Abstract
Bacopa monnieri, commonly known as Brahmi, has been extensively used as a neuromedicine for various disorders such as anxiety, depression and memory loss. Chemical characterization studies revealed the major active constituents of the herb as the triterpenoid saponins, bacosides. Bacoside A, the vital neuroprotective constituent, is composed of four constituents viz., bacoside A3, bacopaside II, jujubogenin isomer of bacopasaponin C (bacopaside X) and bacopasaponin C. B. monnieri extracts as well as bacosides successfully establish a healthy antioxidant environment in various tissues especially in the liver and brain. Free radical scavenging, suppression of lipid peroxidation and activation of antioxidant enzymes by bacosides help to attain a physiological state of minimized oxidative stress. The molecular basis of neuroprotective activity of bacosides is attributed to the regulation of mRNA translation and surface expression of neuroreceptors such as AMPAR, NMDAR and GABAR in the various parts of the brain. Bioavailability as well as binding of neuroprotective agents (such as bacosides) to these receptors is controlled by the Blood Brain Barrier (BBB). However, nano conversion of these drug candidates easily resolves the BBB restriction and carries a promising role in future therapies. This review summarizes the neuroprotective functions of B. monnieri extracts as well as its active compounds (bacoside A, bacopaside I) and the molecular mechanisms responsible for these pharmacological activities.
Keywords: Bacopa monnieri, bacoside A, bacopaside I, neuroprotection, antioxidants, nanoparticles
1. Introduction
Bacopa monnieri is a nootropic herb distributed throughout the warm wetlands of the world. B. monnieri has various medicinal properties, and these medicinal aspects are discussed in several reviews. This article is envisaged in the context of neuroprotection by this herb and its major active constituents. Ancient Vedic scholars frequently used B. monnieri to memorize lengthy sacred hymns and scriptures. In India, in Ayurvedic prescriptions, B. monnieri has been consumed as ‘medhyarasayana’ (in Sanskrit, ‘medhya’ - intellect or cognition, ‘rasayana’ - rejuvenation). Many Ayurvedic preparations prescribed for cognitive dysfunction contain B. monnieri as a prime constituent. In Charaka Samhita (6th century AD), B. monnieri is mentioned as a medicine for the management of mental dysfunctions such as anxiety, poor cognition and lack of concentration [1]. Due to its ability to nourish neurons, B. monnieri is traditionally
used as a neural tonic and memory enhancer. B. monnieri is also known to help attenuating dementia or decline in mental ability [2].
2. Chemistry
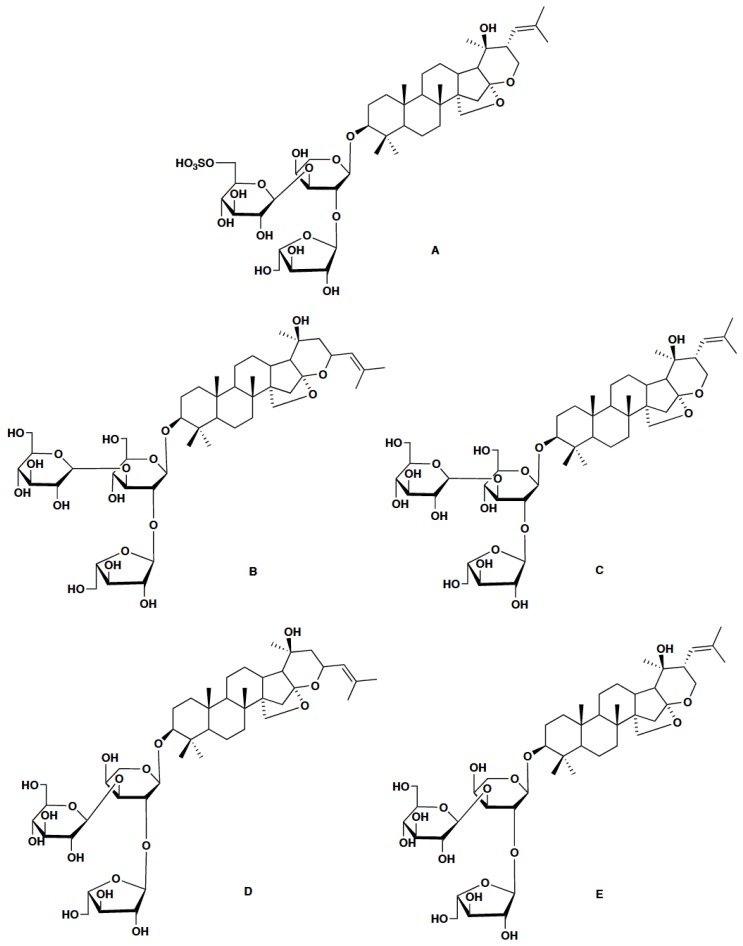
B. monnieri produces various metabolites such as saponins, alkaloids and sterols [3]. The main active constituents of B. monnieri are dammarane-type triterpenoid saponins known as bacosides with jujubogenin or pseudojujubogenin as their aglycone units. Bacosides are known for their nootropic and various other biological activities [4-9]. Another recently identified group of saponins from B. monnieri are bacopasides [10-14]. Bacoside A is the most studied triterpenoid saponin from B. monnieri. Deepak and co-workers reported it as a mixture of four saponins: bacoside A3, bacopaside II, jujubogenin isomer of bacopasaponin C (bacopaside X) and bacopasaponin C [15] (Table 1, Fig. 1). Rastogi et al. reported the major bacosides in B. monnieri as bacopaside I, bacoside A3, bacopaside II, bacopasaponin C isomer and bacopasaponin C, of which the last four saponins constituted bacoside A [16]. B. monnieri accessions, with elite contents of bacoside A and bacopaside I, were recently reported from the southern Western Ghats in India [17].
Table 1.
Bacopaside I and constituents of bacoside A in B. monnieri.
| Name | Aglycone | Sugar Moiety |
|---|---|---|
| Bacopaside I | Pseudojujubogenin | 3-O-[α-L-arabinofuranosyl-(1→2)]- 6-O-sulfonyl-β-D-glucopyranosyl-(1→3)-α-L-arabinopyranoside |
| Bacoside A3 | Jujubogenin | 3-β-[O-β-D-glucopyranosyl(1→3)-O- [α-L-arabinofuranosyl(1→2)-O-β-D-glucopyranosyl)oxy] |
| Bacopaside II | Pseudojujubogenin | 3-O-[α-L-arabinofuranosyl-(1→2)]- β-D-glucopyranosyl-(1→3) β-D-glucopyranoside |
| Bacopaside X (Jujubogenin isomer of Bacopasaponin C) | Jujubogenin | 3-O-[α-L-arabinofuranosyl-(1→2)]- β-D-glucopyranosyl-(1→3)-α-L-arabinopyranoside |
| Bacopasaponin C | Pseudojujubogenin | 3-O-[β-D-glucopyranosyl(1→3){α-L-arabinofuranosyl(1→2)} α-L-arabinopyranoside |
Fig. (1).
A) Bacopaside I and constituents of Bacoside A (B-E), B) Bacoside A3, C) Bacopaside II, D) Bacopaside X, E) Bacopasaponin C.
3. Neuroprotection
In vitro and animal model studies revealed the promising role of B. monnieri in the treatment of epilepsy, anxiety and other neurodegenerative disorders. Oxidative stress is the state where free radicals cause an imbalance in the homeostatic defense mechanisms of the cell [23]. Superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), glutathione-S-transferase (GST) and catalase (CAT) are the free radical-quenching enzymes present in our body. Antioxidant compounds including vitamins A, C, E and phenols also play crucial protective roles [24, 25]. Oxidative stress leads to many diseases, even aging, by degrading ligands, peroxidizing lipids, blocking metabolic pathways, destabilizing DNA strands and denaturing proteins [26, 27]. The metabolically active brain which possesses high levels of pro-oxidant iron and unsaturated lipids is more prone to oxidative stress and lipid peroxidation [28]. Furthermore, due to the BBB, many exogenous antioxidants are not capable of quenching reactive oxygen species in the brain [29].
[These saponins contain three sugar units with either jujubogenin or pseudojujubogenin as their aglycone subunits. Names of sugar units are listed as in the original literature [18-22]].
Saini et al. assessed the activity of B. monnieri against colchicine-induced oxidative stress and found that B. monnieri treatment diminished lipid peroxidation and protein carbonyl levels. Colchicine-induced changes in the activities of acetylcholine esterase (AChE), Na+K+ATPase, SOD, CAT, GPx, GR and GST were all restored to significant levels compared to controls [30]. The administration of B. monnieri ethanolic extract on 6-OHDA-induced lesions in rats showed that both neurobehavioral deficits and enzyme levels were dose-dependently restored by B. monnieri [31]. Singh et al. examined the effect of B. monnieri on paraquat and 1-methyl-4-phenyl-pyridinium iodide-induced toxicities in a dopaminergic SK-N-SH cell line. Normal levels of glutathione, mitochondrial membrane potential and mitochondrial complex I activity were maintained upon B. monnieri treatment. The administration of B. monnieri activated the nuclear factor (erythroid-derived) 2 (Nrf2) pathway by regulating the expression of Keap1 and enhancing glutathione synthesis [32].
Shinomol and Bharath investigated the neuroprotective role of the alcohol extract of B. monnieri against 3-nitropropionic acid (3-NPA)-induced oxidative stress in N27 cells and pre-pubertal male mice. B. monnieri diminished 3-NPA-induced oxidative stress in isolated striatal mitochondria by depleting the levels of malondialdehyde, hydroperoxide, protein carbonyls and reactive oxygen species (ROS). These results recommend B. monnieri as an adjuvant in oxidation-mediated neurodegenerative ailments [33]. Sumathi et al. demonstrated methyl mercury (MeHg) induced activation of GR and inhibition of the activities of SOD, CAT and GPx in the cerebellum of rat brain. These alterations were prevented by the administration of B. monnieri extract [34]. Pretreatment with B. monnieri methanolic extract (mBME) reduced the increased serum level of ALT, AST and creatinine in rats, and provided protection against opioid-induced hepatotoxicity and nephrotoxicity [5]. In another recent study, the neuroprotective role of B. monnieri against decabrominated diphenyl ether (PBDE-209)-induced antioxidant alterations in neonate and young female mice were revealed. B. monnieri significantly preserved the levels of antioxidants and enhanced the activities of antioxidant enzymes in frontal cortex and hippocampus of the brain [35].
Creatine kinase (CK) has three isoforms (CK-MM, MB, BB) which are considered to be sensitive markers in the diagnosis of cardiac and cerebral damage. Anbarasi et al. (2005a) studied the effects of cigarette smoke exposure in rats. The results showed reduction in the CK activity in the heart and brain tissues but marked increase in serum CK activity. This is attributed to free radical-mediated lipid peroxidation, neuronal and cardiac cell membrane damage and leakage of CK into the blood. Bacoside A is known to prevent lipid peroxidation and maintain the structural integrity of cell membranes. It blocked the leakage of CK into the blood and reversed the enzyme level variations in the tissues and blood [36]. The same research group also found that bacoside A treatment inhibited the mitochondrial dysfunction in rat brain induced by cigarette smoke exposure. The mitochondria membrane-stabilizing properties of bacoside A protect the brain, which is evident from the depleted levels of lipid peroxides, cholesterol, and cholesterol/phospholipid (C/P) ratio, and increased levels of mitochondrial enzymes [37]. Further, experimental data revealed the presence of higher levels of serum lactate dehydrogenase (LDH) with a concomitant decrease in the tissue enzyme level in the respective organs upon cigarette smoke exposure. The administration of bacoside A stabilized the cell membranes through its anti-lipid peroxidative effect and these alterations were prevented [38]. In another study, Anbarasi et al. (2005b) evaluated the neuroprotective role of bacoside A against oxidative stress in the brains of rats exposed to cigarette smoke, and found that bacoside A significantly enhanced brain levels of vitamins A, C, E and glutathione. Bacoside A administration inhibited lipid peroxidation and increased the activities of antioxidant enzymes. It also improved the activity of thiol-dependent enzymes like adenosine triphosphatases (ATPases), by protecting the -SH groups in their active sites from free radical-mediated inactivation. Thus bacoside A maintained ionic equilibrium in the brains of cigarette smoke-exposed rats [39].
Bacoside-rich extract from B. monnieri showed antifatigue effect in rats having impairments associated with physical fatigue. Marked reduction in the malondialdehyde (MDA) levels, a significant increase of SOD, CAT and HSP-70 expression in brain, liver and muscle tissues compared with the control group confirmed the antifatigue property of B. monnieri [8]. Rastogi et al. revealed that purified bacosides are efficient in preventing lipofuscin accumulation, enhancing acetylcholine synthesis, monoamine modulation and inhibiting lipid peroxidation. Pro-inflammatory cytokines (interleukin-1β and tumor necrosis factor-α), total nitrite and lipofuscin content in the cortex were significantly reduced, whereas nitric oxide synthetase (iNOS) expression was induced [16]. Ramasamy and co-workers investigated the pharmacological activities of bacoside A, aglycones (jujubogenin, pseudojujubogenin) and their derivatives (ebelin lactone, bacogenin A1) using in silico and in vitro screening methods. The comparative data showed that ebelin lactone has better BBB penetration and highest binding affinity towards M1 and 5-HT2A receptors [40].
Bacopaside I exhibited neuroprotective role by reducing neurological deficits, cerebral infarct volume and edema against injury caused by cerebral ischemia in rats. Oral treatment with bacopaside I markedly increased brain ATP content, antioxidant enzymes, Na+K+ATPase and Ca2+Mg2+ATPase activities. At the same time, bacopaside I decreased MDA content of the brain [41].
4. Molecular expression of receptors and regulators
Neurotransmitters are the chemical messengers that transmit signals from one neuron to another and regulate brain functions through intracellular signaling pathways. Some of the important neurotransmitters are dopamine, noradrenaline, serotonin, neuropeptides, adrenaline, glucocorticoids and acetylcholine [42]. During synaptic signal transmission, neurotransmitters released from the presynaptic neuronal end of one neuron bind to receptors at the postsynaptic membranes of another neuron and produce variations in membrane potential or initiate signaling cascades. Synapses in the brain are of two types: excitatory and inhibitory synapses. In excitatory synapses, neurotransmitters cause depolarization of postsynaptic membranes and glutamate functions as a major neurotransmitter. In contrast, GABA (gamma-aminobutyric acid) and glycine act as major inhibitory neurotransmitters in inhibitory synapses. Glutamate receptors are classified into two - ionotropic or metabotropic glutamate receptors. This classification is based on whether the neurotransmitter binding site and the ion channel are components of the same protein or components of different proteins. Based on the sensitivity to pharmacological agents, ionotropic glutamate receptors are further classified as AMPA-, NMDA-, and kainate-sensitive glutamate receptors. NMDA- and kainate-type receptors are involved in the synaptic plasticity or slower transmission to cause nerve membrane depolarization after glutamate binding. The fast synaptic excitation occurring in the entire brain parts associated with neuronal disorders is controlled primarily by the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs). Therefore, this activity is determined by the number of AMPARs at synapses. Interestingly, it is proved that AMPAR antagonists can block epileptiform activity by inhibiting glutamate-mediated excitation in animal models and in vitro preparations. AMPARs are heterotetrameric protein receptors having four subunits designated either as GluA1–GluA4 or GluR1–GluR4 or GluRA-GluRD. Among them, the GluR2 subunit plays a vital role in synaptic plasticity [43, 44]. The reduction in the AMPAR expression could lead to receptor dysfunction and motor learning deficit in epileptic rats [45]. Since AMPARs mediate the majority of excitatory synaptic neurotransmissions, inhibitors of this class of ionotropic glutamate receptors help to prevent seizures in animal epilepsy models [46, 47].
A fraction of B. monnieri extract (CDRI-08) significantly improved spatial memory with a significant decline in oxidative stress and up-regulation of the AMPAR GluR2 subunit gene expression in the hippocampus of streptozotocin-induced diabetes mellitus type 2 mice [48]. Fmr-1 gene encoded protein, (Fragile X mental retardation protein, FMRP), is a neuronal translational repressor and has been associated with learning, memory and cognition. CDRI-08 administration reversed the memory loss, and this was correlated with significant downregulation of Hif-1α and upregulation of Fmr-1 expression [49]. In another study, the neuroprotective role of B. monnieri methanol extract was investigated in the hippocampus of the temporal lobe of epileptic rats. This study revealed that B. monnieri methanol extract treatment potentiates the therapeutic effect by reversing the alterations in glutamate receptor binding and N-methyl-D-aspartate receptor1 (NMDA R1) gene expression which occurs during epilepsy, thus resulting in reduced glutamate-mediated excitotoxity in the over-stimulated hippocampal neurons [50]. 5-HT2C receptors have been involved in stress, whose alterations were reversed by treatment with B. monnieri in pilocarpine-induced temporal lobe epileptic rats. Similarly, NMDAR functions and IP3 (inositol 1,4,5-trisphosphate) content were modulated with B. monnieri treatment, thereby efficiently balancing the neurotransmitter level in the cerebral cortex [51].
The neuroprotective role of B. monnieri in the prevention of cognitive deficit in schizophrenia in sub-chronic phencyclidine (PCP) rat model has also been studied. PCP administration resulted in the cognitive deficit in rats due to up-regulation of NMDAR1 in CA2/3 and DG. Interestingly, on treatment with B. monnieri prior to PCP administration, NMDAR1 expression was reduced in these brain areas [52]. Reduced expression of vesicular glutamate transporter 1 (VGLUT1), 2 (VGLUT2) and 3 (VGLUT3) is an indication of glutamatergic hypofunction leading to cognitive impairment in schizophrenia. The administration of PCP resulted in low levels of cerebral VGLUT3 accompanied by the cognitive deficit in rats. A standardized extract of B. monnieri containing 20% of bacosides A+B prevented cognitive impairment by increasing VGLUT3 in the prefrontal cortex, striatum and CA1-3 [53]. BME (B. monnieri extract) exhibited neuroprotective properties by mediating hippocampal neurogenesis in rats with an increase in the brain-derived neurotrophic factor (BDNF) level and antioxidant parameters against oxidative stress [54]. Daily administration of BME significantly controls the mechanisms underlying antidepressant-like action by maintaining the normal levels of BDNF, total and phospho-Akt, total and phospho-CREB in the hippocampus and reverses the behavioural changes [55].
The treatment with B. monnieri extract prevented neurochemical alterations related to thioacetamide (TAA) induced hepatic encephalopathy in rats by modulating the expression of two NMDAR subunits - NR2A and NR2B, and their downstream mediators and also normalized the expression of nNOS-apoptotic factors in the cerebellum of rats [56]. The exposure of a flame retardant, PBDE-209, enhanced the expression of NR1 and diminished the binding of REST/NRSF to NR1 promoter in mice. Interestingly, B. monnieri extract modified the glutamatergic system through regulating the expression of NR1 and binding of REST/NRSF to NR1 promoter. Hence it restored the impaired memory caused by PBDE-209 in mice [57].
Scopolamine exposure resulted in a remarkable downregulation of the NMDAR GluN2B subunit expression in prefrontal cortex and hippocampus in mice. The oral administration of B. monnieri extract to scopolamine-treated amnesic mice restored the spatial memory, by the upregulation of GluN2B subunit expression and reduction in the acetylcholinesterase activity in prefrontal cortex as well as hippocampus [58].
Olfactory bulbectomy (OBX) altered the non-spatial short-term memory, spatial working memory and long-term fair memory in mice. OBX decreased the phosphorylation of synaptic plasticity-related signaling proteins: glutamate receptor 1 (GluR1), NR1 subunit of NMDAR, calmodulin-dependent kinase II and reduced the BDNF mRNA in the hippocampus. B. monnieri treatment eliminated OBX-induced cognition dysfunction through upregulation of signaling associated with synaptic plasticity and BDNF transcription thereby preventing neuronal damage in cholinergic systems [59]. Okadaic acid (OKA) resulted in severe memory deficit, oxidative stress, neuroinflammation and neuronal damage accompanied by reduced expression of Nrf2, HO1 and GCLC in rats. Treatment with B. monnieri and melatonin significantly improved memory dysfunction in OKA rats. The supplementation also restored the expression levels of Nrf2, HO1 and GCLC, which led to reduction in oxidative stress, neuroinflammation and neuronal loss. This protective effect of B. monnieri and melatonin in OKA induced memory impairment is mediated through Nrf2 pathway modulation [60].
P-glycoprotein (Pgp) is a major drug efflux transporter which limits drug permeability across the BBB into the central nervous system. Pregnane X Receptor (PXR) is a transcriptional regulator of Pgp and helps to restore Pgp at the BBB. The drug-mediated PXR activation induces up-regulation of Pgp and potentially increases drug penetration through BBB [61]. In silico docking studies showed considerable interaction between pregnane X receptor (PXR) and bacopaside I [62]. Bacopaside II blocked the rhodamine 123 (Rh123) passage across a LLC-GA5-COL150 cell monolayer thereby reduced P-gp efflux ratio of Rh123 compared to control. This study helps to understand herb-drug interactions of bacopaside II upon P-gp substrate drug treatment [63].
Gamma amino butyric acid (GABA) is the primary inhibitory neurotransmitter in the cerebral cortex, which balances neuronal excitation by maintaining the inhibitory excitation. When this balance is disturbed, seizures may ensue. B. monnieri and bacoside A treatments reverse epilepsy associated changes to near control, suggesting that reduced GABA receptors in the cerebral cortex contribute to the epileptic occurrence [64]. B. monnieri extract and bacoside A administration rectified the dopaminergic and cAMP imbalance in hypoglycaemic neonatal rats. B. monnieri extract treatment re-established the altered gene expression parameters of Bax and SOD. Dopamine D1 and D2 receptor subtype expressions were improved whereas cAMP signaling and cell death resulting from oxidative stress were attenuated [65].
Bacopaside I showed antidepressant-like activity. Exposure to chronic unpredictable mild stress (CUMS) increased the level of plasma corticosterone and glucocorticoid receptor expression in mice. Bacopaside I treatment reversed the condition by modifying the hypothalamic-pituitary-adrenal (HPA) axis hyperactivity of CUMS-exposed mice [66]. Aquaporin (AQP) family of water channels which facilitate water flux and CSF movement across the plasma membrane could function as an important target in the treatment of neuropathologies like cerebral edema. Based on the screening conducted in the Xenopus oocyte expression system, it was proved that bacopaside I and bacopaside II can act as selective modulators of Aquaporin-1 (AQP1) channels. Bacopaside I blocked both the water and ion permeability, but bacopaside II selectively inhibited the AQP1 water permeability without impairing the ionic conductance [67]. Bacopaside I attenuated oxygen- and glucose-deprivation (OGD) induced necrosis, apoptosis and neuronal cell damage in an in vitro model of ischemia. The neuroprotective activity of bacopaside I was blocked by the inhibitors of PKC and PI3K, but not by the ERK inhibitor. Bacopaside I restored the level of phospho-Akt (p-Akt), an anti-apoptotic factor. Thus, bacopaside I, via PKC and PI3K/Akt pathways, played a neuroprotective role [68].
Monoamine oxidase inhibitors are potent anti-depressant drugs which prevent oxidative deamination of monoamine type neurotransmitters. Singh et al. evaluated the effect of major constituents (bacopaside I, bacopaside II, bacoside A3, bacopasaponin C, bacosine and bacoside A mixture) of B. monnieri on recombinant human monoamine oxidase (MAO) enzymes. Bacoside A and bacopaside I mixture showed inhibition towards MAO-A and MAO-B enzymes. Bacopaside I selectively inhibited the MAO-A enzyme [69].
5. Nanophytomedicine
Despite promising medical properties, biomolecules suffered from low water solubility, which in turn resulted in their poor bioavailability and less clinical efficacy. Hence, researchers attempted to enhance water solubility and bioavailability of bioactive molecules by loading them in biodegradable polymeric nanoparticles. Other benefits of nanoparticle-drug formulations include minimum patient expenses and low risk of toxicity. Nanoencapsulation improves specificity, efficacy, tolerability and therapeutic potential of drugs [70, 71]. Bioavailability and controlled delivery of drugs related to various diseases were improved successfully by nanoencapsulation [72]. Some of the nanomedicines for diabetes, cancer, AIDS, malaria and tuberculosis were commercialized [73-76].
Neurological disorders are major global health issues, but therapeutics is limited due to drug bioavailability to the central nervous system being controlled and restricted by the BBB [77]. The efficiency of nanomedicine formulation is determined by a suitable polymeric system having higher encapsulation efficiency, maximum bioavailability and high retention time. The encapsulation process with polymeric nanoparticles has more advantages compared to other nanoparticle systems [78]. Polymeric nanoparticles provide controlled drug release, efficient targeted drug delivery, ease of biodegradability and excellent biocompatibility with tissues and cells. They also improve the plasma half-life, stability, solubility and decrease the immunogenicity of drugs [79]. Poly(L-lactic acid) (PLA), poly(D,L-lactide-coglycolide) (PLGA), poly ethylene glycol (PEG), poly(epsilon-caprolactone) (PCL), polyalkylcyanoacrylates, chitosan, gelatin and hyaluronic acid are some of the most commonly used polymers for encapsulation [80].
Poly (lactic-co-glycolic acid) (PLGA) nanoparticles are one of the most promising drug delivery systems for crossing the BBB. PLGA has excellent biocompatibility, and upon exposure to the human physical environment, it is hydrolyzed into lactic and glycolic acids, which are naturally-occurring metabolites [81]. PEGylation contributes to enhancing the aqueous solubility and stability, preventing intermolecular aggregation, reducing immunogenicity and extending the systemic circulation time of a compound. PEG is often linked to PLGA to achieve similar beneficial effects [82]. There are only very limited studies on nanoencapsulation of bacoside A or bacopaside I, particularly in the context of neuroprotection. Jose et al. recently demonstrated the high efficiency of bacoside A-loaded PLGA nanoparticles in delivering bacoside A into the brain, crossing the BBB [83].
Different plant-derived compounds were tested for various bioactivities after nanoparticle conversion. Platinum nanoparticles using B. monnieri (BmE-PtNPs) have neuro-protective activity in 1-methyl 4-phenyl 1,2,3,6 tetrahydro-pyridine (MPTP)-induced experimental Parkinsonism in zebrafish model. MPTP is metabolized to 1-methyl-4-phenyl pyridinium (MPP+) which accumulates in the mitochondria and inhibits complex I activity of the respiratory chain. This leads to ROS generation and neuronal cell death. This study demonstrates that BmE-PtNPs have the ability to oxidize nicotinamide adenine dinucleotide (NAD) similar to mitochondrial complex I. It also functions as an antioxidant by reducing levels of MDA and increasing the levels of dopamine, GSH and activities of GPx, CAT and SOD [84]. Aluminium-induced oxidative stress indicated by a significant increase in the lipid peroxidation levels and decrease of SOD, CAT and GPx was counteracted by the co-administration of B. monnieri stabilized silver nanoparticles (BmSNPs). These findings implicate that BmSNPs can eradicate oxidative stress and prevent tissue damage in aluminium exposed mice [85].
Conclusion
Extensive scientific research on the neuropharmacolo-gical potential of B. monnieri extracts as well as its major triterpenoid saponin constituents is discussed in this mini review. Bacoside A and bacopaside I exhibited neuroprotective role by reducing oxidative stress, inducing expression of antioxidant enzymes and regulating surface expression of various neuroreceptors. This review also provides valuable information on the scope and safe use of B. monnieri secondary metabolite nanoparticles as excellent drugs against neurodegenerative disorders.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Russo A., Borrelli F. Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine. 2005;12(4):305–317. doi: 10.1016/j.phymed.2003.12.008. [http://dx.doi. org/10.1016/j.phymed.2003.12.008]. [PMID: 15898709]. [DOI] [PubMed] [Google Scholar]
- 2.Aguiar S., Borowski T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 2013;16(4):313–326. doi: 10.1089/rej.2013.1431. [http://dx.doi.org/10.1089/rej.2013.1431]. [PMID: 23772955]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Snafi A.E. The pharmacology of Bacopa monniera. A review. Int. J. Pharm. Sci. Res. 2013;4(12):0975–9492. [Google Scholar]
- 4.Kamkaew N., Scholfield C.N., Ingkaninan K., Maneesai P., Parkington H.C., Tare M., Chootip K. Bacopa monnieri and its constituents is hypotensive in anaesthetized rats and vasodilator in various artery types. J. Ethnopharmacol. 2011;137(1):790–795. doi: 10.1016/j.jep.2011.06.045. [http://dx.doi.org/10.1016/j.jep.2011.06.045]. [PMID: 21762768]. [DOI] [PubMed] [Google Scholar]
- 5.Shahid M., Subhan F., Ullah I., Ali G., Alam J., Shah R. Beneficial effects of Bacopa monnieri extract on opioid induced toxicity. Heliyon. 2016;2(2):e00068. doi: 10.1016/j.heliyon.2016.e00068. [http://dx.doi.org/10.1016/ j.heliyon.2016.e00068]. [PMID: 27441247]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janani P., Sivakumari K., Parthasarathy C. Hepatoprotective activity of bacoside A against N-nitrosodiethylamine-induced liver toxicity in adult rats. Cell Biol. Toxicol. 2009;25(5):425–434. doi: 10.1007/s10565-008-9096-4. [http://dx.doi.org/10.1007/s10565-008-9096-4]. [PMID: 18679812]. [DOI] [PubMed] [Google Scholar]
- 7.Janani P., Sivakumari K., Geetha A., Ravisankar B., Parthasarathy C. Chemopreventive effect of bacoside A on N-nitrosodiethylamine-induced hepatocarcinogenesis in rats. J. Cancer Res. Clin. Oncol. 2010;136(5):759–770. doi: 10.1007/s00432-009-0715-0. [http://dx.doi.org/ 10.1007/s00432-009-0715-0]. [PMID: 19916024]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand T., Phani K.G., Pandareesh M.D., Swamy M.S., Khanum F., Bawa A.S. Effect of bacoside extract from Bacopa monniera on physical fatigue induced by forced swimming. Phytother. Res. 2012;26(4):587. doi: 10.1002/ptr.3611. [DOI] [PubMed] [Google Scholar]
- 9.Sharath R., Harish B.G., Krishna V., Sathyanarayana B.N., Swamy H.M. Wound healing and protease inhibition activity of Bacoside-A, isolated from Bacopa monnieri wettest. Phytother. Res. 2010;24(8):1217–1222. doi: 10.1002/ptr.3115. [PMID: 20213670]. [DOI] [PubMed] [Google Scholar]
- 10.Sivaramakrishna C., Rao C.V., Trimurtulu G., Vanisree M., Subbaraju G.V. Triterpenoid glycosides from Bacopa monnieri. Phytochemistry. 2005;66(23):2719–2728. doi: 10.1016/j.phytochem.2005.09.016. [http://dx.doi.org/10. 1016/j.phytochem.2005.09.016]. [PMID: 16293276]. [DOI] [PubMed] [Google Scholar]
- 11.Bhandari P., Kumar N., Singh B., Kaur I. Dammarane triterpenoid saponins from Bacopa monnieri. Can. J. Chem. 2009;87:1230–1234. [http://dx.doi.org/10.1139/V09-111]. [Google Scholar]
- 12.Chakravarty A.K., Sarkar T., Masuda K., Shiojima K., Nakane T., Kawahara N. Bacopaside I and II: two pseudojujubogenin glycosides from Bacopa monniera. Phytochemistry. 2001;58(4):553–556. doi: 10.1016/s0031-9422(01)00275-8. [http://dx.doi.org/10.1016/S0031-9422(01)00275-8]. [PMID: 11576596]. [DOI] [PubMed] [Google Scholar]
- 13.Chakravarty A.K., Garai S., Masuda K., Nakane T., Kawahara N. Bacopasides III-V: three new triterpenoid glycosides from Bacopa monniera. Chem. Pharm. Bull. (Tokyo) 2003;51(2):215–217. doi: 10.1248/cpb.51.215. [http://dx.doi.org/10.1248/cpb.51.215]. [PMID: 12576661]. [DOI] [PubMed] [Google Scholar]
- 14.Garai S., Mahato S.B., Ohtani K., Yamasaki K. Dammarane-type triterpenoid saponins from Bacopa monniera. Phytochemistry. 1996;42(3):815–820. doi: 10.1016/0031-9422(95)00936-1. [http://dx.doi.org/10.1016/0031-9422(95) 00936-1]. [PMID: 8768327]. [DOI] [PubMed] [Google Scholar]
- 15.Deepak M., Sangli G.K., Arun P.C., Amit A. Quantitative determination of the major saponin mixture bacoside A in Bacopa monnieri by HPLC. Phytochem. Anal. 2005;16(1):24–29. doi: 10.1002/pca.805. [http://dx.doi.org/10.1002/pca.805]. [PMID: 15688952]. [DOI] [PubMed] [Google Scholar]
- 16.Rastogi M., Ojha R.P., Prabu P.C., Devi B.P., Agrawal A., Dubey G.P. Prevention of age-associated neurodegeneration and promotion of healthy brain ageing in female Wistar rats by long term use of bacosides. Biogerontology. 2012;13(2):183–195. doi: 10.1007/s10522-011-9367-y. [http://dx.doi.org/10.1007/s10522-011-9367-y]. [PMID: 22143822]. [DOI] [PubMed] [Google Scholar]
- 17.Christopher C., Johnson A.J., Mathew P.J., Baby S. Elite genotypes of Bacopa monnieri, with high contents of Bacoside A and Bacopaside I, from southern Western Ghats in India. Ind. Crops Prod. 2017;98:76–81. [http://dx.doi.org/10.1016/j.indcrop. 2017.01.018]. [Google Scholar]
- 18.Chakravarty A.K., Sarkar T., Masuda K., Shiojima K., Nakane T., Kawahara N. Bacopaside I and II: two pseudojujubogenin glycosides from Bacopa monniera. Phytochemistry. 2001;58(4):553–556. doi: 10.1016/s0031-9422(01)00275-8. [http://dx.doi.org/10.1016/S0031-9422(01)00275-8]. [PMID: 11576596]. [DOI] [PubMed] [Google Scholar]
- 19.Chakravarty A.K., Sarkar T., Masuda K., Shiojima K., Nakane T., Kawahara N. Corrigendum to “Bacopaside I and II: two pseudojujubogenin glycosides from Bacopa monniera”. Phytochemistry. 2002;59(3):365. doi: 10.1016/s0031-9422(01)00275-8. [Phytochemistry, 2001, 58(4), 553–556]. [http://dx.doi.org/10.1016/S0031-9422(01)00475-7]. [DOI] [PubMed] [Google Scholar]
- 20.Rastogi S., Pal R., Kulshreshtha D.K. Bacoside A3--a triterpenoid saponin from Bacopa monniera. Phytochemistry. 1994;36(1):133–137. doi: 10.1016/s0031-9422(00)97026-2. [http://dx.doi.org/10.1016/S0031-9422(00)97026-2]. [PMID: 7764837]. [DOI] [PubMed] [Google Scholar]
- 21.Sivaramakrishna C., Rao C.V., Trimurtulu G., Vanisree M., Subbaraju G.V. Triterpenoid glycosides from Bacopa monnieri. Phytochemistry. 2005;66(23):2719–2728. doi: 10.1016/j.phytochem.2005.09.016. [http://dx.doi.org/ 10.1016/j.phytochem.2005.09.016]. [PMID: 16293276]. [DOI] [PubMed] [Google Scholar]
- 22.Garai S., Mahato S.B., Ohtani K., Yamasaki K. Dammarane-type triterpenoid saponins from Bacopa monniera. Phytochemistry. 1996;42(3):815–820. doi: 10.1016/0031-9422(95)00936-1. [http://dx.doi.org/10.1016/0031-9422(95) 00936-1]. [PMID: 8768327]. [DOI] [PubMed] [Google Scholar]
- 23.Kregel K.C., Zhang H.J. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292(1):R18–R36. doi: 10.1152/ajpregu.00327.2006. [http://dx.doi.org/10.1152/ajpregu.00327. 2006]. [PMID: 16917020]. [DOI] [PubMed] [Google Scholar]
- 24.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [http://dx.doi.org/10.1016/j.biocel.2006.07.001]. [PMID: 16978905]. [DOI] [PubMed] [Google Scholar]
- 25.Rice-Evans C., Miller N., Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [http:// dx.doi.org/10.1016/S1360-1385(97)01018-2]. [Google Scholar]
- 26.De Grey A. The Mitochondrial Free Radical Theory of Aging. Austin, TX: R.G. Landes Company; 1999. [Google Scholar]
- 27.Maxwell S.R.J. Prospects for the use of antioxidant therapies. Drugs. 1995;49(3):345–361. doi: 10.2165/00003495-199549030-00003. [http://dx.doi.org/10.2165/00003495-199549030-00003]. [PMID: 7774511]. [DOI] [PubMed] [Google Scholar]
- 28.Arivazhagan P., Shila S., Kumaran S., Panneerselvam C. Effect of DL-a-lipoic acid in various brain regions of aged rats. Exp. Gerontol. 2002;37:803–811. doi: 10.1016/s0531-5565(02)00015-3. [http://dx.doi.org/10.1016/S0531-5565(02)00015-3]. [PMID: 12175480]. [DOI] [PubMed] [Google Scholar]
- 29.Gilgun-Sherki Y., Melamed E., Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40(8):959–975. doi: 10.1016/s0028-3908(01)00019-3. [http://dx.doi.org/10.1016/S0028-3908(01)00019-3]. [PMID: 11406187]. [DOI] [PubMed] [Google Scholar]
- 30.Saini N., Singh D., Sandhir R. Neuroprotective effects of Bacopa monnieri in experimental model of dementia. Neurochem. Res. 2012;37(9):1928–1937. doi: 10.1007/s11064-012-0811-4. [http://dx.doi.org/10.1007/s11064-012-0811-4]. [PMID: 22700087]. [DOI] [PubMed] [Google Scholar]
- 31.Shobana C., Kumar R.R., Sumathi T. Alcoholic extract of Bacopa monniera Linn. protects against 6-hydroxydopamine-induced changes in behavioral and biochemical aspects: a pilot study. Cell. Mol. Neurobiol. 2012;32(7):1099–1112. doi: 10.1007/s10571-012-9833-3. [http://dx. doi.org/10.1007/s10571-012-9833-3]. [PMID: 22527857]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh M., Murthy V., Ramassamy C. Standardized extracts of Bacopa monniera protect against MPP+- and paraquat-induced toxicity by modulating mitochondrial activities, proteasomal functions, and redox pathways. Toxicol. Sci. 2012;125(1):219–232. doi: 10.1093/toxsci/kfr255. [http://dx.doi.org/10.1093/toxsci/kfr255]. [PMID: 21972102]. [DOI] [PubMed] [Google Scholar]
- 33.Shinomol G.K., Bharath M.M. Muralidhara, Neuromodulatory propensity of Bacopa monnieri leaf extract against 3-nitropropionic acid-induced oxidative stress: in vitro and in vivo evidences. Neurotox. Res. 2012;22(2):102–114. doi: 10.1007/s12640-011-9303-6. [http://dx.doi.org/10.1007/ s12640-011-9303-6]. [PMID: 22203611]. [DOI] [PubMed] [Google Scholar]
- 34.Sumathi T., Shobana C., Christinal J., Anusha C. Protective effect of Bacopa monniera on methyl mercury-induced oxidative stress in cerebellum of rats. Cell. Mol. Neurobiol. 2012;32(6):979–987. doi: 10.1007/s10571-012-9813-7. [http://dx.doi.org/10.1007/s10571-012-9813-7]. [PMID: 22366895]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verma P., Singh P., Gandhi B.S. Neuromodulatory role of Bacopa monnieri on oxidative stress induced by postnatal exposure to decabromodiphenyl ether (PBDE -209) in neonate and young female mice. Iran. J. Basic Med. Sci. 2014;17(4):307–311. [PMID: 24904725]. [PMC free article] [PubMed] [Google Scholar]
- 36.Anbarasi K., Vani G., Balakrishna K., Devi C.S. Creatine kinase isoenzyme patterns upon chronic exposure to cigarette smoke: protective effect of Bacoside A. Vascul. Pharmacol. 2005;42(2):57–61. doi: 10.1016/j.vph.2005.01.003. [http://dx.doi.org/10.1016/j.vph.2005.01.003]. [DOI] [PubMed] [Google Scholar]
- 37.Anbarasi K., Vani G., Devi C.S. Protective effect of bacoside A on cigarette smoking-induced brain mitochondrial dysfunction in rats. J. Environ. Pathol. Toxicol. Oncol. 2005;24(3):225–234. doi: 10.1615/jenvpathtoxoncol.v24.i3.80. [http://dx.doi.org/10.1615/JEnvPathToxOncol.v24.i3.80]. [PMID: 16050806]. [DOI] [PubMed] [Google Scholar]
- 38.Anbarasi K., Sabitha K.E., Devi C.S. Lactate dehydrogenase isoenzyme patterns upon chronic exposure to cigarette smoke: Protective effect of bacoside A. Environ. Toxicol. Pharmacol. 2005;20(2):345. doi: 10.1016/j.etap.2005.03.006. b. [DOI] [PubMed] [Google Scholar]
- 39.Anbarasi K., Vani G., Balakrishna K., Devi C.S. Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sci. 2006;78(12):1378–1384. doi: 10.1016/j.lfs.2005.07.030. [http://dx.doi.org/10.1016/ j.lfs.2005.07.030]. [PMID: 16226278]. [DOI] [PubMed] [Google Scholar]
- 40.Ramasamy S., Chin S.P., Sukumaran S.D., Buckle M.J.C., Kiew L.V., Chung L.Y. In silico and in vitro analysis of bacoside A aglycones and its derivatives as the constituents responsible for the cognitive effects of Bacopa monnieri. PLoS One. 2015;10(5):e0126565. doi: 10.1371/journal.pone.0126565. [http://dx.doi.org/10.1371/journal.pone.0126565]. [PMID: 25965066]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X., Yue R., Zhang J., Shan L., Wang R., Zhang W. Neuroprotective effects of bacopaside I in ischemic brain injury. Restor. Neurol. Neurosci. 2013;31(2):109–123. doi: 10.3233/RNN-120228. [PMID: 23160060]. [DOI] [PubMed] [Google Scholar]
- 42.Roesler R., Schröder N. Cognitive enhancers: focus on modulatory signaling influencing memory consolidation. Pharmacol. Biochem. Behav. 2011;99(2):155–163. doi: 10.1016/j.pbb.2010.12.028. [http://dx. doi.org/10.1016/j.pbb.2010.12.028]. [PMID: 21236291]. [DOI] [PubMed] [Google Scholar]
- 43.Passafaro M., Piëch V., Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat. Neurosci. 2001;4(9):917–926. doi: 10.1038/nn0901-917. [http://dx.doi.org/ 10.1038/nn0901-917]. [PMID: 11528423]. [DOI] [PubMed] [Google Scholar]
- 44.Dingledine R., Borges K., Bowie D., Traynelis S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51(1):7–61. [PMID: 10049997]. [PubMed] [Google Scholar]
- 45.Soman S., Anju T.R., Jayanarayanan S., Antony S., Paulose C.S. Impaired motor learning attributed to altered AMPA receptor function in the cerebellum of rats with temporal lobe epilepsy: ameliorating effects of Withania somnifera and withanolide A. Epilepsy Behav. 2013;27(3):484–491. doi: 10.1016/j.yebeh.2013.01.007. [http://dx.doi.org/10.1016/ j.yebeh.2013.01.007]. [PMID: 23602240]. [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi S., Donevan S.D., Rogawski M.A. Anticonvulsant activity of AMPA/kainate antagonists: comparison of GYKI 52466 and NBOX in maximal electroshock and chemoconvulsant seizure models. Epilepsy Res. 1993;15(3):179–184. doi: 10.1016/0920-1211(93)90054-b. [http://dx.doi.org/ 10.1016/0920-1211(93)90054-B]. [PMID: 7693450]. [DOI] [PubMed] [Google Scholar]
- 47.Rogawski M.A., Kurzman P.S., Yamaguchi S.I., Li H. Role of AMPA and GluR5 kainate receptors in the development and expression of amygdala kindling in the mouse. Neuropharmacology. 2001;40(1):28–35. doi: 10.1016/s0028-3908(00)00112-x. [http://dx.doi.org/10.1016/ S0028-3908(00)00112-X]. [PMID: 11077068]. [DOI] [PubMed] [Google Scholar]
- 48.Pandey S.P., Singh H.K., Prasad S. Alterations in hippocampal oxidative stress, expression of AMPA receptor GluR2 subunit and associated spatial memory loss by Bacopa monnieri extract (CDRI-08) in streptozotocin-induced diabetes mellitus type 2 mice. PLoS One. 2015;10(7):e0131862. doi: 10.1371/journal.pone.0131862. [http://dx.doi.org/10.1371/journal. pone.0131862]. [PMID: 26161865]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rani A., Prasad S. A special extract of Bacopa monnieri (CDRI-08)-restored memory in CoCl2-hypoxia mimetic mice is associated with upregulation of Fmr-1 gene expression in hippocampus. Evid. Based Complement. Alternat. Med. 2015;2015:347978. doi: 10.1155/2015/347978. [http:// dx.doi.org/10.1155/2015/347978]. [PMID: 26413121]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan R., Krishnakumar A., Paulose C.S. Decreased glutamate receptor binding and NMDA R1 gene expression in hippocampus of pilocarpine-induced epileptic rats: neuroprotective role of Bacopa monnieri extract. Epilepsy Behav. 2008;12(1):54–60. doi: 10.1016/j.yebeh.2007.09.021. [http://dx.doi.org/10.1016/j.yebeh.2007.09.021]. [PMID: 18086456]. [DOI] [PubMed] [Google Scholar]
- 51.Krishnakumar A., Anju T.R., Abraham P.M., Paulose C.S. Alteration in 5-HT2C, NMDA receptor and IP3 in cerebral cortex of epileptic rats: restorative role of Bacopa monnieri. Neurochem. Res. 2015;40(1):216–225. doi: 10.1007/s11064-014-1472-2. [http://dx.doi.org/10.1007/s11064-014-1472-2]. [PMID: 25503823]. [DOI] [PubMed] [Google Scholar]
- 52.Piyabhan P., Wetchateng T. Neuroprotective effects of Bacopa monnieri (Brahmi) on novel object recognition and NMDAR1 immunodensity in the prefrontal cortex, striatum and hippocampus of sub-chronic phencyclidine rat model of schizophrenia. J. Med. Assoc. Thai. 2014;97(Suppl. 8):S50–S55. [PMID: 25518293]. [PubMed] [Google Scholar]
- 53.Piyabhan P., Wannasiri S., Naowaboot J. Bacopa monnieri (Brahmi) improved novel object recognition task and increased cerebral vesicular glutamate transporter type 3 in sub-chronic phencyclidine rat model of schizophrenia. Clin. Exp. Pharmacol. Physiol. 2016;43(12):1234–1242. doi: 10.1111/1440-1681.12658. [http://dx.doi.org/10.1111/ 1440-1681.12658]. [PMID: 27562725]. [DOI] [PubMed] [Google Scholar]
- 54.Kumar S., Mondal A.C. Neuroprotective, neurotrophic and anti-oxidative role of Bacopa monnieri on CUS induced model of depression in rat. Neurochem. Res. 2016;41(11):3083–3094. doi: 10.1007/s11064-016-2029-3. [http://dx.doi.org/10.1007/s11064-016-2029-3]. [PMID: 27506204]. [DOI] [PubMed] [Google Scholar]
- 55.Hazra S., Kumar S., Saha G.K., Mondal A.C. Reversion of BDNF, Akt and CREB in hippocampus of chronic unpredictable stress induced rats: effects of phytochemical, Bacopa Monnieri. Psychiatry Investig. 2017;14(1):74–80. doi: 10.4306/pi.2017.14.1.74. [http://dx.doi.org/10. 4306/pi.2017.14.1.74]. [PMID: 28096878]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mondal P., Trigun S.K. Bacopa monnieri extract (CDRI-08) modulates the NMDA receptor subunits and nNOS-apoptosis axis in cerebellum of hepatic encephalopathy rats. Evid. Based Complement. Alternat. Med. 2015;2015:535013. doi: 10.1155/2015/535013. [http://dx.doi. org/10.1155/2015/535013]. [PMID: 26413124]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verma P., Gupta R.K., Gandhi B.S., Singh P. CDRI-08 attenuates REST/NRSF-mediated expression of NMDAR1 gene in PBDE-209-exposed mice brain. Evid. Based Complement. Alternat. Med. 2015;2015:403840. doi: 10.1155/2015/403840. [http://dx.doi.org/10.1155/2015/ 403840]. [PMID: 26413122]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rai R., Singh H.K., Prasad S. A special extract of Bacopa monnieri (CDRI-08) restores learning and memory by upregulating expression of the NMDA receptor subunit GluN2B in the brain of scopolamine-induced amnesic mice. Evid. Based Complement. Alternat. Med. 2015;2015:254303. doi: 10.1155/2015/254303. [http://dx.doi.org/10.1155/ 2015/254303]. [PMID: 26413117]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le X.T., Pham H.T.N., Do P.T., Fujiwara H., Tanaka K., Li F., Van Nguyen T., Nguyen K.M., Matsumoto K. Bacopa monnieri ameliorates memory deficits in olfactory bulbectomized mice: possible involvement of glutamatergic and cholinergic systems. Neurochem. Res. 2013;38(10):2201–2215. doi: 10.1007/s11064-013-1129-6. [http://dx.doi.org/10. 1007/s11064-013-1129-6]. [PMID: 23949198]. [DOI] [PubMed] [Google Scholar]
- 60.Dwivedi S., Nagarajan R., Hanif K., Siddiqui H.H., Nath C., Shukla R. Standardized extract of Bacopa monniera attenuates okadaic acid induced memory dysfunction in rats: effect on Nrf2 pathway. Evid. Based Complement. Alternat. Med. 2013;2013:294501. doi: 10.1155/2013/294501. [http://dx.doi.org/10.1155/2013/294501]. [PMID: 24078822]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bauer B., Hartz A.M., Fricker G., Miller D.S. Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol. Pharmacol. 2004;66(3):413–419. doi: 10.1124/mol.66.3.. [PMID: 15322232]. [DOI] [PubMed] [Google Scholar]
- 62.Evan Prince S., Udhaya L.B., Sunitha P.S., Arumugam G. Reparation of isoniazid and rifampicin combinatorial therapy-induced hepatotoxic effects by Bacopa monnieri. Pharmacology. 2016;98(1-2):29–34. doi: 10.1159/000444856. [http://dx.doi.org/10.1159/000444856]. [PMID: 27007136]. [DOI] [PubMed] [Google Scholar]
- 63.Singh R., Rachumallu R., Bhateria M., Panduri J., Bhatta R.S. In vitro effects of standardized extract of Bacopa monniera and its five individual active constituents on human P-glycoprotein activity. Xenobiotica. 2015;45(8):741–749. doi: 10.3109/00498254.2015.1017752. [http://dx.doi.org/ 10.3109/00498254.2015.1017752]. [PMID: 25869246]. [DOI] [PubMed] [Google Scholar]
- 64.Mathew J., Balakrishnan S., Antony S., Abraham P.M., Paulose C.S. Decreased GABA receptor in the cerebral cortex of epileptic rats: effect of Bacopa monnieri and Bacoside-A. J. Biomed. Sci. 2012;19(1):25. doi: 10.1186/1423-0127-19-25. [http://dx.doi.org/10.1186/1423-0127-19-25]. [PMID: 22364254]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas R.B., Joy S., Ajayan M.S., Paulose C.S. Neuroprotective potential of Bacopa monnieri and Bacoside A against dopamine receptor dysfunction in the cerebral cortex of neonatal hypoglycaemic rats. Cell. Mol. Neurobiol. 2013;33(8):1065–1074. doi: 10.1007/s10571-013-9973-0. [http://dx.doi.org/10.1007/s10571-013-9973-0]. [PMID: 23975094]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zu X., Zhang M., Li W., Xie H., Lin Z., Yang N., Liu X., Zhang W. Zhang, W. Antidepressant-like effect of Bacopaside I in mice exposed to chronic unpredictable mild stress by modulating the hypothalamic-pituitary-adrenal axis function and activating BDNF signaling pathway. Neurochem. Res. 2017;42(11):3233–3244. doi: 10.1007/s11064-017-2360-3. [Liu, X]. [DOI] [PubMed] [Google Scholar]
- 67.Pei J.V., Kourghi M., De Ieso M.L., Campbell E.M., Dorward H.S., Hardingham J.E., Yool A.J. Differential inhibition of water and ion channel activities of mammalian aquaporin-1 by two structurally related bacopaside compounds derived from the medicinal plant Bacopa monnieri. Mol. Pharmacol. 2016;90(4):496–507. doi: 10.1124/mol.116.105882. [http://dx.doi.org/10.1124/mol.116.105882]. [PMID: 27474162]. [DOI] [PubMed] [Google Scholar]
- 68.Le X.T., Nguyet Pham H.T., Van Nguyen T., Minh Nguyen K., Tanaka K., Fujiwara H., Matsumoto K. Protective effects of Bacopa monnieri on ischemia-induced cognitive deficits in mice: the possible contribution of bacopaside I and underlying mechanism. J. Ethnopharmacol. 2015;164:37–45. doi: 10.1016/j.jep.2015.01.041. [http://dx.doi. org/10.1016/j.jep.2015.01.041]. [PMID: 25660331]. [DOI] [PubMed] [Google Scholar]
- 69.Singh R., Ramakrishna R., Bhateria M., Bhatta R.S. In vitro evaluation of Bacopa monniera extract and individual constituents on human recombinant monoamine oxidase enzymes. Phytother. Res. 2014;28(9):1419–1422. doi: 10.1002/ptr.5116. [http://dx.doi.org/10.1002/ptr.5116]. [PMID: 24449518]. [DOI] [PubMed] [Google Scholar]
- 70.Schroeder U., Sommerfeld P., Ulrich S., Sabel B.A. Nanoparticle technology for delivery of drugs across the blood-brain barrier. J. Pharm. Sci. 1998;87(11):1305–1307. doi: 10.1021/js980084y. [http://dx. doi.org/10.1021/js980084y]. [PMID: 9811481]. [DOI] [PubMed] [Google Scholar]
- 71.Leroux J.C., Allémann E., De Jaeghere F., Doelker E., Gurny R. Biodegradable nanoparticles-from sustained release formulations to improved site specific drug delivery. J. Control. Release. 1996;39(2-3):339–350. [http://dx.doi.org/10.1016/0168-3659(95)00164-6]. [Google Scholar]
- 72.Budhian A., Siegel S.J., Winey K.I. Production of haloperidol-loaded PLGA nanoparticles for extended controlled drug release of haloperidol. J. Microencapsul. 2005;22(7):773–785. doi: 10.1080/02652040500273753. [http://dx. doi.org/10.1080/02652040500273753]. [PMID: 16421087]. [DOI] [PubMed] [Google Scholar]
- 73.Mu L., Feng S.S. A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS. J. Control. Release. 2003;86(1):33–48. doi: 10.1016/s0168-3659(02)00320-6. [http:// dx.doi.org/10.1016/S0168-3659(02)00320-6]. [PMID: 12490371]. [DOI] [PubMed] [Google Scholar]
- 74.Damgé C., Maincent P., Ubrich N. Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats. J. Control. Release. 2007;117(2):163–170. doi: 10.1016/j.jconrel.2006.10.023. [http://dx.doi.org/10.1016/ j.jconrel.2006.10.023]. [PMID: 17141909]. [DOI] [PubMed] [Google Scholar]
- 75.Ahmad Z., Pandey R., Sharma S., Khuller G.K. Alginate nanoparticles as antituberculosis drug carriers: formulation development, pharmacokinetics and therapeutic potential. Indian J. Chest Dis. Allied Sci. 2006;48(3):171–176. [PMID: 18610673]. [PubMed] [Google Scholar]
- 76.Lee K.S., Chung H.C. Im, S.A.; Park, Y.H.; Kim, C.S.; Kim, S.B.; Rha, S.Y.; Lee, M.Y.; Ro, J. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008;108(2):241–250. doi: 10.1007/s10549-007-9591-y. [http://dx.doi.org/10.1007/ s10549-007-9591-y]. [PMID: 17476588]. [DOI] [PubMed] [Google Scholar]
- 77.Cai Q., Wang L., Deng G., Liu J., Chen Q., Chen Z. Systemic delivery to central nervous system by engineered PLGA nanoparticles. Am. J. Transl. Res. 2016;8(2):749–764. [PMID: 27158367]. [PMC free article] [PubMed] [Google Scholar]
- 78.van Vlerken L.E., Vyas T.K., Amiji M.M. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm. Res. 2007;24(8):1405–1414. doi: 10.1007/s11095-007-9284-6. [http://dx.doi.org/10.1007/ s11095-007-9284-6]. [PMID: 17393074]. [DOI] [PubMed] [Google Scholar]
- 79.Sharma S., Parmar A., Kori S., Sandhir R. PLGA-based nanoparticles: a new paradigm in biomedical applications. Trends Analyt. Chem. 2016;80:30–40. [http://dx.doi.org/10.1016/j.trac. 2015.06.014]. [Google Scholar]
- 80.Duncan R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003;2(5):347–360. doi: 10.1038/nrd1088. [http://dx.doi.org/10.1038/ nrd1088]. [PMID: 12750738]. [DOI] [PubMed] [Google Scholar]
- 81.Kumari A., Yadav S.K., Yadav S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [http://dx.doi.org/10.1016/ j.colsurfb.2009.09.001]. [PMID: 19782542]. [DOI] [PubMed] [Google Scholar]
- 82.Sah H., Thoma L.A., Desu H.R., Sah E., Wood G.C. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. Int. J. Nanomedicine. 2013;8:747–765. doi: 10.2147/IJN.S40579. [http://dx.doi.org/10.2147/IJN.S40579]. [PMID: 23459088]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jose S., Sowmya S., Cinu T.A., Aleykutty N.A., Thomas S., Souto E.B. Surface modified PLGA nanoparticles for brain targeting of Bacoside-A. Eur. J. Pharm. Sci. 2014;63:29–35. doi: 10.1016/j.ejps.2014.06.024. [http://dx.doi.org/10.1016/j.ejps.2014.06.024]. [PMID: 25010261]. [DOI] [PubMed] [Google Scholar]
- 84.Nellore J., Pauline C., Amarnath K. Bacopa monnieri phytochemicals mediated synthesis of platinum nanoparticles and its neurorescue effect on 1-methyl 4-phenyl 1,2,3,6 tetrahydropyridine-induced experimental parkinsonism in zebrafish. J. Neurodegener. Dis. 2013;2013:972391. doi: 10.1155/2013/972391. [http://dx.doi.org/10.1155/2013/972391]. [PMID: 26317003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mahitha B., Deva Prasad Raju B., Mallikarjuna K. Durga Mahalakshmi, ChN.; Sushmal, N.J. Bacopa monniera stabilized silver nanoparticles attenuates oxidative stress induced by aluminum in albino mice. J. Nanosci. Nanotechnol. 2015;15(2):1101–1109. doi: 10.1166/jnn.2015.8995. [http://dx.doi.org/10.1166/jnn.2015.8995]. [PMID: 26353618]. [DOI] [PubMed] [Google Scholar]