Abstract
Percutaneous mitral valve repair is emerging as a reasonable alternative especially in those with an unfavorable surgical risk profile in the repair of mitral regurgitation. At this time, our understanding of the effects of underlying renal dysfunction on outcomes with percutaneous mitral valve repair and the effects of this procedure itself on renal function is evolving, as more data emerges in this field. The current evidence suggests that the correction of mitral regurgitation via percutaneous mitral valve repair is associated with some degree of improvement in cardiac function, hemodynamics and renal function. The improvement in renal function was more significant for those with greater renal dysfunction at baseline. The presence of Chronic Kidney Disease (CKD) in turn has been associated with poor long-term outcomes including increased mortality and hospitalization among patients who undergo percutaneous mitral valve repair. This was true regardless of the degree of improvement in GFR post repair advanced CKD. The adverse impact of CKD on long-term outcomes was consistent across all studies and was more prominent in those with GFR<30 mL/min/1.73 m2. It is clear that from these contrasting evidences of improved renal function post mitral valve repair but poor long-term outcomes including increased mortality in patients with CKD, that proper patient selection for percutaneous mitral valve repair is key. There is a need to have better-standardized criteria for patients who should qualify to have percutaneous mitral valve replacement with Mitraclip. In this new era of percutaneous mitral valve repair, much work needs to be done to optimize long-term patient outcomes.
Keywords: Percutaneous mitral repair, renal function, review, mitraclip, chronic kidney disease, patient outcomes
1. Introduction
Mitral valve surgery is indicated in patients with severe mitral regurgitation(MR), either due to degenerative valve disease or dilated cardiomyopathy, in order to decrease left ventricular end systolic volume index, left ventricular end diastolic pressure and improve cardiac index [1]. However, in those with an unfavorable surgical risk profile, percutaneous mitral repair is emerging as a reasonable alternative [2]. The Endovascular Valve Edge-to-Edge Repair Study (EVEREST II) randomized trial showed that while the percutaneous MitraClip device (MC; Abbott Vascular, Menlo Park, CA, USA) was less effective than surgery in reducing volume or grade of MR, there were fewer peri-operative adverse events and similar improvement in functional status [3]. Predictors of poor outcomes after percutaneous mitral valve repair include persistent MR post repair, N-terminal pro-brain natriuretic peptide ≥5000 ng/L at baseline, Chronic Obstructive Pulmonary Disease (COPD), history of coronary bypass surgery, history of Congestive Heart Failure (CHF), presence of moderate to severe Tricuspid Regurgitation (TR) and Chronic Kidney Disease (CKD); all of which have been associated with poor long-term outcomes including increased mortality and hospitalization [4-7]. The presence of CKD is a predictor of worse outcomes with Acute Coronary Syndromes (ACS), CHF, and cardiac interventions such as Transcatheter Aortic Valve Replacement (TAVR) [8]. At this time, our understanding of the effects of underlying renal dysfunction on outcomes with percutaneous mitral valve repair and the effects of this procedure on renal function is evolving, as more data emerges in this field. This review summarizes the literature on the effects of underlying CKD on post percutaneous mitral valve repair outcomes, as well as the effects of this procedure on subsequent renal function. While several percutaneous mitral valve repair techniques are in evolution in the US, the MitraClip device which is an edge-to-edge leaflet repair device is the only one approved by the FDA, and therefore further discussion in this review will be dedicated to the MitraClip device system.
2. Mitral regurgitation pathophysiology and percutaneous repair
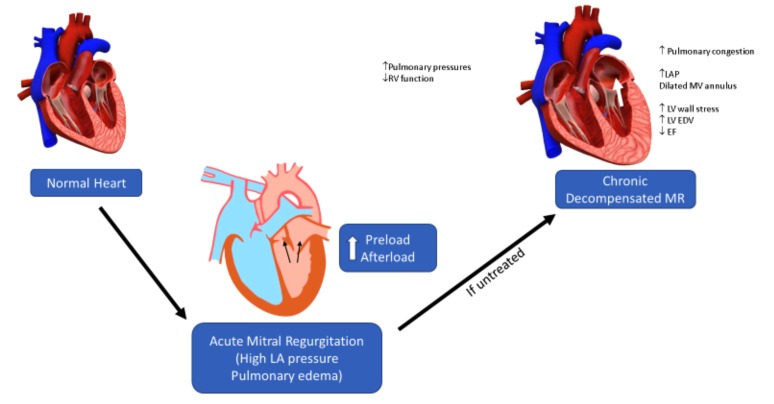
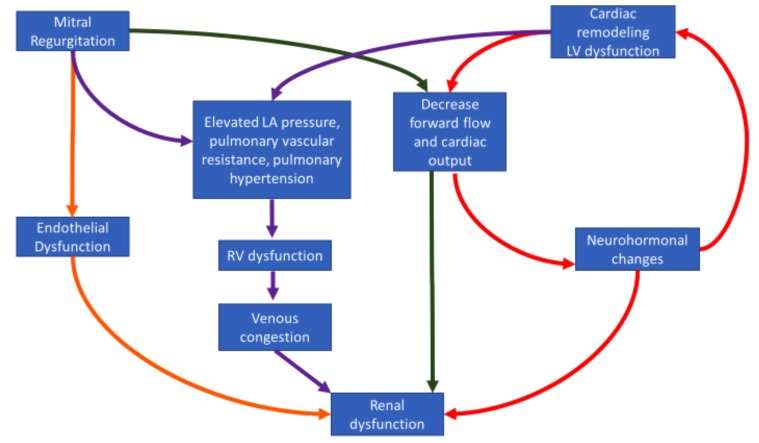
Acute MR results in alteration of both preload and afterload in an attempt to maintain cardiac output. With time, if uncorrected, progression to chronic MR may result in the Left Ventricle [LV] hypertrophies eccentrically as a means to physiologically adapt to normalize preload on individual cardiac sarcomeres [9-11]. With increasing LV chamber enlargement, an alteration in the LV thickness to radius ratio develops which results in LV afterload excess. This afterload excess results in decreased myocardial shortening as compensated chronic MR progresses into decompensated MR. This eventually culminates in decreased stroke volume, as evidenced by invasive hemodynamic measurements in recent studies [12]. The increasing afterload coupled with the reduction in ejection fraction can cause significant alteration of renal function in lieu of decreased renal perfusion from the maladaptive responses intrinsic to decompensated cardio-renal syndrome [13] (Fig. 1). Conversely, CKD can accentuate the degree of mitral regurgitation from its effect on left ventricular hypertrophy secondary to pressure and volume overload with deleterious effects [14]. Please see Figs. (1 and 2).
Fig. (1).
Ventricular remodeling in chronic mitral regurgitation.
Fig. (2).
Pathophysiology of renal dysfunction and mitral regurgitation.
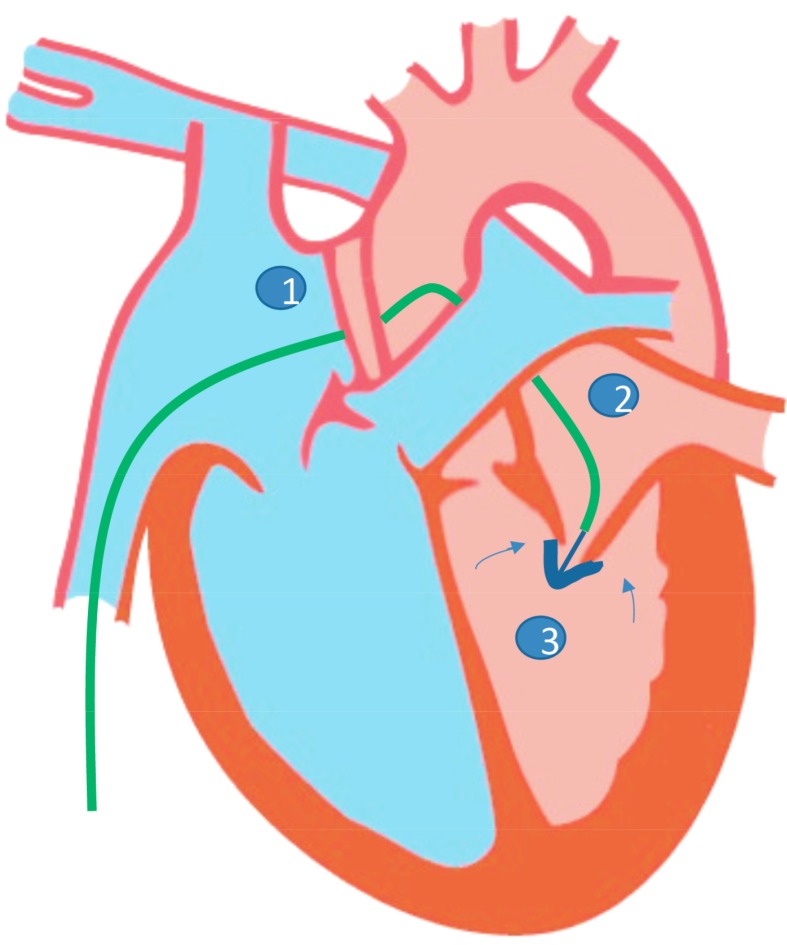
Based on echocardiographic and angio-invasive parameters, different stages of MR have been recognized. Symptoms and treatment options vary depending on the stage, which is beyond the scope of this discussion. However, it is important to note that treatment is specific to the etiology and stage of MR, surgery being a definitive means of therapy to those who merit intervention [15, 16]. However, in patients with multiple comorbidities and with prohibitive surgical risk, transcatheter techniques, particularly MitraClip have been demonstrated to be safe and feasible in multiple studies worldwide. The use of the MitraClip system is performed under general anesthesia using fluoroscopy with both transesophageal and transthoracic echocardiographic guidance. After trans-septal puncture, a guidewire is passed into the left atrium and subsequently the guide catheter. The clip delivery system is introduced into the guide catheter, advanced into the left atrial chamber until it is centered over the mitral orifice. The arms of the clip are opened and advanced into the left ventricle just below the mitral leaflet edges. The clip is closed and pulled back until the mitral leaflets are captured in the arms of the clip. The clip is closed incrementally under real-time echocardiography to optimize the reduction of MR [17] see Fig. (3) for a sample illustration. Current guidelines recommend the use of transcatheter mitral valve repair for severely symptomatic patients (NYHA class III to IV) with chronic severe primary MR (stage D) who have favorable anatomy for the repair procedure and a reasonable life expectancy but who have a prohibitive surgical risk because of severe comorbidities and remain severely symptomatic despite optimal guideline-directed medical treatment for heart failure. As of now, it is not currently approved for chronic secondary MR in the US [15].
Fig. (3).
Conceptual illustration of the mitraclip intervention.
3. Percutaneous mitral valve repair and its effects on renal function
Percutaneous mitral valve repair may be associated with improvement in renal function as shown as proof of concept in small studies. In a study by Rassaf et al. involving 60 patients who underwent MitraClip repair, successful reduction in mitral regurgitation (MR) severity by 2-3 grades, acutely improved renal function [13]. This effect was noted to be more pronounced among those with greater renal dysfunction at baseline. This was quantified as an improvement in the Kidney Disease Outcomes Quality Initiative (KDOQI) stage seen in 20% of patients with preexisting KDOQI stage 3-4 vs. 13% improvement among those with baseline KDOQI stage of 1-2 (p<0.0001) [13]. Subsequently, Wang et al. performed a larger study, analyzing patients with severe MR (grade 3-4) who had CKD at baseline in order to assess the effect of Mitraclip repair on eGFR. The sample population was selected from studies such as EVEREST and the Real World Expanded Multicenter Study of the MitraClip system (REALISM) which consisted of a total of 767 patients analyzed between 61 to 85 years of age, CKD stages 1-5 (excluded those under renal replacement therapy), with MR severity grade 3-4, who underwent MitraClip repair, and were followed for 1 year to assess renal function [18]. In this study, for all stages of CKD, there was a modest improvement in kidney function before discharge. Also, among patients with CKD stages 3-5 who had a reduction of MR by > 2 grades, glomerular filtration rate (GFR) significantly improved at 1 year after mitral valve repair. This is in contrast to patients with CKD stages 1-2 who sometimes exhibited a slight decrease in GFR. Over 36% of patients initially with CKD stage 4-5 improved to stage 3 in one year after MitraClip repair. This study also showed that baseline renal dysfunction correlated with one-year mortality post valve repair, irrespective of the degree of improvement in GFR [18]. Furthermore, an analysis by Kaneko et al. of 78 patients in Germany revealed that 28% of patients had improvement in renal function six months after MitraClip repair and was associated with a significantly higher survival rate [19]. This study, however, did not quantify the effect of baseline GFR/stage of CKD on the subsequent improvement. A recent follow-up analysis of the EVEREST and REALISM cohorts was performed which assessed increase in the forward stroke volume (FSV) as a predictor of clinical outcomes among patients who underwent MitraClip repair. It revealed that among those with >9% increase in forward stroke volume upon discharge, there was a significant improvement in GFR after one year accompanied by a decrease in all-cause mortality after three years [20]. Again, this analysis was not aimed at studying the influence of the baseline stage of CKD on outcomes. However, it demonstrated that the severity of MR at baseline was proportional to the degree of improvement in FSV post-Mitraclip, which in turn corresponded to an improvement in renal function after repair.
4. Underlying mechanisms in the improvement in renal function post mitral valve repair
There are three prevailing possible mechanisms that may explain the improvement of renal function post mitral valve repair. First, MR is associated with characteristic hemodynamic abnormalities, namely increase in left atrial pressures, pulmonary vascular resistance and decrease in cardiac output; all of which lead to the development of worsening heart failure [21]. Increasing right-sided pressures in the long term eventually lead to venous congestion. These hemodynamic changes, specifically venous congestion and decreased cardiac output, leads to renal dysfunction as part of cardiorenal syndrome [22] (Fig. 2). Percutaneous mitral valve repair was associated with acute improvement of these hemodynamic parameters, especially in patients with decompensated heart failure, both by increasing forward cardiac output and decreasing pulmonary and right-sided pressures which in turn can lead to better renal outcomes [20, 23, 24]. These effects on hemodynamics post mitral valve repair are also consistent with the ones found in surgical mitral valve replacement [25]. However, there has been no reported improvement in renal function post-surgical mitral valve repair and on the contrary, surgical mitral valve replacement has been associated with increased incidence of Acute Kidney Injury (AKI) with an associated increase in mortality [26-28]. This was true not only in the context of in-hospital mortality, but also long-term mortality, irrespective of recovery of renal function after the perioperative period. Mechanisms implicated in the pathogenesis of surgical post mitral valve repair associated AKI include the effects of cardiopulmonary bypass, post-operative instability and low cardiac output [28].
A second potential mechanism is the effect of percutaneous mitral valve repair on left ventricular remodeling. In two separate analyses of the EVEREST cohort, there was an observed reduction in Left Ventricular End Diastolic Volume (LVEDV), improvement in Ejection Fraction (EF) and reduced sphericity after MitraClip, especially among patients with preexisting LV dysfunction [29]. Also, there was the same observed decreased in LVEDV and LA volumes for both functional and degenerative MR suggestive of correction of volume overload post-MitraClip [30]. Similar changes in ventricular contractility and function were also observed with surgical mitral valve repair [31, 32]. In one study using three-dimensional speckle tracking echocardiography, there was an improvement of left ventricular global longitudinal strain, as well as right ventricular free wall strain, in as early as 6 months post-MitraClip implantation [33]. Reversal of cardiac remodeling thus may potentially lead to improvement of residual kidney function among this subset of patients by improving long-term hemodynamics and pump function.
Finally, the endothelial function also plays a role in affecting renal function as a consequence of MR. Experimental animal studies have demonstrated the association between worsening mitral regurgitation and endothelial dysfunction [34]. Endothelial dysfunction has long been established as a pathophysiologic mechanism leading to premature cardiovascular disease and worsening renal function [35, 36]. A recent study involving patients who underwent percutaneous MitraClip intervention found evidence of endothelial dysfunction in the form of elevated levels of decompartmentalized hemoglobin which scavenge on nitric oxide, leading to endothelial dysfunction in patients with chronic MR. At three months post mitral valve repair, these levels were found to decrease, and endothelial function improved, which manifested as an increase in flow-mediated vasodilation [37]. This links endothelial dysfunction to MR and its potential effects on end organs like the kidney.
5. Acute Kidney Injury in the setting of mitral valve repair
A recent single-center analysis of 378 patients by Körber et al. 2018 sought to determine the incidence of Acute Kidney Injury (AKI) post percutaneous mitral valve repair and surgical mitral valve repair. They found that there was a significantly lower risk of AKI following percutaneous mitral valve repair compared to surgical mitral valve repair [38]. However, the lack of availability of data on baseline GFR and the degree of mitral regurgitation prior to repair could have influenced the outcomes. Length of procedure and peripheral vascular disease were determined to be predictors of subsequent post-procedural AKI [38]. This study is consistent with prior studies showing the risk of AKI following mitral valve repair and aortic valve repair [39, 40]. However, this was the first study to look into the incidence of AKI post percutaneous mitral valve repair [38]. Recently, another retrospective study of 220 patients who underwent percutaneous mitral valve repair reported the incidence of post-procedural AKI to be approximately 20%. Over 65% of the study population had at least stage 3 CKD. Age, log EuroSCORE, baseline renal function, proBNP levels, serum Hba1c, serum C-reactive protein, diuretic usage and elevated right atrial pressure were the risk factors for post-procedural AKI. The influence of baseline renal function was strong, with 75% of the patients developing AKI having stage 3 CKD stage 3 or worse degrees of baseline GFR before the procedure [41]. The study, however, did not look at the effect of residual MR post repair and the influence of CKD stage on subsequent post procedural AKI, and long-term renal function. Finally, AKI post procedure was associated with poor long-term outcomes such as higher risk of long-term mortality [38, 41]. This is consistent with previous studies showing AKI as a predictor of subsequent mortality in percutaneous coronary interventions and aortic valvular replacements [40, 42]. These data provide snapshots of the incidence and effects of AKI after percutaneous mitral valve replacement, and more prospective large-scale studies are needed to accurately outline the risk factors and modifiable targets for interventions to reduce the burden of AKI with percutaneous mitral valve repairs.
6. Chronic Kidney Disease as a Predictor of Poor Outcomes Post Mitral Valve Repair
The presence of CKD at baseline in patients undergoing mitral valve repair has an adverse impact on long-term outcomes, regardless of the degree of improvement in GFR post repair [43-45]. The strong association between heart failure and CKD is well known, and conceivably the presence of CKD in heart failure was also found to be associated with increased mortality [46]. Heart failure is associated with worsening renal function via several cardiorenal hemodynamic mechanisms [47] and manifests as varying degrees of functional mitral regurgitation brought about by LV remodeling [1].
CKD is associated with adverse outcomes in post-surgical patients and is particularly problematic among patients undergoing cardiac surgery. In a study among patients undergoing Coronary Artery Bypass Graft (CABG) in the Society of Thoracic Surgeons (STS) registry, operative mortality and other complications increased with higher degrees of CKD [48]. The same association between the degree of CKD and adverse outcomes was noted in patients undergoing TAVR [49]. Eventually, this was found to be applicable to patients undergoing mitral valve repair as well, and baseline CKD was implicated in predicting poor outcomes [50, 51]. Addressing long-term outcomes in relation to CKD specific to volume status, a retrospective study involving patients with advanced heart failure and functional MR who underwent surgical mitral valve repair, found that patients with End-Stage Renal Disease (ESRD) on hemodialysis had more favorable outcomes in terms of mortality and readmissions for heart failure exacerbations compared to those with late-stage CKD, not on hemodialysis. This difference in outcomes was attributed to the ability to control volume status more accurately in patients on hemodialysis after surgery, and potentially yielding better outcomes [1].
One of the first studies to provide data on the influence of CKD on outcomes in percutaneous mitral valve repair was a small study involving 41 patients which showed that renal function and hospital stay were inversely related [52]. Another retrospective study analyzing data from 74 patients who had mitral valve repair using MitraClip, found that the presence of CKD was associated with increased mortality (HR 3.29) (CI 1.37 to 7.92) p<0.01 [5]. However, this study was limited by its ability to differentiate outcomes based on the stage of underlying CKD. To address this question, an analysis of a multicenter MitraClip registry involving 173 patients was undertaken which showed that those patients with advanced CKD [GFR<30 cc/min/1.73M2] had an increased rate of mortality when compared to patients with GFR>30 mL/min/1.73 m2 [HR=10, p=0.011]. It was also noted there was no difference in mortality between those with moderate CKD [GFR>30 but <60 cc/min/1.73M2] and with no CKD at all [53]. There was also a trend towards improvement in NYHA functional class among those with CKD, although insignificant, which progressively narrowed down with advancement in CKD stage. However, this study demonstrates a degree of selection bias, as there was a higher proportion of patients with GFR<30 cc/min/1.73M2 with symptom burden of NYHA class II or higher that were included in the study. In the same year, an analysis of the EVEREST cohort, one of the largest studies using the MitraClip device was performed involving over 886 patients. The results of this study revealed that the degree of underlying CKD was independently associated with long-term mortality [HR 3.8, CI: 2.38-6.05] for CKD stages 4-5 (GFR<30 mL/min/1.73 m2) whereas patients with GFR 30-60 mL/min/1.73 m2 had a lower but still significant risk for mortality [HR 2.35, CI: 1.73-3.19] [18]. This is in comparison to a later analysis done by Kaneko et al. in 2016 using a different cohort of 206 patients which demonstrated an increased mortality for those with GFR<30 mL/min/1.73 m2 [HR 4.322, CI: 1.855–10.069, p=0.001] and only a trend towards significance for those with GFR 30-60 mL/min/1.73 m2 [HR 1.868, CI: 0.949–3.679, p=0.071], with the latter likely underpowered to assess mortality among this subset of patients [19]. Subsequent to this study, an analysis of the Getting Reduction of Mitral Insufficiency by Percutaneous Clip Implantation (GRASP) registry using a composite outcome of freedom from death, requirement of surgery for mitral valve dysfunction and having an MR grade >=3 at 12 months (primary efficacy endpoint) was found to be significantly lower in patients with underlying CKD [HR 2.39, CI: 1.19-4.78; p=0.014]. CKD was noted to be an independent predictor of this composite endpoint, whereas calcified leaflets could reliably predict >3+ MR at 12 months [6]. Lastly, a recent abstract presentation by Binita Shah et al. 2018 at the ACC 2018 conference retrospectively studied 5,241 patients in the Society of Thoracic Surgeons/American College of Cardiology (STS/ACC) registry with the primary outcome of a composite of all-cause mortality, stroke and new dialysis. It was found that among male patients with creatinine clearance <=30ml/min, there was a significantly higher composite outcome [OR 2.72, CI 1.26-5.87; p=0.01], and in females, there was an incremental effect observed where the lower the creatinine clearance, the higher the odds for the composite outcome ranging from [OR 6.11, CI 2.13-17.6; p<0.001] for those with creatinine clearance 30-60mL/min to as high as [OR 16.8, CI 4.05-69.6; p<0.001] for those on dialysis [54]. Separate odds ratios were also calculated for 30-day and 1-year mortality based on creatinine clearance which is provided in Table 1. This study so far provides the largest number of patients analyzed looking into the effect of
Table 1.
Showing Relationship of CKD to Mortality among Patients Undergoing Percutaneous Mitral Valve Replacement with Mitraclip.
| Study | Endpoint | GFR<30 | GFR 30-60 | GFR<60 |
|---|---|---|---|---|
| Rodrigo Estévez-Loureiro et al., 2015 Retrospective multicenter UK (n=173) |
Mortality (up to 3 years) | HR 10 [1.7-58.4] p=0.011 (Cox analysis) | ||
| Yohei Ohno et al., 2016 Retrospective (GRASP registry) (n=214) |
Composite of death surgery for mitral valve dysfunc. and MR grade >=3 at 12 months | HR 2.39 [1.19-4.78] p=0.014 (multivariate) | ||
| Kaneko et al., 2016 Retrospective single center Germany (n=206) |
Mortality (up to 1000 days) | HR 4.322 [1.855-10.069] p=0.001 (multivariate) |
HR 1.868 [0.949–3.679] p=0.071 (multivariate) |
|
| Toggweiler, Stefan et al., 2014 Retrospective MitraSWISS registry (n=74) | Mortality (up to 2 years) | HR 3.29 (1.37 to 7.92) p<0.01 (univariate) | ||
| Wang, Andrew et al., 2015 Retrospective EVEREST cohort (n=886) |
Mortality (1 year) | HR 3.8 [2.38-6.05] (cox) | HR 2.35 [1.73–3.19] (cox) | |
| Sha, Binita et al., 2018 Retrospective STS/ACC registry (n=5,241 based on creatinine clearance) | Mortality (30 day) | OR 1.63 [0.99-2.68] p=0.053 (cox) | OR 1.29 [0.78-2.12] p=0.32 (cox) | |
| Sha, Binita et al., 2018 Retrospective STS/ACC registry (n=5,241 based on creatinine clearance) | Mortality (1-year) | 2.6 [1.92-3.51] p<0.001 (cox) | 2.9 [2.01-4.17] p<0.001 (cox) | |
CKD on long-term outcomes post transcatheter mitral valve repair.
At this time, all evidence addressing the effect of renal function on long-term outcomes has been from retrospective data and post-hoc analyses of studies involving Mitraclip repair. This is subject to selection and susceptibility biases inherently, as most samples involve elderly patients with multiple comorbidities who are at baseline high risk for poor outcomes in the long term. This is also inevitable given the inherent nature of using the MitraClip device in patients who are excluded from receiving surgery, because of their extensive comorbidities. In addition to this, the selection criteria for those undergoing MitraClip device repair is still not uniformly standardized. However, the outcomes are consistent across all studies so far in that, advanced CKD with GFR<30 mL/min/1.73 m2 was found to be associated with poorer long-term outcomes, especially survival, with a milder effect on those with moderate CKD (GFR 30-60 mL/min/1.73 m2). This effect is also consistent with various studies involving the effect of CKD on transcatheter aortic valve replacement [55, 56]. More prospective studies with sufficient power are certainly needed to fully address the reciprocal bidirectional relationship between renal function and mitral valve percutaneous procedures. Further studies are also needed to clarify the effect of hemodialysis or peritoneal dialysis on long-term outcomes among patients who undergo MitraClip repair [1].
Conclusion - Proper Patient Selection is Key
After presenting the evidence above, in terms of renal function, proper patient selection for percutaneous mitral valve repair is the key. As noted above, the groups with the greatest recovery of GFR after Mitraclip repair are those with CKD stage 3-5. But on the contrary, it is also almost in this group that long-term risk of post-procedural mortality is the greatest. In addition to this, other criteria must also be taken into consideration especially multiple comorbidities like severe heart failure, hypertension, diabetes and COPD which are widely prevalent in these patients and may influence long-term survival [5, 57]. As a result, there is a need to have standardized criteria for patients who should qualify to have percutaneous mitral valve replacement with Mitraclip. A randomized study RESHAPE-HF was initially underway to address this issue but was terminated due to lack of patient enrollment. In this new era of percutaneous mitral valve repair, much work needs to be done to optimize long-term patient outcomes.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Kainuma S., Taniguchi K., Daimon T., et al. Mitral valve repair for medically refractory functional mitral regurgitation in patients with end-stage renal disease and advanced heart failure. Circulation. 2012;126(11) Suppl. 1:S205–S213. doi: 10.1161/CIRCULATIONAHA.111.077768. [DOI] [PubMed] [Google Scholar]
- 2.Wan B., Rahnavardi M., Tian D.H., et al. A meta-analysis of MitraClip system versus surgery for treatment of severe mitral regurgitation. Ann. Cardiothorac. Surg. 2013;2(6):683. doi: 10.3978/j.issn.2225-319X.2013.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman T., Foster E., Glower D.D., et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 2011;364(15):1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 4.Sürder D., Pedrazzini G., Gaemperli O., et al. Predictors for efficacy of percutaneous mitral valve repair using the MitraClip system: The results of the MitraSwiss registry. Heart. 2013;99(14):1034–1040. doi: 10.1136/heartjnl-2012-303105. [DOI] [PubMed] [Google Scholar]
- 5.Toggweiler S., Zuber M., Sürder D., et al. Two-year outcomes after percutaneous mitral valve repair with the MitraClip system: Durability of the procedure and predictors of outcome. Open Heart. 2014;1(1):e000056. doi: 10.1136/openhrt-2014-000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohno Y., Attizzani G.F., Capodanno D., et al. Association of tricuspid regurgitation with clinical and echocardiographic outcomes after percutaneous mitral valve repair with the MitraClip System: 30-day and 12-month follow-up from the GRASP Registry. Eur. Heart J. Cardiovasc. Imaging. 2014;15(11):1246–1255. doi: 10.1093/ehjci/jeu114. [DOI] [PubMed] [Google Scholar]
- 7.Paranskaya L., D’ancona G., Bozdag‐Turan I., et al. Residual mitral valve regurgitation after percutaneous mitral valve repair with the mitraclip® system is a risk factor for adverse one‐year outcome. Catheter. Cardiovasc. Interv. 2013;81(4):609–617. doi: 10.1002/ccd.24586. [DOI] [PubMed] [Google Scholar]
- 8.Kahn M.R., Robbins M.J., Kim M.C., Fuster V. Management of cardiovascular disease in patients with kidney disease. Nat. Rev. Cardiol. 2013;10(5):261. doi: 10.1038/nrcardio.2013.15. [DOI] [PubMed] [Google Scholar]
- 9.Gaasch W.H., Zile M.R. Left ventricular function after surgical correction of chronic mitral regurgitation. Eur. Heart J. 1991;12(Suppl. B):48–51. doi: 10.1093/eurheartj/12.suppl_b.48. [DOI] [PubMed] [Google Scholar]
- 10.Shah P.M., Adelman A.G., Wigle E.D., et al. The natural and unnatural history of hypertrophic obstructive cardiomyopathy: A multicenter study. Circ. Res. 1974;35(2):II-179. [PubMed] [Google Scholar]
- 11.Zile M.R., Gaasch W.H., Levine H.J. Left ventricular stress-dimension-shortening relations before and after correction of chronic aortic and mitral regurgitation. Am. J. Cardiol. 1985;56(1):99–105. doi: 10.1016/0002-9149(85)90574-0. [DOI] [PubMed] [Google Scholar]
- 12.Corin W.J., Monrad E.S., Murakami T., Nonogi H., Hess O.M., Krayenbuehl H.P. The relationship of afterload to ejection performance in chronic mitral regurgitation. Circulation. 1987;76(1):59–67. doi: 10.1161/01.cir.76.1.59. [DOI] [PubMed] [Google Scholar]
- 13.Rassaf T., Balzer J., Rammos C., et al. Influence of percutaneous mitral valve repair using the MitraClip® system on renal function in patients with severe mitral regurgitation. Catheter. Cardiovasc. Interv. 2015;85(5):899–903. doi: 10.1002/ccd.25705. [DOI] [PubMed] [Google Scholar]
- 14.Parfrey P.S., Harriett J.D., Griffiths S.M., et al. The clinical course of left ventricular hypertrophy in dialysis patients. Nephron. 1990;55(2):114–120. doi: 10.1159/000185937. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura R.A., Otto C.M., Bonow R.O., et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Thorac. Cardiovasc. Surg. 2014;148(1):e1–e32. doi: 10.1016/j.jtcvs.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Vahanian A. Task Force on the Management of Valvular Hearth Disease of the European Society of Cardiology: ESC Committee for Practice Guidelines. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur. Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 17.Feldman T., Wasserman H.S., Herrmann H.C., et al. Percutaneous mitral valve repair using the edge-to-edge technique: Six-month results of the EVEREST Phase I Clinical Trial. J. Am. Coll. Cardiol. 2005;46(11):2134–2140. doi: 10.1016/j.jacc.2005.07.065. [DOI] [PubMed] [Google Scholar]
- 18.Wang A., Sangli C., Lim S., et al. Evaluation of renal function before and after percutaneous mitral valve repair. Circ. Cardiovasc. Interv. 2015;8(1):e001349. doi: 10.1161/CIRCINTERVENTIONS.113.001349. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko H., Neuss M., Schau T., Weissenborn J., Butter C. Interaction between renal function and percutaneous edge-to-edge mitral valve repair using MitraClip. J. Cardiol. 2017;69(2):476–482. doi: 10.1016/j.jjcc.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Kubo S., Nakamura M., Shiota T., et al. Impact of forward stroke volume response on clinical and structural outcomes after percutaneous mitral valve repair with mitraclip. Circ. Cardiovasc. Interv. 2017;10(7):e004909. doi: 10.1161/CIRCINTERVENTIONS.116.004909. [DOI] [PubMed] [Google Scholar]
- 21.Braunwald E. Mitral regurgitation: physiologic, clinical and surgical considerations. N. Engl. J. Med. 1969;281(8):425–433. doi: 10.1056/NEJM196908212810807. [DOI] [PubMed] [Google Scholar]
- 22.Damman K., Navis G., Smilde T.D., et al. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur. J. Heart Fail. 2007;9(9):872–878. doi: 10.1016/j.ejheart.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Biner S., Siegel R.J., Feldman T., et al. Acute effect of percutaneous MitraClip therapy in patients with haemodynamic decompensation. Eur. J. Heart Fail. 2012;14(8):939–945. doi: 10.1093/eurjhf/hfs069. [DOI] [PubMed] [Google Scholar]
- 24.Siegel R.J., Biner S., Rafique A.M., et al. The acute hemodynamic effects of MitraClip therapy. J. Am. Coll. Cardiol. 2011;57(16):1658–1665. doi: 10.1016/j.jacc.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 25.Hoar P.F., Mookerjee A., Stone J.G., Wicks A.E., Malm J.R., Mirsky M.B. Acute hemodynamic alterations after mitral valve replacement with the glutaraldehyde-treated porcine heterograft prosthesis. Ann. Thorac. Surg. 1980;29(5):434–439. doi: 10.1016/s0003-4975(10)61674-8. [DOI] [PubMed] [Google Scholar]
- 26.Landoni G., Zangrillo A., Franco A., et al. Long-term outcome of patients who require renal replacement therapy after cardiac surgery. Eur. J. Anaesthesiol. 2006;23(1):17–22. doi: 10.1017/S0265021505001705. [DOI] [PubMed] [Google Scholar]
- 27.Tang P., Onaitis M., Desai B., et al. Minithoracotomy versus sternotomy for mitral surgery in patients with chronic renal impairment: a propensity-matched study. Innovations (Phila.) 2013;8(5):325–331. doi: 10.1097/IMI.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 28.Loef B.G., Epema A.H., Smilde T.D., et al. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J. Am. Soc. Nephrol. 2005;16(1):195–200. doi: 10.1681/ASN.2003100875. [DOI] [PubMed] [Google Scholar]
- 29.Foster E., Kwan D., Feldman T., et al. Percutaneous mitral valve repair in the initial EVEREST cohort: Evidence of reverse left ventricular remodeling. Circ Cardiovasc Imaging. 2013;6(4):522–530. doi: 10.1161/CIRCIMAGING.112.000098. [DOI] [PubMed] [Google Scholar]
- 30.Grayburn P.A., Foster E., Sangli C., et al. The relationship between the magnitude of reduction in mitral regurgitation severity and left ventricular and left atrial reverse remodeling after mitraclip® therapy. Circulation. 2013;128(15):1667–1674. doi: 10.1161/CIRCULATIONAHA.112.001039. [DOI] [PubMed] [Google Scholar]
- 31.Suri R.M., Schaff H.V., Dearani J.A., et al. Recovery of left ventricular function after surgical correction of mitral regurgitation caused by leaflet prolapse. J. Thorac. Cardiovasc. Surg. 2009;137(5):1071–1076. doi: 10.1016/j.jtcvs.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Husain-Syed F., McCullough P.A., Birk H.W., et al. Cardio-pulmonary-renal interactions. J. Am. Coll. Cardiol. 2015;65(22):2433–2448. doi: 10.1016/j.jacc.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Vitarelli A., Mangieri E., Capotosto L., et al. Assessment of biventricular function by three-dimensional speckle-tracking echocardiography in secondary mitral regurgitation after repair with the MitraClip system. J. Am. Soc. Echocardiogr. 2015;28(9):1070–1082. doi: 10.1016/j.echo.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Moesgaard S.G., Klostergaard C., Zois N.E., et al. Flow‐mediated vasodilation measurements in Cavalier King Charles Spaniels with increasing severity of myxomatous mitral valve disease. J. Vet. Intern. Med. 2012;26(1):61–68. doi: 10.1111/j.1939-1676.2011.00846.x. [DOI] [PubMed] [Google Scholar]
- 35.Cai H., Harrison D.G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87(10):840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 36.Sutton T.A., Fisher C.J., Molitoris B.A. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62(5):1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 37.Rammos C., Zeus T., Balzer J., et al. Percutaneous mitral valve repair in mitral regurgitation reduces cell-free hemoglobin and improves endothelial function. PLoS One. 2016;11(3):e0151203. doi: 10.1371/journal.pone.0151203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Körber M.I., Scherner M., Kuhr K., et al. Acute kidney injury following percutaneous edge-to-edge vs. minimally invasive surgical mitral valve repair: Incidence, predictors and prognostic value. EuroIntervention. 2018;13(14):1645–1651. doi: 10.4244/EIJ-D-17-00131. [DOI] [PubMed] [Google Scholar]
- 39.Chang C.H., Lee C.C., Chen S.W., et al. Predicting acute kidney injury following mitral valve repair. Int. J. Med. Sci. 2016;13(1):19. doi: 10.7150/ijms.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ram P., Mezue K., Pressman G., Rangaswami J. Acute kidney injury post–transcatheter aortic valve replacement. Clin. Cardiol. 2017;40(12):1357–1362. doi: 10.1002/clc.22820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spieker M., Hellhammer K., Katsianos S., et al. Effect of acute kidney injury following percutaneous mitral valve repair on outcome. Am. J. Cardiol. 2018;122(2):316–322. doi: 10.1016/j.amjcard.2018.03.358. [DOI] [PubMed] [Google Scholar]
- 42.Kooiman J., Seth M., Nallamothu B.K., Heung M., Humes D., Gurm H.S. Association between acute kidney injury and in-hospital mortality in patients undergoing percutaneous coronary interventions. Circ. Cardiovasc. Interv. 2015;8(6):e002212. doi: 10.1161/CIRCINTERVENTIONS.114.002212. [DOI] [PubMed] [Google Scholar]
- 43.Lio A., Miceli A., Varone E., et al. Mitral valve repair versus replacement in patients with ischaemic mitral regurgitation and depressed ejection fraction: Risk factors for early and mid-term mortality. Interact. Cardiovasc. Thorac. Surg. 2014;19(1):64–69. doi: 10.1093/icvts/ivu066. [DOI] [PubMed] [Google Scholar]
- 44.Udesh R., Mehta A., Gleason T.G., Wechsler L., Thirumala P.D. Perioperative strokes and early outcomes in mitral valve surgery: a nationwide analysis. J. Cardiothorac. Vasc. Anesth. 2017;31(2):529–536. doi: 10.1053/j.jvca.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Ledwoch J., Bertog S., Wunderlich N., et al. Predictors for prolonged hospital stay after transcatheter mitral valve repair with the MitraClip®. Catheter. Cardiovasc. Interv. 2014;84(4):599–605. doi: 10.1002/ccd.25460. [DOI] [PubMed] [Google Scholar]
- 46.Damman K., Valente M.A., Voors A.A., O’connor C.M., van Veldhuisen D.J., Hillege H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2013;35(7):455–469. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 47.Damman K., Voors A.A., Navis G., van Veldhuisen D.J., Hillege H.L. The cardiorenal syndrome in heart failure. Prog. Cardiovasc. Dis. 2011;54(2):144–153. doi: 10.1016/j.pcad.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 48.Cooper W.A., O’brien S.M., Thourani V.H., et al. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: Results from the Society of Thoracic Surgeons National Adult Cardiac Database. Circulation. 2006;113(8):1063–1070. doi: 10.1161/CIRCULATIONAHA.105.580084. [DOI] [PubMed] [Google Scholar]
- 49.Gupta T., Goel K., Kolte D., et al. Association of chronic kidney disease with in-hospital outcomes of transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2017;10(20):2050–2060. doi: 10.1016/j.jcin.2017.07.044. [DOI] [PubMed] [Google Scholar]
- 50.Lio A., Miceli A., Varone E., et al. Mitral valve repair versus replacement in patients with ischaemic mitral regurgitation and depressed ejection fraction: Risk factors for early and mid-term mortality. Interact. Cardiovasc. Thorac. Surg. 2014;19(1):64–69. doi: 10.1093/icvts/ivu066. [DOI] [PubMed] [Google Scholar]
- 51.Udesh R., Mehta A., Gleason T.G., Wechsler L., Thirumala P.D. Perioperative strokes and early outcomes in mitral valve surgery: A nationwide analysis. J. Cardiothorac. Vasc. Anesth. 2017;31(2):529–536. doi: 10.1053/j.jvca.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 52.Ledwoch J., Bertog S., Wunderlich N., et al. Predictors for prolonged hospital stay after transcatheter mitral valve repair with the MitraClip®. Catheter. Cardiovasc. Interv. 2014;84(4):599–605. doi: 10.1002/ccd.25460. [DOI] [PubMed] [Google Scholar]
- 53.Estévez-Loureiro R., Settergren M., Pighi M., et al. Effect of advanced chronic kidney disease in clinical and echocardiographic outcomes of patients treated with MitraClip system. Int. J. Cardiol. 2015;198:75–80. doi: 10.1016/j.ijcard.2015.06.137. [DOI] [PubMed] [Google Scholar]
- 54.Shah B., Vemulapalli S., Manandhar P., et al. Outcomes after transcatheter mitral valve repair in patients with chronic kidney disease: An analysis of 5,241 patients in the United States. J. Am. Coll. Cardiol. 2018;71(11):A1980. [Google Scholar]
- 55.Dumonteil N., Van Der Boon R.M., Tchetche D., et al. Impact of preoperative chronic kidney disease on short-and long-term outcomes after transcatheter aortic valve implantation: A Pooled-RotterdAm-Milano-Toulouse In Collaboration Plus (PRAGMATIC-Plus) initiative substudy. Am. Heart J. 2013;165(5):752–760. doi: 10.1016/j.ahj.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto M., Hayashida K., Mouillet G., et al. Prognostic value of chronic kidney disease after transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 2013;62(10):869–877. doi: 10.1016/j.jacc.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 57.Neuss M., Schau T., Schoepp M., et al. Patient selection criteria and midterm clinical outcome for MitraClip therapy in patients with severe mitral regurgitation and severe congestive heart failure. Eur. J. Heart Fail. 2013;15(7):786–795. doi: 10.1093/eurjhf/hfs214. [DOI] [PubMed] [Google Scholar]