Abstract
Background:
In women, changes in estrogen levels may increase the incidence and/or symptomatology of de-pression and affect the response to antidepressant treatments. Estrogen therapy in females may provide some mood benefits as a single treatment or might augment clinical response to antidepressants that inhibit serotonin reuptake.
Objective:
We analyzed the mechanisms of estradiol action involved in the regulation of gene expression that modulates sero-tonin neurotransmission implicated in depression.
Method:
Publications were identified by a literature search on PubMed.
Results:
The participation of estradiol in depression may include regulation of the expression of tryptophan hydroxylase-2, monoamine oxidase A and B, serotonin transporter and serotonin-1A receptor. This effect is mediated by estradiol binding to intracellular estrogen receptor that interacts with estrogen response elements in the promoter sequences of tryptophan hy-droxylase-2, serotonin transporter and monoamine oxidase-B. In addition to directly binding deoxyribonucleic acid, estrogen receptor can tether to other transcription factors, including activator protein 1, specificity protein 1, CCAAT/enhancer bind-ing protein β and nuclear factor kappa B to regulate gene promoters that lack estrogen response elements, such as monoamine oxidase-A and serotonin 1A receptor.
Conclusion:
Estradiol increases tryptophan hydroxylase-2 and serotonin transporter expression and decreases the expres-sion of serotonin 1A receptor and monoamine oxidase A and B through the interaction with its intracellular receptors. The understanding of molecular mechanisms of estradiol regulation on the protein expression that modulates serotonin neuro-transmission will be helpful for the development of new and more effective treatment for women with depression.
Keywords: Depression, estradiol, monoamine oxidases, serotonin transporter, serotonin 1A receptor, tryptophan hydroxylase
1. INTRODUCTION
Clinical and epidemiological studies suggest that sex hormones are involved in the development of depression and in response to antidepressant treatment [1, 2]. Supporting this idea, it has been shown that sudden changes in estradiol (E2) levels are associated with depressed mood in women [3] and that estrogen replacement therapy increased a clinical response of elderly depressed women to antidepressants, including selective serotonin reuptake inhibitors (SSRIs), such as, fluoxetine (FLX) and sertraline [4, 5]. E2 treatment alone or in combination with SSRI alleviates depression symptoms in women, suggesting an action of this hormone on the serotonergic system. For example, in ovariectomized (OVX) monkeys, a model of surgical menopause, the long-term loss of E2 leads to a decrease in global serotonin (5-HT) availability and a reduction in gene expression related to 5-HT neural function compared to intact animals [6]; thus, it is possible that E2 positively modulates gene expression that regulates serotonergic neurotransmission implicated in depression. This effect is probably mediated by E2 binding to intracellular estrogen receptor (ER) that interacts with estrogen response elements (ERE) in the promoter sequences of target genes [7], such as 5-HT transporter (SERT or 5-HTT) [8], tryptophan hydroxylase-2 (TPH-2) [9] and monoamine oxidase-B (MAO-B) [10]. In addition to directly binding deoxyribonucleic acid (DNA), ER can tether to other transcription factors, including activator protein 1 (AP-1), specificity protein 1 (Sp1), CCAAT/enhancer binding protein β (C/EBPβ) and nuclear factor kappa B (NF-ҡB) to regulate gene promoters that lack ERE, such as monoamine oxidase-A (MAO-A) [11] and 5-HT1A receptor subtype (5-HT1A) [12].
2. DEPRESSION IN WOMEN
Depression is one of the most common, costly and severe psychopathologies worldwide, primarily characterized by persistent low mood and anhedonia (lack of pleasure in usually enjoyable activities). Symptoms may also include changes in sleep, feelings of guilt or worthlessness, low energy, poor concentration, appetite changes, psychomotor retardation, and thoughts of death or suicide, that result in significant impairment in everyday living [13].
The biological bases of depression are complex and involve multiple interacting disruptions affecting neurons and glial cells within specific brain areas, giving rise to neural network dysfunctions and symptomatology. Many biological processes implicated in depression include altered monoaminergic neurotransmission, reduced neurotrophic support, immune reaction, oxidative stress, altered stress hormone homeostasis and changes in steroid hormone levels [14, 15].
Epidemiologic and clinical data support the notion that women experience more psychiatric problems at some point in their lives compared to men, particularly mood symptoms; for example, unipolar depression is approximately twice as prevalent in women compared to men [1, 2]. In every age group, beginning at age 10, women have a higher prevalence of depression than men. This difference is most pronounced before age 45, when women appear to be at highest risk for experiencing a depressive episode [16].
The fact that gender differences in depression are not shown until puberty suggests that ovarian hormones contribute to depression [17]. Hypothalamic-pituitary-gonadal axis has been of interest to explain this sex-difference in depression risk. Opposite to the circadian release of sex hormones in males, with the onset of menarche at puberty, the female brain is exposed to monthly surges of estrogens and progesterone [16]. It is proposed that certain periods associated with sex hormone fluctuations across the women reproductive life cycle represent “windows of vulnerability” for new-onset and/or recurrent depression [18]. Different hypotheses have been proposed to explain this increased vulnerability in women. Historically, low levels of E2 have been considered the root of depression, but recent evidence suggests that women developing symptoms that need treatment are more vulnerable to drastic changes in hormonal milieu [19]. For example, the high concentration of allopregnanolone, 5α-reduced metabolite of progesterone, at luteal phase seems to precipitate distress and mood alterations in women suffering premenstrual dysphoric disorder (PMDD). Accordingly, stabilization of allopregnanolone levels by means of 5α-reductase inhibitor dutasteride mitigates symptoms of PMDD, with no effects on asymptomatic controls [20]. In addition, depressive symptoms have been identified in a subset of women during postpartum period, in which delivery and placenta extraction reduce hormone levels [21], and in the perimenopausal transition (between 45 to 50 years)¸ a period associated with depression characterized by fluctuations in estrogens and increased follicle-stimulating hormone [22, 23]. Also, it is suggested that depressive episodes related to reproductive events, may predict depression during menopausal years that occur in women over 48 y.o. After menopause, the female brain must once again adapt to the absence of cyclic levels of ovarian hormones and establish a new baseline of homeostasis in order to maintain normal brain function. The inability to rapidly establish a new baseline of neuronal function could lead to increased susceptibility to mood disorders [17].
3. MECHANISM OF E2 ACTION
E2 is mainly synthesized in the ovaries, adrenal gland and Central Nervous System (CNS). This hormone exerts its actions through various mechanisms that are classified as classical and non-classical. In general, non-classical effects of E2 rapidly proceed and occur both at membrane and cytoplasmic levels [24].
ER is widely distributed throughout the brain regions known to be involved in the regulation of mood, such as hypothalamus, hippocampus and raphe nucleus [25]. E2 elicits its effects by interacting with ER, which belongs to the nuclear receptor superfamily of ligand-dependent transcription factors [26]. ER is expressed as two subtypes: ER-α (66 kDa) and ER-β (55 kDa), which are transcribed from different genes [26]. It has been reported that ER-α is a stronger transcriptional activator than ER-β due to differences in the activation function-1 domain in the amino terminus region [27]. It has been shown that ER subtypes are functionally distinct in terms of their ability to activate target genes in the same cell and regulate different physiological and pathological processes.
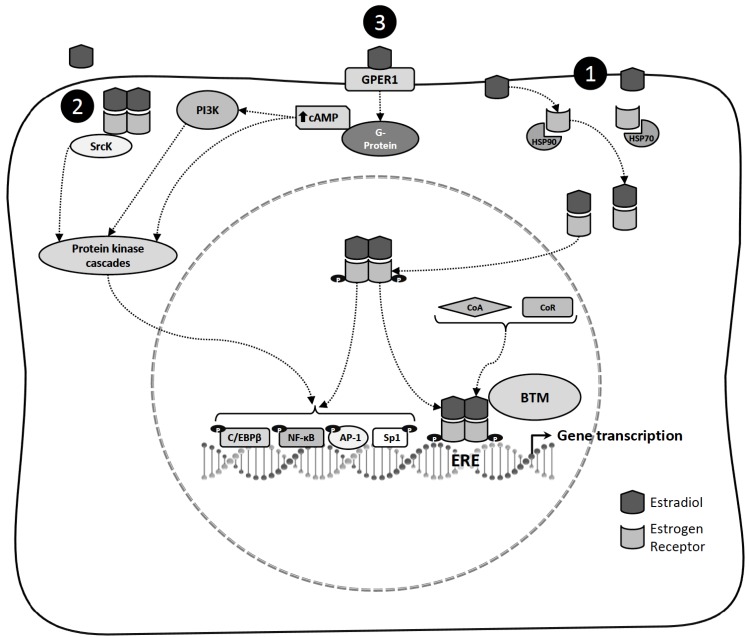
According to the classical model of ER action (Fig. 1), in the absence of ligand, ER is associated with heat shock proteins (HSP70 and HSP90). Hormone interaction induces ER conformational changes that allow dissociation of HSP proteins, promoting dimerization, phosphorylation and high-affinity binding to ERE located within the regulatory regions of target genes [26]. ER modulates target gene transcription by recruiting components of the basal transcriptional machinery and by interacting with coregulatory proteins. Nuclear receptor coregulators are coactivators or corepressors that are required by ER for efficient transcriptional regulation [28]. Nevertheless, ER can also modulate gene expression without directly binding to DNA. The receptors in such cases are tethered through protein-protein interactions to a transcription factor complex that contacts the DNA. By this mechanism, ERs regulate the expression of estrogen-responsive genes that do not contain ERE sequences. The mechanism is used by members of the nuclear receptor superfamily and is referred to as transcriptional cross-talk [29]. Several genes are activated by E2 through the interaction of ERs with transcription factors, including, Sp1 and AP-1 [7]. Other transcription factors that facilitate E2 signaling include NF-ҡB, C/EBPβ, GATA binding protein 1 and signal transducer and activator of transcription 5 [7]. After the separation of phosphorylated ER from the transcription complex, it can be a target for degradation via the ubiquitin-proteasome pathway [30]. ER has dual functions as a nuclear transcription factor and as a modulator of cell signaling pathways. In addition to direct transcriptional effects mediated by nuclear ER, E2 can rapidly activate the protein tyrosine kinase (SrcK)/ mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathways [31]. The SrcK/MAPK signaling pathway can phosphorylate and activate certain nuclear transcription factors, suggesting that activation of this signaling provides an alternate pathway for E2 to regulate gene transcription independent of the direct nuclear transcriptional activity of ER [32].
Fig. (1).
Mechanisms of estradiol action. 1) In the classical model of estradiol action, in the absence of ligand, estrogen receptor is associated with heat shock proteins (HSP70 and HSP90). Estradiol interaction induces estrogen receptor conformational changes that allow dissociation of the HSP, promoting dimerization, phosphorylation and high-affinity binding to estrogen response elements (ERE) located within the regulatory regions of target genes. Estrogen receptor modulates target gene transcription by recruiting components of the basal transcriptional machinery (BTM) and by interacting with coregulatory proteins (Coactivators: CoA or Corepressors: CoR). Estrogen receptor can also modulate the expression of genes without directly binding to deoxyribonucleic acid by tethering to other transcription factors through protein-protein interactions in the nucleus, including specificity protein 1 (Sp1) and activator protein 1 (AP-1), nuclear factor kappa B (NF-ҡB) and CCAAT/enhancer binding protein β (C/EBPβ) to regulate gene promoters that lack canonical ERE sequences. 2) Estrogen receptor localized into the cell membrane or cytoplasm has been proposed to mediate estradiol activation of the protein tyrosine Kinase (SrcK)/mitogen-activated protein kinase (MAPK) signaling pathway, and can phosphorylate and active certain nuclear transcription factors. 3) In the non-classical mechanisms of estradiol action, estradiol binding to G protein-coupled estrogen receptor-1 (GPER1) induces cyclic adenosine monophosphate (cAMP) production and MAPK, and activates phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathways.
The non-classical mechanisms for E2 actions have been documented. These include E2 interaction with receptors located at the cell membrane, growth factors and their receptors. E2 can associate with G protein-coupled estrogen receptor-1 (GPER1, formerly known as GPR30), which is a seven transmembrane domain receptor. It is located in the plasma membrane and cytoplasm. GPER1 binds to heterotrimeric G proteins to activate intracellular signaling cascades [7, 33, 34]. The binding of E2 to ERs at the cell surface can cause mobilization of intracellular calcium, stimulation of adenylate cyclase activity and cyclic adenosine monophosphate (cAMP) production, activation of the MAPK and PI3K signaling pathways and activation of membrane tyrosine kinase receptors [35].
The ability of E2 to regulate the transcription of a particular gene depends on many factors, such as the presence of certain transcription factors in the tissue, the ER expressed subtypes, and the DNA sequence within the promoter region of the gene [7, 24]. Thus, the possible convergence of classical and non-classical actions at multiple response elements provides an extremely fine degree of control for transcription regulation by ERs [7].
4. CHANGES IN E2 LEVELS AND DEPRESSION
The increased vulnerability in some women to depression occurs with the beginning of cyclic changes in gonadal hormone secretion. Although this disorder is common, it is important to note that most women do not experience depression [36-38]. However, changes in endocrine function resulting in unpredictable and dramatic low and high levels of ovarian hormones may predispose women to depressive disorders such as premenstrual dysphoric disorder, postpartum depression and perimenopausal depression [39]. These disorders are associated with reproductive stages involving fluctuations in gonadal hormone levels; specifically, female depression and female affective behaviors are influenced by E2 levels [39]. It has been reported that women with depression show lower levels of E2 during the follicular phase of the menstrual cycle [40]. Bloch et al., 2000 [41] found that induced hypogonadism, with the ovarian hormone suppressor leuprolide acetate, produced depressive symptoms in premenopausal women with a history of postpartum depression, but not in women without a history of postpartum depression. Subsequent treatment with E2 and progesterone for 8 weeks removed these symptoms. This study provides evidence on the pathophysiological relevance of E2 in the onset of postpartum depression episodes. It has been demonstrated that plasma E2 levels are 30% lower in premenopausal women with depression than their matched controls [42]. Interestingly, women who show rapid changes from high to low E2 levels and vice versa as in the transition perimenopause, are those who develop depressive symptoms [22]. Therefore, sudden changes in endogenous E2 levels may precipitate the disorder, or increase the incidence and/or symptomatology of depression in women.
Studies in perimenopausal women with depression demonstrated that E2 therapy may provide some mood benefits [43-46]. The meta-analysis published in 1997 by Zweifel and O´Brien [47] which included 26 studies (for the most part, randomized clinical trial –RCT) showed that hormonal restitution therapy (HRT, estrogens plus progestins) produces a moderate antidepressant effect in menopausal women. A recent systematic review by Rubinow and colleagues (2015) [48] including 24 placebo-controlled RCT provides complementary information about the efficacy of hormonal preparations for affective complaints. This meta-analysis concluded that estrogen replacement therapy (ERT) is more effective than combined HRT to improve mood. Antidepressant efficacy of ERT was confirmed in a small set of studies that included perimenopausal symptomatic women, in which depression diagnosis was established at the baseline. In contrast, ERT was ineffective to reduce depression scores in perimenopausal asymptomatic women, or postmenopausal women (70 y.o.+).
Due to limitations of antidepressant efficacy of E2, new treatments for relieving depression in women are being explored. Among them, the selective estrogen receptor modulators (SERMs) form a drug group whose chemical structure allows them to act as agonists or antagonists in a tissue-dependent manner. SERMs (i.e. tamoxifen, raloxifen) behave as E2 in brain and bone, but are considered ER antagonists in breast. Because of this, they are used as first-line therapy for estrogen-dependent breast cancer. Brain actions of SERMs have led to research their potential as an antidepressant, but at this moment the lack of well-controlled clinical trials limit conclusions about their therapeutic efficacy in depressed women [49]. Preclinical studies are actually being carried out to evaluate new estrogen-related drugs or E2 analogues in animal models of menopause [50]. Notwithstanding the good results in this field, much greater effort is needed to obtain effective treatments for the majority of patients.
Interestingly, E2 also has been used for increasing response to antidepressants in women. Most studies revealed that estrogen replacement therapy might augment clinical response of elderly depressed women to antidepressants, including SSRIs, such as, FLX and sertraline [4,5]. E2 treatment combined with FLX decreases the severity of depressive symptoms in perimenopausal women more effectively than FLX alone [51]. These data suggest that E2 alone and in combination with antidepressants alleviates depressive symptoms in women through a mechanism that involves an action on the 5-HT system [39].
5. ROLE OF 5-HT IN DEPRESSION
The serotonergic neurons of the dorsal and median raphe nucleus in the CNS are involved in the regulation of mood; thereby a deficiency in 5-HT neurotransmission is a leading hypothesis regarding the role of 5-HT in the pathophysiology of depression [52]. 5-HT cannot cross the blood-brain barrier, thus this monoamine is synthesized de novo in the brain from its precursor, tryptophan, an amino acid obtained from the diet. Tryptophan is converted by tryptophan hydroxylase (TPH) to 5-hydroxytryptophan (5-HTP), subsequently, it is converted into 5-HT by the action of 5-HTP decarboxylase. 5-HT is stored in neuronal vesicles and released from serotonergic nerve terminals. Effects of this neurotransmitter on the CNS and Peripheral Nervous System are mediated through several different receptor types (14 5-HT receptors), the binding of 5-HT to these receptors initiates a series of intracellular events that modulate neuronal excitability [53]. The catabolism of 5-HT involves MAO enzymes. 5-HT is transported back into the presynaptic cell by SERT. Once taken up into the presynaptic terminal, 5-HT can be repackaged into secretory vesicles or is metabolized to 5-hydroxyindole-3-acetic acid (5-HIAA) [52, 54].
5-HT synthesis, release, and the interaction with its receptors and reuptake are targets of pharmaceuticals in depression treatment [14]. Antidepressants that influence brain 5-HT availability mainly increase extracellular 5-HT concentrations by blocking the presynaptic reuptake transporter, thus promoting serotonergic transmission at synapse level [53]. Reports indicate that chronic pharmacological depletion of 5-HT with the vesicular monoamine transporter inhibitor reserpine-induced depressive symptoms only in a subset of patients [55]. Studies on women with depression who have recovered following treatment with serotonergic antidepressants demonstrated that acute tryptophan depletion induces the recurrence of depression symptoms [56, 57]. Post-mortem and preclinical studies with positron emission tomography (PET) examining the 5-HT receptors or SERT in vivo provide support for serotonergic dysfunction in depression in humans [58]. The 5-HT1A subtype is implicated in depression and SERT is a target for SSRI, both have been quantified in vivo, in humans by using a high-resolution PET. Several studies have reported decreased 5-HT1A binding potential in depressed individuals compared to healthy individuals [59-61]. These results likely reflect a reduction in 5-HT1A expression, since it has been shown that 5-HT1A mRNA levels were reduced in the hippocampus of subjects (women and men) with a history of depression who died by suicide compared to matched control [62]. Women and men with depression have lower SERT binding potential in the amygdala and midbrain compared to healthy subjects determined by PET [63]. Staley et al., 2006 [64] reported lower SERT availability in depressed women compared to healthy women and depressed men in the diencephalon, measured by single photon emission computed tomography (SPECT) and by a magnetic resonance imaging (MRI) scan. These data suggest that the mechanisms that modulate 5-HT neurotransmission in depression are complex and not precisely defined, but appear to involve modulation of E2.
6. EFFECTS OF E2 ON SEROTONERGIC SYSTEM
The effects of E2 have been observed in brain regions known to be closely involved in mood regulation [17]. Evidence suggests that the modulatory mood effects of E2 should largely depend on changes in serotonergic pathways. Because E2 can regulate gene expression after coupling with ER [65], which is expressed within the midbrain raphe nucleus [66, 67] reservoir of serotonergic neurons, it is possible that E2 modulates the expression of genes that regulate 5HT neurotransmission implicated in depression. E2 has been implicated with increased 5-HT synthesis, decreased 5-HT breakdown and modulation of serotonergic receptors [19].Thus, the close relationship between E2 and the 5-HT system may provide insight as to why some women experience increased susceptibility to mood symptoms during periods of hormonal fluctuation. Currently, little is known about the molecular mechanism underlying the ability of E2 to interact with the serotonergic system; effects of E2 have been primarily studied using in vivo models.
In this regard, long-term loss of E2 in OVX monkeys decreased global of this monoamine availability, was associated with fewer serotonergic neurons, and reduced the expression of TPH-2, SERT and 5-HT1A compared to intact animals [6]. Studies show that E2 treatment in OVX macaques increases TPH-2, MAO-A and MAO-B mRNA expression [9, 68]. It also has been observed that acute administration of E2 decreased 5-HT1A mRNA expression in amygdala, piriform cortex and perirhinal cortex of OVX rats [69]. Besides, E2 increases the SERT gene expression in the dorsal raphe nucleus in female rat brain [70]. Furthermore, by downregulating 5-HT1A auto-receptors and upregulating 5-HT2A receptors, E2 increases 5-HT availability for postsynaptic transmission [71]. Thus, E2 administration results in an overall net increase in 5-HT synthesis and availability.
E2 appears to play a modulatory role in serotonergic neurotransmission; however, it should be noticed that estrogen is probably one of several factors that affect 5-HT. Progesterone can also affect brain functioning and, consequently, mood and behavior. This could be explained because progesterone regulates neurotransmitter synthesis, release and transport and allopregnanolone modulates γ-aminobutyric acid neurotransmission; withdrawal of these hormones across reproductive phases precipitates distress symptoms [72]. Additionally, norepinephrine (NE) and dopamine pathways may play a role in women’s increased susceptibility to depression [19]. This could occur through the regulation of catecholamines synthesis and degradation by E2 [73]. The expression of tyrosine hydroxylase (TH), which catalyzes the conversion of tyrosine to L-dihydroxyphenylalanine, a precursor to NE, is increased by E2 treatment of gonadectomized animals [73-75]. Given that TH is the rate-limiting enzyme for catecholamine synthesis, E2-induced increases in TH could enhance the capacity for NE production [73]. It also has been reported that E2 treatment in OVX rats increases the expression of dopamine β-hydroxylase (DBH), the enzyme that catalyzes the hydroxylation of dopamine into NE [73]. In addition, it was demonstrated in human cell culture that E2 decreases catechol-O-methyltransferase (COMT) protein levels, the enzyme that degrades NE and dopamine [76]. The mechanism by which E2 regulates TH, DBH and COMT is transcriptional, occurring through genomic effects of E2 on ERE found in TH, DBH and COMT promoters [74, 77, 78]. These studies reveal that E2 can increase catecholamines levels by enhancing the capacity for synthesis, while reducing degradation.
Combining animal and human studies, it is apparent that estrogens can regulate mood through actions on the serotonergic system at several levels. Elucidating the mechanisms by which E2 participates in depression is important to enhance its therapeutic potential. We analyzed the mechanisms of E2 action involved in the regulation of the expression of TPH-2, MAOs, SERT and 5-HT1A that modulate 5-HT neurotransmission implicated in depression.
7. REGULATION OF GENES MODULATING 5-HT NEUROTRANSMISSION BY E2
7.1. TPH
The rate-limiting step in 5-HT synthesis is the conversion of tryptophan to 5-HTP, catalyzed by TPH [79]. TPH exists in two isoforms (TPH-1 and -2); TPH-2 is expressed in the brain while TPH-1 is expressed in peripheral tissues, such as the gut, pineal gland, spleen and thymus [80]. Deficient levels of 5-HT, dysfunction and polymorphisms of the TPH-2 are associated with increased vulnerability to depression [81, 82]. Several post-mortem studies have examined the expression of TPH-2 in the dorsal raphe nucleus of depressed or suicide subjects, revealing an increase in TPH-2 mRNA expression using in situ hybridization and a higher number of TPH-immunoreactive neurons in the dorsal raphe nucleus [83, 84]. However, Bonkale and colleagues reported no change in TPH-immunoreactivity of the dorsal raphe nucleus between depressed suicide and control subjects, which indicates no alteration in the TPH-2 protein in depressed suicide subjects [85]. These studies reveal conflicting results regarding the role of TPH-2 in depression. One possible explanation of more TPH-2 protein and less 5-HT is an enzyme variant with less catalytic activity. A TPH-2 variant with 20% of the catalytic activity of the wild type was found in 10% of subjects with unipolar depression, and perhaps other such variants exist in other cases of depression [81]. The increase in the number of TPH-immunoreactivity neurons may reflect a compensatory mechanism where more serotonergic neurons are synthesizing TPH-2 in an attempt to restore a deficit in the synthesis and release of 5-HT in the brain of suicide victims.
It has been determined that TPH-2 protein levels in the dorsal raphe nucleus were reduced by OVX in female macaques [6]. Interestingly, E2 and conjugated equine estrogen treatment increased TPH-2 content, and this effect was blocked by the SERM tamoxifen compared to OVX control animals [86]. Studies indicate that ER-β is strongly expressed within the dorsal raphe nucleus of rodents [87] and primates [88], whereas ER-α is expressed to a small extent in the dorsal raphe nucleus of these species. ER-β is expressed in serotonergic neurons of dorsal raphe nucleus of OVX rats and OVX rats treated with estradiol benzoate, however, the expression of ER-β mRNA between both groups was not significantly different. In contrast, these 5-HT neurons did not express ER-α [89]. Accordingly, studies in OVX female rats show that E2 and a selective ER-β agonist diarylpropionitrile (DPN) enhanced TPH-2 mRNA expression in dorsal raphe nucleus [9, 90]. Furthermore, DPN attenuates despair-like behavior in the forced-swim test (FST) [90], suggesting a prominent participation of ER-β in mood regulation in females. The FST is an animal model commonly used for both the screening of antidepressant drugs [91] and the analysis of the neurobiological bases of depression [92]. In this model, rats are forced to swim, and while at the beginning of the test they show active behaviors (e.g., climbing and swimming) intended to escape from the situation, eventually they adopt a floating posture identified as immobility behavior, which is considered an index of behavioral despair [93]. When antidepressant drugs are administered, a reduction in immobility is produced and this is considered to reflect an antidepressant-like action [92, 93]. Furthermore, it has been described that the way in which antidepressant drugs modify the behavioral profile in the test may indicate the neurotransmission system that it modulates. Thus, drugs that modulate the serotoninergic system reduce immobility behavior and increase swimming in the test, meanwhile those that modulate noradrenergic and dopaminergic systems reduce immobility and increase climbing behavior [94]. In addition, the FST has proven to be sensitive to physiological changes such as steroid level variations [95]. In OVX rats subjected to the FST, it was observed that E2 and FLX decrease the immobility and increase swimming behavior, which was considered an antidepressant-like action [96]. Also, the FST decreased the TPH-2 protein content in dorsal and median raphe nuclei of OVX rats, that was prevented by FLX or E2 administration [96]. This suggests that, similar to FLX, the antidepressant-like effect of E2 involves modulation of the serotonergic system, probably through inhibition of synaptic reuptake of 5-HT and increasing TPH-2 expression in dorsal raphe nucleus [96]. E2 also reduces the synaptic 5-HT uptake by reducing the efficiency of SERT activity as indicated by a study showing that E2 treatment retarded clearance of 5-HT in hippocampal CA3 of OVX rats. According to pharmacologic studies, this effect was possible through the activation of ER-β and GPR30 receptors, however, the involved signaling pathways are unknown [97]. It is assumed that the E2-induced rise in intracellular Ca2+ levels could lead to the activation of protein kinase C which would phosphorylate SERT, causing its removal from the plasma membrane and decreasing 5-HT uptake [98].
Experiments have been conducted to test the hypothesis that E2 regulates TPH2 transcription via ER-β. The serotonergic cell line RN46A-B14 (B14), derived from embryonic rat medullary raphe cells, endogenously expresses ER-β but not ER-α. In these cells that were transiently transfected with a fragment of the human TPH-2 cloned into a luciferase reporter vector (TPH-2-luc), the treatment with E2 or DPN resulted in a dose-dependent increase of TPH-2-luc activity. In contrast, E2 conjugated to bovine serum albumin did not have effects on TPH-2-luc activity. Also, treatment with ICI 182,780, an ER antagonist, blocked E2 or DPN-induced TPH-2-luc activity in transfected B14 cells [9]. These data support the concept that E2 induced TPH-2 transcriptional activity occurs through classical ER-β signaling pathways. A classical ERE half-site has been identified on the TPH-2 promoter; deletion and site-directed mutation of this site abolished the effect of E2 or DPN on TPH-2-luc activity [9]. This finding confirms that the ERE half-site plays an important role in the ER-β mediated regulation of TPH-2 transcriptional activity. In B14 cells numerous putative DNA response elements were found on the TPH-2 promoter, including Sp1, AP-1 and C/EBPβ sites, which appear to play an important role in the regulation of TPH-2 gene transcription by E2 [9, 99]. These studies suggest a direct interaction between E2 and ER-β with the ERE half-site of the TPH-2 promoter.
Although it is possible that ER-α acts on the THP-2 promoter, this has not been studied yet. Thus, it would be important to test the effects of ER-α on TPH-2 transcriptional activity in human serotonergic neurons.
7.2. MAO
MAOs are mitochondrial enzymes that catalyze oxidative deamination of biogenic amines, including 5-HT and catecholamines in the CNS and peripheral tissues [88, 100]. Two isoforms of MAO have been identified, designated types A and B, which have distinct substrate affinity and inhibitor sensitivity [100]. MAO-A and MAO-B are encoded by separate genes [88, 101]. The different promoter organization of MAO-A and MAO-B genes may underlie their different tissue- and cell-specific expression, which raises the possibility of differential regulation by E2. MAO-A has a higher affinity for 5-HT and NE, while MAO-B predominantly metabolizes phenylethylamine and benzylamine, although it also degrades 5-HT and both MAOs can degrade dopamine [88, 102]. MAO-A protein and mRNA are expressed in catecholaminergic neurons and MAO-B protein and mRNA are expressed by serotoninergic neurons of the raphe nucleus, however, MAO-A mRNA also is detected in the dorsal raphe nucleus of primates [103]. These data suggest the coexistence of the isoenzymes in raphe neurons.
Inhibitors of MAO were among the first drugs successfully used for the treatment of depression. The use of irreversible MAO inhibitors began by serendipity since the antitubercular agent iproniazid was found to have antidepressant effects in tuberculosis patients who suffered from depression [104]. Thus, the ability of MAO inhibitors to relieve depression suggests that MAOs dysfunction has been implicated in depression [105].
MAO-A specific distribution volume is significantly elevated in the brain of patients with major depressive disorders compared to healthy individuals [106]. In addition, women of perimenopausal age (41-51 years) showed an elevated MAO-A total distribution volume (an index of MAO-A density, is measurable in vivo in the brain using harmine labeled with carbon 11 positron emission tomography) compared to reproductive age and menopausal age women [107], which is positively correlated with depressive symptoms [106, 108]. In contrast, the MAO-B levels were measured in locus coeruleus and dorsal raphe nucleus of psychiatrically normal individuals and age-matched subjects with major depression; comparison between these groups revealed no significant differences in MAO-B levels [109].
A study conducted on the OVX female macaque model showed that E2 treatment decreased MAO-A mRNA and protein levels in the dorsal raphe nucleus and in hypothalamus; in contrast, E2 did not have effects on MAO-B mRNA and protein expression in the dorsal raphe nucleus [88, 110]. In rats, MAO-A activity decreased with E2 in hypothalamus [111]. However in the locus coeruleus and cerebellum of the rat, MAO-A activity decreased and MAO-B activity increased with E2 [112]. A decrease in the MAOs mRNA expression would be reflected by a decrease in protein content, which in turn may reduce MAOs activity, and this action could elevate extracellular levels of 5-HT and elevate mood.
It is proposed that areas with predominantly ER-β, such as the dorsal raphe nucleus and paraventricular nucleus, showed regulation of MAO-A, but not MAO-B, whereas areas with predominantly ER-α, such as the preoptic area, showed regulation of MAO-B, but not MAO-A. The ventromedial nucleus contains both ER subtypes and exhibits regulation of MAOs by E2 [113]. This suggests that ligand-activated ER-β modulates the expression of MAO-A, whereas MAO-B may be regulated by ER-α. However, there could be an exception to this generalization. The MAO-A promoter does not contain canonical ERE, however, the presence of three Sp1 sites may provide a mechanism for ER regulation of MAO-A expression via Sp1 tethering [11]. Moreover, the MAO-B promoter region has two clusters of overlapping Sp1 sites near the initiation start site and half-ERE sites were found within 2 kb of the promoter region [10]. Sp1 sites could mediate the E2 action indirectly through interaction between ER and Sp1, whereas E2 activated ERs can interact directly with MAO-B promoter by binding to half-ERE.
7.3. SERT
5-HT is released into the synaptic cleft where it exerts its action on pre and postsynaptic serotonergic receptors. SERT is responsible for 5-HT reuptake at the terminals and cell bodies of serotonergic neurons. SERT is a protein with 12 transmembrane domains located at the presynaptic and somatodendritic membranes of most serotonergic neurons [114] and SERT belongs to a large family of sodium-dependent neurotransmitter transporters [115]. This protein is the target of drugs currently used to treat depression named selective SSRI, which increase synaptic 5-HT concentration by blocking SERT activity. Although inhibition of uptake is achieved rapidly, improvement of mood usually occurs in an antidepressant therapy only after 2-3 weeks [116].
Changes in SERT expression are shown in psychopathologies such as depression. In a meta-analysis of 18 studies with 364 depressed patients free from comorbidities or medication and 372 control subjects, SERT reductions were observed in midbrain and amygdala, but there were no differences in other brain regions, such as the brainstem, thalamus, frontal cortex and cingulate cortex [117].
There exist several studies showing that SERT expression is sensitive to HRT. Although there are reports indicating that chronic E2 treatment in OVX animals downregulates SERT mRNA levels in midbrain raphe nucleus [113, 118, 119], most studies agree that there is a direct relationship between HRT or E2 and SERT expression, as indicated by several investigations in macaques in which HTR increased the binding of citalopram (an SSRI) to SERT, the SERT immunofluorescence in fiber tracts and the [3H] 5-HT uptake in brain regions that receive serotonergic projections, such as hypothalamic nuclei [120]. Additionally, acute administration of E2 increases SERT mRNA levels in dorsal raphe nucleus of OVX rats; this effect was blocked by the SERM tamoxifen [70, 121, 122]. It has also been determined that chronic administration of E2 or raloxifene, a SERM that has estrogenic-like effects, increased SERT protein in the OVX monkey dorsal raphe [110]. There are also reports showing that conjugated equine estrogen treatment, a common ERT for menopausal women, increased SERT protein content in the macaque dorsal raphe. It must be noted that macaques have a menstrual cycle similar to that of women [123]. All these studies suggest that ER is involved in mediating E2 action on the regulation of SERT. In contrast, in healthy women, SERT binding potentials did not significantly differ between follicular and luteal phases in dorsal raphe nucleus examined with PET, indicating that the fluctuating levels of steroid hormones during the menstrual cycle are not related to SERT modulation [124].
A direct interaction between ER-β and SERT through protein-protein interaction was proposed, as the signals for ER-β and SERT overlap in the cell bodies of serotonergic neurons [89, 98]. In rodents, ER-α and ER-β are co-localized with SERT in 5-HT neurons of the dorsal raphe nucleus, while in monkeys only ER-β is co-localized with SERT in 5-HT neurons [113, 125]. This differential expression of ERs might contribute to species-related differences in the effects of E2 on SERT mRNA levels in the dorsal raphe nucleus. The increase in SERT mRNA levels may be mediated by a direct action of E2, since a consensus ERE was identified in the SERT promoter region, and ERE is located 1.2 kb upstream of the transcription start site [8]. Although it was determined that ligand-activated ER modulates the promoter activity of some genes through other transcription factors, Sp1, AP-1, NF-ҡB and C/EBPβ sites are located in the SERT gene promoter that may play important roles in the endogenous expression of this gene [8].
7.4. 5-HT1A
The 5-HT1A is a protein with seven transmembrane domains that is coupled to G-protein, inhibits adenylyl cyclase activation and reduces cAMP levels [126]. The 5-HT1A receptors are located in serotonergic neurons of the dorsal and median raphe nuclei (autoreceptors; somatodendritic location) and postsynaptically in the frontal cortex, septum, amygdala, hippocampus and hypothalamus [127]. Activation of the somatodendritic 5-HT1A autoreceptors of raphe nucleus reduces the firing rate of serotonergic neurons [126] and consequent the release of 5-HT from nerve terminals [128].
Treatment with SSRIs and MAOIs during weeks leads to desensitization of the 5-HT1A autoreceptor in raphe dorsal nucleus [129]. It has been determined that postsynaptic 5-HT1A receptors are reduced in cortical regions in depression [130]. Besides, overexpression of the 5-HT1A autoreceptor was implicated in reducing serotonergic neurotransmission, and is associated with depression [131]. Several clinical PET studies examined 5-HT1A receptors in patients with depression, and showed discrepant results. Various authors reported lower 5-HT1A [11C] WAY100635 binding potential in the dorsal raphe nucleus [59, 60, 132], while others reported higher 5-HT1A binding potential [63]. However, most studies in major depression patients that have used binding potential as a measure of 5-HT1A receptor function, have consistently found a decrease in 5-HT1A receptor binding in raphe as well as limbic and cortical regions [133]. The association of depression with a reduction in 5-HT1A receptors seems contradictory, but this could be explained by the fact that most studies did not differentiate between autoreceptors and heteroreceptors, whose role in depression may be opposite [134].
The fluctuating levels of steroid hormones during the menstrual cycle may affect the 5-HT system in animals and humans; however, there are only few in vivo studies that have explored brain neurotransmission in relation to menstrual cycle phases in women. A study in healthy women found no significant differences in 5-HT1A binding potentials between phases of the menstrual cycle examined with PET [124]. In rats, 5-HT1A autoreceptors are located on serotonergic neurons of the dorsal and median raphe nuclei and postsynaptically in the hypothalamus, limbic and cortical areas [135]. Chronic treatment of E2, tamoxifen or raloxifene did not affect 5-HT1A binding and mRNA levels in subregions of the hippocampal formation, prefrontal cortex, cingulated cortex and dorsal raphe nucleus of OVX female rats [136]. In contrast, in OVX female macaques treated with E2, a decrease in 5-HT1A autoreceptors mRNA levels in dorsal raphe nucleus was observed [118]. These differential effects may be determined by inter-specific variations on ER expression. For example, while ER-β appears to be predominant in the serotonergic cells of several species [67, 137], ER-α may only be expressed in murine serotonergic cells [67].
E2 regulates the expression of the 5HT1A in monkey kidney cell line (COS-1) transiently cotransfected with human 5-HT1A promoter, and expression vectors encoding NF-ҡB, ER-α or ER-β and treated with E2, tamoxifen or ICI 164384. E2 and tamoxifen up-regulate the expression of the 5-HT1A via a mechanism involving synergistic activation by NF-ҡB with ER-α. Only ER-α, but not ER-β was able to mediate E2 effects, and mutation analysis showed that the transactivation domain of the p65 subunit of NF-ҡB and activation function 1 of ER-α were essential for regulation of 5-HT1A. The 5-HT1A gene lacks canonical ERE sequences, but the promoter contains two putative NF-ҡB binding sites; thus, the presence of NF-ҡB proteins is critical for synergistic induction by ER-α [12]. These data suggest that E2 may regulate 5-HT1A or autoreceptor expression through interaction between ER-α and NF-ҡB.
Conclusion
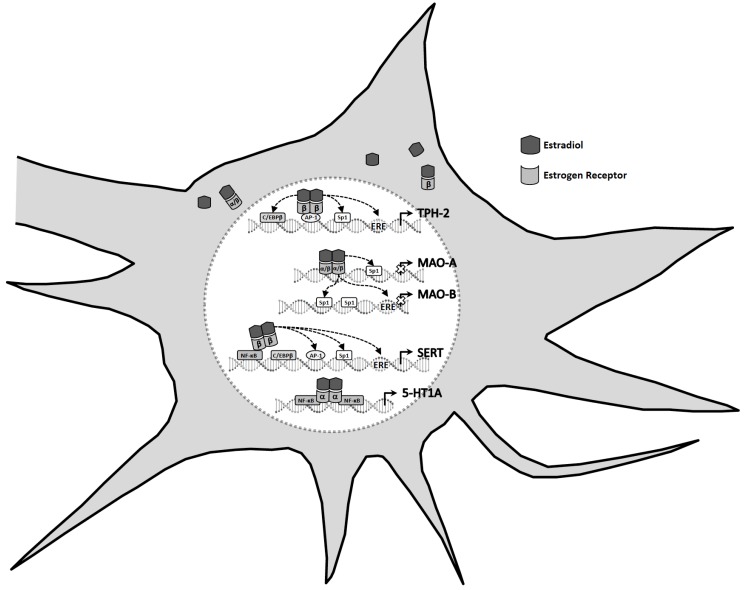
Deficiency in 5-HT neurotransmission and low levels of E2 are involved in the development of depression in women. ERT in depressed perimenopausal women reduces depressive symptoms; in addition, data from few clinical trials support the notion that postmenopausal women may benefit from E2 augmentation therapy to serotonergic antidepressants. Data showed in this review suggest a relationship between E2 and serotonergic neurotransmission system that may be involved in the neurobiological mechanism of depression. E2 modulates the expression of genes that regulate 5HT neurotransmission, such as TPH-2, MAO-A and-B, SERT, and 5-HT1A, which are implicated in depression (Fig. 2). SERT and TPH-2 contain a ERE, MAO-B a half ERE in their promoter regions and may thus be regulated by E2-activated ER-β directly binding to DNA. Also, the promoter regions of SERT, TPH-2 and MAO-B have regulatory elements for multiple transcription factors including AP-1, Sp1, C/EBPβ and NF-ҡB, which might bind activated ER. The MAO-A promoter does not contain canonical ERE, however, the presence of three Sp1 sites may provide a mechanism for E2 regulation of MAO-A expression via Sp1 tethering. The 5-HT1A gene lacks canonical ERE sequences, but the promoter contains two putative NF-ҡB binding sites that could mediate the E2 action indirectly through interaction between ER and NF-ҡB binding.
Fig. (2).
Estradiol regulates the expression of tryptophan hydroxylase-2 (TPH-2), monoamine oxidases (MAOs), serotonin transporter (SERT) and serotonin-1A receptor (5-HT1A) through classical mechanisms. Classically, estradiol-activated estrogen receptor dimers contact estrogen response elements (ERE) in the promoter regions of target genes, such as TPH-2, MAO-B and SERT, to initiate transcription. Besides, estrogen receptor can modulate the expression of genes without directly binding to deoxyribonucleic acid by tethering to other transcription factors, including specificity protein 1 (Sp1) and activator protein 1 (AP-1), nuclear factor kappa B (NF-ҡB) and CCAAT/enhancer binding protein β (C/EBPβ). The MAO-A promoter does not contain canonical ERE, however, the presence of three Sp1 sites may provide a mechanism for estradiol regulation. The 5-HT1A gene lacks canonical ERE, but the promoter contains two putative NF-ҡB binding sites that could mediate the estradiol action indirectly through interaction between estrogen receptor and NF-ҡB binding.
Studies have largely focused on the role of E2 in depression; however, more information about the mechanisms by which E2 participates in the regulation of depressed mood in women is needed. Identification of target genes for E2 implicated in pathophysiology of depression could be a strategy for developing more specific treatments for affective disorders in perimenopausal and postmenopausal women.
Acknowledgements
This work was supported by NC143370.2 from the Instituto Nacional de Psiquiatría “Ramón de la Fuente Muñiz” and Cátedras Consejo Nacional de Ciencia y Tecnología. The authors wish to express their thanks to Dr. Rodolfo Martínez-Mota and Dr. Nicoletta Righini for English language editing.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Marcus S.M., Young E.A., Kerber K.B., Kornstein S., Farabaugh A.H., Mitchell J., Wisniewski S.R., Balasubramani G.K., Trivedi M.H., Rush A.J. Gender differences in depression: findings from the STAR*D study. J. Affect. Disord. 2005;87(2-3):141–150. doi: 10.1016/j.jad.2004.09.008. [http://dx.doi.org/10.1016/j.jad.2004.09.008]. [PMID: 15982748]. [DOI] [PubMed] [Google Scholar]
- 2.Kessler R.C. Epidemiology of women and depression. J. Affect. Disord. 2003;74(1):5–13. doi: 10.1016/s0165-0327(02)00426-3. [http://dx.doi.org/10.1016/S0165-0327(02)00426-3]. [PMID: 12646294]. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt P.J., Rubinow D.R. Sex hormones and mood in the perimenopause. Ann. N. Y. Acad. Sci. 2009;1179:70–85. doi: 10.1111/j.1749-6632.2009.04982.x. [http://dx. doi.org/10.1111/j.1749-6632.2009.04982.x]. [PMID: 19906233]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider L.S., Small G.W., Hamilton S.H., Bystritsky A., Nemeroff C.B., Meyers B.S. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Am. J. Geriatr. Psychiatry. 1997;5(2):97–106. [http://dx.doi.org/10.1097/00019442-199721520-00002]. [PMID: 9106373]. [PubMed] [Google Scholar]
- 5.Schneider L.S., Small G.W., Clary C.M. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am. J. Geriatr. Psychiatry. 2001;9(4):393–399. [http://dx. doi.org/10.1097/00019442-200111000-00007]. [PMID: 11739065]. [PubMed] [Google Scholar]
- 6.Bethea C.L., Smith A.W., Centeno M.L., Reddy A.P. Long-term ovariectomy decreases serotonin neuron number and gene expression in free ranging macaques. Neuroscience. 2011;192:675–688. doi: 10.1016/j.neuroscience.2011.06.003. [http://dx.doi.org/10.1016/j.neuroscience.2011.06.003]. [PMID: 21763405]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Björnström L., Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 2005;19(4):833–842. doi: 10.1210/me.2004-0486. [http://dx.doi. org/10.1210/me.2004-0486]. [PMID: 15695368]. [DOI] [PubMed] [Google Scholar]
- 8.Flattem N.L., Blakely R.D. Modified structure of the human serotonin transporter promoter. Mol. Psychiatry. 2000;5(1):110–115. doi: 10.1038/sj.mp.4000585. [http://dx.doi.org/10.1038/sj.mp.4000585]. [PMID: 10673778]. [DOI] [PubMed] [Google Scholar]
- 9.Hiroi R., Handa R.J. Estrogen receptor-β regulates human tryptophan hydroxylase-2 through an estrogen response element in the 5′ untranslated region. J. Neurochem. 2013;127(4):487–495. doi: 10.1111/jnc.12401. [http:// dx.doi.org/10.1111/jnc.12401]. [PMID: 24033289]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z., Chen K., Shih J.C., Teng C.T. Estrogen-related receptors-stimulated monoamine oxidase B promoter activity is down-regulated by estrogen receptors. Mol. Endocrinol. 2006;20(7):1547–1561. doi: 10.1210/me.2005-0252. [http://dx.doi.org/10.1210/me.2005-0252]. [PMID: 16484337]. [DOI] [PubMed] [Google Scholar]
- 11.Denney R.M., Sharma A., Dave S.K., Waguespack A. A new look at the promoter of the human monoamine oxidase A gene: mapping transcription initiation sites and capacity to drive luciferase expression. J. Neurochem. 1994;63(3):843–856. doi: 10.1046/j.1471-4159.1994.63030843.x. [http://dx.doi.org/10.1046/j.1471-4159.1994.63030843.x]. [PMID: 7519662]. [DOI] [PubMed] [Google Scholar]
- 12.Wissink S., van der Burg B., Katzenellenbogen B.S., van der Saag P.T. Synergistic activation of the serotonin-1A receptor by nuclear factor-κ B and estrogen. Mol. Endocrinol. 2001;15(4):543–552. doi: 10.1210/mend.15.4.0629. [PMID: 11266506]. [DOI] [PubMed] [Google Scholar]
- 13.Owens M., Herbert J., Jones P.B., Sahakian B.J., Wilkinson P.O., Dunn V.J., Croudace T.J., Goodyer I.M. Elevated morning cortisol is a stratified population-level biomarker for major depression in boys only with high depressive symptoms. Proc. Natl. Acad. Sci. USA. 2014;111(9):3638–3643. doi: 10.1073/pnas.1318786111. [http://dx.doi.org/10. 1073/pnas.1318786111]. [PMID: 24550453]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobsen J.P.R., Medvedev I.O., Caron M.G. The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367(1601):2444–2459. doi: 10.1098/rstb.2012.0109. [http://dx.doi.org/10.1098/rstb.2012.0109]. [PMID: 22826344]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soares C.N. Mood disorders in midlife women: understanding the critical window and its clinical implications. Menopause. 2014;21(2):198–206. doi: 10.1097/GME.0000000000000193. [http://dx.doi.org/10.1097/GME.0000000000000193]. [PMID: 24448106]. [DOI] [PubMed] [Google Scholar]
- 16.Joffe H., Cohen L.S. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? 1998:798–811. doi: 10.1016/s0006-3223(98)00169-3. [DOI] [PubMed] [Google Scholar]
- 17.Deecher D., Andree T.H., Sloan D., Schechter L.E. From menarche to menopause: exploring the underlying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology. 2008;33(1):3–17. doi: 10.1016/j.psyneuen.2007.10.006. [http://dx.doi.org/10.1016/j.psyneuen.2007.10. 006]. [PMID: 18063486]. [DOI] [PubMed] [Google Scholar]
- 18.Steiner M., Dunn E., Born L. Hormones and mood: from menarche to menopause and beyond. J. Affect. Disord. 2003;74(1):67–83. doi: 10.1016/s0165-0327(02)00432-9. [http://dx.doi.org/10.1016/S0165-0327(02)00432-9]. [PMID: 12646300]. [DOI] [PubMed] [Google Scholar]
- 19.Lokuge S., Frey B.N., Foster J.A., Soares C.N., Steiner M. Depression in women: windows of vulnerability and new insights into the link between estrogen and serotonin. J. Clin. Psychiatry. 2011;72(11):e1563–e1569. doi: 10.4088/JCP.11com07089. [http://dx.doi.org/10.4088/JCP.11com 07089]. [PMID: 22127200]. [DOI] [PubMed] [Google Scholar]
- 20.Martinez P.E., Rubinow D.R., Nieman L.K., Koziol D.E., Morrow A.L., Schiller C.E., Cintron D., Thompson K.D., Khine K.K., Schmidt P.J. 5α-reductase inhibition prevents the luteal phase increase in plasma allopregnanolone levels and mitigates symptoms in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2016;41(4):1093–1102. doi: 10.1038/npp.2015.246. [http://dx.doi. org/10.1038/npp.2015.246]. [PMID: 26272051]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloch M., Rotenberg N., Koren D., Klein E. Risk factors for early postpartum depressive symptoms. Gen. Hosp. Psychiatry. 2006;28(1):3–8. doi: 10.1016/j.genhosppsych.2005.08.006. [http://dx.doi.org/10.1016/j.genhosppsych.2005. 08.006]. [PMID: 16377359]. [DOI] [PubMed] [Google Scholar]
- 22.Freeman E.W. Associations of depression with the transition to menopause. Menopause. 2010;17(4):823–827. doi: 10.1097/gme.0b013e3181db9f8b. [http://dx.doi.org/ 10.1097/gme.0b013e3181db9f8b]. [PMID: 20531231]. [DOI] [PubMed] [Google Scholar]
- 23.Cohen L.S., Soares C.N., Vitonis A.F., Otto M.W., Harlow B.L. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch. Gen. Psychiatry. 2006;63(4):385–390. doi: 10.1001/archpsyc.63.4.385. [http://dx.doi.org/10.1001/archpsyc.63.4.385]. [PMID: 16585467]. [DOI] [PubMed] [Google Scholar]
- 24.O’Lone R., Frith M.C., Karlsson E.K., Hansen U. Genomic targets of nuclear estrogen receptors. Mol. Endocrinol. 2004;18(8):1859–1875. doi: 10.1210/me.2003-0044. [http://dx.doi.org/10.1210/me.2003-0044]. [PMID: 15031323]. [DOI] [PubMed] [Google Scholar]
- 25.McEwen B.S., Alves S.E. Estrogen actions in the central nervous system. Endocr. Rev. 1999;20(3):279–307. doi: 10.1210/edrv.20.3.0365. [PMID: 10368772]. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson S., Mäkelä S., Treuter E., Tujague M., Thomsen J., Andersson G., Enmark E., Pettersson K., Warner M., Gustafsson J.A. Mechanisms of estrogen action. Physiol. Rev. 2001;81(4):1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [http://dx.doi.org/10.1152/physrev.2001.81.4.1535]. [PMID: 11581496]. [DOI] [PubMed] [Google Scholar]
- 27.Berry M., Metzger D., Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990;9(9):2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [PMID: 2118104]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J., Li Q. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol. Endocrinol. 2003;17(9):1681–1692. doi: 10.1210/me.2003-0116. [http://dx.doi.org/10.1210/me.2003-0116]. [PMID: 12805412]. [DOI] [PubMed] [Google Scholar]
- 29.Göttlicher M., Heck S., Herrlich P. Transcriptional cross-talk, the second mode of steroid hormone receptor action. J. Mol. Med. (Berl.) 1998;76(7):480–489. doi: 10.1007/s001090050242. [http://dx.doi.org/10.1007/s001090050242]. [PMID: 9660166]. [DOI] [PubMed] [Google Scholar]
- 30.Nawaz Z., Lonard D.M., Dennis A.P., Smith C.L., O’Malley B.W. Proteasome-dependent degradation of the human estrogen receptor. Proc. Natl. Acad. Sci. USA. 1999;96(5):1858–1862. doi: 10.1073/pnas.96.5.1858. [http:// dx.doi.org/10.1073/pnas.96.5.1858]. [PMID: 10051559]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Migliaccio A., Piccolo D., Castoria G., Di Domenico M., Bilancio A., Lombardi M., Gong W., Beato M., Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17(7):2008–2018. doi: 10.1093/emboj/17.7.2008. [http://dx.doi.org/10.1093/emboj/17.7.2008]. [PMID: 9524123]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boonyaratanakornkit V., Edwards D.P. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin. Reprod. Med. 2007;25(3):139–153. doi: 10.1055/s-2007-973427. [http://dx.doi.org/10.1055/s-2007-973427]. [PMID: 17447204]. [DOI] [PubMed] [Google Scholar]
- 33.Heldring N., Pike A., Andersson S., Matthews J., Cheng G., Hartman J., Tujague M., Ström A., Treuter E., Warner M., Gustafsson J.A. Estrogen receptors: how do they signal and what are their targets. Physiol. Rev. 2007;87(3):905–931. doi: 10.1152/physrev.00026.2006. [http://dx.doi. org/10.1152/physrev.00026.2006]. [PMID: 17615392]. [DOI] [PubMed] [Google Scholar]
- 34.Lu C.L., Herndon C. New roles for neuronal estrogen receptors. Neurogastroenterol. Motil. 2017;29(7):1–7. doi: 10.1111/nmo.13121. [http://dx.doi.org/ 10.1111/nmo.13121]. [PMID: 28597596]. [DOI] [PubMed] [Google Scholar]
- 35.Vrtačnik P., Ostanek B., Mencej-Bedrač S., Marc J. The many faces of estrogen signaling. Biochem. Med. (Zagreb) 2014;24(3):329–342. doi: 10.11613/BM.2014.035. [http://dx.doi.org/10.11613/BM.2014.035]. [PMID: 25351351]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayward C., Sanborn K. Puberty and the emergence of gender differences in psychopathology. J. Adolesc. Health. 2002;30(4) Suppl.:49–58. doi: 10.1016/s1054-139x(02)00336-1. [http://dx.doi.org/10.1016/S1054-139X(02)00336-1]. [PMID: 11943575]. [DOI] [PubMed] [Google Scholar]
- 37.Kessler R.C., Walters E.E. Epidemiology of DSM-III-R major depression and minor depression among adolescents and young adults in the National Comorbidity Survey. Depress. Anxiety. 1998;7(1):3–14. doi: 10.1002/(sici)1520-6394(1998)7:1<3::aid-da2>3.0.co;2-f. [http://dx.doi.org/10.1002/(SICI)1520-6394(1998)7:1 <3:AID-DA2>3.0.CO;2-F]. [PMID: 9592628]. [DOI] [PubMed] [Google Scholar]
- 38.Walf A.A., Frye C.A. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31(6):1097–1111. doi: 10.1038/sj.npp.1301067. [http://dx.doi.org/10.1038/sj.npp.1301067]. [PMID: 16554740]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borrow A.P., Cameron N.M. Estrogenic mediation of serotonergic and neurotrophic systems: implications for female mood disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;54:13–25. doi: 10.1016/j.pnpbp.2014.05.009. [http://dx.doi.org/10.1016/j.pnpbp.2014.05.009]. [PMID: 24865152]. [DOI] [PubMed] [Google Scholar]
- 40.Holsen L.M., Ph D., Spaeth S.B., Lee J., Ogden L.a, Klibanski A., Whitfield-gabrieli S., Goldstein J.M. Stress Response Circuitry Hypoactivation Related to Hormonal Dysfunction in Women with Major Depression. J. Affect. Disord. 2011;131:379–387. doi: 10.1016/j.jad.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bloch M., Schmidt P.J., Danaceau M., Murphy J., Nieman L., Rubinow D.R. Effects of gonadal steroids in women with a history of postpartum depression. Am. J. Psychiatry. 2000;157(6):924–930. doi: 10.1176/appi.ajp.157.6.924. [http://dx.doi.org/10.1176/appi.ajp.157.6.924]. [PMID: 10831472]. [DOI] [PubMed] [Google Scholar]
- 42.Young E.A., Midgley A.R., Carlson N.E., Brown M.B. Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Arch. Gen. Psychiatry. 2000;57(12):1157–1162. doi: 10.1001/archpsyc.57.12.1157. [http://dx.doi.org/ 10.1001/archpsyc.57.12.1157]. [PMID: 11115329]. [DOI] [PubMed] [Google Scholar]
- 43.Cohen L.S., Soares C.N., Poitras J.R., Prouty J., Alexander A.B., Shifren J.L. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am. J. Psychiatry. 2003;160(8):1519–1522. doi: 10.1176/appi.ajp.160.8.1519. [http://dx.doi.org/10. 1176/appi.ajp.160.8.1519]. [PMID: 12900318]. [DOI] [PubMed] [Google Scholar]
- 44.Soares C.N., Almeida O.P., Joffe H., Cohen L.S.S., de Novaes Soares C., Almedia O., Joff H., Cohen L.S.S. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch. Gen. Psychiatry. 2001;58(6):529–534. doi: 10.1001/archpsyc.58.6.529. [http://dx.doi.org/10.1001/ archpsyc.58.6.529]. [PMID: 11386980]. [DOI] [PubMed] [Google Scholar]
- 45.Fischer B., Gleason C., Asthana S. Effects of hormone therapy on cognition and mood. Fertil. Steril. 2014;101(4):898–904. doi: 10.1016/j.fertnstert.2014.02.025. [http:// dx.doi.org/10.1016/j.fertnstert.2014.02.025]. [PMID: 24680649]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bastos C.P., Pereira L.M., Ferreira-Vieira T.H., Drumond L.E., Massensini A.R., Moraes M.F.D., Pereira G.S. Object recognition memory deficit and depressive-like behavior caused by chronic ovariectomy can be transitorialy recovered by the acute activation of hippocampal estrogen receptors. Psychoneuroendocrinology. 2015;57:14–25. doi: 10.1016/j.psyneuen.2015.03.020. [http://dx.doi.org/10.1016/j.psyneuen.2015.03.020]. [PMID: 25867995]. [DOI] [PubMed] [Google Scholar]
- 47.Zweifel J.E., O’Brien W.H. A meta-analysis of the effect of hormone replacement therapy upon depressed mood. Psychoneuroendocrinology. 1997;22(3):189–212. doi: 10.1016/s0306-4530(96)00034-0. [http://dx.doi.org/10.1016/S0306-4530(96)00034-0]. [PMID: 9203229]. [DOI] [PubMed] [Google Scholar]
- 48.Rubinow D.R., Johnson S.L., Schmidt P.J., Girdler S., Gaynes B. Efficacy of Estradiol in Perimenopausal Depression: So Much Promise and so Few Answers. Depress. Anxiety. 2015;32(8):539–549. doi: 10.1002/da.22391. [http://dx.doi.org/10.1002/da.22391]. [PMID: 26130315]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carranza-Lira S., MacGregor-Gooch A.L., Saráchaga-Osterwalder M. Mood modifications with raloxifene and continuous combined estrogen plus progestin hormone therapy. Int. J. Fertil. Womens Med. 2004;49(3):120–122. [PMID: 15303313]. [PubMed] [Google Scholar]
- 50.Lemini C., García-Albor E., Cruz-López B., Matamoros-Trejo G., Martínez-Mota L. Differential effect of the 17β-aminoestrogens prolame, butolame and pentolame in anxiety and depression models in rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:102–108. doi: 10.1016/j.pnpbp.2015.07.013. [http://dx.doi.org/10.1016/j.pnpbp.2015.07.013]. [PMID: 26239795]. [DOI] [PubMed] [Google Scholar]
- 51.Westlund Tam L., Parry B.L. Does estrogen enhance the antidepressant effects of fluoxetine? J. Affect. Disord. 2003;77(1):87–92. doi: 10.1016/s0165-0327(02)00357-9. [http://dx.doi.org/10.1016/S0165-0327(02)00357-9]. [PMID: 14550939]. [DOI] [PubMed] [Google Scholar]
- 52.Owens M.J., Nemeroff C.B. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin. Chem. 1994;40(2):288–295. [PMID: 7508830]. [PubMed] [Google Scholar]
- 53.Köhler S., Cierpinsky K., Kronenberg G., Adli M. The serotonergic system in the neurobiology of depression: Relevance for novel antidepressants. J. Psychopharmacol. (Oxford) 2016;30(1):13–22. doi: 10.1177/0269881115609072. [http://dx.doi.org/10.1177/0269881115609072]. [PMID: 26464458]. [DOI] [PubMed] [Google Scholar]
- 54.Lasiuk G.C., Hegadoren K.M. The effects of estradiol on central serotonergic systems and its relationship to mood in women. Biol. Res. Nurs. 2007;9(2):147–160. doi: 10.1177/1099800407305600. [http://dx.doi.org/10.1177/ 1099800407305600]. [PMID: 17909167]. [DOI] [PubMed] [Google Scholar]
- 55.Akiskal H.S., McKinney W.T. Jr depressive disorders: Toward a unified hypothesis. Science (80). 1973;182:20–29. doi: 10.1126/science.182.4107.20. [DOI] [PubMed] [Google Scholar]
- 56.Leyton M., Young S.N., Benkelfat C. Relapse of depression after rapid depletion of tryptophan. Lancet. 1997;349(9068):1840–1841. doi: 10.1016/S0140-6736(05)61726-6. [http://dx.doi.org/10.1016/S0140-6736(05)61726-6]. [PMID: 9269238]. [DOI] [PubMed] [Google Scholar]
- 57.Smith K.A., Clifford E.M., Hockney R.A., Clark D.M., Cowen P.J. Effect of tryptophan depletion on mood in male and female volunteers: A pilot study. Psychopharmacology (Berl.) 1997;12:111–117. [Google Scholar]
- 58.Savitz J., Lucki I., Drevets W.C.W. 5-HT(1A) receptor function in major depressive disorder. Prog. Neurobiol. 2009;88(1):17–31. doi: 10.1016/j.pneurobio.2009.01.009. [http://dx.doi.org/10.1016/j.pneurobio.2009.01.009]. [PMID: 19428959]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drevets W.C., Frank E., Price J.C., Kupfer D.J., Holt D., Greer P.J., Huang Y., Gautier C., Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biol. Psychiatry. 1999;46(10):1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [http://dx.doi.org/10.1016/S0006-3223(99)00189-4]. [PMID: 10578452]. [DOI] [PubMed] [Google Scholar]
- 60.Meltzer C.C., Price J.C., Mathis C.A., Butters M.A., Ziolko S.K., Moses-Kolko E., Mazumdar S., Mulsant B.H., Houck P.R., Lopresti B.J., Weissfeld L.A., Reynolds C.F. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29(12):2258–2265. doi: 10.1038/sj.npp.1300556. [http://dx.doi.org/ 10.1038/sj.npp.1300556]. [PMID: 15483563]. [DOI] [PubMed] [Google Scholar]
- 61.Nugent A.C., Bain E.E., Carlson P.J., Neumeister A., Bonne O., Carson R.E., Eckelman W., Herscovitch P., Zarate C.A., Jr, Charney D.S., Drevets W.C. Reduced post-synaptic serotonin type 1A receptor binding in bipolar depression. Eur. Neuropsychopharmacol. 2013;23(8):822–829. doi: 10.1016/j.euroneuro.2012.11.005. [http://dx.doi.org/10.1016/ j.euroneuro.2012.11.005]. [PMID: 23434290]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.López J.F., Chalmers D.T., Little K.Y., Watson S.J.A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol. Psychiatry. 1998;43(8):547–573. doi: 10.1016/s0006-3223(97)00484-8. [http://dx.doi.org/10.1016/S0006-3223(97) 00484-8]. [PMID: 9564441]. [DOI] [PubMed] [Google Scholar]
- 63.Parsey R.V., Olvet D.M., Oquendo M.A., Huang Y-Y., Ogden R.T., Mann J.J. Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006;31(8):1745–1749. doi: 10.1038/sj.npp.1300992. [http://dx.doi.org/10.1038/sj.npp.1300992]. [PMID: 16395308]. [DOI] [PubMed] [Google Scholar]
- 64.Staley J.K., Sanacora G., Tamagnan G., Maciejewski P.K., Malison R.T., Berman R.M., Vythilingam M., Kugaya A., Baldwin R.M., Seibyl J.P., Charney D., Innis R.B. Sex differences in diencephalon serotonin transporter availability in major depression. Biol. Psychiatry. 2006;59(1):40–47. doi: 10.1016/j.biopsych.2005.06.012. [http://dx.doi. org/10.1016/j.biopsych.2005.06.012]. [PMID: 16139815]. [DOI] [PubMed] [Google Scholar]
- 65.Paech K., Webb P., Kuiper G.G., Nilsson S., Gustafsson J., Kushner P.J., Scanlan T.S. Differential Ligand Activation of Estrogen Receptors ERalpha and ERbeta at AP1 Sites Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 66.Alves S.E., Weiland N.G., Hayashi S., McEwen B.S. Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the rat dorsal raphe nucleus. J. Comp. Neurol. 1998;391(3):322–334. [http://dx.doi.org/10.1002/(SICI)1096-9861 (19980216)391:3<322:AID-CNE3>3.0.CO;2-3]. [PMID: 9492203]. [PubMed] [Google Scholar]
- 67.Sheng Z., Kawano J., Yanai A., Fujinaga R., Tanaka M., Watanabe Y., Shinoda K. Expression of estrogen receptors (A, β) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei. sex and species differences. Neurosci. Res. 2004;49:185–196. doi: 10.1016/j.neures.2004.02.011. [PMID: 15140561]. [DOI] [PubMed] [Google Scholar]
- 68.Gundlah C., Lu N.Z., Bethea C.L. Ovarian steroid regulation of monoamine oxidase-A and -B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology (Berl.) 2002;160(3):271–282. doi: 10.1007/s00213-001-0959-0. [http://dx.doi.org/10.1007/s00213-001-0959-0]. [PMID: 11889496]. [DOI] [PubMed] [Google Scholar]
- 69.Osterlund M.K., Hurd Y.L. Acute 17 beta-estradiol treatment down-regulates serotonin 5HT1A receptor mRNA expression in the limbic system of female rats. Brain Res. Mol. Brain Res. 1998;55(1):169–172. doi: 10.1016/s0169-328x(98)00018-7. [http://dx.doi.org/10.1016/S0169-328X(98)00018-7]. [PMID: 9645972]. [DOI] [PubMed] [Google Scholar]
- 70.McQueen J.K., Wilson H., Fink G. Estradiol-17 beta increases serotonin transporter (SERT) mRNA levels and the density of SERT-binding sites in female rat brain. Brain Res. Mol. Brain Res. 1997;45(1):13–23. doi: 10.1016/s0169-328x(96)00233-1. [http://dx.doi.org/10.1016/S0169-328X(96) 00233-1]. [PMID: 9105666]. [DOI] [PubMed] [Google Scholar]
- 71.Soares C.N. Depression in peri- and postmenopausal women: prevalence, pathophysiology and pharmacological management. Drugs Aging. 2013;30(9):677–685. doi: 10.1007/s40266-013-0100-1. [http://dx.doi.org/10.1007/ s40266-013-0100-1]. [PMID: 23801148]. [DOI] [PubMed] [Google Scholar]
- 72.Schiller C.E., Schmidt P.J., Rubinow D.R. Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology (Berl.) 2014;231(17):3557–3567. doi: 10.1007/s00213-014-3599-x. [http://dx. doi.org/10.1007/s00213-014-3599-x]. [PMID: 24846476]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Serova L., Nakashima A., Sabban L. Estradiol stimulates gene expression of norepinephrine biosynthetic enzymes in rat locus coeruleus. Neuroendocrinology. 2002;10595:193–200. doi: 10.1159/000048237. [DOI] [PubMed] [Google Scholar]
- 74.Maharjan S., Serova L., Sabban E.L. Transcriptional regulation of tyrosine hydroxylase by estrogen: opposite effects with estrogen receptors α and β and interactions with cyclic AMP. J. Neurochem. 2005;93(6):1502–1514. doi: 10.1111/j.1471-4159.2005.03142.x. [http://dx.doi.org/10.1111/j.1471-4159. 2005.03142.x]. [PMID: 15935066]. [DOI] [PubMed] [Google Scholar]
- 75.Pau K.Y., Hess D.L., Kohama S., Bao J., Pau C.Y., Spies H.G. Oestrogen upregulates noradrenaline release in the mediobasal hypothalamus and tyrosine hydroxylase gene expression in the brainstem of ovariectomized rhesus macaques. J. Neuroendocrinol. 2000;12(9):899–909. doi: 10.1046/j.1365-2826.2000.00549.x. [http://dx.doi.org/10.1046/j.1365-2826.2000. 00549.x]. [PMID: 10971815]. [DOI] [PubMed] [Google Scholar]
- 76.Jiang H., Xie T., Ramsden D.B., Ho S.L. Human catechol-O-methyltransferase down-regulation by estradiol. Neuropharmacology. 2003;45(7):1011–1018. doi: 10.1016/s0028-3908(03)00286-7. [http://dx.doi.org/10.1016/S0028-3908 (03)00286-7]. [PMID: 14573393]. [DOI] [PubMed] [Google Scholar]
- 77.Bangasser D.A., Wiersielis K.R., Khantsis S. Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Res. 2016;1641(Pt B):177–188. doi: 10.1016/j.brainres.2015.11.021. http://dx.doi.org/10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xie T., Ho S-L., Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol. Pharmacol. 1999;56(1):31–38. doi: 10.1124/mol.56.1.31. [http:// dx.doi.org/10.1124/mol.56.1.31]. [PMID: 10385681]. [DOI] [PubMed] [Google Scholar]
- 79.Grahame-Smith D.G. Tryptophan hydroxylation in brain. Biochem. Biophys. Res. Commun. 1964;16(6):586–592. doi: 10.1016/0006-291x(64)90197-4. [http://dx.doi. org/10.1016/0006-291X(64)90197-4]. [PMID: 5297063]. [DOI] [PubMed] [Google Scholar]
- 80.Walther D.J., Peter J.U., Bashammakh S., Hörtnagl H., Voits M., Fink H., Bader M. Synthesis of Serotonin by a Second Tryptophan Hydroxylase Isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [http://dx.doi.org/10.1126/science.1078197]. [DOI] [PubMed] [Google Scholar]
- 81.Zhang X., Gainetdinov R.R., Beaulieu J.M., Sotnikova T.D., Burch L.H., Williams R.B., Schwartz D.A., Krishnan K.R.R., Caron M.G. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45(1):11–16. doi: 10.1016/j.neuron.2004.12.014. [http://dx.doi.org/10.1016/j.neuron.2004.12.014]. [PMID: 15629698]. [DOI] [PubMed] [Google Scholar]
- 82.Haghighi F., Bach-Mizrachi H., Huang Y.Y., Arango V., Shi S., Dwork A.J., Rosoklija G., Sheng H.T., Morozova I., Ju J., Russo J.J., Mann J.J. Genetic architecture of the human tryptophan hydroxylase 2 Gene: existence of neural isoforms and relevance for major depression. Mol. Psychiatry. 2008;13(8):813–820. doi: 10.1038/sj.mp.4002127. [http://dx.doi.org/10.1038/sj.mp.4002127]. [PMID: 18180764]. [DOI] [PubMed] [Google Scholar]
- 83.Bach-Mizrachi H., Underwood M.D., Tin A., Ellis S.P., Mann J.J., Arango V. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol. Psychiatry. 2008;13:507–513. doi: 10.1038/sj.mp.4002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boldrini M., Underwood M.D., Mann J.J., Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 2005;1041(1):19–28. doi: 10.1016/j.brainres.2005.01.083. [http://dx. doi.org/10.1016/j.brainres.2005.01.083]. [PMID: 15804496]. [DOI] [PubMed] [Google Scholar]
- 85.Bonkale W.L., Murdock S., Janosky J.E., Austin M.C. Normal levels of tryptophan hydroxylase immunoreactivity in the dorsal raphe of depressed suicide victims. J. Neurochem. 2004;88(4):958–964. doi: 10.1046/j.1471-4159.2003.02225.x. [http://dx.doi.org/10.1046/j.1471-4159.2003.02225.x]. [PMID: 14756817]. [DOI] [PubMed] [Google Scholar]
- 86.Bethea C.L., Mirkes S.J., Shively C.A., Adams M.R. Steroid regulation of tryptophan hydroxylase protein in the dorsal raphe of macaques. Biol. Psychiatry. 2000;47(6):562–576. doi: 10.1016/s0006-3223(99)00156-0. [http://dx.doi. org/10.1016/S0006-3223(99)00156-0]. [PMID: 10715363]. [DOI] [PubMed] [Google Scholar]
- 87.Nomura M., Akama K.T., Alves S.E., Korach K.S., Gustafsson J.Å., Pfaff D.W., Ogawa S. Differential distribution of estrogen receptor (ER)-α and ER-β in the midbrain raphe nuclei and periaqueductal gray in male mouse: Predominant role of ER-β in midbrain serotonergic systems. Neuroscience. 2005;130(2):445–456. doi: 10.1016/j.neuroscience.2004.09.028. [http://dx.doi.org/10.1016/j.neuroscience.2004.09.028]. [PMID: 15664701]. [DOI] [PubMed] [Google Scholar]
- 88.Gundlah C., Lu N.Z., Bethea C.L. Ovarian steroid regulation of monoamine oxidase-A and -B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology (Berl.) 2002;160(3):271–282. doi: 10.1007/s00213-001-0959-0. [http://dx.doi.org/10.1007/s00213-001-0959-0]. [PMID: 11889496]. [DOI] [PubMed] [Google Scholar]
- 89.Lu H., Ozawa H., Nishi M., Ito T., Kawata M. Serotonergic neurones in the dorsal raphe nucleus that project into the medial preoptic area contain oestrogen receptor beta. J. Neuroendocrinol. 2001;13(10):839–845. doi: 10.1046/j.1365-2826.2001.00695.x. [http://dx.doi.org/10.1046/j.1365-2826. 2001.00695.x]. [PMID: 11679052]. [DOI] [PubMed] [Google Scholar]
- 90.Donner N.C., Handa R.J. 2009;163:705–718. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Borsini F., Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl.) 1988;94(2):147–160. doi: 10.1007/BF00176837. [http://dx.doi.org/10.1007/BF00176837]. [PMID: 3127840]. [DOI] [PubMed] [Google Scholar]
- 92.Paul I.A., Duncan G.E., Kuhn C., Mueller R.A., Hong J.S., Breese G.R. Neural adaptation in imipramine-treated rats processed in forced swim test: assessment of time course, handling, rat strain and amine uptake. J. Pharmacol. Exp. Ther. 1990;252(3):997–1005. [PMID: 2157002]. [PubMed] [Google Scholar]
- 93.Porsolt R.D., Anton G., Blavet N., Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978;47(4):379–391. doi: 10.1016/0014-2999(78)90118-8. [http://dx.doi.org/10.1016/0014-2999(78)90118-8]. [PMID: 204499]. [DOI] [PubMed] [Google Scholar]
- 94.Detke M.J., Rickels M., Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl.) 1995;121(1):66–72. doi: 10.1007/BF02245592. [http://dx.doi.org/10.1007/BF02245592]. [PMID: 8539342]. [DOI] [PubMed] [Google Scholar]
- 95.Contreras C.M., Martınez-Mota L. Desipramine Restricts Estrous Cycle Oscillations, M.S. in Swimming. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1998;22:1121–1128. doi: 10.1016/s0278-5846(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 96.Yang F-Z., Wu Y., Zhang W-G., Cai Y-Y., Shi S-X. Estradiol or fluoxetine alters depressive behavior and tryptophan hydroxylase in rat raphe. Neuroreport. 2010;21(4):309–312. doi: 10.1097/WNR.0b013e3283377445. [http://dx. doi.org/10.1097/WNR.0b013e3283377445]. [PMID: 20134355]. [DOI] [PubMed] [Google Scholar]
- 97.Benmansour S., Weaver R.S., Barton A.K., Adeniji O.S., Frazer A. Comparison of effects of E2 and progesterone on serotonergic function. Biol. Psychiatry. 2012;71:633–641. doi: 10.1016/j.biopsych.2011.11.023. [PMID: 22225849]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koldzic-Zivanovic N., Seitz P.K., Watson C.S., Cunningham K.A., Thomas M.L. Intracellular signaling involved in estrogen regulation of serotonin reuptake. Mol. Cell. Endocrinol. 2004;226(1-2):33–42. doi: 10.1016/j.mce.2004.07.017. [http://dx.doi.org/10.1016/j.mce.2004.07.017]. [PMID: 15489003]. [DOI] [PubMed] [Google Scholar]
- 99.Chen G.L., Miller G.M. 5′-Untranslated region of the tryptophan hydroxylase-2 gene harbors an asymmetric bidirectional promoter but not internal ribosome entry site in vitro. Gene. 2009;435(1-2):53–62. doi: 10.1016/j.gene.2008.12.019. [http://dx.doi.org/10.1016/j.gene.2008.12.019]. [PMID: 19344641]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Machado-Vieira R., Mallinger A.G. Abnormal function of monoamine oxidase-A in comorbid major depressive disorder and cardiovascular disease: pathophysiological and therapeutic implications. Mol. Med. Rep. 2012;6(5):915–922. doi: 10.3892/mmr.2012.1062. [review]. [PMID: 22948532]. [DOI] [PubMed] [Google Scholar]
- 101.Bach A.W., Lan N.C., Johnson D.L., Abell C.W., Bembenek M.E., Kwan S.W., Seeburg P.H., Shih J.C. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc. Natl. Acad. Sci. USA. 1988;85(13):4934–4938. doi: 10.1073/pnas.85.13.4934. [http://dx.doi.org/10.1073/pnas.85.13.4934]. [PMID: 3387449]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Youdim M.B., Finberg J.P. New directions in monoamine oxidase A and B selective inhibitors and substrates. Biochem. Pharmacol. 1991;41(2):155–162. doi: 10.1016/0006-2952(91)90471-g. [http://dx.doi.org/10.1016/0006-2952(91) 90471-G]. [PMID: 1989626]. [DOI] [PubMed] [Google Scholar]
- 103.Luque J.M., Bleuel Z., Hendrickson A., Richards J.G. Detection of MAO-A and MAO-B mRNAs in monkey brainstem by cross-hybridization with human oligonucleotide probes. Brain Res. Mol. Brain Res. 1996;36(2):357–360. doi: 10.1016/0169-328x(96)88407-5. [http://dx.doi.org/10.1016/0169-328X(96)88407-5]. [PMID: 8965658]. [DOI] [PubMed] [Google Scholar]
- 104.Shulman K.I., Herrmann N., Walker S.E. Current place of monoamine oxidase inhibitors in the treatment of depression. CNS Drugs. 2013;27(10):789–797. doi: 10.1007/s40263-013-0097-3. [http://dx.doi.org/10.1007/s40263-013-0097-3]. [PMID: 23934742]. [DOI] [PubMed] [Google Scholar]
- 105.Duncan J., Johnson S., Ou X.M. Monoamine oxidases in major depressive disorder and alcoholism. Drug Discov. Ther. 2012;6(3):112–122. [PMID: 22890201]. [PubMed] [Google Scholar]
- 106.Meyer J.H., Ginovart N., Boovariwala A., Sagrati S., Hussey D., Garcia A., Young T., Praschak-Rieder N., Wilson A.A., Houle S. Elevated monoamine oxidase a levels in the brain. Arch. Gen. Psychiatry. 2006;63:1209. doi: 10.1001/archpsyc.63.11.1209. [http://dx.doi.org/10.1001/ archpsyc.63.11.1209]. [PMID: 17088501]. [DOI] [PubMed] [Google Scholar]
- 107.Rekkas P.V., Wilson A.A., Lee V.W.H., Yogalingam P., Sacher J., Rusjan P., Houle S., Stewart D.E., Kolla N.J., Kish S., Chiuccariello L., Meyer J.H. Greater monoamine oxidase a binding in perimenopausal age as measured with carbon 11-labeled harmine positron emission tomography. JAMA Psychiatry. 2014;71(8):873–879. doi: 10.1001/jamapsychiatry.2014.250. [http://dx.doi.org/10.1001/jamapsychiatry.2014.250]. [PMID: 24898155]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chiuccariello L., Houle S., Miler L., Cooke R.G., Rusjan P.M., Rajkowska G., Levitan R.D., Kish S.J., Kolla N.J., Ou X., Wilson A.A., Meyer J.H. Elevated monoamine oxidase a binding during major depressive episodes is associated with greater severity and reversed neurovegetative symptoms. Neuropsychopharmacology. 2014;39(4):973–980. doi: 10.1038/npp.2013.297. [http://dx.doi.org/10.1038/npp.2013.297]. [PMID: 24154665]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klimek V., Roberson G., Stockmeier C.A., Ordway G.A. Serotonin transporter and MAO-B levels in monoamine nuclei of the human brainstem are normal in major depression. J. Psychiatr. Res. 2003;37(5):387–397. doi: 10.1016/s0022-3956(03)00045-1. [http://dx.doi.org/10.1016/S0022-3956 (03)00045-1]. [PMID: 12849931]. [DOI] [PubMed] [Google Scholar]
- 110.Smith L.J., Henderson J.A., Abell C.W., Bethea C.L. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport, and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology. 2004;29(11):2035–2045. doi: 10.1038/sj.npp.1300510. [http://dx.doi. org/10.1038/sj.npp.1300510]. [PMID: 15199371]. [DOI] [PubMed] [Google Scholar]
- 111.Ortega-Corona B.G., Valencia-Sánchez A., Kubli-Garfias C., Anton-Tay F., Salazar L.A., Villarreal J.E., Ponce-Monter H. Hypothalamic monoamine oxidase activity in ovariectomized rats after sexual behavior restoration. Arch. Med. Res. 1994;25(3):337–340. [PMID: 7803985]. [PubMed] [Google Scholar]
- 112.Chevillard C., Barden N., Saavedra J.M. Estradiol treatment decreases type A and increases type B monoamine oxidase in specific brain stem areas and cerebellum of ovariectomized rats. Brain Res. 1981;222(1):177–181. doi: 10.1016/0006-8993(81)90955-0. [http://dx.doi.org/10.1016/0006-8993 (81)90955-0]. [PMID: 7296265]. [DOI] [PubMed] [Google Scholar]
- 113.Bethea C.L., Lu N.Z., Gundlah C., Streicher J.M. Diverse actions of ovarian steroids in the serotonin neural system. Front. Neuroendocrinol. 2002;23(1):41–100. doi: 10.1006/frne.2001.0225. [http://dx.doi.org/10.1006/ frne.2001.0225]. [PMID: 11906203]. [DOI] [PubMed] [Google Scholar]
- 114.Olivier B. Serotonin: a never-ending story. Eur. J. Pharmacol. 2015;753:2–18. doi: 10.1016/j.ejphar.2014.10.031. [http://dx.doi.org/10.1016/j.ejphar.2014.10.031]. [PMID: 25446560]. [DOI] [PubMed] [Google Scholar]
- 115.Haase J. Killian, a; Magnani, F.; Williams, C. SERT is regulated by syntaxin I A one potential regulatory protein is the neuronal. Biochem. Soc. Trans. 2001;29:722–728. doi: 10.1042/0300-5127:0290722. [http://dx.doi.org/10. 1042/bst0290722]. [PMID: 11709063]. [DOI] [PubMed] [Google Scholar]
- 116.White K.J., Walline C.C., Barker E.L. Serotonin transporters: implications for antidepressant drug development. AAPS J. 2005;7(2):E421–E433. doi: 10.1208/aapsj070242. [http://dx.doi.org/10.1208/aapsj070242]. [PMID: 16353921]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gryglewski G., Lanzenberger R., Kranz G.S., Cumming P. Meta-analysis of molecular imaging of serotonin transporters in major depression. J. Cereb. Blood Flow Metab. 2014;34(7):1096–1103. doi: 10.1038/jcbfm.2014.82. [http://dx.doi.org/10.1038/jcbfm.2014.82]. [PMID: 24802331]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pecins-Thompson M., Bethea C.L. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience. 1999;89(1):267–277. doi: 10.1016/s0306-4522(98)00326-1. [http:// dx.doi.org/10.1016/S0306-4522(98)00326-1]. [PMID: 10051234]. [DOI] [PubMed] [Google Scholar]
- 119.Zhou W., Koldzic-Zivanovic N., Clarke C.H., de Beun R., Wassermann K., Bury P.S., Cunningham K.A., Thomas M.L. Selective estrogen receptor modulator effects in the rat brain. Neuroendocrinology. 2002;75(1):24–33. doi: 10.1159/000048218. [http://dx.doi.org/10.1159/ 000048218]. [PMID: 11810032]. [DOI] [PubMed] [Google Scholar]
- 120.Lu N.Z., Eshleman A.J., Janowsky A., Bethea C.L. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution, and function in female macaques. Mol. Psychiatry. 2003;8(3):353–360. doi: 10.1038/sj.mp.4001243. [http://dx.doi.org/10.1038/sj.mp.4001243]. [PMID: 12660809]. [DOI] [PubMed] [Google Scholar]
- 121.Sumner B.E., Grant K.E., Rosie R., Hegele-Hartung C., Fritzemeier K-H., Fink G. Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine(2A) receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res. Mol. Brain Res. 1999;73(1-2):119–128. doi: 10.1016/s0169-328x(99)00243-0. [http://dx.doi.org/10.1016/S0169-328X(99)00243-0]. [PMID: 10581405]. [DOI] [PubMed] [Google Scholar]
- 122.Rivera H.M., Oberbeck D.R., Kwon B., Houpt T.A., Eckel L.A. Estradiol increases Pet-1 and serotonin transporter mRNA in the midbrain raphe nuclei of ovariectomized rats. Brain Res. 2009;1259:51–58. doi: 10.1016/j.brainres.2008.12.067. [http://dx.doi.org/10.1016/j.brainres.2008.12.067]. [PMID: 19168037]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shively C.A., Mirkes S.J., Lu N.Z., Henderson J.A., Bethea C.L. Soy and social stress affect serotonin neurotransmission in primates. Pharmacogenomics J. 2003;3(2):114–121. doi: 10.1038/sj.tpj.6500166. [http://dx. doi.org/10.1038/sj.tpj.6500166]. [PMID: 12746737]. [DOI] [PubMed] [Google Scholar]
- 124.Jovanovic H., Karlsson P., Cerin A., Halldin C., Nordström A.L. 5-HT(1A) receptor and 5-HTT binding during the menstrual cycle in healthy women examined with [(11)C] WAY100635 and [(11)C] MADAM PET. Psychiatry Res. 2009;172(1):31–37. doi: 10.1016/j.pscychresns.2008.07.002. [http://dx.doi.org/10.1016/j.pscychresns.2008.07.002]. [PMID: 19118985]. [DOI] [PubMed] [Google Scholar]
- 125.Mitra C., Guha S.R. Serotonin oxidation by type B MAO of rat brain. Biochem. Pharmacol. 1980;29(9):1213–1216. doi: 10.1016/0006-2952(80)90276-2. [http://dx. doi.org/10.1016/0006-2952(80)90276-2]. [PMID: 6772194]. [DOI] [PubMed] [Google Scholar]
- 126.Jabeen H.D. Raphe-hippocampal serotonin neurotransmission in the sex related differences of adaptation to stress: Focus on serotonin-1A receptor. Curr. Neuropharmacol. 2011;9(3):512–521. doi: 10.2174/157015911796558019. [http://dx.doi.org/10.2174/157015911796558019]. [PMID: 22379463]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sharp T., Boothman L., Raley J., Quérée P. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol. Sci. 2007;28(12):629–636. doi: 10.1016/j.tips.2007.10.009. [http://dx.doi. org/10.1016/j.tips.2007.10.009]. [PMID: 17996955]. [DOI] [PubMed] [Google Scholar]
- 128.Invernizzi R., Carli M., Di Clemente A., Samanin R. Administration of 8-hydroxy-2-(Di-n-propylamino)tetralin in raphe nuclei dorsalis and medianus reduces serotonin synthesis in the rat brain: differences in potency and regional sensitivity. J. Neurochem. 1991;56(1):243–247. doi: 10.1111/j.1471-4159.1991.tb02587.x. [http://dx.doi.org/10.1111/j.1471-4159.1991. tb02587.x]. [PMID: 1824782]. [DOI] [PubMed] [Google Scholar]
- 129.Blier P., Ward N.M. Is there a role for 5-HT1A agonists in the treatment of depression? Biol. Psychiatry. 2003;53(3):193–203. doi: 10.1016/s0006-3223(02)01643-8. [http://dx.doi.org/10.1016/S0006-3223(02)01643-8]. [PMID: 12559651]. [DOI] [PubMed] [Google Scholar]
- 130.Bhagwagar Z., Rabiner E.A., Sargent P.A., Grasby P.M., Cowen P.J. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol. Psychiatry. 2004;9(4):386–392. doi: 10.1038/sj.mp.4001401. [http://dx.doi.org/10.1038/sj.mp.4001401]. [PMID: 15042104]. [DOI] [PubMed] [Google Scholar]
- 131.Albert P.R., Le François B., Millar A.M. Transcriptional dysregulation of 5-HT1A autoreceptors in mental illness. Mol. Brain. 2011;4:21. doi: 10.1186/1756-6606-4-21. [http://dx.doi.org/10.1186/1756-6606-4-21]. [PMID: 21619616]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sargent P. a; Kjaer, K.H.; Bench, C.J.; Rabiner, E. a; Messa, C.; Meyer, J.; Gunn, R.N.; Grasby, P.M.; Cowen, P.J. Brain serotonin 1A receptor binding measured by positron emission tomography with [11C]WAY-100635. Effects of depression and antidepressant treatment. Arch. Gen. Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 133.Savitz J.B., Drevets W.C. Neuroreceptor imaging in depression. Neurobiol. Dis. 2013;52:49–65. doi: 10.1016/j.nbd.2012.06.001. [http://dx.doi.org/10.1016/ j.nbd.2012.06.001]. [PMID: 22691454]. [DOI] [PubMed] [Google Scholar]
- 134.Richardson-Jones J.W., Craige C.P., Guiard B.P., Stephen A., Metzger K.L., Kung H.F., Gardier A.M., Dranovsky A., David D.J., Beck S.G., Hen R., Leonardo E.D. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65(1):40–52. doi: 10.1016/j.neuron.2009.12.003. [http://dx.doi.org/10.1016/ j.neuron.2009.12.003]. [PMID: 20152112]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chalmers D.T., Watson S.J. Comparative anatomical distribution of 5-HT1A receptor mRNA and 5-HT1A binding in rat brain--a combined in situ hybridisation/in vitro receptor autoradiographic study. Brain Res. 1991;561(1):51–60. doi: 10.1016/0006-8993(91)90748-k. [http://dx.doi.org/10. 1016/0006-8993(91)90748-K]. [PMID: 1797349]. [DOI] [PubMed] [Google Scholar]
- 136.Landry M., Di Paolo T. Effect of chronic estradiol, tamoxifen or raloxifene treatment on serotonin 5-HT1A receptor. Brain Res. Mol. Brain Res. 2003;112(1-2):82–89. doi: 10.1016/s0169-328x(03)00049-4. [http://dx.doi.org/10. 1016/S0169-328X(03)00049-4]. [PMID: 12670705]. [DOI] [PubMed] [Google Scholar]
- 137.Gundlah C., Lu N.Z., Mirkes S.J., Bethea C.L. Estrogen receptor beta (ERbeta) mRNA and protein in serotonin neurons of macaques. Brain Res. Mol. Brain Res. 2001;91(1-2):14–22. doi: 10.1016/s0169-328x(01)00108-5. [http:// dx.doi.org/10.1016/S0169-328X(01)00108-5]. [PMID: 11457488]. [DOI] [PubMed] [Google Scholar]