | 24% in Japanese and 54% in Koreans. 10%, 24%, 17%, 25% and 44% in 10s, 20s, 30s, 40s and 50s, respectively in Japanese and 45%, 40%, 40%, 80% and 65%, respectively in Koreans |
2008 | [82] |
| Japan | 603 Case control |
Case-control study of prostate cancer (201 cases and 402 control) from the Japan Public Health Center-based Prospective study Mean age of 58 years |
≥1.0 ng/mL in serum (LOD) | 64% in controls and 60% in cases | 2008 | [231] |
| Vietnam, India, Cambodia, Japan and the US | 241 Observational |
Volunteers (63 from Hanoi, Vietnam (20-78 years), 28 from Ho Chi Minh, Vietnam (21-74 years), 39 from Kolkata, India (2—55 years), 32 from Chennai, India, 37 from Cambodia (21-48 years), 26 from Japan (21-54 years) and 16 from the US (23-63 years) | > 0.27 ng/mL in the urine (LOD) | 84% in Hanoi, 61% in Ho Chi Minh, 5% in Kolkata, 9% in Chennai, 27% in Cambodia, 77% in Japan and 69% in the US | 2010 | [232] |
| China | 183 Observational |
Healthy men and women aged 40 ± 13 | > 0.68 nM/mL (LOD) in 24-h urine | 27% in usual diet, 60% after 3-day soy challenge | 2010 | [85] |
| China | 202 Observational |
Community-based healthy subjects aged 20-69 | 24-h urinary S-equol to daidzein ratio > 0.018 | 13% in usual diet 50% after 3-day soy challenge |
2010 | [86] |
| Japan | 377 Case control |
Nested case control study of lung cancer (126 cases and 252 controls), mean age of 57 | > 1.0 nM/mL | 38% in cases and 61% in controls | 2011 | [233] |
| China | 572 Observational |
362 women and 210 men aged 40-65 | Urinary S-equol to daidzein ratio >0.018 in the overnight urine sample | 25% | 2012 | [83] |
| China | 1,130 Case control |
Nested case-control study of coronary heart disease (377 cases and 753 controls) 584 women (mean age of 61) and 546 men (mean age of 63) | Urinary S-equol to daidzein ratio >0.018 | 57% in women and 48% in men | 2012 | [7] |
| Korea | 1,391 Observational |
Selected from a population-based study: the Korean Genome and Epidemiology Study: 748 men and 633 women | > 0.068 μg/L in serum (limit of detection) | 70% (75% in men and 65% in women) | 2012 | [76] |
| Japan | 153 Case control |
153 men without prostate cancer and with prostate specific antigen level 2.5-10 ng/mL, mean age of 66 | Baseline serum >0.5 ng/mL (LOD) | 48% | 2012 | [234] |
| Japan | 500 Observational |
500 women for participants of medical check-up in 5 places in Japan; 25 participants from age-group of 30s, 40s, 50s, and 60s at each location | 20 ng/mL in urine | 39% | 2013 | [26] |
| Japan | 342 Case control |
A nested case-control study between 165 cases who died or were disabled and 177 controls without disability (mean age of 76 in control and 77 in cases) | >1.0 ng/dmL in serum (LOD) | 37% in cases and 52% in controls | 2013 | [235] |
| China | 177 RCT |
Chinese men and women with hypercholesterolemia (5.18 mM/L (200 mg/dL) were randomly assigned to placebo (mean age of 53), 40 mg of daidzein (mean age of 55) and 80 mg of daidzein (mean age of 53) | >1,000 nM/L in urine and log10-transformed S-equol to daidzein ratio > -1.75 after consumption of 150 g tofu for 3 consecutive days (both control and intervention groups) | 60% (69% in women and 51% in men) | 2014 | [168] |
| Japan | 265 Case control |
90 newly diagnosed patients with primary liver cancer and 175 controls; aged 40-69 | > 1.0ng/mL in plasma (LOD) | 44% in cases and 38% in controls | 2015 | [236] |
| Indonesia | 190 Observational |
Postmenopausal Indonesian women aged 47 to 60 (though this is an RCT, equol producing status was evaluated before randomization) |
>5 ng/ml in blood at baseline | 60% | 2015 | [84] |
| Korea | 1,391 Case control |
A nested case-control study of diabetes from the Korean Genome and Epidemiology Study (693 cases and 698 controls) mean age of 54 in women and 51 in men | 0.068 ng/mL in the plasma (LOD) |
65% in women and 75% in men | 2015 | [150] |
| Japan | 144 Case control |
46 cases of premenstrual syndrome and 98 controls aged 20-45 | > 0 .85 nM/mL in the urine after soy challenge test | 24% in cases and 42% in controls | 2016 | [237] |
| China | 573 Observational |
Postmenopausal women with prehypertension without treatment, aged 48-70 | 24-hour urinary log10 S-equol/daidzein ratio > -1.75 in the 24-urine after 7-day administration of 60 mg daidzein. | 53% | 2016 | [238] |
| Japan | 112 Case control |
56 newly diagnosed prostate cancer cases (mean age of 65) and 56 hospital controls(mean age of 64) | > 0.5 ng/mL in blood (LOD) | 34% in cases and 45% in controls | 2016 | [239] |
| Japan | 152 Observational |
Healthy volunteers (61 men and 91 women aged 69 ± 9) without CVD, medication for diabetes, lipids or hypertension | >1000 nM/L in urine after 100 mg/day of isoflavones in previous day | 40% | 2017 | [224] |
| Japan | 274 Observational |
A population-based sample of men aged 40-49 without CVD | >83nM/L, >40nM/L, and >20nM/L in serum | 16%, 22% and 40%, respectively | 2017 | [8] |
| Studies in Western countries | ||||||
| US | 194 Case control |
Case-control study of breast cancer in Asian Americans (Chinese, Filipino and Japanese) from the Log Angeles Asian Breast Cancer Study (97 cases and 97 controls), aged 25-74 | S-equol in plasma > 1nM/L | 49% in controls and 39% in cases | 2004 | [88] |
| UK | 219 Case control |
Case control study of breast cancer from the European Prospective Investigation into Cancer and Nutrition (EPIC) in the UK 333 women aged 45-75 Serum and urine samples available for 219, and 114 subjects, respectively |
Urine S-equol ≥ 1.3ng/mL or serum S-equol ≥ 0.22 ng/mL | 31% (69/219 using serum) 39% (45/114 using urine) |
2004 | [240] |
| Denmark, Germany, Italy, UK, | 117 RCT |
Healthy post-menopausal European women in randomized double-blind, placebo-controlled crossover intervention | >936 nM/L in 24-hour urine during isoflavone intervention | 28.2% | 2005 | [158] |
| US | 313 Observational |
222 Caucasian women (mean age of 41) and 91 Korean American women and girls volunteers (mean age of 36) living in Seattle | Urinary S-equol > 183nM/L after 3-day soy challenge | 36% in Caucasian women and 51% in Korean American women and girls | 2006 | [77] |
| Europe | 1,414 Observational |
Participants of the European Prospective Investigation into Cancer and Nutrition (EPIC) | Not defined but the detection level 0.1 μg/L in serum | S-equol was detected in 38% 16% of the Oxford study population showed S-equol >20 nM/L whereas only 0-2% in the other non-vegetarian EPIC populations |
2007 | [241] |
| US | 200 Observational |
Premenopausal women aged 40-45 recruited from Breast Cancer Screening Program | Urinary S-equol > 87.5 ng/mL (362 nM/L) after 3-day soy challenge | 28% | 2008 | [78] |
| US | 150 RCT |
Postmenopausal American women aged 45-92 without diabetes or CVD. In the intervention group, 60% white, 7% black, 19% Hispanic, and 13% Asian | Consistent producer: >20 nM/L in plasma at all visits in the intervention group, intermittent producer >20 nM/L in plasma at some visits | In the intervention group, consistent producer 26%, Intermittent producer 23% | 2011 | [6] |
| US | 224 Observational |
Recruited from a cohort study of 436 pre-menopausal women enrolled in a longitudinal study of diet and mammographic breast density in Chinese heritage women aged 36-58, migrated from Asia 20 or more years ago. | Urinary S-equol > 30 ng/mL after 3-day soy challenge | 30% | 2013 | [79] |
| US and Australia | 159 Observational |
Healthy adult female and male volunteers (89 in the US and 70 in Australia), aged 21-61 | Log10-transformed urinary equol to daidzein ratio > -1.75 after consumption of 240 mL of soymilk for 3.5 days | 30% in Americans and 29% in Australians | 2013 | [75] |
| US | 355 Observational |
Postmenopausal women or women in menopausal transition aged 45-55 | > S-equol concentration of 0.6 ng/mL in 24-hour urine among women whose urinary concentration of genistein and daidzein >100 ng/mL | 35% | 2015 | [242] |
LOD: Limit of detection, RCT: randomized controlled trial, CVD: cardiovascular disease.
Several studies tested whether supplementation of soy or soy plus pre- or pro-biotics or seaweed induced S-equol production. Wiseman et al. conducted a 10-week RCT of high- (102 mg/day of soy isoflavones) and low- (0.5 mg/day of soy isoflavones) soy intake in the diet in 76 healthy adults. They reported that the 10-week intervention of soy isoflavones did not induce S-equol production [90-97]. Tanaka et al. conducted a feeding experiment of 60 mg/day of soy isoflavones in 28 healthy volunteer males (18 S-equol producers and 10 S-equol non-producers) for 3 months and reported that 2 of the 10 non-producers became S-equol producers after the 3-month intervention [98]. Nettleton et al. conducted a 6-week randomized crossover study of soy (27 g/day of soy protein and 44 mg/day of soy isoflavones and soy plus probiotics) among 20 breast cancer survivors and 20 controls and reported that probiotics supplementation did not affect S-equol producing status [99]. Similarly, Bonorden et al. conducted a 2-month RCT of probiotics among 34 premenopausal women and reported that probiotics supplementation did not affect S-equol producing status [100]. On the other hand, Teas et al. conducted a 7-week randomized crossover study among 15 postmenopausal women of 5 g/day of seaweed powder or placebo with daily soy protein isolate and reported that among S-equol producers (n=5) soy supplementation increased urinary S-equol excretion and soy plus seaweed further increased urinary S-equol excretion [101]. These results from intervention studies, although inconclusive, could imply that some supplements potentially enhance S-equol producing capacity.
3. CHD, Atherosclerosis, Arterial Stiffness, and CV Risk Factors
CHD is the single largest cause of death worldwide, the leading cause of death in developed countries, and one of the leading causes of disease burden in developing countries [102, 103]. In this section, first we review epidemiological studies that reported the association of soy protein and isoflavones with CHD. Second, we review observational studies that reported the association of soy isoflavones with subclinical measures of atherosclerosis and arterial stiffness as well as RCTs that reported the effect of soy isoflavones on these subclinical measures. Third, we review the effect of soy isoflavones on BP and lipids by summarizing systematic reviews and meta-analyses of RCTs reported after the AHA statement in 2006. Fourth, we review the association of soy isoflavones on type 2 diabetes and glucose by summarizing recent studies and systematic reviews. Lastly, we review RCTs of soy isoflavones on CV risk factors in which S-equol was included in a sub-analysis.
3.1. Association of Soy Protein and Isoflavones with CHD
The WHO CARDIAC Study presented ecological evidence to show a significant inverse association of soy isoflavones with CHD mortality by examining 24-hour urinary soy isoflavones among 61 populations in 25 countries [104, 105]. Ho et al. examined an association of soy consumption with CHD death from a population-based case-control study in Hong Kong (1,060 cases for men and 956 cases for women and 10,968 controls) [106]. A relative risk (RR) (95% confidence interval (CI)) associated with CHD death after adjusting for age, physical activity, smoking, alcohol and other dietary factors in soy consumption ≥ 4 times/week compared to that < 1/month was 0.61 (0.42-0.88) for men and 0.60 (0.42-0.87) for women. The Shanghai Women’s Health Study was the first prospective cohort study that reported a significant inverse association of dietary intake of soy protein with CHD [5]. After a mean follow-up of 2.5 years of 64,915 women aged 40-70 years at baseline, 62 incident cases of CHD (43 non-fatal myocardial infarction (MI) and 19 CHD deaths) had occurred. A multivariable-adjusted hazard ratio (HR) (95% CI) in the highest tertile of soy protein intake compared to the lowest was 0.25 (0.10-0.63) for CHD and 0.14 (0.04-0.48) for non-fatal MI. The Japan Public Health Center-Based Study Cohort I was the first prospective cohort study that reported a significant inverse association of dietary intake of soy isoflavones with MI [4]. After a mean follow-up of 12.5 years of 40,462 men and women aged 40-59 years at baseline, 308 incident cases of MI occurred. A multivariable-adjusted HR (95% CI) in the highest quintile of soy isoflavones intake compared to the lowest was 0.37 (0.14-0.98) for women and 0.77 (0.47-1.24) for men. Mean dietary intake of soy isoflavones in the highest and lowest quintile was 45.2 mg/day and 10.6 mg/day, respectively. HRs associated with CHD or MI in these two prospective studies could potentially be overestimated because CV risk factors used for statistical adjustment were either self-reported or lacking (self-reported hypertension in both studies, self-reported lipid medication in the Japanese study, and no adjustment for blood levels of lipids in either study). The European Prospective study Into Cancer and Nutrition Dutch cohort reported no significant association of soy isoflavones with CHD among 16,165 women during a median follow-up of 75 months [107]. The null result is likely to be due to very low dietary intake of soy isoflavones in this population which is less than 1 mg/day (median value).
More recent large epidemiological studies investigating the association of dietary intake of soy isoflavones with CHD have reported conflicting results. The Shanghai Men’s Health Study, a prospective cohort study of 55,274 men with a mean follow-up of 5.4 years, reported that dietary intake of soy isoflavones had a significant positive association with incident CHD [108]. Multivariable-adjusted HRs (95% CI) from the lowest to highest quartiles were 1.00, 1.04 (0.68, 1.59), 1.58 (1.07, 2.33) and 1.42 (0.96, 2.11), respectively (p for trend =0.04). The Singapore Chinese Health Study, a population-based prospective cohort study of ~63,000 Chinese men and women with a mean follow-up of 14.7 years reported a null association of dietary intake of soy isoflavones with CHD mortality [109]. Multivariable-adjusted HRs (95% CI) from the lowest to highest quartiles were 1.00, 0.98 (0.88, 1.09), 0.99 (0.98, 1.11) and 0.99 (0.88, 1.12), respectively (p for trend =0.97). Reasons for these inconsistent results are unknown.
Zhang et al. was the first to report a significant inverse association of incident CHD with S-equol but not with other soy isoflavones or their metabolites. They conducted a case-control study nested within the Shanghai Women’s Health Study and Shanghai Men’s Health Study [7]. There were 536 and 559 incident CHD cases for women (mean follow-up time of 10 years) and men (mean follow-up time of 5 years). Odds ratio (OR) (95% CI) associated with CHD in the highest quartile of urinary S-equol as compared to the lowest after adjusting for total cholesterol, LDL-C, HDL-C and other potential confounders was 0.46 (0.24-0.89) for women and 0.76 (0.39-1.49) for men. Other urinary soy isoflavones or their metabolites showed null associations. The study was significant not only because it was the first study to show a significant inverse association of CHD with S-equol but not with other isoflavones and their metabolites but because the study adjusted for blood levels of lipids and measured biomarkers of soy isoflavones and their metabolites instead of using self-report of health conditions and dietary assessment which are both subject to measurement error.
3.2. Association of Soy Isoflavones with Atherosclerosis and Arterial Stiffness
Measures of subclinical atherosclerosis and arterial stiffness show the cumulative burden of CV risk factors whereas traditional CV risk factors asses risk at one time point [110, 111]. Therefore, subclinical atherosclerosis is generally a significant predictor of future CV events independent of traditional CV risk factors. As measures of subclinical atherosclerosis, we highlight carotid IMT and coronary artery calcification (CAC) as both predict future CV events independent of traditional CV risk factors [112, 113].
Arterial stiffness also predicts future CV events independent of traditional CV risk factors [126-128].
Arterial stiffness has a relatively modest or little association with CV risk factors except for BP and heart rate [129], indicating that it is a different pathophysiology from atherosclerosis although these two are intertwined [130]. Carotid-femoral pulse wave velocity (cfPWV), which predicts future CV events independent of CV risk factors [127], is the gold standard to assess arterial stiffness [131]. cfPWV is widely used in clinical and epidemiological studies in Europe. Brachial-ankle pulse wave velocity (baPWV), which also predicts future CV events independent of CV risk factors [126], is widely used in clinical and epidemiological studies in northeast Asian countries.
3.2.1. Soy Isoflavones and Carotid IMT
Cross-sectional studies in Asia showed a significant inverse association of IMT with soy isoflavones in high-risk and healthy subjects as well as S-equol producers whereas an RCT conducted in the US did not show any effect. The Women’s Isoflavone Soy Health (WISH) trial is an RCT of 25 g/day of soy protein with 91 mg of soy isoflavones or placebo on carotid IMT over a 2.7-year intervention among 350 postmenopausal women aged 45 to 92 without diabetes in the US [6]. The intervention group had a slower IMT progression by 16% without statistical significance: the mean (95% CI) of the progression (μm/year) was 4.77 (3.39-6.16) in the intervention group and 5.68 (4.30-7.06) in the control group (p=0.36). Subgroup analyses showed that among women within 5 years after menopause, the intervention group had a significantly slower progression of IMT compared to the control group: the mean IMT progression (95% CI) (μm/year) (2.16 (-1.10 - 5.43) vs. 6.79 (3.56-10.00), respectively, p=0.05), supporting the timing hypothesis [132-134]. Though S-equol producers had 20% slower IMT progression compared to control group, the difference did not reach statistical significance likely due to a small number of S-equol producers (n=39) (the mean IMT progression (95% CI) (μm/year) (4.56 (1.74-7.39) vs. 5.68 (4.29-7.07), respectively, p=0.49). Curtis et al. conducted a one-year RCT of 850 mg/day of flavon-3-ol with 100 mg/day of soy isoflavones or placebo on carotid IMT and BP among 93 postmenopausal diabetic women [9]. The intervention did not change IMT or BP. Their subgroup analysis showed that in the intervention group, S-equol producers (n=17) compared to non-producers (n=30) had significantly larger reductions in mean arterial and diastolic BPs. Liu et al. conducted a six-month RCT of whole soy, low-fat milk with 63 mg daidzein or low-fat milk only on carotid IMT among 270 postmenopausal women who were S-equol producers [135]. After the 6-month intervention, significant reductions in LDL-C and C-reactive protein (CRP) were observed only in whole soy group. No significant change in IMT was observed in either group. Although these last two RCTs did not observe any significant changes in IMT, an intervention period of 1 year or less is too short to detect changes in IMT [136].
3.2.2. Soy Isoflavones and CAC
We recently reported that S-equol producers compared to non-producers had a significantly lower prevalence of CAC [8]. Our cross-sectional study of 272 middle-aged men in Japan showed that a multivariable-adjusted OR (95% CI) for the presence of CAC in S-equol producers compared to non-producers was 0.10 (0.01-0.90). Importantly, blood levels of dietary sources of soy isoflavones (daidzein and genistein) were not significantly associated with CAC. The results suggest that S-equol is a key factor for anti-atherogenic properties of soy isoflavones, though these findings need to be confirmed in larger studies.
3.2.3. Soy Isoflavones and Arterial Stiffness
A cross-sectional study of 652 men in Japan reported a significant inverse association of dietary intake of soy isoflavones with baPWV after adjusting for traditional CV risk and dietary factors [137]. Another cross-sectional study in Japan reported that among 743 women, S-equol producers had a significantly lower baPWV than in non-producers after adjusting for various risk factors [138]. Using a single-group, pre- and post-test research design, two studies reported the effect of dietary soy isoflavones or S-equol on arterial stiffness. Hoshida et al. administered 50 mg/day of soy isoflavones to 44 pre-menopausal and 11 post-menopausal women in Japan for 2 months and reported that cardio-ankle vascular index (CAVI), an indicator of arterial stiffness, at 2 months was significantly lower in premenopausal women than at baseline [139]. A non-significant result in post-menopausal women was likely to be due to the small sample size. Yoshitaka et al. administered 10 mg/day of S-equol to 74 Japanese women for 12 months and reported that baPWV at 12 months was significantly lower than at baseline [140].
Table 2 summarizes participants, measures of arterial stiffness, soy isoflavones used for intervention, study design, duration of intervention, and major results from RCTs of soy isoflavones or their metabolites on arterial stiffness [9-12, 71, 141-143]. Number of participants were small (n=20 to 105) and duration of intervention was short (24 hours to 1 year). Arterial stiffness was assessed using various methods (systemic arterial compliance, aorto-femoral PWV, cfPWV and CAVI). Six out of the 8 RCTs have shown a significant improvement of arterial stiffness [9-12, 142].
Table 2.
Summary of randomized controlled trials of soy isoflavones and their metabolites on arterial stiffness.
| Subjects | Arterial Stiffness | Intervention | Duration | Change | Blood Pressure | Ref. |
|---|---|---|---|---|---|---|
| 105 post-menopausal women | AF-PWV | 118 mg isoflavones with 40 g of soy | 3 months | 8% reduction (not significant) | No significant change | [141] |
| 80 healthy subjects | AF-PWV | 80 mg biotin or formonoetin – precursor of genistein and daidzein | 6 weeks | 3.5% reduction (significant) | No significant change | [12] |
| 25 subjects | AF-PWV | 1 g of trans-tetrahydordaidzein – metabolites of daidzein | 5 weeks | 9.5% reduction (significant) | Significant reduction. Significant change in PWV remained after adjusting for blood pressure | [11] |
| 21 women | SAC | 80 mg isoflavones | 5-10 weeks | 26% improvement (significant) | No significant change | [142] |
| 54 subjects | CAVI | 10 mg S-equol | 12 weeks | 4% reduction (significant) | No significant change | [71] |
| 93 with diabetics (for PWV, subgroup of 35) |
cfPWV | 90 mg of epicatechin (flavonoid) and 100 mg of soy isoflavones | 1 year | Significant reduction after 12 months but not 6 months | No significant change | [9] |
| 40 post-menopausal women using tibolone (20 equol producers and 20 non-producers) | Augmentation index | 52g of soy protein and 112 mg of soy isoflavones | 8 week cross over trial | No significant change | No significant change | [172] |
| 14 S-equol producers and 14 non producers | cfPWV, EndoPAT Acute effect (24 hours) |
80 mg soy isoflavones, 40 mg S-equol | Cross over trial (baseline, 6 and 24 hours) | Soy isoflavones improved cfPWV in S-equol producers only. S-equol did not improve cfPWV | No significant difference | [10] |
| 17 adults at cardio-metabolic risk (12 women and 5 men) | Augmentation index | 55 mg genistein, 42 mg daidzein, and 4 mg glycitein | 4 weeks | 10% reduction in intervention group vs. 8% increase in control group (significant) | Not reported | [243] |
| 20 adults with moderately elevated resting blood pressure (11 women and 9 men) | cfPWV, augmentation index | 50g/d soya group: 27.5 mg genistein, 54.5 mg daidzein and 53 mg glycitein; 25g/d soya group: 13.8 mg genistein, 27.3 mg daidzein and 26.5 mg glycitein; control: 0 | 6 week cross over trial | No significant difference | Brachial diastolic blood pressure is 1.5 mmHg lower in the 50g/d soy group than control group. | [143] |
Reduction in AF-PWV and CAVI means improvement of arterial stiffness whereas increase in SAC means improvement of arterial stiffness. Change in blood pressure is important because a major determinant of AF-PWV is blood pressure.
AF-PWV: Aorto-femoral pulse wave velocity, cfPWV: carotid-femoral pulse wave velocity, PWV: pulse wave velocity, SAC: Systemic arterial compliance, CAVI: Cardio-ankle vascular index.
Curtis et al. showed that 80 mg/day of soy isoflavones significantly improved cfPWV among 35 women with diabetes. Their subgroup analysis showed that this improvement was observed only among S-equol producers [9]. Hazim et al. administered 80 mg/day of soy isoflavones to 28 men (14 equol producers and 14 non-equol producers) in their placebo-controlled randomized cross-over study with 24 hour durations and showed that soy isoflavones improved cfPWV only among S-equol producers [10]. They also reported that S-equol had no acute effect on cfPWV among non-equol producers evaluating cfPWV before and 2 hours after the administration of 40 mg of S-equol. Usui et al. conducted a 12-week placebo-controlled cross-over trial of 10 mg of S-equol on CAVI among 54 subjects. The study showed that S-equol significantly improved arterial stiffness [71]. Their subgroup analysis by S-equol-producing status showed a significant improvement of CAVI among non-equol producers only. Collectively, these data indicate that soy isoflavones improve arterial stiffness and some evidence suggests that this benefit is due to S-equol.
3.2.4. Effect of Soy Isoflavones on BP and Lipids
Taku et al. conducted a meta-analysis of 14 RCTs (789 participants) of soy isoflavones on BP [49]. They concluded that the ingestion of soy isoflavones significantly decreased systolic BP by 1.9 mmHg compared to the placebo group. This meta-analysis included RCTs published in English, Chinese or Japanese. Liu et al. conducted a meta-analysis of 11 RCTs (1,109 participants) of soy isoflavones on BP [144]. Compared to the placebo group, the isoflavones-treated group had significantly reduced systolic and diastolic BP by 2.5 mmHg and 1.5 mmHg, respectively. Subgroup analyses indicated that the significant reduction in BP occurred only among individuals with hypertension and in younger ages (less than 60 years of age). More recently, Kou et al. conducted a meta-analysis of 12 RCTs (1,551 participants) of soy protein on BP among post-menopausal women [145]. They concluded that ingestion of soy protein significantly reduced systolic BP by 3.03 mmHg and diastolic BP by 0.71 mmHg.. Collectively, soy isoflavones reduced BP moderately but significantly (i.e., 2-3 mmHg in systolic BP). The effect of soy isoflavones on BP corresponds to the effect of decreasing salt intake by 2 g/day [146].
After the AHA statement on soy protein, isoflavones and cardiovascular health in 2006 [33], several meta-analyses of soy protein and soy isoflavones on lipids are reported. Taku et al. conducted a meta-analysis of 11 RCTs (471 participants) and showed that soy protein that contained enriched soy isoflavones significantly decreased serum total cholesterol and LDL-C compared with the same amounts of isoflavone-depleted soy protein [50]. These results suggest that ingesting 102 mg soy isoflavones, independent of the amount of soy protein ingested, for 1-3 months, would lower total cholesterol by 3.9 mg/dL and LDL-C by 5.0 mg/dL. Yang et al. conducted a meta-analysis of 8 RCTs (183 patients with type 2 diabetes) of soy products containing soy isoflavones on lipids in patients with type 2 diabetes [147]. They concluded that the consumption of soy products significantly reduced total cholesterol by 16.2 mg/dL, LDL-C by 11.6 mg/dL and triglycerides by 19.5 mg/dL, and significantly increased HDL-C by 1.9 mg/dL, compared to the placebo groups. Tokede et al. conducted a meta-analysis of 35 RCTs (1,687 participants) of soy products on lipids [34]. They showed significant reductions in LDL-C by 4%, triglycerides by 3% and total cholesterol by 2% and a significant increase in HDL-C by 3%. Their meta-regression analysis showed that baseline levels of total cholesterol, LDL-C and triglycerides were significant determinants of reduction in these lipids levels.
3.2.5. Association of Soy Isoflavones with Type 2 Diabetes and Glucose
Systematic reviews of observational studies show that soy protein and soy isoflavones are associated with lower risk of type 2 diabetes especially in women, whereas meta-analyses of RCTs of soy protein and isoflavones on glucose show no significant effect. Ding et al. analyzed the association of soyfoods (tofu and soy milk) and soy isoflavones with incident type 2 diabetes in a pooled analysis of three large US cohorts (the Nurse’s Health Study, the Nurse’s Health Study II and the Health Professionals Follow-up Study) [148]. They reported no significant association of soyfoods intake with type 2 diabetes but a significant inverse association of soy isoflavones with type 2 diabetes (multivariate-adjusted HR of 0.89 (95%CI: 0.83-0.96) in the highest compared to the lowest consumption of soy isoflavones. Li et al. conducted a systematic review and meta-analysis of observational studies (8 prospective and 2 cross-sectional studies) to examine the association of soyfoods intake with type 2 diabetes [149]. Overall, soyfoods intake was associated with a 23% lower risk of type 2 diabetes. Their subgroup analyses showed a significant inverse association of soyfoods consumption with the risk of type 2 diabetes in women but not men and in Asians but not non-Asians.
Ricci et al. conducted a meta-analysis of 10 RCTs (794 women) of soy isoflavones on glucose metabolism in peri- and post-menopausal non-Asian women [150, 151]. Overall, there was a non-significant reduction in fasting glucose in the intervention group as compared to the placebo group (-2.16 mg/dL, 95% CI: -0.21 to 0.89 mg/dL). Their subgroup analyses showed that (1) RCTs that used genistein alone but not soy isoflavones mixture showed a significant reduction in fasting glucose by -7.15 mg/dl and (2) isoflavones improved Homeostatic Model Assessment of Insulin Resistance, a marker of insulin resistance.
3.3. RCTs of Soy Isoflavones on CV Risk Factors that Reported S-equol Producing Status
We recently conducted a systematic review of RCTs of soy isoflavones where subgroup analyses were conducted by S-equol producing status [152]. Our systematic review identified 28 RCTs of soy isoflavones [9, 45, 153-178]. These RCTs were conducted mostly among women and varied as to the number of participants (32 to 175), duration of intervention (8 weeks to 1 year), dose of isoflavones (33 to 120 mg/day), outcomes (lipids, arterial stiffness, oxidative stress, endothelial function, inflammatory markers, etc.), and definition and rates of S-equol producers.
In these 28 RCTs, supplementation of soy isoflavones significantly improved CV risk factors or markers in 10 RCTs [9, 154, 158, 159, 164, 167, 168, 170, 172, 175]. Of those 10 RCTs, 5 [9, 154, 167, 172, 175] reported that S-equol-producers compared to non-producers had significantly greater improvements. In the rest of 18 RCTs that reported that supplementation of soy isoflavones did not significantly improve CV risk factors or markers, 2 RCTs [160, 163] reported that S-equol producers but not non-producers had significant improvements of CV risk factors or markers. There was no RCT that showed that S-equol-producers compared to non-producers had significantly less improvements of any outcomes. These results may support a hypothesis that S-equol is a key factor to the anti-atherogenic properties of soy isoflavones. The interpretations, however, are limited because (1) these were results from sub-analyses of RCTs and (2) many of the studies did not have enough power to detect differences in outcome between equol-producers and non-producers.
4. Brain
Dementia affects around 50 million people worldwide and about 60% of those affected live in developing countries [179]. Due to the aging population, the prevalence of dementia is expected to nearly triple by 2050. Effective prevention strategies for dementia is of critical importance given that a delayed onset of 5 years reduces the prevalence of dementia by 41% [180]. Epidemiological studies reported that midlife levels of CV risk factors are significantly associated with late-life dementia, typically 20+ years later [181]. However, the association of late-life levels of CV risk factors showed only a weak and non-significant association with cognitive impairment [182]. Recent studies including the authors’ have shown that measures of cumulative burden of CV risk factors, i.e., atherosclerosis (assessed by carotid IMT and CAC) and arterial stiffness (assessed by PWV), are significantly associated with cognitive impairment and dementia [183-185]. Given that soy isoflavones are inversely associated with these measures of atherosclerosis and arterial stiffness, soy isoflavones may prevent cognitive decline and dementia. Moreover, preclinical studies show that soy isoflavones possess properties that may directly prevent dementia and cognitive decline including anti-inflammatory and anti-oxidant properties [186], increase in amyloid β clearance [187], decrease in tau phosphorylation [188], and inhibition of the mitochondrial apoptotic pathway [189].
In this section, first we review prospective cohort studies in the general population that report the association of measures of atherosclerosis and vascular stiffness with cognitive decline and dementia. Second, we review observational studies that report the association of soy isoflavones with cognitive decline and dementia. Third, we review RCTs of soy isoflavones on cognitive performance. Finally, we describe studies that reported the association of S-equol with cognition.
4.1. Prospective Cohort Studies in the General Population that Report the Association of Measures of Atherosclerosis and Vascular Stiffness with Cognition and Dementia
4.1.1. Atherosclerosis and Cognition/Dementia
Prospective cohort studies in the general population in the US, Europe and Asia reported that baseline carotid IMT had a significant association with cognitive decline and incident dementia after multivariable adjustment (Table 3). Both the Cardiovascular Health Study (CHS) [190] and Rotterdam study [191] reported that baseline IMT had a significant positive association with incident dementia after adjusting for age, sex, education, apolipoprotein E and other factors. Importantly, the significant association remained even after adjusting for prevalence of CVD [190] or after excluding subjects with prevalent or incident cases of stroke during the follow-up [191]. As for incident cognitive impairment and cognitive decline, all of the prospective cohort studies reported a significant positive association after adjusting for age, sex, education and CV risk factors [192-199] except for the Atherosclerosis Risk in the Community (ARIC) Study [200, 201]. This discrepancy may be due in part to the difference in IMT assessment; most of the other studies used IMT of the common carotid artery (CCA-IMT) [190-195, 197, 198] whereas ARIC used average IMT of internal, bulb and common carotid arteries [200, 201]. CCA-IMT compared to other segments of IMT has stronger associations with BP and stroke [111, 202, 203] which are important risk factors for dementia and cognitive impairment, thus using average IMT might have obscured the associations. The Framingham Study showed a significant association of IMT with cognitive impairment independent of magnetic-resonance-image (MRI) based ischemic changes in the brain (white matter hyperintensities, silent cerebral infarct), suggesting that IMT may be an independent marker of poor cognitive performance [193].
Table 3.
Association of intima-media thickness with cognition and dementia in prospective cohort studies.
| Study Name, Place and Year Published | Number and Age (Range or Mean) | Follow-up (Year) | Cognition Assessed |
Major Adjusted
Co-variates |
Main Results | Ref. |
|---|---|---|---|---|---|---|
| ARIC US 2001 |
10,963 (47-70) |
6 | Changes in DWR, DSS, and WF | Tertile of IMT had no significant association with changes in cognition. | [200] | |
| CHS US 2005 |
3,602 (74) |
5.4 | Incident dementia (total dementia, Alzheimer’s Disease with or without vascular dementia, and pure Alzheimer’s Disease | Age, race, education, hypertension, diabetes, smoking, income, ApoE, and MMSE | Adjusted hazard ratios (95% CI) of total dementia, Alzheimer’s disease with and without vascular dementia and pure Alzheimer’s disease in the 4th compared to 1st quartile of IMT were 1.6 (1.1-2.2), 1.4 (1.0-2.0), and 1.5 (1.0-2.2). | [190] |
| Rotterdam Study Netherlands 2007 |
6,647 (65.7 ± 6.9) |
9 | Incident dementia (total dementia, Alzheimer’s disease and vascular dementia) | Age, sex, education, BMI, blood pressure, total cholesterol, HDL-C and ApoE | Hazard ratios (95% CI) of dementia, Alzheimer’s disease and vascular dementia of 5th compared to 1st quintile of IMT were 1.50 (1.06-2.12), 1.54 (1.03-2.30) and 1.33 (0.47-3.75) | [191] |
| BLSA US 2009 |
538 (55) |
4 | I-M-C, MMSE, CVLT, BVRT, RCFT, TMT-A, TMT-B, Letter Fluency and Category Fluency | Age, sex, race, education, blood pressure, BMI, total cholesterol, and smoking | Regression coefficients in mixed-effects models associated with IMT were statistically significant for immediate free recall, short-delay free recall, and long-delay free recall in CVLT and long-delay recall in RCFT | [192] |
| Framingham Offspring Study US 2009 |
1,975 (58) | Base-line 1995-98, follow-up 1999-2001 | Classified into 3 factors: verbal memory, executive function and non-verbal memory factors | Age, sex, hypertension, smoking, diabetes, blood pressure, and cardiovascular disease | IMT had significant associations with executive and non-verbal memory factors. | [193] |
| ARIC MRI Study US 2009 |
1,130 (59 ± 4) |
14 | Changes in DWR, DSS, and WF | Age, sex, race, education, diabetes, ApoE | IMT had no significant association with changes in cognition. | [201] |
| INVADE Germany 2010 |
3,367 (67.7) | 2 | 6CIT Incident cognitive impairment was defined as a 6CIT score >7. |
Age, sex, hypertension, education, depression, physical activity baseline 6CIT score and geriatric depression scale | Multivariable-adjusted odd ratio of IMT ≥ 1.0 mm associated with incident cognitive impairment was 1.75 (95%CI: 1.15,2.59) | [194] |
| Tromso Study Norway 2012 |
4,371 (58.6 ± 9.3 for men and 59.5 ± 9.9 for women) | 7 | Verbal memory test, DSST and tapping test | Age, sex, education, physical activity, smoking, blood pressure, total cholesterol, HDL cholesterol, BMI, diabetes, coronary heart disease and depression | IMT has a significant association with DSST but not verbal memory or tapping tests. | [195] |
| EHLS US 2012 |
1,311 (66.8) |
10 | MMSE at baseline and follow-up. TMT-A, TMT-B, DSST, Rey AVLT and VFT at follow-up only | Age, sex, education, hypertension, hemoglobin A1c, HDL-C, history of cardiovascular disease, self-reported lifestyle factors | Multivariable-adjusted hazard ratio of 0.1 mm increase in IMT associated with incident cognitive impairment was 1.09 (95% CI: 1.01-1.18) IMT had a significant positive association with TMT-B but not with TMT-A, DSST, Rey ALVT or VFT |
[196] |
| KLoSHA South Korea 2015 |
348 (72 ± 6) |
5 | Korean versions of the Consortium to Establish a Registry for Alzheimer’s Disease Clinical Assessment Battery and the Mini International Neuropsychiatric Interview. | Age, education, hypertension, baseline MMSE, GDS-K, and CIRS | Multivariable-adjusted hazard ratio of IMT associated with progression of cognitive dysfunction was 1.251 (95%CI: 1.006-1.555) | [197] |
| CARDIA US 2015 |
2,618 (45.3 ± 3.6) |
5 | Rey AVLT, DSST, Stroop test | Age, sex, race, education, smoking, physical activity, BMI, diabetes, hypertension, glomerular filtration rate | 1 SD increase in IMT had significant associations with DSST and Stroop test | [198] |
ARIC: Atherosclerosis Risk in Communities, CHS: Cardiovascular Health Study, BLSA: Baltimore Longitudinal Study of Aging, INVADE: Intervention project on cerebrovascular diseases and dementia in the district of Ebersberg, Bavaria, EHLS: Epidemiology of Hearing Loss Study, KLoSHA: Korean Longitudinal study of health and aging, CARDIA: Coronary Artery Risk Development in Young Adults, DWR: Delayed word recall, DSS: Digit symbol subtest of the Wechsler Adult Intelligence Scale-Revised, WF: The first-letter word fluency, I-M-C: Information-Memory-Concentration test, MMSE: Mini-Mental State Examination, CVLT: California Verbal Learning Test, BVRT: Benton Visual Retention Test, RCFT: Rey Complex Figure Test, TMT-A: Trail-Making test A, TMT-B: Trail-Making Test B, DSST: digit symbol substitution test, 6CIT: 6 Item Cognitive Impairment Test, Rey AVLT: Rey Auditory Verbal Learning Test, VFT: Verbal Fluency test, ApoE: Apolipoprotein E genotype, BMI: Body-mass index, HDL-C: high-density lipoprotein cholesterol, GDS-K: Korean version of geriatric depression scale, CIRS: Cumulative illness rating scale.
Prospective cohort studies indicated that higher CAC was associated with increased risk of dementia. In the CHS Cognition Study, we recently reported that a three-times higher risk of incident dementia was found in women with a CAC score>400 compared to 0 (102 vs. 31 (per 1,000 person-year), p for trend: 0.04), whereas no significant difference was found in men (183). In the Multi-Ethnic Study of Atherosclerosis, the HR of risk of dementia in a CAC score>1,000 was 1.71 (95% CI: 1.07-2.73, p value: 0.03) compared to those who had a CAC score of 0 [204]. Similar result was shown in the Iceland in the Age, Gene/Environment Susceptibility-Reykjavik Study. This cross-sectional study reported that compared to the lowest quartile, the highest quartile of CAC score had 2.34 times higher odds of having dementia (95% CI: 1.31-4.19, p for trend: 0.01) [205]. Overall, epidemiological evidence supports the association of atherosclerosis with dementia and cognitive impairment.
4.1.2. Arterial Stiffness and Cognition/Dementia
Prospective cohort studies in the general population in the US, Europe and Asia reported that baseline measures of arterial stiffness had a significant positive association with cognitive decline and incident dementia after adjusting for age, sex, education, traditional CV risk factors and other co-variates (Table 4). We have recently reported from the CHS Cognition Study (mean age of 78 ± 4 years at baseline) that arterial stiffness was significantly associated with incident dementia over 15-years of follow-up [206]. The Framingham Study also reported a significant association in a younger cohort (mean age of 69 ± 6) at baseline) [207]. In contrast, the Rotterdam Study did not find a significant association [208] which may be partly due to selective attrition (~25% of baseline participants were lost in the follow-up examination). The Rotterdam Study, however, found that increased arterial stiffness was associated with a decline in executive function [208]. Most of the prospective cohort studies reported a significant positive association with incident cognitive impairment and cognitive decline after adjusting for age, sex, education and CV risk factors [207-211].
Table 4.
Association of arterial stiffness with cognition and dementia in prospective cohort studies.
| Study Name, Place and Year Published | Number and Age (Year) | Follow-up (Year) | Cognition Assessed |
Major Adjusted
Co-variates |
Main Results | Ref. |
|---|---|---|---|---|---|---|
| Rotterdam Study Netherland 2007 |
2,767 (70.7 ± 6.0) |
Baseline (1997-99) and follow-up (2002-04) | Incident dementia, MMSE, DST, Stroop test, Word frequency test | Age, sex, education, blood pressure, heart rate, smoking, diabetes, BMI, total cholesterol, HDL-C and carotid IMT | PWV had a significant positive association with Stroop test but not other test or incident dementia. | [208] |
| BLSA US 2008 |
582 (54.3 ± 17.1) |
1.6 | Digits Forward and Backward of WAIS-revised, CVLT, BVRT, TMT A and B, Letter fluency and category fluency tests, Boston naming test, MMSE, Blessed I-M-C test | Age, sex, education, depression heart rate, BMI, smoking, alcohol, and cardiovascular comorbidities | Significant interaction of PWV and age were found for BVRT, CVLT and Blessed I-M-C. Subjects with higher PWV at baseline showed a trajectory of greater decline in performance on each cognitive test. | [209] |
| Health ABC US 2013 |
2,488 (74.2 ± 2.9) |
9 | MMSE (cognitive impairment was defined as a decline of 5 or more points of MMSE) | Age, sex, race, education, ApoE, BMI, HDL-C, diabetes, hypertension and blood pressure | Multivariable-adjusted Odds ratio of cognitive impairment in the highest compared to the lowest tertile of PWV was 1.59 (1.16-2.16) | [210] |
| KLoSH South Korea 2015 |
248 (71.7 ± 6.3) |
5 | Korean versions of the Consortium to Establish a Registry for Alzheimer’s Disease Clinical Assessment Battery and the Mini International Neuropsychiatric Interview. | Age, education, hypertension, baseline MMSE, GDS-K, and CIRS | PWV did not have a significant association with the risk of cognitive impairment | [197] |
| Japan 2015 |
526 (71.7 ± 5.6) |
3.4 | MMSE (cognitive decline was defined as decline of 2 or more points of MMSE) | Age, sex, education, blood pressure, HDL-C, and ApoE | Multivariable-adjusted Odds ratio of cognitive decline in the highest compared to the lowest tertile of PWV was 2.95 (1.29-6.74) | [211] |
| Framingham Study US 2016 |
1,101 (69 ± 6) |
10-year risks | Incident dementia and MCI | Age, sex, education, blood pressure, diabetes, HDL-C, total cholesterol, ApoE4, smoking, prevalent CVD and heart rate. | Multivariable-adjusted Hazard ratio of incident MCI in the top to quintiles compared to the lowest quintile was of PWV was 1.69 (1.04-2.73). Subgroup analyses showed that individuals without diabetes had a significant positive association with incident dementia. | [207] |
| CHS US 2018 |
356 (77.8 ± 3.8) |
Over 15-year follow-up | Incident dementia | Age, sex, race, education, BMI, blood pressure, and ApoE | Multivariable-adjusted Hazard ratio of incident dementia associated with 1 SD increase in PWV was 1.60 (1.20-2.51) | [206] |
BLSA: Baltimore Longitudinal Study of Aging, Health ABC: Health, Aging, and Body Composition, KLoSHA: Korean Longitudinal study of health and aging, CHS: Cardiovascular Health Study, MMSE: Mini-Mental State Examination, DST: Digit subtraction test, WAIS: Wechsler Adult Intelligence Scale, CVLT: California Verbal Learning Test, BVRT: Benton Visual Retention Test, TMT-A: Trail-Making test A, TMT-B: Trail-Making Test B, I-M-C: Information-Memory-Concentration test, MCI: mild cognitive impairment, BMI: Body mass index, HDL-C: high-density lipoprotein cholesterol, IMT: Intima-media thickness, ApoE: Apolipoprotein E genotype, GDS-K: Korean version of geriatric depression scale, CIRS: Cumulative illness rating scale.
Stiffening of arteries reduces the buffering of pulsatile blood flow to vulnerable small cerebral arteries, which causes subclinical brain disease and neurodegeneration (e.g., white matter hyperintensities, ventricular enlargement, small cerebral infarct) [212, 213], which in turn are risk factors for dementia [214]. It should be noted that the CHS Cognition Study reported that the significant association of arterial stiffness with incident dementia remained with little attenuation after further adjusting for white matter hyperintensities, ventricular enlargement and small cerebral infarct [206]. Another potential link between arterial stiffness and dementia is that arterial stiffness accelerates the β-amyloid deposition and progression as well as tau-mediated neurodegeneration [215, 216]. Overall, current evidence supports the notion that arterial stiffness is associated with the risk of cognitive decline and dementia.
4.2. Observational Studies that Report the Association of Soy Isoflavones with Cognition and Dementia
Two recent well-designed prospective cohort studies in Japan reported dietary intake of soy products and isoflavones [217] or dietary pattern characterized by high soy intake [218] had a significant inverse association with cognitive impairment or incident dementia. Nakamoto et al. reported from the National Institute for Longevity Sciences-Longitudinal Study of Aging (403 men and 373 women aged 60-81 years followed for an average of 7.7 years) that multivariate-adjusted ORs (95%CI) for cognitive impairment (defined as a Mini-Mental State Examination score ≤23 at follow-up) associated with one SD increase in total soy products and soy isoflavones were 0.51 (0.32-0.82) and 0.55 (0.32-0.93) in women, respectively. For men OR was 1.03 (0.76-1.40) and 0.89 (0.58-1.37), respectively (217). Ozawa et al. reported from the Hisayama Study (1,006, men and women aged 60-79 years without dementia at baseline followed for an average of 15 years) that dietary pattern high in soybeans, soy products, vegetables, algae, milk and dairy product and low in rice was associated with lower risk of all-cause dementia, Alzheimer’s Disease (AD) and vascular dementia (multivariate-adjusted HRs (95% CI) were 0.66 (0.46-0.95), 0.65 (0.40-1.06) and 0.45 (0.22-0.91), respectively) [217]. Two cross-sectional studies (one in Hong Kong [219] and another in Japan [220]) also reported that a dietary pattern characterized by high intake of soy products had a significant inverse association with cognitive impairment in the elderly.
4.3. RCTs of Isoflavones on Cognition
Because soy isoflavones are structurally similar to estradiol and possess several properties that ameliorate pathophysiology of cognitive impairment and dementia as described earlier [186-189], effects of soy isoflavones on cognition have been a research topic among post-menopausal women. Cheng et al. conducted a meta-analysis of 10 RCTs of soy isoflavones on cognitive function in post-menopausal women (1,024 participants; mean age range: 52-67 years, range of soy isoflavones dose: 60-160 mg/day, treatment duration of 6 weeks to 30 months) [15]. They showed that the intervention group had significantly better scores for summary cognitive function (standardized mean difference (SMD) (95% CI): 0.08 (0.02-0.15)) and visual memory (SMD: 0.10 (0.02-0.18)). Their subgroup analyses showed that the positive effect was more pronounced in women aged 60 years or younger than older women, which support the timing hypothesis [134]. RCTs of soy isoflavones on cognition among men and premenopausal women also showed similar benefits as reported in post-menopausal women [221, 222]. Overall, soy isoflavones have potential roles in improving cognitive function.
4.4. S-equol and Cognitive Decline and Dementia
S-equol may have greater cognitive benefit than dietary sources of soy isoflavones because S-equol possesses higher anti-oxidant properties, greater or similar affinity to ERβ, longer bioavailability and the ability to increase mitochondria activities than dietary sources of soy isoflavones [16, 56, 62, 64, 223]. However, only a few studies in human have reported the association of S-equol with cognition or dementia. A recent cross-sectional study of 152 elderly in Japan reported that S-equol-producers had significantly higher cognitive score (total dementia assessment score: 14.7 ± 0.7 vs. 14.3 ± 0.8, respectively, p=0.02) and lower prevalence of mild cognitive impairment (MCI) than non-producers (8% vs. 21%, respectively, p=0.04) [224]. In their multivariable-adjusted analysis, S-equol producing status had a significant association with both the cognitive score and presence of MCI. In this study in Japan, the prevalence of S-equol producers was 40%. The WISH Trial, an RCT of soy isoflavones on carotid IMT among 350 post-menopausal women with a 30-month intervention [6], also tested whether soy isoflavones had beneficial effects on cognition [225]. There was no significant between-group difference on change from baseline in global cognition (SMD (95% CI): 0.11 (-0.13-0.35). Their subgroup analyses showed that S-equol producer, of which prevalence was 26%, tended to have improved global cognition (SMD (95% CI): 0.34 (-0.04-0.72), p=0.08). Gleason et al., who previously reported that a 6-month intervention of soy isoflavones significantly improved cognitive tests in 30 non-demented elderly men and women [226], conducted an RCT of soy isoflavones on cognition in 65 patients with AD [227]. They showed that there was no significant improvement in cognition yet their secondary analyses revealed that plasma levels of S-equol but not other isoflavones had significant positive associations with scores of verbal fluency and speed dexterity.
5. Future directions of epidemiological and clinical research on the relationship of S-equol and heart as well as cognition/dementia
Very few observational studies have reported the association of S-equol with CHD [7] and cognitive impairment/dementia [224] or atherosclerosis/arterial stiffness [8, 83, 138], where most of studies are cross sectional. Soy isoflavones are regularly consumed only in some Asian countries. Thus, these associations should be investigated rigorously in existing well-designed prospective cohort studies of atherosclerosis/CVD and dementia in northeast Asia. Such studies may answer other questions such as whether the effect of S-equol is stronger in women than in men, whether there is a specific period of time (e.g., peri- to early post-menopausal) that S-equol is effective, and whether beneficial effects of S-equol in observational studies is due to S-equol for phenotypes associated with S-equol producing status.
S-equol is available as nutraceutical supplement [71] and pharmaceutical agent [16]. Thus, well-designed, long-term (≥ 2 years) RCTs of S-equol on atherosclerosis, arterial stiffness as well as cognition should be pursued, probably first, among peri-menopausal and early post-menopausal women in Western countries. Alternatively, after screening for S-equol producers, a well-designed three-group RCT (daidzein to S-equol producers, daidzein to non-producers and placebo) on atherosclerosis, arterial stiffness and cognition could be proposed. In this design the effect of S-equol should be investigated without administering nutraceutical or pharmaceutical agents of S-equol. If the benefit is confirmed in S-equol producers but not non-producers, then we could discern who receives benefit from consuming daidzein. Recently, a real-time quantitative polymerase-chain-reaction method has been developed to detect and quantify S-equol producing bacteria in human stool [228]. This method may lead to precision health as to who receives benefit from consuming dietary sources of soy isoflavones.
After the benefit of S-equol is confirmed in prospective cohort studies in northeast Asia and these small trials above-mentioned, then a large-scale RCT on CHD or cognitive decline/dementia should be conducted.
Conclusion
A series of evidence from observational studies and short-term RCTs reviewed in this article suggests that soy isoflavones, especially S-equol, are anti-atherogenic and improve arterial stiffness and may potentially prevent CHD and cognitive impairment/dementia. Although S-equol is available as nutraceutical supplement and pharmaceutical agent, evidence is not sufficient enough to propose a large-scale long-term RCT of S-equol on CHD or cognitive decline/dementia. To conduct such a large scale trial, more convincing data from observational studies and small but long-term RCTs are urgently needed. Such data should derive from by investigating the association of S-equol at baseline with CHD or cognitive decline/dementia in existing well-designed prospective cohort studies of CHD or cognitive decline/dementia in Asia and by conducting a small but long-term RCTs of S-equol on atherosclerosis, arterial stiffness, and cognitive decline.
Fig. (1).
Chemical structures of the genistein, daidzein, and equol compared with estradiol.
Acknowledgements
The study is funded by the National Institutes of Health grants: RF1AG051615 and R21ES029734.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Messina M. Insights gained from 20 years of soy research. J. Nutr. 2010;140(12):2289S–2295S. doi: 10.3945/jn.110.124107. [DOI] [PubMed] [Google Scholar]
- 2.Anthony M.S., Clarkson T.B., Bullock B.C., Wagner J.D. Soy protein versus soy phytoestrogens in the prevention of diet-induced coronary artery atherosclerosis of male cynomolgus monkeys. Arterioscler. Thromb. Vasc. Biol. 1997;17(11):2524–2531. doi: 10.1161/01.atv.17.11.2524. [DOI] [PubMed] [Google Scholar]
- 3.Clarkson T.B. Soy, Soy phytoestrogens and cardiovascular disease. J. Nutr. 2002;132(3):566S–9. doi: 10.1093/jn/132.3.566S. [DOI] [PubMed] [Google Scholar]
- 4.Kokubo Y., Iso H., Ishihara J., et al. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: The Japan Public Health Center Based (JPHC) study cohort I. Circulation. 2007;116(22):2553–2562. doi: 10.1161/CIRCULATIONAHA.106.683755. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X., Shu X.O., Gao Y-T., et al. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J. Nutr. 2003;133(9):2874–2878. doi: 10.1093/jn/133.9.2874. [DOI] [PubMed] [Google Scholar]
- 6.Hodis H.N., Mack W.J., Kono N., et al. Isoflavone soy protein supplementation and atherosclerosis progression in healthy postmenopausal women. Stroke. 2011;42(11):3168–3175. doi: 10.1161/STROKEAHA.111.620831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X., Gao Y.T., Yang G., et al. Urinary isoflavonoids and risk of coronary heart disease. Int. J. Epidemiol. 2012;41(5):1367–1375. doi: 10.1093/ije/dys130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahuja V., Miura K., Vishnu A., et al. Significant inverse association of equol-producer status with coronary artery calcification but not dietary isoflavones in healthy Japanese men. Br. J. Nutr. 2017;117(2):260–266. doi: 10.1017/S000711451600458X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis P.J., Potter J., Kroon P.A., et al. Vascular function and atherosclerosis progression after 1 y of flavonoid intake in statin-treated postmenopausal women with type 2 diabetes: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2013;97(5):936–942. doi: 10.3945/ajcn.112.043745. [DOI] [PubMed] [Google Scholar]
- 10.Hazim S., Curtis P.J., Schar M.Y., et al. Acute benefits of the microbial-derived isoflavone metabolite equol on arterial stiffness in men prospectively recruited according to equol producer phenotype: a double-blind randomized controlled trial. Am. J. Clin. Nutr. 2016;103(3):694–702. doi: 10.3945/ajcn.115.125690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nestel P., Fujii A., Zhang L. An isoflavone metabolite reduces arterial stiffness and blood pressure in overweight men and postmenopausal women. Atherosclerosis. 2007;192(1):184–189. doi: 10.1016/j.atherosclerosis.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Teede H.J., McGrath B.P., DeSilva L., Cehun M., Fassoulakis A., Nestel P.J. Isoflavones reduce arterial stiffness: A placebo-controlled study in men and postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 2003;23(6):1066–1071. doi: 10.1161/01.ATV.0000072967.97296.4A. [DOI] [PubMed] [Google Scholar]
- 13.Palombo C., Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: Pathophysiologic mechanisms and emerging clinical indications. Vascul. Pharmacol. 2016;77:1–7. doi: 10.1016/j.vph.2015.11.083. [DOI] [PubMed] [Google Scholar]
- 14.Qiu C., Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Natl. Rev. 2015;12(5):267–277. doi: 10.1038/nrcardio.2014.223. [DOI] [PubMed] [Google Scholar]
- 15.Cheng P.F., Chen J.J., Zhou X.Y., et al. Do soy isoflavones improve cognitive function in postmenopausal women? A meta-analysis. Menopause. 2015;22:198–206. doi: 10.1097/GME.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 16.Wilkins H.M., Mahnken J.D., Welch P., et al. A mitochondrial biomarker-based study of S-equol in Alzheimer’s disease subjects: Results of a single-arm, pilot trial. J. Alzheimers Dis. 2017;59(1):291–300. doi: 10.3233/JAD-170077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messina M. Soy and health update: Evaluation of the clinical and epidemiologic literature. Nutrients. 2016;8(12):E754. doi: 10.3390/nu8120754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jia M., Dahlman-Wright K., Gustafsson J.A. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015;29(4):557–568. doi: 10.1016/j.beem.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Wei P., Liu M., Chen Y., Chen D.C. Systematic review of soy isoflavone supplements on osteoporosis in women. Asian Pac. J. Trop. Med. 2012;5(3):243–248. doi: 10.1016/S1995-7645(12)60033-9. [DOI] [PubMed] [Google Scholar]
- 20.Fritz H., Seely D., Flower G., et al. Soy, red clover, and isoflavones and breast cancer: A systematic review. PLoS One. 2013;8(11):e81968. doi: 10.1371/journal.pone.0081968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Die M.D., Bone K.M., Williams S.G., Pirotta M.V. Soy and soy isoflavones in prostate cancer: A systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014;113(5b):E119–E130. doi: 10.1111/bju.12435. [DOI] [PubMed] [Google Scholar]
- 22.Thomas A.J., Ismail R., Taylor-Swanson L., et al. Effects of isoflavones and amino acid therapies for hot flashes and co-occurring symptoms during the menopausal transition and early postmenopause: A systematic review. Maturitas. 2014;78(4):263–276. doi: 10.1016/j.maturitas.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balk E., Chung M., Chew P., et al. Effects of Soy on Health Outcomes. Evidence Report/Technology Assessment No. 126. Rockville, MD: Agency for Healthcare Research and Quality; 2005. [Google Scholar]
- 24.Klein M.A., Nahin R.L., Messina M.J., et al. Guidance from an NIH workshop on designing, implementing, and reporting clinical studies of soy interventions. J. Nutr. 2010;140(6):1192S–204. doi: 10.3945/jn.110.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valentin-Blasini L., Sadowski M.A., Walden D., Caltabiano L., Needham L.L., Barr D.B. Urinary phytoestrogen concentrations in the U.S. population (1999-2000). J. Expo. Anal. Environ. Epidemiol. 2005;15(6):509–523. doi: 10.1038/sj.jea.7500429. [DOI] [PubMed] [Google Scholar]
- 26.Liu W., Tanabe M., Harada K.H., Koizumi A. Levels of urinary isoflavones and lignan polyphenols in Japanese women. Environ. Health Prev. Med. 2013;18(5):394–400. doi: 10.1007/s12199-013-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y., Yoo K.Y., Goodman M.T. Differences in incidence, mortality and survival of breast cancer by regions and countries in Asia and contributing factors. Asian Pac. J. Cancer Prev. 2015;16(7):2857–2870. doi: 10.7314/apjcp.2015.16.7.2857. [DOI] [PubMed] [Google Scholar]
- 28.Kimura T. East meets West: ethnic differences in prostate cancer epidemiology between East Asians and Caucasians. Chin. J. Cancer. 2012;31(9):421–429. doi: 10.5732/cjc.011.10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baber R.J. East is east and West is west: Perspectives on the menopause in Asia and The West. Climacteric. 2014;17(1):23–28. doi: 10.3109/13697137.2013.830607. [DOI] [PubMed] [Google Scholar]
- 30.Cauley J.A., Chalhoub D., Kassem A.M., Fuleihan Gel H. Geographic and ethnic disparities in osteoporotic fractures. Nat. Rev. Endocrinol. 2014;10(6):338–351. doi: 10.1038/nrendo.2014.51. [DOI] [PubMed] [Google Scholar]
- 31.Anderson J.W., Johnstone B.M., Cook-Newell M.E. Meta-analysis of the effects of soy protein intake on serum lipids. N. Engl. J. Med. 1995;333(5):276–282. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 32.Food and Drug Administration Food labeling, health claims, soy protein, and coronary heart disease. Fed. Regist. 1999;57:699–733. [PubMed] [Google Scholar]
- 33.Sacks F.M., Lichtenstein A., Van Horn L., et al. Soy protein, isoflavones, and cardiovascular health. An American Heart Association Science Advisory for Professionals From the Nutrition Committee. Circulation. 2006;113(7):1034–1044. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- 34.Tokede O.A., Onabanjo T.A., Yansane A., Gaziano J.M., Djousse L. Soya products and serum lipids: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015;114(6):831–843. doi: 10.1017/S0007114515002603. [DOI] [PubMed] [Google Scholar]
- 35.Messina M., Nagata C., Wu A.H. Estimated Asian adult soy protein and isoflavone intakes. Nutr. Cancer. 2006;55(1):1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 36.Setchell K.D. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998;68(6) Suppl.:1333s–1346s. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- 37.Clarkson T.B., Anthony M.S., Morgan T.M. Inhibition of postmenopausal atherosclerosis progression: A comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J. Clin. Endocrinol. Metab. 2001;86(1):41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz-Larrea M.B., Mohan A.R., Paganga G., Miller N.J., Bolwell G.P., Rice-Evans C.A. Antioxidant activity of phytoestrogenic isoflavones. Free Radic. Res. 1997;26(1):63–70. doi: 10.3109/10715769709097785. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins D.J., Kendall C.W., Vidgen E., et al. Effect of soy-based breakfast cereal on blood lipids and oxidized low-density lipoprotein. Metabolism. 2000;49(11):1496–1500. doi: 10.1053/meta.2000.17703. [DOI] [PubMed] [Google Scholar]
- 40.Wiseman H., O’Reilly J.D., Adlercreutz H., et al. Isoflavone phytoestrogens consumed in soy decrease F2-isoprostane concentrations and increase resistance of low-density lipoprotein to oxidation in humans. Am. J. Clin. Nutr. 2000;72(2):395–400. doi: 10.1093/ajcn/72.2.395. [DOI] [PubMed] [Google Scholar]
- 41.Jenkins D.J., Kendall C.W., Jackson C-J.C., et al. Effects of high- and low-isoflavone soyfoods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am. J. Clin. Nutr. 2002;76(2):365–372. doi: 10.1093/ajcn/76.2.365. [DOI] [PubMed] [Google Scholar]
- 42.Beavers K.M., Jonnalagadda S.S., Messina M.J. Soy consumption, adhesion molecules, and pro-inflammatory cytokines: A brief review of the literature. Nutr. Rev. 2009;67(4):213–221. doi: 10.1111/j.1753-4887.2009.00191.x. [DOI] [PubMed] [Google Scholar]
- 43.Steinberg D. The LDL modification hypothesis of atherogenesis: An update. J. Lipid Res. 2009;50(Suppl.):S376–S381. doi: 10.1194/jlr.R800087-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vega-Lopez S., Yeum K.J., Lecker J.L., et al. Plasma antioxidant capacity in response to diets high in soy or animal protein with or without isoflavones. Am. J. Clin. Nutr. 2005;81(1):43–49. doi: 10.1093/ajcn/81.1.43. [DOI] [PubMed] [Google Scholar]
- 45.Sen C., Morimoto Y., Heak S., Cooney R.V., Franke A.A., Maskarinec G. Soy foods and urinary isoprostanes: Results from a randomized study in premenopausal women. Food Funct. 2012;3(5):517–521. doi: 10.1039/c2fo10251j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hodgson J.M., Puddey I.B., Croft K.D., Mori T.A., Rivera J., Beilin L.J. Isoflavonoids do not inhibit in vivo lipid peroxidation in subjects with high-normal blood pressure. Atherosclerosis. 1999;145(1):167–172. doi: 10.1016/s0021-9150(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 47.Davies S.S., Roberts L.J., II F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic. Biol. Med. 2011;50(5):559–566. doi: 10.1016/j.freeradbiomed.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts L.J., Morrow J.D. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 2000;28(4):505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 49.Taku K., Lin N., Cai D., et al. Effects of soy isoflavone extract supplements on blood pressure in adult humans: Systematic review and meta-analysis of randomized placebo-controlled trials. J. Hypertens. 2010;28(10):1971–1982. doi: 10.1097/HJH.0b013e32833c6edb. [DOI] [PubMed] [Google Scholar]
- 50.Taku K., Umegaki K., Sato Y., Taki Y., Endoh K., Watanabe S. Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2007;85(4):1148–1156. doi: 10.1093/ajcn/85.4.1148. [DOI] [PubMed] [Google Scholar]
- 51.Setchell K.D., Cole S.J. Method of defining equol-producer status and its frequency among vegetarians. J. Nutr. 2006;136(8):2188–2193. doi: 10.1093/jn/136.8.2188. [DOI] [PubMed] [Google Scholar]
- 52.Marrian G.F., Haslewood G.A. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares’ urine. Biochem. J. 1932;26(4):1227–1232. doi: 10.1042/bj0261227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Setchell K.D., Clerici C. Equol: History, chemistry, and formation. J. Nutr. 2010;140(7):1355S–1362S. doi: 10.3945/jn.109.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Axelson M., Kirk D.N., Farrant R.D., Cooley G., Lawson A.M., Setchell K.D. The identification of the weak oestrogen equol [7-hydroxy-3-(4′-hydroxyphenyl)chroman] in human urine. Biochem. J. 1982;201(2):353–357. doi: 10.1042/bj2010353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hwang J., Wang J., Morazzoni P., Hodis H.N., Sevanian A. The phytoestrogen equol increases nitric oxide availability by inhibiting superoxide production: an antioxidant mechanism for cell-mediated LDL modification. Free Radic. Biol. Med. 2003;34(10):1271–1282. doi: 10.1016/s0891-5849(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 56.Setchell K.D., Brown N.M., Lydeking-Olsen E. The clinical importance of the metabolite sequel-a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002;132(12):3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 57.Joy S., Siow R.C., Rowlands D.J., et al. The isoflavone Equol mediates rapid vascular relaxation: Ca2+-independent activation of endothelial nitric-oxide synthase/Hsp90 involving ERK1/2 and Akt phosphorylation in human endothelial cells. J. Biol. Chem. 2006;281(37):27335–27345. doi: 10.1074/jbc.M602803200. [DOI] [PubMed] [Google Scholar]
- 58.Jackman K.A., Woodman O.L., Chrissobolis S., Sobey C.G. Vasorelaxant and antioxidant activity of the isoflavone metabolite equol in carotid and cerebral arteries. Brain Res. 2007;1141:99–107. doi: 10.1016/j.brainres.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 59.Cheng C., Wang X., Weakley S.M., et al. The soybean isoflavonoid equol blocks ritonavir-induced endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J. Nutr. 2010;140(1):12–17. doi: 10.3945/jn.109.110981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell J.H., Gardner P.T., McPhail D.B., Morrice P.C., Collins A.R., Duthie G.G. Antioxidant efficacy of phytoestrogens in chemical and biological model systems. Arch. Biochem. Biophys. 1998;360(1):142–148. doi: 10.1006/abbi.1998.0951. [DOI] [PubMed] [Google Scholar]
- 61.Arora A., Nair M.G., Strasburg G.M. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch. Biochem. Biophys. 1998;356(2):133–141. doi: 10.1006/abbi.1998.0783. [DOI] [PubMed] [Google Scholar]
- 62.Muthyala R.S., Ju Y.H., Sheng S., et al. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 2004;12(6):1559–1567. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 63.Setchell K.D., Zhao X., Shoaf S.E., Ragland K. The pharmacokinetics of S-(-)equol administered as SE5-OH tablets to healthy postmenopausal women. J. Nutr. 2009;139(11):2037–2043. doi: 10.3945/jn.109.110874. [DOI] [PubMed] [Google Scholar]
- 64.Setchell K.D., Clerici C. Equol: Pharmacokinetics and biological actions. J. Nutr. 2010;140(7):1363S–1368S. doi: 10.3945/jn.109.119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lund T.D., Munson D.J., Haldy M.E., Setchell K.D., Lephart E.D., Handa R.J. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol. Reprod. 2004;70(4):1188–1195. doi: 10.1095/biolreprod.103.023713. [DOI] [PubMed] [Google Scholar]
- 66.Yuan J.P., Wang J.H., Liu X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora--implications for health. Mol. Nutr. Food Res. 2007;51(7):765–781. doi: 10.1002/mnfr.200600262. [DOI] [PubMed] [Google Scholar]
- 67.Rafii F. The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites. 2015;5(1):56–73. doi: 10.3390/metabo5010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jackson R.L., Greiwe J.S., Schwen R.J. Emerging evidence of the health benefits of S-equol, an estrogen receptor beta agonist. Nutr. Rev. 2011;69(8):432–448. doi: 10.1111/j.1753-4887.2011.00400.x. [DOI] [PubMed] [Google Scholar]
- 69.Uchiyama S., Ueno T., Suzuki T. Identification of a newly isolated equol-producing lactic acid bacterium from the human feces (in Japanese: abstract in English). J Intestinal Microbiol. 2007;21:217–220. [Google Scholar]
- 70.Nagata C., Ueno T., Uchiyama S., et al. Dietary and lifestyle correlates of urinary excretion status of equol in Japanese women. Nutr. Cancer. 2008;60(1):49–54. doi: 10.1080/01635580701525885. [DOI] [PubMed] [Google Scholar]
- 71.Usui T., Tochiya M., Sasaki Y., et al. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin. Endocrinol. (Oxf.) 2013;78:365–372. doi: 10.1111/j.1365-2265.2012.04400.x. [DOI] [PubMed] [Google Scholar]
- 72.Maskarinec G., Yamakawa R., Hebshi S., Franke A.A. Urinary isoflavonoid excretion and soy consumption in three generations of Japanese women in Hawaii. Eur. J. Clin. Nutr. 2007;61(2):255–261. doi: 10.1038/sj.ejcn.1602511. [DOI] [PubMed] [Google Scholar]
- 73.Franke A.A., Lai J.F., Pagano I., Morimoto Y., Maskarinec G. Equol production changes over time in pre-menopausal women. Br. J. Nutr. 2012;107(8):1201–1206. doi: 10.1017/S0007114511004223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frankenfeld C.L., Atkinson C., Thomas W.K., et al. High concordance of daidzein-metabolizing phenotypes in individuals measured 1 to 3 years apart. Br. J. Nutr. 2005;94(6):873–876. doi: 10.1079/bjn20051565. [DOI] [PubMed] [Google Scholar]
- 75.Setchell K.D., Brown N.M., Summer S., et al. Dietary factors influence production of the soy isoflavone metabolite s-(-)equol in healthy adults. J. Nutr. 2013;143(12):1950–1958. doi: 10.3945/jn.113.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hong K.W., Ko K.P., Ahn Y., et al. Epidemiological profiles between equol producers and nonproducers: A genomewide association study of the equol-producing phenotype. Genes Nutr. 2012;7(4):567–574. doi: 10.1007/s12263-012-0292-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song K.B., Atkinson C., Frankenfeld C.L., et al. Prevalence of daidzein-metabolizing phenotypes differs between Caucasian and Korean American women and girls. J. Nutr. 2006;136(5):1347–1351. doi: 10.1093/jn/136.5.1347. [DOI] [PubMed] [Google Scholar]
- 78.Atkinson C., Newton K.M., Stanczyk F.Z., Westerlind K.C., Li L., Lampe J.W. Daidzein-metabolizing phenotypes in relation to serum hormones and sex hormone binding globulin, and urinary estrogen metabolites in premenopausal women in the United States. Cancer Causes Control. 2008;19(10):1085–1093. doi: 10.1007/s10552-008-9172-3. [DOI] [PubMed] [Google Scholar]
- 79.Tseng M., Byrne C., Kurzer M.S., Fang C.Y. Equol-producing status, isoflavone intake, and breast density in a sample of U.S. Chinese women. Cancer Epidemiol. Biomarkers Prev. 2013;22(11):1975–1983. doi: 10.1158/1055-9965.EPI-13-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arai Y., Uehara M., Sato Y., et al. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J. Epidemiol. 2000;10(2):127–135. doi: 10.2188/jea.10.127. [DOI] [PubMed] [Google Scholar]
- 81.Morton M.S., Arisaka O., Miyake N., Morgan L.D., Evans B.A. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J. Nutr. 2002;132(10):3168–3171. doi: 10.1093/jn/131.10.3168. [DOI] [PubMed] [Google Scholar]
- 82.Fujimoto K., Tanaka M., Hirao Y., et al. Age-stratified serum levels of isoflavones and proportion of equol producers in Japanese and Korean healthy men. Prostate Cancer Prostatic Dis. 2008;11(3):252–257. doi: 10.1038/sj.pcan.4501030. [DOI] [PubMed] [Google Scholar]
- 83.Cai Y., Guo K., Chen C., et al. Soya isoflavone consumption in relation to carotid intima-media thickness in Chinese equol excretors aged 40-65 years. Br. J. Nutr. 2012;108(9):1698–1704. doi: 10.1017/S0007114511007331. [DOI] [PubMed] [Google Scholar]
- 84.Pusparini Y., Hidayat A. Effect of soy isoflavone supplementation on endothelial dysfunction and oxidative stress in equol-producing postmenopausal women. Endocr. Metab. Immune Disord. Drug Targets. 2015;15(1):71–79. doi: 10.2174/1871530314666141202123309. [DOI] [PubMed] [Google Scholar]
- 85.Liu B., Qin L., Liu A., et al. Prevalence of the equol-producer phenotype and its relationship with dietary isoflavone and serum lipids in healthy Chinese adults. J. Epidemiol. 2010;20(5):377–384. doi: 10.2188/jea.JE20090185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo K., Zhang B., Chen C., et al. Daidzein-metabolising phenotypes in relation to serum lipids and uric acid in adults in Guangzhou, China. Br. J. Nutr. 2010;104(1):118–124. doi: 10.1017/S0007114510000279. [DOI] [PubMed] [Google Scholar]
- 87.Akaza H., Miyanaga N., Takashima N., et al. Comparisons of percent equol producers between prostate cancer patients and controls: Case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn. J. Clin. Oncol. 2004;34(2):86–89. doi: 10.1093/jjco/hyh015. [DOI] [PubMed] [Google Scholar]
- 88.Wu A.H., Yu M.C., Tseng C.C., Twaddle N.C., Doerge D.R. Plasma isoflavone levels versus self-reported soy isoflavone levels in Asian-American women in Los Angeles County. Carcinogenesis. 2004;25(1):77–81. doi: 10.1093/carcin/bgg189. [DOI] [PubMed] [Google Scholar]
- 89.Miyanaga N., Akaza H., Takashima N., et al. Higher consumption of green tea may enhance equol production. Asian Pac. J. Cancer Prev. 2003;4(4):297–301. [PubMed] [Google Scholar]
- 90.Atkinson C., Newton K.M., Bowles E.J., Yong M., Lampe J.W. Demographic, anthropometric, and lifestyle factors and dietary intakes in relation to daidzein-metabolizing phenotypes among premenopausal women in the United States. Am. J. Clin. Nutr. 2008;87(3):679–687. doi: 10.1093/ajcn/87.3.679. [DOI] [PubMed] [Google Scholar]
- 91.Hedlund T.E., Maroni P.D., Ferucci P.G., et al. Long-term dietary habits affect soy isoflavone metabolism and accumulation in prostatic fluid in caucasian men. J. Nutr. 2005;135(6):1400–1406. doi: 10.1093/jn/135.6.1400. [DOI] [PubMed] [Google Scholar]
- 92.Lampe J.W., Skor H.E., Li S., Wahala K., Howald W.N., Chen C. Wheat bran and soy protein feeding do not alter urinary excretion of the isoflavan equol in premenopausal women. J. Nutr. 2001;131(3):740–744. doi: 10.1093/jn/131.3.740. [DOI] [PubMed] [Google Scholar]
- 93.Rowland I.R., Wiseman H., Sanders T.A., Adlercreutz H., Bowey E.A. Interindividual variation in metabolism of soy isoflavones and lignans: Influence of habitual diet on equol production by the gut microflora. Nutr. Cancer. 2000;36(1):27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 94.Frankenfeld C.L., McTiernan A., Tworoger S.S., et al. Serum steroid hormones, sex hormone-binding globulin concentrations, and urinary hydroxylated estrogen metabolites in post-menopausal women in relation to daidzein-metabolizing phenotypes. J. Steroid Biochem. Mol. Biol. 2004;88(4-5):399–408. doi: 10.1016/j.jsbmb.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 95.Lampe J.W., Karr S.C., Hutchins A.M., Slavin J.L. Urinary equol excretion with a soy challenge: Influence of habitual diet. Proc. Soc. Exp. Biol. Med. 1998;217(3):335–339. doi: 10.3181/00379727-217-44241. [DOI] [PubMed] [Google Scholar]
- 96.Lampe J.W., Gustafson D.R., Hutchins A.M., et al. Urinary isoflavonoid and lignan excretion on a Western diet: relation to soy, vegetable, and fruit intake. Cancer Epidemiol. Biomarkers Prev. 1999;8(8):699–707. [PubMed] [Google Scholar]
- 97.Wiseman H., Casey K., Bowey E.A., et al. Influence of 10 wk of soy consumption on plasma concentrations and excretion of isoflavonoids and on gut microflora metabolism in healthy adults. Am. J. Clin. Nutr. 2004;80(3):692–699. doi: 10.1093/ajcn/80.3.692. [DOI] [PubMed] [Google Scholar]
- 98.Tanaka M., Fujimoto K., Chihara Y., et al. Isoflavone supplements stimulated the production of serum equol and decreased the serum dihydrotestosterone levels in healthy male volunteers. Prostate Cancer Prostatic Dis. 2009;12(3):247–252. doi: 10.1038/pcan.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nettleton J.A., Greany K.A., Thomas W., Wangen K.E., Adlercreutz H., Kurzer M.S. Plasma phytoestrogens are not altered by probiotic consumption in postmenopausal women with and without a history of breast cancer. J. Nutr. 2004;134(8):1998–2003. doi: 10.1093/jn/134.8.1998. [DOI] [PubMed] [Google Scholar]
- 100.Bonorden M.J., Greany K.A., Wangen K.E., et al. Consumption of Lactobacillus acidophilus and Bifidobacterium longum do not alter urinary equol excretion and plasma reproductive hormones in premenopausal women. Eur. J. Clin. Nutr. 2004;58(12):1635–1642. doi: 10.1038/sj.ejcn.1602020. [DOI] [PubMed] [Google Scholar]
- 101.Teas J., Hurley T.G., Hebert J.R., Franke A.A., Sepkovic D.W., Kurzer M.S. Dietary seaweed modifies estrogen and phytoestrogen metabolism in healthy postmenopausal women. J. Nutr. 2009;139(5):939–944. doi: 10.3945/jn.108.100834. [DOI] [PubMed] [Google Scholar]
- 102.Finegold J.A., Asaria P., Francis D.P. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int. J. Cardiol. 2013;168(2):934–945. doi: 10.1016/j.ijcard.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Benjamin E.J., Blaha M.J., Chiuve S.E., et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamori Y., Liu L., Ikeda K., Mizushima S., Nara Y., Simpson F.O. Different associations of blood pressure with 24-hour urinary sodium excretion among pre- and post-menopausal women. WHO Cardiovascular Diseases and Alimentary Comparison (WHO-CARDIAC) Study. J. Hypertens. 2001;19(3 Pt 2):535–538. doi: 10.1097/00004872-200103001-00003. [DOI] [PubMed] [Google Scholar]
- 105.Yamori Y. Food factors for atherosclerosis prevention: Asian perspective derived from analyses of worldwide dietary biomarkers. Exp. Clin. Cardiol. 2006;11(2):94–98. [PMC free article] [PubMed] [Google Scholar]
- 106.Ho S.Y., Schooling M., Hui L.L., McGhee S.M., Mak K.H., Lam T.H. Soy consumption and mortality in Hong Kong: Proxy-reported case-control study of all older adult deaths in 1998. Prev. Med. 2006;43(1):20–26. doi: 10.1016/j.ypmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 107.van der Schouw Y.T., Kreijkamp-Kaspers S., Peeters P.H.M., Keinan-Boker L., Rimm E.B., Grobbee D.E. Prospective study on usual dietary phytoestrogen intake and cardiovascular disease risk in western women. Circulation. 2005;111(4):465–471. doi: 10.1161/01.CIR.0000153814.87631.B0. [DOI] [PubMed] [Google Scholar]
- 108.Yu D., Zhang X., Xiang Y.B., et al. Association of soy food intake with risk and biomarkers of coronary heart disease in Chinese men. Int. J. Cardiol. 2014;172(2):e285–e287. doi: 10.1016/j.ijcard.2013.12.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Talaei M., Koh W.P., van Dam R.M., Yuan J.M., Pan A. Dietary soy intake is not associated with risk of cardiovascular disease mortality in Singapore Chinese adults. J. Nutr. 2014;144(6):921–928. doi: 10.3945/jn.114.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Greenland P., Bonow R.O., Brundage B.H., et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: A report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J. Am. Coll. Cardiol. 2007;49(3):378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 111.Bots M.L., Sutton-Tyrrell K. Lessons from the past and promises for the future for carotid intima-media thickness. J. Am. Coll. Cardiol. 2012;60(17):1599–1604. doi: 10.1016/j.jacc.2011.12.061. [DOI] [PubMed] [Google Scholar]
- 112.Naqvi T.Z., Lee M.S. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc. Imaging. 2014;7(10):1025–1038. doi: 10.1016/j.jcmg.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 113.Peters S.A., Bakker M., den Ruijter H.M., Bots M.L. Added value of CAC in risk stratification for cardiovascular events: A systematic review. Eur. J. Clin. Invest. 2012;42(1):110–116. doi: 10.1111/j.1365-2362.2011.02555.x. [DOI] [PubMed] [Google Scholar]
- 114.Goldberger Z.D., Valle J.A., Dandekar V.K., Chan P.S., Ko D.T., Nallamothu B.K. Are changes in carotid intima-media thickness related to risk of nonfatal myocardial infarction? A critical review and meta-regression analysis. Am. Heart J. 2010;160(4):701–714. doi: 10.1016/j.ahj.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 115.Nambi V., Chambless L., Folsom A.R., et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: The ARIC (Atherosclerosis Risk In Communities) study. J. Am. Coll. Cardiol. 2010;55(15):1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Costanzo P., Perrone-Filardi P., Vassallo E., et al. Does carotid intima-media thickness regression predict reduction of cardiovascular events?: A meta-analysis of 41 randomized trials. J. Am. Coll. Cardiol. 2010;56(24):2006–2020. doi: 10.1016/j.jacc.2010.05.059. [DOI] [PubMed] [Google Scholar]
- 117.Lorenz M.W., Markus H.S., Bots M.L., Rosvall M., Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation. 2007;115(4):459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 118.Touboul P.J., Hennerici M.G., Meairs S., et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. . Cerebrovasc Dis (Basel, Switzerland) . 2012;34(4):290–6. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rumberger J.A., Simons D.B., Fitzpatrick L.A., Sheedy P.F., Schwartz R.S. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area: A histopathologic correlative study. Circulation. 1995;92(8):2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 120.Gepner A.D., Young R., Delaney J.A., et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2015;8(1):e002262. doi: 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Folsom A.R., Kronmal R.A., Detrano R.C., et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: The Multi-Ethnic Study of Atherosclerosis (MESA). Arch. Intern. Med. 2008;168(12):1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gepner A.D., Young R., Delaney J.A., et al. Comparison of carotid plaque score and coronary artery calcium score for predicting cardiovascular disease events: The multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. 2017;6(2):e005179. doi: 10.1161/JAHA.116.005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arad Y., Spadaro L.A., Roth M., Newstein D., Guerci A.D. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J. Am. Coll. Cardiol. 2005;46(1):166–172. doi: 10.1016/j.jacc.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 124.Houslay E.S., Cowell S.J., Prescott R.J., et al. Progressive coronary calcification despite intensive lipid-lowering treatment: A randomised controlled trial. Heart. 2006;92(9):1207–1212. doi: 10.1136/hrt.2005.080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Raggi P., Davidson M., Callister T.Q., et al. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering With EBT Scanning (BELLES). Circulation. 2005;112(4):563–571. doi: 10.1161/CIRCULATIONAHA.104.512681. [DOI] [PubMed] [Google Scholar]
- 126.Ohkuma T., Ninomiya T., Tomiyama H., et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: An individual participant data meta-analysis. Hypertension. 2017;69(6):1045–1052. doi: 10.1161/HYPERTENSIONAHA.117.09097. [DOI] [PubMed] [Google Scholar]
- 127.Vlachopoulos C., Aznaouridis K., Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 128.Ben-Shlomo Y., Spears M., Boustred C., et al. Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J. Am. Coll. Cardiol. 2014;63(7):636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cecelja M., Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: A systematic review. Hypertension. 2009;54(6):1328–1336. doi: 10.1161/HYPERTENSIONAHA.109.137653. [DOI] [PubMed] [Google Scholar]
- 130.Zieman S.J., Melenovsky V., Kass D.A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005;25(5):932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 131.Townsend R.R., Wilkinson I.B., Schiffrin E.L., et al. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart Association. Hypertension. 2015;66(3):698–722. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chan Y.H., Lau K.K., Yiu K.H., et al. Isoflavone intake in persons at high risk of cardiovascular events: implications for vascular endothelial function and the carotid atherosclerotic burden. Am. J. Clin. Nutr. 2007;86(4):938–945. doi: 10.1093/ajcn/86.4.938. [DOI] [PubMed] [Google Scholar]
- 133.Zhang B., Chen Y.M., Huang L.L., et al. Greater habitual soyfood consumption is associated with decreased carotid intima-media thickness and better plasma lipids in Chinese middle-aged adults. Atherosclerosis. 2008;198(2):403–411. doi: 10.1016/j.atherosclerosis.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 134.Hodis H.N., Mack W.J. The timing hypothesis and hormone replacement therapy: A paradigm shift in the primary prevention of coronary heart disease in women. Part 2: Comparative risks. J. Am. Geriatr. Soc. 2013;61(6):1011–1018. doi: 10.1111/jgs.12281. [DOI] [PubMed] [Google Scholar]
- 135.Liu Z.M., Ho S.C., Chen Y.M., et al. Whole soy, but not purified daidzein, had a favorable effect on improvement of cardiovascular risks: A 6-month randomized, double-blind, and placebo-controlled trial in equol-producing postmenopausal women. Mol. Nutr. Food Res. 2014;58(4):709–717. doi: 10.1002/mnfr.201300499. [DOI] [PubMed] [Google Scholar]
- 136.Bots M.L., Evans G.W., Riley W.A., Grobbee D.E. Carotid intima-media thickness measurements in intervention studies: Design options, progression rates, and sample size considerations: A point of view. Stroke. 2003;34(12):2985–2994. doi: 10.1161/01.STR.0000102044.27905.B5. [DOI] [PubMed] [Google Scholar]
- 137.Uemura H., Katsuura-Kamano S., Nakamoto M., et al. Inverse association between soy food consumption, especially fermented soy products intake and soy isoflavone, and arterial stiffness in Japanese men. Sci. Rep. 2018;8(1):9667. doi: 10.1038/s41598-018-28038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yoshikata R., Myint K.Z., Ohta H. Relationship between equol producer status and metabolic parameters in 743 Japanese women: Equol producer status is associated with antiatherosclerotic conditions in women around menopause and early postmenopause. Menopause. 2017;24(2):216–224. doi: 10.1097/GME.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 139.Hoshida S., Miki T., Nakagawa T., et al. Different effects of isoflavones on vascular function in premenopausal and postmenopausal smokers and nonsmokers: NYMPH study. Heart Vessels. 2011;26(6):590–595. doi: 10.1007/s00380-010-0103-3. [DOI] [PubMed] [Google Scholar]
- 140.Yoshikata R., Myint K.Z.Y., Ohta H. Effects of equol supplement on bone and cardiovascular parameters in middle-aged japanese women: A prospective observational study. J. Altern. Complement. Med. 2018;24(7):701–708. doi: 10.1089/acm.2018.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Teede H.J., Dalais F.S., Kotsopoulos D., Liang Y-L., Davis S., McGrath B.P. Dietary soy has both beneficial and potentially adverse cardiovascular effects: A placebo-controlled study in men and postmenopausal women. J. Clin. Endocrinol. Metab. 2001;86(7):3053–3060. doi: 10.1210/jcem.86.7.7645. [DOI] [PubMed] [Google Scholar]
- 142.Nestel P.J., Yamashita T., Sasahara T., et al. Soy isoflavones improve systemic arterial compliance but not plasma lipids in menopausal and perimenopausal women. Arterioscler. Thromb. Vasc. Biol. 1997;17(12):3392–3398. doi: 10.1161/01.atv.17.12.3392. [DOI] [PubMed] [Google Scholar]
- 143.Richter C.K., Skulas-Ray A.C., Fleming J.A., et al. Effects of isoflavone-containing soya protein on ex vivo cholesterol efflux, vascular function and blood markers of CVD risk in adults with moderately elevated blood pressure: A dose-response randomised controlled trial. Br. J. Nutr. 2017;117(10):1403–1413. doi: 10.1017/S000711451700143X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu X.X., Li S.H., Chen J.Z., et al. Effect of soy isoflavones on blood pressure: A meta-analysis of randomized controlled trials. Nutr Metab Cardiovas. 2012;22(6):463–470. doi: 10.1016/j.numecd.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 145.Kou T., Wang Q., Cai J., et al. Effect of soybean protein on blood pressure in postmenopausal women: A meta-analysis of randomized controlled trials. Food Funct. 2017;8(8):2663–2671. doi: 10.1039/c6fo01845a. [DOI] [PubMed] [Google Scholar]
- 146.He F.J., Li J., Macgregor G.A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:1325. doi: 10.1136/bmj.f1325. [DOI] [PubMed] [Google Scholar]
- 147.Yang B., Chen Y., Xu T., et al. Systematic review and meta-analysis of soy products consumption in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 2011;20(4):593–602. [PubMed] [Google Scholar]
- 148.Ding M., Pan A., Manson J.E., et al. Consumption of soy foods and isoflavones and risk of type 2 diabetes: a pooled analysis of three US cohorts. Eur. J. Clin. Nutr. 2016;70(12):1381–1387. doi: 10.1038/ejcn.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li W., Ruan W., Peng Y., Wang D. Soy and the risk of type 2 diabetes mellitus: A systematic review and meta-analysis of observational studies. Diabetes Res. Clin. Pract. 2018;137:190–199. doi: 10.1016/j.diabres.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 150.Ko K.P., Kim C.S., Ahn Y., et al. Plasma isoflavone concentration is associated with decreased risk of type 2 diabetes in Korean women but not men: Results from the Korean Genome and Epidemiology Study. Diabetologia. 2015;58(4):726–735. doi: 10.1007/s00125-014-3463-x. [DOI] [PubMed] [Google Scholar]
- 151.Ricci E., Cipriani S., Chiaffarino F., Malvezzi M., Parazzini F. Effects of soy isoflavones and genistein on glucose metabolism in perimenopausal and postmenopausal non-Asian women: A meta-analysis of randomized controlled trials. Menopause. 2010;17(5):1080–1086. doi: 10.1097/gme.0b013e3181dd05a9. [DOI] [PubMed] [Google Scholar]
- 152.Birru R.L., Ahuja V., Vishnu A., et al. The impact of equol-producing status in modifying the effect of soya isoflavones on risk factors for CHD: A systematic review of randomised controlled trials. J. Nutr. Sci. 2016;5:e30. doi: 10.1017/jns.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Badeau R., Jauhiainen M., Metso J., et al. Effect of isolated isoflavone supplementation on ABCA1-dependent cholesterol efflux potential in postmenopausal women. Menopause. 2007;14(2):293–299. doi: 10.1097/01.gme.0000236935.51325.4d. [DOI] [PubMed] [Google Scholar]
- 154.Clerici C., Setchell K.D.R., Battezzati P.M., et al. Pasta naturally enriched with isoflavone aglycons from soy germ reduces serum lipids and improves markers of cardiovascular risk. J. Nutr. 2007;137(10):2270–2278. doi: 10.1093/jn/137.10.2270. [DOI] [PubMed] [Google Scholar]
- 155.Gallagher J.C., Satpathy R., Rafferty K., Haynatzka V. The effect of soy protein isolate on bone metabolism. Menopause. 2004;11(3):290–298. doi: 10.1097/01.gme.0000097845.95550.71. [DOI] [PubMed] [Google Scholar]
- 156.Greany K.A., Nettleton J.A., Wangen K.E., Thomas W., Kurzer M.S. Probiotic consumption does not enhance the cholesterol-lowering effect of soy in postmenopausal women. J. Nutr. 2004;134(12):3277–3283. doi: 10.1093/jn/134.12.3277. [DOI] [PubMed] [Google Scholar]
- 157.Greany K.A., Nettleton J.A., Wangen K.E., Thomas W., Kurzer M.S. Consumption of isoflavone-rich soy protein does not alter homocysteine or markers of inflammation in postmenopausal women. Eur. J. Clin. Nutr. 2008;62(12):1419–1425. doi: 10.1038/sj.ejcn.1602885. [DOI] [PubMed] [Google Scholar]
- 158.Hall W.L., Vafeiadou K., Hallund J., et al. Soy-isoflavone-enriched foods and inflammatory biomarkers of cardiovascular disease risk in postmenopausal women: Interactions with genotype and equol production. Am. J. Clin. Nutr. 2005;82(6):1260–1268. doi: 10.1093/ajcn/82.6.1260. [DOI] [PubMed] [Google Scholar]
- 159.Hallund J., Bugel S., Tholstrup T., et al. Soya isoflavone-enriched cereal bars affect markers of endothelial function in postmenopausal women. Br. J. Nutr. 2006;95(6):1120–1126. doi: 10.1079/bjn20061734. [DOI] [PubMed] [Google Scholar]
- 160.Kreijkamp-Kaspers S., Kok L., Bots M.L., Grobbee D.E., Lampe J.W., van der Schouw Y.T. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am. J. Clin. Nutr. 2005;81(1):189–195. doi: 10.1093/ajcn/81.1.189. [DOI] [PubMed] [Google Scholar]
- 161.Kreijkamp-Kaspers S., Kok L., Grobbee D.E., et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: A randomized controlled trial. JAMA. 2004;292(1):65–74. doi: 10.1001/jama.292.1.65. [DOI] [PubMed] [Google Scholar]
- 162.Ma Y., Chiriboga D., Olendzki B.C., Nicolosi R., Merriam P.A., Ockene I.S. Effect of soy protein containing isoflavones on blood lipids in moderately hypercholesterolemic adults: A randomized controlled trial. J. Am. Coll. Nutr. 2005;24(4):275–285. doi: 10.1080/07315724.2005.10719475. [DOI] [PubMed] [Google Scholar]
- 163.Mangano K.M., Hutchins-Wiese H.L., Kenny A.M., et al. Soy proteins and isoflavones reduce interleukin-6 but not serum lipids in older women: A randomized controlled trial. Nutr. Res. 2013;33(12):1026–1033. doi: 10.1016/j.nutres.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McVeigh B.L., Dillingham B.L., Lampe J.W., Duncan A.M. Effect of soy protein varying in isoflavone content on serum lipids in healthy young men. Am. J. Clin. Nutr. 2006;83(2):244–251. doi: 10.1093/ajcn/83.2.244. [DOI] [PubMed] [Google Scholar]
- 165.Meyer B.J., Larkin T.A., Owen A.J., Astheimer L.B., Tapsell L.C., Howe P.R. Limited lipid-lowering effects of regular consumption of whole soybean foods. Ann. Nutr. Metab. 2004;48(2):67–78. doi: 10.1159/000075592. [DOI] [PubMed] [Google Scholar]
- 166.Nikander E., Tiitinen A., Laitinen K., Tikkanen M., Ylikorkala O. Effects of isolated isoflavonoids on lipids, lipoproteins, insulin sensitivity, and ghrelin in postmenopausal women. J. Clin. Endocrinol. Metab. 2004;89(7):3567–3572. doi: 10.1210/jc.2003-032229. [DOI] [PubMed] [Google Scholar]
- 167.Pipe E.A., Gobert C.P., Capes S.E., Darlington G.A., Lampe J.W., Duncan A.M. Soy protein reduces serum LDL cholesterol and the LDL cholesterol: HDL cholesterol and apolipoprotein B: Apolipoprotein A-I ratios in adults with type 2 diabetes. J. Nutr. 2009;139(9):1700–1706. doi: 10.3945/jn.109.109595. [DOI] [PubMed] [Google Scholar]
- 168.Qin Y., Shu F., Zeng Y., et al. Daidzein supplementation decreases serum triglyceride and uric acid concentrations in hypercholesterolemic adults with the effect on triglycerides being greater in those with the GA compared with the GG genotype of ESR-beta RsaI. J. Nutr. 2014;144(1):49–54. doi: 10.3945/jn.113.182725. [DOI] [PubMed] [Google Scholar]
- 169.Reimann M., Dierkes J., Carlsohn A., et al. Consumption of soy isoflavones does not affect plasma total homocysteine or asymmetric dimethylarginine concentrations in healthy postmenopausal women. J. Nutr. 2006;136(1):100–105. doi: 10.1093/jn/136.1.100. [DOI] [PubMed] [Google Scholar]
- 170.Steinberg F.M., Guthrie N.L., Villablanca A.C., Kumar K., Murray M.J. Soy protein with isoflavones has favorable effects on endothelial function that are independent of lipid and antioxidant effects in healthy postmenopausal women. Am. J. Clin. Nutr. 2003;78(1):123–130. doi: 10.1093/ajcn/78.1.123. [DOI] [PubMed] [Google Scholar]
- 171.Thorp A.A., Howe P.R., Mori T.A., et al. Soy food consumption does not lower LDL cholesterol in either equol or nonequol producers. Am. J. Clin. Nutr. 2008;88(2):298–304. doi: 10.1093/ajcn/88.2.298. [DOI] [PubMed] [Google Scholar]
- 172.Tormala R., Appt S., Clarkson T.B., et al. Equol production capability is associated with favorable vascular function in postmenopausal women using tibolone; no effect with soy supplementation. Atherosclerosis. 2008;198(1):174–178. doi: 10.1016/j.atherosclerosis.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 173.Tormala R., Appt S., Clarkson T.B., et al. Impact of soy supplementation on sex steroids and vascular inflammation markers in postmenopausal women using tibolone: Role of equol production capability. Climacteric. 2008;11(5):409–415. doi: 10.1080/13697130802251344. [DOI] [PubMed] [Google Scholar]
- 174.Tormala R.M., Nikander E., Tiitinen A., Vaisanen-Tommiska M., Ylikorkala O., Mikkola T.S. Serum cholesterol efflux potential in postmenopausal women treated with isolated isoflavones. Menopause. 2006;13(1):96–101. doi: 10.1097/01.gme.0000191210.13115.90. [DOI] [PubMed] [Google Scholar]
- 175.Welty F.K., Lee K.S., Lew N.S., Zhou J. EFfect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch. Intern. Med. 2007;167(10):1060–1067. doi: 10.1001/archinte.167.10.1060. [DOI] [PubMed] [Google Scholar]
- 176.West SG, Hilpert KF, Juturu V, et al. Effects of including soy protein in a blood cholesterol-lowering diet on markers of cardiac risk in men and in postmenopausal women with and without hormone replacement therapy. . J Women's Health . 2005;14(3 ):253–62 . doi: 10.1089/jwh.2005.14.253. [DOI] [PubMed] [Google Scholar]
- 177.Hall W.L., Vafeiadou K., Hallund J., et al. Soy-isoflavone-enriched foods and markers of lipid and glucose metabolism in postmenopausal women: Interactions with genotype and equol production. Am. J. Clin. Nutr. 2006;83(3):592–600. doi: 10.1093/ajcn.83.3.592. [DOI] [PubMed] [Google Scholar]
- 178.Wong W.W., Taylor A.A., Smith E.O., Barnes S., Hachey D.L. Effect of soy isoflavone supplementation on nitric oxide metabolism and blood pressure in menopausal women. Am. J. Clin. Nutr. 2012;95(6):1487–1494. doi: 10.3945/ajcn.111.032045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wolters F.J., Ikram M. Epidemiology of dementia: the burden on society, the challenges for research. In: Perneczky R., editor. Biomarkers for Alzheimer’s Disease Drug Development. New York, NY: Springer New York; 2018. pp. 3–14. [DOI] [PubMed] [Google Scholar]
- 180.Zissimopoulos J., Crimmins E., St Clair P. The value of delaying Alzheimer’s disease onset. Forum Health Econ. Policy. 2014;18(1):25–39. doi: 10.1515/fhep-2014-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chui H.C., Zheng L., Reed B.R., Vinters H.V., Mack W.J. Vascular risk factors and Alzheimer’s disease: Are these risk factors for plaques and tangles or for concomitant vascular pathology that increases the likelihood of dementia? An evidence-based review. Alzheimers Res. Ther. 2012;4(1):1. doi: 10.1186/alzrt98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tan Z.S., Seshadri S., Beiser A., et al. Plasma total cholesterol level as a risk factor for Alzheimer disease: The Framingham Study. Arch. Intern. Med. 2003;163(9):1053–1057. doi: 10.1001/archinte.163.9.1053. [DOI] [PubMed] [Google Scholar]
- 183.Kuller L.H., Lopez O.L., Mackey R.H., et al. Subclinical cardiovascular disease and death, dementia, and coronary heart disease in patients 80+ years. J. Am. Coll. Cardiol. 2016;67(9):1013–1022. doi: 10.1016/j.jacc.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Handy C.E., Desai C.S., Dardari Z.A., et al. The association of coronary artery calcium with noncardiovascular disease: The multi-ethnic study of atherosclerosis. JACC Cardiovasc. Imaging. 2016;9(5):568–576. doi: 10.1016/j.jcmg.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.van Sloten T.T., Protogerou A.D., Henry R.M., Schram M.T., Launer L.J., Stehouwer C.D. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2015;53:121–130. doi: 10.1016/j.neubiorev.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mirahmadi S.M., Shahmohammadi A., Rousta A.M., et al. Soy isoflavone genistein attenuates lipopolysaccharide-induced cognitive impairments in the rat via exerting anti-oxidative and anti-inflammatory effects. Cytokine. 2018;104:151–159. doi: 10.1016/j.cyto.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 187.Bonet-Costa V., Herranz-Perez V., Blanco-Gandia M., et al. Clearing amyloid-beta through ppargamma/apoe activation by genistein is a treatment of experimental Alzheimer’s disease. J. Alzheimers Dis. 2016;51(3):701–711. doi: 10.3233/JAD-151020. [DOI] [PubMed] [Google Scholar]
- 188.Ye S., Wang T.T., Cai B., et al. Genistein protects hippocampal neurons against injury by regulating calcium/calmodulin dependent protein kinase IV protein levels in Alzheimer’s disease model rats. Neural Regen. Res. 2017;12(9):1479–1484. doi: 10.4103/1673-5374.215260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang Y., Cai B., Shao J., et al. Genistein suppresses the mitochondrial apoptotic pathway in hippocampal neurons in rats with Alzheimer’s disease. Neural Regen. Res. 2016;11(7):1153–1158. doi: 10.4103/1673-5374.187056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Newman A.B., Fitzpatrick A.L., Lopez O., et al. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the cardiovascular health study cohort. J. Am. Geriatr. Soc. 2005;53(7):1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- 191.van Oijen M., de Jong F.J., Witteman J.C., Hofman A., Koudstaal P.J., Breteler M.M. Atherosclerosis and risk for dementia. Ann. Neurol. 2007;61(5):403–410. doi: 10.1002/ana.21073. [DOI] [PubMed] [Google Scholar]
- 192.Wendell C.R., Zonderman A.B., Metter E.J., Najjar S.S., Waldstein S.R. Carotid intimal medial thickness predicts cognitive decline among adults without clinical vascular disease. Stroke. 2009;40(10):3180–3185. doi: 10.1161/STROKEAHA.109.557280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Romero J.R., Beiser A., Seshadri S., et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: The Framingham study. Stroke. 2009;40(5):1590–1596. doi: 10.1161/STROKEAHA.108.535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sander K., Bickel H., Forstl H., et al. Carotid- intima media thickness is independently associated with cognitive decline. The INVADE study. Int. J. Geriatr. Psychiatry. 2010;25(4):389–394. doi: 10.1002/gps.2351. [DOI] [PubMed] [Google Scholar]
- 195.Arntzen K.A., Schirmer H., Johnsen S.H., Wilsgaard T., Mathiesen E.B. Carotid atherosclerosis predicts lower cognitive test results: A 7-year follow-up study of 4,371 stroke-free subjects - the Tromso study. Cerebrovasc. Dis. 2012;33(2):159–165. doi: 10.1159/000334182. [DOI] [PubMed] [Google Scholar]
- 196.Zhong W., Cruickshanks K.J., Schubert C.R., et al. Carotid atherosclerosis and 10-year changes in cognitive function. Atherosclerosis. 2012;224(2):506–510. doi: 10.1016/j.atherosclerosis.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Moon J.H., Lim S., Han J.W., et al. Carotid intima-media thickness is associated with the progression of cognitive impairment in older adults. Stroke. 2015;46(4):1024–1030. doi: 10.1161/STROKEAHA.114.008170. [DOI] [PubMed] [Google Scholar]
- 198.Zeki Al Hazzouri A., Vittinghoff E., Sidney S., Reis J.P., Jacobs D.R., Jr, Yaffe K. Intima-media thickness and cognitive function in stroke-free middle-aged adults: findings from the coronary artery risk development in young adults study. Stroke. 2015;46(8):2190–2196. doi: 10.1161/STROKEAHA.115.008994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Cardiovacular Epidemiology Site - epi-c.jp (in Japanese): LIfe Science Publishing Co., Ltd.; http://www.epi-c.jp/
- 200.Knopman D., Boland L.L., Mosley T., et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56(1):42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 201.Knopman D.S., Mosley T.H., Catellier D.J., Coker L.H. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: The ARIC MRI Study. Alzheimers Dement. 2009;5(3):207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 202.Schott L.L., Wildman R.P., Brockwell S., Simkin-Silverman L.R., Kuller L.H., Sutton-Tyrrell K. Segment-specific effects of cardiovascular risk factors on carotid artery intima-medial thickness in women at midlife. Arterioscler. Thromb. Vasc. Biol. 2004;24(10):1951–1956. doi: 10.1161/01.ATV.0000141119.02205.6b. [DOI] [PubMed] [Google Scholar]
- 203.O’Leary D.H., Polak J.F., Kronmal R.A., et al. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke. 1992;23(12):1752–1760. doi: 10.1161/01.str.23.12.1752. [DOI] [PubMed] [Google Scholar]
- 204.Fujiyoshi A., Jacobs D.R., Jr, Fitzpatrick A.L., et al. Coronary artery calcium and risk of dementia in MESA (Multi-Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging. 2017;10(5):e005349. doi: 10.1161/CIRCIMAGING.116.005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Vidal J.S., Sigurdsson S., Jonsdottir M.K., et al. Coronary artery calcium, brain function and structure: The AGES-Reykjavik Study. Stroke. 2010;41(5):891–897. doi: 10.1161/STROKEAHA.110.579581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cui C., Sekikawa A., Kuller L., et al. Aortic stiffness is associated with increased risk of incident dementia in older adults. J. Alzheimers Dis. 2018;66(1):297–306. doi: 10.3233/JAD-180449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pase M.P., Beiser A., Himali J.J., et al. Aortic stiffness and the risk of incident mild cognitive impairment and dementia. Stroke. 2016;47(9):2256–2261. doi: 10.1161/STROKEAHA.116.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Poels M.M., van Oijen M., Mattace-Raso F.U., et al. Arterial stiffness, cognitive decline, and risk of dementia: The Rotterdam study. Stroke. 2007;38(3):888–892. doi: 10.1161/01.STR.0000257998.33768.87. [DOI] [PubMed] [Google Scholar]
- 209.Waldstein S.R., Rice S.C., Thayer J.F., Najjar S.S., Scuteri A., Zonderman A.B. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51(1):99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 210.Zeki Al Hazzouri A., Newman A.B., Simonsick E., et al. Pulse wave velocity and cognitive decline in elders: The Health, Aging, and Body Composition study. Stroke. 2013;44(2):388–393. doi: 10.1161/STROKEAHA.112.673533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Taniguchi Y., Fujiwara Y., Nofuji Y., et al. Prospective study of arterial stiffness and subsequent cognitive decline among community-dwelling older Japanese. J. Epidemiol. 2015;25(9):592–599. doi: 10.2188/jea.JE20140250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Scuteri A., Nilsson P.M., Tzourio C., Redon J., Laurent S. Microvascular brain damage with aging and hypertension: pathophysiological consideration and clinical implications. J. Hypertens. 2011;29(8):1469–1477. doi: 10.1097/HJH.0b013e328347cc17. [DOI] [PubMed] [Google Scholar]
- 213.O’Rourke M.F., Safar M.E. Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension. 2005;46(1):200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 214.Tsao C.W., Himali J.J., Beiser A.S., et al. Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology. 2016;86(7):619–626. doi: 10.1212/WNL.0000000000002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hughes T.M., Kuller L.H., Barinas-Mitchell E.J., et al. Pulse wave velocity is associated with beta-amyloid deposition in the brains of very elderly adults. Neurology. 2013;81(19):1711–1718. doi: 10.1212/01.wnl.0000435301.64776.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nation D.A., Edland S.D., Bondi M.W., et al. Pulse pressure is associated with Alzheimer biomarkers in cognitively normal older adults. Neurology. 2013;81(23):2024–2027. doi: 10.1212/01.wnl.0000436935.47657.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nakamoto M., Otsuka R., Nishita Y., et al. Soy food and isoflavone intake reduces the risk of cognitive impairment in elderly Japanese women. Eur. J. Clin. Nutr. 2018;72(10):1458–1462. doi: 10.1038/s41430-017-0061-2. [DOI] [PubMed] [Google Scholar]
- 218.Ozawa M., Ninomiya T., Ohara T., et al. Dietary patterns and risk of dementia in an elderly Japanese population: The Hisayama Study. Am. J. Clin. Nutr. 2013;97(5):1076–1082. doi: 10.3945/ajcn.112.045575. [DOI] [PubMed] [Google Scholar]
- 219.Chan R., Chan D., Woo J. A cross sectional study to examine the association between dietary patterns and cognitive impairment in older Chinese people in Hong Kong. J. Nutr. Health Aging. 2013;17(9):757–765. doi: 10.1007/s12603-013-0348-5. [DOI] [PubMed] [Google Scholar]
- 220.Okubo H., Inagaki H., Gondo Y., et al. Association between dietary patterns and cognitive function among 70-year-old Japanese elderly: A cross-sectional analysis of the SONIC study. Nutr. J. 2017;16(1):56. doi: 10.1186/s12937-017-0273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Thorp A.A., Sinn N., Buckley J.D., Coates A.M., Howe P.R. Soya isoflavone supplementation enhances spatial working memory in men. Br. J. Nutr. 2009;102(9):1348–1354. doi: 10.1017/S0007114509990201. [DOI] [PubMed] [Google Scholar]
- 222.File S., Jarrett N., Fluck E., Duffy R., Casey K., Wiseman H. Eating soya improves human memory. Psychopharmacology (Berl.) 2001;157(4):430–436. doi: 10.1007/s002130100845. [DOI] [PubMed] [Google Scholar]
- 223.Kelly GE, Joannou GE, Reeder AY, Nelson C, Waring MA. The variable metabolic response to dietary isoflavones in humans. . Proc Soc Exp Biol Med (New York, NY) 1995;208 (1):40–3 . doi: 10.3181/00379727-208-43829. [DOI] [PubMed] [Google Scholar]
- 224.Igase M., Igase K., Tabara Y., Ohyagi Y., Kohara K. Cross-sectional study of equol producer status and cognitive impairment in older adults. Geriatr. Gerontol. Int. 2017;17(11):2103–2108. doi: 10.1111/ggi.13029. [DOI] [PubMed] [Google Scholar]
- 225.Henderson V.W., St John J.A., Hodis H.N., et al. Long-term soy isoflavone supplementation and cognition in women: A randomized, controlled trial. Neurology. 2012;78(23):1841–1848. doi: 10.1212/WNL.0b013e318258f822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gleason C.E., Carlsson C.M., Barnet J.H., et al. A preliminary study of the safety, feasibility and cognitive efficacy of soy isoflavone supplements in older men and women. Age Ageing. 2009;38(1):86–93. doi: 10.1093/ageing/afn227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gleason C.E., Fischer B.L., Dowling N.M., et al. Cognitive Effects of Soy Isoflavones in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2015;47(4):1009–1019. doi: 10.3233/JAD-142958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Vazquez L., Guadamuro L., Giganto F., Mayo B., Florez A.B. Development and use of a real-time quantitative pcr method for detecting and quantifying equol-producing bacteria in human faecal samples and slurry cultures. Front. Microbiol. 2017;8:1155. doi: 10.3389/fmicb.2017.01155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Akaza H., Miyanaga N., Takashima N., et al. Is daidzein non-metabolizer a high risk for prostate cancer? A case-controlled study of serum soybean isoflavone concentration. Jpn. J. Clin. Oncol. 2002;32(8):296–300. doi: 10.1093/jjco/hyf064. [DOI] [PubMed] [Google Scholar]
- 230.Ozasa K., Nakao M., Watanabe Y., et al. Serum phytoestrogens and prostate cancer risk in a nested case-control study among Japanese men. Cancer Sci. 2004;95(1):65–71. doi: 10.1111/j.1349-7006.2004.tb03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kurahashi N., Iwasaki M., Inoue M., Sasazuki S., Tsugane S. Plasma isoflavones and subsequent risk of prostate cancer in a nested case-control study: The Japan Public Health Center. J. Clin. Oncol. 2008;26(36):5923–5929. doi: 10.1200/JCO.2008.16.8807. [DOI] [PubMed] [Google Scholar]
- 232.Kunisue T., Tanabe S., Isobe T., Aldous K.M., Kannan K. Profiles of phytoestrogens in human urine from several Asian countries. J. Agric. Food Chem. 2010;58(17):9838–9846. doi: 10.1021/jf102253j. [DOI] [PubMed] [Google Scholar]
- 233.Shimazu T., Inoue M., Sasazuki S., et al. Plasma isoflavones and the risk of lung cancer in women: a nested case-control study in Japan. Cancer Epidemiol. Biomarkers Prev. 2011;20(3):419–427. doi: 10.1158/1055-9965.EPI-10-1025. [DOI] [PubMed] [Google Scholar]
- 234.Miyanaga N., Akaza H., Hinotsu S., et al. Prostate cancer chemoprevention study: An investigative randomized control study using purified isoflavones in men with rising prostate-specific antigen. Cancer Sci. 2012;103(1):125–130. doi: 10.1111/j.1349-7006.2011.02120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hozawa A., Sugawara Y., Tomata Y., et al. Relationship between serum isoflavone levels and disability-free survival among community-dwelling elderly individuals: Nested case-control study of the Tsurugaya project. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68(4):465–472. doi: 10.1093/gerona/gls198. [DOI] [PubMed] [Google Scholar]
- 236.Michikawa T., Inoue M., Sawada N., et al. Plasma isoflavones and risk of primary liver cancer in Japanese women and men with hepatitis virus infection: A nested case-control study. Cancer Epidemiol. Biomarkers Prev. 2015;24(3):532–537. doi: 10.1158/1055-9965.EPI-14-1118. [DOI] [PubMed] [Google Scholar]
- 237.Takeda T., Ueno T., Uchiyama S., Hiramatsu K., Shiina M. Relation between premenstrual syndrome and equol-production status. J. Obstet. Gynaecol. Res. 2016;42(11):1575–1580. doi: 10.1111/jog.13073. [DOI] [PubMed] [Google Scholar]
- 238.Liu Z.M., Ho S.C., Chen Y.M., Liu J., Woo J. Cardiovascular risks in relation to daidzein metabolizing phenotypes among Chinese postmenopausal women. PLoS One. 2014;9(2):e87861. doi: 10.1371/journal.pone.0087861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Nagata Y., Sugiyama Y., Fukuta F., et al. Relationship of serum levels and dietary intake of isoflavone, and the novel bacterium Slackia sp. strain NATTS with the risk of prostate cancer: A case-control study among Japanese men. Int. Urol. Nephrol. 2016;48(9):1453–1460. doi: 10.1007/s11255-016-1335-7. [DOI] [PubMed] [Google Scholar]
- 240.Grace P.B., Taylor J.I., Low Y.L., et al. Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European prospective investigation of cancer and nutrition-norfolk. Cancer Epidemiol. Biomarkers Prev. 2004;13(5):698–708. [PubMed] [Google Scholar]
- 241.Peeters P.H., Slimani N., van der Schouw Y.T., et al. Variations in plasma phytoestrogen concentrations in European adults. J. Nutr. 2007;137(5):1294–1300. doi: 10.1093/jn/137.5.1294. [DOI] [PubMed] [Google Scholar]
- 242.Newton K.M., Reed S.D., Uchiyama S., et al. A cross-sectional study of equol producer status and self-reported vasomotor symptoms. Menopause. 2015;22(5):489–495. doi: 10.1097/GME.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 243.Reverri E.J., LaSalle C.D., Franke A.A., Steinberg F.M. Soy provides modest benefits on endothelial function without affecting inflammatory biomarkers in adults at cardiometabolic risk. Mol. Nutr. Food Res. 2015;59(2):323–333. doi: 10.1002/mnfr.201400270. [DOI] [PMC free article] [PubMed] [Google Scholar]